Abstract
Studies about environmental burdens often explore overall community risk. Increasing evidence suggests, however, differential burdens by gender and age. The purpose of the authors’ research was to determine if gender-related difference exists among children in a region plagued with poor air quality and if increased exposure to pollutants from a major goods movement rail yard influences the relationship. Using a cross-sectional study design, the authors provided respiratory screening for children at two elementary schools. Compared to females, males were at significantly greater odds of exhibiting elevated fractional exhaled nitric oxide (FeNO) but less likely to exhibit reduced lung volume. Even in an area of overall poor air quality, the authors found that male children were a vulnerable subpopulation for greater elevated FeNO, while females were at increased risk for reduced lung capacity. Understanding differential burdens in vulnerable subpopulations is critical to providing timely and responsive strategies targeted towards health-based prevention and intervention activities.
Introduction
Interest is increasing in studying gender-related differences associated with air pollution studies (Hwang, Chen, Lin, Wu, & Leo Lee, 2015). While it is well established that younger age is a risk factor for poorer respiratory health (Pope, 2000; Schwartz, 2004), recent epidemiological evidence suggests differing effects by gender; however, the results are far from consistent (Clougherty, 2010). Also, most studies have focused on the effects of traffic-related air pollution exposure, but limited consideration has been given to emissions from major transportation goods movement facilities, such as rail yards (Castaneda et al., 2008; Gehring et al., 2002; Spencer-Hwang et al., 2014).
Risk may stem both from pollutants emitted as well as the characteristics of the individual pollutants and ultimately result in different adverse health impacts depending on the gender of the exposed. Since some locations are burdened by several sources of pollution, research is also needed to assess the cumulative health impact on residents living in close proximity to these local sources (Fox, 2002). Indeed, no research exists on the potential adverse health impacts on children living in close proximity to a major rail yard located in an already polluted area and if effects differ by gender.
Gender-related air pollution studies have reported mixed findings (Clougherty, 2010). Many studies have linked chronic exposure to air pollution with a wide range of respiratory health outcomes, including retarded lung function and growth, asthma onset and exacerbation, wheezing, respiratory infections, cough, and other related symptoms among children aged 0–18 years of age. In a study conducted by Peters and co-authors (1999), researchers identified gender-influenced differences among children in grades 4, 7, and 10 across 12 communities in Southern California and found stronger relationships between ambient air pollutants (nitrogen dioxide, ozone, and particulate matter 2.5 μm in diameter or less) and reduced lung volume among girls. In that study, an association was identified for boys, but not as strong as the association found for girls. In a Canadian study of children aged 0–14 years of age, girls were more likely to be hospitalized with a respiratory ailment with increased exposure to ambient air pollutants such as carbon monoxide and nitrogen dioxide (Luginaah, Fung, Gorey, Webster, & Wills, 2005).
In contrast to these findings indicating an increased risk for females, a number of researchers have found stronger and significant adverse respiratory health outcomes for males (Delfino et al., 2004; Gehring et al., 2002), while researchers in a study conducted in Mexico City found no clear gender differences (Rojas-Martinez et al., 2007).
The inland region of Southern California may provide a unique opportunity for health research examining this issue, given the perennially poor air quality experienced by San Bernardino County’s residents, combined with the existence of several local major freight rail yards. The air quality problem is exacerbated in the inland community of San Bernardino as the prevailing winds transport air pollutants eastward from Los Angeles. Air pollution becomes trapped by the mountains surrounding the inland region, which leads to high concentrations of pollutants when coupled with the routinely stagnant air flow and temperature inversions. Thus San Bernardino is regularly at or near the bottom of U.S. air quality rankings for ozone and fine particulate air pollution in the U.S. according to the U.S. Environmental Protection Agency and the American Lung Association (ALA).
In this article, we utilize data collected as part of the Environmental Railyard Research Impacting Community Health (ENRRICH) Project, a community health outcomes study designed to better understand the health risks among local residents living in close proximity to the San Bernardino Railyard (SBR). A series of health risk assessments conducted by the California Air Resource Board (CARB) across the 18 major rail yards indicates that SBR may pose the greatest health risk to its surrounding communities as it is the largest source of air pollution immediately adjacent to densely populated areas (Castaneda et al., 2008). The purpose of this investigation was to determine if a gender-related difference exists for adverse respiratory health outcomes among children living and attending school in close proximity to SBR compared with children living in the same region but farther away from the rail yard.
Materials and Methods
Study Location
SBR is located in a densely populated area in inland Southern California, characterized by poor air quality for ozone and fine particulate matter air pollution. This region has experienced an increase in population growth while facing severe social challenges and a weakened economy. It is home to predominantly young, low-income, Hispanic populations.
Air pollution emission sources at this 24/7 rail yard facility include diesel locomotives, on-road and off-road loading equipment and associated machinery, and typical roadway vehicles. Diesel particulate matter (DPM) is the dominant air pollutant, although air toxics (e.g., benzene and 1,3-butadiene) are also emitted in small amounts (Castaneda et al., 2008). CARB has estimated the SBR’s combined DPM emissions and other significant non-rail-yard (mobile and stationary) sources within a one-mile radius of the facility at 33 tons per year (Castaneda et al., 2008).
Study Design
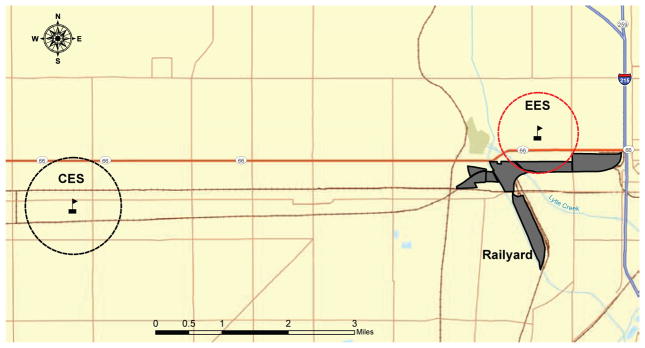
We used a cross-sectional design to compare two sociodemographically similar schools (Figure 1) matched by a GIS-derived sociodemographic profile: one located 500 m downwind from SBR, the exposure elementary school (EES); and the second, the control elementary school (CES), located seven miles west of SBR.
FIGURE 1. Map of the Study Area.
This map illustrates the location of the exposure elementary school (EES) and the control elementary school (CES), relative to the San Bernardino Railyard and in relation to the transportation infrastructure (railroads and roadways). The dashed circles identify the areas where two-thirds (approximately 67%) of the participating students at each school reside.
After obtaining Loma Linda University institutional review board and school district approval, an explanatory letter, consent form, and a short questionnaire were sent to the parents of the children. An assembly in the form of a theatrical play with an air quality theme was conducted to encourage students to tell their parents/guardians about the study and return active parental permission slips and a parent-completed child health questionnaire, resulting in 74% of children screened. School-based respiratory health screenings were conducted during late February 2012, with students from grades K–5 screened throughout the school day.
Screening Clinics
Trained technicians using standardized methods collected peak expiratory flow (PEF) and fractional exhaled nitric oxide (FeNO), along with height and weight to determine each child’s body mass index (BMI) (CDC, 2011). Screenings were conducted in partnership with the San Bernardino County Breathmobile Program (SCBP), a no-cost mobile pediatric asthma disease management program staffed by a physician, a licensed vocational nurse, a clinic assistant, and a respiratory therapist specially trained in asthma case management. Children who exhibited respiratory values outside normal PEF range, or per the parental survey had asthma, received additional spirometry testing by the SCBP staff and were offered free follow-up medical care.
PEF
PEF was assessed using a peak flow meter. The highest of three readings was used in analyses after having been transformed into the percentage of the predicted PEF according to the child’s height based on manufacturer’s guidelines.
FeNO Determination
Nitric oxide in exhaled breath reflects the redox state of the airway and has been proposed as a biomarker of lung tissue injury and inflammation (Dweik et al., 2010). FeNO was measured once with the child in a sitting position using a NIOX MINO instrument.
Potential Confounders
Potential confounders included the following variables:
From the parental questionnaire. Sociodemographic, residential, and health history information was collected including gender (male/female), age (years), grade (kindergarten, 1st, 2nd, 3rd, 4th, 5th), race (non-Hispanic white, non-Hispanic black, Hispanic, other), furry pets in the home (yes/no), time spent outdoors (<12 hrs. per week, 12–24 hrs. per week, >24 hrs. per week), exposure to environmental tobacco smoke (ETS) (yes/no), type of home heating system (gas, wood burning stove/fireplace, coal/oil, other), length of time at current address (months), physician diagnosis of asthma for their child (yes/no), use of asthma inhaler medication (yes/no), and lack of access to medical care (yes/no).
Census-derived neighborhood characteristics. Using GIS, we created several variables to characterize population density, housing indicators, and median household income at the census block group (BG) level using 2010 census figures and definitions. These neighborhood-level indicators were assigned to study subjects from both schools according to their residential BG.
Traffic-related air pollution exposures. We modeled proximity to major roadways as a proxy for residential exposure and school exposure to traffic emissions. Distance from the subjects’ residence to nearest major roads (freeway, highway, and arterials) was estimated through GIS mapping methods described previously (McConnell et al., 2006; Newcomb & Li, 2008; Rioux, Gute, Brugge, Peterson, & Parmenter, 2010; Wilhelm et al., 2008).
DPM exposure variables. To account for exposures to DPM emissions from local sources, we used data from the Multiple Air Toxics Exposure Study III (MATES-III), a regional emissions gridded inventory of air toxics emissions developed by the South Coast Air Quality Management District (Ospital, Cassmassi, & Chico, 2008). A GIS raster data set replicated the spatial coverage and resolution of the MATES-III inventory and the combined DPM (kg/day) air emissions from local stationary, on-road, and off-road sources were computed for each 2-km x 2-km grid cell and linked to participant geocoded residential addresses to assign total DPM emission. Background, non-rail-yard-related DPM emissions (both stationary and mobile sources) were found to be similar for EES and CES sites.
Statistical Analysis
Descriptive and summary estimates (i.e., percentages, means, medians, and standard deviations/variances) were assessed with Chi-square tests and t-tests. The association of gender with respiratory health measures, PEF and FeNO, was assessed using logistic regression models that facilitated the calculation of the odds ratios (OR) and 95% confidence intervals (95% CI). FeNO values were dichotomized into elevated inflammation (≥20 parts per billion) versus normal, based on cutpoints previously identified (Dweik et al., 2011). PEF values were dichotomized into decreased lung function (<80% of predicted value) versus normal, based on cutpoints previously described by ALA. The dichotomized respiratory health endpoints (PEF and FeNO) were assessed individually as outcomes of interest within the logistic regression models. The final model for independent variables included gender, school, age, race, exposure to ETS, time spent outdoors, median household income, total diesel pollution, and proximity to nearest major road. Health endpoints were the dependent variables. For the intraschool comparison, males were compared with females within school. For the interschool comparison, males were compared with males and females with females between the two schools. An additional sensitivity analysis was conducted to exclude the students from the model who had not lived at their current address for at least six months. All statistical analyses were conducted using SAS version 9.4.
Results
General Characteristics and Distribution of Respiratory Outcomes of Participants
A total of 1,066 children (561 females and 505 males) participated in our study. The majority of the children screened were Hispanic (81%) (Table 1), and most lived fewer than two miles from their school (Figure 1). No significant differences were identified between males and females for most of the potential confounding factors including age, race, BMI, time spent outdoors, exposure to ETS, neighborhood median household income, and background (non-rail-yard-related) diesel exposure. Significant differences were identified for parent-reported doctor asthma diagnosis, use of asthma inhaler medications, and PEF and FeNO breath test results. Approximately 42% (n = 452, 43% males and 42% females) of the children screened were identified as having respiratory health issues. Children were identified with a respiratory health issue based on prior asthma diagnosis, use of asthma inhaler medication, or poor PEF or FeNO results. The proportion of children identified as having a respiratory health issue did not significantly differ by gender for all schools combined. When both schools were compared, however, 48% of the EES females compared to 35% of CES females (Chi-square p-value < .01) were identified as having respiratory challenges while no significant difference was found for males (EES = 47% and CES = 40%, Chi-square p-value > .10).
TABLE 1.
Demographics and Baseline Characteristics of Children Participating in the Environmental Railyard Research Impacting Community Health Study
| Demographic | All Subjects (N = 1,066) # (%) | Control Elementary School (n = 531) | Exposure Elementary School (n = 535) | ||
|---|---|---|---|---|---|
| Males (n = 249) # (%) | Females (n = 282) # (%) | Males (n = 256) # (%) | Females (n = 279) # (%) | ||
| Age, years (mean ± SD ) | 8.00±1.8 | 8.03±1.8 | 7.99±1.8 | 7.99±1.8 | 7.98±1.8 |
| Race/ethnicity | |||||
| White | 50 (4.7) | 13 (5.2) | 13 (4.6) | 12 (4.7) | 12 (4.3) |
| Hispanic | 865 (81.1) | 199 (79.9) | 224 (79.4) | 211 (82.4) | 231 (82.8) |
| African-American | 54 (5.1) | 13 (5.2) | 24 (8.5) | 10 (3.9) | 7 (2.5) |
| Other | 95 (9.0) | 23(9.2) | 20 (7.1) | 23 (9.0) | 29 (10.4) |
| Grade | |||||
| Kindergarten | 146 (13.7) | 41 (16.5) | 43 (15.2) | 27 (10.5) | 35 (12.5) |
| 1st | 175 (16.4) | 28 (11.2) | 37 (13.1) | 59 (23.0) | 51 (18.3) |
| 2nd | 196 (18.4) | 40 (16.1) | 54 (19.1) | 49 (19.1) | 53 (19.0) |
| 3rd | 175 (16.4) | 46 (18.5) | 49 (17.4) | 35 (13.7) | 45 (16.1) |
| 4th | 193 (18.1) | 58 (23.3) | 46 (16.3) | 44 (17.2) | 45 (16.1) |
| 5th | 181 (17.0) | 36 (14.5) | 53 (18.8) | 42 (16.4) | 50 (17.9) |
| Body mass index (kg/m2) | |||||
| Underweight (<18.5 kg/m2) | 43 (4.0) | 18 (7.2) | 12 (4.3) | 2 (0.8) | 11 (3.9) |
| Normal (18.5–24.9 kg/m2) | 590 (55.4) | 132 (53.0) | 155 (55.0) | 139 (54.3) | 164 (58.8) |
| Overweight (25.0–29.9 kg/m2) | 174 (16.3) | 35 (14.1) | 52 (18.4) | 41 (16.0) | 46 (16.5) |
| Obese (>30 kg/m2) | 259 (24.3) | 64 (25.7) | 63 (22.3) | 74 (28.9) | 58 (20.8) |
| Time spent outdoors | |||||
| <12 hours per week | 399 (37.4) | 85 (34.1) | 113 (40.1) | 88 (34.4) | 113 (40.5) |
| 12–24 hours per week | 399 (37.4) | 100 (40.2) | 108 (38.3) | 105 (41.0) | 86 (30.8) |
| >24 hours per week | 172 (16.1) | 33 (13.3) | 41 (14.5) | 45 (17.6) | 53 (19.0) |
| Lived with smoker | |||||
| Yes | 209 (19.6) | 60 (24.1) | 54 (19.1) | 45 (17.6) | 50 (17.9) |
| Medical services needed but couldn’t access | |||||
| Yes | 12 (1.2) | 3 (1.2) | 4 (1.4) | 2 (0.8) | 3 (1.1) |
| Parent-reported doctor-diagnosed asthma | |||||
| Yes | 136 (12.8) | 34 (13.7) | 43 (15.2)* | 38 (14.8) | 21 (7.5) |
| Use of asthma inhaler | |||||
| Yes | 130 (13.5) | 36 (14.5) | 37 (13.1)* | 40 (15.6) | 17 (6.1) |
| PEFa | |||||
| <80% of predicted mean ± SD | 225 (21.1) | 48 (19.3) | 88 (31.2) | 40 (15.6) | 49 (17.6)* |
| (L/min) | 208±61.1 | 211±59.8 | 192±57.7** | 226±66.4 | 204±56.1** |
| NIOX (FeNOa) | |||||
| FeNO ≥20 parts per billion (ppb) | 174 (16.3) | 57 (22.9) | 32 (11.3) | 48 (18.8) | 37 (13.3)** |
| Mean ± SD (ppb) | 13.3±14.9 | 14.7±15.6 | 11.9±14.7 | 14.2±16.1 | 12.5±13.2 |
| Median household income | |||||
| Mean ± SD | 43,779±13,620 | 38,415±12,448 | 39,030±12,675 | 48,821±12,910 | 48,856±12,628 |
| Non-rail-yard-related diesel exposure (mobile and stationary sources) | |||||
| Mean ± SD (kg/day) | 6.51±1.33 | 6.11±1.34 | 6.23±1.91 | 6.85±0.81 | 6.83±0.68 |
Note. Some columns do not add to 100% because of missing data. For continuous outcomes, comparison by Student’s t-test. For categorical outcomes, comparison by Chi-square test.
PEF = peak expiratory flow; FeNO = fractional exhaled nitric oxide.
p < .01.
p < .001.
Associations Between Rail-Yard-Related Exposures and Respiratory Outcomes
Logistic regression results (Tables 2–4) for both schools combined showed that males had significantly greater odds (OR: 1.74, 95% CI: 1.20–2.54) of exhibiting elevated FeNO values than females. Males had significantly lower odds (OR: 0.69, 95% CI: 0.49–0.96), however, for exhibiting low PEF values (Table 2). In comparing the respiratory outcomes among the male students by school, students at the EES were at greater odds of elevated FeNO values and low PEF compared to the males at the CES; however, the results were not statistically significant (Table 3). When comparing only the females between the two schools, we found that the students at the EES had greater odds for elevated FeNO values and significantly greater odds for low PEF (OR: 2.24, 95% CI: 1.30–3.85). A within-school analysis comparing males with females was performed to assess the effect of increased proximity to the rail yard on the potential gender-related adverse respiratory health outcomes. At both schools, males had greater odds than females of exhibiting elevated FeNO values, within the adjusted models (Table 4). Males were less likely than females, however, to exhibit low PEF. These gender-related adverse respiratory health outcomes were magnified at the EES. For males, elevated FeNO values of increased odds of 1.66 vs. 1.93 were observed, as was a decrease in odds for low PEF from 0.83 to a significantly protective effect of 0.59.
TABLE 2.
Logistic Regression Modeling Results of Children Experiencing Adverse Respiratory Related Health Outcomes: Overall Males Compared to Females
| Result | All Subjects | Sensitivity Analysisc | |||||
|---|---|---|---|---|---|---|---|
| Crude | Adjustedd | Adjustedd | |||||
| Health outcomes | n | Events | ORb (95% CIb) | OR (95% CI) | n | Events | OR (95% CI) |
| PEFa <80% | 1,065 | 225 | 0.65 (0.48–0.88) | 0.69 (0.49–0.96) | 853 | 164 | 0.76 (0.53–1.08) |
| FeNOa ≥20 parts per billion | 1,052 | 174 | 1.87 (1.34–2.61) | 1.74 (1.20–2.54) | 843 | 133 | 1.73 (1.17–2.54) |
PEF = peak expiratory flow; FeNO = fractional exhaled nitric oxide.
OR = odds ratio; 95% CI = 95% confidence interval.
Sensitivity analysis included only subjects residing more than six months at their current address.
Model = sex (1 = males, 0 = females), school, age, race, environmental tobacco smoke, time spent outdoors, median household income, total diesel pollution, and proximity to nearest major road.
TABLE 4.
Logistic Regression Modeling Results of Children Experiencing Adverse Respiratory Related Health Outcomes: Gender-Specific Intraschool Comparison
| Variables | All Subjects | Sensitivity Analysise | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude | Adjustedd | Adjustedd | |||||||
| Gender | School | Health Outcomes | n | Events | ORc (95% CIc) | OR (95% CI) | n | Events | OR (95% CI) |
| Males with females | CESa only | PEFb <80% | 535 | 89 | 0.87 (0.55–1.37) | 0.83 (0.50–1.38) | 437 | 72 | 0.87 (0.51–1.47) |
| FeNOb ≥20 parts per billion (ppb) | 527 | 85 | 1.51 (0.95–2.41) | 1.66 (0.95–2.89) | 432 | 61 | 1.58 (0.89–2.80) | ||
| Males with females | EESa only | PEF <80% | 531 | 136 | 0.53 (0.35–0.79) | 0.59 (0.37–0.92) | 417 | 92 | 0.64 (0.39–1.04) |
| FeNO ≥20 ppb | 525 | 89 | 2.32 (1.45–3.72) | 1.93 (1.14–3.26) | 411 | 72 | 1.92 (1.12–3.32) | ||
EES = exposure elementary school; CES = control elementary school.
PEF = peak expiratory flow; FeNO = fractional exhaled nitric oxide.
OR = odds ratio; 95% CI = 95% confidence interval.
Model = sex (1 = males, 0 = females), age, race, environmental tobacco smoke, time spent outdoors, median household income, total diesel pollution, and proximity to nearest major road.
Sensitivity analysis included only subjects residing more than six months at their current address.
TABLE 3.
Logistic Regression Modeling Results of Children Experiencing Adverse Respiratory Related Health Outcomes: Gender-Specific Interschool Comparison
| Variables | All Subjects | Sensitivity Analysise | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude | Adjustedd | Adjustedd | |||||||
| Gender | School | Health Outcomes | n | Events | ORc (95% CI c) | OR (95% CI) | n | Events | OR (95% CI) |
| Males only | EESa with CESa | PEFb <80% | 504 | 88 | 1.29 (0.81–2.05) | 1.59 (0.80–3.17) | 403 | 69 | 1.42 (0.66–3.04) |
| FeNOb ≥20 parts per billion (ppb) | 497 | 105 | 1.29 (0.84–1.98) | 1.44 (0.75–2.77) | 399 | 78 | 1.55 (0.77–3.12) | ||
| Females only | EES with CES | PEF <80% | 561 | 137 | 2.13 (1.43–3.17) | 2.24 (1.30–3.85) | 451 | 95 | 1.73 (0.93–3.23) |
| FeNO ≥20 ppb | 554 | 69 | 0.84 (0.51–1.39) | 1.37 (0.73–2.58) | 451 | 55 | 1.35 (0.70–2.59) | ||
EES = exposure elementary school; CES = control elementary school.
PEF = peak expiratory flow; FeNO = fractional exhaled nitric oxide.
OR = odds ratio; 95% CI = 95% confidence interval.
Model = school (1 = EES, 0 = CES), age, race, environmental tobacco smoke, time spent outdoors, median household income, total diesel pollution, and proximity to nearest major road.
Sensitivity analysis included only subjects residing more than six months at their current address.
Discussion
In our study, we observed differing adverse respiratory health endpoints by gender. Moreover, in a region characterized by poor background-level air quality, both genders were at risk for experiencing adverse respiratory health outcomes. Compared to females, males were significantly more likely to exhibit elevated FeNO values, indicative of airway inflammation, but significantly less likely to experience low PEF. While males in both schools had greater odds of exhibiting elevated FeNO values, the gender-related differences in adverse respiratory health endpoints were enhanced for the school next to the rail yard; males were significantly more likely to exhibit elevation in FeNO values and females were more likely to exhibit reduced lung volume. Since previous studies lacked consistent results on gender-related adverse health outcomes associated with air pollutants, our study provides further support for the studies supporting the existence of gender-related differences in adverse respiratory outcomes associated with air pollutants.
Unique Exposure Environment/Setting
Our data suggest that the unique setting of the San Bernardino area, with the air pollutants transported from the Los Angeles basin by the prevailing winds, along with exposure to a major local rail yard source, could enhance potential gender differences in respiratory health outcomes. Recent evidence indicates that the concentration of quinones, the key agents in the toxicity of polycyclic aromatic hydrocarbons (PAHs), increases significantly in an eastward direction from Santa Monica/Long Beach toward the inland valleys of Southern California, and that 90% of these organic compounds measure at receptor sites inland are generated photolytically during atmospheric transport (Eiguren-Fernandez et al., 2008). Both the EES and CES schools are exposed to the toxically enhanced organic species as they travel inland, which our data suggest indeed impact the respiratory health of students. In addition, students at the EES are exposed to a local source of air pollution from this major freight rail yard, thus further exacerbating the respiratory health impacts experienced by inland area students.
Of note, the constituents in the rail yard–related emissions are likely to differ from those in traffic-related air pollution. Railyard emissions are associated with combustion from diesel engines as well as other emission sources associated with the specific daily functions of the rail yard (e.g., arriving and departing trains, fueling of equipment and trains, maintenance) (ENVIRON International, 2007). It has been shown that the diesel engines emit large quantities of fine particulate matter, PAHs, volatile organic carbons, carbon dioxide, carbon monoxide, and nitrogen oxides. Diesel exhaust also contains other carcinogens including benzene, formaldehyde, and arsenic. It’s therefore possible that EES pollutants may differ in their chemical composition from those associated with roadway traffic. The arrival and departure of trains may produce shavings of tiny metal particles as metal-to-metal friction is taking place. Indeed, a study conducted of the subway system in New York City has identified a microenvironment for heavy metal exposures with higher iron, manganese, and chromium in airway samples due to steel dust exposure (Chillrud et al., 2005). Additionally, another study has found gender-related differences in adverse health responses linked to heavy metal exposures (Llop, Lopez-Espinosa, Rebagliato, & Ballester, 2013; Vahter, Akesson, Liden, Ceccatelli, & Berglund, 2007).
Implications of Study Findings
The findings from our study have a number of implications especially for school and local health professionals in areas with air quality issues, but also for key government officials and local institutions. Local health professionals should be aware of the potential impact on the respiratory health of residents living in regions with high background levels of air pollutants, especially with the added burden of exposure to rail-yard-associated emissions and that these adverse respiratory health endpoints may differ by gender.
Given the fact that air pollution has been shown to promote initial development of asthma in children, routine asthma screening should be offered to all children attending schools in areas burdened with complex air pollution (McConnell et al., 2010). Health professionals have a responsibility to consider the impact of these environmental conditions on growing children, especially on children from low-income minority households who often already live stressful lives and are, according to emerging evidence, at even greater risk of adverse respiratory health effects (Islam et al., 2011; Shankardass et al., 2009). Chen and co-authors (2008) have reported that chronic traffic-related air pollution and stress interact to predict biologic and clinical outcomes in asthma that are stronger than either factor alone. Proximity to a major air emissions source like the rail yard may further increase difficulty in asthma management for children complicated by differential gender presentation. A better understanding of the gender-related difference in response to different types of air pollutant exposure may lead to novel prevention and treatment strategies for children residing in areas with multisource air pollution.
In addition to implications for school and health officials, implications also exist for government officials and institutional agencies serving these high-impact areas. Indeed, the needs of these types of affected communities should be addressed by taking a Health in All Policies (HiAP) approach (National Association of County and City Health Officials, 2013; Spencer-Hwang et al., 2014). Coordinated efforts should focus on responsive policy development and implementation to ensure beneficial effects moving toward prevention, as well as delivery of health services to address already emerging needs within the community. As imports into the U.S. continue to grow and expansion of inland ports is a reality, results of this study should provide caution toward protecting children who are among the most vulnerable of the affected communities and allow them a chance at an environment where they can live and thrive (Landrigan & Etzel, 2014).
Strengths and Limitations
Our study has a number of strengths that merit discussion. Rather than merely relying on the self-reporting of individuals participating in the study, biological measurements were obtained, including tests on airway inflammation (FeNO) as well as lung function (PEF). The comparison school was sociodemographically matched to the exposure school to allow for a robust comparison. The schools were in close proximity to each other and therefore subject to virtually the same levels of regional air pollution, allowing us to assess the additional rail-yard-related risk in an environment already burdened by poor background-level air quality. Finally, a high overall study participation rate (74%) allowed us to obtain a large sample of participants for whom we had complete data.
Our research also had some limitations. One, school location, rather than actual personal exposure measurements, was used as a surrogate of exposure. Additionally, due to the cross-sectional design of our study, a causal association between FeNO and PEF outcomes and exposure to DPM between CES and EES students cannot be established. Future research studies should attempt to collect individual longitudinal exposures on children as has been done in adults (Spira-Cohen, Chen, Kendall, Lall, & Thurston, 2011; van Roosbroeck et al., 2006), such as having participants carry personal monitoring equipment for an extended period of time. Another limitation was the difficulty of isolating the exposures to rail-yard-related (on-site) emissions given the presence of other (off-site) local sources of pollution. The 2008 CARB report has documented, however, that the SBR accounts for 66% of the combined on-site and off-site DPM emissions and adjustments were made to take that into account in our model.
Conclusion
In summary, emerging epidemiological evidence indicating the existence of gender-related differences in adverse respiratory health outcomes is supported by the findings from this research study (Brunekreef et al., 1997; Delfino et al., 2004; Llop et al., 2013; Vahter et al., 2007). We also found that increased exposure to air pollution from a major goods movement rail yard additionally contributes (adversely) to enhancing gender-related respiratory health outcomes, even in an area plagued with poor background air quality. Further research is warranted to better understand the impact of air pollution on the gender-related respiratory differences and for potential development of novel prevention and treatment strategies.
Acknowledgments
This research was funded by the South Coast Air Quality Management District/BP West Coast Products Oversight Committee, LLC, grant #659005 also supported by NIH #1P20MD006988. We would like to thank the Arrowhead Regional Center Breathmobile for kindly collaborating with Project ENRRICH to assist in screening the children and the Aerocrine Corporation for their technical assistance and donation of additional NIOX tests. We are also grateful to Dr. Xinqiu Zhang from the South Coast Air Quality Management District for kindly providing the Multiple Air Toxics Exposure Study-III emissions data used in the analyses.
Contributor Information
Rhonda Spencer-Hwang, School of Public Health, Loma Linda University.
Sam Soret, School of Public Health, Loma Linda University.
Mark Ghamsary, School of Public Health, Loma Linda University.
Nico Rizzo, School of Public Health, Loma Linda University.
Marti Baum, Department of Pediatrics, Loma Linda University Medical Center.
David Juma, School of Public Health, Loma Linda University.
Susanne Montgomery, School of Behavioral Health, Loma Linda University.
References
- Brunekreef B, Janssen NA, de Hartog J, Harssema H, Knape M, van Vliet P. Air pollution from truck traffic and lung function in children living near motorways. Epidemiology. 1997;8(3):298–303. doi: 10.1097/00001648-199705000-00012. [DOI] [PubMed] [Google Scholar]
- Castaneda H, Yang E, Mahmood A, Cutts S, Mitchell A, Dolney N, Schwehr B, Servin A, Zelinka S. Health risk assessment for the BNSF San Bernardino rail yard. 2008 Retrieved from http://www.arb.ca.gov/railyard/hra/bnsf_sb_final.pdf.
- Centers for Disease Control and Prevention. Children’s BMI tool for schools. 2011 Retrieved from http://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/tool_for_schools.html.
- Chen E, Schreier HM, Strunk RC, Brauer M. Chronic traffic-related air pollution and stress interact to predict biologic and clinical outcomes in asthma. Environmental Health Perspectives. 2008;116(7):970–975. doi: 10.1289/ehp.11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chillrud SN, Grass D, Ross JM, Coulibaly D, Slavkovich V, Epstein D, Sax SN, Pederson D, Johnson D, Spengler JD, Kinney PL, Simpson HJ, Brandt-Rauf P. Steel dust in the New York City subway system as a source of manganese, chromium, and iron exposures for transit workers. Journal of Urban Health. 2005;82(1):33–42. doi: 10.1093/jurban/jti006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clougherty J. A growing role for gender analysis in air pollution epidemiology. Environmental Health Perspectives. 2010;118(2):167–176. doi: 10.1289/ehp.0900994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Quintana PJ, Floro J, Gastanaga VM, Samimi BS, Kleinman MT, Liu LJ, Bufalino C, Wu CF, McLaren CE. Association of FEV1 in asthmatic children with personal and microenvironmental exposure to airborne particulate matter. Environmental Health Perspectives. 2004;112(8):932–941. doi: 10.1289/ehp.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor DR American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels for Clinical Applications. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. American Journal of Respiratory and Critical Care Medicine. 2011;184(5):602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dweik RA, Sorkness RL, Wenzel S, Hammel J, Curran-Everett D, Comhair SA, Bleecker E, Busse W, Calhoun WJ, Castro M, Chung KF, Israel E, Jarjour N, Moore W, Peters S, Teague G, Gaston B, Erzurum SC National Heart, Lung, and Blood Institute Severe Asthma Research Program. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. American Journal of Respiratory and Critical Care Medicine. 2010;181(10):1033–1041. doi: 10.1164/rccm.200905-0695OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiguren-Fernandez A, Miguel A, Di Stefano E, Schmitz D, Cho A, Thurairatnam S, Avol E, Froines J. Atmospheric distribution of gas- and particle-phase quinones in southern California. Aerosol Science and Technology. 2008;42(10):854–861. [Google Scholar]
- ENVIRON International. Air dispersion modeling assessment of air toxic emissions from BNSF San Bernardino rail yard. 2007 Retrieved from http://www.arb.ca.gov/railyard/hra/env_sb_admrpt.pdf.
- Fox MA. Evaluating cumulative risk assessment for environmental justice: A community case study. Environmental Health Perspectives. 2002;110(Suppl 2):203–209. doi: 10.1289/ehp.02110s2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring U, Cyrys J, Sedlmeir G, Brunekreef B, Bellander T, Fischer P, Bauer CP, Reinhardt D, Wichmann HE, Heinrich J. Traffic-related air pollution and respiratory health during the first two years of life. European Respiratory Journal. 2002;19(4):690–698. doi: 10.1183/09031936.02.01182001. [DOI] [PubMed] [Google Scholar]
- Hwang BF, Chen YH, Lin YT, Wu XT, Leo Lee Y. Relationship between exposure to fine particulates and ozone and reduced lung function in children. Environmental Research. 2015;137:382–390. doi: 10.1016/j.envres.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Islam T, Urman R, Gauderman WJ, Milam J, Lurmann F, Shankardass K, Avol E, Gilliland F, McConnell R. Parental stress increases the detrimental effect of traffic exposure on children’s lung function. American Journal of Respiratory and Critical Care Medicine. 2011;184(7):822–827. doi: 10.1164/rccm.201104-0720OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Etzel RA. Children’s environmental health. New York: Oxford University Press; 2014. [Google Scholar]
- Llop S, Lopez-Espinosa MJ, Rebagliato M, Ballester F. Gender differences in the neurotoxicity of metals in children. Toxicology. 2013;311(1–2):3–12. doi: 10.1016/j.tox.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Luginaah I, Fung K, Gorey K, Webster G, Wills C. Association of ambient air pollution with respiratory hospitalization in a government-designated area of concern: The case of Windsor, Ontario. Environmental Health Perspectives. 2005;113(3):290–296. doi: 10.1289/ehp.7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R, Berhane K, Yao L, Jerrett M, Lurmann F, Gilliland F, Kunzli N, Gauderman J, Avol E, Thomas D, Peters J. Traffic, susceptibility, and childhood asthma. Environmental Health Perspectives. 2006;114(5):766–772. doi: 10.1289/ehp.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R, Islam T, Shankardass K, Jerrett M, Lurmann F, Gilliland F, Gauderman J, Avol E, Kunzli N, Yao L, Peters J, Berhane K. Childhood incident asthma and traffic-related air pollution at home and school. Environmental Health Perspectives. 2010;118(7):1021–1026. doi: 10.1289/ehp.0901232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Association of County and City Health Officials. Health in all policies. 2013 Retrieved from http://www.naccho.org/topics/environmental/HiAP/
- Newcomb P, Li J. Predicting admissions for childhood asthma based on proximity to major roadways. Journal of Nursing Scholarship. 2008;40(4):319–325. doi: 10.1111/j.1547-5069.2008.00245.x. [DOI] [PubMed] [Google Scholar]
- Ospital J, Cassmassi J, Chico T. Multiple air toxics exposure study in the south coast air basin: MATES-III. Final report. 2008 Retrieved from http://www.aqmd.gov/home/library/air-quality-data-studies/health-studies/mates-iii/mates-iii-final-report.
- Peters JM, Avol E, Gauderman WJ, Linn WS, Navidi W, London SJ, Margolis H, Rappaport E, Vora H, Gong H, Jr, Thomas DC. A study of twelve southern California communities with differing levels and types of air pollution. II. Effects on pulmonary function. American Journal of Respiratory and Critical Care Medicine. 1999;159(3):768–775. doi: 10.1164/ajrccm.159.3.9804144. [DOI] [PubMed] [Google Scholar]
- Pope CA., 3rd Epidemiology of fine particulate air pollution and human health: Biologic mechanisms and who’s at risk? Environmental Health Perspectives. 2000;108(Suppl 4):713–723. doi: 10.1289/ehp.108-1637679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux CL, Gute DM, Brugge D, Peterson S, Parmenter B. Characterizing urban traffic exposures using transportation planning tools: An illustrated methodology for health researchers. Journal of Urban Health. 2010;87(2):167–188. doi: 10.1007/s11524-009-9419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Martinez R, Perez-Padilla R, Olaiz-Fernandez G, Mendoza-Alvarado L, Moreno-Macias H, Fortoul T, McDonnell W, Loomis D, Romieu I. Lung function growth in children with long-term exposure to air pollutants in Mexico City. American Journal of Respiratory and Critical Care Medicine. 2007;176(4):377–384. doi: 10.1164/rccm.200510-1678OC. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Air pollution and children’s health. Pediatrics. 2004;113(4 Suppl):1037–1043. [PubMed] [Google Scholar]
- Shankardass K, McConnell R, Jerrett M, Milam J, Richardson J, Berhane K. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proceedings of the National Academy of Sciences of the USA. 2009;106(30):12406–12411. doi: 10.1073/pnas.0812910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Hwang R, Montgomery S, Dougherty M, Valladares J, Rangel S, Gleason P, Soret S. Experiences of a rail yard community: Life is hard. Journal of Environmental Health. 2014;77(2):8–17. [PMC free article] [PubMed] [Google Scholar]
- Spira-Cohen A, Chen LC, Kendall M, Lall R, Thurston GD. Personal exposures to traffic-related air pollution and acute respiratory health among Bronx schoolchildren with asthma. Environmental Health Perspectives. 2011;119(4):559–565. doi: 10.1289/ehp.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M, Akesson A, Liden C, Ceccatelli S, Berglund M. Gender differences in the disposition and toxicity of metals. Environmental Research. 2007;104(1):85–95. doi: 10.1016/j.envres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- van Roosbroeck S, Wichmann J, Janssen NA, Hoek G, van Wijnen JH, Lebret E, Brunekreef B. Long-term personal exposure to traffic-related air pollution among school children, a validation study. Science of the Total Environment. 2006;368(2–3):565–573. doi: 10.1016/j.scitotenv.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Meng YY, Rull RP, English P, Balmes J, Ritz B. Environmental public health tracking of childhood asthma using California health interview survey, traffic, and outdoor air pollution data. Environmental Health Perspectives. 2008;116(9):1254–1260. doi: 10.1289/ehp.10945. [DOI] [PMC free article] [PubMed] [Google Scholar]