Abstract
Background
Asymptomatic individuals account for a majority of sudden cardiac deaths (SCDs). Development of effective, low-cost, and non-invasive SCD risk stratification tools are necessary.
Methods and Results
Participants from the Atherosclerosis Risk in Communities study and Cardiovascular Health Study (n=20,177; age 59.3±10.1 years; age range 44–100; 56% female; 77% white) were followed for 14.0 years (median). Five ECG markers of global electrical heterogeneity (GEH) (sum absolute QRST integral, spatial QRST angle, spatial ventricular gradient (SVG) magnitude, SVG elevation, and SVG azimuth) were measured on standard 12-lead ECGs. Cox proportional hazards and competing risks models evaluated associations between GEH ECG parameters and SCD. A SCD competing risks score was derived using demographics, comorbidities, and GEH parameters. SCD incidence was 1.86 per 1,000 person-years. After multivariable adjustment, baseline GEH parameters and large increases in GEH parameters over time were independently associated with SCD. Final SCD risk scores included age, sex, race, diabetes, hypertension, coronary heart disease, and stroke, and GEH parameters as continuous variables. When GEH parameters were added to clinical/demographic factors, the C-statistic increased from 0.777 to 0.790 (p=0.008), the risk score classified 10-year SCD risk as high (>5%) in 7.2% of participants, 10% of SCD victims were appropriately reclassified into a high-risk category, and only 1.4% of SCD victims were inappropriately reclassified from high- to intermediate-risk. Net reclassification index was 18.3%.
Conclusions
Abnormal electrophysiological substrate quantified by GEH parameters is independently associated with SCD in the general population. Addition of GEH parameters to clinical characteristics improves SCD risk prediction.
Keywords: electrocardiography, sudden cardiac death, risk score
Journal Subject Terms: Sudden Cardiac Death, Risk Factors, Epidemiology, Electrophysiology, Electrocardiology (ECG)
Despite advances in the treatment and prevention of cardiovascular disease and reduction in total cardiovascular mortality, the incidence of sudden cardiac death (SCD) remains high. In the United States, 180,000–450,000 people per year die suddenly1, and in up to half of SCDs, cardiac arrest is the first manifestation of cardiovascular disease2. Effective, low-cost, non-invasive, and readily available tools to identify individuals at increased SCD risk are therefore necessary to optimally target primary prevention interventions and decrease SCD incidence. In the general population, SCD is primarily related to coronary heart disease (CHD)2 and ventricular tachyarrhythmias3,4. Fundamental studies in electrophysiology have demonstrated that susceptibility to ventricular arrhythmias is characterized by heterogeneity in myocardial activation and recovery times4,5 and action potential morphology6,7 which can be detected on QRST integral maps8,9. Non-invasive assessment of cardiac electrical heterogeneity is therefore a promising method of assessing SCD risk.
In the 1930s, Wilson developed the concept of an arithmetically summed area under the QRS complex and T-wave as a measure of the net electrical effect produced by local variations in the duration of the excited state10. Wilson calculated the vectorial sum of the QRS- and T-vectors, defined as the spatial ventricular gradient (SVG), in order to determine the direction along which non-uniformity in excitation and repolarization were greatest10 and the duration of the excited state was shortest11. Subsequent experimental and theoretical investigations demonstrated that the SVG is related to global heterogeneity of both action potential duration and morphology7. The concept underlying the SVG was extended to the spatial QRS-T angle, the three-dimensional angle between the QRS- and T-vectors12, and the sum absolute QRST integral (SAI QRST), a scalar analogue of the SVG calculated as the absolute value of the area under the QRS complex and T-wave13–15. These electrocardiographic parameters have been associated with ventricular arrhythmia in high-risk individuals14,16, but their association with SCD in the general population and their utility in SCD risk stratification remain unclear.
We hypothesized that markers of myocardial global electrical heterogeneity (GEH) (SVG, spatial QRS-T angle, and SAI QRST) would be independently associated with SCD and that they would improve SCD risk prediction in the general population beyond clinical and demographic characteristics.
Methods
Study populations
To obtain widely generalizable results we merged 2 large, bi-racial, prospective, community-dwelling adult cohorts. The Atherosclerosis Risk in Communities (ARIC) study is an ongoing, prospective cohort study assessing risk factors, progression, and outcomes of atherosclerosis in 15,792 community participants (45% male, 74% white) aged 45–64 years recruited from 4 United States communities between 1987–1989. Details of ARIC enrollment and study procedures have been previously described17. Black participants in the Washington and Minnesota cohorts (n=55), and participants with reported race other than white or black (n=48), uninterpretable ECGs (n=344), or missing covariates (n=736) were excluded. The final ARIC study population included 14,609 participants.
The Cardiovascular Health Study (CHS) is an ongoing, prospective cohort study assessing risk factors, progression, and outcomes of CHD and stroke in 5,888 community participants aged 65–100 years (42% male, 85% white) recruited from 4 United States communities. During 1989–1990 5,201 participants were enrolled, and in 1992–1993 a second cohort of 687 African-Americans was recruited. Details of CHS enrollment and study procedures have been previously published18. After excluding participants with reported race other than white or black (n=39), uninterpretable ECGs (n=87), or missing covariates (n=194), the final CHS study population included 5,568 participants.
Together the two cohorts included 20,177 adults (mean age 59.3±10.1 years; range 44–100 years; 44.1% male; 77.3% white). Both studies were approved by the institutional review boards of all participating institutions, and all participants gave informed consent. Definitions of covariates and incident non-fatal cardiovascular events are provided in the Supplemental Methods.
ECG recording, analysis, and measurement of GEH parameters
Recording and processing of 12-lead ECGs were identical in ARIC and CHS. Standard 10-second 12-lead ECGs were digitally acquired at a sampling rate of 500Hz and amplitude resolution of 1μV using MAC Personal Computer electrocardiographs (Marquette Electronics, Milwaukee, WI) and were automatically processed with the GE Magellan research utility (GE Marquette, Milwaukee, WI) to measure amplitudes and intervals. 12-lead ECGs were digitally recorded at study enrollment and during follow-up: yearly in the CHS cohort and triennially in the ARIC cohort. To evaluate longitudinal ECG changes, we analyzed ECGs at up to 10 visits in CHS participants and at up to 4 visits in ARIC participants.
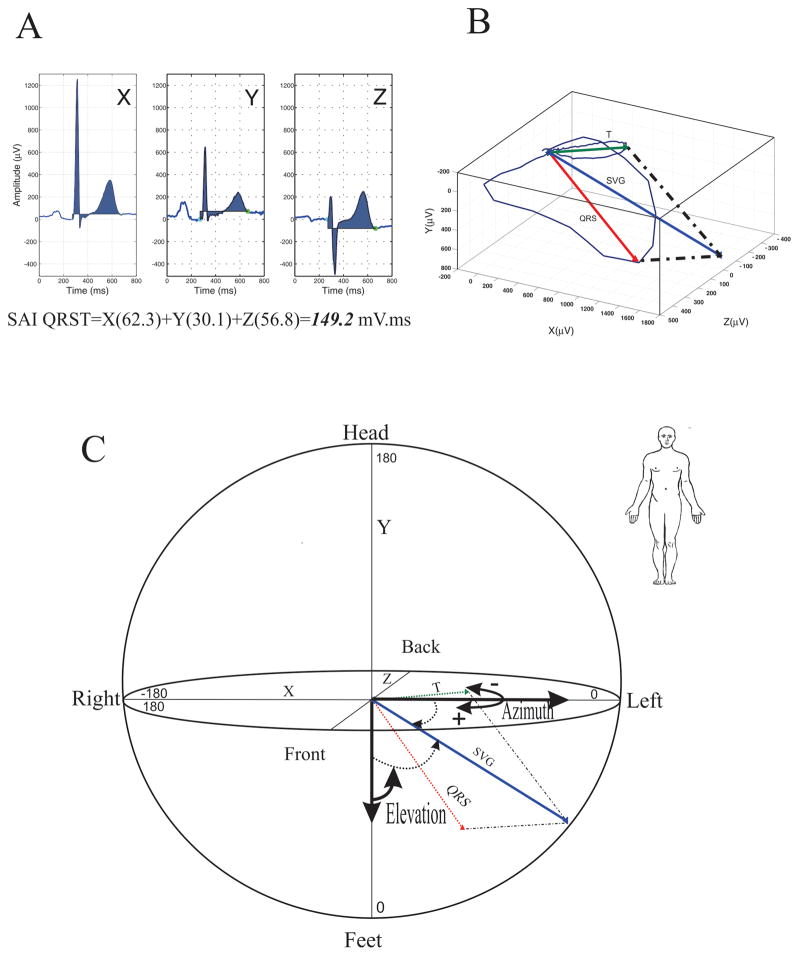
A detailed description of GEH parameter measurement is provided in the Supplemental Methods. SAI QRST was measured as the arithmetic sum of areas under the QRST curve as previously described13,15 (Figure 1A). Spatial mean QRS-T angle was defined as the three-dimensional angle between the mean QRS-vector and the mean T-vector (Figure 1B) as previously described12. SVG represents a vector in three-dimensional space defined by the vectorial sum of the QRS-vector and the T-vector (Figure 1B). The magnitude, azimuth and elevation of the SVG vector were measured (Figure 1C).
Figure 1.
Measurement of GEH parameters. A:SAI QRST represents the sum of the area under the QRS complex and T-wave using the isoelectric line as the reference (shaded area). X-, Y-, and Z-lead QRS-T complexes and their calculated SAI QRST results are shown. B:Spatial QRS-T angle represents the angle between the QRS-vector and T-vector in three-dimensional space. C:SVG is a vector defined as the vectorial sum of the QRS-vector and the T-vector. SVG magnitude is the length of the SVG-vector. SVG azimuth is the angle of the SVG-vector projected onto the XY (horizontal) plane, and SVG elevation is the angle of the SVG-vector projected in the XZ (vertical) plane.
Heart rate, corrected QT interval (QTc), and QRS duration were measured by the GE 12SL algorithm (GE Marquette, Milwaukee, WI). Sex-specific Cornell product was calculated for assessment of ECG-left ventricular hypertrophy19.
Patient follow-up and SCD adjudication
Follow-up of ARIC participants included annual telephone calls, local hospital surveillance, three triennial visits through 1998, and searching the Social Security Death Index (SSDI); details of follow-up have been previously reported20. CHS follow-up included semi-annual alternating phone calls and clinic visits through 1999 with twice-yearly phone calls thereafter, review of Medicare hospitalization records, and searching the SSDI; details of follow-up have been previously reported21.
The primary outcome of this analysis was SCD, which was similarly adjudicated in ARIC and CHS. SCD was defined as a sudden pulseless condition presumed due to a ventricular tachyarrhythmia in a previously stable individual without evidence of a non-cardiac cause of cardiac arrest. We a priori sought to exclude cases with non-arrhythmic characteristics, including those with evidence of progressive hypotension or advanced decompensated heart failure (HF) before death. All SCD events in this analysis occurred out of the hospital or in a hospital Emergency Department. A detailed description of SCD adjudication is provided in the Supplemental Methods.
Participants were censored at time of loss to follow-up or death if cause of death was not SCD. Administrative censoring occurred on July 31, 2006 for CHS and December 31, 2001 for ARIC.
Statistical analysis
A detailed description of our statistical methods is provided in the supplementary materials. In brief, we conducted the following analyses:
Association between GEH parameters and baseline characteristics
Minimally adjusted linear regression was used to determine associations between baseline demographic, clinical, and traditional ECG characteristics and GEH parameters. Circular variables (SVG azimuth and elevation) were analyzed using circular statistics.
Association between GEH parameters and SCD
Cox proportional hazards and competing risks models quantified associations between individual GEH parameters treated as continuous variables and SCD. Given that there are no prior data demonstrating the association between GEH parameters and SCD in the general population, we constructed 4 models that were designed to assess the magnitude and significance of association between each GEH parameter and SCD as additional potential confounders were sequentially added. Model 1 adjusted for demographic characteristics (age, gender, race, and study cohort/center). Model 2 additionally adjusted for prevalent cardiovascular disease and traditional cardiovascular risk factors (CHD, HF, stroke, atrial fibrillation (AF), beta-blockers, creatinine, body-mass-index, hypertension, anti-hypertensive medications, DM, smoking status, alcohol intake, total cholesterol, high-density-lipoprotein cholesterol, triglycerides, and physical activity index). Model 3 further adjusted for ECG parameters associated with SCD (heart rate, QTc, QRS duration, sex-specific Cornell product, and bundle branch block (BBB) or intra-ventricular conduction delay (IVCD)). Model 4 evaluated whether the association of GEH parameters with SCD remained significant over time and included all baseline covariates included in Model 3, time-updated GEH parameters, time-updated traditional ECG measurements, and time-updated incident non-fatal cardiovascular events (AF, HF, CHD, and stroke). As information on baseline left ventricular ejection fraction (LVEF) was not available in ARIC participants, sensitivity analyses evaluated the effect of adding LVEF into fully-adjusted and time-updated models in 4,954 CHS participants. Subgroup analysis was performed in Model 3 to determine significant interactions between GEH parameters and clinical characteristics.
Definition of abnormal GEH parameters
Gender- and race-specific thresholds defining abnormal GEH parameter values were selected using Youden’s index22 to maximize the sum of sensitivity and specificity. A competing risks model was constructed to determine the incremental SCD risk associated with multiple “abnormal” GEH parameters.
Longitudinal changes in GEH parameters over time and SCD risk
Mixed effect multilevel models (adjusted by age, sex, and race with participants nested within study center nested within cohort) were constructed to determine whether GEH parameters changed over time. To investigate whether longitudinal changes in GEH parameters were independently associated with SCD, the interaction with time was assessed in time-updated Cox models to test the assumption of proportionality for the hazard of time-updated variables over time. In addition, separate Cox proportional hazards models were used to determine if large increases in GEH parameters between study visits 1–3 were associated with SCD. Schoenfeld residuals confirmed that the proportional hazards assumption was valid in all Cox proportional hazards models.
Risk score development
We constructed 2 SCD risk scores using Fine and Gray’s competing risks model to test the incremental predictive value of adding GEH parameters as continuous variables to clinical characteristics. A combination of clinical guidance and backward selection was used to select covariates for inclusion in the final risk score models. To allow wider applicability of the risk score we initially assessed clinically important covariates from Model 3 (see Supplemental Methods). The final clinical-only SCD risk score included known SCD risk factors: age, gender, race, CHD, stroke, diabetes, and hypertension.
The combined clinical+GEH score was developed using initially covariates from the clinical-only score, all 5 GEH parameters, and all significant interaction terms. Backwards selection was then performed with a cut-off p-value of 0.10. The final model included age, gender, race, CHD, stroke, diabetes, hypertension, SAI QRST, spatial QRS-T angle, SVG elevation, and interaction terms (SAI QRST*age, QRS-T angle*age, QRS-T angle*race, QRS-T angle*diabetes, QRS-T angle*hypertension, and SVG azimuth*gender). Weighting of each variable’s contribution to SCD risk was determined by relative size of effect estimates. A cumulative incidence function was used to assign 10-year SCD risk to each participant based on their individual clinical-only and clinical+GEH risk scores.
All statistical analyses were performed using STATA 14 (StataCorp LP, College Station, TX) and Oriana-Circular Statistics Version 4 (Kovach Computing Services, Pentraeth, Wales, UK).
Results
Associations of baseline clinical and traditional ECG characteristics with GEH parameters
Baseline characteristics and incident non-fatal cardiovascular events are shown in Table 1. Associations between baseline clinical and ECG characteristics and the 5 measures of GEH are shown in Supplemental Tables 1–3. Abnormal LVEF was positively associated with all GEH parameters. Prevalent CHD was positively associated with all GEH parameters except SVG magnitude, for which a strong inverse association was observed.
Table 1.
Baseline characteristics and incident non-fatal cardiovascular events.
| Characteristic | Combined n=20,177 | ARIC n=14,609 | CHS n=5,568 |
|---|---|---|---|
| Age, years | 59.3±10.1 | 54.1±5.76 | 72.8±5.6 |
| Female, n(%) | 11,274(55.9) | 8,067(55.2) | 3,207(57.6) |
| White, n(%) | 15,590(77.3) | 10,873(74.4) | 4,717(84.72) |
| Diabetes, n(%) | 2,613(13.0) | 1,693(11.6) | 920(16.5) |
| Hypertension, n(%) | 8,250(40.9) | 4,985(34.1) | 3,265(58.6) |
| Anti-hypertensive medications, n(%) | 7,030(34.8) | 4,388(30.0) | 2,642(47.5) |
| CHD, n(%) | 1,762(8.73) | 674(4.61) | 1,088(19.5) |
| Heart failure, n(%) | 914(4.5) | 662(4.5) | 252(4.5) |
| Stroke, n(%) | 469(2.32) | 243(1.7) | 226(4.1) |
| Atrial fibrillation, n(%) | 183(0.9) | 32(0.2) | 151(2.71) |
| Current smoking, n(%) | 4,452(22.1) | 3,789(25.9) | 663(11.9) |
| Body-mass-index, kg/m2 | 27.4±5.2 | 27.7±5.3 | 26.7±4.7 |
| Total cholesterol, mg/dL | 213.8±41.1 | 214.8±41.8 | 211.3±39.3 |
| HDL cholesterol, mg/dL | 52.4±16.8 | 51.7±17.2 | 54.2±15.8 |
| Triglycerides, mg/dL | 133.6±86.8 | 131.6±90.5 | 139.0±75.8 |
| Beta-blockers, n(%) | 1,987(9.9) | 1,269(8.7) | 718(12.9) |
| Alcohol consumption, g/wk | 40.8±114.5 | 42.4±95.3 | 36.7±153.7 |
| Creatinine, g/dL | 1.10±0.42 | 1.11±0.43 | 1.06±0.40 |
| Abnormal LVEF*, n(%) | 183 (3.4) | N/A | 183(3.4) |
| Heart rate, bpm | 66±11 | 66±10 | 65±11 |
| Corrected QT, ms | 418.2±20.7 | 416.2±19.0 | 423.5±23.8 |
| QRS duration, ms | 92.7±14.6 | 92.2±12.3 | 92.5±12.8 |
| BBB/IVCD, n(%) | 2,328(11.5) | 1,672(11.4) | 656(11.8) |
| Sex-adjusted Cornell Product, mV* ms | 1,514±705 | 1,457±619 | 1,664±874 |
| Ventricular pacing, n(%) | 47(0.2) | 2(0.01) | 45(0.81) |
| Incident Non-Fatal Events | Incidence per 1,000 person-years(95%CI) | ||
|---|---|---|---|
| Combined | ARIC | CHS | |
| Incident heart failure | 11.22(10.82–11.64) | 5.73(5.40–6.08) | 28.03(26.76–29.37) |
| Incident atrial fibrillation | 9.34(8.97–9.72) | 4.72(4.42–5.04) | 24.53(23.29–25.83) |
| Incident stroke | 6.44(6.14–6.75) | 3.26(3.02–3.53) | 15.76(14.83–16.74) |
| Incident CHD | 14.87(14.39–15.37) | 10.18(9.73–10.65) | 31.62(30.14–33.19) |
n(% total) for categorical variables; mean±SD for continuous variables.
CHS only (n=4,953).
LVEF=left ventricular ejection fraction; CHD=coronary heart disease; BBB=bundle branch block; IVCD=intraventricular conduction delay
Association between GEH parameters and SCD
Among ARIC participants, over median follow-up of 14.1 years, 291 SCDs occurred (incidence 1.48 (95%CI 1.32–1.66) per 1,000 person-years). Among CHS participants, over median follow-up of 13.1 years, 195 SCDs occurred (incidence 3.00 (95%CI 2.61–3.45) per 1,000 person-years). In the combined cohort, over median follow-up of 14.0 years, 486 SCDs occurred (incidence 1.86 (95%CI 1.70–2.03) per 1,000 person-years). SCD accounted for 7.56% of all deaths in the combined cohort.
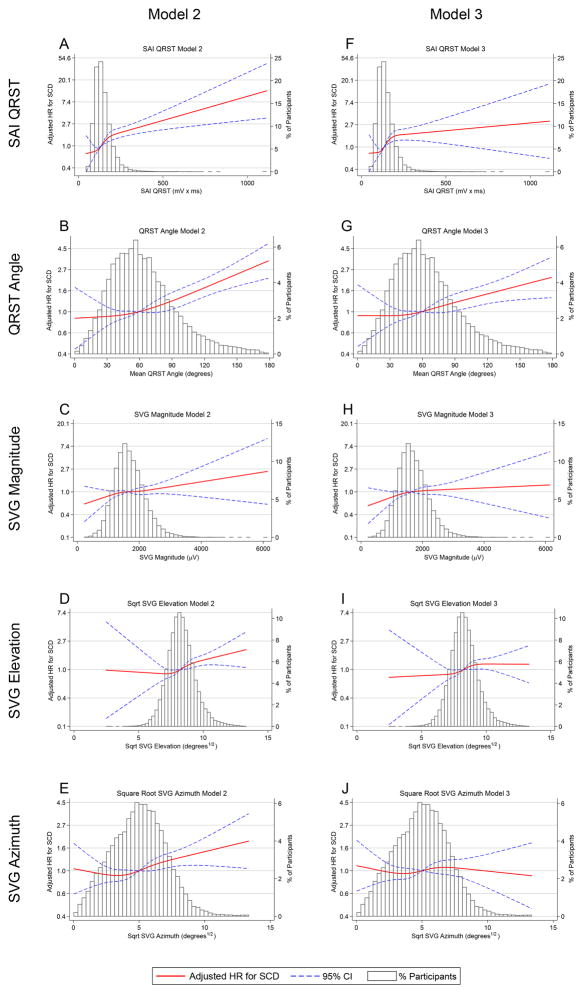
Table 2 shows associations between GEH parameters and SCD risk in Cox proportional hazards Models 1–4. In Model 2 all 5 GEH parameters were associated with SCD. Figure 2 shows the hazard of SCD over the range of GEH parameter values, relative to the mean value, when quadratic splines were used to characterize the relationship between GEH parameters and SCD risk; in adjusted analyses there was a dose-response relationship between GEH parameters and SCD. Further adjustment for baseline ECG parameters (Model 3) and time-updated ECG/GEH measurements and incident non-fatal cardiovascular outcomes (Model 4) revealed minimal change in the magnitude of association between SAI QRST, QRS-T angle, and SVG magnitude and SCD, although the associations between SVG elevation/azimuth and SCD attenuated. In sensitivity analyses exploring the importance of adding LVEF to fully-adjusted models (Table 2), the addition of baseline LVEF did not substantially change the magnitude or significance of association between any GEH parameter and SCD. Competing risks models revealed similar associations between GEH parameters and SCD (Supplemental Table 4).
Table 2.
Associations of GEH parameters with SCD per 1 SD change in parameter in Cox regression models
| SAI QRST | QRS-T Angle | SVG Magnitude | SVG Elevation | SVG Azimuth | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Merged | HR(95%CI) | P-value | HR(95%CI) | P-value | HR(95%CI) | P-value | HR(95%CI) | P-value | HR(95%CI) | P-value |
| Model 1 | 1.26(1.20–1.33) | <0.0001 | 1.55(1.43–1.68) | <0.0001 | 1.03(0.95–1.13) | 0.482 | 1.26(1.15–1.37) | <0.0001 | 1.30(1.20–1.42) | <0.0001 |
| Model 2 | 1.21(1.15–1.28) | <0.0001 | 1.30(1.20–1.41) | <0.0001 | 1.10(1.01–1.20) | 0.032 | 1.19(1.09–1.29) | <0.0001 | 1.14(1.05–1.24) | 0.002 |
| Model 3 | 1.16(1.07–1.25) | <0.0001 | 1.21(1.10–1.32) | <0.0001 | 1.09(1.00–1.19) | 0.048 | 1.11(1.02–1.22) | 0.015 | 1.01(0.92–1.11) | 0.761 |
| Model 4 | 1.16(1.07–1.25) | <0.0001 | 1.29(1.17–1.43) | <0.0001 | 1.15(1.05–1.25) | 0.002 | 1.01(0.92–1.11) | 0.807 | 1.05(0.95–1.17) | 0.309 |
|
| ||||||||||
| ARIC | ||||||||||
| Model 1 | 1.33(1.24–1.43) | <0.0001 | 1.70(1.54–1.88) | <0.0001 | 0.99(0.88–1.11) | 0.871 | 1.30(1.17–1.44) | <0.0001 | 1.30(1.16–1.46) | <0.0001 |
| Model 2 | 1.23(1.14–1.31) | <0.0001 | 1.39(1.25–1.54) | <0.0001 | 1.08(0.96–1.21) | 0.200 | 1.18(1.06–1.32) | 0.002 | 1.11(0.99–1.24) | 0.065 |
| Model 3 | 1.17(1.07–1.29) | 0.001 | 1.30(1.16–1.46) | <0.0001 | 1.04(0.93–1.17) | 0.477 | 1.11(1.00–1.24) | 0.054 | 0.98(0.87–1.11) | 0.747 |
| Model 4 | 1.06(0.97–1.15) | 0.180 | 1.37(1.23–1.54) | <0.0001 | 1.08(0.96–1.21) | 0.210 | 1.05(0.94–1.17) | 0.390 | 1.09(0.96–1.24) | 0.207 |
|
| ||||||||||
| CHS | ||||||||||
| Model 1 | 1.27(1.16–1.39) | <0.0001 | 1.40(1.22–1.61) | <0.0001 | 1.10(0.96–1.26) | 0.157 | 1.20(1.04–1.37) | 0.011 | 1.34(1.17–1.54) | <0.0001 |
| Model 2 | 1.22(1.10–1.34) | <0.0001 | 1.18(1.03–1.36) | 0.017 | 1.13(0.99–1.29) | 0.067 | 1.17(1.02–1.34) | 0.028 | 1.18(1.03–1.35) | 0.014 |
| Model 3 | 1.13(0.98–1.31) | 0.098 | 1.05(0.90–1.23) | 0.534 | 1.14(1.00–1.31) | 0.050 | 1.08(0.93–1.24) | 0.308 | 1.02(0.87–1.20) | 0.777 |
| Model 4 | 1.29(1.13–1.49) | <0.0001 | 1.14(0.97–1.33) | 0.123 | 1.23(1.08–1.41) | 0.002 | 0.97(0.85–1.12) | 0.717 | 0.98(0.83–1.16) | 0.811 |
| Sensitivity Analysis to evaluate effect of LVEF on association with SCD in CHS Participants* | ||||||||||
|
Model 4 w/o
LVEF |
1.34(1.15–1.55) | <0.0001 | 1.15(0.98–1.36) | 0.109 | 1.24(1.08–1.43) | 0.002 | 0.98(0.85–1.14) | 0.800 | 0.97(0.82–1.16) | 0.773 |
| With LVEF | 1.30(1.12–1.51) | 0.001 | 1.12(0.95–1.32) | 0.182 | 1.23(1.07–1.42) | 0.003 | 0.99(0.85–1.14) | 0.853 | 0.99(0.83–1.18) | 0.923 |
Model 1-adjusted for age, sex, race, study center/cohort.
Model 2-further adjusted for CHD, HF, stroke, AF, beta-blockers, creatinine, body-mass-index, hypertension, anti-hypertensive medications, diabetes, smoking, alcohol intake, total cholesterol, HDL cholesterol, triglycerides, physical activity index.
Model 3-further adjusted for ECG characteristics: heart rate, corrected QT interval, QRS duration, Cornell product, bundle branch block or intraventricular conduction delay
Model 4-further adjusted for time-updated ECG/GEH measurements and time-updated incident non-fatal cardiovascular outcomes (AF, HF, CHD, stroke)
Restricted to 4,954 CHS participants with baseline LVEF available CHD=coronary heart disease; HF=heart failure; AF=atrial fibrillation; LVEF=left ventricular ejection fraction
Figure 2.
Multivariable adjusted hazard ratios with 95%CI for SCD associated with SAI QRST (A,F), QRS-T angle (B,G), SVG magnitude (C,H), SVG elevation (D,I), and SVG azimuth (E,J), modeled as continuous variables using quadratic splines in Models 2 (A–E) and 3 (F–J). Plotted HRs represent the hazard at a given value of the covariate relative to the hazard at the average value of the covariate.
Longitudinal changes in GEH parameters and SCD risk
Mixed effects models revealed that over time values of SAI QRST, QRS-T angle, SVG elevation, and SVG azimuth increased, while values of SVG magnitude decreased, in a statistically significant manner (Supplemental Table 5). Importantly, the overall magnitude of changes in GEH parameters, however, were small: e.g. <1 degree of QRS-T angle and <1mV*ms of SAI QRST between two visits (median 2.8 years). In time-updated Cox models there were no significant interactions between time and time-updated SAI QRST (p=0.436), QRS-T angle (p=0.189), SVG magnitude (p=0.083), SVG elevation (p=0.982), or SVG azimuth (p=0.534), and the assumption of proportional hazards was confirmed in all time-updated Cox regression models. Therefore, there was no evidence of change in the association between SCD risk and GEH parameters over time—small changes in GEH parameters over time were not associated with additional SCD risk.
However, large, sudden increases in GEH parameters (≥50% for SAI QRST, SVG magnitude, elevation and azimuth, and ≥3-fold for QRS-T angle) were independently associated with SCD after adjustment for all other covariates, incident non-fatal CVD events, and baseline GEH parameter values (Supplemental Table 6 and Supplemental Figure 1). There was a dose-dependent increase in SCD proportional to the increase in GEH parameter values between baseline and study visits 2 or 3.
Subgroup analyses
The strength of association between SAI QRST and spatial QRS-T angle and SCD decreased with increasing age (Supplemental Table 7). QRS-T angle had a stronger association with SCD in white participants and participants free of HTN or DM. SAI QRST had a stronger association with SCD in females. There was no significant interaction with ventricular pacing or the presence BBB/IVCD.
Dichotomized GEH parameters and SCD risk
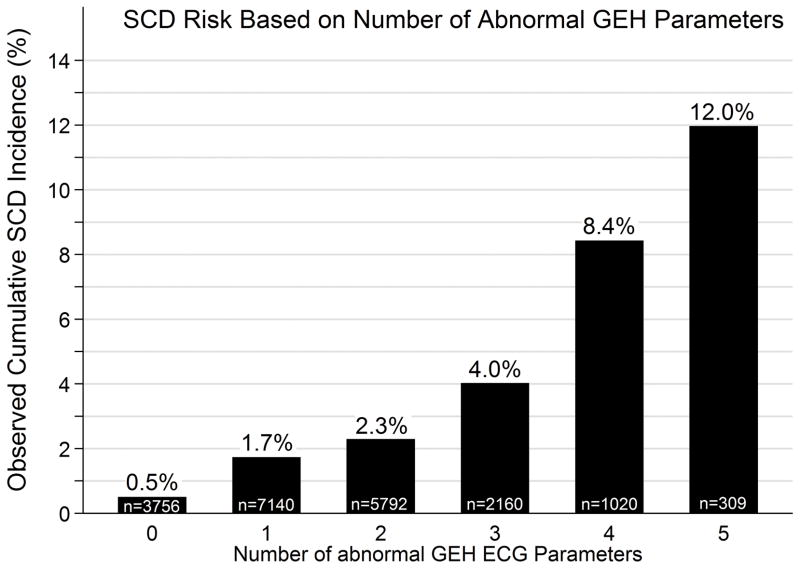
The optimal gender- and race-stratified cutoff points for GEH parameters, as calculated by Youden’s index, are reported in Supplemental Table 8. As the number of abnormal GEH parameters increased from 0 to 5, the rate of SCD increased from 0.5% to 12.0% (Figure 3), and the percent of all deaths due to SCD increased from 2.4% to 17.6% (Supplemental Figure 2). In an unadjusted competing risks model, participants with 5 abnormal GEH parameters had a sub- HR for SCD of 25.4 (95%CI 14.6–44.1, p<0.0001) compared to those with 0 abnormal parameters.
Figure 3.
SCD incidence based on number of abnormal dichotomized GEH parameters with each parameter given equal weight.
Development of SCD risk scores
Table 3 describes the final SCD risk scores. Significant improvement in SCD risk prediction was seen with the addition of GEH parameters and appropriate interaction terms. The clinical-only risk score predicted 10-year cumulative SCD incidence between 0.44% and 42.14%, whereas the clinical+GEH risk score assigned participants to a wider range of 10-year SCD risk (0.05% to 55.12%). The clinical+GEH score C-statistic was significantly higher than the clinical-only C-statistic (0.790 vs 0.777, p=0.008), although the magnitude of the difference was small. Additionally, in spite of increased complexity, goodness of fit of the clinical+GEH score was better than that of the clinical-only score as shown by a smaller AIC. The full clinical+GEH SCD risk score equation and an interactive risk calculator are available in the online supplemental materials and online at http://www.ecgpredictscd.org/.
Table 4.
Ten-year competing risks for SCD as predicted by clinical-only and clinical+GEH models
| 10-year risk from Clinical+GEH score | ||||
|---|---|---|---|---|
| <0.5% | 0.5–5% | >5% | Total | |
| 10-year risk from Clinical-only score | ||||
| <0.5% | ||||
| Participants in category (% total cohort) | 1,921(9.52%) | 472(2.34%) | 4(0.02%) | 2,397(11.88%) |
| SCD events (% of all SCDs) | 5(1.03%) | 1(0.21%) | 0(0.00%) | 6(1.23%) |
| Nonevents (non-SCD) (% all non-SCDs) | 1,916(9.73%) | 471(2.39%) | 4(0.02%) | 2,397(12.14%) |
| Proportion of all deaths due to SCD in category | 6.67% | 4.35% | 0.00% | 6.12% |
| Non-sudden fatal CHD (% all deaths in category) | 10(13.33%) | 5(21.74%) | 0(0.00%) | 15(15.31%) |
| Non-CHD death (% all deaths in category) | 60(80.00%) | 17(73.91%) | 0(0.00%) | 77(78.57%) |
| Proportion of all deaths not due to SCD in category | 93.33% | 95.65% | 0.00% | 93.88% |
| All-cause death (% total in category) | 75(3.90%) | 23(4.87%) | 0(0.00%) | 98(4.09%) |
| 0.5–5% | ||||
| Participants in category (% total cohort) | 2,948(14.61%) | 13,191(65.38%) | 401(1.99%) | 16,540(81.97%) |
| SCD events (% of all SCDs) | 10(2.06%) | 288(59.26%) | 49(10.08%) | 347(71.40%) |
| Nonevents (non-SCD) (% all non-SCDs) | 2,938(14.92%) | 12,903(65.53%) | 352(1.79%) | 16,193(82.24%) |
| Proportion of all deaths due to SCD in this category | 2.21% | 6.12% | 18.49% | 6.40% |
| Non-sudden fatal CHD (% all deaths in category) | 78(17.22%) | 1,403(29.81%) | 108(40.75%) | 1,589(29.29%) |
| Non-CHD death (% all deaths in category) | 365(80.57%) | 3,016(64.07%) | 108(40.75%) | 3,489(64.31%) |
| Proportion of all deaths not due to SCD in category | 97.79% | 93.88% | 81.51% | 93.60% |
| All-cause death (% total in category) | 453(15.37%) | 4,707(35.68%) | 265(66.08%) | 5,425(32.80%) |
| >5% | ||||
| Participants in category (% total cohort) | 0(0.0%) | 201(1.00%) | 1,039(5.15%) | 1,240(6.15%) |
| SCD events (% of all SCDs) | 0(0.0%) | 8(1.65%) | 125(25.72%) | 133(27.37%) |
| Nonevents (non-SCD) (% all non-SCDs) | 0(0.0%) | 193(0.98%) | 914(4.64%) | 1,107(5.62%) |
| Proportion of all deaths due to SCD in this category | 0.0% | 7.27% | 15.72% | 14.70% |
| Non-sudden fatal CHD (% all deaths in category) | 0(0.0%) | 47(42.73%) | 326(41.01%) | 373(41.22%) |
| Non-CHD death (% all deaths in category) | 0(0.0%) | 55(50.00%) | 344(43.27%) | 399(44.09%) |
| Proportion of all deaths not due to SCD in category | 0.0% | 92.73% | 84.28% | 85.30% |
| All-cause death (% total in category) | 0(0.0%) | 110(54.73%) | 795(76.52%) | 905(72.98%) |
| Total | ||||
| Participants in category (% total cohort) | 4,869(24.13%) | 13,864(68.71%) | 1,444(7.16%) | 20,177(100.00%) |
| SCD events (% of all SCDs) | 15(3.09%) | 297(61.11%) | 174(35.80%) | 486(100.00%) |
| Nonevents (non-SCD) (% all non-SCDs) | 4,854(24.65%) | 13,567(68.90%) | 1,270(6.45%) | 19,691(100.00%) |
| Proportion of all deaths due to SCD in this category | 2.84% | 6.14% | 16.42% | 7.56% |
| Non-sudden fatal CHD (% all deaths in category) | 88(16.67%) | 1,455(30.06%) | 434(40.94%) | 1,977(30.76%) |
| Non-CHD death (% all deaths in category) | 425(80.49%) | 3,088(63.80%) | 452(42.64%) | 3,965(61.68%) |
| Proportion of all deaths not due to SCD in category | 97.16% | 93.86% | 83.58% | 92.44% |
| All-cause death (% total in category) | 528(10.84%) | 4,840(34.91%) | 1,060(73.41%) | 6,428(31.86%) |
Performance of the Clinical+GEH SCD risk score
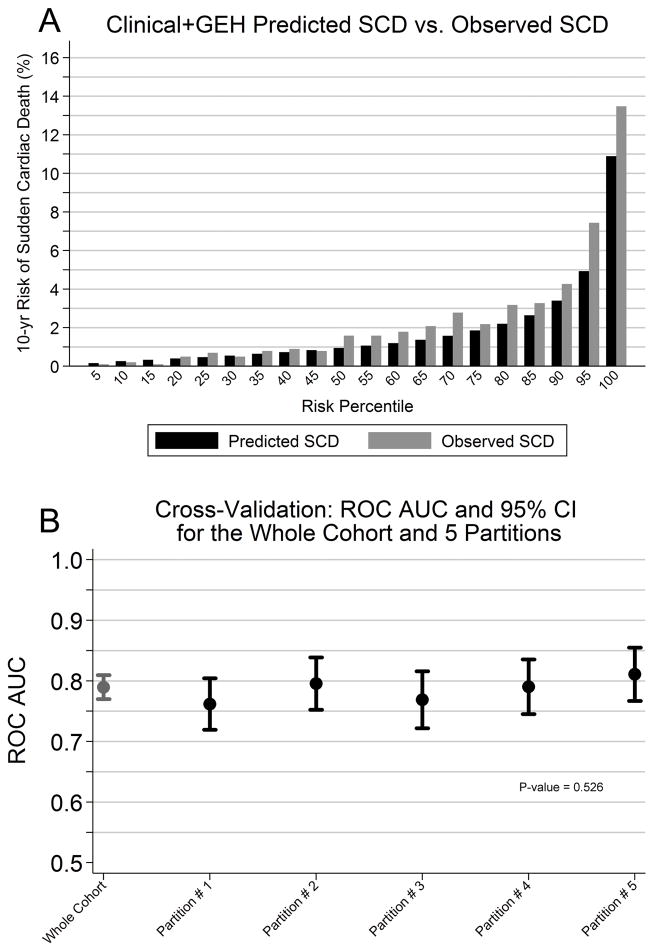
Internal cross validation and calibration
The clinical+GEH risk score was well calibrated for SCD events, with similar predicted and observed rates of SCD (Supplemental Table 9 and Figure 4A). No significant differences between C-statistics in the 5 internal cross-validation partitions were observed for the clinical-only risk score (C-statistic ranged 0.746–0.806, p=0.29) or the clinical+GEH risk score (C-statistic ranged 0.762–0.811, p=0.53). Figure 4B shows results of cross-validation of the clinical+GEH score in 5 partitions of the study cohort.
Figure 4.
Clinical+GEH risk score performance. A:Clinical+GEH risk score calibration. Predicted and observed SCD incidence are shown. B:Clinical+GEH risk score internal cross-validation.
Stratification capacity
Risk stratification capacity of the clinical+GEH score is shown in Table 4 and Supplemental Tables 10 and 11. Compared to the clinical-only score, the clinical+GEH score classified twice as many participants (24.1 vs. 11.9%) as low-risk (<0.5% 10-year SCD risk), fewer participants (68.7 vs. 82.0%) as intermediate-risk (1–5% 10-year SCD risk), and more participants (7.2 vs. 6.2%) as high-risk (>5% 10-year SCD risk). Overall, 35.8% of SCD victims were identified as high-risk by the clinical+GEH risk score, while the clinical-only score identified only 27.4% of SCD victims as high-risk. Only 6.5% of participants without SCD events were identified as high-risk by the clinical+GEH score.
Reclassification improvement
Table 4 also demonstrates that with the addition of GEH parameters 50/486 (10.3%) SCD victims were appropriately reclassified into a higher-risk category, and almost all of these participants (49 out of 50) were appropriately reclassified from intermediate-risk to high-risk. Overall, 14.9% of SCD-free participants were appropriately reclassified from intermediate-risk to low-risk. Only 8/486 (1.7%) SCD victims were inappropriately reclassified from high-risk to intermediate-risk, and no SCD victims were inappropriately reclassified from high-risk to low-risk. Net reclassification index (NRI) was 18.3% with an event NRI of 6.6% and a non-event NRI of 11.7%.
The addition of GEH parameters improved SCD-specific risk prediction as well (Table 4); the proportion of all deaths due to SCD decreased from 6.1% to 2.8% in in the low-risk groups and increased from 14.7% to 16.4% in the high-risk groups. Amongst participants with a predicted 10-year SCD risk of >10%, one out of every four deaths was SCD (Supplemental Table 10).
Classification tests
A high-risk clinical+GEH score predicted SCD with 35.8% sensitivity, 93.6% specificity, 98.3% negative predictive value (NPV), and 12.1% positive predictive value. A combined high- or intermediate-risk score predicted SCD with improved sensitivity (96.9%) at the cost of reduced specificity (24.7%), and retained a very high NPV (99.7%).
Clinical+GEH risk score performance in subgroups
As shown in Supplemental Table 12, the risk score also performed well in the subgroup of patients with abnormal intraventricular conduction (BBB/IVCD; n=2,328). In this group, assessment of GEH appropriately reclassified 29.4% of SCD victims (all intermediate-risk to high-risk), and no patients with SCD were inappropriately reclassified into a lower-risk category. Importantly, 64.1% of SCD victims were appropriately identified as a high-risk. In a small subgroup of participants with ECGs analyzed during ventricular-pacing (n=47), assessment of GEH appropriately reclassified 33% of SCD victims and identified 5/6 (83%) SCD victims (Supplemental Table 13).
Discussion
Analysis of this large, community based, bi-racial, prospective cohort of >20,100 participants with a wide age range revealed several important findings. First, we demonstrated an independent association of GEH with SCD. GEH ECG parameters remained independently associated with SCD after adjustment for multiple known SCD risk factors, time-updated ECG measurements, and time-updated incident non-fatal cardiovascular events. GEH parameters selectively predicted SCD over non-sudden fatal CHD and non-cardiac death in competing risks models, suggesting that abnormal GEH parameters selectively identified participants with abnormal electrophysiological substrate rather than simply identifying a sicker population with structural heart disease. Moreover, each GEH parameter provided additive information on SCD risk. The complementary nature of SVG, QRS-T angle, and SAI QRST is expected, as each of these measures represent distinct ways of quantifying GEH. Large increases in GEH parameters over short periods of time were also associated with increased SCD risk. Decreases in GEH parameters were not associated with reduced SCD risk, but given that relatively few participants experienced large decreases in GEH parameters we may be underpowered to detect a reduction in SCD risk in these subgroups.
Importantly, there was no significant change in the magnitude or significance of association between GEH parameters and SCD when LVEF was included in models, suggesting that although GEH parameters are associated with LVEF, their association with SCD is independent of the degree of LV dysfunction. The finding that GEH parameters are independently associated with SCD highlights the importance of including electrophysiological markers in SCD risk stratification models and future investigation of heritable genetic mechanisms underlying increased GEH.
Secondly, we developed a competing risks SCD risk score which combined clinical/demographic SCD risk factors with GEH parameters. The risk score identified a small subgroup of individuals with a high risk of SCD over 10-years of follow-up amongst a study population with an overall low SCD risk. The risk score was cross-validated internally. Our results open a new avenue for risk stratification and primary prevention of SCD, although validation in prospective studies is needed, and the optimal management/treatment of high-risk individuals requires further study.
SCD risk score development and performance
Development of an accurate and easily deployable SCD risk score is an important goal23, and no SCD risk scores are available for use in the general population. Several SCD risk scores have been proposed (including the MUSIC score24, MUSTT score25, and Duke score26), yet none has performed well enough for widespread clinical application. Many SCD risk scores are specific for patients with reduced LVEF, and an important limitation is that factors associated with SCD are also associated with non-sudden death due to progressive HF. Importantly, our SCD risk score considered competing risks of non-sudden fatal CHD and non-cardiac death. This approach allowed us to demonstrate that our risk score is specific for SCD over other modes of death, likely due to our ability to identify abnormal electrophysiological substrate with inclusion of GEH parameters.
Our risk score performed well in identifying individuals with a high-risk of SCD from an overall low-risk community population. Population screening tests are often designed to be highly sensitive at the expense of specificity, as initial false-positive tests are accepted if specific confirmatory tests are available. In the case of SCD, however, there are no specific tests available which could confirm the results of a highly sensitive and poorly specific screening test. Thus, for screening for and risk stratification of SCD in the general population, a highly specific test is desirable.
It is important to consider that abnormal GEH by itself is not responsible for onset of ventricular arrhythmias and/or SCD. Even with myocardial electrophysiological substrate favorable for ventricular arrhythmias, a triggering event is required. Thus, it is not surprising that there might be significant delay in the onset of SCD even in patients with significantly abnormal GEH parameters. This offers an opportunity for intervention.
Our finding of improved reclassification of SCD risk in a small subgroup of participants with ventricularly-paced-ECGs or BBB/IVCD supports Wilson’s hypothesis that variability in GEH can be detected independent of the ventricular activation sequence10. The SVG reflects heterogeneity of activation (and secondary heterogeneity of repolarization) across the myocardium which is independent of the myocardial activation sequence. It is often assumed that ventricularly-paced ECGs do not provide useful information beyond the presence of pacing. Our results, although limited by small numbers, tend to refute this assumption. Ventricular pacing introduces electrical dyssynchrony and heterogeneity which are reflected by larger GEH parameters compared to ECGs recorded during native ventricular activation. Despite the overall pacing-induced increase in GEH, however, assessment of GEH parameters during pacing still improved SCD reclassification in this subgroup. Further study of GEH in patients with ventricular pacing is warranted.
Clinical application of the SCD risk score
Beyond treatment of cardiovascular risk factors, there are no accepted interventions specifically for primary prevention of SCD in the general population. Prediction and prevention of SCD, however, are interconnected, as development of effective SCD prevention requires the availability of effective SCD risk prediction tools. Although measurement of GEH parameters is not currently readily available, existing 12-lead ECG systems could easily report their values with minimal additional software modification. A two-step strategy, however, may also be reasonable: the clinical-only SCD score could be easily evaluated without the need for lab tests or an ECG, and individuals with elevated clinical-only SCD risk could then undergo further risk stratification via measurement of GEH ECG parameters.
Individuals identified as extremely high-risk for SCD (such as those with 10-year SCD risk >10%) might be appropriate candidates for future randomized control trials investigating expanded indications for implantable cardioverter-defibrillators. Those with more modest risk might be targeted for aggressive diagnosis and treatment of subclinical heart disease. Patients with large increases in GEH parameters in the short-term might also be similarly targeted with aggressive cardiovascular risk modification.
There is growing recognition27 that approximately half of sudden cardiac arrest (SCA) victims have warning symptoms before their index event, but most symptoms are ignored, likely because only 1/3rd of SCA victims have been previously diagnosed with cardiovascular disease. Implementation of our risk score would enable early identification of individuals at increased SCD risk, which, in turn, could also improve education and awareness of SCD risk factors and warning signs. Simply having high-risk individuals recognize their increased SCD risk could improve SCA survival by increasing rates of seeking medical attention at the onset of cardiac symptoms27.
Strengths and Limitations
We developed our SCD risk score in 2 large, bi-racial, prospective cohorts encompassing a wide age range. Our risk score is therefore likely to be widely generalizable. However, the study does have limitations. The definition of covariates was slightly different in each cohort. We adjusted for multiple SCD confounders, but it is possible that residual confounding influenced the results. Information on LVEF, which has been associated with SCD, was only available in the CHS cohort and therefore could not be included in the final risk score. However, addition of LVEF in time-updated models did not alter the association between GEH parameters and SCD. One possible reason for this observation is that LVEF is normal in the vast majority of the general population. Additionally, although HF was associated with SCD in minimally adjusted analyses, after multivariable adjustment, HF was not a significant predictor of SCD in the study population, and according to our procedure for backward selection it was removed from the final risk score.
Patients who experienced SCA but who were successfully resuscitated and survived to hospital discharge were not considered cases of SCD in this analysis. However, as survival of out-of-hospital cardiac arrest is rare27 it is unlikely that this significantly impacted the results. Finally, although all deaths were thoroughly adjudicated, we cannot determine if some SCDs were primarily due to bradyarrhythmias as opposed to ventricular tachyarrhythmias. However, as sudden cardiac arrest due to bradyarrhythmia is frequently due to pause-dependent polymorphic ventricular tachycardia, GEH might still be associated with SCD in these patients, and this phenomenon requires further study.
Supplementary Material
Table 3.
Competing risks scores for SCD.
|
|
||||
|---|---|---|---|---|
| Clinical-only Score | Clinical+GEH Score | |||
|
|
||||
| Sub-HR(95%CI) | β-coefficient | Sub-HR(95%CI) | β-coefficient | |
|
|
||||
| Age, per 10 years | 1.131(1.032–1.241) | 0.1234 | 2.027(1.644–2.500) | 0.7066 |
| Female | 0.495(0.411–0.596) | −0.7034 | 0.373(0.228–0.612) | −0.9851 |
| White | 0.663(0.544–0.809) | −0.4106 | 0.361(0.226–0.577) | −1.0180 |
| Diabetes | 2.132(1.739–2.615) | 0.7572 | 3.233(2.035–5.136) | 1.1734 |
| Hypertension | 1.711(1.397–2.096) | 0.5370 | 2.329(1.439–3.769) | 0.8452 |
| Coronary Heart Disease | 3.647(2.930–4.540) | 1.2939 | 3.095(2.503–3.828) | 1.1298 |
| Stroke | 2.070(1.503–2.852) | 0.7278 | 1.920(1.397–2.641) | 0.6525 |
| Sqrt SVG Elevation | -- | -- | 1.127(1.036–1.227) | 0.1199 |
| QRS-T Angle, per 10° increase | -- | -- | 1.480(1.273–1.722) | 0.3923 |
| SAI QRST, per 100 mV*ms increase | -- | -- | 2.491(1.433–4.328) | 0.9126 |
| SAI QRST*age | -- | -- | 0.902(0.833–0.977) | −0.1030 |
| QRS-T Angle*age | -- | -- | 0.945(0.923–0.968) | −0.0566 |
| QRS-T Angle*race | -- | -- | 1.095(1.034–1.159) | 0.0908 |
| QRS-T Angle*diabetes | -- | -- | 0.948(0.902–0.997) | −0.0534 |
| QRS-T Angle*HTN | -- | -- | 0.953(0.904–1.004) | −0.0481 |
| Sqrt SVG Azimuth*female | -- | -- | 1.092(1.010–1.182) | 0.0882 |
|
| ||||
| Akaike Information Criterion | 9121 | 9028 | ||
| C-statistic (95%CI); comparison p=0.008 | 0.777(0.757–0.797) | 0.790(0.770–0.809) | ||
| Partial ROC AUC for 2% false-positive fraction (comparison p=0.006) | 0.0017(0.0013–0.0021) | 0.0022(0.0017–0.0027) | ||
| Predicted Cumulative Incidence/10-years | 0.44% to 42.14% | 0.05% to 55.12% | ||
Clinical Perspectives.
Despite advances in treatment and primary prevention of cardiovascular disease, sudden cardiac death (SCD) incidence remains high, and SCD is frequently the first manifestation of cardiovascular disease. Development of a non-invasive, inexpensive, and easy-to-use SCD risk score for use in the general population is an important goal. In this study, we assessed 5 electrocardiographic (ECG) measures of myocardial global electrical heterogeneity (GEH)—sum absolute QRST integral, spatial QRS-T angle, and spatial ventricular gradient (magnitude, azimuth, and elevation)—in 20,177 participants in the community-based ARIC and CHS studies. We demonstrated that baseline GEH ECG parameters and large increases in GEH parameters over time were independently associated with SCD, and that assessment of multiple GEH ECG parameters provided additive/complementary information on SCD risk. We developed a novel SCD risk score which used readily available clinical characteristics (age, gender, race, hypertension, diabetes, stroke, and coronary heart disease) and GEH ECG parameters. We demonstrated that the risk score was highly specific for SCD, and that addition of GEH ECG parameters to clinical characteristics significantly improved SCD risk prediction, likely because GEH ECG parameters identified participants with myocardial electrical substrate favorable for ventricular arrhythmias. Our study represents an important step forward in understanding SCD risk factors, and identifying people in the general population with elevated SCD risk who might be targeted for future SCD risk reduction strategies. The genetic basis of abnormal myocardial electrical heterogeneity, as expressed by abnormal GEH ECG parameters, also warrants further study.
Acknowledgments
The authors thank the staff and participants of the ARIC and CHS studies for their important contributions.
Funding Sources: The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, HHSN268201100012C). CHS is supported by NHLBI contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant U01HL080295, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by R01AG023629 from the National Institute on Aging. A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. This work was supported by 1R01HL118277 (LGT), R01HL116747 (NS), and R01HL111089 (NS).
Footnotes
Disclosures: Johns Hopkins University (LGT) holds US patent “Methods for determining risk of ventricular arrhythmia”, which was among the methods used to measure SAI QRST (not licensed).
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics—2015 Update: A Report From the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Myerburg R, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation. 2012;125:1043–52. doi: 10.1161/CIRCULATIONAHA.111.023846. [DOI] [PubMed] [Google Scholar]
- 3.Pouleur AC, Barkoudah E, Uno H, Skali H, Finn PV, Zelenkofske SL, Belenkov YN, Mareev V, Velazquez EJ, Rouleau JL, Maggioni AP, Køber L, Califf RM, McMurray JJ, Pfeffer MA, Solomon SD VALIANT Investigators. Pathogenesis of sudden unexpected death in a clinical trial of patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. Circulation. 2010;122:597–602. doi: 10.1161/CIRCULATIONAHA.110.940619. [DOI] [PubMed] [Google Scholar]
- 4.Vassallo JA, Cassidy DM, Kindwall KE, Marchlinski FE, Josephson ME. Nonuniform recovery of excitability in the left ventricle. Circulation. 1988;78:1365–1372. doi: 10.1161/01.cir.78.6.1365. [DOI] [PubMed] [Google Scholar]
- 5.Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–488. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 6.Geselowitz DB. The ventricular gradient revisited: relation to the area under the action potential. IEEE Trans BiomedEng. 1983;30:76–77. doi: 10.1109/tbme.1983.325172. [DOI] [PubMed] [Google Scholar]
- 7.Plonsey R. A contemporary view of the ventricular gradient of Wilson. J Electrocardiol. 1979;12:337–341. doi: 10.1016/s0022-0736(79)80001-1. [DOI] [PubMed] [Google Scholar]
- 8.Abildskov JA, Green LS, Evans AK, Lux RL. The QRST deflection area of electrograms during global alterations of ventricular repolarization. JElectrocardiol. 1982;15:103–107. doi: 10.1016/s0022-0736(82)80001-0. [DOI] [PubMed] [Google Scholar]
- 9.Hubley-Kozey CL, Mitchell LB, Gardner MJ, Warren JW, Penney CJ, Smith ER, Horacek BM. Spatial features in body-surface potential maps can identify patients with a history of sustained ventricular tachycardia. Circulation. 1995;92:1825–38. doi: 10.1161/01.cir.92.7.1825. [DOI] [PubMed] [Google Scholar]
- 10.Wilson FN, Macleod AG, Barker PS, Johnston FD. The determination and the significance of the areas of the ventricular deflections of the electrocardiogram. Am Heart J. 1934;10:46–61. [Google Scholar]
- 11.Hurst JW. Thoughts about the ventricular gradient and its current clinical use (Part I of II) Clin Cardiol. 2005;28:175–80. doi: 10.1002/clc.4960280404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oehler A, Feldman T, Henrikson CA, Tereshchenko LG. QRS-T Angle: A Review. Annals Noninvasive Electrocardiol. 2014;19:534–42. doi: 10.1111/anec.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tereshchenko LG, Cheng A, Fetics BJ, Butcher B, Marine JE, Spragg DD, Sinha S, Dalal D, Calkins H, Tomaselli GF, Berger RD. A new electrocardiogram marker to identify patients at low risk for ventricular tachyarrhythmias: sum magnitude of the absolute QRST integral. J Electrocardiol. 2011;44:208–216. doi: 10.1016/j.jelectrocard.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tereshchenko LG, McNitt S, Han L, Berger RD, Zareba W. ECG marker of adverse electrical remodeling post-myocardial infarction predicts outcomes in MADIT II study. PLoS One. 2012;7:e51812. doi: 10.1371/journal.pone.0051812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sur S, Han L, Tereshchenko LG. Comparison of sum absolute QRST integral, and temporal variability in depolarization and repolarization, measured by dynamic vectorcardiography approach, in healthy men and women. PLoS One. 2013;8:e57175. doi: 10.1371/journal.pone.0057175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tereshchenko LG, Cheng A, Fetics BJ, Marine JE, Spragg DD, Sinha S, Calkins H, Tomaselli GF, Berger RD. Ventricular arrhythmia is predicted by sum absolute QRST integralbut not by QRS width. J Electrocardiol. 2010;43:548–552. doi: 10.1016/j.jelectrocard.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The ARIC Investigators. The Atherosclerosis Risk in Community (ARIC) Study: Design and Objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 18.Mittelmark MB, Psaty BM, Rautaharju PM, Fried LP, Borhani NO, Tracy RP, Gardin JM, O’Leary DH. Prevalence of cardiovascular diseases among older adults. The Cardiovascular Health Study. Am J Epidemiol. 1993;137:311–7. doi: 10.1093/oxfordjournals.aje.a116678. [DOI] [PubMed] [Google Scholar]
- 19.Molloy TJ, Okin PM, Devereux RB, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage-duration product. J Am Coll Cardiol. 1992;20:1180–6. doi: 10.1016/0735-1097(92)90376-x. [DOI] [PubMed] [Google Scholar]
- 20.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 21.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 22.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Fishman GI, Chugh SS, DiMarco JP, Albert CM, Anderson ME, Bonow RO, Buxton AE, Chen PS, Estes M, Jouven X, Kwong R, Lathrop DA, Mascette AM, Nerbonne JM, O’Rourke B, Page RL, Roden DM, Rosenbaum DS, Sotoodehnia N, Trayanova NA, Zheng ZJ. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122:2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vazquez R, Bayes-Genis A, Cygankiewicz I, Pascual-Figal D, Grigorian-Shamagian L, Pavon R, Gonzalez-Juanatey JR, Cubero JM, Pastor L, Ordonez-Llanos J, Cinca J, de Luna AB. The MUSIC Risk score: a simple method for predicting mortality in ambulatory patients with chronic heart failure. EurHeart J. 2009;30:1088–1096. doi: 10.1093/eurheartj/ehp032. [DOI] [PubMed] [Google Scholar]
- 25.Buxton AE, Lee KL, Hafley GE, Pires LA, Fisher JD, Gold MR, Josephson ME, Lehmann MH, Prystowsky EN, Investigators M. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J Am Coll Cardiol. 2007;50:1150–7. doi: 10.1016/j.jacc.2007.04.095. [DOI] [PubMed] [Google Scholar]
- 26.Atwater BD, Thompson VP, Vest RN, III, Shaw LK, Mazzei J, Al-Khatib SM, Hranitzky PM, Bahnson TD, Velazquez EJ, Califf RM, Lee KL, Roe MT. Usefulness of the Duke Sudden Cardiac Death Risk Score for Predicting Sudden Cardiac Death in Patients With Angiographic (>75% Narrowing) Coronary Artery Disease. Am J Cardiol. 2009;104:1624–1630. doi: 10.1016/j.amjcard.2009.07.042. [DOI] [PubMed] [Google Scholar]
- 27.Marijon E, Uy-Evanado A, Dumas F, Karam N, Reinier K, Teodorescu C, Narayanan K, Gunson K, Jui J, Jouven X, Chugh SS. Warning Symptoms Are Associated With Survival From Sudden Cardiac ArrestWarning Symptoms and Sudden Cardiac Arrest. Ann Intern Med. 2016;164:23–29. doi: 10.7326/M14-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.