Abstract
Diclofenac (DCLF) is a widely used non-steroidal anti-inflammatory drug that is associated with idiosyncratic, drug-induced liver injury (IDILI) in humans. The mechanisms of DCLF-induced liver injury are unknown; however, patients with certain inflammatory diseases have an increased risk of developing IDILI, which raises the possibility that immune mediators play a role in the pathogenesis. DCLF synergizes with the cytokines tumor necrosis factor-alpha (TNF) and interferon-gamma (IFN) to cause hepatocellular apoptosis in vitro by a mechanism that involves activation of the endoplasmic reticulum (ER) stress response pathway and of the mitogen-activated protein kinases, c-Jun N-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK). DCLF also causes an increase in intracellular calcium (Ca++) in hepatocytes, but the role of this in the cytotoxic synergy between DCLF and cytokines is unknown. We tested the hypothesis that Ca++ contributes to DCLF/cytokine-induced cytotoxic synergy. Treatment of HepG2 cells with DCLF led to an increase in intracellular Ca++ at 6 and 12 h, and this response was augmented in the presence of TNF and IFN at 12 h. The intracellular Ca++ chelator BAPTA/AM reduced cytotoxicity and caspase-3 activation caused by DCLF/cytokine cotreatment. BAPTA/AM also significantly reduced DCLF-induced activation of the ER stress sensor, protein kinase RNA-like ER kinase (PERK), as well as activation of JNK and ERK. Treatment of cells with an inositol trisphosphate receptor antagonist almost completely eliminated DCLF/cytokine-induced cytotoxicity and decreased DCLF-induced activation of PERK, JNK, and ERK. These findings indicate that Ca++ contributes to DCLF/cytokine-induced cytotoxic synergy by promoting activation of the ER stress-response pathway and JNK and ERK.
Keywords: idiosyncratic drug-induced liver injury, calcium, ER stress, MAPK, BAPTA/AM, caspase, tumor necrosis factor, interferon-gamma, diclofenac, non-steroidal anti-inflammatory drugs
Drug-induced liver injury (DILI) is the leading cause of acute liver failure in the United States and the most common adverse event associated with failure to obtain U.S. Food and Drug Administration approval for new drugs (Aithal et al., 2011). Most DILI reactions are dose-dependent and predictable using routine animal testing; however, a subset of DILI reactions is idiosyncratic. Idiosyncratic DILI (IDILI) reactions are typically rare but sometimes severe and are the most common cause of post-marketing warnings and withdrawal of drugs from the pharmaceutical market.
IDILI is a poorly understood phenomenon, but susceptibility to these reactions is likely due to actions of the drug in the context of environmental and genetic factors specific to patients (Boelsterli, 2002). Along with antibiotics, non-steroidal anti-inflammatory drugs (NSAIDs) are the most frequent causes of IDILI (Unzueta and Vargas, 2013). The frequency and severity of IDILI among drugs differ within this pharmacologic class (Teoh and Farrell, 2003), and patients with certain underlying diseases are susceptible to IDILI induced by some NSAIDs but not others. A retrospective cohort study found that rheumatoid arthritis was a risk factor for NSAID-induced idiosyncratic hepatotoxicity (García Rodríguez et al., 1994). These observations further suggest that both patient-specific factors as well as drug-specific actions are important determinants of susceptibility.
Diclofenac (DCLF) is one of the most widely used NSAIDs worldwide, although its use has been restricted in the United States due to association with IDILI. The mechanisms of DCLF-induced hepatotoxicity are unknown, but immune mediators might play a role. Interestingly, osteoarthritis was found to be a risk factor for IDILI induced by DCLF in particular (Banks et al., 1995). These observations suggest a role for inflammation in IDILI caused by NSAIDs, particularly DCLF.
Studies in rodents also revealed a role for immune mediators in DILI caused by various drugs, including DCLF (Deng et al., 2006, 2008; Dugan et al., 2011, Shaw et al., 2009a, b; Zou et al., 2009). When rodents were administered a non-hepatotoxic dose of the inflammagen, lipopolysaccharide, in combination with a non-hepatotoxic dose of DCLF, they developed pronounced hepatocellular injury (Deng et al., 2006). Similar animal models employing other IDILI-associated drugs revealed a critical role for the proinflammatory cytokines, tumor necrosis factor-alpha (TNF), and interferon-gamma (IFN), in the pathogenesis of liver injury (Dugan et al., 2011; Hassan et al., 2008; Shaw et al., 2009a, b; Zou et al., 2009). Gene expression analysis of the livers from rodents treated with DCLF revealed increased expression of various genes involved in both the TNF and IFN signaling pathways, including TNF receptor superfamily member 1 a, signal transducer and activator of transcription-1 (STAT-1), and the tumor suppressor protein p53 (Deng et al., 2008). The protein products of these genes are known to promote apoptosis (Gorina et al., 2005; Hussain and Harris, 2006; Shen and Pervaiz, 2006). These findings in animals suggest that DCLF can synergize with immune mediators to cause death of hepatocytes and might explain why humans with certain underlying inflammatory diseases are more susceptible to toxicity from DCLF.
In vitro, DCLF synergized with inflammatory cytokines including TNF to kill human primary hepatocytes (Cosgrove et al., 2009). Similarly, DCLF synergized with TNF to cause death of HepG2 cells, and this depended on caspase activation and activation of the mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase (JNK) (Fredriksson et al., 2011). In addition, IFN treatment enhanced cytotoxicity mediated by DCLF/TNF treatment, and this required activation of caspases, JNK, and extracellular signal-regulated kinase (ERK) (Maiuri et al., 2015). Fredriksson et al. (2014) demonstrated that DCLF treatment caused activation of the endoplasmic reticular (ER) stress sensors, inositol requiring enzyme-1, and protein kinase RNA-like endoplasmic reticulum kinase (PERK), and this was followed by upregulation of the proapoptotic transcription factor CCAAT/-enhancer-binding protein homologous protein (CHOP). Silencing of the ER stress mediators PERK and CHOP using siRNA reduced apoptosis induced by DCLF/TNF treatment (Fredriksson et al., 2014). These studies in vitro provided insight into the pathways activated in response to DCLF that promote a cytotoxic interaction with TNF. However, how DCLF/cytokine treatment promotes the activation of these stress-response pathways and how the pathways interact with each other in causing cell death remain unknown.
It is been reported that DCLF treatment induces increases in intracellular calcium (Ca++) in rat and human hepatocytes, and this contributed to cytotoxicity induced by DCLF in these cell types (Bort et al., 1999; Lim et al., 2006). Increases in intracellular Ca++ are known to promote the activation of MAPKs and also activation of the ER stress response pathway (Bollo et al., 2010; Kim and Sharma, 2004). In this study, we tested the hypothesis that Ca++ contributes to DCLF/cytokine-induced cytotoxic synergy by promoting ER stress and activation of JNK, ERK, STAT-1, and caspase 3. In addition, we explored the interdependence of DCLF-induced JNK, ERK, and STAT-1 activation.
MATERIALS AND METHODS
Materials
All drugs were purchased from Sigma-Aldrich (St. Louis, Missouri) unless otherwise noted. Recombinant human TNF and IFN were purchased from Millipore (Billerica, Massachusetts). Phosphate-buffered saline (PBS), Dulbecco’s Modified Eagles Medium (DMEM), Ca++-free DMEM, fetal bovine serum (FBS), fluo-3/AM, Antibiotic-Antimycotic (ABAM), and 0.25% Trypsin-EDTA were obtained from Life Technologies (Carlsbad, California). The phosphorylated PERK antibody was purchased from Santa Cruz Biotechnology (Dallas, Texas). All other antibodies were from Cell Signaling Technology (Beverly, Massachusetts).
Cell culture
Human hepatoma HepG2 cells (American Type Culture Collection, Manassas, Virginia) were chosen because they respond similar to primary human hepatocytes with regard to the cytotoxic interaction between DCLF and cytokines (Cosgrove et al., 2009). Although HepG2 cells have low expression of phase 1 drug metabolizing enzymes compared with primary human hepatocytes, they have similar expression of phase II enzymes compared with primary human hepatocytes (Westerink and Schoonen, 2007a, b). Importantly, HepG2 cells metabolize DCLF to both acylglucuronide and hydroxyl metabolites (Fredriksson et al., 2011), which are the metabolites that have been suggested to mediate DCLF-induced hepatotoxicity (Boelsterli, 2003). Notably, evidence supporting a role for metabolic bioactivation in DCLF-mediated hepatotoxicity in human patients is lacking (Aithal, et al., 2000).
Cells were grown in 25-cm2 tissue culture-treated flasks, maintained in DMEM supplemented with 10% FBS and 1% ABAM (complete DMEM) and cultured at 37°C in 95% air and 5% CO2 in a humidified incubator. They were passaged when they reached ∼80% confluence.
Experimental design and cytotoxicity assessment
HepG2 cells were plated at a density of 4 × 104 cells per well in black-walled, 96-well, tissue culture plates, and allowed to attach overnight before treatment with compounds. DCLF was reconstituted in sterile water. Cells were treated with 250-μM DCLF or its vehicle, and simultaneously with TNF (10 ng/ml) and/or IFN (10 ng/ml) or their vehicle (PBS). The concentrations selected were based on previous concentration response studies published in Maiuri et al. (2015). It was demonstrated that treatment of cells with 250 μM DCLF in combination with TNF (10 ng/ml) caused a robust cytotoxic response in HepG2 cells that was enhanced by IFN (10 ng/ml), whereas treatment of cells with each component individually did not result in cell death (Maiuri et al., 2015). The cytokine concentrations chosen for this study are within 10-fold of the concentrations found in serum of human patients undergoing an inflammatory response (Pinsky et al., 1993; Taudorf et al., 2007). In addition, a previous time course study revealed that cytotoxicity in response to DCLF/cytokine treatment begins near 18 h and progresses until at least 24 h (Maiuri et al., 2015). Cells treated with DCLF/cytokine combinations were also incubated in the presence or absence of the intracellular Ca++ chelator acetoxymethyl-1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA/AM, 10 μM, 4 h pretreatment) or the IP3 receptor antagonist 2-aminophenoxydiphenyl borate (2-APB, 100 μM, addition simultaneous with DCLF/cytokines). The concentration of BAPTA/AM chosen was based on the observation that concentrations of BAPTA/AM ranging from 5 to 10 μM are effective in reducing intracellular free Ca++ in stimulated HepG2 cells (Choi et al., 2014; Huang et al., 2009; Liu et al., 2004). Cells were exposed to the drug/cytokine/inhibitor combination for 24 h, and cytotoxicity was evaluated by measuring release of lactate dehydrogenase (LDH) from the cells into culture medium using the Homogeneous Membrane Integrity Assay kit from Promega (Madison, Wisconsin). BAPTA/AM and 2-APB were reconstituted in dimethyl sulfoxide (DMSO). DMSO (0.1%) was used as the vehicle control in all experiments involving treatment with BAPTA/AM or 2-APB.
To examine the involvement of extracellular Ca++ in the cytotoxic interaction between DCLF and cytokines, DCLF/cytokine combinations were prepared in Ca++-free medium. At the time of drug treatment, complete DMEM was replaced with Ca++-free medium, which was prepared using FBS-free and Ca++-free DMEM supplemented with sodium pyruvate (1 mM) and l-glutamine (4 mM).
To determine if iron or reactive oxygen species (ROS) are involved in the cytotoxic interaction between DCLF and cytokines, DCLF/cytokine combinations were incubated in the presence or absence of the iron chelator, deferoxamine (DF), or the membrane permeable ROS scavenger, Tempol.
Measurement of intracellular Ca++
HepG2 cells were plated in 12-well tissue culture plates at a density of 6 × 105 cells per well and allowed to attach overnight. 18 h after plating, HepG2 cells were treated with 250 μM DCLF or its vehicle, alone or in combination with TNF, IFN, or both. After treatment, cells were trypsinized and incubated with the Ca++-sensitive dye, fluo-3/AM, for 30 min. Fluo-3 fluorescence was measured using a BD FACSCanto II flow cytometer (Beckman Coulter, Brea, California). Data analysis was performed using FlowJo software (version 8.8.7, Treestar Software, Ashland, Oregon).
Caspase-3 activity
Caspase-3 activity was measured using the Caspase-3 Fluorometric Assay Kit purchased from R&D Systems (Minneapolis, Minnesota). HepG2 cells were plated at 1.2 × 106 cells per well in 6-well tissue culture plates. They were treated with DCLF alone or in combination with TNF and/or IFN and also in the presence or absence of BAPTA/AM or 2-APB. For all studies involving BAPTA/AM, cells were pretreated with BAPTA/AM for 4 h prior to the addition of DCLF and cytokines. For all studies involving 2-APB, cells were treated with 2-APB simultaneously with DCLF and cytokines. Cells were lysed and centrifuged after 24 h of exposure. 50 μl of lysate were added to black-walled, 96-well plates and incubated with assay reaction buffer and fluorogenic substrate for 1 h. The plate was then read in a fluorescence plate reader at an excitation wavelength of 400 nm and an emission wavelength of 505 nm.
Protein isolation
Cells (1.2 × 106 per well) were plated in 6-well tissue culture plates and allowed to adhere overnight. They were exposed to 250 μM DCLF and its vehicle alone or in combination with TNF and/or IFN for 18 h. For some experiments, cells treated with DCLF/cytokine combinations were also incubated in the presence of BAPTA/AM, 2-APB, or the JNK inhibitor, SP600125. SP600125 was prepared in DMSO and 0.1% DMSO was used as the vehicle control in all experiments involving treatment with SP600125. Cells were rinsed with cold PBS followed by addition of 150 µl of radioimmunoprecipitation assay buffer containing HALT protease and phosphatase inhibitor cocktails (Thermo Scientific, Rockford, Illinois). Cells were scraped, collected, placed in microcentrifuge tubes, and incubated on ice for 10 min. During the 10-min incubation, the tubes were vortexed intermittently. Lysates were centrifuged for 25 min at 20 000 × g. The supernatant fluids containing whole cell protein were collected and stored at −80°C until use. Protein concentrations were quantified using the bicinchoninic acid assay (Thermo Scientific).
Western analysis
For detection of phosphorylated JNK (pJNK), phosphorylated ERK (pERK), phosphorylated PERK (pPERK), and phosphorylated STAT-1 (pSTAT-1) in whole cell lysates, 25 μg protein were loaded onto precast NuPAGE 12% Bis-Tris gels (Life Technologies), and subjected to electrophoresis. Proteins were transferred onto polyvinylidene fluoride membranes (Millipore). Membranes were blocked for 1 h with 5% bovine serum albumin (BSA) reconstituted in 1% tris-buffered saline (TBS) containing 0.1% tween-20 (TBSt). They were then probed with antibodies directed against pJNK, pERK, pPERK, pSTAT-1 (tyrosine 701), pSTAT-1 (serine 727), and α-tubulin. Primary antibodies were diluted in 2% BSA in TBSt. Membranes were incubated with primary antibodies overnight at 4°C, after which they were washed with TBSt followed by the addition of secondary antibody. Goat anti-rabbit or goat anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibody was diluted in 5% BSA in TBSt at a concentration of 1:2500 for pJNK and 1:5000 for all others. Clarity Western ECL substrate (Bio-Rad, Hercules, California) was used to visualize HRP, and the substrate was developed on HyBlot CL film (Denville Scientific, Metuchen, New Jersey). All images were quantified by performing densitometry using Image J software.
Statistical analysis
All results are expressed as mean ± standard error of the mean (SEM). Data were subjected to log transformation as necessary to achieve equal variance and normality. Data were analyzed by either a 1-way or 2-way analysis of variance (ANOVA), as appropriate. For 1-way and 2-way ANOVAs, the Holm-Sidak post hoc test was used for multiple, pair-wise comparisons between treatment groups. The criterion for significance was set at α = 0.05.
RESULTS
DCLF Promotes an Increase in Cytosolic Free Ca++
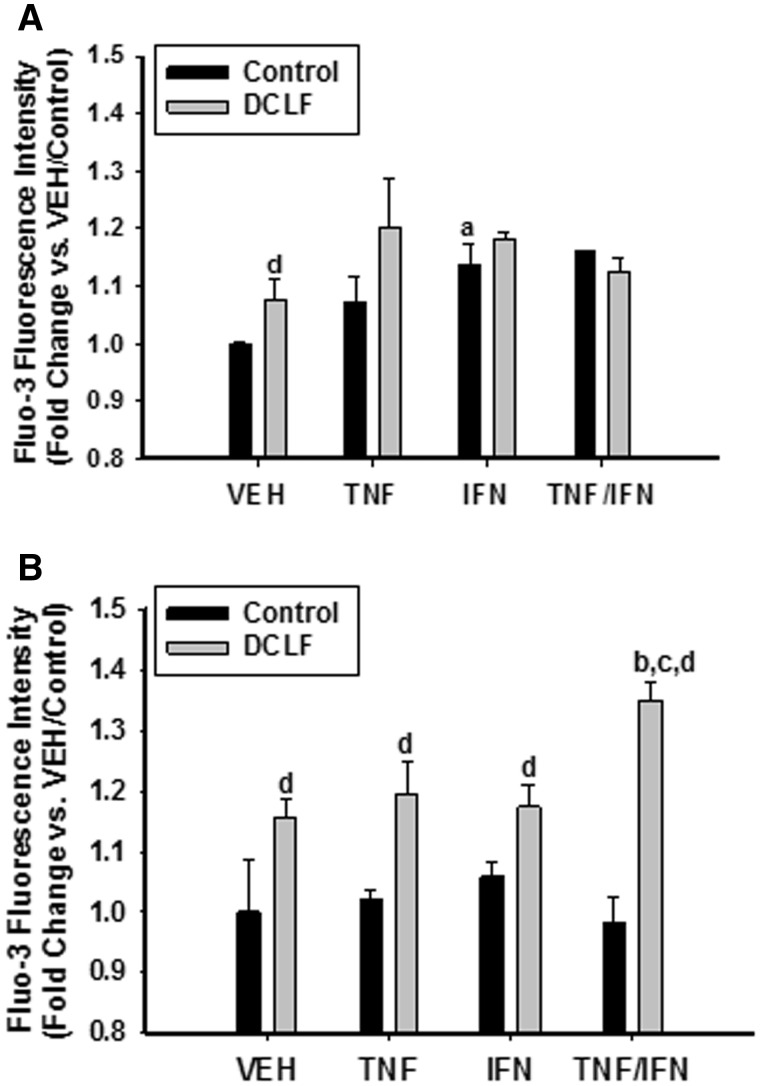
Ca++ levels were measured at 2 times prior to the onset of cytotoxicity. Treatment of cells with TNF did not promote an increase in intracellular calcium at the times examined. Treatment with IFN promoted an increase in intracellular Ca++ at 6 h but not at 12 h. Treatment with DCLF caused an increase in intracellular Ca++ at 6 h that was still apparent at 12 h. Interestingly, the DCLF-induced increase in intracellular Ca++ at 12 h was enhanced by treatment with TNF/IFN (Figure 1).
FIG. 1.
DCLF treatment caused an increase in intracellular Ca++. HepG2 cells were exposed to DCLF (250 μM) or its VEH, alone or in combination with TNF (10 ng/ml) and/or IFN (10 ng/ml) for (A) 6 or (B) 12 h. Cells were then removed from the plate with trypsin and incubated with the Ca++ indicator fluo-3 for 30 min. Intracellular Ca++ levels were quantified by measuring fluo-3 fluorescence intensity by flow cytometry. a, significantly different from corresponding bar in the VEH group. b, significantly different from corresponding bar in the TNF group. c, significantly different from corresponding bar in the IFN group. d, significantly different from Control within a cytokine treatment group. Data are represented as mean ± SEM of at least 3 experiments. Abbreviations: VEH, vehicle; TNF, tumor necrosis factor-alpha; IFN, interferon-gamma; DCLF, diclofenac.
An Intracellular Ca++ Chelator Reduces Cytotoxicity Mediated by DCLF/Cytokine Cotreatment
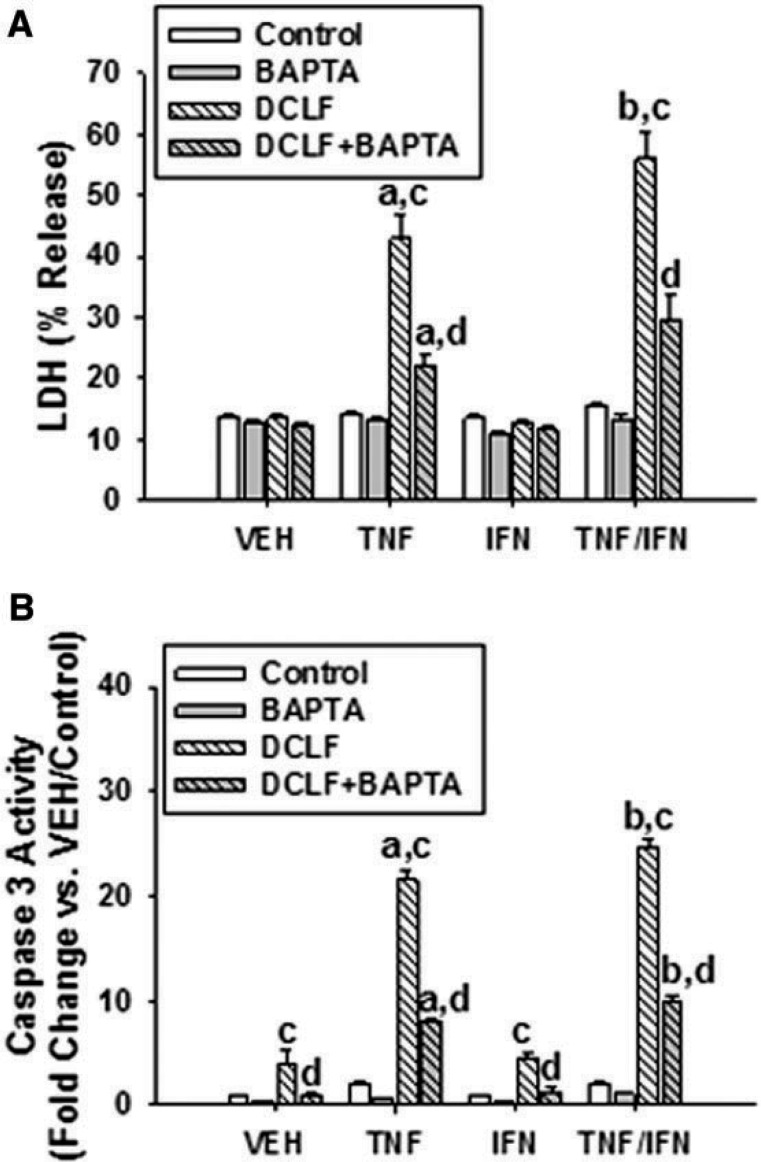
Consistent with previous observations, treatment with DCLF by itself did not result in cell death (LDH release) (Figure 2A). Similarly, treatment with the cytokines individually or in combination did not result in cytotoxicity. DCLF synergized with TNF to cause LDH release from cells. Although DCLF did not synergize with IFN alone, IFN enhanced the cytotoxic interaction between DCLF and TNF. Pretreatment of cells with the intracellular Ca++ chelator BAPTA/AM had no effect on LDH release from VEH/Control-treated cells but markedly reduced cytotoxicity induced by DCLF/TNF treatment, as well as the IFN-mediated enhancement of DCLF/TNF-induced cytotoxicity (Figure 2A).
FIG. 2.
Treatment with BAPTA/AM, a membrane-permeable Ca++ chelator, reduced cytotoxicity mediated by DCLF/cytokine cotreatment. HepG2 cells were pretreated with VEH (0.1% DMSO) or BAPTA/AM (10 μM) for 4 h. Cells were then treated with DCLF (250 µM) alone or in combination with TNF (10 ng/ml) and/or IFN (10 ng/ml), and (A) cytotoxicity or (B) caspase-3 activity was measured 24 h later. a, significantly different from corresponding bar within VEH. b, significantly different from corresponding bar within TNF. c, significantly different from Control within a cytokine group. d, significantly different from DCLF without BAPTA/AM within a cytokine group. Data are represented as mean ± SEM of at least 3 experiments. Abbreviations: VEH, vehicle; TNF, tumor necrosis factor-alpha; IFN, interferon-gamma; LDH, lactate dehydrogenase; DCLF, diclofenac; BAPTA/AM, acetoxymethyl-1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid.
DCLF/TNF-induced cytotoxicity and the IFN-mediated enhancement of this cytotoxicity are caspase-dependent (Fredriksson et al., 2011; Maiuri et al., 2015). BAPTA/AM pretreatment markedly reduced DCLF/cytokine-induced caspase-3 activation, suggesting that Ca++ released from an intracellular source contributes to DCLF/cytokine-induced apoptosis (Figure 2B). In contrast, incubating cells in culture medium depleted of Ca++ did not significantly alter the DCLF/cytokine-induced cytotoxic interaction, suggesting that extracellular Ca++ is not important in the cytotoxic interaction (Supplementary Figure 1).
BAPTA/AM has been reported to have iron-chelating activities (Britigan and Rasmussen, 1998); accordingly, a potential role for iron in DCLF/cytokine-induced cytotoxicity was assessed. Furthermore, since ROS can be generated through iron-catalyzed reactions, we also evaluated a potential contribution of ROS to the DCLF/cytokine interaction. Cytotoxicity induced by treatment with DCLF/cytokines was unaffected by inclusion of either deferoxamine to chelate iron or Tempol to scavenge ROS (Supplementary Figure 2).
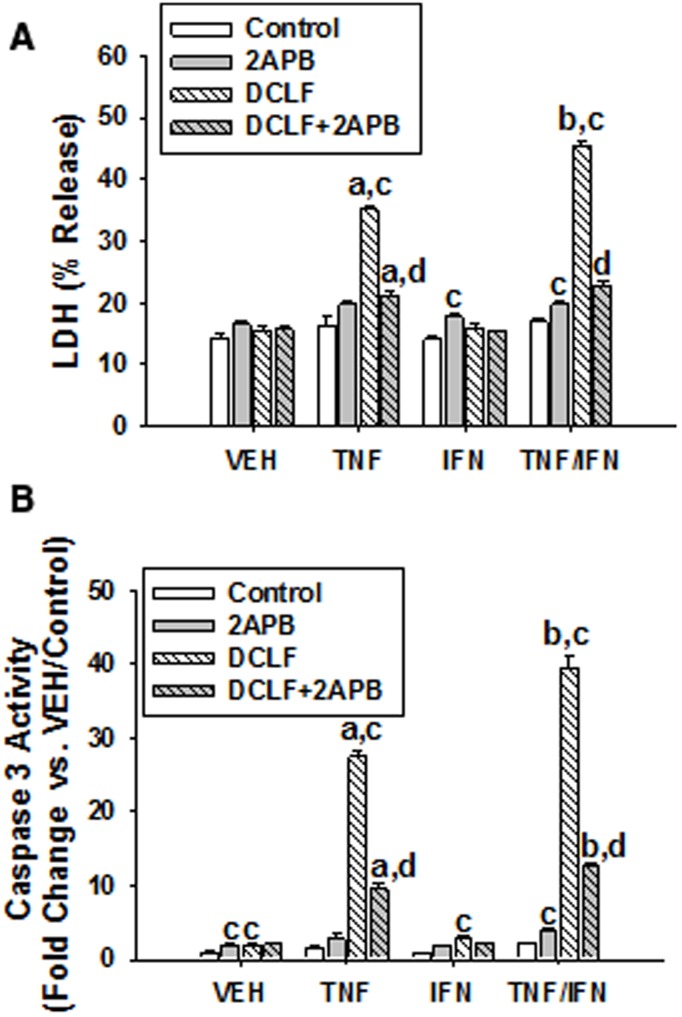
An IP3 Receptor Antagonist Reduces Cytotoxicity Induced by DCLF/Cytokine Cotreatment
The ER is widely known for its role in intracellular Ca++ sequestration, and Ca++ can be released from the ER via activation of IP3 receptors located on the ER membrane. Treatment of HepG2 cells with 2-APB, an IP3 receptor antagonist, almost completely eliminated DCLF/TNF-induced cytotoxicity as well as the IFN-mediated enhancement of cytotoxicity (Figure 3A). In addition, treatment of HepG2 cells with 2-APB markedly reduced DCLF/cytokine-induced caspase-3 activation (Figure 3B).
FIG. 3.
Treatment with 2-APB, an IP3 receptor antagonist, reduced cytotoxicity induced by DCLF/cytokine cotreatment. HepG2 cells were treated with VEH (0.1% DMSO) or 2-APB (100 μM) and treated simultaneously with DCLF (250 µM) alone or in combination with TNF (10 ng/ml) and/or IFN (10 ng/ml). (A) Cytotoxicity or (B) caspase-3 activity was measured 24 h later. a, significantly different from corresponding bar within VEH. b, significantly different from corresponding bar within TNF treatment group. c, significantly different from Control within a cytokine group. d, significantly different from DCLF without 2-APB within a cytokine group. Data are represented as mean ± SEM of at least 3 experiments. Abbreviations: VEH, vehicle; TNF, tumor necrosis factor-alpha; IFN, interferon-gamma; LDH, lactate dehydrogenase; DCLF, diclofenac; APB, aminophenoxydiphenyl borate.
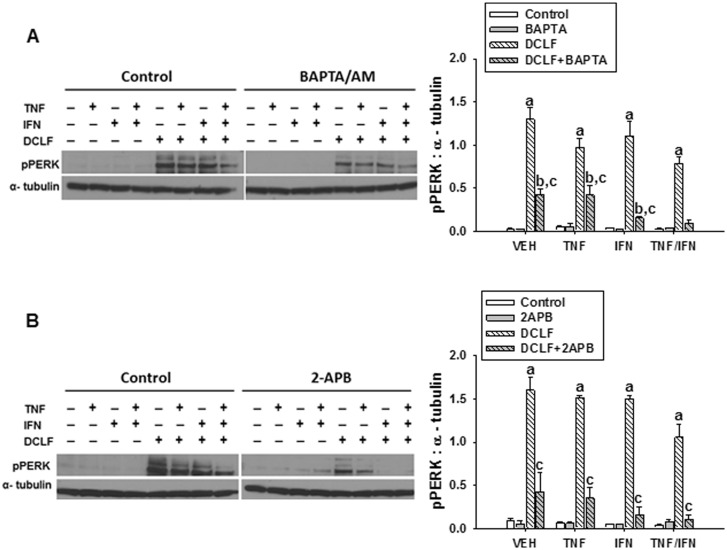
Ca++ Contributes to DCLF-Mediated Activation of the ER Stress Sensor, PERK
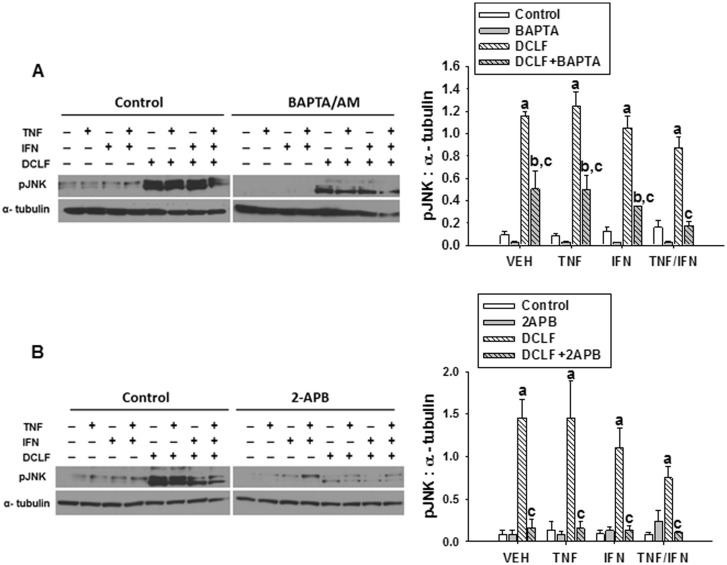
The ER stress pathway and intracellular Ca++ dysregulation are intricately linked phenomena. ER stress is known to promote elevations in intracellular Ca++, and this can in turn promote persistent activation of the ER stress pathway leading to apoptosis (Fribley et al., 2009). We evaluated whether Ca++ contributes to DCLF-mediated, persistent ER stress. Treatment with cytokines alone did not cause activation (phosphorylation) of PERK (Figures 4A and B). Treatment with DCLF led to phosphorylation of PERK, and this was unaffected by addition of TNF and/or IFN. Treatment with either BAPTA/AM (Figure 4A) or 2-APB (Figure 4B) significantly decreased the activation of PERK.
FIG. 4.
Ca++ contributes to DCLF-mediated activation of the ER stress sensor, PERK. HepG2 cells were treated with VEH (0.1% DMSO), (A) BAPTA/AM (10 μM, 4 h before addition of DCLF/cytokines) or (B) 2-APB (100 μM, simultaneous addition with DCLF/cytokines) and treated with sterile water (Control) or DCLF (250 µM) alone or in combination with TNF (10 ng/ml) and/or IFN (10 ng/ml). Proteins were collected 18 h after drug treatment. pPERK and α-tubulin levels were detected via western analysis. a, significantly different from Control group within a cytokine treatment. b, significantly different from BAPTA/AM (A) or 2-APB (B) within a cytokine treatment group. c, significantly different from DCLF within a cytokine treatment. Western analysis of proteins from cells treated with and without BAPTA/AM or 2-APB was performed simultaneously. Data are represented as mean ± SEM of at least 3 experiments. Abbreviations: VEH, vehicle; DCLF, diclofenac; pPERK, phosphorylated protein kinase RNA-like endoplasmic reticulum kinase; BAPTA/AM, acetoxymethyl-1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; APB, aminophenoxydiphenyl borate.
Ca++ Contributes to DCLF-Mediated JNK Activation
DCLF/TNF-mediated cytotoxicity and the IFN-mediated enhancement of that cytotoxicity are JNK-dependent processes (Fredriksson et al., 2011; Maiuri et al., 2015). DCLF caused activation of JNK at 18 hours, consistent with previous findings, and this effect was unaltered by cytokine treatment (Figures 5A and B). DCLF/cytokine-mediated JNK activation was reduced by pretreatment with BAPTA/AM (Figure 5A) and by treatment with 2-APB (Figure 5B).
FIG. 5.
Ca++ contributes to DCLF-mediated JNK activation. HepG2 cells were treated with VEH (0.1% DMSO), (A) BAPTA/AM (10 μM, 4 h before addition of DCLF/cytokines) or (B) 2-APB (100 μM, simultaneous addition with DCLF/cytokines) and treated with sterile water (Control) or DCLF (250 µM) alone or in combination with TNF (10 ng/ml) and/or IFN (10 ng/ml). Proteins were collected 18 h after drug treatment. pJNK and α-tubulin levels were detected via western analysis. a, significantly different from Control group within a cytokine treatment. b, significantly different from BAPTA/AM (A) or 2-APB (B) within a cytokine treatment group. c, significantly different from DCLF within a cytokine treatment. Western analysis of proteins from cells treated with and without BAPTA/AM or 2-APB was performed simultaneously. Data are represented as mean ± SEM of at least 3 experiments. Abbreviations: VEH, vehicle; DCLF, diclofenac; pJNK, phosphorylated c-Jun N-terminal kinase; BAPTA/AM, acetoxymethyl-1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; APB, aminophenoxydiphenyl borate.
Ca++ Contributes to DCLF-Mediated ERK Activation
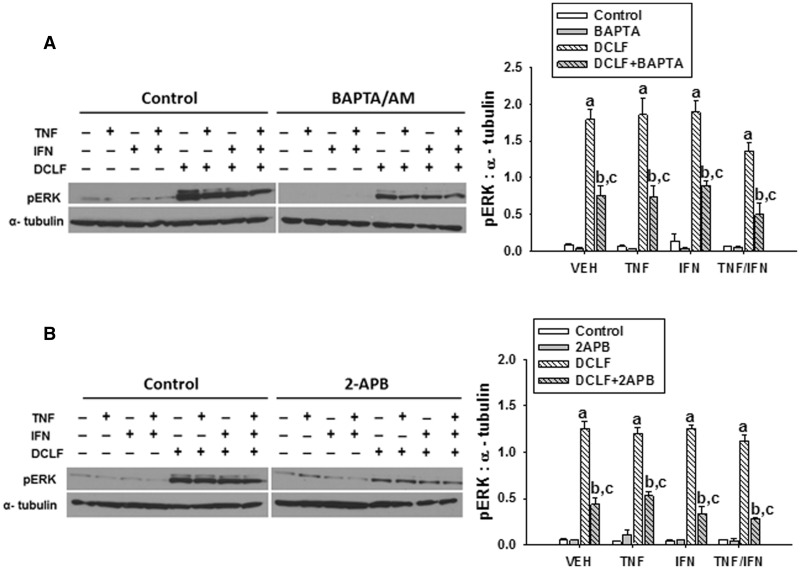
The IFN-mediated enhancement of DCLF/TNF-induced cytotoxicity depends on ERK (Maiuri et al., 2015). DCLF treatment promoted strong activation of ERK that was unaffected by cytokine treatment (Figure 6), confirming our earlier observation. Pretreatment of cells with BAPTA/AM significantly reduced ERK activation induced by DCLF (Figure 6A). Similarly, treatment of HepG2 cells with 2-APB markedly reduced DCLF-mediated activation of ERK (Figure 6B).
FIG. 6.
Ca++ contributes to DCLF-mediated ERK activation. HepG2 cells were treated with VEH (0.1% DMSO), (A) BAPTA/AM (10 μM, 4 h before addition of DCLF/cytokines) or (B) 2-APB (100 μM, simultaneous addition with DCLF/cytokines) and treated with sterile water (Control) or DCLF (250 µM) alone or in combination with TNF (10 ng/ml) and/or IFN (10 ng/ml). Proteins were collected 18 h after drug treatment. pERK and α-tubulin were detected via western analysis. a, significantly different from Control group within a cytokine treatment. b, significantly different from BAPTA/AM (A) or 2-APB (B) within a cytokine treatment group. c, significantly different from DCLF within a cytokine treatment. Western analysis of proteins from cells treated with and without BAPTA/AM or 2-APB was performed simultaneously. Data are represented as mean ± SEM of at least 3 experiments. Abbreviations: VEH, vehicle; DCLF, diclofenac; pERK, phosphorylated extracellular signal-regulated kinase; BAPTA/AM, acetoxymethyl-1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; APB, aminophenoxydiphenyl borate.
Ca++ Contributes to DCLF/IFN-Mediated Phosphorylation of STAT-1 at Ser 727
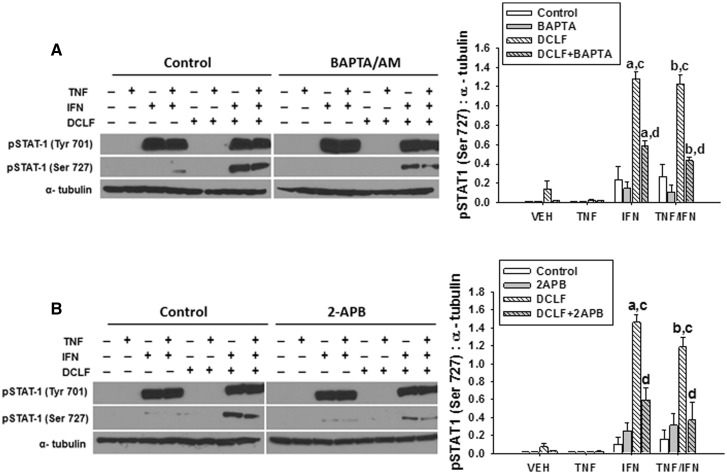
Janus kinase (JAK)-mediated phosphorylation of STAT-1 at Tyr 701 and ERK-mediated phosphorylation of STAT-1 at Ser 727 are required for maximal activation of STAT-1 and STAT-1-mediated apoptosis (Varinou et al., 2003). We demonstrated previously that DCLF-mediated ERK activation promotes phosphorylation of STAT-1 at Ser 727 in the presence of IFN and that the IFN-mediated enhancement of DCLF/TNF-induced cytotoxicity is driven by ERK (Maiuri et al., 2015). Since Ca++ contributed to DCLF-mediated ERK activation, we evaluated whether Ca++ also contributes to DCLF/IFN-induced phosphorylation of STAT-1 at Ser 727. As reported previously by Maiuri et al. (2015), treatment with IFN led to phosphorylation of Tyr 701 of STAT-1 in the absence and presence of DCLF, but Ser 727 of STAT-1 was only phosphorylated in the presence of both IFN and DCLF (Figure 7). Treatment of HepG2 cells with either BAPTA/AM or 2-APB significantly reduced DCLF/IFN-mediated phosphorylation of STAT-1 at Ser 727 without affecting phosphorylation of STAT-1 at Tyr 701 (Figure 7).
FIG. 7.
Ca++ contributes to DCLF/IFN-mediated phosphorylation of STAT-1 at Ser 727. HepG2 cells were treated with VEH (0.1% DMSO), (A) BAPTA/AM (10 μM, 4 h before addition of DCLF/cytokines) or (B) 2-APB (100 μM, simultaneous addition with DCLF/cytokines) and treated with sterile water (Control) or DCLF (250 µM) alone or in combination with TNF and/or IFN. Proteins were collected 18 h after drug treatment. pSTAT-1 (Tyr 701), pSTAT-1 (Ser 727), and α-tubulin levels were detected via western analysis. a, significantly different from corresponding bar in VEH group. b, significantly different from corresponding bar in TNF group. c, significantly different from Control within a cytokine group. d, significantly different from DCLF within a cytokine group. Western analysis of proteins from cells treated with and without BAPTA/AM or 2-APB was performed simultaneously. Data are represented as mean ± SEM of at least 3 experiments. Abbreviations: VEH, vehicle; DCLF, diclofenac; pSTAT-1, phosphorylated signal transducer and activator of transcription-1; Tyr, tyrosine; Ser, serine; BAPTA/AM, acetoxymethyl-1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; APB, aminophenoxydiphenyl borate.
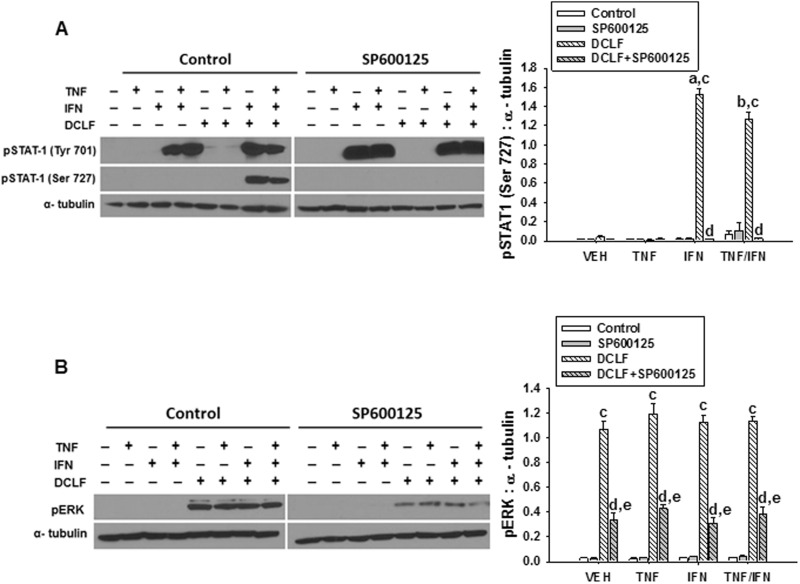
JNK Promotes DCLF/IFN-Mediated Phosphorylation of STAT-1 at Ser 727 Via Activation of ERK
Activation of JNK and ERK both contributed to the IFN-mediated enhancement of DCLF/TNF-induced cytotoxicity (Maiuri et al., 2015). ERK contributed to the phosphorylation of STAT-1 at Ser 727 (Maiuri et al., 2015), but the role of JNK in this response is unknown, as is whether there is interdependence of JNK and ERK activation. Treatment of HepG2 cells with the JNK inhibitor SP600125 prevented DCLF/IFN-mediated phosphorylation of STAT-1 at Ser 727 (Figure 8A). Moreover, treatment with SP600125 significantly reduced DCLF-induced activation of ERK (Figure 8B).
FIG. 8.
JNK promotes DCLF/IFN-mediated phosphorylation of STAT-1 at Ser 727 via activation of ERK. HepG2 cells were treated with VEH (0.1% DMSO) or SP600125 (20 μM) and simultaneously treated with sterile water (Control) or DCLF (250 µM) alone or in combination with TNF and/or IFN. Whole cell lysates were collected 18 h after treatment. (A) pSTAT-1 (Tyr 701), pSTAT-1 (Ser 727), α-tubulin and (B) pERK and α-tubulin levels were detected via western analysis. a, significantly different from corresponding bar in VEH group. b, significantly different from corresponding bar in TNF group. c, significantly different from Control within a cytokine group. d, significantly different from DCLF within a cytokine group. e, significantly different from SP600125 within a cytokine group. Western analysis of proteins from cells treated with and without SP600125 was performed simultaneously. Data are represented as mean ± SEM of at least 3 experiments. Abbreviations: VEH, vehicle; DCLF, diclofenac; pSTAT-1, phosphorylated signal transducer and activator of transcription-1; Tyr, tyrosine; Ser, serine; pERK, phosphorylated extracellular signal-regulated kinase.
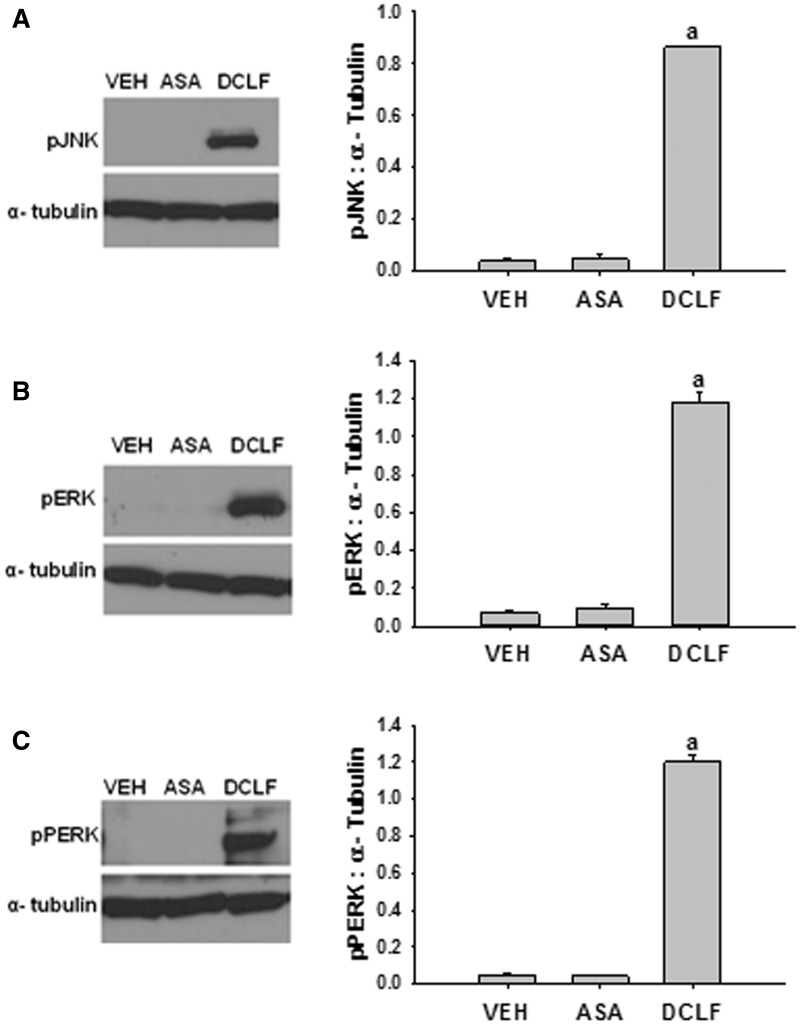
Aspirin Does Not Promote Activation of JNK or ERK, or the ER Stress Sensor, PERK
The results above suggest that DCLF-induced activation of JNK, ERK, and the ER stress sensor PERK is required for cytotoxic synergy mediated by DCLF/cytokine cotreatment. Aspirin is an NSAID that is not associated with human IDILI, and it does not synergize with cytokines to kill HepG2 cells (Maiuri et al., 2015). To evaluate the specificity of the IDILI-associated drug DCLF in activating these pathways, we evaluated whether aspirin treatment promotes activation of JNK, ERK, and PERK. The concentration of aspirin chosen relative to its maximal plasma concentration observed in human patients (Cmax) is comparable to that chosen for DCLF relative to its Cmax (Brandon et al., 1986; Xu et al., 2008). Treatment of HepG2 cells with aspirin did not result in activation of any of these factors (Figure 9).
FIG. 9.
Aspirin does not promote activation of JNK, ERK, or PERK. HepG2 cells were treated with VEH (0.1% DMSO), ASA (2 mM), or DCLF (250 µM). Protein was collected 18 h after treatment. (A) pJNK, (B) pERK, and (C) pPERK levels were measured via western analysis. a, significantly different from all treatment groups. Data are represented as mean ± SEM of at least 3 experiments. Abbreviations: VEH, vehicle; ASA, aspirin; pJNK, phosphorylated c-Jun N-terminal kinase; pERK, phosphorylated extracellular signal-regulated kinase; pPERK, phosphorylated protein kinase RNA-like endoplasmic reticulum kinase.
DISCUSSION
We and others have shown that DCLF synergizes with cytokines to cause cytotoxicity in human primary hepatocytes (Cosgrove et al., 2009) and HepG2 cells (Fredriksson et al., 2011; Maiuri et al., 2015) by a mechanism involving the MAPKs, JNK, and ERK. Moreover, Fredriksson et al. (2014) reported that DCLF caused activation of the ER stress pathway in HepG2 cells as early as 2 h after treatment, and this response was unaffected by TNF but was required for DCLF/TNF-induced cytotoxic synergy. In addition, DCLF induced a delayed increase in intracellular Ca++ in transformed human hepatocytes after 6 h of exposure (Lim et al., 2006). Moreover, others have shown that cytokine treatment can also promote an increase in intracellular Ca++ (Chang et al., 2004; Delmotte et al., 2012). Similarly, we demonstrated in HepG2 cells that DCLF promotes a delayed increase in intracellular Ca++, and this increase was enhanced by treatment with TNF/IFN (Figure 1). Since ER stress is strongly associated with promoting increases in intracellular Ca++, and since DCLF treatment can induce both of these responses in liver cells, we hypothesized that Ca++ contributes to DCLF/cytokine-induced cytotoxic synergy.
Chelation of intracellular Ca++ markedly reduced cytotoxicity and caspase-3 activation induced by DCLF/cytokine treatment (Figure 2), whereas removal of extracellular Ca++ did not affect the cytotoxic interaction (Supplementary Figure 1). These findings suggest that Ca++ released from an intracellular source underlies the cytotoxic interaction mediated by DCLF/cytokine cotreatment.
Ca++ is primarily stored in the ER, but it can also be stored in other intracellular compartments including the mitochondria (Berridge et al., 1998). Ryanodine receptors and IP3 receptors are the most well-characterized Ca++ channels localized to the ER membrane. Moreover, IP3 receptor activation is associated with Ca++-mediated apoptosis via the intrinsic (mitochondrial) pathway (Deniaud et al., 2008; Verrier et al., 2004). 2-APB, a commonly used IP3 receptor antagonist, greatly reduced the cytotoxic interaction mediated by DCLF/TNF cotreatment, prevented the IFN-mediated enhancement of cytotoxicity, and reduced DCLF/cytokine-induced caspase-3 activation (Figure 3), suggesting that these responses require IP3 receptor activation. This receptor is typically activated by IP3 released from membranes by phospholipase C. Whether DCLF can activate a phospolipase C isozyme or directly activate the IP3 receptor remains to be determined.
Activation of PERK, a component of the ER stress-response pathway, contributed to cytotoxicity mediated by DCLF/TNF (Fredriksson et al., 2014). Although treatment with TNF did not affect the activation of PERK (Fredriksson et al., 2014), the participation of IFN in activation of PERK had not been investigated. As observed with TNF, IFN did not modulate the activation of PERK in response to DCLF treatment (Figure 4). It is well understood that ER stress can cause increases in intracellular Ca++. Conversely, elevated intracellular Ca++ can engage in a feedback amplification loop, thereby promoting persistent activation of the ER stress pathway (Timmins et al., 2009). Treatment with either BAPTA/AM or 2-APB reduced DCLF-induced PERK activation. These findings indicate that intracellular free Ca++ contributes to persistent ER stress in response to DCLF exposure.
DCLF/cytokine-induced cytotoxic synergy requires JNK (Fredriksson et al., 2011; Maiuri et al., 2015). JNK is activated in response to a variety of stressors, including TNF exposure, UV radiation, ROS, ER stress, and increased intracellular Ca++ (Kim and Sharma, 2004; Seki et al., 2012). The kinetics of the activation of JNK can vary depending on the inducer, and the duration of JNK activation is critical to determining the fate of a cell. For instance, TNF promotes transient activation of JNK, which is associated with cell survival. Other stressors that induce persistent activation of JNK are associated with caspase activation and apoptosis (Seki et al., 2012). TNF modestly activated JNK at 12 h after treatment in HepG2 cells, and this response was transient in the absence of DCLF. In contrast, in the presence of DCLF, JNK activation persisted until at least 18 h (Maiuri et al., 2015). The mechanism by which DCLF promotes persistent activation of JNK appears to involve ER stress and elevated intracellular Ca++, since BAPTA/AM and 2-APB reduced activation of JNK in response to DCLF (Figure 5). Since 2-APB also greatly reduced cytotoxicity induced by DCLF/cytokine cotreatment, these results are consistent with our previous findings which suggested that JNK is necessary for DCLF/cytokine-induced cytotoxic synergy (Maiuri et al., 2015).
Ca++ can lead to activation of JNK via several routes, one of which involves activation of Ca++/calmodulin-dependent protein kinase II (CaMKII) in response to ER stress. CaMKII can directly phosphorylate apoptosis signal-regulating kinase 1, a MAPK kinase kinase (MAPKKK) that promotes downstream sustained activation of JNK (Brnjic et al., 2010). Taken together, these findings indicate that DCLF-mediated activation of JNK requires availability of Ca++. Furthermore, IP3-mediated release of Ca++ from the ER drives DCLF-induced JNK activation.
The IFN-mediated enhancement of DCLF/TNF-induced cytotoxicity involves ERK (Maiuri et al., 2015). DCLF treatment caused activation of ERK as early as 12 h; this persisted until after 18 h and was unaffected by TNF and/or IFN treatment (Maiuri et al., 2015). The observation that both BAPTA/AM and 2-APB reduced ERK activation (Figure 6) suggests that Ca++ released from the ER via IP3 receptors contributes to ERK activation induced by DCLF. It remains unclear exactly how Ca++ causes activation of ERK; however, in some cell types, Ca++ can promote activation of ERK via activation of the upstream MAPKKK, Ras (Li et al., 2005).
Activation of STAT-1 plays an important role in IFN-dependent apoptosis (Cao et al., 2015). Dual phosphorylation of STAT-1 is required for maximal activation (Varinou et al., 2003), and this occurred in cells treated with DCLF/IFN but not in cells treated with IFN or DCLF alone (Maiuri et al., 2015 and Figure 7). Not surprisingly, treatment of HepG2 cells with IFN caused phosphorylation of STAT-1 at Tyr 701 (Maiuri et al., 2015 and Figure 7) presumably via activation of JAK (Schroder et al., 2004). DCLF in the presence of IFN promoted phosphorylation of STAT-1 at Ser 727 via activated ERK (Maiuri et al., 2015). Consistent with their effects on DCLF-induced ERK activation, treatment with either BAPTA/AM or 2-APB reduced DCLF/IFN-induced phosphorylation of STAT-1 at Ser 727 (Figure 7). These results indicate that cytoplasmic-free Ca++ contributes to STAT-1 activation induced by DCLF/IFN cotreatment. Interestingly, treatment with BAPTA/AM or 2-APB did not affect IFN-induced phosphorylation of STAT-1 at Tyr 701 (Figure 7).
JNK can also phosphorylate STAT-1 at Ser 727 (Zhang et al., 2004). Indeed, treatment of HepG2 cells with a JNK inhibitor eliminated the IFN-mediated enhancement of DCLF/TNF-induced cytotoxicity, suggesting that, along with ERK, JNK drives the IFN component of the DCLF/cytokine interaction (Maiuri et al., 2015). Treatment with the JNK inhibitor SP600125 eliminated DCLF/IFN-induced phosphorylation of STAT-1 at Ser 727 without affecting IFN-mediated phosphorylation of STAT-1 at Tyr 701 (Figure 8A). These results indicate that in addition to ERK, JNK mediates activation of STAT-1 in response to DCLF/IFN and raise the question, does JNK contribute to the activation of ERK in response to DCLF treatment? The kinetics of DCLF-induced JNK activation were similar to the kinetics of DCLF-induced ERK activation (Maiuri et al., 2015). Treatment with SP600125 reduced DCLF-induced ERK activation (Figure 8B), suggesting that JNK is involved in the activation of ERK in response to DCLF treatment but is not solely responsible for it. In addition, these results raise the possibility that JNK contributes to the phosphorylation of STAT-1 at Ser 727 by promoting the activation of ERK.
We and others have shown that aspirin, an NSAID not associated with IDILI, does not synergize with cytokines to kill primary human hepatocytes (Cosgrove et al., 2009) or HepG2 cells (Maiuri et al., 2015). Unlike DCLF, treatment with aspirin did not induce activation of PERK, JNK, or ERK (Figure 9). Thus, in the absence of activation of these pathways, no cytotoxicity was observed, and when they were activated, cytotoxicity occurred. These observations lend support to the conclusion that activation of PERK, JNK, and ERK plays a critical role in the cytotoxic DCLF/cytokine interaction and support the possibility that NSAID-mediated IDILI involves activation of ER stress and MAPK pathways in hepatocytes.
The molecular mechanisms by which DCLF initiates activation of the ER stress pathway remain unknown. It is possible that DCLF directly or indirectly promotes activation of phospholipase C leading to formation of IP3, which causes IP3 receptor (IP3R)-mediated release of Ca++ from the ER. This could lead to elevated cytoplasmic Ca++ and ER stress. Another possibility is that DCLF inhibits proteasomal degradation of proteins, leading to accumulation of proteins in the ER. Indeed, several IDILI-associated drugs can cause ER stress by inhibiting the ubiquitin-proteasome pathway. For instance, efavirenz, ritonavir, and lopinavir, antiretroviral drugs associated with IDILI, induced ER stress in primary human and transformed hepatocytes by inhibiting the proteasome (Apostolova, 2013; Kao et al., 2012). Moreover, several protease inhibitors used for the treatment of HIV induced activation of CHOP, activating transcription factor-4, and several other ER stress markers in human HepG2 cells by inhibiting the proteasome (Parker et al., 2005). Additional studies are needed to elucidate the molecular mechanisms underlying DCLF-induced activation of the ER stress pathway.
It was shown previously that a large concentration of DCLF promotes mitochondrial permeability transition leading to death of hepatocytes in vitro, and this was initiated by an increase in intracellular Ca++ (Lim et al., 2006). Although the role of mitochondrial permeability transition in the cytotoxic interaction between DCLF and cytokines has not been examined directly, results from a previous study suggest that it might be involved. Specifically, the observation that siRNA-mediated silencing of components of the apoptosome protected HepG2 cells from DCLF/TNF-induced cytotoxicity raised the possibility that mitochondrial permeability transition occurs, releasing cytochrome c into the cytosol to initiate the intrinsic pathway of apoptosis (Fredriksson et al., 2011). Furthermore, in our study Ca++ release contributed to sustained activation of JNK, a phenomenon that is well known to promote mitochondrial permeability transition and cell death. Therefore, our findings are consistent with what has been demonstrated previously with regard to DCLF-mediated induction of mitochondrial permeability transition and its role in death of hepatocytes.
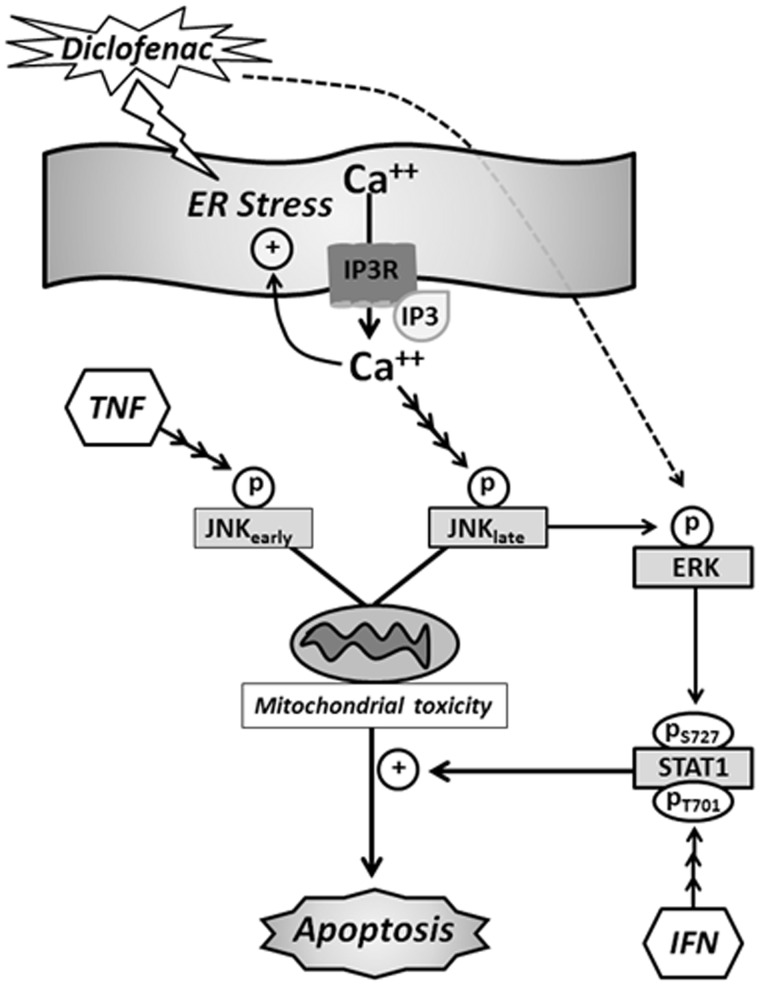
Collectively, these findings indicate that availability of Ca++ in the cytoplasm, likely due to release from the ER via IP3 receptor stimulation, underlies most, if not all, aspects of DCLF/cytokine-induced cytotoxic synergy. This raises the possibility that increased intracellular Ca++ contributes to hepatocellular injury that occurs in cases of human IDILI. Finally, results from this study together with previous findings tie together critical components of the mechanism underlying the cytotoxic interaction mediated by DCLF and cytokines (summarized in Figure 10).
FIG. 10.
Proposed mechanism of DCLF/cytokine-induced cytotoxic synergy. DCLF treatment causes early activation of the ER stress response pathway (Fredriksson et al., 2014). ER stress results in release of Ca++ from the ER via IP3 receptors, leading to an increase in cytoplasmic free Ca++ (Deniaud et al., 2008). Ca++ released from the ER during ER stress can participate in a feedback amplification loop, leading to persistent ER stress, which is known to be associated with apoptosis (Timmins et al., 2009). Consistent with its induction of the ER stress pathway, DCLF treatment caused an increase in intracellular Ca++. Although not shown in the diagram, TNF/IFN treatment caused a delayed enhancement of the DCLF-mediated increase in intracellular Ca++. The increase in intracellular free Ca++ contributes to the cytotoxic DCLF/cytokine interaction not only by contributing to persistent ER stress but also by activating JNK and ERK. TNF treatment causes modest, early activation of JNK that is transient in the absence of DCLF but persistent in its presence (Maiuri et al., 2015). Persistent activation of JNK is essential for DCLF/TNF-induced apoptosis presumably by contributing to mitochondrial permeability transition (Fredriksson et al., 2011; Lim et al., 2006).DCLF-induced activation of JNK contributes to activation of ERK which plays a role in the IFN-mediated enhancement of DCLF/TNF-induced cytotoxicity (Maiuri et al., 2015). IFN alone leads to phosphorylation of STAT-1 at Tyr 701 which allows translocation of STAT-1 from the cytoplasm to the nucleus. Translocation allows STAT-1 to be phosphorylated at Ser 727 (Sadzak et al. 2008), leading to full activation. In our model, dual phosphorylation of STAT-1 occurs through exposure to IFN only in the presence of DCLF and is mediated through ERK which is activated in response to DCLF. Full STAT-1 activation enhances apoptosis caused by the interaction of DCLF and TNF. Abbreviations: ER, endoplasmic reticulum; IP3R, inositol trisphosphate receptor; Ca++, calcium; JNK, c-Jun N-terminal kinase; TNF, tumor necrosis factor-alpha; ERK, extracellular signal-regulated kinase; STAT-1, signal transducer and activator of transcription; S727, serine 727; T701, tyrosine 701; IFN, interferon gamma.
Supplementary Material
ACKNOWLEDGMENTS
We thank Robert Crawford for his assistance with measuring intracellular Ca++ by flow cytometry.
FUNDING
The National Institutes of Health [grants RO1DK061315 and T32 GM092715 (A.R.M)] and a Colgate Palmolive Award for Research in Alternative Methods from the Society of Toxicology.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
REFERENCES
- Aithal G., Day C., Leathart J., Daly K. (2000). Relationship of polymorphism in CYP2C9 to genetic susceptibility to diclofenac-induced hepatitis. Pharmacogenetics. 10, 511–518. [DOI] [PubMed] [Google Scholar]
- Aithal G., Watkins P., Andrade R., Larrey D., Molokhia M., Takikawa H., Hunt C., Wilke R., Avigan M., Kaplowitz N., et al. (2011). Case definition and phenotype standardization in drug-induced liver injury. Clin. Pharmacol. Ther. 89, 806–815. [DOI] [PubMed] [Google Scholar]
- Apostolova N., Gomez-Sucerquia L., Alegre F., Funes H., Victor V., Barrachina M., Blas-Garcia A., Esplugues J. (2013). ER stress in human hepatic cells treated with efavirenz: mitochondria again. Hepatology 59, 780–789. [DOI] [PubMed] [Google Scholar]
- Banks A., Zimmerman H., Ishak K., Harter J. (1995). Diclofenac-associated hepatotoxicity: analysis of 180 cases reported to the Food and Drug Administration as adverse reactions. Hepatology 22, 820–827. [PubMed] [Google Scholar]
- Berridge M., Bootman M., Lipp P. (1998). Calcium—a life and death signal. Nature 395, 645–648. [DOI] [PubMed] [Google Scholar]
- Boelsterli U. (2002). Mechanisms of NSAID-induced hepatotoxicity: focus on nimesulide. Drug Saf. 25, 633–648. [DOI] [PubMed] [Google Scholar]
- Boelsterli U. (2003). Diclofenac-induced liver injury: a paradigm of idiosyncratic drug-induced liver injury. Toxicol. Appl. Pharmacol. 192, 307–322. [DOI] [PubMed] [Google Scholar]
- Bollo M., Paredes R., Holstein D., Zheleznova N., Camacho P., Lechleiter J. (2010). Calcineurin interacts with PERK and dephosphorylates calnexin to relieve ER stress in mammals and frogs. PLoS One 5, e11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bort R., Ponsoda X., Jover R., Gómez-Lechón M., Castell J. (1999). Diclofenac toxicity to hepatocytes: a role for drug metabolism in cell toxicity. J. Pharmacol. Exp. Ther. 288, 65–72. [PubMed] [Google Scholar]
- Brandon R., Eadie M., Curran A., Nolan P., Presneill J. (1986). A new formulation of aspirin: bioavailability and analgesic efficacy in migraine attacks. Cephalalgia 6, 19–27. [DOI] [PubMed] [Google Scholar]
- Britigan B., Rasmussen G. (1998). Binding of iron and inhibition of iron-dependent oxidative cell injury by the “calcium chelator” 1,2-bis(2-aminophenoxy)ethane N,N,N’,N’-tetraacetic acid (BAPTA). Biochem. Pharmacol. 55, 287–295. [DOI] [PubMed] [Google Scholar]
- Brnjic S., Olofsson M., Havelka A., Linder S. (2010). Chemical biology suggests a role for calcium signaling in mediating sustained JNK activation during apoptosis. Mol. Biosyst. 6, 767–774. [DOI] [PubMed] [Google Scholar]
- Cao Z., Zheng Q., Li G., Hu X., Feng S., Xu G., Zhang K. (2015). STAT1-mediated down-regulation of Bcl-2 expression is involved in IFN-γ/TNF-α-induced apoptosis in NIT-1 cells. PLoS One 10, e0120921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang I., Cho N., Kim S., Kim J., Kim E., Woo J., Nam J., Kim S., Lee M. (2004). Role of calcium in pancreatic islet cell death by IFN-gamma/TNF-alpha. J. Immunol. 172, 7008–7014. [DOI] [PubMed] [Google Scholar]
- Choi A., Choi J., Hwang K., Jeong Y., Wonchae C., Kyung-Sik Y., Yoohun H., Sung S., Jang H., Eui-Ju Y., et al. (2014). Licochalcone A induces apoptosis through endoplasmic reticulum stress via a phospholipase Cγ1 -, Ca2+-, and reactive oxygen species-dependent pathway in HepG2 human hepatocellular carcinoma cells. Apoptosis 19, 682–697. [DOI] [PubMed] [Google Scholar]
- Cosgrove B., King B., Hasan M., Alexopoulos L., Farazi P., Hendriks B., Griffith L., Sorger P., Tidor B., Xu J., et al. (2009). Synergistic drug-cytokine induction of hepatocellular death as an in vitro approach for the study of inflammation-associated idiosyncratic drug hepatotoxicity. Toxicol. Appl. Pharmacol. 237, 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmotte P., Yang B., Thompson M., Pabelick C., Prakash Y., Sieck G. (2012). Inflammation alters regional mitochondrial Ca2+ in human airway smooth muscle cells. Am. J. Physiol. Cell Physiol. 303, C244–C256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X., Liguori M., Sparkenbaugh E., Waring J., Blomme E., Ganey P., Roth R. (2008). Gene expression profiles in livers from diclofenac-treated rats reveal intestinal bacteria-dependent and -independent pathways associated with liver injury. J. Pharmacol. Exp. Ther. 327, 634–644. [DOI] [PubMed] [Google Scholar]
- Deng X., Stachlewitz R., Liguori M., Blomme E., Waring J., Luyendyk J., Maddox J., Ganey P., Roth R. (2006). Modest inflammation enhances diclofenac hepatotoxicity in rats: role of neutrophils and bacterial translocation. J. Pharmacol. Exp. Ther. 319, 1191–1199. [DOI] [PubMed] [Google Scholar]
- Deniaud A., Sharaf el dein O., Maillier E., Poncet D., Kroemer G., Lemaire C., Brenner C. (2008). Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene 27, 285–299. [DOI] [PubMed] [Google Scholar]
- Dugan C., Fullerton A., Roth R., Ganey P. (2011). Natural killer cells mediate severe liver injury in a murine model of halothane hepatitis . Toxicol. Sci. 120, 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson L., Herpers B., Benedetti G., Matadin Q., Puigvert J., de Bont H., Dragovic S., Vermeulen N., Commandeur J., Danen E., et al. (2011). Diclofenac inhibits tumor necrosis factor-alpha-induced nuclear factor-kappaB activation causing synergistic hepatocyte apoptosis. Hepatology 53, 2027–2041. [DOI] [PubMed] [Google Scholar]
- Fredriksson L., Wink S., Herpers B., Benedetti G., Hadi M., de Bont H., Groothuis G., Luijten M., Danen E., de Graauw M., et al. (2014). Drug-induced endoplasmic reticulum and oxidative stress responses independently sensitize toward TNFα-mediated hepatotoxicity. Toxicol. Sci. 140, 144–159. [DOI] [PubMed] [Google Scholar]
- Fribley A., Zhang K., Kaufman R. (2009). Regulation of apoptosis by the unfolded protein response. Methods Mol. Biol. 559, 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García Rodríguez L., Williams R., Derby L., Dean A., Jick H. (1994). Acute liver injury associated with nonsteroidal anti-inflammatory drugs and the role of risk factors. Arch. Intern. Med. 154, 311–316. [DOI] [PubMed] [Google Scholar]
- Gorina R., Petegnief V., Chamorro A., Planas A. (2005). AG490 prevents cell death after exposure of rat astrocytes to hydrogen peroxide or proinflammatory cytokines: involvement of the Jak2/STAT pathway. J. Neurochem. 92, 505–518. [DOI] [PubMed] [Google Scholar]
- Hassan F., Morikawa A., Islam S., Tumurkhuu G., Dagvadorj J., Koide N., Naiki Y., Mori I., Yoshida T., Yokochi T. (2008). Lipopolysaccharide augments the in vivo lethal action of doxorubicin against mice via hepatic damage. Clin. Exp. Immunol. 151, 334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Chen J., Wu C., Chen C., Tang N., Ho Y., Lo C., Lin J., Chung J., Lin J. (2009). Capsaicin-induced apoptosis in human hepatoma HepG2 cells. Anticancer Res. 29, 165–174. [PubMed] [Google Scholar]
- Hussain S., Harris C. (2006). p53 biological network: at the crossroads of the cellular-stress response pathway and molecular carcinogenesis. J. Nippon Med. Sch. 73, 54–64. [DOI] [PubMed] [Google Scholar]
- Kao E., Shinohara M., Feng M., Lau M., Ji C. (2012). Human immunodeficiency virus protease inhibitors modulate Ca2+ homeostasis and potentiate alcoholic stress and injury in mice and primary mouse and human hepatocytes. Hepatology 56, 594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Sharma R. (2004). Calcium-mediated activation of c-Jun NH2-terminal kinase (JNK) and apoptosis in response to cadmium in murine macrophages. Toxicol. Sci. 81, 518–527. [DOI] [PubMed] [Google Scholar]
- Li D., Liu J., Mao Y., Xiang H., Wang J., Ma W., Dong Z., Pike H., Brown R., Reed J. (2005). Calcium-activated RAF/MEK/ERK signaling pathway mediates p53-dependent apoptosis and is abrogated by alpha B-crystallin through inhibition of RAS activation. Mol. Biol. Cell. 16, 4437–4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M., Lim P., Gupta R., Boelsterli U. (2006). Critical role of free cytosolic calcium, but not uncoupling, in mitochondrial permeability transition and cell death induced by diclofenac oxidative metabolites in immortalized human hepatocytes. Toxicol. Appl. Pharmacol. 217, 322–331. [DOI] [PubMed] [Google Scholar]
- Liu Q., Ulrike M., Flügel D., Kietzmann T. (2004). Induction of plasminogen activator inhibitor I gene expression by intracellular calcium via hypoxia-inducible factor-1. Blood 104, 3993–4001. [DOI] [PubMed] [Google Scholar]
- Maiuri A., Breier A., Gora L., Parkins R., Ganey P., Roth R. (2015). Cytotoxic synergy between cytokines and NSAIDs associated with idiosyncratic hepatotoxicity is driven by mitogen-activated protein kinases. Toxicol. Sci. 146, 265–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R., Flint O., Mulvey R., Elosua C., Wang F., Yang W., Noor M. (2005). Endoplasmic reticulum stress links dyslipidemia to inhibition of proteasome activity and glucose transport by HIV protease inhibitors. Molecular Pharmacology 67, 1909–1919. [DOI] [PubMed] [Google Scholar]
- Pinsky M., Vincent J., Alegre M., Dupont E. (1993). Serum cytokine levels in human septic shock. Chest 103, 565–575. [DOI] [PubMed] [Google Scholar]
- Sadzak I., Schiff M., Gattermeler I., Glinitzer R., Sauer I., Saalmuller A., Yang E., Schaljo B., Kovarik P. (2008). Recruitment of Stat1 to chromatin is required for interferon-induced serine phosphorylation of Stat1 transactivation domain. PNAS. 105, 8944–8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K., Hertzog P., Ravasi T., Hume D. (2004). Interferon-γ: an overview of signals, mechanisms and functions. J. Leukocyte Biol. 75, 163–189. [DOI] [PubMed] [Google Scholar]
- Seki E., Brenner D., Karin M. (2012). A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology 124, 307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Ganey P., Roth R. (2009a). Tumor necrosis factor alpha is a proximal mediator of synergistic hepatotoxicity from trovafloxacin/lipopolysaccharide coexposure. J. Pharmacol. Exp. Ther. 328, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Ditewig A., Waring J., Liguori M., Blomme E., Ganey P., Roth R. (2009b). Coexposure of mice to trovafloxacin and lipopolysaccharide, a model of idiosyncratic hepatotoxicity, results in a unique gene expression profile and interferon gamma-dependent liver injury. Toxicol. Sci. 107, 270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Pervaiz S. (2006). TNF receptor superfamily-induced cell death: redox-dependent execution. FASEB J. 20, 1589–1598. [DOI] [PubMed] [Google Scholar]
- Taudorf S., Krabbe K., Berg R., Pedersen B., Møller K. (2007). Human models of low-grade inflammation: bolus versus continuous infusion of endotoxin. Clin. Vaccine Immunol. 14, 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh N., Farrell G. (2003). Hepatotoxicity associated with non-steroidal anti-inflammatory drugs. Clin. Liver Dis. 7, 401–413. [DOI] [PubMed] [Google Scholar]
- Timmins J., Ozcan L., Seimon T., Li G., Malagelada C., Backs J., Backs T., Bassel-Duby R., Olson E., Anderson M., et al. (2009). Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J. Clin. Invest. 119, 2925–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unzueta A., Vargas H. (2013). Nonsteroidal anti-inflammatory drug-induced hepatotoxicity . Clin. Liver Dis. 17, 643–656. [DOI] [PubMed] [Google Scholar]
- Varinou L., Ramsauer K., Karaghiosoff M., Kolbe T., Müller M., Decker T. (2003). Phosphorylation of the Stat1 transactivation domain is required for full-fledged IFN-gamma-dependent innate immunity. Immunity 19, 793–802. [DOI] [PubMed] [Google Scholar]
- Verrier F., Deniaud A., Lebras M., Métivier D., Kroemer G., Mignotte B., Jan G., Brenner C. (2004). Dynamic evolution of the adenine nucleotide translocase interactome during chemotherapy-induced apoptosis. Oncogene 23, 8049–8064. [DOI] [PubMed] [Google Scholar]
- Westerink W., Schoonen W. (2007a). Cytochrome p450 enzyme levels in HepG2 cells and cryopreserved primary human hepatocytes and their induction in HepG2 cells. Toxicol. in vitro 21, 1581–1591. [DOI] [PubMed] [Google Scholar]
- Westerink W., Schoonen W. (2007b). Phase II enzyme levels in HepG2 cells and cryopreserved primary human hepatocytes and their induction in HepG2 cells. Toxicol. in vitro 21, 1592–1602. [DOI] [PubMed] [Google Scholar]
- Xu J., Henstock P., Dunn M., Smith A., Chabot J., Graff D. (2008). Cellular imaging predictions of clinical drug-induced liver injury. Toxicol. Sci. 105, 97–105. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Cho Y., Peterson B., Zhu F., Dong Z. (2004). Evidence of STAT1 phosphorylation modulated by MAPKs, MEK1 and MSK1. Carcinogenesis 25, 1165–1175. [DOI] [PubMed] [Google Scholar]
- Zou W., Beggs K., Sparkenbaugh E., Jones A., Younis H., Roth R., Ganey P. (2009). Sulindac metabolism and synergy with tumor necrosis factor-alpha in a drug-inflammation interaction model of idiosyncratic liver injury. J. Pharmacol. Exp. Ther. 331, 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.