Abstract
NY-ESO-1 is a “cancer-testis” antigen expressed in many cancers. ISCOMATRIX is a saponin-based adjuvant that induces antibody and T cell responses. We performed a placebo-controlled clinical trial evaluating the safety and immunogenicity of recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant. Forty-six evaluable patients with resected NY-ESO-1-positive tumors received three doses of vaccine intramuscularly at monthly intervals. The vaccine was well tolerated. We observed high-titer antibody responses, strong delayed-type hypersensitivity reactions, and circulating CD8+ and CD4+ T cells specific for a broad range of NY-ESO-1 epitopes, including known and previously unknown epitopes. In an unplanned analysis, vaccinated patients appeared to have superior clinical outcomes to those treated with placebo or protein alone. The vaccine is safe and highly potent immunologically.
NY-ESO-1 is a “cancer-testis” antigen (Ag) that is frequently expressed in a variety of cancers but not normal adult tissues apart from testis (1). Spontaneous humoral and cellular immune responses against NY-ESO-1 can occur in patients (pts) with NY-ESO-1-positive tumors (1), and several HLA class I- and II-restricted peptides have been defined as the targets (2–12). This immunogenicity and its tissue distribution make NY-ESO-1 a good candidate Ag for immunotherapy. Clinical vaccine trials using HLA-A2-restricted synthetic peptides from NY-ESO-1 have shown that these can be administered safely and generate T cell responses and may have clinical benefit (5).‡‡,§§ However, to maximize vaccine efficacy, an immune response against a broad range of HLA class I and II epitopes is required. Vaccination with the full-length Ag has the potential to broaden this response, without the need to restrict the target pt population by HLA type. ISCOMATRIX adjuvant (IMX) is a saponin-based adjuvant that is well tolerated and induces strong Ab and T cell responses (13–15). Its ability to induce cellular immune responses makes it a particularly attractive adjuvant for cancer vaccination. A vaccine combining NY-ESO-1 and IMX therefore had the potential to generate broad-based immunity against a wide variety of tumor types.
We performed a double-blind placebo-controlled dose escalation clinical trial using recombinant NY-ESO-1 protein formulated with IMX. The vaccine was administered every 4 weeks for three doses to pts with NY-ESO-1-positive cancers and minimal residual disease. The objectives were to determine the safety and tolerability of increasing doses of NY-ESO-1 IMX vaccine and to measure cellular and humoral immune responses after vaccination.
Methods
Vaccine Production. Recombinant NY-ESO-1 protein was produced by using a bacterial expression system and purified chromatographically (R.M., S.G., G.R., L. Cohen, D. Ryan, W. Woods, M. Rubira, J.C., I.D.D., A. Sjolander, et al., unpublished work). NY-ESO-1 protein was formulated in a fixed ratio of IMX. All processes were performed under cGMP conditions.
Study Protocol. The trial had two parts: an initial dose escalation phase to determine safety, followed by accrual at one dose to evaluate immunogenicity. No cancer response endpoints were planned. Pts had minimal residual cancer (no detectable disease or small volume locoregional disease only) that expressed NY-ESO-1 Ag by immunohistochemistry or RT-PCR, and a 5-year risk of relapse exceeding 25%. Pts were excluded if alternative effective therapy was available or if they were immunodeficient. The protocol was approved by the Human Research Ethics Committees of participating centers and was performed under the Australian Clinical Trial Notification scheme. All pts provided written informed consent. The trial was monitored independently by Kendle Australia (Victoria, Australia).
Three intramuscular injections were administered at 4-week intervals. Pts were evaluable for safety after one vaccine dose and for immune responses after three.
Five pt cohorts were studied. Dose escalation was undertaken with three pts in each of cohorts A, B, and C who received 10, 30, and 100 μg of protein combined with 12, 36, and 120 μg of IMX, respectively. These cohorts are referred to here as: 10+IMX, 30+IMX, and 100+IMX. Once safety was established, cohort C was expanded to include a total of 20 pts, 10 of whom expressed HLA-A2 and 10 of whom did not. Cohort D was the same, except they received 100 μg of NY-ESO-1 protein without IMX (“Protein 100 alone”). In order to control for the potential immune impact of the delayed-type hypersensitivity (DTH) protein, eight pts in cohorts C and D were randomized to sterile saline placebo (two HLA-A2 positive and two HLA-A2 negative from each cohort), constituting a fifth cohort (“Placebo”).
Toxicity. Tolerability and safety endpoints were determined by using the National Cancer Institute Common Toxicity Criteria Scale (Version 2.0, April 30, 1999).
Immunological Testing and Interpretation. Immunogenicity endpoints were determined by using standardized assays. An immunological response was defined as a positive response in any of the following assays.
(i) Serology. NY-ESO-1-specific antibodies were measured by ELISA at baseline and on study days 14, 42, 70, and 86. Plates were coated with vaccine protein, and bound Ab was detected with horseradish peroxidase-labeled goat anti-human IgG (Kirkegaard & Perry Laboratories). Results were expressed as reciprocal titer. The lower limits of detection and quantitation of this assay were 2,000 and 5,000, respectively. Based on examination of blinded data, pts with pretreatment titers >5,000 were deemed to have a preexisting response. Pts were deemed to have had a positive humoral response to vaccination if they developed a titer >5,000 and had no preexisting response.
(ii) DTH tests. DTH testing was performed before and after vaccination (day 84). One microgram of recombinant NY-ESO-1 protein was administered intradermally. Induration and erythema were measured 2 days later. Based on an examination of blinded data, preexisting reactivity was defined as a baseline induration of >5 mm. A positive response to vaccination was recorded if the second DTH reading was >5 mm and at least double the baseline reading.
(iii) T cell assays. When the protocol was initiated, T cell assays were available only for HLA-A2-positive pts by using the NY-ESO-1157–165 peptide SLLMWITQC. Two flow-cytometric assays were used: intracellular cytokine staining (ICS) (16) and HLA-peptide tetramer binding (17). Peripheral blood mononuclear cells (5 × 106) were pulsed with the NY-ESO-1157–165 peptide SLLMWITQC at 0.3 μM in the presence of the reducing agent 1 mM Tris (2-carboxyethyl) phosphine hydrochloride (Pierce) for 30 min (18). The cells were then washed and cultured in a 24-well plate in 2 ml of RPMI medium 1640 containing 10% FCS (CSL) and IL-2 10 international units/ml, harvested on day 7, and assayed for intracellular IFN-γ expression against T2 cells pulsed with the NY-ESO-1 peptide SLLMWITQC. Binding of the appropriate tetramer was measured on the same population. An assay was accepted only if all negative and positive controls were within limits. A positive assay was one in which all controls passed and a population of reactive T cells could be defined on the FACS plot. In most cases, this represented ≥0.1% IFN-γ+ CD8+ T cells. Because the assay involves in vitro prestimulation, a positive response should be interpreted as indicating the presence of T cells ex vivo and not as a quantitative measure.
Further examination of CD8+ and CD4+ T cell responses to undefined T cell epitopes was performed by using peptide 18-mers overlapping by 12 aa (as stimulating Ags) and 13-mers overlapping by 11 aa (as screening Ags), by using the same method described above except that no tris(2-carboxyethyl) phosphine hydrochloride or T2 cells were used. Eighteen-mers were individually synthesized, and 13-mers were synthesized as pin-peptides (Chiron Mimotopes, Victoria, Australia). Individual 18-mer-stimulated cultures were first tested against the 18-mer. Positive cultures were then screened with appropriate 13-mers after a total of 11–13 days of culture. Positive results were confirmed by independent assays by stimulation with and titration of the relevant 13-mer peptides.
(iv) Preparation of DTH-infiltrating lymphocytes. A 4-mm punch biopsy of skin from the DTH site was minced in RPMI medium 1640 with 10% FCS (CSL). Single-cell suspensions were stimulated with 1 μg/ml phytohemagglutinin (Sigma) and cocultured with irradiated autologous peripheral blood mononuclear cells with 10 international units/ml IL-2 (Cetus) and 10 ng/ml IL-7 (PeproTech, Rocky Hill, NJ). Medium was replenished each 2–3 days. Established T cells were screened by using autologous Epstein–Barr virus-transformed B cells pulsed with NY-ESO-1 peptides in an ICS assay.
Statistical Design. The study was powered to show safety but not to enable comparisons between groups. If the true rate of toxicity (more than Common Toxicity Criteria grade 2) was 25%, the probability of correctly observing such toxicity in at least one pt of 16 in the 100+IMX dose level was 99.0%. The probability of incorrectly concluding there was no toxicity was 1%.
Results
Pt Characteristics. Forty-six pts were evaluable for assessment of immune response. Most had resected melanoma. Five were replaced because of either disease progression (2), noncompliance with the study protocol (2), or voluntary withdrawal (1) (see Tables 1 and 2, which are published as supporting information on the PNAS web site).
Toxicity. The vaccine was well tolerated. A maximum tolerated dose was not determined, although there was a trend to more adverse events (AEs) in the 100+IMX dose level. The most common AE was pain at the injection site, which was severe (Grade 3) in only three pts. The dose was reduced to 30% in those pts, and their subsequent injections were well tolerated. Other toxicities were less than or equal to grade 2 and included fatigue, myalgia, pyrexia, and headache (see Table 3, which is published as supporting information on the PNAS web site).
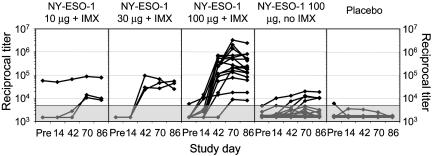
Immune Responses. (i) Antibody responses to NY-ESO-1. Three pts had preexisting immunity to NY-ESO-1 on the basis of Ab titer, and none showed a significant increase in Ab titer after vaccination (Fig. 1). Excluding these, all 20 who received a full course of NY-ESO-1 IMX vaccine developed a positive Ab response regardless of dose level, whereas only four of 16 (25%) of those who received NY-ESO-1 protein without adjuvant (Protein 100 alone) developed an Ab response. There was a clear dose–response relationship between vaccine dose and Ab titer, and the addition of IMX adjuvant greatly enhanced Ab responses (Fig. 1). No placebo pt developed a positive Ab response. The specificity of the Ab responses was confirmed in some pts by using two additional methods: ELISA assay using a different source of NY-ESO-1 protein (19) (kindly performed by Lisa Stockert, Ludwig Institute), and Western blot assays that showed that pt sera recognized NY-ESO-1 recombinant protein (data not shown).
Fig. 1.
Ab responses by treatment dose level. Each curve represents an individual pt within that dose level. y axis is reciprocal titer on a logarithmic scale. Shaded area indicates titers below the limit of quantitation (<5,000).
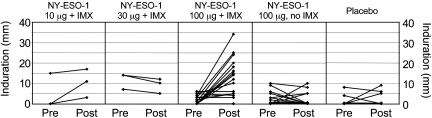
(ii) DTH responses to NY-ESO-1. DTH responses to intradermal protein were commonly seen in vaccinated pts, particularly those in the 100+IMX cohort (Fig. 2). Biopsy of these DTH reactions showed dermal lymphoid infiltrates predominantly comprised of CD4+ T cells and a lesser population of CD8+ cells (not shown). Addition of the IMX adjuvant significantly enhanced these DTH responses to the injected DTH material: 10 of 16 pts in the 100+IMX cohort responded, compared to only one of 16 who received protein alone (Fig. 2). Furthermore, there was good correlation with antibody responses in the 100+IMX cohort, because all pts who developed DTH responses also developed Ab responses. In contrast, the one seropositive pt who received protein alone did not develop a DTH response.
Fig. 2.
DTH measurements (Induration) 2 days after intradermal injection of 1 μg of NY-ESO-1 protein without IMX. Each curve represents an individual pt within that dose level. Pre, before vaccination; Post, after three vaccinations at day 86.
A total of nine of 46 pts had preexisting DTH responses. One was in the placebo cohort, and eight were in various vaccine cohorts. These responses did not increase in any pt and subsequently became negative in three. Another pt in the placebo cohort developed 9-mm induration and 30-mm erythema after the second DTH injection. Neither of the two pts in the placebo cohort with detectable DTH reactivity developed an Ab response. In view of this, the significance of this reactivity to intradermally injected protein is uncertain, because all published reports of spontaneous immunity to NY-ESO-1 are based on detection of Ab.
(iii) Characterization of the specificity of lymphocytes isolated from the DTH site. To confirm the specificity of the DTH response, lymphocytes were obtained from a skin biopsy of a DTH site in pt 7 (100+IMX dose level). CD8+ and CD4+ T cells specific for NY-ESO-1 peptides were identified in this sample (Fig. 6, which is published as supporting information on the PNAS web site), indicating that at least part of the DTH reactivity was attributable to NY-ESO-1 specificity.
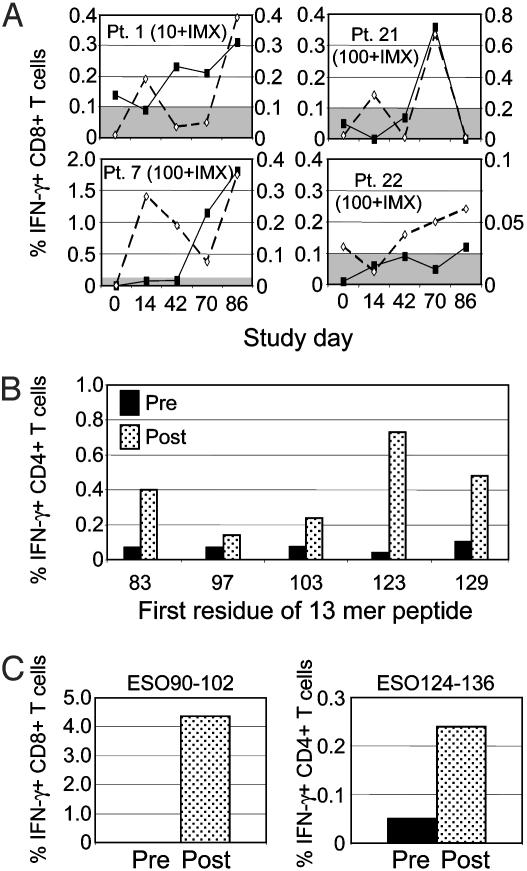
(iv) Peripheral blood CD8+ and CD4+ T cell responses to NY-ESO-1. At the time this study was initiated, only HLA-A2-restricted CD8+ T cell epitopes had been described for NY-ESO-1. Immune monitoring was therefore designed to evaluate responses to the NY-ESO-1157–165 epitope SLLMWITQC, by using either tetramers or ICS. These responses were seen in relatively few pts: one of three HLA-A2-positive pts in cohort 10+IMX and three of eight HLA-A2-positive pts in cohort 100+IMX (Fig. 3A). Pts 1 and 22 had evidence of prior immunity, as defined by a detectable pretreatment Ab titer, so responses to this epitope were considered to have been induced by the vaccine in only two pts (pts 7 and 21). Both were treated at the 100+IMX dose level. An additional pt (pt 39), who received protein alone without IMX, also reacted weakly to this epitope by tetramer staining and by ICS; however, to identify this reactivity a longer period of culture (10–13 days) was required after a single restimulation (data not shown).
Fig. 3.
Time course of T cell responses. (A) CD8+ T cell responses in four HLA-A2+ pts with detectable responses against NY-ESO-1157–165 epitope SLLMWITQC. Solid line with closed symbol indicates ICS assay (left y axis). Dashed line with open symbol indicates tetramer assay (right y axis). Shaded area indicates cutoff for positive ICS response (0.1%). (B) Induction of CD4+ T cell responses in pt 14. Filled bars, before treatment; stippled bars, after three vaccinations. (C) Induction of CD8+ and CD4+ T cell responses in pt 13. Filled bars, before treatment; stippled bars, after three vaccinations.
These responses were less frequent than anticipated. During the course of the trial, additional NY-ESO-1 epitopes recognized by both CD4+ and CD8+ T cells were described by others. A method using overlapping peptides was used to test for responses to other determinants. This method did not rely on preexisting knowledge of potential epitopes and took advantage of the presence of serum proteases in vitro to process longer peptides to shorter fragments capable of binding to both HLA class I and II molecules.
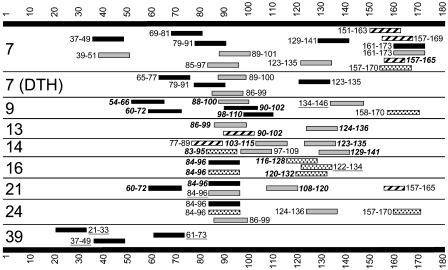
Using this method, a broad range of epitopes recognized by both CD4+ and CD8+ T cells was detected in all pts tested to date (Fig. 4). Many of these were proven to be induced by vaccination (Fig. 3 A–C); peptide sequences where this was shown to be the case are shown in bold italics (Fig. 4). Spontaneous responses to some of these epitopes have been described previously in cancer pts (7), indicating that the NY-ESO-1 IMX vaccine is capable of inducing T cell responses against epitopes that are naturally presented by tumors. In addition, we observed T cell responses to a variety of previously undescribed epitopes. For example, the CD4+ T cell response to peptide 124–136 in pt 13 (Fig. 3C) overlaps with a peptide containing a previously described HLA-DR4/DR7 epitope (20) and could be blocked using an anti-HLA-DR Ab (data not shown). However, this pt expressed HLA-DR3 and DR5, suggesting that, in this instance, the peptide was presented by a different HLA-DR specificity to that previously reported (7).
Fig. 4.
Peripheral blood T cell HLA class I- and II-restricted responses to overlapping 13-mer NY-ESO-1 peptides. Large bars and numbers represent the protein and amino acids of NY-ESO-1. Dark bars, HLA class I responses; light bars, HLA class II responses; filled bars, previously undescribed epitopes; striped bars, class I epitopes definitely or possibly described previously; dotted bars, class II epitopes definitely or possibly described previously; numbers in bold italics, responses proven to be induced by vaccination; underlined numbers, proven preexisting responses; numbers neither in bold italic nor underlined, unable to ascertain whether preexisting or induced. Numbers to the left indicate the pt number. 7 (DTH) to responses observed in T cells derived from a DTH injection site in pt 7. All pts shown had resected melanoma and received treatment at the 100+IMX dose level, except pt 39, who received NY-ESO-1 protein without IMX. Pts 7, 9, 21, and 39 were HLA-A2-positive, and pts 13, 14, 16, and 24 were HLA-A2-negative.
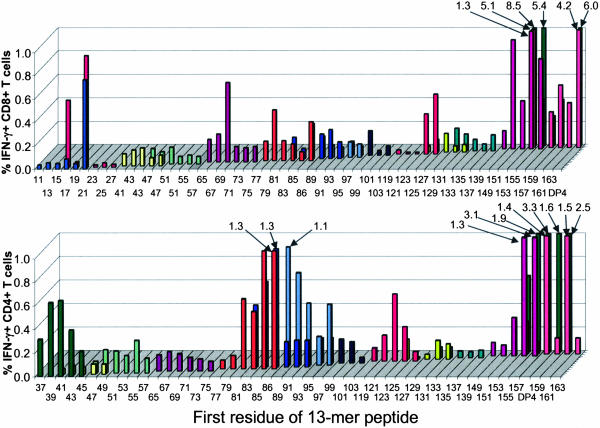
Fig. 5 illustrates the breadth of the T cell immune response in blood from a single pt from cohort 100+IMX. Circulating CD8+ and CD4+ T cells specific for numerous NY-ESO-1 epitopes were observed and included previously described HLA-A2 and HLA-DP4 epitopes as well as previously undescribed class I and II epitopes. T cell responses to 18-mer peptides were confirmed in separate assays by stimulating the cultures with appropriate 13-mer peptides. The finer details of these responses, including the minimal peptide sequences and HLA restricting elements, are beyond thescope of this clinical report and are reported separately (see Table 4, which is published as supporting information on the PNAS web site) (21).
Fig. 5.
Detailed results from pt 7. Vertical axis represents percent CD8+ (Upper) or CD4+ (Lower) T cells positive for IFN-γ. Values >1.0% are represented by the numbers and arrows. Screening 13-mer peptides used in the ICS assay are listed on the x axis by the number of the first amino acid residue. Stimulating 18-mer peptides used for culturing T cells are coded by color on the y axis and cover the same regions as the 13-mers. DP4, HLA-DP4 epitope (157–170).
Clinical Responses in Melanoma Pts. Although this study was not designed to assess clinical endpoints, the impression emerged that most melanoma pts who relapsed were in the groups that were characterized by poorer immune responses, i.e., those who received either placebo or protein alone without adjuvant. Forty-two melanoma pts completed vaccination. With a median followup of 748 days, 16 have relapsed: five of seven placebo pts, nine of 16 who received protein alone and two of 19 who received NY-ESO-1 with IMX. The following covariates were analyzed retrospectively in case imbalance between groups had affected this result: pathological stage at study entry; primary lesion thickness; age; sex; time since diagnosis; estimated risk of relapse at study entry; number of recurrences before entry; and time since last resection. None were found to be significant; however, the small sample size may influence this result.
Discussion
The vaccine comprised of recombinant NY-ESO-1 protein with IMX adjuvant was safe, well tolerated, and effective at inducing a broad integrated cellular and humoral response to NY-ESO-1. This included T cell responses to naturally processed HLA class I and II epitopes as well as previously undescribed epitopes. IMX induced substantially greater DTH and Ab responses to NY-ESO-1.
Immune responses were induced in pts both with and without detectable preexisting responses, including some whose only prior exposure to the NY-ESO-1 Ag was within a small primary tumor. This indicates that the vaccine could induce primary immunity against NY-ESO-1 rather than simply amplifying preexisting memory responses. Every pt who received the 100-μg dose of protein with IMX (100+IMX cohort) showed evidence of an immune response. In view of this efficacy for priming immune responses to both class I and II determinants, we have performed additional laboratory studies that focus on the capacity of IMX to facilitate presentation and crosspresentation of NY-ESO-1 protein by human dendritic cells of various types. These studies have provided insights into antigen-processing pathways in dendritic cells (M. Schnurr, Q.C., A. Shin, W.C., T. Toy, C. Jenderek, S.G., L.M., D. Drane, I.D.D., E.M., and J.S.C., unpublished work).
The protocol-defined strategy for monitoring CD8+ T cell responses was based around a cluster of HLA-A2-restricted epitopes centered on amino acids 157–165 (SLLMWITQC). When the study was initiated, no other class I or II epitopes had been described. We were surprised to find that relatively few pts responded to this epitope. In contrast, CD4+ and CD8+ T cell responses to numerous other epitopes were found both in skin and blood (Figs. 2, 3, 4, 5), suggesting that the NY-ESO-1157–165 epitope may not be immunodominant. Alternatively, Ag-specific cells may have been poorly distributed in peripheral blood and thus T cells specific for this epitope were not prevalent in that compartment. If so, the DTH response may provide a better measure of immunity to the whole protein, especially because this response relies on the integrity of the entire pathway of Ag uptake, processing, and presentation. Minor contamination with Escherichia coli proteins could also contribute to this response. This concern was somewhat allayed by the identification of NY-ESO-1 peptide-specific T cells from DTH lesions (Figs. 4 and 6) and by the low level of DTH reactivity in pts immunized with NY-ESO-1 protein alone.
Very few cancer immunotherapy studies have used recombinant protein. The trials that did have used Ags that are either normal cellular proteins or viral Ags, either alone or fused with granulocyte/macrophage colony-stimulating factor (22), keyhole limpet hemocyanin (23), or other molecules (24–28). One clinical trial using a recombinant cancer-testis Ag involved the MAGE-3 protein formulated in the adjuvant SBAS-2, containing MPL and QS21 (29). In that study, the vaccine was also well tolerated. Ab responses were reported, but cellular responses were reported for only one pt (30). Another study using recombinant MAGE-3 protein with or without the AS02B adjuvant showed that Ab responses and HLA-DP4-restricted T cell responses were induced in most pts receiving the protein with adjuvant (31). CD8+ T cell responses to MAGE-3 peptides were induced in two pts. The NY-ESO-1 IMX vaccine is therefore unique in terms of its potency and consistency and the breadth of the immune response generated. Further optimization of its efficacy by exploring other routes or schedules of administration remains possible.
The use of a recombinant protein has provided the opportunity to define additional class I and II NY-ESO-1 epitopes. This will assist evaluation of future methods for optimizing immunization of cancer pts using NY-ESO-1 and provides additional epitopes and methods for the evaluation of immune responses in future studies. Another cancer-testis Ag, LAGE-1, is highly homologous to NY-ESO-1 and shares some conserved Ab and T cell epitopes (32, 33). It is possible that the NY-ESO-1 vaccine may represent more than a monovalent vaccine and thus might also be applicable to tumors expressing LAGE-1.
This trial provides a sound immunological basis for proceeding to clinical efficacy studies in pts who have evaluable NY-ESO-1-expressing cancers. Although not designed to assess clinical impact, a preliminary observation of this trial was that pts receiving effective vaccination relapsed less frequently than pts receiving NY-ESO-1 protein alone or placebo. These clinical data should be interpreted with caution, because pts were not randomized between the NY-ESO-1 IMX vaccine and NY-ESO-1 alone treatment groups, and this analysis was retrospective and unplanned. However, this observation justifies further clinical trials to evaluate the use of NY-ESO-1 IMX vaccine for the adjuvant treatment of melanoma.
Supplementary Material
Acknowledgments
We thank the following colleagues for their contributions: C. Barrow, R. Basser, J. Bennet, J. Boyle, T. Burgess, G. Cartwright, L. Cohen, J. Davis, D. Drane, P. Gardiner, S. Gibbs, H. Goldie, G. Hartel, J. Haynes, H. Kalnins, A. Kypridis, K. Lillie, K.-A. Masterman, A. McKenzie, M. McNamara, C. Millar, L. Pugliese, M. Rubira, D. Ryan, D. Santiago, M. Scanlan, J. Skipper, P. Stewart, and E. Stockert. ISCOMATRIX is a trademark of ISCOTEC AB, a CSL Limited company. I.D.D. is supported in part by an Australian National Health and Medical Research Council Career Development Award. W.C. is supported by Wellcome Trust International Senior Research Fellow Fellowship 066646/Z/01/Z. This clinical study was sponsored and funded by the Ludwig Institute for Cancer Research. Certain proprietary rights to NY-ESO-1 have been licensed by the Ludwig Institute to CSL Limited, who participated in the clinical study through the provision of clinical reagents and additional logistical support.
Abbreviations: Ag, antigen; ICS, intracellular cytokine staining; IMX, ISCOMATRIX; pt, patient; DTH, delayed-type hypersensitivity.
Footnotes
Davis, I., Cebon, J., Parente, P., Shackleton, M., Hopkins, W., Masterman, K.-A., Jefford, M., Chen, Q., Jackson, H., Chen, W., et al. (2002) Proc. Am. Assoc. Cancer Res., p. 2774 (abstr.).
Biskamp, M., Jaeger, E., Karbach, J., Neumann, A., Jaeger, D., Gnjatic, S., Pugliese, L., Hoffman, E. W., Old, L. J. & Knuth, A. (2003) Proc. Am. Soc. Clin. Oncol., p. 706 (abstr.).
References
- 1.Scanlan, M. J., Simpson, A. J. & Old, L. J. (2004) Cancer Immun. 4, 1-15. [PubMed] [Google Scholar]
- 2.Jäger, E., Chen, Y. T., Drijfhout, J. W., Karbach, J., Ringhoffer, M., Jäger, D., Arand, M., Wada, H., Noguchi, Y., Stockert, E., et al. (1998) J. Exp. Med. 187, 265-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang, R.-F., Johnston, L., Zeng, G., Topalian, S. L., Schwartzentruber, D. J. & Rosenberg, S. A. (1998) J. Immunol. 161, 3598-3606. [PubMed] [Google Scholar]
- 4.Jäger, E., Nagata, Y., Gnjatic, S., Wada, H., Stockert, E., Karbach, J., Dunbar, P. R., Lee, S. Y., Jungbluth, A., Jäger, D., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 4760-4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jäger, E., Gnjatic, S., Nagata, Y., Stockert, E., Jäger, D., Karbach, J., Neumann, A., Rieckenberg, J., Chen, Y. T., Ritter, G., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 12198-12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jäger, E., Jäger, D., Karbach, J., Chen, Y. T., Ritter, G., Nagata, Y., Gnjatic, S., Stockert, E., Arand, M., Old, L. J., et al. (2000) J. Exp. Med. 191, 625-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gnjatic, S., Nagata, Y., Jäger, E., Stockert, E., Shankara, S., Roberts, B. L., Mazzara, G. P., Lee, S. Y., Dunbar, P. R., Dupont, B., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 10917-10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zarour, H. M., Storkus, W. J., Brusic, V., Williams, E. & Kirkwood, J. M. (2000) Cancer Res. 60, 4946-4952. [PubMed] [Google Scholar]
- 9.Zeng, G., Touloukian, C. E., Wang, X., Restifo, N. P., Rosenberg, S. A. & Wang, R. F. (2000) J. Immunol. 165, 1153-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng, G., Wang, X., Robbins, P. F., Rosenberg, S. A. & Wang, R. F. (2001) Proc. Natl. Acad. Sci. USA 98, 3964-3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jäger, E., Karbach, J., Gnjatic, S., Jäger, D., Maeurer, M., Atmaca, A., Arand, M., Skipper, J., Stockert, E., Chen, Y.-T., et al. (2002) Cancer Immun. 2, 12-24. [PubMed] [Google Scholar]
- 12.Zarour, H. M., Maillere, B., Brusic, V., Coval, K., Williams, E., Pouvelle-Moratille, S., Castelli, F., Land, S., Bennouna, J., Logan, T., et al. (2002) Cancer Res. 62, 213-218. [PubMed] [Google Scholar]
- 13.Barr, I. G. & Mitchell, G. F. (1996) Immunol. Cell Biol. 74, 8-25. [DOI] [PubMed] [Google Scholar]
- 14.Ennis, F. A., Cruz, J., Jameson, J., Klein, M., Burt, D. & Thipphawong, J. (1999) Virology 259, 256-261. [DOI] [PubMed] [Google Scholar]
- 15.Kersten, G., Drane, D., Pearse, M., Jiskot, W. & Coulter, A. (2004) in Novel Vaccination Strategies, ed. Kaufmann, S. (Wiley–VCH, Weinheim, Germany), pp. 173-196, in press.
- 16.Jung, T., Schauer, U., Heusser, C., Neumann, C. & Rieger, C. (1993) J. Immunol. Methods 159, 197-207. [DOI] [PubMed] [Google Scholar]
- 17.Jackson, H. M., Dimopoulos, N., Chen, Q., Luke, T., Tai, T. Y., Maraskovsky, E., Old, L., Davis, I. D., Cebon, J. & Chen, W. (2004) J. Immunol. Methods, in press. [DOI] [PubMed]
- 18.Chen, W., Yewdell, J. W., Levine, R. L. & Bennink, J. R. (1999) J. Exp. Med. 189, 1757-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stockert, E., Jäger, E., Chen, Y. T., Scanlan, M. J., Gout, I., Karbach, J., Arand, M., Knuth, A. & Old, L. J. (1998) J. Exp. Med. 187, 1349-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gnjatic, S., Atanackovic, D., Jäger, E., Matsuo, M., Selvakumar, A., Altorki, N. K., Maki, R. G., Dupont, B., Ritter, G., Chen, Y. T., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 8862-8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen, Q., Jackson, H., Parente, P., Luke, T., Rizkalla, M., Yai, T. Y., Zhu, H.-C., Mifsud, N. A., Dimopoulos, N., Masterman, K.-A., et al. (2004) Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 22.Tao, M. H. & Levy, R. (1993) Nature 362, 755-758. [DOI] [PubMed] [Google Scholar]
- 23.Timmerman, J. M. & Levy, R. (2000) J. Immunol. 164, 4797-4803. [DOI] [PubMed] [Google Scholar]
- 24.Karanikas, V., Hwang, L. A., Pearson, J., Ong, C. S., Apostolopoulos, V., Vaughan, H., Xing, P. X., Jamieson, G., Pietersz, G., Tait, B., et al. (1997) J. Clin. Invest. 100, 2783-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez, G., Crombet, T., Catala, M., Mirabal, V., Hernandez, J. C., Gonzalez, Y., Marinello, P., Guillen, G. & Lage, A. (1998) Ann. Oncol. 9, 431-435. [DOI] [PubMed] [Google Scholar]
- 26.Fernando, G. J., Murray, B., Zhou, J. & Frazer, I. H. (1999) Clin. Exp. Immunol. 115, 397-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fong, L., Brockstedt, D., Benike, C., Breen, J. K., Strang, G., Ruegg, C. L. & Engleman, E. G. (2001) J. Immunol. 167, 7150-7156. [DOI] [PubMed] [Google Scholar]
- 28.Staib, L., Birebent, B., Somasundaram, R., Purev, E., Braumuller, H., Leeser, C., Kuttner, N., Li, W., Zhu, D., Diao, J., et al. (2001) Int. J. Cancer 92, 79-87. [PubMed] [Google Scholar]
- 29.Marchand, M., Punt, C. J., Aamdal, S., Escudier, B., Kruit, W. H., Keilholz, U., Hakansson, L., van Baren, N., Humblet, Y., Mulders, P., et al. (2003) Eur. J. Cancer 39, 70-77. [DOI] [PubMed] [Google Scholar]
- 30.Zhang, Y., Chaux, P., Stroobant, V., Eggermont, A. M. M., Corthals, J., Maillere, B., Thielemans, K., Marchand, M., Boon, T. & van der Bruggen, P. (2003) J. Immunol. 171, 219-225. [DOI] [PubMed] [Google Scholar]
- 31.Atanackovic, D., Altorki, N. K., Stockert, E., Williamson, B., Jungbluth, A. A., Ritter, E., Santiago, D., Ferrara, C. A., Matsuo, M., Selvakumar, A., et al. (2004) J. Immunol. 172, 3289-3296. [DOI] [PubMed] [Google Scholar]
- 32.Lethé, B., Lucas, S., Michaux, L., De Smet, C., Godelaine, D., Serrano, A., De Plaen, E. & Boon, T. (1998) Int. J. Cancer 76, 903-908. [DOI] [PubMed] [Google Scholar]
- 33.Rimoldi, D., Rubio-Godoy, V., Dutoit, V., Lienard, D., Salvi, S., Guillaume, P., Speiser, D., Stockert, E., Spagnoli, G., Servis, C., et al. (2000) J. Immunol. 165, 7253-7261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.