Abstract
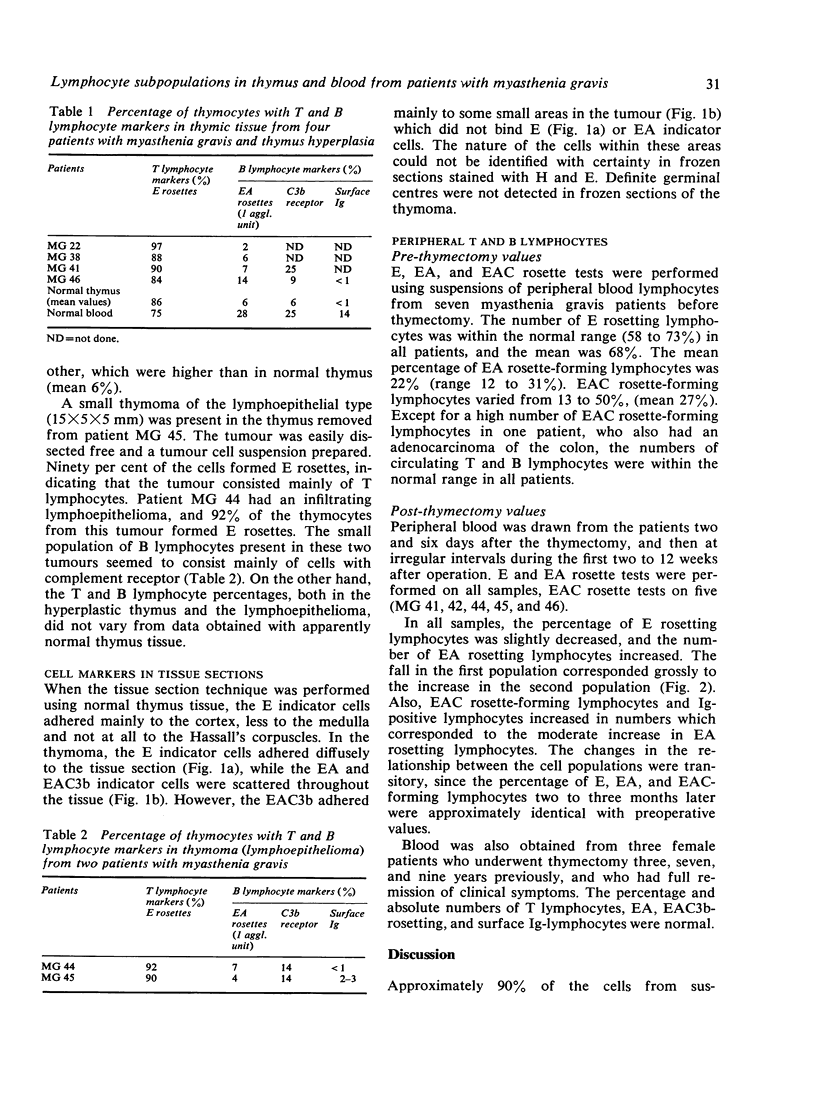
Cell suspensions were prepared from hyperplastic thymic tissue and lymphoepithelioma from patients with myasthenia gravis and from presumed normal thymic tissue obtained at cardiac surgery. The mononuclear cells were examined for surface markers. The mean percentages of both T lymphocytes and Fc receptor-carrying lymphocytes were similar in the three groups, whereas there was an increase in C receptor-carrying lymphocytes in the samples from myasthenic patients. Sections from the thymus gland were examined for T and B markers. In the hyperplastic thymus and in lymphoepithelioma, the T lymphocytes were distributed diffusely throughout the cortex and the medulla; in the normal thymus they were predominant in the cortex. The mean percentage of T and B lymphocytes in peripheral blood from patients with myasthenia gravis was normal. Thymectomy involved a transitory decrease in T lymphocytes with a corresponding increase in B lymphocytes.
Full text
PDF

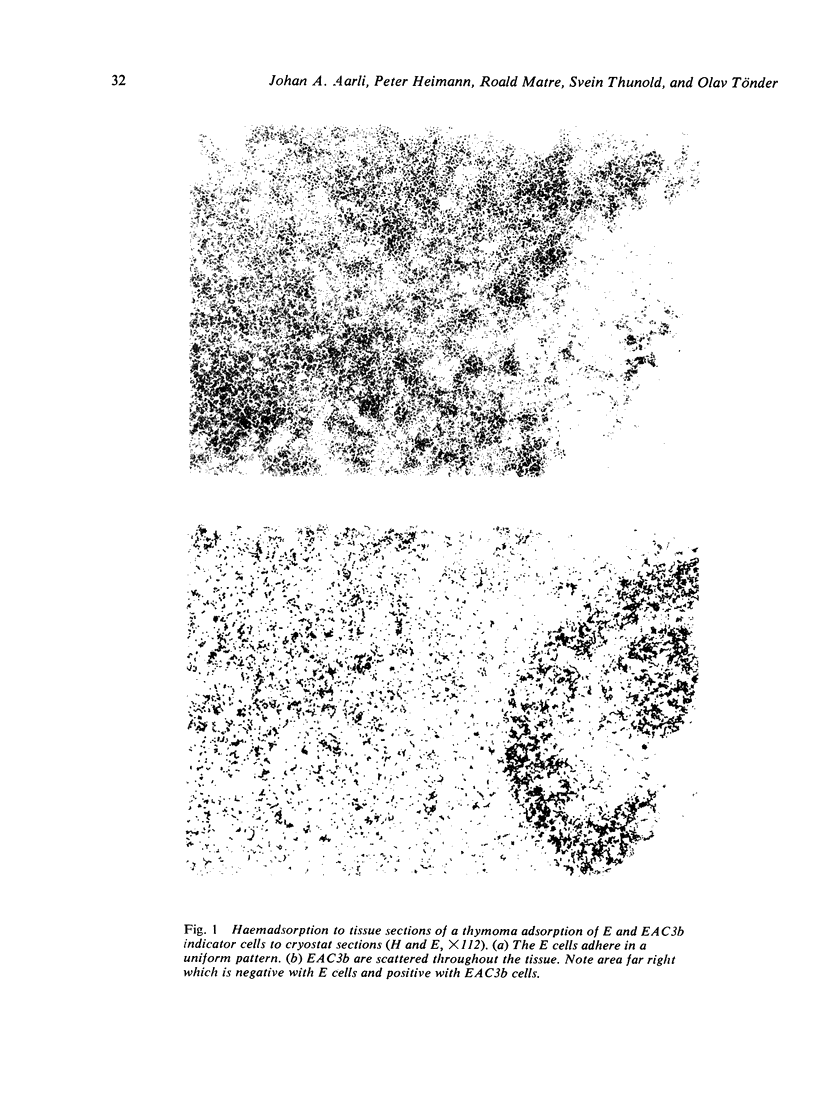

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
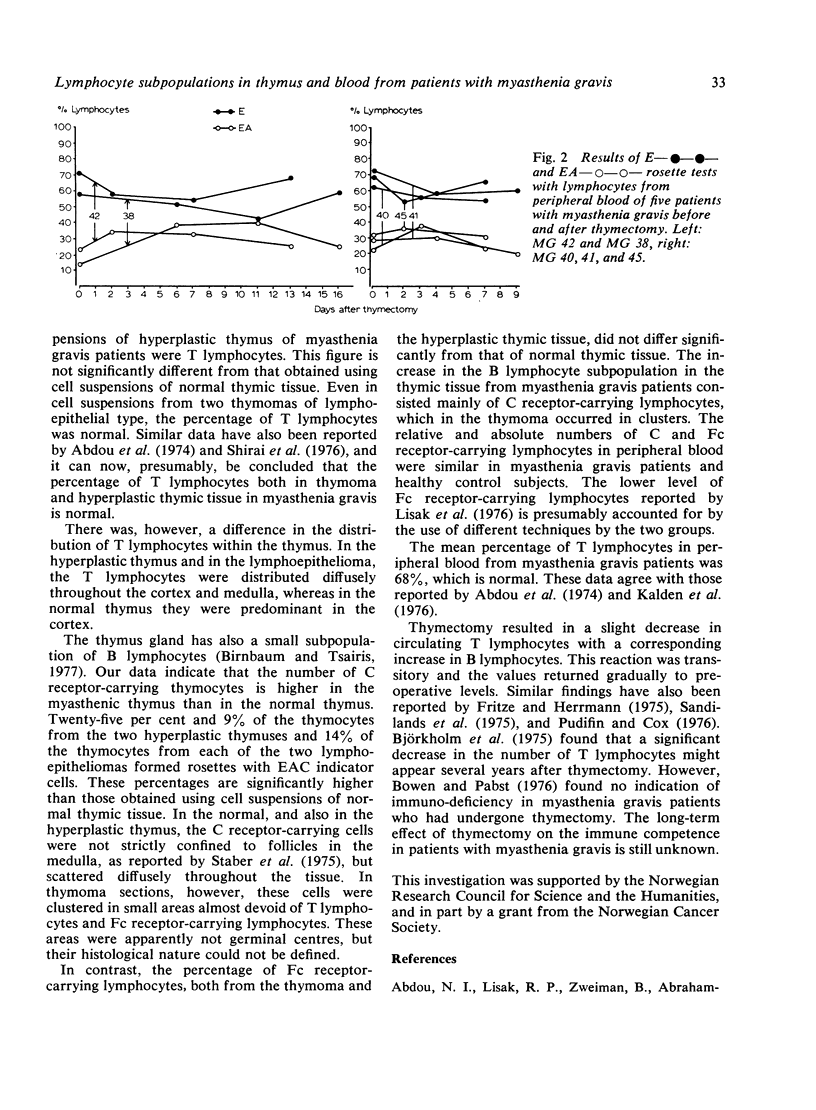
- Abdou N. I., Lisak R. P., Zweiman B., Abrahamsohn I., Penn A. S. The thymus in myasthenia gravis. Evidence for altered cell populations. N Engl J Med. 1974 Dec 12;291(24):1271–1275. doi: 10.1056/NEJM197412122912403. [DOI] [PubMed] [Google Scholar]
- Beutner E. H., Sepulveda M. R., Barnett E. V. Quantitative studies of immunofluorescent staining. Relationships of characteristics of unabsorbed antihuman IgG conjugates to their specific and non-specific staining properties in an indirect test for antinuclear factors. Bull World Health Organ. 1968;39(4):587–606. [PMC free article] [PubMed] [Google Scholar]
- Birnbaum G., Tsairis P. Thymic lymphocytes in myasthenia gravis. Ann Neurol. 1977 Apr;1(4):331–333. doi: 10.1002/ana.410010404. [DOI] [PubMed] [Google Scholar]
- Björkholm M., Holm G., Johansson B., Mellstedt H. T-Lymphocyte deficiency following adult thymectomin man. Scand J Haematol. 1975 May;14(3):210–215. [PubMed] [Google Scholar]
- Bowen T., Pabst H. F. Immunologic response in vitro after thymechtomy in patients with myasthenia gravis. Can Med Assoc J. 1976 Oct 9;115(7):619–622. [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Fritze D., Herrmann C., Jr Leukocyte and lymphoid cell counts in myasthenia gravis. Neurology. 1975 Mar;25(3):251–254. doi: 10.1212/wnl.25.3.251. [DOI] [PubMed] [Google Scholar]
- Hallberg T., Gurner B. W., Coombs R. R. Opsonic adherence of sensitized ox red cells to human lymphocytes as measured by rosette formation. Int Arch Allergy Appl Immunol. 1973;44(4):500–513. doi: 10.1159/000230956. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalden J. R., Lohmann E., Peter H. H., Hilger C. Antibody-dependent cellular cytotoxicity and B-and T-cell activity in the peripheral blood of myasthenia gravis patients. Ann N Y Acad Sci. 1976;274:421–433. doi: 10.1111/j.1749-6632.1976.tb47703.x. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. M., Lennon V. A., Seybold M. E., Whittingham S. Experimental autoimmune myasthenia gravis and myasthenia gravis: biochemical and immunochemical aspects. Ann N Y Acad Sci. 1976;274:254–274. doi: 10.1111/j.1749-6632.1976.tb47691.x. [DOI] [PubMed] [Google Scholar]
- Lisak R. P., Abdou N. I., Zweiman B., Zmijewski C., Penn A. S. Aspects of lymphocyte function in myasthenia gravis. Ann N Y Acad Sci. 1976;274:402–410. doi: 10.1111/j.1749-6632.1976.tb47701.x. [DOI] [PubMed] [Google Scholar]
- Matre R., Tönder O. Complement receptors in human renal glomeruli. Scand J Immunol. 1976;5(4):437–441. doi: 10.1111/j.1365-3083.1976.tb00298.x. [DOI] [PubMed] [Google Scholar]
- Pudifin D. J., Cox J. Lymphocyte subpopulations after thymectomy. Lancet. 1976 Jun 12;1(7972):1299–1299. doi: 10.1016/s0140-6736(76)91770-0. [DOI] [PubMed] [Google Scholar]
- Richman D. P., Patrick J., Arnason B. G. Cellular immunity in myasthenia gravis. Response to purified acetylcholine receptor and autologous thymocytes. N Engl J Med. 1976 Mar 25;294(13):694–698. doi: 10.1056/NEJM197603252941304. [DOI] [PubMed] [Google Scholar]
- Sandilands G. P., Gray K., Cooney A., Anderson J. R., Simpson J. A., Behan P. O. Letter: Rosette tests following thymectomy. Lancet. 1975 Jan 18;1(7899):171–172. doi: 10.1016/s0140-6736(75)91479-8. [DOI] [PubMed] [Google Scholar]
- Shirai T., Miyata M., Nakase A., Itoh T. Lymphocyte subpopulation in neoplastic and non-neoplastic thymus and in blood of patients with Myasthenia gravis. Clin Exp Immunol. 1976 Oct;26(1):118–123. [PMC free article] [PubMed] [Google Scholar]
- Staber F. G., Fink U., Sack W. Letter: B lymphocytes in the thymus of patients with myasthenia gravis. N Engl J Med. 1975 May 8;292(19):1032–1033. [PubMed] [Google Scholar]
- Tonder O., Morse P. A., Jr, Humphrey L. J. Similarities of Fc receptors in human malignant tissue and normal lymphoid tissue. J Immunol. 1974 Oct;113(4):1162–1169. [PubMed] [Google Scholar]



