Abstract
Aceruloplasminemia is an autosomal recessive disorder of iron metabolism caused by mutations in the ceruloplasmin gene. Its prevalence is 1 in 2,000,000 people in Japan. This is a disorder of neurodegeneration with iron accumulation in the brain revealed by MRI. The iron overload induces oxidative stress and generation of reactive oxygen species, which triggers a cascade of pathological events that lead to neuronal death. Intravenous administration of an iron chelator, deferoxamine has been proposed as a method of inhibiting the accumulation of iron.
The patient was a 46-year-old Japanese woman. She was diagnosed at the age of 33 years. Deferoxamine was administrated for 6 months but was discontinued due to adverse effects. On admission at the age of 46, psychomotor excitement was acute in onset. The extrapyramidal symptoms reflected iron deposition in the basal ganglia and substantia nigra in the midbrain. Ataxia and a wide-based gate reflected iron deposition in the dentate nuclei of the cerebellum. An antibiotic, minocycline at 150 mg/day successfully ameliorated the clinical symptoms.
Minocycline, a second generation tetracycline, has a direct radical scavenging property due to its chemical structure. It has been reported that minocycline is similar to deferoxamine in its ability to chelate iron. Minocycline is also involved in preventing the upregulation of proinflammatory cytokines. The iron-chelating, antioxidant, and anti-inflammatory effects of minocycline were involved in this case.
INTRODUCTION
Miyajima et al first characterized an adult-onset, autosomal recessive, neurodegenerative disorder resembling Parkinson disease (PD) associated with near-absent circulating serum ceruloplasmin levels in 1987.1 This new disease, aceruloplasminemia, revealed 1 role of ceruloplasmin as an essential ferroxidase critical for iron homeostasis.2 Aceruloplasminemia is an autosomal recessive iron metabolism disorder caused by mutations in the ceruloplasmin gene.3 The prevalence of aceluroplasminemia is 1 in 2,000,000 people in Japan.4 While all aceruloplasminemia patients have evidence of abnormal iron homeostasis with marked parenchymal iron metabolism, most have a mild normochromic, normocytic anemia with a decreased serum iron concentration.5 The elevated serum ferritin suggests a systemic iron overload syndrome, yet affected patients had low transferrin saturation and mild anemia.1 A variety of symptoms are exhibited due to the accumulation of excess iron. Diabetes mellitus (DM), retinitis pigmentosa, and central nervous symptoms are the main symptoms of aceruloplasminemia. Patients usually present in the fourth or fifth decade of life with neurologic signs.6 These neurologic features are advanced in most patients and associated with significant iron accumulation in the basal ganglia and dentate nucleus of the brain.6 Aceruloplasminemia is one of the disorders causing neurodegeneration with brain iron accumulation (NBIA).7 The diagnosis of NBIA is made on the basis of the combination of representative clinical features along with MRI evidence of iron accumulation.7 Pathophysiologic hallmarks of NBIA include the disease-defining deposition of iron, presence of neuroaxonal spheroids, and variable accumulation of α-synuclein-positive Lewy bodies and/or tau pathology.7 Some studies have included neurodegenerative disorders including Alzheimer diseases (AD), Parkinson disease (PD), amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS), which are known to feature brain accumulation of iron or associated with altered iron metabolism.8 Iron deposition has been observed in the cortex and amyloid plaques of patients with AD, substantia nigra in PD, and spinal cord in ALS.9 The overlap of NBIA disorders with common neurodegenerative disorders has generated significant interest and suggests an element of shared pathophysiology.10 Many of the NBIA syndromes links to the common idiopathic neurodegenerative diseases.11–13
The administration of an iron chelator, before onset or in the early stages of this disorder, is considered to be effective in treatment of aceruloplasminemia. The intravenous administration of deferoxamine has been proposed as a method of inhibiting the iron accumulation. Deferoxamine is a high-affinity iron chelator that combines with a ferric iron molecule in 1:1 molar ratio. After crossing the blood–brain barrier, deferoxamine has been shown to promote the excretion of excess iron in patients.14
In this report, we describe a female patient with aceruloplasminemia presenting as psychomotor excitement and neurological symptoms. An antibiotic, minocycline at 150 mg/day, successfully ameliorated the symptoms. To our knowledge, this is the first published case report of successful treatment of aceruloplasminemia with minocycline.
CASE REPORT
The patient was a 46-year-old Japanese woman. She presented with anemia, an unsteady gait, and cognitive dysfunction. Extrapyramidal symptoms and cerebellar ataxia were observed as the central nervous system symptoms. The patient's birth was uneventful, and she developed normally. Anemia was detected at the age of 21 years. She first had glycosuria at 23 years of age. One year later, insulin therapy was started for DM, and the insulin requirement gradually increased in a postpartum period. DM was caused by dysfunction of the pancreas due to iron accumulation, suggesting the replacement β cells by adipose tissue. Retinitis pigmentosa was detected at the age of 25 years. Her 1-year younger sister had been diagnosed aceruloplasminemia. Based on a positive family history and identification of the homozygous mutation of the ceruloplasmin gene on exon 12 by a genetic study, aceruloplasminemia was diagnosed at the age of 33 years. The concentrations of serum ferritin and iron were 630 ng/mL and 42 μg/dL, respectively. Deferoxamine was administered for 6 months but was discontinued because of severe dizziness and hearing dysfunction. At the time, the serum ferritin and iron were 459 ng/mL and 42 μg/dL, respectively. She had been unable to control her diet, and her blood sugar control had regressed since she was 40-year old. On admission at the age of 46 years, she talked incoherently. Psychomotor excitement was acute in onset and psychotic features included dysphoria, delusional ideas, and thought disturbance. She had a 1-year history of difficulty walking with frequent falls and dizziness. Her family noted a decline in social functioning compared with that of 3 years prior. She required assistance for most daily activities. Neuropsychiatric symptoms regressed gradually from 1 year previous. Her Mini Mental State Examination (MMSE) score was 22.
General findings were as follows: her height and weight were 1.61 m and 60.5 kg (BMI: 23.4), her blood pressure was 129/83 mm Hg and pulse was 90 bpm and regular. Neurologically, the patient was awake and without dysarthria. She presented resting and intentional tremor, mild limb rigidity, and bradykinesia. Gait tests revealed that the patient was unable to stand with her feet together without falling backward. She was unable to walk unassisted, and the gait was wide-based. She experienced dizziness. Extraocular movements were full, without nystagmus or diplopia. Dysdiadochokinesis of the bilateral hands was mild. Muscle strength and deep tendon reflexes were preserved. Babinski sign was not present. Rigidity was mild in the bilateral elbow joints.
Head MRI on axial T2-weighted images showed low signal intensities in the bilateral lentiform nuclei, thalamus, caudate nuclei, cerebellar dentate nuclei, red nuclei, and substantia nigra in the midbrain. Low intensities that looked like linear bodies were visible on the surfaces of the brain hemispheres (Figure 1). Decreased striatal DAT binding is shown in Figure 2. The extrapyramidal symptoms reflected the iron deposition in the basal ganglia (Figures 1 and 2). Ataxia and the wide-based gait reflected iron deposition in the dentate nuclei of the cerebellum (Figure 1). Abdominal CT showed iron accumulation in hepatocytes (Figure 3).
FIGURE 1.
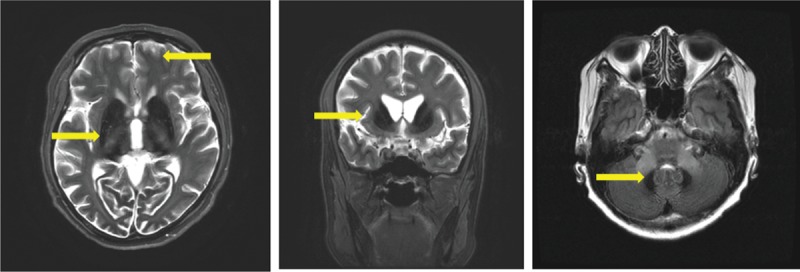
Magnetic resonance imaging (T2/FLAIR): iron deposition detected as a low-intensity signal. There were low-signal intensities in the bilateral lentiform nuclei, caudate nuclei, thalamus, and cerebellar dentate nuclei. In addition, linear low intensities were exhibited on the surfaces of the brain hemispheres. (Arrows indicate the low intensities).
FIGURE 2.

Dopamine transporter scintigraphy: DAT transporter imaging showed reduced uptake in the putamen on both the right and left sides.
FIGURE 3.
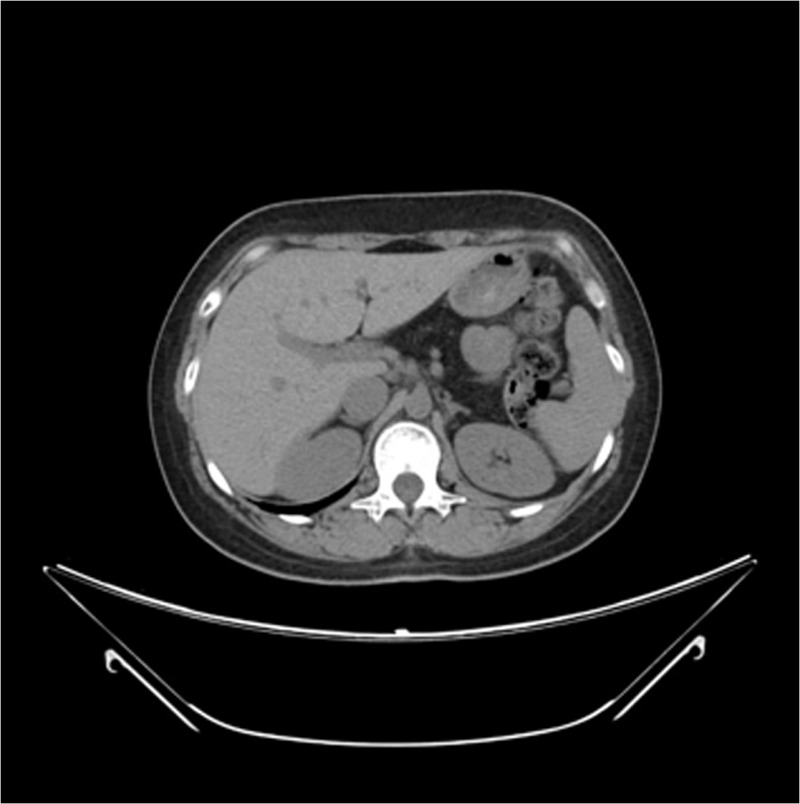
Abdominal CT: CT showed iron accumulation in hepatocytes. The pancreas was not detected (adipose tissue was replaced by iron accumulation). CT = computed tomography.
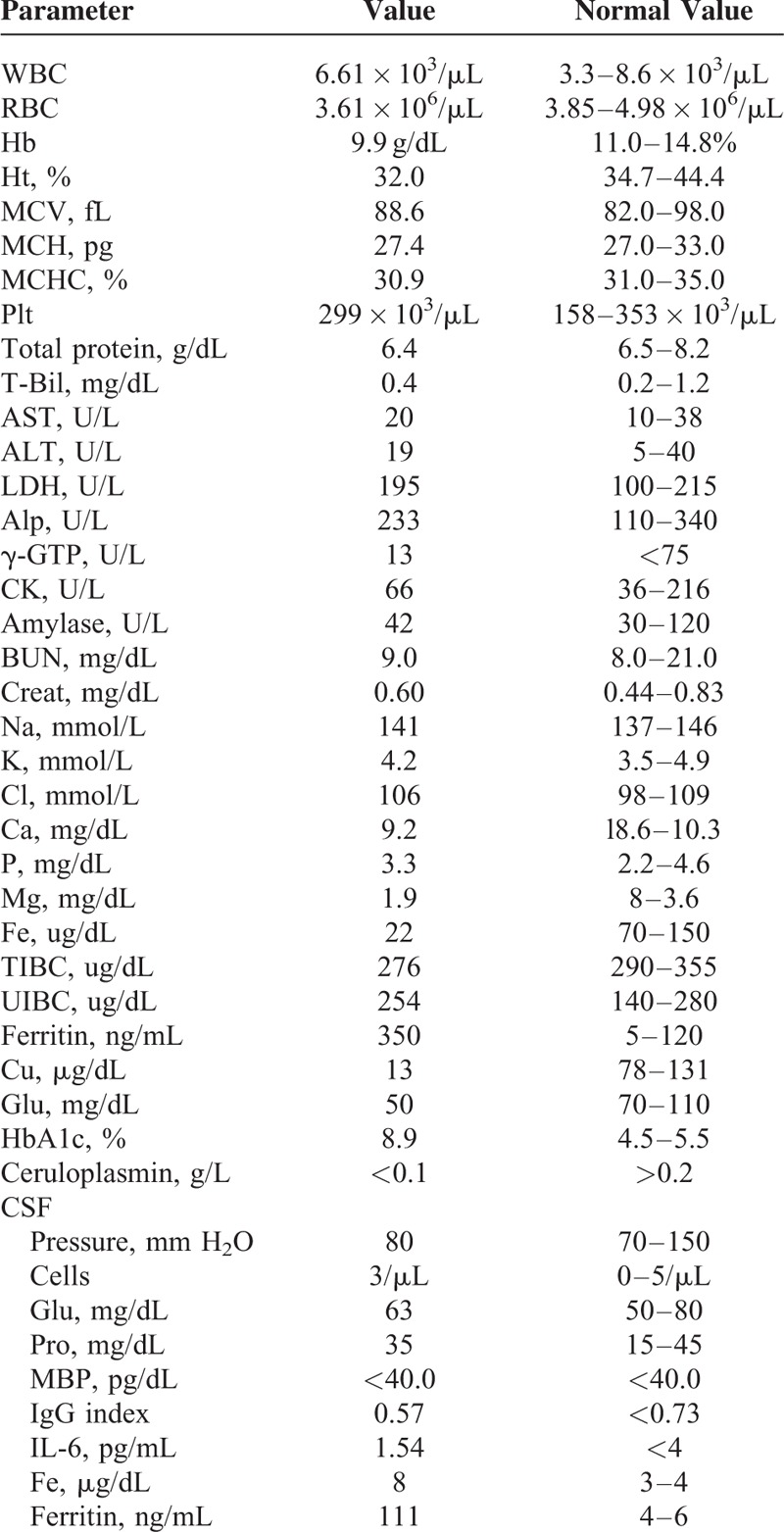
Laboratory examinations of serum and urine and cerebrospinal fluid are shown in Table 1. These findings include microcytic anemia, decreased serum iron and copper concentrations, and increased TIBC, UIBC, and serum ferritin concentrations. CSF ferritin was also increased massively. An elevated serum ferritin suggests a systemic iron overload syndrome, and the laboratory findings showed a marked decrease in ceruloplasmin.
TABLE 1.
Laboratory Examinations
PROGRESSION
Her psychiatric symptoms were evaluated by the Brief Psychiatric Rating Scale (BPRS). The score was 80.15 The neurological symptoms rated by the Unified Parkinson Disease Rating Scale16 and the International Cooperative Ataxia Rating Scale (ICARS) were 32 and 62, respectively. Lithium carbonate (400 mg/day) and aripiprazole (30 mg/day) were started. Psychomotor excitement was less than on admission but still continued. Another iron chelator, deferasirox, was approved in 2008 in Japan. A previous study reported that oral administration of deferasirox as iron chelation therapy improved the clinical symptoms.17 We considered the administration deferasirox to interrupt the cycle of iron overload and iron-induced tissue damage. However, her family refused because it is necessary to continue the medication for a long period, requires a large co-pay not covered by health insurance, and has adverse effects. The psychiatric symptoms were not improved, but extrapyramidal symptoms including salivation regressed. Therefore, aripiprazole and lithium carbonate were discontinued. Acute bronchitis and sinusitis occurred, and a regimen of 150 mg/day minocycline was initiated with her consent. The ethical approval was not necessary as it did not involve any experimental treatment. One week later, her mood was more stable than on admission, and the tendency toward aggression disappeared. Minocycline is apparently effective for treatment of thought disturbance and psychiatric and neurological symptoms generally. Her BPRS was decreased to 26. Her walk gradually became stable, and she could walk with the assistance of a walker. However, wearing a protective helmet was still needed. Later on she was able to walk by herself, and staggering was also reduced. She no longer needed the helmet or assistance to walk. UPDRS Part 3 and ICARS scores were 20 and 27, respectively. The clinical course is shown in Figure 4. One year after discharge, the clinical improvement has been maintained, and there is no worsening of psychiatric and neurological symptoms. Her walk is even more stable than at discharge. However, the MR imaging in the range of resolution was not improved despite the clinical improvement after minocycline administration (Figure 5). Written informed consent was obtained from the patient for the publication of this report.
FIGURE 4.
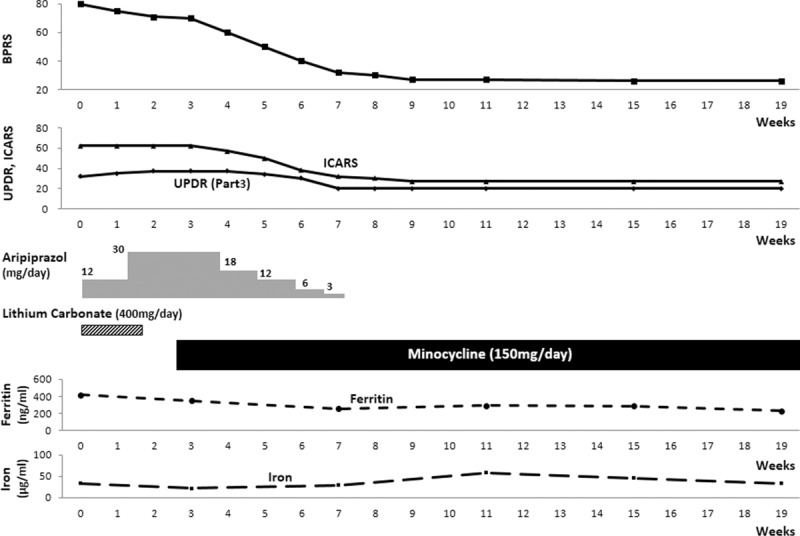
Clinical course: an antibiotic, minocycline at 150 mg/day, ameliorated psychiatric and neurological symptoms. BPRS = Brief Psychiatric Rating Scale, ICARS = International Cooperative Ataxia Rating Scale, UPDR = Unified Parkinson Disease Rating Scale.
FIGURE 5.
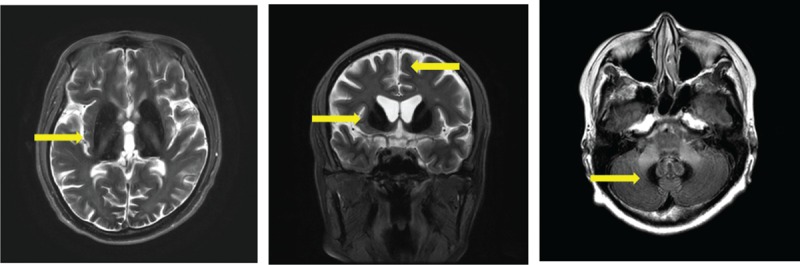
Magnetic resonance imaging (T2W1/FLAIR): 1 year after starting minocycline administration, the same low signal intensities are seen in the bilateral lentiform nuclei, caudate nuclei, thalamus, and cerebellar dentate nuclei as before treatment. (Arrows indicate the low intensities).
DISCUSSION
The imaging sign that is a neuropathological hallmark of aceruloplasminemia is intracellular iron overload, which is thought to lead to neuronal cell death.2 In aceruloplasminemia, abnormal iron-loaded globular structures are observed in the brain tissue.3 Abnormal low intensities reveal iron deposition in the brain. In this case, the finding that head MRI on axial T2-weighted images showed low signal intensities in the basal ganglia and dentate nucleus and decreased striatal DAT binding (Figure 2) indicate severe destruction of the basal ganglia. Low intensities that look like linear bodies were also detected on the surfaces of the brain hemispheres (Figure 1). It is supposed that the pathology may have been present for a long time, indicating that the neuropathologic process had worsened and extended beyond the basal ganglia to the cerebral cortex.18 This observation is consistent with the finding that patients with AD onset at an early age have a greater neocortical iron burden than late-onset patients.19 Cortical iron elevation is a feature of AD and might contribute to the oxidative damage observed in AD brains.19 In this case, the changes in the serum ferritin level since she was diagnosed did not have a direct relationship with neurological and psychiatric symptoms (Figure 4); on the other hand, the CSF ferritin level was abnormally elevated on admission. CSF ferritin is supposed to be an index of the brain iron load, and an elevated CSF ferritin in AD was reported to be an indication of cognitive decline.20 Thus, lowering CSF ferritin levels might be expected to be a treatment for neurodegenerative disorders, and deferoxamine has been used clinically to slow the progression associated with iron-induced AD and PD.21
Minocycline is a semisynthetic, second-generation tetracycline analog that effectively crosses the blood–brain barrier, and is effective against gram-positive and gram-negative infections. Tetracycline antibiotics have been reported to chelate metal ions including iron. Minocycline, a second-generation tetracycline, has a direct radical scavenging property based on its chemical structure.22 Kraus et al23 reported that minocycline is similar to the metal ion chelator deferoxamine. Administration of minocycline induced effects similar to those of deferoxamine, which was used 13 years ago in this case, but was abandoned due to side effects. Treatment of the cognitive impairment of aceruloplasminemia with minocycline needs to be evaluated.
Iron is a bioactive metal essential for normal brain functions and an essential substance for the neural development in the central nervous system, myelination, neurotransmitter synthesis, catabolism, and the electron transport system in neuronal cells. On the other hand, iron is easily oxidized when it is not bound to protein, an unstable state that produces free radicals such as hydroxyl radicals by the Fenton reaction.24 The brain is particularly vulnerable to oxidative damage because of its high oxygen utilization and its high concentration of oxidizable polyunsaturated fatty acids.25 Therefore, the iron concentration in the brain is usually critically regulated by several iron metabolism molecules, including ferritin and ceruloplasmin against oxidative stress.26 Ceruloplasmin, an amino oxidase, also known as ferroxidase oxidizes ferrous iron to ferric iron. Therefore, ceruloplasmin is not only a ferroxidase but also a scavenger of ROS; in other words, the antioxidant activity of ceruloplasmin can be ascribed mainly to its ferroxidase activity, which inhibits ferrous ion-stimulated lipid peroxidation and formation of hydroxyl radicals in the Fenton reaction. Ultimately, this iron overload induces oxidative stress in the brain in aceruloplasminemia. Oxidative stress and generation of reactive oxygen species (ROS) are followed by lipid and protein peroxidation. Previous studies demonstrated a marked excess in plasma lipid peroxidation in patients with aceruloplasminemia, which is consistent with a potential role for this process in iron-mediated tissue damage, an abnormality that was markedly decreased by deferoxamine therapy.27 Minocycline has a direct radical scavenging property that is consistent with its chemical structure, which includes a multiply substituted phenol ring similar to a-tocopherol (vitamin E). The effective reaction is of a phenol ring of radical scavengers with a phenol-derived free radical that is relatively stable and unreactive. Minocycline can therefore be considered to belong to the class of phenolic antioxidants.23 It is also suggested that the direct antioxidant properties of minocycline may contribute to neuroprotective effects in this patient. There is enough evidence to show that enhanced oxidative stress is a major cause of neuronal cell death in aceruloplasminemia.28 The pathology of aceruloplasminemia, abnormal astrogliosis, and iron-loaded globular structures is observed28 and also seen in microglial cells.29
Minocycline has been reported to inhibit microglial inflammatory responses in various neurodegenerative diseases such as HD, PD, and MS and has been shown to delay motor function alterations in models of HD, ALS, and PD.30 Neuroinflammation is supposed one of the important causative factors of neurodegenerative disorders.31 In neuroinflammation, it is known that microglia and astrocytes are activated following nerve injury.32 Some studies claim that minocycline inhibits both microglial and astrocytic activation in neuroinflammation.33–35 Minocycline may act in this context as a general inhibitor of proinflammatory cytokine activity. Proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 are produced by microglial cells, astrocytes, neutrophils, and macrophages.36 This activity of minocycline could inhibit microglial activation, but could also prevent inflammatory mechanisms within astrocytes.36 The chronic inflammation is closely related to clinical symptoms of neurodegenerative diseases, which are associated with altered iron metabolism and free radical injury.8 Age is a major risk factor for neurodegenerative diseases.37 The age-related increase in brain iron concentration occurs in conjunction with other risk factors, such as neuroinflammation and metabolic dysfunction.26,38 The anti-inflammatory effects of minocycline have been attributed to the compound's antioxidant activity.37 The clinical improvement in this case may be related to reciprocal and synergic actions induced by minocycline.
Miyaoka et al reported the antipsychotic effects of minocycline in patients with schizophrenia and suggested that minocycline might be a safe and effective adjunct to antipsychotic medications, that is, that augmentation with minocycline may prove to be a viable strategy for “boosting” antipsychotic efficacy and for treating schizophrenia.39 In this case, psychotic excitement was ameliorated, similar to reported cases. Minocycline is also considered to have a potential for treatment of major depression through its effects on neurogenesis and antiglutamate excitotoxicity because decreased neuronal survival and inflammatory reactions are important causative factors of major depression.30,40,41 It is noteworthy minocycline does not exert significant direct effects on different neuroreceptors, including monoaminergic and N-methyl-d-aspartic acid (NMDA) receptors.41 However, it is reported that minocycline can regulate several processes involved in neurodevelopment and in neural plasticity.41 The clinical improvement in this case could be considered that minocycline provides a potential approach for preventing or treating neuropsychiatric symptoms associated with aceruloplasminemia and inhibiting the progress.
CONCLUSION
Despite controversies about the efficacy of minocycline, its relative safety and tolerability might lead to clinical improvements in patients of aceruloplasminemia. Adverse events associated with the administration of minocycline were not observed.
Footnotes
Abbreviations: AD = Alzheimer disease, ALS = amyotrophic lateral sclerosis, BPRS = Brief Psychiatric Rating Scale, CSF = cerebrospinal fluid, DAT = dopamine transporter, DM = diabetes mellitus, ICARS = International Cooperative Ataxia Rating Scale, MMSE = Mini-Mental State Evaluation, MS = multiple sclerosis, NMDA = N-methyl-d-aspartic acid, PD = Parkinson disease, ROS = reactive oxygen species, UPDRS = Unified Parkinson Disease Rating Scale.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Xu X, Pin S, Gathinji M, et al. Aceruloplasminemia: an inherited neurodegenerative disease with impairment of iron homeostasis. Ann N Y Acad Sci 2004; 1012:299–305. [DOI] [PubMed] [Google Scholar]
- 2.Miyajima H, Nishimura Y, Mizoguchi K, et al. Familial apoceruloplasmin deficiency associated with blepharospasm and retinal degeneration. Neurology 1987; 37:761–767. [DOI] [PubMed] [Google Scholar]
- 3.Kaneko K, Yoshida K, Arima S, et al. Astrocytic deformity and globular structures are characteristic of the brains of patients with aceruloplasminemia. J Neuropathol Exp Neurol 2002; 61:1069–1077. [DOI] [PubMed] [Google Scholar]
- 4.Miyajima H, Takahashi Y, Kono S. Aceruloplasminemia, inherited disorder of iron metabolism. Biometals 2003; 16:205–213. [DOI] [PubMed] [Google Scholar]
- 5.Hellman NE, Gitlin JD. Ceruloplasmin metabolism and function. Gitlin Annu Rev Nutr 2002; 22:439–458. [DOI] [PubMed] [Google Scholar]
- 6.Morita H, Ikeda S, Yamamoto K, et al. Hereditary ceruloplasmin deficiency with hemosiderosis: a clinopathological study of Japanese family. Ann Neurol 1995; 37:646–656. [DOI] [PubMed] [Google Scholar]
- 7.Kruer MC. The neuropathology of neurodegeneration with brain iron accumulation. Int Rev Neurobiol 2013; 110:165–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerlach M, Ben-Shachar D, Riederer P, et al. Altered brain metabolism of iron as a cause of neurodegenerative diseases? J Neurochem 1994; 63:793–807. [DOI] [PubMed] [Google Scholar]
- 9.Harris ZL, Takahashi Y, Miyajima H, et al. Aceruloplasminemia: molecular characterization of this disorder of iron metabolism. Proc Natl Acad Sci U S A 1995; 92:2539–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider SA, Bhatia KP. Excess iron harms the brain: the syndromes of neurodegeneration with brain iron accumulation (NBIA). J Neural Transm 2013; 120:695–703. [DOI] [PubMed] [Google Scholar]
- 11.Kruer MC, Paisán-Ruiz C, Boddaert N, et al. Defective FA2H leads to a novel form of neurodegeneration with brain iron accumulation (NBIA). Ann Neurol 2010; 68:611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bras JM, Singleton A. Genetic susceptibility in Parkinson's disease. Biochim Biophys Acta 2009; 1792:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stankiewicz J, Panter SS, Neema M, et al. Iron in chronic brain disorders: imaging and neurotherapeutic implications. Neurotherapeutics 2007; 4:371–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Summers MR, Jacobs A, Tudway D, et al. Studies in deferoxamine and ferrioxamine metabolism in normal and iron-loaded subjects. Br J Haematol 1978; 179:542–555. [DOI] [PubMed] [Google Scholar]
- 15.Overall E, Donald R, Gorham E. The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812. [Google Scholar]
- 16.Goetz CG, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord 2007; 22:41–47. [DOI] [PubMed] [Google Scholar]
- 17.Skidmore FM, Drago V, Foster P, et al. Aceruloplasminemia with progressive atrophy without brain iron overload: treatment with oral chelation. J Neurol Neurosurg Psychiatry 2008; 79:467–470. [DOI] [PubMed] [Google Scholar]
- 18.Kaneko K, Hineno A, Yoshida K, et al. Extensive brain pathology in a patient with aeruloplasminemia with a prolonged duration of illness. Hum Pathol 2012; 13:451–456. [DOI] [PubMed] [Google Scholar]
- 19.van Rooden S, Doan NT, Versluis MJ, et al. 7T T2∗- weighted magnetic resonance imaging reveals cortical phase differences between early- and late-onset Alzheimer's disease. Neurobiol Aging 2014; 36:20–26. [DOI] [PubMed] [Google Scholar]
- 20.Anyton S, Faux NG, Bush AI, et al. Ferritin levels in the cerebrospinal fluid predict Alzheimer's disease outcomes and are regulated by APOE and Alzheimer's Disease Neuroimaging Intiative. Nat Commun 2015; 6:6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith MA, Harris PL, Sayre LM, et al. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci U S A 2015; 94:9866–9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caswell AH, Hutchison JD. Selectivity of cation chelation to tetracyclines: evidence for special conformation of calcium chelate. Biochem Biophys Res Commun 1971; 43:625–630. [DOI] [PubMed] [Google Scholar]
- 23.Klaus RL, Pasoeczny R, Lariosa-Willingham K, et al. Antioxidant properties of minocycline: neuroprotection in an oxidative stress assay and direct radical-scavenging activity. J Neurochem 2005; 94:819–827. [DOI] [PubMed] [Google Scholar]
- 24.Halliwell B. Oxidative stress and neurogeneration: where are we now? J Neurochem 2006; 97:1634–1658. [DOI] [PubMed] [Google Scholar]
- 25.Valko D, Leibfritz J, Moncol MTD, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39:44–84. [DOI] [PubMed] [Google Scholar]
- 26.Zecca L, Youdin MBH, Riedere P, et al. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci 2004; 5:863–873. [DOI] [PubMed] [Google Scholar]
- 27.Miyajima H, Takahashi Y, Serizawa M, et al. Increased plasma lipid peroxidation in patients with aceruloplasminemia. Free Radic Biol Med 1996; 20:757–760. [DOI] [PubMed] [Google Scholar]
- 28.Kaneko K, Yoshida K, Arima S, et al. Astrocytic deformity and globular structures are characteristic of the brains of patients with aceruloplasminemia. J Neuropathol Exp Neurol 2002; 61:1069–1077. [DOI] [PubMed] [Google Scholar]
- 29.Harris ZL, Klomp LWJ, Gitlin AJD. Aceruloplasminemia: an inherited neurodegenerative disease with impairment of iron homeostasis. Am J Clin Nutr 1998; 67:972–977. [DOI] [PubMed] [Google Scholar]
- 30.Kim HS, Suh YH. Minocycline and neurodegenerative diseases. Behav Brain Res 2009; 196:168–179. [DOI] [PubMed] [Google Scholar]
- 31.Yrjanheikki J, Tikka T, Keinanen R, et al. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A 1999; 96: 13496–13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garrison CJ, Dougherty PM, Kajander KC, et al. Staining of glial fibrillary acidic protein (GFAP) in lumbar spinal cord increases following as sciatic nerve construction injury. Brain Res 1991; 565:1–7. [DOI] [PubMed] [Google Scholar]
- 33.Ledeboer A, Sloane EM, Milligan ED, et al. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain 2005; 115:71–83. [DOI] [PubMed] [Google Scholar]
- 34.Ryu JK, Franciosi S, Sattayaprasert P, et al. Minocycline inhibits neuronal death and glial activation induced by beta-amyloid peptide in rat hippocampus. Glia 2004; 48:85–90. [DOI] [PubMed] [Google Scholar]
- 35.Sung CS, Cheng CH, Wen ZH, et al. Minocycline and fluorocitrate suppress spinal nociceptive signaling in intrathecal IL-1β-induced thermal hyperalgesic rats. Glia 2012; 60:2004–2017. [DOI] [PubMed] [Google Scholar]
- 36.Robinson CR, Zhang H, Doughrty PM. Astrocytes, but not microglia, are activated in oxaliplatin and bortezomib-induced peripheral neuropathy in the rat. Neuroscience 2014; 274:308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reeve A, Simcox E, Turnbull D. Ageing and Parkinson's disease: Why is advancing age the biggest risk factor? Ageing Res Rev 2014; 14:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moos T, Nielsen TR, Skjorringe T, et al. Iron trafficking inside the brain. J Neurochem 2007; 103:1730–1740. [DOI] [PubMed] [Google Scholar]
- 39.Miyaoka T, Yasukawa R, Yasuda H, et al. Clinical potential of minocycline for schizophrenia. CNS Neurol Disord Drug Targets 2008; 7:376–381. [DOI] [PubMed] [Google Scholar]
- 40.Pae CU, Marks DM, Han C, et al. Does minocycline have antidepressant effect? Biomed Pharmacother 2008; 62:308–311. [DOI] [PubMed] [Google Scholar]
- 41.Chaves C, Zyardi AW, Hallak J. Minocycline as a potential treatment in the early stages of schizophrenia: a translational approach. Ther Targets Neurol Dis 2015; 2:1–4. [Google Scholar]


