Abstract
One of the most important issues facing cartilage tissue engineering is the inability to move technologies into the clinic. Despite the multitude of review articles on the paradigm of biomaterials, signals, and cells, it is reported that 90% of new drugs that advance past animal studies fail clinical trials (1). The intent of this review is to provide readers with an understanding of the scientific details of tissue engineered cartilage products that have demonstrated a certain level of efficacy in humans, so that newer technologies may be developed upon this foundation. Compared to existing treatments, such as microfracture or autologous chondrocyte implantation, a tissue engineered product can potentially provide more consistent clinical results in forming hyaline repair tissue and in filling the entirety of the defect. The various tissue engineering strategies (e.g., cell expansion, scaffold material, media formulations, biomimetic stimuli, etc.) used in forming these products, as collected from published literature, company websites, and relevant patents, are critically discussed. The authors note that many details about these products remain proprietary, not all information is made public, and that advancements to the products are continuously made. Nevertheless, by fully understanding the design and production processes of these emerging technologies, one can gain tremendous insight into how to best use them and also how to design the next generation of tissue engineered cartilage products.
1. Introduction
An adequate therapy for the long-term repair of cartilage lesions has yet to be developed. Being largely avascular and with low cellularity, articular cartilage has a limited ability to heal itself. Despite possessing remarkable mechanical properties, the tissue can develop defects following long-term wear or acute trauma. Defects in the highly organized matrix can progressively deteriorate through mechanisms of stress concentration and cell signaling cascades. Ultimately, the tissue loses mechanical integrity, breaks, thins, loses lubrication, and no longer functions in cushioning bone-to-bone contact – imparting great physical pain to the patient.
Focal lesions are the ideal indication for the repair of articular cartilage. The prevalence of focal lesions is difficult to estimate. In 2005, an estimated 27 million people in the U.S. had osteoarthritis (2). In one study, 60% of all arthroscopies revealed the presence of articular lesions (36% being Outerbridge Grade III and IV lesions) and, of these, 67% were characterized as focal lesions (3). From a surgical perspective, an estimated 250,000 articular cartilage repair procedures (involving chondroplasty, microfracture, mosaicplasty, and autologous chondrocyte implantation (ACI)) are performed annually in the U.S. (4). These cartilage repair therapies, however, do not consistently produce hyaline repair tissue, fill the entirety of the defect, and integrate repair tissue with adjacent native tissue.
To overcome these limitations, a number of cell-based, tissue engineered cartilage products have recently entered clinical trials in the U.S. and abroad. In this review, tissue engineered cartilage is defined as a construct formed by following the paradigm of integrating chondrocytes, signals, and scaffolds. The scaffolds can be exogenously provided or endogenously produced by the cells; the latter are usually referred to as scaffold-free or scaffold-less approaches if no exogenous scaffold is provided. Acellular scaffolds, considered an augmented form of microfracture, are not included in this definition. Tissue grafts including osteochondral autografts and allografts, as well as their particulated forms such as DeNovo® NT from Zimmer, are also not considered tissue engineered cartilage. Finally, using this definition, injection of passaged chondrocytes into a cartilage defect is also not considered tissue engineering. Through systematic design, tissue engineered cartilage can be manipulated in vitro to enhance its biochemical and biomechanical properties. Complete fill and good integration can be achieved by manipulating construct shape, the use of adhesives and other fixation methods, and other strategies. Tissue engineering offers a promising solution for the long-term treatment of cartilage lesions.
The first section of this review aims to provide a description of current repair therapies and the tissue engineered cartilage products – BioCart™II, Bioseed®-C, CaReS®, Cartipatch®, Chondrosphere®, Hyalograft® C, INSTRUCT, NeoCart®, NOVOCART® 3D, MACI, and RevaFlex™. Table 1 lists the construct specifications and Table 2 lists the products’ clinical indications, current status, and clinical trials. The second section aims to discuss the tissue engineering strategies used in product fabrication, identify current challenges, and suggest future directions. The authors note that the information in this review was gathered from published literature, company websites, and relevant patents. Owing to this and the fact that there may be a plethora of proprietary details not publicly available, the current status of the products may not be adequately reflected. In reviewing the details on the science behind each product, one quickly realizes that improvements can be made on five areas. These include 1) defining and optimizing the chondrocyte cell source, 2) understanding tissue-scaffold interaction and scaffold degradation, 3) identifying and applying novel stimuli, 4) understanding construct maturation, biomechanics, and functionality, and 5) improving implantation, fixation, and rehabilitation methods. The current challenges and future directions in these five areas, along with challenges in commercialization, are discussed in Perspectives. Thus, by understanding the general details of how these clinically used tissue engineered products are fabricated, one can gain insight as to how to best use them and how to design the next generation of tissue engineered cartilage.
Table 1.
List of cell-based, tissue engineered cartilage products, their approximate production specifications, and their implantation method
| Product | No. of Surgeries |
Chondrocytes | Scaffold | Seeding Density (areal) |
Construct Size |
3D Culture Duration |
Implantati on Technique |
Fixation Technique |
|---|---|---|---|---|---|---|---|---|
| Biocart™II | 2 | Autologous (unspecified passage) |
Freeze-dried fibrin/ hyaluronan |
500,000 cells/cm2 |
NA | 3-4 days | Cut to size using template |
Fibrin glue |
| Bioseed®-C | 2 | Autologous (unspecified passage) |
Polyglactin/ polydiaxanon, fibrin carrier |
3-5 million/cm2 |
3×2×0.2, 3×2×0.11, 3×3×0.11, 5×2×0.11 cm |
NA | Cut to size | Transosseous sutures, sewn, or fibrin glue |
| Cartipatch® | 2 | Autologous, up to P3 |
Agarose- alginate hydrogel |
10 million cells/mL |
1.0, 1.4, or 1.8 cm in diameter, 4 mm thick |
NA | Use of drills to create matching defects and constructs |
Press fit, fibrin glue optional |
| Chondrosphere® | 2 | Autologous (unspecified passage) |
Scaffold-free | 200,000 cells/sphe- roid, 10-70 spheroids/ cm2 |
500-800 μm in diameter |
2 weeks | Injected to defect bed |
Spheroids naturally adhere |
| Hyalograft® C | 2 | Autologous, up to P3 |
Non-woven mesh of hyaluronic acid-based microfibers |
1 million cells/cm2 |
2×2 cm (×2) | 2 weeks | Use of punches to create matching defects and constructs |
Press fit, fibrin glue optional |
| MACI | 2 | Autologous, P1-P3 |
Collagen I/III scaffold from porcine peritoneum |
0.5-1 million cells/cm2 |
4×5 cm (×2) | 3-4 days | Cut to size using template |
Fibrin glue |
| NeoCart® | 2 | Autologous (unspecified passage) |
Honeycomb bovine type I collagen scaffold, cell carrier |
NA | NA | 3-5 weeks | Cut to size | Collagen/PEG glue |
| NOVOCART® 3D | 2 | Autologous, P1 |
Bilayer type I collagen sponge containing chondroitin sulfate |
0.5-3 million cells/cm2 |
NA | 2 days | Use of punches to create matching defects and constructs |
Sutures or pins |
| RevaFlex™ | 1 | Allogeneic, juvenile (unknown passage) |
Scaffold-free | NA | 2.2-2.5 cm diameter |
> 40 days | Cut to size using template |
Fibrin glue |
| CaReS® | 2 | Autologous, primary |
Type I collagen hydrogel |
NA (possibly 20,000 cells/mL) (2500- 3,333 cells/cm2 |
3.4 cm diameter, 6- 8 mm thick |
10-13 days | Cut to size | Fibrin glue |
| INSTRUCT | 1 | Autologous, primary chondrocytes + bone marrow cells |
PEOT/PBT 3D printed scaffold |
NA (possibly 240,000 cells/cm2) |
0.4 cm diameter, 4 mm thick |
Immediate | NA | NA |
Table 2.
Clinical status of the reviewed cartilage products
| Product | Completed Clinical Studies | Ongoing Clinical Studies/Current Status | Clinical Indication |
|---|---|---|---|
| Biocart™II | - Prospective, preliminary study (n=8) (26) - Phase II study (n=40) (NCT00729716) (29) |
- Future trials not available | 1.5-7.5 cm2, up to 6 mm deep, symptomatic single contained lesion of femoral condyle/trochlea, can treat OCD |
| Bioseed-C | - Over 3,000 patients treated since 2002 - Randomized study comparing to ACI (n=21) (37) - Prospective study (n=79) (30, 36) |
- Available in some European countries - Future trials not available |
Grade III to IV focal chondral and osteochondral lesion |
| Cartipatch® | - Prospective, multi-center study (n=17) (39) - Phase III study (n=58) (NCT00560664) (41, 270) |
- Phase III study terminated (NCT00945399); future trials not available |
2.5-7.5 cm2, up to 10 mm deep, Grade III or IV isolated femoral osteochondral lesion, can treat OCD |
| Chondrosphere® | - Over 7,200 patients treated since 2004 - Prospective, multi-center study (n=42) (47) - Prospective, investigator-initiated trial (n=37) (49) |
- Phase II study to finish Nov. 2017 with 5- year outcome measures (NCT01225575) - Phase III study to finish Dec. 2020 with 5-year outcome measures (NCT01222559) |
1-4 cm2, up to 6 mm deep, Grade III or IV isolated single chondral lesion on femoral condyle |
| Hyalograft® C | - Over 5,000 patients treated since 1999 (53) |
- Withdrawn from market; future trials not available |
1.0-5.0 cm2, Grade III or IV chondral lesion of the femoral condyle/trochlea |
| MACI | - Study comparing to microfracture (n=60) (72) - Study comparing to C-ACI (n=91) (73) - Phase III study (n=144) (NCT00719576) (69) |
- Approved in the EU but production stopped |
≥3.0 cm2, Grade III or IV focal chondral lesion of femoral condyle/trochlea |
| NeoCart® | - Phase I study (n=8) (82) - Phase II study comparing to microfracture (n=30) (NCT00548119) (80) |
- Phase III study (n=245) to finish July 2017 with 1-year outcome measures (NCT01066702) |
symptomatic articular cartilage lesion of femur/trochlea |
| NOVOCART® 3D | - Over 6,000 patients treated since 2003 - Prospective study (n=23) (91) - Other prospective studies (n=30-41) (86, 93, 94) - Study on treatment of OCD (95) |
- Phase III study (n=233) to finish July 2018 with 2-year outcome measures (NCT01957722) - Non-interventional study (n=80) to finish Sept. 2019 with 3-year outcome measures (NCT02348697) - Phase III study (n=261) to finish June 2019 with 5-year outcome measures (NCT01656902) |
2-6 cm2, isolated articular cartilage lesions of femoral condyle |
| RevaFlex™ | - Phase I/II study (n=12) (102) | - Phase III study (n=225) to finish July 2019 with 5-year outcome measures (NCT01400607) |
<5 cm2 lesion of the distal femur |
| CaReS® | - Prospective, multi-center study (n=116) (109) - Retrospective study comparing to microfracture (n=20) (116) |
- available in select European countries, Turkey, Iran, and China - Future trials not available |
3.5-14 cm2, Grades III or IV lesion, can treat OCD |
| INSTRUCT | - Phase II study (n=40) finished June 2014 with 2-year outcome measures (127) (NCT01041885) |
- Future trials not available | symptomatic articular cartilage defects in knee |
2. Current cartilage repair therapies
Chondroplasty (76.6%) and microfracture (22.0%) account for the majority of the procedures performed on articular cartilage in the knee (4, 5). However, these cartilage repair options may have several shortcomings (6-11). Chondroplasty, used only when wear is minor, has acceptable short-term but potentially poor long-term results (6, 7). In microfracture, the defect is cleaned and the bone punctured to induce bleeding, resulting in a fibrocartilaginous repair tissue formed by multipotent marrow cells. Despite favorable short-term outcomes, studies have indicated deteriorating repair tissue quality after 1.5-5 years (8-11). Failure has been attributed to the inferior biochemical and biomechanical properties of the fibrocartilaginous fill (12) and alterations of the subchondral bone (13). On the other hand, mosaicplasty with osteochondral auto/allografts has been reported to offer better clinical results than microfracture (14, 15). However, issues with failure at the osseous region, lateral integration, and deterioration of graft edges have contributed to graft failure in 15-55% of patients after 10 years (16-18). Furthermore, mosaicplasty with autografts is limited to treating lesions less than 4 cm2 to minimize donor site morbidity. Although fresh allografts can be used to treat large defects, they are also limited in supply.
Autologous chondrocyte implantation (ACI) has been emerging as a superior, long-term treatment option (16, 19). When compared to multipotent marrow cells, passaged articular chondrocytes possess greater innate potential to form hyaline-like cartilage (20). When compared to mature osteochondral grafts, the immature neocartilage fill formed by ACI may have a better ability to integrate and conform to the defect. Despite these advantages, a 10-year follow-up study showed graft failure in up to 25% of ACI-treated patients (21). Although use of a collagen membrane instead of a periosteal flap drastically reduced complications such as hypertrophy, only 75% of patients reported good to excellent Cincinnati scores with this newer technique at a 2-year follow-up (22) (see Table 3 for common knee evaluation outcome measures). In addition, ACI commonly resulted in a fibrocartilaginous or fibrous fill, with only 15-30% of patients developing hyaline-like repair tissue in some studies (23, 24). These inconsistencies may arise from cell passaging, patient age, and surgical discrepancies. However, the procedure is also inherently inconsistent owing to a lack of control; for example, how many cells are retained, where they adhere, and how they form neocartilage is uncontrolled. Off-label use of first injecting the cell suspension to a collagen sponge represents a desire by physicians to place the cells more consistently into the defect (25). Currently, a number of cartilage products with pre-seeded cells have been developed to address the limitations of current practices. Although these products are often described as second- or third-generation ACI, they actually represent the first generation of tissue engineered cartilage products.
Table 3.
Common outcome measures in clinical studies involving cartilage repair
| Outcome Measure | Purpose |
|---|---|
| International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form |
To measure knee symptoms, sports and daily activities, and function |
| Knee Injury and Osteoarthritis Outcome Score (KOOS) and KOOS Physical Function Short Form (KOOS-PS) |
To measure patients’ opinions about their knee and associated problems |
| Cincinnati knee rating system (and modified versions) | To measure patient’s symptoms and perception of knee function with a focus on physical abilities |
| Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) |
To measure pain, stiffness, and physical function; widely used to evaluate knee or hip osteoarthritis |
| Lysholm Knee Scoring Scale | To measure patient’s evaluation of knee function; originally designed to assess ligament injuries |
| Tegner Activity Scale | To measure activity level; developed to complement the Lysholm scale. |
| Visual Analog Scale (VAS) for Pain Short Form 36 (SF-36) |
To measure patient pain on an analog scale from 1-100 To measure patient-reported general health |
| International Cartilage Repair System (ICRS) Cartilage Injury Evaluation |
Includes questions from SF-36, IKDC, Lysholm, and Tegner evaluation forms |
| Bern and O’Driscoll Scores | To histologically evaluate the quality of cartilage repair tissue relative to native articular cartilage |
| Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) |
To use different MRI variables to describe the constitution of the cartilage repair tissue and the surrounding structures |
3. Tissue engineered articular cartilage products
3.1. BioCart™II (Histogenics, Waltham, MA)
Biocart™II was first developed by Prochon Biotech, Ltd. until the company’s acquisition by Histogenics in 2011. The product is a fibrinogen/hyaluronic acid scaffold seeded with expanded autologous chondrocytes. Cells were expanded in the presence of autologous serum and 10 ng/mL fibroblast growth factor 2 variant (FGF2v) (26). FGF2v has been shown to increase cell proliferation rates and maintain the chondrocytic phenotype during expansion (27). The scaffold was described to be composed of homologous human fibrinogen (Omrix Biopharamceuticals, New York, NY) copolymerized with recombinant hyaluronan (Ferring Pharmaceuticals, Switzerland) with the use of thrombin and freeze-dried to yield a sponge-like, 3D structure (26). A company patent indicated that hyaluronic acid not only provides bioactivity to the matrix but also imparts viscoelastic properties to the scaffold (28). Cells were injected into the scaffold with a syringe at 500,000 cells/cm2 (26). The construct was cultured for 3-4 days prior to the implantation date. BioCart™II was ready for implantation 3 to 4 weeks after the first surgery (26).
During the operation, as described (26), the lesion was debrided and a template of the defect created with aluminum foil. Whether bleeding was avoided was not made clear. The BioCart™II construct was then cut to size using the template, press fit into the defect, and fixed with fibrin glue applied to the border of the defect. BioCart™II was described to contain some self-adhesive properties due to its high fibrinogen content. Rehabilitation involved continuous passive motion (CPM) for 4 weeks up to 30° range of motion. Touch-weight bearing was prescribed from day 2 to week 6 followed by increases in weight until free walking without assists at 12 weeks.
A Phase I trial (n=8) demonstrated the feasibility of BioCart™II for treating femoral lesions and osteochondritis dissecans (26). Defects were 1 to 8 cm2 (mean=3 cm2) and no deeper than 5 mm (mean=4.4 mm). IKDC scores improved at 6 and 12 months, while Lysholm scores improved at 6 months (see Table 3 for scoring methods). MRI at 12 months showed 75-100% fill in all patients, an intact surface (i.e., no damage and fissures) in 75% (6/8) of patients, and one case of construct delamination at the bone-cartilage interface. Integration was described as stable via MRI analysis. Another clinical study (n=31, defect size=3.3±1.9 cm, all femoral condylar defects) showed that BioCart™II treatment increased IKDC and MOCART scores at 12 months (29). Full results of the Phase II trial have not yet been published and plans for continuation toward Phase III trials have not been made available.
3.2. Bioseed®-C (BioTissue Technologies GmbH, Freiburg, Germany)
Bioseed®-C consists of a polyglactin 910/poly-p-dioxanone fleece scaffold seeded with a solution of fibrin and expanded autologous chondrocytes (30). During the first surgery, 250 mg healthy cartilage is collected along with 100 mL blood (31). Cells are expanded in presence of autologous serum for 3 weeks (31) to an unknown passage number. In a preclinical horse study, cells were expanded to P3 or P4 in RPMI, 1% horse serum, 5% FBS, and penicillin/streptomycin (32). The product comes in a variety of rectangular sizes (3×2×0.2, 3×2×0.11, 3×3×0.11, 5×2×0.11 cm) and is seeded with 28.8 × 106 cells per unit, which correlates to a seeding density of 2.9-4.8 × 106/cm2 (33). Fibrin glue is used as a cell carrier. The polymeric fleece has an interconnected porosity of about 90%. In the preclinical horse study, constructs were cultured for 3 weeks prior to implantation in Ham’s F-12 medium, 5% horse serum, penicillin/streptomycin, and 50 μg/mL ascorbic acid (32). Whether this stage is used in the actual product is unclear. On the company website, scaffold degradation is stated to occur within 6 months (34). The implant is ready 4.5 weeks after the first surgery (33).
For implantation, the defect zone is debrided down to the subchondral bone and the construct cut to size (31). For deep defects, two constructs may be layered. The construct is either fixed into place using a transosseous suture (Erggelet) technique, a regular suture, or fibrin glue. The procedure can be done arthroscopically or arthrotomically depending on the defect size and location (35). Rehabilitation involved CPM and loading of 15% bodyweight until for 6 weeks (30). After week 13, patients progressively increased loads until full weightbearing; mild exercise was allowed after 6 months.
Bioseed®-C has been used to treat more than 3000 patients since 2002 (33). A Phase II clinical study (n=79) with 2- and 4-year outcome measures showed significant beneficial effects with Bioseed®-C treatment (30, 36). At 2 years, significant increase in KOOS scores over baseline was observed (36). Histology of second-look biopsies were said to show good integration and formation of cartilaginous repair tissue. At 4 years, patients (n=50) showed significant improvements in ICRS, IKDC, KOOS, Lysholm, and Noyes scores compared to baseline values (30). MRI showed complete filling in 72.7%, moderate filling in 25%, and less than 50% filling in 0.3% of patients. In another clinical study (n=21), Bioseed®-C was compared to ACI (periosteal flap method) in a randomized trial (37). No significant differences were found in IKDC, SF-36, Tegner, and MRI scores at 12 and 24 months. However, the ACI method scored better in knee functionality (Lysholm and Gillquist) tests. Complication rates with Bioseed®-C treatment was similar to that of ACI (38). Bioseed®-C is currently available in Europe.
3.3. Cartipatch® (Tissue Bank of France, Lyon, France)
Cartipatch® is an agarose-alginate hydrogel seeded with expanded autologous chondrocytes. As described (39), chondrocytes were expanded to a maximum of passage 3 (P3) in medium consisting of DMEM/Ham’s F-12 (1:1), 10% autologous serum, ascorbic acid (50 mg/L), and gentamicin/Fungizone. Use of antibiotics and Fungizone was discontinued after P1. Cells were then suspended in medical grade, ultrapurified agarose-alginate (GelForCel; Tissue Bank of France, Paris, France) at a minimum concentration of 10 million cells/mL and cast into plugs 10, 14, or 18 mm in diameter. The cell embedding technique is presumably similar to that commonly used with low-melt agarose.
For implantation, the defect was drilled to a depth up to 4 mm using specially-designed drill bits to match the construct size (39). The size and number of constructs used (up to three) were preplanned to provide the best coverage of the defect. Implanted Cartipatch® constructs were generally level with the surrounding tissue. Fibrin glue was used in one case to improve construct stability.
A prospective, multi-center study (n=17) showed positives results with Cartipatch® treatment (39). Because the operation involved removal of subchondral bone, Cartipatch® was largely used to treat osteochondritis dissecans (76% of patients). The defects ranged from 1.0 to 5.1 cm2 (mean=3 cm2). At 2 years, patient IKDC scores significantly improved over baseline. Second-look arthroscopy revealed the repair cartilage as “normal and nearly normal” in 85% (11/13) of patients, level with surrounding tissue in 77% (10/13) of patients, and smooth in 38% (5/13) of patients. Histological analysis of biopsies showed hyaline cartilage in 62% (8/13) of patients, while others developed fibrocartilage. A Phase III trial (n=58) comparing Cartipatch® to mosaicplasty was completed on August 2013 with 2-year outcome measures (IKDC, MRI, arthroscopy with biopsy) (40). Published histological results at 2 years showed that mosaicplasty resulted in superior repair tissue quality (41), prompting the authors to note that 2 years was insufficient for the construct to fully develop into cartilage tissue. A Phase III trial comparing Cartipatch® to microfracture was scheduled for completion for September 2014 but had been terminated (42).
3.4. Chondrosphere®/ACT3D/Athrocell 3D (co.don AG, Teltow, Germany)
Chondrospheres® (also known as ACT3D-CS or ARTHROCELL 3D®) are small spheroids of neocartilage composed of expanded autologous chondrocytes and their associated matrix. Autologous chondrocytes are expanded in the presence of human serum and in the absence of antibiotics, fungicide, or exogenous growth factors (43). A company patent indicates the potential use of DMEM/Ham’s F-12 (1:1) and 10% autologous serum for expansion (44). Passage number is kept to a minimum (45). For spheroid formation, one study described seeding 200,000 cells per well of hydrogel-coated, 96-well plates (46). These wells were possibly concavely tapered (44). After 2 weeks of culture, the spheroids grew to 600-800 μm in diameter and were then used for implantation (47). During 3D culture, the chondrocytes began a process of redifferentiation and secreted an abundance of cartilage-specific matrix. Penicillin and streptomycin was stated not to be used because it slowed cell expansion, delayed spheroid formation, and decreased the expression of chondrogenic markers (48). Spheroids could be fused at 1 week to form larger neotissue depending on the patient’s needs (45, 46), although this strategy does not seem to be used in recent clinical studies. The spheroid outer layer was stated to contain a zone of proliferating and migratory cells that facilitate integration with other spheroids and adjacent native tissue (47, 48). The Chondrosphere® product is available 5-10 weeks after the first surgery (49).
During the operation, Chondrospheres® were suspended in a saline solution and evenly placed with a syringe into the debrided defect. Generally, subchondral bleeding is avoided or the bleeding is stopped before implantation (49). Information on a planned Phase III clinical trial recommends a dose of 10-70 spheroids/cm2 in the defect (50). One study involving 37 patients reported the use of 14-170 spheroids/cm2 (average = 60 spheroids/cm2) in defects ranging from 1-12 cm2 (average = 4.4 cm2) (49). No glue or overlying material was used, as the spheroids naturally adhered to the defect bed within 20 minutes. Rehabilitation involved knee immobilization for 48 h followed by 6 weeks of CPM and partial weightbearing exercises (49).
Since 2004, Chondrosphere® has been used to treat defects of the knee, ankle, shoulder, and hip (47) and, as of 2015, has treated over 7,200 patients in Germany (43). However, few clinical studies have been published. One publication briefly described a prospective, multi-center study (total n=42) in treating patellofemoral lesions 1.5-10 cm2 in size (47). At a 2-year follow-up, Chondrosphere® treatment was reported to improve range of motion, pain levels, and Lysholm scores, while IKDC and WOMAC scores remained unchanged. Second-look arthroscopy of nine patients performed at 3 months was reported to reveal filled defects, excellent integration, and a smooth repair tissue surface (47). In an investigator-initiated trial (n=37), Chondrosphere® treatment was shown to significantly improve patient evaluation scores (IKDC, VAS for pain, Lysholm, Tegner, and SF-36) over baseline values at 1-year follow-up (49). Fast filling of the defect was reported, as indicated by MOCART, although baseline values were not reported. A Phase III trial (n=102) comparing Chondrosphere® to microfracture is estimated to finish September 2017 with 2-year outcome measures (KOOS) and December 2020 with 5-year outcome measures (KOOS, MOCART, arthroscopy with biopsy, Bern, ICRS/IKDC, Lysholm) (50).
3.5. Hyalograft® C (Anika Therapeutics, Bedford, MA)
Hyalograft® C is hyaluronic acid-based scaffold seeded with expanded autologous chondrocytes. The product was developed as early as 1998 by Fidia Advanced Biopolymers (Abano Terme, Italy), which was then acquired by Anika Therapeutics in 2009. As described (51-53), primary chondrocytes were expanded up to P3 in medium consisting of Ham’s F-12, 10% fetal calf serum (FCS), 1% penicillin/streptomycin, 1% L-glutamine, 1 ng/mL TGF-α1 or TGF-β1, 1 ng/mL insulin, 1 ng/mL EGF, and 10 ng/mL bFGF (51, 52). After chondrocyte expansion, 8 million cells in 0.4 mL medium (now containing 50 mg/mL ascorbic acid) were seeded to two 2×2 cm Hyaff®11 scaffolds. The next day, medium was added to submerge the construct. The medium was changed twice a week for 2 weeks before implantation. The Hyaff®11 scaffold has been described as a non-woven, 3D mesh of 20 μm diameter fibers with 10-400 μm pore size and 80% porosity (54). The fibers were composed of hyaluronic acid with 90-100% of its carboxyl groups substituted with benzyl esters (55). Upon esterification, these hydrophobic polymeric chains can undergo aggregation, allowing the material to be processed into various forms including extrusion into microfibers. The Hyalograft® C product has been reported to be available for implantation 6 weeks after the first surgery (56).
Several in vitro studies have characterized various aspects of chondrocyte-seeded Hyaff®11 scaffolds. Two days after cell seeding, 92-96% of initial chondrocytes were reported to have adhered to the scaffold (57). At day 33 post-seeding, cells were evenly distributed over the scaffold surface but appear to only penetrate ~200 μm inwards (57). As early as day 7 of culture, chondrocytes in the scaffold were able to redifferentiate in the 3D environment, as shown by increasing collagen type II expression. Chondrocytes continuously exhibited proliferation within the scaffold up to day 21 (58). Such proliferation may be beneficial in contributing to initial cell infiltration and neocartilage growth but raises concerns for potential overgrowth (53). Collagen I expression was present in cell-seeded Hyaff®11 constructs up to 21 days of culture (58). More recent in vitro studies have shown that use of a perfusion bioreactor can improve cell penetration into Hyaff®11 scaffolds (59, 60). The bioreactor also substantially increased construct size and matrix content (59).
The operation, as previously described (61), involves the use of diameter-adjustable drills to debride the lesion to a predetermined diameter and thickness of 2 mm, as to avoid subchondral bleeding. The same instrument is used to punch out one or multiple circular Hyalograft® C constructs from two 2×2 cm stock constructs to fill the defect. The constructs are press fit into the defect without additional fixation. Rehabilitation involved immobilization for 24 h and CPM from 0-30° until full range after 4 weeks (62). Crutch-assisted, non weight-bearing ambulation was ordered for 6 weeks; touch-down weight-bearing to full weight-bearing was prescribed from weeks 7 to 12. Moderate exercise started at 3-6 months.
Clinical efficacy of Hyalograft®C has been comprehensively reviewed in a European Medicines Agency report (53). From 1999 to 2011, Hyalograft®C have been used in 5348 patients and a total of 28 studies (n=793) have reported clinical results. Hyalograft®C treatment was shown to generally improve patient evaluation scores over baseline and, in some cases, was superior over microfracture treatment. Biopsies taken at various time points showed hyaline tissue in 53%, fibrocartilage in 22%, and mixed cartilage in 25% of patients (n=68). MOCART scoring at 24 months reported complete defect repair and complete integration in 68% and 86% of patients (n=118), respectively. Overall failure rate was found to be 9.3% in 551 patients. Scaffold degradation was said to occur within 10 months in the vast majority of patients. Despite supportive evidence of clinical efficacy, several objections were raised, including quality control and lack of randomization typical of Phase III studies. Commercialization was stopped in 2013.
3.6. Matrix-induced Autologous Chondrocyte Implantation (MACI; Vericel, Cambridge, MA)
MACI was first developed and marketed in 1999 by Verigen AG (Leverkusen, Germany) until its acquisition by Genzyme in 2005, by Sanofi 2011, and then by Vericel Corporation (Cambridge, MA) in 2014. In a European Medicines Agency (EMA) report, which approved the marketing of MACI in the European Union, the product is described as a 14.5 cm2 type I/III collagen membrane (ACI-Maix™; Matricel, Herzogenrath, Germany) seeded with expanded autologous chondrocytes (63). Chondrocytes were described to be passaged to either P2 or P3 in medium consisting of DMEM, 9% FBS, and 45 μg/mL gentamycin. Cells are then seeded to a 4×5 cm ACI-Maix™ scaffold (5.5 cm2 is used for product testing) at a density of 0.5-1 million cells/cm2. Constructs have been reportedly cultured for an additional 3 (64) or 4 (65) days before implantation. The ACI-Maix™ scaffold is derived from decellularized porcine peritoneal tissue and largely consists of collagen and elastin (63). The resulting scaffold has a bilayer structure with a porous side to allow cell infiltration and a non-porous side for cell retention, as shown by scanning electron microscopy (65, 66). The porous side is placed facing the defect bed. Detailed characterization of the scaffold structure could not be found. The approximate dried scaffold thickness has been reported to be 400 μm (65). Histology of the pre-implanted MACI product shows a fairly inhomogeneous infiltration of cells into the scaffold ranging from 10 to 200 μm deep (65, 67, 68). Upon receiving the implant, cells were shown to already exhibit characteristics of redifferentiation, such as rounded cell morphologies and increased collagen II, aggrecan, and S-100 expression (65). The MACI product is available 4-8 weeks after the first surgery.
During the operation, the lesion was debrided without breaching the subchondral bone (69). A foil piece was used to template the defect and the MACI product is cut to size according to the template (70). A thin layer of fibrin glue was used at the base and edges of the defect for fixation (69), although previous studies have used suture fixation (70). Because the scaffold is thinner than the defect thickness, it resides depressed relative to adjacent cartilage. Rehabilitation was based on a 4-phase program (71) and consisted of 1) restricted CPM and weightbearing (0-6 weeks), 2) gradual progress toward full range of motion and full weightbearing exercises (7-12 weeks), 3) light exercises (3-6 months), and 4) normal activity (6-12 months) (69).
Several studies have shown clinical efficacy of MACI. In a randomized, open-labeled, multi-center Phase III trial (n=144) (69), where only lesions > 3 cm2 were treated, MACI scored high in KOOS pain and function than microfracture at a 2-year follow up. In the MACI group, no cases were identified as treatment failures. Another study (n=60) showed that several outcome measures (Lysholm, Tegner, and patient and surgeon ICRS scores) improved significantly with MACI than with microfracture at 2 years (72). In a study comparing MACI to C-ACI (i.e., ACI with a collagen sponge, n=91), both groups showed improved clinical results but were not different from one another at 1-year follow-up (73). Interestingly, 4 of 11 (36%) MACI biopsies showed fibrocartilage, as also supported by other studies (74, 75), indicating a potential need for improvement. In a 5-year follow-up study (n=21) with only MACI-treated patients, 19 patients exhibited improved clinical scores and two exhibited graft failure; 76% of grafts had normal filling, as assessed by MRI. Other long-term clinical studies found similar results (76-78). Although MACI has gained EU market approval, its facilities closed in Europe and the technology was sold to Vericel. Market approval in the U.S. remains to be determined.
3.7. NeoCart® (Histogenics, Waltham, MA)
NeoCart® is comprised of a honeycomb bovine type I collagen scaffold seeded with expanded autologous chondrocytes. The company website describes 2-3 weeks are needed for cell isolation, expansion, and seeding of the 3D scaffold; 1 week culture is needed in a biomimetic bioreactor; and 2-4 weeks are needed for static culture before implantation (79). The bioreactor involves hydrostatic pressure, perfusion, and hypoxia as biomimetic stimuli (80). Data presented in a company patent showed that cyclic hydrostatic pressure (0.5 MPa, 0.5 Hz) applied during the first 6 days of culture increased S-GAG production (81). Culture in hypoxia (2% O2) also improved GAG production, while perfusion (5 μL/min) was said to increase cell proliferation (81). To create the construct, the patent mentions first suspending the cells in a thermoreversible collagen gel at a concentration of 5-10×106 cells/mL before seeding it to a freeze-dried, type I collagen sponge (81). The construct was reported to be available, on average, 67 days after the first surgery (82).
During the operation, the defect is debrided and bleeding avoided (82). The construct is cut to size and fixed into the defect with a collagen/PEG-based adhesive (CT-3 glue; Histogenics) applied to the underlying and adjacent tissue. The CT-3 glue, stated to be 10× stronger than fibrin glue (83), was found to be the preferred strategy of fixation after two failed surgeries that involved the use of suture fixation (82). Rehabilitation followed the standard protocol for microfracture: toe-touch weightbearing for 6 weeks, a CPM regimen, and restriction of sports activities for 6 months (82).
A Phase I and Phase II study have shown beneficial results of NeoCart®. In the Phase I trial (n=8), NeoCart® implantation significantly improved pain scores and range of motion over baseline values at 24 months (82). Five patients showed improvement in all categories; six patients showed good fill (>66%); and four patients demonstrated an organized collagen matrix characteristic of native cartilage, as indicated by MRI. In the Phase II trial, NeoCart® (n=21) significantly improved KOOS, IKDC, and VAS scores over microfracture (n=9) at 24 months (80). Advantages of NeoCart® were recorded as early as six months. In addition, a greater percentage of patients treated with NeoCart® (76%) were considered “therapeutic responders” than those treated with microfracture (22%) at 12 months. A randomized Phase III trial (n=245) comparing NeoCart® to microfracture is estimated to finish July 2017 with 1-year outcome measures (KOOS, IKDC, and MRI) (84).
3.8. NOVOCART® 3D (TETEC, Melsungen, Germany)
NOVOCART® 3D consists of a biphasic type I collagen scaffold seeded with autologous chondrocytes. Isolated chondrocytes are expanded in monolayer in the presence of homologous serum until P1 (67). No antibiotics of antimycotics are used (85). One layer of the bilayer scaffold is a dense, cell-impermeable membrane derived from bovine pericardium (86). This membrane is lyophilized with a collagen sponge layer, allowing the two layers to be firmly connected. A company patent describes the collagen sponge layer as consisting of type I collagen and chondroitin sulfate with pores 130 to 200 μm in diameter arranged in columns (87). In one study, the scaffold is seeded with 1.45 million cells/cm2 and cultured for 2 days (67). The patent indicates the possible use of a solution of collagen VI as a cell-carrier prior to seeding (87). Histology of the pre-implanted construct shows dense, homogeneous cell distribution in the porous layer and devoid of cells in the dense layer (67). The product can be manufactured and delivered in 3 weeks (85).
The construct, implanted via miniarthrotomy, was attached to the healthy cartilage using absorbable Vicryl sutures or pins when there was no healthy cartilage adjacent to the implant (88, 89). Rehabilitation followed a standard program (90) involving CPM, toe-touch weightbearing for 4 weeks, and full weightbearing by 8 weeks (91).
Over 6,000 patients in Europe have undergone successful treatment with NOVOCART® 3D since 2003 (92). A prospective clinical study (n=23) showed that NOVOCART® 3D treatment led to increased IKDC, Tegner, Noyes, and MOCART scores over baseline at 2-year follow-up (91). Defect filling was described as complete or “slightly incomplete” in most cases. Other clinical studies have also shown increased IKDC, VAS, and MOCART scores over baseline at 1- or 2-year follow-up (86, 93, 94). NOVOCART® 3D coupled with a cancellous bone autograft has been used to treat osteochondritis dissecans; after 2- to 5-year follow-up, patients (n=26) exhibited improved Lysholm, Cincinnati, and MOCART scores (95). However, some of these studies have reported graft hypertrophy in 20-27% of patients at 2-years (91, 93, 94), indicating a need for long-term evaluation. A Phase III trial (n=233) comparing NOVOCART® 3D to microfracture is estimated to finish in July 2018 with 2-year outcome measures (KOOS pain and function); the estimated study completion date is 2021 (96). A non-interventional study (n=80) to evaluate the safety and efficacy of NOVOCART® is estimated to finish in September 2019 with 3-year outcome measures (IKDC, number of adverse events) (97). Finally, another Phase III trial (n=261) comparing NOVOCART® 3D plus and microfracture is estimated to finish in June 2019 with 5-year outcome measures (IKDC, KOOS, MOCART, SF-36 scores, and others) (92).
3.9. RevaFlex™/DeNovo® ET (Isto Technologies, St. Louis, MO)
DeNovo® ET has been renamed RevaFlex™ since Isto Technologies obtained full control of its development in 2013. RevaFlex™ is a scaffold-free neocartilage disc composed of allogeneic juvenile chondrocytes and their associated matrix. Juvenile chondrocytes have been shown to have better proliferation and matrix secretion capabilities than adult chondrocytes (98, 99). Although current production methods are unknown, published studies describe a method that involves expansion of chondrocytes and growth of a 3D neocartilage construct in a single step (98, 100). Primary chondrocytes were seeded onto an unknown surface at 500,000-1,000,000 cells/cm2 and cultured for 44-63 days to form a 3D neocartilage construct (98). Whether cell expansion occurs after the initial seeding is unknown. As a culture medium, HL-1 or a proprietary, chemically-defined medium, displaying growth characteristics similar to HL-1, was used. The medium was supplemented with 50 μg/mL ascorbate after 3 days. An earlier study described use of 10% serum that was gradually reduced to 0% (10% at day 3 to 5% at day 7 and to less than 2% at day 10) (100). Initial serum concentrations were said to be necessary for sufficient cell adhesion, while the transition to serum-free medium significantly increased sGAG and collagen type II production. Construct growth and dimensions were also described in this study. At day 90 of culture, construct thickness ranged from 0.75 to 1.5 mm. At an unspecified time during the process, the neocartilage discs were transferred to larger diameter culture plates as lateral growth continuously occurred – 150 day constructs were 50% wider than 90 day constructs. In another study, the mechanical integrity was qualitatively described as robust enough to be picked up by forceps at day 20, to hold sutures at day 30, and be implanted at day 40 (101). Because the cell source is allogeneic, only one surgery is required and the construct could theoretically be available at any time.
During the operation, the defect site is first debrided and bleeding avoided or hemostasis achieved with fibrin glue (102). A foil piece is used to create a template of the defect. The RevaFlex™ construct, 2.2-2.5 cm in diameter, is cut to size using the template as a guide and fixed into the defect with a thin layer of fibrin glue. It is recommended that the construct sit 0.5 mm below the surface relative to the surrounding native tissue. A recommended post-operative regimen consists of 4-6 weeks of protected or non-weight bearing with continuous passive motion and rehabilitative exercises.
Several animal studies have supported the ability of this construct to treat cartilage lesions. In an ovine model study, allogeneic neocartilage constructs (2.5 cm in diameter, 0.2-0.3 mm thick, cultured for 107-130 days) were cut to 0.5 cm diameter discs and implanted into partial thickness defects (0.55 cm diameter and 400-500 μm deep) in the femoral condyle using resorbable sutures as fixation (103). At 8 and 12 weeks, 70-80% of the animals (n=13) exhibited graft survival. Cases of failure were attributed to problems in post-operative leg immobilization. Of the survived constructs, histological analysis showed 70-100% cell viability in the neocartilage, partial filling, fair integration, and a layer of fibrous tissue covering the neocartilage construct. In a longer-term caprine model study, human neocartilage constructs were implanted into 6 mm condylar and 4 mm trochlear, full-thickness defects in each animal (n=8, two defects per animal) using fibrin glue (Tisseel; Baxter, Deerfield, IL) fixation (104). After 6 weeks immobilization followed by 18 weeks uninhibited movement, only 50% of the constructs survived, which was attributed to the difficulty of maintaining leg immobilization. In that study, the grafts were xenogeneic. Xenogeneic cartilage constructs have been previously shown to elicit a significant immune reaction (105), although such was not described here. The majority of the eight surviving grafts were described as completely filling the defect, having a smooth surface, and exhibiting partial integration.
In a Phase I/II study, 12 patients with 1-5 cm2 femoral or trochlear lesions were treated with RevaFlex™ (102). At 1 year, a second-look arthroscopy revealed the repair as normal or nearly normal in 67% (6/9) and “level” with surrounding tissue in 78% (7/9) of patients. The remaining 22% (2/9) had repair cartilage 75% of the lesion depth. Patient-reported evaluations and histology of biopsies were conducted but not reported. In all studies, immunological and histological analysis showed no adverse immune response, supporting in vitro findings that allogeneic chondrocytes may be immune-privileged (106). A Phase III trial (n=225) comparing RevaFlex™ to microfracture is estimated to finish July 2019 with 5-year outcome measures (KOOS, IKDC, various questionnaires) (107).
3.10. CaReS® (Arthro Kinetics Biotechnology, Krems, Austria)
The Cartilage Regeneration System (CaReS®) implant consists of a type I collagen hydrogel embedded with primary autologous chondrocytes. To form the constructs, isolated primary chondrocytes were suspended in 2× buffer solution, mixed with a rat tail-derived type I collagen solution (6 mg/mL) in 0.1% acetic acid at a 1:1 volume ratio, and gelled at 37°C (108, 109). At physiological pH and temperature, acid-extracted collagen solutions are able to form a hydrogel (110). The cell density used in CaReS® was unspecified and may be patient-dependent. Preclinical studies have indicated the use of 20,000 cells/mL (108, 111). A cell-free version of this collagen gel is sold as CaReS®-1S. Constructs formed are 3.4 cm in diameter and 6-8 mm thick (109), although the company website indicates any size can be made. Constructs are approximately twice the thickness of human cartilage, as about 50% of its water content can be lost during the implantation procedure (109). Other publications by the same authors have used a compressed collagen gel for increased mechanical stability (112, 113); whether this is used in the product is unknown. After gelation, constructs are cultured in vitro for 10-13 days in the presence of autologous serum and are then ready for implantation (109).
Although CaReS® has a relatively low cell density compared to other products, the high cellularity needed for sufficient neotissue formation can be achieved through in vivo cell proliferation and migration. Primary chondrocytes were shown to expand a factor of 33 in CaReS® gels after 6 weeks of in vitro culture (111). Although in this study evidence of chondrocyte dedifferentiation was observed, the chondrocytes maintained their differentiated state when implanted in vivo. On the other hand, P2 chondrocytes did not exhibit such chondrogenic phenotype in vivo, justifying the use of primary cells in the CaReS® product. Some evidence of cell migration from the host tissue into the collagen gel has also been shown in several in vivo animal models (108, 114, 115). However, the origin of these cells and whether they contribute to the formation of hyaline repair tissue remain unclear.
During the operation, the cartilage defect is first debrided to the subchondral bone (109). The CaReS® constructs were prepared using a punch 1 mm wider than the defect and molded into the defect with blunt forceps. Fixation was achieved by application of fibrin glue to the defect base. Rehabilitation involves immobilization for 48 hours, continuous passive motion for 6 weeks, and partial weight bearing up to 12 weeks.
A prospective, multi-center study (n=116) of patients treated with CaReS® from 2003-2008 supports its safety and clinical efficacy (109). Mean follow-up time was 30 months and mean defect size was 5.4 cm2. IKDC, global pain, SF-36, and IKDC functional knee score significantly increased from baseline with treatment. Total adverse events were low and fell below those seen in ACI. Unfortunately, second-look arthroscopy, MRI analysis, or biopsies were not reported, giving little information on repair tissue fill and quality. However, histological analysis of these constructs at 1 year in a minipig model indicates that they promote hyaline cartilage formation (108). When compared to microfracture, a small retrospective study (n=20) indicated no differences between the two treatment options (116). CaReS® has been used in select European countries and is also approved in Iran, Turkey, and China.
3.11. INSTRUCT (CellCoTec, Bilthoven, Netherlands)
INSTRUCT is a poly(ethylene oxide-terephtalate)/poly(butylene terephtalate) (PEOT/PBT) scaffold seeded with primary autologous chondrocytes and bone marrow cells. Although details of the scaffold are unknown, one study described the use of a Bioplotter device (Envisiontec, Germany) to create a porous lattice structure with approximately 170 μm diameter fibers, 200 μm pore size, and 56% porosity (117). The softer PEOT functions to support the chondrogenic phenotype, while the PBT increases material stiffness (118). The scaffolds were said to be “mechanically functional” (119), with one study showing a “compressive dynamic stiffness” of 10 MPa (117). Scaffold degradation occurs via hydrolysis of ester groups and oxidation and scaffolds are said to be resorbed within 12 months (119). Cell seeding is conducted at point-of-care. The patient’s cartilage biopsy and bone marrow aspirate are inserted into a semi-automated machine, termed the INSTRUCT cell processor, that isolates the chondrocytes, mixes them with marrow cells, adds fibronectin, and seeds the cell mixture to the scaffolds (120). Fibronectin was said to facilitate cell aggregation and enhance matrix formation (117). The cell seeding density is unknown and may be patient-dependent. A density of 60 million cells/mL (i.e., 3 million cells seeded per 0.4 cm diameter, 4 mm thick scaffolds) has been used in in vitro (117, 118). The cell processor is able to produce a usable implant within 1 hour (120). Details on the surgical procedure could not be found.
Different scaffold compositions and structures have been explored to determine the parameters that best support neocartilage formation. PEOT/PBT 2D films with a higher PEOT mass ratio were found to support a rounded chondrocyte morphology, no proliferation, and higher collagen II:collagen I gene expression; films with a higher PBT mass ratio supported a flat cell morphology, cell adhesion, and proliferation (121, 122). Similarly, chondrocytes cultured in 3D scaffolds with the composition 1000PEG-70:30 (PEG molecular weight = 1000 g/mol; 70% PEOT and 30% PBT by mass) secreted more sulfated glycosaminoglycans (GAGs) than those in 300PEG-55:45 scaffolds after 4 weeks of in vitro culture (117). Different scaffold structures have also been compared. A 3D printed scaffold with a lattice structure supported more neocartilage growth than in a salt-leached, porous scaffold, most likely due to the former’s pores being larger and more accessible (123). When comparing two 3D printed scaffolds of different porosities, the scaffolds with lower porosity (56%) supported more GAG production than scaffolds with higher porosity (74%) (these less porous scaffolds were said to exhibit mechanical properties matching those of native tissue) (117).
Neocartilage formation within these scaffolds has been assessed. SEM images of 1 day-old constructs show chondrocytes largely aggregated within the pores with few cells adhered to the scaffold (117). After 4 weeks of in vitro culture, neocartilage completely filled the pore space. After 4 weeks subcutaneous implantation in nude mice, the neotissue formed was described to be largely hyaline. However, after 3 months in vivo in a rabbit osteochondral defect, the scaffold (0.4 cm diameter, 4 mm thick) formed repair tissue described as fibrous tissue, incompletely differentiated mesenchyme, and cartilage (123). Bleeding in the osteochondral model may have led to fibrous tissue formation. Acellular salt-leached, porous scaffolds in the same osteochondral model was shown to support a top layer of hyaline neocartilage formation, indicating the scaffold’s potential chondroinductive properties (124).
Scaffold degradation occurs through hydrolysis and oxidation. In vitro hydrolysis experiments show more rapid degradation of scaffolds with a higher PEOT content (125). When implanted subcutaneously in mini-pigs for 52 weeks, 300PEG-55:45 scaffolds remained largely intact, while 2000PEG-80:20 scaffolds largely fragmented to < 1 μm particles (122). Molecular weight of polymer chains decreased 34% and 90%, respectively. In the latter, macrophages dominated the cellular response, but cell viability in the region was reportedly high. After 3 months in a rabbit osteochondral or mouse subcutaneous model, 300PEG-55:45 and 1000PEG-70:30 scaffolds seemed to exhibit minimal degradation (117, 123). Ideally, long-term analysis of PEOT/PBT degradation, specifically in an ectopic defect, would better demonstrate its degradation profile and biocompatibility. The company website states scaffolds resorb within 12 months (119).
A Phase II trial (n=40), single group assignment, open-label) was completed June 2014 with 2-year outcome measures (KOOS, IKDC, VAS, histopathology, and MRI) (126). In the study, patients showed improvement in VAS, IKDC, and all KOOS scores over baseline at 1-year follow-up (127). Biopsies taken at the 1 year showed 72% (21/29) of patients with hyaline cartilage and 97% (28/29) with either fibrocartilage or hyaline cartilage. Full publication of the results or additional clinical trials could not be found.
4. Tissue engineering strategies used in current clinical products
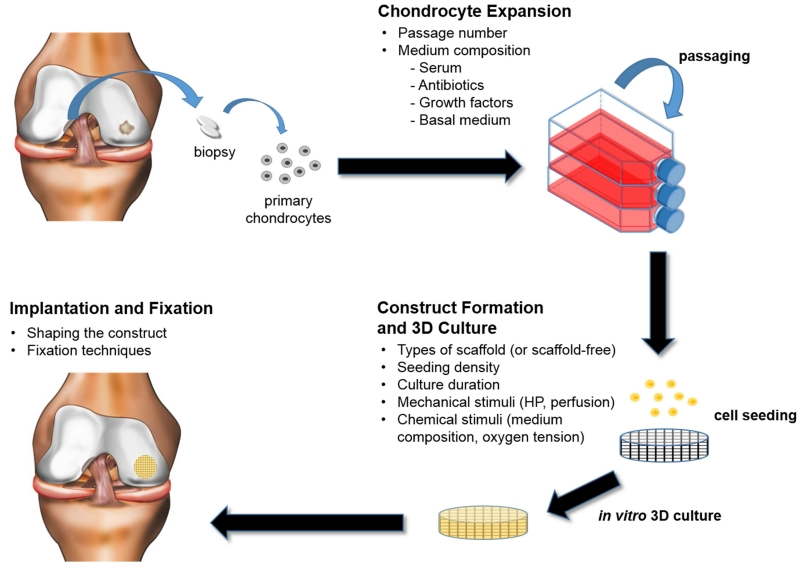
This section of the review compares the different tissue engineering strategies used during each stage of the tissue engineering paradigm. The paradigm consists of 1) identifying a cell source (i.e., primary or passaged articular chondrocytes), 2) forming the construct either using scaffold or scaffold-free approaches, and 3) culturing the construct in vitro, where biomimetic stimuli can be further applied, before implantation (Figure 1). Construct maturation, implantation, fixation, and rehabilitation are also included in this section. For each topic, advantages and disadvantages of different strategies are discussed, current challenges identified, and future directions suggested.
Figure 1.
The classical tissue engineering paradigm used for the fabrication of the reviewed cartilage products. Various factors can be manipulated in each stage, as listed.
Any comparisons made should not be construed to advocate one product over another. Because there is probably proprietary information unknown to us, the comparisons below may not reflect fully the final products. The ultimate aim of this section is to provide insights to clinically translatable strategies for tissue engineering articular cartilage.
4.1. Chondrocyte expansion
Most products involve a chondrocyte expansion stage, except for CaReS® and INSTRUCT which use primary chondrocytes. To obtain sufficient cell numbers for therapy, 200-300 mg autologous biopsies from less loaded regions of the knee are first digested to yield primary chondrocytes. As human articular cartilage contains 1000-8000 cells/mg tissue (128, 129), a biopsy can yield 0.2-2.4 million cells. Seeding with 0.5-5 million cells/cm2 in lesions 1-12 cm2 in size would require 0.5-60 million cells. Assuming that the cell expansion factor after each passage is four, although this is highly dependent on the initial seeding density, then primary chondrocytes need to be passaged one to four times to reach sufficient numbers.
A caveat of chondrocyte expansion is the rapid loss of the chondrogenic phenotype. To mitigate chondrocyte dedifferentiation, one can minimize the passage number (or cell expansion factor) or optimize the expansion medium components to best maintain the chondrogenic phenotype (e.g., serum, antibiotics, and growth factors). Table 4 lists the expansion medium composition used in the reviewed products. How these factors affect the chondrocyte phenotype and ultimately neocartilage formation is described next.
Table 4.
Cell expansion medium composition (blank entries indicate the information was not available to the authors). The last column represents additional supplements that were added to the existing expansion medium to formulate the medium used for 3D culture. Cell passage numbers are indicated in Table 1.
| Product | Base Medium | Serum | Antibiotics | Growth Factors | Ascorbate | Other Additives |
3D Culture |
|---|---|---|---|---|---|---|---|
| Biocart™II | Autologous serum | FGF-2 variant (10 ng/mL) |
|||||
| Bioseed-C | Autologous serum | ||||||
| Cartipatch® | DMEM/Ham’s F-12 (1:1) |
10% autologous serum |
Gentamycin fungizone (discontinued after P1 |
50 μg/mL | |||
| Chondrosphere® | DMEM/Ham’s F-12 (1:1) |
Autologous serum | None | None | |||
| Hyalograft® C | Ham’s F-12 | 10% FCS | Penicillin streptomycin |
TGF-B1 (1ng/mL), FGF-2 (5ng/mL), EGF (1ng/mL) |
1 ng/mL insulin 1% L-glutamine |
+ 50 mg/mL ascorbic acid |
|
| MACI | DMEM | 9% FBS | Gentamycin | ||||
| NeoCart® | |||||||
| NOVOCART® 3D | Allogeneic serum | None | |||||
| RevaFlex™ | HL-1 or similar | 10% serum to serum-free |
Yes | 50 μg/mL |
4.1.1. Chondrocyte passage number (or cell expansion factor)
Of the reviewed products, few details on the passage number and no information on the cell expansion factor can be found. Passage number refers to the number of times chondrocytes are serially passaged in monolayer. The cell expansion factor is calculated by dividing the final cell yield by the initial number of primary chondrocytes seeded for expansion. An important note is that cell expansion factor does not correlate with passage number. Cartipatch®, Hyalograft® C, and MACI indicate use up to P3 cells, while NOVOCART® 3D indicates use of only P1 cells. Unfortunately, the other products lack this description. Nevertheless, given the calculations above, use of P2 and P3 chondrocytes can be generally inferred for most products, with infrequent use of P1 and P4 chondrocytes. Whether passage number in these ranges can affect the quality of neocartilage formation is discussed below.
The hallmarks of chondrocyte dedifferentiation include a progression from rounded to fibroblastic cell morphologies, an increase in cell size, and decreased secretion of cartilage-specific matrix (130, 131). Microarray analysis comparing P0 and P2 chondrocytes shows changes in 137 genes (132). Changes generally follow a downregulation of chondrogenic genes including SZP, BMP-2, TGF-β1, FGFR3, COMP, aggrecan, collagen II, collagen XI, collagen IX, and SOX 9 and an upregulation of fibroblastic or mesenchymal genes including collagen I, collagen X, and collagen III, tenascin, and versican (133-142). These changes occur rapidly within the first passage (133, 135, 137, 138), with one study showing changes within 4 days (135). Gene expression changes from P0 to P1 also appear the largest among these studies. From P1 to P4, the incremental changes between each passage are less pronounced, but a decreasing trend in chondrogenic gene expression exists. When comparing P1 and P4 chondrocytes, large differences can be found. However, when comparing P2 and P3 chondrocytes, significant differences are less commonly found. Nevertheless, these results indicate that cells of each passage possess a different gene expression profile. Whether these differences are carried over to protein synthesis and, thus, affect neocartilage formation is discussed below.
Chondrocyte redifferentiation can be induced by prolonged 3D culture (e.g., pellet culture, alginate encapsulation, suspension culture, culture within a scaffold, etc.), where chondrocytes largely cease proliferating (143), change gene expression that is generally conducive to redifferentiation, and secrete cartilage-specific matrix. Upon 3D culture, chondrocytes of different passage numbers in the low range (approximately P1 to P4) have been shown to upregulate chondrogenic genes to similar levels (137, 144-147). Therefore, among the reviewed products, the use of different passage numbers seems to be of little consequence in affecting neocartilage formation. On the other hand, high-passage chondrocytes (> P4) have been shown to lose their ability to partially or completely redifferentiate (133, 144, 146, 148, 149). However, defining this critical passage number is difficult because of several confounding factors. For one, P4 cells from one study most likely have undergone a significantly different cell expansion number than P4 cells from another study, making the passage number inadequate in expressing how many times the cells have divided. Furthermore, differences in the cell source, such as age (98, 128) and the use of exogenous stimuli (145, 150), during expansion can affect chondrocyte dedifferentiation and redifferentiation. Therefore, comparisons among studies are difficult and one cannot assume use of P1 or P4 chondrocytes will lead to the same clinical outcome. Therefore, it should be critical to report both the passage number, as well as the cell expansion factor. Finally, ideally, authors of clinical studies should acquire the cell expansion factor of each graft from the manufacturer to determine potential correlations with clinical outcome, such as is sometimes done with lesion size and patient age.
4.1.2. Expansion medium compositions
The expansion medium compositions used in the reviewed products remain largely proprietary, though some information can be gleaned from early studies. Known differences among the media used include the use of serum, antibiotics, growth factors, basal medium, and other supplements.
4.1.2.1. Serum
Without additional growth factors, serum is necessary to induce chondrocyte proliferation in monolayers (151, 152). MACI and Hyalograft® C use fetal bovine serum (FBS) and NOVOCART® 3D uses allogeneic serum, while the majority use autologous serum – all typically at 10% (v/v). Only RevaFlex™ uses a largely serum-free medium. Despite the majority of products using serum for cell expansion, there is a large body of literature supporting the use of serum-free media. One disadvantage of serum is that its composition is source-dependent and its use may confound product quality. FBS batch-to-batch composition is potentially more consistent than the serum composition among individual patients. On the other hand, the use of autologous serum can eliminate risk of disease transmission. Secondly, serum may adversely affect the cell’s chondrogenic potential. As articular cartilage is non-vascularized, a serum-free environment may more closely mimic in vivo conditions. Early studies have shown serum to prevent chondrogenesis in limb buds during development (153, 154). Currently, use of serum-free medium is standard for culturing chondrocytes in 3D (e.g., pellets, hydrogels, suspension culture, etc.), as supported by studies showing more hyaline-like matrix production in serum-free 3D cultures (155-157).
Many studies have demonstrated the feasibility of expanding chondrocytes in serum-free medium. Such a medium is supplemented with growth factors (e.g., FGF2, PDGF-BB, TGF-β1, IGF-1, BMP-2, EGF etc.) and other additives (e.g., ITS+ and Ham’s F-12 medium) to promote cell growth and proliferation (158-160). Matrix-coated culture flasks have also been used to promote cell adhesion and provide chondroinductive cues (161, 162).
Whether use of a serum-free expansion medium is clinically beneficial remains unclear. Chondrocyte expansion in serum-free medium promotes chondrogenic gene expression, such as SOX-9, as assessed in the cells immediately after monolayer culture (155, 160, 163). When chondrocytes were expanded with or without serum and then cultured in 3D (e.g., pellets, hydrogels, suspension cultures, etc.) in serum-free medium, the latter group expressed enhanced chondrogenic markers (160, 162, 164) and formed neocartilage with higher compressive properties (165). However, other studies have shown no difference in the type II collagen (165) and GAG (160) content of the neocartilage formed by chondrocytes expanded with or without serum. The most compelling evidence that serum may be an insignificant factor comes from a clinical study which reported that presence or absence of serum during chondrocyte expansion did not affect the clinical outcome in patients treated with a MACI-like graft (166). Therefore, although several studies have indicated a beneficial effect of using a serum-free medium for expansion, further studies may need to justify this switch.
4.1.2.2. Antibiotics and antimycotics
Chondrosphere® and NOVOCART® 3D do not use any antibiotics or antimycotics during production, while most other products have indicated use of these agents. A Cartipatch® study mentioned discontinuing such agents after P1 (39). In a study related to Chondrosphere®, antibiotics were mentioned to inhibit neocartilage matrix synthesis (48). However, no other supporting studies could be found. It is known that antibiotics and antimycotics can induce global changes in the proteome (167). In addition, antibiotics have been shown to inhibit proliferation of musculoskeletal cells in vitro (168). Furthermore, antibiotics are known to reduce the proliferation and differentiation capacity of embryonic stem cells (169). Some researchers have argued that the use of antimicrobials can potentially hide latent infections (170). However, mycoplasma tests are routinely conducted for each cartilage product. Finally, not using antibiotics can simplify safety concerns for allergic patients. Whether these benefits outweigh the risk of contamination remains open to debate.
4.1.2.3. Growth factors during 2D expansion
During expansion, exogenous supplementation of growth factors aims to either increase cell proliferation, especially in serum-free medium, or enhance the chondrogenic phenotype. The former is beneficial in minimizing construct production times, while the latter may allow the formation of more functional neocartilage. Various growth factors and their effects on chondrocytes have been thoroughly reviewed elsewhere (171, 172). Here, select agents are briefly described. Of the reviewed products, FGF-2 variant (10 ng/mL) has been used in BioCart™II, while FGF-2 (10 ng/mL), EGF (1 ng/mL), and TGF-β1 (1 ng/mL) have been used in Hyalograft® C. FGF-2 has been generally used to promote cell proliferation and stem cell renewal (173). For chondrocyte expansion, FGF-2 has not only been shown to enhance proliferation but also maintain the chondrogenic phenotype (150, 174, 175). Specifically, FGF-2-expanded chondrocytes were more differentiated immediately after monolayer expansion and formed neocartilage with higher matrix content. EGF has also been shown to enhance chondrocyte proliferation in monolayer (152, 176). Although EGF is generally known to suppress chondrogenic differentiation and matrix secretion, it may also have anabolic effects (177). TGF-β1 can also act as a mitogen for chondrocytes (151, 152, 176, 178). TGF-β1 plays an important role in cartilage development, is extensively used in the chondrodifferentiation of stem cells (179, 180), and promotes matrix synthesis in 3D-cultured chondrocytes (148, 181). Use of TGF-β1 may not always be beneficial, as addition of anti-TGFβ antibody during chondrocyte expansion led to enhanced chondrogenesis in subsequent pellet cultures (182). Other pro-mitogenic or pro-chondrogenic growth factors (e.g., IGF-1, PDGF-BB, TGF-β2, TGF-β3, BMP-2, BMP-7, BMP-14, etc.) may also have beneficial effects during monolayer expansion. Synergism between two or more growth factors may be key in finding the optimal expansion medium. For example, a cocktail of TGF-β1, FGF-2, and PDGF-BB (TFP) has been shown to promote chondrocyte proliferation and the chondrogenic phenotype (128, 165, 183, 184). TFP-expanded chondrocytes formed neocartilage with higher GAG and collagen content, as well as compressive and tensile properties than those formed by FGF-2-expanded cells (165). Finding optimal growth factor cocktails for chondrocyte expansion remains a relevant and ongoing field of research. However, the expanded use of growth factors in tissue engineering of clinically relevant products will necessitate overcoming additional regulatory hurdles.
4.1.2.4. Basal medium and other additives
Of the reviewed products, the basal media that have been used include Dulbecco’s Modified Eagle Medium (DMEM), Ham’s F-12, HL-1, and DMEM/Ham’s F-12 (1:1) mixtures. DMEM is a standard cell culture medium containing amino acids, salts, vitamins, and glucose. Compared to DMEM, Ham’s F-12 contains additional supplements including B vitamins, linoleic and lipoic acid, copper, zinc, hypoxanthine, putrescine, and thymidine. HL-1 medium is composed of a modified DMEM/Ham’s F-12 base with additional supplements including insulin, transferrin, selenium, testosterone, ethanolamine, various fatty acids, and proprietary stabilizing proteins. These nutrient components play important cellular roles and some are known to specifically affect chondrocyte biology and neocartilage matrix formation, such as copper (185) and putrescine (186, 187). Insulin-transferrin-selenium (ITS) is often added to reduced-serum or serum-free medium to support cell culture. ITS has been shown to mitigate dedifferentiation and lead to the formation of neocartilage with greater matrix content (188, 189). One study showed that insulin, rather than transferrin or selenium, exerted a significant effect on chondrocyte matrix metabolism (190). Glucose concentration has been shown to significantly affect the chondrogenic potential of expanded chondrocytes, with lower glucose being more beneficial (191). Unfortunately, glucose or pyruvate concentrations in the expansion medium are rarely specified in literature. No study could be found that directly compared the effects of different basal media on chondrocyte expansion and neocartilage formation; such a study would be informative.
4.2. Construct formation and 3D culture
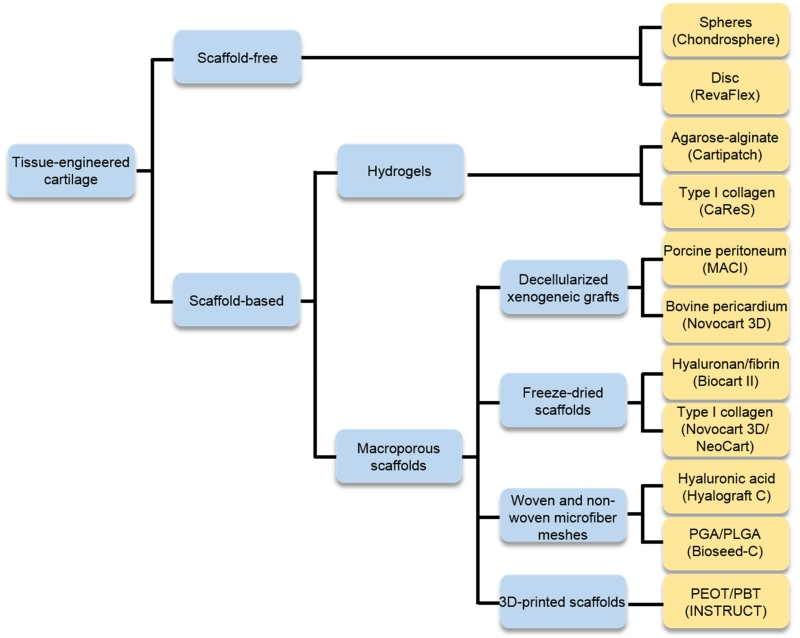
Integration of passaged articular chondrocytes into a 3D construct can be achieved through a scaffold-based or scaffold-free approach (Figure 2). Advantages and disadvantages of each will be outlined. For products using a scaffold-based approach, the different types of scaffolds used and their properties will be described. Because neocartilage properties are largely influenced by the cells, the cell seeding density and cell distribution in these products will also be briefly discussed. Finally, the tissue engineering strategies used during 3D culture will be described.
Figure 2.
The reviewed products use either scaffold-free or scaffold-based approaches. For the scaffold-based products, the various types of scaffolds are listed. NeoCart® and Bioseed®-C may use a collagen and fibrin gel, respectively, as a cell carrier.
4.2.1. Scaffold-free constructs
Chondrosphere® and RevaFlex™ are both scaffold-free constructs. The versatility and advantages of a scaffold-free approach have been recently reviewed (192, 193). In general, scaffold-free, engineered cartilage constructs can be fabricated by culturing chondrocytes at high density in wells or molds. Over hours, the cells adhere to one another and, over weeks, secrete an abundance of cartilage-specific matrix, subsequently forming solid neotissues. Initial chondrocyte self-aggregation is mediated by cell-matrix (e.g., α10β1-collagen II (194)) and cell-cell (e.g., cadherins (195)) interactions. Once aggregated, the cells can continue to secrete cartilage-specific matrix over many weeks. Scaffold-free neocartilage formed by primary bovine articular chondrocytes steadily increased in GAG and collagen content over 8 weeks (195). By week 1, these constructs can be gently handled (195) and by week 4, under a specific regimen of chemical stimulation, they can reach biomechanical properties on par with native juvenile cartilage (196).
There are several advantages of a scaffold-free over a scaffold-based approach. For one, matrix formation is not hindered by potentially harmful surface chemistries and degradation products of a scaffold; instead, it may more closely follow mechanisms similar to cartilage development (i.e., via mesenchymal condensation). Secondly, the construct is completely biological, thus potentially having fewer biocompatibility issues. Finally, the high cellularity may help integration.
In the case of Chondrosphere®, the authors noted spheres were designed to be less than 800 μm to avoid the 800-1000 μm diffusion limit seen in tumor spheroid cultures (48). However, this limit is probably also dependent on cell type, cell density, and tissue permeability, as 1.5-2 mm thick neocartilage discs can be achieved without necrotic centers (100, 197).
In the case of Chondrosphere®, the scaffold-free spheroids can adhere to, conform to, and fill the defect over time. The high cellularity and immature matrix of the 2-week-old neocartilage may contribute to these properties. For example, complete filling and integration within the defect has been reported, although the spheroids cover only a fraction of the defect area (47). In an in vitro explant model, histological observations show spheroid adherence to human cartilage explants within 45 minutes (48). After 3 weeks, spheroids flattened on the explant, presumably due to cell migration and matrix secretion. When spheroids were used to fill a defect in a human cartilage explant and then subcutaneously implanted in SCID mice (46), the spheroids were observed to continuously secrete matrix from 4-24 weeks, adapt to the shape of the defect, and integrate with adjacent native tissue. In a pig model study, Chondrospheres® were described to regenerate tibiofemoral, full-thickness defect sites with no visible gaps, suggesting good integration (47). Finally, a second-look arthroscopy performed on nine patients 3 months after Chondrosphere® implantation showed good filling of the defect with smooth, hyaline-like tissue and no visible gaps at the defect boundary (47). These studies not only provide preliminary evidence that Chondrospheres® are able to fill and integrate with the defect site over time, but also that scaffold-free neocartilage can be a responsive tissue that can grow in size, conform to the defect site, and integrate with adjacent native cartilage.
Although also a scaffold-free construct, RevaFlex™ differs significantly from Chondrosphere®. RevaFlex™ or similar constructs were grown for months in vitro and appeared relatively stiff (100). Unlike Chondrosphere®, these constructs did not naturally adhere to the defect and required fixation with sutures or glue. Initial fixation seemed a significant issue, as in vivo animal model studies required extensive leg immobilization and graft failure (20-50%) was attributed to incomplete immobilization (103, 104). On the other hand, advantages of implanting a more mature neocartilage construct are that construct growth can be consistently controlled and manipulated to enhance certain properties. In addition, more mature neocartilage grafts have higher initial mechanical properties, allowing for potentially faster rehabilitation. Histologically, these constructs (100) resemble native tissue more closely than many other products, affirming their potential as promising tissue engineered cartilage grafts.
4.2.2. Scaffold-based constructs
In a scaffold-based approach, expanded chondrocytes are seeded into or onto a porous scaffold, cultured in vitro (0-35 days), and then implanted. Depending on the scaffold material, the seeded chondrocytes can either adhere to the scaffold, aggregate within the pores, or exhibit both behaviors. These chondrocytes then form neocartilage in the pores and spaces left over by the degrading or remodeled scaffold. Scaffolds used in cartilage tissue engineering can fall in two general categories: hydrogels (microporous scaffolds) or macroporous scaffolds (Figure 2). They can be composed of biopolymers, synthetic polymers, or a mix.
4.2.2.1. Decellularized xenogeneic grafts
Both the MACI and NOVOCART® 3D products use decellularized xenogeneic grafts as scaffolds, specifically porcine peritoneum and bovine pericardium, respectively. These scaffolds and others, such as porcine small intestine submucosa (SIS), dermis, and porcine urinary bladder, belong to a class of decellularized tissue grafts that have presumably innate healing properties and have been developed for general regenerative applications (198). Harvested xenogeneic tissue is treated with solvents, detergents, salts, acids, bases, and enzymes to yield a largely collagenous scaffold with minimal antigens (199). However, compared to a simple freeze-dried collagen sponge, these scaffolds could possess much greater bioactive properties. The native collagen architecture (i.e., collagen fiber diameter, alignment, surface chemistry, pore size, hierarchical structure, etc.) can, in a more biomimetic fashion, promote cell adhesion, proliferation, and growth (200). In addition, decellularized porcine peritoneum was shown to retain active cytokines, such as TGF-β1, to further promote tissue regeneration (201, 202). However, the main justification for using porcine peritoneum in MACI is because of its bilayer structure – the porous layer is suitable for cell attachment, while the dense layer is suitable for cell retention. Similarly, the dense bovine pericardium layer of NOVOCART® 3D also functions to retain cells. Whether these decellularized xenogeneic grafts provide any functionality beyond their structural role remains to be shown. Finally, other key advantages of xenogeneic grafts are that they have the potential to be more readily remodeled and to have degradation products less harmful than other biopolymer or synthetic scaffolds.
4.2.2.2. Biopolymers
Biopolymer scaffolds also possess some bioactivity and can be readily degraded into less harmful byproducts. NOVOCART® 3D and NeoCart® use a freeze-dried type I collagen sponge. Manipulating the freeze-drying conditions and ice crystal formation can provide some control over pore size and shape. Freezing to “warm” temperatures (e.g., −10°C) provided larger pores, while “annealing” the ice crystals also increased pore size (203). Constant rate cooling (0.9°C/min) provided more uniform pores than quick freezing (204). Anisotropic pores can also be created (205), which may be useful in recapitulating the cellular organization in native cartilage. Biocart™II uses a freeze-dried fibrin/hyaluronan scaffold but information on its pore architecture could not be found. Hyalograft® C also uses a hyaluronan-based scaffold. The bioactive effects of hyaluronic acid on chondrocytes have bene largely beneficial. Hyaluronic-coated surfaces promote stem cell chondrogenesis (206) and soluble hyaluronic acid can stimulate the production of cartilage matrix (207, 208). Hyaluronic acid-coated PGA scaffolds induced seeded chondrocytes to secrete more matrix than those on uncoated scaffolds (209). Degradation of the reviewed biopolymer scaffolds has not been thoroughly characterized, but is expected to occur following enzyme-mediated mechanisms. Hyalograft® C was noted to degrade within 10 months (53). Other biopolymer scaffolds based on freeze-dried collagen I/GAG, chitosan, fibrin, and gelatin have also been used throughout the literature for engineering cartilage, each having its own unique properties and advantages (210, 211). Because of their versatility and biocompatibility, biopolymer scaffolds remain the most promising scaffold type to be used for cartilage tissue engineering.
4.2.2.3. Hydrogels
Cartipatch® is a hydrogel construct composed of alginate-agarose. Use of hydrogels for cartilage engineering has been previously reviewed (212, 213). Upon encapsulation, chondrocytes were able to express redifferentiation markers and secrete cartilage matrix in this 3D environment (214). Chondrocytes can remain metabolically active within agarose gels for more than 8 months (214). Compressive properties of chondrocyte-embedded agarose constructs were said to have reached properties on par with native tissues (215). However, tensile properties were lacking (216). Although hydrogels provide a 3D environment, chondrocytes are isolated and devoid of cell-cell interactions, which are critical in chondrogenesis (217) and present during neocartilage formation (195). The secreted matrix is also seemingly disconnected, as the network only forms when the pericellular matrix of individual cells coalesces. Whether this process of neocartilage formation can form a robust, interconnected matrix needs to be further established. Other than agarose or alginate, various other biopolymer hydrogels (e.g., collagen, fibrin, chitosan, and hyaluronic acid) and synthetic hydrogels (e.g., PEG) have been used in cartilage engineering (212, 213). Through creative modification of polymer chain moieties, functional groups (e.g., adhesion molecules, degradation sites, drug molecules) can be added to tailor the gel to have a variety of properties to meet specific needs of the application. Despite the ease of use of hydrogels and a plethora of chemical modifications available, whether quality neocartilage can develop within these microporous confines still needs to be clearly addressed.
4.2.2.4. Synthetic Polymers
Both Bioseed®-C and INSTRUCT use synthetic polymer scaffolds made of polglactin 910/poly-p-dioxanone (Ethisorb®) and PEOT/PBT, respectively. Both degrade primarily through hydrolysis. Polyglactin 910, short for PGA/PLLA (90/10), have been extensively used in vivo in various devices. This material has been said to degrade in ~3 weeks in vitro (218). However, in in vivo conditions, Bioseed®-C exhibited complete degradation in about 12 weeks (219). Chondrocytes have been shown to undergo typical redifferentiation on Ethisorb scaffolds (220); in a rabbit animal model study, up to 70% of implants showed hyaline-like repair tissue (218). PEOT/PBT scaffolds also support cell adhesion and chondrocyte redifferentiation, as reported earlier. However, the scaffold resorption time of 12 months, as stated on the company website, is much slower than that of Bioseed®-C. In vitro hydrolysis experiments show more rapid degradation of scaffolds with a higher PEOT content (125). When implanted subcutaneously in mini-pigs for 52 weeks, 300PEG-55:45 scaffolds remained largely intact, while 2000PEG-80:20 scaffolds largely fragmented to < 1 μm particles (122). After 3 months in a rabbit osteochondral or mouse subcutaneous model, 300PEG-55:45 and 1000PEG-70:30 scaffolds seemed to exhibit minimal degradation (117, 123). Though these scaffolds may release harmful degradation products, no adverse events in clinical studies were attributed to them. Therefore, synthetic polymers remain a viable strategy for providing a mechanically robust and easily customizable scaffold to repair cartilage defects.
4.2.3. Co-culture of cells
In cartilage engineering, co-cultures generally consist of primary chondrocytes mixed with a less differentiated cell type, such as a passaged chondrocytes or stem cells. The former are expected to induce the latter toward more complete chondrodifferentiation than through the application of exogenous stimuli alone. The latter provide the high cellularity needed for neotissue formation. Of the reviewed products, INSTRUCT uses a co-culture approach, where primary chondrocytes are mixed with bone marrow cells. Studies have shown co-cultures of primary chondrocytes and mesenchymal stem cells (MSCs) formed pellets with similar or higher matrix content than those formed by only primary chondrocytes (221, 222). Interestingly, contrary to the standard hypothesis, it was shown that MSCs served only to stimulate chondrocyte proliferation, as they themselves underwent eventual apoptosis (223-225). Co-cultures of primary chondrocytes with passaged chondrocytes, embryonic stem cells, or skin stem cells have also shown promising results in generating hyaline neocartilage (226, 227). Whether using these co-culture systems is more clinically beneficial than simply using passaged chondrocytes as a cell source remains to be established.
4.2.4. Exogenous stimuli in 3D culture
Only NeoCart® has specifically used any exogenous stimuli, other than growth factors, during 3D culture to further enhance construct properties. These stimuli include hydrostatic pressure, hypoxia, and perfusion.
Hydrostatic pressure is a predominant force in the knee. Hydrostatic pressure has been shown to increase cartilage-specific matrix synthesis in 2D or 3D cultured chondrocytes (197, 228-230) and even stem cells (231). In a NeoCart® patent, data presented showed that cyclic hydrostatic pressure (0.5 MPa, 0.5 Hz) applied during the first 6 days of culture increased S-GAG production (81). The mechanotransduction pathways, however, are unclear and may involve stretch-activated Ca2+ channels (232), TRPV4 (233), or the cell cilia (234). Studies that elucidate how hydrostatic pressure can increase matrix synthesis will provide critical information toward how to best use this stimulus in commercial applications. A challenge in implementing mechanical stimulation, such as hydrostatic pressure, to tissue engineered constructs is that large-scale and consistent application is technically and logistically difficult. However, because mechanical stimulation is integral in cartilage development, it remains a significant area of research for engineering functional cartilage.
Hypoxia, characterized by an oxygen tension of 2% or less, occurs in the standard environment of articular cartilage. Hypoxia has been shown to increase matrix synthesis in 2D and 3D cultured chondrocytes (235, 236) and help induce chondrogenesis in stem cells (237). In the NeoCart® construct, hypoxia (2% O2) was shown to increase GAG production (81). The size difference of micropellets cultured with or without hypoxia is significant (238). However, not only does hypoxia activate pathways for matrix synthesis, but also the expression of lysyl oxidase, subsequent increases in collagen crosslinks, and, ultimately, biomechanical properties of the neocartilage (196). Hypoxia could be easily applied to any tissue engineered construct to potentially improve its properties.
Direct perfusion, achieved via systems that force medium flow through the scaffold, has been shown to increase matrix content in the NeoCart® constructs (at 5 μL/min) (81), scaffold-based constructs (239, 240), and scaffold-free constructs (241). These benefits can arise either from increased access to nutrients or from the mechanical stimulation of fluid flow. The construction of a complex bioreactor system may limit its common practice in laboratories, although commercial perfusion bioreactors (e.g., C9-x Cartigen) are available. More studies are needed to establish whether perfusion is a necessary strategy for engineering cartilage.
Many other exogenous stimuli can be applied to neocartilage constructs in 3D culture to enhance their biochemical and biomechanical properties. Mechanical stimulation such as fluid-induced shear (242), compression (243), tension, ultrasound (244), and osmotic pressure (245) have induced increases in matrix synthesis by chondrocytes. Recently, chemicals and enzymes that directly induce biophysical modifications to the engineered tissue have also been introduced, such as chondroitinase ABC (246) and lysyl oxidase-like protein (196), to augment existing bioactive stimuli such as cytochalasin D (247), kartogenin (248), and growth factors (249). These molecules that act through a biophysical mechanism can have broad spectrum effects, i.e., they can beneficially modify matrices of not only articular cartilage, but also other collagenous tissues. Their potential for commercial use has yet to be explored. A discussion of current and novel stimuli can be found in many available reviews on cartilage engineering.
4.2.5. Construct maturation
After implantation, the engineered cartilage constructs will continue to mature, ideally, to a state of full biomechanical functionality. Maturation of the implanted neocartilage will involve a multitude of natural processes, including matrix secretion, matrix remodeling, and development of collagen cross-links.
The maturation process of a tissue engineered neocartilage construct in vivo is difficult to assess. When chondrocytes are first seeded to the scaffold, the cells are either adhered to each other (e.g., in non-adherent scaffolds such as NOVOCART® 3D or in scaffold-free constructs), adhered to the scaffold (e.g., MACI and Hyalograft® C), encapsulated within a hydrogel (e.g., Cartipatch® and CaReS® or if a cell carrier is used), or follow a mixture of each. Chondrocyte adherence to a relatively large surface within a scaffold could result in proliferation until inhibited by contacts with other cells or signals. Then chondrocytes begin secreting cartilage-specific matrix (collagens, GAGs, and other molecules). Chondrocytes of each product, under varying 3D environments, will form tissue that follows different maturation processes.
Maturation of scaffold-free cartilage has been characterized extensively (195). The first week after cell seeding is characterized primarily by collagen VI production and intense N-cadherin staining, reflective of cartilage development after cell condensation. GAG production increases significantly after day 14, while collagen production, after an initial surge after day 1, increases significantly after day 28. After several weeks, histology reveals a neocartilage morphology largely representing that of native tissue (e.g., cells residing in lacunae and organized in zones). Another maturation process of neocartilage formation is the production of collagen cross-links, which contributes to the increase in tissue mechanical properties over time (196). These in vitro descriptions of maturation may not reflect those in vivo. Animal studies should be performed to establish correlations between in vitro and in vivo construct properties. Imaging or other non-invasive modalities need to be improved to assess long-term in vivo maturation of the implanted construct in humans. Unfortunately, studies characterizing in detail the maturation process of neocartilage in the reviewed products have yet to be published.
Details on the fate of the implanted chondrocytes have not been presented in the reviewed clinical trials. This is largely because few follow-ups include tissue biopsy, particularly since doing so results in a defect. While this is an inherent limitation in clinical trials of cartilage repair products, no cell tracking studies in animal models could be found either. It is generally assumed that chondrocytes do not migrate out of the implant since cartilage matrix is dense and native chondrocytes are largely non-migratory. However, these assumptions may not hold true for manipulated chondrocytes within engineered matrices. Studies should be performed to verify cell fate for both scientific and regulatory purposes.
As with cell fate, the absence of biopsies during follow-up render difficult the biomechanical evaluation of the reviewed products’ neocartilage after implantation. This is of concern since many of these products begin as soft materials unable to bear physiological levels of load. Increasingly, biomechanical assays are regularly employed in cartilage tissue engineering studies (250-252). Inasmuch as the biomechanical milieu of implanted neocartilage is exceedingly strenuous, such tests should be standard when assessing construct properties in pre-clinical animal studies. Furthermore, these mechanical tests should be standardized to allow researchers to better correlate implant characteristics with clinical functionality.
4.3. Implantation, fixation, and rehabilitation
4.3.1. Construct shape and size
Fitting the construct into the defect is critical for its stabilization and integration. The construct can be cut to match the defect shape, typically using a piece of foil as a template, such as in BioCart™ II, Bioseed®-C, MACI, NeoCart®, RevaFlex™, and CaReS®. Alternatively, the defect can be shaped to fit the construct. Special drills and punches have been developed to create defect shapes matching that of the construct, such as in Cartipatch®, Hyalograft® C, and NOVOCART® 3D. Because most products are soft, pliable implants, they can be shaped more easily to match the size and curvature of the defect. The need to create a shape-specific construct can be obviated by using multiple, small constructs to create a larger construct in situ. This strategy is used by Chondrosphere® and others (253, 254). Stiffer constructs, such as Cartipatch®, RevaFlex™, and INSTRUCT are less able to conform to the defect shape and may require the proper shape to be engineered into the implant. Equally important may be matching the thickness of the implant to adjacent native cartilage. However, such a need has not been fully confirmed, as it has been shown that implants depressed relative to native cartilage still attain good repair fill (102).Unfortunately, the construct thickness of many other products are not known. This quantitative information is needed to better understand the design criteria for construct thickness.
At present, there is no bridge therapy between focal defects and unicompartmental prosthetics. Should constructs be fabricated that are large enough to cover multiple defects or to even replace entire joint compartments, a high degree of shape-specificity will be required. 3D printing of custom-shaped constructs or molds is a promising approach toward creating anatomical shapes unique to each individual (255-260).
4.3.2. Fixation
Implants must remain stable in the defect to restore function. Sutures offer the strongest fixation strategy and are commonly used in the ACI procedure and implantation of Bioseed®-C and NOVOCART® 3D. However, proud sutures can rub against the opposing articular surface resulting in fissures and cell loss reminiscent of early osteoarthritis (261). In response, transosseous sutures (Erggelet technique) are recommended for Bioseed®-C, though this technique is more complex. Fibrin glue, popular because of its ease of use and suitability for arthroscopy, is used for BioCart™II, MACI, RevaFlex™, and CaReS®. Since chondrocytes proliferate and migrate within fibrin gels (262, 263), integration is not hindered. It is worth noting that fixation methods are often designed concomitantly with products. For example, Chondrosphere®, Cartipatch®, and Hyalograft® C do not require either sutures or glues since they are press-fit to the defect site. Another example is NeoCart®, which uses a proprietary CT-3 glue composed of collagen and PEG.
With the progression toward larger and stiffer implants, new fixation methods will be required, as these constructs can be more prone to delamination. Although delamination can also be due to a lack of post-surgery leg immobilization, as was thought to be the case for RevaFlex™ implants (104), semi-rigid and flat implants may not easily stay on the curved defect surface. Delamination was not an issue in subsequent clinical studies, perhaps due to patient compliance, but better methods for affixing stiff implants to the defect would certainly be more forgiving during rehabilitation.
A promising fixation method is to create an osteochondral construct that can be press-fit into the defect. The bone phase would serve as a strong anchor for the cartilage phase. Cartipatch® (4 mm thick), CaReS® (6-8 mm thick), and INSTRUCT (4 mm thick), are current examples, though, interestingly, these products are all single phase materials. The most obvious future step would be to develop a biphasic graft that promotes bone or neocartilage formation in the correct regions (264). Fabrication of a large and stiff neocartilage construct would most likely use an osteochondral approach (258).
4.3.3. Rehabilitation
In the majority of clinical trials related to the reviewed products, rehabilitation followed approximately the standard for microfracture (71) and ACI. Four phases of rehabilitation were described (69) after the initial 24-48 hours of immobilization. From 0 to 6 weeks, patients underwent restricted range of motion and weight-bearing exercises (e.g., toe-touch weight-bearing) for the purpose of protecting the new repair tissue and restoring joint homeostasis. In some studies, CPM was often employed to 30° flexion for the first 4 weeks. When patients could achieve 120° flexion and good quadriceps contraction, they began the second phase (weeks 6-12) to restore full range of motion and improve muscle strength. When patients could walk 1-2 miles or ride a bike for 30 min, they proceeded to the third phase (week 12-26) to increase strength and endurance and to reintroduce activities. When patients could reach 80-90% strength with no pain or swelling, they moved to the final phase (weeks 26-52), in which they could participate in full unrestricted activity. High impact sports were not recommended within 12-18 months of surgery.
Ideally, the rehabilitation program would match gradual increases in weight-bearing activities to increases in repair tissue biomechanical properties in such a way to 1) optimally promote healing and 2) return the patient to normal activities in the minimum time. Thus, the rehabilitation program used for microfracture or ACI may not be optimally suitable for tissue engineered cartilage, as all three result in repair tissue with temporally distinctive biomechanical properties. For example, as MACI may represent a more mature graft than ACI, it was shown that an accelerated rehabilitation program benefited MACI-treated patients more than a traditional program (265). In finding an optimal program, one of the most significant but poorly understood question is to what extent in vivo biomechanical stimuli can improve healing. This is important because weight-bearing exercises could be performed to induce compressive strains known to stimulate matrix synthesis (266). Repetitive passive flexion, which is known to induce intermittent intraarticular pressure (267), could be used to induce hydrostatic pressures known to induce matrix synthesis by chondrocytes (197, 228-230). Therefore, more beneficial rehabilitation programs, tailored to each product, can be established for tissue engineered cartilage.
5. Perspectives
The recent wave of cell-based articular cartilage products in clinical trials in the U.S. and abroad indicates a growing recognition that current repair techniques can be improved by using a tissue engineering approach. Through a detailed account of how these products are fabricated, one can gain insight to the key strategies, current challenges, and future directions in five areas: 1) defining and optimizing the chondrocyte cell source, 2) understanding tissue-scaffold interaction and scaffold degradation, 3) identifying and applying novel stimuli, 4) understanding construct maturation, biomechanics, and functionality, and 5) improving implantation, fixation, and rehabilitation methods. These perspectives, along with a brief description of commercial challenges, are summarized below and will hopefully provide a broad roadmap to move the field forward.
In the chondrocyte expansion stage, cells only need to be passaged 1 to 4 times to reach sufficient numbers given the seeding density of current products. Because P1-P4 cells each have different gene expression profiles, whether passage number in these ranges can affect clinical efficacy should be more thoroughly investigated. To mitigate chondrocyte dedifferentiation, passage number can be minimized or the expansion medium formulation can be improved. The latter remains an important research objective. The use of serum-free medium and growth factor cocktails can, as supported by literature, improve the chondrogenic phenotype during expansion and lead to superior neocartilage formation. However, serum-free medium is not widely employed in the reviewed products. Finally, published studies should specify in detail the culture medium composition and the cell expansion factor, not necessarily passage number, to gain clearer insight how these factors can affect the chondrocyte phenotype and subsequent neocartilage formation.
In the construct formation stage, either scaffold-based or scaffold-free approaches have been employed; both systems have their own advantages and disadvantages. When using a scaffold, pore size and interconnectivity need to be sufficient to allow for even cell distribution, as evidence indicates uneven cellular distribution in some products. Chondrocyte aggregation within the pores rather than adhesion to the scaffold may be more beneficial in forming neocartilage. The scaffold degradation and remodeling rates need to be sufficiently investigated. This is especially important for synthetic polymeric scaffolds, as in vivo degradation may not mirror that observed in vitro. Hydrogel scaffolds may pose challenges as cell-cell contacts are hindered. In a scaffold-free approach, large cell numbers are required to form neocartilage constructs; these immature neotissues have a higher cell density than native cartilage and, therefore, may require long-term evaluation for potential complications such as hypertrophy. However, as an advantage, the formation of scaffold-free neocartilage may more closely mimic the cartilage developmental process, as it develops into tissue that has the most resemblance to native cartilage.
In the construct 3D culture stage, various stimuli other than hypoxia, perfusion, and hydrostatic pressure can be applied to enhance the construct biochemical and biomechanical properties. The application of chemical stimuli (e.g., growth factors, catabolic enzymes, collagen cross-linking promoting agents, and other chemical factors) has been shown to enhance the properties of engineered cartilage constructs. Other forms of mechanical stimuli (e.g., compression, tension, fluid-induced shear, etc.) can also have similar beneficial effects. Application of these stimuli will require longer 3D culture durations (1 to several weeks); long culture durations also have the advantage of ensuring consistent neocartilage growth. Most products, however, have a short culture duration to minimize wait times between surgeries. As only a couple of the reviewed products apply exogenous stimuli during this stage, much potential exists in improving current clinical products through the above strategies.
During the construct implantation stage, fitting the construct into the defect is critical for its stability and integration. Constructs can be cut to shape to match the defect or vice versa. Alternatively, multiple smaller constructs can be used to fill a larger defect. Constructs of softer material, which represent the majority of the reviewed products, can more easily conform to the shape of the defect, whereas stiffer or large constructs require stringent shape-specificity. To meet such shape-specificity, 3D printing may prove essential in creating anatomical patient-specific constructs. Shaping the constructs has not been a significant issue with the current products. However, these challenges will be more significant in the future when fabricating stiffer and larger constructs.
Construct fixation typically involves fibrin glue, while a few products utilize sutures. Of the current products, graft delamination rates appear to be relatively rare, indicating current fixation methods may so far be adequate for the current products. However, future efforts to fabricate stiff or large constructs will face more challenging fixation issues. This can potentially be addressed through the use of osteochondral constructs or innovative chondroinductive tissue glues.
The ideal rehabilitation program should match weight-bearing activities to the repair tissue biomechanical properties. Currently, products follow a rehabilitation program largely used for microfracture or ACI, which may not be optimal for tissue engineered constructs, as their temporal biomechanical properties differ. A program tailored for each product is ideal, although this will require time and a large patient pool for studies.
The non-scientific hurdles in commercializing a cell-based engineered cartilage product are substantial. From comparing studies presented in this review, it becomes evident that it typically takes over 10 years to transition from the bench to Phase III trials, which themselves can take over 5 years. Other than time-associated costs, pre-clinical trials using large animals (e.g., goat, sheep, or horse) are required to obtain an Investigational New Drug (IND) to start clinical trials. Undoubtedly, clinical trials are also quite expensive, as Phase I/II clinical trials typically involve 10-40 patients, while Phase III trials involve more than 200 patients. Furthermore, FDA approval is not necessarily followed by reimbursement from insurance companies, which may require separate standards than the FDA. Other insights to the commercialization of tissue engineered cartilage products are described elsewhere (268, 269).
Process control and release criteria are important in the commercialization process. However, there is limited knowledge of assays used for process control for the reviewed products. In an EMA report, the MACI product release specifications included visual inspection, viability, minimum cell number detection, identity, potency, and sterility (63). Identity was determined with RT-PCR of chondrocytic marker HAPLN1 and the synovial/fibroblastic marker MFAP5, while potency was determined with measuring aggrecan mRNA expression. In the report, it was requested that a new potency test be established to better assess the ability to form functional cartilage, indicating some difficulty in identifying a suitable potency assay. For constructs that will be implanted immediately after being seeded with cells, mRNA quantification may be the suitable assay. For constructs that will undergo prolonged 3D in vitro culture, such tests may include measuring construct biomechanical properties and matrix content (e.g., collagen and GAG content).
The field of cartilage tissue engineering continues to be dynamic and expanding as novel stimuli, innovative fabrication strategies, and improved surgical methods that best allow hyaline repair tissue formation are discovered. The strategies used in current, tissue engineered cartilage products provide a valuable foundation for the research and development of the next generation of improved products.
Acknowledgements
The authors would like to acknowledge support from the following grants: the National Institutes of Health (NIH) grant R01AR067821 and California Institute for Regenerative Medicine (CIRM) grant TR3-05709.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
Kyriacos A. Athanasiou is on the scientific advisory board for Histogenics Corporation (2011-present). He was on the scientific advisory board for Prochon Biotech Ltd. (2009-2011). He has served as a consultant for multiple companies including DePuy and Arthrex. In 1993, he was a co-founder of Osteobiologics Inc., which was acquired by Smith and Nephew in 2006.
Conflict of Interest
The authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- 1.Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. Clinical development success rates for investigational drugs. Nature biotechnology. 2014;32(1):40–51. doi: 10.1038/nbt.2786. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14(3):177–82. doi: 10.1016/j.knee.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 4.McCormick F, Harris JD, Abrams GD, Frank R, Gupta A, Hussey K, et al. Trends in the surgical treatment of articular cartilage lesions in the United States: an analysis of a large private-payer database over a period of 8 years. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2014;30(2):222–6. doi: 10.1016/j.arthro.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Montgomery SR, Foster BD, Ngo SS, Terrell RD, Wang JC, Petrigliano FA, et al. Trends in the surgical treatment of articular cartilage defects of the knee in the United States. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 2014;22(9):2070–5. doi: 10.1007/s00167-013-2614-9. [DOI] [PubMed] [Google Scholar]
- 6.Hubbard MJ. Articular debridement versus washout for degeneration of the medial femoral condyle. A five-year study. The Journal of bone and joint surgery British volume. 1996;78(2):217–9. [PubMed] [Google Scholar]
- 7.Aaron RK, Skolnick AH, Reinert SE, Ciombor DM. Arthroscopic debridement for osteoarthritis of the knee. The Journal of bone and joint surgery American volume. 2006;88(5):936–43. doi: 10.2106/JBJS.D.02671. [DOI] [PubMed] [Google Scholar]
- 8.Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2006;14(11):1119–25. doi: 10.1016/j.joca.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Gobbi A, Karnatzikos G, Kumar A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 2014;22(9):1986–96. doi: 10.1007/s00167-013-2676-8. [DOI] [PubMed] [Google Scholar]
- 10.Goyal D, Keyhani S, Lee EH, Hui JH. Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2013;29(9):1579–88. doi: 10.1016/j.arthro.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 11.Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. The American journal of sports medicine. 2009;37(10):2053–63. doi: 10.1177/0363546508328414. [DOI] [PubMed] [Google Scholar]
- 12.Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. The American journal of sports medicine. 2002;30(1):2–12. doi: 10.1177/03635465020300011601. [DOI] [PubMed] [Google Scholar]
- 13.Orth P, Cucchiarini M, Kohn D, Madry H. Alterations of the subchondral bone in osteochondral repair--translational data and clinical evidence. European cells & materials. 2013;25:299–316. doi: 10.22203/ecm.v025a21. discussion 4-6. [DOI] [PubMed] [Google Scholar]
- 14.Krych AJ, Harnly HW, Rodeo SA, Williams RJ., 3rd Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee: a retrospective comparative study. The Journal of bone and joint surgery American volume. 2012;94(11):971–8. doi: 10.2106/JBJS.K.00815. [DOI] [PubMed] [Google Scholar]
- 15.Gudas R, Kalesinskas RJ, Kimtys V, Stankevicius E, Toliusis V, Bernotavicius G, et al. A prospective randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2005;21(9):1066–75. doi: 10.1016/j.arthro.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. J Bone Joint Surg Br. 2012;94(4):504–9. doi: 10.1302/0301-620X.94B4.27495. [DOI] [PubMed] [Google Scholar]
- 17.Gross AE, Kim W, Las Heras F, Backstein D, Safir O, Pritzker KP. Fresh osteochondral allografts for posttraumatic knee defects: long-term followup. Clinical orthopaedics and related research. 2008;466(8):1863–70. doi: 10.1007/s11999-008-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demange M, Gomoll AH. The use of osteochondral allografts in the management of cartilage defects. Current reviews in musculoskeletal medicine. 2012;5(3):229–35. doi: 10.1007/s12178-012-9132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saris DB, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. The American journal of sports medicine. 2009;37(Suppl 1):10S–9S. doi: 10.1177/0363546509350694. [DOI] [PubMed] [Google Scholar]
- 20.Somoza RA, Welter JF, Correa D, Caplan AI. Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue engineering Part B, Reviews. 2014;20(6):596–608. doi: 10.1089/ten.teb.2013.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minas T, Von Keudell A, Bryant T, Gomoll AH. The John Insall Award: A minimum 10-year outcome study of autologous chondrocyte implantation. Clinical orthopaedics and related research. 2014;472(1):41–51. doi: 10.1007/s11999-013-3146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gooding CR, Bartlett W, Bentley G, Skinner JA, Carrington R, Flanagan A. A prospective, ranomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: Periosteum covered versus type I/III collagen covered. Knee. 2006;13(3):203–10. doi: 10.1016/j.knee.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87(5):640–5. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 24.Shekkeris A, Perera J, Bentley G, Flanagan A, Miles J, Carrington R, et al. HISTOLOGICAL RESULTS OF 406 BIOPSIES FOLLOWING ACI/MACI PROCEDURES FOR OSTEOCHONDRAL DEFECTS IN THE KNEE. Journal of Bone & Joint Surgery, British Volume. 2012;94-B(SUPP XXXVI):12. [Google Scholar]
- 25.Behrens P, Bitter T, Kurz B, Russlies M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)--5-year follow-up. Knee. 2006;13(3):194–202. doi: 10.1016/j.knee.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Nehrer S, Chiari C, Domayer S, Barkay H, Yayon A. Results of chondrocyte implantation with a fibrin-hyaluronan matrix: a preliminary study. Clinical orthopaedics and related research. 2008;466(8):1849–55. doi: 10.1007/s11999-008-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yayon A, Neria E, Blumenstein S, Stern B, Barkai H, Zak R, et al. BIOCART™II A NOVEL IMPLANT FOR 3D RECONSTRUCTION OF ARTICULAR CARTILAGE. Journal of Bone & Joint Surgery, British Volume. 2006;88-B(SUPP II):344. [Google Scholar]
- 28.Yayon A, Azachi M, Gladnikoff M, Prochon Biotech Ltd Freeze-dried fibrin matrices and methods for preparation thereof patent US 08618258. 2013 Dec 31; inventors. assignee. 2013.
- 29.Eshed I, Trattnig S, Sharon M, Arbel R, Nierenberg G, Konen E, et al. Assessment of cartilage repair after chondrocyte transplantation with a fibrin-hyaluronan matrix--correlation of morphological MRI, biochemical T2 mapping and clinical outcome. European journal of radiology. 2012;81(6):1216–23. doi: 10.1016/j.ejrad.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 30.Kreuz PC, Muller S, Freymann U, Erggelet C, Niemeyer P, Kaps C, et al. Repair of focal cartilage defects with scaffold-assisted autologous chondrocyte grafts: clinical and biomechanical results 48 months after transplantation. The American journal of sports medicine. 2011;39(8):1697–705. doi: 10.1177/0363546511403279. [DOI] [PubMed] [Google Scholar]
- 31.GmbH BT, editor. Bioseed-C for Articular Cartilage Regeneration [brochure] Freiburg, Germany: n.d. [Google Scholar]
- 32.Barnewitz D, Endres M, Kruger I, Becker A, Zimmermann J, Wilke I, et al. Treatment of articular cartilage defects in horses with polymer-based cartilage tissue engineering grafts. Biomaterials. 2006;27(14):2882–9. doi: 10.1016/j.biomaterials.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 33.PRODUCTS: BIOSEED®-C - INTRODUCTION. 2015 Nov 16; Available from: http://www.biotissue.de/en-ProductsHealthProfessionalsBioSeedCIntroduction.html.
- 34.BIOSEED®-C - INTRODUCTION. 2015 Nov 16; Available from: http://www.biotissue.de/en-ProductsPatientsBioSeedCIntroduction.html.
- 35.Erggelet C, Sittinger M, Lahm A. The arthroscopic implantation of autologous chondrocytes for the treatment of full-thickness cartilage defects of the knee joint. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2003;19(1):108–10. doi: 10.1053/jars.2003.50025. [DOI] [PubMed] [Google Scholar]
- 36.Ossendorf C, Kaps C, Kreuz PC, Burmester GR, Sittinger M, Erggelet C. Treatment of posttraumatic and focal osteoarthritic cartilage defects of the knee with autologous polymer-based three-dimensional chondrocyte grafts: 2-year clinical results. Arthritis research & therapy. 2007;9(2):R41. doi: 10.1186/ar2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeifang F, Oberle D, Nierhoff C, Richter W, Moradi B, Schmitt H. Autologous chondrocyte implantation using the original periosteum-cover technique versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. The American journal of sports medicine. 2010;38(5):924–33. doi: 10.1177/0363546509351499. [DOI] [PubMed] [Google Scholar]
- 38.Niemeyer P, Pestka JM, Kreuz PC, Erggelet C, Schmal H, Suedkamp NP, et al. Characteristic complications after autologous chondrocyte implantation for cartilage defects of the knee joint. The American journal of sports medicine. 2008;36(11):2091–9. doi: 10.1177/0363546508322131. [DOI] [PubMed] [Google Scholar]
- 39.Selmi TA, Verdonk P, Chambat P, Dubrana F, Potel JF, Barnouin L, et al. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. The Journal of bone and joint surgery British volume. 2008;90(5):597–604. doi: 10.1302/0301-620X.90B5.20360. [DOI] [PubMed] [Google Scholar]
- 40.Comparison of Autologous Chondrocyte Implantation Versus Mosaicoplasty: a Randomized Trial (Cartipatch) 2015 Nov 16; Available from: https://clinicaltrials.gov/ct2/show/NCT00560664.
- 41.Barnouin L, Mainard D, Tan N, Laganier L, Robert H. 23rd Annual Meeting of the European Tissue Repair Society: Histological Outcomes of CARTIPATCH® Phase III Clinical Trial: Autologous Chondrocytes Implantation Versus Mosaicplasty for Cartilage Repair. Wound Repair and Regeneration. 2013;21(6):A56. [Google Scholar]
- 42.Comparison of Microfracture Treatment and CARTIPATCH® Chondrocyte Graft Treatment in Femoral Condyle Lesions. 2015 Nov 16; Available from: https://clinicaltrials.gov/ct2/show/NCT00945399.
- 43.Frequently Asked Questions for Investors. 2015 Nov 16; Available from: http://www.codon.de/investors/faq-for-investors.html?L=1.
- 44.Libera J, Anderer U, Fritsch K-G, Josimovic-Alasevic O, Codon Aktiengesllschaft Method for in vitro production of three-dimensional vital cartilage tissue and use thereof as transplant material patent US 07887843. 2011 Feb 15; inventors. assignee. 2011.
- 45.Libera JB, DE) Anderer, Ursula (Berlin, DE) Fritsch, Karl-gerd (Berlin, DE) Josimovic-alasevic, Olivera (Berlin, DE) Co.don Aktiengesllschaft (Teltow, DE) United States patent 7887843 Method for in vitro production of three-dimensional vital cartilage tissue and use thereof as transplant material. 2011 inventor. assignee.
- 46.Schubert T, Anders S, Neumann E, Scholmerich J, Hofstadter F, Grifka J, et al. Long-term effects of chondrospheres on cartilage lesions in an autologous chondrocyte implantation model as investigated in the SCID mouse model. International journal of molecular medicine. 2009;23(4):455–60. doi: 10.3892/ijmm_00000151. [DOI] [PubMed] [Google Scholar]
- 47.Libera J, Ruhnau K, Baum P, Lüthi U, Schreyer T, Meyer U, et al. Cartilage Engineering. In: Meyer U, Handschel J, Wiesmann HP, Meyer T, editors. Fundamentals of Tissue Engineering and Regenerative Medicine. Springer Berlin Heidelberg; 2009. pp. 233–42. [Google Scholar]
- 48.Anderer U, Libera J. In vitro engineering of human autogenous cartilage. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2002;17(8):1420–9. doi: 10.1359/jbmr.2002.17.8.1420. [DOI] [PubMed] [Google Scholar]
- 49.Fickert S, Gerwien P, Helmert B, Schattenberg T, Weckbach S, Kaszkin-Bettag M, et al. One-Year Clinical and Radiological Results of a Prospective, Investigator-Initiated Trial Examining a Novel, Purely Autologous 3-Dimensional Autologous Chondrocyte Transplantation Product in the Knee. Cartilage. 2012;3(1):27–42. doi: 10.1177/1947603511417616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Efficacy and Safety Study of co.Don Chondrosphere to Treat Cartilage Defects. 2015 Nov 16; Available from: https://clinicaltrials.gov/ct2/show/NCT01222559.
- 51.Marcacci M, Kon E, Zaffagnini S, Reggiani L, Neri M, Iacono F. Cell-Based Cartilage Repair Using the Hyalograft Transplant. In: Williams R III, editor. Cartilage Repair Strategies: Humana Press; 2007. pp. 207–18. [Google Scholar]
- 52.Marcacci M, Kon E, Grigolo B, Delcogliano M, Filardo G, Neri MP. 8.3 The clinician view. Osteoarthritis and Cartilage. 15:B11–B3. [Google Scholar]
- 53.European Medicines Agency (EMA) Committee for Advanced Therapies (CAT) Withdrawal assessment report: Hyalograft C autograft. 2013. [Google Scholar]
- 54.Solchaga LA, Dennis JE, Goldberg VM, Caplan AI. Hyaluronic acid-based polymers as cell carriers for tissue-engineered repair of bone and cartilage. J Orthopaed Res. 1999;17(2):205–13. doi: 10.1002/jor.1100170209. [DOI] [PubMed] [Google Scholar]
- 55.Brun P, Abatangelo G, Radice M, Zacchi V, Guidolin D, Gordini DD, et al. Chondrocyte aggregation and reorganization into three-dimensional scaffolds. J Biomed Mater Res. 1999;46(3):337–46. doi: 10.1002/(sici)1097-4636(19990905)46:3<337::aid-jbm5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 56.Kon E, Gobbi A, Filardo G, Delcogliano M, Zaffagnini S, Marcacci M. Arthroscopic Second-Generation Autologous Chondrocyte Implantation Compared With Microfracture for Chondral Lesions of the Knee Prospective Nonrandomized Study at 5 Years. Am J Sport Med. 2009;37(1):33–41. doi: 10.1177/0363546508323256. [DOI] [PubMed] [Google Scholar]
- 57.Aigner J, Tegeler J, Hutzler P, Campoccia D, Pavesio A, Hammer C, et al. Cartilage tissue engineering with novel nonwoven structured biomaterial based on hyaluronic acid benzyl ester. J Biomed Mater Res. 1998;42(2):172–81. doi: 10.1002/(sici)1097-4636(199811)42:2<172::aid-jbm2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 58.Girotto D, Urbani S, Brun P, Renier D, Barbucci R, Abatangelo G. Tissue-specific gene expression in chondrocytes grown on three-dimensional hyaluronic acid scaffolds. Biomaterials. 2003;24(19):3265–75. doi: 10.1016/s0142-9612(03)00160-1. [DOI] [PubMed] [Google Scholar]
- 59.Pei M, Solchaga LA, Seidel J, Zeng L, Vunjak-Novakovic G, Caplan AI, et al. Bioreactors mediate the effectiveness of tissue engineering scaffolds. Faseb J. 2002;16(10):1691–+. doi: 10.1096/fj.02-0083fje. [DOI] [PubMed] [Google Scholar]
- 60.Santoro R, Olivares AL, Brans G, Wirz D, Longinotti C, Lacroix D, et al. Bioreactor based engineering of large-scale human cartilage grafts for joint resurfacing. Biomaterials. 2010;31(34):8946–52. doi: 10.1016/j.biomaterials.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 61.Marcacci M, Zaffagnini S, Kon E, Visani A, Iacono F, Loreti I. Arthroscopic autologous condrocyte transplantation: technical note. Knee Surg Sport Tr A. 2002;10(3):154–9. doi: 10.1007/s00167-001-0275-6. [DOI] [PubMed] [Google Scholar]
- 62.Brix MO, Stelzeneder D, Chiari C, Koller U, Nehrer S, Dorotka R, et al. Treatment of Full-Thickness Chondral Defects With Hyalograft C in the Knee: Long-term Results. The American journal of sports medicine. 2014;42(6):1426–32. doi: 10.1177/0363546514526695. [DOI] [PubMed] [Google Scholar]
- 63.European Medicines Agency (EMA) Committee for Medicinal Products for Human Use (CHMP) Assessment report. MACI; 2013. [Google Scholar]
- 64.Edwards PK, Ackland TR, Ebert JR. Accelerated weightbearing rehabilitation after matrix-induced autologous chondrocyte implantation in the tibiofemoral joint: early clinical and radiological outcomes. The American journal of sports medicine. 2013;41(10):2314–24. doi: 10.1177/0363546513495637. [DOI] [PubMed] [Google Scholar]
- 65.Zheng MH, Willers C, Kirilak L, Yates P, Xu J, Wood D, et al. Matrix-induced autologous chondrocyte implantation (MACI): biological and histological assessment. Tissue engineering. 2007;13(4):737–46. doi: 10.1089/ten.2006.0246. [DOI] [PubMed] [Google Scholar]
- 66.Zheng MH, Hinterkeuser K, Solomon K, Kunert V, Pavlos NJ, Xu J. Collagen-Derived Biomaterials in Bone and Cartilage Repair. Macromolecular Symposia. 2007;253(1):179–85. [Google Scholar]
- 67.Albrecht C, Tichy B, Nurnberger S, Hosiner S, Zak L, Aldrian S, et al. Gene expression and cell differentiation in matrix-associated chondrocyte transplantation grafts: a comparative study. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2011;19(10):1219–27. doi: 10.1016/j.joca.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 68.Gigante A, Bevilacqua C, Ricevuto A, Mattioli-Belmonte M, Greco F. Membrane-seeded autologous chondrocytes: cell viability and characterization at surgery. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 2007;15(1):88–92. doi: 10.1007/s00167-006-0115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saris D, Price A, Widuchowski W, Bertrand-Marchand M, Caron J, Drogset JO, et al. Matrix-Applied Characterized Autologous Cultured Chondrocytes Versus Microfracture: Two-Year Follow-up of a Prospective Randomized Trial. The American journal of sports medicine. 2014;42(6):1384–94. doi: 10.1177/0363546514528093. [DOI] [PubMed] [Google Scholar]
- 70.Abelow SP, Guillen P, Ramos T. Arthroscopic Technique for Matrix-Induced Autologous Chondrocyte Implantation for the Treatment of Large Chondral Defects in the Knee and Ankle. Operative Techniques in Orthopaedics. 2006;16(4):257–61. [Google Scholar]
- 71.Steadman JR, Rodkey WG, Briggs KK. Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. The journal of knee surgery. 2002;15(3):170–6. [PubMed] [Google Scholar]
- 72.Basad E, Ishaque B, Bachmann G, Sturz H, Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 2010;18(4):519–27. doi: 10.1007/s00167-009-1028-1. [DOI] [PubMed] [Google Scholar]
- 73.Bartlett W, Skinner JA, Gooding CR, Carrington RWJ, Flanagan AM, Briggs TWR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee. Journal of Bone and Joint Surgery-British Volume. 2005;87B(5):640–5. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 74.Enea D, Cecconi S, Busilacchi A, Manzotti S, Gesuita R, Gigante A. Matrix-induced autologous chondrocyte implantation (MACI) in the knee. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 2012;20(5):862–9. doi: 10.1007/s00167-011-1639-1. [DOI] [PubMed] [Google Scholar]
- 75.Zheng MH, Willers C. REVIEW ON CLINICALLY FAILED CASES OF MATRIX INDUCED AUTOLOGOUS CHONDROCYTE IMPLANTATION (MACI) Journal of Bone & Joint Surgery, British Volume. 2012;94-B(SUPP XXIII):173. [Google Scholar]
- 76.Ebert JR, Robertson WB, Woodhouse J, Fallon M, Zheng MH, Ackland T, et al. Clinical and magnetic resonance imaging-based outcomes to 5 years after matrix-induced autologous chondrocyte implantation to address articular cartilage defects in the knee. The American journal of sports medicine. 2011;39(4):753–63. doi: 10.1177/0363546510390476. [DOI] [PubMed] [Google Scholar]
- 77.Ebert JR, Smith A, Edwards PK, Hambly K, Wood DJ, Ackland TR. Factors predictive of outcome 5 years after matrix-induced autologous chondrocyte implantation in the tibiofemoral joint. The American journal of sports medicine. 2013;41(6):1245–54. doi: 10.1177/0363546513484696. [DOI] [PubMed] [Google Scholar]
- 78.Clar H, Pascher A, Kastner N, Gruber G, Robl T, Windhager R. Matrix-assisted autologous chondrocyte implantation into a 14cm(2) cartilage defect, caused by steroid-induced osteonecrosis. The Knee. 2010;17(3):255–7. doi: 10.1016/j.knee.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 79.Product and platform: NeoCart. 2015 Nov 16; Available from: http://www.histogenics.com/products-platform/neocart.
- 80.Crawford DC, DeBerardino TM, Williams RJ., 3rd NeoCart, an autologous cartilage tissue implant, compared with microfracture for treatment of distal femoral cartilage lesions: an FDA phase-II prospective, randomized clinical trial after two years. The Journal of bone and joint surgery American volume. 2012;94(11):979–89. doi: 10.2106/JBJS.K.00533. [DOI] [PubMed] [Google Scholar]
- 81.Mizuno S, Kusanagi A, Tarrant LJB, Tokuno T, Smith RL. 2013 US2013273121-A1. A61K-009/00 201370 System useful for repairing cartilage, comprises lyophilized acellular collagen matrix containing pores and bioactive agent disposed within the pores patent US2013273121-A1. 2013 Oct 17; inventors; MIZUNO S (MIZU-Individual) KUSANAGI A (KUSA-Individual) TARRANT L J B (TARR-Individual) TOKUNO T (TOKU-Individual) SMITH R L (SMIT-Individual), assignee.
- 82.Crawford DC, Heveran CM, Cannon WD, Jr., Foo LF, Potter HG. An autologous cartilage tissue implant NeoCart for treatment of grade III chondral injury to the distal femur: prospective clinical safety trial at 2 years. The American journal of sports medicine. 2009;37(7):1334–43. doi: 10.1177/0363546509333011. [DOI] [PubMed] [Google Scholar]
- 83.OneMed Forum 2013 Conference Presentation; 4/15/2016; http://www.onemedplace.com/forum/presentations/sf2013/pdf/12753.pdf. viewed. [Google Scholar]
- 84.Confirmatory Study of NeoCart in Knee Cartilage Repair. 2015 Nov 16; Available from: https://clinicaltrials.gov/ct2/show/NCT01066702.
- 85.What is NOVOCART® 3D? 2015 Nov 16; Available from: http://www.tetec-ag.com/cps/rde/xchg/cw-tetec-en-int/hs.xsl/7321.html.
- 86.Pietschmann MF, Horng A, Niethammer T, Pagenstert I, Sievers B, Jansson V, et al. Cell quality affects clinical outcome after MACI procedure for cartilage injury of the knee. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 2009;17(11):1305–11. doi: 10.1007/s00167-009-0828-7. [DOI] [PubMed] [Google Scholar]
- 87.Mollenhauer J. IMPLANT AND THERAPEUTIC COMPOSITION FOR TREATING DAMAGE AND/OR DISEASES RELATING TO THE HUMAN ANIMAL MUSCULOSKELETAL SYSTEM. 2012 inventor. [Google Scholar]
- 88.Novocart Autologous Chondrocyte Transplantation BBRaun.com. B. Braun Melsungen AG; 2014. BBRaun.com cited 2014. Available from: http://www.bbraun.com/cps/rde/xchg/bbraun-com/hs.xsl/products.html?prid=PRID00004555. [Google Scholar]
- 89.Niethammer TR, Muller PE, Safi E, Ficklscherer A, Rossbach BP, Jansson V, et al. Early resumption of physical activities leads to inferior clinical outcomes after matrix-based autologous chondrocyte implantation in the knee. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 2013 doi: 10.1007/s00167-013-2583-z. [DOI] [PubMed] [Google Scholar]
- 90.Hambly K, Bobic V, Wondrasch B, Van Assche D, Marlovits S. Autologous chondrocyte implantation postoperative care and rehabilitation: science and practice. The American journal of sports medicine. 2006;34(6):1020–38. doi: 10.1177/0363546505281918. [DOI] [PubMed] [Google Scholar]
- 91.Zak L, Albrecht C, Wondrasch B, Widhalm H, Vekszler G, Trattnig S, et al. Results 2 Years After Matrix-Associated Autologous Chondrocyte Transplantation Using the Novocart 3D Scaffold: An Analysis of Clinical and Radiological Data. The American journal of sports medicine. 2014;42(7):1618–27. doi: 10.1177/0363546514532337. [DOI] [PubMed] [Google Scholar]
- 92.Safety and Effectiveness Study to Evaluate NOVOCART® 3D Plus Compared to the Microfracture to Treat Articular Cartilage Defects of the Knee (N3D) 2015 Nov 16; Available from: https://clinicaltrials.gov/ct2/show/NCT01656902.
- 93.Niethammer TR, Pietschmann MF, Horng A, Rossbach BP, Ficklscherer A, Jansson V, et al. Graft hypertrophy of matrix-based autologous chondrocyte implantation: a two-year follow-up study of NOVOCART 3D implantation in the knee. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 2014;22(6):1329–36. doi: 10.1007/s00167-013-2454-7. [DOI] [PubMed] [Google Scholar]
- 94.Pietschmann MF, Niethammer TR, Horng A, Gulecyuz MF, Feist-Pagenstert I, Jansson V, et al. The incidence and clinical relevance of graft hypertrophy after matrix-based autologous chondrocyte implantation. The American journal of sports medicine. 2012;40(1):68–74. doi: 10.1177/0363546511424396. [DOI] [PubMed] [Google Scholar]
- 95.Ochs BG, Muller-Horvat C, Albrecht D, Schewe B, Weise K, Aicher WK, et al. Remodeling of articular cartilage and subchondral bone after bone grafting and matrix-associated autologous chondrocyte implantation for osteochondritis dissecans of the knee. The American journal of sports medicine. 2011;39(4):764–73. doi: 10.1177/0363546510388896. [DOI] [PubMed] [Google Scholar]
- 96.NOVOCART®3D for Treatment of Articular Cartilage of the Knee (N3D) 2015 Nov 16; cited. Available from: https://clinicaltrials.gov/ct2/show/NCT01957722.
- 97.Non-Interventional Study to Evaluate Safety and Efficacy of NOVOCART 3D in Patients With Cartilage Defects (NISANIK) 2015 Nov 16; Available from: https://clinicaltrials.gov/ct2/show/NCT02348697.
- 98.Adkisson HDt, Martin JA, Amendola RL, Milliman C, Mauch KA, Katwal AB, et al. The potential of human allogeneic juvenile chondrocytes for restoration of articular cartilage. The American journal of sports medicine. 2010;38(7):1324–33. doi: 10.1177/0363546510361950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bonasia DE, Martin JA, Marmotti A, Amendola RL, Buckwalter JA, Rossi R, et al. Cocultures of adult and juvenile chondrocytes compared with adult and juvenile chondral fragments: in vitro matrix production. The American journal of sports medicine. 2011;39(11):2355–61. doi: 10.1177/0363546511417172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adkisson HD, Gillis MP, Davis EC, Maloney W, Hruska KA. In vitro generation of scaffold independent neocartilage. Clinical orthopaedics and related research. 2001;(391 Suppl):S280–94. doi: 10.1097/00003086-200110001-00026. [DOI] [PubMed] [Google Scholar]
- 101.Adkisson HDSL, MO) Barnes-jewish, Hospital (St. Louis, MO) United States patent 6645764 Neocartilage and methods of use. 2003 inventor. assignee.
- 102.McCormick F, Cole BJ, Nwachukwu B, Harris JD, Adkisson HD, Iv, Farr J. Treatment of Focal Cartilage Defects With a Juvenile Allogeneic 3-Dimensional Articular Cartilage Graft. Operative Techniques in Sports Medicine. 2013;21(2):95–9. [Google Scholar]
- 103.Lu Y, Adkisson HD, Bogdanske J, Kalscheur V, Maloney W, Cheung R, et al. In vivo transplantation of neonatal ovine neocartilage allografts: determining the effectiveness of tissue transglutaminase. The journal of knee surgery. 2005;18(1):31–42. doi: 10.1055/s-0030-1248155. [DOI] [PubMed] [Google Scholar]
- 104.Lewis PB, McCarty LP, 3rd, Yao JQ, Williams JM, Kang R, Cole BJ. Fixation of tissue-engineered human neocartilage constructs with human fibrin in a caprine model. The journal of knee surgery. 2009;22(3):196–204. doi: 10.1055/s-0030-1247749. [DOI] [PubMed] [Google Scholar]
- 105.Arzi B, DuRaine GD, Lee CA, Huey DJ, Borjesson DL, Murphy BG, et al. Cartilage immunoprivilege depends on donor source and lesion location. Acta biomaterialia. 2015;23:72–81. doi: 10.1016/j.actbio.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Adkisson HD, Milliman C, Zhang X, Mauch K, Maziarz RT, Streeter PR. Immune evasion by neocartilage-derived chondrocytes: Implications for biologic repair of joint articular cartilage. Stem cell research. 2010;4(1):57–68. doi: 10.1016/j.scr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 107.Neocartilage Implant to Treat Cartilage Lesions of the Knee. 2015 Nov 16; Available from: https://clinicaltrials.gov/ct2/show/NCT01400607.
- 108.Schneider U, Schmidt-Rohlfing B, Gavenis K, Maus U, Mueller-Rath R, Andereya S. A comparative study of 3 different cartilage repair techniques. Knee Surg Sport Tr A. 2011;19(12):2145–52. doi: 10.1007/s00167-011-1460-x. [DOI] [PubMed] [Google Scholar]
- 109.Schneider U, Rackwitz L, Andereya S, Fensky F, Reichert J, Loer I, et al. A Prospective Multicenter Study on the Outcome of Type I Collagen Hydrogel-Based Autologous Chondrocyte Implantation (CaReS) for the Repair of Articular Cartilage Defects in the Knee. Am J Sport Med. 2011;39(12):2558–65. doi: 10.1177/0363546511423369. [DOI] [PubMed] [Google Scholar]
- 110.Wallace DG, Rosenblatt J. Collagen gel systems for sustained delivery and tissue engineering. Advanced drug delivery reviews. 2003;55(12):1631–49. doi: 10.1016/j.addr.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 111.Muller-Rath R, Gavenis K, Andereya S, Mumme T, Schmidt-Rohlfing B, Schneider U. A novel rat tail collagen type-I gel for the cultivation of human articular chondrocytes in low cell density. Int J Artif Organs. 2007;30(12):1057–67. doi: 10.1177/039139880703001205. [DOI] [PubMed] [Google Scholar]
- 112.Mueller-Rath R, Gavenis K, Andereya S, Mumme T, Albrand M, Stoffel M, et al. Condensed cellular seeded collagen gel as an improved biomaterial for tissue engineering of articular cartilage. Bio-Med Mater Eng. 2010;20(6):317–28. doi: 10.3233/BME-2010-0645. [DOI] [PubMed] [Google Scholar]
- 113.Gavenis K, Mueller-Rath R, Andereya S, Schneider U, Schmidt-Rohlfing B. The Treatment of Focal Cartilage Defects Using a Compressed, Cell-Free Collagen Type-I Gel Seeded with Bmp-7 Loaded Polylactide Microspheres in the Gottinger Minipig. Int J Artif Organs. 2008;31(7):610. [Google Scholar]
- 114.Gavenis K, Schmidt-Rohlfing B, Andereya S, Mumme T, Schneider U, Mueller-Rath R. A Cell-Free Collagen Type I Device for the Treatment of Focal Cartilage Defects. Artif Organs. 2010;34(1):79–83. doi: 10.1111/j.1525-1594.2009.00776.x. [DOI] [PubMed] [Google Scholar]
- 115.Gavenis K, Schneider U, Maus U, Mumme T, Muller-Rath R, Schmidt-Rohlfing B, et al. Cell-free repair of small cartilage defects in the Goettinger minipig: which defect size is possible? Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 2012;20(11):2307–14. doi: 10.1007/s00167-011-1847-8. [DOI] [PubMed] [Google Scholar]
- 116.Petri M, Broese M, Simon A, Liodakis E, Ettinger M, Guenther D, et al. CaReS (R) (MACT) versus microfracture in treating symptomatic patellofemoral cartilage defects: a retrospective matched-pair analysis. Journal of Orthopaedic Science. 2013;18(1):38–44. doi: 10.1007/s00776-012-0305-x. [DOI] [PubMed] [Google Scholar]
- 117.Hendriks JA, Moroni L, Riesle J, de Wijn JR, van Blitterswijk CA. The effect of scaffold-cell entrapment capacity and physico-chemical properties on cartilage regeneration. Biomaterials. 2013;34(17):4259–65. doi: 10.1016/j.biomaterials.2013.02.060. [DOI] [PubMed] [Google Scholar]
- 118.Moroni L, Hendriks JA, Schotel R, de Wijn JR, van Blitterswijk CA. Design of biphasic polymeric 3-dimensional fiber deposited scaffolds for cartilage tissue engineering applications. Tissue engineering. 2007;13(2):361–71. doi: 10.1089/ten.2006.0127. [DOI] [PubMed] [Google Scholar]
- 119.Mechanically Functional Scaffold Technology. 2015 Nov 16; Available from: http://www.cellcotec.com/technology/mechanically-functional-scaffold/
- 120.The INSTRUCT Products. 2015 Nov 16; Available from: http://www.cellcotec.com/technology/instruct-products/
- 121.Mahmood TA, Miot S, Frank O, Martin I, Riesle J, Langer R, et al. Modulation of chondrocyte phenotype for tissue engineering by designing the biologic-polymer carrier interface. Biomacromolecules. 2006;7(11):3012–8. doi: 10.1021/bm060489+. [DOI] [PubMed] [Google Scholar]
- 122.Lamme EN, Druecke D, Pieper J, May PS, Kaim P, Jacobsen F, et al. Long-term evaluation of porous PEGT/PBT implants for soft tissue augmentation. Journal of biomaterials applications. 2008;22(4):309–35. doi: 10.1177/0885328207075552. [DOI] [PubMed] [Google Scholar]
- 123.Emans PJ, Jansen EJ, van Iersel D, Welting TJ, Woodfield TB, Bulstra SK, et al. Tissue-engineered constructs: the effect of scaffold architecture in osteochondral repair. Journal of tissue engineering and regenerative medicine. 2013;7(9):751–6. doi: 10.1002/term.1477. [DOI] [PubMed] [Google Scholar]
- 124.Jansen EJ, Pieper J, Gijbels MJ, Guldemond NA, Riesle J, Van Rhijn LW, et al. PEOT/PBT based scaffolds with low mechanical properties improve cartilage repair tissue formation in osteochondral defects. Journal of biomedical materials research Part A. 2009;89(2):444–52. doi: 10.1002/jbm.a.31986. [DOI] [PubMed] [Google Scholar]
- 125.Deschamps AA, Grijpma DW, Feijen J. Poly(ethylene oxide)/poly(butylene terephthalate) segmented block copolymers: the effect of copolymer composition on physical properties and degradation behavior. Polymer. 2001;42(23):9335–45. [Google Scholar]
- 126.INSTRUCT for Repair of Knee Cartilage Defects. 2015 Nov 16; cited. Available from: https://clinicaltrials.gov/ct2/show/NCT01041885.
- 127.Hendriks J, Verdonk P, Widuchowski J, Snow M, Weiss W, Kruczyński J, et al. First clinical experience with INSTRUCT – a single surgery, autologous cell based technology for cartilage repair; European Society of Sports Traumatology, Knee Surgery and Arthroscopy Congress; Amsterdam. May 14-17, 2014. [Google Scholar]
- 128.Barbero A, Grogan S, Schafer D, Heberer M, Mainil-Varlet P, Martin I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2004;12(6):476–84. doi: 10.1016/j.joca.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 129.Stenhamre H, Slynarski K, Petren C, Tallheden T, Lindahl A. Topographic variation in redifferentiation capacity of chondrocytes in the adult human knee joint. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008;16(11):1356–62. doi: 10.1016/j.joca.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 130.Schulze-Tanzil G. Activation and dedifferentiation of chondrocytes: implications in cartilage injury and repair. Annals of anatomy = Anatomischer Anzeiger: official organ of the Anatomische Gesellschaft. 2009;191(4):325–38. doi: 10.1016/j.aanat.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 131.Demoor M, Ollitrault D, Gomez-Leduc T, Bouyoucef M, Hervieu M, Fabre H, et al. Cartilage tissue engineering: molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochimica et biophysica acta. 2014;1840(8):2414–40. doi: 10.1016/j.bbagen.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 132.Ma B, Leijten JC, Wu L, Kip M, van Blitterswijk CA, Post JN, et al. Gene expression profiling of dedifferentiated human articular chondrocytes in monolayer culture. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21(4):599–603. doi: 10.1016/j.joca.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 133.Dell’Accio F, De Bari C, Luyten FP. Molecular markers predictive of the capacity of expanded human articular chondrocytes to form stable cartilage in vivo. Arthritis and rheumatism. 2001;44(7):1608–19. doi: 10.1002/1529-0131(200107)44:7<1608::AID-ART284>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 134.Schnabel M, Marlovits S, Eckhoff G, Fichtel I, Gotzen L, Vecsei V, et al. Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2002;10(1):62–70. doi: 10.1053/joca.2001.0482. [DOI] [PubMed] [Google Scholar]
- 135.Barlic A, Drobnic M, Malicev E, Kregar-Velikonja N. Quantitative analysis of gene expression in human articular chondrocytes assigned for autologous implantation. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2008;26(6):847–53. doi: 10.1002/jor.20559. [DOI] [PubMed] [Google Scholar]
- 136.Cheng T, Maddox NC, Wong AW, Rahnama R, Kuo AC. Comparison of gene expression patterns in articular cartilage and dedifferentiated articular chondrocytes. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2012;30(2):234–45. doi: 10.1002/jor.21503. [DOI] [PubMed] [Google Scholar]
- 137.Darling EM, Athanasiou KA. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2005;23(2):425–32. doi: 10.1016/j.orthres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 138.Hamada T, Sakai T, Hiraiwa H, Nakashima M, Ono Y, Mitsuyama H, et al. Surface markers and gene expression to characterize the differentiation of monolayer expanded human articular chondrocytes. Nagoya journal of medical science. 2013;75(1-2):101–11. [PMC free article] [PubMed] [Google Scholar]
- 139.Kaps C, Frauenschuh S, Endres M, Ringe J, Haisch A, Lauber J, et al. Gene expression profiling of human articular cartilage grafts generated by tissue engineering. Biomaterials. 2006;27(19):3617–30. doi: 10.1016/j.biomaterials.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 140.Karlsen TA, Shahdadfar A, Brinchmann JE. Human primary articular chondrocytes, chondroblasts-like cells, and dedifferentiated chondrocytes: differences in gene, microRNA, and protein expression and phenotype. Tissue engineering Part C, Methods. 2011;17(2):219–27. doi: 10.1089/ten.TEC.2010.0200. [DOI] [PubMed] [Google Scholar]
- 141.Lin Z, Fitzgerald JB, Xu J, Willers C, Wood D, Grodzinsky AJ, et al. Gene expression profiles of human chondrocytes during passaged monolayer cultivation. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2008;26(9):1230–7. doi: 10.1002/jor.20523. [DOI] [PubMed] [Google Scholar]
- 142.Stokes DG, Liu G, Coimbra IB, Piera-Velazquez S, Crowl RM, Jimenez SA. Assessment of the gene expression profile of differentiated and dedifferentiated human fetal chondrocytes by microarray analysis. Arthritis and rheumatism. 2002;46(2):404–19. doi: 10.1002/art.10106. [DOI] [PubMed] [Google Scholar]
- 143.Caron MM, Emans PJ, Coolsen MM, Voss L, Surtel DA, Cremers A, et al. Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2012;20(10):1170–8. doi: 10.1016/j.joca.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 144.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30(1):215–24. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 145.Mandl EW, van der Veen SW, Verhaar JA, van Osch GJ. Multiplication of human chondrocytes with low seeding densities accelerates cell yield without losing redifferentiation capacity. Tissue engineering. 2004;10(1-2):109–18. doi: 10.1089/107632704322791754. [DOI] [PubMed] [Google Scholar]
- 146.Schulze-Tanzil G, de Souza P, Villegas Castrejon H, John T, Merker HJ, Scheid A, et al. Redifferentiation of dedifferentiated human chondrocytes in high-density cultures. Cell and tissue research. 2002;308(3):371–9. doi: 10.1007/s00441-002-0562-7. [DOI] [PubMed] [Google Scholar]
- 147.Huey DJ, Athanasiou KA. Alteration of the fibrocartilaginous nature of scaffoldless constructs formed from leporine meniscus cells and chondrocytes through manipulation of culture and processing conditions. Cells, tissues, organs. 2013;197(5):360–71. doi: 10.1159/000346252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Giovannini S, Diaz-Romero J, Aigner T, Mainil-Varlet P, Nesic D. Population doublings and percentage of S100-positive cells as predictors of in vitro chondrogenicity of expanded human articular chondrocytes. Journal of cellular physiology. 2010;222(2):411–20. doi: 10.1002/jcp.21965. [DOI] [PubMed] [Google Scholar]
- 149.Stokes DG, Liu G, Dharmavaram R, Hawkins D, Piera-Velazquez S, Jimenez SA. Regulation of type-II collagen gene expression during human chondrocyte de-differentiation and recovery of chondrocyte-specific phenotype in culture involves Sry-type high-mobility-group box (SOX) transcription factors. The Biochemical journal. 2001;360(Pt 2):461–70. doi: 10.1042/0264-6021:3600461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mandl EW, van der Veen SW, Verhaar JA, van Osch GJ. Serum-free medium supplemented with high-concentration FGF2 for cell expansion culture of human ear chondrocytes promotes redifferentiation capacity. Tissue engineering. 2002;8(4):573–80. doi: 10.1089/107632702760240490. [DOI] [PubMed] [Google Scholar]
- 151.Guerne PA, Sublet A, Lotz M. Growth factor responsiveness of human articular chondrocytes: distinct profiles in primary chondrocytes, subcultured chondrocytes, and fibroblasts. Journal of cellular physiology. 1994;158(3):476–84. doi: 10.1002/jcp.1041580312. [DOI] [PubMed] [Google Scholar]
- 152.Vivien D, Galera P, Lebrun E, Loyau G, Pujol JP. Differential effects of transforming growth factor-beta and epidermal growth factor on the cell cycle of cultured rabbit articular chondrocytes. Journal of cellular physiology. 1990;143(3):534–45. doi: 10.1002/jcp.1041430319. [DOI] [PubMed] [Google Scholar]
- 153.Parker CL, Paulsen DF, Rosebrock JA, Hooper WC. Inhibition of chondrogenesis by normal mouse serum in cultured chick limb cells. Experimental cell research. 1980;130(1):21–30. doi: 10.1016/0014-4827(80)90038-5. [DOI] [PubMed] [Google Scholar]
- 154.Hattori T, Ide H. Limb bud chondrogenesis in cell culture, with particular reference to serum concentration in the culture medium. Experimental cell research. 1984;150(2):338–46. doi: 10.1016/0014-4827(84)90577-9. [DOI] [PubMed] [Google Scholar]
- 155.Stewart MC, Saunders KM, Burton-Wurster N, Macleod JN. Phenotypic stability of articular chondrocytes in vitro: the effects of culture models, bone morphogenetic protein 2, and serum supplementation. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2000;15(1):166–74. doi: 10.1359/jbmr.2000.15.1.166. [DOI] [PubMed] [Google Scholar]
- 156.van Osch GJ, van der Veen SW, Verwoerd-Verhoef HL. In vitro redifferentiation of culture-expanded rabbit and human auricular chondrocytes for cartilage reconstruction. Plastic and reconstructive surgery. 2001;107(2):433–40. doi: 10.1097/00006534-200102000-00020. [DOI] [PubMed] [Google Scholar]
- 157.Lee J, Lee JY, Lee E, Kim JH, Son Y. Serum Deprivation Induces Efficient Chondrogenic Differentiation of Fully Dedifferentiated Human Costal Chondrocytes. Tissue Eng Regen Med. 2009;6(4-11):957–62. [Google Scholar]
- 158.Kim BS, Yoo SP, Park HW. Tissue engineering of cartilage with chondrocytes cultured in a chemically-defined, serum-free medium. Biotechnology letters. 2004;26(9):709–12. doi: 10.1023/b:bile.0000024093.94151.d8. [DOI] [PubMed] [Google Scholar]
- 159.Kato Y, Gospodarowicz D. Growth requirements of low-density rabbit costal chondrocyte cultures maintained in serum-free medium. Journal of cellular physiology. 1984;120(3):354–63. doi: 10.1002/jcp.1041200314. [DOI] [PubMed] [Google Scholar]
- 160.Mandl EW, Jahr H, Koevoet JL, van Leeuwen JP, Weinans H, Verhaar JA, et al. Fibroblast growth factor-2 in serum-free medium is a potent mitogen and reduces dedifferentiation of human ear chondrocytes in monolayer culture. Matrix biology: journal of the International Society for Matrix Biology. 2004;23(4):231–41. doi: 10.1016/j.matbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 161.Grogan SP, Chen X, Sovani S, Taniguchi N, Colwell CW, Jr., Lotz MK, et al. Influence of cartilage extracellular matrix molecules on cell phenotype and neocartilage formation. Tissue engineering Part A. 2014;20(1-2):264–74. doi: 10.1089/ten.tea.2012.0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ho ST, Yang Z, Hui HP, Oh KW, Choo BH, Lee EH. A serum free approach towards the conservation of chondrogenic phenotype during in vitro cell expansion. Growth factors. 2009;27(5):321–33. doi: 10.1080/08977190903137595. [DOI] [PubMed] [Google Scholar]
- 163.Malpeli M, Randazzo N, Cancedda R, Dozin B. Serum-free growth medium sustains commitment of human articular chondrocyte through maintenance of Sox9 expression. Tissue engineering. 2004;10(1-2):145–55. doi: 10.1089/107632704322791790. [DOI] [PubMed] [Google Scholar]
- 164.Jakob M, Demarteau O, Schafer D, Hintermann B, Dick W, Heberer M, et al. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. Journal of cellular biochemistry. 2001;81(2):368–77. doi: 10.1002/1097-4644(20010501)81:2<368::aid-jcb1051>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 165.Murphy MK, Huey DJ, Reimer AJ, Hu JC, Athanasiou KA. Enhancing post-expansion chondrogenic potential of costochondral cells in self-assembled neocartilage. PloS one. 2013;8(2):e56983. doi: 10.1371/journal.pone.0056983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Steinwachs MR, Kreuz PC, Huber C. The first prospective clinical outcome study of ACT by use of serum-free medium for cell cultivation; Presented at the 6th Symposium of the International Cartilage Repair Society San Diego; Jan. 8-11, 2006. [Google Scholar]
- 167.Mathieson W, Kirkland S, Leonard R, Thomas GA. Antimicrobials and in vitro systems: antibiotics and antimycotics alter the proteome of MCF-7 cells in culture. Journal of cellular biochemistry. 2011;112(8):2170–8. doi: 10.1002/jcb.23143. [DOI] [PubMed] [Google Scholar]
- 168.Rathbone CR, Cross JD, Brown KV, Murray CK, Wenke JC. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. Journal of Orthopaedic Research. 2011;29(7):1070–4. doi: 10.1002/jor.21343. [DOI] [PubMed] [Google Scholar]
- 169.Cohen S, Samadikuchaksaraei A, Polak JM, Bishop AE. Antibiotics reduce the growth rate and differentiation of embryonic stem cell cultures. Tissue engineering. 2006;12(7):2025–30. doi: 10.1089/ten.2006.12.2025. [DOI] [PubMed] [Google Scholar]
- 170.Coecke S, Balls M, Bowe G, Davis J, Gstraunthaler G, Hartung T, et al. Guidance on good cell culture practice. a report of the second ECVAM task force on good cell culture practice. Alternatives to laboratory animals: ATLA. 2005;33(3):261–87. doi: 10.1177/026119290503300313. [DOI] [PubMed] [Google Scholar]
- 171.Forriol F. Growth factors in cartilage and meniscus repair. Injury. 2009;40(Suppl 3):S12–6. doi: 10.1016/S0020-1383(09)70005-1. [DOI] [PubMed] [Google Scholar]
- 172.Vinatier C, Mrugala D, Jorgensen C, Guicheux J, Noel D. Cartilage engineering: a crucial combination of cells, biomaterials and biofactors. Trends in biotechnology. 2009;27(5):307–14. doi: 10.1016/j.tibtech.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 173.Dvorak P, Dvorakova D, Hampl A. Fibroblast growth factor signaling in embryonic and cancer stem cells. FEBS letters. 2006;580(12):2869–74. doi: 10.1016/j.febslet.2006.01.095. [DOI] [PubMed] [Google Scholar]
- 174.Mandl EW, Jahr H, Koevoet JLM, van Leeuwen JPTM, Weinans H, Verhaar JAN, et al. Fibroblast growth factor-2 in serum-free medium is a potent mitogen and reduces dedifferentiation of human ear chondrocytes in monolayer culture. Matrix Biology. 2004;23(4):231–41. doi: 10.1016/j.matbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 175.Yang KG, Saris DB, Geuze RE, Helm YJ, Rijen MH, Verbout AJ, et al. Impact of expansion and redifferentiation conditions on chondrogenic capacity of cultured chondrocytes. Tissue engineering. 2006;12(9):2435–47. doi: 10.1089/ten.2006.12.2435. [DOI] [PubMed] [Google Scholar]
- 176.Ribault D, Khatib AM, Panasyuk A, Barbara A, Bouizar Z, Mitrovic RD. Mitogenic and metabolic actions of epidermal growth factor on rat articular chondrocytes: modulation by fetal calf serum, transforming growth factor-beta, and tyrphostin. Archives of biochemistry and biophysics. 1997;337(2):149–58. doi: 10.1006/abbi.1996.9767. [DOI] [PubMed] [Google Scholar]
- 177.Shepard JB, Jeong JW, Maihle NJ, O’Brien S, Dealy CN. Transient anabolic effects accompany epidermal growth factor receptor signal activation in articular cartilage in vivo. Arthritis research & therapy. 2013;15(3):R60. doi: 10.1186/ar4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.van der Kraan P, Vitters E, van den Berg W. Differential effect of transforming growth factor beta on freshly isolated and cultured articular chondrocytes. The Journal of rheumatology. 1992;19(1):140–5. [PubMed] [Google Scholar]
- 179.Hwang NS, Kim MS, Sampattavanich S, Baek JH, Zhang Z, Elisseeff J. Effects of three-dimensional culture and growth factors on the chondrogenic differentiation of murine embryonic stem cells. Stem cells. 2006;24(2):284–91. doi: 10.1634/stemcells.2005-0024. [DOI] [PubMed] [Google Scholar]
- 180.Stevens MM, Marini RP, Martin I, Langer R, Prasad Shastri V. FGF-2 enhances TGF-beta1-induced periosteal chondrogenesis. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2004;22(5):1114–9. doi: 10.1016/j.orthres.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 181.Elder BD, Athanasiou KA. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2009;17(1):114–23. doi: 10.1016/j.joca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Narcisi R, Signorile L, Verhaar JA, Giannoni P, van Osch GJ. TGFbeta inhibition during expansion phase increases the chondrogenic re-differentiation capacity of human articular chondrocytes. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2012;20(10):1152–60. doi: 10.1016/j.joca.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 183.Hendriks J, Riesle J, Vanblitterswijk CA. Effect of stratified culture compared to confluent culture in monolayer on proliferation and differentiation of human articular chondrocytes. Tissue Eng. 2006;12(9):2397–405. doi: 10.1089/ten.2006.12.2397. [DOI] [PubMed] [Google Scholar]
- 184.Barbero A, Ploegert S, Heberer M, Martin I. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis and rheumatism. 2003;48(5):1315–25. doi: 10.1002/art.10950. [DOI] [PubMed] [Google Scholar]
- 185.Makris EA, MacBarb RF, Responte DJ, Hu JC, Athanasiou KA. A copper sulfate and hydroxylysine treatment regimen for enhancing collagen cross-linking and biomechanical properties in engineered neocartilage. Faseb J. 2013;27(6):2421–30. doi: 10.1096/fj.12-224030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Facchini A, Borzi RM, Olivotto E, Platano D, Pagani S, Cetrullo S, et al. Role of polyamines in hypertrophy and terminal differentiation of osteoarthritic chondrocytes. Amino acids. 2012;42(2-3):667–78. doi: 10.1007/s00726-011-1041-9. [DOI] [PubMed] [Google Scholar]
- 187.Takano T, Takigawa M, Suzuki F. Role of polyamines in expression of the differentiated phenotype of chondrocytes in culture. Medical biology. 1981;59(5-6):423–7. [PubMed] [Google Scholar]
- 188.Chua KH, Aminuddin BS, Fuzina NH, Ruszymah BH. Insulin-transferrin-selenium prevent human chondrocyte dedifferentiation and promote the formation of high quality tissue engineered human hyaline cartilage. European cells & materials. 2005;9:58–67. doi: 10.22203/ecm.v009a08. discussion. [DOI] [PubMed] [Google Scholar]
- 189.Liu X, Liu J, Kang N, Yan L, Wang Q, Fu X, et al. Role of insulin-transferrin-selenium in auricular chondrocyte proliferation and engineered cartilage formation in vitro. International journal of molecular sciences. 2014;15(1):1525–37. doi: 10.3390/ijms15011525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cigan AD, Nims RJ, Albro MB, Esau JD, Dreyer MP, Vunjak-Novakovic G, et al. Insulin, ascorbate, and glucose have a much greater influence than transferrin and selenous acid on the in vitro growth of engineered cartilage in chondrogenic media. Tissue engineering Part A. 2013;19(17-18):1941–8. doi: 10.1089/ten.tea.2012.0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Heywood HK, Nalesso G, Lee DA, Dell’accio F. Culture expansion in low-glucose conditions preserves chondrocyte differentiation and enhances their subsequent capacity to form cartilage tissue in three-dimensional culture. BioResearch open access. 2014;3(1):9–18. doi: 10.1089/biores.2013.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.DuRaine GD, Brown WE, Hu JC, Athanasiou KA. Emergence of scaffold-free approaches for tissue engineering musculoskeletal cartilages. Annals of biomedical engineering. 2015;43(3):543–54. doi: 10.1007/s10439-014-1161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Athanasiou KA, Eswaramoorthy R, Hadidi P, Hu JC. Self-organization and the self-assembling process in tissue engineering. Annual review of biomedical engineering. 2013;15:115–36. doi: 10.1146/annurev-bioeng-071812-152423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gigout A, Jolicoeur M, Nelea M, Raynal N, Farndale R, Buschmann MD. Chondrocyte aggregation in suspension culture is GFOGER-GPP- and beta1 integrin-dependent. The Journal of biological chemistry. 2008;283(46):31522–30. doi: 10.1074/jbc.M804234200. [DOI] [PubMed] [Google Scholar]
- 195.Ofek G, Revell CM, Hu JC, Allison DD, Grande-Allen KJ, Athanasiou KA. Matrix development in self-assembly of articular cartilage. PloS one. 2008;3(7):e2795. doi: 10.1371/journal.pone.0002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Makris EA, Responte DJ, Paschos NK, Hu JC, Athanasiou KA. Developing functional musculoskeletal tissues through hypoxia and lysyl oxidase-induced collagen cross-linking. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(45):E4832–41. doi: 10.1073/pnas.1414271111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hu JC, Athanasiou KA. The effects of intermittent hydrostatic pressure on self-assembled articular cartilage constructs. Tissue engineering. 2006;12(5):1337–44. doi: 10.1089/ten.2006.12.1337. [DOI] [PubMed] [Google Scholar]
- 198.Kulig KM, Luo X, Finkelstein EB, Liu XH, Goldman SM, Sundback CA, et al. Biologic properties of surgical scaffold materials derived from dermal ECM. Biomaterials. 2013;34(23):5776–84. doi: 10.1016/j.biomaterials.2013.02.055. [DOI] [PubMed] [Google Scholar]
- 199.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32(12):3233–43. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Teodori L, Costa A, Marzio R, Perniconi B, Coletti D, Adamo S, et al. Native extracellular matrix: a new scaffolding platform for repair of damaged muscle. Frontiers in physiology. 2014;5:218. doi: 10.3389/fphys.2014.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hoganson DM, Owens GE, O’Doherty EM, Bowley CM, Goldman SM, Harilal DO, et al. Preserved extracellular matrix components and retained biological activity in decellularized porcine mesothelium. Biomaterials. 2010;31(27):6934–40. doi: 10.1016/j.biomaterials.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 202.Wu Z, Tang Y, Fang H, Su Z, Xu B, Lin Y, et al. Decellularized scaffolds containing hyaluronic acid and EGF for promoting the recovery of skin wounds. Journal of materials science Materials in medicine. 2015;26(1):5322. doi: 10.1007/s10856-014-5322-1. [DOI] [PubMed] [Google Scholar]
- 203.Haugh MG, Murphy CM, O’Brien FJ. Novel freeze-drying methods to produce a range of collagen-glycosaminoglycan scaffolds with tailored mean pore sizes. Tissue engineering Part C, Methods. 2010;16(5):887–94. doi: 10.1089/ten.TEC.2009.0422. [DOI] [PubMed] [Google Scholar]
- 204.O’Brien FJ, Harley BA, Yannas IV, Gibson L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials. 2004;25(6):1077–86. doi: 10.1016/s0142-9612(03)00630-6. [DOI] [PubMed] [Google Scholar]
- 205.Schoof H, Apel J, Heschel I, Rau G. Control of pore structure and size in freeze-dried collagen sponges. J Biomed Mater Res. 2001;58(4):352–7. doi: 10.1002/jbm.1028. [DOI] [PubMed] [Google Scholar]
- 206.Kujawa MJ, Caplan AI. Hyaluronic acid bonded to cell-culture surfaces stimulates chondrogenesis in stage 24 limb mesenchyme cell cultures. Developmental biology. 1986;114(2):504–18. doi: 10.1016/0012-1606(86)90214-9. [DOI] [PubMed] [Google Scholar]
- 207.Responte DJ, Natoli RM, Athanasiou KA. Identification of potential biophysical and molecular signalling mechanisms underlying hyaluronic acid enhancement of cartilage formation. Journal of the Royal Society, Interface / the Royal Society. 2012;9(77):3564–73. doi: 10.1098/rsif.2012.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Schwartz Z, Griffon DJ, Fredericks LP, Lee HB, Weng HY. Hyaluronic acid and chondrogenesis of murine bone marrow mesenchymal stem cells in chitosan sponges. American journal of veterinary research. 2011;72(1):42–50. doi: 10.2460/ajvr.72.1.42. [DOI] [PubMed] [Google Scholar]
- 209.Kang JY, Chung CW, Sung JH, Park BS, Choi JY, Lee SJ, et al. Novel porous matrix of hyaluronic acid for the three-dimensional culture of chondrocytes. International journal of pharmaceutics. 2009;369(1-2):114–20. doi: 10.1016/j.ijpharm.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 210.Johnstone B, Alini M, Cucchiarini M, Dodge GR, Eglin D, Guilak F, et al. Tissue engineering for articular cartilage repair--the state of the art. European cells & materials. 2013;25:248–67. doi: 10.22203/ecm.v025a18. [DOI] [PubMed] [Google Scholar]
- 211.Bhardwaj N, Devi D, Mandal BB. Tissue-engineered cartilage: the crossroads of biomaterials, cells and stimulating factors. Macromolecular bioscience. 2015;15(2):153–82. doi: 10.1002/mabi.201400335. [DOI] [PubMed] [Google Scholar]
- 212.Balakrishnan B, Banerjee R. Biopolymer-based hydrogels for cartilage tissue engineering. Chemical reviews. 2011;111(8):4453–74. doi: 10.1021/cr100123h. [DOI] [PubMed] [Google Scholar]
- 213.Kim IL, Mauck RL, Burdick JA. Hydrogel design for cartilage tissue engineering: a case study with hyaluronic acid. Biomaterials. 2011;32(34):8771–82. doi: 10.1016/j.biomaterials.2011.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Buschmann MD, Gluzband YA, Grodzinsky AJ, Kimura JH, Hunziker EB. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 1992;10(6):745–58. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- 215.Ng KW, Lima EG, Bian L, O’Conor CJ, Jayabalan PS, Stoker AM, et al. Passaged adult chondrocytes can form engineered cartilage with functional mechanical properties: a canine model. Tissue engineering Part A. 2010;16(3):1041–51. doi: 10.1089/ten.tea.2009.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Huang AH, Yeger-McKeever M, Stein A, Mauck RL. Tensile properties of engineered cartilage formed from chondrocyte- and MSC-laden hydrogels. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008;16(9):1074–82. doi: 10.1016/j.joca.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.DeLise AM, Fischer L, Tuan RS. Cellular interactions and signaling in cartilage development. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2000;8(5):309–34. doi: 10.1053/joca.1999.0306. [DOI] [PubMed] [Google Scholar]
- 218.Rotter N, Aigner J, Naumann A, Planck H, Hammer C, Burmester G, et al. Cartilage reconstruction in head and neck surgery: comparison of resorbable polymer scaffolds for tissue engineering of human septal cartilage. J Biomed Mater Res. 1998;42(3):347–56. doi: 10.1002/(sici)1097-4636(19981205)42:3<347::aid-jbm2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 219.Perka C, Sittinger M, Schultz O, Spitzer RS, Schlenzka D, Burmester GR. Tissue engineered cartilage repair using cryopreserved and noncryopreserved chondrocytes. Clinical orthopaedics and related research. 2000;(378):245–54. doi: 10.1097/00003086-200009000-00035. [DOI] [PubMed] [Google Scholar]
- 220.Kaps C, Frauenschuh S, Endres M, Ringe J, Haisch A, Lauber J, et al. Gene expression profiling of human articular cartilage grafts generated by tissue engineering. Biomaterials. 2006;27(19):3617–30. doi: 10.1016/j.biomaterials.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 221.Tsuchiya K, Chen GP, Ushida T, Matsuno T, Tateishi T. The effect of coculture of chondrocytes with mesenchymal stem cells on their cartilaginous phenotype in vitro. Mat Sci Eng C-Bio S. 2004;24(3):391–6. [Google Scholar]
- 222.Gruber HE, Deepe R, Hoelscher GL, Ingram JA, Norton HJ, Scannell B, et al. Human adipose-derived mesenchymal stem cells: direction to a phenotype sharing similarities with the disc, gene expression profiling, and coculture with human annulus cells. Tissue engineering Part A. 2010;16(9):2843–60. doi: 10.1089/ten.TEA.2009.0709. [DOI] [PubMed] [Google Scholar]
- 223.Wu L, Leijten JC, Georgi N, Post JN, van Blitterswijk CA, Karperien M. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue engineering Part A. 2011;17(9-10):1425–36. doi: 10.1089/ten.TEA.2010.0517. [DOI] [PubMed] [Google Scholar]
- 224.Acharya C, Adesida A, Zajac P, Mumme M, Riesle J, Martin I, et al. Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. Journal of cellular physiology. 2012;227(1):88–97. doi: 10.1002/jcp.22706. [DOI] [PubMed] [Google Scholar]
- 225.Wu L, Prins HJ, Helder MN, van Blitterswijk CA, Karperien M. Trophic effects of mesenchymal stem cells in chondrocyte co-cultures are independent of culture conditions and cell sources. Tissue engineering Part A. 2012;18(15-16):1542–51. doi: 10.1089/ten.TEA.2011.0715. [DOI] [PubMed] [Google Scholar]
- 226.Hendriks JAA, Miclea RL, Schotel R, de Bruijn E, Moroni L, Karperien M, et al. Primary chondrocytes enhance cartilage tissue formation upon co-culture with a range of cell types. Soft Matter. 2010;6(20):5080–8. [Google Scholar]
- 227.de Windt TS, Hendriks JAA, Zhao X, Vonk LA, Creemers LB, Dhert WJA, et al. Concise Review: Unraveling Stem Cell Cocultures in Regenerative Medicine: Which Cell Interactions Steer Cartilage Regeneration and How? Stem Cell Transl Med. 2014;3(6):723–33. doi: 10.5966/sctm.2013-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Smith RL, Rusk SF, Ellison BE, Wessells P, Tsuchiya K, Carter DR, et al. In vitro stimulation of articular chondrocyte mRNA and extracellular matrix synthesis by hydrostatic pressure. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 1996;14(1):53–60. doi: 10.1002/jor.1100140110. [DOI] [PubMed] [Google Scholar]
- 229.Mizuno S, Tateishi T, Ushida T, Glowacki J. Hydrostatic fluid pressure enhances matrix synthesis and accumulation by bovine chondrocytes in three-dimensional culture. Journal of cellular physiology. 2002;193(3):319–27. doi: 10.1002/jcp.10180. [DOI] [PubMed] [Google Scholar]
- 230.Elder BD, Athanasiou KA. Effects of temporal hydrostatic pressure on tissue-engineered bovine articular cartilage constructs. Tissue engineering Part A. 2009;15(5):1151–8. doi: 10.1089/ten.tea.2008.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ogawa R, Mizuno S, Murphy GF, Orgill DP. The effect of hydrostatic pressure on three-dimensional chondroinduction of human adipose-derived stem cells. Tissue engineering Part A. 2009;15(10):2937–45. doi: 10.1089/ten.tea.2008.0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mouw JK, Imler SM, Levenston ME. Ion-channel regulation of chondrocyte matrix synthesis in 3D culture under static and dynamic compression. Biomechanics and modeling in mechanobiology. 2007;6(1-2):33–41. doi: 10.1007/s10237-006-0034-1. [DOI] [PubMed] [Google Scholar]
- 233.O’Conor CJ, Leddy HA, Benefield HC, Liedtke WB, Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(4):1316–21. doi: 10.1073/pnas.1319569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wann AK, Zuo N, Haycraft CJ, Jensen CG, Poole CA, McGlashan SR, et al. Primary cilia mediate mechanotransduction through control of ATP-induced Ca2+ signaling in compressed chondrocytes. Faseb J. 2012;26(4):1663–71. doi: 10.1096/fj.11-193649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Thoms BL, Dudek KA, Lafont JE, Murphy CL. Hypoxia promotes the production and inhibits the destruction of human articular cartilage. Arthritis and rheumatism. 2013;65(5):1302–12. doi: 10.1002/art.37867. [DOI] [PubMed] [Google Scholar]
- 236.Coyle CH, Izzo NJ, Chu CR. Sustained hypoxia enhances chondrocyte matrix synthesis. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2009;27(6):793–9. doi: 10.1002/jor.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Meretoja VV, Dahlin RL, Wright S, Kasper FK, Mikos AG. The effect of hypoxia on the chondrogenic differentiation of co-cultured articular chondrocytes and mesenchymal stem cells in scaffolds. Biomaterials. 2013;34(17):4266–73. doi: 10.1016/j.biomaterials.2013.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Babur BK, Ghanavi P, Levett P, Lott WB, Klein T, Cooper-White JJ, et al. The interplay between chondrocyte redifferentiation pellet size and oxygen concentration. PloS one. 2013;8(3):e58865. doi: 10.1371/journal.pone.0058865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Pazzano D, Mercier KA, Moran JM, Fong SS, DiBiasio DD, Rulfs JX, et al. Comparison of chondrogensis in static and perfused bioreactor culture. Biotechnology progress. 2000;16(5):893–6. doi: 10.1021/bp000082v. [DOI] [PubMed] [Google Scholar]
- 240.Tran SC, Cooley AJ, Elder SH. Effect of a mechanical stimulation bioreactor on tissue engineered, scaffold-free cartilage. Biotechnology and bioengineering. 2011;108(6):1421–9. doi: 10.1002/bit.23061. [DOI] [PubMed] [Google Scholar]
- 241.Gilbert E, Mosher M, Gottipati A, Elder S. A Novel Through-Thickness Perfusion Bioreactor for the Generation of Scaffold-Free Tissue Engineered Cartilage. Processes. 2014;2(3):658–74. [Google Scholar]
- 242.Alford AI, Yellowley CE, Jacobs CR, Donahue HJ. Increases in cytosolic calcium, but not fluid flow, affect aggrecan mRNA levels in articular chondrocytes. Journal of cellular biochemistry. 2003;90(5):938–44. doi: 10.1002/jcb.10715. [DOI] [PubMed] [Google Scholar]
- 243.Jeon JE, Schrobback K, Hutmacher DW, Klein TJ. Dynamic compression improves biosynthesis of human zonal chondrocytes from osteoarthritis patients. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2012;20(8):906–15. doi: 10.1016/j.joca.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 244.Hasanova GI, Noriega SE, Mamedov TG, Guha Thakurta S, Turner JA, Subramanian A. The effect of ultrasound stimulation on the gene and protein expression of chondrocytes seeded in chitosan scaffolds. Journal of tissue engineering and regenerative medicine. 2011;5(10):815–22. doi: 10.1002/term.384. [DOI] [PubMed] [Google Scholar]
- 245.Xu X, Urban JP, Tirlapur UK, Cui Z. Osmolarity effects on bovine articular chondrocytes during three-dimensional culture in alginate beads. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18(3):433–9. doi: 10.1016/j.joca.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 246.Responte DJ, Arzi B, Natoli RM, Hu JC, Athanasiou KA. Mechanisms underlying the synergistic enhancement of self-assembled neocartilage treated with chondroitinase-ABC and TGF-beta1. Biomaterials. 2012;33(11):3187–94. doi: 10.1016/j.biomaterials.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Newman P, Watt FM. Influence of cytochalasin D-induced changes in cell shape on proteoglycan synthesis by cultured articular chondrocytes. Experimental cell research. 1988;178(2):199–210. doi: 10.1016/0014-4827(88)90391-6. [DOI] [PubMed] [Google Scholar]
- 248.Zhang J, Wang JH. Kartogenin induces cartilage-like tissue formation in tendon-bone junction. Bone Res. 2014;2 doi: 10.1038/boneres.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hunziker EB, Lippuner K, Keel MJ, Shintani N. An educational review of cartilage repair: precepts & practice--myths & misconceptions--progress & prospects. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2015;23(3):334–50. doi: 10.1016/j.joca.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 250.Allen KD, Athanasiou KA. Viscoelastic characterization of the porcine temporomandibular joint disc under unconfined compression. Journal of biomechanics. 2006;39(2):312–22. doi: 10.1016/j.jbiomech.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 251.Natoli RM, Skaalure S, Bijlani S, Chen KX, Hu J, Athanasiou KA. Intracellular Na(+) and Ca(2+) modulation increases the tensile properties of developing engineered articular cartilage. Arthritis and rheumatism. 2010;62(4):1097–107. doi: 10.1002/art.27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Olvera D, Daly A, Kelly DJ. Mechanical Testing of Cartilage Constructs. Methods in molecular biology. 2015;1340:279–87. doi: 10.1007/978-1-4939-2938-2_20. [DOI] [PubMed] [Google Scholar]
- 253.Bhumiratana S, Eton RE, Oungoulian SR, Wan LQ, Ateshian GA, Vunjak-Novakovic G. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(19):6940–5. doi: 10.1073/pnas.1324050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Nover AB, Jones BK, Yu WT, Donovan DS, Podolnick JD, Cook JL, et al. A puzzle assembly strategy for fabrication of large engineered cartilage tissue constructs. Journal of biomechanics. 2016 doi: 10.1016/j.jbiomech.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Koo S, Hargreaves BA, Gold GE, Dragoo JL. Fabrication of custom-shaped grafts for cartilage regeneration. Int J Artif Organs. 2010;33(10):731–7. [PMC free article] [PubMed] [Google Scholar]
- 256.Grayson WL, Chao PH, Marolt D, Kaplan DL, Vunjak-Novakovic G. Engineering custom-designed osteochondral tissue grafts. Trends in biotechnology. 2008;26(4):181–9. doi: 10.1016/j.tibtech.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Cohen DL, Malone E, Lipson H, Bonassar LJ. Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue engineering. 2006;12(5):1325–35. doi: 10.1089/ten.2006.12.1325. [DOI] [PubMed] [Google Scholar]
- 258.Roach BL, Hung CT, Cook JL, Ateshian GA, Tan AR. Fabrication of tissue engineered osteochondral grafts for restoring the articular surface of diarthrodial joints. Methods. 2015;84:103–8. doi: 10.1016/j.ymeth.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Pomerantseva I, Bichara DA, Tseng A, Cronce MJ, Cervantes TM, Kimura AM, et al. Ear-Shaped Stable Auricular Cartilage Engineered from Extensively Expanded Chondrocytes in an Immunocompetent Experimental Animal Model. Tissue engineering Part A. 2016;22(3-4):197–207. doi: 10.1089/ten.tea.2015.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Saxena V, Kim M, Keah NM, Neuwirth AL, Stoeckl BD, Bickard K, et al. Anatomic Mesenchymal Stem Cell-Based Engineered Cartilage Constructs for Biologic Total Joint Replacement. Tissue engineering Part A. 2016;22(3-4):386–95. doi: 10.1089/ten.tea.2015.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hunziker EB, Stahli A. Surgical suturing of articular cartilage induces osteoarthritis-like changes. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008;16(9):1067–73. doi: 10.1016/j.joca.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Tompkins M, Adkisson HD, Bonner KF. DeNovo NT Allograft. Operative Techniques in Sports Medicine. 2013;21(2):82–9. [Google Scholar]
- 263.Kirilak Y, Pavlos NJ, Willers CR, Han R, Feng H, Xu J, et al. Fibrin sealant promotes migration and proliferation of human articular chondrocytes: possible involvement of thrombin and protease-activated receptors. International journal of molecular medicine. 2006;17(4):551–8. [PubMed] [Google Scholar]
- 264.Yousefi AM, Hoque ME, Prasad RG, Uth N. Current strategies in multiphasic scaffold design for osteochondral tissue engineering: A review. Journal of biomedical materials research Part A. 2015;103(7):2460–81. doi: 10.1002/jbm.a.35356. [DOI] [PubMed] [Google Scholar]
- 265.Ebert JR, Robertson WB, Lloyd DG, Zheng MH, Wood DJ, Ackland T. A Prospective, Randomized Comparison of Traditional and Accelerated Approaches to Postoperative Rehabilitation following Autologous Chondrocyte Implantation: 2-Year Clinical Outcomes. Cartilage. 2010;1(3):180–7. doi: 10.1177/1947603510362907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Davisson T, Kunig S, Chen A, Sah R, Ratcliffe A. Static and dynamic compression modulate matrix metabolism in tissue engineered cartilage. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2002;20(4):842–8. doi: 10.1016/S0736-0266(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 267.O’Driscoll SW, Giori NJ. Continuous passive motion (CPM): theory and principles of clinical application. Journal of rehabilitation research and development. 2000;37(2):179–88. [PubMed] [Google Scholar]
- 268.Sridharan B, Sharma B, Detamore MS. A Road Map to Commercialization of Cartilage Therapy in the United States of America. Tissue engineering Part B, Reviews. 2015 doi: 10.1089/ten.teb.2015.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lee JK, Responte DJ, Cissell DD, Hu JC, Nolta JA, Athanasiou KA. Clinical translation of stem cells: insight for cartilage therapies. Critical reviews in biotechnology. 2014;34(1):89–100. doi: 10.3109/07388551.2013.823596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Robert H, Potel JF, Dubrana F, Servien E, Bussiere C, Mainard D, et al. Essai clinique de phase III: implantation de chondrocytes autologues cultivés dans un gel Cartipatch® versus Mosaïcplastie dans le traitement des lésions chondrales des condyles fémoraux. Revue de Chirurgie Orthopédique et Traumatologique. 2013;99(8, Supplement):e17–e8. [Google Scholar]