Abstract
Numerous studies of ASD have attempted to link behavioral phenotypes to genetic findings, but reliance on cross-sectional behavioral data in samples that span wide age ranges may have limited this endeavor because ASD behaviors are not static within individuals across development. This study uses quantitative methods to describe specific aspects of changes in ASD-related and more general behaviors in order to yield trajectories that could be used in place of single time-point data as behavioral phenotypes in neurobiological studies of both ASD and other overlapping conditions. Building on previous analyses [Anderson et al, 2014], we examined trajectories of parent-reported social-communication deficits, social adaptive functioning, and two types of repetitive behaviors, repetitive sensory motor (RSM) behaviors and insistence on sameness (IS) behaviors, in a relatively large sample of participants referred for possible autism at age 2 years and followed into young adulthood (n=85). A strength of this sample was the diverse range of outcomes, including young adults with intellectual disability and persistent ASD related difficulties, those with IQs in the borderline or average range who continued to experience functional impairment related to ASD, and a small group of young adults (n=8) with IQs in the average range who were judged to be functioning at typical age appropriate levels at age 19 years, despite a previous childhood diagnosis of ASD.
Keywords: Autism spectrum disorder, behavior phenotype, trajectory, social-communication, repetitive sensory motor, insistence on sameness
Introduction
There have been multiple attempts in recent years to link behavioral phenotypes to molecular genetics within Autism Spectrum Disorders (ASD) [e.g. Brune et al., 2006; Bernier et al., 2014; Hu & Steinberg, 2009; Merikangas et al., 2014]. In behavioral genetics studies, primarily with typical populations, there have been consistent findings of associations between various parent-reported behaviors generally associated with ASD and concordance between identical twins, even those who score at the extremes [Reiersen et al., 2008]. In studies of selected populations with different genetic patterns, a few associations have been found, for example, between 16p11.2 and a shift downward from a normal distribution of IQ [Hanson et al, 2014], as well as between general social communication-behavioral deficits and CHD8 mutations [Bernier et al., 2014] and between ASD traits and Fragile X [Abbeduto, McDuffie, Thurman, 2014]. Findings of links between activation in various areas of brain and behavioral characteristics, including both observational measures of social behavior [Monk et al, 2009] and more general parent-report measures of social-behavioral difficulties [Swartz et al., 2013], have been more prominent, but have been limited by inconsistent replications across measures and areas of the brain [see Di Martino et al., 2014].
One of the most striking and puzzling aspects of ASD is the heterogeneity in outcomes, not just in terms of overall independence, but also in terms of changes over time in behaviors that define ASD [Fountain, Winter, & Bearman, 2012; Richler, Huerta, Bishop, & Lord, 2010; Troyb et al, 2014]. Because these behaviors change over time, it is potentially quite problematic to try to link cross-sectional behavioral data to genetics, since findings of relationships become dependent on the specific point in time at which the behavior was measured. This may help explain why attempts to increase genetic homogeneity via reduced phenotypic heterogeneity have proven less fruitful than had originally been hoped [see Chaste et al., 2014]. However, as we have developed a better understanding of how to characterize at least some of these behavioral clusters that define ASD [Bishop et al., 2013; Lecavalier, 2006; Gotham, Pickles, & Lord, 2012], and as longitudinal data become more available, the possibility of using trajectories of change as behavioral phenotypes becomes an option. The purpose of this paper is to use quantitative methods to describe specific aspects of changes in ASD-related and more general behaviors in a relatively large sample of participants referred for possible autism at age 2 years and followed into young adulthood. The hope is that these trajectories could be used as behavioral phenotypes in neurobiological studies of both ASD and other overlapping conditions.
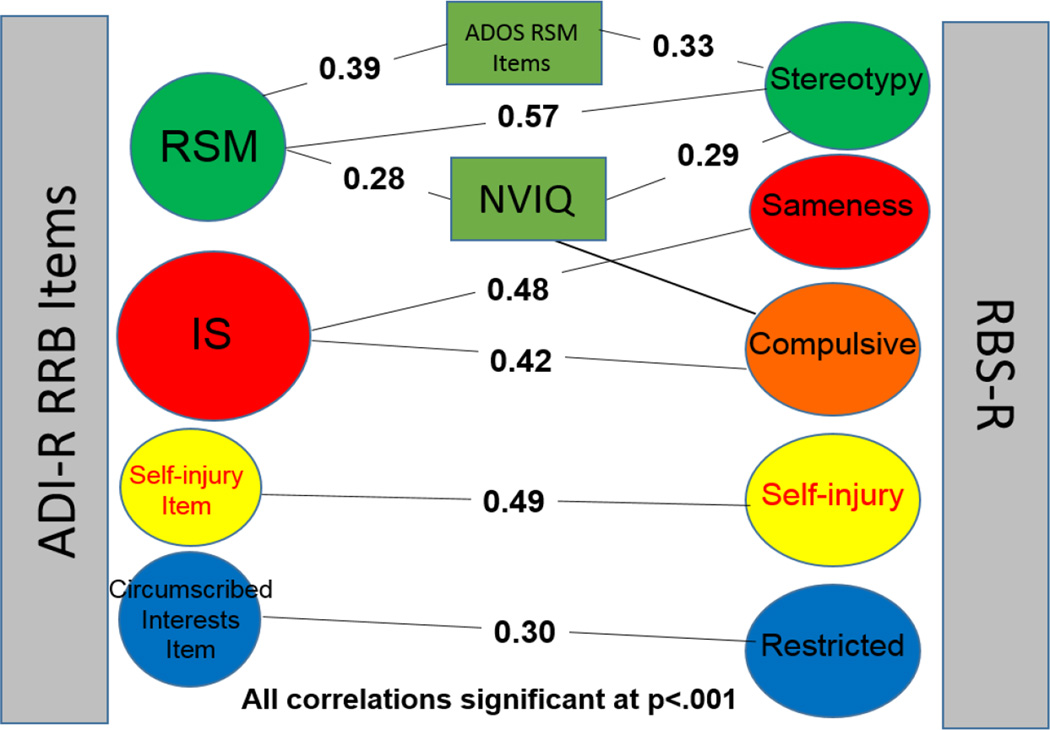
A substantial literature now exists that attempts to define how various repetitive and sensory-related interests and behaviors (referred to as RRBs) cluster in individuals with ASD [Lam, Bodfish, & Piven, 2008; Richler, et al., 2010; Bishop et al., 2006; Szatmari et al., 2006]. Many of these analyses are based on either the Autism Diagnostic Interview-Revised [ADI-R; Rutter, LeCouteur & Lord, 2003] or the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000), but a recent paper [Bishop et al., 2013] showed good convergence between the ADOS, ADI-R and a more scalable, parent questionnaire, the Repetitive Behavior Scale-Revised [RBSR; Bodfish, Symons, & Lewis, 1999]. This is particularly encouraging because the instruments rely on different methods of reporting and yet show good agreement for four areas within RRBs: Repetitive Sensory Motor Behaviors (RSM), Insistence on Sameness (IS), Self-injury and Circumscribed Interests. Figure 1 depicts these relationships.
Figure 1.
Relationships among ADI-R, RBS-R and ADOS items
In the present paper, the focus is on the two areas in RRBs that have emerged as sub-categories in factor analyses of RRBs in multiple samples using different measures, that begin in early development, and that often continue into adulthood: RSM and IS. Previous analyses of these areas in the same sample from ages 2 to 9 had shown three classes of trajectories within ASD for RSM: one group which started high even at age 2 and increased steadily from 2 to 9 (25%); the largest group (50%), which started moderately high and decreased gradually from 2 to 9, and another group that started low and remained low throughout childhood (25%). Within IS, three trajectory groups were identified, a smaller group (13%) with consistently mild symptoms, an increasing group (71%), which showed relatively modest increases between the ages of 2 and 5 years, and a moderate group (16%). In this paper, we look at trajectories of the same clusters into young adulthood.
Identifying measures of social-communicative behavior in ASD that predict outcomes has been more difficult than for RRBs. Parent reports of social communicative behaviors are differentially related to a child’s language level, IQ and more general behavior problems for various instruments [Hus, Bishop, Gotham, Huerta, & Lord, 2013; Hus, & Lord, 2014; Charman et al., 2007] and so, in analyses, these factors must be taken into account. Different ADOS trajectories in Social Affect have been identified in the present sample up to age 12, with nearly 80% of the sample remaining at the same level of severity from age 2 onward into adolescence [Gotham et al., 2012]. In the present paper, we look both at a Social Communication Deficits trajectory from the ADI-R from 2 to 19 years, and also at changes in the social skills domain of the Vineland Adaptive Behavior Scales as a measure of social adaptation [Klin et al., 2007]. We use independent measures of outcome at age 19, based on directly assessed intelligence and interviews (ADOS), self and parent-reported use of support services, as well as clinician judgment to create trajectory groups with markedly different functioning as young adults [See Anderson, Liang & Lord, 2014].
Materials and Methods
Participants were consecutive referrals of children under 37 months old with suspected ASD or a non-ASD developmental disorders to four North Carolina-based, state-funded autism centers (n = 113) and a specialty autism clinic at the University of Chicago (n = 79). Of the 213 original participants, three-quarters received ASD diagnoses at the initial age 2 visit [Anderson et al., 2007]. By the age 19 assessment, two thirds (n=142) of the original sample and their families were still participating to some extent, with 120 participating in the age 19 in-person assessment. Attrition was not related to gender, diagnosis, gender, or IQ at the initial assessment, but African American families with less education were lost to the study at a higher rate than Caucasian families and families with more education.
This study includes all 85 youths (92% male) who were diagnosed with an ASD in early childhood and seen at age 19. The average ages at the first and last assessments were 2 years, 5 months (SD = 0.43) and 19 years, 1 month (SD = 1.08) respectively. Ethnic minorities, most of whom were African American, accounted for 24% of the sample, with a mix of children from rural and urban areas (North Carolina = 49%; Chicago = 51%). Participants with profound cognitive impairment (nonverbal IQ <25 at age 2) who received non-ASD diagnoses at age 2 but later received ASD diagnoses were excluded from the current analyses.
Procedures
Children and families completed a battery of face to face diagnostic and psychometric tests when the children were 2, 3, 5, 9 and 19 years, free of charge. Additional time points (e.g., ages 11 and 14) were available for some measures (VABS/Vineland II), and some children were seen at slightly different or additional time points due to follow up visits. At each face-to-face assessment, with the exception of age 3, an overall best estimate consensus diagnosis of autism spectrum disorder, other non-ASD diagnosis, or typical development/no diagnosis was based on all available information obtained during the assessment. At the age 19 assessment, a ‘typical’ diagnosis required overall global functioning in the normal range in terms of a clinical judgment using social adjustment, restricted and repetitive behaviors, independence, and comorbid symptoms [see Anderson et al., 2014]. Details about the measures administered as part of the larger study, including all those considered in making best-estimate diagnoses, are described in other reports [e.g., Lord et al., 2006, Anderson et al., 2014]. Below we provide descriptions of the measures used in the current analyses.
Measures
The Autism Diagnostic Interview-Revised [ADI-R; Rutter, Le Couteur, Lord, 2003] is a semi-structured standardized parent interview designed to differentiate children with ASD from those with non-ASD developmental disorders. The diagnostic algorithm uses scores based on historical information (i.e., “Most abnormal 4–5” scores or “Ever” scores), but because this study was focused on trajectories, we constructed scores for each time point based on current behaviors. As shown in Table I, a social-communication score for each time point was calculated by summing the “Current” item scores in the Nonverbal Communication and Reciprocal Social Interaction Domains of the ADI-R algorithm for all items that were included in interviews at each time of administration (ages 2, 3, 4, 5, 9, 19). For example, eye contact was not included because it is not coded for older children or adults as a “current behavior”, and verbal items were not included because not all participants were verbal at all time points. Current social-communication deficit scores had a possible range of 0–30, where higher scores indicated greater abnormality. Two separate repetitive behavior scores were calculated for each time point, one representing repetitive sensory motor (RSM) behaviors, and one representing insistence on sameness (IS) behaviors (see Richler et al., 2010). RSM and IS scores had possible score ranges from 0–10 and 0–8, respectively, where higher scores indicated greater abnormality.
Table I.
Current ADI-R Symptom Domains (Based on “Current” item scores; scores of 3 converted to 2)
Social Communication (15 items; possible score range from 0–30)
|
Repetitive Sensory Motor (5 items; possible score range from 0–10)
|
Insistence on Sameness (4 items, possible score range from 0–8)
|
Verbal IQ (VIQ) and nonverbal IQ (NVIQ) scores were derived from various psychometric tests following a pre-determined hierarchy of difficulty and appropriateness (see Anderson et al., 2014). Social adaptation was assessed using the Vineland Adaptive Behavior Scales or Vineland II [Sparrow, Cicchetti, & Balla, 2005], a standardized, semi-structured, parent interview which yields domain scores in the areas of communication, daily living skills, and social skills, as well as an adaptive behavior composite. We used the standard score for social skills as a measure of social adaptation [Klin et al, 2007].
Analyses
Building on previous analyses (Anderson et al, 2014), the sample was divided into three groups based on IQ, diagnostic symptoms and independent functioning at age 19: ASD and VIQ<70 (n=53), ASD and VIQ≥70 (n=24), and Very Positive Outcome (VPO) (n=8). Growth curve analyses with SAS MIXED procedure was used to examine changes in ADI-R social-communication, ADI-R Repetitive Sensory Motor (RSM), ADI-R Insistence on Sameness, Verbal IQ, Nonverbal IQ, and Vineland Adaptive Behavior Composite standard scores for the three groups. A separate intercept and slope was calculated for each child as a control for high correlations among repeated measures on the same individuals over time. The three groups were compared with respect to relative starting point at age 2 (intercept) and rate and pattern of change (linear and quadratic slopes). Other covariates included age at testing; VIQ was run as a covariate for all the non-IQ measures and was significant for each of them (Vineland Social Adaptation, ADI-R Social Communication Deficits, ADI-R RSM) except IS, but did not change the slopes or any of the group differences and so is not represented further. Other measures that were included in previous analyses as covariates (gender, maternal education, ethnicity, occurrence of seizures, any psychopharmacological medication use), but had little effect (i.e., no evidence of main effects or interactions approaching significance; Anderson, Maye & Lord, 2011) were not included in order to maintain a reasonable number of parameters, given the sample sizes.
The estimates for both the covariance and beta parameters were obtained by restricted maximum likelihood methods so that results would be less biased [Verbeke & Molenberghs, 2000]. To test for group differences in slopes and intercepts, we used t statistics for each parameter, calculated as the ratio of the parameter estimate divided by the standard error. To examine whether rate of change in each measure over time differed significantly from 0, we used t tests for linear combinations of variables representing slopes.
Effect size for changes in mean scores over time was calculated using the standardized mean difference (SMD) method: SMD = (Time 1 behavior score – Time 2 behavior score)/pooled SD [Cohen, 1988]. We used the widely accepted guidelines of Cohen (1988) for interpreting the effect size, where 0.2 is small, 0.5 is medium, and 0.8 is large. Effect sizes in the present study were generally large, in part because variances were very low within each group. The fact that the variances were so low is also important in terms interpreting the validity of these three subgroups.
Results
Previous Analyses
Previous analyses of the same sample included χ Squares to test for differences in means [Anderson et al, 2014]. The three groups did not differ in age at first or last testing, site, ethnicity, percent males, marital status of caregivers, maternal education, diagnosis in preschool years (autism, PDD-NOS) or seizures (ever). Participants in the ASD IQ<70 were more likely to have taken psychometric medications (68%) and to have received early intervention between ages 2 and 3 (93%) compared to the ASD IQ≥ 70 (38% medication; 54% early intervention) versus the VPO group (none ever medicated; 100% early intervention). At age 2, there were differences between the ASD IQ<70 group and the two other, higher IQ groups in verbal and nonverbal IQ, adaptive scores, ADI-R social communication scores, RSM and IS, but these variables did not differ between the ASD ≥ 70 group and VPO. VPO was used as the reference group in the following analyses in order to compare the difference between a very positive outcome and continued functioning limited by ASD, with or without intellectual disabilities.
Trajectories in intellectual functioning
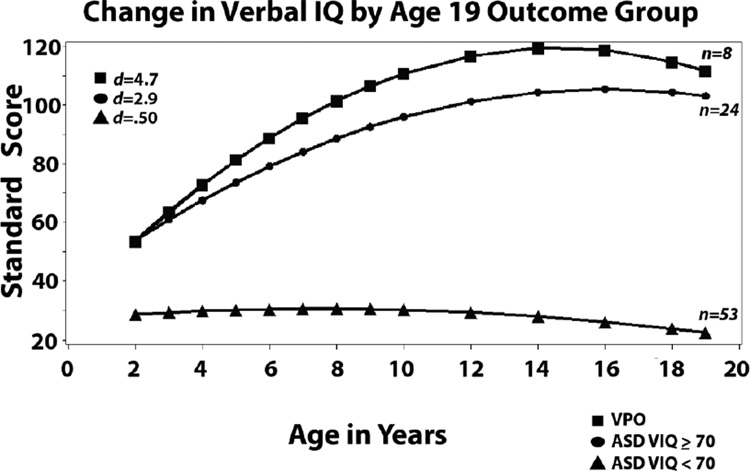
As shown in Table II and Figure 2, not surprisingly given the groupings we created, the two higher IQ groups (M VIQs of 53; M NVIQ’s 81–83) differed from the less cognitively able group (ASD VIQ<70) on VIQ (M VIQ = 29) and NVIQ (M NVIQ =61) at age 2. Trajectories for VIQ for the two more able groups were quadratic, reflecting steady, quite remarkable improvements in verbal reasoning skills beginning at age 2 and continuing into the teen years followed by stable functioning at or above average levels. The difference in trajectory (age X group: b=0.11, SE=.06) between the VPO group and the ASD VIQ ≥70 group approached significance, at p<.10, which, given the small sample sizes, is a call for consideration of a possible effect in further studies, but needs to be replicated. Mean VIQ for the less cognitively able group (ASD VIQ < 70) did not change significantly from 2 to 19. It is important to note that the IQ differences at outcome are tautological because we defined the groups in part on the basis of verbal IQ. What is interesting here are the trajectories from early development, which were striking in their degree of improvement beginning from age 2 for about a third of our sample.
Table II.
Changes in Cognitive and Adaptive Abilities from Age 2 to 19 by Outcome Group
| Predictors | Verbal IQ | Nonverbal IQ | Social Adaptation |
|---|---|---|---|
| Coefficient (S.E.) | Coefficient (S.E.) | Coefficient (S.E.) | |
| Fixed Effects | |||
| Intercept | 53.65 (5.99) *** | 82.74 (5.26) *** | 64.37 (2.58) *** |
| Age at Testing | .87 (.11) *** | .16 (.04) *** | .31 (.07) *** |
| Group: | |||
| Very Positive (VPO)1 |
------ | ------ | ------ |
| VIQ ≥ 70 ASD | .30 (6.95) | 1.37 (6.09) | 4.34 (3.02) |
| VIQ ≤ 70 ASD | −25.01 (6.44) *** | −22.03 (5.64)*** | −2.12 (2.78) |
| Linear Slopes: | |||
| Age* VPO | ------ | ------ | ------ |
| Age* VIQ ≥ 70 ASD |
−.26 (.13) * | −.06 (.04) * | −.34 (.09)*** |
| Age* VIQ ≤ 70 ASD |
−.82 (.12)*** | −.29 (.04)*** | −.78 (.08)*** |
| Quadratic Slopes:3 | |||
| Age2 | −.29 (.05)*** | ------2 | −.07(.03)* |
| Age2* VPO | ------ | ------ | ------ |
| Age2* VIQ ≥ 70 ASD |
.11 (.06) + | ------ | .01 (.04)** |
| Age2* VIQ ≤ 70 ASD |
.25 (.05)*** | ------ | .03 (.04)*** |
| Variance | Variance | Variance | |
| Random Effects | |||
| Intercept | 24.73*** | 159.31*** | 12.87* |
| Slope | .26*** | .004** | .002*** |
N=85
p<.001,
p<.01,
p<.05,
p < .10
Dashes indicate reference group.
Dashes indicate parameter was omitted from final model.
Quadratic slope parameters are multiplied by 100.
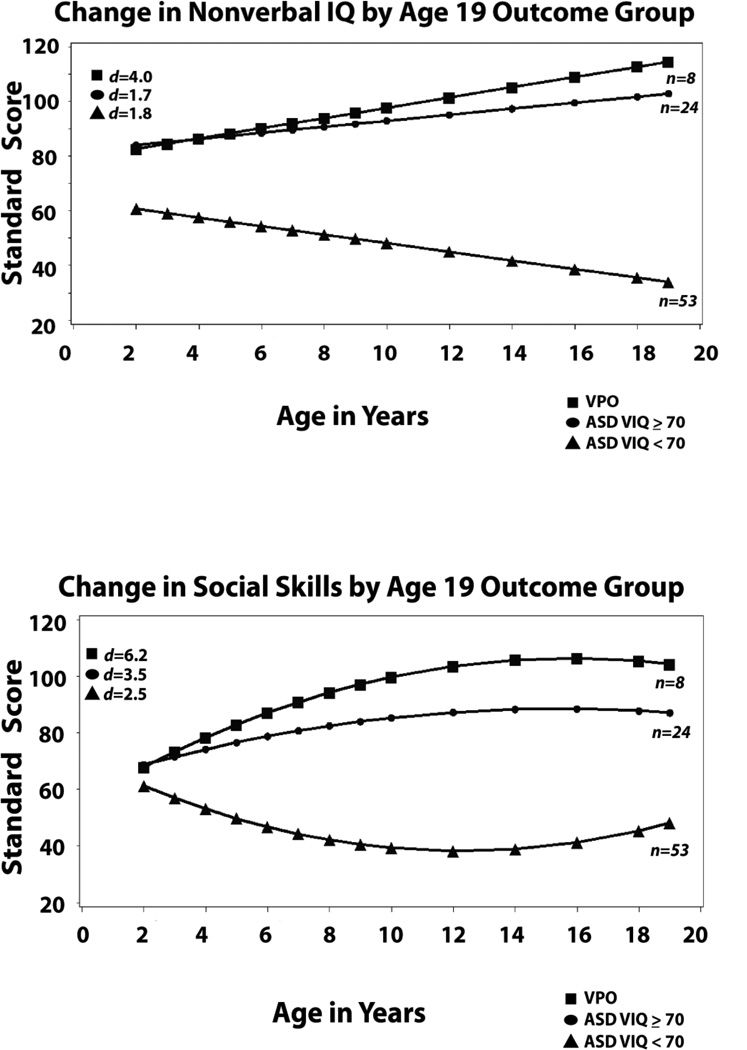
Figure 2.
Developmental trajectories from 2 to 19 grouped by outcome
Trajectories for nonverbal IQ followed a linear pattern, significantly different from 0 for all three groups. Slopes again differed for the two cognitively able groups and the less cognitively able participants, with steady increases over time for both the VPO and ASD VIQ ≥ 70 groups up to scores at average or above, and steady decreases for the ASD VIQ<70 group, with means moving from the mild range of intellectual disability to the moderate to severe range of intellectual disability [see Bishop, Farmer, & Thurm, 2014].
Social Adaptation scores (Vineland II VABS social domain)
Most interesting about the adaptive scores was that there was no group difference at intercept at age 2 at all, despite the relatively large differences on other measures and quadratic changes in all three groups. Not surprising, given our definition of outcome in terms of independence, are the relatively high (i.e., average range) adaptive scores of the VPO. However, what was surprising is the slope of the increase to a mean of 97 by age 9 and 101 at 19 from a much lower mean score at age 2 (SS = 65). In contrast, the other more cognitively able group (ASD VIQ ≥ 70) made much slower progress from the same starting point to a mean of 80 at age 9 and 78 at 19. The change for the ASD VIQ<70 group was quite different, with steady decreases in standard scores into elementary school years and then a gradual increase into young adulthood. These patterns were all significantly different from each other and from no change. Although VIQ was a significant covariate, it did not change the slopes or the group differences.
Changes in ADI-R social-communication scores
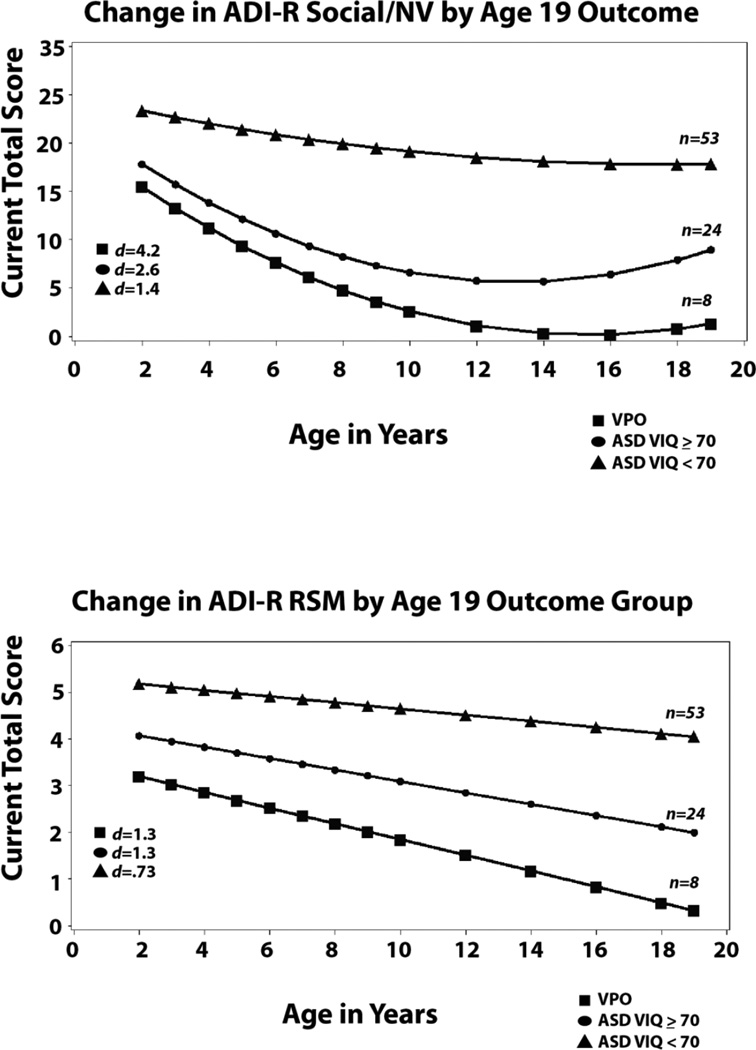
The ADI-R social-communication domain scores ranged from 0 to 30 possible, with higher scores indicating more abnormality (see Table I). As shown in Table III, intercepts for the less able group differed from VPO and the more cognitively able group did not differ from VPO scores at 2; scores for the less cognitively able group decreased gradually following a quadratic pattern. Scores for the more cognitively able ASD group also decreased significantly but followed a linear pattern and were significantly different from the quadratic pattern of the VPO participants. It appears that the divergence of the VPO group and the more cognitively able group who continued to have ASD occurred later, after age 9, compared to the scores for the Vineland Social Adaptation. Because we do not have ADI-R scores between 9 and 19, we cannot say when this change occurred for this measure.
Table III.
Changes in ASD Core Features from Age 2 to 19 by Outcome Group
| Predictors |
ADI-R Social Communication |
ADI-R RSM | ADI-R IS |
|---|---|---|---|
| Coefficient (S.E.) | Coefficient (S.E.) | Coefficient (S.E.) | |
| Fixed Effects | |||
| Intercept | 15.48 (1.86) *** | 3.20 (.75) *** | 1.28 (.51) * |
| Age at Testing | −.19 (.04) *** | −.01 (.0) ** | −.00 (.00) |
| Group: | |||
| Very Positive (VPO)1 |
------ | ------ | ------ |
| VIQ ≥ 70 ASD | 2.34 (2.17) | .87 (.87) | .79 (.59) |
| VIQ ≤ 70 ASD | 7.90 (2.00) *** | 1.99 (.80)*** | .35 (.55) |
| Linear Slopes: | |||
| Age* VPO | ------ | ------ | ------ |
| Age* VIQ ≥ 70 ASD |
.10 (.05) * | .00 (.00) | .00 (.00) |
| Age* VIQ ≤ 70 ASD |
.13 (.05)** | .01 (.01)+ | .01 (.003)** |
| Quadratic Slopes:3 | |||
| Age2 | .06 (.01)** | ------2 | ------2 |
| Age2* VPO | ------ | ------ | ------ |
| Age2* VIQ ≥ 70 ASD |
.01 (.02) | ------ | ------ |
| Age2* VIQ ≤ 70 ASD |
−.04 (.02)* | ------ | ------ |
| Variance | Variance | Variance | |
| Random Effects | |||
| Intercept | 13.04*** | 3.07*** | 1.31*** |
| Slope | .0002 | .0001* | .0002*** |
N=85
p<.01,
p<.05,
p < .10
Dashes indicate reference group.
Dashes indicate parameter was omitted from final model.
Quadratic slope parameters are multiplied by 100.
Changes in ADI-R Repetitive Behaviors (RRBs)
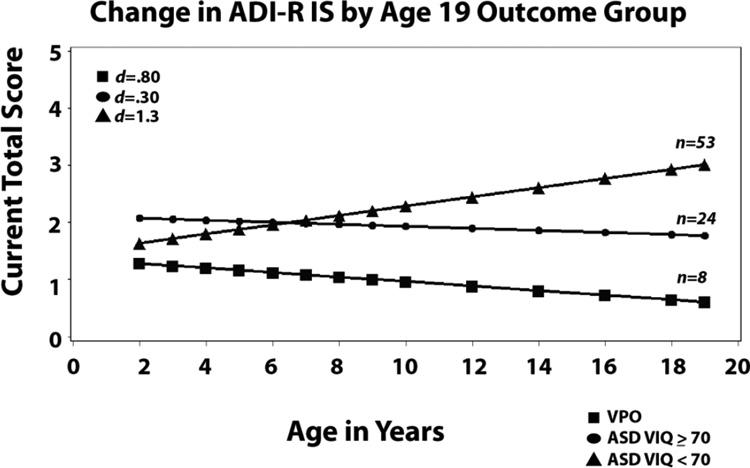
Two subdomains of RRBs, Repetitive Sensory Motor behaviors (RSM) and Insistence on Sameness (IS) based on “current items” from each administration of the ADI-R were analyzed separately (see Table I). For RSM, there were significant differences at intercept between VPO who had the fewest RSM symptoms and ASD VIQ<70 who had the most. As shown in Table III and Figure 2, linear slopes for all three groups indicated very gradual declines. Changes in these behaviors were much different than the quite marked changes in social communication deficits and social adaptation and for VIQ and NVIQ for both of the higher ability groups.
For Insistence on Sameness (IS), the only slope that changed significantly was for the participants with ASD VIQ<70, where IS symptoms increased over time. There were no differences in intercept at 2, but differences widened across the three groups as the children grew older. These groups were significantly different in other analyses [Anderson et al., 2014] using slightly different items (for those analyses, we used all relevant items available at 2 and 3 for RRBs and then a different set at 19; rather than the single set of items available at all ages used here)
Discussion
The present results offer geneticists and neuroscientists a number of behavioral phenotypes that may provide a new approach to finding meaningful associations between behavior and outcomes in ASD. We hope that this approach may also be useful to studies of the genetics of disorders that overlap with ASD, including individuals who do not necessarily meet standard ASD criteria.
One value of these trajectories is to help us determine which behaviors can be sensibly treated as categorical and to identify those that are so continuous that division into subgroups is arbitrary or inappropriate. Analyses of the distinctiveness of trajectories based on outcome provide us with evidence that, at least by school age, simple bimodal groupings of children with ASD can be created using verbal IQ, nonverbal IQ, social adaptive skills and parent-reported social-communication deficits. The trajectories for more cognitively able and less cognitively able children with ASD in these groups are clearly different from each other, though sometimes linear (NVIQ, ADI-R RSM and IS) and sometimes quadratic (VIQ, Vineland Social Adaptation, ADI-R Social Deficits). In addition, for Social Adaptation, Social-Communication Deficits and both forms of repetitive behavior, Repetitive Sensory Motor and Insistence on Sameness, trajectories from preschool to adulthood were different for the small group of participants with Very Positive Outcomes compared to young adults with ASD who also had quite strong cognitive abilities. This offers the possibility of treating the VPO as a behavioral phenotype within ASD. Individuals with early diagnoses of ASD who have Very Positive Outcomes comprise a group that is not only defined by cognitive strengths, but also by early [in language; Tek, Mesite, Fein, Naigles, 2014, in RSM; Anderson et al, 2014;] and later [in social behavior and insistence on sameness; Troyb et al, 2014] trajectories of improvement. How many other potential behavioral phenotypes, based on outcome, will eventually be defined for ASD, likely depends on the samples that we study and the variables we happen to measure. In any case, it is encouraging that there is a statistically differentiable group of young adults with VPOs who have different trajectories on standardized measures, such as IQ and adaptive functioning, as well as social communication skills, than most individuals with ASD with higher IQ.
These and other analyses of both the same data and other samples indicate that significant changes in parent-reported and directly observed behavior occur in individuals with ASD even into later adolescence and adulthood [Anderson et al, 2011; Howlin, Moss, Savage, Rutter, 2013; Smith, Maenner, Seltzer, 2012]. Therefore, attempts to link behavioral phenotypes with genetics may benefit from moving beyond cross-sectional data. In the present study, several of the trajectories were clearly differentiated by age 9, suggesting that for IQ, social adaptation, and RSM, longitudinal data from early into middle childhood may be sufficient to find effects. More detailed analysis of changes in receptive and expressive language in the same sample [Pickles, Anderson, Lord, 2014] found that, though there was one small group of “late bloomers” whose trajectories changed between 3 and 5, overall, there was little change in language trajectory after age 5. This is not to say that there were not later changes in language development, but rather that there were no changes in relative level of delay after preschool years. On the other hand, the Fountain et al (2012) study, with a much larger sample, but with much more restricted data, did find a significant number of what they called “ bloomers” who had changes slightly later in preschool and early school age years. This calls attention to limitations in the present study, which include a sample skewed to more frequently having intellectual disability compared to more recent U.S. samples [Center for Disease Control and Prevention, 2014], as well as our relatively small sample sizes here, particularly for the Very Positive Outcome group.
One explanation for why IQ often emerges as being associated with genetics findings is because of its relative stability in school age populations compared to other behavioral constructs (though even IQ is not entirely stable; Bishop, Farmer, Thurm, 2014). Other characteristics such as onset of seizures or attainment of early milestones are also appealing in this regard, though we have learned over the years that parent retrospective reports of these phenomena are also subject to developmental factors and a potential source of misinterpretation [Hus, Taylor, & Lord; Jones et al, 2014]. From the data presented here, however, it is clear that attempts to draw links between genetics and behaviors described for children across a wide range of ages, without some attention to trajectory and level of development within individual children, may be misleading in a way that controlling for age effects at the time of data collection cannot address. This could also be why measures of whether RRBs ever occurred, as reported in the ADI-R, compared to current reports, has been more commonly used in linkage studies. Nevertheless, because probands are still of different ages and at different points in trajectories in those studies, the difficulty is not completely avoided even when using a historical measure [Jones et al, 2014]. In a previous paper about a different sample [Bishop, Richler, & Lord, 2006], we proposed that it was not necessarily as important to know IF a behavior was present (e.g., RSM or IS), but rather WHEN it was present, because of the clear interaction with NVIQ and age that exists for RRBs. For example, repetitive sensory motor behaviors in an intellectually able young child with autism may have a different meaning than repetitive sensory motor behavior that persists in an older child with or without significant delays.
Limited sample size and specific characteristics of this sample, in terms of including only children who were referred for possible autism at age 2, mean that it will be important to assess the degree to which similar trajectories are found in other populations. Because we grouped the sample by outcome, the “end point” of the VPO group was predetermined, which also means that the slopes were different than previous analyses where we grouped children by how their trajectories clustered without specifying an endpoint [Hus Bal, Kim, Cheong & Lord, 2015; Gotham et al, 2012; Richler et al, 2010]. What these trajectories do offer, however, are potential baselines to which individual slopes can be compared. It is possible that being “off” any of the usual trajectories for children with ASD, with or without intellectual disability, might be particularly useful as a unique phenotype for children with specific genetic or other etiologies. To this end, further refinement of the ASD diagnostic domains of social-communication or RRBs into more specific areas of impairment will be useful for understanding where the trajectories of children with non-ASD diagnoses, such as language disorders, intellectual disability, or Attention Deficit/Hyperactivity Disorder, meaningfully diverge from children with ASD of the same age and level of cognitive ability.
A number of recent papers have described different trajectories in early development of ASD [Chawarska et al., 2014; Lord, Luyster, Guthrie, & Pickles, 2012; Luyster, Powell, Tager-Flusberg, & Nelson, 2014; Mayo, Chlebowski, Fein & Eigsti, 2013; Yoder, Stone, Walden, & Malesa, 2009]. These papers have primarily begun documenting autism-related behaviors in children under age 2 and followed them into preschool years. They have shown that trajectories in very young children are even more variable within shorter periods of time than across much longer age spans. One difference is that most of these children did not have intellectual disabilities and many had milder symptoms than the two year-olds in our sample. One major conclusion is that the cases of “regressive autism” are not as well defined as previously expected, and that the course of early development in ASD is better characterized by variation in timing of the acquisition of prosocial behaviors, the emergence of “positive” autism symptoms, including repetitive sensory motor behaviors, plateaus in the communicative development, and fairly frequent loss of social attention and engagement [Ozonoff et al, 2010]. Nevertheless, other aspects of variation in these trajectories [Charwarska et al., 2014], particularly if they are shown to interact with responsiveness to treatment, are also of interest in terms of plasticity and neurobiological function.
In summary, our findings offer a number of potential behavior phenotypes for geneticists and other neurobiologists interested in autism and related disorders and developmental delays. Early trajectories in verbal IQ and nonverbal IQ are associated with what, by adulthood and even by middle childhood, will be very significant differences in functioning, but which do not account completely for differences in outcome and ASD. Linear trajectories in repetitive sensory motor behaviors also separate individuals with ASD with higher cognitive abilities by adulthood from those with lower IQs. Trajectories of change from preschool to school age years in social adaptation, and in social deficits and insistence on sameness into teen years and young adulthood, are related to significant differences in independent function and lack of comorbidity, at least with a sample of individuals with early diagnoses of ASD and no intellectual disability in adulthood. As we showed earlier in Figure 1, we are also hopeful, at least for restricted and repetitive behaviors, that measures less time consuming and difficult to administer than the ADI-R, such as the RBS-R [Bodfish Symons & Lewis,1999], may present more scalable opportunities to measure such changes. The Vineland Adaptive Behavior Scales (Sparrow et al, 2005) also offers a parent reported measure of social adaptation that can provide critical information about social development. This information is not the same as a detailed description of autism-specific social deficits, such as in the ADI-R, but is very useful. The Autism Diagnostic Observation Schedule [ADOS 2: Lord et al, 2000], because it is not dependent on parent report and it is much less affected by level of verbal and cognitive function, does offer this potential [Gotham et al, 2012] but is limited as well by the need for administration by a trained examiner. To date, other potentially useful instruments describing social and communicative functioning in ASD and related disorders, such as the Social Responsiveness Scale, 2nd Edition [SRS 2: Constantino & Gruber. 2012] and the Children’s Communication Checklist-2 [Norbury, Nash, Baird, Bishop, 2004], have not yet been shown to be sufficiently specific in describing social function to be valid measures of social skills or deficits [Aldridge, Gibbs, Schmidhofer, Williams, 2012; Charman et al, 2007; Hus et al, 2013]. It has been much more difficult to find instruments that provide empirically supported factors of social communication and restricted, repetitive behavior in ASD samples that are not highly correlated with other, than we would have expected [Berument et al.,1999; Frazier et al., 2014], so we cannot just choose items that sound like they correspond to the different domains and assume that they are independent of the other domain, age, IQ or behavior problems [Hus et al, 2013]. On the other hand, given the scalability of instruments such as the SRS and CCC-2, it is certainly worth testing if they have value in representing trajectories in more general behavior dysfunction and delay that, in themselves, may be quite important. Thus, there is much to be learned in the future, but also, we hope, some clear directions for use of behavioral phenotypes now.
Acknowledgments
This research was supported by 5R01MH081873-05 and Simons Foundation CRA #336363 to Catherine Lord and MCHB R40MC274730100 to Somer Bishop. We thank the families and participants in the longitudinal study for their patience and time and graciousness. We also thank Melanie Cabrera, Amarelle Hamo, Marcella Bello and Caroline Carberry for help with the manuscript preparation and Shanping Qiu for data support. Dr. Bishop receives payment for workshops related to the ADOS and ADI-R and Dr. Anderson serves as a consultant to Western Psychological Services regarding translations of the ADOS.
Footnotes
Dr. Lord receives royalties from Western Psychological Services for publication of the ADI-R, SCQ and ADOS/ADOS-2 and Dr. Bishop receives royalties from Western Psychological Services for publication of the ADOS-2; all royalties received related to this project were given to a not for profit agency per agreement with Weill Cornell and University of Michigan Conflict of Interest Review.
References
- Abbeduto L, McDuffie A, Thurman AJ. The fragile X syndrome-autism comorbidity: what do we really know? Front Genet. 2014;5(355):1–10. doi: 10.3389/fgene.2014.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge FJ, Gibbs VM, Schmidhofer K, Williams M. Investigating the clinical usefulness of the Social Responsiveness Scale (SRS) in a tertiary level, autism spectrum disorder specific assessment clinic. J Autism Dev Disord. 2012;42(2):294–300. doi: 10.1007/s10803-011-1242-9. [DOI] [PubMed] [Google Scholar]
- Anderson DK, Liang JW, Lord C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J Child Psychol Psychiatry. 2014;55(5):485–494. doi: 10.1111/jcpp.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DK, Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Welch K, Pickles A. Patterns of growth in verbal abilities among children with autism spectrum disorder. J Consult Clin Psychol. 2007;75(4):594–604. doi: 10.1037/0022-006X.75.4.594. [DOI] [PubMed] [Google Scholar]
- Anderson DK, Maye MP, Lord C. Changes in maladaptive behaviors from midchildhood to young adulthood in autism spectrum disorder. Am J Intellect Dev Disabil. 2011;116(5):381–397. doi: 10.1352/1944-7558-116.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier R, Glozio C, Xiong B, Stessman HA, Coe BP, Penn O, Witherspoon K, Gerdts J, Baker C, Vulto-van Silfhout AT, Schuurs-Hoeijmakers JH, Fichera M, Bosco P, Buono S, Alberti A, Failla P, Peeters H, Steyaert J, Vissers Le, Francescatto L, Mefford HC, Rosenfeld JA, Bakken T, O’Roak BJ, Pawlus M, Moon R, Shendure J, Amaral DG, Lein E, Rankin J, Romano C, de Vries BB, Katsanis N, Eichler EE. Disruptive CHD8 Mutations Define a Subtype of Autism Early in Development. Cell. 2014;158(2):263–276. doi: 10.1016/j.cell.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: Diagnostic validity. Br J Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Bishop SL, Farmer C, Thurm A. Measurement of nonverbal IQ in autism spectrum disorder: scores in young adulthood compared to early childhood. J Autism Dev Disord. 2014 doi: 10.1007/s10803-014-2250-3. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SL, Hus V, Duncan A, Huerta M, Gotham K, Pickles A, Kreiger A, Buja A, Lund S, Lord C. Subcategories of restricted and repetitive behaviors in children with autism spectrum disorders. J Autism Dev Disord. 2013;43(6):1287–1297. doi: 10.1007/s10803-012-1671-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SL, Richler J, Lord C. Association between restricted and repetitive behaviors and nonverbal IQ in children with autism spectrum disorders. Child Neuropsychol. 2006;4(5):247–267. doi: 10.1080/09297040600630288. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Symons FW, Lewis MH. The Repetitive Behavior Scale, Western Carolina Center Research Reports. 1999 [Google Scholar]
- Brune C, Kim SJ, Salt J, Leventhal B, Lord C, Cook E. 5-HTTLPR genotype-specific phenotype in children and adolescents with autism. Am J Psychiatry. 2006;163(12):2148–2156. doi: 10.1176/ajp.2006.163.12.2148. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63(2):1–21. [PubMed] [Google Scholar]
- Charman T, Baird G, Simonoff E, Loucas T, Chandler S, Meldrum D, Pickles A. Efficacy of three screening instruments in the identification of autistic-spectrum disorders. Br J Psychiatry. 2007;191:554–59. doi: 10.1192/bjp.bp.107.040196. [DOI] [PubMed] [Google Scholar]
- Chaste P, Klei L, Sanders SJ, Hus V, Murtha MT, Lowe JK, Willsey AJ, Moreno-De-Luca D, Yu TW, Fombonne E, Geschwind D, Grice DE, Ledbetter DH, Mane SM, Martin DM, Morrow EM, Walsh CA, Sutcliffe JS, Lese Martin C, Beaudet AL, Lord C, State MW, Cook EH, Jr, Devlin B, Jr, et al. Biol Psychiatry. 2014 In Press. [Google Scholar]
- Chawarska K, Shic F, Macari S, Campbell DJ, Brian J, Landa R, Hutman T, Nelson CA, Ozonoff S, Tager-Flusberg H, Young GS, Zwaigenbaum L, Cohen IL, Charman T, Messinger DS, Klin A, Johnson S, Bryson S. 18-Month Predictors of Later Outcomes in Younger Siblings of Children With Autism Spectrum Disorder: A Baby Siblings Research Consortium Study. J Am Acad Child Adolesc Psychiatry. 2014;53(12):1317–1327. doi: 10.1016/j.jaac.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Constantino JN, Gruber CP. Social Responsiveness Scale-Second Edition (SRS-2) Torrance, CA: Western Psychological Services; 2012. [Google Scholar]
- Di Martino A, Yan CG, Li Q, Denio E, Castellanos FX, Alaerts K, Anderson JS, Assaf M, Bookheimer SY, Dapretto M, Deen B, Delmonte S, Dinstein I, Ertl-Wagner B, Fair DA, Gallagher L, Kennedy DP, Keown CL, Keysers C, Lainhart JE, Lord C, Luna B, Menon V, Minshew NJ, Monk CS, Mueller S, Muller RA, Nebel MB, Nigg JT, O’Hearn K, Pelphrey KA, Peltier JS, Wenderoth N, Wiggins JL, Mostofsky SH, Milham MP. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Molecular Psychiatry. 2014;19:659–667. doi: 10.1038/mp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain C, Winter AS, Bearman PS. Six developmental trajectories characterize children with autism. Pediatrics. 2012;129(5):e1112–e1120. doi: 10.1542/peds.2011-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, Ratliff KR, Gruber C, Zhang Y, Law PA, Constantino JN. Confirmatory factor analytic structure and measurement invariance of quantitative autistic trains measured by the social responsiveness scale-2. Autism. 2014;18(1):31–44. doi: 10.1177/1362361313500382. [DOI] [PubMed] [Google Scholar]
- Gotham K, Pickles A, Lord C. Trajectories of autism severity in children using standardized ADOS scores. Pediatrics. 2012;130(5):e1278–e1284. doi: 10.1542/peds.2011-3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson E, Nasir RH, Fong A, Lian A, Hundley R, Shen Y, Wu BL, Hoim IA, Miller DT 16p11.2 Study Group Clinicians. Cognitive and behavioral characteristics of 16p11.2 delection syndrome. J Dev Behav Pediatr. 2010;31(8):649–57. doi: 10.1097/DBP.0b013e3181ea50ed. [DOI] [PubMed] [Google Scholar]
- Howlin P, Moss P, Savage S, Rutter M. Social outcomes in mid- to later adulthood among individuals diagnosed with autism and average nonverbal IQ as children. J Am Acad Child Adolesc Psychiatry. 2013;52(6):572–81. doi: 10.1016/j.jaac.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Hu VW, Steinberg ME. Novel clustering of items from the Autism Diagnostic Interview-Revised to define phenotypes within autism spectrum disorders. Autism Res. 2009;2(2):67–77. doi: 10.1002/aur.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V, Bishop S, Gotham K, Huerta M, Lord C. Factors influencing scores on the social responsiveness scale. J Child Psychol Psychiatry. 2013;54(2):216–24. doi: 10.1111/j.1469-7610.2012.02589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus Bal V, Kim SH, Cheong D, Lord C. Daily Living Skills in individuals with ASD from 2 to 22. Autism; 2015. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V, Lord C. The Autism Diagnostic Observation Schedule, Module 4: Revised Algorithm and Standardized Severity Scores. J Autism Dev Disord. 2014;44(8):1996–2012. doi: 10.1007/s10803-014-2080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, Risi S, Wexler D, Anderson D, Corsello C, Pickles A, Lord C. How interview questions are placed in time influences caregiver descriptions of social communication symptoms on the ADI-R. J Child Psychol Psychiatry. 2014 doi: 10.1111/jcpp.12325. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Saulnier CA, Sparrow SS, Cicchetti DV, Volkmar FR, Lord C. Social and communication abilities and disabilities in higher functioning individuals with autism spectrum disorders: The Vineland and the ADOS. J Autism Dev Disord. 2007;37(4):748–759. doi: 10.1007/s10803-006-0229-4. [DOI] [PubMed] [Google Scholar]
- Lam KS, Bodfish JW, Piven J. Evidence for three subtypes of repetitive behavior in autism that differ in familiality and association with other symptoms. J Child Psycho Psychiatry. 2008;49(11):1193–1200. doi: 10.1111/j.1469-7610.2008.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecavalier L. Behavioral and emotional problems in young people with pervasive developmental disorders: Relative prevalence, effects of subject characteristics, and empirical classification. J Autism Dev Disord. 2006;36(8):1101–1114. doi: 10.1007/s10803-006-0147-5. [DOI] [PubMed] [Google Scholar]
- Lord C, Luyster R, Guthrie W, Pickles A. Patterns of developmental trajectories in toddlers with autism spectrum disorder. J Consult Clin Psychol. 2012;80(3):477–89. doi: 10.1037/a0027214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Arch Gen Psychiatry. 2006;63(6):694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Luyster RJ, Powell C, Tager-Flusberg H, Nelson CA. Neural measures of social attention across the first years of life: Characterizing typical development and markers of autism risk. Dev Cogn Neurosci. 2014;8:131–143. doi: 10.1016/j.dcn.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo J, Chlebowski C, Fein DA, Eigsti IM. Age at first words predicts cognitive ability and adaptive skills in children with ASD. J Autism Dev Disord. 2013;43(2):253–64. doi: 10.1007/s10803-012-1558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas AK, Segurado R, Heron EA, Anney RJ, Paterson AD, Cook EH, Pinto D, Scherer SW, Szatmari P, Gill M, Corvin AP, Gallagher L. The phenotypic manifestations of rare genic CNVs in autism spectrum disorder. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.150. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risis S, Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47(2):764–72. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury CF, Nash M, Baird G, Bishop D. Using a parental checklist to identify diagnostic groups in children with communication impairment: a validation of Children’s Communication Checklist—2. Int J Lang Commun Disord. 2004;39(3):345–64. doi: 10.1080/13682820410001654883. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, Rogers SJ, Rozga A, Sangha S, Sigman M, Steinfeld MB, Young GS. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry. 2010;49(3):256–66. [PMC free article] [PubMed] [Google Scholar]
- Pickles A, Anderson DK, Lord C. Heterogeneity and plasticity in the development of language: a 17-year follow-up of children referred early for possible autism. J Child Psychol Psychiatry. 2014;55(12):1354–62. doi: 10.1111/jcpp.12269. [DOI] [PubMed] [Google Scholar]
- Reiersen AM, Constantino JN, Grimmer M, Martin NG, Todd RD. Evidence for shared genetic influences on self-reported ADHD and autistic symptoms in young adult Australian twins. Twin Res Hum Genet. 2008;11(6):579–85. doi: 10.1375/twin.11.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richler J, Huerta M, Bishop SL, Lord C. Developmental trajectories of restricted and repetitive behaviors and interests in children with autism spectrum disorders. Dev Psychopathol. 2010;22:55–69. doi: 10.1017/S0954579409990265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter ML, LeCouteur A, Lord C. Autism Diagnostic Interview –Revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Smith LE, Maenner MJ, Seltzer MM. Developmental trajectories in adolescents and adults with autism: the case of daily living skills. J Am Acad Child Adolesc Psychiatry. 2012;51(6):622–31. doi: 10.1016/j.jaac.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales. 2nd. Circle Pines, MN: American Guidance Service; 2005. [Google Scholar]
- Swartz JR, Wiggins JL, Carrasco M, Lord C, Monk CS. Amygdala habituation and prefrontal functional connectivity in youth with autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2013;52(1):84–93. doi: 10.1016/j.jaac.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari P, Georgiades S, Bryson S, Zwaigenbaum L, Roberts W, Mahoney W, Goldberg J, Tuff L. Investigating the structure of the restricted, repetitive behaviours and interests domain of autism. J Child Psychol Psychiatry. 2006;47(6):582–590. doi: 10.1111/j.1469-7610.2005.01537.x. [DOI] [PubMed] [Google Scholar]
- Tek S, Mesite L, Fein D, Naigles L. Longitudinal analyses of expressive language development reveal two distinct language profiles among young children with autism spectrum disorders. J Autism Dev Disord. 2014;44(1):75–89. doi: 10.1007/s10803-013-1853-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyb E, Orinstein A, Tyson J, Eigsti IM, Naigles L, Fein D. Restricted and repetitive behaviors in individuals with a history of ASDs who have achieved optimal outcomes. J Autism Dev Disord. 2014;44(12):3168–84. doi: 10.1007/s10803-014-2182-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer-Verlag; 2000. [Google Scholar]
- Yoder P, Stone WL, Walden T, Malesa E. Predicting social impairment and ASD diagnosis in younger siblings of children with autism spectrum disorder. J Autism Dev Disord. 2009;39(10):1381–1391. doi: 10.1007/s10803-009-0753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]