Abstract
We report that Emx2 homeogene is expressed at the mRNA and protein levels in the adult mouse olfactory neuroepithelium. As expected for a transcription factor, Emx2 is present in the nucleus of immature and mature olfactory sensory neurons. However, the protein is also detected in the axonal compartment of these neurons, both in the olfactory mucosa axon bundles and in axon terminals within the olfactory bulb. Emx2 axonal staining is heterogeneous, suggesting an association with particles. Subcellular fractionations of olfactory bulb synaptosomes, combined with chemical lesions of olfactory neurons, confirm the presence of Emx2 in axon terminals. Significant amounts of Emx2 protein cosediment with high density synaptosomal subfractions containing eukaryotic translation initiation factor 4E (eIF4E). Nonionic detergents and RNase treatments failed to detach eIF4E and Emx2 from these high-density fractions enriched in vesicles and granular structures. In addition, Emx2 and eIF4E can be coimmunoprecipitated from olfactory mucosa and bulb extracts and interact directly, as demonstrated in pull-down experiments. Emx2 axonal localization, association with high-density particles and interaction with eIF4E strongly suggest that this transcription factor has new nonnuclear functions most probably related to the local control of protein translation in the olfactory sensory neuron axons. Finally, we show that two other brain-expressed homeoproteins, Otx2 and Engrailed 2, also bind eIF4E, indicating that several homeoproteins may modulate eIF4E functions in the developing and adult nervous system.
Homeoproteins constitute a class of transcription factors essential for the embryonic development of all tissues, including the nervous system (1, 2). However, in addition to their developmental expression, many of them are also expressed or reexpressed at late developmental stages and throughout adulthood, suggesting the existence of adult functions. Some of these putative functions may have little to do with development, whereas others are clearly associated with developmental events taking place in the adult.
Emx2 is a homeoprotein likely to support developmental functions in the adult as it is involved in the regulation of neurogenesis in the adult subventricular zone, is expressed in neuronal progenitors in the hippocampus dentate gyrus, and is up-regulated in the hippocampus together with Pax6 and Mash1 upon fibroblast growth factor infusion after induced ischemia in the mouse (3–5). The subventricular zone and the dentate gyrus are two regions where adult neurogenesis takes place. A third region is the olfactory epithelium in which the olfactory sensory neurons (OSNs) are continuously renewed (6). Accordingly, there is a permanent turnover of the OSN axons in the olfactory nerve and of the synapses between the OSN axon terminals and their postsynaptic targets, i.e., mitral and tufted cells in the olfactory bulb at the level of the glomeruli (7, 8).
The expression of Emx2 in the embryonic olfactory epithelium (9, 10) and the impairment of olfactory system development in the Emx2-invalidated mouse (11, 12) has incited us to study the expression of Emx2 mRNA and protein in the adult olfactory system. We report that Emx2 is expressed in the adult olfactory epithelium and that the protein is not only present in the OSN nuclei at the level of the epithelium but also transported into their axon and axon terminals. Moreover, Emx2, in the olfactory mucosa and within the terminals, forms a stable complex with the mRNA cap-binding protein eukaryotic translation initiation factor 4E (eIF4E), suggesting that it may have a function in the regulation of mRNA transport or translation.
Methods
DNA Constructs. Mouse Emx1, Emx2 (pCMV-Emx2), and human Otx2 cDNAs were provided by A. Simeone (King's College, London). Emx1 cDNA was inserted in pEGFP-N1 (Clontech) for COS-7 cell transfection (pEmx1-N1). eIF4E cDNA was obtained by RT-PCR from total-brain RNA and cloned in pGEM-T vector (Promega). For GST-Emx2, GST-Otx2, and GST-eIF4E production, Emx2, Otx2, and eIF4E cDNA were subcloned into pGEX-derived plasmid (Amersham Pharmacia). pTL-hemagglutinin (HA)-Emx2, allowing the expression of HA-tagged Emx2 (HA-Emx2) in COS-7 cells, was obtained by subcloning Emx2 cDNA into the pTL plasmid (13) in fusion with an oligonucleotide linker at the 5′ extremity of Emx2 cDNA encoding an HA tag.
Protein Production and COS-7 Cell Transfections. GST and GST fusion proteins were produced by following instructions provided by Amersham Pharmacia. For eIF4E purification, GST-eIF4E-bound beads were treated with the PreScission protease for 4 h at 4°C. The beads were centrifuged, and the supernatant containing pure eIF4E was collected. Purified proteins were quantified by using the Bradford method (Bio-Rad). COS-7 cells were electroporated with pEmx1-N1, pCMV-Emx2, or pTL-HA-Emx2 as described in ref. 13 and cultured for 48 h before lysis in immunoprecipitation (IP) buffer (50 mM Tris·HCl, pH 7.5/150 mM NaCl/30 mM EDTA/1% Triton X-100/1× Roche Complete protease inhibitor) for IPs or in Laemmli buffer for Western blot analyses.
Animals. B6D2F1 adult female mice (Elevage Janvier, Le Genest, France) were used. Specific lesions of olfactory sensory receptors were provoked by a single i.p. injection of 3-methyl-indol (3-MI; 150 mg/kg in vegetal oil) (14). These animals and sham-treated mice were killed 12 days after injection. Animal handling followed European Community recommendations.
Immunoprecipitations and GST Pull-Down. COS-7 cells or tissues were lysed in IP buffer. Extracts were centrifuged at 12,000 × g for 8 min at 4°C to eliminate nuclei and cellular debris. Supernatants were precleared with protein A Sepharose beads previously loaded with 1 μg/ml BSA and 10 μg/ml yeast tRNA. Precleared supernatants were incubated with anti-eIF4E (1/100; Cell Signaling Technology, Beverly, MA) or anti-Emx2 (1/100) antibody for 3 h and washed four times with IP buffer. Proteins were eluted from the beads with Laemmli buffer and analyzed by Western blot (pan-Emx antibody, 1/1,000; anti-eIF4E antibody, 1/1,000; anti-HA antibody, 1/800; Roche). For GST pull-down experiments, 0.5 μg of eIF4E was incubated for 1 h at room temperature with 1 μg GST, GST-Emx2, GST-Otx2, or GST-Engrailed2 (15) in pull-down buffer (0.4 M KCl/1× PBS/1% Nonidet P-40). After four washes in pull-down buffer, the bead-attached proteins were analyzed by PAGE and Western blot.
Subcellular Fractionation and Biochemical Analyses. Synaptosome preparation and fractionation were performed as described in ref. 16, with slight modifications (see Fig. 2C). Briefly, freshly dissected olfactory bulbs were disrupted in synaptosome buffer (0.32 M sucrose/5 mM Hepes/mixture of Roche protease inhibitors). Nuclei were pelleted at 1,700 × g for 8 min, and the supernatant was centrifuged at 12,000 × g to yield a “crude synaptosome pellet.” Synaptosomes were lysed by hypotonic shock and centrifuged at 33,000 × g to pellet mitochondria and postsynaptic densities (LP1). Supernatant (LS1) was centrifuged at 165,000 × g to obtain the high-density organelle pellet (LP2). Soluble proteins from synaptosomes were present in the resulting supernatant (LS2). Treatments with 100 mM N-octylglucoside, 0.2% saponin, 20 μg/ml RNase A, or 1 M NaCl were performed on the LS1 before centrifugation.
Fig. 2.
Emx2 is present in the axon of the OSNs and associates with high-density fractions. (A) Colocalization of Emx2 (in red) and OMP (in green) proteins in the olfactory bulb of adult mice. The Emx2 protein is detected in OMP-positive axons within the glomeruli. (Scale bar, 30 μm.) (B) Localization of Emx2 (in red), Synaptophysin (in blue), and OMP (in green) proteins within a glomerulus. High-magnification confocal images of the same field show that Emx2 immunoreactivity is restricted to OMP-positive axons (B1, B3, and B4) and that it is highly punctated (B1). Emx2-positive patches are observed in the vicinity of synaptophysin-labeled structures, but the two stainings never overlap, indicating that the Emx2-labeled structures present in axon terminals do not correspond to synaptic vesicle clusters. (Scale bar, 4 μm.) (C) Schematic representation of the biochemical protocol used to study the subcellular localization of Emx2 in the olfactory bulb (modified from ref. 16). (D) Western blot analysis of LP1 (membrane- and postsynaptic density-enriched; 33% of total LP1 was loaded) and LP2 (synaptic vesicle-enriched; total LP2 was loaded) and LS2 (soluble fraction from synaptosomes; 25% of LS2 was loaded). LP1 and LP2 fractions display a band corresponding to Emx2 (33 kDa), the intensity of which is higher in LP2. No Emx2 is detected in the LS2 fraction. (E and F) Presence of Emx2 in the LP2 fraction from untreated (E) or sham and 3-MI-treated (F) mice. (E) Incubation of pan-Emx antibody with 20 μg of GST-Emx2 abolished the staining in the LP2 fraction, whereas similar incubation with GST did not. (F) 3-MI treatment (3-MI) strongly reduces Emx2 levels in the LP2 fraction. (G) Colocalization of Emx2 (in red) and OMP (in green) in the olfactory bulb of sham and 3-MI-treated mice. Note the correlated decrease of Emx2 and OMP in the glomeruli of 3-MI-treated mice, as illustrated in the high-magnification view of a glomerulus (G3 and G6 show insets merged from G1 and G2 and G4 and G5, respectively). The two proteins still colocalize in 3-MI-treated mice, confirming the axonal localization of Emx2 in the glomeruli. (Scale bar, 30 μm.)
In Situ Hybridization. Five nonoverlapping oligonucleotide probes were designed to cover the most specific parts of the mRNA sequence of Emx1, Emx2, and olfactory marker protein (OMP) (see Table 1, which is published as supporting information on the PNAS web site). The probes were labeled with 35S and hybridized to cryostat sections as described elsewhere (17).
Immunohistochemistry. Anti-Emx1 and anti-Emx2 antibodies were produced by immunization of rabbits (Animal Pharm Services, Healdsburg, CA) with GST-Emx1 and GST-Emx2 proteins. The rabbit pan-Emx and the goat anti-OMP antibodies were provided by G. Corte (National Institute for Cancer Research, Genoa, Italy) (18) and F. Margolis (University of Maryland, Baltimore, MD) (19), respectively. Mice deeply anaesthetized with pentobarbital were fixed by intracardiac perfusion of either 1% or 4% paraformaldehyde in 0.1 M phosphate buffer. The brain and the olfactory mucosa were dissected, postfixed for 1 h at 4°C in the same fixative, rinsed in PBS, and cryoprotected overnight in 15% sucrose prepared in PBS. Fourteen-micrometer cryostat sections were taken on slides, rinsed several times in PBS, pretreated sequentially with 0.5% hydrogen peroxide in PBS and 1% glycine in PBS, and saturated in PBS containing 5% FCS supplemented with 0.5% BSA and 0.2% Triton X-100. Anti-Emx1 and anti-Emx2 antibodies were incubated overnight at 1/1,000 in the saturation solution, whereas the pan-Emx antibody, the anti-synaptophysin monoclonal antibody (Synaptic Systems, Goettingen, Germany), and the goat anti-OMP were used at 1/2,000 in the same conditions. In some experiments, slides were heated at 70°C overnight in PBS before the saturation step. The secondary antibodies used were Cy3-conjugated donkey anti-rabbit, FITC-conjugated donkey anti-goat, Cy5-conjugated donkey anti-mouse (Jackson Laboratories) and horseradish peroxidase-conjugated anti-rabbit (Envision System, DAKO).
Results
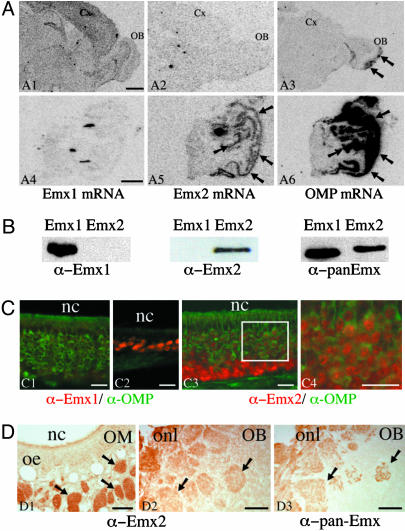
Emx2 mRNA Is Expressed in the Adult Olfactory Mucosa. The expression pattern of Emx2 and Emx1 mRNAs in the adult olfactory system were examined by radioactive in situ hybridization on cryostat sections and autoradiography. To ensure the specific detection of Emx2 and to avoid crosslabeling with Emx1, which is a closely related gene, we used multiple 35S-labeled oligonucleotides designed against the most divergent regions of Emx1 and Emx2. This approach, using probes of the same specific activity and very close hybridization features, also has the advantage of allowing the comparison of expression levels across distinct mRNA species (17). As shown in Fig. 1A, significant amounts of Emx1 mRNA were detected in the cortex, olfactory bulb, and hippocampus (Fig. 1 A1), but no specific signal was detected in the olfactory mucosa (Fig. 1 A4).
Fig. 1.
Emx2 is expressed in the adult mouse OSNs. (A) In situ hybridization of Emx1 (A1 and A4), Emx2 (A2 and A5), and OMP (A3 and A6) mRNAs by using 35S-labeled oligonucleotide probes and film autoradiography (10 days for Emx1 and Emx2 and 4 days for OMP) was performed on brain (A1–A3) and olfactory mucosa (A4–A6) cryostat sections. Emx1 mRNA is detected in the olfactory bulb (OB) and cortex (Cx) but not in the olfactory mucosa (A4). Emx2 mRNA is not detected in the olfactory bulb or cortex (A2) but is visualized in the olfactory mucosa (A5, arrows) with a pattern similar to that of OMP mRNA (A6, arrows). As reported in refs. 26 and 44, OMP mRNA is also detected in the olfactory nerve and axon terminals in the olfactory bulb (A3, arrows). (Scale bar, 1 mm.) (B) Characterization of the anti-Emx1, anti-Emx2, and pan-Emx antibodies on extracts of COS-7 cells overexpressing Emx1 or Emx2. Anti-Emx1 and anti-Emx2 antibodies detect Emx1 and Emx2, respectively, whereas pan-Emx antibody recognizes Emx1 and Emx2. (C) Colocalization of Emx1 or Emx2 (in red) with OMP (in green) in olfactory mucosa cryostat sections fixed with low paraformaldehyde. The Emx1-specific antibody labels the nucleus of small cell clusters observed mainly in thin areas of the olfactory epithelium (C2) and very rarely in the principal pluristratified epithelium containing most OSNs (C1). The Emx2-specific antibody labels the nucleus of virtually all OSN (C3). Emx2 is found in the nuclei of immature and mature neurons. Immature OMP-negative neurons in the basal part of the epithelium display a higher nuclear labeling (C3) than the mature OMP-positive neurons in the medial and apical part of the epithelium (C4, which is the area indicated by a square in C3). nc, nasal cavity. (Scale bar, 20 μm.) (D) Immunoperoxidase detection of Emx2 in olfactory mucosa (OM) and bulb (OB). Anti-Emx2 antibody labels the axonal tracts (D1, arrows) and glomeruli (D2, arrows). The same localization was obtained with the pan-Emx antibody (D3). oe, olfactory epithelium; onl, olfactory nerve layer. (Scale bar, 80 μm.)
Low levels of Emx2 mRNA signal were detected in forebrain structures (i.e., in the hippocampus) as reported in ref. 4, but no Emx2 mRNA was detected in the cortex or olfactory bulb (Fig. 1 A2). In strong contrast, a striking signal was detected in the sensory olfactory mucosa (Fig. 1 A5), with a pattern similar to that of the OMP mRNA (Fig. 1 A6), known to be exclusively expressed in OSNs (20).
These data demonstrate that Emx2 mRNA is present in the neuronal layer of adult olfactory mucosa, where Emx1 mRNA is either not present or present at much lower levels. In contrast, the Emx1 gene is expressed in the adult olfactory bulb, whereas Emx2 is not expressed or only expressed at very low levels.
Emx2 Homeoprotein Is Present in the Axonal Tracts and Terminal Fields of the OSNs. To characterize the expression patterns of these two genes in the olfactory system at the protein level, we raised two polyclonal antibodies by immunizing rabbits with recombinant mouse Emx1 or Emx2 fused to GST. The specificity of the two antibodies was verified on extracts of COS-7 cells transfected with either Emx1- or Emx2-expressing plasmids. Specificity was verified by Western blot (Fig. 1B) and immunohistochemistry (Fig. 5, which is published as supporting information on the PNAS web site). In the same set of experiments, a third antibody, previously produced against human EMX1 (18), was tested. Fig. 1B illustrates that this antibody, pan-Emx, recognizes both Emx1 and Emx2.
On olfactory mucosa cryostat sections fixed with 1% paraformaldehyde, the Emx1-specific antibody labeled the nucleus of rare and small clusters of cells mainly localized in thin regions of the olfactory epithelium (Fig. 1C2). Emx1 staining was virtually absent in the main, pseudostratified layer of the olfactory epithelium, which contains mostly immature and mature OSNs (Fig. 1C1). By contrast, the anti-Emx2 antibody labeled the nucleus of the vast majority of, if not all, the OSNs within the olfactory epithelium (Fig. 1C3). This nuclear staining appears more intense in immature neurons than in mature ones, as identified by OMP expression (Figs. 1 C3 and C4). These Emx1 and Emx2 immunohistochemical localizations thus correlate with our in situ hybridization studies and confirm that Emx2 is the main Emx protein expressed in the OSNs.
A significant Emx2 staining, appearing stronger in 4% paraformaldehyde-fixed sections treated by transient heating at 70°C, was observed in all olfactory axonal bundles within the olfactory mucosa and glomeruli in the olfactory bulb (Fig. 1 D1 and D2). It is noteworthy that the heating procedure almost completely abolished the Emx2 nuclear labeling. Similar patterns of labeling of the axonal tracts and glomeruli were obtained with the pan-Emx antibody (Fig. 1D3), which was thereafter preferred to the anti-Emx2 antibody because of its high efficiency in immunofluorescence and Western blotting.
In contrast and independent of the fixation and pretreatment protocol, no significant axonal labeling was ever observed with our anti-Emx1 antibody (Fig. 6, which is published as supporting information on the PNAS web site), although this antibody stained the nucleus of many cells in the cortex and olfactory bulb, as expected from the Emx1 mRNA pattern of expression (Fig. 1 A).
These data, along with the in situ hybridization results, demonstrate that Emx2 mRNA and protein are expressed in the adult olfactory epithelium and suggest that the protein is transported into the OSN axons, including their terminals within the olfactory bulb glomeruli. This finding does not preclude that the small amount of Emx1 expressed in the olfactory epithelium is also subject to axonal transport, as proposed by Briata and colleagues (18).
Emx2 Is Transported in the Axon of the OSNs. To evaluate whether the anti-Emx2 or anti-pan-Emx staining is truly axonal and does not reflect the expression of Emx2 by other cell types, for example axon-ensheating cells, we colocalized Emx2 with OMP. Indeed, OMP is a soluble protein specifically expressed by mature OSNs and present in their cell bodies and axons. As illustrated in the glomeruli of Fig. 2A, axonal Emx2 immunoreactivity colocalizes with that of OMP. We further colocalized Emx2 and OMP with the synaptophysin presynaptic marker and observed some Emx2 staining in proximity of synaptophysin labeled structures, strongly suggesting that Emx2 is located in the vicinity of synaptic vesicles (i.e., in the axon terminals), but probably not associated with these vesicles (Fig. 2B). Furthermore, Emx2 staining appeared punctated, suggesting an association of Emx2 with subcellular particulate structures (Fig. 2B1).
To verify this association, synaptosomes were prepared from olfactory bulbs and fractionated as described in ref. 16 and Fig. 2C. As shown in Fig. 2D, a substantial amount of Emx2 is present in the LP2 fraction, enriched in synaptic vesicles and other synaptic high-density structures. Although the blot of Fig. 2D was done with the pan-Emx antibody, which also interacts with Emx1, the protein present in the terminals is essentially Emx2. This identity is confirmed by the presence of a single band of the expected size (33 kDa), our immunocytochemical and in situ hybridization data (see above), and the loss of staining on the blot after preincubation of pan-Emx antibody with GST-Emx2 (Fig. 2E).
To confirm that LP2 Emx2 originated from the olfactory epithelium, the same fractionation was done after i.p. 3-MI injection, which specifically provokes a chemical lesion of the olfactory mucosa OSNs (14). Fig. 2F demonstrates that the 3-MI lesion reduces the amount of Emx2 in the LP2 fraction. The data of Fig. 2G further demonstrate that in 3-MI-treated mice Emx2 and OMP immunoreactivities are reduced in parallel in the glomeruli, confirming the colocalization of the two proteins in the olfactory axons. Taken together, these data clearly demonstrate that the protein found in the LP2 fraction is Emx2 and that this protein is located in the OSN axons.
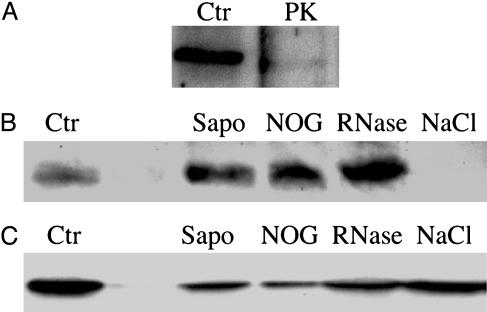
Axonal Emx2 Protein Is Associated with High-Density Particles. To characterize the axonal structures with which Emx2 protein associates, lysed synaptosomal LS1 fractions were submitted to a series of chemical and enzymatic treatments before centrifugation at 165,000 × g and subsequent analysis for the presence of Emx2 in the LP2 pellet. Emx2 was almost entirely degraded upon proteinase K treatment, demonstrating that the protein is directly accessible to the protease and, hence, not localized within vesicles (Fig. 3A). Importantly, treatments with the nonionic detergent N-octyl-glucoside or with saponin did not dissociate Emx2 from the high-density structures, strongly suggesting that Emx2 is not simply adsorbed to vesicles or lipid rafts in the synaptosomal fraction (Fig. 3B). In contrast, high-salt treatment (1 M NaCl) completely dissociated Emx2 protein from the LP2 high-density structures. Taken together, these biochemical analyses indicates that Emx2 is likely associated with nonvesicular high-density structures through electrostatic interactions.
Fig. 3.
Emx2 and eIF4E associate with high-density structures in the olfactory bulb. Western blot analysis of Emx2 (A and B) or eIF4E (C) in LP2 after pretreatment of LS1 with proteinase K (PK), saponin (Sapo), N-octyl-glucoside, RNase A, or 1 M NaCl. LP2 obtained from the equivalent of four olfactory bulbs was loaded on each lane. Ctr, LP2 obtained from untreated LS1.
The high-density, nonvesicular structures expected to be retrieved in LP2 fractions may contain the so-called “mRNA granules” involved in the transport of RNAs and proteins (i.e., RNA-binding proteins) in neuronal processes (21–23). This, and the RNA-binding properties of some homeoproteins, plus the presence of specific mRNAs in olfactory axons and terminals (24–27), prompted us to investigate whether the association of Emx2 with the high-density structures sedimenting in LP2 was lost upon RNase treatment. As illustrated in Fig. 3B, treating the LS1 fraction with RNase before high-speed centrifugation failed to dissociate Emx2 from the particulate fraction, suggesting that the association of Emx2 with these structures does not require RNA.
The recent demonstration that eIF4E binds two homeoproteins, Bicoid and proline-rich homeodomain (PRH) (28, 29), led us to investigate by Western blot the presence of eIF4E in the LP2 fractions pretreated or not with detergent, RNase, or high salt. Interestingly, like Emx2, eIF4E is present in the LP2 fraction, and none of the two proteins is dissociated from high-density structures by N-octyl-glucoside, saponin, or RNase treatments (Fig. 3C). In contrast, and as opposed to Emx2, eIF4E is not dissociated from the high-density structures upon high-salt treatment (Fig. 3C).
It can be concluded from these experiments that Emx2 associates with high-density structures distinct from vesicles and that the latter association does not involve Emx2 binding to RNA molecules. The strikingly similar behavior of eIF4E suggests that Emx2 may bind eIF4E in the olfactory axons and that the two proteins are present in particles within the olfactory axons with high molecular mass.
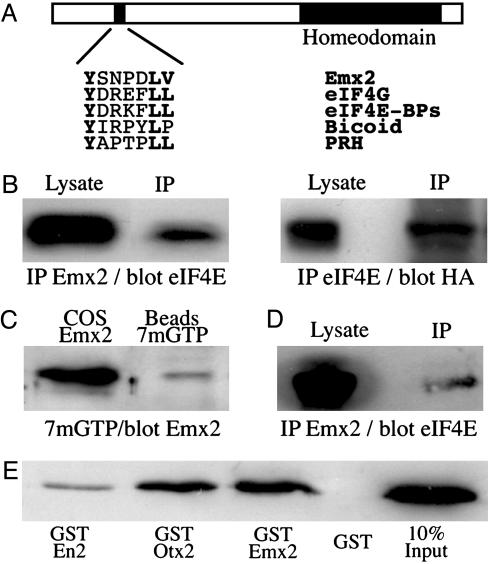
Emx2 Interacts with eIF4E. The above data and the presence in the Emx2 sequence of a putative eIF4E-binding motif previously identified in several proteins interacting with eIF4E, including Bicoid and PRH (Fig. 4A and refs. 28–34), led us to investigate a possible Emx2/eIF4E interaction. We first demonstrated that a fraction of endogenous eIF4E is coimmunoprecipitated with Emx2 overexpressed in COS-7 cells and vice versa (Fig. 4B). To verify whether this interaction exists in vivo, we purified eIF4E from olfactory mucosa extracts by using 7mGTP-coated beads (Fig. 4C). The 7mGTP motif mimics the mRNA cap normally recognized by this factor, and, as expected, eIF4E was retained on the beads (Fig. 7, which is published as supporting information on the PNAS web site). Interestingly, Emx2 was also retained, suggesting an interaction with eIF4E in the OSNs. In another experiment, Emx2 was immunoprecipitated from olfactory mucosa or olfactory bulb extracts and tested for the presence of coimmunoprecipitated eIF4E. In both olfactory mucosa, which contains many OSN axonal tracts as seen on Fig. 1D1 (Fig. 7), and bulb (Fig. 4D), Emx2 coimmunoprecipitated with a fraction of eIF4E. Finally, similar to Bicoid and PRH (28, 29), Emx2 directly interacts with eIF4E, as demonstrated in a pull-down experiment (Fig. 4E).
Fig. 4.
Emx2 directly interacts with eIF4E in vivo and in vitro. (A) Schematic representation of the Emx2 protein, with its homeodomain and its putative eIF4E-binding site, and comparison of the known consensus eIFE4-binding motifs in eIF4G, eIF4E-BPs, Bicoid, and PRH with the putative motif in Emx2. (B) Cytosolic extracts from COS-7 cells transfected with HA-Emx2 were immunoprecipitated with anti-Emx2 (Left) or anti-eIF4E (Right) antibodies. Five percent of total extract (Lysate) and total beads (IP) were analyzed by Western blotting for the presence of eIF4E or HA-Emx2. (C) Cytosolic extracts from olfactory mucosa were incubated with 7 methyl GTP-Sepharose beads. Emx2 expressed in COS-7 (COS Emx2) was used as a positive control size marker. eIF4E (Fig. 7) and Emx2 were retrieved on the beads. (D) Cytosolic extracts from olfactory bulb were immunoprecipitated with the anti-Emx2 antibody. Cell extracts (5% of total extract was loaded; Lysate) and beads (IP) were analyzed by Western blotting for the presence of eIF4E. eIF4E was coimmunoprecipitated with Emx2. (E) Purified recombinant eIF4E was incubated with equal amounts of recombinant GST, GST-Emx2, GST-Otx2, or GST-Engrailed2. The anti-eIF4E Western blot shows that all but GST are specifically retained by eIF4E. Ten percent of input was loaded as a positive control size marker.
Taken together, these data demonstrate that Emx2 is transported into the OSN axon, where it is associated with eIF4E in high-density particles.
Discussion
We demonstrate that the Emx2 homeoprotein is present in two distinct cellular compartments of the adult OSNs: the nucleus and the axon. The rather conventional nuclear localization of Emx2 is probably related to the functions of this DNA-binding protein as a transcriptional regulator. The prominent axonal localization of Emx2, however, was not expected and raises the question of its functional significance. Because of the association of Emx2 with eIF4E and of the presence of mRNAs in the OSN axons (see below), a distinct possibility is that Emx2 regulates the local translation of mRNAs in this neuronal compartment.
It has long been thought that all neuronal proteins were synthesized only within the cell bodies and, to a lesser extent, the dendrites of vertebrate neurons but not in their axons, which were supposedly free of mRNAs and other components of the protein synthesis machinery. However, several mRNA species have now been detected in a variety of developing or mature mammalian axons (24–26, 35–38). Evidence of local mRNA translation in vertebrate axons also accumulates. In Xenopus and chick, for example, mRNAs and components of the translation machinery are present in the growth cones of navigating axons (37, 39, 40), and protein synthesis in the cones is necessary for decision making in response to extracellular cues (39–41). Interestingly, also in a lower vertebrate (Xenopus nerve-muscle coculture), brain-derived neurotrophic factor-induced potentiation of neurotransmitter secretion in a developing synapse requires presynaptic protein translation (42). In mammals, protein synthesis was observed in the axons of severed adult sensory neurons (43), and a recent study demonstrates that, after lesion, importin-β is translated from a dormant axonal mRNA and transported back to the cell body (38).
The mouse olfactory tract is one of the rare systems in which relatively high amounts (i.e., detectable by in situ hybridization) of at least two mRNA species, the olfactory receptor (OR) and OMP-encoding mRNAs, have been observed in the axons and terminals of adult mammalian axons (24–26, 44). The local translation of these or other mRNAs in OSN axon terminals might contribute to synaptic plasticity, like in the case of the developing neuro-muscular junction (42), or serve in guiding the growth cones of newly generated OSNs, given that the OSN population is continuously renewed in the adult mouse (6, 8). The latter hypothesis, built on the evidence that axonal guidance requires protein synthesis (39, 40), is of particular interest in view of the presence of the OR mRNAs in OSN axon terminals. Indeed, it appears that the OR protein itself participates in OSN growth cone guidance and, in particular, in the convergence of all OSN axons expressing the same OR toward two or a few specific target glomeruli, possibly through homophilic interactions (45–50).
The direct interaction between Emx2 and eIF4E demonstrated in this report indicates that Emx2 could regulate protein synthesis initiation by eIF4E in OSN axons. Emx2 could, for example, decrease the affinity of eIF4E for the m7GTP cap of mRNAs, as reported for the PRH homeoprotein (29) or inhibit the eIF4E-dependent translation initiation of selected mRNAs, as shown for Bicoid homeoprotein and caudal mRNA in the Drosophila embryo (28, 51). Fractionation and biochemical analyses shown here suggest that Emx2 is associated and co-transported with eIF4E within high-density particles. We do not yet know whether these Emx2-eIF4E granules also contain the axonal mRNAs, because different types of transport particles or granules can coexist in the same cell (52). Whereas some granules transport mRNAs, RNA-binding proteins, and elements of the translation machinery yet lack eIF4E (31), others are devoid of mRNAs yet contain aminoacyl-tRNA synthetases and elongation factors, such as EF1 (52). Therefore, the composition of the particles carrying eIF4E and Emx2 will have to be clarified. An important issue will be to determine whether eIF4E can be dissociated from the Emx2-containing granules, hence allowing translation initiation (53).
The pull-down experiments of Fig. 4E demonstrate that Otx2 and Engrailed 2, two homeoproteins expressed in the developing and adult central nervous system (54–56), also bind eIF4E. It is noteworthy that these homeoproteins and several others (i.e., Otx1 and Emx1) show a nonnuclear and in some instances dendritic localization in selected classes of neurons (10, 57, 58, and our unpublished observations). It is possible, as proposed for Otx homeoproteins (57, 58), that this cytoplasmic retention prevents homeoprotein transcriptional activity until a specific trigger allows their nuclear import. This model is not contradictory with the idea that the interaction between homoproteins and eIF4E, as demonstrated in the case of Bicoid and PRH, regulates eIF4E functions, including protein translation, both in the perikaryon and in distal cell compartments, such as axons and dendrites. The identification of a short consensus domain mediating the binding of Bicoid and PRH to eIF4E and the conservation of this sequence in many other homeoproteins (29) suggest that such regulatory functions might exist for many homeoproteins.
In this report we have concentrated on cell autonomous homeoprotein activity. However, given that homeoproteins have the unsuspected ability to transfer between cells, an investigation into the possibility that translation regulation might also be nonautonomous (59), thus giving to this class of transcription factor the status of signaling proteins, is necessary. Finally, this nonnuclear homeoprotein activity, be it cell automous or non-cell autonomous, might shed a new light on the several physiological or pathological circumstances in which homeoproteins have been implicated (60–62).
Supplementary Material
Acknowledgments
We thank Drs. G. Corte and F. Margolis for providing us with the anti-human EMX1 and anti-OMP antibodies, respectively; Dr. A. Simeone for the gift of the Emx2 and Otx2 plasmids; Drs. C. Goridis, A. Joliot, and A. Di Nardo for critical reading of this manuscript; and Drs. M. L Montesinos, M. Volovitch, P.-M. Lledo, and T. Galli for helpful discussions. This work was supported by L'Action Concertée Incitative Grant 0220504, Association pour la Recherche sur le Cancer Grant 5168, and by a grant from the Institut Universitaire de France (to A.T.). S.N. is a fellow of the Ligue Contre le Cancer Foundation.
Abbreviations: OSN, olfactory sensory neuron; eIF4E, eukaryotic translation initiation factor 4E; OMP, olfactory marker protein; 3-MI, 3-methyl-indol; PRH, proline-rich homeodomain; IP, immunoprecipitation; HA, hemagglutinin.
References
- 1.Reichert, H. & Simeone, A. (1999) Curr. Opin. Neurobiol. 9, 589-595. [DOI] [PubMed] [Google Scholar]
- 2.Dasen, J. S., Liu, J. P. & Jessell, T. M. (2003) Nature 425, 926-933. [DOI] [PubMed] [Google Scholar]
- 3.Gangemi, R. M., Daga, A., Marubbi, D., Rosatto, N., Capra, M. C. & Corte, G. (2001) Mech. Dev. 109, 323-329. [DOI] [PubMed] [Google Scholar]
- 4.Galli, R., Fiocco, R., De Filippis, L., Muzio, L., Gritti, A., Mercurio, S., Broccoli, V., Pellegrini, M., Mallamaci, A. & Vescovi, A. L. (2002) Development (Cambridge, U.K.) 129, 1633-1644. [DOI] [PubMed] [Google Scholar]
- 5.Nakatomi, H., Kuriu, T., Okabe, S., Yamamoto, S., Hatano, O., Kawahara, N., Tamura, A., Kirino, T. & Nakafuku, M. (2002) Cell 110, 429-441. [DOI] [PubMed] [Google Scholar]
- 6.Mackay-Sim, A. & Kittel, P. (1991) J. Neurosci. 11, 979-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu, F., Greer, C. A. & Shepherd, G. M. (2000) J. Comp. Neurol. 422, 489-495. [DOI] [PubMed] [Google Scholar]
- 8.Gogos, J. A., Osborne, J., Nemes, A., Mendelsohn, M. & Axel, R. (2000) Cell 103, 609-620. [DOI] [PubMed] [Google Scholar]
- 9.Simeone, A., Gulisano, M., Acampora, D., Stornaiuolo, A., Rambaldi, M. & Boncinelli, E. (1992) EMBO J. 11, 2541-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mallamaci, A., Iannone, R., Briata, P., Pintonello, L., Mercurio, S., Boncinelli, E. & Corte, G. (1998) Mech. Dev. 77, 165-172. [DOI] [PubMed] [Google Scholar]
- 11.Pellegrini, M., Mansouri, A., Simeone, A., Boncinelli, E. & Gruss, P. (1996) Development (Cambridge, U.K.) 122, 3893-3898. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida, M., Suda, Y., Matsuo, I., Miyamoto, N., Takeda, N., Kuratani, S. & Aizawa, S. (1997) Development (Cambridge, U.K.) 124, 101-111. [DOI] [PubMed] [Google Scholar]
- 13.Joliot, A., Trembleau, A., Raposo, G., Calvet, S., Volovitch, M. & Prochiantz, A. (1997) Development (Cambridge, U.K.) 124, 1865-1875. [DOI] [PubMed] [Google Scholar]
- 14.Setzer, A. K. & Slotnick, B. (1998) Physiol. Behav. 65, 479-487. [DOI] [PubMed] [Google Scholar]
- 15.Foucher, I., Montesinos, M. L., Volovitch, M., Prochiantz, A. & Trembleau, A. (2003) Development (Cambridge, U.K.) 130, 1867-1876. [DOI] [PubMed] [Google Scholar]
- 16.Huttner, W. B., Schiebler, W., Greengard, P. & De Camilli, P. (1983) J. Cell Biol. 96, 1374-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broide, R. S., Trembleau, A., Ellison, J. A., Cooper, J., Lo, D., Young, W. G., Morrison, J. H. & Bloom, F. E. (2004) Brain Res. 1000, 211-222. [DOI] [PubMed] [Google Scholar]
- 18.Briata, P., Di Blas, E., Gulisano, M., Mallamaci, A., Iannone, R., Boncinelli, E. & Corte, G. (1996) Mech. Dev. 57, 169-180. [DOI] [PubMed] [Google Scholar]
- 19.Keller, A. & Margolis, F. L. (1975) J. Neurochem. 24, 1101-1106. [DOI] [PubMed] [Google Scholar]
- 20.Danciger, E., Mettling, C., Vidal, M., Morris, R. & Margolis, F. (1989) Proc. Natl. Acad. Sci. USA 86, 8565-8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siomi, M. C., Zhang, Y., Siomi, H. & Dreyfuss, G. (1996) Mol. Cell. Biol. 16, 3825-3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang, H. L., Eom, T., Oleynikov, Y., Shenoy, S. M., Liebelt, D. A., Dictenberg, J. B., Singer, R. H. & Bassell, G. J. (2001) Neuron 31, 261-275. [DOI] [PubMed] [Google Scholar]
- 23.Duchaine, T. F., Hemraj, I., Furic, L., Deitinghoff, A., Kiebler, M. A. & DesGroseillers, L. (2002) J. Cell Sci. 115, 3285-3295. [DOI] [PubMed] [Google Scholar]
- 24.Vassar, R., Chao, S. K., Sitcheran, R., Nunez, J. M., Vosshall, L. B. & Axel, R. (1994) Cell 79, 981-991. [DOI] [PubMed] [Google Scholar]
- 25.Ressler, K. J., Sullivan, S. L. & Buck, L. B. (1994) Cell 79, 1245-1255. [DOI] [PubMed] [Google Scholar]
- 26.Wensley, C. H., Stone, D. M., Baker, H., Kauer, J. S., Margolis, F. L. & Chikaraishi, D. M. (1995) J. Neurosci. 15, 4827-4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singer, M. S., Hughes, T. E., Shepherd, G. M. & Greer, C. A. (1998) NeuroReport 9, 3745-3748. [DOI] [PubMed] [Google Scholar]
- 28.Niessing, D., Blanke, S. & Jackle, H. (2002) Genes Dev. 16, 2576-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Topisirovic, I., Culjkovic, B., Cohen, N., Perez, J. M., Skrabanek, L. & Borden, K. L. (2003) EMBO J. 22, 689-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen, N., Sharma, M., Kentsis, A., Perez, J. M., Strudwick, S. & Borden, K. L. (2001) EMBO J. 20, 4547-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krichevsky, A. M. & Kosik, K. S. (2001) Neuron 32, 683-696. [DOI] [PubMed] [Google Scholar]
- 32.Wilhelm, J. E., Hilton, M., Amos, Q. & Henzel, W. J. (2003) J. Cell Biol. 163, 1197-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura, A., Sato, K. & Hanyu-Nakamura, K. (2004) Dev. Cell 6, 69-78. [DOI] [PubMed] [Google Scholar]
- 34.Nelson, M. R., Leidal, A. M. & Smibert, C. A. (2004) EMBO J. 23, 150-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohr, E., Fehr, S. & Richter, D. (1991) EMBO J. 10, 2419-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trembleau, A., Morales, M. & Bloom, F. E. (1994) J. Neurosci. 14, 39-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bassell, G. J., Zhang, H., Byrd, A. L., Femino, A. M., Singer, R. H., Taneja, K. L., Lifshitz, L. M., Herman, I. M. & Kosik, K. S. (1998) J. Neurosci. 18, 251-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanz, S., Perlson, E., Willis, D., Zheng, J. Q., Massarwa, R., Huerta, J. J., Koltzenburg, M., Kohler, M., van-Minnen, J., Twiss, J. L. & Fainzilber, M. (2003) Neuron 40, 1095-1104. [DOI] [PubMed] [Google Scholar]
- 39.Campbell, D. S. & Holt, C. E. (2001) Neuron 32, 1013-1026. [DOI] [PubMed] [Google Scholar]
- 40.Ming, G. L., Wong, S. T., Henley, J., Yuan, X. B., Song, H. J., Spitzer, N. C. & Poo, M. M. (2002) Nature 417, 411-418. [DOI] [PubMed] [Google Scholar]
- 41.Brittis, P. A., Lu, Q. & Flanagan, J. G. (2002) Cell 110, 223-235. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, X. & Poo, M. M. (2002) Neuron 36, 675-688. [DOI] [PubMed] [Google Scholar]
- 43.Zheng, J. Q., Kelly, T. K., Chang, B., Ryazantsev, S., Rajasekaran, A. K., Martin, K. C. & Twiss, J. L. (2001) J. Neurosci. 21, 9291-9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krishna, N. S., Getchell, M. L., Buiakova, O. I., Margolis, F. L. & Getchell, T. V. (1995) NeuroReport 6, 817-821. [DOI] [PubMed] [Google Scholar]
- 45.Singer, M. S., Shepherd, G. M. & Greer, C. A. (1995) Nature 377, 19-20. [DOI] [PubMed] [Google Scholar]
- 46.Mombaerts, P., Wang, F., Dulac, C., Chao, S. K., Nemes, A., Mendelsohn, M., Edmondson, J. & Axel, R. (1996) Cell 87, 675-686. [DOI] [PubMed] [Google Scholar]
- 47.Wang, F., Nemes, A., Mendelsohn, M. & Axel, R. (1998) Cell 93, 47-60. [DOI] [PubMed] [Google Scholar]
- 48.Treloar, H. B., Feinstein, P., Mombaerts, P. & Greer, C. A. (2002) J. Neurosci. 22, 2469-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bozza, T., Feinstein, P., Zheng, C. & Mombaerts, P. (2002) J. Neurosci. 22, 3033-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reed, R. R. (2004) Cell 116, 329-336. [DOI] [PubMed] [Google Scholar]
- 51.Dubnau, J. & Struhl, G. (1996) Nature 379, 694-699. [DOI] [PubMed] [Google Scholar]
- 52.Carson, J. H., Kwon, S. & Barbarese, E. (1998) Curr. Opin. Neurobiol. 8, 607-612. [DOI] [PubMed] [Google Scholar]
- 53.Smart, F. M., Edelman, G. M. & Vanderklish, P. W. (2003) Proc. Natl. Acad. Sci. USA 100, 14403-14408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kelley, C. G., Lavorgna, G., Clark, M. E., Boncinelli, E. & Mellon, P. L. (2000) Mol. Endocrinol. 14, 1246-1256. [DOI] [PubMed] [Google Scholar]
- 55.Simeone, A., Puelles, E. & Acampora, D. (2002) Curr. Opin. Genet. Dev. 12, 409-415. [DOI] [PubMed] [Google Scholar]
- 56.Simon, H. H., Bhatt, L., Gherbassi, D., Sgado, P. & Alberi, L. (2003) Ann. N.Y. Acad. Sci. 991, 36-47. [PubMed] [Google Scholar]
- 57.Zhang, Y. A., Okada, A., Lew, C. H. & McConnell, S. K. (2002) Mol. Cell Neurosci. 19, 430-446. [DOI] [PubMed] [Google Scholar]
- 58.Baas, D., Bumsted, K. M., Martinez, J. A., Vaccarino, F. M., Wikler, K. C. & Barnstable, C. J. (2000) Brain Res. Mol. Brain Res. 78, 26-37. [DOI] [PubMed] [Google Scholar]
- 59.Prochiantz, A. & Joliot, A. (2003) Nat. Rev. Mol. Cell. Biol. 4, 814-819. [DOI] [PubMed] [Google Scholar]
- 60.Faiella, A., Brunelli, S., Granata, T., D'Incerti, L., Cardini, R., Lenti, C., Battaglia, G. & Boncinelli, E. (1997) Eur. J. Hum. Genet. 5, 186-190. [PubMed] [Google Scholar]
- 61.Abate-Shen, C. (2003) Cancer Cell 4, 329-330. [DOI] [PubMed] [Google Scholar]
- 62.Cillo, C., Cantile, M., Faiella, A. & Boncinelli, E. (2001) J. Cell Physiol. 188, 161-169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.