Abstract
Ethanol causes pancreatic damage by an unknown mechanism. Previously, we demonstrated that a sustained rise of the cytosolic Ca2+ concentration ([Ca2+]i) causes pancreatic acinar cell injury. Here we have investigated the effects of ethanol and its metabolites on Ca2+ signaling in pancreatic acinar cells. Most cells exposed to ethanol (up to 850 mM) showed little or no increase in [Ca2+]i (and never at concentrations <50 mM). During sustained exposure to 850 mM ethanol, acetylcholine (ACh) evoked a normal [Ca2+]i elevation and following ACh removal there was a normal and rapid recovery to a low resting level. The oxidative metabolite acetaldehyde (up to 5 mM) had no effect, whereas the nonoxidative unsaturated metabolite palmitoleic acid ethyl ester (10–100 μM, added on top of 850 mM ethanol) induced sustained, concentration-dependent increases in [Ca2+]i that were acutely dependent on external Ca2+ and caused cell death. These actions were shared by the unsaturated metabolite arachidonic acid ethyl ester, the saturated equivalents palmitic and arachidic acid ethyl esters, and the fatty acid palmitoleic acid. In the absence of external Ca2+, releasing all Ca2+ from the endoplasmic reticulum by ACh (10 μM) or the specific Ca2+ pump inhibitor thapsigargin (2 μM) prevented such Ca2+ signal generation. We conclude that nonoxidative fatty acid metabolites, rather than ethanol itself, are responsible for the marked elevations of [Ca2+]i that mediate toxicity in the pancreatic acinar cell and that these compounds act primarily by releasing Ca2+ from the endoplasmic reticulum.
Excessive ethanol consumption is a principal cause of the common human disease acute pancreatitis (1). In severe cases, extensive pancreatic necrosis may occur, leading to multiple organ failure and death in up to one half of affected individuals (1). The speed and extent of necrosis distinguish acute pancreatitis from the chronic, progressive toxicity of ethanol in other organs (2, 3), as well as from the more indolent disease chronic pancreatitis (4). Even if common mechanisms operate, this relatively sudden pattern of necrosis remains unexplained. Recent data have implicated fatty acid ethyl esters (FAEEs), nonoxidative metabolites of ethanol, which accumulate at higher concentrations within the pancreas than elsewhere after ethanol excess and which induce pancreatic injury by an unknown mechanism (5–7).
Cytosolic Ca2+ signals are crucial for the control of pancreatic secretion (8, 9), but such signals, particularly when they are sustained, can also be toxic (10–12). In pancreatic acinar cells, Ca2+ signals are primarily generated by release of Ca2+ from the endoplasmic reticulum (ER) (9) via Ca2+ release channels controlled by inositol trisphosphate, cyclic ADP ribose, nicotinic acid adenine dinucleotide phosphate, and Ca2+ itself (13, 14). Physiological Ca2+ signals consist of repetitive cytosolic Ca2+ spikes, always initiated in the apical pole (8, 9) and mostly confined to this region (8, 13) by the perigranular mitochondria (9, 15). In contrast, hyperstimulation elicits a sustained, global [Ca2+]i rise that in turn induces pronounced premature intracellular digestive enzyme activation and vacuolization (11), nuclear factor κB activation (16), cytokine expression (17, 18), and acinar cell death (1, 17), characteristic changes of acute pancreatitis. Similar changes are induced by naturally occurring bile salts (19, 20) and pancreatic duct obstruction (21), which also induce acute pancreatitis.
Identification of the critical role of abnormal Ca2+ signaling in several models of acute pancreatitis led us to test the hypothesis that either ethanol or its metabolites induce acute pancreatic injury by initiating sustained, global [Ca2+]i rises (22). We now show that ethanol alone and the oxidative metabolite acetaldehyde have minimal or no effects on Ca2+ signaling, whereas the nonoxidative metabolite palmitoleic (C16:1) acid (POA) ethyl ester (POAEE) induces a profound, concentration-dependent elevation of [Ca2+]i that results in acinar cell necrosis. This action depends on extracellular Ca2+ and is shared by other FAEEs, both unsaturated and saturated, including palmitic (16:0), arachidonic (20:4), and arachidic (20:0) acid ethyl esters. Additionally, POA (C16:1), released from the deesterification of POAEE by intracellular hydrolases (23), produces reversible, concentration-dependent, global, and sustained [Ca2+]i elevations by emptying the ER Ca2+ store. We conclude that nonoxidative fatty acid metabolites mediate ethanol toxicity by inducing abnormal, sustained [Ca2+]i elevations that cause pancreatic acinar cell injury.
Materials and Methods
Cell Preparation and Solutions. Freshly isolated pancreatic acinar cells and acinar cell clusters of two or three cells were prepared from the pancreas of adult CD1 mice by using collagenase (Worthington) as in our previous work (9, 11, 13–15). All experiments were performed at room temperature (23–25°C), and all cells were used within 4 h of isolation. The extracellular solution contained 140 mM NaCl, 4.7 mM KCl, 1.13 mM MgCl2, 1 mM CaCl2, 10 mM d-glucose, and 10 mM Hepes (adjusted to pH 7.35 by NaOH); in some experiments CaCl2 was omitted (Ca2+-free extracellular solution). Where stated, 1 mM EGTA was added to the Ca2+-free solution (all chemicals were the highest grade available from Sigma).
[Ca2+]i Measurement. Isolated cells were loaded with Fura 2-acetoxymethyl ester (AM) (5 μM, Sigma) or Fluo 4-AM (2.5 μM, Molecular Probes) for 20–40 min at room temperature, followed by washing and incubation for up to 30 min for intracellular deesterification of the dyes. Cells were then perifused on the stage of a Nikon Diaphot inverted microscope, by using extracellular solutions with or without Ca2+ (see above). The fluorescence intensity of the cells loaded with Fura 2-AM was analyzed by using a tardis system running on MagiCal computer hardware (Visitech, Sunderland, U.K.), to determine [Ca2+]i changes in response to ethanol and its metabolites (all the highest grade available from Sigma). These included the oxidative metabolite acetaldehyde, the nonoxidative metabolites palmitic, palmitoleic, arachidic, and arachidonic acid ethyl esters, and POA under specified conditions. For Fura-2 recording, alternate excitation wavelengths of 340 and 380 nm were used, with a 540-nm filter for emission. Background subtraction was carried out independently at each of the two excitation wavelengths, and the system was calibrated with cells that were loaded with dye and exposed to solutions containing either 5 mM EGTA or 10 mM CaCl2 in the presence of 20 μM ionomycin (Sigma) (24), by using a dissociation constant of 150 nM for Ca2+-Fura at room temperature.
Confocal imaging of the cells loaded with Fluo 4-AM was also performed by using a Zeiss LSM510 system to assess the effects of POA. The fluorescence of Fluo 4 was excited with an argon 488-nm laser line, with emitted light collected by using an LP505 filter. The images collected were composed of 256 × 256 pixels, and the optical section was selected to be 5–6 μm.
Cell Toxicity Measurement. Isolated cells were incubated for 1 h at room temperature with selected ethanol metabolites. Some cells were also preincubated with the membrane-permeant Ca2+ chelator 1,2-bis(O-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA)-AM (20 μM) for 10 min, to abrogate large rises of [Ca2+]i. Cells were then incubated in 1 μM propidium iodide for 10 min and observed by confocal microscopy (Zeiss LSM510; excitation 363 nm, emission >400 nm) to detect necrotic changes (25).
Mitochondrial membrane potential changes were assessed by loading cells with 100 nM tetramethyl rhodamine methyl ester for 30 min at 37°C to measure fluorescence in the perigranular mitochondrial region as described in ref. 11 (excitation 488 nm, emission >550 nm). POA (10–50 μM) was applied for 15 min, after which the protonophore carbonylcyanide 3-chlorophenylhydrazone (10 μM) was added to induce complete mitochondrial depolarization (11).
Results
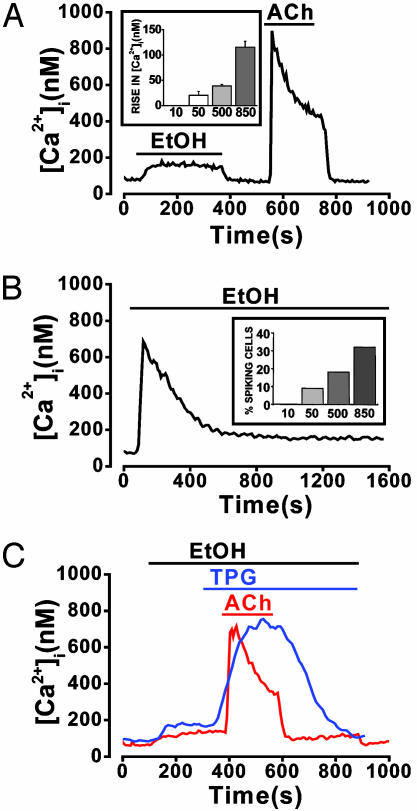
Effects of Ethanol on [Ca2+]i. Ethanol, at a concentration of 10 mM, induced no change in [Ca2+]i. At higher concentrations (50–850 mM) continuous ethanol perifusion elicited modest, variable responses. In the majority of cells (177 of 261, 68%), ethanol, even at a concentration of 850 mM, produced only small, sustained increases in [Ca2+]i (115 ± 12 nM) that were immediately reversible upon washout (Fig. 1A). For comparison, Fig. 1 A also shows a normal response to acetylcholine (ACh) (10 μM). At lower ethanol concentrations (50–500 mM), the increases in [Ca2+]i were tiny (Fig. 1 A Inset) and not always sustained. For example, of the 63 of 77 (82%) cells that responded to 500 mM ethanol with a very small nonspiking increase in [Ca2+]i, only 36 (47%) showed a sustained elevation, whereas [Ca2+]i returned to basal levels within 5 min during continued ethanol perifusion in the remaining 27 (35%) cells.
Fig. 1.
Ethanol evokes small or transient cytosolic Ca2+ signals. Shown are the typical effects of ethanol on [Ca2+]i. (A) Small sustained [Ca2+]i increase elicited in the majority of cells (177 of 261 cells, 68%) by perifusion with 850 mM ethanol. For comparison, a normal response to ACh (10 μM), obtained after ethanol washout, is also included. Inset shows the dependence of mean sustained [Ca2+]i rise on ethanol concentration (10–850 mM) (n between 15 and 36 for each concentration tested). (B) Transient increase in [Ca2+]i induced by 850 mM ethanol, seen in a minority of cells (84 of 261 cells, 32%), followed by a very small, sustained elevation over baseline. Inset shows the percentage of cells showing a transient [Ca2+]i increase (spike) plotted as a function of the ethanol (10–850 mM) concentration. (C) Typical recordings showing that 850 mM ethanol does not affect cytosolic Ca2+ signals elicited by ACh (10 μM, 22 of 22 cells) or TPG (2 μM, 8 of 8 cells) in the absence of external Ca2+.
In a minority of cells (84 of 261, 32%) perifused with 850 mM ethanol, a sharp, transient “spike” increase in [Ca2+]i (533 ± 30 nM) was observed that declined to a smaller resting level comparable to that of the small sustained increase found in nonspiking cells (Fig. 1B). This transient increase also occurred with lower concentrations of ethanol, but the probability of obtaining such a spike was diminished in a concentration-dependent manner (Fig. 1B Inset).
During continuous exposure of acinar cells to ethanol (850 mM), in the absence of external Ca2+, the Ca2+ release response to ACh (10 μM) was normal, and removal of ACh resulted in a normal rapid reduction of [Ca2+]i to the prestimulation level (Fig. 1C). This result shows that the ACh signal-transduction pathway was fully functional and also that the ER Ca2+ pumps were not blocked by the high ethanol concentration. Thapsigargin (TPG) (2 μM) also elicited a normal [Ca2+]i rise during continued exposure to ethanol (850 mM) (Fig. 1C), and because this occurred in the absence of external Ca2+, the source must have been the internal ER Ca2+ store, which therefore could not have been emptied by ethanol. These data indicate that acute exposure to even a very high ethanol concentration (850 mM) leaves the Ca2+ handling capacity of the acinar cells surprisingly intact.
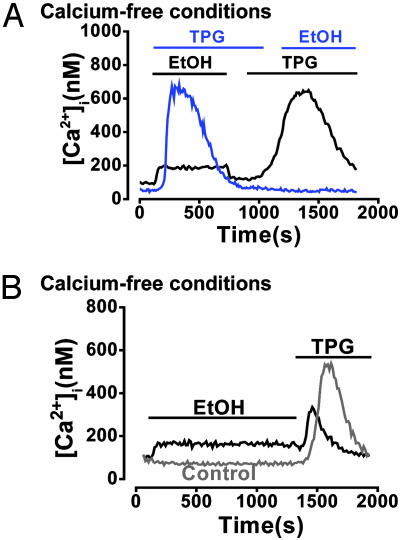
Prior depletion of intracellular Ca2+ stores with 2 μM TPG under Ca2+-free conditions abolished the response to 850 mM ethanol (11 of 11 cells; Fig. 2A). However, when ethanol was applied 10 min before TPG, the converse was not true (17 of 17 cells; Fig. 2 A). A more prolonged application (20 min) of 850 mM ethanol in a Ca2+-free solution significantly diminished the [Ca2+]i increase subsequently elicited by 2 μM TPG [Fig. 2B; 145 ± 35 nM (n = 12) after ethanol compared with 498 ± 47 nM (n = 16) in control cells not exposed to ethanol].
Fig. 2.
Ethanol releases Ca2+ from a TPG-sensitive (ER) intracellular store. (A) In the absence of external Ca2+, prior depletion of ER stores with 2 μM TPG abolished the normally occurring [Ca2+]i response to 850 mM ethanol (blue trace; 11 of 11 cells). Prior addition of ethanol, however, did not abolish a subsequent response to TPG (black trace; 17 of 17 cells). (B) Typical recordings showing that in the absence of external Ca2+, prolonged (20 min) exposure to ethanol results in a significant (*, P < 0.05) reduction of the [Ca2+]i rise elicited by TPG compared with time-matched controls (n = 12 and 16, respectively).
Effects of Ethanol Metabolites on [Ca2+]i. Acetaldehyde (5 mM), an oxidative metabolite of ethanol implicated in alcoholic liver disease (2), produced no detectable [Ca2+]i increase in six separate preparations. We therefore turned our attention to nonoxidative ethanol metabolites.
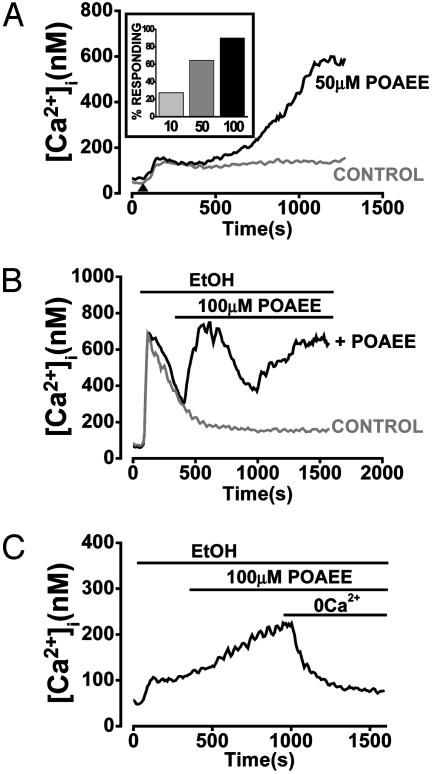
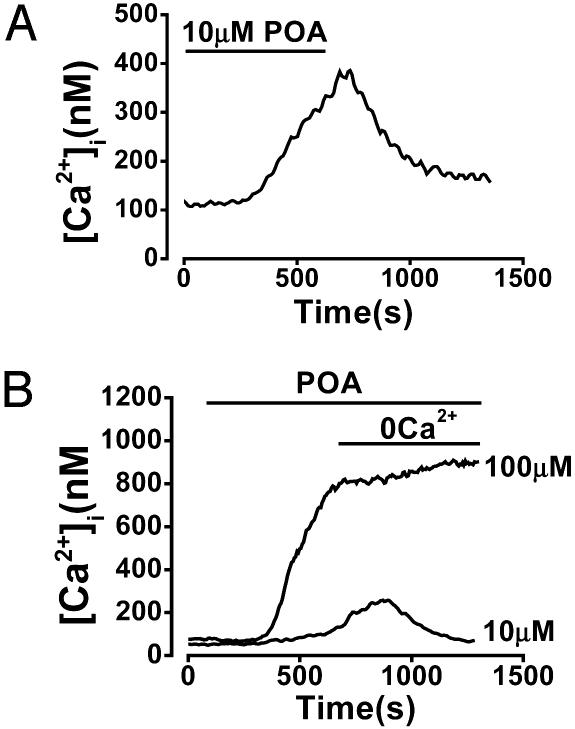
The lowest concentration of ethanol found to maintain FAEEs in aqueous solution was 850 mM. Therefore, this ethanol concentration was used throughout all subsequent experiments with these substances. Under these conditions, POAEE (10–100 μM), an unsaturated nonoxidative metabolite of ethanol, induced a concentration-dependent and sustained increase in [Ca2+]i (Fig. 3 A and B), reaching 546 ± 36 nM at the highest concentration tested (100 μM). This elevation was maintained for the duration of the experimental protocol (up to 20 min; Fig. 3 A and B), and the probability of obtaining such a response was directly related to the applied POAEE concentration (Fig. 3A Inset). The [Ca2+]i increase was reversible when extracellular Ca2+ was withdrawn from the perfusion medium in 20 of 22 cells tested (Fig. 3C). The control (850 mM ethanol) traces in Fig. 3 A and B show the typical ethanol effects demonstrated in Fig. 1 A and B.
Fig. 3.
POAEE evokes a large [Ca2+]i rise, which is sustained in the presence of external Ca2+. (A) POAEE (50 μM, in the presence of 850 mM ethanol) elicits a substantial [Ca2+]i increase compared with the small elevation typically observed in response to 850 mM ethanol alone (control). Inset shows that the probability of cells responding to POAEE, in the presence of ethanol, depends on the POAEE concentration (10–100 μM). (B) In a “minority” cell that shows a single transient (spike) response to ethanol (control trace) (see Fig. 1B), POAEE (100 μM, in the presence of 850 mM ethanol) elicits a continuing quasi-sinusoidal [Ca2+]i elevation. (C) Typical recording showing that the sustained [Ca2+]i increase elicited by 100 μM POAEE (in the presence of 850 mM ethanol) is reversed upon withdrawal of external Ca2+ (20 of 22 cells).
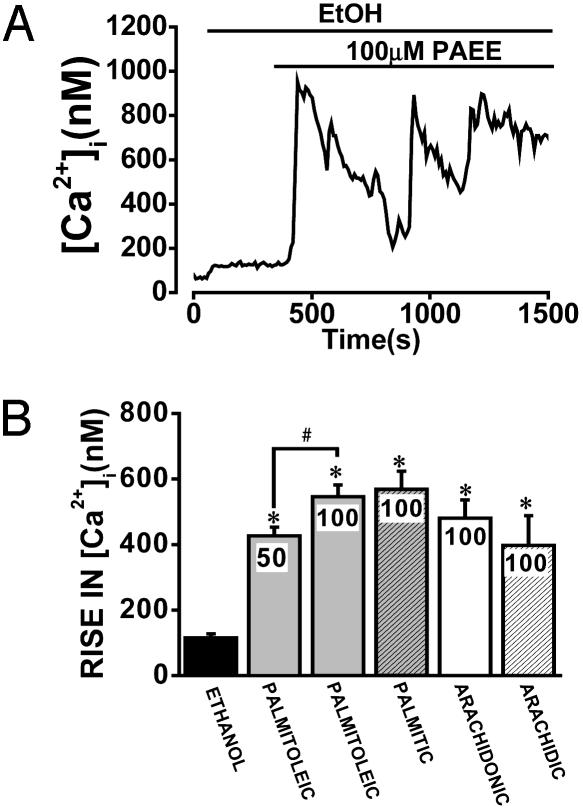
Palmitic acid ethyl ester (100 μM), a saturated nonoxidative metabolite of ethanol, induced a sustained [Ca2+]i elevation that was not significantly different from that of an equivalent concentration of POAEE (Fig. 4 A and B). The ability of FAEEs to increase [Ca2+]i was not confined to C16 fatty acid carbon chain length, because both the unsaturated arachidonic acid ethyl ester and the saturated arachidic acid ethyl ester, derived from C20 fatty acids, elicited similar [Ca2+]i elevations (Fig. 4B).
Fig. 4.
The saturated FAEE palmitic acid ethyl ester elicits a marked and sustained (oscillating) [Ca2+]i increase. (A) Typical recording showing that palmitic acid ethyl ester induces a large, quasi-sinusoidal, and sustained [Ca2+]i rise in the presence of ethanol (850 mM). (B) Mean data showing the comparative increases in [Ca2+]i elicited by 850 mM ethanol alone and by various FAEEs in the presence of 850 mM ethanol (n = 14–49 cells for each ester; *, P < 0.05 versus ethanol alone; #, P < 0.05 for 50 μM versus 100 μM POAEE).
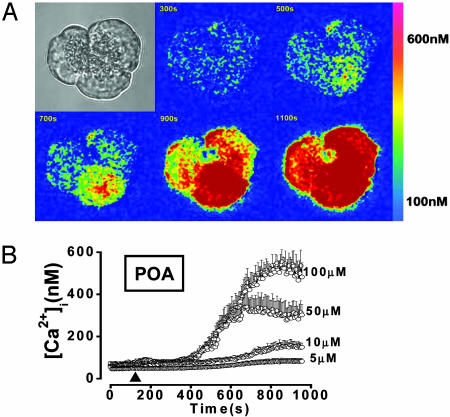
Effects of POA on [Ca2+]i. POA (5–100 μM in aqueous solution without ethanol) induced a concentration-dependent, slowly rising, and sustained (up to 30 min) increase in [Ca2+]i (Fig. 5 A and B), reaching 557 ± 46 nM at the highest concentration tested (100 μM; 12 of 12 cells responded). The response to POA was always reversible on washout (six of six cells; Fig. 6A).
Fig. 5.
POA evokes a global and sustained [Ca2+]i rise. Effects of different POA concentrations on [Ca2+]i. (A) Shown is the typical pattern of [Ca2+]i changes in an acinar cell triplet (transmitted light image in upper left corner) perifused with 100 μM POA. An increase in [Ca2+]i is denoted by a change from a “cold” color (blue) to a “warmer” color (through yellow to red) (see color scale on the right). The Ca2+ signal starts in the bottom cell and then spreads, first to the cell on the right and then also to the cell on the left. (B) Dose dependence of mean [Ca2+]i elevations (vertical bars = SEM) in response to 5, 10, 50, and 100 μM POA in the presence of external Ca2+.
Fig. 6.
Reversibility of POA effect. The POA-elicited rise in [Ca2+]i can or cannot be reversed by removal of external Ca2+ depending on stimulus intensity. (A) Typical effect of washout of 10 μM POA 10 min after the start of perifusion, resulting in return of [Ca2+]i to the basal level. (B) Typical effect of withdrawal of external Ca2+ (and addition of 1 mM EGTA) 10 min after the start of perifusion with 10 μM POA, resulting in return of [Ca2+]i to the basal level. In contrast, the [Ca2+]i rise elicited by 100 μM POA could not be reversed by removal of external Ca2+.
When external Ca2+ was removed early in the response to 10 μM POA (often still during the ascending [Ca2+]i phase), the increase in [Ca2+]i was completely reversed (Fig. 6B; five of five cells). However, when external Ca2+ was removed later, during the plateau phase of the response, [Ca2+]i did not return to the baseline in 20 of 22 cells. Moreover, no reversal of the induced [Ca2+]i increase was observed when extracellular Ca2+ was removed after 10 min of perifusion with 100 μM POA (Fig. 6B; nine of nine cells).
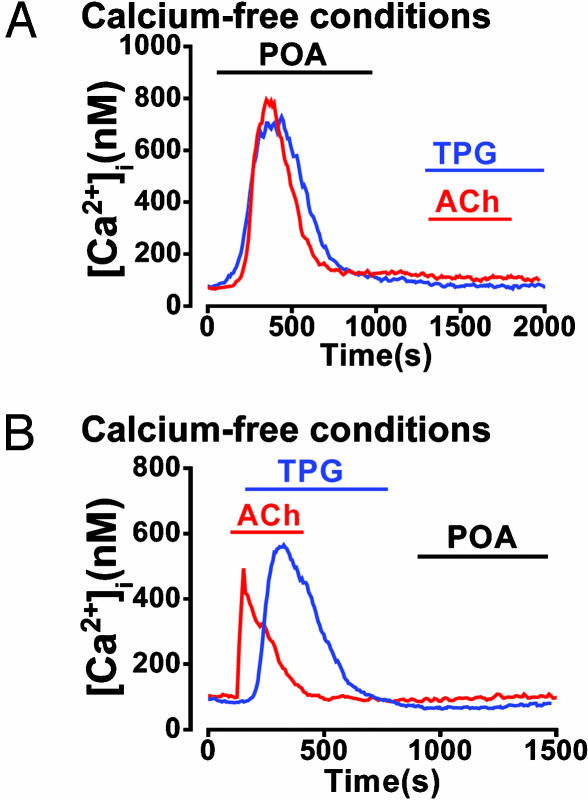
In the nominal absence of external Ca2+ (no EGTA), POA (50 μM) elicited a transient [Ca2+]i rise; i.e., [Ca2+]i returned to basal levels within 10 min (Fig. 7A), most likely because of depletion of intracellular stores. Consistent with this interpretation, a subsequent application of ACh (10 μM) or TPG (2 μM) produced no further Ca2+ release (Fig. 7A; 11 of 11 cells and 10 of 10 cells, respectively). Similarly, when ACh (10 μM) or TPG (2 μM) were applied first to induce depletion of internal Ca2+ stores, subsequent application of 50 μM POA induced no further [Ca2+]i change (Fig. 7B; 12 of 12 cells and 9 of 9 cells, respectively).
Fig. 7.
POA releases Ca2+ from a TPG-sensitive store (ER). Effects of POA, TPG, and ACh on Ca2+ store depletion in a Ca2+-free external solution. (A) Typical effect of 50 μM POA on Ca2+ release, producing a large but transient [Ca2+]i elevation, after which ACh (10 μM) or TPG (2 μM) did not produce further Ca2+ release. (B) Effects of ACh and TPG stimulation, which produced their characteristic transient elevations in [Ca2+]i, after which 50 μM POA induced no further Ca2+ release.
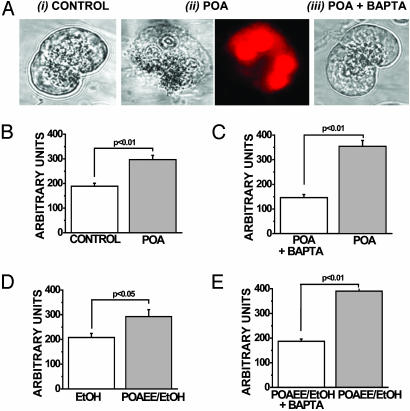
Effects of Ethanol Metabolites on Cell Survival. Incubation with 100 μM POA for 1 h induced significant cellular necrosis, as seen directly by inspection of light-transmitted images (Fig. 8A) and also shown by an increase in fluorescence intensity from nuclear uptake of propidium iodide, compared with time-matched controls in the absence of POA (Fig. 8 A and B; n = 9). Pretreatment of the cells with the membrane-permeant Ca2+ chelator BAPTA-AM prevented the deleterious effects of POA (Fig. 8 A and C). POAEE (100 μM) in the presence of 850 mM ethanol also significantly increased the probability of cell necrosis (very similar images to those shown for POA; Fig. 8A) compared with time-matched controls exposed to ethanol alone (Fig. 8D; n = 9). Again, the deleterious effects of POAEE were prevented by pretreatment of the cells with BAPTA-AM (Fig. 8E).
Fig. 8.
POAEE and POA induce Ca2+-dependent cellular necrosis. (A) Typical light-transmitted and fluorescence images of normal control-isolated acinar cells after a 1-h incubation in normal Hepes solution (i), cells exposed to 100 μM POA (also showing propidium iodide staining of the four nuclei) (ii), and cells exposed to 100 μM POA after prior incubation with 20 μM BAPTA-AM (iii). Similar necrotic changes, observed in light-transmitted and fluorescence images, occurred after exposure of acinar cells to 100 μM POAEE and 850 mM ethanol together (as compared with ethanol alone) that were also prevented by BAPTA pretreatment. (B) Fluorescence intensity after propidium iodide staining of cells after a 1-h exposure to 100 μM POA compared with controls (n = 9). (C) Protective effect of prior incubation with 20 μM BAPTA-AM on POA effect (n = 9). (D) Fluorescence intensity after propidium iodide staining of cells after a 1-h exposure to 100 μM POAEE and 850 mM ethanol together compared with ethanol (850 mM) alone (n = 9). (E) Protective effect of prior incubation with 20 μM BAPTA-AM on the POAEE-induced effect (n = 9).
In a separate series of experiments assessing changes in the electrical potential across the inner mitochondrial membrane by measuring tetramethyl rhodamine methyl ester fluorescence (11), exposure of the cells to POA (50 μM) for 15 min evoked complete mitochondrial depolarization, because no further fluorescence change was evoked by subsequent application of the protonophore carbonylcyanide 3-chlorophenylhydrazone (10 μM) (nine of nine cells). However, 10 μM POA induced only a partial depolarization (50 ± 4% of maximal effect; n = 10), because subsequent addition of carbonylcyanide 3-chlorophenylhydrazone elicited a further, complete depolarization.
Discussion
FAEEs elicited large and sustained [Ca2+]i elevations (Figs. 3 and 4), a previously identified critical early step in the development of pancreatic acinar cell injury (11, 12). The [Ca2+]i increases were concentration-dependent and reversible upon removal of extracellular Ca2+ (Fig. 3C). The deesterification product POA, rapidly formed from the intracellular hydrolysis of FAEEs in vivo (23), also induced substantial, concentration-dependent [Ca2+]i increases (Figs. 5 and 6); however, at high concentrations of POA these increases were remarkably resistant to reversal upon removal of extracellular Ca2+ (Fig. 6B).
In contrast, ethanol itself had surprisingly small effects on cellular Ca2+ homeostasis. The [Ca2+]i changes elicited by even a very high ethanol concentration (850 mM) were typically very small (Figs. 1, 2, 3, 4), although large, but transient, responses were observed in a minority of cells (Figs. 1B and 3B). Ethanol, at a concentration of <500 mM, had hardly any effect on [Ca2+]i (Fig. 1), which is remarkable because blood ethanol levels in intoxicated humans typically reach only 50–100 mM (26).
The marked [Ca2+]i elevation elicited by FAEEs or POA was toxic, as seen directly by inspection of light-transmitted images and also demonstrated by a significantly enhanced propidium iodide uptake, which could be prevented by the membrane-permeant Ca2+ chelator BAPTA-AM (Fig. 8). Furthermore POA, at a high concentration (50 μM), caused complete mitochondrial depolarization, which would prevent mitochondrial ATP formation, accounting for the irreversible [Ca2+]i elevation caused by a high concentration of this fatty acid (Fig. 6B).
Our use of ethanol to render FAEEs soluble in the perifusion fluid has parallels with the extracellular environment of intoxicated humans, in whom blood FAEE concentrations may build up to levels of 5–50 μM during alcohol intoxication (5, 26, 27). Although the direct effects of ethanol on [Ca2+]i are relatively small, they may contribute to pancreatic injury. It is known that alcohols sensitize the acinar cell to the effects of hyperstimulation (28, 29), whereas ethanol alone appears not to result in significant pancreatic injury (30). However, within several hours of an ethanol infusion in the rat, temporary pancreatic edema, acinar cell trypsinogen activation, and apical vacuolization occur (7). These changes are associated with the formation of FAEEs within the pancreas (6, 7), and when oxidative ethanol metabolism is inhibited, FAEE concentrations rise, and the resultant injury is more severe (6, 7). Acute pancreatic injury has also been observed after infusions of FAEEs (5); furthermore, the addition of unsaturated fat together with ethanol to animal diets results in premature digestive enzyme activation, acinar cell injury, an inflammatory cell infiltrate, focal necrosis, and the development of chronic injury (30). On the other hand, acetaldehyde did not elicit any changes in [Ca2+]i in our experiments. Oxidative ethanol metabolism in the pancreas may be protective, at least in the short term, by preventing formation of locally toxic FAEEs (6, 7). Similarly, FAEEs, but not acetaldehyde, promote pancreatic fibrosis, at least in part by inhibiting degradation of extracellular matrix proteins (31).
Abnormal, prolonged, global elevations of [Ca2+]i initiate premature intracellular digestive enzyme activation and vacuolization, early changes of pancreatic acinar cell injury, both in vitro within isolated living pancreatic acinar cells exposed to hyperstimulation (11, 12), TPG (11), or bile salts (19, 20) and in vivo within the duct-obstructed pancreas (21). The abnormal [Ca2+]i elevations may induce Ca2+ release from zymogen granules, which prompts unfolding and premature activation of digestive enzymes (11, 32). Characteristic accompanying changes include cytoskeletal disruption (33, 34), basolateral secretion (35), nuclear factor κB activation (16), and up-regulation of acinar cell cytokine expression (17, 18). These changes are followed in vivo by pancreatic swelling, leukocyte infiltration, and acinar cell necrosis (1, 5–7, 35, 36) but can be prevented in vitro and in vivo by attenuation of the [Ca2+]i elevations with the calcium chelator BAPTA (11, 12, 21). Our elucidation of the effects of FAEEs on pancreatic acinar cell Ca2+ signaling supports the hypothesis that these molecules mediate pancreatic injury from ethanol by provoking an abnormal, prolonged [Ca2+]i elevation (22), an effect primarily caused by release of Ca2+ from the unified ER store in the acinar cells (37) that in turn triggers Ca2+ entry (8). Abrogation of the toxic effects of FAEEs, as well as POA, by prior incubation with the Ca2+ chelator BAPTA (Fig. 8) confirms mediation by abnormal [Ca2+]i elevations, rather than by nonspecific actions.
The pancreas is capable of synthesizing more FAEEs than any other human organ (27). In acutely intoxicated subjects, the pancreas may contain concentrations in excess of 100 μM of both unsaturated and saturated FAEEs of various carbon chain lengths, including the C16 and C20 fatty acid compounds evaluated in this study. Within the cell, FAEEs readily bind to the inner mitochondrial membrane (38) and undergo hydrolysis (23), producing fatty acids that are important fuels but in excess dissipate energy either by altering ion channel function or by uncoupling oxidative phosphorylation (39). High levels of fatty acids, such as POA, are thus likely to be generated preferentially in pancreatic tissue (23) as a result of ethanol-induced production of FAEEs by means of the nonoxidative pathway, exerting effects both on mitochondrial function and cellular Ca2+ handling as demonstrated here. Moreover, cytosolic Ca2+ overload typically causes massive mitochondrial Ca2+ uptake, which further impairs oxidative phosphorylation (40, 41).
The sustained [Ca2+]i elevation elicited by hyperstimulation (11) or exposure to bile salts (19, 20) was shown to be acutely dependent on Ca2+ entry, therefore appearing to be a crucial element in the initiation of pancreatic autodigestion (11, 42). Ca2+ entry is important for the FAEE action, because the [Ca2+]i rise elicited by these compounds can be reversed by removal of external Ca2+ (Fig. 3C). These data agree with our earlier proposal that a store-operated Ca2+ entry channel (43) could be an attractive target for pharmacological preventive action (11, 42). However, the global sustained [Ca2+]i elevations elicited by higher POA concentrations can continue in the absence of external Ca2+ (Fig. 6B), probably because of lack of ATP (complete mitochondrial depolarization) and/or inhibition, malfunction, or destruction of the plasma membrane Ca2+ pump (44). Ca2+ channel blockade would therefore have no effect on such responses. This finding indicates the magnitude of the challenge presented by severe acute pancreatitis from excess ethanol.
Acknowledgments
This work was supported by Programme, Cooperative, and Component Grants from the Medical Research Council (U.K.). M.G.T.R. received a fellowship from the Royal College of Surgeons of England. O.H.P. is a Medical Research Council Research Professor.
Abbreviations: ACh, acetylcholine; AM, acetoxymethyl ester; BAPTA, 1,2-bis(O-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; ER, endoplasmic reticulum; [Ca2+]i, free ionized cytosolic calcium concentration; FAEE, fatty acid ethyl ester; POA, palmitoleic acid; POAEE, palmitoleic acid ethyl ester; TPG, thapsigargin.
References
- 1.Neoptolemos, J. P., Raraty, M., Finch, M. & Sutton, R. (1998) Gut 42, 886-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoek, J. B., Cahill, A. & Pastorino, J. G. (2002) Gastroenterology 122, 2049-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lieber, C. S. (1995) N. Engl. J. Med. 333, 1058-1065. [DOI] [PubMed] [Google Scholar]
- 4.Etemad, B. & Whitcomb, D.C. (2001) Gastroenterology 120, 682-707. [DOI] [PubMed] [Google Scholar]
- 5.Werner, J., Laposata, M., Fernández-del Castillo, C., Saghir, M., Iozzo, R. V., Lewandrowski, K. B. & Warshaw, A. L. (1997) Gastroenterology 113, 286-294. [DOI] [PubMed] [Google Scholar]
- 6.Werner, J., Saghir, M., Fernández-del Castillo, C., Warshaw, A. L. & Laposata, M. (2001) Surgery 129, 736-744. [DOI] [PubMed] [Google Scholar]
- 7.Werner, J., Saghir, M., Warshaw, A. L., Lewandrowski, K. B., Laposata, M., Iozzo, R. V., Carter, E. A., Schatz, R. J. & Fernández-del Castillo, C. (2002) Am. J. Physiol. 283, G65-G73. [DOI] [PubMed] [Google Scholar]
- 8.Ashby, M. & Tepikin, A. V. (2002) Physiol. Rev. 82, 701-734. [DOI] [PubMed] [Google Scholar]
- 9.Park, M. K., Lee, M. & Petersen, O. H. (2004) Cell Calcium 35, 367-379. [DOI] [PubMed] [Google Scholar]
- 10.Nicotera, P., Bellomo, G. & Orrenius, S. (1992) Annu. Rev. Pharmacol. Toxicol. 32, 449-470. [DOI] [PubMed] [Google Scholar]
- 11.Raraty, M., Ward, J., Erdemli, G., Vaillant, C., Neoptolemos, J. P., Sutton, R. & Petersen, O. H. (2000) Proc. Natl. Acad. Sci. USA 97, 13126-13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruger, B., Albrecht, E. & Lerch, M. M. (2000) Am. J. Pathol. 157, 43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cancela, J. M., Van Coppenolle, F., Galione, A., Tepikin, A. V. & Petersen, O. H. (2002) EMBO J. 21, 909-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashby, M. C., Craske, M., Park, M. K., Gerasimenko, O. V., Burgoyne, R. D., Petersen, O. H. & Tepikin, A. V. (2002) J. Cell Biol. 158, 283-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tinel, H., Cancela, J. M., Mogami, H., Gerasimenko, J. V., Gerasimenko, O. V., Tepikin, A. V. & Petersen, O. H. (1999) EMBO J. 18, 4999-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han, B. & Logsdon, C. D. (2000) Am. J. Physiol. 278, C344-C351. [DOI] [PubMed] [Google Scholar]
- 17.Gukovskaya, A. S., Gukovsky, I., Zaninovic, V., Song, M., Sandoval, D., Gukovsky, S. & Pandol, S. J. (1997) J. Clin. Invest. 100, 1853-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gukovskaya, A. S., Mouria, M., Gukovsky, I., Reyes, C., Kasho, V. N., Faller, L. D. & Pandol, S. J. (2002) Gastroenterology 122, 106-118. [DOI] [PubMed] [Google Scholar]
- 19.Voronina, S., Longbottom, R., Sutton, R., Petersen, O. H. & Tepikin, A. (2002) J. Physiol. 540, 49-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, J. Y., Kim, K. H., Lee, J. A., Namkung, W., Sun, A.-Q., Ananthanarayanan, M., Suchy, F. J., Shin, D. M., Muallem, S. & Lee, M. G. (2002) Gastroenterology 122, 1941-1953. [DOI] [PubMed] [Google Scholar]
- 21.Mooren, F. C., Hlouschek, V., Finkes, T., Turi, S., Weber, I. A., Singh, J., Domschke, W., Schnekenburger, J., Kruger, B. & Lerch, M. M. (2003) J. Biol. Chem. 278, 9361-9369. [DOI] [PubMed] [Google Scholar]
- 22.Ward, J. B., Petersen, O. H., Jenkins, S. A. & Sutton, R. (1995) Lancet 346, 1016-1019. [DOI] [PubMed] [Google Scholar]
- 23.Diczfalusy, M. A., Björkhem, I., Einarsson, C., Hillebrant, C.-G. & Alexson, S. E. H. (2001) J. Lipid Res. 42, 1025-1032. [PubMed] [Google Scholar]
- 24.Grynkiewicz, G., Poenie, M. & Tsien, R. Y. (1985) J. Biol. Chem. 260, 3440-3450. [PubMed] [Google Scholar]
- 25.Gerasimenko, J. V., Gerasimenko, O. V., Palejwala, A., Tepikin, A. V., Petersen, O. H. & Watson, A. J. (2002) J. Cell Sci. 115, 485-497. [DOI] [PubMed] [Google Scholar]
- 26.Soderberg, B. L., Salem, R. O., Best, C. A., Cluette-Brown, J. E. & Laposata, M. (2003) Am. J. Clin. Pathol. 119, Suppl., S94-S99. [DOI] [PubMed] [Google Scholar]
- 27.Laposata, E. A. & Lange, L. G. (1986) Science 231, 497-499. [DOI] [PubMed] [Google Scholar]
- 28.Katz, M., Carangelo, R., Miller, L. J. & Gorelick, F. (1996) Am. J. Physiol. 270, G171-G175. [DOI] [PubMed] [Google Scholar]
- 29.Lu, Z., Karne, S., Kolodecik, T. & Gorelick, F. S. (2002) Am. J. Physiol. 282, G501-G507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kono, H., Nakagami, M., Rusyn, I., Connor, H. D., Stefanovic, B., Brenner, D. A., Mason, R. P., Arteel, G. E. & Thurman, R. G. (2001) Am. J. Physiol. 280, G1178-G1186. [DOI] [PubMed] [Google Scholar]
- 31.Lugea, A., Gukovsky, I., Gukovskaya, A. S. & Pandol, S. J. (2003) Gastroenterology 125, 1845-1859. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen, T., Chin, W.-C. & Verdugo, P. (1998) Nature 395, 908-912. [DOI] [PubMed] [Google Scholar]
- 33.O'Konski, M. S. & Pandol, S. J. (1990) J. Clin. Invest. 86, 1649-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beil, M., Leser, J., Lutz, M. P., Gukovskaya, A., Seufferlein, T., Lynch, G., Pandol, S. J. & Adler, G. (2002) Am. J. Physiol. 282, G450-G460. [DOI] [PubMed] [Google Scholar]
- 35.Gaisano, H. Y., Lutz, M. P., Leser, J., Sheu, L., Lynch, G., Tang, L., Tamori, Y., Trimble, W. S. & Salapatek, A. M. (2001) J. Clin. Invest. 108, 1597-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gukovskaya, A. S., Vaquero, E., Zaninovic, V., Gorelick, F. S., Lusis, A. J., Brennan, M. L., Holland, S. & Pandol, S. J. (2002) Gastroenterology 122, 974-984. [DOI] [PubMed] [Google Scholar]
- 37.Petersen, O. H., Tepikin, A. V. & Park, M. K. (2001) Trends Neurosci. 24, 271-276. [DOI] [PubMed] [Google Scholar]
- 38.Lange, L. G. & Sobel, B. E. (1983) J. Clin. Invest. 72, 724-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Penzo, D., Tagliapietra, C., Colonna, R., Petronilli, V. & Bernardi, P. (2002) Biochem. Biophys. Acta 1555, 160-165. [DOI] [PubMed] [Google Scholar]
- 40.Di Lisa, F. & Bernardi, P. (1998) Mol. Cell. Biochem. 184, 379-391. [PubMed] [Google Scholar]
- 41.Kroemer, G. & Reed, J. C. (2000) Nat. Med. 6, 513-519. [DOI] [PubMed] [Google Scholar]
- 42.Parekh, A. (2000) Proc. Natl. Acad. Sci. USA 97, 12933-12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parekh, A. & Penner, R. (1997) Physiol. Rev. 77, 901-930. [DOI] [PubMed] [Google Scholar]
- 44.Orrenius, S., Zhivotovsky, B. & Nicotera, P. (2003) Nat. Rev. Mol. Cell Biol. 4, 552-565. [DOI] [PubMed] [Google Scholar]