Abstract
The incidence of skin cancer is equivalent to the incidence of malignancies in all other organs combined. The main risk factor for this disease is overexposure of the skin to solar ultraviolet (UV) radiation. UV irradiation induces inflammation, oxidative stress, DNA damage and suppression of the immune system in the skin, which together contribute to carcinogenesis. The use of dietary phytochemicals shows great promise as a complementary and alternative strategy for skin cancer prevention. Grape seed proanthocyanidins (GSPs) have been tested extensively for their anti-skin cancer effect using in vivo animal models. Supplementation of an AIN76A control diet with GSPs (0.2% and 0.5%, w/w) significantly inhibits UV radiation-induced skin tumor development as well as malignant transformation of papillomas to carcinoma in mice. The inhibition of UVB-induced skin tumor development by GSPs is mediated through inter-related mechanisms of action including: (i) inhibition of inflammation, (ii) rapid repair of damaged DNA, and (iii) stimulation of immune system. Additionally, the chemopreventive effects of GSPs involve DNA repair-dependent functional activation of antigen presenting cells and stimulation of CD8+ effector T cells. These effects of GSPs could be useful in attenuation of the adverse effects of UV radiation and may have health benefits in humans.
Keywords: skin cancer, cyclobutane pyrimidine dimers, dendritic cells, T cells, ultraviolet radiation
1 Introduction
Cutaneous malignancies, including melanoma and non-melanoma, are common in fair-skinned individuals and particularly Caucasians. Skin cancer is a major burden on the health-care system due in part to its high incidence and in part due to the cost of treatment. The incidence of cutaneous malignancy is equivalent to that of malignancies in all other organs combined [1] and more than 2.00 million new cases of skin cancers are diagnosed each year in the USA. As many cases of skin cancers are not registered in national registries, the number of skin cancers may be higher. The cost of treating skin cancers in the USA is >$3.0 billion annually [2]. Solar ultraviolet (UV) radiation has been implicated in the pathogenesis of various skin diseases, including melanoma and non-melanoma skin cancers, through clinical, laboratory and epidemiological studies. Both overexposure and chronic UV irradiation of the skin have been shown to induce inflammation, oxidative stress, DNA damage and suppression of the immune system which, together, underlie the development of skin cancers. The cumulative effects of UV exposure on the skin have been identified as a critical factor for disease initiation, including photoaging and skin cancer [3]. Many of the drugs that have been developed to treat cancer are not suitable for chemoprevention due to toxicities and the development of resistance over time; thus, alternative strategies are needed. Such chemopreventive strategies can include the use of agents that can block, retard or reverse the processes that contribute to cancer risk. It is clear from the adverse effects of UV radiation that any agent or phytochemical which can block or inhibit UV radiation (UVR)-induced inflammation, oxidative stress, and DNA damage as well as stimulate the immune system would be an ideal agent for the prevention of skin cancer. There has been considerable interest in the use of plant-based products (phytochemicals) and particularly those that are widely distributed in plant foods (dietary phytochemicals) to prevent the risk of skin cancers. These phytochemicals occur in fresh fruits, vegetables, seeds, nuts, flowers and bark. For more than two decades, research has focused on the identification and screening of phytochemicals, particularly dietary phytochemicals, for the prevention of UV radiation-induced skin cancer risk using in vitro and in vivo animal models. The most studied phytochemicals are green tea polyphenols, proanthocyanidins from grape seeds, silymarin from milk thistle, curcumin and resveratrol, all of which have been shown to possess substantial anti-skin carcinogenic activities [4–8]. This review article summarizes the chemopreventive effects of proanthocyanidins from grape seeds against UVR-induced skin cancer risk that have been described based on the use of in vitro and in vivo model systems with a focus on the elucidation of their effects on the various molecular events that contribute to the process of photocarcinogenesis.
2. Grape seed proanthocyanidins (GSPs): chemical composition and source
The seeds of grapes (Vitis vinifera) are a byproduct of the industrial production of grape juice and wine. Seeds are a potent source of proanthocyanidins or procyanidins, which are a class of phenolic compounds and acquire the form of oligomers or polyhydroxy flavan-3-ol units. The author has played a lead role in the evaluation of the chemopreventive effects of dietary GSPs against photocarcinogenesis and the chemical composition of GSPs used in his laboratory is as follows: 89% total proanthocyanidins, which are present in the form of dimers (6.6%), trimers (5.0%), tetramers (2.9%) and oligomers (74.8%) of monomeric catechins, such as (+)-catechins and (+)-epicatechins. Monomeric flavanols are detected at the level of 6.6% in the GSPs [9–11]. This product is available commercially through Kikkoman Corporation, Japan, and other commercial vendors as well.
3 Dietary GSPs prevent UVR-induced skin tumor development and their progression to carcinomas in mice
The anti-photocarcinogenic effects of GSPs have been studied extensively in the author’s laboratory using in vitro and in vivo animal models [7]. In animal models, the GSPs are administered as a dietary supplement in which the GSPs are incorporated into an AIN76A control diet, which is available commercially.
The effect of dietary GSPs on photocarcinogenesis was evaluated using the SKH-1 hairless mouse model. These in vivo animal experiments indicate that dietary GSPs (0.2 and 0.5%, w/w) prevents the risk of photocarcinogenesis in a dose-dependent manner [7]. The risk was evaluated in terms of the percentage of mice with tumors, tumor multiplicity and size of the growing tumors in UVB-exposed mice provided the GSPs-supplemented diet as compared to UVB-exposed mice that received the un-supplemented control diet. The process of carcinogenesis can be subdivided into distinct stages. To determine whether the GSPs act during the initiation stage and/or the promotion stage of carcinogenesis, three different protocols were used in which GSPs were given only at the initiation stage; only at the promotional stage of tumor development, i.e., GSPs were given seven days after continuous UVB exposures; or throughout photocarcinogenesis protocol, i.e., a complete photocarcinogenesis protocol in which dietary GSPs were given from the beginning till the end of the photocarcinogenesis protocol, as detailed earlier [7]. Similar dose-dependent effects of GSPs on the percentage of mice with tumors, tumor multiplicity and size of the tumors in UVB-irradiated mice were observed using all three protocols, which show chemopreventive efficacy of GSPs.
The development of carcinomas requires the malignant transformation of benign papillomas. To determine the effects of GSPs on this process, the studies of photocarcinogenesis in the presence and absence of dietary GSPs were continued for 30 weeks. The results showed that dietary GSPs significantly inhibited malignant transformation of papillomas to carcinomas in mice [7]. The exact mechanism of this inhibitory effect is not known. It is known that transformation of benign papillomas to carcinomas requires additional genetic and epigenetic changes in the tumor cells, which can be achieved through the use of free radical-generating agents or genotoxic substances [12–14]. The continuous exposure to UV radiation would provide the free radical stimulation and/or genetic/epigenetic instability. Thus, the ability of the GSPs to prevent the transformation of papillomas to carcinomas suggests that the GSPs might prevent UVB-induced free radical-mediated enhancement of genetic/epigenetic instability.
4 Mechanisms of action of GSPs: Molecular and cellular targets
4.1 Anti-inflammatory effects of GSPs
The characteristic effects of chronic and sustained exposure to UV radiation on the generation of inflammatory responses and inflammatory mediators have been implicated in skin diseases, including the risk of skin tumor development. UV-induces inflammatory biomarkers, such as erythema, edema and hyperplastic responses and these are considered to play crucial roles in skin tumor promotion [15]. UV radiation induces overexpression of cyclooxygenase-2 (COX-2) and a subsequent increase in the production of prostaglandin (PG) metabolites in the skin and this is a characteristic response of skin cells to either acute or chronic exposure to UV radiation. COX-2 is a rate-limiting enzyme for the generation of PG metabolites from arachidonic acid [16], and COX-2 expression has been linked to the pathophysiology of both inflammation and cancer [17]. Overexpression of COX-2 in chronically UVB-irradiated skin, as well as in UVB-induced premalignant lesions and squamous- and basal-cell carcinomas of the skin, has been reported in several studies [18, 19]. Mechanistic studies have revealed that dietary intake of GSPs resulted in significant inhibition of UV radiation-induced COX-2 expression and prostaglandin metabolites production in mouse skin as well as inhibition of other inflammatory mediators, such as infiltration of leukocytes and myeloperoxidase induction [20].
The concept that GSPs act, at least in part, by ameliorating the UVB-induced inflammatory responses is further suggested by the finding of lower levels of pro-inflammatory cytokines, such as TNF-α, interleukin (IL)-1β and IL-6, in the UVB-exposed skin of mice that received GSPs treatment than the UVB-exposed skin of control mice. As elevated levels of proinflammatory cytokines have been implicated in skin cancer risk [15, 21, 22], the higher levels of pro-inflammatory cytokines in the untreated mice would be expected to contribute to the tumor promotion process and thus the development of skin tumors would be expected to occur earlier and progress more rapidly. This trend was observed in those mice that were not given GSPs in the diet [20]. The dietary administration of GSPs significantly inhibited UVB-induced expression of proinflammatory cytokines in skin tumors as well as mouse skin and this may have further contributed to inhibition of skin tumor initiation, growth and development.
4.2 Dietary GSPs stimulate repair of damaged DNA in UV-exposed skin: Role of the xeroderma pigmentosum complementation group A (XPA)
It is well established that UV irradiation induces DNA damage in the skin and that a predominant characteristic of this damage is the formation of cyclobutane pyrimidine dimers (CPD). The UV-induced formation of CPDs has been recognized as a molecular trigger of initiation of skin cancer (23–25). Moreover, reduction or repair of CPDs through the application of DNA repair enzymes reduces the risk of UV-induced skin tumor development (25, 26). There are several UV-induced DNA repair mechanisms but nucleotide excision repair (NER) has been identified as the most important DNA repair pathway in mammalian cells (27). The xeroderma pigmentosum complementation group A (XPA) gene encodes a protein that plays a key role in the NER pathway (28). The importance of the ability to repair UV-induced DNA damage, and specifically the role of XPA and the NER pathways, is indicated by the early occurrence and high incidence of skin cancer on sunlight-exposed skin in patients with XPA-deficiency (29). To investigate the possible cause of prevention of photocarcinogenesis by dietary GSPs, Vaid and colleagues [30] studied the effect of dietary GSPs (0.5%, w/w) on UVB-induced DNA damage in the mouse skin and the animals were sacrificed either immediately after UV exposure or 48 h after UVB exposure. The levels of CPDs in skin samples were then measured using immunohistochemical or immune-cytochemical analyses. Of importance, there was no difference in the levels of CPDs immediately after UV exposure of the skin in the groups of mice whether treated or not treated with GSPs, which eliminates the speculation that dietary GSPs might act, at least in part, through a filtering effect on UV radiation. In the skin samples collected at 48 h after UVB exposure, the number of CPD-positive cells was significantly lower (P<0.001) in the mice receiving dietary GSPs as compared to the mice that were exposed to UVB but did not receive GSPs in the diet. This observation was further verified by analysis of genomic DNA in skin samples obtained from different treatment groups in the same study [30].
The finding that treatment with GSPs enhances the repair of UVB-induced DNA damage in the form CPDs prompted further studies to determine whether the repair of UV-induced CPDs by GSPs is mediated through induction of genes encoding components of the NER pathway. Real-time PCR data revealed that treatment of mice with GSPs stimulates the levels of NER genes (e.g., XPA, XPC, and DDB2, etc.) in UVB-exposed skin of mice as compared to the levels in the UVB-exposed skin of mice that were not fed GSPs [30]. This indicates that this effect of GSPs on NER genes may contribute in the rapid repair of damaged DNA in mouse skin. In a complementary approach, the effect of GSPs on UVB-induced DNA damage was compared in human XPA-deficient cells obtained from patients suffering from XP disease and XPA-proficient cells from normal healthy persons [30]. The XPA complementation type represents the most severe phenotype, because the XPA gene is the most crucial component in the repair process and, thus, cells lacking the XPA gene are deficient in NER (29). Vaid and colleagues [30] demonstrated that treatment of GSPs was able to repair UV-induced CPDs in XPA-proficient cells but was not able to repair CPDs in XPA-deficient cells. These observations indicate that repair of UV-induced DNA damage by GSPs is mediated through the XPA-dependent mechanism. Importantly, XPA has been shown to be part of the core incision complex of the NER system [31]. These findings have important implications for the chemoprevention of skin cancer by GSPs, and identify a new mechanism by which GSPs prevent UV-induced skin tumor development in animal models.
4.3 Dietary GSPs prevent UV radiation-induced suppression of immune system
The immunosuppressive effect of UV radiation, particularly the UVB (290–320 nm) spectrum, is well known, and UVB-induced suppression of the immune system has been implicated in the risk of non-melanoma skin cancers, including both squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) [32, 33]. The role of the immunosuppressive effect of UV radiation on skin cancer risk is supported by several observations concerning the incidence of skin cancer in immunosuppressed individuals. Chronically immunosuppressed patients living in regions of intense sun exposure experience an exceptionally high risk of skin cancer [34] and the incidence of skin cancers has been found to be increased among organ transplant recipients. This increased risk of skin cancer in transplant patient is associated with long-term immunosuppressive therapy [35–38]. In animals, it has been found that an enhanced immune response is associated with a lower risk of skin tumor development on chronic UV exposure [39]. These observations can be interpreted as suggesting that protection from UV radiation-induced immunosuppression may be an important strategy in the management of skin cancer.
To determine whether dietary GSPs inhibit photocarcinogenesis in mice by preventing UVB-induced suppression of the immune system, a contact hypersensitivity (CHS) model has been used. CHS is considered to be a prototypic T-cell mediated immune response. It represents a delayed-type hypersensitivity response and is induced by epicutaneous application of a skin contact sensitizer, such as 2, 4-dinitrofluorobenzene. The chemopreventive effect of dietary GSPs on UVB-induced immunosuppression was evaluated using the CHS in inbred C3H/HeN mice [40, 41]. The CHS response was first assessed in mice that did not receive a GSPs-supplemented diet and that were UVB (180 mJ/cm2) irradiated as compared with mice that were not UVB irradiated. The significantly lower (P<0.001) CHS response in the mice that were UVB-irradiated, confirmed the immunosuppressive effect of the UV radiation. The provision of dietary GSPs by supplementation of the AIN76A control diet (0.5% or 1.0% GSPs, w/w) resulted in significant amelioration (P<0.001) of the UVB-induced suppression of CHS response [40]. The results of this study not only indicated that supplementation of the diet with GSPs is capable of protecting mice from UVB-induced immunosuppression but also provided evidence that dietary GSPs can protect the animals from UVB-induced immunosuppression for some time after the consumption of GSPs has ceased.
4.4 Dietary GSPs stimulate the functional activity of antigen presenting cells of the skin in UV-irradiated skin
The primary and major antigen-presenting cells in the skin are the epidermal Langerhans cells (LC). UV-induced photodamage of LC is considered to be an important mediator of UV-induced immune suppression [42, 43]. There also is evidence indicating that DNA repair mechanisms are related directly to the functional activity of LCs in the stimulation of T cells and the induction of immune responses [44, 45]. For example, a decrease in CPD-positive LCs is correlated with an overall increase in LC function as assessed by induction of CHS response and the production of IFNγ by T cells [44]. As described above, the repair or removal of CPDs in UV-exposed skin requires a functional NER mechanism and can be inhibited by mutation of the XPA gene [30, 44]. Vaid and colleagues [46] have demonstrated that dietary GSPs prevent UV-induced suppression of the CHS response to 2, 4-dinitrofluorobenzene in wild-type mice but this effect of GSPs was not observed in XPA-deficient mice, implicating the involvement of XPA or NER mechanisms in the prevention of UV-induced immunosuppression by GSPs. The authors have further verified these observation using bone marrow-derived dendritic cells (BM-DC). For this purpose, BM-DC from GSPs-treated or untreated mice were exposed to UV radiation. The cells were harvested immediately or 24 h after UV irradiation and CPD-positive cells detected using immune-cytostaining [46]. Treatment of UV-irradiated BM-DC with GSPs resulted in repair of CPDs; however, GSPs were not able to repair UV-induced DNA damage in the DCs obtained from XPA-deficient mice. Additionally, dietary GSPs were found to enhance the repair of UV-induced DNA damage in the form of CPDs in epidermal DCs in wild-type mice, but this effect of GSPs was not observed in the epidermal DCs of UV-exposed skin of XPA-deficient mice [46]. These pre-clinical observations suggest that GSPs-mediated DNA repair in DCs is mediated through an effect on XPA and that this is an important contributing mechanism in their prevention of UV-induced immunosuppression. The role of XPA in the repair of UV-induced DNA damage also has been shown by Li and colleagues [47].
The chemopreventive effects of GSPs on the dysregulation of immune responses in UVR-induced immunosuppression have been studied extensively [40, 48, 49]. Dietary GSPs have been shown to inhibit the UVR-induced suppression of the CHS response to contact sensitizer through the enhanced production of IL-12 in the UV-exposed mouse skin. IL-12 has been recognized as a cytokine which has the ability to repair UV-induced formation of CPDs and the mechanisms underlying the effects of IL-12 on DNA repair have been discussed extensively previously [40, 48, 49]. The secretion or production of specific cytokines by DCs plays a major role in their ability to stimulate specific populations of T cells and, hence, to shape the immune response. DCs can produce both IL-12 (immunostimulatory) and IL-10 (immunosuppressive) cytokines under different conditions [50–53]. UV-irradiation has been shown to suppress the production of IL-12 while increasing the levels of IL-10 in the skin [54–56]. The reduction in the production of IL-12 by the DCs after UV exposure may be a mechanism by which UV radiation stimulates the development of tolerogenic DCs [57, 58]. Dietary GSPs enhanced the production of IL-12 and IFNγ while reducing the levels of IL-10 in DCs obtained from UVB-exposed wild-type mice. However, this ameliorating effect of GSPs was not found in the DCs obtained from UVB-exposed XPA-deficient mice. These findings suggest that GSPs can restore regulated production of IL-12 and IL-10 in UV-irradiated DCs, and it would be anticipated that this would contribute to the ability of GSPs to restore the function of DCs in terms of their ability to activate T-cell subpopulations. In similar experiments carried out under identical conditions, GSPs enhanced the function of DCs in terms of their ability to enhance the proliferative capacity of effector T cells. GSPs also enhanced the production of IFNγ while suppressing the levels of IL-10 and IL-4 by the DCs, which further verifies that dietary GSPs enhance the functional activity of DCs. In contrast, GSPs failed to activate DCs obtained from UV-exposed XPA-deficient mice [46]. These outcomes suggest that GSPs prevent UVB-induced immunosuppression by the repair of CPDs in DCs that is associated with the restoration of the functional activation of UVB-irradiated DCs.
4.5 Dietary GSPs and T-cell activation
In parallel, the roles of GSPs on T-cell development in UV-irradiated mice have been assessed using adoptive transfer experiments. This approach allowed efforts to identify the T-cell subpopulations responsible for the transfer of the GSPs-induced prevention of immune suppression. Donor C3H/HeN mice (inbred) that were provided dietary GSPs or the control diet were irradiated with UVB and sensitized with contact sensitizer as detailed by Vaid and colleagues [46]. CD8+ T cells were then positively selected from the regional lymph nodes using the MACS system. These purified CD8+ T cells were injected i.v. into naïve mice and the recipient mice were then challenged immediately by application of 2, 4-dinitrofluorobenzene [49]. Those naïve mice which have received CD8+ effector T cells from GSPs-treated, UVB-exposed donor mice showed a greater CHS response than the naïve mice that had received cells from UVB-exposed mice that had not been treated with GSPs. This suggests that (i) the prevention of UVB-induced immunosuppression by GSPs is transferable to naïve mice by CD8+ T cells, and (ii) treatment of mice with GSPs results in activation and development of the CD8+ T-cell subpopulation and that these cells play a role in the higher CHS response in the GSPs-treated UVB-irradiated mice [49]. In parallel, the role of CD4+ T cells was examined and the results indicated that the GSPs prevention of UVB-induced suppression was also associated with diminishment of the development of regulatory CD4+ T cells. There are conflicting opinions as to whether the CD8+ or the CD4+ T-cell subpopulation mediates the CHS response. Studies by Hauser [59] and Kondo et al. [60] suggest that CD4+ T cells are the critical effector cells in the CHS response, whereas other investigators [61–64] provide evidence that CD8+ T cells are involved.
4.6 Cytokine profile of the effector T cells in response to UVB and GSPs treatment in mice
An alternative approach to analysis of the T-cell mediated immune responses is analysis of the cytokine profiles. The functional activation of CD8+ T cells by dietary GSPs was determined by analyzing the levels of Th1 and Th2 cytokine profiles [49]. The levels of IFNγ (>5-fold) and IL-2 (8-fold) were considerably higher in the supernatants of CD8+ T cells prepared from the GSPs- treated, UVB-exposed mice than in the supernatants of cells from mice that were exposed to UVB but not treated with GSPs. In contrast, Th2 cytokines were hardly detectable in the supernatants of CD8+ T cells obtained either from the GSPs-treated, UVB-exposed mice or the CD8+ T cells from the mice that were exposed to UVB but not treated with GSPs. The significantly higher levels of Th1 cytokines in the mice that were treated with GSPs suggests that the activation of CD8+ T cells by the dietary GSPs may play a significant role in the immune response in the GSPs-treated, UVB-exposed mice. Thus, the studies by Vaid and colleagues [49] indicate that the CD8+ T cells are the critical effector cells, a finding that is in accordance with the findings of other investigators [62, 64]. This property of GSPs can be used as an alternative strategy for augmentation of the induction of CD8+ effector T cells and suppression of the development of CD4+ regulatory T cells, which may lead to the prevention of skin cancer risk.
5 Bioavailability and toxicity of dietary GSPs
The attempts have been made to determine the bioavailability of GSPs in vivo models. Studies revealed that proanthocyanidins are not absorbed as such in the gut [65], but they were detected in the form of monomers and dimers in human plasma [66, 67]. The distribution of grape seed flavanols and their metabolites was studied in rat after 1 and 2 h of an acute intake of GSPs using LC-ESI-TOF/MS. Flavanols and their metabolites were detected in plasma, liver and maternal placenta [68]. Serra et al [69] studied the bioavailability of GSPs after a long term consumption of GSPs using rats as a model. Glucuronidated conjugates and methyl glucuronidated conjugates were the main flavanols metabolites quantified in plasma. Dimers were also detected in free form in plasma. Similar studies were also conducted by Prasain et al [70] in rats wherein the metabolites of GSPs were identified and detected using LC-MS/MS operating in the multiple reaction monitoring mode. Monomeric catechins and their methylated metabolites, and proanthocyanidins up to trimers were detected in blood samples. The (+)-catechins and (−)-epicatechin monomers were also identified in the brain. These observation suggest that GSPs catechins cross the blood brain barrier. These studies indicate the bioavailability of GSPs in its metabolite forms and they may be responsible for the anti-cancer effects.
During long-term animal experiments, there were no apparent sign of toxicities or adverse health effects of dietary GSPs were noted in animals in terms of their normal body growth and development or abnormal behavior [7]. Further, the genotoxic nature of GSPs has not been reported.
6 Dietary GSPs: prospects in prevention of skin cancer in humans
As the incidence and risk of UVR-induced skin cancer is on the rise and the available options for its treatment and prevention are limited, new strategies are urgently needed to control this malignancy. Dietary phytochemicals can be considered as complementary and alternative approach for the prevention and treatment of skin cancers based on evidence of lack of toxicity and demonstrated anti-cancer activities. An understanding of the mechanisms of action of phytochemicals also is of importance due to the world-wide interest on the use of naturally occurring dietary supplements as chemopreventive agents or as complementary and alternative medicine for this purpose. Studies have revealed that dietary GSPs has the ability to inhibit UVR-induced adverse effects, such as inflammatory mediators, DNA damage and suppression of immune system. Based on these characteristics of GSPs, it is suggested that regular intake of GSPs as a dietary supplement may help to reduce the risk of skin cancer in humans and in particular those individuals who have fair skin and frequently exposed to UV sunlight. The simple and easy way to consume this product on regular basis is in the form of pills or dietary supplement available commercially. Based on the exposure frequency, exposure time and intensity of UVR at the time of exposure, the quantity or amount of GSPs consumption need to be adjusted. Alternatively, the supplementation of the sunscreens or skin care lotions with GSPs may provide an effective strategy for the prevention of UV light induced adverse effects in humans. Moreover, the use of GSPs in combination with already available cancer therapeutic drugs may offer an enhanced ability to simultaneously target other cancer-related targets thereby improving their efficacy. As summarized in this review and the accompanying Figure-1, GSPs possess adequate anti-skin cancer properties and has well-defined molecular and cellular targets that are responsible for its chemopreventive effects.
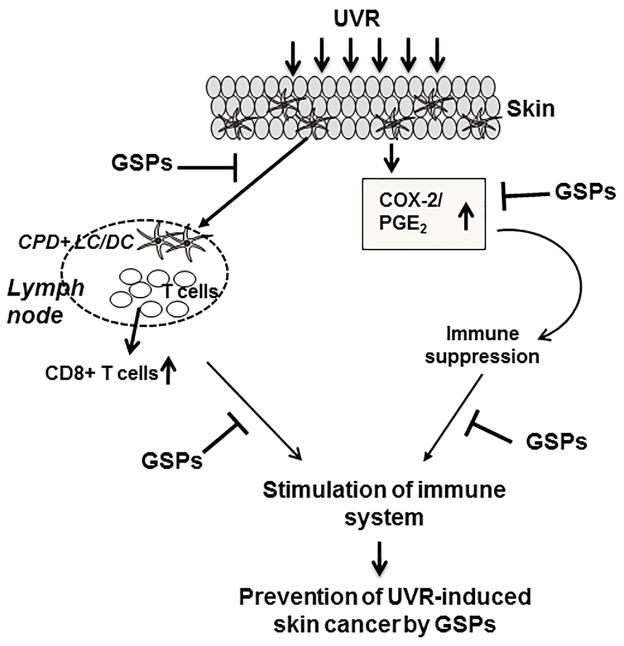
Figure 1.
Exposure of the skin to UVR causes DNA damage in antigen presenting cells (e.g., LC/DC) of the skin in terms of formation of CPDs and also enhances the expression levels of inflammatory mediators, such as of COX-2/PGE2, etc.. These events lead to the suppression of immune system. Regular intake of dietary GSPs stimulates the repair of CPDs in LC/DC in UV-exposed skin which leads to the functional activation of DCs and stimulation of CD8+ effector T cells. GSPs treatment also reduce the expression of inflammatory mediators responsible for the suppression of immune reactions and thus protect the immune system. Together, these mechanisms of action of the GSPs enhance or stimulate the immune system in mice, which ultimately protects them from UVR-induced skin cancer risk. CPD, cyclobutane pyrimidine dimers; LC, Langerhans cells, DC, dendritic cells.
Acknowledgments
The work reported from Dr. Katiyar’s research laboratory was financially supported from the funds from National Cancer Institute/NIH (CA104428) and Veterans Administration Merit Review Award (1I01BX001410). Grateful thanks are due to our former and current research staff members for their outstanding contribution in the related studies. The content of this publication does not necessarily reflect the views or policies of the funding sources.
Abbreviations
- BM-DC
bone marrow-derived dendritic cells
- CHS
contact hypersensitivity
- COX-2
cyclooxygenase-2
- CPD
cyclobutane pyrimidine dimers
- DC
dendritic cells
- GSPs
grape seed proanthocyanidins
- IL
interleukin
- LC
Langerhans cells
- NER
nucleotide excision repair
- UV
ultraviolet
- XPA
xeroderma pigmentosum complementation group A
Footnotes
Conflict of Interest: The author has declared no conflict of interest.
References
- 1.Narayanan DL, Saladi RN, Fox JL. Ultraviolet radiation and skin cancer. Int J Dermatol. 2010;49:978–986. doi: 10.1111/j.1365-4632.2010.04474.x. [DOI] [PubMed] [Google Scholar]
- 2.Housman TS, Feldman SR, Williford PM, Fleischer AB, Jr, et al. Skin cancer is among the most costly of all cancers to treat for the Medicare population. J Am Acad Dermatol. 2003;48:425–429. doi: 10.1067/mjd.2003.186. [DOI] [PubMed] [Google Scholar]
- 3.Young AR. Cumulative effects of ultraviolet radiation on the skin: Cancer and photoaging. Semin Dermatol. 1990;9:25–31. [PubMed] [Google Scholar]
- 4.Baliga MS, Katiyar SK. Chemoprevention of photocarcinogenesis by selected dietary botanicals. Photochem Photobiol Sci. 2006;5:243–253. doi: 10.1039/b505311k. [DOI] [PubMed] [Google Scholar]
- 5.Joi A, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meeran SM, Akhtar S, Katiyar SK. Inhibition of UVB-induced skin tumor development by drinking green tea polyphenols is mediated through DNA repair and subsequent inhibition of inflammation. J Invest Dermatol. 2009;129:1258–1270. doi: 10.1038/jid.2008.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mittal A, Elmets CA, Katiyar SK. Dietary feeding of proanthocyanidins from grape seeds prevents photocarcinogenesis in SKH-1 hairless mice: Relationship to decreased fat and lipid peroxidation. Carcinogenesis. 2003;24:1379–1388. doi: 10.1093/carcin/bgg095. [DOI] [PubMed] [Google Scholar]
- 8.Vaid M, Katiyar SK. Molecular mechanisms of inhibition of photocarcinogenesis by silymarin, a phytochemical from milk thistle (Silybum marianum L. Gaertn.) Int J Oncol. 2010;36:1053–1060. doi: 10.3892/ijo_00000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi J, Yu J, Pohorly JE, Kakuda Y. Polyphenolics in grape seeds-biochemistry and functionality. J Med Food. 2003;6:291–299. doi: 10.1089/109662003772519831. [DOI] [PubMed] [Google Scholar]
- 10.Prieur C, Rigaud J, Cheynier V, Moutounet M. Oligomeric and polymeric procyanidins from grape seeds. Phytochemistry. 1994;36:781–789. [Google Scholar]
- 11.Yamakoshi J, Saito M, Kataoka S, Kikuchi M. Safety evaluation of proanthocyanidins-rich extract from grape seeds. Food Chemical Toxicol. 2002;40:599–607. doi: 10.1016/s0278-6915(02)00006-6. [DOI] [PubMed] [Google Scholar]
- 12.Slaga TJ, Klein-Szanto AJ, Triplett LL, Yotti LP, et al. Skin tumor-promoting activity of benzoyl peroxide, a widely used free radical-generating compound. Science. 1981;213:1023–1025. doi: 10.1126/science.6791284. [DOI] [PubMed] [Google Scholar]
- 13.Hennings H, Shores R, Wenk ML, Spangler EF, et al. Malignant conversion of mouse skin tumours is increased by tumour initiators and unaffected by tumour promoters. Nature. 1983;304:67–69. doi: 10.1038/304067a0. [DOI] [PubMed] [Google Scholar]
- 14.O’Connell JF, Klein-Szanto AJ, DiGiovanni DM, Fries JW, et al. Enhanced malignant progression of mouse skin tumors by the free-radical generator benzoyl peroxide. Cancer Res. 1986;46:2863–2865. [PubMed] [Google Scholar]
- 15.Mukhtar H, Elmets CA. Photocarcinogenesis: mechanisms, models and human health implications. Photochem Photobiol. 1996;63:355–447. doi: 10.1111/j.1751-1097.1996.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 16.Langenbach R, Loftin CD, Lee C, Tiano H. Cyclooxygenase-deficient mice. A summary of their characteristics and susceptibilities to inflammation and carcinogenesis. Ann NY Acad Sci. 1999;889:52–61. doi: 10.1111/j.1749-6632.1999.tb08723.x. [DOI] [PubMed] [Google Scholar]
- 17.Chapple KS, Cartwright EJ, Hawcroft G, Tisbury A, et al. Localization of cyclooxygenase-2 in human sporadic colorectal adenomas. Am J Pathol. 2000;156:545–553. doi: 10.1016/S0002-9440(10)64759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buckman SY, Gresham A, Hale P, Hruza G, et al. COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis. 1998;19:723–729. doi: 10.1093/carcin/19.5.723. [DOI] [PubMed] [Google Scholar]
- 19.Vanderveen EE, Grekin RC, Swanson NA, Kragballe K. Arachidonic acid metabolites in cutaneous carcinomas. Arch Dermatol. 1986;122:407–412. doi: 10.1001/archderm.122.4.407. [DOI] [PubMed] [Google Scholar]
- 20.Sharma SD, Katiyar SK. Dietary grape seed proanthocyanidins inhibit UVB-induced cyclooxygenase-2 expression and other inflammatory mediators in UVB-exposed skin and skin tumors of SKH-1 hairless mice. Pharm Res. 2010;27:1092–1102. doi: 10.1007/s11095-010-0050-9. [DOI] [PubMed] [Google Scholar]
- 21.Scott KA, Moore RJ, Arnott CH, East N, Thompson RG, Scallon BJ, Shealy DJ, Balkwill FR, et al. An anti-tumor necrosis factor-alpha antibody inhibits the development of experimental skin tumors. Mol Cancer Ther. 2003;2:445–451. [PubMed] [Google Scholar]
- 22.Tron VA, Rosenthal D, Sauder DN. Epidermal interleukin-1 is increased in cutaneous T-cell lymphoma. J Invest Dermatol. 1988;90:378–381. doi: 10.1111/1523-1747.ep12456433. [DOI] [PubMed] [Google Scholar]
- 23.Kripke ML, Cox PA, Alas LG, Yarosh DB. Pyrimidine dimers in DNA initiated systemic immunosuppression in UV-irradiated mice. Proc Natl Acad Sci USA. 1992;89:7516–7520. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yarosh D, Alas LG, Yee V, Oberyszyn A, et al. Pyrimidine dimer removal enhanced by DNA repair liposomes reduces the incidence of UV skin cancer in mice. Cancer Res. 1992;52:4227–4231. [PubMed] [Google Scholar]
- 25.Yarosh D, Klein J, O’Connor A, Hawk J, Rafal E, et al. Effect of topically applied T4 endonuclease V in liposomes on skin cancer in xeroderma pigmentosum: a randomised study. Xeroderma Pigmentosum Study Group. Lancet. 2001;357:926–929. doi: 10.1016/s0140-6736(00)04214-8. [DOI] [PubMed] [Google Scholar]
- 26.de Vries A, van Oostrom CT, Hofhuis FM, Dortant PM, et al. Increased susceptibility to ultraviolet-B and carcinogens of mice lacking the DNA excision repair gene XPA. Nature. 1995;377:169–173. doi: 10.1038/377169a0. [DOI] [PubMed] [Google Scholar]
- 27.Cline SD, Hanawalt PC. Who’s on first in the cellular response to DNA damage? Nat Rev Mol Cell Biol. 2003;4:361–372. doi: 10.1038/nrm1101. [DOI] [PubMed] [Google Scholar]
- 28.Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 29.Bootsma D, Kraemer KH, Cleaver JE, Hoeijmakers JHJ. In: The Genetic Basis of Human Cancer. Vogelstein B, Kinzler KW, editors. New York: McGraw Hill; 1998. pp. 245–274. [Google Scholar]
- 30.Vaid M, Sharma SD, Katiyar SK. Proanthocyanidins inhibit photocarcinogenesis through enhancement of DNA repair and xeroderma pigmentosum group A-dependent mechanism. Cancer Prev Res. 2010;3:1621–1629. doi: 10.1158/1940-6207.CAPR-10-0137. [DOI] [PubMed] [Google Scholar]
- 31.Camenisch U, Dip R, Schumacher SB, Schuler B. Recognition of helical kinks by xeroderma pigmentosum group A protein triggers DNA excision repair. Nat Struct Mol Biol. 2006;13:278–284. doi: 10.1038/nsmb1061. [DOI] [PubMed] [Google Scholar]
- 32.Urbach F. Incidences of nonmelanoma skin cancer. Dermatol Clin. 1991;9:751–755. [PubMed] [Google Scholar]
- 33.Yoshikawa T, Rae V, Bruins-Slot W, vand-den-Berg JW, et al. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J Invest Dermatol. 1990;95:530–536. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- 34.Kinlen L, Sheil A, Peta J, Doll R. Collaborative United Kingdom–Australia study of cancer in patients treated with immunosuppressive drugs. Br J Med. 1979;II:1461–1466. doi: 10.1136/bmj.2.6203.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cowen EW, Billingsley EM. Awareness of skin cancer by kidney transplant patients. J Am Acad Dermatol. 1999;40:697–701. doi: 10.1016/s0190-9622(99)70149-0. [DOI] [PubMed] [Google Scholar]
- 36.Otley CC, Pittelkow MR. Skin cancer in liver transplant recipients. Liver Transpl. 2000;6:253–262. doi: 10.1053/lv.2000.6352. [DOI] [PubMed] [Google Scholar]
- 37.Fortina AB, Caforio AL, Piaserico S, Alaibac M, et al. Skin cancer in heart transplant recipients: frequency and risk factor analysis. J Heart Lung Transplant. 2000;19:249–255. doi: 10.1016/s1053-2498(99)00137-0. [DOI] [PubMed] [Google Scholar]
- 38.DiGiovanna JJ. Posttransplantation skin cancer: scope of the problem, management and role for systemic retinoid chemoprevention. Transplant Proc. 1998;30:2771–2775. doi: 10.1016/s0041-1345(98)00806-9. [DOI] [PubMed] [Google Scholar]
- 39.Beissert S, Bluestone JA, Mindt I, Voskort M, et al. Reduced UV-induced carcinogenesis in mice with a functional disruption in B7-mediated costimulation. J Immunol. 1999;63:6725–6731. [PubMed] [Google Scholar]
- 40.Sharma SD, Katiyar SK. Dietary grape-seed proanthocyanidin inhibition of ultraviolet B-induced immune suppression is associated with induction of IL-12. Carcinogenesis. 2006;27:95–102. doi: 10.1093/carcin/bgi169. [DOI] [PubMed] [Google Scholar]
- 41.Meeran SM, Mantena SK, Katiyar SK. Prevention of ultraviolet radiation-induced immunosuppression by (−)-epigallocatechin-3-gallate in mice is mediated through interleukin 12-dependent DNA repair. Clinical Cancer Res. 2006;12:2272–2280. doi: 10.1158/1078-0432.CCR-05-2672. [DOI] [PubMed] [Google Scholar]
- 42.Toews GB, Bergstresser PR, Streilein JW, Sullivan S. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol. 1980;124:445–453. [PubMed] [Google Scholar]
- 43.Cooper KD, Oberhelman L, Hamilton TA, Baadsgaard O, et al. UV exposure reduces immunization rates and promotes tolerance to epicutaneous antigens in humans: relationship to dose, CD1a-DR+ epidermal macrophage induction, and Langerhans cell depletion. Proc Natl Acad Sci USA. 1992;89:8497–8501. doi: 10.1073/pnas.89.18.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vink AA, Moodycliffe AM, Shreedhar V, Ullrich SE, et al. The inhibition of antigen-presenting activity of dendritic cells resulting from UV irradiation of murine skin is restored by in vitro photorepair of cyclobutane pyrimidine dimers. Proc Natl Acad Sci USA. 1997;94:5255–5260. doi: 10.1073/pnas.94.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vink AA, Strickland FM, Bucana C, Cox PA, et al. Localization of DNA damage and its role in altered antigen-presenting cell function in ultraviolet-irradiated mice. J Exp Med. 1996;183:1491–1500. doi: 10.1084/jem.183.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaid M, Singh T, Prasad R, Elmets CA, et al. Bioactive grape proanthocyanidins enhance immune reactivity in UV-irradiated skin through functional activation of dendritic cells in mice. Cancer Prev Res. 2013;6:242–252. doi: 10.1158/1940-6207.CAPR-12-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z, Musich PR, Serrano MA, Dong Z, et al. XPA-mediated regulation of global nucleotide excision repair by ATR is p53-dependent and occurs primarily in S-phase. PLoS ONE. 2011;6:e28326. doi: 10.1371/journal.pone.0028326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katiyar SK. Grape seed proanthocyanidins and skin cancer prevention: Inhibition of oxidative stress and protection of immune system. Mol Nutr Food Res. 2008;52:S71–S76. doi: 10.1002/mnfr.200700198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaid M, Singh T, Li A, Katiyar N, et al. Proanthocyanidins inhibit UV-induced immunosuppression through IL-12-dependent stimulation of CD8+ effector T cells and inactivation of CD4+ T cells. Cancer Prev Res. 2010;4:238–247. doi: 10.1158/1940-6207.CAPR-10-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morelli AE, Thomson AW. Dendritic cells: regulators of alloimmunity and opportunities for tolerance induction. Immunological Rev. 2003;196:125–146. doi: 10.1046/j.1600-065x.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 51.Wakkach A, Fournier N, Brun V, Breittmayer JP, et al. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 52.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: The alternative approaches. Ann Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 53.Corinti S, Albanesi C, la Sala A, Pastore S, et al. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166:4312–4318. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 54.Loser K, Apelt J, Voskort M, Mohaupt M, et al. IL-10 controls ultraviolet-induced carcinogenesis in mice. J Immunol. 2007;179:365–371. doi: 10.4049/jimmunol.179.1.365. [DOI] [PubMed] [Google Scholar]
- 55.Katiyar SK. Interleukin-12 and photocarcinogenesis. Toxicol Appl Pharmacol. 2007;224:220–227. doi: 10.1016/j.taap.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmitt DA, Owen-Schaub L, Ullrich SE. Effect of IL-12 on immune suppression and suppressor cell induction by ultraviolet radiation. J Immunol. 1995;154:5114–5120. [PubMed] [Google Scholar]
- 57.Ullrich SE. Mechanism involved in the systemic suppression of antigen-presenting cell function by UV irradiation. Keratinocyte-derived IL-10 modulates antigen-presenting cell function of splenic adherent cells. J Immunol. 1994;152:3410–3416. [PubMed] [Google Scholar]
- 58.Ullrich SE, Schmitt DA. The role of cytokines in UV-induced systemic immune suppression. J Dermatol Sci. 2000;23:S10–S12. doi: 10.1016/s0923-1811(99)00073-0. [DOI] [PubMed] [Google Scholar]
- 59.Hauser C. Cultured epidermal Langerhans cells activate effector T cells for contact sensitivity. J Invest Dermatol. 1990;95:436–440. doi: 10.1111/1523-1747.ep12555587. [DOI] [PubMed] [Google Scholar]
- 60.Kondo S, Beissert S, Wang B, Fujisawa H, et al. Hyporesponsiveness in contact hypersensitivity and irritant contact dermatitis in CD4 gene targeted mouse. J Invest Dermatol. 1996;106:993–1000. doi: 10.1111/1523-1747.ep12338505. [DOI] [PubMed] [Google Scholar]
- 61.Bour H, Peyron E, Gaucherand M, Garrigue JL, et al. Major histocompatibility complex class I-restricted CD8+ T cells and class II-restricted CD4+ T cells, respectively, mediate and regulate contact sensitivity to dinitrofluorobenzene. Eur J Immunol. 1995;25:3006–3010. doi: 10.1002/eji.1830251103. [DOI] [PubMed] [Google Scholar]
- 62.Xu H, DiIulio NA, Fairchild RL. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: interferon gamma-producing (Tc1) effector CD8+ T cells and interleukin (IL) 4/IL-10-producing (Th2) negative regulatory CD4+ T cells. J Exp Med. 1996;183:1001–1012. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anderson C, Hehr A, Robbins R, Hasan R, et al. Metabolic requirements for induction of contact hypersensitivity to immunotoxic polyaromatic hydrocarbons. J Immunol. 1995;155:3530–3537. [PubMed] [Google Scholar]
- 64.Gocinski BL, Tigelaar RE. Roles of CD4+ and CD8+ T cells in murine contact sensitivity revealed by in vivo monoclonal antibody depletion. J Immunol. 1990;144:4121–4128. [PubMed] [Google Scholar]
- 65.Manach C, Williamson G, Morand C, Scalbert A, et al. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 66.Holt RR, Lazarus SA, Sullards MC, Zhu QY, et al. Procyanidin dimer B2 [epicatechin-(4β-8)-epicatechin] in human plasma after the consumption of a flavanol-rich cocoa. Am J Clin Nutr. 2002;76:798–804. doi: 10.1093/ajcn/76.4.798. [DOI] [PubMed] [Google Scholar]
- 67.Sano A, Yamakoshi J, Tokutake S, Tobe K, et al. Procyanidin B1 is detected in human serum after intake of proanthocyanidin-rich grape seed extract. Biosci Biotechnol Biochem. 2003;67:1140–1143. doi: 10.1271/bbb.67.1140. [DOI] [PubMed] [Google Scholar]
- 68.Arola-Arnal A, Oms-Oliu G, Crescenti A, del Bas JM, et al. Distribution of grape seed flavanols and their metabolites in pregnant rats and their fetuses. Mol Nutr Food Res. 2013;57:1741–1752. doi: 10.1002/mnfr.201300032. [DOI] [PubMed] [Google Scholar]
- 69.Serra A, Bladé C, Arola L, Macià A, et al. Flavanol metabolites distribute in visceral adipose depots after a long-term intake of grape seed proanthocyanidin extract in rats. Br J Nutr. 2013;110:1411–1420. doi: 10.1017/S0007114513000706. [DOI] [PubMed] [Google Scholar]
- 70.Prasain JK, Peng N, Dai Y, Moore R, et al. Liquid chromatography tandem mass spectrometry identification of proanthocyanidins in rat plasma after oral administration of grape seed extract. Phytomedicine. 2009;16:233–243. doi: 10.1016/j.phymed.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]