High levels of methylesterification of pectin allow for enhanced cell expansion, partitioning of resources to leaf growth, and increased leaf area for photosynthesis, which in turn enhance plant growth.
Abstract
Photosynthesis occurs in mesophyll cells of specialized organs such as leaves. The rigid cell wall encapsulating photosynthetic cells controls the expansion and distribution of cells within photosynthetic tissues. The relationship between photosynthesis and plant growth is affected by leaf area. However, the underlying genetic mechanisms affecting carbon partitioning to different aspects of leaf growth are not known. To fill this gap, we analyzed Arabidopsis plants with altered levels of pectin methylesterification, which is known to modulate cell wall plasticity and plant growth. Pectin methylesterification levels were varied through manipulation of cotton Golgi-related (CGR) 2 or 3 genes encoding two functionally redundant pectin methyltransferases. Increased levels of methylesterification in a line over-expressing CGR2 (CGR2OX) resulted in highly expanded leaves with enhanced intercellular air spaces; reduced methylesterification in a mutant lacking both CGR-genes 2 and 3 (cgr2/3) resulted in thin but dense leaf mesophyll that limited CO2 diffusion to chloroplasts. Leaf, root, and plant dry weight were enhanced in CGR2OX but decreased in cgr2/3. Differences in growth between wild type and the CGR-mutants can be explained by carbon partitioning but not by variations in area-based photosynthesis. Therefore, photosynthesis drives growth through alterations in carbon partitioning to new leaf area growth and leaf mass per unit leaf area; however, CGR-mediated pectin methylesterification acts as a primary factor in this relationship through modulation of the expansion and positioning of the cells in leaves, which in turn drive carbon partitioning by generating dynamic carbon demands in leaf area growth and leaf mass per unit leaf area.
Photosynthesis is the major source of carbon (C) for plant growth. However, there is no clear correlation between area-based photosynthesis and plant growth, and studies have shown that the relationship between photosynthesis and plant growth is affected by leaf area growth parameters. A strong positive correlation exists between relative growth rate and leaf growth parameters such as leaf area per unit leaf mass (specific leaf area, SLA), leaf area per unit plant mass (leaf area ratio), and investment of C in leaf growth (leaf mass ratio; Shipley, 2002; Lambers et al., 2008; Poorter et al., 2009). In fact, modeled partitioning coefficients obtained from the Arabidopsis leaf area growth model showed that plant growth is more sensitive to changes in C partitioning to leaf thickening and leaf area growth than to changes to area-based photosynthesis (Weraduwage et al., 2015). The term “thickening” was used to indicate increased mass per unit leaf area (LMA), whether that results from increased thickness in the normal sense of more depth to the leaf between adaxial and abaxial surfaces or from increased cell density. LMA is the reciprocal of SLA. While LMA has been shown to positively and linearly correlate with investment to leaves in the form of anatomical and chemical composition changes, SLA shows a hyperbolic relationship with such changes (Poorter et al., 2009). Therefore, we will mainly focus on LMA throughout this work. Weraduwage et al. (2015) concluded that even though photosynthetic C is required for growth, the degree of growth is determined by the amount of C partitioned to leaf area growth and LMA (Weraduwage et al., 2015). However, how and to what degree this variation in leaf expansion and LMA is affected by cell wall properties of leaf mesophyll cells is not known. In addition, the underlying mechanisms that drive C partitioning to favor either leaf area growth or LMA, and the genes that can influence changes in C demands toward these two processes, are yet to be found.
Leaves are the major photosynthetic organs in plants and regulation of cell expansion and growth is crucial for the development of leaf architecture. Leaf growth can occur in the form of leaf area or LMA (Lambers et al., 2008; Weraduwage et al., 2015). The size and shape of cells in the leaf mesophyll can have a significant impact on: (1) projected or effective leaf surface area, which is the leaf area capable of intercepting light; (2) leaf mass per unit leaf area or LMA; (3) CO2 conductance from the external environment through intercellular air spaces into chloroplasts in mesophyll cells; and (4) surface area of mesophyll cells and chloroplasts exposed to intercellular airspaces (Honda and Fisher, 1978; Evans et al., 1994; Galmés et al., 2013). The plant cell wall controls expansion and positioning of cells of photosynthetic tissues and therefore, changes in cell wall properties will have a direct impact on the above properties. Although there is evidence for changes in leaf architecture as a result of alterations in genes that affect the synthesis and properties of the cell wall (Burton et al., 2000; Kim and Delaney, 2002; Pilling et al., 2004; Wolf et al., 2009; Kim et al., 2015), an in-depth analysis of effects on leaf gas exchange and overall plant growth under such circumstances has not yet been conducted.
The plant cell wall is composed mainly of polysaccharides, and among these, pectin is considered a critical element that controls cell wall elasticity and expansion. Pectin is synthesized in the Golgi apparatus and secreted in a highly methylesterified form. Methylesterification of galacturonic acid (GalUA) is catalyzed by pectin methyltransferases (Wolf et al., 2009; Kim et al., 2015). The level of pectin methylesterification in the cell wall is controlled by methylesterases and inhibitors of methylesterases, which modify the levels of methylation and demethylation of GalUA in the pectin backbone (Wolf et al., 2009; Kim et al., 2015). Removal of methyl groups by methylesterases allows carboxyl groups of GalUA to form Ca2+- and Mg2+-mediated intermolecular linkages that lead to hardening of pectin (Burton et al., 2000; Heldt and Piechulla, 2010; Kim et al., 2015). Indeed, over-expression of pectin methylesterase inhibitors results in longer root cells (Lionetti et al., 2007). Therefore, the degree of methylation and demethylation of pectin determines the delicate balance between cell wall extensibility and rigidity with consequent effects on growth and shape of cells (Burton et al., 2000; Pilling et al., 2004; Lionetti et al., 2007; Wolf et al., 2009; Xiao and Anderson, 2013; Kim et al., 2015). Consistent with this notion, over-expression of pectin methyltransferases, CGR2 or CGR3, led to enhanced rosette size and fresh weight in Arabidopsis (Kim et al., 2015). However, a double knockout of CGR2 and CGR3 genes, named cgr2/3, showed reduced cell expansion and overall plant growth; both phenotypes correlated with reduced levels of pectin methylesterification compared to wild type (Kim et al., 2015). To our knowledge, the effect of pectin methylation on leaf growth parameters, photosynthesis, and respiration through its effect on leaf architecture has not been studied.
The goal of this study was to use the Arabidopsis model system developed by Kim et al. (2015) with altered levels of pectin methyltransferases, CGR2 or CGR3 (single gene modifiers found to positively or negatively affect cell expansion as a function of their cellular availability) to investigate how changes in cell-wall plasticity affect C assimilation, leaf respiration, and overall plant growth through effects on leaf area growth and mesophyll structure. We examined whether the changes in overall plant growth in Arabidopsis with altered CGR2 and CGR3 could result from changes in leaf area growth and LMA or changes in area-based photosynthesis to explore the relationship between photosynthesis and growth. Based on the differences in rosette size reported previously (Kim et al., 2015), we hypothesized that area-based photosynthesis rates would be lower in a CGR2 over-expressor (CGR2OX) but larger in the mutant lacking both CGR2 and 3 genes (cgr2/3). As CGR2 and CGR3 affect pectin methylation and cell expansion in leaves, we also hypothesized that plant growth would be enhanced in the CGR2OX line and reduced in the cgr2/3 mutant mainly as a result of altered LMA. Indeed, through analysis of leaf and plant growth, gas exchange, and growth modeling, we found that CGR2 and CGR3 have significant impacts on photosynthesis and respiration through alteration of mesophyll structure and LMA. Plant growth was enhanced in the CGR2OX line but reduced in the cgr2/3 mutant. This variation in plant growth was as a result of differences in C partitioning and not as a result of changes in photosynthetic rate per unit leaf area. These observations support a novel (to our knowledge) model that changes in cell wall plasticity due to changes in pectin composition influence C partitioning between leaf area growth and LMA, thereby regulating the relationship between photosynthesis and plant growth.
RESULTS
CGR2OX and cgr2/3 Mutant Lines Retained Altered Cell Wall Composition
Cell wall composition of Col-0 (wild type), cgr2/3 complemented with CGR2 (cgr2com), CGR2OX, and cgr2/3 was analyzed to determine whether the previously observed changes in methylesterification and modification of cell wall polysaccharides by Kim et al. (2015) were retained in the mutant lines. Similar to what was seen by Kim et al. (2015), crystalline cellulose content was lower in cgr2/3 mutant line and it was greater in CGR2OX than that in Col-0 (Supplemental Fig. S1A). In addition, the content of neutral sugars such as fucose, arabinose, and glucose was significantly higher in cgr2/3 than Col-0, as was shown by Kim et al. (2015; Supplemental Fig. S1B). The uronic-acid content and the amount of methylesters per uronic acid were greater in CGR2OX compared to wild type; the uronic-acid content was lower in cgr2/3 (Supplemental Fig. S1C). These data support that the cell wall composition, including pectin and the degree of methylesterification, is altered in the CGR2OX and cgr2/3 mutant lines in response to altered CGR2 and CGR3 gene expression, as previously reported in Kim et al. (2015).
Enhanced Pectin Methylesterification Increased Leaf Area while a Decrease in Pectin Methylesterification Reduced Leaf Area
Hypocotyl length and cotyledon and leaf areas were measured to determine how altered expression of CGR2 and CGR3 affects early seedling growth and expansion of the leaf blade.
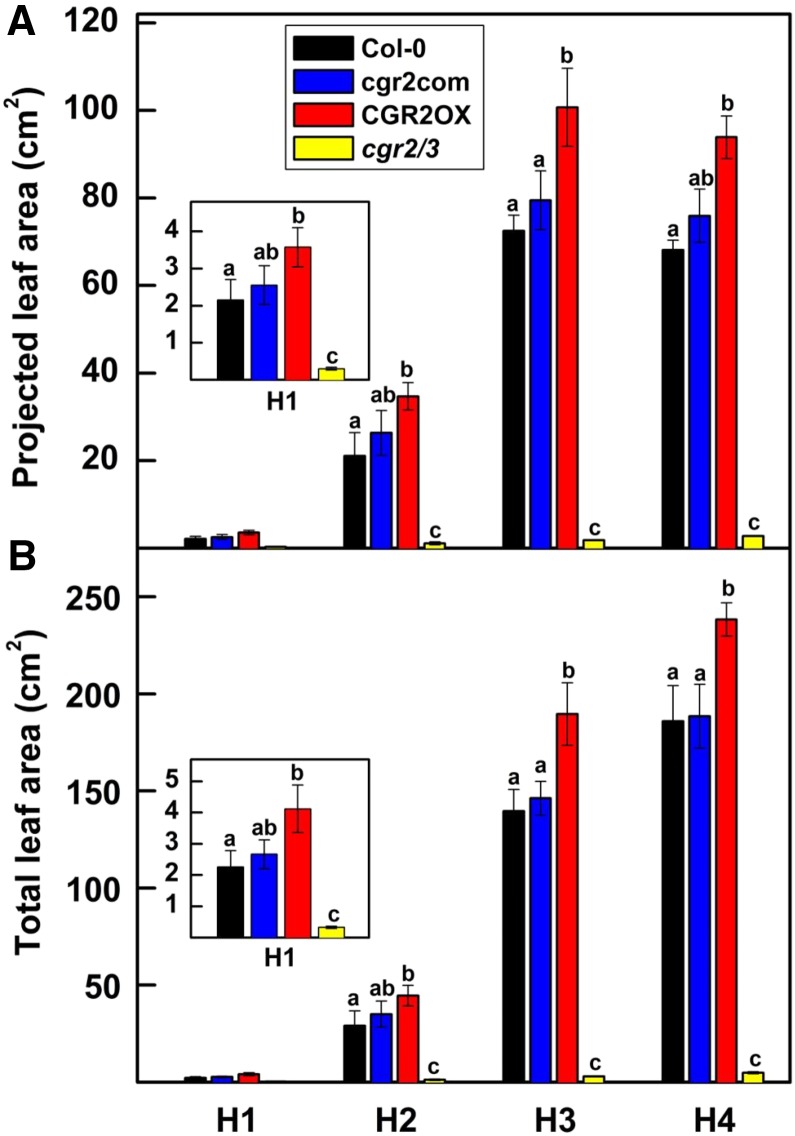
Similar to earlier results (Kim et al., 2015), hypocotyls of CGR2OX were significantly longer than wild type, while those of cgr2/3 were shorter (Supplemental Figs. S2A and S3A). Cotyledon area was also smaller in cgr2/3 than Col-0 (Supplemental Fig. S3B). The CGR2OX mutant produced rosettes with greater projected and total leaf area compared to Col-0 throughout the lifecycle (Fig. 1, Supplemental Fig. S2, B and C). In contrast, a significant reduction in projected and total leaf area was observed in cgr2/3. The degree of leaf overlap measured by the ratio between projected and total leaf area was not different between the transgenic lines and Col-0 (Fig. 1). Thus, functional availability of CGR2 and CGR3 has a significant impact on leaf area growth.
Figure 1.
Projected and total leaf area over time in Arabidopsis with altered CGR2 and CGR3 expression. A, Projected leaf area and B, total leaf area at four different harvests (H) is presented for Col-0, cgr2com, CGR2OX, and cgr2/3. H1, H2, H3, and H4 correspond to 29, 49, 63, and 82 DAS, respectively. For clarity, data for H1 is presented as insets in (A) and (B). Values represent the mean ± se and n = 5 plants per line. Statistical differences at α = 0.05 are marked with lower-case letters.
Genetic Depletion of CGR2 and CGR3 Results in Thin Leaves with Small, Densely Packed Mesophyll Cells
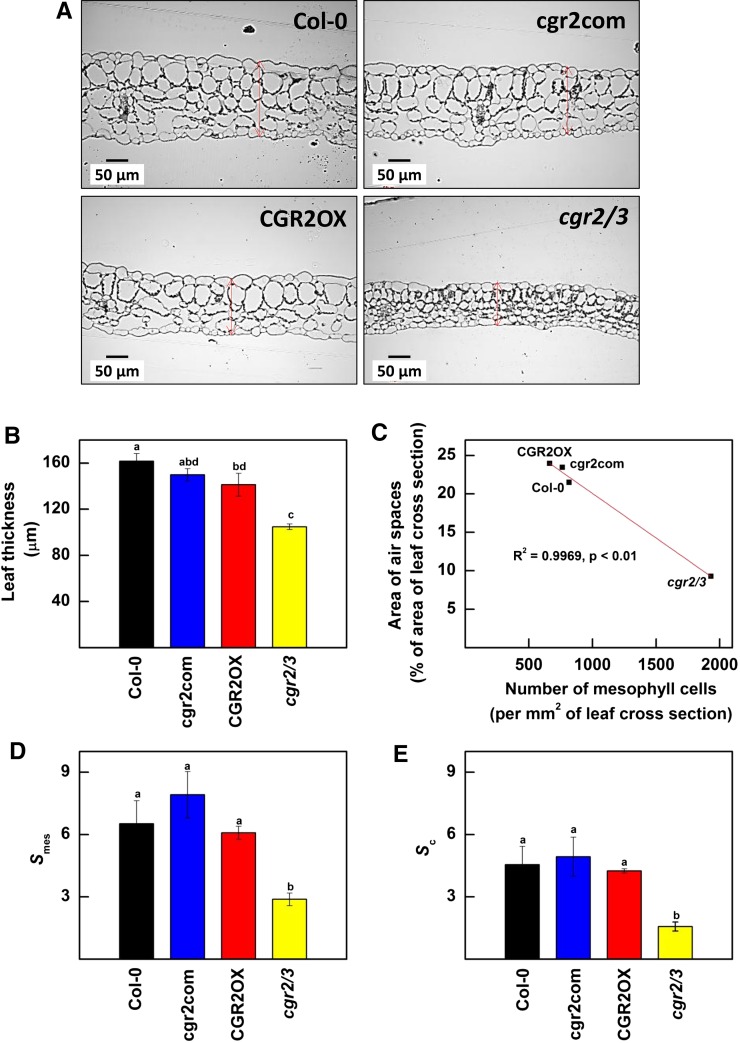
Leaf area and thickness are generally negatively correlated (Lambers et al., 2008; Weraduwage et al., 2015). To determine whether changes in leaf area in CGR2OX and cgr2/3 were accompanied by changes in leaf thickness, we measured leaf thickness. Interestingly, leaves of cgr2/3 were both thin and of small leaf area (Fig. 2, A and B); this trend was observed throughout the lifecycle (data not shown). During early vegetative growth [34 d after seeding (DAS)], CGR2OX produced thinner leaves with a tendency toward more area than Col-0. Nonetheless, the CGR2OX leaves were thicker than those of cgr2/3.
Figure 2.
Leaf thickness and anatomy in Arabidopsis with altered CGR2 and CGR3 expression. A, Representative micrographs of leaf cross sections; B, leaf thickness; C, the relationship between the number of mesophyll cells and size of the intercellular air spaces in the leaf mesophyll; D, the surface area of mesophyll cells exposed to intercellular air spaces per unit leaf area (Smes); and E, the surface area of chloroplasts exposed to intercellular air spaces per unit leaf area (Sc) are presented for Col-0, cgr2com, CGR2OX, and cgr2/3. In (A), leaf thickness is denoted by red double arrows. Data were obtained from 34-d old leaves. In (B), (D), and (E), values represent the mean ± se and n = 4 plants per line. In (C), n = 4 plants per line were used to obtain the mean values for the area of air spaces as a % of area of leaf cross section and the number of mesophyll cells per mm2 of leaf cross section. Statistical differences at α = 0.05 are marked with lower-case letters.
Next, we determined whether CGR2- and CGR-mediated pectin methylesterification was correlated with changes in mesophyll architecture. CGR2OX had larger palisade cells and 3 to 4 mesophyll cell layers compared to Col-0, which produced 4 to 5 layers of comparatively smaller palisade cells (Fig. 2A). However, the mesophyll cells of cgr2/3 were significantly smaller and more numerous than in Col-0 (Fig. 2A), which led to a marked reduction in intercellular airspaces in the leaf mesophyll (Fig. 2C). Consequently, a significant negative correlation was observed between the number of cells and intercellular air spaces in the leaf mesophyll (Fig. 2C). Enhanced cell density and the reduction in intercellular air spaces in cgr2/3 compared to the other Arabidopsis lines also led to a significant reduction in mesophyll cell surface area (Smes) and chloroplast surface area (Sc) exposed to intercellular air spaces per unit leaf area (Fig. 2, D and E).
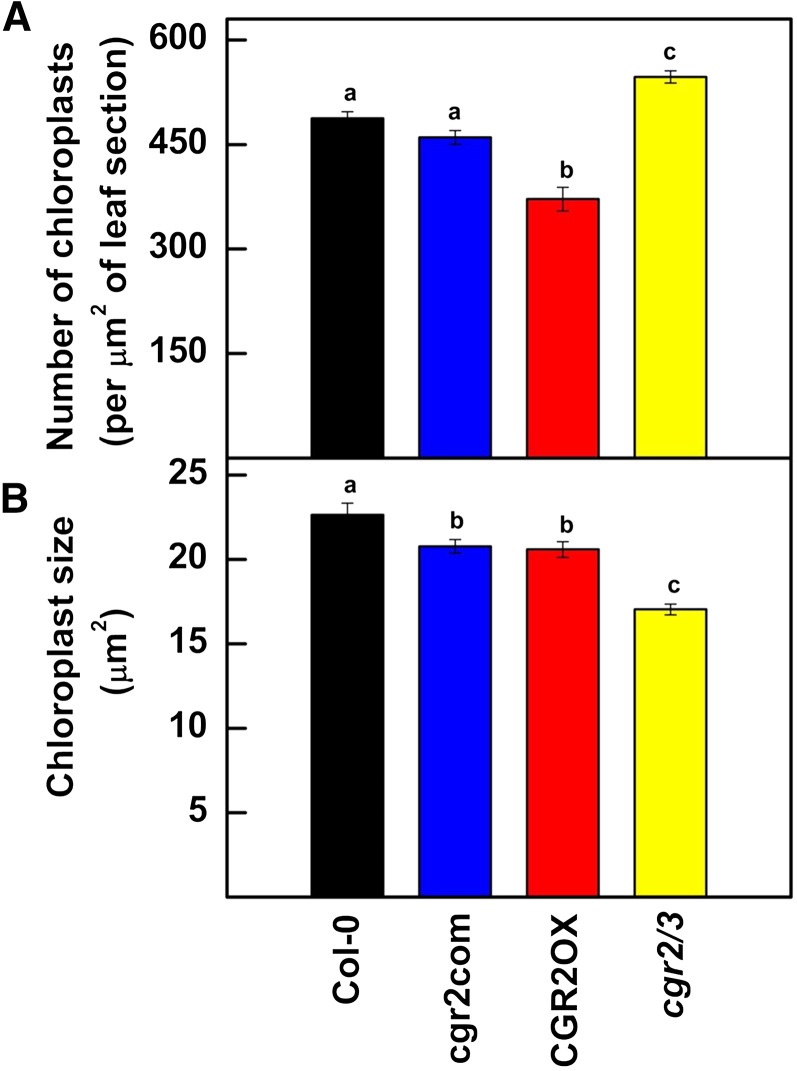
Interestingly also, the number of chloroplasts per unit area of a leaf cross section was lower in CGR2OX and greater in cgr2/3 compared to Col-0 (Fig. 3A). However, the average size of chloroplasts was smaller in all transgenic lines compared to Col-0 (Fig. 3B). The data indicate that the reduction in Sc in cgr2/3 was not a result of a reduction in chloroplast area per unit leaf area, but instead was linked to the significant reduction in intercellular air spaces in the leaf mesophyll. Therefore, our results show that variations in the levels of methylesterification of the cell wall significantly impact leaf thickness, cell density, and mesophyll architecture.
Figure 3.
Abundance and size of chloroplasts in Arabidopsis with altered CGR2 and CGR3 expression. A, Number of chloroplasts per unit area of leaf cross section; B, Size of chloroplasts in 34-d-old leaves is shown for Col-0, cgr2com, CGR2OX, and cgr2/3. Values represent the mean ± se and n = 12. Statistical differences at α = 0.05 are marked with lower-case letters.
Changes in Mesophyll Architecture due to Alterations in CGR-Expression Affected Area-Based Photosynthesis and Respiration
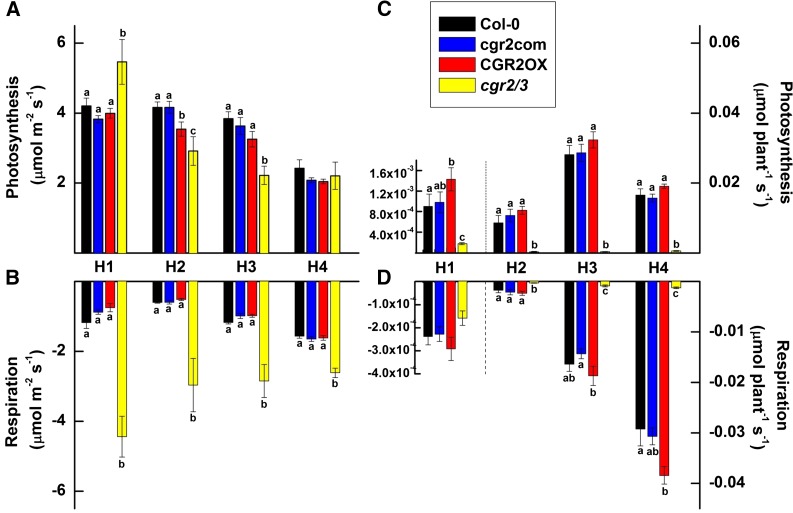
We next aimed to establish whether changes in mesophyll architecture as well as projected and total leaf areas affect net C assimilation in mutant lines with altered CGR-expression. To do this, we measured area- and plant-based photosynthesis and respiration. During early vegetative growth, at around 29 DAS, area-based photosynthesis in cgr2/3 was significantly higher than the other lines (Fig. 4A). Afterward, cgr2/3 showed lower area-based photosynthesis rates compared to Col-0 throughout the vegetative growth phase (Fig. 4A). Area-based photosynthesis in CGR2OX was lower than Col-0 around 49 DAS, but remained higher compared to cgr2/3.
Figure 4.
Photosynthesis and respiration over time in Arabidopsis with altered CGR2 and CGR3 expression. A, Area-based photosynthesis, B, area-based nighttime respiration, C, photosynthesis on a whole plant basis, and D, night-time respiration on a whole plant basis at four different harvests (H) is shown for Col-0, cgr2com, CGR2OX, and cgr2/3. Photosynthesis (C gain) is shown as a positive number (A, C) while respiration (C loss) is shown as a negative number (B, D). H1, H2, H3, and H4 correspond to 29, 49, 63, and 82 DAS, respectively. For clarity, the scale of the Y-axis for H1 in (C) and (D) is presented on the left-hand-side. Values represent the mean ± se and n = 5 plants per line. Statistical differences at α = 0.05 are marked with lower-case letters.
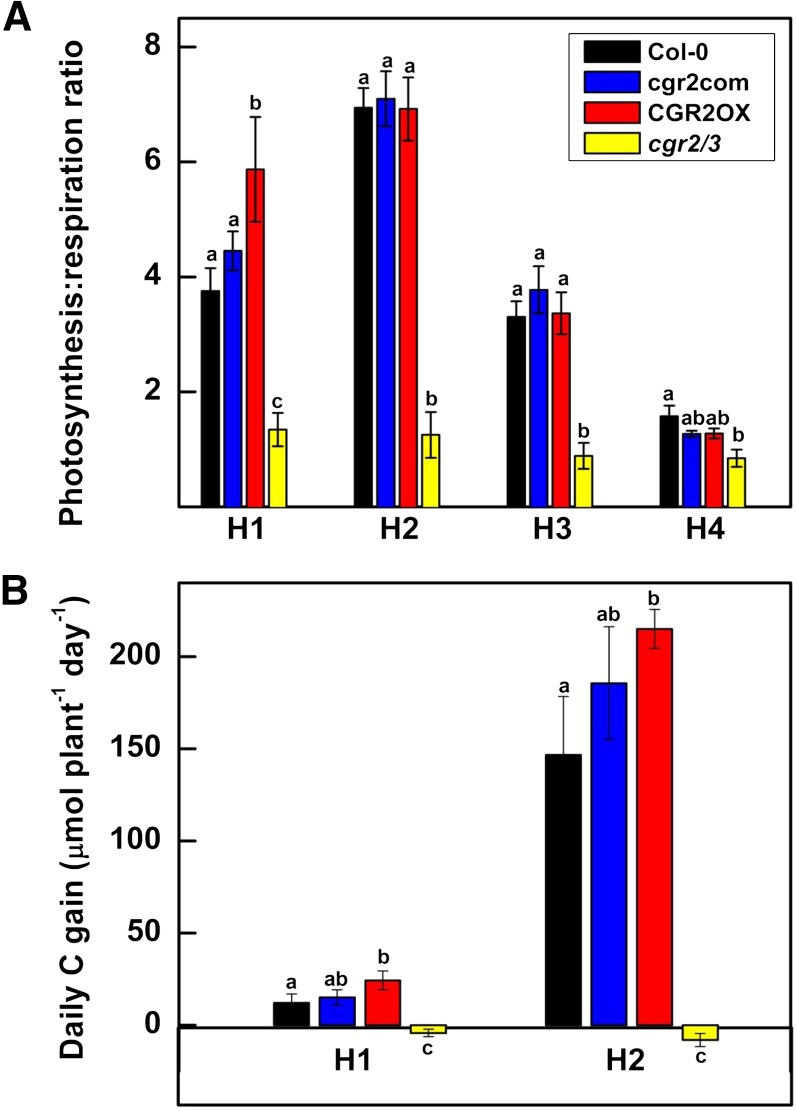
We also established that area-based respiration in cgr2/3 was 2 to 5 times greater than Col-0 (Fig. 4B). Thus, photosynthesis/respiration ratio remained significantly smaller in cgr2/3 throughout the lifecycle (Fig. 5A). Although not statistically significant at the 0.05 significance level, CGR2OX showed a trend toward lower area-based respiration (Fig. 4B) and therefore, its photosynthesis/respiration ratio appeared to be greater in young rosette leaves at 29 DAS (Fig. 5A). Increased area-based respiration in cgr2/3 and lower area-based respiration in CGR2OX correlated well with the differences in the number of mesophyll cells in these lines (Fig. 2C). In general, plant-based photosynthesis in CGR2OX was similar to Col-0 with an upward trend, even though area-based photosynthesis was smaller (Fig. 4, A and C); this was mainly due to the larger projected leaf area in CGR2OX (Fig. 1A). However, owing to its smaller projected leaf area, plant-based photosynthesis in cgr2/3 was much lower than Col-0 (Fig. 4C). Plant-based respiration was greater in CGR2OX, and smaller in cgr2/3 than in Col-0 (Fig. 4D), which could be explained by differences in total leaf area (Fig. 1B).
Figure 5.
Daily C gain in Arabidopsis with altered CGR2 and CGR3 expression. A, Photosynthesis/respiration ratio at four different harvests (H); B, The daily C gain at two different harvests for Col-0, cgr2com, CGR2OX, and cgr2/3 is given. H1, H2, H3, and H4 correspond to 29, 49, 63, and 82 DAS, respectively. Values represent the mean ± se and n = 5 plants per line. Statistical differences at α = 0.05 are marked with lower-case letters.
Daily C gain in rosettes was greater in CGR2OX compared to Col-0 when measured at 29 and 49 DAS, while it was significantly smaller in cgr2/3 (Fig. 5B). The upward trend in daily C gain observed in CGR2OX was a result of its higher plant-based photosynthetic rates due to greater projected leaf area (Figs. 1A and 4C). The decrease in C gain in cgr2/3 resulted from reduced plant-based photosynthesis due to the smaller projected leaf area and smaller photosynthesis/respiration ratio compared to CGR2OX (Figs. 1A, 4C, and 5A). Together these results support the hypothesis that by controlling leaf area and mesophyll architecture, pectin methylesterification is a critical factor for regulation of whole-plant rates of photosynthesis and respiration.
Suppression of Pectin Methylesterification Led to a Reduction in CO2 Availability for Photosynthesis
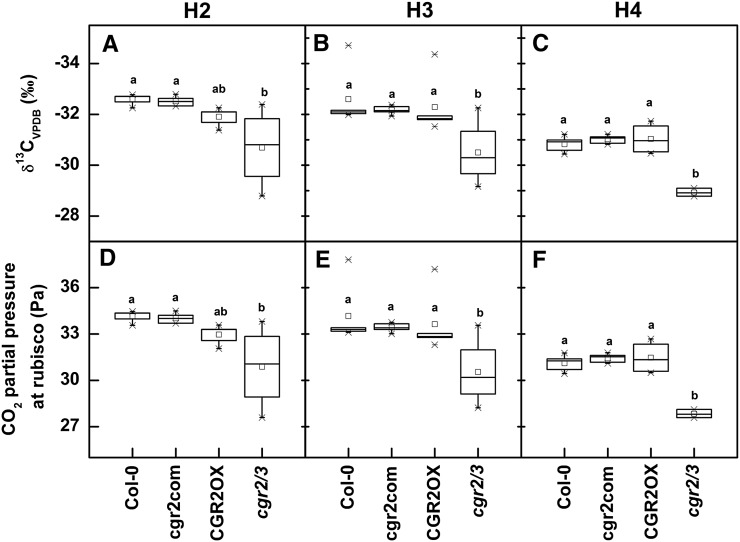
The differences in chloroplast number and size could not account for the large differences observed in area-based photosynthesis between cgr2/3 and the other three Arabidopsis lines (Fig. 3, A and B). Therefore, to investigate whether lower area-based photosynthesis rates in cgr2/3 at harvests 2, 3, and 4 were a result of a reduction in CO2 availability owing to altered mesophyll architecture, the degree of 13C discrimination was analyzed in leaf tissue of the four Arabidopsis lines. The 13C content of cgr2/3 was greater, consistent with less discrimination against 13CO2 during photosynthesis compared to the other lines (Fig. 6, A–C). The average CO2 partial pressure at Rubisco calculated using the δ13CVPDB (Vienna-Pee-Dee Belemnite, VPDB) values showed CO2 availability to be significantly lower in cgr2/3 (Fig. 6, D–F). These data are in agreement with the hypothesis that there is a greater resistance to CO2 diffusion into chloroplasts of cgr2/3, owing to lower surface areas of mesophyll cells and chloroplasts facing intercellular air spaces per unit leaf area as a result of enhanced cell density and reduced intercellular air spaces (Fig. 2, C–E). Therefore, reduction in CGR2 and CGR3 availability appears to have a strong negative impact on area-based photosynthesis owing to reduction in CO2 availability to chloroplasts, most likely as a result of altered mesophyll architecture.
Figure 6.
Degree of resistance to CO2 diffusion through the leaf mesophyll over time in Arabidopsis with altered CGR2 and CGR3 expression. A, B, C, The δ13CVPDB value calculated as the ratio of 13C to 12C isotopes in leaf tissue relative to a VPDB value; D, E, F, CO2 partial pressure at Rubisco at three different harvests (H) is shown for Col-0, cgr2com, CGR2OX, and cgr2/3. H2, H3, and H4 correspond to 49, 63, and 82 DAS, respectively. Values represent the mean ± se and n = 5 plants per line. Statistical differences at α = 0.05 are marked with lower-case letters.
A Decrease in Pectin Methylesterification Increased LMA, and Reduced Plant Growth and Relative Growth Rates
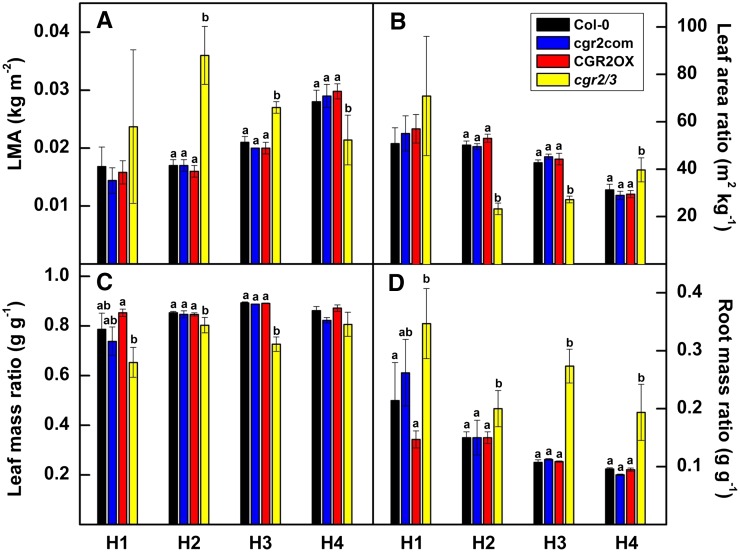
We next analyzed the effects of altered expression of CGR2 and CGR3 on plant growth by measuring leaf growth parameters, plant biomass, and relative growth rates. We found that LMA was higher in cgr2/3 during the early to late vegetative phases, around 29 to 63 DAS (Fig. 7A). In addition, LMA in CGR2OX remained significantly lower than in cgr2/3. Leaf area per unit plant mass (leaf area ratio) and leaf mass ratio (mass of leaf per unit plant mass) were significantly smaller in cgr2/3 than other lines (Fig. 7, B and C). In contrast, root mass ratio (mass of root per unit plant mass) was larger in cgr2/3 than other lines (Fig. 7D).
Figure 7.
Variations in leaf growth parameters over time in Arabidopsis with altered CGR2 and CGR3 expression. A, LMA, B, leaf area ratio, C, leaf mass ratio, and D, root mass ratio at four different harvests (H) for Col-0, cgr2com, CGR2OX, and cgr2/3 is presented. H1, H2, H3, and H4 correspond to 29, 49, 63, and 82 DAS, respectively. Values represent the mean and n = 5 plants per line. Statistical differences at α = 0.05 are marked with lower-case letters.
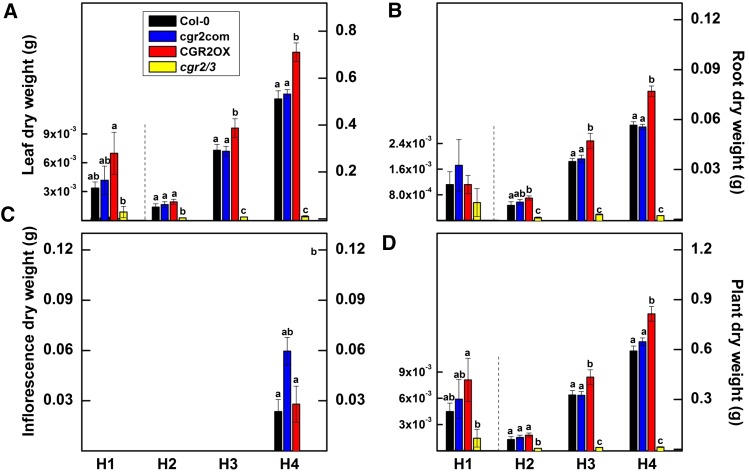
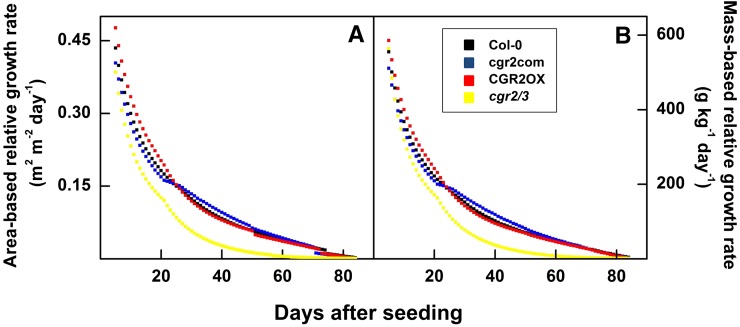
Interestingly, CGR2OX maintained the greatest leaf, root, and plant biomass throughout the lifecycle (Fig. 8, A–D). In contrast, cgr2/3 plants exhibited a significant reduction in leaf, root, and plant dry weight throughout the lifecycle. Collectively, these data reveal that alterations in CGR2 and CGR3 expression can have a significant effect not only on leaf growth, but also on overall plant growth. Therefore, while an increase in pectin methylesterification enhances plant biomass, a decrease in pectin methylesterification reduces it. Modeled relative growth rates revealed that both area-based (Fig. 9A) and mass-based (Fig. 9B) relative growth rates were higher in CGR2OX and smaller in cgr2/3 than Col-0 during most part of the lifecycle. In summary, alterations in CGR2 and CGR3 have a significant impact on important leaf growth parameters such as LMA, and biomass partitioning to leaf growth, and also affect plant growth in terms of biomass accumulation and relative growth rates. LMA showed a negative correlation with plant growth and relative growth rates.
Figure 8.
Variations in dry weight over time in Arabidopsis with altered CGR2 and CGR3 expression. A, Leaf, B, root, C, inflorescence, and D, whole plant dry weight at four different harvests (H) for Col-0, cgr2com, CGR2OX, and cgr2/3 is given. H1, H2, H3, and H4 correspond to 29, 49, 63, and 82 DAS, respectively. For clarity, the scale of the Y-axis for H1 in (A), (B) and (C) is presented on the left-hand-side. Values represent the mean ± se and n = 5 plants per line. Statistical differences at α = 0.05 are marked with lower-case letters.
Figure 9.
Modeled relative growth rates over time in Arabidopsis with altered CGR2 and CGR3 expression. A, Area-based relative growth rates and B, mass-based relative growth rates simulated from days 5 to 84 using the Arabidopsis leaf area growth model fitted with data obtained from Col-0, cgr2com, CGR2OX, and cgr2/3 are presented.
Enhanced Pectin Methylesterification and C Partitioning to Leaf Area Growth
The Arabidopsis leaf area growth model parameterized with measurements obtained from the four Arabidopsis lines was used to estimate how much C was partitioned to new leaf area growth and LMA, as a proportion of net assimilated C that is available for growth. These values, referred to as partitioning coefficients (Table I), would allow determination of whether changes in CGR2 and CGR3 availability could affect C partitioning to growth or respiratory processes. The proportions of C partitioned to leaf growth in total (C partitioned to area + LMA), and to root growth found by 14CO2 feeding experiments at 29 DAS, were used as an initial estimate when setting the partitioning coefficients to leaf growth for the early vegetative growth phase (Supplemental Fig. S4; Table I). Partitioning coefficients obtained from growth modeling for CGR2OX revealed that, compared to the other lines, the CGR2 over-expression line partitioned more of the C available for leaf growth toward leaf area growth and less toward LMA, especially during the early vegetative phase (Table I). In contrast, we found that in cgr2/3 a significantly greater proportion of C was allocated to LMA rather than to leaf area growth (Table I). The differences in C partitioning to leaf area growth and LMA between Col-0 and CGR2OX and cgr2/3 were most prominent during early vegetative phases of growth.
Table I. Comparison of modeled partitioning coefficients during different growth phases for Arabidopsis lines with altered pectin methyltransferase expression.
| Arabidopsis line |
Organ/process to which C is partitioned |
Partitioning coefficients | ||
|---|---|---|---|---|
| Early vegetative growth phase |
Late vegetative growth phase | Reproductive growth phase | ||
| Col-0 | Inflorescence growth | 0.00 | 0.02 | 0.30 |
| Root growth | 0.06 | 0.13 | 0.12 | |
| LMA | 0.17 | 0.10 | 0.10 | |
| Leaf area growth | 0.77 | 0.75 | 0.48 | |
| cgr2com | Inflorescence growth | 0.00 | 0.03 | 0.40 |
| Root growth | 0.03 | 0.12 | 0.12 | |
| LMA | 0.19 | 0.10 | 0.10 | |
| Leaf area growth | 0.78 | 0.75 | 0.38 | |
| CGR2OX | Inflorescence growth | 0.00 | 0.03 | 0.30 |
| Root growth | 0.04 | 0.15 | 0.15 | |
| LMA | 0.16 | 0.11 | 0.10 | |
| Leaf area growth | 0.80 | 0.71 | 0.45 | |
| cgr2/3 | Inflorescence growth | 0.00 | 0.00 | 0.10 |
| Root growth | 0.10 | 0.25 | 0.25 | |
| LMA | 0.24 | 0.18 | 0.10 | |
| Leaf area growth | 0.66 | 0.57 | 0.55 | |
Partitioning coefficients for partitioning of net assimilated C to inflorescence and root growth, LMA, and leaf area growth during the early and late vegetative phases and the reproductive phase for Col-0, cgr2com, CGR2OX, and cgr2/3 were obtained from the Arabidopsis leaf area growth model. The durations of the early and late vegetative phases and the reproductive phase for each line is as follows: for Col-0, 5–51 DAS, 52–74 DAS, and 75–90 DAS; for cgr2com, 5–48 DAS, 49–70 DAS, and 71–90 DAS; for CGR2OX, 5–49 DAS, 50–72 DAS, and 73–90 DAS; and for cgr2/3, assuming flower initiation started at 82 DAS, 5–55 DAS, 56–81 DAS, and 82–90 DAS.
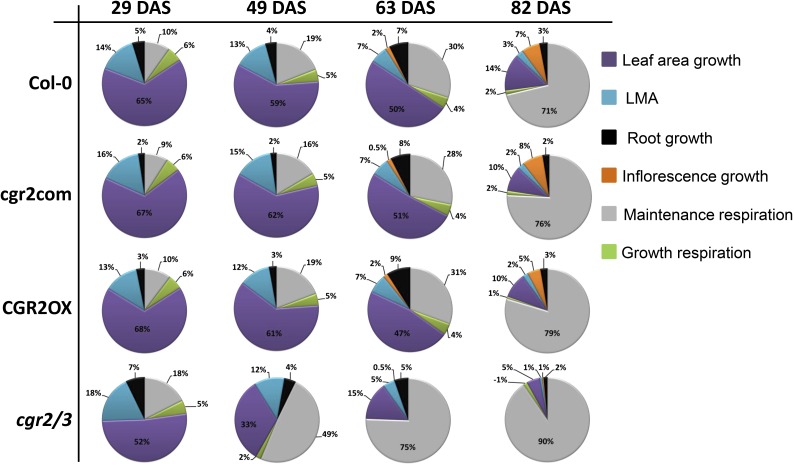
The patterns of C partitioning in transgenic lines seen in Table I were further investigated by determining the amounts of C being partitioned to specific growth processes as well as respiratory processes as a proportion of the total available C in the plant (Fig. 10). CGR2OX still partitioned a greater proportion of total available C to leaf area growth and a lesser proportion to LMA compared to the other lines; cgr2/3 showed an opposite trend to that seen in CGR2OX (Fig. 10). The maintenance respiratory cost increased as the plant aged. Toward the end of its lifecycle (82 DAS), close to 75% of assimilated C was consumed in maintenance respiration (Fig. 10). While the maintenance costs in CGR2OX were somewhat larger than Col-0 toward the end of the lifecycle, a significant enhancement in the proportion of C spent in maintenance respiration could be seen in cgr2/3 throughout the lifecycle (Fig. 10). Collectively, these data provide evidence to support that alteration in CGR2 and CGR3 expression has a significant impact on determining the proportions of C partitioned to new leaf area growth versus LMA, and to maintenance respiration in plants.
Figure 10.
Modeled C partitioning to growth and respiration in Arabidopsis with altered CGR2 and CGR3 expression. Carbon partitioned to support growth in terms of leaf area and LMA, and inflorescence growth, root growth, and maintenance and growth respiration is presented as percentages of the daily available C at 29, 49, 63, and 82 DAS. Data were derived from the Arabidopsis leaf area growth model fitted with data obtained from Col-0, cgr2com, CGR2OX, and cgr2/3.
Growth in CGR2OX and cgr2/3 was Affected More by Alterations in C Partitioning than by Changes in Area-Based Photosynthesis
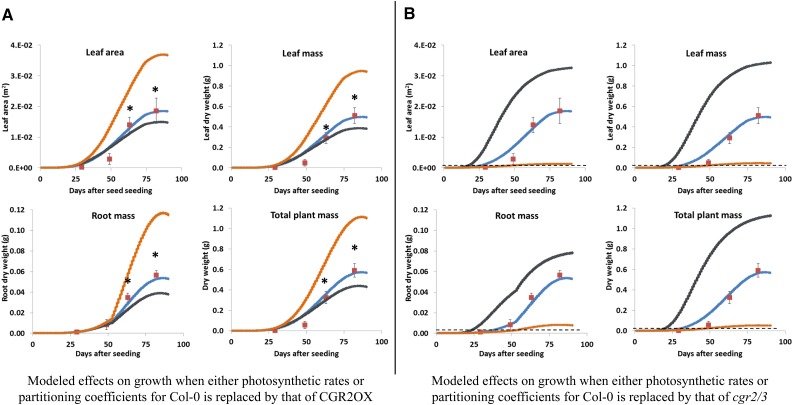
To determine whether the alterations in growth observed in CGR2OX and cgr2/3 were a result of alterations in area-based photosynthesis (Fig. 4A) or partitioning coefficients to leaf area growth and LMA growth (Table I), the values for these parameters in the growth model for Col-0 were substituted separately by corresponding values from the two transgenic lines. Interestingly, by keeping the partitioning coefficients the same, substitution of area-based photosynthesis rates for 1 to 90 d with those of CGR2OX resulted in a small reduction in modeled growth in Col-0 whereas cgr2/3 caused a large increase in growth (gray lines in Fig. 11).
Figure 11.
Comparison of the effects of altered area-based photosynthesis and C partitioning coefficients on plant growth in Arabidopsis with altered CGR2 and CGR3 expression. Modeled effects on leaf area and leaf, root, and plant dry weights when either photosynthetic rates or partitioning coefficients spanning the entire growth cycle for Col-0 are replaced by those of (A) CGR2OX and (B) cgr2/3 are presented. In (A) and (B), blue lines represent modeled growth for Col-0, and red squares represent measured data points (mean ± sd) for Col-0 at 29, 49, 63, and 82 DAS. Gray lines represent modeled data for Col-0 when its area-based photosynthesis rates are replaced by that of either CGR2OX (A) or cgr2/3 (B). Orange lines represent modeled data for Col-0 when its partitioning coefficients (Table I) are replaced by that of either CGR2OX (A) or cgr2/3 (B) without making any changes to area-based photosynthesis rates of Col-0. In (A), asterisks represent measured data for CGR2OX at 63 and 82 DAS. In (B), the dotted line indicates the upper limit of measured data for cgr2/3.
However, by keeping area-based photosynthesis the same, substituting the partitioning coefficients of Col-0 with that of CGR2OX had a strikingly large positive effect on modeled growth; cgr2/3 partitioning coefficients had a significantly large negative effect on growth that mirrored the observed differences in growth in the CGR2 over-expression line and the CGR2 and CGR3 double mutant line (orange lines in Fig. 11). These observed changes in growth could be achieved by substituting the partitioning coefficients of only the early vegetative growth phase (data not shown). The data show that the observed changes plant growth in CGR2OX and cgr2/3 were not a result of differences in photosynthetic rates, but a result of differences in C partitioning to growth in terms of leaf area and LMA, especially during early vegetative growth. Thus, our data support the hypothesis that the magnitude of plant growth is determined by how photosynthetic C is partitioned to new leaf area growth and LMA.
DISCUSSION
In this study, we investigated how modification of cell wall plasticity via the genetic manipulation of pectin methyltransferases, CGR2 and CGR3, affects C assimilation, leaf respiration, and overall plant growth through effects on leaf area growth and mesophyll structure. We also wanted to further understand the relationship between photosynthesis and plant growth by determining whether the changes in overall plant growth in Arabidopsis with altered cellular availability of CGR2 and CGR3 were a result of changes in growth in terms of leaf area and LMA or due to changes in area-based photosynthesis. We found that over-expression of CGR2 had a significant positive effect on leaf area and plant growth. On the other hand, suppression of CGR2 and CGR3 expression caused a marked increase in LMA and a reduction in leaf area and plant growth. Altered expression of CGR2 and CGR3 also had a significant impact on photosynthesis and respiration through altered mesophyll architecture. The differences in plant growth in Arabidopsis with altered CGR2 and CGR3 levels were linked to changes in C partitioning to leaf area growth and LMA rather than altered area-based photosynthesis rates. These findings establish that CGR2- and CGR3-mediated pectin methylesterification affects mesophyll architecture, C accumulation, C partitioning, leaf and plant growth, and the relationship between photosynthesis and plant growth.
CGR2 and CGR3 Directly Affect Leaf Mass per Unit Area by Altering Both Leaf Thickness and the Mass of Leaf per Unit Volume of Leaf Tissue
Often, thicker leaves are associated with larger LMA due to an increase in the number of cell layers, which can increase leaf mass per unit leaf area (Lambers et al., 2008; Poorter et al., 2009; Villar et al., 2013; Weraduwage et al., 2015). However, a negative correlation exists between leaf mass density or LMD (mass of leaf per unit volume of leaf tissue) and leaf thickness, and both parameters can affect LMA as follows: LMD (kg m−3) = leaf mass/(leaf area × leaf thickness) and LMA (kg m−2) = LMD × leaf thickness (Witkowski and Lamont, 1991; Lambers et al., 2008; Poorter et al., 2009; Villar et al., 2013). Therefore, leaf thickening or an increase in LMA can occur due to an increase in leaf thickness or LMD. While thicker leaves have been shown to be associated with larger mesophyll cells (elongated, especially in the depth direction) and larger air spaces, LMD has been associated with smaller proportions of intercellular air spaces and smaller cells (Tsuge et al., 1996; Poorter et al., 2009; Villar et al., 2013). In addition to being affected by anatomical changes, LMA and LMD can also be determined by variations in cellular and chemical composition (Cunningham et al., 1999; Lambers et al., 2008). For example, plants with slow growth rates possess a larger number of smaller cells with thicker cell walls, and a greater proportion of lignin and other cell wall polysaccharides per unit leaf area that can increase LMD and LMA (Cunningham et al., 1999; Lambers et al., 2008). A key aspect of cellular mass is represented by organelles, and plants with higher growth rates may possess large cells with a lower number of chloroplasts, which results in lower LMA (Lambers et al., 2008).
Interestingly, while both CGR2OX and cgr2/3 had thinner leaves compared to Col-0, cgr2/3 showed a significant increase in LMA. Such increase in cgr2/3 can be attributed to increased LMD as a result of enhanced cell density and reduction of intercellular air spaces in the leaf mesophyll. Smaller cells can lead to concentration of cellular metabolites, which can also lead to increased LMD. In addition, cgr2/3 also possessed a larger number of chloroplasts per unit leaf area. The proportions of cell wall components per unit leaf area are also likely greater in cgr2/3 compared to the other backgrounds. Therefore, collectively the data clearly show that although cgr2/3 had thinner leaves, enhanced LMD led to greater leaf mass per unit area (or lower SLA) in cgr2/3. Conversely, LMA in CGR2OX was significantly smaller than cgr2/3. Leaves of CGR2OX were also thicker than cgr2/3 and contained larger intercellular airspaces, and larger cells with a lower number of chloroplasts. Therefore, our results support that variation in LMA between CGR2OX and cgr2/3 resulted from the combination of increased leaf thickness and reduced leaf mass density.
The significant alterations in mesophyll architecture observed during this study are linked to changes in CGR2 and CGR3 expression. Based on the model that reduced methylation of pectin may enhance Ca2+-mediated cross linking of GalUA in cell walls, thereby preventing normal cell expansion in cgr2/3 (Burton et al., 2000; Wolf et al., 2009; Kim et al., 2015), we propose that the larger number of smaller mesophyll cells observed in cgr2/3 during this study is likely linked to the hardening of the cell wall and consequent restriction of cell wall expansion. This in turn is likely to cause an increase of the cell number per unit leaf area in cgr2/3 compared to Col-0. In addition, cross linking between GalUA and Ca2+ in cell wall pectin is required for cellular adhesion (Caffall and Mohnen, 2009). Also a reduction in GalUA-Ca2+ complex formation in Solanum lycopersicum resulted in large air spaces in the fruit pericarp caused by dramatic reductions in the middle-lamellae-mediated cell-to-cell adhesion (Caffall and Mohnen, 2009). The observed decrease in air spaces in cgr2/3 and the dense distribution of the cells (hence thinner leaves) are likely due to enhanced cell-to-cell adhesion because of the lower degree of methylesterified pectin in cgr2/3 compared to Col-0. In contrast, enhanced methylation of pectin promotes cell expansion (Kim et al., 2015); this resulted in the observed presence of wider palisade cells and a lower number of cell layers (hence thinner leaves) in CGR2OX than Col-0. The number of cells and area of airspaces in CGR2OX differed only marginally from Col-0. However, CGR2OX did have a smaller number of smaller chloroplasts compared to Col-0, which may be an indication of lower LMA. Whether over-expression of CGR2 has a dosage effect, and over-expression of both CGR2 and CGR3 would result in larger and thicker leaves with lower cell density remains to be investigated.
Thinner leaves usually correlate with larger SLA or smaller LMA (Lambers et al., 2008; Weraduwage et al., 2015). However, based on the negative correlation between LMD and leaf thickness, it is likely that LMD would start to increase should leaf thickness decrease below a certain optimum, thereby resulting in an increase in LMA. This was demonstrated in cgr2/3, where suppression of CGR2 and CGR3 resulted in leaves that were too thin, leading to higher cell density and LMA. This can have significant negative effects on leaf gas exchange properties and daily C gain, as discussed below. The above phenotype was rescued by complementing with CGR2, as seen in cgr2com or by over-expressing CGR2 as in CGR2OX. Thus, the degree of CGR2- and CGR3-mediated pectin methyl esterification can directly affect LMA by altering both leaf thickness and leaf mass density.
CGR2 and CGR3 Affect Area-Based Respiration and Daily C Gain by Altering LMD and LMA
In general, leaves growing under high light develop thicker leaves with multiple cell layers and higher LMA (lower SLA), and incurs higher area-based respiration rates corresponding to their greater mass per unit leaf area (Mitchell et al., 1999; Lambers et al., 2008). In contrast, plants grown under low light have lower LMA (higher SLA), which optimizes light capture and lowers area-based respiration rates (Lambers et al., 2008). This assists in maximizing daily C gain and compensates for lower area-based photosynthesis rates when growing under low light conditions (Lambers et al., 2008). Although cgr2/3 produced thinner leaves, the marked enhancement in area-based respiration positively correlated with enhanced leaf cell density and higher LMA. Higher area-based respiratory rates led to smaller photosynthesis/respiration ratio, and when coupled with smaller projected leaf area, they had a significant negative impact on daily carbon gain. Thus, pectin methylesterification affects area-based respiration rates through alterations in leaf cell density, LMD, and LMA and consequently affect photosynthetic efficiency in plants.
CGR2 and CGR3 Directly Affect Area-Based Photosynthesis by Altering Mesophyll Architecture
Diffusion of CO2 into chloroplasts occurs along the path that poses the least resistance through the cell wall, plasma membrane, chloroplast envelope, and into the chloroplast stroma (Terashima et al., 2006). Therefore, Sc represents the active area through which CO2 diffuses in to the chloroplast stroma (Terashima et al., 2006). A decrease in Sc leads to a reduction in CO2 concentration at Rubisco and therefore to a reduction in the carboxylation/oxygenation ratio (Terashima et al., 2006). Parameters that affect CO2 diffusion into the chloroplast stroma are thickness of the mesophyll cell wall, internal conductance to CO2 diffusion, and stomatal conductance (Terashima et al., 2006). Sc has been shown to be positively correlated with internal conductance, while the latter is negatively correlated with cell wall thickness (Terashima et al., 2006).
In contrast to our original hypothesis, area-based photosynthesis rates were significantly lower in cgr2/3 for most of the lifecycle, even though this mutant possessed smaller projected leaf area and denser leaves. This study revealed that this reduction in area-based photosynthesis in cgr2/3 was not a result of differences in chloroplast number and size, but, mainly a result of a reduction in CO2 partial pressure at Rubisco due to restrictions imposed on CO2 diffusion by increased cell density and reduced airspaces in the leaf mesophyll that lowered Sc. Preliminary investigations revealed that stomatal conductance is not negatively impacted in the mutant lines examined during this study (data not shown). Therefore, the observed reduction in area-based photosynthetic rates is most probably due to a cumulative effect of lower Sc, higher internal resistance and greater cell wall thickness per unit area. The greater area-based photosynthesis rates in cgr2/3 observed during early stages of vegetative development may have been a result of leaves and cell walls being comparatively thinner in young plants. Sc values and CO2 partial pressure at Rubisco in cgr2/3 were restored to the levels of Col-0 in cgr2com owing to alterations brought about by CGR2 expression in its leaf anatomy: thicker leaves with less dense mesophyll and more intercellular air spaces. Sc and CO2 partial pressure in CGR2OX were similar to that of Col-0. The lower area-based photosynthesis in CGR2OX may have resulted from thinner but larger leaves, which may have then resulted in less photosynthetic machinery per unit leaf area. Correspondingly, chloroplast number and size were lower in CGR2OX. These data emphasize the importance of pectin methylesterification in fine-tuning cell expansion and size and cell-to-cell adhesion qualities in building a leaf mesophyll optimized for CO2 exchange. While thin leaves are associated with lower LMA (or enhanced SLA), it is important to maintain an optimum thickness to maintain sufficient intercellular air spaces, which would maintain high internal conductance, Smes, and Sc, and therefore higher carboxylation rates.
Pectin Methylesterification Mediates the Relationship between Photosynthesis and Plant Growth through Alterations in C Partitioning to Leaf Area Growth and Thickening
As hypothesized, suppression of CGR2- and CGR3-mediated pectin methylesterification had a profound negative effect on leaf area growth while over-expression of CGR2 significantly enhanced leaf area growth. Suppression of CGR2 and CGR3 also led to a reduction in plant dry weight. Over-expression of CGR2 enhanced plant dry weight. Growth modeling revealed that the observed variations in growth were not caused by changes in area-based photosynthesis. In fact, high rates of area-based photosynthesis measured in cgr2/3 during early growth were found to be sufficient to drive growth higher than that seen in Col-0. Thus, growth had the opposite trend (CGROX > Col-0 > cgr2/3) compared with area-based photosynthetic rate (cgr2/3 > Col-0 > CGROX). To account for enhanced overall plant growth, the amount of net assimilated C available for growth has to increase, which will depend on the total amount of C assimilated and the amount of C respired to support maintenance processes (Weraduwage et al., 2015). Our study showed that CGR2 and CGR3, through pectin methylesterification, are capable of affecting total C assimilation by directly altering leaf area growth and by altering maintenance respiratory costs through direct effects on LMA. We also showed that although CGR2 and CGR3 affect area-based photosynthetic rates, the latter is not a major factor affecting overall plant growth.
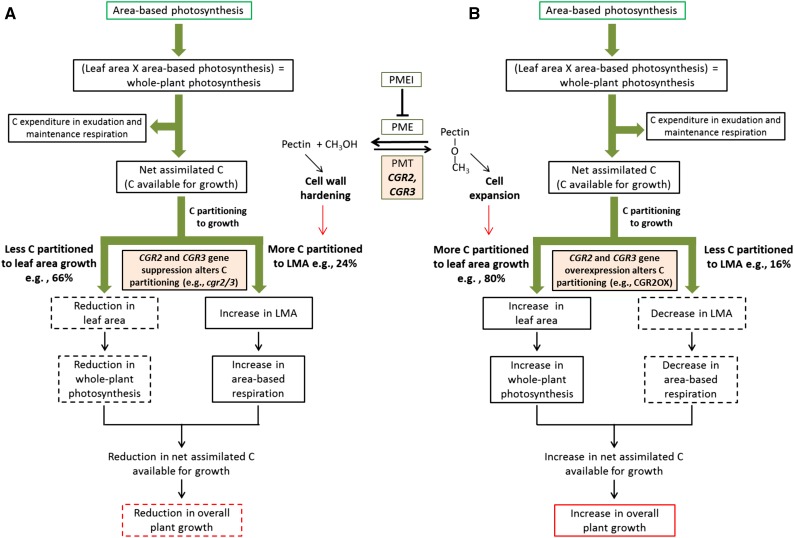
A reduction in pectin methylesterification by reduced expression of CGR2 and CGR3 seems to enhance cell-wall hardening and cell-to-cell adhesion through increased cross linking between GalUA and Ca2+, favoring an increase in LMA against cell expansion and posing a greater demand for C for LMA growth compared to area growth (Fig. 12A). Hence less C will be utilized in area growth of new leaves and more C will be utilized in increasing LMA, leading to a smaller projected leaf area (Fig. 12A). Smaller projected leaf area yields lower whole plant C assimilation. In contrast, an increase in pectin methylesterification via enhanced expression of CGR2 and CGR3 seems to reduce cell wall hardening and cell-to-cell adhesion, and thereby favors cell expansion and area growth against increases in LMA; it poses a greater demand for C for leaf area growth compared to that for LMA growth (Fig. 12B). Hence, more C will be utilized in area growth of new leaves, and a lower proportion C will be utilized in LMA growth, leading to larger projected leaf area and enhanced whole-plant C assimilation (Fig. 12B). Suppression of CGR2 and CGR3 increases respiratory costs through higher LMA, resulting in lower photosynthesis/respiration ratio, lower daily C gain, reduced net assimilated C available for growth, and ultimately reduced plant growth (Fig. 12A); over-expression of CGR2 yields opposite physiological effects (Fig. 12B).
Figure 12.
CGR2- and CGR3-mediated pectin methylesterification regulates the relationship between photosynthesis and plant growth. A schematic diagram outlining how (A) suppression or (B) over-expression of CGR2 and CGR3 gene expression affects the relationship between photosynthesis and plant growth in Arabidopsis is presented. Suppression of CGR2 and CGR3 reduces the degree of pectin methylation, which leads to an increase in cell-to-cell adhesion, cation-mediated cross linking of GalUA, and consequent hardening of cell walls. CGR2 over-expression increases the degree of pectin methylation, which allows cell expansion through reduced cell-to-cell adhesion and cation-mediated cross linking. We propose that pectin methyltransferase enzyme, through its ability to directly alter cell expansion, determines the amount of C partitioned to leaf area growth versus growth in terms of LMA. For example, while more C is partitioned to growth in terms of LMA in the CGR2 and CGR3 double knockout mutant, in the CGR2 over-expression line more C is partitioned to leaf area growth. An increase in LMA leads to enhanced area-based respiration and a reduction in leaf area contribute to a reduction in whole plant photosynthesis. Collectively, this results in a reduction in C available for growth and consequently a decrease in overall plant growth; an opposite trend is seen in CGR2OX. Thus, CGR2 and CGR3, through their ability to alter the degree of methylated pectin in the cell wall of the mesophyll cells, determine how photosynthate is utilized to grow the plant. In other words, CGR2- and CGR3-mediated pectin methylesterification affects the relationship between photosynthesis and plant growth by regulating the proportions of C that is partitioned to leaf area growth and LMA. Final overall growth mainly depends on the expression patterns of CGR2 and CGR3 and how much C is partitioned to area growth and LMA and not on area-based photosynthesis. PME: pectin methylesterase; PMEI: PME inhibitor; PMT: pectin methyltransferase. CH3OH is methanol.
There may be other genes that also impact partitioning of carbon toward leaf area growth or LMA. One example is the xyloglucan endotransglucosylase/hydrolase (XTH) gene family. Reduced expression of XTH8 and XTH31 gene expression led to reduced leaf width, length, and leaf area (Miura and Hasegawa, 2010). The leaf mesophyll of these mutant lines consisted of a large number of small mesophyll cells per unit area (Miura and Hasegawa, 2010). Suppression of XTH21 led to a reduction in cellulose, and xyloglucan content with subsequent reductions in both leaf and plant size (Liu et al., 2007). One of the major roles of XTH enzymes is cleavage of cross linkages between cellulose and hemicellulose microfibrils that promotes wall loosening and cell expansion. This may explain the above leaf phenotypes in xth8, xth21, and xth31 mutant lines (Liu et al., 2007; Miura and Hasegawa, 2010). Thus, through its effects on cell wall properties, XTH genes may be able to alter C partitioning to leaf area and LMA and thereby affect overall plant growth similar to the role played by CGR genes.
In summary, the results from this study are in agreement with findings from Weraduwage et al. (2015) that optimization of leaf area growth and plant growth can be achieved by increased partitioning of C to leaf area growth with concomitant reduction in partitioning to growth in terms of LMA especially during the early vegetative growth phase (Weraduwage et al., 2015), and that photosynthesis drives growth through alterations in C partitioning to leaf area and LMA growth. In this study, we found that CGR2 and CGR3 directly affect this relationship by altering the degree of cell expansion and/or adhesion, thereby driving C partitioning by generating C demands for leaf area growth and for growth in terms of LMA.
CONCLUSION
Our study supports the model that qualitative and quantitative changes in pectin in the cell wall have a significant impact on area-based photosynthesis and respiration through alterations in leaf thickness, mesophyll cell density, and area of the leaf blade. Such changes in mesophyll architecture directly altered LMA and consequently area-based respiration. CGR2 and CGR3 affected area-based photosynthesis by altering CO2 availability at Rubisco. These genes could affect plant-based photosynthesis by enabling enhanced light capture through larger leaf area. These data emphasizes the importance of pectin methylesterification in fine-tuning cell expansion and size, and adhesion qualities when building an optimum leaf to maximize photosynthesis under different environmental conditions. While thin leaves are associated with reduced LMA, it is important to maintain an optimum thickness to maintain sufficient intercellular air spaces that would maintain high internal conductance as reflected in Smes and Sc and therefore higher carboxylation rates. CGR2 and CGR3, which directly alter the size of the leaf blade and mesophyll architecture, may find applications in the future to improve light interception in crop plant canopies.
Over-expression of CGR2 had a significant positive effect on leaf area and plant growth. Suppression of CGR2 and CGR3 caused a marked increase in LMA, and a reduction in leaf area and plant growth. These differences resulted through changes in C partitioning to leaf area growth and growth in terms of LMA and not as a result of altered area-based photosynthesis rates. Therefore, photosynthesis drives plant growth through alterations in C partitioning to new leaf area growth and LMA growth, and this study discovered that pectin methylesterification affects this relationship by directly altering the degree of cell expansion and positioning in the leaves to create dynamic carbon demands in leaf area growth and leaf mass thereby driving C partitioning for these processes. Collectively, these results uncover an unexpected connection between cell wall composition and photosynthesis, and support the novel (to our knowledge) model that through its role in modulation of organ development, the cell wall plasticity is a key factor influencing photosynthetic processes in land plants.
MATERIALS AND METHODS
Growth Analysis
Wild-type Arabidopsis (Col-0), cgr2/3 (loss of function double mutant line of CGR2 and CGR3), CGR2OX (CGR2 over-expression line), and cgr2com (cgr2/3 complemented by CGR2) (Kim et al., 2015) were used in the study. Gene accession numbers for CGR2 and CGR3 are At3g49720 and At5g65810, respectively. Plants were grown hydroponically in 1/2-strength Hoagland’s solution under a light intensity of 120 μmol m−2 s−1, using 8-h photoperiods with respective day- and night-time temperatures of 22°C and 20°C, and 60% relative humidity. The hydroponics system was housed in a model no. GC-20 Bigfoot Series Growth Chamber (BioChambers, Winnipeg, Manitoba, Canada).
Plant growth parameters including projected and total leaf area, leaf, root, and inflorescence dry weights, relative growth rates, specific leaf area (SLA), leaf mass per unit leaf area (LMA), leaf area ratio, and leaf, root, and inflorescence mass ratios were measured as described in Weraduwage et al. (2015). When calculating SLA, LMA, leaf area ratio, and leaf, root, and inflorescence mass ratios, the total leaf area, and organ and plant mass corresponding to each harvest was used: SLA (m2 kg−1) = total leaf area/leaf mass; LMA (kg m−2) = leaf mass/total leaf area; leaf area ratio (m2 kg−1) = total leaf area/total plant mass; and leaf, root or inflorescence mass ratio (g g−1) = leaf, root, or inflorescence mass/total plant mass. Growth measurements were obtained throughout the lifecycle of the plant at 2–3-week intervals at 29, 49, 63, and 82 d after seeding (DAS). The 100-seed weight prior to stratification and sowing, cotyledon area, and hypocotyl length was also measured.
Determination of Leaf Thickness, Mesophyll Cell Density, Smes, Sc, and Chloroplast Size and Abundance
Leaves of similar age from 34- and 51-d-old rosettes were used for anatomical measurements. Leaves were harvested and processed for leaf cross sectioning as described in Weraduwage et al. (2015), at the Center for Advanced Microscopy, Michigan State University. Thin (500-nm) leaf sections (PTXL Ultramicrotome; RMC Boeckeler Instruments, Tucson, AZ) were observed under a SpectralView FV1000 Confocal Laser Scanning Microscope (Olympus, Melville, NY), and the distance between the adaxial and abaxial leaf surfaces was measured to determine leaf thickness.
Micrographs of leaf cross sections were used to determine the number of palisade and spongy parenchyma cells, the total number of cells, and the number of mesophyll cell layers in a 20,000 μm2 area of a leaf cross section. These data were later expressed per mm2. ImageJ (http://imagej. nih.gov/ij/) software was utilized to measure the total area of intercellular air spaces, total length of mesophyll cells, total length of mesophyll cells exposed to intercellular air spaces (Lmes), and the total length of chloroplasts exposed to intercellular air spaces (Lc). The latter three measurements were utilized to determine the surface area of mesophyll cells exposed to intercellular air spaces (Smes) and the surface area of chloroplast exposed to intercellular air spaces (Sc) per unit leaf area as described in Evans et al. (1994).
A series of z-stack images of leaves of 34-d-old plants was acquired using an inverted laser scanning confocal microscope (model no. LSM510 META, Carl Zeiss; http://www.zeiss.com). Chloroplasts were imaged using an ECPlan-Neofluar 40×/1.30 oil M27 objective (Carl Zeiss) at a 512 × 512 pixel resolution using an excitation wavelength of 514 nm and an emission wavelength of 673 nm. The different planes acquired were then projected on the same image and transformed in binary using Image J. The resulting number of chloroplasts per unit area and average chloroplast size was also measured using ImageJ.
Gas Exchange Measurements, 14CO2 Feeding Experiments, and Analysis of Leaf 13C Discrimination
Whole rosette photosynthesis and nighttime dark respiration were measured at 2–3 week intervals at 29, 49, 63, and 82 DAS. A custom-built rosette-gas-exchange chamber connected to a model no. LI-6400 portable gas exchange system (LI-COR Biosciences, Lincoln, NE) was used to obtain photosynthesis and respiratory measurements. Conditions in the Arabidopsis rosette gas-exchange chamber during photosynthesis measurements were: a leaf temperature of 22°C; [CO2] of 400 μmol mol−1; and 120 μmol m−2 s−1 light intensity. During night-time respiration measurements, the leaf temperature was maintained at 20°C. Flow rate was set at 500 μmol s−1 when making measurements from 49- and 63-d-old plants. For 29- and 82-d-old plants, flow rate was set at 300 and 700 μmol s−1, respectively, to better control humidity.
After the initial measurements of photosynthesis, 29-d-old Arabidopsis rosettes were fed with 14CO2 for 10 min. After a 2-h chase period, leaf and root tissue were harvested separately into scintillation vials. Radioactivity was determined as disintegrations per min (DPM) by liquid scintillation counting. The DPM values recorded from roots and rosettes and the total DPM were used to calculate the proportion of C present in these organs.
The content ratio of 13CO2 to 12CO2 in Arabidopsis freeze-dried leaf material was measured at the Stable Isotope Ratio Facility for Environmental Research, University of Utah (Salt Lake City, UT), by mass spectrometry. These data were used to calculate the δ13CVPDB value, which is the 13C to 12C isotopic ratio of leaves relative to the 13C to 12C isotopic ratio of the Vienna-Pee-Dee Belemnite (VPDB) standard (Farquhar et al., 1982). The δ13CVPDB values were used to calculate the CO2 partial pressure at Rubisco using Eq. 12 given in Farquhar et al. (1982). Values for the various factors are as follows: δ-value of CO2 in the air at present = −8.4‰ (http://www.esrl.noaa.gov); partial pressure of CO2 in the air = 39 Pa; 13C discrimination associated with the diffusion of CO2 through air = 4.4‰; and 13C discrimination in the carboxylation reaction = 27‰ (Farquhar et al., 1989).
Analysis of Cell Wall Polysaccharides in Leaves
Preparation of alcohol-insoluble residues and uronic acid and methyl ester assays were performed based on the methodology described in Kim et al. (2015). The matrix polysaccharide (hemicellulose) and crystalline cellulose composition, and soluble sugar content in leaves, were measured at the Great Lakes Bioenergy Research Center Cell Wall Facility, Michigan State University (East Lansing, MI) as described in Kim et al. (2015).
Parameterization of the Arabidopsis Leaf Area Growth Model
Development, parameterization, and execution of the Arabidopsis leaf area growth model were described in detail in Weraduwage et al. (2015). This model is a tool to determine partitioning coefficients of C that could give rise to the growth patterns observed in different plants (Weraduwage et al., 2015). In the model, plant growth is divided into four growth stages: (1) heterotrophic phase (1–4 DAS); (2) early vegetative phase; (3) late vegetative phase; and the (4) reproductive phase. Ninety days of plant growth are simulated in the model using a fixed time step of 1 h and the photoperiod is set at 8 h. As described in detail in Weraduwage et al. (2015), the following were used as inputs of the model: weight of storage reserves, ratio of mobilized storage reserves to stored reserves, initial leaf area, initial leaf mass, projected to total leaf area ratio, net photosynthesis rate per unit leaf area, proportion of C stored as starch during the day, leaf maintenance and growth respiratory coefficients, photoperiod, and lengths of growth phases.
Values for area-based photosynthesis obtained during the four harvests were fitted with a polynomial third-order equation to extrapolate area-based photosynthesis through the entire 90 d of the lifecycle. Similarly, measured projected to total leaf area ratios during the four harvests were fitted with a polynomial second-order equation to extrapolate the values over 90 d. These extrapolated values were used in the model as input photosynthesis values and projected to total leaf area ratios over the life span. The model also takes both growth and maintenance respiration into consideration. The model considers maintenance respiration of a plant/particular organ to be proportional to the corresponding dry mass and growth respiration to be proportional to the growth rate (Penning de Vries et al., 1974; Amthor, 1984; Thomas et al., 1993; Lambers et al., 2008). The model has set values for maintenance coefficients (amount of C respired to maintain the existing mass of the plant or specific organ, μmol C g−1 s−1), and for growth coefficients (amount of C respired per unit increase in mass, g g−1) based on values provided in the literature (Amthor, 1984; Mariko, 1988) and assumes that growth respiration coefficients of leaf growth in terms of area and LMA are the same throughout the lifecycle. The net amount of C available for growth and maintenance processes of the plant is determined as the product of area-based photosynthetic rate and the projected leaf area. Next, C consumed in maintenance respiration and exudation is subtracted to determine the net assimilation rate, which provides the amount of C available for growth. The model then simulates the partitioning of C available for growth to leaf area growth, growth in terms of LMA, and root and inflorescence growth based on the set partitioning coefficients that are adjustable to facilitate fitting of the data. Partitioning coefficients vary based on the growth phases. The increase in mass during growth in terms of leaf area and LMA, and root and inflorescence growth, is calculated by subtracting the C consumed in growth respiration from the total C allocated for growth of that particular organ. Accordingly, the model simulates the increase in leaf area and mass, and the mass of the inflorescence and roots. The model calculated SLA, LMA, and leaf, root, and inflorescence mass ratios over time based on the equations given above.
To fit the modeled data to measured data, 16 partitioning coefficient parameters (partitioning to the inflorescence, roots, leaf area and LMA, for each of the four growth phases: germination phase, early and late vegetative phases, and reproductive phase) were adjusted so that the modeled and measured leaf area, mass of inflorescence, root, and leaf matched (Weraduwage et al., 2015). C partitioning data obtained by feeding Arabidopsis rosettes at 29 DAS with 14CO2 were used as initial estimates that were fine-tuned to fit the data. Sensitivity of the model to changes in the inputs was tested in Col-0 by simulating an increase or decrease of 1% in C partitioned to growth processes, and other model inputs, and noting the resulting response in output. The model was considered sensitive, in cases where more than 1% variation occurred in model outputs because of changing a particular input.
Statistical Analyses of Experimental Data
Four to five biological replicates per line were used to collect experimental data at a given time point. Statistical analyses were carried out using SPSS 22 (IBM, Armonk, NY). The effects of altered CGR2 and CGR3 expression on plant growth and gas exchange were compared using a univariate general linear model and the differences between means were tested by subjecting data to one-way ANOVA at α = 0.05, followed by a Fisher’s Least Significant Difference Test. Measured data presented in figures represent the mean ± standard error (SE).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers At3g49720 and At5g65810.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Cell wall composition in Arabidopsis with altered CGR2 and CGR3 expression.
Supplemental Figure S2. Hypocotyl and rosette size in Arabidopsis with altered CGR2 and CGR3 expression.
Supplemental Figure S3. Early growth phenotypes in Arabidopsis with altered CGR2 and CGR3 expression.
Supplemental Figure S4. Partitioning of photosynthetic C to shoots and roots in Arabidopsis with altered CGR2 and CGR3 expression.
Supplementary Material
Acknowledgments
We are grateful to Drs. Sean E. Weise (Department of Biochemistry and Molecular Biology), Cliff Foster (the Cell Wall Facility, Great Lakes Bioenergy Research Center), Alicia Withrow and Melinda Frame (Center for Advanced Microscopy) of Michigan State University (East Lansing, MI), and Dr. Suvankar Chakraborty (Stable Isotope Ratio Facility for Environmental Research) of the University of Utah for their support. We thank Jim Klug and Cody Keilen (Growth Chamber Facility) of Michigan State University for their assistance and all members of the Sharkey and Brandizzi labs for their support.
Glossary
- DAS
days after seeding
- DPM
disintegrations per min
- LMA
mass per unit leaf area
- LMD
leaf mass density
- SLA
specific leaf area
- VPDB
Vienna-Pee-Dee Belemnite
Footnotes
Funding for this research was provided by the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy (award no. DE-FG02-91ER20021) and in part by the Department of Energy Great Lakes Bioenergy Research Center (Department of Energy Office of Science no. BER DE-FC02-07ER64494). Partial salary support for T.D.S. and F.B. came from Michigan AgBioResearch.
Articles can be viewed without a subscription.
References
- Amthor JS. (1984) The role of maintenance respiration in plant growth. Plant Cell Environ 7: 561–569 [Google Scholar]
- Burton RA, Gibeaut DM, Bacic A, Findlay K, Roberts K, Hamilton A, Baulcombe DC, Fincher GB (2000) Virus-induced silencing of a plant cellulose synthase gene. Plant Cell 12: 691–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffall KH, Mohnen D (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res 344: 1879–1900 [DOI] [PubMed] [Google Scholar]
- Cunningham SA, Summerhayes B, Westoby M (1999) Evolutionary divergences in leaf structure and chemistry, comparing rainfall and soil nutrient gradients. Ecol Monogr 69: 569–588 [Google Scholar]
- Evans JR, von Caemmerer S, Setchell BA, Hudson GS (1994) The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of rubisco. Aust J Plant Physiol 21: 475–495 [Google Scholar]
- Farquhar G, Ehleringer J, Hubick K (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40: 503–537 [Google Scholar]
- Farquhar G, O’Leary M, Berry J (1982) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Funct Plant Biol 9: 121–137 [Google Scholar]
- Galmés J, Ochogavía JM, Gago J, Roldán EJ, Cifre J, Conesa MÀ (2013) Leaf responses to drought stress in Mediterranean accessions of Solanum lycopersicum: anatomical adaptations in relation to gas exchange parameters. Plant Cell Environ 36: 920–935 [DOI] [PubMed] [Google Scholar]
- Heldt H-W, Piechulla B (2010) Plant Biochemistry, Ed 4 Academic Press, London, UK [Google Scholar]
- Honda H, Fisher JB (1978) Tree branch angle: maximizing effective leaf area. Science 199: 888–890 [DOI] [PubMed] [Google Scholar]
- Kim HS, Delaney TP (2002) Over-expression of TGA5, which encodes a bZIP transcription factor that interacts with NIM1/NPR1, confers SAR-independent resistance in Arabidopsis thaliana to Peronospora parasitica. Plant J 32: 151–163 [DOI] [PubMed] [Google Scholar]
- Kim S-J, Held MA, Zemelis S, Wilkerson C, Brandizzi F (2015) CGR2 and CGR3 have critical overlapping roles in pectin methylesterification and plant growth in Arabidopsis thaliana. Plant J 82: 208–220 [DOI] [PubMed] [Google Scholar]
- Lambers H, Chapin F, Pons T (2008) Plant Physiological Ecology, Ed 2 Springer, New York, NY [Google Scholar]
- Lionetti V, Raiola A, Camardella L, Giovane A, Obel N, Pauly M, Favaron F, Cervone F, Bellincampi D (2007) Overexpression of pectin methylesterase inhibitors in Arabidopsis restricts fungal infection by Botrytis cinerea. Plant Physiol 143: 1871–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YB, Lu SM, Zhang JF, Liu S, Lu YT (2007) A xyloglucan endotransglucosylase/hydrolase involves in growth of primary root and alters the deposition of cellulose in Arabidopsis. Planta 226: 1547–1560 [DOI] [PubMed] [Google Scholar]
- Mariko S. (1988) Maintenance and constructive respiration in various organs of Helianthus annuus L. and Zinnia elegans L. Bot Mag 101: 73 [Google Scholar]
- Mitchell KA, Bolstad PV, Vose JM (1999) Interspecific and environmentally induced variation in foliar dark respiration among eighteen southeastern deciduous tree species. Tree Physiol 19: 861–870 [DOI] [PubMed] [Google Scholar]
- Miura K, Hasegawa PM (2010) Sumoylation and other ubiquitin-like post-translational modifications in plants. Trends Cell Biol 20: 223–232 [DOI] [PubMed] [Google Scholar]
- Penning de Vries FW, Brunsting AHM, van Laar HH (1974) Products, requirements and efficiency of biosynthesis: a quantitative approach. J Theor Biol 45: 339–377 [DOI] [PubMed] [Google Scholar]
- Pilling J, Willmitzer L, Bücking H, Fisahn J (2004) Inhibition of a ubiquitously expressed pectin methyl esterase in Solanum tuberosum L. affects plant growth, leaf growth polarity, and ion partitioning. Planta 219: 32–40 [DOI] [PubMed] [Google Scholar]
- Poorter H, Niinemets U, Poorter L, Wright IJ, Villar R (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol 182: 565–588 [DOI] [PubMed] [Google Scholar]
- Shipley B. (2002) Trade-offs between net assimilation rate and specific leaf area in determining relative growth rate: relationship with daily irradiance. Funct Ecol 16: 682–689 [Google Scholar]
- Terashima I, Hanba YT, Tazoe Y, Vyas P, Yano S (2006) Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J Exp Bot 57: 343–354 [DOI] [PubMed] [Google Scholar]
- Thomas RB, Reid CD, Ybema R, Strain BR (1993) Growth and maintenance components of leaf respiration of cotton grown in elevated carbon dioxide partial pressure. Plant Cell Environ 16: 539–546 [Google Scholar]
- Tsuge T, Tsukaya H, Uchimiya H (1996) Two independent and polarized processes of cell elongation regulate leaf blade expansion in Arabidopsis thaliana (L.) Heynh. Development 122: 1589–1600 [DOI] [PubMed] [Google Scholar]
- Villar R, Ruiz-Robleto J, Ubera JL, Poorter H (2013) Exploring variation in leaf mass per area (LMA) from leaf to cell: an anatomical analysis of 26 woody species. Am J Bot 100: 1969–1980 [DOI] [PubMed] [Google Scholar]
- Weraduwage SM, Chen J, Anozie FC, Morales A, Weise SE, Sharkey TD (2015) The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front Plant Sci 6: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski ETF, Lamont B (1991) Leaf specific mass confounds leaf density and thickness. Oecologia 88: 486–493 [DOI] [PubMed] [Google Scholar]
- Wolf S, Mouille G, Pelloux J (2009) Homogalacturonan methyl-esterification and plant development. Mol Plant 2: 851–860 [DOI] [PubMed] [Google Scholar]
- Xiao C, Anderson CT (2013) Roles of pectin in biomass yield and processing for biofuels. Front Plant Sci 4: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.