Abstract
We have recently characterized the role of lipocalin 2 (Lcn2) as a new adipose-derived cytokine in the regulation of adaptive thermogenesis via a non-adrenergic pathway. Herein, we explored a potential non-adrenergic mechanism by which Lcn2 regulates thermogenesis and lipid metabolism. We found that Lcn2 is a retinoic acid target gene, and retinoic acid concurrently stimulated UCP1 and Lcn2 expression in adipocytes. Lcn2 KO mice exhibited a blunted effect of all-trans-retinoic acid (ATRA) on body weight and fat mass, lipid metabolism, and retinoic acid signaling pathway activation in adipose tissue under the high fat diet-induced obese condition. We further demonstrated that Lcn2 is required for the full action of ATRA on the induction of UCP1 and PGC-1α expression in brown adipocytes and the restoration of cold intolerance in Lcn2 KO mice. Interestingly, we discovered that Lcn2 KO mice have decreased levels of retinoic acid and retinol in adipose tissue. The protein levels of STRA6 responsible for retinol uptake were significantly decreased in adipose tissue. The retinol transporter RBP4 was increased in adipose tissue but decreased in the circulation, suggesting the impairment of RBP4 secretion in Lcn2 KO adipose tissue. Moreover, Lcn2 deficiency abolished the ATRA effect on RBP4 expression in adipocytes. All the data suggest that the decreased retinoid level and action are associated with impaired retinol transport and storage in adipose tissue in Lcn2 KO mice. We conclude that Lcn2 plays a critical role in regulating metabolic homeostasis of retinoids and retinoid-mediated thermogenesis in adipose tissue.
Keywords: adipose tissue, cytokine, energy metabolism, lipid metabolism, retinoid, lipocalin 2
Introduction
Adipose tissue plays a central role in metabolic homeostasis, inflammation, and insulin resistance. The main functions of white adipose tissue (WAT)5 are to regulate lipid storage and mobilization, glucose homeostasis, and inflammation; many WAT functions in metabolism and insulin sensitivity are exerted by adipose-derived adipokines and cytokines (1). In contrast to WAT, brown adipose tissue (BAT) burns fat to generate heat via the uncoupling protein 1 (UCP1)-mediated non-shivering thermogenic mechanism (2). Recent discovery of metabolically active BAT in adult humans suggests a potential alternative mechanism for metabolic regulation (3). A decline in adipose tissue thermogenic activity has been associated with metabolic dysregulation in obesity and diabetes. Thus, activating thermogenic function of adipose tissue is an attractive therapeutic approach for obesity and obesity-related metabolic complications in humans.
UCP1 is fully responsible for thermogenesis in brown adipocytes (4). It is well established that the β-adrenergic-cAMP-PKA pathway is an important mediator of sympathetic activation of UCP1 transcription and thermogenesis. The activation of p38 MAPK (p38 mitogen-activated protein kinases), a key component of adrenergic pathway, is essential for PGC-1α and UCP1 expression by adrenergic stimulation. Increasing sympathetic nervous system tone is known as one of the therapeutic approaches to activate BAT thermogenesis and the color of WAT becoming beige, thereby promoting weight loss. However, this approach causes non-selective activation of the sympathetic nervous system leading to cardiovascular side effects, which prevent the therapeutic use of adrenergic activators. Therefore, recent attempts have been aimed at identifying nonadrenergic activators of BAT and WAT thermogenesis and oxidative metabolism as potential novel therapeutic targets for obesity and diabetes (5). However, mechanisms for nonadrenergic activation of thermogenesis and oxidative metabolism remain largely unknown. Some physiological and pharmacological factors have been known to activate UCP1 and adipose tissue thermogenesis via a nonadrenergic mechanism, including thiazolidinedione and retinoic acid.
Adipose tissue is a central organ for retinoid storage and metabolism, and it is also the main target tissue of retinoid action. Many metabolic functions such as lipid and energy homeostasis, adipogenesis, inflammatory response, and insulin sensitivity have been attributed to retinoids (6–8). Retinoic acid, a major active retinoid form, modulates oxidative metabolism and thermogenesis by serving as a ligand for specific nuclear receptors, RARs and RXRs, allowing for their activation (9, 10). In the context of adaptive thermogenesis, retinoic acid is a key regulator of BAT thermogenic activation. Because of the presence of the retinoic acid-response element (RARE) and peroxisome proliferator-activated receptor-response element in the promoter of the Ucp1 gene (11–13), retinoic acid is a direct and strong non-adrenergic inducer of Ucp1 gene transcription (14–18). The requirement for retinoids is increased during prolonged cold exposure; vitamin A deficiency significantly impairs thermogenic function (19), leading to the reduced survival time of animals during cold exposure (20).
Lipocalin 2 (Lcn2) is a 25-kDa secreted protein that belongs to the Lipocalin supergene family of hydrophobic ligand-binding proteins (21, 22). We and others have previously identified Lcn2 as a new adipokine, and Lcn2 is significantly up-regulated in adipose tissue of obese rodents (23, 24). We have also shown that mice lacking Lcn2 developed significantly increased body fat mass, adipocyte hypertrophy, and adipose inflammation upon a high fat diet (HFD) feeding (25, 26). Moreover, we discovered that Lcn2 deficiency impairs adaptive thermogenesis via an uncharacterized non-adrenergic mechanism (27). Studies have demonstrated that the Lcn2 gene promoter region contains multiple transcription factor binding sites and nuclear receptor response elements, including RARE (28, 29). Lcn2 has also been identified as a putative mediator of retinoic acid (RA) effect on apoptosis in human sebocytes (30). All the above information led us to investigate the role of Lcn2 in regulating retinoid metabolism and action on metabolic homeostasis and thermogenesis of adipose tissue. We found that Lcn2 deficiency significantly disrupts retinoid homeostasis in adipose tissue and blunts the retinoic acid effect on energy metabolism and BAT thermogenesis.
Experimental Procedures
Animals and Experimental Design
Lcn2-deficient mice were kindly provided by Dr. Alan Aderem, Institute for Systems Biology, Seattle, WA. Heterozygous mating scheme was used to generate WT and Lcn2−/− mice for the experiments as described previously (25). Animals were housed in a specific pathogen-free facility at the University of Minnesota. All animal studies were conducted with the approval of the University of Minnesota Animal Care and Use Committee. All experiments conformed to the National Institutes of Health guidelines for laboratory animal care.
Four-week-old Lcn2-deficient male mice were housed with a 12-h light-dark cycle and fed a HFD (fat calories, 60%; Bio-Serv F3282; New Brunswick, NJ) for 12 weeks. Age- and sex-matched wild-type (WT) mice served as controls. The inguinal and epididymal WAT and BAT were collected and used for gene expression and retinoid analyses (n = 7–8 per genotype).
At 16 weeks of age, HFD-fed WT and Lcn2 KO mice were treated with all-trans-retinoic acid (ATRA) (Sigma) at a dose of 50 mg/kg body weight via gavage daily for 24 days based on previous publications (31–34). WT and Lcn2 KO controls were treated with vehicle (1% ethanol). During the 24 days of ATRA treatment, HFD feeding was continued until the end of the experiment. Body weight was recorded daily until euthanization. Blood and tissues were collected, weighed, frozen in liquid nitrogen, and stored at −80 °C for use.
For thermogenesis studies, chow diet-fed WT and Lcn2 KO mice at 14 weeks of age were subjected to gavage of ATRA (10 mg/kg body weight/day; ATRA was dissolved in 1% ethanol and 99% water) for 2 weeks. Mice treated with the vehicle (1% ethanol in water) served as controls. The rectal temperature was recorded on days 7 and 14 of ATRA treatment using the Micro Therma Thermometer (Braintree Scientific, Braintree, MA) at an ambient temperature of 22 °C. Following the measurement of body temperature on day 14, the mice were exposed to 4 °C for 4 h. Rectal temperature of mice was measured every 30 min. After cold exposure, mice were sacrificed immediately; blood and tissue samples were collected for various assessments.
Primary Adipocyte Differentiation and Treatment
Stromal-vascular (SV) cells were isolated from inguinal WAT and interscapular BAT of WT and Lcn2-deficient mice for differentiation as described previously (26, 27). When reaching confluence in culture medium containing Dulbecco's modified Eagle's medium (DMEM, Invitrogen) with l-glutamine and 25 mm glucose, 10% FBS (Atlanta Biological, Lawrenceville, GA), and 100 units/ml penicillin/streptomycin (Invitrogen), the preadipocytes from inguinal WAT were then incubated for 72 h in culture medium further supplemented with 0.25 mm isobutylmethylxanthine (Sigma), 0.5 μm dexamethasone (Sigma), and 0.85 μm insulin. For the brown preadipocytes, 0.125 mm indomethacin (Sigma) and 1 nm triiodothyronine (Sigma) were added to this medium to allow for the induction of differentiation. Subsequently, the cells were maintained in culture medium supplemented with 0.85 μm insulin for 4–5 days until fully differentiated with massive accumulation of fat droplets.
LC/MS/MS Analysis of Retinoids
Tissue levels of ATRA were determined by ultra high performance liquid chromatography tandem mass spectrometry (LC/MS/MS) using a Waters Xevo TQ MS ACQUITY UPLC system (Waters). For this analysis, we only employed LC/MS-grade acetonitrile and LC/MS-grade water purchased from Thermo Fisher (Pittsburgh, PA). ATRA was purchased from Sigma. Penta-deuterated ATRA was employed as an internal standard and was purchased from Toronto Research Chemicals (North York, Ontario, Canada). Retinoid concentrations were verified spectrophotometrically using published ϵ values (35). Tissue homogenates were extracted using the two-step acid-base extraction described by Kane et al. (36). ATRA was detected and quantified using the multiple reaction monitoring mode employing the following transitions: ATRA, m/z 301.16 → 123.00; penta-deuterated ATRA, m/z 306.15 → 127.03; and 9-cis-RA, m/z 301.16 → 123.00.
Analysis of All-trans-RA and 9-cis-RA Binding to Lcn2
Cell Culture and Transfection
3T3-L1 cells were plated at a density such that the cells would be about 80% confluent at 24 h after plating. Cells were transfected with a mouse Lcn2-FLAG DNA construct expressed under the control of CMV promoter in the pCAGGS vector (kindly provided by Dr. Yukio Nakamura from RIKEN BioResource Center, Japan). Cells were transfected with 10 μg of DNA/10-cm plate using a DNA/Lipofectamine transfection reagent (Invitrogen) at a ratio of 1:4 according to the protocol recommended by the manufacturer. At 48 h after transfection, the culture media were harvested.
Purification of Lcn2-FLAG
Cell culture media containing FLAG-tagged Lcn2 was added to the anti-FLAG® M2 affinity column (Sigma) according to the manufacturer's protocol, and bound protein was eluted with 0.1 m glycine, 0.2 m NaCl, pH 3.5. Fractions of 1 ml were collected into tubes containing 20 μl of neutralization buffer (2 m Tris, pH 8.0). Fractions were analyzed for the presence of proteins using the Bradford assay. The purified proteins were detected by SDS-PAGE and Western blot analysis. The target proteins were pooled and concentrated on an Amicon stirred cell with a YM10 membrane (Millipore) and dialyzed against 100 times the volume of 20 mm NaPi, 0.1 m NaCl, 1 mm EDTA, pH 7.5.
Protein Concentration Determination
Protein concentration was determined using the extinction coefficient of Lcn2, 29,930 m−1 cm−1 at A280 (33) along with Beer's law (A = ϵlc), where A is the absorbance; ϵ is the extinction coefficient; l is the path length, and c is the concentration. The A280 was determined using a Cary 50 Bio UV-visible spectrophotometer (Varian Analytical Instruments).
All-trans-RA and 9-cis-RA Binding
For titration with RA, the Trp and Tyr fluorescence of the Lcn2 was excited at 280 nm and detected at 340 nm with slits set to 5 nm. Measurements were performed with an FluoroMax-2 spectrofluorometer (Instruments S.A., Inc.) using a 5 × 5 cm2 quartz cuvette equipped with a small magnetic stirrer, at room temperature. 500 μl of a 1 μm solution of Lcn2 in the assay buffer was titrated with 0.5 mm RA in 50% N,N-dimethylformamide, as prepared above, in 1-μl increments up to 10 μl followed by 2-μl additions until the total volume of RA added reached 20 μl or 19.2 μm. After each addition, the sample was allowed to stir for 1 min in the dark before the sample was read. The instrument read-out was set to have an integration time of 0.1 s, 1% S.E., and 10 maximum trials.
Retinoic Acid Extinction Coefficient
Because of the inner filter effects of RA at 280 nm, the extinction coefficient for RA in the assay buffer was determined for correction purposes (Breustedt). Again using Beer's law, the A280 of a known concentration of a RA was determined using a spectrophotometer, and the extinction coefficient was calculated. For this experiment, the RA was tested in the same conditions as the assay, but instead of LCN2, 5 μm N-acetyltryptophanamide (Sigma) was used. The A280 values of 10 samples of 9.8 μm RA, using the solution of N-acetyl-tryptophanamide in the assay buffer as a blank, were averaged before applying to Beer's law.
This extinction coefficient (ϵapp) was used to correct the fluorescence for the data analysis using the following formula: F = Fapp × 10[caret](ϵapp × cL), where Fapp is the apparent fluorescence determined from the spectrofluorometer read-out; ϵapp is the apparent extinction coefficient of RA determined from the above experiment, and cL is the concentration of RA at each titration step (37).
Data Analysis
Using the starting volume (v = 500 μl), RA concentration (L = 500 μm), and Lcn2 concentration (p = 1 μm), an Excel spread sheet with columns A–K was used to perform the data calculations for each titration as follows: A, total volume (μl) (v + B); B, RA volume added (μl); C, final concentration RA at each point (μm) (L·B/A); D, apparent fluorescence; E, corrected fluorescence (D·10[caret](3558·C·10[caret]−6)); F, dilution factor = total volume/starting volume (A/first A); G, fluorescence adjusted (E/F); H, ΔF (first G − G); I, % total fluorescence change (H/last H); J, bound Lcn2 (μm) [P·I]; K, free RA (μm) (C–J). The percent total fluorescence change versus free RA was plotted, and the data were fitted by non-linear least squares regression to determine the apparent KD value using GraphPad Prism® (version 4.0a). The standard error between six apparent KD values was also calculated.
RNA Isolation and Relative Quantitative RT-PCR
Total RNA was prepared from frozen tissues with TRIzol reagent (Invitrogen). cDNA was synthesized from DNase-treated total RNA (1 μg) using a Superscript II reverse transcriptase kit (Invitrogen). Quantitative amplification by PCR was carried out using SYBR Green quantitative PCR master mix (SABiosciences, Frederick, MD) by a StepOne real time PCR system (Applied Biosystem, Foster City, CA). For quantification, β-actin mRNA served as an endogenous control. The ΔΔCt method was used to calculate the results. The primer sequences for amplifying the target genes and the GenBankTM accession number are summarized in supplemental Table S1.
Western Blot Analysis
Tissue samples were homogenized and solubilized in RIPA buffer (Sigma). Cell lysates were prepared in lysis buffer containing 25 mm Tris-HCl, pH 7.5, 0.5 mm EDTA, 25 mm sodium chloride, 10 mm sodium fluoride, 1 mm sodium vanadate, 1% Nonidet P-40, and protease inhibitor mixtures (Roche Diagnostics). Protein concentrations of homogenized samples and cell lysates were measured using bicinchoninic acid method (Pierce). Equal amounts of proteins were separated on SDS-PAGE and immunoblotted with primary antibodies against Lcn2 and UCP1 (R&D Systems, Minneapolis, MN), hormone-sensitive lipase (HSL), phospho-HSL (Ser-563), RARα, β-actin (Cell Signaling Technology, Danvers, MA), and STRA6 and RBP4 (Abcam, Cambridge, MA) according to the recommendations of the manufacturers. After incubation with primary antibodies, the membranes were incubated with secondary antibodies conjugated to horseradish peroxidase. ECL Western blotting detection systems (GE Healthcare) were used to detect antibody reactivity.
Statistical Analysis
A test of normal distribution of the variants was performed showing that the variants were normally distributed before analysis of variance, and Student's t test was used for the data analysis. Results were expressed as means ± S.E. of mean (S.E.). Differences in the levels of retinoids and gene expression in adipose tissues between HFD-fed WT and Lcn2-deficient mice were evaluated using the two-tailed Student's t test. Differences in parameters of body weight, fat mass, and gene expressions between groups treated and untreated with ATRA were evaluated by analysis of variance. A p value of less than 0.05 was considered significant.
Results
ATRA Is a Potent Inducer of Lcn2 and UCP1 in Adipocytes
Because of the presence of a non-canonical RARE and peroxisome proliferator-activated receptor response element in the promoter of the Ucp1 gene (11–13), ATRA is known to be a direct and strong inducer of Ucp1gene transcription (14–18). Our previous studies have shown that Lcn2 is an important regulator of BAT thermogenic function independently of an adrenergic mechanism (27). A non-canonical RARE was also found in the promoter region of Lcn2 gene, suggesting that Lcn2 is a target gene of retinoic acid action and may connect to retinoic acid stimulation of adipose tissue thermogenesis. To determine how retinoic acid regulates Lcn2 and UCP1 expression in adipose tissue, we investigated the regulation of Ucp1 and Lcn2 expression by ATRA in differentiated brown and white adipocytes.
Because the Ucp1 expression is regulated by the activation of various transcription factors, including peroxisome proliferator-activated receptor, RAR, and cyclic AMP-response elements (38), we compared the expression of UCP1 by RA with that by norepinephrine and thiazolidinedione. We found that ATRA stimulated much higher levels of Ucp1 gene expression than norepinephrine did in differentiated brown adipocytes (Fig. 1A). ATRA also enhanced UCP1 protein expression in differentiated brown adipocytes (Fig. 1B). In differentiated brown (Fig. 1C), inguinal (Fig. 1D), and epididymal (Fig. 1E) adipocytes, 24-h treatment of RA induced Lcn2 protein expression in a dose-dependent manner; RA treatment for 24 h also stimulated Lcn2 secretion in brown adipocytes (Fig. 1C). Moreover, administration of ATRA via oral gavage for 14 days led to a significant increase in serum levels of Lcn2 in normal mice (Fig. 1F). These data together with the structural characteristics of Lcn2 directed us to study the role of Lcn2 in retinoic metabolism and retinoic acid function in thermogenesis.
FIGURE 1.
RA regulation of UCP1 and Lcn2 expression in adipocytes. Ucp1 gene expression by norepinephrine (NE) and all-trans-retinoic acid (RA) in differentiated brown adipocytes (A) is shown. The UCP1 protein expression by RA in differentiated brown adipocytes (B) is shown. The induction of Lcn2 protein expression and secretion by RA in differentiated brown adipocytes (C) is shown. The induction of Lcn2 protein expression by RA in differentiated inguinal (D) and epididymal adipocytes (E) is shown. Serum Lcn2 in HFD-fed mice with or without RA treatment for 24 days (F) is shown. The data are represented as mean ± S.E. **, p < 0.01 versus control.
Lcn2 Deficiency Attenuates ATRA Effect on Body Weight, Fat Mass, and Lipid Metabolism in HFD-induced Obesity
To test the hypothesis that Lcn2 mediates the action of retinoic acid on energy metabolism, we investigated how Lcn2 deficiency impacts the effect of retinoic acid on reducing body weight and adiposity in HFD-induced obese mice. WT and Lcn2 KO mice were fed a HFD for 12 weeks followed by oral gavage of ATRA (50 mg/kg body weight) for 24 days. As we expected, ATRA administration for 24 days led to a significant loss of body weight in WT mice; ATRA-treated WT mice had significantly lower body weights than control WT mice as indicated by change in body weight (Fig. 2A). However, in Lcn2 KO mice, the ATRA effect on change in body weight was significantly reduced (Fig. 2A). Likewise, ATRA treatment significantly reduced body fat mass, including inguinal and brown adipose tissue in WT mice, but not Lcn2 KO mice (Fig. 2B). Similar changes were also perceived in liver weights of WT but not Lcn2−/− mice in response to ATRA treatment.
FIGURE 2.
Effect of ATRA on body weight, adiposity, and lipid metabolism in Lcn2 KO mice. Body weight (A), tissue weight (B), and the mRNA expression of genes involved in lipid metabolism (C–E) and HSL phosphorylation (F) in epididymal (Epi) WAT, (G) in inguinal (Ing) WAT, and (H) in BAT of HFD-fed mice with or without ATRA treatment for 24 days (n = 4 for WT control, n = 5 for KO control, n = 5 for WT with RA, and n = 6 for KO with RA) are shown. The gene expression data are represented as mean ± S.E. The data of body weight and HSL phosphorylation are presented as means ± S.D. The experiments were repeated three times with three different sets of mice, yielding similar results. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus vehicle (Veh); #, p < 0.05; ##, p < 0.01; ###, p < 0.001 versus WT mice. AU, arbitrary units.
Next, we examined the effect of ATRA treatment on the expression of genes involved in lipid metabolism in epididymal and inguinal adipose depots in HFD-induced WT and Lcn2 KO obese mice, including the following: fatty acid synthase (Fasn); peroxisome proliferator-activated receptor γ (Pparg); the transcription factor sterol regulatory element-binding factor 1 (Srebf1); stearoyl-CoA desaturase 1 (Scd1); lipoprotein lipase (Lpl); hormone-sensitive lipase (HSL, Lipe); and aP2. Under the non-treated condition, Lcn2 KO mice exhibited the down-regulated expression of Pparg, Srebf1, Scd1, and Lpl genes in epididymal adipose tissue but not inguinal adipose tissue compared with WT mice (Fig. 2, C and D). The expression of all the examined genes involved in lipid metabolism was markedly induced by ATRA treatment in epididymal WAT of WT mice, whereas ATRA treatment had no or little effect on the expression of these genes with the exception for Pparg and Scd1 in inguinal adipose tissue of WT mice (Fig. 2, C and D). The down-regulation of Pparg gene expression and a trend toward decreased expression of Srebf1 and Lpl genes were also observed in BAT of Lcn2 KO mice compared with WT mice (Fig. 2E). In BAT, ATRA treatment simulated the expression of Fasn and Scd1 genes; this effect of ATRA was blunted in Lcn2 KO mice (Fig. 2E). Additionally, ATRA treatment markedly stimulated the phosphorylation of HSL in epididymal and inguinal WAT as well as BAT of WT mice (Fig. 2, E–H). Intriguingly, ATRA-stimulated HSL phosphorylation was diminished in Lcn2 KO mice (Fig. 2, F–H).
To determine the effect of Lcn2 deficiency on retinoic acid signaling activation, the retinoic acid-induced expression of RARs and RAR target genes, including RARα, RARβ, RXRα, cytosolic phosphoenolpyruvate carboxykinase (Pck1), acyl-coenzyme A oxidase 1, palmitoyl (Acox1), solute carrier family 25 (mitochondrial carnitine/acylcarnitine translocase), and member 20 (Slc25a20), was examined in epididymal and inguinal adipose tissues. As illustrated in Fig. 3, A–C, the mRNA expression levels of RARα and RXRα were significantly lower in epididymal but not inguinal adipose tissue and BAT of Lcn2 KO mice compared with WT controls. After 24 days of ATRA treatment, mRNA expression of RARα and RARβ as well as RAR target genes Pck1, Acox1, and Slc25a20 was up-regulated in epididymal and inguinal adipose tissues as well as BAT in WT mice (Fig. 3, A–F). However, the effect of ATRA in up-regulating the expression of Rara, Rarb, and Rxra genes was significantly blunted in epididymal and inguinal adipose tissue of Lcn2 KO mice (Fig. 3, A–F). In BAT, the gene expression of Rara but not Rarb and Rxra was significantly stimulated by ATRA; Lcn2 deficiency blunted the ATRA effect on Rxra expression (Fig. 3C). These results suggest that retinoic acid action and signaling activation is impaired in the absence of Lcn2 and that this effect selectively occurs in epididymal adipose tissue.
FIGURE 3.
Effect of ATRA on RA signaling activation in adipose tissue in Lcn2 KO mice. The mRNA expression of RARs and RXR (A–C) as well as RA-responsive genes (D–F) in epididymal (Epi) WAT, inguinal (Ing) WAT, and BAT of HFD-fed WT and Lcn2 KO mice treated with or without ATRA for 24 days (n = 4 for WT control, n = 5 for KO control, n = 5 for WT with RA, and n = 6 for KO with RA) is shown. The data are represented as mean ± S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus vehicle (Veh); #, p < 0.05; ##, p < 0.01; ###, p < 0.001 versus WT mice. AU, arbitrary units.
Lcn2 Deficiency Attenuates ATRA Effect on Thermogenic Activation of BAT
Our previous studies have demonstrated that Lcn2-deficient mice had impaired BAT thermogenic activation (25, 27). We then sought to test the hypothesis that Lcn2 mediates RA-induced non-adrenergic activation of thermogenesis. In the first experiment, we determined whether Lcn2 is required for ATRA-stimulated thermogenesis. Mice fed a chow diet were given ATRA via oral gavage for 14 days, followed by a cold tolerance test. Although WT mice had increased body temperature after 7 and 14 days of ATRA treatment, this effect of ATRA was not observed in Lcn2 KO mice (Fig. 4A). In the cold tolerance test, ATRA was not able to completely rescue cold-intolerance in Lcn2 KO mice when exposed to 4 °C for 4 h (Fig. 4B). In the second experiment, we further tested the impact of Lcn2 deficiency on ATRA-stimulated thermogenesis in HFD-induced obese mice. WT and Lcn2 KO mice were fed a HFD for 12 weeks followed by an oral gavage of ATRA for 14 days. We found that the UCP1 protein expression was decreased in BAT of HFD-fed Lcn2 KO mice compared with WT controls (Fig. 4C). ATRA treatment strongly induced the UCP1 protein expression in BAT of HFD-fed WT mice. However, ATRA was unable to completely restore the UCP1 protein levels in BAT of Lcn2 KO mice (Fig. 4C). To more directly test the effect of RA on thermogenic activation of brown adipocytes in the absence of Lcn2, we performed an in vitro experiment using primary differentiated brown adipocytes. As we reported previously, WT and Lcn2 KO brown SV cells from BAT were able to differentiate into brown adipocytes to a similar extent (27). Because insulin has been reported to induce UCP1 expression (39), WT and Lcn2 KO brown adipocytes at day 8 of differentiation were treated with or without ATRA in the presence or absence of insulin for 24 h. ATRA or insulin alone significantly induced the mRNA expression of Ucp1 in WT brown adipocytes, whereas Lcn2 KO brown adipocytes had much lower levels of Ucp1 gene expression in the basal and ATRA- or insulin-stimulated conditions (Fig. 4D). The combination of ATRA and insulin caused a synergistic increase in Ucp1 mRNA expression in WT brown adipocytes, but this induction was markedly attenuated in Lcn2 KO brown adipocytes (Fig. 4D). Consistently, Lcn2 KO brown adipocytes had significantly lower levels of Pgc1a gene expression compared with WT cells in the basal condition and conditions with the treatment of ATRA, insulin, or ATRA and insulin in combination (Fig. 4E). These results suggest that brown adipocytes have reduced response to ATRA stimulation of BAT thermogenic activation in the absence of Lcn2.
FIGURE 4.
Effect of ATRA on thermogenesis in Lcn2 KO mice. WT and Lcn2 KO mice on an RCD at the age of 14 weeks were given ATRA via oral gavage at the dose of 10 mg/kg body weight per day for 14 days, followed by the acute cold exposure to 4 °C for 4 h. Body temperature was measured at ambient temperature of 22 °C after 7 and 14 days of ATRA treatment (A) and at 4 °C during cold tolerance test (B) (n = 7 for WT control, n = 4 for WT with RA, n = 5 for KO control, and n = 7 for KO with RA). The cold-tolerant experiments were repeated twice with two different sets of mice, yielding similar results. The UCP1 protein expression in BAT of HFD-fed WT and Lcn2 KO mice with 24 days of ATRA treatment (C) is shown. The mRNA expression of Ucp1 (D) and Pgc1α (E) by insulin (INS) and RA treatment in differentiated brown adipocytes is shown. The data of body temperature and UCP1 protein are presented as mean ± S.D. The gene expression data are presented as mean ± S.E. AU, arbitrary unit. *, p < 0.05; **, p < 0.01; ***, p < 0.001, WT versus KO; &, p < 0.05, versus vehicle (Veh); #, p < 0.05; ##, p < 0.01, WT-RA versus KO-RA; §, p < 0.05, KO-Veh versus KO-RA. Con, control; AU, arbitrary units.
Lcn2 Has a Binding Affinity for RA and Lcn2 Deficiency Disrupts Retinoid Homeostasis in BAT
Next, we sought to understand the molecular mechanism by which Lcn2 regulates RA action on energy metabolism and BAT thermogenesis. Because of the structural characteristics of Lcn2 with a hydrophobic pocket, we hypothesized that Lcn2 may serve as a retinoic acid-binding protein and play a role in mediating retinoic acid delivery and function. To test the hypothesis, we performed an Lcn2-RA binding assay. In a previous ligand binding study with RA, Lcn2 exhibited weak binding of ATRA with a Kd value between 2.8 and 5.6 μm (37). However, in that study, Lcn2 used for the titration experiment was bacterially produced. To avoid the possible effect of bacterial production on the biological activity of Lcn2, we developed techniques for expression and purification of active Lcn2 from NIH 3T3 cells. Lcn2 was devoid of any bound fatty acids as assessed by GC-MS analysis of protein extracts (data not shown). The solutions of NIH 3T3 cell-produced Lcn2 were titrated with all-trans-RA and 9-cis-RA. Our results showed that Lcn2 binds ATRA with a Bmax of 1.27 ± 0.06 and a Kd of 4.243 ± 0.54 μm (Fig. 5A) and 9-cis-RA with a Bmax of 1.13 ± 0.06 and a Kd of 2.07 ± 0.43 (Fig. 5B). Our results are very consistent with that from a previous study (37), suggesting that Lcn2 displays a very weak binding of retinoic acid. Moreover, we have employed a sensitive LC/MS/MS method for measuring adipose tissue ATRA levels as indicated in Fig. 5, C and D. Using this method, we determined the ATRA levels in BAT and serum ATRA levels in Lcn2 KO mice fed a chow diet. As shown in Fig. 5E, ATRA levels in BAT were significantly decreased (p = 0.028) in Lcn2 KO mice compared with WT mice (n = 8) (Fig. 5F). Serum levels of ATRA had no change in Lcn2 KO mice with the p value of 0.06 (p = 0.06). These data suggest that Lcn2 deficiency disrupts retinoid homeostasis in BAT.
FIGURE 5.
Ligand binding of Lcn2 with RA, RA levels in Lcn2 KO mice, and RA responsiveness in Lcn2 KO adipocytes. Fluorescence titration of Lcn2 with all-trans-retinoic acid and 9-cis-retinoic acid is shown. Protein fluorescence was measured at 25 °C in 100 mm NaCl, 1 mm EDTA, 20 mm sodium phosphate, pH 7.5. Titration of 1 μm Lcn2 with all-trans-RA (A) and 9-cis-RA (B) is shown. Fluorescence of retinoic acid was excited at 280 nm and detected at 340 nm. LC/MS/MS profiles for purified all-trans-RA and purified all-trans-RA-D5 (internal standard) (C) and all-trans-RA and all-trans-RA-D5 in an extract obtained from BAT from WT mice (D) are shown. Mean levels of all-trans-retinoic acid in BAT (E) and serum (F) in WT and Lcn2 KO mice on RCD (n = 8 for WT mice; n = 7 for KO mice) are shown. The induction of RARα nuclear translocation by a 1-h treatment of RA in primary adipocytes isolated from epididymal WAT of WT and Lcn2 KO mice (G) is shown. The induction of mRNA expression of RA-responsive genes STRA6 and CRABPII by 24 h of treatment of ATRA in differentiated brown adipocytes (H) is shown. The data are presented as mean ± S.E. Veh, vehicle. **, p < 0.05 versus control. AtRA-D5, all-trans-retinoic acid-D5; rfu, relative fluorescence units.
To further determine whether decreased retinoic acid action on thermogenesis and metabolism in vivo in mice is due to decreased transport of retinoic acid into the cell, we tested whether the addition of exogenous ATRA directly to Lcn2 KO adipocytes in vitro could rescue RAR signaling activation. Primary adipocytes were isolated from epididymal adipose tissue of regular chow diet (RCD)-fed WT and Lcn2 KO mice and were treated with ATRA for 1 h. As illustrated in Fig. 5G, ATRA treatment for 1 h caused a redistribution of RARα from the cytoplasm to the nucleus, and this action of ATRA was similarly effective in WT and Lcn2 KO adipocytes. Additionally, we determined the effect of Lcn2 deficiency on the ATRA responsiveness in differentiated adipocytes. The expression of the RA-responsive genes STRA6 and CRABPII was similarly up-regulated in differentiated brown adipocytes treated with ATRA for 24 h (Fig. 5H). These data suggest that Lcn2 deficiency does not affect the uptake or transport of ATRA into the cell.
Lcn2 Deficiency Alters Retinoid Homeostasis in White Adipose Tissue
Because 70% of the retinoic acid pool in adipose tissue is produced locally through the oxidation of retinol (41) and only 30% of the retinoic acid pool is derived from plasma retinoic acid, it seemed unlikely that the defect in Lcn2 function in transporting circulating retinoic acid into BAT is the major contributor to decreased ATRA levels in Lcn2 KO BAT. We therefore sought other possible reasons to explain the decreased ATRA and RA action in Lcn2 KO adipose tissue. It is known that endogenous retinoic acid is derived from vitamin A (retinol), which is mainly stored and metabolized in the liver. In addition to liver, adipose tissue is an important site for the storage and metabolism of retinoids (42). After ester hydrolysis, retinol is mobilized from liver stores to meet the retinoid needs of other tissues by binding to retinol-binding protein 4 (RBP4), the specific plasma transport protein for retinol (43). In retinoid-responsive tissues/cells, retinol is reversibly oxidized to retinaldehyde and then RA by alcohol dehydrogenases and retinaldehyde dehydrogenases, respectively. To understand better the role of Lcn2 in retinoid homeostasis and whether altered retinoid metabolism may be attributed to decreased ATRA levels and retinoic acid signaling in Lcn2 KO adipocytes, we extended our investigation of retinoid metabolism in white adipose tissue. First, we measured the retinoid levels in white adipose tissue and liver in Lcn2 KO mice. As shown in Table 1, the levels of retinol and retinyl ester were not affected in liver but were significantly reduced in adipose tissue (p = 0.03) of Lcn2 KO mice compared with WT mice.
TABLE 1.
Retinoid levels in liver and adipose tissue
| Retinoid | Liver |
p value | Adipose tissue |
p value | ||
|---|---|---|---|---|---|---|
| WT | Lcn2−/− | WT | Lcn2−/− | |||
| Retinol (μg/g tissue) | 15.16 ± 4.36 | 14.57 ± 2.48 | 0.75 | 0.59 ± 0.13 | 0.46 ± 0.07 | 0.034 |
| Retinyl ester (μg/g tissue) | 1510.13 ± 375.16 | 1286 ± 171.51 | 0.16 | 0.14 ± 0.18 | 0.05 ± 0.06 | 0.025 |
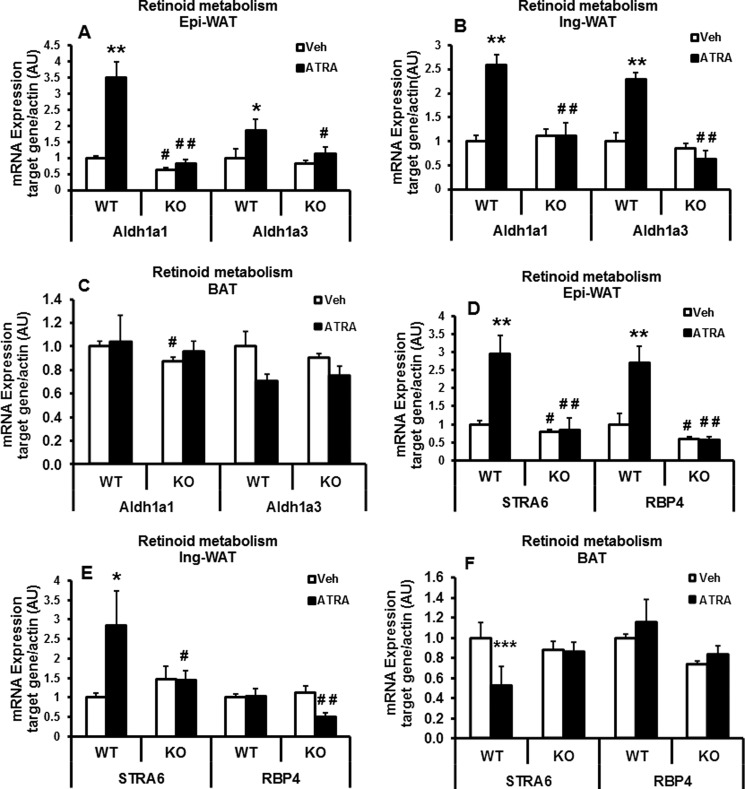
Next, we assessed the levels of genes encoding proteins involved in retinol transport, uptake, and metabolism in the cells to further explore the metabolic state of retinoids in Lcn2 KO adipose tissue. As indicated in Fig. 6, A and B, the mRNA levels of retinal dehydrogenase family 1, subfamily A1 (Aldh1a1), the key enzyme in the irreversible conversion of retinal into retinoic acid, were decreased significantly in epididymal WAT of Lcn2 KO versus WT control mice upon 15 weeks of HFD feeding (Fig. 6A), although no significant difference in the expression of Aldh1a1was observed in inguinal WAT (Fig. 6B). There was also no significant change in the expression levels of Aldh1a3 in either epididymal or inguinal WAT of Lcn2 KO mice compared with WT mice (Fig. 6, A and B). After 24 days of ATRA treatment, mRNA expression levels of Aldh1a1 and Aldh1a3 were increased in both epididymal and inguinal adipose tissue in WT but not Lcn2 KO mice (Fig. 6, A and B). The mRNA expression of Aldh1a1 was also decreased in BAT of Lcn2 KO mice. But ATRA treatment had no significant effect on Aldh1a1 and Aldh1a3 gene expression in BAT of both WT and Lcn2 KO mice (Fig. 6C).
FIGURE 6.
mRNA expression of genes involved in retinoid metabolism and transport in adipose tissue of Lcn2 KO mice on HFD. The mRNA expression of retinoid-metabolizing enzymes (A–C) and retinol transport proteins (D–F) in epididymal (Epi) WAT, inguinal (Ing) WAT, and BAT of HFD-fed mice with or without ATRA treatment for 24 days (n = 4 for WT control, n = 5 for KO control, n = 5 for WT with RA, and n = 6 for KO with RA) is shown. The data are presented as mean ± S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus WT mice. #, p < 0.05; ##, p < 0.01; Veh, vehicle; AU, arbitrary unit.
RBP4 and STRA6 (stimulated by retinoic acid gene 6), an RA-responsive gene encoding a cell-surface receptor for retinol-binding protein, play critical roles in the regulation of retinol transport and uptake. RBP4 is responsible for the transport of retinol between tissues, whereas STRA6 has been reported to be an important regulator of retinol uptake by cells (44–46). We found that the mRNA expression levels of STRA6 and RBP4 were decreased selectively in epididymal adipose tissue, and there was a trend toward a decrease in RBP4 mRNA expression in BAT of Lcn2 KO mice versus WT mice (Fig. 6, D–F). In WT mice, ATRA treatment for 24 days led to a significant increase in the mRNA expression of STRA6 and RBP4 genes in epididymal adipose tissue (Fig. 6D) and an increase in STRA6 in inguinal adipose tissue (Fig. 6D). This effect of ATAR was not observed in Lcn2 KO mice. ATRA treatment had no significant effect on STRA6 and RBP4 expression in BAT (Fig. 6F). Moreover, expression levels of STRA6 protein were also significantly decreased in primary adipocytes (Fig. 7A) and SV cells (Fig. 7B) isolated from epididymal adipose tissue of HFD-fed Lcn2 KO mice. Interestingly, we found that the protein levels of RBP4 were increased in adipose tissue and liver of HFD-fed Lcn2 KO mice compared with WT mice (Fig. 7, C and D). Using Western blotting, RBP4 in serum was detected as two bands (Fig. 7E). It is likely that these two bands may represent two different forms of RBP4 because it has been reported that there are at least two forms of RBP4, i.e. full-length and C-terminal proteolyzed RBP4 present in the circulation (47–49). Interestingly, serum levels of RBP4 were significantly reduced in HFD-fed Lcn2 KO mice (Fig. 7E), suggesting that RBP4 may accumulate in the liver and adipose tissue, but it is not released into the circulation for transport of retinol to tissues in Lcn2 KO mice.
FIGURE 7.
Expression levels of retinol transport proteins in adipocytes of Lcn2 KO mice. The protein expression of STRA6 in primary adipocytes (A) and SV cells (B) isolated from epididymal (Epi)-WAT of HFD-fed mice (n = 4 for each genotype group) is shown. The protein expression of RBP4 in epididymal WAT (C), liver (D), and serum (E) of HFD-fed mice (n = 8 for WT mice and n = 7 for KO mice) is shown. The induction of ATRA on RBP4 protein expression in primary epididymal adipocytes isolated from HFD-fed WT and Lcn2 KO mice (F) is shown. The expression and secretion of RBP4 by ATRA in differentiated inguinal (Ing) adipocytes from RCD-fed WT and Lcn2 KO mice (G) are shown. The data are presented as mean ± S.E.
In the next experiment, we determined whether RA regulates RBP4 protein levels and whether this regulation is affected by Lcn2 deficiency. Primary adipocytes isolated from epididymal adipose tissue of WT and Lcn2 KO mice were treated with ATRA. As shown in Fig. 7F, a 4-h treatment of ATRA increased RBP4 protein levels in WT adipocytes in a dose-dependent fashion. Consistent with the observation of increased RBP4 levels in Lcn2 KO epididymal adipose tissue, Lcn2 KO adipocytes had a higher level of RBP4 protein than WT cells in the basal condition. However, Lcn2 KO epididymal adipocytes had a diminished response to ATRA stimulation in RBP4 protein levels. Similar to epididymal adipose tissue and primary epididymal adipocytes, differentiated Lcn2 KO inguinal adipocytes expressed higher levels of RBP4 protein than WT cells in the basal state. ATRA treatment for 24 h significantly increased RBP4 protein expression and secretion in WT cells (Fig. 7G). However, ATRA failed to stimulate RBP4 expression and secretion in Lcn2 KO inguinal adipocytes (Fig. 7G). These results suggest that the defective retinol metabolism may contribute to lower intracellular ATRA levels and decreased RA signaling activity in Lcn2 KO adipose tissue.
Discussion
Our previous studies have demonstrated that Lcn2 deficiency significantly impairs thermogenesis and lipid homeostasis in male and female mice without affecting β-adrenergic signaling activation (25–27, 50). In this study, we explored the potential non-adrenergic mechanism by which Lcn2 regulates energy and lipid metabolism in obesity. We showed that both Lcn2 and Ucp1 are ATRA target genes. ATRA is a potent inducer of UCP1 expression and as well as Lcn2 expression in adipocytes. Lcn2 deficiency impairs ATRA effects on facilitating weight loss, reducing fat mass, inducing Ucp1 gene expression, and promoting BAT thermogenic activation in HFD-induced obese mice. Importantly, we found that RA-induced expression of retinol transport proteins (STRA6 and RBP4) and HSL phosphorylation is blunted, suggesting that retinol transport activity and retinyl ester hydrolysis are decreased in Lcn2-deficient adipocytes. Lcn2-deficient adipose tissue displays significantly decreased levels of retinol, retinyl ester, and ATRA compared with adipose tissue from WT mice. Using a fluorescence-based binding assay, Lcn2 shows weak binding of either ATRA or 9-cis-RA. These results collectively suggest that defective retinoid metabolism in adipose tissue may contribute to decreased retinoic acid biosynthesis and action in Lcn2-deficient mice.
Lipocalin 2 is a secreted protein with the structure similar to fatty acid-binding proteins, both of which have a clearly defined hydrophobic binding pocket (51). In our previous studies, we have characterized the role of Lcn2 as an important regulator of energy metabolism in HFD-induced obesity. We showed that food intake, ambulatory activity, VO2, and respiratory quotient were not significantly different between WT and Lcn2 KO mice (25). However, we found that cold-induced adaptive thermogenesis is impaired in Lcn2-deficient mice with normal catecholamine release and norepinephrine-stimulated p38 MAPK phosphorylation and UCP1 expression. We concluded that thermogenic defects are likely the major contributor to HFD-induced obesity in Lcn2 KO mice (25). In an attempt to understand the non-adrenergic mechanism underlying Lcn2-regulated thermogenesis, we connected Lcn2 to RA-mediated thermogenesis for a number of reasons. First, ATRA has been known to be a potent inducer of Ucp1 transcription and thermogenesis. Second, the promoter region of the Lcn2 gene contains an RARE, and RA induces Lcn2 expression. Third, the literature indicates that Lcn2 mediates the effect of 13-cis-RA on apoptosis (30). In this study, we found that ATRA concurrently stimulates UCP1 and Lcn2 expression in adipocytes. Consistent with previous reports (31, 34), our results showed that ATRA treatment significantly reduces body weight and fat mass in WT mice. The mechanism for ATRA effect on weight loss is likely related to its action on promoting thermogenesis and energy expenditure. Fat storage in WATs largely depends on a balance between the rate of lipogenesis and lipolysis/fat oxidation. Although ATRA stimulates lipogenic gene expression in adipose tissue, our data showed that ATRA also significantly up-regulates HSL phosphorylation and lipolysis as well as Ucp1 expression. This suggests that ATRA promotes the lipogenesis-lipolysis-fatty acid oxidation feed-forward loop. Fatty acids that are synthesized from lipogenesis are mostly oxidized in adipocytes under the ATRA stimulation. Interestingly, our data from in vivo studies demonstrated that Lcn2 deficiency blunts the effect of ATRA on the weight loss and the reduction of subcutaneous and brown fat mass. In addition, the stimulatory effect of ATRA on lipid metabolism was significantly affected in Lcn2 KO mice. Specifically, ATRA-induced up-regulation of genes involved in lipogenesis and lipolysis in adipose tissue, especially in epididymal adipose tissue, was blunted in Lcn2 KO mice. Lcn2 deficiency also diminishes ATRA-induced HSL phosphorylation in epididymal and inguinal adipose tissue as well as BAT. Moreover, we observed that ATRA administration to HFD-induced obese mice for 24 days failed to activate UCP1 expression in BAT and to increase body temperature as well as rescue cold intolerance in Lcn2 KO mice. These results led us to conclude that Lcn2 is a modulator of ATRA action on lipid metabolism and thermogenesis in adipose tissue. Our results from in vitro studies that Lcn2 KO brown adipocytes have significantly lower levels of Ucp1 and Pgc1α expression in the basal and ATRA-stimulated conditions further support this conclusion. The decreased brown fat markers in differentiated Lcn2 KO brown adipocytes are not associated with the defective differentiation capacity of SV cells as we previously reported that Lcn2 deficiency does not significantly affect the capacity of brown SV cell differentiation into adipocytes (27).
It is well established that retinoic acid exerts its biological functions primarily by controlling the activation of nuclear transcription factors, including RARs and RXRs, thereby regulating the expression of their target genes (9, 10). We demonstrated that the expression levels of RARα and RXRα were significantly decreased in epididymal adipose tissue of Lcn2 KO mice under the HFD-fed condition. The ATRA induction of the expression of the RA-responsive genes such as Pck1, Acox1, and Slc25a20 was significantly diminished in Lcn2 KO mice. These data indicate that RA signaling pathway activation and ATRA-induced transcriptional activity are impaired in Lcn2 KO adipocytes. Interestingly, the effect of Lcn2 deficiency on ATRA-induced expression of retinoic acid-responsive genes and genes involved in lipid metabolism was more profound in epididymal WAT than that in inguinal WAT. This suggests that Lcn2 effect on retinoid metabolism is fat depot-selective.
We then attempted to understand how Lcn2 deficiency affects retinoic acid action and signaling activation. Because of the unique hydrophobic structure of Lcn2, we hypothesized that Lcn2 may function as a retinoic acid-binding protein in transporting RA in the circulation. In a previous report, bacterially produced Lcn2 had no ability to bind retinol but had a very low affinity for ATRA (37), which excludes the possibility for Lcn2 to serve as a retinol-binding protein. In this study, we used NIH 3T3 cell-produced Lcn2 to reproducibly show that the binding of Lcn2 with ATRA is weak. This result does not seem to support our original hypothesis that Lcn2 is a carrier protein for circulating retinoic acid. To further test this hypothesis, we measured ATRA levels in BAT and serum of Lcn2−/− mice fed an RCD. Our data showed significantly lower RA levels in BAT but no significant difference in circulating ATRA (p = 0.063) levels in Lcn2 KO mice. These data also do not strongly establish Lcn2 as a transport of circulating retinoic acid. Unlike the observations from in vivo studies, Lcn2 KO adipocytes had a full response to ATRA when it was added directly to the culture media in terms of its stimulatory effect on RARα nuclear translocation and the expression of retinoic acid target genes STRA6 and CRABPII. Although Lcn2 KO brown adipocytes have lower levels of Ucp1 and Pgc1α expression as well as p38 MAPK phosphorylation in both basal and ATRA-stimulated states, they do show an ATRA-induced up-regulation of UCP1 and PGC-1α expression. These data further support that the uptake and intracellular transport of ATRA do not require Lcn2; the significantly decreased ATRA levels Lcn2 KO adipose tissue may not arise due to a defect in circulating ATRA transport or uptake into the tissue.
We next determined whether Lcn2 deficiency affects retinoid metabolism and whether altered retinoid homeostasis contributes to the defective lipid metabolism and thermogenesis in Lcn2 KO mice. The control of retinol delivery from the liver, the main storage site for retinoid, to responsive tissues/cells is critical in determining the rate of de novo biosynthesis of retinoic acid and, consequently, also retinoic acid action in peripheral tissues, including adipose tissue. The regulation of retinol transport and uptake into responsive tissues has been known to involve STRA6, a membrane receptor for retinol-binding protein 4 (RBP4), mediating retinol uptake into cells that express Stra6 (45, 46). Once taken up into adipocytes, retinol is re-esterified and stored in the form of retinyl ester within adipose tissue lipid droplets. HSL has been shown to be the physiologically important retinyl ester hydrolase within adipose tissue (40, 52). Upon stimulation of HSL, retinyl esters are hydrolyzed to retinol, which can be metabolized by a number of short chain dehydrogenase/reductase family members to retinaldehyde, which in turn can be oxidized to retinoic acid by a number of aldehyde dehydrogenases (ALDH1A1, ALDH2A1, and ALDH3A1). Interestingly, we found that the long term treatment of ATRA significantly induces HSL phosphorylation (activation) in epididymal WAT and inguinal WAT, suggesting both an increase in retinyl ester hydrolysis and de novo retinoic acid synthesis. Interestingly, this effect of ATRA on HSL phosphorylation was diminished in Lcn2 KO mice, suggesting the impaired retinyl ester hydrolysis that may partly contribute to the decreased ATRA levels in adipose tissue in Lcn2 KO mice.
The level of retinyl ester storage in adipocytes is an important determinant of the rate of retinoic acid biosynthesis in adipose tissue. RBP4 and STRA6 play critical roles in regulating the transport and uptake of circulating retinol into adipose tissue, thereby affecting retinyl ester storage in adipose tissue. When we evaluated retinoid levels and enzymes involved in retinol metabolism and retinoic acid synthesis in adipose tissue, we found that retinol and retinyl ester levels were significantly decreased in inguinal adipose tissue of Lcn2 KO mice compared with WT mice. Moreover, the mRNA expression of Alhd1a3, the key enzyme for retinoic acid biosynthesis, was significantly down-regulated in epididymal and brown adipose tissue; STRA6 protein levels were also decreased in adipose tissue. Most intriguingly, RBP4 protein levels were increased in adipose tissue, but serum levels of RBP4 protein were significantly reduced in HFD-fed Lcn2 KO mice, suggesting that RBP4 secretion may be impaired in Lcn2 KO adipocytes. Moreover, ATRA-stimulated RBP4 protein expression and secretion was blunted in Lcn2 KO adipocytes. Taken together, these data suggest that retinol transport and uptake into adipose tissue is impaired in Lcn2-deficient adipose tissue, giving rise to decreased levels of retinyl ester stores. This decrease in retinyl ester storage combined with impaired retinyl ester hydrolysis is likely responsible for the decreased retinoic acid synthesis in adipose tissue and the diminished retinoic acid effect on thermogenic activation in Lcn2 KO mice. Additionally, the decreased RA biosynthesis and RA signaling could be the direct cause of the decreased STRA6 expression in Lcn2 KO adipocytes as STRA6 is an RA-responsive gene.
In summary, we found that Lcn2 is a retinoic acid-responsive gene that modulates ATRA effects on lipid metabolism and thermogenesis in obesity. Our in vivo and in vitro studies have demonstrated that Lcn2 deficiency reduces RBP4 secretion/STRA6 expression, impairs circulating ATRA levels, alters retinol transport and metabolism in adipose tissue, and reduces ATRA levels in adipose tissue. We suggest that these effects ultimately account for the disruption of retinoid-mediated thermogenesis in Lcn2-deficient mice. Our results provide novel insights into the role of Lcn2 as a regulator in retinoid homeostasis and retinoid-mediated thermogenesis in the pathogenesis of obesity. This will enable better understanding of regulatory mechanisms for non-adrenergic thermogenesis and will help in the development of novel therapeutic strategies for the treatment of obesity and diabetes.
Author Contributions
H. G. designed and performed the experiments, analyzed the data, and wrote the article. R. F. designed and performed the experiments, analyzed the data, and wrote the article. S. M. O., H. J., and Y. Z. performed the experiments. W. S. B. conceived and designed the experiments, analyzed the data, and edited the article. D. A. B. conceived and designed the experiments, analyzed the data, and edited the article. X. C. researched, conceived and designed the experiments, analyzed the data, and wrote the article. X. C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This work was supported by National Institutes of Health Grants R01DK080743 from NIDDK (to X. C.) and P30DK050456 from NIDDK (to Minnesota Obesity Center). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.

This article contains supplemental Table S1.
- WAT
- white adipose tissue
- ATRA
- all-trans-retinoic acid
- BAT
- brown adipose tissue
- RA
- retinoic acid
- RARE
- retinoic acid-response element
- HFD
- high fat diet
- RAR
- retinoic acid receptor
- RXR
- retinoid X receptor
- SV
- stromal-vascular
- HSL
- hormone-sensitive lipase
- RCD
- regular chow diet.
References
- 1. Ouchi N., Parker J. L., Lugus J. J., and Walsh K. (2011) Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 11, 85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cannon B., and Nedergaard J. (2004) Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 [DOI] [PubMed] [Google Scholar]
- 3. Ravussin E., and Galgani J. E. (2011) The implication of brown adipose tissue for humans. Annu. Rev. Nutr. 31, 33–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matthias A., Ohlson K. B., Fredriksson J. M., Jacobsson A., Nedergaard J., and Cannon B. (2000) Thermogenic responses in brown fat cells are fully UCP1-dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty Scid-induced thermogenesis. J. Biol. Chem. 275, 25073–25081 [DOI] [PubMed] [Google Scholar]
- 5. Villarroya F., and Vidal-Puig A. (2013) Beyond the sympathetic tone: the new brown fat activators. Cell Metab. 17, 638–643 [DOI] [PubMed] [Google Scholar]
- 6. Bonet M. L., Ribot J., and Palou A. (2012) Lipid metabolism in mammalian tissues and its control by retinoic acid. Biochim. Biophys. Acta 1821, 177–189 [DOI] [PubMed] [Google Scholar]
- 7. Berry D. C., and Noy N. (2012) Signaling by vitamin A and retinol-binding protein in regulation of insulin responses and lipid homeostasis. Biochim. Biophys. Acta 1821, 168–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pino-Lagos K., Guo Y., and Noelle R. J. (2010) Retinoic acid: a key player in immunity. Biofactors 36, 430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Repa J. J., Hanson K. K., and Clagett-Dame M. (1993) All-trans-retinol is a ligand for the retinoic acid receptors. Proc. Natl. Acad. Sci. U.S.A. 90, 7293–7297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ziouzenkova O., and Plutzky J. (2008) Retinoid metabolism and nuclear receptor responses: new insights into coordinated regulation of the PPAR-RXR complex. FEBS Lett. 582, 32–38 [DOI] [PubMed] [Google Scholar]
- 11. Larose M., Cassard-Doulcier A. M., Fleury C., Serra F., Champigny O., Bouillaud F., and Ricquier D. (1996) Essential cis-acting elements in rat uncoupling protein gene are in an enhancer containing a complex retinoic acid response domain. J. Biol. Chem. 271, 31533–31542 [DOI] [PubMed] [Google Scholar]
- 12. Rabelo R., Reyes C., Schifman A., and Silva J. E. (1996) A complex retinoic acid response element in the uncoupling protein gene defines a novel role for retinoids in thermogenesis. Endocrinology 137, 3488–3496 [DOI] [PubMed] [Google Scholar]
- 13. Sears I. B., MacGinnitie M. A., Kovacs L. G., and Graves R. A. (1996) Differentiation-dependent expression of the brown adipocyte uncoupling protein gene: regulation by peroxisome proliferator-activated receptor γ. Mol. Cell. Biol. 16, 3410–3419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Puigserver P., Vázquez F., Bonet M. L., Picó C., and Palou A. (1996) In vitro and in vivo induction of brown adipocyte uncoupling protein (thermogenin) by retinoic acid. Biochem. J. 317, 827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alvarez R., de Andrés J., Yubero P., Viñas O., Mampel T., Iglesias R., Giralt M., and Villarroya F. (1995) A novel regulatory pathway of brown fat thermogenesis. Retinoic acid is a transcriptional activator of the mitochondrial uncoupling protein gene. J. Biol. Chem. 270, 5666–5673 [DOI] [PubMed] [Google Scholar]
- 16. Teruel T., Hernandez R., Benito M., and Lorenzo M. (2003) Rosiglitazone and retinoic acid induce uncoupling protein-1 (UCP-1) in a p38 mitogen-activated protein kinase-dependent manner in fetal primary brown adipocytes. J. Biol. Chem. 278, 263–269 [DOI] [PubMed] [Google Scholar]
- 17. Kumar M. V., Sunvold G. D., and Scarpace P. J. (1999) Dietary vitamin A supplementation in rats: suppression of leptin and induction of UCP1 mRNA. J. Lipid Res. 40, 824–829 [PubMed] [Google Scholar]
- 18. Serra F., Bonet M. L., Puigserver P., Oliver J., and Palou A. (1999) Stimulation of uncoupling protein 1 expression in brown adipocytes by naturally occurring carotenoids. Int. J. Obes. Relat. Metab. Disord. 23, 650–655 [DOI] [PubMed] [Google Scholar]
- 19. Bonet M. L., Oliver J., Picó C., Felipe F., Ribot J., Cinti S., and Palou A. (2000) Opposite effects of feeding a vitamin A-deficient diet and retinoic acid treatment on brown adipose tissue uncoupling protein 1 (UCP1), UCP2 and leptin expression. J. Endocrinol. 166, 511–517 [DOI] [PubMed] [Google Scholar]
- 20. Sundaresan P. R., Winters V. G., and Therriault D. G. (1967) Effect of low environmental temperature on the metabolism of vitamin A (retinol) in the rat. J. Nutr. 92, 474–478 [DOI] [PubMed] [Google Scholar]
- 21. LaLonde J. M., Bernlohr D. A., and Banaszak L. J. (1994) The up-and-down β-barrel proteins. FASEB J. 8, 1240–1247 [DOI] [PubMed] [Google Scholar]
- 22. Flower D. R. (2000) Beyond the superfamily: The lipocalin receptors. Biochim. Biophys. Acta 1482, 327–336 [DOI] [PubMed] [Google Scholar]
- 23. Yan Q. W., Yang Q., Mody N., Graham T. E., Hsu C. H., Xu Z., Houstis N. E., Kahn B. B., and Rosen E. D. (2007) The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes 56, 2533–2540 [DOI] [PubMed] [Google Scholar]
- 24. Zhang J., Wu Y., Zhang Y., Leroith D., Bernlohr D. A., and Chen X. (2008) The role of lipocalin 2 in the regulation of inflammation in adipocytes and macrophages. Mol. Endocrinol. 22, 1416–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo H., Jin D., Zhang Y., Wright W., Bazuine M., Brockman D. A., Bernlohr D. A., and Chen X. (2010) Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes 59, 1376–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo H., Bazuine M., Jin D., Huang M. M., Cushman S. W., and Chen X. (2013) Evidence for the regulatory role of lipocalin 2 in high-fat diet-induced adipose tissue remodeling in male mice. Endocrinology 154, 3525–3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Y., Guo H., Deis J. A., Mashek M. G., Zhao M., Ariyakumar D., Armien A. G., Bernlohr D. A., Mashek D. G., and Chen X. (2014) Lipocalin 2 regulates brown fat activation via a nonadrenergic activation mechanism. J. Biol. Chem. 289, 22063–22077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shen F., Hu Z., Goswami J., and Gaffen S. L. (2006) Identification of common transcriptional regulatory elements in interleukin-17 target genes. J. Biol. Chem. 281, 24138–24148 [DOI] [PubMed] [Google Scholar]
- 29. Garay-Rojas E., Harper M., Hraba-Renevey S., and Kress M. (1996) An apparent autocrine mechanism amplifies the dexamethasone- and retinoic acid-induced expression of mouse lipocalin-encoding gene 24p3. Gene 170, 173–180 [DOI] [PubMed] [Google Scholar]
- 30. Nelson A. M., Zhao W., Gilliland K. L., Zaenglein A. L., Liu W., and Thiboutot D. M. (2008) Neutrophil gelatinase-associated lipocalin mediates 13-cis retinoic acid-induced apoptosis of human sebaceous gland cells. J. Clin. Invest. 118, 1468–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berry D. C., and Noy N. (2009) All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor β/δ and retinoic acid receptor. Mol. Cell. Biol. 29, 3286–3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim S. C., Kim C. K., Axe D., Cook A., Lee M., Li T., Smallwood N., Chiang J. Y., Hardwick J. P., Moore D. D., and Lee Y. K. (2014) All-trans-retinoic acid ameliorates hepatic steatosis in mice by a novel transcriptional cascade. Hepatology 59, 1750–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marzan C. V., Kupumbati T. S., Bertran S. P., Samuels T., Leibovitch B., Mira-y-Lopez R., Ossowski L., and Farias E. F. (2011) Adipocyte derived paracrine mediators of mammary ductal morphogenesis controlled by retinoic acid receptors. Dev. Biol. 349, 125–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mercader J., Ribot J., Murano I., Felipe F., Cinti S., Bonet M. L., and Palou A. (2006) Remodeling of white adipose tissue after retinoic acid administration in mice. Endocrinology 147, 5325–5332 [DOI] [PubMed] [Google Scholar]
- 35. Barua A. B., and Furr H. C. (1998) Properties of retinoids. Structure, handling, and preparation. Mol. Biotechnol. 10, 167–182 [DOI] [PubMed] [Google Scholar]
- 36. Kane M. A., Folias A. E., Wang C., and Napoli J. L. (2008) Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal. Chem. 80, 1702–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Breustedt D. A., Schönfeld D. L., and Skerra A. (2006) Comparative ligand-binding analysis of 10 human lipocalins. Biochim. Biophys. Acta 1764, 161–173 [DOI] [PubMed] [Google Scholar]
- 38. Collins S., Yehuda-Shnaidman E., and Wang H. (2010) Positive and negative control of Ucp1 gene transcription and the role of beta-adrenergic signaling networks. Int. J. Obes. 34, suppl. 1, 28–33 [DOI] [PubMed] [Google Scholar]
- 39. Valverde A. M., Arribas M., Mur C., Navarro P., Pons S., Cassard-Doulcier A. M., Kahn C. R., and Benito M. (2003) Insulin-induced up-regulated uncoupling protein-1 expression is mediated by insulin receptor substrate 1 through the phosphatidylinositol 3-kinase/akt signaling pathway in fetal brown adipocytes. J. Biol. Chem. 278, 10221–10231 [DOI] [PubMed] [Google Scholar]
- 40. Ström K., Gundersen T. E., Hansson O., Lucas S., Fernandez C., Blomhoff R., and Holm C. (2009) Hormone-sensitive lipase (HSL) is also a retinyl ester hydrolase: evidence from mice lacking HSL. FASEB J. 23, 2307–2316 [DOI] [PubMed] [Google Scholar]
- 41. Kurlandsky S. B., Gamble M. V., Ramakrishnan R., and Blaner W. S. (1995) Plasma delivery of retinoic acid to tissues in the rat. J. Biol. Chem. 270, 17850–17857 [DOI] [PubMed] [Google Scholar]
- 42. Tsutsumi C., Okuno M., Tannous L., Piantedosi R., Allan M., Goodman D. S., and Blaner W. S. (1992) Retinoids and retinoid-binding protein expression in rat adipocytes. J. Biol. Chem. 267, 1805–1810 [PubMed] [Google Scholar]
- 43. Blaner W. S. (1989) Retinol-binding protein: the serum transport protein for vitamin A. Endocr. Rev. 10, 308–316 [DOI] [PubMed] [Google Scholar]
- 44. Blaner W. S. (2007) STRA6, a cell-surface receptor for retinol-binding protein: the plot thickens. Cell Metab. 5, 164–166 [DOI] [PubMed] [Google Scholar]
- 45. Kawaguchi R., Yu J., Honda J., Hu J., Whitelegge J., Ping P., Wiita P., Bok D., and Sun H. (2007) A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 315, 820–825 [DOI] [PubMed] [Google Scholar]
- 46. Isken A., Golczak M., Oberhauser V., Hunzelmann S., Driever W., Imanishi Y., Palczewski K., and von Lintig J. (2008) RBP4 disrupts vitamin A uptake homeostasis in a STRA6-deficient animal model for Matthew-Wood syndrome. Cell Metab. 7, 258–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang Q., Eskurza I., Kiernan U. A., Phillips D. A., Blüher M., Graham T. E., and Kahn B. B. (2012) Quantitative measurement of full-length and C-terminal proteolyzed RBP4 in serum of normal and insulin-resistant humans using a novel mass spectrometry immunoassay. Endocrinology 153, 1519–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kiernan U. A., Tubbs K. A., Nedelkov D., Niederkofler E. E., and Nelson R. W. (2002) Comparative phenotypic analyses of human plasma and urinary retinol binding protein using mass spectrometric immunoassay. Biochem. Biophys. Res. Commun. 297, 401–405 [DOI] [PubMed] [Google Scholar]
- 49. Nedelkov D., Kiernan U. A., Niederkofler E. E., Tubbs K. A., and Nelson R. W. (2005) Investigating diversity in human plasma proteins. Proc. Natl. Acad. Sci. U.S.A. 102, 10852–10857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jin D., Guo H., Bu S. Y., Zhang Y., Hannaford J., Mashek D. G., and Chen X. (2011) Lipocalin 2 is a selective modulator of peroxisome proliferator-activated receptor-γ activation and function in lipid homeostasis and energy expenditure. FASEB J. 25, 754–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Flower D. R., North A. C., and Attwood T. K. (1993) Structure and sequence relationships in the lipocalins and related proteins. Protein Sci. 2, 753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wei S., Lai K., Patel S., Piantedosi R., Shen H., Colantuoni V., Kraemer F. B., and Blaner W. S. (1997) Retinyl ester hydrolysis and retinol efflux from BFC-1β adipocytes. J. Biol. Chem. 272, 14159–14165 [DOI] [PubMed] [Google Scholar]