Abstract
STUDY QUESTION
Does a single intrauterine infusion of human chorionic gonadotropin (hCG) at the time corresponding to a Day 3 embryo transfer in oocyte donors induce favorable molecular changes in the endometrium for embryo implantation?
SUMMARY ANSWER
Intrauterine hCG was associated with endometrial synchronization between endometrial glands and stroma following ovarian stimulation and the induction of early decidual markers associated with stromal cell survival.
WHAT IS KNOWN ALREADY
The clinical potential for increasing IVF success rates using an intrauterine hCG infusion prior to embryo transfer remains unclear based on previously reported positive and non-significant findings. However, infusion of CG in the non-human primate increases the expression of pro-survival early decidual markers important for endometrial receptivity, including α-smooth muscle actin (α-SMA) and NOTCH1.
STUDY DESIGN, SIZE, DURATION
Oocyte donors (n=15) were randomly assigned to receive an intrauterine infusion of 500 IU hCG (n=7) or embryo culture media vehicle (n=8) 3 days following oocyte retrieval during their donor stimulation cycle. Endometrial biopsies were performed 2 days later, followed by either RNA isolation or tissue fixation in formalin and paraffin embedding.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Reverse transcription of total RNA from endometrial biopsies generated cDNA, which was used for analysis in the endometrial receptivity array (ERA; n = 5/group) or quantitative RT–PCR to determine relative expression of ESR1, PGR, C3 and NOTCH1. Tissue sections were stained with hematoxylin and eosin followed by blinded staging analysis for dating of endometrial glands and stroma. Immunostaining for ESR1, PGR, α-SMA, C3 and NOTCH1 was performed to determine their tissue localization.
MAIN RESULTS AND THE ROLE OF CHANCE
Intrauterine hCG infusion was associated with endometrial synchrony and reprograming of stromal development following ovarian stimulation. ESR1 and PGR were significantly elevated in the endometrium of hCG-treated patients, consistent with earlier staging. The ERA did not predict an overall positive impact of intrauterine hCG on endometrial receptivity. However, ACTA2, encoding α-SMA was significantly increased in response to intrauterine hCG. Similar to the hCG-treated non-human primate, sub-epithelial and peri-vascular α-SMA expression was induced in women following hCG infusion. Other known targets of hCG in the baboon were also found to be increased, including C3 and NOTCH1, which have known roles in endometrial receptivity.
LIMITATIONS, REASONS FOR CAUTION
This study differs from our previous work in the hCG-treated non-human primate along with clinical studies in infertile patients. Specifically, we performed a single intrauterine infusion in oocyte donors instead of either continuous hCG via an osmotic mini-pump in the baboon or infusion followed by blastocyst-derived hCG in infertile women undergoing embryo transfer. Therefore, the full impact of intrauterine hCG in promoting endometrial receptivity may not have been evident.
WIDER IMPLICATIONS OF THE FINDINGS
Our findings suggest a potential clinical benefit for intrauterine hCG prior to embryo transfer on Day 3 in counteracting endometrial dyssynchrony from ovarian stimulation and promoting expression of markers important for stromal survival. Finally, there were no obvious negative effects of intrauterine hCG treatment.
STUDY FUNDING/COMPETING INTEREST(S)
Funding for this work was provided by NICHD R01 HD042280 (A.T.F.) and NICHD F30 HD082951 (M.R.S.). C.S. and P.D.-G are co-inventors of the patented ERA, which is owned by IGENOMIX SL and was used in this study, and C.S. is a shareholder in IGENOMIX SL. M.R.-A. is employed by IGENOMIX SL. No other authors have any conflicts of interest to report.
TRIAL REGISTRATION NUMBER
This study was registered with ClinicalTrials.gov (NCT01786252).
TRIAL REGISTRATION DATE
5 February 2013.
DATE OF FIRST PATIENT'S ENROLLMENT
10 May 2013.
Keywords: hCG, endometrium, oocyte donors, controlled ovarian stimulation, embryo implantation, decidualization, endometrial receptivity, Notch, complement
Introduction
Embryo implantation defects contribute to 70% of all pregnancy losses and are responsible for the low efficacy of embryo transfer during in vitro fertilization (IVF) protocols, necessitating transfer of multiple embryos (Macklon et al., 2002; Koot et al., 2012). Impaired endometrial receptivity remains one of the most significant barriers to the establishment of pregnancy, and its causes are often multifactorial in nature. Failed endometrial receptivity results from disease processes, including endometriosis (Klemmt et al., 2006; Lessey et al., 2013), and may be induced iatrogenically as a result of ovarian stimulation such as in assisted reproductive technology protocols (Paulson et al., 1990).
The developing primate embryo secretes chorionic gonadotropin (CG) at the time of implantation. Human chorionic gonadotropin (hCG) has been shown to enhance endometrial receptivity in both women (Licht et al., 1998) and non-human primates (Fazleabas et al., 1999; Sherwin et al., 2007; Banerjee and Fazleabas, 2010). Intrauterine hCG administration induces endometrial stromal expression of α-smooth muscle actin (α-SMA) and NOTCH1 in the baboon, both of which are essential for cell survival and differentiation during decidualization (Fazleabas et al., 1999; Kim et al., 1999; Jasinska et al., 2006; Afshar et al., 2012a,b). Mansour et al. demonstrated that an intrauterine 500 IU hCG administration prior to embryo transfer on Day 3 post-oocyte retrieval significantly increased both implantation and clinical pregnancy rates (Mansour et al., 2011). Several subsequent studies on the response to intrauterine hCG administration have been reported, with positive (Santibanez et al., 2014) and non-significant (Hong et al., 2014; Wirleitner et al., 2015) clinical findings, leaving the clinical potential and utility of intrauterine hCG administration for improving pregnancy rates with IVF unresolved.
The mechanisms by which exogenous administration of intrauterine hCG might improve implantation rates are unknown. Additionally, the task of identifying mechanisms of intrauterine hCG in promoting a receptive endometrium in infertile women undergoing IVF remains difficult. We have previously demonstrated the importance of hCG treatment on human endometrial histology and prevention of apoptosis (Lovely et al., 2005). In the current study, we performed a single intrauterine infusion of hCG in oocyte donors on Day 3 following oocyte retrieval to determine the potential role of intrauterine hCG in endometrial function following ovarian stimulation. The dose of 500 IU hCG and the infusion time point were based on the study published by Mansour et al. (2011), which showed a positive effect on implantation rates following embryo transfer. Administration of intrauterine luteal phase hCG to oocyte donors in this study was used to further define hCG-regulated molecular mechanisms during the putative implantation window. Additionally, we evaluated the expression of selected genes with known roles in endometrial receptivity that we had previously reported to be regulated by hCG in the baboon model (Fazleabas et al., 1999; Sherwin et al., 2007; Banerjee and Fazleabas, 2010). We show from these studies that there is potential clinical benefit for intrauterine hCG prior to embryo transfer in an IVF setting.
Materials and Methods
Patient population and ovarian stimulation cycle
A total of 15 oocyte donors were recruited to participate in this study by clinicians at The Fertility Center (Grand Rapids, MI). The study was approved by the Institutional Review Board of Michigan State University. After informed consent was obtained, patients were blindly randomized to either the vehicle (n = 8) or hCG (n = 7) treatment groups, using simple randomization by the oocyte donor coordinator at The Fertility Center. All oocyte donors underwent a GnRH agonist (GnRa) long protocol with leuprolide acetate for luteinizing hormone suppression plus injectable gonadotropins for ovarian stimulation. There was no significant difference in the length of stimulation or total gonadotropin dosage between the two groups. Final oocyte maturity was induced with i.m. hCG (5000 or 10 000 IU; P = 0.6084 between treatment groups, Table I) followed by ultrasound-guided transvaginal oocyte retrieval 35.5 h later. Ethical considerations preclude the collection of endometrial biopsies from women undergoing embryo transfer following ovarian stimulation due to the potential harm to the establishment of pregnancy; therefore, oocyte donors were used in this study to determine hCG-regulated mechanisms during the implantation window. For identifying the molecular mechanisms contributing to a potential clinical benefit of hCG infusion prior to embryo transfer, oocyte donors represent a homogeneous population of healthy, fertile women who have undergone extensive screening prior to their acceptance into the oocyte donor program at The Fertility Center. Additionally, these women are subjected to similar clinical procedures (including ovarian stimulation and transvaginal ultrasound-guided oocyte retrieval) leading up to the time point of intrauterine hCG infusion. Sample sizes were selected based on our previous work in the hCG-treated non-human primate and in primary endometrial stromal cells isolated from women (Kim et al., 1998; Fazleabas et al., 1999). Additionally, power analysis was performed based on α-SMA response between treatment groups with α set at 0.05 and power was determined to be 0.93 using a standard power analysis calculator (DSS Research). Inclusion criteria were based on ASRM Practice Guidelines for oocyte donation (Practice Committee of American Society for Reproductive and Practice Committee of Society for Assisted Reproductive, 2013). Donors with irregular menses, using an intrauterine device, or previously diagnosed polycystic ovary syndrome were excluded from this study. Further, patients displaying signs of ovarian hyperstimulation syndrome or early menses after ovarian stimulation were removed from the study. The flow of women through the study is shown in Supplementary Figure S1.
Table I.
Demographics and clinical data (represented as mean ± standard error) for patients participating in this study.
| Vehicle (n = 8) | hCG (n = 7) | |
|---|---|---|
| Age | 26.3 ± 1.35 | 25.0 ± 0.976 |
| BMI | 23.3 ± 0.764 | 25.3 ± 1.44 |
| Gravidity | 1.63 ± 0.461 | 1.43 ± 0.429 |
| Parity | 1.25 ± 0.313 | 1.14 ± 0.404 |
| Max E2 level prior to oocyte retrieval | 4428 ± 504 | 4097 ± 421 |
| Average ovulatory hCG provided | 7500 ± 945 | 6429 ± 922 |
| Cycle length (days) | 13.3 ± 1.49 | 12.3 ± 0.756 |
| Oocytes retrieved | 20.5 ± 2.87 | 19.1 ± 3.76 |
No significant differences between treatment groups were observed in any of the measured parameters.
Experimental design
Three days following oocyte retrieval (corresponding to a Day 3 embryo transfer), oocyte donors were placed in the lithotomy position. A speculum was utilized to visualize the cervix. A catheter was passed through the cervical os, allowing for intrauterine injection of 50 µl IVF-grade culture media (Global media, LifeGlobal Group, Guelph, ON, Canada; LGGG) with or without 500 IU hCG (Novarel, Ferring Pharmaceuticals Inc, Parsippany, NJ, USA). Clinically, intrauterine hCG infusion in IVF patients would occur prior to embryo transfer, therefore, the use of IVF culture media as a diluent is ideal. Patients remained in lithotomy position for several minutes following infusion to prevent leakage. Two days following intrauterine injection of vehicle or hCG (corresponding to the peri-implantation period), an endometrial biopsy was performed using a sterile pipelle and the tissue was stored in Hank's Balanced Salt Solution (Life Technologies, Grand Island, NY) on ice for further processing.
Tissue processing
Endometrial tissue was rinsed in chilled PBS followed by division into three groups: (i) 10% neutral buffered formalin-fixed and paraffin embedded (FFPE), (ii) snap frozen in liquid nitrogen and stored at −80°C and (iii) incubated 24 h in RNAlater (Life Technologies) at 4°C followed by RNA isolation for the endometrial receptivity array (ERA) as previously described (Diaz-Gimeno et al., 2011).
Endometrial staging and immunostaining
FFPE tissues were sectioned at 6 µm thickness for both hematoxylin and eosin (H&E) staining and immunohistochemistry. For H&E staining, sections underwent dewaxing, rehydration in graded alcohol series, H&E staining, dehydration and mounting. All H&E-stained endometrial biopsies were analyzed in a blinded manner by B.A.L. for endometrial dating and glandular and stromal development. Criteria for endometrial dating included the presence or absence of sub-nuclear vacuoles, which is one of the more reproducible features of the Noyes dating criteria (Noyes et al., 1950). For the purposes of statistical analysis, the most advanced elements in each of the two endometrial compartments were considered. For immunostaining, formalin-fixed paraffin embedded were sectioned at 6 µm and placed on microscope slides (Thermo Fisher Scientific, Waltham, MA, USA; 12-550-15). Each tissue section was dewaxed, rehydrated in graded alcohol series, followed by heat-mediated antigen retrieval in citrate buffer (Antigen unmasking solution, H-3300; Vector Laboratories, Burlingame, CA). Slides were blocked for 1 h in 10% normal horse serum (Vector Laboratories; S-2000) in PBS then incubated overnight at 4°C in one of the following primary antibodies: mouse anti-ERα (750 ng/ml; Vector Laboratories; VP-E613), rabbit anti-PR (12 µg/ml; DAKO, Carpinteria, CA; A0098), rabbit anti-ERK1/2 (80 ng/ml; Cell Signaling, Danvers, MA; cs4695), rabbit anti-phospho-ERK1/2 (50 ng/ml; Cell Signaling; cs-4376), rabbit anti-α-smooth muscle actin (233 ng/ml; DAKO; M0851), rabbit anti-NOTCH1 (1 µg/ml; Santa Cruz Biotechnology, Santa Cruz, CA; sc-6014-R) or goat anti-C3 (400 ng/ml; Santa Cruz Biotechnology; sc-14612). Subsequently, sections were incubated in respective biotinylated secondary antibodies for anti-mouse, anti-rabbit or anti-goat (Vector Laboratories; BA-9200, BA-1000, BA-9500) followed by HRP-conjugated streptavidin. Detection for immunoreactivity was achieved using the DAB Substrate Kit (Vector Laboratories; SK-4100) producing brown staining. Staining intensity of each section was quantified by image analysis software ImageJ (NIH) resulting in a Digital HSCORE (D-HSCORE), ranging from 0 to 255, of staining intensity as previously reported (Fuhrich et al., 2013).
RNA isolation and real-time qPCR
Snap frozen samples were homogenized in TRIzol reagent (Life Technologies), followed by extraction of total RNA. Total RNA (1 µg) was reverse transcribed to cDNA using High Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Foster City, CA). Gene expression levels were measured using the ViiA 7 qPCR System (Applied Biosystems) with SYBR Green PCR Master Mix (Applied Biosystems). Ribosomal Protein L17 (RPL17) was used for normalization, and the primer sequences used in this study are provided in Supplementary Table SI.
Endometrial receptivity array
Total RNA was extracted as described above (five patients/group). RNA quality was determined using RNA Labchip and analyzed using an A2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). An RNA integrity number ≥7 was set for establishing good-quality RNA. Sample preparation and hybridization were performed as previously described (Ruiz-Alonso et al., 2013). Briefly, first-strand cDNA was transcribed from 200 ng total RNA using T7-oligo(dT) promoter primers. Subsequent sample in vitro transcription and Cy-3 labeling were performed with the Low Input Quick Amp Labeling Kit (Agilent Technologies), yielding complementary RNA (cRNA). Fragmented cRNA was hybridized to the custom ERA array (Diaz-Gimeno et al., 2011) for 17 h at 65°C followed by washing, scanning using Axon 4100A scanner (Molecular Devices, Sunnyvale, CA) and data extraction using Genepix Pro 6.0 software (Molecular Devices), producing GPR files for further gene expression analysis. GPR files were analyzed by the ERA computational predictor, obtaining an endometrial diagnosis as ‘receptive’ or ‘non-receptive’ with a sensitivity of 0.997 and specificity of 0.885 (Diaz-Gimeno et al., 2011).
Transcriptomic analysis
Hierarchical clustering and principal component analysis (PCA) were performed with ERA gene expression data to analyze the systematic patterns of variations in the data and the hCG treatment effect in gene expression. Prcomp and hclust functions in Stats R Library were used for PCA and hierarchical analysis, respectively, to visualize sample dissimilarities. Differential gene expression analysis between hCG-treated samples versus non-treated samples was performed using limma R function from Bioconductor Release 3.1 (Ritchie et al., 2015).
Statistical analysis
Statistical analysis of endometrial glandular and stromal staging between vehicle and hCG-treated patients was performed using two-way ANOVA with Sidak's multiple comparisons test. For detection of significant differences between treatment groups, a non-parametric Mann–Whitney U-test was used. Significance was defined as P < 0.05. All statistical analysis was performed using GraphPad Prism 6.0 (GraphPad Software).
Results
Intrauterine hCG infusion is associated with endometrial synchronization due to delayed stromal advancement
Patient demographics are provided in Table I, and there were no significant differences between the two groups in terms of age, parity, cycle length, maximum estradiol level prior to oocyte retrieval or number of oocytes retrieved. Of 15 oocyte donors, 10 had been pregnant prior to their participation in this study. The five nulligravid patients were presumably normal without signs or symptoms of gynecological disorders and were evenly distributed between the two treatment groups through randomization (two in vehicle and three in hCG group). All endometrial biopsies collected from both vehicle and hCG-treated oocyte donors on luteal Day 7 demonstrated characteristic secretory phase histological findings (Table II). Consistent with previous reports, endometrial staging in oocyte donors undergoing ovarian stimulation revealed significant endometrial dyssynchrony between glandular and stromal development with glandular lag observed (Table II). Specifically, the glandular and stromal compartments in the vehicle-treated patients were staged at luteal Day 4-5 and 6-7 (P = 0.043), respectively. In comparison, endometrial synchrony, with no significant differences between glandular and stromal staging (P = 0.979), was present in the hCG-administered group. Interestingly, hCG administration resulted in a significant delay in stromal advancement with a mean stromal staging of luteal Day 3-4 compared with 6-7 in vehicle-treated patients (P = 0.004).
Table II.
Blinded histological staging of endometrial biopsies from oocyte donors on Day 7 post-ovulation induction after intrauterine infusion of hCG or vehicle on Day 3.
| Vehicle (n = 8) |
hCG (n = 7) |
||||
|---|---|---|---|---|---|
| Patient ID | Gland | Stroma | Patient ID | Gland | Stroma |
| H1422 | 2-3 | 7 | H1417 | 2-3 | 6 |
| H1423 | 2-3 | 7 | H1381 | 2-3 | 2-3 |
| H1353 | 2-4 | 2-4 | H1382 | 2-3 | 2-3 |
| H1405 | 3-4 | 3-4 | H1386 | 2-3 | 2-3 |
| H1339 | 3-4 | 7-8 | H1390 | 3-4 | 3-4 |
| H1340 | 4-5 | 7-8 | H1378 | 4-5 | 4-5 |
| H1352 | 5-7 | 5-7 | H1393 | 7 | 2-3 |
| H1348 | 6-7 | 6-7 | |||
| Mean | 4-5a | 6-7b | 4a | 3-4a | |
Vehicle-treated endometrium displayed significantly different glandular and stromal staging (P = 0.043), which was not present in the hCG-treated patients (P = 0.980). Additionally, stromal staging was significantly decreased (P = 0.004) in the hCG-treated patients. Numbers shown represent luteal day or range of days as histologically staged based on tissue morphology in a blinded manner. Statistically significant differences of each column are indicated by different lettered superscripts.
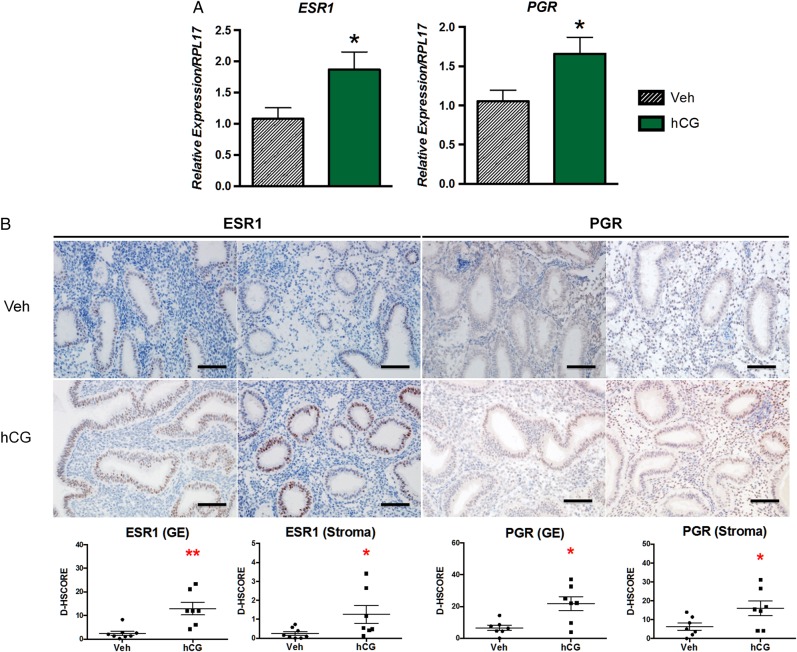
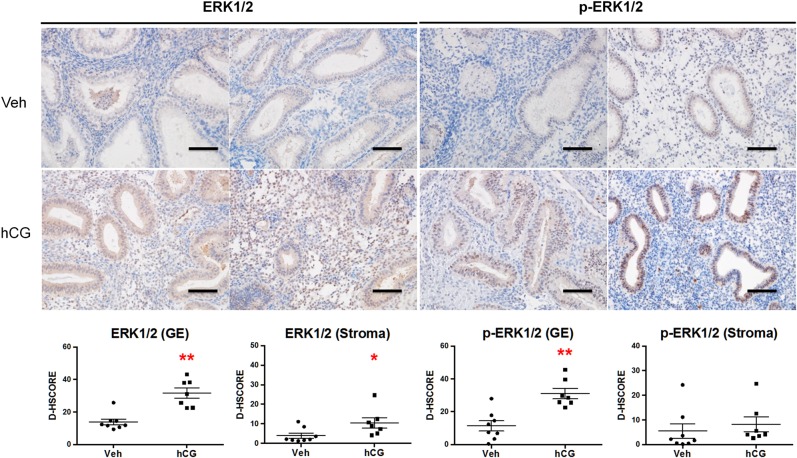
Endometrial dating has been correlated with predicted expression profiles of steroid receptors ESR1 and PGR (Garcia et al., 1988). Based on quantitative RT–PCR analysis, we found significantly elevated ESR1 and PGR mRNA levels in hCG-treated patients (Fig. 1A). Immunostaining corroborated this finding with significantly increased ESR1 and PGR protein levels in both endometrial compartments of the hCG-treated patients (Fig. 1B). The magnitude of ESR1 expression based on the D-HSCORE in the glandular epithelium of hCG-treated patients was approximately 10-fold greater than in the stroma. We chose to validate that an endometrial response to intrauterine hCG infusion was achieved in our patients by immunostaining for ERK1/2 and phospho-ERK1/2 (Fig. 2). We have previously shown that hCG induces phosphorylation of ERK1/2 in endometrial epithelial cells isolated from both women (Banerjee et al., 2009) and the non-human (Srisuparp et al., 2003). As expected, glandular epithelial ERK1/2 phosphorylation was significantly increased in response to intrauterine hCG, and hCG treatment was associated with elevated epithelial and stromal ERK1/2 expression.
Figure 1.
Expression of steroid hormone receptors, ESR1 and PGR, detected by quantitative RT–PCR and immunohistochemistry after intrauterine infusion of hCG or vehicle. (A) Both ESR1 and PGR mRNA expression were significantly increased in hCG-treated patients. (B) Consistent with mRNA expression, both endometrial glandular epithelial and stromal compartments from hCG-treated patients expressed significantly elevated protein levels of both ESR1 and PGR. Staining intensity for glandular epithelial (GE) and stromal compartments was quantified by image analysis software ImageJ (D-HSCORE). *P < 0.05, **P < 0.01; Scale bar = 100 µm.
Figure 2.
Validation of endometrial hCG effect by the induction of epithelial ERK1/2 phosphorylation. Immunostaining for ERK1/2 and phospho-ERK1/2 was performed in oocyte donors after intrauterine infusion of vehicle or hCG. Intrauterine hCG resulted in significantly elevated levels of ERK1/2 in both endometrial glandular epithelium (GE) and stroma. Phosphorylation of ERK1/2 was significantly increased in GE in response to hCG but not in the stroma. Staining intensity for GE and stromal compartments was quantified by image analysis software ImageJ (D-HSCORE) for statistical analysis. *P < 0.05, **P < 0.01; Scale bar = 100 µm.
Intrauterine hCG does not significantly impact overall endometrial receptivity but induces pre-decidual marker expression of α-SMA
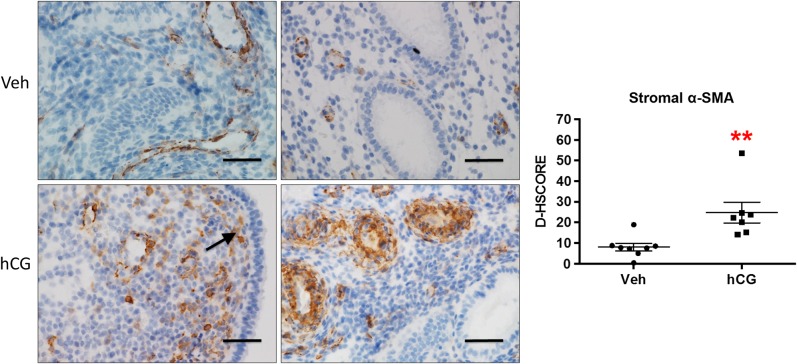
The ERA did not detect an effect of intrauterine hCG administration on the 238 genes selected compared with vehicle treatment (Supplementary Table SII). ACTA2, the gene encoding α-SMA, was significantly increased in the hCG-treated patients (fold change: 1.98) prior to adjustment for false discovery rate but was not statistically significant after this adjustment. We have previously shown that hCG specifically induces stromal α-SMA in the baboon endometrium and in isolated primary cells (Kim et al., 1998; Fazleabas et al., 1999). We hypothesized that tissue composition differences from endometrial sampling and in total RNA isolated for the ERA contributed to the lack of significance following false discovery adjustment. Therefore, we performed immunohistochemistry to determine α-SMA protein localization and found a similar staining pattern to what we had previously reported in the baboon (Fazleabas et al., 1999). α-SMA was induced in the endometrial sub-epithelial stromal cells in response to hCG treatment and was essentially absent in the vehicle-treated group (Fig. 3). Additionally, peri-vascular stromal α-SMA expression was enhanced in the hCG-treated group and was associated with the physiological decidual response, which occurs normally during the luteal phase.
Figure 3.
Immunolocalization of endometrial α-SMA in response to intrauterine hCG infusion in oocyte donors. Intrauterine hCG resulted in significantly increased stromal expression of α-SMA compared with vehicle controls. Similar to the non-human primate, α-SMA expression localized primarily to the sub-luminal epithelial (arrow) and peri-vascular stromal cells. Staining intensity was quantified by image analysis software ImageJ (D-HSCORE) for statistical analysis. **P < 0.01; Scale bar = 50 µm.
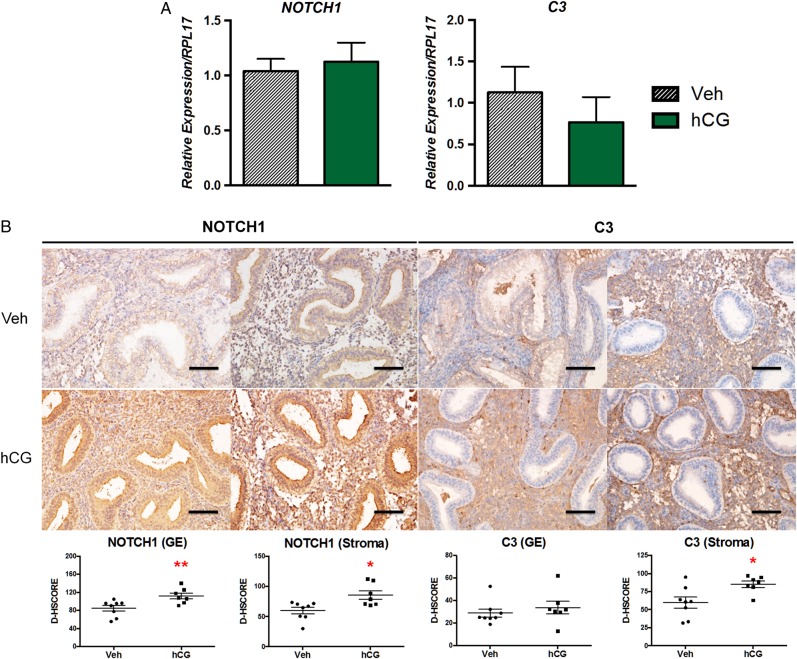
Targets of intrauterine hCG in the non-human primate, NOTCH1 and C3, are also increased in women
Our previous work in the hCG-treated baboon provided a wealth of information regarding regulated processes critical during the implantation period (Fazleabas et al., 1999; Sherwin et al., 2007). NOTCH1 is induced in the baboon and human uterine fibroblast (HuF) cells in response to hCG (Sherwin et al., 2007; Afshar et al., 2012a,b). The Notch signaling pathway regulates many important developmental processes and, specifically, NOTCH1 is a key regulator in coordinating decidualization (Afshar et al., 2012a,b; Su et al., 2015). In the current study, intrauterine hCG infusion resulted in a significant increase in both glandular and stromal NOTCH1 protein levels (P = 0.006 and 0.02, respectively); however, NOTCH1 mRNA expression was unchanged between treatment groups (Fig. 4), which is consistent with previous data (Afshar et al., 2012a,b). C3 is an important mediator of the innate immune response and is expressed in the secretory endometrium (Sayegh et al., 1996). Additionally, C3 is further induced in the endometrial stroma in response to hCG in the baboon and in women receiving a single IM injection of hCG on luteal Day 8 (Sherwin et al., 2007; Palomino et al., 2013). Intrauterine hCG infusion in women resulted in significantly higher stromal C3 protein levels (Fig. 4, P = 0.021). Glandular C3 was minimal in both groups, consistent with our findings in the baboon. C3 mRNA was not significantly changed between groups (consistent with the ERA findings), which may have resulted from differences in quantities of glandular epithelial versus stromal cells present in whole tissue mRNA extracts.
Figure 4.
Expression of NOTCH1 and C3 in the endometrium of oocyte donors following intrauterine hCG infusion. (A) Intrauterine hCG did not significantly impact mRNA expression levels of NOTCH1 or C3 by quantitative RT–PCR. (B) Both endometrial glandular epithelial (GE) and stromal compartments from hCG-treated patients expressed significantly elevated levels of NOTCH1 protein. Consistent with our findings in the non-human primate, intrauterine infusion of hCG significantly increased endometrial stromal C3 protein expression while there was no significant impact on C3 expression in the GE. Staining intensity for GE and stromal compartments was quantified by image analysis software ImageJ (D-HSCORE) for statistical analysis. *P < 0.05, **P < 0.01; Scale bar = 100 µm.
Discussion
The main objective of our study was to determine the potential mechanisms responsible for the improved clinical outcomes reported in response to intrauterine hCG infusion prior to embryo transfer on Day 3 post-retrieval (Mansour et al., 2011; Santibanez et al., 2014). Oocyte donors represented a homogeneous population of young, fertile women to study the effects of intrauterine hCG following ovarian stimulation and gauge its potential impact on endometrial receptivity. Endometrial staging revealed that endometrial dyssynchrony between glands and stroma was reduced with hCG treatment due to delayed stromal development. Endometrial advancement and luteal phase shortening due to ovarian stimulation have been previously reported extensively in the literature (Garcia et al., 1984; Benadiva and Metzger, 1994; Meyer et al., 1999; Basir et al., 2001; Devroey et al., 2004), and endometrial advancement of more than 3 days on the day of oocyte retrieval has been associated with no clinical pregnancies in stimulated cycles (Ubaldi et al., 1997). Further, we do not expect that the use of a GnRH agonist in our study, versus a GnRH antagonist, contributed to the effect of hCG in reducing endometrial dyssynchrony, as endometrial dyssynchrony and the degree of endometrial advancement is not different between either protocol (Saadat et al., 2004).
Our previously reported studies in the hCG-treated non-human primate in women (Fazleabas et al., 1999; Afshar et al., 2012a,b) are now validated in women. Endometrial stromal α-SMA induction occurs during the early stages of pregnancy, in response to hCG, and at the initiation of decidualization (Christensen et al., 1995; Fazleabas et al., 1999; Kim et al., 1999). Its expression is critical for preventing stromal apoptosis (Lovely et al., 2005; Jasinska et al., 2006) and regulates differentiation during decidualization, including the secretion of the decidual marker protein, insulin-like growth factor binding protein-1 (Kim et al., 1999; Jasinska et al., 2006). In the current study, intrauterine hCG infusion induced α-SMA expression, particularly in sub-luminal epithelial and peri-vascular stromal cells, similar to the baboon (Fazleabas et al., 1999). Previously, we described a role for hCG in preventing stromal apoptosis during luteal phase support in women (Lovely et al., 2005), which also occurred in the setting of actin destabilization (Jasinska et al., 2006). We identified NOTCH1 as a target of hCG in the baboon (Afshar et al., 2012a,b), which has been shown to act as an important arbiter of cell survival and fate (Miele and Osborne, 1999). Similar to our studies in the baboon (Afshar et al., 2007, 2012a,b), intrauterine hCG administration significantly induced NOTCH1 protein expression in both the endometrial stroma and glandular epithelium. NOTCH1 mRNA levels were not significantly changed between vehicle and hCG-treated patients, which was consistent with our previous findings (Afshar et al., 2012a,b).
Complement C3 represents another hCG target in the non-human primate (Sherwin et al., 2007) as well as in women (Palomino et al., 2013). Although it is a well-characterized mediator of the innate immune response (Ricklin et al., 2010), the role of C3 during the implantation window remains ill defined, but C3 deficiency is consistent with increased embryo loss during pregnancy in the mouse (Chow et al., 2009). C3 has been described as an estrogen receptor target in rodent endometrial epithelial cells (Sundstrom et al., 1989). C3 is induced in the endometrial stroma of the hCG-treated baboon as well as women (Sherwin et al., 2007; Palomino et al., 2013), which is consistent with the current study. Because many of the events surrounding embryo implantation are pro-inflammatory in nature (Granot et al., 2012), it is not surprising that the embryo would favor the secretion of such mediators, and it is likely that its stimulation may serve to prime the immune response for pregnancy.
PGR acts downstream of, or in synergy with, hCG to coordinate expression of many of the stromal mediators affected in our study (Fig. 5). Antagonism of PGR in the baboon-simulated pregnancy model resulted in a drastic reduction in the expression of both α-SMA (Banaszak et al., 2000) and NOTCH1 (Afshar et al., 2012a,b) with hCG treatment. Additionally, progesterone appears to favor γ-secretase-mediated cleavage of the full-length NOTCH1 receptor to its transcriptionally active form in the presence of hCG (Afshar et al., 2012a,b). The effect of PGR antagonism was further assessed in endometrial explants from women during the mid-luteal phase treated with hCG, where a PGR antagonist impaired stromal C3 induction (Palomino et al., 2013).
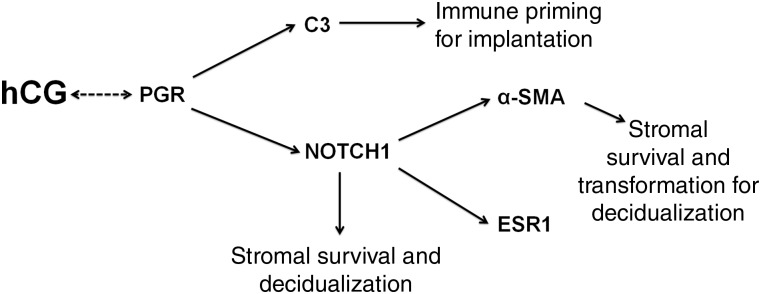
Figure 5.
Working hypothesis of mechanisms by which intrauterine hCG regulates key signaling mechanisms in the endometrial stroma. PGR coordinates hCG-induced responses and may in itself be regulated by hCG based on the current study. C3 acts downstream of PGR to modulate the endometrial immune response for implantation. NOTCH1 can directly act to promote stromal survival for decidualization or regulate α-SMA, which is necessary for both stromal survival and transformation to the secretory decidual phenotype.
Our study failed to show a difference in the overall endometrial receptivity profile characterized by the ERA, which may account for some of the differences in clinical outcomes between the studies conducted in IVF patients receiving intrauterine hCG infusions. Additionally, some of the genes targeted in this array were significantly altered in our CG-treated baboon model but were unchanged in this current study, such as LIF and SOD2 (Sherwin et al., 2007). The inability of our study to validate some of the previously reported hCG-regulated genes and demonstrate a role for hCG in the ERA may be due to the absence of continuous hCG administration in the current study, which may have reduced our ability to detect a more significant impact of intrauterine hCG infusion. Specifically, in the current study, a single intrauterine infusion of 500 IU hCG was followed by an endometrial biopsy 2 days later as opposed to the continuous infusion over the course of 4 days with immediate tissue collection as performed in our non-human primate model (Fazleabas et al., 1999). Additionally, endogenous production of hCG by the morula may have complemented the effect of intrauterine hCG infusion, contributing to the significantly improved IVF outcomes with a Day 3 infusion before embryo transfer (Bonduelle et al., 1988; Mansour et al., 2011).
Our current findings, combined with previous reports, indicate that a more substantial beneficial effect of a single intrauterine infusion of hCG at Day 3 may occur in the presence of an embryo. Continuous embryonic derived hCG may act to sustain the initial effect of a loading dose of exogenous hCG which may have a positive impact on IVF outcomes. It stands to reason that insufficient time for hCG action prior to embryo implantation along with stromal advancement precluded a positive impact of hCG infusion prior to Day 6 blastocyst transfer (Hong et al., 2014). Additionally, the absence of continuous hCG production from the embryo on Day 3 prior to embryo transfer on Day 5 may be the reason for the lack of improved pregnancy outcomes in that study (Wirleitner et al., 2015).
Finally, the positive effect of hCG infusion on Day 3 may be beneficial in fresh cycles to counteract luteal phase defect associated with ovarian stimulation, particularly since endometrial advancement is present to a greater extent earlier in the luteal phase (Garcia et al., 1984). The desired effect of hCG in reducing uterine dyssynchrony may be unnecessary in natural cycles without ovarian stimulation because luteal defects are not incurred (Benadiva and Metzger, 1994). On the basis of the current study, a single intrauterine infusion of hCG in women may play a role in delaying stromal advancement following ovarian stimulation and promoting stromal cell survival for decidualization. However, an epithelial effect of hCG administration was achieved based on ERK1/2 phosphorylation, and given the role for endometrial epithelial–stromal communication during implantation (Hantak et al., 2014), we cannot rule out an additional possible role for hCG-driven epithelial proliferation in preventing stromal advancement to maintain endometrial synchrony. A growing body of evidence describes the importance of decidualization for successful implantation (Gellersen and Brosens, 2014), and, therefore, there is potential for a positive clinical impact of intrauterine hCG infusion. Future studies to determine the impact of intrauterine hCG prior to embryo transfer in an infertile population are warranted.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
M.R.S., R.W.S. and A.T.F. were responsible for experimental design, molecular analysis, interpretation of data and manuscript preparation. J.E.Y., W.G.D., V.I.S. and R.E.L. conducted clinical oversight and sample collection. B.A.L., C.S., P.D.-G. and M.R.-A. were responsible for data acquisition and analysis. All authors actively participated in manuscript revision for final submission.
Funding
Funding for this work was kindly provided by NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Research Project Grant R01 HD042280 (to A.T.F.) and NICHD Ruth L. Kirschstein National Research Service Award F30 HD082951 (to M.R.S.).
Conflict of interest
C.S. and P.D.-G are co-inventors of the patented ERA, which is owned by IGENOMIX SL and was used in this study, and C.S. is a shareholder in IGENOMIX SL. M.R.-A. is employed by IGENOMIX SL. No other authors have any conflicts of interest to report.
Supplementary Material
Acknowledgements
We thank Christine Dixon, R.N., Susan Ferguson, M.S. and Robin Strouse, R.N. for their assistance in study coordination and sample collection and Samantha Bond for assistance with the molecular analysis.
References
- Afshar Y, Stanculescu A, Miele L, Fazleabas AT. The role of chorionic gonadotropin and Notch1 in implantation. J Assist Reprod Genet 2007;24:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshar Y, Jeong JW, Roqueiro D, DeMayo F, Lydon J, Radtke F, Radnor R, Miele L, Fazleabas A. Notch1 mediates uterine stromal differentiation and is critical for complete decidualization in the mouse. FASEB J 2012a;26:282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshar Y, Miele L, Fazleabas AT. Notch1 is regulated by chorionic gonadotropin and progesterone in endometrial stromal cells and modulates decidualization in primates. Endocrinology 2012b;153:2884–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaszak S, Brudney A, Donnelly K, Chai D, Chwalisz K, Fazleabas AT. Modulation of the action of chorionic gonadotropin in the baboon (Papio anubis) uterus by a progesterone receptor antagonist (ZK 137. 316). Biol Reprod 2000;63:820–825. [DOI] [PubMed] [Google Scholar]
- Banerjee P, Fazleabas AT. Endometrial responses to embryonic signals in the primate. Int J Dev Biol 2010;54:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee P, Sapru K, Strakova Z, Fazleabas AT. Chorionic gonadotropin regulates prostaglandin E synthase via a phosphatidylinositol 3-kinase-extracellular regulatory kinase pathway in a human endometrial epithelial cell line: implications for endometrial responses for embryo implantation. Endocrinology 2009;150:4326–4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basir GS, O WS, Ng EH, Ho PC. Morphometric analysis of peri-implantation endometrium in patients having excessively high oestradiol concentrations after ovarian stimulation. Hum Reprod 2001;16:435–440. [DOI] [PubMed] [Google Scholar]
- Benadiva CA, Metzger DA. Superovulation with human menopausal gonadotropins is associated with endometrial gland-stroma dyssynchrony. Fertil Steril 1994;61:700–704. [PubMed] [Google Scholar]
- Bonduelle ML, Dodd R, Liebaers I, Van Steirteghem A, Williamson R, Akhurst R. Chorionic gonadotrophin-beta mRNA, a trophoblast marker, is expressed in human 8-cell embryos derived from tripronucleate zygotes. Hum Reprod 1988;3:909–914. [DOI] [PubMed] [Google Scholar]
- Chow WN, Lee YL, Wong PC, Chung MK, Lee KF, Yeung WS. Complement 3 deficiency impairs early pregnancy in mice. Mol Reprod Dev 2009;76:647–655. [DOI] [PubMed] [Google Scholar]
- Christensen S, Verhage HG, Nowak G, de Lanerolle P, Fleming S, Bell SC, Fazleabas AT, Hild-Petito S. Smooth muscle myosin II and alpha smooth muscle actin expression in the baboon (Papio anubis) uterus is associated with glandular secretory activity and stromal cell transformation. Biol Reprod 1995;53:598–608. [DOI] [PubMed] [Google Scholar]
- Devroey P, Bourgain C, Macklon NS, Fauser BC. Reproductive biology and IVF: ovarian stimulation and endometrial receptivity. Trends Endocrinol Metab 2004;15:84–90. [DOI] [PubMed] [Google Scholar]
- Diaz-Gimeno P, Horcajadas JA, Martinez-Conejero JA, Esteban FJ, Alama P, Pellicer A, Simon C. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril 2011;95:50–60, 60e51–15. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Donnelly KM, Srinivasan S, Fortman JD, Miller JB. Modulation of the baboon (Papio anubis) uterine endometrium by chorionic gonadotrophin during the period of uterine receptivity. Proc Natl Acad Sci USA 1999;96:2543–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrich DG, Lessey BA, Savaris RF. Comparison of HSCORE assessment of endometrial beta3 integrin subunit expression with digital HSCORE using computerized image analysis (ImageJ). Anal Quant Cytopathol Histpathol 2013;35:210–216. [PMC free article] [PubMed] [Google Scholar]
- Garcia JE, Acosta AA, Hsiu JG, Jones HW Jr. Advanced endometrial maturation after ovulation induction with human menopausal gonadotropin/human chorionic gonadotropin for in vitro fertilization. Fertil Steril 1984;41:31–35. [DOI] [PubMed] [Google Scholar]
- Garcia E, Bouchard P, De Brux J, Berdah J, Frydman R, Schaison G, Milgrom E, Perrot-Applanat M. Use of immunocytochemistry of progesterone and estrogen receptors for endometrial dating. J Clin Endocrinol Metab 1988;67:80–87. [DOI] [PubMed] [Google Scholar]
- Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev 2014;35:851–905. [DOI] [PubMed] [Google Scholar]
- Granot I, Gnainsky Y, Dekel N. Endometrial inflammation and effect on implantation improvement and pregnancy outcome. Reproduction 2012;144:661–668. [DOI] [PubMed] [Google Scholar]
- Hantak AM, Bagchi IC, Bagchi MK. Role of uterine stromal-epithelial crosstalk in embryo implantation. Int J Dev Biol 2014;58:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KH, Forman EJ, Werner MD, Upham KM, Gumeny CL, Winslow AD, Kim TJ, Scott RT Jr. Endometrial infusion of human chorionic gonadotropin at the time of blastocyst embryo transfer does not impact clinical outcomes: a randomized, double-blind, placebo-controlled trial. Fertil Steril 2014;102:1591–1595. e1592. [DOI] [PubMed] [Google Scholar]
- Jasinska A, Strakova Z, Szmidt M, Fazleabas AT. Human chorionic gonadotropin and decidualization in vitro inhibits cytochalasin-D-induced apoptosis in cultured endometrial stromal fibroblasts. Endocrinology 2006;147:4112–4121. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jaffe RC, Fazleabas AT. Comparative studies on the in vitro decidualization process in the baboon (Papio anubis) and human. Biol Reprod 1998;59:160–168. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jaffe RC, Fazleabas AT. Insulin-like growth factor binding protein-1 expression in baboon endometrial stromal cells: regulation by filamentous actin and requirement for de novo protein synthesis. Endocrinology 1999;140:997–1004. [DOI] [PubMed] [Google Scholar]
- Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril 2006;85:564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koot YE, Teklenburg G, Salker MS, Brosens JJ, Macklon NS. Molecular aspects of implantation failure. Biochim Biophys Acta 2012;1822:1943–1950. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Lebovic DI, Taylor RN. Eutopic endometrium in women with endometriosis: ground zero for the study of implantation defects. Semin Reprod Med 2013;31:109–124. [DOI] [PubMed] [Google Scholar]
- Licht P, Losch A, Dittrich R, Neuwinger J, Siebzehnrubl E, Wildt L. Novel insights into human endometrial paracrinology and embryo-maternal communication by intrauterine microdialysis. Hum Reprod Update 1998;4:532–538. [DOI] [PubMed] [Google Scholar]
- Lovely LP, Fazleabas AT, Fritz MA, McAdams DG, Lessey BA. Prevention of endometrial apoptosis: randomized prospective comparison of human chorionic gonadotropin versus progesterone treatment in the luteal phase. J Clin Endocrinol Metab 2005;90:2351–2356. [DOI] [PubMed] [Google Scholar]
- Macklon NS, Geraedts JP, Fauser BC. Conception to ongoing pregnancy: the ‘black box’ of early pregnancy loss. Hum Reprod Update 2002;8:333–343. [DOI] [PubMed] [Google Scholar]
- Mansour R, Tawab N, Kamal O, El-Faissal Y, Serour A, Aboulghar M, Serour G. Intrauterine injection of human chorionic gonadotropin before embryo transfer significantly improves the implantation and pregnancy rates in in vitro fertilization/intracytoplasmic sperm injection: a prospective randomized study. Fertil Steril 2011;96:1370–1374. e1371. [DOI] [PubMed] [Google Scholar]
- Meyer WR, Novotny DB, Fritz MA, Beyler SA, Wolf LJ, Lessey BA. Effect of exogenous gonadotropins on endometrial maturation in oocyte donors. Fertil Steril 1999;71:109–114. [DOI] [PubMed] [Google Scholar]
- Miele L, Osborne B. Arbiter of differentiation and death: notch signaling meets apoptosis. J Cell Physiol 1999;181:393–409. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Hertig AI, Rock J. Dating the endometrial biopsy. Fertil Steril 1950;1:3–25. [DOI] [PubMed] [Google Scholar]
- Palomino WA, Argandona F, Azua R, Kohen P, Devoto L. Complement C3 and decay-accelerating factor expression levels are modulated by human chorionic gonadotropin in endometrial compartments during the implantation window. Reprod Sci 2013;20:1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson RJ, Sauer MV, Lobo RA. Embryo implantation after human in vitro fertilization: importance of endometrial receptivity. Fertil Steril 1990;53:870–874. [DOI] [PubMed] [Google Scholar]
- Practice Committee of American Society for Reproductive M, Practice Committee of Society for Assisted Reproductive T. Recommendations for gamete and embryo donation: a committee opinion. Fertil Steril 2013;99:47–62. [DOI] [PubMed] [Google Scholar]
- Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol 2010;11:785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015;43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Alonso M, Blesa D, Diaz-Gimeno P, Gomez E, Fernandez-Sanchez M, Carranza F, Carrera J, Vilella F, Pellicer A, Simon C. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril 2013;100:818–824. [DOI] [PubMed] [Google Scholar]
- Saadat P, Boostanfar R, Slater CC, Tourgeman DE, Stanczyk FZ, Paulson RJ. Accelerated endometrial maturation in the luteal phase of cycles utilizing controlled ovarian hyperstimulation: impact of gonadotropin-releasing hormone agonists versus antagonists. Fertil Steril 2004;82:167–171. [DOI] [PubMed] [Google Scholar]
- Santibanez A, Garcia J, Pashkova O, Colin O, Castellanos G, Sanchez AP, De la Jara JF. Effect of intrauterine injection of human chorionic gonadotropin before embryo transfer on clinical pregnancy rates from in vitro fertilisation cycles: a prospective study. Reprod Biol Endocrinol 2014;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayegh RA, Tao XJ, Awwad JT, Isaacson KB. Localization of the expression of complement component 3 in the human endometrium by in situ hybridization. J Clin Endocrinol Metab 1996;81:1641–1649. [DOI] [PubMed] [Google Scholar]
- Sherwin JR, Sharkey AM, Cameo P, Mavrogianis PM, Catalano RD, Edassery S, Fazleabas AT. Identification of novel genes regulated by chorionic gonadotropin in baboon endometrium during the window of implantation. Endocrinology 2007;148:618–626. [DOI] [PubMed] [Google Scholar]
- Srisuparp S, Strakova Z, Brudney A, Mukherjee S, Reierstad S, Hunzicker-Dunn M, Fazleabas AT. Signal transduction pathways activated by chorionic gonadotropin in the primate endometrial epithelial cells. Biol Reprod 2003;68:457–464. [DOI] [PubMed] [Google Scholar]
- Su RW, Strug MR, Joshi NR, Jeong JW, Miele L, Lessey BA, Young SL, Fazleabas AT. Decreased notch pathway signaling in the endometrium of women with endometriosis impairs decidualization. J Clin Endocrinol Metab 2015;100:E433–E442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom SA, Komm BS, Ponce-de-Leon H, Yi Z, Teuscher C, Lyttle CR. Estrogen regulation of tissue-specific expression of complement C3. J Biol Chem 1989;264:16941–16947. [PubMed] [Google Scholar]
- Ubaldi F, Bourgain C, Tournaye H, Smitz J, Van Steirteghem A, Devroey P. Endometrial evaluation by aspiration biopsy on the day of oocyte retrieval in the embryo transfer cycles in patients with serum progesterone rise during the follicular phase. Fertil Steril 1997;67:521–526. [DOI] [PubMed] [Google Scholar]
- Wirleitner B, Schuff M, Vanderzwalmen P, Stecher A, Okhowat J, Hradecky L, Kohoutek T, Kralickova M, Spitzer D, Zech NH. Intrauterine administration of human chorionic gonadotropin does not improve pregnancy and life birth rates independently of blastocyst quality: a randomised prospective study. Reprod Biol Endocrinol 2015;13:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.