Abstract
Varicella-zoster virus (VZV) establishes latency in sensory ganglia and causes herpes zoster upon reactivation. These investigations in a nonobese diabetic severe combined immunodeficient mouse-human neural cell model showed that VZV infected both neurons and glial cells and spread efficiently from cell to cell in vivo. Neural cell morphology and protein synthesis were preserved, in contrast to destruction of epithelial cells by VZV. Expression of VZV genes in neural cells was characterized by nuclear retention of the major viral transactivating protein and a block in synthesis of the predominant envelope glycoprotein. The attenuated VZV vaccine strain retained infectivity for neurons and glial cells in vivo. VZV gene expression in differentiated human neural cells in vivo differs from neural infection by herpes simplex virus, which is characterized by latency-associated transcripts, and from lytic VZV replication in skin. The chimeric nonobese diabetic severe combined immunodeficient mouse model may be useful for investigating other neurotropic human viruses.
Varicella-zoster virus (VZV) is a human alphaherpesvirus that causes varicella and herpes zoster (1–4). VZV has a linear, double-stranded DNA genome encoding at least 70 proteins, including immediate early (IE) regulatory proteins that control viral gene transcription, early genes, such as viral kinases, and late genes, which are predominantly viral glycoproteins that comprise the virion envelope. Like all alphaherpesviruses, VZV establishes latency in neural cells within sensory ganglia. VZV reactivation results in herpes zoster, associated with postherpetic neuralgia in the elderly and dissemination to lungs, liver, and brain in immunocompromised individuals (5). Although most VZV morbidity results from its capacity to infect neural cells, investigating neurotropism has been difficult because the virus is a highly human-specific pathogen (2, 4). Herpes simplex virus 1 produces latency-associated antisense mRNA transcripts (LATs) in neuronal cell nuclei, and viral proteins are not detected in human autopsy specimens or animal models of persistent infection (6). In contrast, VZV has no LAT sequence, and transcription or translation of VZV genes encoding the IE major transactivating protein, IE62, the IE63 coregulatory protein, and ORFs 4, 21, 29, 40, and 66 has been reported in ganglia (2, 4, 7–18).
Recently, Uchida et al. (19) described proliferation and differentiation of human CNS stem cells into neurons and glial cells in mice with nonobese diabetic severe combined immunodeficiency (NOD-SCID). Transfer of these cells into the lateral ventricle results in site-appropriate engraftment within the subventricular zone (SVZ), self-renewal, migration, and differentiation to human neurons and glia. The expanding populations migrate throughout the brain from the SVZ to the olfactory bulb, express human neuronal and glial cell markers, and develop axonal processes. Skin and thymus/liver xenografts in SCID mice permit analysis of VZV tropism for human epidermal and dermal cells and T cells within their intact tissue microenvironments in vivo (20–24). Therefore, we used the chimeric NOD-SCID mouse–human neural cell model to investigate VZV infection of differentiated neural cells in vivo. We compared the parent Oka virus (pOka), a low passage clinical isolate, to the vaccine Oka virus (vOka), a pOka derivative used to make live attenuated varicella vaccines, because it is not known whether vOka has reduced infectivity for neural cells (25–27). We also evaluated a VZV deletion mutant, rOkaΔ70, that lacks one copy of the gene encoding IE63, because neural cell expression of IE63 has been prominent in autopsy and animal studies (9, 13, 15). This mutant, and two other single-copy ORF63/70 variants, exhibits no deficiencies in cell culture and is as virulent as the parent virus in human skin xenografts in SCID mice (24),
These experiments in the NOD-SCID mouse–human neural cell model showed that VZV infection with pOka, vOka, and rOkaΔ70 was characterized by efficient spread in human neurons and glial cells, without disrupting cell morphology or expression of neural cell adhesion molecules (hNCAMs), glial fibrillary acidic protein (hGFAP), or neuron-specific class III β-tubulin in vivo. VZV infection of human neural cells exhibited a distinctive intracellular distribution of the IE regulatory proteins and a block in synthesis of the major envelope glycoprotein, gE, compared to lytic VZV infection of epidermal and dermal cells in vivo.
Materials and Methods
NOD-SCID Mice with Neural Cell Xenografts. Processing of fetal brain tissue and the isolation of human CNS stem cells by flow cytometry were carried out as described (19, 28). Fetal brain tissues of 16–20 gestational weeks were used. Sorted CD133+CD24-/lo cells were plated at a density of 105 cells per ml in human neurosphere culture media consisting of X-vivo 15 medium (BioWhittaker), N-2 supplement (GIBCO), and 0.2 mg/ml heparin supplemented with basic fibroblast growth factor (20 ng/ml), epidermal growth factor (20 ng/ml), and leukemia inhibitory factor (10 ng/ml). Sorted cells form neurospheres within 10–14 days in culture. Neurospheres were passaged by enzymatic dissociation into single cells for 5–10 min in collagenase (0.5 mg/ml in PBS containing 0.1% human serum albumin). Neurosphere cultures were passaged every 10–14 days. Cryoanesthetized neonatal mice (<24 h) were placed on a stereotaxic device (Stoelting) and injected with 2 μl of cells (105 cells) into both lateral ventricles (19). Human brain tissue was obtained in accordance with state and federal guidelines.
VZV Infection of NOD-SCID Mice. NOD-SCID mice transplanted with human neurosphere cells (StemCells Inc.) were inoculated with VZV-infected human melanoma cells at 4–6 months after transplant. Viruses tested were pOka, live attenuated vOka (Merck), and rOkaΔ70, a deletion mutant with only one copy of the gene encoding IE63 (24). Melanoma cells were grown in tissue culture medium (DMEM, GIBCO) supplemented with heat-inactivated FCS. VZV-infected melanoma cells were trypsinized when 75% of cells showed cytopathic changes; cells from one T75 flask were resuspended in 1 ml of PBS. The inoculum for mouse injection was 10 μl of this virus per cell suspension, containing ≈2.0 × 105 cells and ≈2.0–8.0 × 103 plaque-forming units of infectious virus. Narcotized animals were placed on a stereotaxic device (Stoelting), and VZV-infected cell preparation (10 μl) was injected into each lateral ventricle. Stereotaxic coordinates were 0.4 mm posterior to Bregma, 2.2 mm deep, and ±1.0 mm lateral (29). Animals were cared for according to Animal Welfare Act PL 94-279, with protocols approved by the Stanford University Administrative Panel on Laboratory Animal Care.
Immunohistochemistry. The murine mAb and polyclonal antisera used to evaluate cellular and viral protein expression included: anti-human nuclei, mouse mAb, human-specific nuclear stain, 1:200 (Chemicon) (30); anti-hNCAM (CD56), mouse mAb to two isoforms, 145 and 185 kDa, of human neural cell adhesion molecule, 1:200 (Zymed) (31); anti-HMB45, mouse mAb to human melanosome antigen in the cytoplasm of melanoma cells, 1:500 (Dako) (32); anti-hGFAP, mouse mAb, cytoplasmic staining of human glial cells, 1:2,000 (StemCells Inc.); anti-neuronal class III β-tubulin, rabbit antiserum to neuron-specific class III β-tubulin in mouse and human neurons, 1:500 (Covance, Princeton, NJ) (33); anti-active caspase-3, rabbit antiserum to active human or mouse caspase-3, 1:200 (Becton Dickinson-Pharmingen) (34); and anti-nestin, rabbit antiserum to human nestin intermediate filaments expressed in developing CNS cells, 1:500 (Chemicon). Primary antibodies to VZV-infected cell proteins were rabbit antisera to IE62 and IE63 (a gift from Paul Kinchington, University of Pittsburgh, Pittsburgh), ORF47 kinase (20), and gE (35). GK is a high titer human polyclonal VZV antiserum. Secondary antibodies were donkey antisera, conjugated with FITC (green), Cy3 (red), or Cy5 (blue) and diluted 1:500 (Jackson ImmunoResearch).
Confocal Analysis of Mouse Brain Sections. Three weeks after VZV inoculation, mice were perfused with PBS followed by 4% paraformaldehyde (PFA) in PBS. Mouse brains were removed and fixed in 4% PFA/PBS for 2 days and dehydrated in a 30% sucrose/PBS solution for 3 days. Fixed brains were sectioned sagitally at 40-μm thickness with a freezing microtome. Sections were stored at -20°C in cryoprotectant solution. Each mouse brain yielded at least eight sections containing human neural cells. Brain sections were rinsed with Tris-buffered saline (TBS) and blocked for 30 min at room temperature in TBS with 10% donkey serum and 0.3% Triton X-100 (TBS+). Sections were incubated at 4°C overnight with primary antibodies, diluted in TBS+, washed, and incubated at 4°C overnight with the secondary fluorochrome-conjugated antibodies (Jackson ImmunoResearch) diluted in TBS+. Sections were washed, wet-mounted by using polyvinyl alcohol-1,4 diazabicyclo [2.2.2] octane (36), and evaluated with a Zeiss510 confocal laser-scanning device attached to a Zeiss-Axiovert microscope and LSM510 software. Appropriate gain and black-level settings were determined on control tissues stained with secondary antibodies alone. Upper and lower thresholds were set by using the range indicator function to minimize data loss caused by saturation.
Skin Xenografts. As described (22), skin implants were inoculated with VZV-infected MRC-5 cells (50 μl) 4–6 weeks after implantation and harvested 3 weeks later. Sections were blocked in TBS (10% goat serum/0.3% Triton X-100) overnight, incubated in TBS (3% goat serum/0.3% Triton X-100) with primary antisera and washed. Slides were incubated in TBS (3% goat serum/0.3% Triton X-100) with biotin-labeled goat secondary antibody (1:1,000) (Vector Laboratories), washed with TBS, and incubated in TBS with diluted alkaline phosphatase-conjugated streptavidin (1:400) (Jackson ImmunoResearch). Slides were treated with FastRed (Sigma) and counterstained with hematoxylin.
VZV-Infected Melanoma Cells. Infected melanoma cells were fixed in 4% paraformaldehyde, washed with PBS, incubated overnight at 4°C in PBS (10% donkey serum/0.3% Triton X-100), and incubated in PBS (3% donkey serum/0.3% Triton X-100) with primary antisera at 4°C overnight. Slides were washed and incubated in PBS (3% donkey serum/0.3% Triton X-100) with fluorochrome-conjugated secondary antibodies (Jackson ImmunoResearch), washed, and mounted with Vectashield plus 4′, 6-diamidino-2-phenylindole.
Results
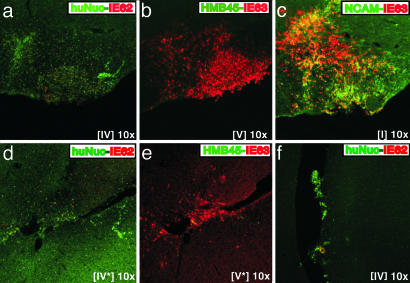
Infection of Human Neural Cells by pOka and vOka in Vivo. Extensive expression of IE62 and IE63 proteins was detected by 3 weeks after inoculation of VZV-infected melanoma cells into the ventricles of NOD-SCID mice with human neural cell xenografts (Fig. 1). Alternate mouse brain sections containing human neural cells were stained with antibodies against IE62 or IE63 (red), in combination with markers that stained human cell nuclei, hNCAM, or the melanoma cell marker HMB45 (green). IE62 and IE63 proteins were detected whether the infecting virus was the clinical isolate, pOka (Fig. 1 a–c) or the attenuated varicella vaccine strain, vOka (Fig. 1 d and e). VZV-infected cells were evident within the mouse brain in regions containing cells that stained with anti-human nuclei mAb (huNuc) (Fig. 1 a and d) and anti-hNCAM (Fig. 1c). Dual expression of IE63 and hNCAM was detected in many cells (Fig. 1c). Cells expressing IE proteins did not stain with the melanoma marker HMB45 (Fig. 1 b and e). Invasion of mouse brain by melanoma cells was not observed and anti-hNCAM did not react with melanoma cells. IE62 and IE63 expression in engrafted cells expressing the human nuclei marker or hNCAM was confirmed in 11 alternate sections examined after pOka inoculation and eight alternate sections after vOka inoculation.
Fig. 1.
Infection of human neural cells by VZV pOka and vOka in vivo. Alternate mouse brain sections prepared 3 weeks after infection with pOka (a–c and f) or vOka (d and e) were stained with anti-human nuclei mAb (green/FITC) in combination with polyclonal rabbit antiserum to IE62 (red/Cy3) (a, d, and f) the melanoma cell marker anti-HMB45 mAb (green/FITC) in combination with polyclonal rabbit antisera to IE63 (red/Cy3) (b and e), or anti-hNCAM mAb (green/FITC) in combination with polyclonal rabbit antisera to IE63 (red/Cy3) (c). Roman numerals indicate section numbers.
Residual melanoma cells were found within ventricles in a few sections. These cells were identified with anti-human nuclei mAb (Fig. 1f) and anti-HMB45 (data not shown). A few surviving melanoma cells expressed IE62 protein (Fig. 1f). Mouse brain tissue beyond the areas of hNCAM-positive or human nuclei-positive cells showed no reactivity with antibodies to human nuclei, HMB45, IE62, or IE63.
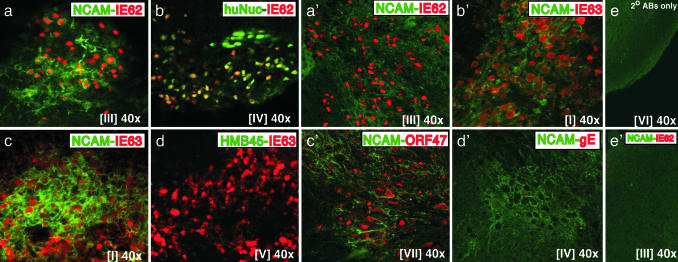
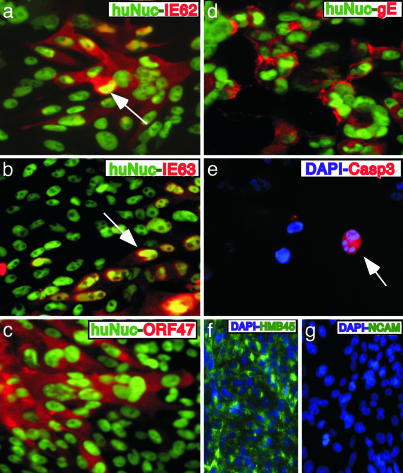
Expression of IE62 and IE63 in VZV-Infected Human Neural Cells. The IE62 and IE63 regulatory proteins showed different patterns of intracellular localization within human neural cells, as illustrated for pOka-infected cells (Fig. 2). IE62 exhibited a distinct nuclear localization (red) and colocalized with the human nuclei marker (green) (Fig. 2b). In cells showing dual expression of IE62 (red) and hNCAM (green), which were sectioned in a plane that included nuclei and membranes, IE62 expression was separated from the cell membranes expressing hNCAM by a cytoplasmic zone in which IE62 was not detected (Fig. 2a). In dual positive cells, IE63 staining extended to the hNCAM-expressing cell membranes, with no zone of separation, suggesting that IE63 was present in the cytoplasm as well as in nuclei (Fig. 2 c and d). In these and other experiments, secondary antibodies did not crossreact with VZV-infected human neural cell xenografts (Fig. 2e).
Fig. 2.
Expression of IE62, IE63, ORF47 kinase, and gE in VZV-infected human neural cells in vivo. Mouse brain sections prepared 3 weeks after infection with pOka (a–e) or rOkaΔ70 (a′–e′) were stained with anti-hNCAM mAb (green/FITC) (a, c, and a′–e′), anti-human nuclei mAb (green/FITC) (b), or the melanoma cell marker, anti-HMB45 mAb (green/FITC) (d), in combination with polyclonal rabbit antisera to IE62 (red/Cy3) (a, b, and a′), IE63 (red/Cy3) (c, d, and b′), ORF47 (red/Cy3) (c′), or gE (red/Cy3) (d′). No nonspecific staining was detected with secondary antibodies within the same region (e) or anti-hNCAM and anti-IE62 primary antibodies (e′). Roman numerals indicate section numbers.
Expression of IE62 and IE63 in Human Neural Cells Infected with rOkaΔ70. IE63 is an essential VZV protein encoded by identical genes, ORF63/70. Our hypothesis was that VZV neurotropism might require both copies. However, IE62 and IE63 proteins showed the same patterns of intracellular localization after inoculation of the single-copy OR63 mutant, rOkaΔ70, compared to pOka or vOka, which have two copies of ORF63. IE62 and IE63 (red) were expressed extensively in hNCAM-positive cells (green) within rOkaΔ70-infected xenografts. IE62 localized to the nuclei, as it did in human neural cells infected with pOka or vOka (Fig. 2a′). As observed with pOka and vOka, IE63 was detected diffusely in the cytoplasm and nuclei (Fig. 2b′). Primary antibodies did not crossreact with mouse brain (Fig. 2e′). These patterns were confirmed in 13 mouse brain sections examined after rOkaΔ70 inoculation.
Expression of Early and Late VZV Proteins in Human Neural Cells. The VZV ORF47 protein kinase, a viral serine/threonine kinase, is expressed during the second, or early, phase of the VZV replication cycle (37). ORF47 protein was detected abundantly within the cytoplasm and nuclei of human neural cells in vivo, as illustrated with rOkaΔ70 (Fig. 2c′). VZV glycoprotein E (gE) is an essential late protein that is expressed on cell membranes during lytic infection and is a predominant component of the virion envelope (2, 35). VZV gE was detected very rarely in human neural cells. Limited gE expression was observed in only a few cells within regions that contained abundant hNCAM-positive cells (Fig. 2d′) and that showed IE62, IE63, and ORF47 protein expression on alternate sections. These observations indicated that VZV replication was blocked at the third, or late, phase in infected neural cells.
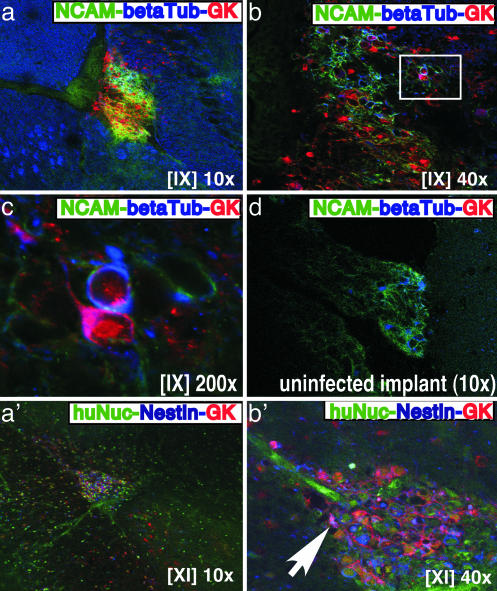
Analysis of Human Neural Cells Infected with VZV. Alternate sections were stained for hNCAM (green), neuron-specific class III β-tubulin (blue), which is present in the cytoplasm of human and mouse neurons, and VZV proteins, using the polyclonal human anti-VZV antiserum, GK (red) (Fig. 3 a–c). Mouse neurons expressing β-tubulin III were detected in mouse brain tissue surrounding the human neural cell xenograft (Fig. 3a). Because anti-β-tubulin III also stained melanoma cells, the triple stain included anti-hNCAM, which did not crossreact with mouse brain or melanoma cells. Two infected cells have been highlighted that expressed hNCAM (green) on the cell surface, β-tubulin III (blue) in the cytoplasm, and VZV proteins (red) (Fig. 3 b and c). One of these cells had VZV protein expression visible along an axonal structure and over the neural cell body. The polyclonal anti-VZV antiserum, GK, did not crossreact with an uninfected human neural cell xenograft that contained hNCAM-positive cells (Fig. 3d).
Fig. 3.
Analysis of human neural cells infected with VZV in vivo.(a–c, a′, and b′) Mouse brain sections prepared 3 weeks after infection with rOkaΔ70 were triple-stained with anti-hNCAM mAb (green/FITC), polyclonal rabbit anti-serum to β-tubulin III (blue/Cy5), and human antiserum against VZV (GK) (red/Cy3). (d) A control uninfected neural cell xenograft section showed staining with anti-hNCAM and anti-β-tubulin III but not anti-VZV antiserum (GK). (a′ and b′) Triple staining with anti-human nuclei (green/FITC), polyclonal rabbit antiserum to nestin (blue/Cy5), and anti-VZV antiserum GK (red/Cy3) is shown. Roman numerals indicate section numbers.
These sections showed VZV protein expression in cells that did not express hNCAM, indicating that other neural cell types were infected with VZV in vivo. Nestin is a cytoplasmic marker for early-stage, undifferentiated human neural cells. When sections were stained for human nuclei (green), nestin (blue), and VZV proteins (red) (Fig. 3 a′ and b′), coexpression of nestin and VZV proteins, resulting in a purple signal, was observed, indicating that VZV could infect immature human neural cells in vivo (Fig. 3b′).
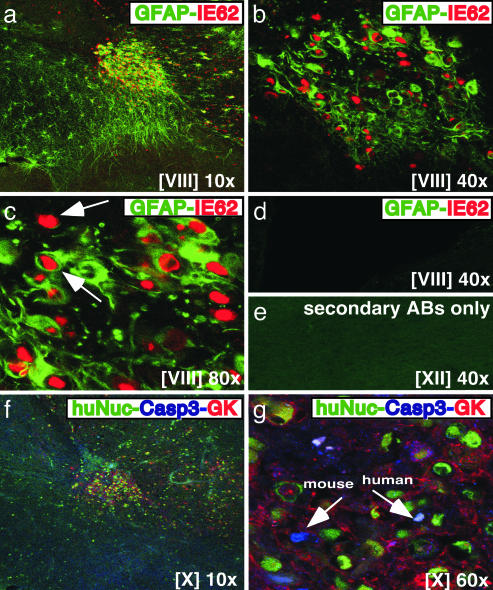
Neural cell xenografts in the NOD-SCID mice contained cells that expressed hGFAP, found in glial cell cytoplasm (Fig. 4). When sections were stained with hGFAP and IE62, cells that expressed hGFAP (green) and IE62 (red) were identified within the same area at low magnification (Fig. 4a). Higher magnifications showed that many hGFAP-positive cells were infected with VZV in vivo (Fig. 4 b and c). As was observed in hNCAM-positive cells, IE62 showed a distinct nuclear localization in hGFAP-positive cells (Fig. 4 b and c). Some IE62-positive cells were not hGFAP positive, which was because of the presence of VZV-infected neurons, expressing hNCAM and β-tubulin III, within this region. Primary antibodies to hGFAP and IE62 did not cause nonspecific staining (Fig. 4d); anti-hGFAP antiserum did not crossreact with melanoma cells.
Fig. 4.
Analysis of human neural cells infected with VZV for expression of GFAP and evidence of apoptosis. (a–d) Mouse brain sections prepared 3 weeks after infection with rOkaΔ70 were stained with anti-hGFAP mAb (green/FITC) and polyclonal rabbit antiserum to IE62 (red/Cy3). (d) No nonspecific staining was observed in an uninfected xenograft. (f and g) Triple staining was done with anti-human nuclei mAb (green/FITC), polyclonal rabbit antiserum against caspase 3 active form (blue/Cy5), and anti-VZV antiserum, GK (red/Cy3). (e) Secondary antibodies did not stain nonspecifically. Roman numerals indicate section numbers.
Apoptosis within VZV-Infected Human Neural Cell Xenografts. To analyze whether infected human neural cells showed evidence of apoptosis, sections were stained with antibodies against human nuclei (green), caspase 3 active form (blue), and VZV proteins, using polyclonal anti-VZV antiserum, GK (red) (Fig. 4 f and g). Only a few VZV-infected human neural cells expressed caspase 3; a few mouse brain cells also stained with anti-caspase 3, which is not human specific (Fig. 4g). Secondary antibodies did not crossreact with VZV-infected human neural cells when the same area was stained on an alternate section (Fig. 4e).
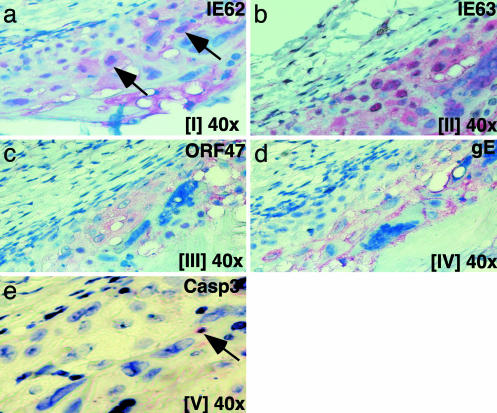
VZV Protein Expression in Infected Skin Xenografts. VZV causes lytic infection of human dermal and epidermal cells in skin xenografts in vivo (20–24, 38). To compare VZV protein expression in human neural and skin cells in vivo, skin xenografts were collected 3 weeks after VZV infection, and alternate sections were examined for IE62, IE63, ORF47 kinase, or gE synthesis (Fig. 5). In contrast to neural cell xenografts, IE62 was detected in both the cytoplasm and nuclei of VZV-infected skin cells and was present in small, dense bodies within the nucleus (Fig. 5a). Intense IE63 expression was prominent in nuclei of VZV-infected cells; cytoplasmic expression was also observed, although much less than in neural cells (Fig. 5b). The ORF47 protein kinase was abundant and predominantly cytoplasmic (Fig. 5c). In contrast to VZV-infected human neural cells, gE expression was extensive, with high concentrations along the plasma membranes of VZV-infected skin cells (Fig. 5d). Some skin cells expressed caspase 3 active form, suggesting apoptosis (Fig. 5e).
Fig. 5.
Analysis of VZV protein expression in infected skin xenografts. Alternate sections of human skin prepared 3 weeks after infection with rOka were stained with polyclonal antiserum against IE62 (a), IE63 (b), ORF47 (c), gE (d), or caspase 3 active form (red/FastRed) (e); counterstain was hematoxylin/eosin. Roman numerals indicate section numbers.
VZV Protein Expression in Infected Melanoma Cells. IE62, IE63, ORF47 protein, and gE expression was evaluated 4 days after VZV infection of melanoma cells. During this lytic infection, IE62 was abundant in cytoplasm, in contrast to its nuclear localization in VZV-infected neural cells in vivo. IE62 was present within distinct small, dense nuclear bodies within nuclei, as in VZV-infected skin cells (Fig. 6a). IE63 showed substantial nuclear localization, as in VZV-infected skin, and in contrast to its extensive cytoplasmic distribution in VZV-infected neural cells (Fig. 6b). ORF47 protein was cytoplasmic (Fig. 6c). As in VZV-infected skin cells, abundant gE expression was detected along plasma membranes and more diffusely in cytoplasm (Fig. 6d). Uninfected melanoma cells were induced to undergo apoptosis with UV light, as a positive control for anti-caspase 3 antibody (Fig. 6e); melanoma cells stain positive for HMB45 (Fig. 6f); and absence of hNCAM expression by human melanoma cells was confirmed (Fig. 6g).
Fig. 6.
VZV protein expression in infected melanoma cells in vitro. (a–d) Melanoma cells infected with rOka were stained with anti-human nuclei (green/FITC) and polyclonal rabbit antiserum against IE62 (a), IE63 (b), ORF47 (c), or gE (red/Texas red) (d). (e) Melanoma cells induced to undergo apoptosis by UV light were stained with polyclonal antiserum to caspase 3 active form (red/Texas red); counterstain 4′,6-diamidino-2-phenylindole (DAPI) (blue). Melanoma cells were stained with anti-HMB45 mAb (green/FITC) and counterstain DAPI (blue/Cy5) (f), or anti-hNCAM mAb (green/FITC) and counterstain DAPI (blue) (g).
Discussion
VZV infection in the chimeric NOD-SCID mouse–human neural cell model provided a unique opportunity to examine the initial encounter between VZV and human neural cells in vivo. These experiments demonstrated that VZV spreads efficiently between neural cells in vivo, without changing cellular morphology or expression of neural cell proteins, including hNCAM, hGFAP, and β-tubulin III. VZV gene expression in neural cells differed dramatically from VZV infection of epidermal and dermal cells in human skin in vivo, which is associated with cell destruction and release of infectious virions (20–24). IE62, the major viral transactivating protein and an important component of the virion tegument (38, 39), was restricted to the nuclei of neurons and glial cells, whereas IE62 localized to the cytoplasm of infected skin cells. IE63 was abundant in the cytoplasm of neurons and glial cells, whether expressed by low-passage pOka or the single-copy rOkaΔ70 recombinant, which differed from its predominant nuclear expression in VZV-infected skin cells. In contrast, the essential late glycoprotein, gE, was detected in only a very few neural cells, which was strikingly different from the extensive expression of gE in skin cells. The block in glycoprotein synthesis in neural cells occurred despite synthesis of the ORF47 protein kinase, which phosphorylates IE62, IE63, and gE, and is required for VZV infection of skin and T cells in vivo (20, 37, 40). The patterns of IE62, IE63, ORF47, and gE synthesis and distribution found with lytic VZV infection of skin were observed in cultured melanoma cells, which also produce infectious VZ virions in vitro. These observations support the concept that infection of neural cells by VZV, in which viral gene expression occurs, but is limited to IE and early genes in neural cells, differs fundamentally from herpes simplex virus, in which viral proteins are not detected in human autopsy specimens (6).
In this analysis of VZV neuropathogenesis, human neural cells were infected with VZV in vivo and fixed in situ before removal of tissues. The observation that IE proteins were expressed in neural cells in the NOD-SCID mouse–human neural cell model while gE synthesis was reduced serves to validate reports about VZV gene transcription and translation in neural cells within human sensory ganglia obtained at autopsy and in animal models (2, 4, 7–18). Conclusions from autopsy specimens have been questioned because VZV gene expression might be triggered during the time required to obtain tissues, and infection of nonhuman neural cells in animals may not reproduce the VZV pathogenesis in the human host accurately. VZV gene expression was similar in human neurons, glial cells, and early-stage, less differentiated neural cells in vivo. Thus, VZV appears to regulate viral proteins and abort the full replicative cycle in various types of cells of neural origin. The conditions for blocking late gene expression were established even though VZV gained access to neural cells by transfer from cells with the capacity to produce infectious virus, rather than by transport along nerve cell axons. The generalized nature of this genetic program is also suggested by VZV infection of guinea pig enteric neurons in vitro, in which ORF62 and early genes were transcribed, but gE mRNA was not detected (7). Importantly, although VZV-specific T cell immunity is needed to control VZV reactivation as zoster, these experiments in the NOD-SCID mouse model suggest that the nonlytic interaction of VZV with neural cells is established independently of adaptive host immunity. It will be of interest to evaluate modulation of viral gene expression by innate neural cell responses in this model.
Expression of the IE63 regulatory protein was extensive and predominantly cytoplasmic in the NOD-SCID mouse–human neural cell model, as has been reported in autopsy and animal studies (9, 13, 15). Cytoplasmic retention of IE63 could interfere with efficient transcription of VZV glycoprotein genes in neural cells (3, 4, 40). A single copy of ORF63 was sufficient to support VZV infection of neural cells in vivo. In these experiments, IE62 was restricted to the nuclei of neurons and glial cells, rather than to the cytoplasm, as was found in an autopsy study (13). This difference in IE62 localization could reflect an earlier phase of VZV infection of neural cells, as opposed to prolonged latency during which further modulation of virus–host cell interactions may occur. Both patterns contrast with the distribution of IE62 to cytoplasm and nuclei in productively infected skin cells in vivo. Either IE62 nuclear exclusion or retention can block VZV replication, because IE62 is the key transactivating factor for viral gene transcription in the nucleus, and a major component of the virion tegument, which is assembled in the cytoplasm (2, 39).
Although the genetic basis of vOka attenuation has not been determined, vOka and pOka genomes have nucleotide differences predicted to change amino acid residues in all classes of viral proteins (2, 41, 42). Although vOka can cause herpes zoster, it seems to reactivate less often than wild-type VZV (25, 26). Herpes zoster may be less common after vaccination because initial access of vOka to neural cells is reduced by limited skin replication, or vOka reactivation and secondary viral infection of skin is less efficient, rather than because of an intrinsic attenuation of vOka neurotropism. Whereas vOka has a diminished capacity to replicate in skin, vOka remains unattenuated in T cells in vivo (23). Our initial experiments suggest that deriving vOka by passage of pOka in fibroblasts does not appear to have altered infectivity for neural cells, which can be further explored in the NOD-SCID mouse–human neural cell model.
In summary, our observations provide evidence for unique patterns of VZV gene expression in human neural cells. These experiments support the concept that the chimeric NOD-SCID mouse–human neural cell model will be valuable for investigating the neurotropism of other human viruses, such as HIV, in differentiated human neural cells in vivo.
Acknowledgments
We thank Dr. Brent Harris, Dartmouth Medical School, Hanover, NH, for expert technical assistance. This work was supported by National Institute of Allergy and Infectious Diseases Grants AI-36884 and AI-053846 and Deutsche Forschungsgemeinschaft Fellowship BA 2035/1-1 (to A.B.).
Abbreviations: VZV, varicella-zoster virus; IE, immediate early; NOD-SCID, nonobese diabetic severe combined immunodeficiency; pOka, parent Oka virus; vOka, vaccine Oka virus; hNCAM, human neural cell adhesion molecule; hGFAP, human glial fibrillary acidic protein; TBS, Tris-buffered saline; gE, glycoprotein E.
References
- 1.Arvin, A. M. (2001) in Field's Virology, eds. Knipe, D. M. & Howley, P. M. (Lippincott-Williams & Wilkins, Philadelphia), pp. 2731-2767.
- 2.Cohen, J. & Strauss, S. (2001) in Field's Virology, eds. Knipe, D. M. & Howley, P. M. (Lippincott-Williams & Wilkins, Philadelphia), pp. 2547-2586.
- 3.Kinchington, P. R. (1999) Front. Biosci. 4, 200-211. [DOI] [PubMed] [Google Scholar]
- 4.Silverstein, S. & Straus, S. E. (2000) in Varicella-Zoster Virus: Virology and Clinical Management, eds. Arvin, A. M. & Gershon, A. A. (Cambridge Univ. Press, Cambridge, U.K.), pp. 123-141.
- 5.Gilden, D. H., Kleinschmidt-DeMasters, B. K., LaGuardia, J. J., Mahalingam, R. & Cohrs, R. J. (2000) N. Engl. J. Med. 2, 635-645. [DOI] [PubMed] [Google Scholar]
- 6.Roizman, B. & Knipe, D. M. (2001) in Field's Virology, eds. Knipe, D. M. & Howley, P. M. (Lippincott-Williams & Wilkins, Philadelphia), pp. 2399-2459.
- 7.Chen, J. J., Gershon, A. A. Li, Z. S., Lungu, O. & Gershon, M. D. (2003) J. Med. Virol. 70, 71-82. [DOI] [PubMed] [Google Scholar]
- 8.Cohrs, R. J., Gilden, D. H., Kinchington, P. R., Grinfeld, E. & Kennedy, P. G. E. (2003) J. Virol. 77, 6660-6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debrus, S., Sadzot-Delvaux, C., Nikkels, A. F., Piette, J. & Rentier, B. (1995) J. Virol. 69, 3240-3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy, P. G., Grinfeld, E. & Gow, J. W. (1998) Proc. Natl. Acad. Sci. USA 95, 4658-4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy, P. G., Grinfeld, E. & Bell, J. E. (2000) J. Virol. 74, 11893-11898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy, P. G., Grinfeld, E., Bontems, S. & Sadzot-Delvaux, C. (2001) Virology 289, 218-223. [DOI] [PubMed] [Google Scholar]
- 13.Lungu, O., Panagiotidis, C. A., Annuziato, P. W., Gershon, A. A. & Silverstein, S. J. (1998) Proc. Natl. Acad. Sci. USA 95, 7080-7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahalingam, R., Wellish, M., Wolf, W., Dueland, A. N., Cohrs, R., Vafai, A. & Gilden, D. (1990) N. Engl. J. Med. 323, 627-631. [DOI] [PubMed] [Google Scholar]
- 15.Maghalingam, R., Wellish, M., Cohrs, R., Debrus, S., Piette, J., Renier, B. & Gilden, D. H. (1996) Proc. Natl. Acad. Sci. USA 93, 2122-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meier, J. L., Holman, R. P., Croen, K. D., Smialek, J. E. & Straus, S. E. (1993) Virology 193, 193-200. [DOI] [PubMed] [Google Scholar]
- 17.Pevenstein, S. R., Williams, R. K., McChesney, D., Mont, E. K., Smialek, J. E. & Straus, S. E. (1999) J. Virol. 73, 10514-10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato, H., Pesnicak, L. & Cohen, J. L. (2002) J. Virol. 76, 3575-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uchida, N., Buck, D. W., He, D., Reitsma, M. J., Masek, M., Phan, T. V., Tsukamoto, A. S., Gage, F. H. & Weissman, I. L. (2000) Proc. Natl. Acad. Sci. USA 97, 14720-14725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Besser, J., Sommer, M. H., Zerboni, L., Bagowski, C., Ito, H., Moffat, J., Ku, C.-C. & Arvin, A. M. (2003) J. Virol. 77, 5964-5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito, H., Sommer, M. H., Zerboni, L. H., He, H., Boucaud, D., Hay, J., Ruyechan, W. & Arvin, A. M. (2003) J. Virol. 77, 489-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moffat, J. F., Stein, M. D., Kaneshima, H. & Arvin, A. M. (1995) J. Virol. 69, 5236-5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moffat, J., Zerboni, L., Stein, M., Grose, C., Kaneshima, H. & Arvin, A. M. (1998) J. Virol. 72, 965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sommer, M. H., Zagha, E., Serrano, O. K., Ku, C. C., Zerboni. L., Baiker, A., Santos, R., Spengler, M., Lynch, J., Grose, C., et al. (2001) J. Virol. 75, 8224-8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Annunziato, P. A. & Gershon, A. A. (2000) in Varicella-Zoster Virus: Virology and Clinical Management, eds. Arvin, A. M. & Gershon, A. A. (Cambridge Univ. Press, New York), pp. 460-476.
- 26.Gershon, A. A., LaRussa, P., Hardy, I., Steinberg, S. & Silverstein, S. (1992) J. Infect. Dis. S63-S68. [DOI] [PubMed]
- 27.Takahashi, M., Otsuka, T., Okuno, Y., Asano, Y. & Yazaki, T. (1974) Lancet 2, 1288-1290. [DOI] [PubMed] [Google Scholar]
- 28.Tamaki, S., Eckert, K., He, D., Sutton, R., Dohse, M., Jain, G., Tushinski, R., Reitsma, M., Harris, B., Tsukamoto, A., et al. (2002) J. Neurosci. Res. 69, 976-986. [DOI] [PubMed] [Google Scholar]
- 29.Davidson, B. L., Stein, C. S., Heth, J. A., Martins, I., Koitin, R. M., Derksen, T. A., Zabner, J., Ghodsi, A. & Chiorini, J. A. (2000) Proc. Natl. Acad. Sci. USA 97, 3428-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vescovi, A. L., Parati, E. A., Gritti, A., Poulin, P., Ferrario, M., Wanke, E., Frolichsthal-Schoeller, P., Cova, L., Arcellana-Panlilio, M., Colombo, A., et al. (1999) Exp. Neurol. 156, 71-83. [DOI] [PubMed] [Google Scholar]
- 31.Schol, D. J., Mooi, W. J., van der Gugten, A. A., Wagenaar, S. S. & Hilgers, J. (1988). Int. J. Cancer S2, 33-40. [DOI] [PubMed] [Google Scholar]
- 32.Leong, A. S. & Millos, J. (1989) Surg. Pathol. 2, 137-141. [Google Scholar]
- 33.Lee, M. K., Tuttle, J. B., Rebhuhn, L. I., Cleveland, D. W. & Frankfurter, A. (1990) Cell Motil. Cytoskeleton 17, 118-132. [DOI] [PubMed] [Google Scholar]
- 34.Patel, T., Gores, G. J. & Kaufmann, S. H. (1996) FASEB J. 10, 587-597. [DOI] [PubMed] [Google Scholar]
- 35.Mo, C., Lee, J., Sommer, M., Grose, C. & Arvin, A. M. (2002) Virology 304, 176-186. [DOI] [PubMed] [Google Scholar]
- 36.Valnes, K. & Brandtzaeg, P. (1985) J. Histochem. Cytochem. 33, 755-761. [DOI] [PubMed] [Google Scholar]
- 37.Kenyon, T. K., Lynch, J., Hay, J., Ruyechan, W. & Grose, C. (2001) J. Virol. 75, 8854-8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato, B., Ito, H., Hinchliffe, S., Sommer, M. H., Zerboni, L. & Arvin, A. M. (2003) J. Virol. 77, 5607-5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinchington, P. R., Fite, K. & Turse, S. E. (2000) J. Virol. 74, 2265-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lynch, J. M., Kenyon, T. K., Grose, C., Hay, J. & Ruyechan, W. T. (2002) Virology 302, 71-82. [DOI] [PubMed] [Google Scholar]
- 41.Argaw, T., Cohen, J. I., Klutch, M., Lekstrom, K., Yoshikawa, T., Asano, Y. & Krause, P. R. (2002) J. Infect. Dis. 81, 1153-1157. [DOI] [PubMed] [Google Scholar]
- 42.Gomi, Y., Imagawa, T., Takahashi, M. & Yamanishi, K. (2000) J. Med. Virol. 61, 497-503. [DOI] [PubMed] [Google Scholar]