Abstract
Background/Objectives
Vitamin D exerts metabolic activities. We investigated whether the 25-hydroxy vitamin D [25(OH)D] is associated with liver fat content (LFC) and non-alcoholic fatty liver disease (NAFLD) in a middle-aged, elderly Chinese population.
Subject/Methods
A total of 2,960 participants (954 men and 2,006 women) aged over 45 years old were enrolled. Each participant underwent a standard interview, anthropometric measurements and laboratory examinations. Vitamin D deficiency and insufficiency was diagnosed when serum 25(OH) D level was < 50 and 50–75nmol/L. An ultrasound quantitative method was used to assess the LFC.
Results
Among the 2,960 participants, 1,982 (67.0%) subjects had vitamin D deficiency, 769 (26.0%) had vitamin D insufficiency, and 209 (7%) had normal vitamin D. Male subjects with vitamin D deficiency and insufficiency had significantly higher LFC than those with normal 25(OH)D (P = 0.034), while the LFC values showed no significant difference among the female subjects with vitamin D sufficiency, insufficiency and deficiency (P = 0.396). Univariate correlation analysis showed that 25(OH)D had a significantly negative association with LFC in men (r = -0.085, P = 0.009), but not in women. After adjusting for age, cigarette smoking, examination season, serum calcium, PTH and all possible confounders that displayed significant associations with LFC in univariate correlation analysis, serum 25(OH)D remained associated with LFC in middle-aged and elderly Chinese men.
Conclusion
Serum 25(OH)D level was inversely associated with LFC in middle-aged and elderly Chinese men.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a pathological condition that consists of a spectrum of liver diseases caused by macrovesicular accumulation of triglycerides within hepatocytes (hepatic steatosis). Currently, NAFLD is thought to be the hepatic manifestation of insulin resistance and correlated with metabolic syndrome, diabetes and cardiovascular diseases [1]. The prevalence of NAFLD is approximately 10–30% in the general population, and it is still increasing worldwide [2,3]. Detection of novel risk factors could promote our understanding of the NAFLD pathogenesis, and facilitate early diagnosis and treatment of this disease clinically.
Vitamin D plays an important role in calcium and phosphorus metabolism, and recent studies have focused on the extra-skeletal effects of vitamin D. Vitamin D has been implicated in many metabolic disorders, such as type 2 diabetes, insulin resistance, metabolic syndrome and cardiovascular diseases [4–7], and experimental studies showed that Vitamin D can improve free fatty acid (FFA)-induced insulin resistance in peripheral tissues and hepatocytes [8].
Several recent studies had shown a relationship between presence of NAFLD and serum 25-hydroxy vitamin D [25(OH)D] level [9]. However, most of the previous studies used common ultrasound method to diagnose NAFLD, which provided no information on the severity of liver steatosis. The quantitative association between liver fat content (LFC) and serum 25(OH)D has not been fully studied.
In the current study, we measured LFC by a ultrasound quantitative method in a large-scale middle-aged and elderly Chinese population, and investigated whether liver fat accumulation was associated with serum 25(OH)D level.
Subjects and Methods
Subjects
The Changfeng Study is a community-based study of chronic diseases among middle-aged and elderly individuals, which has been described previously [10].A total of 4659participants aged over 45 years oldwereinitially recruitedfrom October 2010 to July 2012. Exclusion criteria were incomplete data of serum 25(OH)D or LFC (n = 472), alcohol abuse (>20 g/day for male, >10 g/day for female) [11] (n = 581), chronic viral hepatitis (HbsAg or anti-HCVAb positive) (n = 261), history of viral, autoimmune or drug-induced chronic hepatitis, cirrhosis, or liver cancer (n = 385) Finally, 2960 subjects (954 males and 2006 females) were included in the analysis.
The study was approved by the ethics committee of Zhongshan Hospital at Fudan University, and it was done in accordance with the guidelines of Declaration of Helsinki. Each subject provided written informed consent. Interviews, physical examinations and ultrasound scans were performed at the Changfeng Community Health Service Center.
Ultrasound quantitative examinations
Trained ultrasonographists who were unaware of clinical data performed ultrasound examinations. Ultrasound images were captured using a GE logiq P5 scanner (GE Healthcare, Milwaukee, WI, USA), analyzed using a NIH image software (ImageJ 1.41o, NationalInstitutes of Health, Bethesda, MD) and standardized using a tissue-mimicking phantom (Model 057; Computerized Imaging Reference Systems, Norfolk, VA). LFC was calculated using the equation: LFC (%) = 62.592 * standardized US hepatic/renal ratio + 168.076*standardized US hepatic attenuation rate—27.863 [12,13].
Other Measurements
Participants were required not to alter their diets or physical activity levels for at least 3 days before the test. Trained investigators interviewed all participants to evaluate the medical history and lifestyle. The time of examination was divided into four subgroups: (1) spring (March-May), (2) summer (June-August), (3) autumn (September-November), and (4) winter (December-February). Weight and height were measured while each participant was clothed in a light gown. The body mass index (BMI) was calculated asweight divided by height squared (kg/m2). Waist circumference (WC) was measured midway between the lowest rib margin and the iliac crest in a standing position. The resting blood pressure was measured three times using a standardized sphygmomanometer after five minutes of rest, and the mean value was used for the analysis. All of the subjects were examined after an over-night fast. Total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), liver enzymes, and serum calcium and phosphate were measured using a model 7600 automated bio-analyser (Hitachi, Tokyo, Japan). The level of low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation. The fasting blood glucose (FBG) and 2h postload blood glucose (PBG) following a 75-g oral glucose challenge were measured using the glucose oxidase method. Hemoglobin A1c (HbA1c) was measured by high performance liquid chromatography (HPLC) (BIO-RAD II TURBO), which was standardized to the National Glycated Haemoglobin Standardization Program (NGSP). The serum 25(OH)D, parathyroid hormone (PTH) and fasting insulin (FINS) were measured by electrochemiluminescence immunoassay using an immunoassay analyzer (Roche Cobas-6001, Switzerland). The homeostasis model assessment insulin resistance index (HOMA-IR) was obtained by applying the following formula: HOMA-IR = fasting insulin (mU/L) × fasting glucose (mmol/L)/22.5[14].
Diagnosis of metabolic syndrome
Metabolic syndrome was defined as follows: waist circumference≥90 cm for males, and _≥80 cm for females, antihypertensive treatment or blood pressure≥130/85 mmHg, antidiabetic treatment or FBG≥5.6 mmol/L, TG ≥1.7 mmol/L, and HDL cholesterol <1.03 mmol/L for males and <1.29 mmol/L for females [15].
Statistical analyses
All statistical analyses were performed using SPSS software version15.0 (SPSS, Chicago, IL).The data were presented as the mean±SD, except for skewed variables, which were presented asthe median with the interquartile range (25–75%) given in parentheses. One-way analysis of variance or the Mann–Whitney U-test was used for the comparisons of continuous data among groups, whereas the Chi-squared test was used for the comparisons of categorical variables. The correlations of LFC with all anthropometric and biochemical parameters were analyzed by Spearman correlation analysis. All subjects in the community were divided into three subgroups according the level of serum 25(OH)D: Vitamin D sufficiency (> 75nmol/L), insufficiency (50–75nmol/L)and deficiency(<50nmol/L) [16]. Independent associations between the 25(OH)D categories and LFC were assessed using five multivariate linear regression models, after adjusting for age, cigarette smoking, examination season, serum calcium, PTH and all variables that displayed significant associations with LFC in univariate correlation analysis. A two-tailed P-value < 0.05 was considered statistically significant.
Results
General characteristics of the study population
A total of2960 participants (954 men and 2006 women), 46–96 years of age, were enrolled for the analyses. Their baseline characteristics are given in Table 1. The mean BMI was 24.2±3.3kg/m2 and median LFC was 5.3% (2.4%-11.6%). Among the 2960 participants, 1982 (67.0%) subjects had vitamin D deficiency, 769 (26.0%) had vitamin D insufficiency, and 209 (7%) had normal vitamin D. Compared with subjects with sufficient vitamin D, subjects with decreased serum 25(OH)D were younger, more obese, and had significantly higher blood glucose, fasting insulin, cholesterol, LDL-c, bone turn-over biomarkers (PTH and ALP), and LFC (All p value <0.05). The prevalence of NAFLD in subjects with vitamin D sufficiency, insufficiency and deficiency was 24.4%, 31.2% and 33.8%, respectively (P = 0.016).
Table 1. Characteristics of the study participants according to vitamin D levels.
| Vitamin D | ||||
|---|---|---|---|---|
| Sufficiency (>75nmol/L) | Insufficiency (50–75nmol/L) | Deficiency (<50nmol/L) | P value | |
| N | 209 | 769 | 1982 | |
| Gender (M/F) | 120/89 | 304/465 | 530/1452 | <0.001 |
| Smoking, n(%) | 29 (13.9%) | 113 (14.7%) | 239 (12.1%) | 0.223 |
| Examination season | <0.001 | |||
| Spring, n (%) | 791 (39.9%) | 223 (29.0%) | 37 (17.7%) | - |
| Summer, n (%) | 405 (20.4%) | 216 (28.1%) | 73 (34.9%) | - |
| Autumn, n(%) | 398 (20.1%) | 225 (29.3%) | 67 (32.1%) | - |
| Winter, n(%) | 388 (19.6%) | 105 (13.7%) | 32 (15.3%) | - |
| Age | 65.27±9.86 | 64.57±9.65 | 62.82±9.73** | <0.001 |
| BMI | 23.46±2.86 | 24.19±3.22** | 24.25±3.41** | 0.005 |
| WC | 81.91±8.76 | 83.44±9.36* | 82.99±9.73 | 0.118 |
| SBP | 134.71±20.20 | 135.14±18.02 | 136.29±19.97 | 0.256 |
| DBP | 75.36±10.15 | 75.18±9.50 | 76.52±9.90 | 0.003 |
| FBG | 5.0(4.8–5.5) | 5.2(4.9–5.8)** | 5.2(4.8–5.8)** | 0.001 |
| PBG | 6.6(5.5–7.8) | 6.9(5.8–8.7)** | 6.7(5.5–8.6)* | 0.011 |
| HbA1c | 5.6(5.4–5.9) | 5.7(5.4–6.1)* | 5.7(5.4–6.0)** | 0.022 |
| FINS | 6.7(4.9–9.7) | 7.9(5.5–11.3) | 8.2(5.5–11.9)* | 0.040 |
| HOMA-IR | 1.79±1.99 | 2.45±2.78 | 2.75±4.98* | 0.024 |
| TG | 1.29(0.96–1.69) | 1.46(1.1–2.0) | 1.44(1.06–2.05) | 0.173 |
| TC | 4.88±0.97 | 5.08±0.89 ** | 5.16±0.94** | <0.001 |
| HDL-c | 1.43±0.39 | 1.42±0.36 | 1.44±0.37 | 0.185 |
| LDL-c | 2.78±0.75 | 2.90±0.77 | 2.94±0.81** | 0.017 |
| Cr | 73(61–87) | 67(58–80) ** | 63(55–73)** | <0.001 |
| Ca | 2.42(2.34–2.50) | 2.4(2.3–2.5) | 2.38(2.3–2.46) | 0.558 |
| P | 1.10(0.98–1.20) | 1.1(1.0–1.2) | 1.15(1.04–1.24) | 0.612 |
| PTH | 32.3(26.3–41.5) | 37.3(29.5–47.9) ** | 43.7(33.8–55.0)** | <0.001 |
| ALT | 16(13–20) | 16(12–22) | 15(12–21) | 0.627 |
| AST | 20(18–24) | 20(17–24) | 20(17–23) | 0.758 |
| ALP | 68(59–79) | 71(61–83.5) | 72(61–86)** | 0.009 |
| GGT | 22(17–31) | 22(17–32) | 22(17–32) | 0.508 |
| LFC | 3.83(1.90–8.99) | 5.06(2.10–11.42) | 5.59(2.58–11.76)** | 0.002 |
| NAFLD, % | 24.4 | 31.2 | 33.8 | 0.016 |
Data are expressed as the means ± SD, percentages or median (25th to 75th percentiles).
*P < 0.05
**P < 0.01 vs. Vitamin D sufficiency group.
Association between serum 25(OH)D and NAFLD
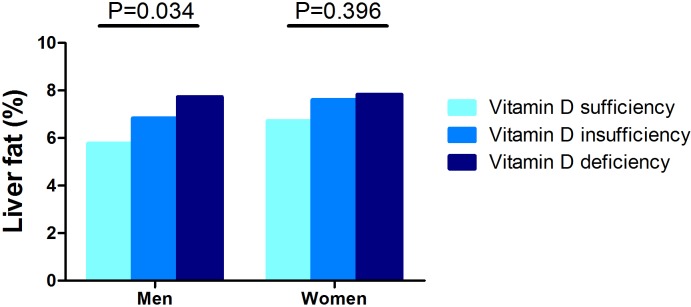
By taking the gender difference of vitamin D status into consideration [17], we stratified all analyses by sex. As shown in Fig 1, male subjects with vitamin D deficiency and insufficiency had significantly higher LFC than those with normal 25(OH)D (P = 0.034), while the LFC values showed no significant difference among the female subjects with vitamin D sufficiency, insufficiency and deficiency (P = 0.396). Linear correlation analyses also showed that 25(OH)D had a significantly negative association with LFC in men (r = -0.085, P = 0.009), but not in women (r = -0.014, P = 0.523) in our middle-aged and elderly study population (Table 2).
Fig 1.
The average LFC (%) in male (left) and female (right) subjects with different vitamin D status with a serum 25(OH)D <50nmol/L, = 50–75nmol/L and >75nmol/L. The liver fat content was significantly higher in male subjects with vitamin D deficiency and insufficiency, but not in female subjects.
Table 2. Correlations between LFC, 25(OH)D and other metabolic parameters.
| Men | Women | |||
|---|---|---|---|---|
| r | P | r | P | |
| age | -0.095 | 0.003 | -0.026 | 0.243 |
| Smoking | 0.045 | 0.164 | -0.002 | 0.936 |
| Season | 0.006 | 0.855 | 0.001 | 0.952 |
| BMI | 0.364 | <0.001 | 0.335 | <0.001 |
| WC | 0.349 | <0.001 | 0.297 | <0.001 |
| SBP | 0.038 | 0.024 | 0.118 | <0.001 |
| DBP | 0.111 | 0.001 | 0.156 | <0.001 |
| TC | 0.117 | <0.001 | 0.062 | 0.005 |
| TG | 0.194 | <0.001 | 0.288 | <0.001 |
| HDL | -0.185 | <0.001 | -0.225 | <0.001 |
| LDL | 0.089 | 0.006 | 0.000 | 0.990 |
| FBG | 0.105 | 0.001 | 0.186 | <0.001 |
| PBG | 0.201 | <0.001 | 0.262 | <0.001 |
| HbAlc | 0.096 | 0.005 | 0.211 | <0.001 |
| FINS | 0.067 | 0.038 | 0.298 | <0.001 |
| HOMA-IR | 0.053 | 0.101 | 0.265 | <0.001 |
| Cr | -0.078 | 0.016 | -0.070 | 0.002 |
| Ca | -0.010 | 0.758 | 0.007 | 0.755 |
| P | 0.046 | 0.152 | -0.011 | 0.614 |
| 25(OH)D | -0.085 | 0.009 | -0.014 | 0.523 |
| PTH | -0.101 | 0.002 | 0.007 | 0.762 |
| Menopause | - | - | 0.034 | 0.126 |
Univariate analyses on the associations between metabolic parameters and NAFLD
As shown in Table 2, LFC was significantly associated with BMI, all components of metabolic syndrome (waist circumference, blood pressure, serum TG, HDL-c, FBG, and PBG), serum insulin and HbA1c in both male and female subjects (all p <0.05). In parallel to the change of 25(OH)D, serum PTH (r = -0.101, P = 0.002) and creatine levels (r = -0.078, P = 0.016) were also negatively associated with LFC in men, but in women, LFC had no significantly associations with bone metabolic parameters, including serum calcium, phosphate, PTH, and 25(OH)D.
Independent contribution of 25(OH)D in variability in LFC
In unadjusted linear regression analysis, 25(OH) was significantly correlated with LFC (stdβ = -0.084, P = 0.010). In multiple regression analyses, after adjustment for age and cigarette smoking (model 1), 25(OH)D was still significantly associated with LFC (stdβ = -0.074, P = 0.024). Parameter estimates of these correlations remained significant after successively adjusting for examination season (model 2), serum calcium, PTH and creatine (model 3), and then in addition BMI (model 4). In model 5, the significant negative correlation between LFC and 25(OH)D remained after additional adjustment for all components of metabolic syndrome, including WC, FBG, SBP, TG and HDL-c, and insulin resistance level measured by HOMA-IR (stdβ = -0.074, P = 0.016) (Table 3).
Table 3. Multivariate regression analysis for the association between liver fat content (dependent variable) and 25(OH)D categories (independent variables) in different models in men.
| Liver fat content | ||||
|---|---|---|---|---|
| Std β | β | 95%CI | P | |
| Unadjusted | -0.084 | -0.954 | (-1.675, -0.233) | 0.010 |
| Model 1 | -0.074 | -0.836 | (-1.562, -0.111) | 0.024 |
| Model 2 | -0.076 | -0.859 | (-1.595, -0.123) | 0.022 |
| Model 3 | -0.092 | -1.051 | (-1.789, -0.312) | 0.005 |
| Model 4 | -0.083 | -0.938 | (-1.626, -0.249) | 0.008 |
| Model 5 | -0.074 | -0.859 | (-1.558, -0.160) | 0.016 |
Model 1: adjusted for age and cigarette smoking
Model 2: Model 1 plus season
Model3: Model 2 plus serum creatine, calcium and PTH
Model4: Model 3 plus BMI
Model 5: Model 4 plus all components of metabolic syndrome (waist circumference, FBG, SBP, TG and HDL-c) and HOMA-IR.
Discussion
In this study, we found that 1) individual vitamin D deficiency or insufficiency was associated with impaired glucose and lipid metabolism and higher prevalence of metabolic syndrome; 2) serum 25(OH)D concentration had an independent and negative association with LFC and the presence of NAFLD in middle-aged and elderly Chinese men, but not in women. To the best of our knowledge, this is the first study to investigate the relationship between serum 25(OH)D and LFC which is measured by quantitative ultrasonography.
At present, ultrasonography remains the most common method for the diagnosis of liver steatosis, because it is simple, non-invasive, inexpensive, and widely available [18]. However, the conventional ultrasonography is limited due to its poor sensitivity and inability to quantify LFC [19]. While the current accurate quantitative modalities, such as liver biopsy or proton magnetic resonance spectroscopy ([1H]-MRS), can not be carried out routinely in the large-scale cross-sectional studies due to either the invasiveness [20] or the demanding technologies and expensive costs [18, 21]. Therefore, there are still few epidemiological evidences on the quantitative correlation between LFC and serum 25(OH)D level until now. Recently, several new techniques have been developed to facilitate the measurement of LFC in large-scale community population, including the controlled attenuation parameter (CAP) [22] and backscatter coefficient [23], but these methods depend on the specialized equipment that was not routinely used in clinic. In the current community study, we used a method of computer-aided ultrasound measurement of LFC based on the ultrasound hepatic/renal ratio and hepatic attenuation rate under traditional ultrasonic apparatus [12]. The accuracy of the quantitative ultrasound examination has been validated using [1H]-MRS as a reference [12] and was consistent with both [1H]-MRS(r = 0.89, P<0.001) and histology-determined steatosis grade (r = 0.79, P<0.001) [13]. It has also been used in our previous community studies [24–26]. Therefore, we can provide reliable quantitative relation between individual liver fat content and vitamin D status.
Recent laboratory studies have shown a complex network of interactions among adipose, liver and bone tissues [27]. Vitamin D is one of the primary biological regulators of calcium and phosphates homeostasis in the body [28]. With the wide tissue distribution of the vitamin D receptor (VDR), vitamin D can function the same way as other steroid hormones in the whole body [29]. Previous studies have revealed the role of 25(OH)D in regulating the glucose and lipid metabolism [30–32], and the cardiovascular protective action of vitamin D [33]. In accordance with the previous studies, we also found thatlow 25(OH)D was associated with impaired glucose and lipid metabolism such as higher BMI, blood pressure, blood glucose, HbA1c, FINS, HOMA-IR, TC and LDL-C levels.
NAFLD is regarded as the liver manifestation of the metabolic syndrome [1].Several studies have reported the association between low serum 25(OH)D concentrations and NAFLD in humans. Targher et al. have compared 60 consecutive patients with biopsy-proven NAFLD and 60 healthy controls of comparable age, sex and BMI, and the NAFLD patients had a remarkable decrease in serum 25(OH)D concentrations [34]. Zhai HL et al. showed a negative correlation between vitamin D level and NAFLD diagnosed by common ultrasonography in a large-scale community study [35]. A meta-analysis of 17 cross-sectional and case-control studies concluded that NAFLD patients were 1.26 times more likely to be vitamin D deficient [36]. Recently, Nelson JE et al. also reported a relationship between vitamin D deficiency and increased risk of non-alcoholic steatohepatitis diagnosed by liver biopsy [37]. Our study further expand the knowledge on the association between vitamin D status and NAFLD, and importantly evaluated the independently inverse relationship between the quantitative value of LFC and serum 25(OH)D level.
The mechanisms underlying the inverse relation between NAFLD and 25(OH)D are still not clear. On the one hand, vitamin D was converted to 25(OH)D in the liver for its active form [38], However, most of NAFLD patients in our study had normal liver enzyme, albumin level and coagulation function, which indicated a normal liver synthesis function of 25(OH)D. On the other hand, vitamin D also had extra-skeletal effects on metabolic organs, which may indirectly affect the hepatic lipid metabolism [28]. The role of vitamin D in adipokine activity is a current active area of research, Roth et al. has investigated the effect of vitamin D deficiency on adiponectin, leptin, resistin, TNF-α, and IL-6 [39], and it may worsen NAFLD by up-regulation of hepatic inflammatory and oxidative stress genes. In addition, it is reported that vitamin D receptor was widely expressed in the liver, and the negative association between the severity of liver histology and vitamin D receptor expression in NASH patients indicated that vitamin D might have a direct role in the progression of NAFLD [40].
Interestingly, we found a relationship between LFC and 25(OH)D in middle-aged and elderly men, but not in women. The gender difference has also been found in our previous study on the association between LFC and bone mineral density in the same community population [41]. The difference between men and women in the features of age-related bone metabolism, liver lipid metabolism and sex hormone levels could be potential explanations. However, we cannot deduce the mechanisms of the gender difference in the association between LFC and 25(OH)D in our current study, which need to be elucidated further.
Our study has some limitations. Firstly, the present study was across-sectional study, and did not permit the identification of causal relationships between LFC and 25(OH)D, which need to be further evaluated in longitudinal studies. Secondly, in a study of our current size, it was not possible to obtain liver biopsies to investigate the association of 25(OH)D with liver fibrosis or inflammation. Thirdly, the estrogen and androgen levels have not been measured in the current study, which might be responsible for the gender difference in the relationship between 25(OH)D and NAFLD.
Conclusion
Serum 25(OH)D was inversely associated with LFC in middle-aged and elderly Chinese men. Vitamin D replacement may have benefits against NAFLD and liver fat accumulation, which need to be validated by further well-designed clinical trials in the future.
Data Availability
Our study was supported from several national and local government research funds. According to the relevant policy of data management, we were forbidden to provide our data to a third party. Data will be available upon request from Xin Gao at zhongshan_endo@126.com.
Funding Statement
This work was supported by grants from the National Key Basic Research Program of China (grant no. 2012CB524906 to X. Gao), URL www.973.gov.cn/Default_3.aspx; the Shanghai Municipal Health Project Grant (grant no. 2013ZYJB0802 to X. Gao); the Shanghai Health and Family Planning Commission Foundation (Grant Nos. 12GWZX0103 and 2013SY005 to X. Gao); the Shanghai Hospital Development Center Foundation (SHDC12012201 to X. Gao). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221–31. [DOI] [PubMed] [Google Scholar]
- 2.Williams R. Global challenges in liver disease. Hepatology. 2006;44(3):521–6. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9(6):524–30. 10.1016/j.cgh.2011.03.020 [DOI] [PubMed] [Google Scholar]
- 4.Anderson J, May H, Horne B, Bair T, Hall N, Carlquist J, et al. Relation of Vitamin D Deficiency to Cardiovascular Risk Factors, Disease Status, and Incident Events in a General Healthcare Population. American Journal of Cardiology. 2010;106(7):963–8. 10.1016/j.amjcard.2010.05.027 [DOI] [PubMed] [Google Scholar]
- 5.Kendrick J, Targher G, Smits G, Chonchol M. 25-Hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2009;205(1):255–60. 10.1016/j.atherosclerosis.2008.10.033 [DOI] [PubMed] [Google Scholar]
- 6.Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Shaw JE, Zimmet PZ, et al. Low serum 25-hydroxyvitamin D is associated with increased risk of the development of the metabolic syndrome at five years: results from a national, population-based prospective study (The Australian Diabetes, Obesity and Lifestyle Study: AusDiab). J Clin Endocrinol Metab. 2012;97(6):1953–61. 10.1210/jc.2011-3187 [DOI] [PubMed] [Google Scholar]
- 7.Kim MK, Il Kang M, Won Oh K, Kwon HS, Lee JH, Lee WC, et al. The association of serum vitamin D level with presence of metabolic syndrome and hypertension in middle-aged Korean subjects. Clin Endocrinol (Oxf). 2010;73(3):330–8. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Q, Hou F, Guo Z, Liang M, Wang G, Zhang X. 1,25-Dihydroxyvitamin D improved the free fatty-acid-induced insulin resistance in cultured C2C12 cells. Diabetes-Metabolism Research and Reviews. 2008;24(6):459–64. 10.1002/dmrr.873 [DOI] [PubMed] [Google Scholar]
- 9.Bhatt S, Nigam P, Misra A, Guleria R, Pasha M. Independent associations of low 25 hydroxy vitamin D and high parathyroid hormonal levels with nonalcoholic fatty liver disease in Asian Indians residing in north India. Atherosclerosis. 2013;230(1):157–63. 10.1016/j.atherosclerosis.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 10.Gao X, Hofman A, Hu Y, Lin H, Zhu C, Jeekel J, et al. The Shanghai Changfeng Study: a community-based prospective cohort study of chronic diseases among middle-aged and elderly: objectives and design. European Journal of Epidemiology. 2010;25(12):885–93. 10.1007/s10654-010-9525-6 [DOI] [PubMed] [Google Scholar]
- 11.Farrell GC, Chitturi S, Lau GK, Sollano JD; Asia-Pacific Working Party on NAFLD. Guidelines for theassessment and management of nonalcoholic fatty liver disease in the Asia-Pacific region: executivesummary. J Gastroenterol Hepatol 2007;22:775–7. [DOI] [PubMed] [Google Scholar]
- 12.Xia M, Yan H, He W, Li X, Li C, Yao X, et al. Standardized Ultrasound Hepatic/Renal Ratio and Hepatic Attenuation Rate to Quantify Liver Fat Content: An Improvement Method. Obesity. 2012;20(2):444–52. 10.1038/oby.2011.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia MF, Bian H, Yan HM, Lin HD, Chang XX, Li XM, et al. Assessment of Liver Fat Content Using Quantitative Ultrasonography to Evaluate Risks for Metabolic Diseases. Obesity (Silver Spring).2015;23:1929–37. [DOI] [PubMed] [Google Scholar]
- 14.Sundström J, Arnlöv J, Stolare K, Lind L.Blood pressure-independent relations of left ventricular geometry to the metabolic syndrome and insulin resistance: a population-based study. Heart. 2008;94(7):874–8. [DOI] [PubMed] [Google Scholar]
- 15.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome: a new worldwide definition. A consensus statement from the International Diabetes Federation. Diabet Med 2006;23:469–80. [DOI] [PubMed] [Google Scholar]
- 16.Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20(11):1807–20. 10.1007/s00198-009-0954-6 [DOI] [PubMed] [Google Scholar]
- 17.Verdoia M, Schaffer A, Barbieri L, Di Giovine G, Marino P, Suryapranata H, et al. Impact of gender difference on vitamin D status and its relationship with the extent of coronary artery disease.Nutr Metab Cardiovasc Dis. 2015;25(5):464–70. 10.1016/j.numecd.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 18.Castera L, Vilgrain V, Angulo P. Noninvasive evaluation of NAFLD. Nat RevGastroenterol Hepatol 2013;10:666–75. [DOI] [PubMed] [Google Scholar]
- 19.Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ.Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol 2009;51:1061–7. 10.1016/j.jhep.2009.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005;128:1898–906. [DOI] [PubMed] [Google Scholar]
- 21.McPherson S, Jonsson JR, Cowin GJ, O'Rourke P, Clouston AD, Volp A, et al. Magnetic resonance imaging and spectroscopy accurately estimate the severity of steatosis provided the stage of fibrosis is considered. J Hepatol 2009;51:389–97. 10.1016/j.jhep.2009.04.012 [DOI] [PubMed] [Google Scholar]
- 22.Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, et al. Controlled attenuation parameter(CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol 2010;36:1825–35. 10.1016/j.ultrasmedbio.2010.07.005 [DOI] [PubMed] [Google Scholar]
- 23.Lin SC, Heba E, Wolfson T, Ang B, Gamst A, Han A, et al. Non invasive Diagnosis of Nonalcoholic Fatty Liver Disease and Quatification of Liver Fat Using a New Quantitative Ultrasound Technique. Clin Gastroenterol Hepatol. 2015;13:1337–45. 10.1016/j.cgh.2014.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia MF, Ling Y, Bian H, Lin HD, Yan HM, Chang XX, et al. I148M variant of PNPLA3 increases the susceptibility to non-alcoholic fatty liver disease caused by obesity and metabolic disorders. Aliment Pharmacol Ther. 2016. March;43(5):631–42. 10.1111/apt.13521 [DOI] [PubMed] [Google Scholar]
- 25.Li X, Xia M, Ma H, Hofman A, Hu Y, Yan H, et al. Liver fat content is associated with increased carotid atherosclerosis in a Chinese middle-aged and elderly population: the Shanghai Changfeng study. Atherosclerosis 2012; 224: 480–5. 10.1016/j.atherosclerosis.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 26.Li X, Xia M, Ma H, Hu Y, Yan H, He W, et al. Liver fat content, evaluated through semi-quantitative ultrasound measurement, is associated with impaired glucose profiles: a community-based study in Chinese. PLOS ONE 2013; 8: e65210 10.1371/journal.pone.0065210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musso G, Paschetta E, Gambino R, Cassader M, Molinaro F. Interactions among bone, liver, andadipose tissue predisposing to diabesity and fatty liver. Trends Mol Med. 2013;19:522–35. 10.1016/j.molmed.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 28.Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health.Am J Clin Nutr. 2008;88(2):491S–499S. [DOI] [PubMed] [Google Scholar]
- 29.Feldman D, Pike JW, Glorieux FH, eds. Vitamin D. San Diego, CA:Elsevier Academic Press, 2005. [Google Scholar]
- 30.Kayaniyil S, Vieth R, Harris SB, Retnakaran R, Knight JA, Gerstein HC, et al. Association of 25(OH)D and PTH with metabolic syndrome and its traditional and nontraditional components. J Clin Endocrinol Metab. 2011;96(1):168–75. 10.1210/jc.2010-1439 [DOI] [PubMed] [Google Scholar]
- 31.Lu L, Yu Z, Pan A, Hu FB, Franco OH, Li H, et al. Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care. 2009;32(7):1278–83. 10.2337/dc09-0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jafari T, Fallah AA, Barani A. Effects of vitamin D on serum lipid profile in patients with type 2 diabetes: A meta-analysis of randomized controlled trials.Clin Nutr. 2016. March 15 pii: S0261-5614(16)00090-X. 10.1016/j.clnu.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 33.Pilz S, Verheyen N, Grübler MR, Tomaschitz A, März W.Vitamin D and cardiovascular disease prevention. Nat Rev Cardiol. 2016. May 6 10.1038/nrcardio.2016.73 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Targher G, Bertolini L, Scala L, Cigolini M, Zenari L, Falezza G, et al. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2007;17(7):517–24. [DOI] [PubMed] [Google Scholar]
- 35.Zhai HL, Wang NJ, Han B, Li Q, Chen Y, Zhu CF, et al. Low vitamin D levels and non-alcoholic fatty liver disease, evidence for their independent association in men in East China: a cross-sectional study (Survey on Prevalence in East China for Metabolic Diseases and Risk Factors (SPECT-China)).Br J Nutr. 2016. February 18:1–8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Eliades M, Spyrou E, Agrawal N, Lazo M, Brancati FL, Potter JJ, et al. Meta-analysis: vitamin D and non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;38(3):246–54. 10.1111/apt.12377 [DOI] [PubMed] [Google Scholar]
- 37.Nelson JE, Roth CL, Wilson LA, Yates KP, Aouizerat B, Morgan-Stevenson V, et al. Vitamin D Deficiency Is Associated With Increased Risk of Non-alcoholic Steatohepatitis in Adults With Non-alcoholic Fatty Liver Disease: Possible Role for MAPK and NF-κB? Am J Gastroenterol. 2016. March 22 10.1038/ajg.2016.51 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lips P. Vitamin D Physiology. Prog Biophys Mol Biol 2006; 92: 4–8. [DOI] [PubMed] [Google Scholar]
- 39.Roth CL, Elfers CT, Figlewicz DP, Melhorn SJ, Morton GJ, Hoofnagle A, et al. Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty liver disease and increases hepatic resistin and Toll-like receptor activation. HEPATOLOGY 2012;55:1103–11. 10.1002/hep.24737 [DOI] [PubMed] [Google Scholar]
- 40.Barchetta I, Carotti S, Labbadia G, Gentilucci UV, Muda AO, Angelico F, et al. Liver vitamin D receptor, CYP2R1, and CYP27A1 expression: relationship with liver histology and vitamin D3 levels in patients with nonalcoholic steatohepatitis or hepatitis C virus. Hepatology. 2012;56(6):2180–7. 10.1002/hep.25930 [DOI] [PubMed] [Google Scholar]
- 41.Xia MF, Lin HD, Yan HM, Bian H, Chang XX, Zhang LS, et al. The association of liver fat content and serum alanine aminotransferase with bone mineral density in middle-aged and elderly Chinese men and postmenopausal women. J Transl Med. 2016;14:11 10.1186/s12967-016-0766-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Our study was supported from several national and local government research funds. According to the relevant policy of data management, we were forbidden to provide our data to a third party. Data will be available upon request from Xin Gao at zhongshan_endo@126.com.