Abstract
Because immune responses within the tumor microenvironment may be important predictors of tumor biology, correlations of specific types of tumor infiltrating lymphocytes (TILs) with clinical variables and outcomes were determined in 278 previously untreated patients with head and neck cancer (HNSCC).
Methods
Infiltrating levels of CD4 (helper T cells), CD8 (cytotoxic/suppressor T cells), FoxP3, regulatory T cells), CD68 (myeloid derived suppressor cells) and CD1a (Langerhan) cells were retrospectively measured by immunohistology in tissue mircoarrays. Cox models tested associations with patient outcomes after adjusting for all known prognostic factors. Median follow up was 36.6 months.
Results
Higher CD4 and CD8 TIL levels were associated with improved overall (HR 0.77 [0.65 –0.93] p=.005 and HR 0.77 [0.64–0.94] p=.008 respectively), and relapse free survival (p=.03, and .05 respectively). After controlling for prognostic factors, higher CD4 levels predicted improved overall and disease specific survival (p=.003 and .004 respectively).
Conclusions
The findings suggest that TILs are a significant independent prognostic factor and potential biomarker for HNSCC that differ by treatment.
Keywords: cancer, tumor infiltrating lymphocytes, prognosis
Introduction
Immune responses within the tumor microenvironment are increasingly important predictors of tumor biology and outcome. Numbers of tumor infiltrating lymphocytes (TILs), their function, and location in the microenvironment of head and neck squamous cancer appear important and may differ significantly by tumor site and extent. Emerging evidence suggests that degree of T cell infiltration of primary tumors consistently predicts favorable outcomes in a number of tumor types1–5. Recent studies that have characterized immune infiltrates in the tumor microenvironment of head and neck squamous carcinomas (HNSCC) have generally suggested improved patient survival is associated with high levels of intratumoral immune cell infiltrates6–15. Similar findings have also been reported in the prognostically favorable subgroup of HPV-16 related oropharyngeal cancers14,16,17. Correlations with outcome have varied with some studies suggesting that cytotoxic/suppressor T cells (CD8) are important while others suggest that helper T lymphocytes (CD4) are associated with favorable prognosis while infiltrates of CD68 positive cells are associated with poorer prognosis. The CD68 marker generally identifies myeloid derived suppressor cells, however there is some potential cross reactivity with other monocytes and tumor associated fibroblasts. The clinical usefulness of these findings as predictive or prognostic factors has been limited due to variability in the methods of assessment of the types of lymphocytes, their microenvironment location, their functional activity and heterogeneity among the small numbers of patients studied. To extend and confirm these observations, we undertook a, study of immune cell tumor infiltrates in 278 patients treated in a uniform fashion to better determine correlations with clinical prognostic variables and to evaluate the overall impact of specific TIL populations on patient outcomes.
Methods
Patient Population
From November 2008 through June 2012, a total of 513 patients were screened and 92% of subjects signed an institutionally approved, written, informed consent to permit biologic specimen collection and complete a baseline questionnaire of demographics, epidemiologic characteristics, and behavior modules. Comorbidity data were abstracted from medical records and graded using the validated Adult Comorbidity Evaluation of 27 conditions organized by 12 systems. Formalin-fixed paraffin-embedded (FFPE) tissue blocks from diagnostic biopsies were collected and detailed clinical data updated annually until death or when patients were lost to follow-up. A total of 354 of subjects had blocks available with sufficient tissue to create a tissue microarray (TMA). Some subjects were excluded because of uncommon tumor sites or were lost to follow up (1 subject). The final patient cohort included 278 subjects with demographics and clinical characteristics listed in Table 1. All patients were discussed at our multidisciplinary tumor board where standardized treatment recommendations were made (Table 2). New tumor events and status (disease free, recurrence, persistent disease, or second primary) were updated at each scheduled patient visit and annually through medical record review. Deaths were confirmed through the Social Security Death Index. There were 67 death events recorded (40 due to HNSCC) and 62 tumor recurrence events among the 278 patients. Median patient follow up for living patients was 36.6 months (range 16–64 months) and was the same when calculated using inverse Kaplan-Meier method.
Table 1.
Patient Characteristics
| Variable | Level | N (%) |
|---|---|---|
| Age Category | <60 | 149 (54%) |
| 60–80 | 114 (41%) | |
| >80 | 15 (4%) | |
| Gender | Male | 202 (73%) |
| Female | 76 (27%) | |
| Historical HN Cancer | No | 273 (98%) |
| Yes | 5 (2%) | |
| Stage | 0/1 | 42 (15%) |
| 2 | 35 (13%) | |
| 3 | 42 (15%) | |
| 4 | 159 (57%) | |
| Disease Site | Larynx | 48 (17%) |
| Oral Cavity | 138 (50%) | |
| Oropharynx | 83 (30%) | |
| Hypopharynx | 9 (3%) | |
| ACE Comorbidities Score | none | 81 (29%) |
| mild | 126 (45%) | |
| moderate | 48 (17%) | |
| severe | 23 (8%) | |
| BMI Category, n=277 | underweight (<18.5) | 10 (4%) |
| normal weight (18.5–24.9) | 92 (33%) | |
| overweight (25–29.9) | 101 (36%) | |
| obese (30+) | 74 (27%) | |
| HPV status, n=257 | negative | 168 (65%) |
| positive | 89 (35%) | |
| Drinker | never | 26 (9%) |
| current | 185 (67%) | |
| former (quit >12 months) | 67 (24%) | |
| Smoker (cigarettes) | never | 77 (28%) |
| current | 118 (42%) | |
| former (quit >12 months) | 83 (30%) |
Table 2.
Primary Treatment According to Tumor Site and Stage
| Oral Cavity | Oropharynx | Larynx | Hypopharynx | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Stage I/II | Stage III/IV | Stage I/II | Stage III/IV | Stage I/II | Stage III/IV | Stage I/II | Stage III/IV | ||
| Surgery | 57 | 75 | 1 | 11 | 4 | 16 | 0 | 1 | 165 |
| Chemoradiation | 0 | 2 | 0 | 63 | 1 | 16 | 0 | 3 | 85 |
| Radiation | 0 | 0 | 5 | 2 | 9 | 0 | 0 | 0 | 16 |
| Palliation | 0 | 4 | 0 | 1 | 0 | 2 | 0 | 5 | 12 |
| Total | 57 | 81 | 6 | 77 | 14 | 34 | 0 | 9 | 278 |
Surgery=“surgery alone” (84), “surgery + adjuvant radiation” (41), “surgery + adjuvant chemoradiation” (40)
Chemoradiation= “chemoradiation alone” (85)
Radiation=“radiation alone” (16)
Palliation= “no curative treatment before death” (11), “no modality (alive with >1 yr follow-up)” (1)
Immunohistology
Representative hematoxylin-eosin stained slides from each block were cut and reviewed by an expert (JM) to confirm histology and screened for >70% cellularity and minimal necrosis and the block marked for tissue sampling. Blocks with insufficient histology or size were excluded resulting in 273 subjects with TIL data, 263 with CD1a gene expression data and 257 with HPV DNA testing results. Triplicate 0.7mm diameter cores for each patient sample were selectively punched/extracted and transferred to a recipient tissue array block18.
TMA sections were incubated in hot-air oven at 65°C overnight, deparaffinized, rehydrated with xylene, graded alcohols, and buffer immersion steps. Antigen retrieval was carried out by heat-induced epitope retrieval method (Supplementary provided for specific details for each antibody). The slides were incubated in a preheated pressure cooker with Citrate buffer pH6 or Tris-EDTA buffer pH9 and blocked with horse serum (30 minutes at 25°C). Immunohistochemical staining was completed on a DAKO autostainer using liquid streptavidin biotin horseradish peroxidase and DBA (DAKO labeled avidin-biotin-peroxidase kits) as chromogens. Deparaffinized sections were stained with six monoclonal antibodies at the following titrations: CD4-1:250 (Abcam Ab846); CD8-1:40 (Nova Castra VP-C320); FoxP3 -1:200 (Abcam Ab20034); CD104 -1:50 (Beta-4 integrin, eBioscience 439-9b); CD68 -1:100 (Dako M0814) and CD1a -1:500, (Abcam Ab-108309). Appropriate negative (without primary antibodies) and positive (tonsillar tissue and various carcinomas) controls were stained concurrently on the same slides.
Quantification of tumor infiltrating lymphocytes and CD1a expression
The stained TMA slides were assessed by a technician blinded to patient clinical status and treatment outcome. The TMA slides were digitally imaged, scanned, and retrieved with Aperio ImageScope v.12 software. Grid software (Meazure, C Thing Software 2.01) was used to overlay each tissue core image prior to counting cells. CD104 staining (beta-4 integrin) for each core was examined first to locate and confirm the extent and location of carcinoma within the tissue cores. Only cores consisting of >50% tumor parenchyma were counted. The TILs stained with CD4, CD8, FoxP3, and CD68 antibodies in each core were manually counted at 200× magnification (20× objective lens). (Supplementary Figure). Only TILs infiltrating in tumor parenchyma were quantified since it has proven to be most reproducible and representative immune response parameter for TILs6,16. Mean counts per core of triplicate samples for each patient were calculated and used in statistical analysis. Although some non-uniformity of staining can result from differences in primary tissue processing and fixation, a major advantage of using a tissue microarray for these studies is that all staining conditions are uniform for each antibody tested which limits variability due to immunohistologic staining techniques, heat extraction timing, and antibodies.
The expression of CD1a in the tissue microarrays was graded semi-quantitatively by pattern and number of positive cells19–21. CD1a expression in tumor nodules was both cytoplasmic and membranous and located primarily in the basal layers. In tumor stroma, the expression was primarily membranous on inflammatory cells. Grading in tumor was 0 for no expression, 1+ for modest expression mostly in basal cells and 2+ if uniform expression on many cells (>10/core) with moderate intensity. Grading in stroma was 0 if no cells were stained, 1+ if only occasional cells (1–10/core) with membrane staining and 2+ if there were many cells (>10) with membranous staining.
Human Papilloma Virus testing
Adequate tumor DNA was available from 257 patient specimens to determine HPV status. HPV positive tumors were identified by an ultrasensitive method using real-time competitive polymerase chain reaction and matrix-assisted laser desorption/ionization time of flight mass spectroscopy with separation of products on a matrix loaded silicon chip array, as previously described22,23.
Statistical Methods
Mean counts per tumor core were used in analysis for CD4, CD8, FoxP3, and CD68. CD1a was analyzed using an ordinal score according to grading scheme described above. Clinical variables studied are listed in Table 1. All clinical variables were categorized (Table 1) and tests were performed for associations using the nonparametric Kruskal-Wallis test (for TILs) or chi-square test (for CD1a) using p-value estimations by Monte Carlo simulation.
Time-to-event outcomes were defined from date of initial diagnosis to date of last follow-up, recurrence (relapse free survival [RFT]) or death (overall survival [OST] and disease specific survival [DST]). The Kaplan-Meier method and log-rank tests were used to illustrate univariable outcomes based on high or low levels of each biomarker dichotomized at the median. Single variable and multivariable Cox proportional hazard models were used to test associations between TILs, CD1a expression and time-to-event outcomes treating each biomarker as a continuous (TILs) or ordinal category (CD1a). Hazard ratios are presented for increases of 10 cells. Multivariable models included covariate adjustments for age, stage, disease site, HPV status, treatment and smoking history. The interrelationship of treatment modality in these models was explored using multivariable Cox proportional hazard models including an interaction term for primary treatment groups and biomarkers. All statistical analyses were conducted in SASv9.3 and graphed in R (v3.0.3).
Results
Immune infiltrates and clinical variables
The association of levels of each lymphocyte subset with tumor stage, tumor site, HPV status, tobacco and alcohol use, comorbidities and age was determined (Table 3). CD8 and CD68 levels differed by clinical tumor stage while levels of all subsets, including CD1a, differed significantly by tumor site. This was most notable for oropharynx cancers where higher levels of CD4 (p=0.002), CD8 (p<0.0001), FoxP3 (<0.0001) and CD68 (p=0.02) positive cells were observed. When separating oropharynx cancers by HPV status, HPV+ oropharynx cancers still differed significantly from the other disease sites (p=0.006, <0.0001, <0.0001, and 0.01 for CD4, CD8, FoxP3 and CD68, respectively). There were only 15 HPV− oropharynx cancers in our sample rendering our evaluation of that subset of oropharynx cancers less powerful, however lower levels compared to HPV+ oropharynx were observed. As expected, levels of CD4, CD8, FoxP3 and CD68 infiltrates were higher in HPV+ cases (p=0.02, p<0.0001, p<0.0001, and p=0.01 respectively) compared to HPV− tumors in the combined cohort. Among oropharynx cases alone, infiltrates still tended to be higher in HPV+ cases, most notably for CD8 (p=0.05, p=0.002, p=0.08, and p=0.10, respectively for CD4, CD8, FoxP3 and CD68). Interestingly, CD4, CD8 and FoxP3 infiltrates tended to be higher in patients with low comorbidity scores (p=0.15, p=0.02 and p=0.01, respectively). Likewise, levels of these subsets also tended to be higher in never smokers compared to former or current smokers (p=0.03, p=0.07, and p=0.06, respectively).
Table 3.
Associations between Immune Cell Infiltrates and Clinical Variables
| CD4 | CD8 | FOXP3 | CD68 | CD1A tumor | CD1A stroma | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | p-value | Median | p-value | Median | p-value | Median | p-value | 0 (%) | 1 (%) | 2 (%) | p-value | 0 (%) | 1 (%) | 2 (%) | p-value | ||
| Age | <60 | 11.3 | 0.67 | 6.8 | 0.04 | 11.0 | 0.25 | 13.0 | 0.58 | 42 | 40 | 18 | 0.87 | 75 | 16 | 9 | 0.78 |
| 60–80 | 10.0 | 12.5 | 9.3 | 16.0 | 44 | 39 | 17 | 74 | 17 | 9 | |||||||
| >80 | 13.3 | 7.0 | 7.3 | 13.5 | 56 | 28 | 17 | 67 | 22 | 11 | |||||||
| Stage | 1 | 10.3 | 0.20 | 7.3 | 0.04 | 13.0 | 0.33 | 15.5 | 0.03 | 44 | 48 | 8 | 0.56 | 68 | 20 | 12 | 0.73 |
| 2 | 17.3 | 9.5 | 6.7 | 16.0 | 38 | 42 | 19 | 77 | 15 | 8 | |||||||
| 3 | 11.0 | 5.7 | 8.3 | 9.0 | 41 | 41 | 18 | 86 | 9 | 5 | |||||||
| 4 | 10.0 | 8.5 | 11.9 | 15.0 | 47 | 34 | 20 | 72 | 18 | 10 | |||||||
| Disease Site | larynx | 9.7 | 0.002 | 4.0 | <0.001 | 6.0 | <0.001 | 4.0 | 0.02 | 57 | 36 | 7 | 0.01 | 86 | 14 | . | 0.81 |
| oral cavity | 9.0 | 7.0 | 7.3 | 14.0 | 45 | 42 | 13 | 74 | 16 | 10 | |||||||
| oropharynx | 25.0 | 24.5 | 27.3 | 18.0 | 39 | 24 | 37 | 68 | 21 | 11 | |||||||
| hypopharynx | 11.5 | 1.3 | 3.0 | 3.0 | . | 100 | . | 100 | . | . | |||||||
| Comorbidities | none | 14.2 | 0.15 | 9.5 | 0.02 | 13.1 | 0.01 | 12.0 | 0.93 | 36 | 41 | 23 | 0.27 | 77 | 11 | 13 | 0.18 |
| mild | 11.5 | 8.0 | 11.3 | 16.0 | 45 | 39 | 16 | 66 | 23 | 10 | |||||||
| moderate | 5.3 | 5.7 | 7.3 | 10.5 | 50 | 35 | 15 | 90 | 10 | . | |||||||
| severe | 5.8 | 1.4 | 4.2 | 14.0 | 67 | 25 | 8 | 83 | 17 | . | |||||||
| HPV | Negative | 10.3 | 0.005 | 5.7 | <0.001 | 8.0 | <0.001 | 10.5 | 0.004 | 42 | 43 | 14 | 0.004 | 76 | 16 | 8 | 0.58 |
| Positive | 18.5 | 13.7 | 18.0 | 18.0 | 49 | 24 | 27 | 69 | 22 | 9 | |||||||
| Smoking | never | 14.3 | 0.03 | 9.0 | 0.07 | 12.8 | 0.06 | 13.0 | 0.50 | 44 | 41 | 15 | 0.64 | 65 | 20 | 15 | 0.30 |
| current | 10.3 | 5.7 | 7.2 | 14.0 | 44 | 37 | 19 | 83 | 11 | 6 | |||||||
| former | 10.0 | 8.0 | 12.6 | 17.5 | 44 | 38 | 19 | 73 | 21 | 6 | |||||||
| Alcohol Use | never | 8.0 | 0.22 | 4.5 | 0.30 | 6.0 | 0.35 | 9.0 | 0.84 | 68 | 26 | 5 | 0.29 | 63 | 21 | 16 | 0.77 |
| current | 10.7 | 7.7 | 9.7 | 15.0 | 42 | 40 | 18 | 77 | 15 | 8 | |||||||
| former | 16.6 | 8.5 | 10.7 | 13.5 | 38 | 38 | 23 | 72 | 21 | 8 | |||||||
| Treatment | Surgery | 10.3 | 0.24 | 7.3 | <0.001 | 8.0 | <0.001 | 13.0 | 0.23 | 43 | 43 | 14 | 0.01 | 75 | 16 | 10 | 0.98 |
| Chemoradiation | 16.0 | 21.3 | 14.5 | 14.5 | 56 | 17 | 28 | 69 | 22 | 8 | |||||||
| Radiation | 26.8 | 5.7 | 20.8 | 19.0 | 25 | 50 | 25 | 75 | 13 | 13 | |||||||
| Palliation | 5.8 | 1.3 | 3.2 | 11.0 | 33 | 50 | 17 | 83 | 17 | . | |||||||
Immune infiltrates and treatment outcomes
Overall, when patients were grouped by high or low levels of immune cell infiltrates in their tumors, higher levels of each subset except CD68 showed significantly improved overall, relapse free and disease specific survival (Figure 1). Prognostic Cox models for association of patient outcomes with TIL markers as single variables and after controlling for age, tumor stage, tumor site, comorbidities, HPV status, treatment and tobacco use were created (Table 4). Higher CD4 and CD8 TIL levels were associated with improved overall survival OS (HR 5 0.77; 95% confidence interval [CI] 5 0.65–0.93; p 5.005 and HR 50.77; 95% CI 50.64–0.94; p 5 .008, respectively), and RFS (p 5.03, and p 5 .05, respectively). In the multivariable Cox model controlling for age, clinical stage, disease site, comorbidities, HPV status, treatment and smoking, higher CD4 levels remained significant for improved overall and disease specific survival (p=0.003 and 0.004, respectively). Higher levels of CD8 and FoxP3 infiltrates were borderline significantly associated with improved overall survival but not for relapse free or disease specific outcomes.
Figure 1.
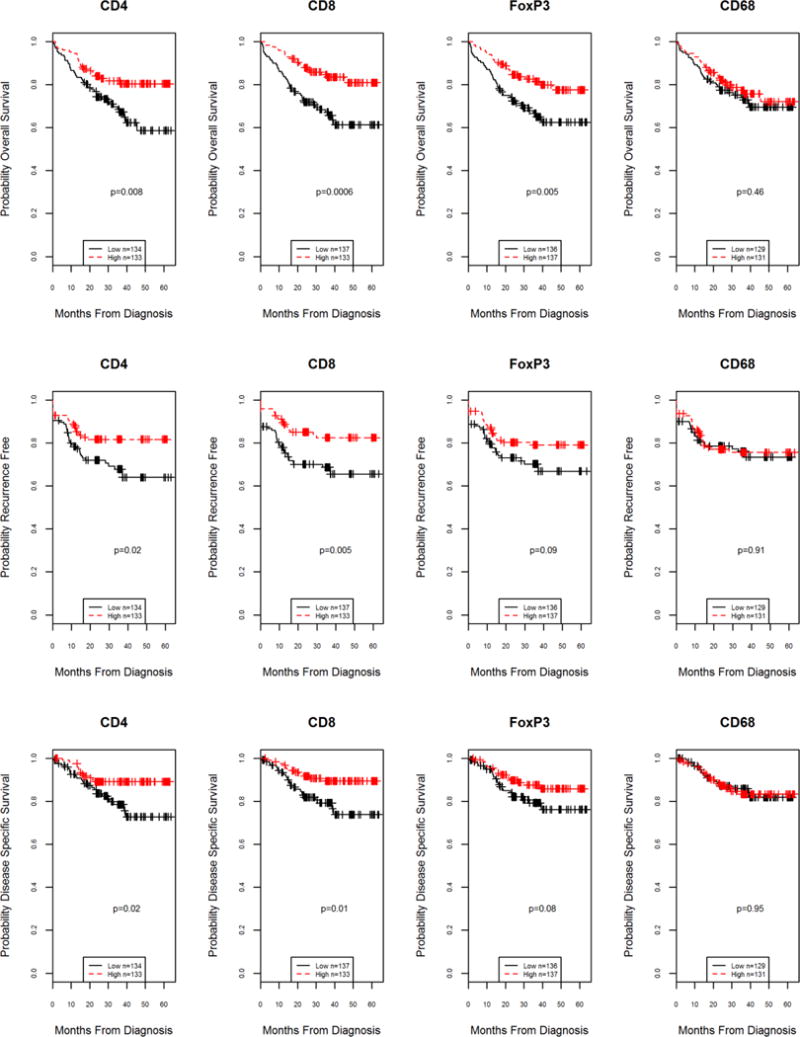
Kaplan-Meier survival curves for overall, recurrence free and disease specific survival according to lymphocyte subset levels above (high) or below (low) the median value. Significant differences (log-rank testing) were found in all outcomes for CD4 and CD8 infiltrate levels. FoxP3 levels were also significant for overall survival.
Table 4.
Cox Model Results for Association of Immune Cell Infiltrates and Outcomes
| Single Variable Results | OS | RFT | DST | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | n | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| CD4 | 10 cell increase | 267 | 0.77 (0.65, 0.93) | 0.005 | 0.83 (0.70, 0.99) | 0.03 | 0.73 (0.56, 0.94) | 0.02 |
| CD8 | 10 cell increase | 270 | 0.77 (0.64, 0.94) | 0.008 | 0.85 (0.72, 1.00) | 0.05 | 0.80 (0.64, 1.01) | 0.06 |
| FOXP3 | 10 cell increase | 272 | 0.82 (0.70, 0.96) | 0.01 | 0.92 (0.81, 1.04) | 0.18 | 0.89 (0.76, 1.06) | 0.19 |
| CD68 | 10 cell increase | 263 | 0.95 (0.81, 1.12) | 0.56 | 1.02 (0.87, 1.20) | 0.77 | 1.01 (0.83, 1.24) | 0.91 |
| CD1A_tumor | uniform | 52 | 0.68 (0.34, 1.35) | 0.27 | 0.56 (0.26, 1.24) | 0.15 | 0.41 (0.14, 1.20) | 0.10 |
| Modest,mostly basal | 90 | 0.84 (0.48, 1.47) | 0.54 | 0.84 (0.48, 1.47) | 0.54 | 0.94 (0.47, 1.89) | 0.86 | |
| none | 105 | ref | ref | ref | ||||
| CD1A_stroma | many cells + | 15 | 0.23 (0.03, 1.71) | 0.15 | 0.49 (0.12, 2.05) | 0.33 | 0.34 (0.05, 2.53) | 0.29 |
| occasional cells + | 30 | 0.94 (0.42, 2.14) | 0.89 | 0.80 (0.33, 1.91) | 0.61 | 0.60 (0.18, 2.01) | 0.41 | |
| no cells + | 122 | ref | ref | ref | ||||
| Multivariable Results | OS | RFT | DST | |||||
| Variable | n | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| CD4 | 10 cell increase | 267 | 0.70 (0.55, 0.89) | 0.003 | 0.85 (0.70, 1.03) | 0.09 | 0.56 (0.38, 0.83) | 0.004 |
| CD8 | 10 cell increase | 270 | 0.81 (0.67, 0.99) | 0.04 | 0.90 (0.75, 1.07) | 0.22 | 0.85 (0.68, 1.07) | 0.16 |
| FOXP3 | 10 cell increase | 272 | 0.83 (0.69, 1.00) | 0.05 | 0.94 (0.81, 1.09) | 0.41 | 0.87 (0.71, 1.08) | 0.21 |
| CD68 | 10 cell increase | 263 | 1.04 (0.87, 1.24) | 0.65 | 1.10 (0.93, 1.31) | 0.27 | 1.14 (0.92, 1.41) | 0.22 |
| CD1A_tumor | uniform | 52 | 0.94 (0.44, 1.99) | 0.87 | 0.52 (0.22, 1.25) | 0.15 | 0.44 (0.13, 1.51) | 0.19 |
| Modest, mostly basal | 90 | 0.93 (0.51, 1.70) | 0.82 | 0.82 (0.46, 1.48) | 0.51 | 0.89 (0.42, 1.90) | 0.76 | |
| none | 105 | ref | ref | ref | ||||
| CD1A_stroma | many cells + | 15 | 0.22 (0.03, 1.73) | 0.15 | 0.58 (0.13, 2.63) | 0.48 | 0.42 (0.05, 3.41) | 0.41 |
| occasional cells + | 30 | 1.11 (0.44, 2.78) | 0.83 | 0.99 (0.38, 2.56) | 0.98 | 1.17 (0.30, 4.59) | 0.82 | |
| no cells + | 122 | ref | ref | ref | ||||
Hazard ratios for continuous measures are all presented for each 10 cell increase.
Single variable models are Cox proportional hazards models with biomarker alone as a predictor.
Multivariable models are Cox proportional hazards models with biomarker as a predictor controlling for age, clinical stage, disease site, comorbidities, HPV, treatment and smoking in the model.
Definitions: OS=Overall Survival. RFS=Recurrence Free Survival. DSS=Disease Specific Survival. HR: Hazard Ratio. 95% CI: 95% confidence interval.
Survival outcomes differ by tumor site
Because of the association of oropharyngeal cancers with HPV-16, survival was better for patients with oropharyngeal cancers than the other sites. We had previously noted that the association of peripheral blood T cell subsets and TILs with prognosis differed for patients with oral cavity or oropharyngeal cancers6,16,23. Therefore, we also performed a separate exploratory analysis of the patients with laryngeal, oral cavity, and HPV+ oropharyngeal cancers separately, however the results should be interpreted with caution because of smaller subsets size and confounding of interactions between treatment, tumor site and biology. For larynx cancer, CD68 infiltrates were significantly associated with worse RFS and DSS as univariables and with worse RFS (HR 51.53; 95% CI 5 1.02–2.31; p 5 .04) in multivariable analysis. Multivariable analysis of the oral cavity cohort showed CD8 infiltrates were significantly associated with improved overall survival (HR 5 0.70; 95% CI 5 0.49–0.99; p 5 .04) and there was a trend for improved DSS (p=0.15). Multivariable analysis of the oropharyngeal cohort showed CD4 and FoxP3 infiltrates were significantly associated with improved OS (HR 5 0.92; 95% CI 5 0.86–0.99; p 5.02 and HR 5 0.95; 95% CI 50.91–0.99; p 5 .03 for CD4 and FoxP3, respectively) and there were trends for both infiltrates with improved RFS and DSS (p < .10). These associations and respective effect sizes remained similar after examining only the HPV-positive oropharyngeal cohort (OS: HR = 0.93; 95% CI 50.86–1.01; p = .07 and HR = 0.96; 95% CI = 0.92–1.00; p = .07 for CD4 and FoxP3, respectively).
For the other clinical variables among patients with oral cavity cancer, only CD4 infiltrates were associated with HPV status, however, only 16 patients with oral cavity cancer were HPV-positive (p = .04). Among patients with oropharyngeal cancer, HPV status was at least marginally associated with all infiltrates, and most strongly for CD8 (p = .05, p 5.002, p = .08, p = .09 for CD4, CD8, FoxP3, and CD68, respectively). Other associations between clinical variables infiltrates among patients with oropharyngeal cancer included an association of stage with CD68 infiltrates (p = .04) and associations of age with CD8 and FoxP3 (p = .002 and p = .01, respectively). These associations remained when the oropharyngeal HPV-positive subset alone was analyzed. CD68 infiltrates were higher in patients with lower stage and patients diagnosed at a younger age (<60 years old) were more likely to have lower CD8 and FoxP3 infiltrate levels. In addition, among patients with HPV-positive oropharyngeal cancer, we observed that younger patients were less likely to have CD1a-positive cells in the stroma (p = .01) and higher comorbidities was associated with higher CD8 infiltrates (p = .05).
Effects of treatment and immune infiltrates
To determine if treatment influenced the associations of immune infiltrates with outcomes, and whether TIL levels might be prognostic within treatment groups, patients were grouped by primary treatment and outcomes analyzed with respect to T cell infiltrates. We used an interaction model as a way to test our prior hypothesis that there would be a modulating effect between treatment type and the relationship between TILs and prognosis. The overall survival interaction model did not provide enough evidence to prove statistically that there was a modulating effect for CD4 or FoxP3 (p value for interaction term = 0.07 and 0.15 respectively), but, nonetheless, a trend was observed and it appears that CD4 and FoxP3 behave in a similar manner as CD8 which was significantly modulated by treatment type (p = .04). These differences in associations were less evident in RFS and DSS. Separate Kaplan-Meier plots and log-rank tests of overall survival by level of infiltrates are presented for surgical and chemoradiation patients in Figure 2. High and low strata for plots were calculated separately for each treatment group using the treatment-specific median as a cutoff. Significant benefit for overall survival was evident for patients with infiltrates above the median for CD4, CD8 and Foxp3 infiltrates but not CD68 in patients undergoing chemoradiation. In contrast, for patients undergoing surgery, modest improvements in overall survival were evident for patients with high CD8 infiltrates (Figure 2). It should be noted that treatment was influenced by tumor site since most oral cavity patients were treated with surgery and most oropharyngeal cancer patients received chemoradiation The multivariable interaction model included adjustment for disease site and its possible effect modification (interaction) with treatment type, although this is not represented in the Kaplan-Meier plots of Figure 2.
Figure 2.
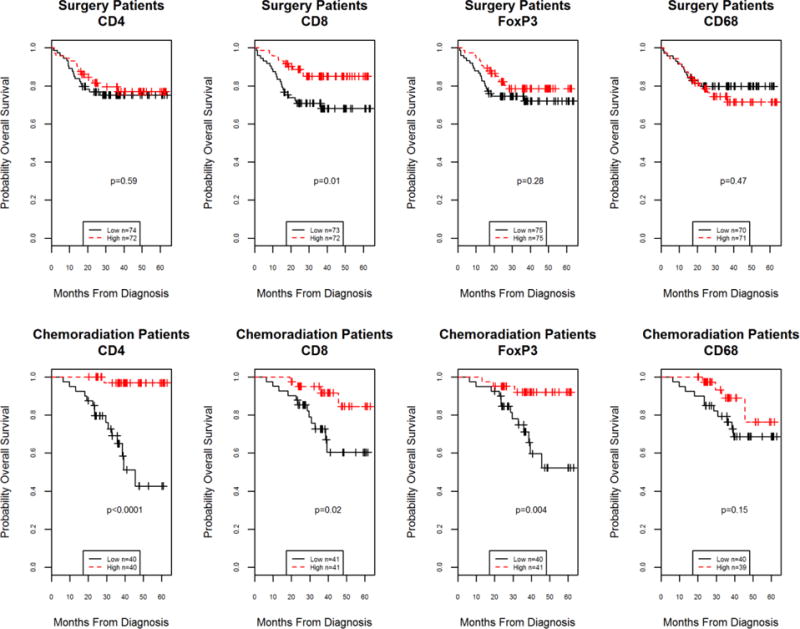
Kaplan-Meier curves for overall survival for above (high) or below (low) the median value within treatment type of of tumor infiltrating lymphocyte subset levels according to primary treatment modality. Surgery patient survival illustrated in upper panels and chemoradiation patient survival illustrated in lower panels. Among surgical patients, high levels of CD8 cells were significant for prognosis (log-rank testing) while for chemoradiation patients, high levels of CD4, CD8 and FoxP3 T-cell infiltrates were significant (log-rank testing). Low and high strata were defined separately for surgery and chemoradiation modality cohorts by median cutoff within treatment set.
Correlations among immune infiltrates
Levels of immune cell subsets were positively correlated with each other (p < 0.0001, Spearman rho range: 0.42–0.51), except for CD68 which showed weaker, but still significant correlations with CD4, CD8 and FoxP3 but no correlation with CD1a expressing cells in the tumor or stroma except for a weak correlation with CD8 levels (p = 0.0016, Spearman rho: 0.24) in stroma.
Discussion
The major finding in this large cohort study was the demonstration that multiple subsets of TILs were strong prognostic factors for overall survival in previously untreated patients with HNSCC. This finding confirms prior results by a number of investigators11 and extends them to include multiple subsets of T lymphocytes. In other tumor types, CD8 infiltrates that have functionally been characterized as cytotoxic/suppressor T lymphocytes have been most often associated with favorable prognosis3,24,25, while FoxP3 infiltrates have been associated with either improved4,27,28, or worse outcomes29. FoxP3 cells represent a functional subset of regulatory (generally suppressive) T lymphocytes that are a subset of CD4 (helper) T lymphocytes. In HNSCC, improved survival has been most closely associated with CD8 infiltrates9,11,16,17,30, whereas intratumoral FoxP3 infiltrates have been variably associated with improved7,11 neutral or negative impact on survival7,13,30,31. CD4 infiltrates have been associated with favorable outcomes less commonly, even though FoxP3 cells are considered a subset of CD4 cells6,11,32.
Interestingly, in the HPV-positive oropharyngeal subset, higher than expected levels of FoxP3 infiltrates were noted that were associated with increasing age. This might have been due to co-staining of CD8-positive FoxP3 memory cells that have been associated with aging. The multivariable analysis of the subset of HPV-positive oropharyngeal cases also confirmed correlations of CD4 infiltrates with OS but did not see the strong correlations of CD8 infiltrates that we previously reported in a small trial of patients with oropharyngeal cancer treated with neoadjuvant chemotherapy16. When median TIL levels were compared among various smaller cohorts (Table 3), it is also interesting to note that levels of FoxP3 cells were also slightly higher than CD4 levels in stage I patients and former smokers. This is somewhat paradoxical because FoxP3 cells are typically a subset of CD4 cells. Although these differences could be due to co-staining of some CD8-positive FoxP3 cells, it is more likely merely because of reporting median values and the small number of patients in these subsets. However, the number of patients analyzed for the oropharyngeal tumor site was reasonably large and the slightly higher levels of FoxP3 cells could alternatively reflect the abundant intermingling of stromal infiltrating cells with the tumor parenchyma and variability in counting these cells in such small tumor cores rather than differences in immunohistologic staining.
Most investigators have reported generally higher levels of TILs in patients with HPV+ cancers and those levels have been associated with better survival11,14,16,33. Whether this is due to immune reactivity in the tumor microenvironment or other more favorable genetic or molecular characteristics of HPV+ oropharyngeal cancers remains unknown. Previous smaller studies demonstrated that CD8 infiltrates were prognostically important in both HPV+ and HPV− oropharynx cancers, and that CD68 infiltrates were most important in oral cavity cancers6,16. This is of interest since the CD68 antibody marks a subset of myeloid derived cells that are functionally thought to be immunosuppressive. The current results validate these findings in a large cohort and demonstrate that higher levels of all T cell subsets (except CD68) are associated with HPV+ cancers and may account for the strong association of these levels with improved outcomes. Whether higher TIL levels indicate a beneficial immune response in HPV-related cancers that accounts for improved survivorship or if high TILs are merely a result of abundant lymphoid tissue in the pharynx is unknown.
We attempted to minimize this confounding factor by focusing our assessment only on cells within tumor parenchyma. Better outcomes associated with the higher TIL levels noted in patients with HPV-positive cancers may also be a reflection of the correlations of TIL levels we demonstrated with lower patient co-morbidity scores, and less frequent smoking which are often characteristics of HPV-related oropharyngeal cancer. This is the first demonstration of strong correlations of TILs with these patient characteristics since these have not typically been assessed in other studies of immune competence in HNSCC.
Others have also noted that correlations with outcome are influenced by type of treatment and stage of disease8. Although not reported in head and neck cancer, levels of TILs have been directly associated with favorable response to chemotherapy in breast cancer2,24,34,36. Importantly, we confirmed that the levels of T cell infiltrates differed significantly by tumor site, tumor stage and by HPV status. We also extended this analysis to primary treatment modality and demonstrated that the prognostic significance of individual T cell infiltrates differed by treatment modality with generally strong and favorable prognostic significance of higher T cell infiltrates, particularly CD4 infiltrates, for patients treated with chemoradiation. In contrast, our findings suggested that CD8 infiltrates were the more important favorable prognostic factor for patients treated with primary surgery and infiltration of CD68 tumor associated macrophage infiltrates were a negative although not statistically significant prognostic factor. These findings are consistent with recent preliminary findings in oral cavity cancer patients since most of the patients in our large cohort that had primary surgery were patients with oral cavity cancers6.
Because factors like tumor site, stage and treatment could confound correlations of TILs with outcomes, it was important to perform multivariable analysis adjusting for tumor site, stage and treatment. Some caution is warranted because of possible “over fitting” of the data due to the large number of variables analyzed and the number of survival “events”. However, we believe this analysis is the first to demonstrate the significant effects of both treatment and tumor site on the association of TILs and prognosis. The results will need to be confirmed in additional cohorts. Treatment and tumor site are often strongly interrelated since most oral cavity cancers are treated with primary surgery, most oropharyngeal cancers are treated with chemoradiation and laryngeal cancers treated with either surgery, radiation or combined chemoradiation. Nonetheless, our findings were still significant after adjusting for site and treatment. These data support the conclusion that specific TILs are likely independent prognostic factors that could be useful as a variable in risk stratification models that include treatment.
With the evolution of personalized medicine, the current data lend further support to the concept that the immune system is important in outcomes for patients with HNSCC. Differences by treatment modality suggest that the immune system may be particularly important in chemotherapy and radiation approaches. It may be that effective cytoreduction by these modalities relies on the immune system more than a modality such as surgical excision. We have previously suggested this by measuring levels of T cell subsets in pretreatment peripheral blood and demonstrating significant correlations with tumor response to neoadjuvant chemotherapy36,37. Taken together, such findings may be particularly relevant to increasing interest in use of immunomodulating chemotherapy and radiation as primary therapy for common tumor sites such as larynx and oropharynx. Although still speculative, patients lacking significant TILs, particularly CD8 infiltrates, might benefit from primary surgical management with or without addition of developing immunotherapeutic approaches.
It is somewhat remarkable that we found significant associations of TILs with survival outcomes using direct quantitation of infiltrating cells within 0.7 mm tissue array microcores. Few investigators have used tissue microarrays for assessing immune cell infiltrates in head and neck8,9,14 or other cancers28,38. Most assessments have been done using whole sections and have been semi-quantitative or have used automated quantification of immunohistochemical markers. Most have measured both stromal and tumor parenchymal infiltrates and some have used specific locations of infiltrates such as the tumor invasive margin39. None have demonstrated stronger correlations with outcomes or significant associations with tumor site and treatment than this study has shown. In particular, the demonstration of these correlations in view of the use of biopsy specimens, the random selection of tumor site for core harvesting and unbiased counting of the TMAs by a technician blinded as to outcomes underscores the conclusion that correlations of the degree of infiltration are strong enough to overcome issues such as sampling error. Some variability was overcome by limiting our quantification to cells infiltrating tumor parenchyma. This approach, however, could miss important immunologic characteristics occurring in the stromal microenvironment where B lymphocytes and monocytes are more prevalent40. For some tumors such as breast cancer, stromal infiltrates seem particularly important36. There is evidence that cellular crosstalk via cytokines and prostaglandins can influence both numbers and function of intratumoral lymphocytes, particularly FoxP39,41–44 and that activation of cytotoxic lymphocytes is under complex immune checkpoint regulation in the microenvironment46. There is also new evidence that B cell populations may also be important46. We did not measure B cell populations in this study based on our prior work where we found no correlations of B cell infiltrates with prognosis.32
Conclusions
The search for HNSCC biomarkers that define biologic behavior and response to therapy has intensified with the identification of the unique HPV-related subset of oropharyngeal cancers. Precision medicine demands identification of individual biologic characteristics that could improve therapy selection. Our current findings lend additional support to the important role of host immunity in prognosis and indicate that the degree of immune cell infiltrate in the tumor microenvironment is a significant independent prognostic factor that deserves further study as a potentially useful biomarker in patients with HNSCC.
Supplementary Material
Acknowledgments
The authors thank the many investigators in the University of Michigan Head and Neck Specialized Program of Research Excellence for their contributions to patient recruitment, assistance in data collection and encouragement including Carol R. Bradford, MD, Thomas E. Carey, PhD, Douglas B. Chepeha, MD, Sonia Duffy, PhD, Avraham Eisbruch, MD, Joseph Helman, DDS, Kelly M. Malloy, MD, Jonathan McHugh, MD, Scott A. McLean, MD, Tamara H. Miller, RN, Jeff Moyer, MD, Mark E. Prince, MD, Nancy Rogers, RN, Matthew E. Spector, MD, Nancy E. Wallace, RN, Heather Walline, PhD, Brent Ward, DDS, and Francis Worden, MD. We greatly thank our patients and their families who tirelessly participated in our survey and specimen collections. This work was supported by the University of Michigan Head and Neck Specialized Program of Research Excellence NIH/NCI P50CA097248 and NIH/NIDCD T32 DC005356.
References
- 1.Adams S, Gray RJ, Demaria S, et al. Prognostic Value of Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancers From Two Phase III Randomized Adjuvant Breast Cancer Trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27) doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31(7):860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 3.Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29(15):1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 4.Haas M, Dimmler A, Hohenberger W, et al. Stromal regulatory T-cells are associated with a favourable prognosis in gastric cancer of the cardia. BMC Gastroenterol. 2009;9(1):65. doi: 10.1186/1471-230X-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angell H, Galon J. From the immune contexture to the Immunoscore: The role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25(2):261–267. doi: 10.1016/j.coi.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Wolf GT, Chepeha DB, Bellile E, et al. Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: A preliminary study. Oral Oncol. 2015;51(1):90–95. doi: 10.1016/j.oraloncology.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell M, Cooper AC, Dhayal S, et al. Differential effects of interleukin-13 and interleukin-6 on Jak/STAT signaling and cell viability in pancreatic β-cells. Islets. 2013;5(2):95–105. doi: 10.4161/isl.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Distel LV, Fickenscher R, Dietel K, et al. Tumour infiltrating lymphocytes in squamous cell carcinoma of the oro- and hypopharynx: Prognostic impact may depend on type of treatment and stage of disease. Oral Oncol 2009. 2009;45(10):e167–e174. doi: 10.1016/j.oraloncology.2009.05.640. [DOI] [PubMed] [Google Scholar]
- 9.Pretscher D, Distel LV, Grabenbauer GG, et al. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+ B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer. 2009;9:292. doi: 10.1186/1471-2407-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badoual C, Hans S, Rodriguez J, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12(2):465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 11.Badoual C, Hans S, Merillon N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV associated head and neck cancer. Cancer Res. 2012;73:128–138. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 12.Berinstein NL, Wolf GT, Naylor PH, et al. Increased lymphocyte infiltration in patients with head and neck cancer treated with the IRX-2 immunotherapy regimen. Cancer Immunol Immunother. 2012;61(6):771–782. doi: 10.1007/s00262-011-1134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balermpas P, Michel Y, Wagenblast J, et al. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer. 2014;110(2):501–509. doi: 10.1038/bjc.2013.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward MJ, Thirdborough SM, Mellows T, et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br J Cancer. 2014;110(2):489–500. doi: 10.1038/bjc.2013.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung AC, Guihard S, Krugell S, et al. CD8-alpha T-cell infiltration in human papillomavirus-related oropharyngeal carcinoma correlates with improved patient prognosis. Int J Cancer. 2013;132:E26–E36. doi: 10.1002/ijc.27776. [DOI] [PubMed] [Google Scholar]
- 16.Wansom D, Light E, Thomas D, et al. Infiltrating lymphocytes and human papillomavirus-16–associated oropharyngeal cancer. Laryngoscope. 2012;122(1):121–127. doi: 10.1002/lary.22133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nordfors C, Grün N, Tertipis N, et al. CD8+ and CD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur J Cancer. 2013;49(11):2522–2530. doi: 10.1016/j.ejca.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Nocito A, Kononen J, Kallioniemi OP, et al. Tissue microarrays (TMAS) for high-throughput molecular pathology research. Int J Cancer. 2001;94(1):1–5. doi: 10.1002/ijc.1385. [DOI] [PubMed] [Google Scholar]
- 19.Goldman S, Baker E, Weyant RJ, et al. Peritumoral CD1a-positive dendritic cells are associated with improved survival in patients with tongue carcinoma. Arch Otolaryngol Head Neck Surg. 1998;124(6):641–646. doi: 10.1001/archotol.124.6.641. [DOI] [PubMed] [Google Scholar]
- 20.Colmone A, Li S, Wang C. Activating transcription factor/cAMP response element binding protein family member regulated transcription of CD1A. J Immunol. 2006;177(10):7024–7032. doi: 10.4049/jimmunol.177.10.7024. [DOI] [PubMed] [Google Scholar]
- 21.Gogolak P, Rethi B, Szatmari I, et al. Differentiation of CD1a- and CD1a+ monocyte-derived dendritic cells is biased by lipid environment and PPAR-γ. Blood. 2007;109(2):643–652. doi: 10.1182/blood-2006-04-016840. [DOI] [PubMed] [Google Scholar]
- 22.Yang H, Yang K, Khafagi A, et al. Sensitive detection of human papillomavirus in cervical, head/neck, and schistosomiasis-associated bladder malignancies. Proc Natl Acad Sci U S A. 2005;102(21):7683–7688. doi: 10.1073/pnas.0406904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26(19):3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dewyer NA, Wolf GT, Light E, et al. Circulating CD4-positive lymphocyte levels as predictor of response to induction chemotherapy in patients with advanced laryngeal cancer. Head Neck. 2014;36:9–14. doi: 10.1002/hed.23263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anitei M-G, Zeitoun G, Mlecnik B, et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res. 2014;20(7):1891–1899. doi: 10.1158/1078-0432.CCR-13-2830. [DOI] [PubMed] [Google Scholar]
- 26.Fridman WH, Galon J, Pagès F, et al. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 2011;71(17):5601–5605. doi: 10.1158/0008-5472.CAN-11-1316. [DOI] [PubMed] [Google Scholar]
- 27.Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: The paradox of colorectal cancer. Cancer Immunol Immunother. 2011;60(7):909–918. doi: 10.1007/s00262-011-1046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salama P, Phillips M, Grieu F, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27(2):186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 29.deLeeuw RJ, Kost SE, Kakal J, et al. The Prognostic Value of FoxP3+ Tumor-Infiltrating Lymphocytes in Cancer: A Critical Review of the Literature. Clin Cancer Res. 2012;18(11):3022–3029. doi: 10.1158/1078-0432.CCR-11-3216. [DOI] [PubMed] [Google Scholar]
- 30.Näsman A, Romanitan M, Nordfors C, et al. Tumor infiltrating CD8 + and Foxp3 + Lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in Tonsillar cancer. PLoS One. 2012;7(6):e38711. doi: 10.1371/journal.pone.0038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun DS, Zhao MQ, Xia M, et al. The correlation between tumor-infiltrating Foxp3+ regulatory T cells and cyclooxygenase-2 expression and their association with recurrence in resected head and neck cancers. Med Oncol. 2012;29(2):707–713. doi: 10.1007/s12032-011-9903-2. [DOI] [PubMed] [Google Scholar]
- 32.Wolf GT, Hudson JL, Peterson K, et al. Lymphocyte subpopulations infiltrating squamous carcinomas of the head and neck: correlations with extent of tumor and prognosis. Otolaryngol Head Neck Surg. 1986;95(2):142–152. doi: 10.1177/019459988609500203. [DOI] [PubMed] [Google Scholar]
- 33.Rajjoub S, Basha SR, Einhorn E, et al. Prognostic significance of tumor-infiltrating lymphocytes in oropharyngeal cancer. Ear Nose Throat J. 2007;86(8):506–511. [PubMed] [Google Scholar]
- 34.Brown JR, Wimberly H, Lannin DR, et al. Multiplexed Quantitative Analysis of CD3, CD8, and CD20 Predicts Response to Neoadjuvant Chemotherapy in Breast Cancer. Clin Cancer Res. 2014;20(23):5995–6005. doi: 10.1158/1078-0432.CCR-14-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 36.Denkert C, von Minckwitz G, Brase JC, et al. Tumor-Infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy With or Without Carboplatin in Human Epidermal Growth Factor Receptor 2-Positive and Triple-Negative Primary Breast Cancers. J Clin Oncol. 2014;33(9):983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 37.Bradford CR, Kumar B, Bellile E, et al. Biomarkers in advanced larynx cancer. Laryngoscope. 2014;124:179–187. doi: 10.1002/lary.24245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee AM, Clear AJ, Calaminici M, et al. Number of CD4+ Cells and Location of Forkhead Box Protein P3 – Positive Cells in Diagnostic Follicular Lymphoma Tissue Microarrays Correlates With Outcome. J Clin Oncol. 2006;24(31):5052–5059. doi: 10.1200/JCO.2006.06.4642. [DOI] [PubMed] [Google Scholar]
- 39.Galon J, Franck P, Marincola FM, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miles FL, Sikes R. Insidious changes in stromal matrix fuel cancer progression. Mol Cancer Res. 2014;12(3):297–312. doi: 10.1158/1541-7786.MCR-13-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whiteside TL. Regulatory T cell subsets in human cancer: are they regulating for or against tumor progression? Cancer Immunol Immunother. 2014;63(1):67–72. doi: 10.1007/s00262-013-1490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iuchi T, Teitz-Tennenbaum S, Huang J, et al. Interleukin-21 augments the efficacy of T-cell therapy by eliciting concurrent cellular and humoral responses. Cancer Res. 2008;68(11):4431–4441. doi: 10.1158/0008-5472.CAN-07-5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woodford D. An Inflammatory Cytokine Milieu is Prominent in Premalignant Oral Lesions, but Subsides when Lesions, Progress to Squamous Cell Carcinoma. J Clin Cell Immunol. 2014;05(3) doi: 10.4172/2155-9899.1000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oshimori N, Oristian D, Fuchs E. TGF-β Promotes Heterogeneity and Drug Resistance in Squamous Cell Carcinoma. Cell. 2015;160(5):963–976. doi: 10.1016/j.cell.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33 doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Q, Lao X, Pan Q, et al. Adoptive Transfer of Tumor Reactive B Cells Confers Host T-Cell Immunity and Tumor Regression. Clin Cancer Res. 2011;17(15):4987–4995. doi: 10.1158/1078-0432.CCR-11-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


