Abstract
To study the genetic basis of heat tolerance at anthesis, a set of chromosome segment substitution lines (CSSLs) derived from Sasanishiki (japonica ssp. heat susceptible) and Habataki (indica spp. heat tolerant) were used for analysis across three high temperature environments. Spikelet fertility (SF), daily flowering time (DFT) and pollen shedding level (PSL) under high temperature (HT) were assessed. Eleven related QTLs were detected, of which, two QTLs qSFht2 and qSFht4.2 for spikelet fertility were identified on chromosomes 2 and 4. Four QTLs qDFT3, qDFT8, qDFT10.1 and qDFT11 for daily flowering time were detected on chromosomes 3, 8, 10 and 11. The other five QTLs qPSLht1, qPSLht4.1, qPSLht5, qPSLht7 and qPSLht10.2 on chromosomes 1, 4, 5, 7 and 10, respectively, were found had effects both on spikelet fertility and pollen shedding level. Of the 11 QTLs, 8 were overlapped with QTLs reported by others, 3 QTLs qPSLht4.1, qPSLht7 and qPSLht10.2 identified in this study were novel. The stability of qPSLht4.1 was further verified at different temperatures, which could be used to improve the pollen shedding and pollen growth on stigma for rice heat-tolerance breeding.
Keywords: rice (Oryza sativa L.), heat tolerance, anthesis stage, CSSLs, QTL, qPSLht4.1
Introduction
Rice is one of the most important food crops in the world (Khush 2005). The high yield of rice plays an important role in food security. However, the high frequency of drought, floods, plant diseases, insect pests and high temperature stress have brought a huge challenge for rice production (Khush 1999, Zhang 2007). Due to the influence of human activities, global warming has become a big concern (Crowley 2000). Over the past 100 years, the global average temperature has increased by 0.6°C and is projected to continue to rise at a rapid rate, with another 0.5–2.8°C increase predicted by the end of 21st century (Meehl et al. 2005, Van Vuuren et al. 2008). Therefore, breeding more rice varieties incorporated with heat tolerance is necessary to cope with the future climate change.
Rice responses to high temperature differ with the developmental stages. On the whole, the reproductive stage, especially the flowering stage, is the most sensitive stage to high temperature (Satake and Yoshida 1978). High temperature over 35°C at anthesis and lasting for more than 1h causes high spikelet sterility, leading to seriously yield losses (Jagadish et al. 2007). It has been proved that the poor anther dehiscence is the main cause of spikelet sterility induced by high temperature, because poor anther dehiscence results into few germinated pollen grains on the stigma (Jagadish et al. 2010b, Matsui and Omasa 2002, Prasad et al. 2006). In addition, daily flowering time is also shown to be connected with the heat tolerance. The varieties which carrying the Early-Morning Flowering (EMF) trait can avoid the high temperature at anthesis thereby mitigating high temperature-induced spikelet sterility (Ishimaru et al. 2010). The QTL qEMF3, identified recently from wild rice Oryza officinalis, showed the potential to shift flower opening time of cultivars to earlier in the morning (Hirabayashi et al. 2015).
To uncover the genetic basis of heat tolerance at flowering stage in rice, several independent studies have been conducted and many quantitative trait loci (QTLs) have been identified (Ye et al. 2012). However, there are few reports about the confirmation and fine mapping of the identified QTL for heat tolerance. It is known that the mapping populations and accurate phenotyping technology are essential for QTL mappings (Ashikari and Matsuoka 2006, Tuberosa 2012). Although many kinds of mapping populations, including DH, RILs, F2, BC1F1 and BIL population have been widely used to detect the QTLs associated with heat tolerance (Ye et al. 2012), CSSLs and near-isogenic lines (NILs) are rarely used. As a permanent mapping population, these two kinds of populations are ideally suited for quantitative traits QTL detection (Ashikari and Matsuoka 2006).
In general, indica rice cultivars show much better heat-tolerance than japonica cultivars. To explore QTLs controlling heat-tolerance at anthesis, a set of CSSLs derived from the backcross between indica donor cultivar ‘Habataki’ and japonica recipient cultivar ‘Sasanishiki’ were employed for studying the traits across three environments. As to the evaluation index for heat tolerance identification, spikelet fertility or some related secondary traits have been usually used. Little attention was paid to seeking other traits associated with high temperature stress. In this study, three traits i.e. spikelet fertility, daily flowering time and pollen shedding level were introduced for identification of heat tolerance. Some main effect QTLs were detected and the overlapping QTLs from the present study were compared with other researches. The results would provide a clue to understand the genetics of rice heat tolerance at flowering stage.
Materials and Methods
Plant materials
Sasanishiki, an elite japonica variety, was selected as the recurrent parent, and Habataki, an indica elite variety, was used as the donor. The development of CSSLs through backcrossing and simple sequence repeat (SSR) marker selection has been described previously by Ando et al. (2008). Of all the 39 CSSLs, 37 lines (SL401–SL437) were used in this investigation, except SL438 and SL439, which showed very low seed-set percentage under normal growth conditions. Each CSSL contained a major segment and a variable number of minor segments inherited from Habataki in the Sasanishiki genetic background. The selected CSSLs population covered most of the genome, except for four small regions on chromosomes 4 (defined by Bb38P21a), 8 (defined by RM1148), 10 (defined by RM7492) and 12 (defined by RM6998 and RM2197), as described by Ando et al. (2008).
Field experiment
The 33 CSSLs (SL401–SL433) and their parents, Sasanishiki and Habataki, were sown at Nanchang, Jiangxi Province, China on May 15, 2013, and transplanted in a randomized complete-block design. Each plot consisted of ten rows separated by 30 cm, with each row consisting of 8 plants, separated by 20 cm, and the management of the field experiments was conducted according to the normal procedures for rice. At maturity, 15 panicles were harvested for seed setting rate investigation.
Cement floor experiment
In 2014, 37 CSSLs (SL401–SL437) and their parents were also planted at Nanchang (Jiangxi province, China) with three sowing dates (May 18, May 25 and June 1). The field experiment and management were consisted with that of 2013. However, at booting stage 10 plants of each CSSL were re-transplanted to plastic boxes (32 cm × 23 cm) filled with paddy soil, one box with two plants. After that, potted plants were transferred to the cement roof of the laboratory on the third floor. To avoid the high temperature stress before anthesis, shade nets were used to cover the plants at a height of 3m from the floor. The plants were watered every 2–3 days to avoid soil drought. Spraying water to the cement floor every early morning and evening was conducted to lower the ambient temperature.
Air temperature curve was measured with a copper-constantan thermocouple (Zeda Instruments Co. LTD, Hangzhou, China). On August 5, all of CSSLs and the two parents reached full heading stage, and the daily maximum temperature of the cement floor was above 42°C. Then two plastic boxes of each line were moved to another place of the same floor without the shade net in the afternoon after 16:00.
15 panicles per each line which just started blooming were chosen for experiment in the next day, August 6. The spikelets flowered before or after high temperature stress were all carefully removed on the afternoon of August 5 and August 6, respectively. The experiment was repeated another time in August 7 with the same design. Plants were moved to the shade net and raised for 3d, and then transferred back to the field.
Verfication test
Materials (Sasanishiki and SL412) were planted in the field before and after high temperature stress trials. In order to imitate the natural flowering patterns, the plants were moved to climatic chambers at about 10:00 to 11:00 before flowering. We planted the materials in late seasons to avoid heat stress in August. The spikelets which have flowered were cut off on the afternoon of the first day. After flowering on the second day, spikelets which have not flowered were also cut out. Thus, all of the retained spikelets have undergone the heat stress and the seed setting rate was examined.
Daily flowering time
The daily flowering time was determined for all CSSLs and two parents in cement floor experiment. The spikelets were considered open when anthers protruded from the glumes. Anthesis timing was investigated for 2 days starting from 6:00 to 14:00 on August 6 and August 7. Data from field and shade net were not collected due to the low variation of flowering patterns in the mapping population.
Pollen shedding level
To examine the dehiscence of anthers affected by the high temperature, the pollen shedding level was introduced as the identification indicator. In the cement floor experiment, pollen shedding level was investigated by eyes after flowering later than 14:00. The observation was repeated in two days, August 6 and August 7. A total of five grades of pollen shedding level were classified according to the anther appearances and shapes after flowering: I) all of anthers are white and not curly; II) the majority of anthers are white, no anthers curly; III) the majority of anthers are white, a portion of anthers curly; IV) a portion of anthers are yellow and curly; V) the majority of anthers are yellow and curly.
Spikelet fertility
Spikelet fertility was calculated by the ratio of number of filled grains and total number of observed florets. The spikelet fertility under different environment was used as indicator of tolerance to high temperature for the QTL mapping.
Microscopic observations
To examine the pollen tube growth, at least 10 pistils from spikelets which have been flowered were collected from 13:00 to 15:00, and fixed in Carnoy’s solution. As described by Endo et al. (2009), the pistils were transferred to 1N NaOH, incubated at 56°C for at least 6–10 min and then placed on the glass slide for observation. Pistils were carefully washed using purified water, subsequently stained with 2% aniline blue. Images were captured under UV illumination.
Statistical analysis
The linkage map utilized consisted of 166 DNA markers covering the 12 chromosomes, as described by (Ando et al. 2008). Means for each replication in three environments were calculated for each trait and used in data analysis. The existence of a QTL was declared when the average value of a trait was significantly different between a CSSL and the recurrent parent, Sasanishiki, according to one-way analysis of variance at a probability level of 0.001. From the identity of the CSSLs, QTLs could be assigned to the substituted chromosomal segments. When QTLs were detected on overlapping chromosome segments in multiple CSSLs, their location could be narrowed down. Otherwise, the QTL could be located on the non-overlapping chromosome segments (Paterson et al. 1990). The QTL nomenclature followed the recommendations of (McCouch 2008). Correlation analysis was performed to detect the association between the spikelet fertility of CSSLs population in three environments using SPSS software. The additive effect of each QTL was also calculated (Eshed and Zamir 1995). The positive additive effect indicates that Habataki contributes to the positive allele, whereas the negative additive effect indicates that positive allele is contributed by Sasanishiki.
Results
High temperature treatment at the flowering stage
To preliminarily evaluate the heat tolerance variation of CSSLs derived from Habataki and Sasanishiki, the field experiment was conducted in 2013, Nanchang. Most of the CSSLs headed at the period on August 7 to August 15, when the daily maximum temperature varied from 35°C to 39°C, and the high temperature of 39°C lasted more than one week (Supplemental Fig. 1a). Considering the temperature fluctuations and heading dates differ from CSSLs, three sowing dates were arranged in 2014. However, extraordinary weather occurred during heading stage, the frequent rainy and low temperature made it difficult to identify the phenotype under heat stress. Thus, the cement floor experiment was carried out on 6 August and 7 August. The temperature was increased by 5–7°C under the cement floor environment compared to the field at flowering time (10:00 to 14:00). In this period, the cement floor temperature varied from 36°C to 43°C on 6 August, and 35.8°C to 41.4°C on 7 August, while the maximum temperature in the field was not above 36°C in both of the two days (Supplemental Fig. 1b). Moreover, the temperature rising and falling were more rapid in cement floor experiment than in the field (Supplemental Fig. 1b), which made it effective to create a high temperature environment in flowering time and recover to a normal temperature after flowering.
Performances of CSSLs and their parents under high temperature
The spikelet fertility under normal temperature was not listed, because SL401–SL437 and two parents showed quite high seed setting percentages over the past years. The significant differences of spikelet fertility between two parents were exhibited under high temperature in three environments (Table 1). The donor parent Habataki showed good spikelet fertility under three environments, however, the recurrent parent Sasanishiki showed sharp reduction of seed setting percentages. In the field experiment of 2013, the highest and the lowest temperatures (39°C and 29°C, respectively) lasted for nearly two weeks covering flowering stage. The spikelet fertility of Sasanishiki was less than 10%, while Habataki showed 83.7%. The value of CSSLs ranged from 2.7% to 39.1% with the mean of 20.1%. The similar variations were also observed in 2014 (Table 1, Supplemental Fig. 2). The CSSL population segregation for spikelet fertility was also examined, which distributed continuously in the three environments (Fig. 1a, 1b). The significant positive correlations of spikelet fertility between three environments were also observed (Supplemental Table 1). These results indicated that the indica variety Habataki contained several segments which could enhance heat tolerance of the japonica variety Sasanishiki to different extents.
Table 1.
Phenotype data of spikelet fertility under high temperature for CSSLs and parents (Sasanishiki and Habataki) across three environments
| Environment | Parents | CSSLs population | |||
|---|---|---|---|---|---|
|
|
|
||||
| Sasanishiki (%) | Habataki (%) | Mean (%) | Min (%) | Max (%) | |
| 2013 | 8.4 ± 3.9 | 83.7 ± 6.5** | 20.1 | 2.7 | 39.1 |
| 2014/8/6 | 42.3 ± 6.1 | 87.3 ± 5.7** | 74.7 | 9.0 | 88.3 |
| 2014/8/7 | 51.6 ± 1.9 | 92.3 ± 12.1** | 84.6 | 19.4 | 93.0 |
mean the significance levels of 0.01 between Habataki and the recurrent parent Sasanishiki.
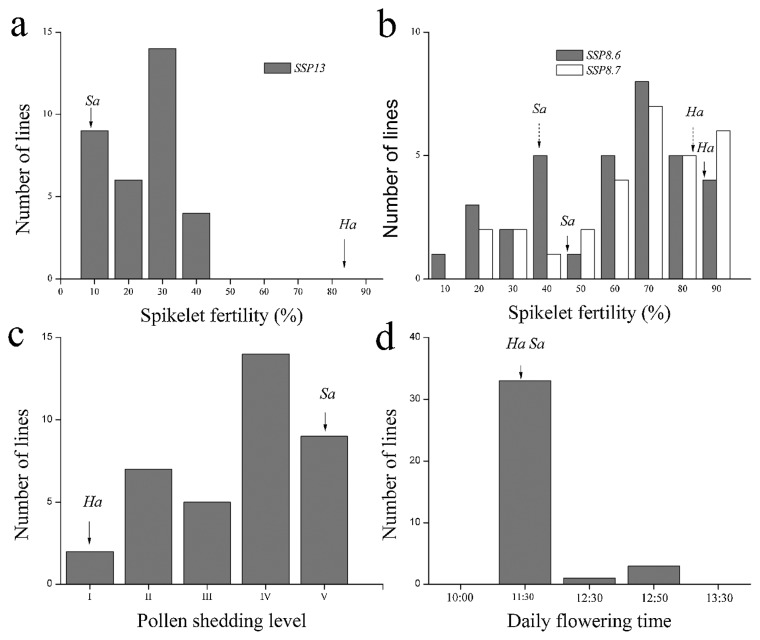
Fig. 1.
Frequency distributions of spikelet fertility, pollen shedding level and daily flowering time of the CSSLs population in 2013 (a) and 2014 (b, c and d). SSP13, spikelet fertility in 2013; SSP8.6 and SSP8.7 means spikelet fertility of 2014/8/6 and 2014/8/7 experiments, respectively; Spikelet fertility of parents in two experiments were distinguished by solid arrow (2014/8/7) and dotted arrow (2014/8/6) in Fig. 1b; Ha, Habataki; Sa, Sasanishiki.
The pollen shedding level (PSL) showed continuously distribution and nearly 90% CSSLs exhibited better PSL than Sasanishiki. The rest 10% CSSLs exhibited obviously improved PSL under high temperature, which showed I or II level (Fig. 1c). For daily flowering time (DFT), the two parents showed no significant differences. However, the starting time of flowering was delayed more than 1h in 4 CSSLs, SL409, SL428, SL433 and SL435 (Fig. 1d, Table 2).
Table 2.
QTLs for spikelet fertility under high temperature detected in Sasanishiki/Habataki CSSLs population across three environments
| QTL | Chr. | Flanking maker interval | SF (%) | Add (a) (%) | Environment | CSSL | DFT | PSL |
|---|---|---|---|---|---|---|---|---|
| qSFht2 | 2 | RM1234–RM3850 | 30.7 | 11.1 | 2013 | SL407 | 11:30 | IV |
| 80.7 | 6.8 | 2014/8/6 | ||||||
| 80.8 | 5.3 | 2014/8/7 | ||||||
| qPSLht4.1 | 4 | RM7585–Bb38P21a | 37.9 | 14.7 | 2013 | SL412 | 11:30 | II |
| 87.0 | 9.9 | 2014/8/6 | ||||||
| 93.0 | 11.3 | 2014/8/7 | ||||||
| qSFht4.2 | 4 | RM3916–RM2431 | 39.1 | 15.3 | 2013 | SL414 | 11:30 | IV |
| 82.0 | 7.4 | 2014/8/6 | ||||||
| 85.0 | 7.3 | 2014/8/7 | ||||||
| qPSLht5 | 5 | RM1248–RM4915 | 28.9 | 10.2 | 2013 | SL415 | 11:30 | I |
| 83.3 | 8.1 | 2014/8/6 | ||||||
| 86.8 | 8.3 | 2014/8/7 | ||||||
| qDFT3 | 3 | RM3766–RM3513 | 14.0 | −26.5 | 2014/8/6 | SL409 | 12:50 | V |
| 20.0 | −25.2 | 2014/8/7 | ||||||
| qDFT8 | 8 | RM5891–RM4997 | 31.0 | −18.1 | 2014/8/6 | SL428 | 12:30 | IV |
| 42.0 | −14.2 | 2014/8/7 | ||||||
| qDFT10.1 | 10 | RM6737–RM6673 | 16.0 | −25.6 | 2014/8/6 | SL433 | 12:50 | V |
| 21.1 | −24.6 | 2014/8/7 | ||||||
| qDFT11 | 11 | RM1355–RM2191 | 9.0 | −29.1 | 2014/8/6 | SL435 | 12:50 | V |
| 19.4 | −25.5 | 2014/8/7 | ||||||
| qPSLht1 | 1 | RM1196–RM6581 | 24.5 | 8.0 | 2013 | SL402 | 11:30 | I |
| 32.3 | −17.4 | 2014/8/6 | ||||||
| 20.0 | −25.2 | 2014/8/7 | ||||||
| qPSLht7 | 7 | RM6394–RM1364 | 79.0 | 5.9 | 2014/8/6 | SL423 | 11:30 | II |
| 78.4 | 4.1 | 2014/8/7 | ||||||
| 88.3 | 10.6 | 2014/8/6 | SL424 | 11:30 | II | |||
| 74.2 | 1.9 | 2014/8/7 | ||||||
| qPSLht10.2 | 10 | RM7492–RM1859 | 85.3 | 9.1 | 2014/8/6 | SL431 | 11:30 | II |
| 84.9 | 7.3 | 2014/8/7 |
SF, spikelet fertility; Add (a), additive effect, positive additive effect means Habataki allele increasing the trait values; DFT, daily flowering time; PSL, pollen shedding level.
QTL mapping
According to one-way analysis of variance (P ≤ 0.001), a total of 12 CSSLs exhibited significant spikelet fertility differences under at least two environments compared to Sasanishiki. Among them, 4 CSSLs SL407, SL412, SL414 and SL415 showed significant increase of spikelet fertility in three experiments (Table 2, Supplemental Fig. 2). Compared with Sasanishiki, the spikelet fertilities for 4 CSSLs SL409, SL428, SL433 and SL435 were significantly declined in two experiments of 2014; however, the related QTLs were not detected in 2013. One CSSL SL402 exhibited significant increase in 2013, while significant reduction was detected in two experiments of 2014. Moreover, the spikelet fertilities of three CSSLs SL423, SL424 and SL431 were significantly increased only in the experiments of 2014 (Table 2, Supplemental Fig. 2).
The flowering time of the three lines SL409, SL433 and SL435 was about 80 minutes later than that of Sasanishiki, while 1h delayed was observed in SL428. Additionally, these four lines all showed very bad pollen shedding level and significant reduction of spikelet fertility (Table 2). It is suggested that the poor pollen shedding of these lines might be caused by delayed flowering time, which led to a higher temperature at anthesis, and thus caused the spikelet fertility to decrease.
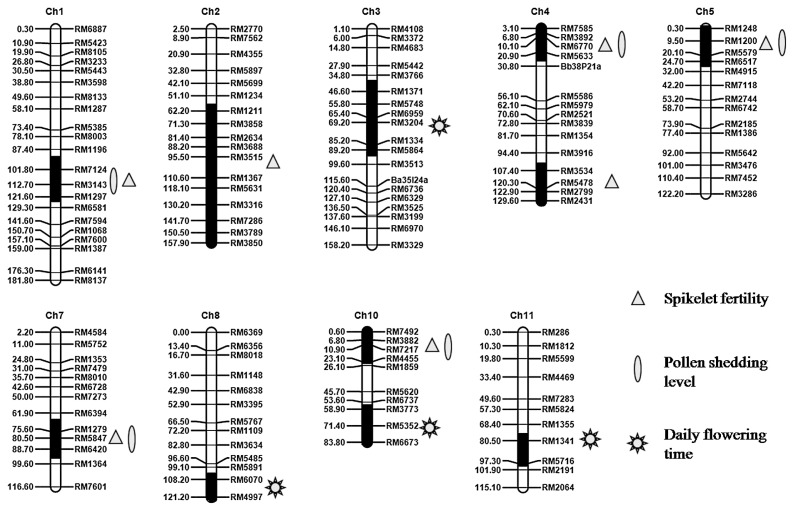
Moreover, the 6 CSSLs, SL402, SL412, SL415, SL423, SL424 and SL431 exhibited better pollen shedding level. It was noticed that SL423 and SL424 harbored one overlapping segment on chromosome 7, thus the QTL—qPSLht7 was limited to a region between RM6394 and RM1364. In addition, 5 QTLs (qPSLht1, qPSLht4.1, qPSLht5, qPSLht7 and qPSLht10.2) had effects on both spikelet fertility and pollen shedding level. The above 11 QTLs were further mapped on rice chromosome genetic map and distributed on nine rice chromosomes (Fig. 2).
Fig. 2.
Genetic linkage map for CSSLs population, showing location of QTLs for spikelet fertility, pollen shedding level and daily flowering time under high temperature. The black regions indicate the target QTL locations. The marker distance (cM) is presented on the left side of each chromosome, and the marker names are shown on the right side.
Validation of a large effect QTL qPSLht4.1
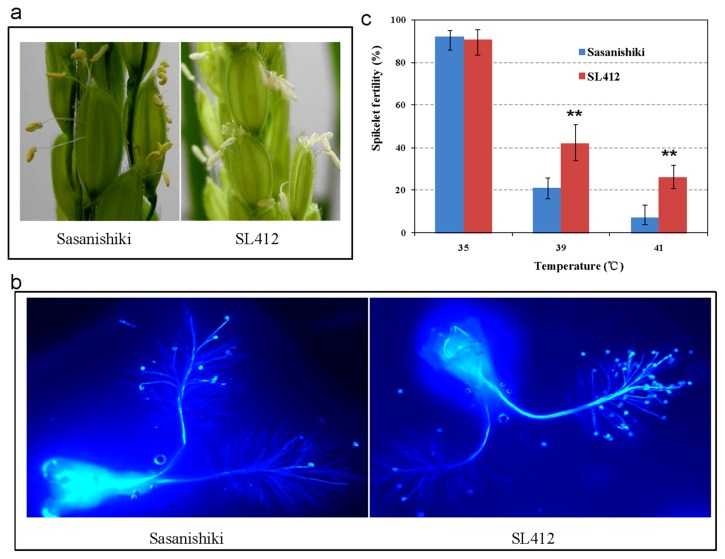
SL412, which was found to contain a large effect QTL— qPSLht4.1 for heat tolerance, showed obviously better spikelet fertility in all three high temperature experiments (Table 2, Supplemental Fig. 2). This line only carried one substituted segment on the short arm of chromosome 4 (RM7585-Bb38P21) compared to the recurrent parent, Sasanishiki (Ando et al. 2008). To investigate the potential physiological mechanisms for spikelet fertility increase contributed by qPSLht4.1, the anther properties and pollen germination on stigma were investigated. After high temperature treating in the cement floor experiments of 2014, the majority of anthers in Sasanishiki were yellow and curly after flowering (Fig. 3a), which meant there would be fewer number of pollens which dropped to the stigmas for fertilization. On the contrary, the pollens shed thoroughly and the anthers became white in SL412 (Fig. 3a). As expected, the pollen germination rate on stigma in SL412 was obviously higher than in Sasanishiki under two environments in 2014 (Fig. 3b). It is concluded that SL412 has good heat tolerance and the corresponding QTL—qPSLht4.1 has potential breeding value.
Fig. 3.
Comparison of heat tolerance between Sasanishiki and SL412. (a) Flowering of Sasanishiki and SL412 after high temperature treating. (b) Fewer (Sasanishiki) and more (SL412) germinated pollens on stigmas after high temperature treatment. (c) Comparision of spikelet fertility between SL412 and Sasanishiki exposed to temperatures of 35°C, 39°C and 41°C.
To further investigate qPSLht4.1 effect, the temperature gradient experiments were performed using manual climatic chambers. The big differences of spikelet fertility were emerged with temperature increasing. With the treatment under the moderate high temperature 35°C for one hour just before flowering, both of SL412 and Sasanishiki showed normal seed setting (Fig. 3c). When the temperature reached 39°C, sharp reduction of spikelet fertility was observed in both materials, but the seed-set percentage of SL412 reduced from the normal to 40.5%, about 20% higher than that of Sasanishiki (Fig. 3c). Furthermore, suffered from the extreme high temperature environment of 41°C, SL412 could maintain approximately 25% setting percentage, while the recurrent parent Sasanishiki was nearly sterile (Fig. 3c). The significant differences of spikelet fertility between SL412 and Sasanishiki can be repeatedly observed under various high temperature conditions, which suggested the stable effect of QTL—qPSLht4.1.
Discussion
Physiology mechanisms for heat tolerance at anthesis
Global warming has a significant effect on rice production. Though rice originates from the tropics, high temperatures of more than 35°C during the reproductive stages reduce rice production. Especially, during flowering period, high temperature causes low seed setting and low yield (http://irri.org/). One of the most popular viewpoint is that the reduced spikelet fertility is caused by lower pollen germination on stigma when suffered to heat stress at anthesis, and variation in spikelet fertility is highly correlated with the proportion of spikelets with 20 germinated pollen grains on the stigma (Jagadish et al. 2010b, Rang et al. 2011). In addition, high temperature can cause bad anther dehiscence, especially in heat sensitive varieties (Prasad et al. 2006, Satake and Yoshida 1978). The heat tolerant variety N22 possessed better anther dehiscence characters (Rang et al. 2011) and can maintain high pollen productions under high temperature (Prasad et al. 2006). It seemed that lower pollen germination on stigma was caused by reduced anther dehiscence, which led to decreased pollen production (Prasad et al. 2006, Rang et al. 2011). In this study, we reported five QTLs for pollen shedding level (Fig. 2). The 5 PSL QTLs showed potential in improving spikelet fertility of Sasanishiki. However, there are some exceptions: the two CSSLs SL407 and SL414 both had bad pollen shedding level during flowering under high temperature, whereas the spikelet fertilities were high; moreover, SL402 possessed good pollen shedding properties, but the spikelet fertility was found reduced in 2014 (Table 2). These results indicate that the anther dehiscence is not the only necessary factor in heat resistance and there are some other factors to consider as well.
QTL mapping for heat tolerance at anthesis
To better uncover the genetic mechanisms for heat tolerance at anthesis, we conducted repeated trails in three environments of high temperature. Four QTLs qSFht2, qPSLht4.1, qSFht4.2 and qPSLht5 were stably identified in three environments, showing the potential applications on improving heat tolerance at flowering stage. The two QTLs qPSLht7 and qPSLht10.2 that were only identified in 2014 could be used as candidates for heat tolerance improvement at anthesis. In addition, the QTL—qPSLht1 was found improved spikelet fertility in 2013, while reduced in 2014 experiments. This abnormality might be caused by stress escapes, the related CSSL–SL402 has been reported delayed about 3 days for heading (Ando et al. 2008).
Generally, the heat tolerant alleles tend to derived from heat tolerant parents; however, this is not always the case. Using 181 RILs derived from Bala (tolerant) × Azucena (suceptible), Jagadish et al. (2010a) identified 10 QTLs for spikelet fertility related traits under heat stress at anthesis. The most significant heat-responsive QTL—qtl1.1, contributed by Bala and explaining up to 18% of the phenotypic variation, while 3 positive QTLs were found donated by heat sensitive parent, Azucena (Jagadish et al. 2010a). Ye et al. (2012) identified two major heat tolerant QTLs (qHFSF1.1 and qHFSF4.1) at flowering stage. These two major QTL could explain 12.6% (qHTSF1.1) and 17.6% (qHTSF4.1) of the variation in spikelet fertility under high temperature. Tolerant allele of qHTSF1.1 was from the susceptible parent IR64, and that of qHTSF4.1 was from tolerant parent N22. In our research, the tolerant parent, Habataki, also donated negative alleles of four QTLs (qPSLht1, qDFT3, qDFT8, qDFT10.1 and qDFT11) (Table 2).
Previously, QTLs for heat tolerance at flowering stage have been mapped on all chromosomes except chromosome 6 and 7 by using various rice populations and high temperature treatment methods (Ye et al. 2012). In our study, qPSLht7 identified on chromosome 7 was a new site in QTL mapping for rice heat tolerance. By comparing the QTL locations reported by others, 8 QTLs identified in this study were overlapped with published QTLs (Supplemental Table 2). Further fine mapping and cloning of these QTLs will accelerate the breeding progress to improve the heat tolerance of new rice varieties. A large amount of heat tolerant putative QTLs identified in different mapping populations were overlapped or adjacent with each other (Supplemental Table 2), suggesting the heat tolerant alleles widely exist in cultivated rice. However, there are obvious differences on heat tolerance between the indica and japonica subspecies. It is likely that the cultivars have been suffered to various geographical environments selection pressures, thus leading to allelic variations.
The pleiotropy of heat tolerant QTLs
The heat tolerance is a complex quantitative trait, many environmental factors can influence the results of QTL mapping, such as humidity (Weerakoon et al. 2008), wind speed (Ishimaru et al. 2012), CO2 (Madan et al. 2012) and water stress (Rang et al. 2011). Therefore, improving the accuracy of phenotype identification is essential for map-based cloning. Spikelet fertility decrease was the most important heat sensitive indicator and was usually used for QTL mapping of heat tolerance (Ye et al. 2012). In fact, the spikelet fertility was just a final phenotype of heat injury. It might be controlled by several early traits which happened in the process of double fertilization, such as size of anther basal pores (Matsui and Kagata 2003), pollen production (Prasad et al. 2006), anther dehiscence (Rang et al. 2011) and pollen germination (Jagadish et al. 2010b). However, researches using these traits for QTL mapping were rare. In this study, eleven QTLs related to heat tolerance were detected, of which, five QTLs qPSLht1, qPSLht4.1, qPSLht5, qPSLht7 and qPSLht10.2 had effects both on spikelet fertility and pollen shedding level. Four daily flowering time QTLs qDFT3, qDFT8, qDFT10.1 and qDFT11 also had pleiotropic effects on spikelet fertility and pollen shedding level (Table 2, Fig. 2). It is possible that these QTLs should be useful for genetic improvement of heat tolerance (Table 2).
Flowering in the early morning is an interesting trait for heat tolerance, which can mitigate heat-induced spikelet sterility at the flowering stage by escaping heat stress during the daytime (Ishimaru et al. 2010). However, the underlying molecular mechanisms remain largely unknown. By using backcrossed inbred lines derived from a cross between O. rufipogon and O. sativa, QTLs for flower opening time have been detected on chromosomes 4, 5, and 10 (Thanh et al. 2010). The O. rufipogon alleles of the three QTLs theoretically contributed to the 30 min advancement of flower opening time in O. sativa (Thanh et al. 2010). Most recently, Hirabayashi et al. (2015) identified two QTLs for flower opening time using an F2 populations derived from EMF20 and Nanjing 11. One of them, qEMF3 could shift flower opening time by 1.5–2.0 h earlier for Nanjing 11 in temperate Japan and IR64 in the Philippine tropics (Hirabayashi et al. 2015). However, qEMF3 was only defined by a single SSR marker. It seemed that this QTL was overlapped or adjacent with qDFT3 identified in our research (Table 2). Furthermore, a major QTL for thermo-tolerance in African rice (Oryza glaberrima), TT1 was cloned and well functionally studied recently (Li et al. 2015). TT1 was involved in degradation of ubiquitinated proteins during heat treatment, and has potential to improve heat tolerance of japonica rice during seeding and adult stages (Li et al. 2015). Since TT1 was also located in the same region of qDFT3, whether TT1 was the target gene of qDFT3 or qEMF3 remains to be studied. Usually, the daily flowering time of indica spp. is earlier than japonica spp., but it is a little later in Sasanishiki than in Habataki, with no significance in this research. It is speculated that there may exist some phenotypic mechanisms delaying flowering time of Habataki, such as seed shape or thickness of glumes.
Improvement of heat tolerance in rice
The 3 QTLs qPSLht4.1, qPSLht7 and qPSLht10.2 identified in our study are novel. One of them, qPSLht4.1 should be a major QTL, because of the relatively large addictive effect and can be repeatedly detected in three environments (Table 2). For heat resistant varieties breeding, some progresses have been made, such as utilizing the EMF trait from O. glaberrima and screening heat tolerant rice from improved and traditional rice varieties (http://irri.org/). Compared to identification of EMF trait, the progeny selection of heat resistant plants in a traditional crossing program needs testing the heat tolerance in ‘hot and dry’ and ‘hot and humid’ countries (http://irri.org/), which requires high labor and economic costs. Therefore, using maker assisted selection (MAS) breeding strategy is essential. Although dozens of putative QTLs for heat tolerance at anthesis have been identified, the effect stability of target QTL needs to be further confirmed. Recently, a previously reported major QTL (qHTSF4.1) donated by heat tolerant variety N22 was narrowed down to about 1.2 Mb on chromosome 4 (Ye et al. 2015), which was different from qPSLht4.1 and qSFht4.2 of this study according to their mapping results. In the current research, the CSSLs were used for genetic analysis of heat tolerance at anthesis in rice for the first time. Compared to other permanent mapping populations, such as RILs and DH, using CSSLs to map QTLs can eliminate the interference of genetic background, thus QTLs with minor effects could be detected (Ebitani. et al. 2005). In this study, 7 and 4 QTLs were detected in two and three environments, respectively, which were more than other researches (Chen et al. 2008, Jagadish et al. 2010a, Xiao et al. 2011). Furthermore, one large effect QTL—qPSLht4.1 was validated. These findings would help in understanding the genetic basis and enhancing the breeding level of heat tolerant cultivars in rice.
Supplementary Information
Acknowledgements
We thank Tsuyu Ando, Toshio Yamamoto and Masahiro Yano et al. for construction of the CSSLs population. This study was supported by Jiangxi Provincial Project for Culturing Leading Researchers of Major Areas of Science and Technology (060006), the key Program for Science and Technology of Jiangxi Province, China (20133ACF60001), and the National Natural Science Foundation of China (31460343).
Literature Cited
- Ando, T., Yamamoto, T., Shimizu, T., Ma, X.F., Shomura, A., Takeuchi, Y., Lin, S.Y. and Yano, M. (2008) Genetic dissection and pyramiding of quantitative traits for panicle architecture by using chromosomal segment substitution lines in rice. Theor. Appl. Genet. 116: 881–890. [DOI] [PubMed] [Google Scholar]
- Ashikari, M. and Matsuoka, M. (2006) Identification, isolation and pyramiding of quantitative trait loci for rice breeding. Trends Plant Sci. 11: 344–350. [DOI] [PubMed] [Google Scholar]
- Cao, L., Zhao, J., Zhan, X., Li, D., He, L. and Cheng, S. (2003) Mapping QTLs for heat tolerance and correlation between heat tolerance and photosynthetic rate in rice. Chin. J. Rice Sci. 17: 223–227. [Google Scholar]
- Chen, Q., Yu, S., Li, C. and Mou, T. (2008) Identification of QTLs for heat tolerance at flowering stage in rice. Sci. Agric. Sin. 41: 315–321. [Google Scholar]
- Crowley, T.J. (2000) Causes of climate change over the past 1000 years. Science 289: 270–277. [DOI] [PubMed] [Google Scholar]
- Ebitani, T., Takeuchi, Y., Nonoue, Y., Yamamoto, T., Takeuchi, K. and Yano, M. (2005) Construction and evaluation of chromosome segment substitution lines carrying overlapping chromosome segments of indica rice cultivar ‘Kasalath’ in a genetic background of japonica elite cultivar ‘Koshihikari’. Breed. Sci. 55: 65–73. [Google Scholar]
- Endo, M., Tsuchiya, T., Hamada, K., Kawamura, S., Yano, K., Ohshima, M., Higashitani, A., Watanabe, M. and Kawagishi-Kobayashi, M. (2009) High temperatures cause male sterility in rice plants with transcriptional alterations during pollen development. Plant Cell Physiol. 50: 1911–1922. [DOI] [PubMed] [Google Scholar]
- Eshed, Y. and Zamir, D. (1995) An introgression line population of Lycopersicon pennellii in the cultivated tomato enables the identification and fine mapping of yield-associated QTL. Genetics 141: 1147–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi, H., Sasaki, K., Kambe, T., Gannaban, R.B., Miras, M.A., Mendioro, M.S., Simon, E.V., Lumanglas, P.D., Fujita, D., Takemoto-Kuno, Y.et al. (2015) qEMF3, a novel QTL for the early-morning flowering trait from wild rice, Oryza officinalis, to mitigate heat stress damage at flowering in rice, O. sativa. J. Exp. Bot. 66: 1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru, T., Hirabayashi, H., Ida, M., Takai, T., San-Oh, Y.A., Yoshinaga, S., Ando, I., Ogawa, T. and Kondo, M. (2010) A genetic resource for early-morning flowering trait of wild rice Oryza officinalis to mitigate high temperature-induced spikelet sterility at anthesis. Ann. Bot. 106: 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru, T., Hirabayashi, H., Kuwagata, T., Ogawa, T. and Kondo, M. (2012) The early-morning flowering trait of rice reduces spikelet sterility under windy and elevated temperature conditions at anthesis. Plant Prod. Sci. 15: 19–22. [Google Scholar]
- Jagadish, S.V.K., Craufurd, P.Q. and Wheeler, T.R. (2007) High temperature stress and spikelet fertility in rice (Oryza sativa L.). J. Exp. Bot. 58: 1627–1635. [DOI] [PubMed] [Google Scholar]
- Jagadish, S.V.K., Cairns, J., Lafitte, R., Wheeler, T.R., Price, A. and Craufurd, P.Q. (2010a) Genetic analysis of heat tolerance at anthesis in rice. Crop Sci. 50: 1633–1641. [Google Scholar]
- Jagadish, S.V.K., Muthurajan, R., Oane, R., Wheeler, T.R., Heuer, S., Bennett, J. and Craufurd, P.Q. (2010b) Physiological and proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.). J. Exp. Bot. 61: 143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khush, G.S. (1999) Green revolution: preparing for the 21st century. Genome 42: 646–655. [PubMed] [Google Scholar]
- Khush, G.S. (2005) What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol. Biol. 59: 1–6. [DOI] [PubMed] [Google Scholar]
- Li, X.M., Chao, D.Y., Wu, Y., Huang, X., Chen, K., Cui, L.G., Su, L., Ye, W.W., Chen, H., Chen, H.C.et al. (2015) Natural alleles of a proteasome α2 subunit gene contribute to thermotolerance and adaptation of African rice. Nat. Genet. 47: 827–833. [DOI] [PubMed] [Google Scholar]
- Madan, P., Jagadish, S.V.K., Craufurd, P.Q., Fitzgerald, M., Lafarge, T. and Wheeler, T.R. (2012) Effect of elevated CO2 and high temperature on seed-set and grain quality of rice. J. Exp. Bot. 63: 3843–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui, T. and Omasa, K. (2002) Rice (Oryza sativa L.) cultivars tolerant to high temperature at flowering: anther characteristics. Ann. Bot. 89: 683–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui, T. and Kagata, H. (2003) Characteristics of floral organs related to reliable self-pollination in rice (Oryza sativa L.). Ann. Bot. 91: 473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCouch, S.R. (2008) Gene nomenclature system for rice. Rice 1: 72–84. [Google Scholar]
- Meehl, G.A., Washington, W.M., Collins, W.D., Arblaster, J.M., Hu, A., Buja, L.E., Strand, W.G. and Teng, H. (2005) How much more global warming and sea level rise? Science 307: 1769–1772. [DOI] [PubMed] [Google Scholar]
- Paterson, A.H., DeVerna, J.W., Lanini, B. and Tanksley, S.D. (1990) Fine mapping of quantitative trait loci using selected overlapping recombinant chromosomes, in an interspecies cross of tomato. Genetics 124: 735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad, P.V.V., Boote, K.J., Allen, L.H., Sheehy, J.E. and Thomas, J.M.G. (2006) Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crops Res. 95: 398–411. [Google Scholar]
- Rang, Z.W., Jagadish, S.V.K., Zhou, Q.M., Craufurd, P.Q. and Heuer, S. (2011) Effect of high temperature and water stress on pollen germination and spikelet fertility in rice. Environ. Exp. Bot. 70: 58–65. [Google Scholar]
- Satake, T. and Yoshida, S. (1978) High temperature-induced sterility in indica rices at flowering. Jpn. J. Crop Sci. 47: 6–17. [Google Scholar]
- Thanh, P.T., Phan, P.D.T., Ishikawa, R. and Ishii, T. (2010) QTL analysis for flowering time using backcross population between Oryza sativa Nipponbare and O. rufipogon. Genes Genet. Syst. 85: 273–279. [DOI] [PubMed] [Google Scholar]
- Tuberosa, R. (2012) Phenotyping for drought tolerance of crops in the genomics era. Front Physiol. 3: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vuuren, D.P., Meinshausen, M., Plattner, G.-K., Joos, F., Strassmann, K.M., Smith, S.J., Wigley, T.M.L., Raper, S.C.B., Riahi, K., de la Chesnaye, F.et al. (2008) Temperature increase of 21st century mitigation scenarios. Proc. Natl. Acad. Sci. USA 105: 15258–15262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerakoon, W.M.W., Maruyama, A. and Ohba, K. (2008) Impact of humidity on temperature-induced grain sterility in rice (Oryza sativa L). J. Agron. Crop Sci. 194: 135–140. [Google Scholar]
- Xiao, Y.H., Pan, Y., Luo, L.H., Zhang, G.L., Deng, H.B., Dai, L.Y., Liu, X.L., Tang, W.B., Chen, L.Y. and Wang, G.L. (2011) Quantitative trait loci associated with seed set under high temperature stress at the flowering stage in rice (Oryza sativa L.). Euphytica 178: 331–338. [Google Scholar]
- Ye, C.R., Argayoso, M.A., Redoña, E.D., Sierra, S.N., Laza, M.A., Dilla, C.J., Mo, Y., Thomson, M.J., Chin, J., Delaviña, C.B.et al. (2012) Mapping QTL for heat tolerance at flowering stage in rice using SNP markers. Plant Breed. 131: 33–41. [Google Scholar]
- Ye, C.R., Tenorio, F.A., Redona, E.D., Morales-Cortezano, P.S., Cabrega, G.A., Jagadish, K.S. and Gregorio, G.B. (2015) Fine-mapping and validating qHTSF4.1 to increase spikelet fertility under heat stress at flowering in rice. Theor. Appl. Genet. 128: 1507–1517. [DOI] [PubMed] [Google Scholar]
- Zhang, Q.F. (2007) Strategies for developing Green Super Rice. Proc. Natl. Acad. Sci. USA 104: 16402–16409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, T., Yang, L., Jiang, K.F., Huang, M., Sun, Q., Chen, W.F. and Zheng, J.K. (2008) QTL mapping for heat tolerance of the tassel period of rice. Mol. Plant Breed. 6: 867–873. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.