Abstract
Granulysin, a cationic protein expressed by human natural killer cells and cytotoxic T lymphocytes, is a mediator for drug-induced Stevens-Johnson syndrome and graft-versus-host disease. Some 15 kDa granulysin are processed into 9 kDa forms and sequestered in cytolytic granules, while others are constitutively secreted into body fluids. Both 9 and 15 kDa granulysin have been shown to be a serum marker for cell-mediated immunity. Furthermore, 15 kDa is able to activate monocyte differentiation. However, its antimicrobial properties have not been clearly addressed. Here, we report a novel method to prepare both the soluble 9 and 15 kDa granulysin and show that the 15 kDa form is more effective than the 9 kDa form in exerting specific antimicrobial activity against Pseudomonas aeruginosa within a range of few micromolars. We also show that the 15 kDa granulysin is able to hyperpolarize the membrane potential and increase membrane permeability of treated bacteria. Interestingly, the bactericidal activity and membrane permeability of the granulysins were markedly reduced at lower pH (pH 5.4) as a result of probable increase in hydrophobicity of the granulysins. Additionally, we’ve also shown the granulysin to inhibit biofilm formation by P. aeruginosa. These results suggest that the 15 kDa granulysin exhibits a novel mechanism in bacteria killing in a way that’s different from most antimicrobial peptides. Our novel granulysin preparation methodology will be useful for further study of action mechanisms of other antimicrobial, cytotoxic and immunomodulating properties in granulysin-mediated diseases.
Introduction
Worldwide emergence of multiple-drug-resistant (MDR) bacteria has led to the urgent need for the development of new antibiotics [1]. Antimicrobial peptides and proteins (AMPs) are important components of the host innate defensive system that inhibits invading pathogens [2–4]. As a result, AMPs are considered to be potent alternatives to conventional antibiotics. Although AMPs possess diverse secondary structures, their surfaces are amphipathic with cationic and hydrophobic residues on opposite sides within a hydrophobic environment. These AMPs have various modes of actions that differ from conventional antibiotics [4–6]. The positive-charged residues of AMPs promote selectivity for negatively charged components on microbial surfaces, whereas the hydrophobic regions of AMPs facilitate the interactions with the bacterial membrane. Disruption of membrane integrity and subsequent condensation of cytoplasmic components usually occur in the AMP-treated bacteria while inhibition of intracellular components can also occur without membrane damage [4, 7]. Various targets of AMPs have been extensively proposed, such as the outer surface lipid, outer membrane protein, inner membrane, inner membrane protein, intracellular protein, and nucleic acids [7–10].
Granulysin is a cytotoxic and proinflammatory protein produced by the human cytolytic T-lymphocytes and natural killer cells [11]. It is co-expressed with perforin and granzymes in cytolytic granules and released via receptor-mediated granule exocytosis [12]. The 9 kDa granulysin is derived from a 15 kDa precusor by truncation of both the N- and C-termini [13]. The recombinant 9 kDa granulysin has been shown to kill a variety of microbes such as bacteria, fungi, yeast, parasites and several tumor cell lines [11]. It can also kill extracellular Mycobacterium tuberculosis (MTB) by inducing lesions on the cell surface and damaging the intracellular MTB with perforin [12]. It also acts as a chemo-attractant for T lymphocytes, monocytes, and other inflammatory cells, and activation function in the expression of a number of cytokines, including RANTES/CCL5, MCP-1, MCP-3, MIP-1α/CCL3, IL-10, IL-1, IL-6 and IFN-α [14]. Additionally, it has been shown to be relevant to other clinical diseases including infection, cancer, transplantation, autoimmunity, skin afflictions and reproductive complications [11]. These reports reveal that granulysin plays an important role in immunomodulation and diseases, and may potentially be a therapeutic target. However, most of the studies have focused on the 9 kDa form instead of the 15 kDa form with the exception that the 15 kDa form has been shown to be an important mediator of drug-induced Stevens-Johnson syndrome and graft-versus-host disease (GVHD) [15–18].
Although the 9 kDa granulysin prepared by denaturation and refolding has been shown to exert antimicrobial activity on Gram-positive bacteria, such as Staphylococcus aureus and Listeria monocytogenes, and Gram-negative bacteria, such as Escherichia coli and Salmonella typhimurium, at the concentration of 10–25 μM [12, 19–21], large-scale production of bioactive soluble protein is still not successfully available due to bactericidal activity and formation of inclusion bodies in E. coli [13, 19, 22]. Furthermore, the 15 kDa form is generally regarded as a precursor form without much cytotoxicity against bacterial and mammalian cells [23, 24]. The commercially-available granulysin containing tags at the termini may be detrimental to protein function [25]. In this study, we have successfully developed an over-expression system in E. coli to produce soluble and bioactive recombinant 15 and 9 kDa forms of granulysin without denaturation/refolding procedures. The 15 kDa granulysin exerts antimicrobial activity preferentially against Pseudomonas aeruginosa and alters the membrane potential and permeability, but does not disrupt structural integrity. However, the antimicrobial activity is affected by salts, divalent cations and changes in pH. Additionally, it also inhibits the viability and formation of biofilms by P. aeruginosa. These results may benefit studies on bactericidal activity and cytotoxicity mediated by granulysin.
Materials and Methods
Cloning, expression and purification of human granulysin
The cDNAs encoding the 15 kDa human granulysin (Asn1-Leu130 from 520 mRNA transcript) and 9 kDa granulysin (Gly1-Arg74) [16] were tagged with DNA fragments containing HindIII-tagged PreScission™ Protease (GE Healthcare, Buckinghamshire, UK) recognition sequence (CTGGAAGTTCTGTTCCAGGGGCCC) and XhoI at the 5’ and 3’ends, respectively, and cloned into pGEM-T (Promega, WI, USA). In addition, DNA fragments containing His×6 peptide and maltose-binding protein (MBP) sequence were also constructed in pGEM-T with NdeI and HindIII at the 5’ and 3’ends, respectively. Both fragments were subsequently subcloned into the expression vector pET-22b (Merck Millipore, Darmstadt, Germany) through the NdeI/HindIII and HindIII/XhoI sites. Both 15 kDa and 9 kDa granulysins were expressed in E. coli BL21-CodonPlus(DE3)-RIL (Agilent, CA, USA) at 30°C overnight in the presence of 0.5 mM isopropyl-β-D-thiogalactopyranoside (IPTG). The crude cell lysate was passed through phosphate cellulose chromatography (P11, Whatman, Kent, England) using a 0.2–1 M NaCl gradient in 20 mM Tris-HCl, pH 7.4 and further purified by a HisTrap™ HP column chromatography (GE Healthcare, Uppsala, Sweden) using a 20–250 mM imidazole gradient in 20 mM HEPES, pH 7.4, 2 M NaCl. The granulysin in the soluble fraction was released and separated from maltose-binding protein by PreScission™ Protease and fast protein liquid gel filtration chromatography (FPLC Superose™ 12, GE Healthcare, Uppsala, Sweden) in 20 mM HEPES, pH 7.4, 0.15 M NaCl, 100 mM imidazole. The granulysins were further purified to homogeneity by HiTrap™ SP FF (GE Healthcare, Uppsala, Sweden) cation-exchange column chromatography using 0.15–1 M NaCl gradient in 20 mM HEPES, pH 7.4, and finally dialyzed against phosphate-buffered saline (PBS), pH 7.4, and stored at -70°C before use. The molecular masses of 9 and 15 kDa granulysins were determined by direct nanospray infusion of protein solutions. The isotopically resolved spectra acquired from orbitrap were further deconvoluted with the Xtract algorithm to determine the molecular weight [26].
Antimicrobial Activity Assay
The Gram-negative bacteria Escherichia coli K-12 (M61655), Pseudomonas aeruginosa PAO1 (ATCC BAA-47™), Klebsiella pneumoniae (ATCC 13884), Salmonella typhimurium (ATCC 14028), Yersinia enterocolitica (ATCC 23715), Serratia marcescens (ATCC 8100) were separately cultured in Luria-Bertani broth (Merck Millipore, Darmstadt, Germany) and plated on Luria-Bertani agar. The Gram-positive bacteria Listeria innocua (ATCC 33090), Staphylococcus aureus (ATCC 6538P) and Enterococcus faecalis (ATCC 29212) were cultured and plated in tryptic soy broth/agar (BD, MD, USA). The bacteria were grown overnight, washed, and diluted 1:500 in 10 mM sodium phosphate, pH 7.5. Forty five μL of bacteria (ca. 1×105 colony-forming unit (cfu)) were mixed with 5 μL of granulysin and incubated at 37°C for 3 hr. Serial dilutions of each granulysin-treated bacteria were prepared and plated for the determination of the remaining cfu. At least three independent experiments were performed for each assay to determine the average value with standard deviation.
Confocal laser scanning microscopy (CLSM) and transmission electron microscopy (TEM)
P. aeruginosa PAO1 cells (ca. 1×107 cfu) were cultured in an 8-well Lab-Tek™ II Chamber Slide™ (Thermo, MA, USA) and incubated with 2 μM 15 kDa granulysin at 37°C for 180 min in 10 mM sodium phosphate, pH 7.5. The cells were fixed with 4% paraformaldehyde, permeabilized by 0.5% Triton X-100, and incubated with anti-granulysin antibody RF10 (MBL, Chicago, USA) (1:1000) and Alexa Fluor 488-conjugated goat anti-mouse secondary antibody (Molecular Probes, OR, USA) (1:1000) for the localization of granulysin. Propidium iodide (Sigma, Missouri, USA) (1:1000) was also employed to stain bacterial DNAs inside the cytosol. After washing with PBS-T (PBS with 1% Tween 20), the images for granulysin (green) and DNA (blue) were taken by LSM 700 confocal laser-scanning microscope (Carl Zeiss, Jena, Germany) and merged [27]. The bacterial cells (ca. 1×107 cfu in 95 μL) were incubated with 2 μM 15 kDa granulysin at 37°C for 3 hr in 10 mM sodium phosphate, pH 7.5, fixed by 4% paraformaldehyde and 1% osmium tetraoxide in 0.1 M cacodylate buffer, pH 7.4, and embedded in epoxy resin. Thin sections were double-stained with uranyl acetate and lead citrate, and the morphologies were observed under JEM 1200-EX transmission electron microscope (JEOL, Tokyo, Japan) [28].
Membrane potential and permeability assays
P. aeruginosa PAO1 cells were collected from mid-log-phase culture, washed in HEPES buffer (5 mM HEPES, pH 7.2, and 20 mM glucose), and re-suspended in the same buffer with the addition of 0.2 mM EDTA to an OD600 of 0.05. The bacteria were incubated with 0.4 μM DiSC3(5) (3,3’-dipropylthiadicarbocyanine iodide, Molecular Probes, OR, USA) in the dark for 2 hr at room temperature with gentle agitation (150 rpm). The osmotic gradient was equilibrated to a final concentration of 0.1 M KCl. The granulysin and SMAP-29 were added to a 200 μL cell suspension in a High Precision Cell cuvette (Hellma Analytics, Mülheim, Germany). The fluorescence intensity was determined by an FP-8500 fluorescence spectrophotometer (Jasco, Tokyo, Japan) with an excitation wavelength of 622 nm and an emission wavelength of 670 nm [28]. Alternatively, the membrane potential of bacteria was also determined by the BacLight™ Membrane Potential Kit (Molecular Probes, OR, USA) as described by the manufacturer. Microbes were collected, washed and re-suspended to an concentration of 1×106 per mL in PBS and stained with 30 μM DiOC2(3) (3,3’-diethyloxa-carbocyanine iodide) for 5 min. The samples were then incubated with antimicrobial agents for 30 min at room temperature and flow cytometric measurements were performed immediately thereafter by FACSCanto flow cytometer (BD Biosciences, CA, USA). The mean value of red fluorescence intensity was divided by that of green fluorescence and expressed in fluorescence ratio (red/green) [29]. With respect to permeability, microbes were collected, washed and re-suspended in 10 mM sodium phosphate, pH 7.5, to an OD600 of 0.05. One μM SYTOX® Green (Molecular Probes, OR, USA) was added into a 100 μL cell suspension in a dark 96-well plate for 30 min in the dark before addition of granulysin. The fluorescence intensity of SYTOX® Green bound to cytosolic DNA was determined by a SpectraMax M2 microplate reader (Molecular Devices, CA, USA) with an excitation wavelength of 485 nm and an emission wavelength of 520 nm [30].
Hydrophobicity Assay
A small volume of 8-Anilino-1-naphthalenesulfonic acid (ANS, Sigma Missouri, USA) solution at the concentrations as indicated (0 to 30 μM) was added to a 200 μL granulysin (6 μg/mL) in PBS in the dark at 20°C. The fluorescence of ANS was determined by a temperature-controlled FP-8500 fluorescence spectrophotometer (Jasco, Tokyo, Japan), which was excited at 370 nm and measured between 400 nm to 600 nm. The free form ANS exerts a maximum emission at 520 nm, however, it exhibits a blue shift of 470 nm once it is bound to hydrophobic protein [26].
Biofilm formation assay and viability assay
One hundred μL of P. aeruginosa PAO1 (ca. 1×108 cfu/mL) was distributed in flat-bottom 96-well microplate in the presence of granulysin in M63 minimal medium at 37°C for 24 hr. Subsequently, the planktonic cells were removed and the adherent biofilms were washed three times with water. One hundred and twenty five μL of 0.1% (w/v) crystal violet (Sigma, Missouri, USA) was added and incubated for 30 min. Excess crystal violet was removed and washed three times with water. The crystal violet remained in the biofilms were dissolved in 125 μL of 95% (v/v) ethanol and quantified by SpectraMax M2 microplate reader (Molecular Devices, CA, USA) [31]. Student’s t-test was used to evaluate the significant difference between biofilms with and without AMP treatment and a value of p<0.001 was considered significant. The viabilities of bacteria in the biofilms were assessed using LIVE/DEAD® BacLight™ Bacterial Viability Kit (Molecular Probes, OR, USA). Two hundred μL of P. aeruginosa PAO1 cell suspension (ca. 1×108 cfu/mL) was distributed on chamber slides in the presence of granulysin at the concentration as indicated in M63 minimal medium at 37°C for 24 hr. The planktonic cells were removed and the adherent bacteria in the biofilm were washed three times with PBS and stained with two component dyes (SYTO 9 and propidium iodide in a 1:1 mixture) in PBS according to manufacturer’s instructions. The excitation/emission maxima for these two dyes were 480/500 nm for SYTO 9 live cell staining (green) and 490/635 nm for propidium iodide dead cell staining (red), respectively [31]. Fluorescence images were taken by LSM 700confocal laser-scanning microscope (Carl Zeiss, Jena, Germany).
Results
Preparation of soluble granulysin from E. coli having antimicrobial activity against Pseudomonas aeruginosa
The recombinant human 15 kDa granulysin fused with maltose-binding protein (MBP) was expressed in E. coli and purified to homogeneity by several column chromatographies (S1A–S1D Fig). The method was completely different from previously published reports which exploited the denaturation and renaturation of the protein from inclusion bodies [12, 19–21]. The actual molecular weights of 9 and 15 kDa granulysin were 4 and 6 Daltons less than those of predicted values according to their amino acid compositions, respectively (S1E Fig). These results indicate that the recombinant 9 kDa and 15 kDa granulysin may possess 2 and 3 pairs of disulfide bonds, respectively.
Bactericidal activities of granulysin against P. aeruginosa
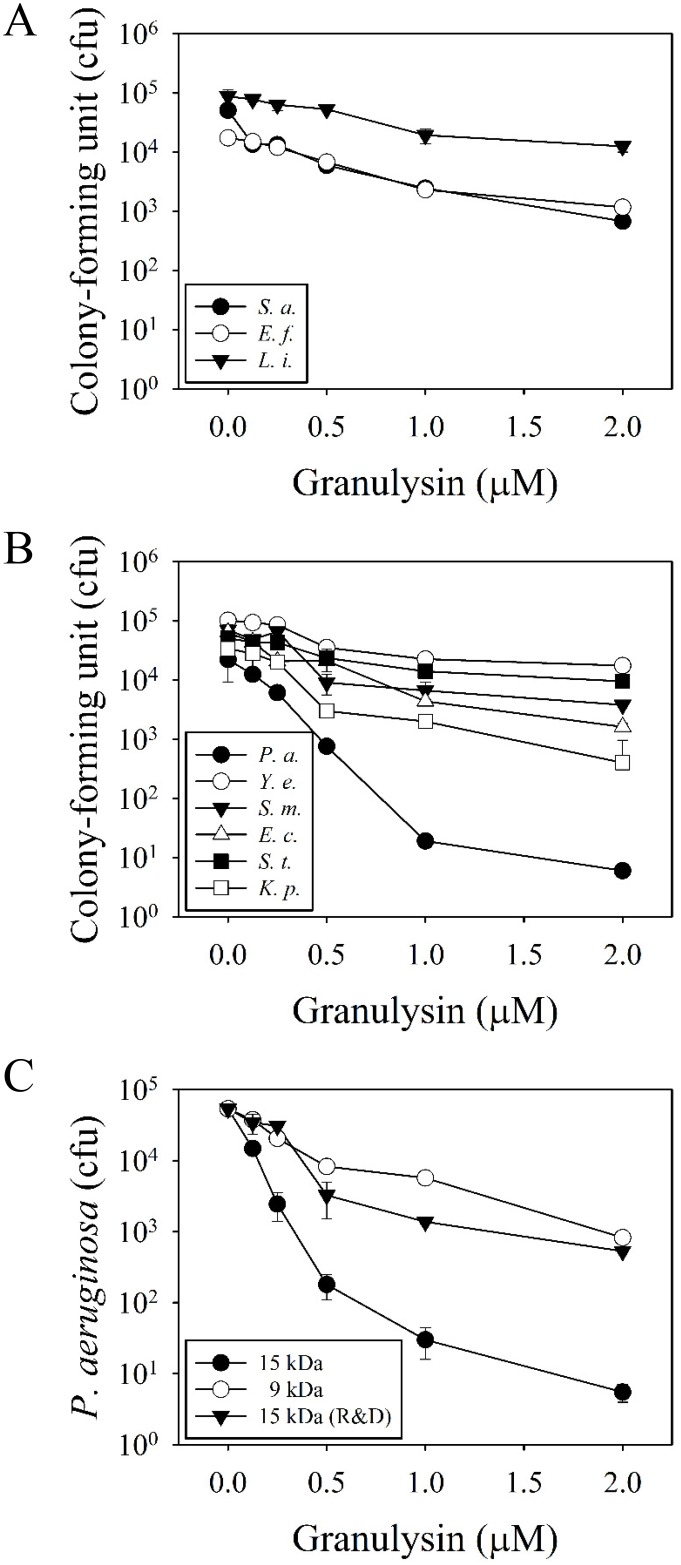
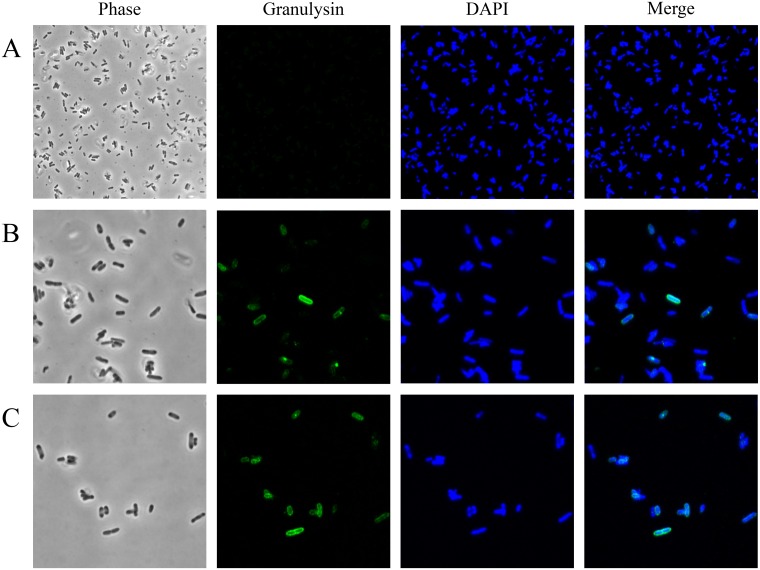
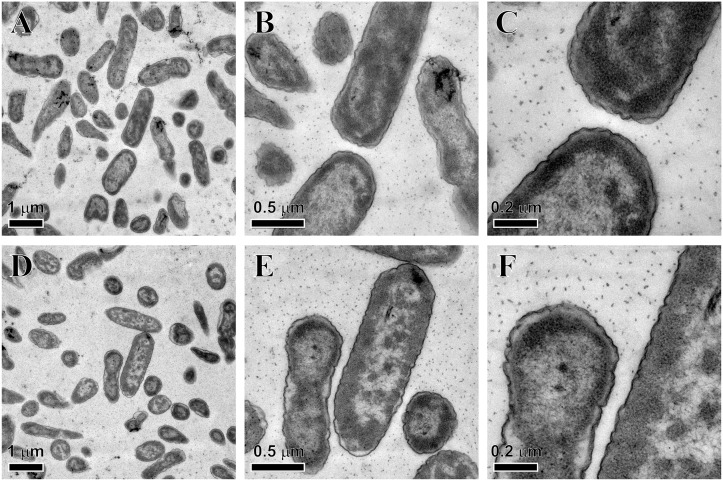
Among the antimicrobial spectrum of 15 kDa granulysin, P. aeruginosa PAO1 was the most susceptible bacteria (104-folds reduction in cfu at 2 μM), while the other bacteria either Gram-positive (S. aureus, E. faecalis and L. innocua) or Gram-negative (Y. enterocolitica, S. marcescens, E. coli, S. typhimurium and K. pneumoniae) were much less susceptible (Fig 1A and 1B). The 15 kDa granulysin was also much more effective than 9 kDa granulysin and the commercially-available 15 kDa granulysin (R&D) in the reduction of cfu (Fig 1C). The P. aeruginosa PAO1 showed resistance to the actions of ampicillin, kanamycin, and vancomycin (unpublished data). We found that the 15 kDa granulysin was only detected at the surface of bacteria, but not in the cytosol because a clearly green-fluorescence zone surrounding the DAPI-stained cytosol in blue was found even after 3 hr treatment (Fig 2). In contrast to most AMPs, the granulysin (2 μM) did not dramatically change the morphology of P. aeruginosa PAO1, by membrane blebbing/damage, cytoplasm condensation and component leakage as demonstrated by transmission electron microscopy (TEM) (Fig 3). These results suggest that granulysin binds to the bacterial surface and triggers a bactericidal pathway rather than entering the cytosol.
Fig 1. Bactericidal activities of granulysin.
Antimicrobial activities of 15 kDa granulysin on Gram-positive (A) and Gram-negative (B) bacteria. Microbes (5–10×104 cfu) were incubated with 15 kDa granulysin at 37°C in 10 mM sodium phosphate, pH 7.5, for 3 hr and plated on agar plates for the determination of remaining cfu. Comparison on the antimicrobial activity against P. aeruginosa among 15 kDa, 9 kDa granulysin and a commercial 15 kDa granulysin (R&D) were also employed (C). S. a., Staphylococcus aureus; E. f., Enterococcus faecalis; L. i., Listeria innocua; P. a., Pseudomonas aeruginosa; Y. e., Yersinia enterocolitica; S. m., Serratia marcescens; E. c., Escherichia coli; S. t., Salmonella typhimurium; K. p., Klebsiella pneumonia.
Fig 2. Localization of 15 kDa granulysin on Pseudomonas aeruginosa PAO1.
Approximately 1×107 microbes were incubated with 2 μM 15 kDa granulysin for 0 min (A), 60 min (B) and 180 min (C), respectively. Immunocytochemistry was performed using RF10 mAb for granulysin staining and DAPI for DNA staining. Images were taken using confocal laser-scanning microscopy (CLSM).
Fig 3. Morphology of Pseudomonas aeruginosa PAO1 after 15 kDa granulysin treatment.
Approximately 1×107 microbes were treated with 2 μM 15 kDa granulysin for 0 min (A-C) and 180 min (D-F) and observed by transmission electron microscopy (TEM).
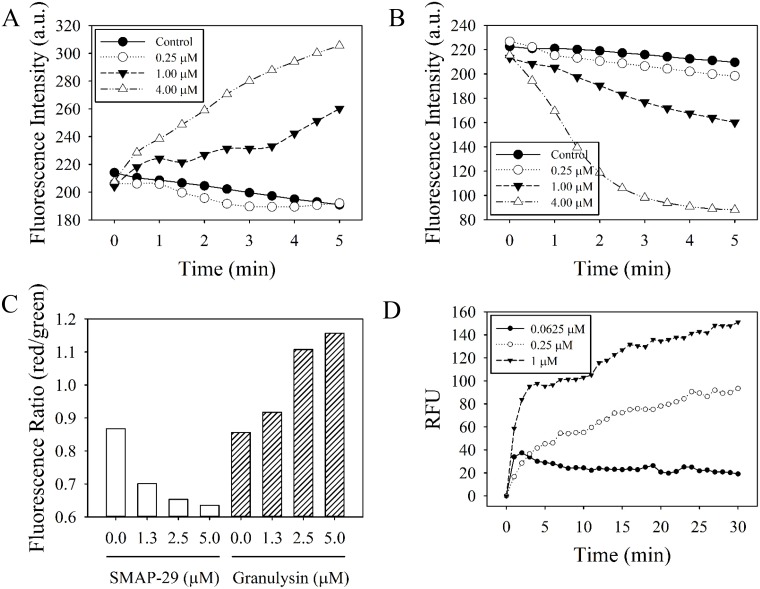
Similar to most AMPs, the sheep myeloid antimicrobial peptide, SMAP-29, induced depolarization of bacterial membrane potential because the DiSC3(5) dye was released into the surrounding medium that caused the increase of fluorescence intensity in a dose-dependent manner (Fig 4A). However, the 15 kDa granulysin hyperpolarized the membrane potential due to fluorescent intensity decrease after granulysin treatment (Fig 4B). The depolarization of membrane potential by SMAP-29 and hyperpolarization by 15 kDa granulysin were further confirmed by another membrane potential indicator DiOC2(3) using flow cytometry. The emission of fluorescence shifted to red as the dye molecules aggregate at higher cytosolic concentrations under higher membrane potentials caused by 15 kDa granulysin, while it shifted to green by SMAP-29 (Fig 4C). The membrane permeability of the bacteria markedly increased a few minutes after granulysin treatment (1 μM) as a result of the green fluorescent SYTOX® Green being internalized into the cytosol and complexed with bacterial DNA (Fig 4D). Taken together, these results demonstrate that granulysin effectively hyperpolarizes membrane potential, increases membrane permeability, and activates the bactericidal pathway.
Fig 4. Effect of 15 kDa granulysin on the membrane potential and permeability of Pseudomonas aeruginosa PAO1.
Membrane potential of SMAP-29- (A) and 15 kDa granulysin- (B) treated microbes were determined in the presence of DiSC3(5). Fluorescence intensity was monitored at an excitation wavelength of 622 nm and an emission wavelength of 670 nm. Data plotted are representative average values of three independent trials. a.u., absorbance unit. Membrane potential of treated microbes were also measured in the presence of DiOC2(3) by flow cytometry (C). Red/green fluorescence ratio was calculated using population mean fluorescence intensities for bacteria in the presence of SMAP-29 or 15kDa granulysin. Membrane permeability of microbes was monitored in the presence of SYTOX™ Green at 485 nm and 520 nm for excitation and emission wavelength, respectively (D). Data plotted are normalized with values of untreated sample and representative average values of three independent trials. RFU, relative fluorescence unit.
Components of sera affecting bactericidal activity of granulysin
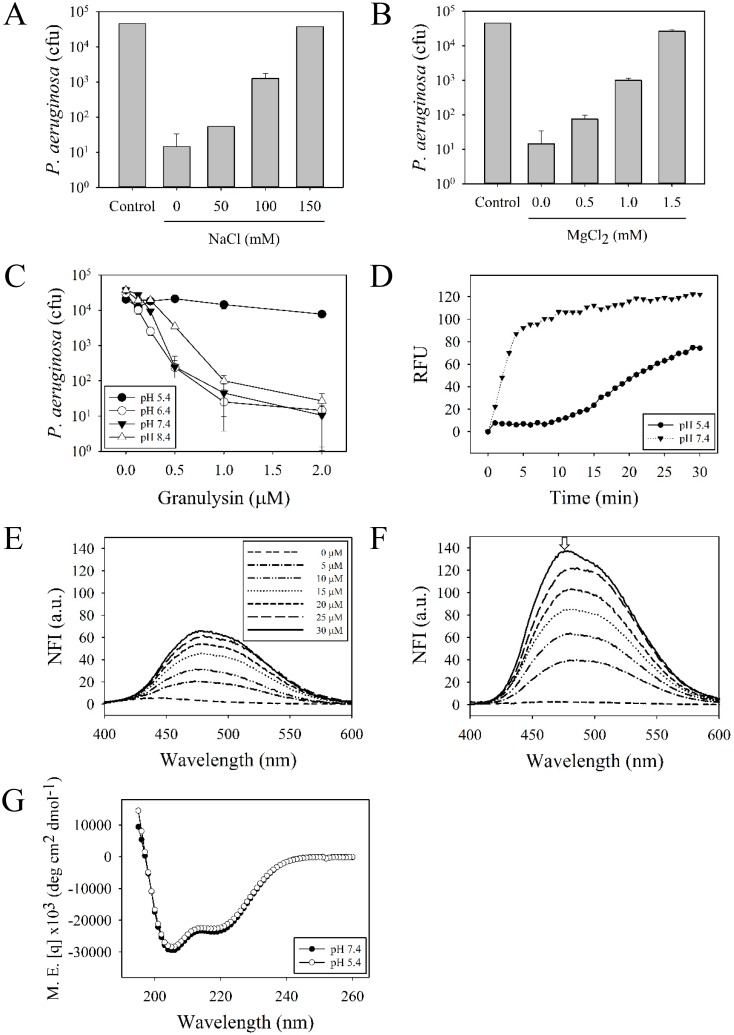
To determine whether the antimicrobial activity of granulysin is still effective in body fluids, other tissues, or affected by components in sera, we examined the factors such as salts, divalent cations and pH values. The results showed that the antimicrobial activity was repressed by 150 mM NaCl or 1.5 mM MgCl2, which is similar to the concentrations in sera (125 mM for NaCl and 1.25 mM for MgCl2) (Fig 5A and 5B). In addition, the granulysin retained its antimicrobial activity close to neutral pH values (6.4 to 8.4), but was inactive at pH 5.4 (Fig 5C). The increase in membrane permeability was also repressed in the acidic environment (pH 5.4) (Fig 5D). These results suggest that granulysin may exert its function in a neutral environment that lacks body fluids. To further investigate the possible effects of an acidic environment on the structure of granulysin, the changes of hydrophobicity were measured by the emission spectra of a hydrophobic fluorescent dye 8-anilino-1-naphthalenesulfonate (ANS). The ANS exhibited an emission maximum at 520 nm in free form at pH 7.4, and a blue shift to 470 nm once it binds to the hydrophobic granulysin at pH 5.4 (Fig 5E and 5F). However, the CD spectra of 15 kDa granulysin in pH 7.4 and 5.4 showed that they possess almost identical secondary structures (Fig 5G). These results suggest that an acidic environment renders granulysin hydrophobic and reduces the bactericidal activity without changing the secondary structure.
Fig 5. Effect of cations and pH on the antimicrobial activity, membrane permeability against Pseudomonas aeruginosa PAO1 and secondary structure of 15 kDa granulysin.
The antimicrobial activities of 2 μM 15 kDa granulysin were determined in the presence of NaCl (A) and MgCl2 (B) at the concentrations indicated. Microbes were incubated with granulysin at different pH values and the remaining colony-forming units were determined (C). SYTOX™ Green was added to monitor the change in bacterial membrane permeability in the presence of 1 μM granulysin at pH 5.4 and pH 7.4, respectively (D). Fluorescence emission spectra of 8-Anilino-1-naphthalenesulfonic acid (ANS) was excited at 370 nm and measured between 400 and 600 nm in the presence of 1 μM granulysin at pH 7.4 (E) and pH 5.4 (F). Arrow indicates the blue shift (470 nm) of emission spectra of ANS-granulysin hydrophobic complex. Data plotted are normalized by the fluorescence intensity of ANS alone at pH 7.4 or pH 5.4, respectively. NFI, normalized fluorescence intensity. Circular dichroism spectrum of 20 μM 15 kDa granulysin in 20 mM HEPES, 50 mM NaCl, pH 7.4 or pH 5.4 (G). M.E., molar ellipticity.
Inhibition of biofilm formation by granulysin
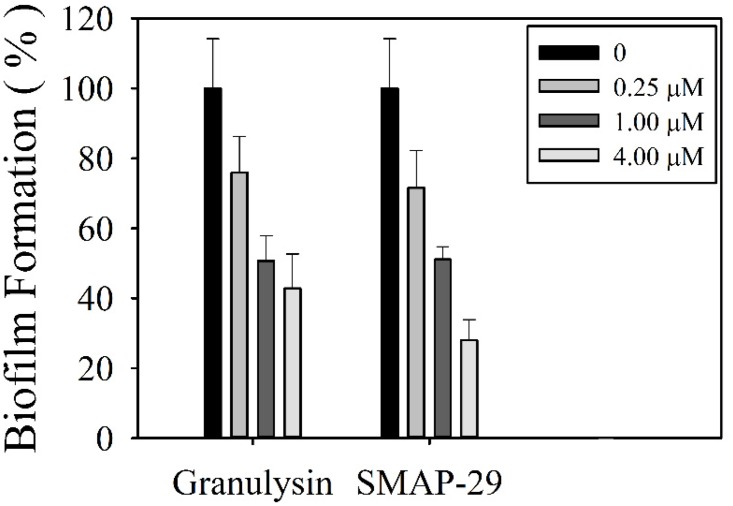
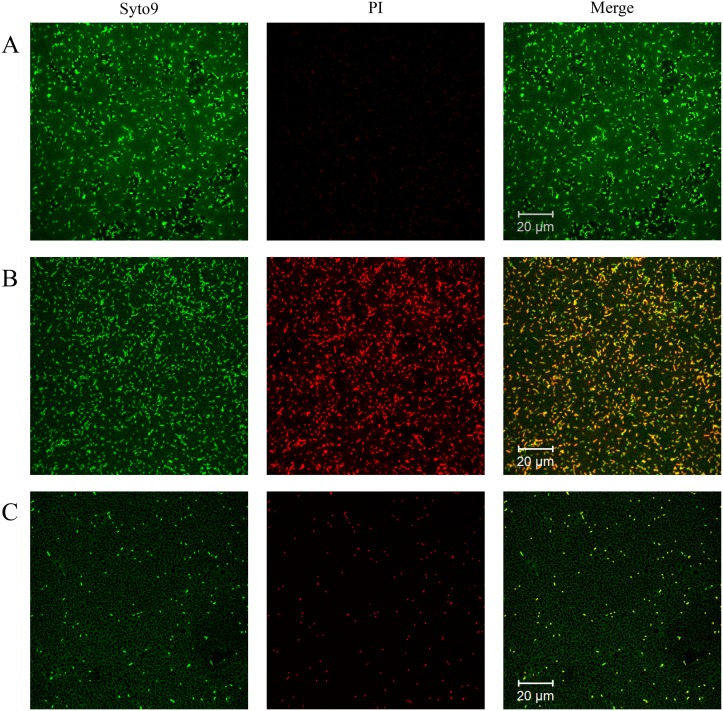
To further evaluate the efficacy of granulysin in the treatment of bacterial infection, the effect of 15 kDa granulysin on the formation of biofilm was examined where the bacteria can be protected from actions of antibiotics and AMPs. Both 15 kDa granulysin and SMAP-29 markedly reduced biofilm formation by P. aeruginosa in M63 minimal medium in a dose-dependent manner (Fig 6). The P. aeruginosa PAO1 was able to grow in a static state and form a homogenous biofilm on chamber slides for 24 hr with robust viability. However, there remained a homogenous biofilm but with less viability in the presence of 0.25 μM granulysin as visualized by CLSM using SYTO-9 and propidium iodide to stain live and dead cells, respectively (Fig 7A and 7B). The remaining bacteria, however, were unable to establish a homogenous biofilm and lost the ability to colonize surfaces in the presence of 4 μM granulysin (Fig 7C). By contrast, 15 kDa granulysin had no effect on the viability of one day-old preformed biofilms (data not shown). These data demonstrate that granulysin is not only able to kill planktonic bacteria but also inhibits biofilm formation under the doses employed as demonstrated by crystal violet staining (Fig 6A and 6B).
Fig 6. Effect of 15 kDa granulysin on the biofilm formation of Pseudomonas aeruginosa PAO1.
Microbes were grown in M63 minimal media in the presence of SMAP-29 and 15 kDa granulysin at 37°C for 24 hr and quantified by crystal violet staining and measured at the absorbance of 600 nm. Error bars represent the standard errors of the means. The asterisks indicate samples that are significantly different from control. (Student’s t-tests; p≤0.01).
Fig 7. Viability of bacteria in the biofilm of Pseudomonas aeruginosa PAO1 after 15 kDa granulysin treatment.
Microbes were visualized by staining with SYTO-9 (green fluorescence for living bacteria) and propidium iodide (red fluorescence for dead bacteria) in the presence of 0 (A), 0.25 μM (B), and 4 μM granulysin (C) at 37°C for 24 hr. Images were obtained by confocal laser scanning microscope (CLSM).
Discussion
In addition to antimicrobial activity, granulysin plays important roles in tissue transplantation and immune-mediated skin diseases [11, 32]. The 15 kDa granulysin has been shown to be responsible for disseminated keratinocyte death in SJS/TEN [16]. It is also significantly elevated and correlate with the severity of GVHD [17].
Progress in research on the cytotoxic mechanisms of granulysin has been hindered by the lack of protein abundance. Currently, the 15 kDa granulysin products are available for purchase from two companies, R&D Systems and Novus Biologicals (Abnova). The protein from R&D Systems contains a 10-histidine tag at the C-terminus and the other from Novus Biologicals contains a glutathione S-transferase (GST) tag at the N-terminus. These added tags may affect their function [25]. Insect cell systems and cell-free E. coli expression systems have also been employed to express the 15 kDa granulysin, but with high cost and poor yield [16, 25]. Alternatively, both 9 and 15 kDa granulysins can be collected from the culture media of Pichia pastoris after secretion, and followed by purification using affinity and cation exchange chromatography. The downside is that the bactericidal activity of 15 kDa granulysin (50–100 μM) is much less effective than that of the 9 kDa granulysin (1–2 μM) [33]. Productions of recombinant cytotoxic protein are usually prepared by the denaturation/refolding of insoluble proteins in the inclusion bodies of E. coli. However, the yield can be limited, the procedure is time-consuming and the antimicrobial activity is low (10–50 μM) [13, 19, 22]. In this study, we successfully constructed an expression vector which contains His×6, maltose binding protein (MBP), PreScission™ protease recognition site, 9/15 kDa granulysin, and expressed the fusion protein in E. coli. The soluble granulysin was released from MBP-fused protein by the PreScission™ protease and purified by a few column chromatography steps. The merit of this study is that no protein denaturation/refolding, animal cell culture and in vitro cell-free synthesis were employed. The recombinant 15 kDa granulysin possesses strong antimicrobial activity against P. aeruginosa at 2 μM.
Both the 9 kDa granulysin refolded from E. coli inclusion bodies, and the 15 kDa granulysin collected from P. pastoris culture media exerted broad antimicrobial spectra, but showed low activity against Gram-positive, -negative bacteria and fungi, like S. aureus, L. monocytogenes, E. coli, S. typhimurium and Candida albicans at 10–50 μM [19–21, 33]. Interestingly, the soluble 15 kDa granulysin prepared from MBP-fused protein in this study exerted strong and specific antimicrobial activity against P. aeruginosa. This may suggest that some specific receptors on P. aeruginosa may be responsible for the recognition of granulysin. The outer membrane lipoprotein OprI of P. aeruginosa is responsible for the recognition of cationic α-helical antimicrobial peptide [26], but it was not recognized by the 15 kDa granulysin because the bactericidal activity of granulysin was not repressed by the presence of excess amounts of recombinant OprI or anti-OprI antibodies (data not shown). O-linked glycosylation was found in both 9 and 15 kDa granulysin obtained from the secreted culture media of P. pastoris, but it was not required for their antimicrobial activities [33]. The molecular weights of 9 and 15 kDa granulysin prepared from E. coli were 8800 and 14952 Daltons, respectively. They were 4 and 6 Daltons less than those of predicted values according to their amino acid sequences. No glycosylation occurred in both forms (S1E Fig). Our results indicate that the recombinant 9 kDa granulysin possesses 2 pairs of disulfide bonds, which is in good agreement with the crystal structure of 9 kDa granulysin, Cys54-Cys117 and Cys81-Cys92 [34]. The 15 kDa granulysin is shown to be tethered by 3 pairs of disulfide bonds, Cys28/Cys30-Cys123, Cys54-Cys117 and Cys81-Cys92.
Depolarization or disruption of cytoplasmic membrane renders the bound fluorescent dye DiSC3(5) to be released into medium leading to increased intensity of fluorescence [35]. Similar to most AMPs, the sheep myeloid antimicrobial peptide (SMAP-29) exhibited membrane potential depolarization (Fig 4A) once the bacterial membrane was permeabilized or disrupted [30]. On the contrary, the 15 kDa granulysin hyperpolarized membrane potential in a dose-dependent manner detected by fluorescence spectrometry and flow cytometry using different dye indicators, DiSC3(5) and DiOC2(3), as shown in Fig 4B and 4C. Although membrane potential depolarization is considered to be an initial event of membrane injury, the hyperpolarization has also been reported as an adaptation related to bacterial viability [24, 29, 36, 37]. Hyperpolarization has also been associated with the formation of superoxide radicals [37], which are implicated in membrane integrity and cell viability [24, 29]. The hyperpolarization of membrane potential induced by the 15 kDa granulysin indicates that it may have a novel bactericidal mechanism which is different from those of conventional AMPs.
The AMPs exert host defense activity against invading pathogens in various environments containing salts, divalent cations, serum proteins and changes in pH. Here, we found the antimicrobial activities of granulysin against P. aeruginosa to be greatly inhibited by the presence of divalent cations or by the acidic environment at pH 5.4 (Fig 5A–5C). The pH values in living organisms usually vary with tissues such as the neutrophil phagolysosomes or inflammatory condition-induced acidic pH values between 4.5 and 6.5 [38]. On the other hand, the bloodstream and mucosal surfaces maintain neutral pH values of 7.2 to 7.5 [39]. The antimicrobial activities of human β-defensin-3 and LL-37 against P. aeruginosa at acidic pH values are less effective than neutral environments [40]. Our results reveal that granulysin may exhibit its bactericidal activity only in neutral environments devoid of body fluids such as the skin.
Amphipathicity of an AMP including hydrophobicity and net charge is crucial for its antimicrobial activity [41, 42]. The hydrophobicity of an amphipathic peptide facilitates its interaction with bacterial hydrophobic membrane. However the positive charges of AMPs promote selectivity for negatively charged components on microbial surfaces. Both properties are essential for its function. The hydrophobicity of a peptide is increased in vitro if the environment becomes hydrophobic, as in the presence of 30~50% trifluoroethanol [43]. The antimicrobial activity of a peptide against P. aeruginosa varies with the extent of hydrophobicity in certain ranges [44, 45]. Our previous results demonstrated that α-helical cationic antimicrobial peptide, SMAP-29, was able to interact with the bacterial outer membrane receptor protein, OprI, and to render it hydrophobic to induced membrane fusion [26]. Interestingly, we found that both membrane permeability and antimicrobial activity of 15 kDa granulysin were markedly inhibited in an acidic environment (pH5.4), which increased the hydrophobicity of granulysin as shown in Fig 5C–5F. These results suggest that the electrostatic interactions between amphipathic AMPs and specific receptors are important prior to fusion with bacterial outer membrane through the hydrophobic sides of AMP.
The 9 kDa granulysin is cytotoxic to tumor cells through the apoptotic pathway by the release of cytochrome c and apoptosis-inducing factor (AIF), and also by activation of caspases and endonucleases [46–50]. However, the cytotoxicity of the 15 kDa granulysin remains controversial. Chung et al. showed that the 15 kDa granulysin, but not 9 kDa granulysin, in blister fluids of patients with SJS/TEN can exert cytotoxic activity against keratinocyte that leads to the severity of the skin-associated disease [16]. However, Clayberger et al. demonstrated that the 15 kDa granulysin from insect cells can induce the differentiation from monocytes to immature dendritic cells (iDC) but without cytotoxicity in U937 tumor cells [23]. The discrepancy in cytotoxicity may be due to the following reasons. First, granulysin may possess selective cytotoxicity toward different cell types. Second, granulysin needs the aid of other components such as perforin, sFasL, and granzyme B, to exert the cytotoxic events although it is the key mediator of cytotoxicity [16].
In this study, we provide a simple way to express and purify soluble 15 kDa granulysin which exhibited specific antimicrobial activity against P. aeruginosa. The 15 kDa granulysin hyperpolarized membrane and permeated the membrane through a novel mechanism to kill bacteria which is different from most AMPs. Our results render the study possible for the action mechanisms of antimicrobial activity and cytotoxicity to different human cell types in transplantation and skin-associated diseases.
Supporting Information
FPLC chromatographies of 15 kDa granulysin by HisTrap™ HP (A), Superose™ 12 (B), and HiTrap™ SP FF (C) column and analysis of proteins from column eluates by 15% SDS-PAGE and Coomassie Blue staining (D). Lane 1, crude lysate; Lane 2, eluates of phosphate cellulose (P11) chromatography; Lane 3, eluates of HisTrap™ HP column chromatography; Lane 4, digestion product of PreScission™ Protease; Lane 5, eluates of Superose™ 12 gel filtration chromatography; Lane 6, eluates of HiTrap™ SP FF column chromatography. Arrow indicates the purified 15 kDa granulysin. The molecular masses of recombinant 9 and 15 kDa granulysin were determined by ESI-MS spectra (E).
(PDF)
Acknowledgments
We thank the Proteomics Core Facility, Flow Cytometry Core Facility and Electron Microscope Core Facility of the Institute of Biomedical Sciences, Academia Sinica, for assistance in liquid chromatography-tandem mass spectrometry analysis, flow cytometric analysis and morphological study, respectively.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a grant from the Academia Sinica Thematic Research Program (AS-102-TP-B04), and funds from Academia Sinica and Ministry of Science and Technology (104-0210-01-09-02), Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gammon K. Drug discovery: Leaving no stone unturned. Nature. 2014;509(7498):S10–2. 10.1038/509S10a . [DOI] [PubMed] [Google Scholar]
- 2.Bowdish DM, Davidson DJ, Hancock RE. A re-evaluation of the role of host defence peptides in mammalian immunity. Curr Protein Pept Sci. 2005;6(1):35–51. . [DOI] [PubMed] [Google Scholar]
- 3.Brown KL, Hancock RE. Cationic host defense (antimicrobial) peptides. Curr Opin Immunol. 2006;18(1):24–30. 10.1016/j.coi.2005.11.004 . [DOI] [PubMed] [Google Scholar]
- 4.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–95. 10.1038/415389a . [DOI] [PubMed] [Google Scholar]
- 5.Boix E, Nogues MV. Mammalian antimicrobial proteins and peptides: overview on the RNase A superfamily members involved in innate host defence. Mol Biosyst. 2007;3(5):317–35. 10.1039/b617527a . [DOI] [PubMed] [Google Scholar]
- 6.Schroder JM, Harder J. Antimicrobial skin peptides and proteins. Cell Mol Life Sci. 2006;63(4):469–86. 10.1007/s00018-005-5364-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3(3):238–50. 10.1038/nrmicro1098 . [DOI] [PubMed] [Google Scholar]
- 8.Huang YC, Lin YM, Chang TW, Wu SJ, Lee YS, Chang MD, et al. The flexible and clustered lysine residues of human ribonuclease 7 are critical for membrane permeability and antimicrobial activity. J Biol Chem. 2007;282(7):4626–33. 10.1074/jbc.M607321200 . [DOI] [PubMed] [Google Scholar]
- 9.Nguyen LT, Haney EF, Vogel HJ. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011;29(9):464–72. 10.1016/j.tibtech.2011.05.001 . [DOI] [PubMed] [Google Scholar]
- 10.Nicolas P. Multifunctional host defense peptides: intracellular-targeting antimicrobial peptides. FEBS J. 2009;276(22):6483–96. 10.1111/j.1742-4658.2009.07359.x . [DOI] [PubMed] [Google Scholar]
- 11.Krensky AM, Clayberger C. Biology and clinical relevance of granulysin. Tissue Antigens. 2009;73(3):193–8. 10.1111/j.1399-0039.2008.01218.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282(5386):121–5. . [DOI] [PubMed] [Google Scholar]
- 13.Pena SV, Hanson DA, Carr BA, Goralski TJ, Krensky AM. Processing, subcellular localization, and function of 519 (granulysin), a human late T cell activation molecule with homology to small, lytic, granule proteins. J Immunol. 1997;158(6):2680–8. . [PubMed] [Google Scholar]
- 14.Deng A, Chen S, Li Q, Lyu SC, Clayberger C, Krensky AM. Granulysin, a cytolytic molecule, is also a chemoattractant and proinflammatory activator. J Immunol. 2005;174(9):5243–8. . [DOI] [PubMed] [Google Scholar]
- 15.Ashokkumar C, Ningappa M, Ranganathan S, Higgs BW, Sun Q, Schmitt L, et al. Increased expression of peripheral blood leukocyte genes implicate CD14+ tissue macrophages in cellular intestine allograft rejection. Am J Pathol. 2011;179(4):1929–38. 10.1016/j.ajpath.2011.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008;14(12):1343–50. 10.1038/nm.1884 . [DOI] [PubMed] [Google Scholar]
- 17.Nagasawa M, Isoda T, Itoh S, Kajiwara M, Morio T, Shimizu N, et al. Analysis of serum granulysin in patients with hematopoietic stem-cell transplantation: its usefulness as a marker of graft-versus-host reaction. Am J Hematol. 2006;81(5):340–8. 10.1002/ajh.20570 . [DOI] [PubMed] [Google Scholar]
- 18.Sarwal MM, Jani A, Chang S, Huie P, Wang Z, Salvatierra O Jr., et al. Granulysin expression is a marker for acute rejection and steroid resistance in human renal transplantation. Hum Immunol. 2001;62(1):21–31. . [DOI] [PubMed] [Google Scholar]
- 19.Ernst WA, Thoma-Uszynski S, Teitelbaum R, Ko C, Hanson DA, Clayberger C, et al. Granulysin, a T cell product, kills bacteria by altering membrane permeability. J Immunol. 2000;165(12):7102–8. . [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Choice E, Kaspar A, Hanson D, Okada S, Lyu SC, et al. Bactericidal and tumoricidal activities of synthetic peptides derived from granulysin. J Immunol. 2000;165(3):1486–90. . [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Zhong C, Shi L, Guo Y, Fan Z. Granulysin induces cathepsin B release from lysosomes of target tumor cells to attack mitochondria through processing of bid leading to Necroptosis. J Immunol. 2009;182(11):6993–7000. 10.4049/jimmunol.0802502 . [DOI] [PubMed] [Google Scholar]
- 22.Rudolph R, Lilie H. In vitro folding of inclusion body proteins. FASEB J. 1996;10(1):49–56. . [PubMed] [Google Scholar]
- 23.Clayberger C, Finn MW, Wang T, Saini R, Wilson C, Barr VA, et al. 15 kDa granulysin causes differentiation of monocytes to dendritic cells but lacks cytotoxic activity. J Immunol. 2012;188(12):6119–26. 10.4049/jimmunol.1200570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yount NY, Kupferwasser D, Spisni A, Dutz SM, Ramjan ZH, Sharma S, et al. Selective reciprocity in antimicrobial activity versus cytotoxicity of hBD-2 and crotamine. Proc Natl Acad Sci U S A. 2009;106(35):14972–7. 10.1073/pnas.0904465106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finn MW, Clayberger C, Krensky AM. Expression and purification of 15 kDa granulysin utilizing an insect cell secretion system. Protein Expr Purif. 2011;75(1):70–4. 10.1016/j.pep.2010.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang TW, Wang CF, Huang HJ, Wang I, Hsu ST, Liao YD. Key Residues of Outer Membrane Protein OprI Involved in Hexamer Formation and Bacterial Susceptibility to Cationic Antimicrobial Peptides. Antimicrob Agents Chemother. 2015;59(10):6210–22. 10.1128/AAC.01406-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen HL, Su PY, Chang YS, Wu SY, Liao YD, Yu HM, et al. Identification of a novel antimicrobial peptide from human hepatitis B virus core protein arginine-rich domain (ARD). PLoS Pathog. 2013;9(6):e1003425 10.1371/journal.ppat.1003425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu X, Shan A, Ma Z, Xu W, Wang J, Chou S, et al. Bactericidal efficiency and modes of action of the novel antimicrobial peptide T9W against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2015;59(6):3008–17. 10.1128/AAC.04830-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanhauteghem D, Janssens GP, Lauwaerts A, Sys S, Boyen F, Cox E, et al. Exposure to the proton scavenger glycine under alkaline conditions induces Escherichia coli viability loss. PLoS One. 2013;8(3):e60328 10.1371/journal.pone.0060328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang TW, Lin YM, Wang CF, Liao YD. Outer membrane lipoprotein Lpp is Gram-negative bacterial cell surface receptor for cationic antimicrobial peptides. J Biol Chem. 2012;287(1):418–28. 10.1074/jbc.M111.290361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasamiravaka T, Vandeputte OM, Pottier L, Huet J, Rabemanantsoa C, Kiendrebeogo M, et al. Pseudomonas aeruginosa Biofilm Formation and Persistence, along with the Production of Quorum Sensing-Dependent Virulence Factors, Are Disrupted by a Triterpenoid Coumarate Ester Isolated from Dalbergia trichocarpa, a Tropical Legume. PLoS One. 2015;10(7):e0132791 10.1371/journal.pone.0132791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krensky AM, Clayberger C. Granulysin: a novel host defense molecule. Am J Transplant. 2005;5(8):1789–92. 10.1111/j.1600-6143.2005.00970.x . [DOI] [PubMed] [Google Scholar]
- 33.Guo Y, Luan G, Shen G, Wu L, Jia H, Zhong Y, et al. Production and characterization of recombinant 9 and 15 kDa granulysin by fed-batch fermentation in Pichia pastoris. Appl Microbiol Biotechnol. 2013;97(17):7669–77. 10.1007/s00253-012-4602-2 . [DOI] [PubMed] [Google Scholar]
- 34.Pena SV, Krensky AM. Granulysin, a new human cytolytic granule-associated protein with possible involvement in cell-mediated cytotoxicity. Semin Immunol. 1997;9(2):117–25. 10.1006/smim.1997.0061 . [DOI] [PubMed] [Google Scholar]
- 35.Wu M, Maier E, Benz R, Hancock RE. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry. 1999;38(22):7235–42. 10.1021/bi9826299 . [DOI] [PubMed] [Google Scholar]
- 36.Sanchez E, Garcia S, Heredia N. Extracts of edible and medicinal plants damage membranes of Vibrio cholerae. Appl Environ Microbiol. 2010;76(20):6888–94. 10.1128/AEM.03052-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spindler EC, Hale JD, Giddings TH Jr., Hancock RE, Gill RT. Deciphering the mode of action of the synthetic antimicrobial peptide Bac8c. Antimicrob Agents Chemother. 2011;55(4):1706–16. 10.1128/AAC.01053-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall JE, Guyton AC. Textbook of medical physiology. 12th ed ed. Philadelphia, Pa.; London: Saunders; 2011. [Google Scholar]
- 39.Waugh A, Grant A, Chambers G. Ross and Wilson anatomy and physiology in health and illness. 11th ed ed. Edinburgh: Churchill Livingstone Elsevier; 2010. [Google Scholar]
- 40.Abou Alaiwa MH, Reznikov LR, Gansemer ND, Sheets KA, Horswill AR, Stoltz DA, et al. pH modulates the activity and synergism of the airway surface liquid antimicrobials beta-defensin-3 and LL-37. Proc Natl Acad Sci U S A. 2014;111(52):18703–8. 10.1073/pnas.1422091112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, Mant CT, Farmer SW, Hancock RE, Vasil ML, Hodges RS. Rational design of alpha-helical antimicrobial peptides with enhanced activities and specificity/therapeutic index. J Biol Chem. 2005;280(13):12316–29. 10.1074/jbc.M413406200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Epand RM, Vogel HJ. Diversity of antimicrobial peptides and their mechanisms of action. Biochim Biophys Acta. 1999;1462(1–2):11–28. . [DOI] [PubMed] [Google Scholar]
- 43.Kozlov SA, Vassilevski AA, Feofanov AV, Surovoy AY, Karpunin DV, Grishin EV. Latarcins, antimicrobial and cytolytic peptides from the venom of the spider Lachesana tarabaevi (Zodariidae) that exemplify biomolecular diversity. J Biol Chem. 2006;281(30):20983–92. 10.1074/jbc.M602168200 . [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Guarnieri MT, Vasil AI, Vasil ML, Mant CT, Hodges RS. Role of peptide hydrophobicity in the mechanism of action of alpha-helical antimicrobial peptides. Antimicrob Agents Chemother. 2007;51(4):1398–406. 10.1128/AAC.00925-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wieprecht T, Dathe M, Beyermann M, Krause E, Maloy WL, MacDonald DL, et al. Peptide hydrophobicity controls the activity and selectivity of magainin 2 amide in interaction with membranes. Biochemistry. 1997;36(20):6124–32. 10.1021/bi9619987 . [DOI] [PubMed] [Google Scholar]
- 46.Anderson DH, Sawaya MR, Cascio D, Ernst W, Modlin R, Krensky A, et al. Granulysin crystal structure and a structure-derived lytic mechanism. J Mol Biol. 2003;325(2):355–65. . [DOI] [PubMed] [Google Scholar]
- 47.Kaspar AA, Okada S, Kumar J, Poulain FR, Drouvalakis KA, Kelekar A, et al. A distinct pathway of cell-mediated apoptosis initiated by granulysin. J Immunol. 2001;167(1):350–6. . [DOI] [PubMed] [Google Scholar]
- 48.Okada S, Li Q, Whitin JC, Clayberger C, Krensky AM. Intracellular mediators of granulysin-induced cell death. J Immunol. 2003;171(5):2556–62. . [DOI] [PubMed] [Google Scholar]
- 49.Pardo J, Perez-Galan P, Gamen S, Marzo I, Monleon I, Kaspar AA, et al. A role of the mitochondrial apoptosis-inducing factor in granulysin-induced apoptosis. J Immunol. 2001;167(3):1222–9. . [DOI] [PubMed] [Google Scholar]
- 50.Saini RV, Wilson C, Finn MW, Wang T, Krensky AM, Clayberger C. Granulysin delivered by cytotoxic cells damages endoplasmic reticulum and activates caspase-7 in target cells. J Immunol. 2011;186(6):3497–504. 10.4049/jimmunol.1003409 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FPLC chromatographies of 15 kDa granulysin by HisTrap™ HP (A), Superose™ 12 (B), and HiTrap™ SP FF (C) column and analysis of proteins from column eluates by 15% SDS-PAGE and Coomassie Blue staining (D). Lane 1, crude lysate; Lane 2, eluates of phosphate cellulose (P11) chromatography; Lane 3, eluates of HisTrap™ HP column chromatography; Lane 4, digestion product of PreScission™ Protease; Lane 5, eluates of Superose™ 12 gel filtration chromatography; Lane 6, eluates of HiTrap™ SP FF column chromatography. Arrow indicates the purified 15 kDa granulysin. The molecular masses of recombinant 9 and 15 kDa granulysin were determined by ESI-MS spectra (E).
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.