Abstract
Throughout history, muscle research has led to numerous scientific breakthroughs that have brought insight to a more general understanding of all biological processes. Potentially one of the most influential discoveries was the role of the second messenger calcium and its myriad of handling and sensing systems that mechanistically control muscle contraction. In this review we will briefly discuss the significance of calcium as a universal second messenger along with some of the most common calcium binding motifs in proteins, focusing on the EF-hand. We will also describe some of our approaches to rationally design calcium binding proteins to palliate, or potentially even cure cardiovascular disease. Considering not all failing hearts have the same etiology, genetic background and co-morbidities, personalized therapies will need to be developed. We predict designer proteins will open doors for unprecedented personalized, and potentially, even generalized medicines as gene therapy or protein delivery techniques come to fruition.
Calcium – The universal second messenger
Both prokaryotes and eukaryotes universally utilize the coordination of Ca2+ to modulate biological functions. Typically the “resting” free intracellular concentration of Ca2+ within a living cell is approximately four orders of magnitude lower than that of the surrounding aqueous or interstitial environments [1]. Cells have developed a plethora of ion pumps, exchangers, pores, buffers and organelles necessary to maintain a low free intracellular Ca2+ concentration, generally in the range of tens to hundreds of nM. The myriad of protein systems used to work against this large chemical gradient and maintain intracellular Ca2+ at very low levels is energetically costly [2]. Ultimately, changes in both the extracellular, and especially the intracellular Ca2+ concentrations, can have a profound impact on cellular behavior [3].
The first demonstration that Ca2+ is essential for a biological process was discovered by Dr. Sydney Ringer circa 1883 [4]. In a series of experiments (and insightful mishaps in 1882), Ringer and his colleagues discerned Ca2+ was a necessary component of physiological solutions required to sustain cardiac muscle contraction. Very soon afterwards, extracellular Ca2+ was demonstrated to strongly influence several cellular behaviors and functions [3]. However, it took almost a century (and much debate) before the work of many biochemists, cell biologists and physiologists studying muscle clearly showed intracellular Ca2+ was a bona fide biological signal [5]. Today, it is widely acknowledged that every physiological and cellular change in our bodies beginning from the initiation of life to death, is either directly controlled or at a minimum, influenced by changes in intracellular Ca2+ [3, 6].
There are a vast number of plasmalemmal and intracellular receptors that upon receiving a stimulus trigger a rise in intracellular Ca2+ [2]. Depending on the integration of all the stimuli that a cell is receiving, the intracellular Ca2+ level may: 1) remain constant; 2) adjust the “baseline”; 3) pulse with wave fronts that may propagate through the cell (spiral, chevron, etc. that can collide with interference patterns); and 4) globally rise in amplitude with a particular duration and frequency of occurrence. The shape, amplitude and duration of the intracellular Ca2+ signal depends on the stimulus, its signal strength, frequency, cell type and integration with other signaling processes. In addition to the mechanisms that increase intracellular Ca2+, the ability of the cell to extrude Ca2+ and/or refresh the intracellular Ca2+ stores also plays a central role in shaping the specific Ca2+ signature of a stimulus [7]. There are also receptors and signaling pathways that instead of raising intracellular Ca2+, work just the opposite, and lower available Ca2+ within the cell [8]. Additionally, there are numerous Ca2+ sensors and buffers within different cells that also play a pivotal role in defining the amplitude and duration of the Ca2+ signal. Due to the expansive combination of receptors and Ca2+ handling machinery, there is a vast and wide range of Ca2+ signals that can be generated by different cell types and even within a particular cell. The contractile cells of the heart (myocytes) are an excellent example of a cellular system that routinely modulates its intracellular Ca2+ to dictate functional outcomes [9].
Calcium Signaling and Cardiac Muscle Contraction
It was the intense and deep study of the three types of muscle (cardiac, skeletal and smooth) that began to shed light on the universal significance and control mechanisms of Ca2+ signaling. Whereas the basic mechanisms that control the myocyte’s intracellular Ca2+ transient are well understood and can be found in most biochemical and nearly all physiological textbooks, there are specific details and nuances that are still being discovered [10]. In general, intracellular free Ca2+ rises and then falls in a highly coordinated and timely manner across every cardiac myocyte to control the rhythmic contraction and relaxation cycle of the heart. For example, it is generally thought that during ventricular filling (relaxed heart), the free concentration of Ca2+ within the myocytes is lowered and then maintained at ~70 to 125 nM. This low concentration of Ca2+ is below the threshold required to initiate contraction. Upon an action potential, cytosolic Ca2+ rapidly increases, reaching free concentrations of 0.3 to 0.5 µM and as high as ~1.5 µM during maximal stimulation [9]. The amplitude of the Ca2+ signal within the myocyte under normal conditions is sufficient to initiate less than half the maximal contraction of the myocyte or muscle preparation [11]. Thus, there is a large contractile reserve that can be tapped into to increase cardiac contractility (or vice versa).
The myocyte can alter its contractility through several neural-hormonal influences that change the Ca2+ signal in both amplitude and duration [9]. The archetype of this phenomenon is the “fight or flight” response on the heart. Interestingly, as early as 1928 the effects of adrenaline on the heart was demonstrated to be dependent on extracellular Ca2+ and suggested to function via changes in intracellular Ca2+ [3]. Both the amplitude and duration of the Ca2+ signal can also be adjusted by the frequency of stimulation (i.e. the force-frequency response) [12]. Ultimately, the behavior of nearly all the electrical and Ca2+ handling proteins in the heart can be post-translationally modified to tune the Ca2+ signal [13]. Depending on the composition of the electrical and Ca2+ handling proteins (as occurs in different species), the duration of the Ca2+ transient can be drastically different (although changes in amplitude are less pronounced) [9]. Besides contraction, cytosolic and more spatially constrained Ca2+ signals (as occur in caveolae) also influence energetics, transcription, hypertrophy and many other cellular functions [14]. Ultimately, all of these Ca2+-dependent processes require a Ca2+ sensor (i.e. Ca2+ binding protein) to either translate or relay the Ca2+ signal into a cellular response. There is plenty of evidence that at least the contractile response of cardiac muscle can change without corresponding alterations in the Ca2+ signal [13]. Thus, the heart can also modulate contractility via altering the Ca2+ sensing machinery of the myocyte [15]. Through protein engineering of Ca2+ binding proteins, we are attempting to modulate the response of the heart to Ca2+ in order to palliate, and potentially cure, various cardiovascular diseases.
Classes of Ca2+ Binding Proteins
There is an ample and diverse array of Ca2+ binding proteins used by cells to decode the information carried by the Ca2+ signal. Depending on the location, concentration and type of Ca2+ sensors in the cell, different cellular outcomes can arise. Although there are other types, three major classes of Ca2+ binding motifs have evolved independently to sense Ca2+ signals: the annexin fold, the C2 domain, and the EF-hand.
The Annexin Fold
The annexin family of proteins in eukaryotes bind negatively-charged phospholipids in a reversible, Ca2+-dependent manner. The roles for annexins in the cell are varied, including Ca2+ homeostasis, endocytosis, exocytosis, cell migration, and cellular scaffolding [16]. Several of the annexins are expressed in cardiac myocytes, where they play a role in modulating the Ca2+ signal [17]. Annexins have a highly variable N-terminal region that regulates membrane association, but all family members share a conserved "core" Ca2+-binding, C-terminal domain consisting of either four or eight repeats that form α-helices and bind Ca2+ with moderate affinity. The variable N-terminal domain is used in a number of protein-protein interactions including dimerization, and can also be post-translationally modified by myristoylation or phosphorylation. The effects of such post-translational modifications vary widely between annexins, as do the free Ca2+ concentrations required for their activity, allowing each annexin isoform to be tuned to respond to specific Ca2+ signals. Much is to be learned on how precisely this is accomplished. There are at least 160 different proteins that contain the annexin fold [16].
The C2 Domain
Another type of Ca2+-binding motif is the C2 domain, an 8-stranded antiparallel β-sandwich connected by variable loops that are thought to confer functional specificity. Three loops located at the top of the domain bind two to three Ca2+ ions cooperatively but with low affinity [18]. As for annexins, many proteins containing the C2 domain also bind the cell membrane and in most cases their action is Ca2+-dependent. The C2 domain is generally found in proteins involved in signal transduction or membrane trafficking. Phospholipase C and protein kinase C isoforms both contain C2 domains and are involved in several signaling cascades in the heart [19]. Throughout eukaryotes, more than 200 proteins have been found to contain the C2 domain [20].
The EF-Hand Motif
By far, the most common Ca2+-binding motif in eukaryotes is the EF-hand (Figure 1) [21]. The canonical EF-hand is formed by a helix-loop-helix motif in which one Ca2+ ion is chelated by side chain oxygen atoms in a flexible 12-residue loop, and the flanking α-helices are oriented perpendicular to one another [22]. Most EF-hands occur in pairs connected by a short β-sheet, which contributes to the high cooperativity of the adjacent Ca2+ binding sites [23]. However, a small number of proteins contain an odd number of EF-hand motifs typically used for dimerization rather than Ca2+ binding [24].
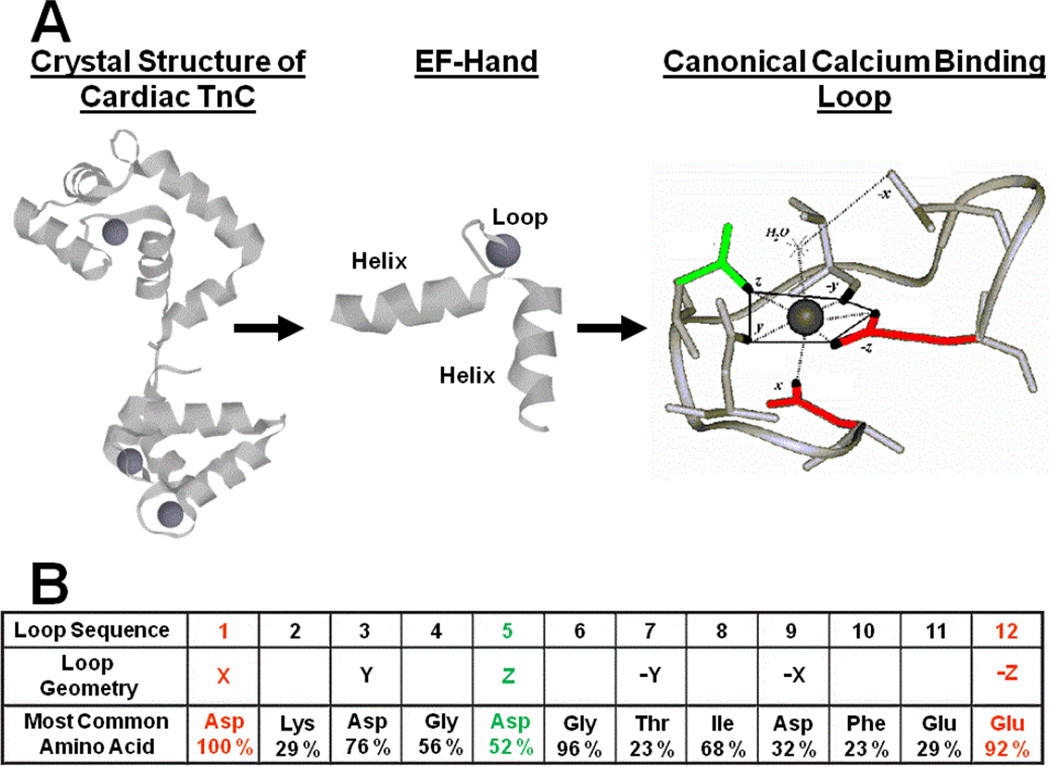
Figure 1. The EF-hand is the most common Ca2+ binding motif used to decode the Ca2+ signal.
Panel A depicts the crystal structure of cardiac TnC with its three functional EF-hands bound by Ca2+ (grey balls). Each EF-hand is a simple helix-loop-helix, where the loop chelates Ca2+ with pentagonal bipyrimidal geometry mimicking Ca2+’s hydration shell. Panel B shows the most common amino acids found within the 12 residue EF-hand loop [22]. Note, the first and twelfth amino acids (red) are essential for Ca2+ binding, whereas an Asp at position 5 (green) increased Mg2+ binding.
The Ca2+ binding loop of the classical EF-hand coordinates Ca2+ with seven oxygens in a pentagonal bipyrimidal arrangement, mimicking the hydration shell around the solvated ion [23]. Residues at positions 1, 3, 5, 7, 9, and 12 in the loop work together to non-covalently chelate the Ca2+ ion. In most EF-hands, aspartate occupies position 1, whereas a glutamate in position 12 provides bidentate oxygen ligands (Figure 1). In addition, a highly conserved glycine in position 6 allows the loop to bend around the bound Ca2+ ion. The identity of the intervening amino acids can be highly variable; proteins that are identical at positions 1, 3, 6 and 12 may still have vastly different affinities [23].
Although the EF-hand motif is generally considered to be highly selective for Ca2+, the loop is also capable of binding other divalent ions and heavy metals with low to extremely high affinity. Furthermore, environmental conditions such as pH, ionic strength and temperature may or may not alter cation binding [23, 25]. In the cell, the most prevalent competitor for EF-hand sites is Mg2+, which is present at concentrations of up to 2 mM [26]. In contrast to Ca2+, Mg2+ has a smaller ionic radius and has a strong preference for coordination by six ligands in an octahedral geometry. Many EF-hand loops are flexible enough to adjust to the smaller Mg2+ but bind with 103–104 lower affinity compared to Ca2+. The lower Mg2+ affinity is primarily caused by a slower rate of Mg2+ association to the EF-hand due to a substantially slower rate of Mg2+ desolvation compared to that of Ca2+ [3]. In order for the cations to bind a protein, the water shell around the ion must be entirely or partially replaced by the chelating residues of the protein. Mg2+ is chelated by the same ligands as Ca2+ at positions 1, 3, 5, and 7 in the loop, however the glutamate at position 12 only provides a single oxygen ligand or is displaced entirely by a water molecule [22, 27]. Some EF-hand proteins undergo a large conformational change when Ca2+ binds, but not upon Mg2+ binding, and it has been suggested that this lack of bidentate coordination from the glutamate at position 12 is the cause [27].
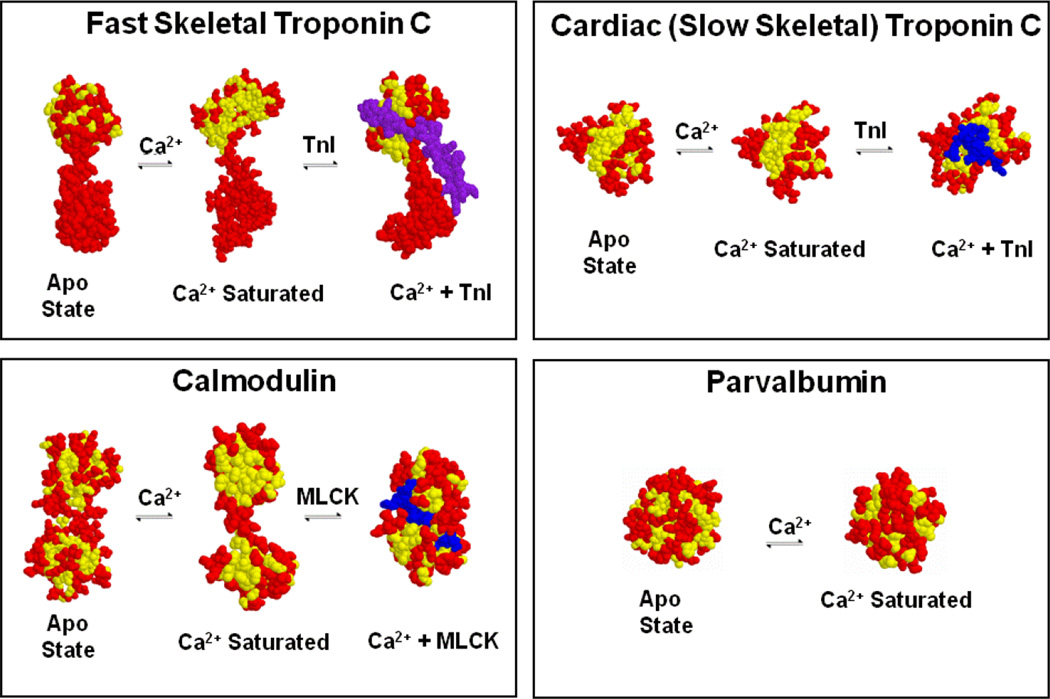
EF-hand proteins play three major roles in the cell: structural integrity, Ca2+ buffering and/or Ca2+-dependent regulation (Figure 2). The well-known EF-hand containing proteins calmodulin (CaM) and troponin C (TnC) can play all three of these roles in muscle. Certain EF-hand proteins have Ca2+ and/or Mg2+ sensitivities below resting ion levels and thus are nearly always occupied by Ca2+ or Mg2+ [23]. In some of these cases, through cation dependent protein-protein interactions, the EF-hand protein remains bound and an integral, structural member of a larger protein system, as exemplified by the C-terminal domain of TnC [28]. Ca2+ buffering proteins help to control the free Ca2+ in a cell by their relatively slow Ca2+ exchange, while the regulatory proteins relay or transduce a Ca2+ signal into action. The archetype Ca2+ buffer, alpha parvalbumin, also has a relatively high affinity for both Ca2+ and Mg2+, but its structure does not appreciably change between the apo and Ca2+-bound states (Figure 2) [29, 30]. Thus, alpha parvalbumin is generally thought to only buffer Ca2+. This lack of protein-protein interaction may not be the case for the close relative of alpha parvalbumin, oncomodulin (a.k.a. beta parvalbumin) [31]. In contrast, the regulatory proteins, such as the N-terminal domain of TnC and both domains of CaM, generally undergo a large conformational change upon Ca2+ binding, which allows these proteins to interact with additional target proteins acting as “Ca2+-dependent switches”. Whereas CaM has hundreds of potential targets in a cell, TnC only binds a single target, troponin I (TnI) [32, 33]. Depending on the concentration of these Ca2+-dependent switches, they can also become substantial “sinks” or buffers themselves for rises in cellular Ca2+. For instance, the concentration of CaM in the myocyte is in the 10 µM range (4 Ca2+ binding sites), whereas TnC is in the 70 µM range (3 Ca2+ binding sites in cardiac isoforms and 4 in the skeletal isoforms). These non-negligible concentrations of switches in the heart have a substantial influence on the shape of the free Ca2+ transient [34]. Thus, the various cation binding properties of EF-hands allow proteins to perform structural, buffer and switch-like functions.
Figure 2. Ca2+-dependent switches and buffers in muscle.
Unlike parvalbumin, TnC and CaM expose a hydrophobic “pocket” upon Ca2+ binding that is then utilized to bind target proteins, TnI in the case of TnC and as an example, myosin light chain kinase (MLCK) for CaM. Only the regulatroy, N-terminal domain is depicted for TnC. Yellow amino acids are shown in yellow, whereas all other amino acids are shown in red with the target in blue.
Ca2+ Binding Properties of EF Hand Proteins
Of all the Ca2+ binding motifs, the EF-hand has been the most extensively studied. Utilizing this simple motif, different EF-hand proteins bind Ca2+ with affinities that can differ by over a million fold (nM to mM) [23]. Within the Ca2+ binding loop alone, there are hundreds of unique EF-hand sequences that can be found in nature (Figure 1B) [35]. Adding the variability of the helices to the mix, the number of unique EF-hand sequences grows into the thousands. To date, there are no algorithms that can predict the Ca2+ binding properties of EF-hand proteins, making it challenging to re-engineer or modulate function in a specific manner.
Due to the high variability within the loops, it was initially thought that the amino acid composition of the loop alone dictated the Ca2+ affinity. Although the loop sequence had a strong impact on the Ca2+ binding properties of isolated EF-hand peptides, one cannot simply exchange the Ca2+ binding loops between EF-hand containing proteins and expect the chimeric protein to adopt the Ca2+ binding properties of the parent protein [36–38]. However, this is not to say that the loop has no impact on the overall Ca2+ binding properties of the proteins. For instance, moderate to high affinity Ca2+ binding requires an Asp residue at the 1st and a Glu residue at the 12th positions of the loop (Figure 1) [39]. We and others have demonstrated that having an Asp residue at the 5th position increases Mg2+ affinity for that EF-hand, decreasing overall Ca2+ sensitivity via increased Mg2+ competition (Figure 1) [36, 40, 41]. Depending on the protein, altering the other chelating residues can also have a subtle to moderate effect on the Ca2+ affinity [42, 43]. In the end, residues throughout the entire protein (yes, even outside of the EF-hand) can have a profound influence on Ca2+ binding [44].
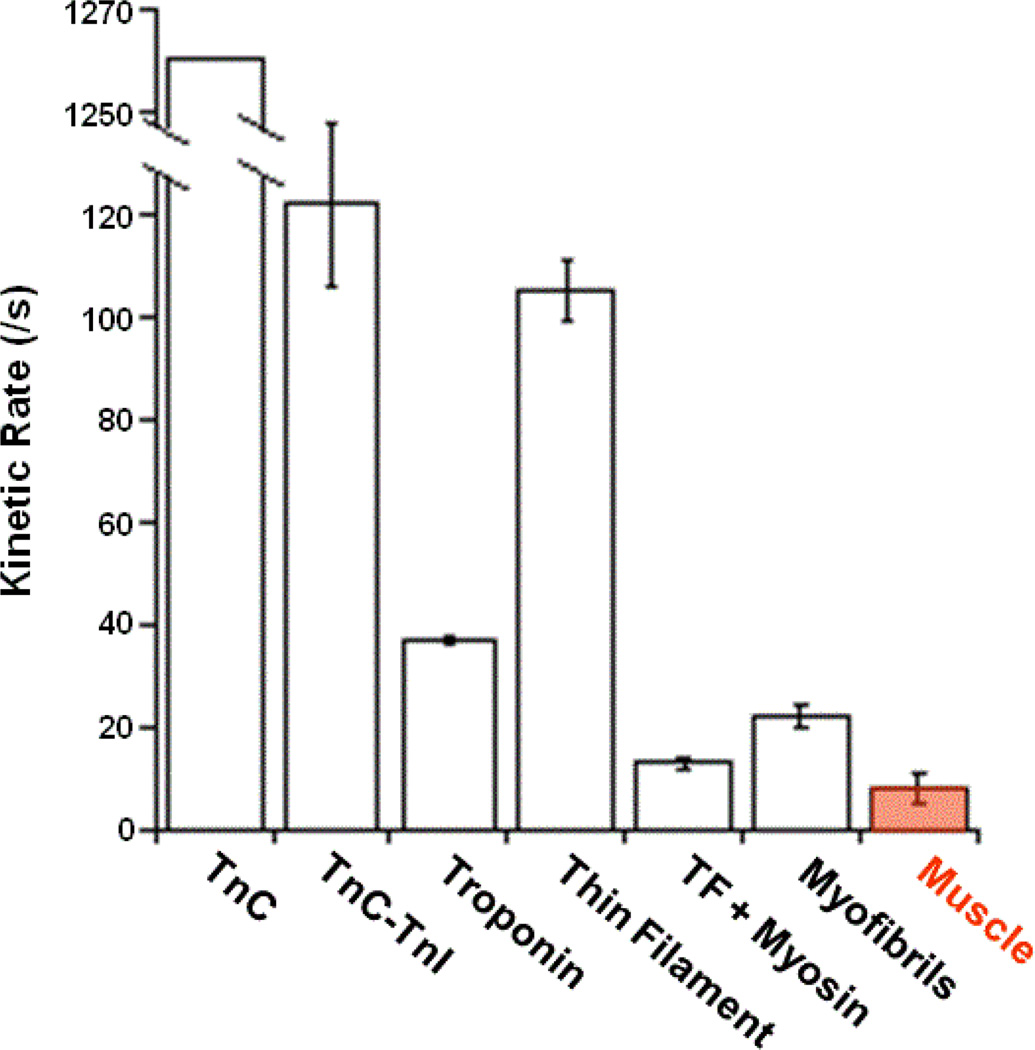
Many EF-hand proteins alter their structure upon Ca2+ binding, which alters their interaction with one or more target proteins (Figure 2). In general, mutations that hinder the ability of the protein to change its structure upon Ca2+ binding can drastically impair Ca2+ affinity [45]. Protein-protein interactions add another complication to predicting the Ca2+ binding properties of an EF-hand protein. Typically the binding of an EF-hand protein to its target protein enhances the overall Ca2+ sensitivity of the system, often by orders of magnitude [15]. However, there are several examples where target interactions actually decrease the Ca2+ sensitivity of the EF-hand protein, as can occur with CaM binding to IQ motif containing peptides [46]. Unlike traditional CaM binding motifs that require Ca2+-bound CaM to initiate an interaction, IQ motifs typically bind Ca2+-free CaM [47]. We are also learning that target protein interactions away from the direct EF-hand interaction can also have a large impact on how the EF-hand protein senses and releases Ca2+ [28]. Cardiac TnC is an excellent example that clearly demonstrates Ca2+ binding to a single EF-hand is a function of the state of the system rather than solely determined by the composition of the EF-hand protein alone. That is, the Ca2+ binding properties of TnC can change over an order of magnitude going from the isolated protein, to the Tn complex, to the thin filament, to myofibrils, to muscle and in most cases depending if there is a strongly bound myosin attached to actin (Figure 3) [15]. Understanding how the system behaves has helped us to engineer TnCs that when combined with gene therapy approaches may one day be used to treat various cardiovascular diseases [48, 49].
Figure 3. Comparison of Ca2+ dissociation rates from cardiac TnC in systems of increasing complexity with that of muscle relaxation.
The rate of Ca2+ dissociaiton from the single regulatory EF-hand of cardiac TnC is extremely state dependent [15]. This figure highlights the fact that the loop sequence alone is not the dominating factor that determines the Ca2+ binding properties of an EF-hand or its system.
Reformulating Cardiac Troponin C
Cardiovascular disease is the leading cause of death throughout the world. This seems preposterous considering we know so much about how the heart and vascular systems function in both health and disease, from in vivo performance to the minute workings of the isolated proteins. Considering the importance of the Ca2+ signal to cardiac function and performance, a great deal of effort has been put into altering the Ca2+ signal to halt or reverse disease [50, 51]. However, since the Ca2+ signal controls so many processes in the myocyte (contraction only being one), long term alteration of the Ca2+ signal leads to detrimental consequences. Much of this results from altering the Ca2+ signal via modulating specific steps in the beta adrenergic pathway. Although enhancing the beta adrenergic response can acutely improve the quality of life, overstimulation and long term use leads to excessive remodeling, hypertrophy, increased risk of arrhythmias and shortening of life span [52]. Interestingly, in the process of developing pharmaceuticals to modulate the L-type Ca2+ channel, it was discovered that some of these compounds sensitized the contractile apparatus to Ca2+ through increasing the Ca2+ sensitivity of TnC [53, 54]. Since then, several compounds have been discovered that enhance contraction of the heart via TnC. Unfortunately, none of these compounds have high affinity or specificity for cardiac TnC, can prolong relaxation and begin to bind and alter the function of other proteins such as CaM and phosphodiesterases, limiting their efficacy and making them potentially dangerous [55, 56]. However, a more targeted pharmaceutical designed against fast skeletal TnC shows promise as a therapeutic for a plethora of skeletal muscle weakness [57]. Alternatively, we have taken a direct route to enhance Ca2+ binding of TnC through protein engineering [25, 48, 49].
Unfortunately, there is no off-the-shelf “cook-book” that one can peruse to quickly whip up an EF-hand recipe that will bind Ca2+ with specific kinetics and thus altered affinities. One of the only reproducible and highly effective ways to alter Ca2+ binding to classical EF-hands is to replace the first or last chelating residues in the loop with another amino acid (typically Ala) [39]. Although this is an excellent method to “destroy and inactivate” an EF-hand, it is not a satisfactory approach to retain the switch-like properties or fine tune EF-hand Ca2+ binding. There have been several hypotheses put forth to explain the diversity of EF-hand Ca2+ sensitivities and kinetics. One of the earliest was the acid-pair hypothesis that suggested the number and specific geometry of negatively charged residues within the chelating positions of the loop controlled Ca2+ sensitivity in a predictable manner [36]. Subsequently, the “gateway” hypothesis, suggested that the size and chemical properties of the ninth chelating residue in the loop controlled the kinetics of cation exchange [58]. Utilizing CaM as a model system, we directly tested these hypotheses and disappointingly demonstrated these particular rules were insufficient to predict mutational outcomes in Ca2+ sensitivity, selectivity or kinetics of the N-terminal domain of CaM [41, 42]. Interestingly, these mutations tended to alter the rate of Ca2+ association more so than the rate of Ca2+ dissociation, similar to mutations that increased the negative surface charge of another EF-hand protein, calbindin [59]. The short coming of these theories lie in not considering the diversity and complexity of how the other parts of the EF-hand protein (and binding partners) influence the loop residues and thus Ca2+ chelation. It will be extremely challenging, if not impossible, to establish a set of simple rules that all EF-hand proteins follow to control their Ca2+ sensitivities and kinetics. However, within a protein family such as CaM and TnC, there may be some general principles that can be discerned and manipulated [6, 25, 40–42, 44, 48, 49, 60–64].
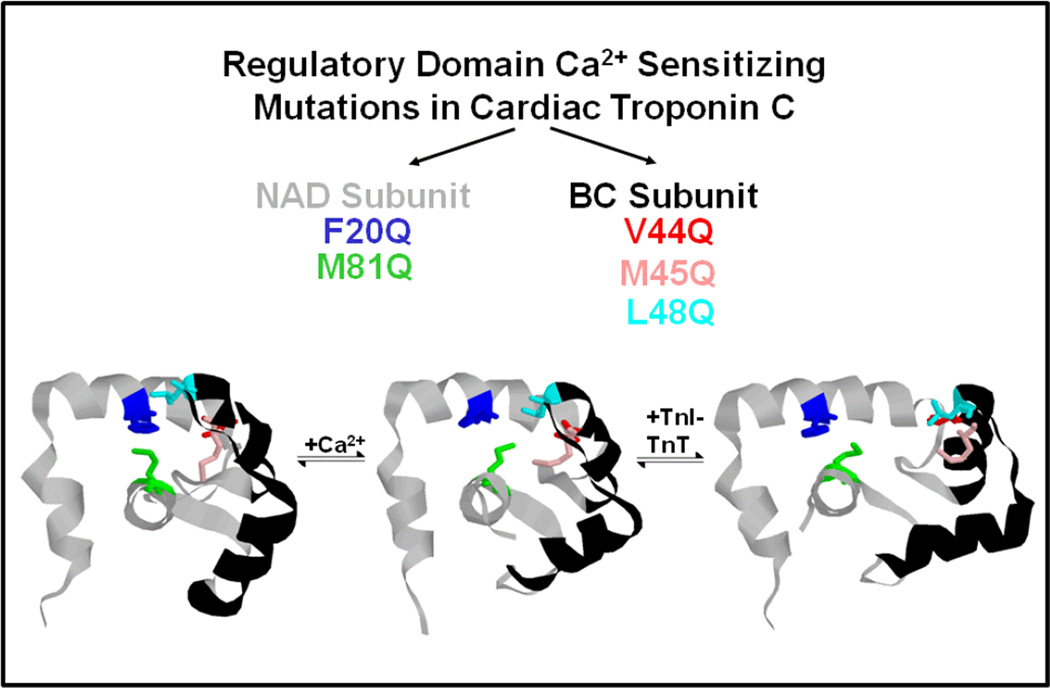
As mentioned above, Ca2+-dependent switches typically display a large structural change upon Ca2+ binding (Figure 2). In general, the apo (and often Mg2+ bound) structure of the protein is compact, burying several hydrophobic amino acids within its core. Occluding hydrophobic residues from the aqueous environment is thought to be energetically favorable considering “oil and water do not like to mix”. However, upon Ca2+ binding, a large hydrophobic surface area becomes exposed to the cytosol, which is thought to be energetically costly to Ca2+ binding [65]. Thus, minimizing this oily patch was predicted to enhance Ca2+ binding, potentially keeping the “pocket” open for longer periods of time – precisely what we wanted to do in order to test whether the kinetics of Ca2+ dissociation from TnC played a role in modulating the rate of muscle relaxation (yes it can) [66]. We individually mutated every hydrophobic residue throughout the N-terminal, regulatory domain of skeletal TnC and found that although the Ca2+ sensitivity could be altered over three orders of magnitude, surprisingly, there was no correlation between the Ca2+ affinity and the solvent accessibility of the mutated residues in either the absence or presence of Ca2+ (or the difference between the two states) [44]. Thus, the change in solvent exposure of a hydrophobic residue is not the sole determinant in how a residue affects Ca2+ binding. Instead, altering the ability of the protein to achieve its “open” state upon Ca2+ binding, as well as stabilizing the beta sheet, specific subdomains within the protein and the hinges for structural movement play a more central role in setting the Ca2+ sensitivity of TnC (Figure 4). Recent, molecular dynamics simulations have confirmed some of our original hypotheses [67].
Figure 4. Designing Ca2+ sensitizing mutations into the regulatory domain of cardiac TnC.
In the apo state, there is a cluster of hydrophobic residues in the NAD and BC subunits of the regulatory domain of cardiac TnC that interact with one another to keep the hydrophobic pocket closed. Upon Ca2+ binding the residues in the two domains begin to come apart and are far removed from one another upon TnI binding. Individually exchanging these hydrophobic residues with polar Gln help to increase pocket opening upon Ca2+ binding, in some cases (L48Q) substantially increasing overall Ca2+ sensitivity that carries through upon TnI binding [64].
For the most part, the majority of foundational work that one would utilize to rationally design cardiac TnC has been collected with isolated proteins, or with proteins that negligibly change structure. TnC does not function in isolation, but is an integral part of the Tn complex anchored tightly to the thin filament. Thus, a functional cardiac TnC must be able to stably form the Tn complex, anchor to the thin filament and via changes in Ca2+ concentration, reversibly bind the C-terminal domain of TnI (which ultimately controls the position of tropomyosin and the strong binding of myosin to actin). The behavior of the reconstituted thin filament is one of the best and relatively simple biochemical systems where all these processes can be systematically manipulated. Furthermore, the Ca2+-dependent behavior of the thin filament is an excellent predictor of how a particular perturbation of the thin filament will alter mechanical force [64]. Using this system, we and others have demonstrated that post-translational modification of several of the thin filament proteins (even at several different locations within a particular protein) can either increase or decrease the Ca2+ sensitivity and kinetics of the thin filament and force development/relaxation in a like manner [61, 68–71]. Interestingly, many disease associated mutations aberrantly alter the Ca2+ binding properties of the thin filament [48, 62]. Thus, designer TnCs should change the force response of cardiac muscle without altering the normal regulation of the thin filament (primarily the beta adrenergic response, i.e. Ser 23/24 TnI phosphorylation).
Working with both CaM and skeletal TnC, we were able to build a substantial database of point mutations that altered the N-terminal Ca2+ binding properties of these proteins. Considering cardiac TnC is very similar in structure and amino acid sequence to CaM and skeletal TnC, we reasoned that analogous mutations in these protein systems would behave similarly. Excitingly, and with high fidelity, we were able to generate a series of cardiac TnC mutants with a broad range of Ca2+ sensitivities and kinetics [25, 48]. Through combining mutations, we were successful at designing TnCs that could be finely tuned with precise Ca2+ affinities that could be used to counteract the aberrant Ca2+ sensitivities brought on by disease associated mutations in the other troponin subunits (TnI and TnT). Interestingly, not all of the mutations that affected the isolated protein carried this property through to the reconstituted thin filament, highlighting the importance of studying systems with physiological significance that contain additional proteins, which too can influence Ca2+ binding [64].
Excitingly, we and others have recently demonstrated that a Ca2+ sensitizing mutation in cardiac TnC (L48Q) enhances the contraction of healthy and diseased cardiac myocytes, and more significantly, improves in vivo contraction therapeutically after a myocardial infarction [49, 72]. This can be achieved in mice without the detrimental consequences observed with other positive inotropes such as adversely affecting cardiac morphology, electrical activity, beta-adrenergic response, relaxation, diastolic function, intracellular Ca2+ or survival [49]. Studies are currently underway to test this gene therapy approach in a larger myocardial infarction model in sheep. Besides enhancing contraction, there are several cardiac diseases where decreasing cardiac contractility may be warranted, such as occurs in hypertrophic and restrictive cardiomyopathies. We have specifically engineered Ca2+ desensitized TnCs for these diseases as well [48]. With the expanding impact of viral gene delivery and gene editing (CRISPR) technologies as viable gene therapies, and the promise of alternative protein delivery systems, protein engineering is at the precipice of substantially impacting cardiovascular medicine.
Conclusion
This review highlights the universal nature of Ca2+ signaling and the importance of Ca2+ binding proteins to sense these signals and translate or relay cellular and physiological outcomes. Muscle research has been at the forefront of pushing these ideas to the broader biological fields. In an attempt to understand the governing rules that dictate Ca2+ binding to EF-hand proteins, we have designed several cardiac TnC constructs that have been demonstrated to have a therapeutic potential. By no means has our work been limited to TnC, as we have also designed several parvalbumins and CaMs that also have potential to help the failing heart and vasculature [73–75]. Considering the importance of Ca2+ sensing to all of life’s processes, it is not farfetched to begin to design Ca2+ binding proteins to physiologically and therapeutically augment any organ system.
Acknowledgments
This effort was supported by NIH grant R56 HL091986 (J.P.D.) and by an American Heart Association Grant in Aid (M.T.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Case RM, Eisner D, Gurney A, Jones O, Muallem S, Verkhratsky A. Evolution of calcium homeostasis: from birth of the first cell to an omnipresent signalling system. Cell calcium. 2007;42:345–350. doi: 10.1016/j.ceca.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ. Elementary and global aspects of calcium signalling. The Journal of physiology. 1997;499(Pt 2):291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell AK. Intracellular Calcium Its Universal Role as a Regulator. New York: John Wiley & Sons Ltd.; 1983. [Google Scholar]
- 4.Ringer S. A third contribution regarding the Influence of the Inorganic Constituents of the Blood on the Ventricular Contraction. The Journal of physiology. 1883;4:222–225. doi: 10.1113/jphysiol.1883.sp000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rall JA. Mechanism of Muscular Contraction. New York: Springer-Verlag; 2014. [Google Scholar]
- 6.Lee RS, Tikunova SB, Kline KP, Zot HG, Hasbun JE, Minh NV, Swartz DR, Rall JA, Davis JP. Effect of Ca2+ binding properties of troponin C on rate of skeletal muscle force redevelopment. Am J Physiol Cell Physiol. 2010;299:C1091–C1099. doi: 10.1152/ajpcell.00491.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nature reviews. Molecular cell biology. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 8.Thorneloe KS, Nelson MT. Ion channels in smooth muscle: regulators of intracellular calcium and contractility. Canadian journal of physiology and pharmacology. 2005;83:215–242. doi: 10.1139/y05-016. [DOI] [PubMed] [Google Scholar]
- 9.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annual review of physiology. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 10.Roof SR, Biesiadecki BJ, Davis JP, Janssen PM, Ziolo MT. Effects of increased systolic Ca(2+) and beta-adrenergic stimulation on Ca(2+) transient decline in NOS1 knockout cardiac myocytes. Nitric Oxide. 2012;27:242–247. doi: 10.1016/j.niox.2012.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Troponin: Regulator of Muslce Contraction. New York: Nova Biomedical; 2014. [Google Scholar]
- 12.Janssen PM. Myocardial contraction-relaxation coupling. American journal of physiology. Heart and circulatory physiology. 2010;299:H1741–H1749. doi: 10.1152/ajpheart.00759.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biesiadecki BJ, Davis JP, Ziolo MT, Janssen PM. Tri-modal regulation of cardiac muscle relaxation; intracellular calcium decline, thin filament deactivation, and cross-bridge cycling kinetics. Biophys Rev. 2014;6:273–289. doi: 10.1007/s12551-014-0143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berridge MJ. Calcium signalling remodelling and disease. Biochemical Society transactions. 2012;40:297–309. doi: 10.1042/BST20110766. [DOI] [PubMed] [Google Scholar]
- 15.Davis JP, Tikunova SB. Ca(2+) exchange with troponin C and cardiac muscle dynamics. Cardiovascular research. 2008;77:619–626. doi: 10.1093/cvr/cvm098. [DOI] [PubMed] [Google Scholar]
- 16.Gerke V, Moss SE. Annexins: from structure to function. Physiological reviews. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 17.Camors E, Monceau V, Charlemagne D. Annexins and Ca2+ handling in the heart. Cardiovascular research. 2005;65:793–802. doi: 10.1016/j.cardiores.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Nalefski EA, Falke JJ. The C2 domain calcium-binding motif: structural and functional diversity. Protein science : a publication of the Protein Society. 1996;5:2375–2390. doi: 10.1002/pro.5560051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jalili T, Takeishi Y, Song G, Ball NA, Howles G, Walsh RA. PKC translocation without changes in Galphaq and PLC-beta protein abundance in cardiac hypertrophy and failure. The American journal of physiology. 1999;277:H2298–H2304. doi: 10.1152/ajpheart.1999.277.6.H2298. [DOI] [PubMed] [Google Scholar]
- 20.Rizo J, Sudhof TC. C2-domains, structure and function of a universal Ca2+-binding domain. The Journal of biological chemistry. 1998;273:15879–15882. doi: 10.1074/jbc.273.26.15879. [DOI] [PubMed] [Google Scholar]
- 21.Lewit-Bentley A, Rety S. EF-hand calcium-binding proteins. Current opinion in structural biology. 2000;10:637–643. doi: 10.1016/s0959-440x(00)00142-1. [DOI] [PubMed] [Google Scholar]
- 22.Gifford JL, Walsh MP, Vogel HJ. Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. The Biochemical journal. 2007;405:199–221. doi: 10.1042/BJ20070255. [DOI] [PubMed] [Google Scholar]
- 23.Linse S, Forsen S. Determinants that govern high-affinity calcium binding. Advances in second messenger and phosphoprotein research. 1995;30:89–151. doi: 10.1016/s1040-7952(05)80005-9. [DOI] [PubMed] [Google Scholar]
- 24.Maki M, Kitaura Y, Satoh H, Ohkouchi S, Shibata H. Structures, functions and molecular evolution of the penta-EF-hand Ca2+-binding proteins. Biochimica et biophysica acta. 2002;1600:51–60. doi: 10.1016/s1570-9639(02)00444-2. [DOI] [PubMed] [Google Scholar]
- 25.Tikunova SB, Davis JP. Designing calcium-sensitizing mutations in the regulatory domain of cardiac troponin C. The Journal of biological chemistry. 2004;279:35341–35352. doi: 10.1074/jbc.M405413200. [DOI] [PubMed] [Google Scholar]
- 26.Permyakov EA, Kretsinger RH. Cell signaling, beyond cytosolic calcium in eukaryotes. Journal of inorganic biochemistry. 2009;103:77–86. doi: 10.1016/j.jinorgbio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Grabarek Z. Insights into modulation of calcium signaling by magnesium in calmodulin, troponin C and related EF-hand proteins. Biochimica et biophysica acta. 2011;1813:913–921. doi: 10.1016/j.bbamcr.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis JP, Norman C, Kobayashi T, Solaro RJ, Swartz DR, Tikunova SB. Effects of thin and thick filament proteins on calcium binding and exchange with cardiac troponin C. Biophysical journal. 2007;92:3195–3206. doi: 10.1529/biophysj.106.095406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pauls TL, Cox JA, Berchtold MW. The Ca2+(-)binding proteins parvalbumin and oncomodulin and their genes: new structural and functional findings. Biochimica et biophysica acta. 1996;1306:39–54. doi: 10.1016/0167-4781(95)00221-9. [DOI] [PubMed] [Google Scholar]
- 30.Henzl MT, Tanner JJ. Solution structure of Ca2+-free rat alpha-parvalbumin. Protein science : a publication of the Protein Society. 2008;17:431–438. doi: 10.1110/ps.073318308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin Y, Henzl MT, Lorber B, Nakazawa T, Thomas TT, Jiang F, Langer R, Benowitz LI. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nature neuroscience. 2006;9:843–852. doi: 10.1038/nn1701. [DOI] [PubMed] [Google Scholar]
- 32.Tidow H, Nissen P. Structural diversity of calmodulin binding to its target sites. The FEBS journal. 2013;280:5551–5565. doi: 10.1111/febs.12296. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi T, Solaro RJ. Calcium, thin filaments, and the integrative biology of cardiac contractility. Annual review of physiology. 2005;67:39–67. doi: 10.1146/annurev.physiol.67.040403.114025. [DOI] [PubMed] [Google Scholar]
- 34.Shannon TR, Wang F, Puglisi J, Weber C, Bers DM. A mathematical treatment of integrated Ca dynamics within the ventricular myocyte. Biophysical journal. 2004;87:3351–3371. doi: 10.1529/biophysj.104.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakayama S, Kretsinger RH. Evolution of the EF-hand family of proteins. Annual review of biophysics and biomolecular structure. 1994;23:473–507. doi: 10.1146/annurev.bb.23.060194.002353. [DOI] [PubMed] [Google Scholar]
- 36.Reid RE, Hodges RS. Co-operativity and calcium/magnesium binding to troponin C and muscle calcium binding parvalbumin: an hypothesis. Journal of theoretical biology. 1980;84:401–444. doi: 10.1016/s0022-5193(80)80013-0. [DOI] [PubMed] [Google Scholar]
- 37.Henzl MT, Ndubuka K. Low-affinity signature of the rat beta-parvalbumin CD site. Evidence for remote determinants. Biochemistry. 2007;46:23–35. doi: 10.1021/bi061421h. [DOI] [PubMed] [Google Scholar]
- 38.Persechini A, Stemmer PM, Ohashi I. Localization of unique functional determinants in the calmodulin lobes to individual EF hands. The Journal of biological chemistry. 1996;271:32217–32225. doi: 10.1074/jbc.271.50.32217. [DOI] [PubMed] [Google Scholar]
- 39.Putkey JA, Sweeney HL, Campbell ST. Site-directed mutation of the trigger calcium-binding sites in cardiac troponin C. The Journal of biological chemistry. 1989;264:12370–12378. [PubMed] [Google Scholar]
- 40.Davis JP, Rall JA, Reiser PJ, Smillie LB, Tikunova SB. Engineering competitive magnesium binding into the first EF-hand of skeletal troponin C. The Journal of biological chemistry. 2002;277:49716–49726. doi: 10.1074/jbc.M208488200. [DOI] [PubMed] [Google Scholar]
- 41.Tikunova SB, Black DJ, Johnson JD, Davis JP. Modifying Mg2+ binding and exchange with the N-terminal of calmodulin. Biochemistry. 2001;40:3348–3353. doi: 10.1021/bi0021333. [DOI] [PubMed] [Google Scholar]
- 42.Black DJ, Tikunova SB, Johnson JD, Davis JP. Acid pairs increase the N-terminal Ca2+ affinity of CaM by increasing the rate of Ca2+ association. Biochemistry. 2000;39:13831–13837. doi: 10.1021/bi001106+. [DOI] [PubMed] [Google Scholar]
- 43.Henzl MT, Sirianni AG, Markus LA, Davis CM. Site-directed mutagenesis of rat alpha-parvalbumin: replacement of canonical CD-site residues with their non-consensus counterparts from rat beta-parvalbumin. Biophysical chemistry. 2015;197:25–39. doi: 10.1016/j.bpc.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Tikunova SB, Rall JA, Davis JP. Effect of hydrophobic residue substitutions with glutamine on Ca(2+) binding and exchange with the N-domain of troponin C. Biochemistry. 2002;41:6697–6705. doi: 10.1021/bi011763h. [DOI] [PubMed] [Google Scholar]
- 45.Tan RY, Mabuchi Y, Grabarek Z. Blocking the Ca2+-induced conformational transitions in calmodulin with disulfide bonds. The Journal of biological chemistry. 1996;271:7479–7483. doi: 10.1074/jbc.271.13.7479. [DOI] [PubMed] [Google Scholar]
- 46.Black DJ, Selfridge JE, Persechini A. The kinetics of Ca(2+)-dependent switching in a calmodulin-IQ domain complex. Biochemistry. 2007;46:13415–13424. doi: 10.1021/bi700774s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Kleerekoper QK, Xiong LW, Putkey JA. Intrinsically disordered PEP-19 confers unique dynamic properties to apo and calcium calmodulin. Biochemistry. 2010;49:10287–10297. doi: 10.1021/bi100500m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu B, Lee RS, Biesiadecki BJ, Tikunova SB, Davis JP. Engineered troponin C constructs correct disease-related cardiac myofilament calcium sensitivity. The Journal of biological chemistry. 2012;287:20027–20036. doi: 10.1074/jbc.M111.334953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shettigar V, Zhang B, Little SC, Salhi HE, Hansen BJ, Li N, Zhang J, Roof SR, Ho H-T, Brunello L, Lerch JK, Weisleder N, Fedorov VV, Accornero F, Rafael-Fortney JA, Gyorke S, Janssen PML, Biesiadecki BJ, Ziolo MT, Davis JP. Rationally engineered Troponin C modulates in vivo cardiac function and performance in health and disease. Nature Communications. 2016 doi: 10.1038/ncomms10794. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hasenfuss G, Teerlink JR. Cardiac inotropes: current agents and future directions. Eur Heart J. 2011;32:1838–1845. doi: 10.1093/eurheartj/ehr026. [DOI] [PubMed] [Google Scholar]
- 51.Overgaard CB, Dzavik V. Inotropes and vasopressors: review of physiology and clinical use in cardiovascular disease. Circulation. 2008;118:1047–1056. doi: 10.1161/CIRCULATIONAHA.107.728840. [DOI] [PubMed] [Google Scholar]
- 52.Curfman GD. Inotropic therapy for heart failure--an unfulfilled promise. N Engl J Med. 1991;325:1509–1510. doi: 10.1056/NEJM199111213252111. [DOI] [PubMed] [Google Scholar]
- 53.Modulation of Cardiac Calcium Sensitivity: A new approach to increasing the strength of the heart. New York: Oxford University Press Inc.; 1993. [Google Scholar]
- 54.Schiereck P, De Beer E, Van Heijst B, Janssen P, Van Andel A, Jennekens F, Sontrop A, Bavinck A. Ca2+ channel antagonists enhance tension in skinned skeletal and heart muscle fibres. European journal of pharmacology. 1993;249:317–324. doi: 10.1016/0014-2999(93)90528-p. [DOI] [PubMed] [Google Scholar]
- 55.Hwang PM, Sykes BD. Targeting the sarcomere to correct muscle function. Nat Rev Drug Discov. 2015 doi: 10.1038/nrd4554. In Press. [DOI] [PubMed] [Google Scholar]
- 56.Kass DA, Solaro RJ. Mechanisms and use of calcium-sensitizing agents in the failing heart. Circulation. 2006;113:305–315. doi: 10.1161/CIRCULATIONAHA.105.542407. [DOI] [PubMed] [Google Scholar]
- 57.Russell AJ, Hartman JJ, Hinken AC, Muci AR, Kawas R, Driscoll L, Godinez G, Lee KH, Marquez D, Browne WFt, Chen MM, Clarke D, Collibee SE, Garard M, Hansen R, Jia Z, Lu PP, Rodriguez H, Saikali KG, Schaletzky J, Vijayakumar V, Albertus DL, Claflin DR, Morgans DJ, Morgan BP, Malik FI. Activation of fast skeletal muscle troponin as a potential therapeutic approach for treating neuromuscular diseases. Nature medicine. 2012;18:452–455. doi: 10.1038/nm.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drake SK, Falke JJ. Kinetic tuning of the EF-hand calcium binding motif: the gateway residue independently adjusts (i) barrier height and (ii) equilibrium. Biochemistry. 1996;35:1753–1760. doi: 10.1021/bi952335c. [DOI] [PubMed] [Google Scholar]
- 59.Linse S, Brodin P, Johansson C, Thulin E, Grundstrom T, Forsen S. The role of protein surface charges in ion binding. Nature. 1988;335:651–652. doi: 10.1038/335651a0. [DOI] [PubMed] [Google Scholar]
- 60.Little SC, Biesiadecki BJ, Kilic A, Higgins RS, Janssen PM, Davis JP. The rates of Ca2+ dissociation and cross-bridge detachment from ventricular myofibrils as reported by a fluorescent cardiac troponin C. The Journal of biological chemistry. 2012;287:27930–27940. doi: 10.1074/jbc.M111.337295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu B, Lopez JJ, Biesiadecki BJ, Davis JP. Protein kinase C phosphomimetics alter thin filament ca(2+) binding properties. PLoS One. 2014;9:e86279. doi: 10.1371/journal.pone.0086279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu B, Tikunova SB, Kline KP, Siddiqui JK, Davis JP. Disease-related cardiac troponins alter thin filament Ca2+ association and dissociation rates. PLoS One. 2012;7:e38259. doi: 10.1371/journal.pone.0038259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Norman C, Rall JA, Tikunova SB, Davis JP. Modulation of the rate of cardiac muscle contraction by troponin C constructs with various calcium binding affinities. American journal of physiology. Heart and circulatory physiology. 2007;293:H2580–H2587. doi: 10.1152/ajpheart.00039.2007. [DOI] [PubMed] [Google Scholar]
- 64.Tikunova SB, Liu B, Swindle N, Little SC, Gomes AV, Swartz DR, Davis JP. Effect of calcium-sensitizing mutations on calcium binding and exchange with troponin C in increasingly complex biochemical systems. Biochemistry. 2010;49:1975–1984. doi: 10.1021/bi901867s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pearlstone JR, Borgford T, Chandra M, Oikawa K, Kay CM, Herzberg O, Moult J, Herklotz A, Reinach FC, Smillie LB. Construction and characterization of a spectral probe mutant of troponin C: application to analyses of mutants with increased Ca2+ affinity. Biochemistry. 1992;31:6545–6553. doi: 10.1021/bi00143a026. [DOI] [PubMed] [Google Scholar]
- 66.Luo Y, Davis JP, Tikunova SB, Smillie LB, Rall JA. Myofibrillar determinants of rate of relaxation in skinned skeletal muscle fibers. Advances in experimental medicine and biology. 2003;538:573–581. doi: 10.1007/978-1-4419-9029-7_51. discussion 581-572. [DOI] [PubMed] [Google Scholar]
- 67.Kekenes-Huskey PM, Lindert S, McCammon JA. Molecular basis of calcium-sensitizing and desensitizing mutations of the human cardiac troponin C regulatory domain: a multi-scale simulation study. PLoS Comput Biol. 2012;8:e1002777. doi: 10.1371/journal.pcbi.1002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nixon BR, Liu B, Scellini B, Tesi C, Piroddi N, Ogut O, Solaro RJ, Ziolo MT, Janssen PM, Davis JP, Poggesi C, Biesiadecki BJ. Tropomyosin Ser-283 pseudo-phosphorylation slows myofibril relaxation. Arch Biochem Biophys. 2013;535:30–38. doi: 10.1016/j.abb.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nixon BR, Thawornkaiwong A, Jin J, Brundage EA, Little SC, Davis JP, Solaro RJ, Biesiadecki BJ. AMP-activated protein kinase phosphorylates cardiac troponin I at Ser-150 to increase myofilament calcium sensitivity and blunt PKA-dependent function. The Journal of biological chemistry. 2012;287:19136–19147. doi: 10.1074/jbc.M111.323048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nixon BR, Walton SD, Zhang B, Brundage EA, Little SC, Ziolo MT, Davis JP, Biesiadecki BJ. Combined troponin I Ser-150 and Ser-23/24 phosphorylation sustains thin filament Ca(2+) sensitivity and accelerates deactivation in an acidic environment. Journal of molecular and cellular cardiology. 2014;72:177–185. doi: 10.1016/j.yjmcc.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salhi HE, Walton SD, Hassel NC, Brundage EA, de Tombe PP, Janssen PM, Davis JP, Biesiadecki BJ. Cardiac troponin I tyrosine 26 phosphorylation decreases myofilament Ca2+ sensitivity and accelerates deactivation. Journal of molecular and cellular cardiology. 2014;76:257–264. doi: 10.1016/j.yjmcc.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feest ER, Steven Korte F, Tu AY, Dai J, Razumova MV, Murry CE, Regnier M. Thin filament incorporation of an engineered cardiac troponin C variant (L48Q) enhances contractility in intact cardiomyocytes from healthy and infarcted hearts. Journal of molecular and cellular cardiology. 2014;72:219–227. doi: 10.1016/j.yjmcc.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang W, Barnabei MS, Asp ML, Heinis FI, Arden E, Davis J, Braunlin E, Li Q, Davis JP, Potter JD, Metzger JM. Noncanonical EF-hand motif strategically delays Ca2+ buffering to enhance cardiac performance. Nature medicine. 2013;19:305–312. doi: 10.1038/nm.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang J, Shettigar V, Zhang GC, Kindell DG, Liu X, Lopez JJ, Yerrimuni V, Davis GA, Davis JP. Engineering Parvalbumin for the Heart: Optimizing the Mg Binding Properties of Rat beta-Parvalbumin. Front Physiol. 2011;2:77. doi: 10.3389/fphys.2011.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Lierop JE, Wilson DP, Davis JP, Tikunova S, Sutherland C, Walsh MP, Johnson JD. Activation of smooth muscle myosin light chain kinase by calmodulin. Role of LYS(30) and GLY(40) The Journal of biological chemistry. 2002;277:6550–6558. doi: 10.1074/jbc.M111404200. [DOI] [PubMed] [Google Scholar]