Abstract
The clade B/intracellular serpins protect cells from peptidase-mediated injury by forming covalent complexes with their targets. SERPINB12 is expressed in most tissues, especially at cellular interfaces with the external environment. This wide tissue distribution pattern is similar to that of granzyme A (GZMA). Since SERPINB12 inhibits trypsin-like serine peptidases, we determined whether it might also neutralize GZMA. SERPINB12 formed a covalent complex with GZMA and inhibited the enzyme with typical serpin slow-binding kinetics. SERPINB12 also inhibited Hepsin (HPN). SERPINB12 may function as an endogenous inhibitor of these peptidases.
Graphical abstract
SERPINB12 is a Slow-binding Inhibitor of Granzyme A and Hepsin: Jason Z. Niehaus, Mark T. Miedel, Misty Good, Allyson N. Wyatt, Stephen C. Pak, Gary A. Silverman, Cliff J. Luke
SERPINB12 is one of 13 human clade B/intracellular serpins 1, 2. Unlike standard mechanism inhibitors, serpins neutralize their target peptidases using a unique suicide-substrate mechanism that employs a mobile reactive-site loop (RSL) 3, 4. Proteolytic cleavage of the RSL relieves strain on the metastable serpin fold, leading to a major conformational re-arrangement that distorts the peptidase active site and traps it in a covalent complex with the inhibitor 5. SERPINB12 contains an Arg residue at the canonical P1 position in the RSL and inhibits the trypsin-like peptidases, plasmin and trypsin, but not thrombin or urokinase-type plasminogen activator 1. One function the clade B/intracellular serpins is to protect cells from intracellular peptidase activity 6, 7. For example, the granzyme B (GZMB) inhibitor, SERPINB9, protects lymphoid and antigen-presenting cells from cell death by neutralizing enzyme that leaks from lytic granules in CD8+ cytotoxic T lymphocytes and natural killer (NK) cells, or that is taken up with perforin from the extracellular space 6, 8, 9. Based on these observations and the wide tissue distribution of SERBINB12 1, 10, we sought to determine whether this serpin might serve a similar role by inhibiting the lytic granule-associated trypsin-like peptidase, granzyme A (GZMA).
Serpins are slow-binding inhibitors 11-14. The hallmarks of their activity are 1) the formation of a covalent serpin-peptidase complex, 2) a stoichiometry of inhibition (SI)∼1 and 3) a second order rate constant (kass) ∼ 104 M-1 s-1 11-13. Previously, we showed that a recombinant 6× His-tag-SERPINB12 fusion protein inhibited trypsin with an SI=2 and a kass = 2.5 × 105 M-1 s-1 1. The yields of this recombinant serpin were low, so we substituted in an N-terminal GST tag. GST-SERPINB12 (hereafter referred to as SERPINB12) showed a thermal denaturation profile (a measure of the metastable active serpin), and an SI=2 and kass = 2.2 × 105 M-1 s-1 for human trypsin that were comparable to those described for 6×His-tag-SERPINB12 (Figure S1) 1.
The sine qua non of the serpin inhibitory mechanism is the formation of covalent inhibitor-enzyme complex 11-13, 15. We analyzed a mixture of the purified SERPINB12 and/or GZMA by SDS-PAGE under reducing conditions. Companion gels were blotted and probed with anti-SERPINB12 or anti-GZMA monoclonal antibodies (Mab). Both antibodies detected a SERPINB12-GZMA complex of the appropriate molecular mass (Figure 1). Of note, most GZMA forms a disulfide-linked homo-dimer with two active sites 16, 17. SDS-PAGE under non-reducing conditions yielded a molecular mass species consistent with one SERPINB12 molecule binding to the GZMA dimer (not shown). Under these conditions, however, the presence of higher order aggregates containing only GZMA or SERPINB12 precluded a precise determination of whether two SERPINB12 molecules formed complexes with the GZMA dimer.
Figure 1.
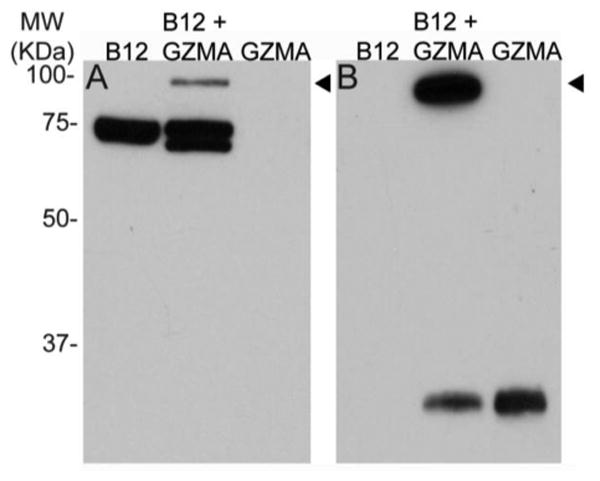
SERPINB12 (B12) forms SDS-stable complexes with granzyme GZMA. SERPINB12 and/or GZMA were incubated at a ∼5:1 serpin:peptidase ratio, separated by SDS-PAGE, and probed with a SERPINB12 (A) or GZMA (B) specific Mab. Higher molecular mass SERPINB12-GZMA complexes (arrowheads) were detected by both Mab.
The RSL of SERPINB12 contains Arg-Ser residues at both the P1-P1′ (canonical cleavage site) and the P3′-P4′ positions, respectively 1.
Analysis of the SERPINB12-trypsin complex by mass spectrometry shows that this enzyme also cleaves after the P3′ Arg, which renders the serpin inactive and accounts for the SI∼2 1. We pre-incubated different concentrations of SERPINB12 with a constant amount GZMA and plotted the fractional enzyme activity versus the [I]/[E] to obtain an SI∼2 (Figure 2A). Interestingly, analysis of the complex by mass spectrometry showed that GZMA, unlike trypsin, only cleaved the RSL after the canonical P1 Arg (Figure 3A & C). This finding suggested that following formation of the SERPINB12-GZMA acyl-enzyme intermediate, at least half of the complex partitions down the substrate, rather than the inhibitory pathway. This type of branched pathway has been well described for other serpin-target protease interactions and reflects a competition between the rate of RSL insertion and trapping of the protease versus the rate of peptidase de-acylation and escape from the serpin trap 18. An alternative explanation, is that binding of SERPINB12 to one GZMA active site yielded conformational changes in the dimer that precluded further inhibitory rearrangements from occurring after binding to a second SERPINB12 molecule. This mechanism would convert the second SERPINB12 molecule to a simple substrate and account perfectly for the SI∼2. In vivo, the lack of inhibition by a second SERPINB12 molecule would be unlikely to alter the final fate for the SERPINB12-GZMA dimer complex, as misfolding induced by one productive interaction would lead to the rapid elimination of the complex by endogenous proteostasis pathways.
Figure 2.
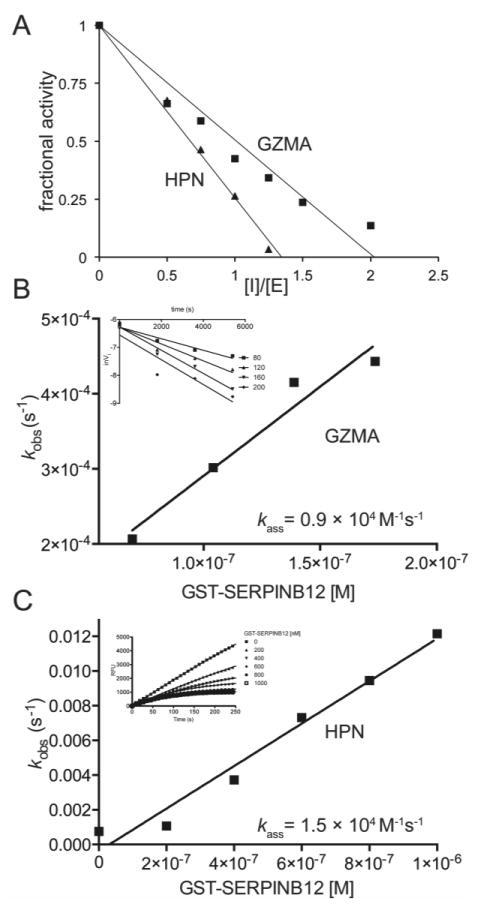
Representative analyses of the SERPINB12 stoichiometry of inhibition (SI) with GZMA and HPN (A) and the kass determination for GZMA (B) and HPN (C). The SI for the SERPINB12-GZMA or -HPN interaction was 2 and 1.3, respectively. The kass for the SERPINB12 inhibition of GZMA (0.9 × 104 M-1 s-1) or HPN is (1.5 × 104 M-1 s-1) were from representative experiments. The kass in the text were the means ± SD from at least three experiments.
Figure 3.
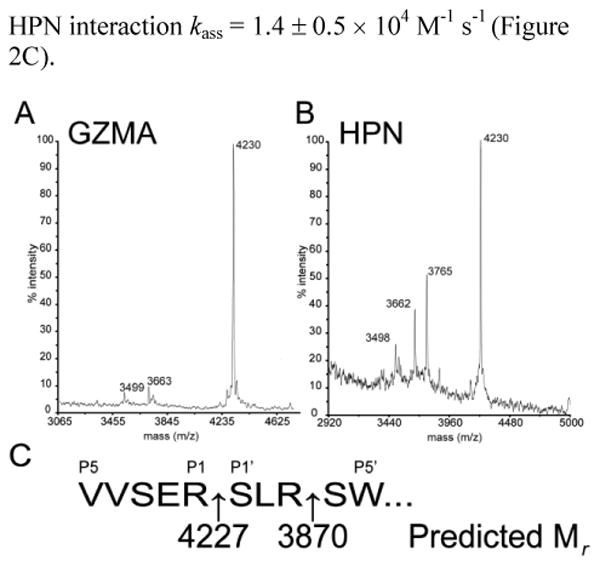
Mapping of the GZMA (A) or HPN (B) meidatedSERPINB12 RSL cleavage sites (P1-P1′) by mass spectrometry of the released C-terminal RSL peptide. (C) Schematic of the of SERPINB12 RSL (P5-P5′) with predicted peptidase cleavage sites and expected masses of the respective C-terminal peptides.
The second-order rate constant (kass) for the SERPINB12-GZMA interaction was determined using the discontinuous assay for low rates of inhibition 11. The kass = 8.9 ± 1.5 × 103 M-1 s-1 (Figure 2B). Taken together, these data showed that SERPINB12 was a slow-binding GZMA inhibitor.
Hepsin (HPN) is a type II transmembrane serine protease that preferentially cleaves after Arg residues, and via alternative splicing, is also found in cytosol instead of the plasma membrane 19, 20. SERPINB12 also formed a covalent complex with HPN (Figure S2). The SI for the interaction was ∼ 1.3 (Figure 2A), and HPN cleaved the RSL of SERPINB12 after the canonical P1 Arg (Figure 3B & C). Under pseudo-first order conditions using the progress-curve method 11, 12, 14, the SERPINB12-HPN interaction kass = 1.4 ± 0.5 × 104 M-1 s-1 (Figure 2C).
GZMA was originally detected in the lytic granules of CD8+ cytotoxic T lymphocytes and NK cells, where it plays a role in inducing target cell death 21-23, possibly via cleavage of the SET complex and release of the DNA nucleases, NM23-H1 and Trex1 (reviewed in 24). However, recent reports show that GZMA has a broad expression pattern including epithelia of the gastrointestinal and respiratory tracts 25. GZMA's broad expression pattern overlaps significantly with that of SERPINB12 10, 25. Immunofluorescence confocal microscopy of human small intestinal epithelial cells shows that GZMA has a diffuse cytoplasmic distribution, whereas SERPINB12 has a more apical pattern (Figure S3). While there appears to be some overlap, these proteins occupied separate subcellular compartments. This result was anticipated, as the presence of both proteins in the same compartment under steady-state conditions would lead to the constitutive inactivation of GZMA. The function of GZMA in epithelial cells is unknown, but could play a role in the innate defenses against microbial pathogens at mucosal surfaces 26. Similar to Serpinb6b and GZMA, as well as SERPINB9 and GZMB, SERPINB12 would be available to protect the cells if any of the GZMA becomes misdirected to a sensitive subcellular site 6, 8, 9, 27.
The biological functions of HPN are understood incompletely. HPN may serve as a scavenger receptor or an activator of growth factors (e.g., cleavage of pre-hepatocyte growth factor) 28. HPN is overexpressed also in prostate cancer 29, ovarian cancer 30, and renal cell carcinoma 31; where it has been implicated in tumor progression and metastasis 32, 33. Although HPN is mainly a type II transmembrane serine protease 34, it can occupy an intracellular niche via alternative splicing, which occurs predominately in the kidney, brain and lung 19. Immunofluorescence confocal microscopy of human bronchial epithelial cells shows that HPN and SERPIN12 co-localize to the cytoplasm of the same bronchial epithelial cells, but appeared to reside in different subcellular compartments, as was observed with GZMA and SERPINB12 (Figure S4). Taken together these studies suggested that SERPINB12 might be a cytoprotective factor by serving as an endogenous slow-binding inhibitor of GZMA and HPN.
Supplementary Material
Acknowledgments
Funding Sources: Supported by NIH grants T32 AR052282 and DK081422.
Footnotes
Notes: The authors declare no competing financial interests.
Supporting information: Material and methods, Figures S1-S4 (PDF).
References
- 1.Askew YS, Pak SC, Luke CJ, Askew DJ, Cataltepe S, Mills DR, Kato H, Lehoczky J, Dewar K, Birren B, Silverman GA. SERPINB12 is a novel member of the human ov-serpin family that is widely expressed and inhibits trypsin-like serine proteinases. J Biol Chem. 2001;276:49320–49330. doi: 10.1074/jbc.M108879200. [DOI] [PubMed] [Google Scholar]
- 2.Silverman GA, Whisstock JC, Askew DJ, Pak SC, Luke CJ, Cataltepe S, Irving JA, Bird PI. Human clade B serpins (ov-serpins) belong to a cohort of evolutionarily dispersed intracellular proteinase inhibitor clades that protect cells from promiscuous proteolysis. Cell Mol Life Sci. 2004;61:301–325. doi: 10.1007/s00018-003-3240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverman GA, Whisstock JC, Bottomley SP, Huntington JA, Kaiserman D, Luke CJ, Pak SC, Reichhart JM, Bird PI. Serpins flex their muscle: I. Putting the clamps on proteolysis in diverse biological systems. J Biol Chem. 2010;285:24299–24305. doi: 10.1074/jbc.R110.112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whisstock JC, Silverman GA, Bird PI, Bottomley SP, Kaiserman D, Luke CJ, Pak SC, Reichhart JM, Huntington JA. Serpins flex their muscle: II. Structural insights into target peptidase recognition, polymerization, and transport functions. J Biol Chem. 2010;285:24307–24312. doi: 10.1074/jbc.R110.141408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huntington JA, Read RJ, Carrell RW. Structure of a serpin-protease complex shows inhibition by deformation. Nature. 2000;407:923–926. doi: 10.1038/35038119. [DOI] [PubMed] [Google Scholar]
- 6.Bird CH, Sutton VR, Sun J, Hirst CE, Novak A, Kumar S, Trapani JA, Bird PI. Selective regulation of apoptosis: the cytotoxic lymphocyte serpin proteinase inhibitor 9 protects against granzyme B-mediated apoptosis without perturbing the Fas cell death pathway. Mol Cell Biol. 1998;18:6387–6398. doi: 10.1128/mcb.18.11.6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luke CJ, Pak SC, Askew YS, Naviglia TL, Askew DJ, Nobar SM, Vetica AC, Long OS, Watkins SC, Stolz DB, Barstead RJ, Moulder GL, Bromme D, Silverman GA. An intracellular serpin regulates necrosis by inhibiting the induction and sequelae of lysosomal injury. Cell. 2007;130:1108–1119. doi: 10.1016/j.cell.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bird CH, Christensen ME, Mangan MS, Prakash MD, Sedelies KA, Smyth MJ, Harper I, Waterhouse NJ, Bird PI. The granzyme B-Serpinb9 axis controls the fate of lymphocytes after lysosomal stress. Cell Death Differ. 2014 doi: 10.1038/cdd.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang M, Park SM, Wang Y, Shah R, Liu N, Murmann AE, Wang CR, Peter ME, Ashton-Rickardt PG. Serine protease inhibitor 6 protects cytotoxic T cells from self-inflicted injury by ensuring the integrity of cytotoxic granules. Immunity. 2006;24:451–461. doi: 10.1016/j.immuni.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Niehaus JZ, Good M, Jackson LE, Ozolek JA, Silverman GA, Luke CJ. Human SERPINB12 Is an Abundant Intracellular Serpin Expressed in Most Surface and Glandular Epithelia. J Histochem Cytochem. 2015 doi: 10.1369/0022155415600498. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horvath AJ, Lu BG, Pike RN, Bottomley SP. Methods to measure the kinetics of protease inhibition by serpins. Methods in enzymology. 2011;501:223–235. doi: 10.1016/B978-0-12-385950-1.00011-0. [DOI] [PubMed] [Google Scholar]
- 12.Schechter NM, Plotnick MI. Measurement of the kinetic parameters mediating protease-serpin inhibition. Methods. 2004;32:159–168. doi: 10.1016/s1046-2023(03)00207-x. [DOI] [PubMed] [Google Scholar]
- 13.Gettins PG. Serpin structure, mechanism, and function. Chem Rev. 2002;102:4751–4804. doi: 10.1021/cr010170+. [DOI] [PubMed] [Google Scholar]
- 14.Morrison JF, Walsh CT. The behavior and significance of slow-binding enzyme inhibitors. Adv Enzymol Relat Areas Mol Biol. 1988;61:201–301. doi: 10.1002/9780470123072.ch5. [DOI] [PubMed] [Google Scholar]
- 15.Longas MO, Finlay TH. The covalent nature of the human antithrombin III--thrombin bond. Biochem J. 1980;189:481–489. doi: 10.1042/bj1890481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell JK, Goetz DH, Mahrus S, Harris JL, Fletterick RJ, Craik CS. The oligomeric structure of human granzyme A is a determinant of its extended substrate specificity. Nat Struct Biol. 2003;10:527–534. doi: 10.1038/nsb944. [DOI] [PubMed] [Google Scholar]
- 17.Hink-Schauer C, Estebanez-Perpina E, Kurschus FC, Bode W, Jenne DE. Crystal structure of the apoptosis-inducing human granzyme A dimer. Nat Struct Biol. 2003;10:535–540. doi: 10.1038/nsb945. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence DA, Olson ST, Muhammad S, Day DE, Kvassman JO, Ginsburg D, Shore JD. Partitioning of serpin-proteinase reactions between stable inhibition and substrate cleavage is regulated by the rate of serpin reactive center loop insertion into beta-sheet A. J Biol Chem. 2000;275:5839–5844. doi: 10.1074/jbc.275.8.5839. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Yu Z, Zhao X, Shen SH. Identification and characterization of hepsin/-TM, a non-transmembrane hepsin isoform. Biochim Biophys Acta. 2005;1681:157–165. doi: 10.1016/j.bbaexp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Barre O, Dufour A, Eckhard U, Kappelhoff R, Beliveau F, Leduc R, Overall CM. Cleavage specificity analysis of six type II transmembrane serine proteases (TTSPs) using PICS with proteome-derived peptide libraries. PLoS ONE. 2014;9:e105984. doi: 10.1371/journal.pone.0105984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beresford PJ, Xia Z, Greenberg AH, Lieberman J. Granzyme A loading induces rapid cytolysis and a novel form of DNA damage independently of caspase activation. Immunity. 1999;10:585–594. doi: 10.1016/s1074-7613(00)80058-8. [DOI] [PubMed] [Google Scholar]
- 22.Pardo J, Balkow S, Anel A, Simon MM. The differential contribution of granzyme A and granzyme B in cytotoxic T lymphocyte-mediated apoptosis is determined by the quality of target cells. Eur J Immunol. 2002;32:1980–1985. doi: 10.1002/1521-4141(200207)32:7<1980::AID-IMMU1980>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 23.Shresta S, Graubert TA, Thomas DA, Raptis SZ, Ley TJ. Granzyme A initiates an alternative pathway for granule-mediated apoptosis. Immunity. 1999;10:595–605. doi: 10.1016/s1074-7613(00)80059-x. [DOI] [PubMed] [Google Scholar]
- 24.Lieberman J. Granzyme A activates another way to die. Immunological reviews. 2010;235:93–104. doi: 10.1111/j.0105-2896.2010.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Ponten F. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 26.Arias MA, Jimenez de Bagues MP, Aguilo N, Menao S, Hervas-Stubbs S, de Martino A, Alcaraz A, Simon MM, Froelich CJ, Pardo J. Elucidating sources and roles of granzymes A and B during bacterial infection and sepsis. Cell Rep. 2014;8:420–429. doi: 10.1016/j.celrep.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Kaiserman D, Stewart SE, Plasman K, Gevaert K, Van Damme P, Bird PI. Identification of Serpinb6b as a Species-specific Mouse Granzyme A Inhibitor Suggests Functional Divergence between Human and Mouse Granzyme A. J Biol Chem. 2014;289:9408–9417. doi: 10.1074/jbc.M113.525808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herter S, Piper DE, Aaron W, Gabriele T, Cutler G, Cao P, Bhatt AS, Choe Y, Craik CS, Walker N, Meininger D, Hoey T, Austin RJ. Hepatocyte growth factor is a preferred in vitro substrate for human hepsin, a membrane-anchored serine protease implicated in prostate and ovarian cancers. Biochem J. 2005;390:125–136. doi: 10.1042/BJ20041955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magee JA, Araki T, Patil S, Ehrig T, True L, Humphrey PA, Catalona WJ, Watson MA, Milbrandt J. Expression profiling reveals hepsin overexpression in prostate cancer. Cancer Res. 2001;61:5692–5696. [PubMed] [Google Scholar]
- 30.Tanimoto H, Yan Y, Clarke J, Korourian S, Shigemasa K, Parmley TH, Parham GP, O'Brien TJ. Hepsin, a cell surface serine protease identified in hepatoma cells, is overexpressed in ovarian cancer. Cancer Res. 1997;57:2884–2887. [PubMed] [Google Scholar]
- 31.Betsunoh H, Mukai S, Akiyama Y, Fukushima T, Minamiguchi N, Hasui Y, Osada Y, Kataoka H. Clinical relevance of hepsin and hepatocyte growth factor activator inhibitor type 2 expression in renal cell carcinoma. Cancer Sci. 2007;98:491–498. doi: 10.1111/j.1349-7006.2007.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xuan JA, Schneider D, Toy P, Lin R, Newton A, Zhu Y, Finster S, Vogel D, Mintzer B, Dinter H, Light D, Parry R, Polokoff M, Whitlow M, Wu Q, Parry G. Antibodies neutralizing hepsin protease activity do not impact cell growth but inhibit invasion of prostate and ovarian tumor cells in culture. Cancer Res. 2006;66:3611–3619. doi: 10.1158/0008-5472.CAN-05-2983. [DOI] [PubMed] [Google Scholar]
- 33.Tang X, Mahajan SS, Nguyen LT, Beliveau F, Leduc R, Simon JA, Vasioukhin V. Targeted inhibition of cell-surface serine protease Hepsin blocks prostate cancer bone metastasis. Oncotarget. 2014;5:1352–1362. doi: 10.18632/oncotarget.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leytus SP, Loeb KR, Hagen FS, Kurachi K, Davie EW. A novel trypsin-like serine protease (hepsin) with a putative transmembrane domain expressed by human liver and hepatoma cells. Biochemistry. 1988;27:1067–1074. doi: 10.1021/bi00403a032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


