The phytohormone cytokinin is an essential negative regulator of the arsenic detoxification machinery and is thus a key factor in plant tolerance and adaptation to arsenic.
Abstract
The presence of arsenic in soil and water is a constant threat to plant growth in many regions of the world. Phytohormones act in the integration of growth control and stress response, but their role in plant responses to arsenic remains to be elucidated. Here, we show that arsenate [As(V)], the most prevalent arsenic chemical species in nature, causes severe depletion of endogenous cytokinins (CKs) in the model plant Arabidopsis (Arabidopsis thaliana). We found that CK signaling mutants and transgenic plants with reduced endogenous CK levels showed an As(V)-tolerant phenotype. Our data indicate that in CK-depleted plants exposed to As(V), transcript levels of As(V)/phosphate-transporters were similar or even higher than in wild-type plants. In contrast, CK depletion provoked the coordinated activation of As(V) tolerance mechanisms, leading to the accumulation of thiol compounds such as phytochelatins and glutathione, which are essential for arsenic sequestration. Transgenic CK-deficient Arabidopsis and tobacco lines show a marked increase in arsenic accumulation. Our findings indicate that CK is an important regulatory factor in plant adaptation to arsenic stress.
Since the inception of life, arsenic in the biosphere has been a constant challenge to the survival of life forms. Although all organisms on earth have developed strategies to cope with this extremely toxic metalloid (Rosen, 2002; Tripathi et al., 2007), arsenic currently poses a major worldwide environmental problem (Naujokas et al., 2013).
Arsenate [As(V)] is the most abundant chemical form of arsenic. Due to its structural similarity to phosphate (Pi), it is easily incorporated into plants and other organisms through Pi transporters. Once taken up by the cell, As(V) is rapidly reduced to arsenite [As(III)] by As(V) reductases. As(III) is either extruded from the cytoplasm or is sequestered by phytochelatins (PCs) and other related thiol-containing compounds and is compartmentalized into vacuoles (Tripathi et al., 2007).
In plants, when As(V) is perceived, Pi transporters are rapidly downregulated, and thiol compound accumulation increases concomitantly to cope with the metalloid. These two responses are key As(V) tolerance strategies for natural plant populations (Meharg and Macnair 1992; Bleeker et al., 2006). Accumulation of PC and other related thiol-containing compounds is widely used by plants for heavy metal detoxification. PCs are synthesized from glutathione (GSH) by the enzyme PHYTOCHELATIN SYNTHASE1 (PCS1). As(V) induces PCS1, together with two other genes involved in GSH biosynthesis: γ-GLUTAMYLCYSTEINE SYNTHETASE and GLUTATHIONE SYNTHETASE (GSH2; Sung et al., 2009). In addition, As(III) activates GSH and PC accumulation; As(V) reductase activity is thus essential for arsenic tolerance (Bleeker et al., 2006). Arabidopsis thaliana ARSENATE REDUCTASE QTL1 (AtARQ1, At2g21045; also termed HAC1; Chao et al., 2014), an As(V) reductase-encoding gene, is also transcriptionally regulated by As(V) (Sánchez-Bermejo et al., 2014). Arsenic tolerance thus requires coordinated expression of several As(V)-responsive genes involved in As(V) reductase activity and PC accumulation.
Exposure to stress arrests plant growth. When plants perceive As(V), for example, root growth is immediately inhibited (Li et al., 2007; Shri et al., 2009; Yoon et al., 2015). Root meristem activity can be restored by removing As(V) from the medium. Root growth recovery depends on the function of an As(V) reductase that is essential for arsenic detoxification (Sánchez-Bermejo et al., 2014), which supports the idea that plants are able to integrate arsenic tolerance mechanisms into developmental programs. In general, phytohormones are involved in reconfiguration of developmental patterns in response to abiotic stress (Peleg and Blumwald, 2011; Zwack and Rashotte, 2015), although their role in arsenic-mediated growth control is currently unknown.
Here, we characterized the involvement of the hormone cytokinin (CK) in the As(V) response. We found that As(V) provokes severe depletion of endogenous CK levels, leading to activation of As(V) tolerance mechanisms, including As(V) reductase activity and PC and GSH accumulation, all critical for arsenic detoxification. CK is therefore an essential component of plant strategies that lead to As(V) tolerance.
RESULTS
CK-Deficient Plants Have an Enhanced As(V) Tolerance Phenotype
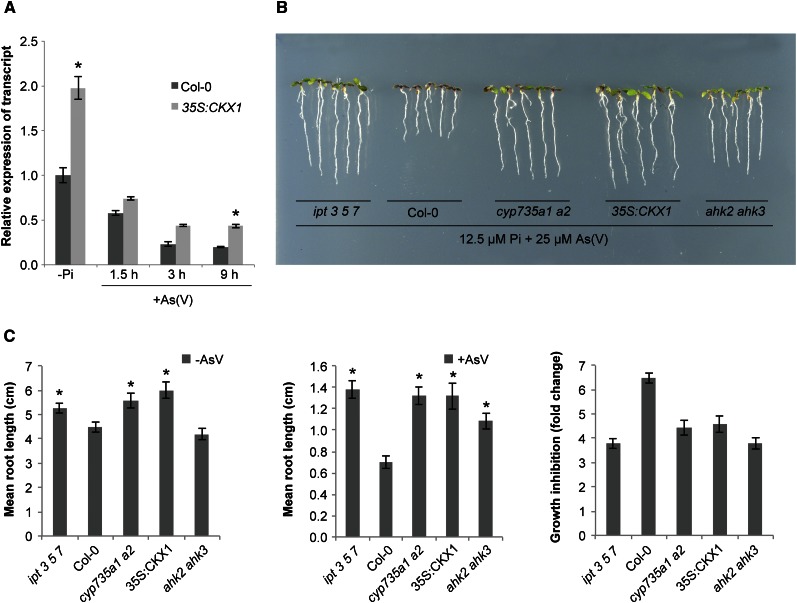
In response to As(V), expression of the As(V)/Pi-transporter gene PHT1;1 is quickly repressed, which provides an efficient strategy for As(V) tolerance (Castrillo et al., 2013). CK acts as a negative regulator of PHT1;1 expression in response to Pi starvation (Martín et al., 2000; Franco-Zorrilla et al., 2002; Wang et al., 2006). To determine the effect of CK on PHT1;1 repression in response to As(V), we performed a time course analysis of PHT1;1 transcript accumulation in As(V)-exposed plants. We used Arabidopsis (Arabidopsis thaliana) lines that constitutively express the CYTOKININ OXIDASE/DEHYDROGENASE1 (CKX1) gene (35S:CKX1; Werner et al., 2003); all bioactive CK levels are strongly decreased in these plants (Werner et al., 2003, 2010; Nishiyama et al., 2011). qRT-PCR analysis showed that in the absence of As(V), CK deficiency increased PHT1;1 expression relative to that observed in wild-type plants, in line with previous studies. After As(V) exposure, CKX1-overexpressing lines rapidly repressed PHT1;1 expression (2.7-fold) to a similar degree or even more than in wild-type plants (Fig. 1A). Absolute mRNA levels for PHT1;1 after As(V) exposure are thus similar in CKX1-overexpressing and wild-type plants.
Figure 1.
Reduction of endogenous CK confers As(V) tolerance. A, qRT-PCR analysis of PHT1;1 expression in wild-type plants (Col-0) and in a 35S:CKX1-overexpressing line grown in +Pi medium for 7 d, transferred to −Pi medium for 3 d, then transferred to −Pi liquid medium for the indicated time, alone (−Pi) or with 30 μm As(V). Values show mean ± sd (n = 3). B, As(V) tolerance phenotypes of ipt 3 5 7, wild-type (Col-0), cyp735a1 a2, 35S:CKX1, and ahk2 ahk3 plants grown on 12.5 μm Pi supplemented with 25 μm As(V) for 10 d. C, Root length of indicated mutant, transgenic, and wild-type plants grown alone (left) or with As(V) (center) were measured in plants growth as described in B. Right, root growth inhibition (fold change) by As(V) compared to growth of corresponding plant types in the absence of As(V). Values show mean ± sd (n = 5). Means are significantly different (Student’s t test, *P < 0.01).
As CK is defined as a negative regulator of several stress responses (Tran et al., 2007; Srivastava et al., 2009; Werner et al., 2010; Nishiyama et al., 2011; Jeon and Kim, 2013), we examined the As(V) tolerance phenotype using CK-depleted transgenic lines and mutants. Since CKX1-overexpressing lines and CK signaling mutants show increased root length (Werner et al., 2003, 2010; Riefler et al., 2006), we compared root growth inhibition in the presence of As(V) (Fig. 1B) relative to root length in the corresponding plants grown in As(V)-free media (Supplemental Fig. S1). The fold change in growth reduction between these conditions showed that growth arrest was lower in all mutant and transgenic lines, with lower CK levels than wild-type ecotype Columbia (Col-0) plants (Fig. 1, B and C). These lines included the 35S:CKX1 transgenic line as well as the triple mutant ipt 3 5 7 and the cyp735a1 a2 double mutant (both with impaired CK biosynthesis), and the double receptor mutant ahk2/ahk3 with reduced CK sensitivity (Fig. 1, B and C). Relative root growth inhibition in the CK mutants was <4.5-fold, while it was >6-fold in wild-type plants. Similar PHT1;1 mRNA levels in wild-type and CK mutants, with less As(V) sensitivity in the latter, suggest that other mechanisms involved in arsenic tolerance must be under CK control.
As(V) Reduces Endogenous CK Levels
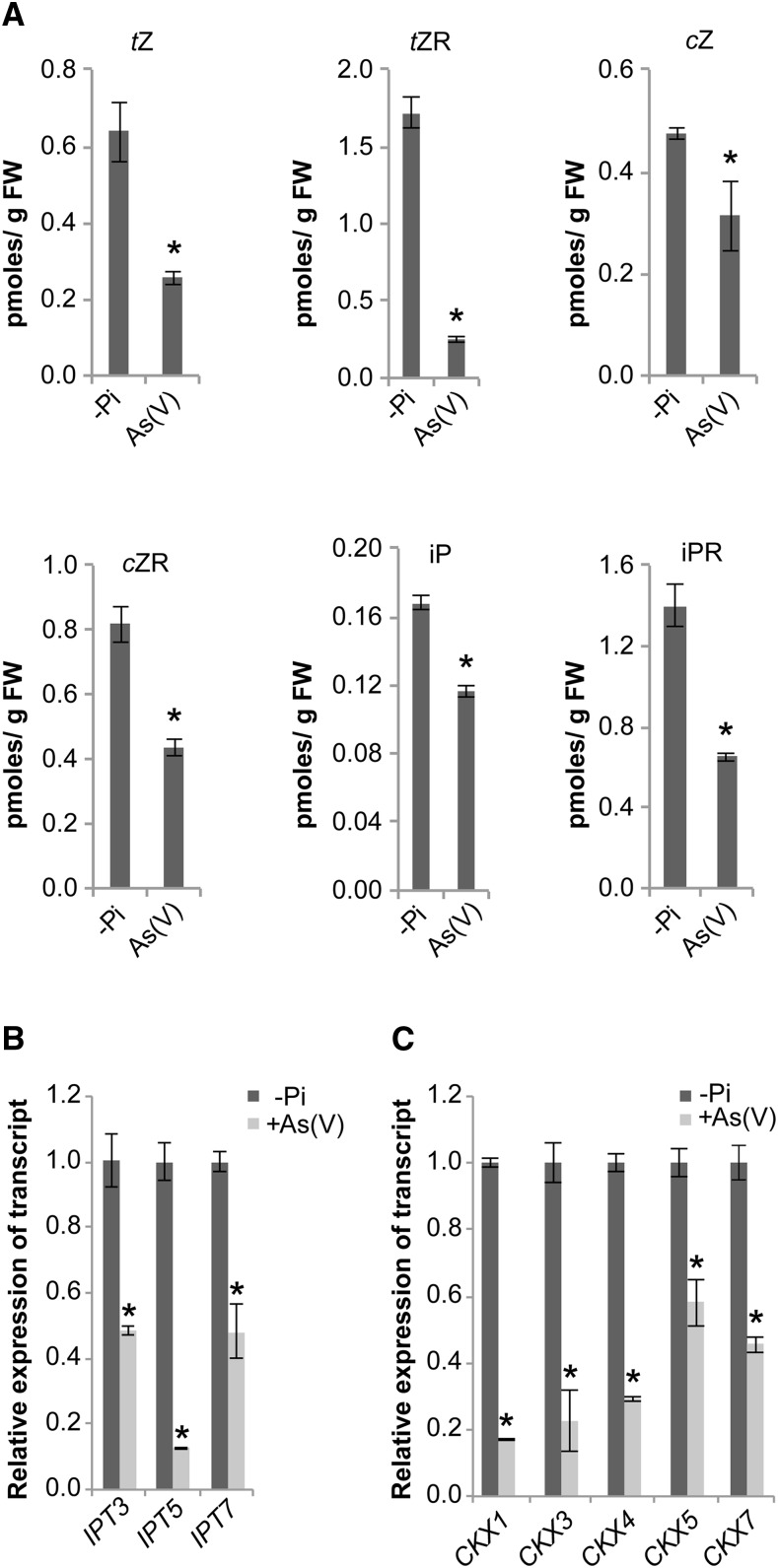
The arsenic tolerance phenotype shown by CK-depleted transgenic lines and CK signaling mutants led us to test the As(V) effect on endogenous CK levels. Quantification of biologically active CK metabolites (iP, tZ, cZ) and their ribosides (iPR, tZR, cZR) in As(V)-exposed Arabidopsis plants showed that concentrations of all species were significantly reduced after As(V) exposure, particularly of tZ-type species (Fig. 2A). The As(V) tolerance phenotype of the cyp735a1 a2 double mutant, in which tZ-type CK are specifically reduced (Kiba et al., 2013), was similar to that of CKX1-overexpressing lines, in which the level of all CK metabolites is lower (Fig. 1, B and C). Consistent with lower CK levels, the IPT genes (IPT3, IPT5, and IPT7) responsible for biosynthesis of the bulk of CK during vegetative growth were markedly down-regulated after As(V) exposure (Fig. 2B). Expression of five CKX family members (CKX1, CKX3, CKX4, CKX5, and CKX7) was also repressed, which might be the consequence of lower CK synthesis (Fig. 2C). As(V) thus reduces CK levels, particularly of the tZ type, which suggests the biological relevance of CK depletion for As(V) tolerance in plants.
Figure 2.
As(V) depletes endogenous CKs. A, Wild-type plants (Col-0) were grown in +Pi medium for 7 d, transferred to -Pi medium for 3 d, and then to -Pi liquid medium alone or with 30 μm As(V) for 6 h. CK levels were analyzed as pmol/g fresh weight (FW) of whole plants. Values show mean ± sd (n = 3). Means are significantly different (ANOVA, *P < 0.05). B and C, As(V) inhibits expression of CK metabolism genes. qRT-PCR analysis of IPT (B) and CKX (C) gene expression. Treatment was as in A. Values show mean ± sd (n = 3), t test *P < 0.05.
To further understand the basis of CK depletion in response to As(V), we compared a transcriptome profile of the As(V) response (Castrillo et al., 2013) with a meta-analysis of CK microarray data (Brenner and Schmülling, 2015). We found that As(V) down-regulates a significant number of genes that are considered CK core-inducible (36 of 65 TOP65CytK), including CK biosynthesis genes and response regulator genes (Table 1; Supplemental Table S1). As(V) repression of three CK core responsive genes was confirmed by qRT-PCR expression analysis (Supplemental Fig. S2). These data showed that the CK receptor gene CRE1/AHK4 was also significantly down-regulated in response to As(V), which would probably result in lower CK signaling. Indeed, reduced expression of numerous CK-responsive genes supports this idea (Supplemental Table S1; Supplemental Fig. S2).
Table I. As(V) down-regulates CK core response genes. The total number of up- and down-regulated genes after As(V) exposure (1.5 or 8 h; Castrillo et al., 2013) was compared with 65 genes identified as CK core response genes (TOP65CytK) from a meta-analysis of microarray data (Brenner and Schmülling, 2015). Numbers for observed/expected indicate As(V)-regulated genes that overlap with CK core response genes. The expected number of overlapping genes was calculated assuming random distribution. Enrichment was assessed using a hypergeometric test with Bonferroni correction; significant overrepresentation is in italics (P < 0.05).
| TOP65CytoK | |||||
|---|---|---|---|---|---|
| Array | Gene Expression | No. of Genes | Observed | Expected | P-Value |
| As(V) -1.5 h | Up | 1936 | 3 | 3.39 | 1 |
| Down | 1518 | 14 | 2.66 | 1.19E-06 | |
| As(V) -8 h | Up | 1263 | 3 | 2.62 | 1 |
| Down | 926 | 32 | 1.92 | 3.93E-31 | |
The Plant Detoxification Machinery Is Modulated by Endogenous CK
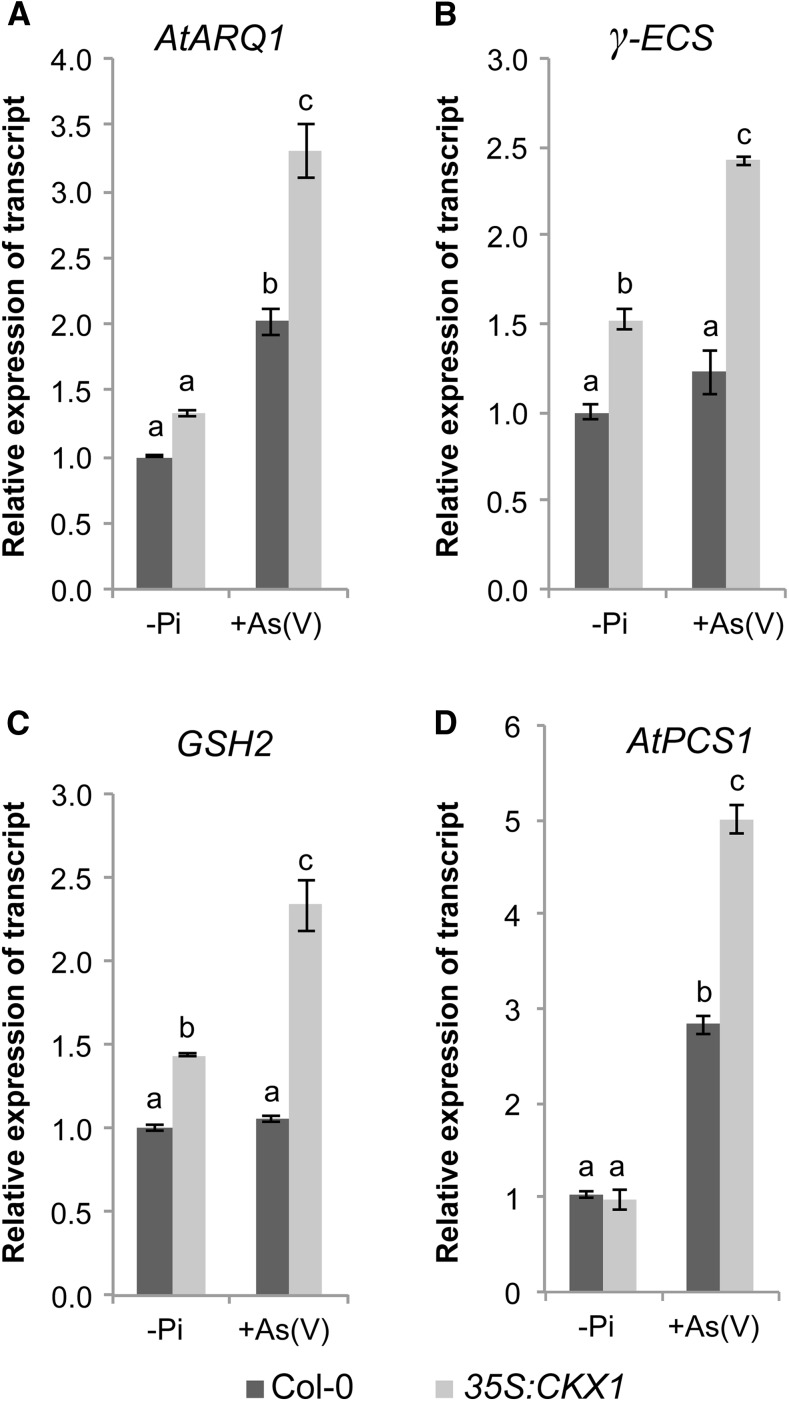
To identify the molecular mechanism that underlies As(V) tolerance in response to CK depletion, we tested whether CK deficiency alters essential As(V) resistance mechanisms in plants. As(V) repression of the As(V)/Pi transporter gene, an essential strategy in As(V) tolerance, was very efficient in the CKX1-overexpressing lines; however, Pi transporter transcript levels were higher in the As(V)-treated CKX1-overexpressing line than in wild-type plants (Fig. 1A). Alternative mechanisms must therefore be invoked to explain the increased As(V) tolerance of low CK-accumulating plants. To determine whether CKs are involved in regulation of the detoxification machinery, we analyzed expression of the biothiol biosynthetic genes γ-GLUTAMYLCYSTEINE SYNTHETASE, GSH2, and AtPCS1 in wild-type and CKX1-overexpressing plants (Fig. 3). Since As(III) is necessary for PC biosynthesis and accumulation, the action of As(V) reductases is essential for As(V) tolerance in plants (Bleeker et al., 2006); we thus also analyzed expression of the recently identified As(V) reductase gene AtARQ1/HAC1 (Chao et al., 2014; Sánchez-Bermejo et al., 2014). qRT-PCR expression analysis of wild-type plants showed that PCS1 and AtARQ1 transcript accumulation was induced after 6-h As(V) exposure. In the CKX1-overexpressing line, the As(V) responsiveness of AtARQ1 and other PC biosynthetic genes (γ−ECS, GSH2, and AtPCS1) was increased compared to wild-type controls (Fig. 3). In accordance with these findings, microarray data for CK-treated Arabidopsis seedlings showed that, in response to this phytohormone, AtPCS1 and AtARQ1 are down-regulated 4.0- and 4.4-fold, respectively (Brenner et al., 2005). In the CKX1-overexpressing line, these genes were not up-regulated in the absence of As(V) (Fig. 3). Nonetheless, transcripts altered in response to CK do not always behave as opposites in CK-deficient plants (Brenner and Schmülling, 2012). These results thus indicate that CK negatively regulates the expression of genes involved in biothiol accumulation and that the As(V) tolerance phenotype of the CKX1-overexpressing plants is due at least in part to the up-regulation of genes related to the As(V) detoxification machinery.
Figure 3.
Genes involved in thiol compound accumulation are up-regulated in CK-depleted transgenic plants. A to D, qRT-PCR expression analysis of AtARQ1 (A), γ−ECS (B) GSH2 (C), and AtPCS1 (D) in Col-0 and 35S:CKX1. Plants were grown in +Pi medium for 7 d, transferred to −Pi medium for 3 d, and then to −Pi liquid medium alone (−Pi) or with 30 μm As(V) [+As(V)] for 6 h. Expression was analyzed in whole seedlings except for AtARQ1, for which only roots were used. Values show mean ± sd (n = 3). Means labeled with the same letter indicate no significant difference, based on Tukey’s posthoc test (α = 0.05).
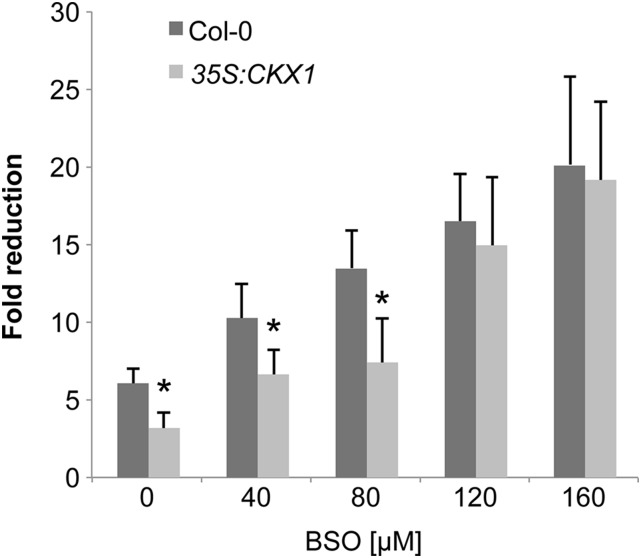
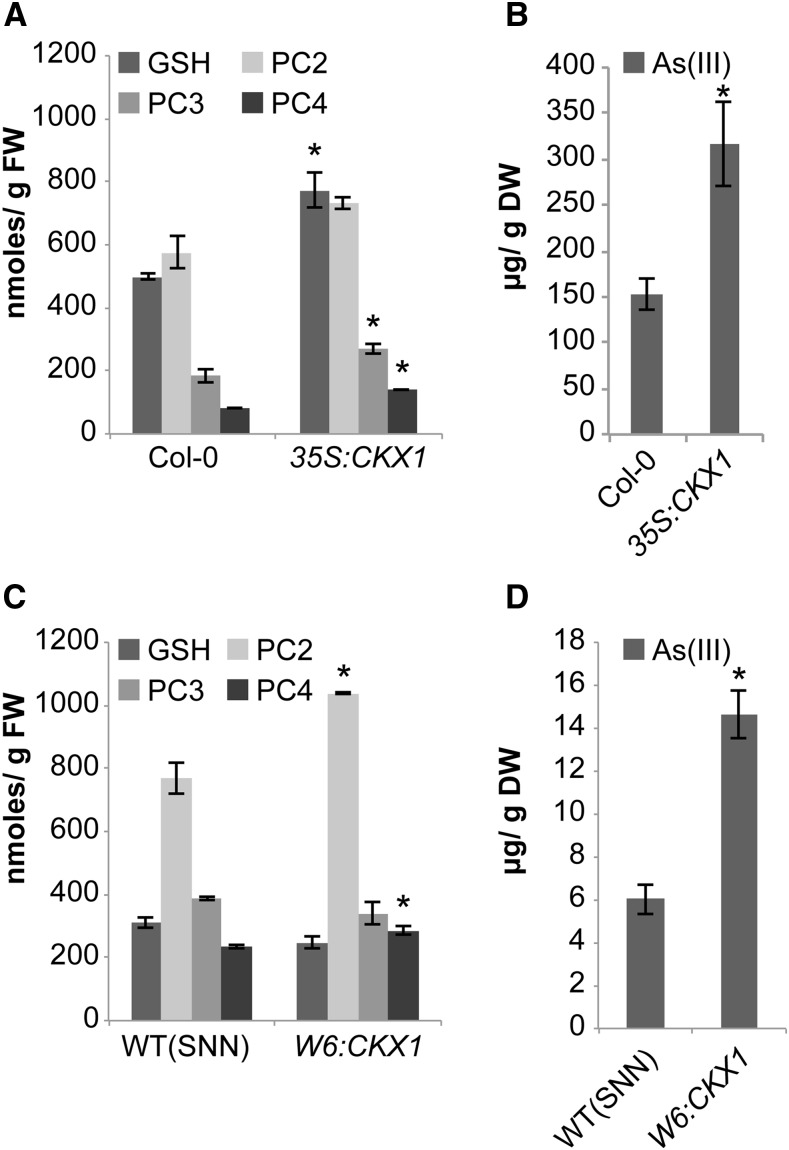
The contribution of PC accumulation to the As(V) tolerance phenotype shown by the CKX1-overexpressing line was evaluated by analyzing the effect of l-buthionine sulphoximine (BSO) on As(V) sensitivity in wild-type and CKX1-overexpressing plants. BSO is a specific inhibitor of γ−ECS activity and thus particularly effective for PC depletion. Wild-type Arabidopsis plants exposed to this compound perfectly mimic the metal sensitivity of PC biosynthetic mutants (Howden et al., 1995). Coinciding with previous observations (Ortega-Villasante et al., 2005), we found that BSO had no effect on root growth in the absence of As(V), even at the highest concentration tested (Supplemental Fig. S3). In the presence of the metalloid, increased BSO concentrations greatly enhanced As(V) sensitivity; we therefore analyzed root growth inhibition after brief exposure to As(V). In plants exposed to BSO, As(V) sensitivity increased preferentially in the CKX1-overexpressing lines compared to wild-type plants (Fig. 4 ;Supplemental Fig. S3). In the presence of As(V) and at relatively high BSO concentrations, CKX1-overexpressing and wild-type plants showed similar root growth. As(V) exposure in wild-type and the CKX1-overexpressing line showed that CK depletion led to a clear increase in PC2, PC3, and PC4 and their precursor, GSH (Fig. 5A), and that these plants accumulated more than twice as much As(III) as wild-type plants (Fig. 5B). These observations strongly support the concept that severe depletion of endogenous CK leads to enhanced PC accumulation, which suggests that the higher arsenic tolerance in the CKX1-overexpressing line is caused to a great extent by the higher PC levels in these plants. Once arsenic is perceived, CK depletion modulates Arabidopsis detoxification capacity and is thus a key factor in plant tolerance and adaptation to As(V).
Figure 4.
As(V) sensitivity to BSO increased preferentially in CK-depleted plants. Plants were grown on Johnson medium with 12.5 μm Pi in a vertical position for 5 d and then transferred to the same medium with 20 μm As(V) alone or with increasing concentrations of BSO. Root growth inhibition (fold change) was determined by quantifying root elongation after 3 d of As(V) exposure alone or with BSO. Bars show mean ± sd (n ≥ 15). *P < 0.0001 (Student’s t test).
Figure 5.
Phytochelatins, GSH, and As(III) accumulation increased in response to CK depletion in Arabidopsis and tobacco. GSH, PCs PC2, PC3, PC4 A, and As(III) (B) levels were determined in Col-0 and 35S:CKX1 Arabidopsis seedlings. Quantification of thiols (C) and As(III) (D) in wild-type (SNN) and W6::CKX1 tobacco roots. Phytochelatins and As(III) were quantified as described in “Methods.” Values show mean ± sd (n = 3). *P < 0.05 (Student’s t test). FW, fresh weight; DW, dry weight.
Various strategies have been used to enhance arsenic tolerance and accumulation in plants (Dhankher et al., 2006; Zhu and Rosen, 2009; Song et al., 2010). Our data suggest CK depletion as an alternative approach to increase the efficiency of arsenic accumulation. CK reduction also leads to a notable increase in root biomass, which could contribute to better arsenic extraction from soil (Werner et al., 2010). A CK decrease thus confers two important characteristics for arsenic hyperaccumulation: increased levels of thiol-containing compounds and enhancement of total root area. To analyze potential applications of CK reduction for arsenic phytoaccumulation in other plant species, we measured PC levels and As(III) accumulation in CKX1-expressing tobacco roots (Fig. 5, C and D). Since constitutive CKX1 expression provokes pleiotropic effects in the aerial part of the plant, we analyzed tobacco plants that express the CKX1 gene under the control of the WRKY6 promoter (Werner et al., 2010). These plants are particularly suitable for such experiments, as this promoter is mainly root specific and As(V) responsive (Castrillo et al., 2013). Similar to Arabidopsis, root-specific CKX1 expression in response to As(V) provoked an increase in total PC levels in tobacco roots, particularly of PC2 (Fig. 5C). As(III) quantification in CKX1-expressing tobacco plants showed that root-specific CK depletion led to a 2-fold As(III) increase compared to wild-type plants (Fig. 5D). These results suggest CK depletion as a suitable strategy to enhance arsenic accumulation in different plant species.
DISCUSSION
The biological role of phytohormones in plant tolerance to arsenic is currently unknown. Transcriptome profiling of a few plant species indicates that several phytohormones might be involved in plant growth regulation in response to arsenic (Yu et al., 2012; Fu et al., 2014), although downstream targets remain to be determined. Here, we found that CK modulates arsenic detoxification in plants. CKX1-overexpressing lines depleted of endogenous CK, plants with reduced CK synthesis, and CK signaling mutants showed a clear arsenic tolerance phenotype. CK acts as a negative regulator of plant responses to several forms of stress. The arsenic tolerance phenotype of the CK-deficient plants and signaling mutants nonetheless suggests that CK coordinates arsenic detoxification mechanisms. As(V) tolerance is achieved mainly through the combined action of two specific responses, prevention of As(V) uptake by suppression of PHT1;1, the most active As(V)/Pi-transporter (Castrillo et al., 2013), and through detoxification by As(III) complex formation with thiol compounds and sequestration into the vacuoles (Schmöger et al., 2000). Although CK negatively regulates the As(V)/Pi transporter (Martín et al., 2000), As(V)-mediated down-regulation of PHT1;1 was somewhat more efficient in the CK-depleted lines than in wild-type plants. PHT1;1 transcript levels after As(V) exposure were similar in CK-depleted transgenic and wild-type plants, however, which implies that the effect of CK depletion on PHT1;1 expression is unlikely to be responsible for the increased As(V) tolerance of CKX1-overexpressing lines. Repression of As(V)/Pi transporters in response to As(V) is controlled by the WRKY6 transcription factor (Castrillo et al., 2013). Our results support the idea that As(V) down-regulation of the Pi transporter is controlled preferentially by WRKY6 action. In contrast, As(V) reductase gene expression and thiol compound accumulation was increased in CK-depleted plants, which suggests that CK controls activation of the detoxification machinery responsible for As(V) tolerance.
As(V) caused a significant reduction in endogenous CK levels, which coincides with reduced expression of CK synthesis genes (Fig. 2B). Our analyses showed that As(V) reduces the expression of CK-inducible genes (Table I). As(V) efficiently represses the CYP735A gene, responsible for biosynthesis of tZ-type CK in roots (Kiba et al., 2013; Ramireddy et al., 2014). In addition, the arsenic-tolerant phenotype of the Arabidopsis cyp735a1 a2 double mutant was identical to that of the CKX1-overexpressing line (Fig. 1, B and C). tZ-type CKs therefore appear to have a major role in the repression of arsenic tolerance mechanisms, probably because this CK species is synthesized in roots (Kiba et al., 2013), where biothiols are essential for As(III) sequestration.
Endogenous CK depletion in response to As(V) alters the expression of genes involved in arsenic detoxification. CK negative regulation is not limited to genes involved in GSH and PC biosynthesis, but also affects expression of the As(V) reductase gene AtARQ1. As(III) and As(V) increase the intracellular thiol content in arsenic hyperaccumulator plants (Bleeker et al., 2006); As(V) reductase activity is therefore a prerequisite for arsenic tolerance and accumulation. CK also represses sulfur transporters (Maruyama-Nakashita et al., 2004), and As(III) causes severe sulfur starvation and induces expression of sulfur transporters (Reid et al., 2013). These data support the idea that sulfur is critical for increased biosynthesis of PC and other thiol-related compounds, which leads to arsenic accumulation. The use of an inhibitor of GSH and PC biosynthesis showed that the As(V) tolerance phenotype in CK-depleted Arabidopsis plants is due in great measure to increased accumulation of thiol compounds. Although these compounds have a limited role in some arsenic hyperaccumulator plants (Webb et al., 2003), As(III) is the major arsenic species that accumulates in hyper- and nonaccumulator plant vacuoles. Enhanced As(V) reductase gene expression in response to CK depletion is therefore a critical prerequisite for arsenic accumulation in both plant types. Reduction of endogenous CK levels in response to As(V) is thus a central factor in arsenic tolerance and accumulation in plants.
Hitherto only a few transgenic approaches have been used to coordinate expression of key genes for arsenic phytoremediation (Dhankher et al., 2002; Zhu and Rosen, 2009). Efficient use of a system-wide approach that integrates all responses to increase arsenic accumulation would require identification of the master regulatory genes that underlie the response to arsenic. The Arabidopsis and tobacco CKX1-overexpressing plants used in this study accumulate more cadmium as well as nutrient elements, associated with their increased root biomass and possibly with deregulated expression of the respective transporter genes (Werner et al., 2010). Our results show that CK reduction not only increases root biomass but also promotes activation of the metal(loid) detoxification machinery, a major cause of As(III) accumulation. We suggest CK depletion as a strategy to increase arsenic accumulation in nonhyperaccumulator plants.
Our findings show that CK acts as a negative regulator of the response to As(V). Reduction of CK levels in response to the metalloid stimulates the detoxification machinery that leads to As(III) accumulation. Further experiments are needed to determine how plants sense the amount of biothiol elicited by CK depletion to coordinate As(V) uptake and detoxification. Identification of CK as an integral part of the As(V) response offers a new understanding of how plants integrate phytohormone signaling pathways with adaptation to stress and provides an additional molecular component with which to engineer arsenic tolerance in plants.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
We used Arabidopsis (Arabidopsis thaliana) seeds of Col-0, 35S:CKX1 (Werner et al., 2003), the CK receptor mutant ahk2 ahk3 (ahk2-5/ahk3-7; Riefler et al., 2006), and the CK biosynthetic mutants ipt3 5 7 (ipt3-2/ipt5-3/ipt7-1; Miyawaki et al., 2006) and cyp735a1 a2 (cyp735a1-1/cyp735a2-2; Kiba et al., 2013). The last two lines were kindly provided by Tatsuo Kakimoto (Laboratory of Plant Growth and Development, Osaka University Japan) and Hitoshi Sakakibara (RIKEN Center for Sustainable Resource Science, Yokohama, Japan), respectively. For tobacco (Nicotiana tabacum) experiments, we used cultivar SNN as the wild type and transgenic line WRKY6:CKX1#29 (Werner et al., 2010). Arabidopsis seedlings were grown on Johnson medium (Johnson et al., 1957) without Pi or supplemented with Pi (KH2PO4) alone or with As(V) (NaH2AsO4·7H2O) at distinct concentrations, depending on the experiment. For all molecular, phenotypic, and biochemical analyses, Arabidopsis plants were grown in a growth chamber on a 16-h-light/8-h-dark regime (24°C/21°C).
Tobacco seeds were germinated on Murashige and Skoog (MS) with 3% Suc plates and cultivated in long-day conditions (16-h light/8-h dark) at 22°C for 10 d. Seedlings at a similar developmental stage were then transferred to a hydroponic system containing 0.1-strength Hoagland solution (Krämer et al., 1996) and cultivated for another 4 weeks. Medium was aerated and renewed every five days.
Phenotypic Analysis
For As(V) tolerance analysis, Arabidopsis plants were grown on Johnson medium with 12.5 μm Pi alone or with 25 μm As(V) in a vertical position for 10 d. To determine root growth retardation in response to As(V), main root length was measured in these plants using ImageJ software (Schneider et al., 2012).
To analyze the effect of BSO on As(V) tolerance, Arabidopsis plants were grown on Johnson medium with 12.5 μm Pi in a vertical position for 5 d. Plants were then transferred to the same medium with 20 μm As(V) alone or with increasing BSO concentrations. Root growth inhibition (fold change) was determined by quantifying root elongation after 3 d of As(V) exposure, alone or with BSO, and comparing it with roots of plants grown at corresponding BSO concentrations without As(V). Root elongation was measured using ImageJ software.
Arsenic Quantification
For arsenic quantification experiments in Arabidopsis, plants were grown and treated using a method that accurately determines arsenic uptake kinetics and accumulation (Narang et al., 2000). Arabidopsis plants were grown on plates containing Johnson media solidified with 0.4% bacto-agar with 30 μm Pi and covered with 0.4 mm-pore nylon mesh. Seeds were sown onto the mesh and cultured for 7 d, after which plants were treated with PI and PII buffers (Narang et al., 2000). Arsenic accumulation experiments were performed in 50 mL pots using 5 μm As(V) for 24 h. Plants were dried (60°C, 3 d) prior to arsenic quantification.
To determine arsenic accumulation in tobacco plants were grown in hydroponic conditions (4 weeks), washed with deionized water, grown for another 2 d in Pi-deficient conditions, then exposed to As(V) in medium containing 0.1 mm CaCl2, 5 μm As(V), 5 mm 2-(N-morpholino) ethanesulfonic acid, pH 5.75 (24 h). Plants were placed in ice-cold buffer [0.1 mm CaCl2, 1 mm KH2PO4, 5 mm 2-(N-morpholino) ethanesulfonic acid, pH 5.75] for 1 h. Shoot and root samples were harvested separately and dried (60°C, 3 d).
Arsenic species from Arabidopsis and tobacco samples were extracted with 1:1 methanol:water using an ultrasonic homogenizer SONOPULS HD 2200 (30%, 60 s). Extracts were centrifuged (5500g, 10 min) and supernatant filtered through a 22-μm nylon syringe before injection into the HPLC for arsenic speciation. Diluted samples were injected onto an anion-exchange column PRP-X100 (250 × 4.1 mm, particle size 10 μm; Hamilton, Reno, NV). Arsenic species were eluted in 10 mm HPO4−2/H2PO4−1, 2% (v/v) MeOH mobile phase, at a 1.5 mL min−1 flow rate. To test the efficiency of the method, three replicates of the extracts were digested individually with HNO3–H2O2 to compare total arsenic content (ICP-MS) with the concentration sum of all arsenic species (LC-ICP-MS) in the initial extracts. As(III) was quantified on an As(III) calibration curve; As(V) in the sample was used as an internal normalization standard.
Analytical quality control was used for total arsenic content and arsenic species were validated by analyzing certified reference material (ref. numbers SRM 1568a; NIST and CRM-627; BCR, Belgium). Good agreement was found for both reference materials (<10% uncertainty considering a 95% confidence level). For extraction recovery, samples were spiked with known amounts of distinct arsenic species, with recoveries of 80% to 100%.
Quantification of Biothiols
For biothiol quantification, Arabidopsis and tobacco plants were grown as described for arsenic quantification. An alkaline-reducing procedure was used to extract biothiols from Arabidopsis seedlings exposed to As(V) using the procedure described by LeBlanc et al. (2013) with minor modifications. Frozen samples (0.1 mg) were ground with 30 μL 50 mm N-acetyl Cys as an internal standard and homogenized with 270 μL reducing reagent (2 mg mL−1 NaBH4 in 0.1 m NaOH). The mixture was transferred to 1.5-mL Eppendorf tubes, acidified with 50 μL 10 m HCl, centrifuged (12,000g, 15 min, 4°C), and supernatant transferred to chromatographic vials under dim light for HPLC analysis.
For tobacco samples, we used an acidic extraction procedure (Sobrino-Plata et al., 2014). Frozen root tissue (100 mg) was homogenized in 0.125 n HCl (270 μL) with 30 μL 50 mm N-acetyl-Cys as an internal standard. Homogenized samples were centrifuged (12,000g, 15 min, 4°C) and supernatant used for HPLC analysis.
Biothiol profiles were analyzed by HPLC (Sobrino-Plata et al., 2009). Extracts (100 μL) were injected into a Mediterranea SEA18 column (250 x 4.6 mm; Teknokroma, Sant Cugat del Vallés, Spain), using an Agilent Technologies 1200 series HPLC system (Santa Clara, CA). Thiols were detected after postcolumn derivatization with Ellman reagent and quantified against the N-acetyl Cys internal standard. Biothiols were identified by comparing retention times of peaks from each sample with those of commercially available standards; Cys and GSH were purchased from Sigma-Aldrich (St. Louis, MO) and PC2, PC3 and PC4 from AnaSpec (Fremont, CA).
CK Quantification
For quantification of CKs, Arabidopsis seedlings were grown in Johnson medium (+Pi) for 7 d and transferred to Pi-lacking medium for 3 d to activate expression of the As(V)/Pi transporters. Pi-starved plants were then exposed to 30 μm As(V) in liquid medium (-Pi) for 6 h. Fresh samples were collected and frozen in liquid N2. Frozen samples (250 mg) were ground in a mortar with liquid N2 and homogenized with 4 mL precooled (−20°C) methanol:water:formic acid (15:4:1, v/v/v). Deuterium-labeled CK internal standards ([2H5]Z, [2H5]tZR, [2H6]iP, [2H6]iPR, [2H7] BA, and [2H7]BAR; Olchemim Ltd, Olomouc, Czech Republic) were added (40 μL stock solution of 50 ng/mL of each standard in methanol) and extracted as described (Sudre et al., 2013). CKs were quantified using a High performance liquid chromatography–electrospray ionization tandem mass spectrometry (HPLC-ESI-MS/MS) system as described (Sudre et al., 2013). Significant differences (P < 0.05) among treatments were calculated using one-way ANOVA and the Least Significatn Difference Fisher post hoc test. Statistical test were performed using the statistical package Statistica 6.0 (StatSoft, Tulsa, OK).
Gene Expression Analysis by qRT-PCR
Gene expression analysis was performed with plants grown as for CK quantification. Quantitative real-time reverse transcription-PCR was performed on three independent biological samples as described (Aguilar-Martínez et al., 2007). RNA extracted with TRI Reagent (Sigma) was used for cDNA synthesis using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) following the manufacturer’s instructions. cDNA was diluted ten times in water and qRT-PCR performed using SYBR Green reagent. Each sample was normalized using EF1α (for primer sequences, see Supplemental Table S2).
Supplemental Data
The following supplemental materials are available.
Supplemental Table S1. TOP65CytK genes that overlap with As(V)-down-regulated genes.
Supplemental Table S2. Primers used for qRT-PCR analysis.
Supplemental Figure S1. Phenotypes of ipt 3 5 7, wild-type (Col-0), cyp735a1 a2, 35S:CKX1, and ahk2 ahk3 plants grown on 12.5 μm Pi for 10 d.
Supplemental Figure S2. CK-responsive genes are down-regulated in response to As(V). qRT-PCR expression analysis of ARR9, AHK4, and CYP82F1 in Col-0.
Supplemental Figure S3. As(V) sensitivity to BSO increased preferentially in CK-depleted plants.
Supplementary Material
Acknowledgments
We thank Carmen L. Torán for critical reading of the manuscript, Cristihan González and Yolanda Leo del Puerto for technical assistance, and Catherine Mark for editorial assistance.
Glossary
- As(III)
arsenite
- As(V)
arsenate
- BSO
l-buthionine sulphoximine
- CK
cytokinin
- Col-0
ecotype Columbia
- GSH
glutathione
- HPLC
High Performance Liquid Chromatography
- PC
phytochelatin
- Pi
phosphate
- qRT-PCR
References
- Aguilar-Martínez JA, Poza-Carrión C, Cubas P (2007) Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 19: 458–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeker PM, Hakvoort HW, Bliek M, Souer E, Schat H (2006) Enhanced arsenate reduction by a CDC25-like tyrosine phosphatase explains increased phytochelatin accumulation in arsenate-tolerant Holcus lanatus. Plant J 45: 917–929 [DOI] [PubMed] [Google Scholar]
- Brenner WG, Romanov GA, Köllmer I, Bürkle L, Schmülling T (2005) Immediate-early and delayed cytokinin response genes of Arabidopsis thaliana identified by genome-wide expression profiling reveal novel cytokinin-sensitive processes and suggest cytokinin action through transcriptional cascades. Plant J 44: 314–333 [DOI] [PubMed] [Google Scholar]
- Brenner WG, Schmülling T (2012) Transcript profiling of cytokinin action in Arabidopsis roots and shoots discovers largely similar but also organ-specific responses. BMC Plant Biol 12: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner WG, Schmülling T (2015) Summarizing and exploring data of a decade of cytokinin-related transcriptomics. Front Plant Sci 6: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillo G, Sánchez-Bermejo E, de Lorenzo L, Crevillén P, Fraile-Escanciano A, Tc M, Mouriz A, Catarecha P, Sobrino-Plata J, Olsson S, et al. (2013) WRKY6 transcription factor restricts arsenate uptake and transposon activation in Arabidopsis. Plant Cell 25: 2944–2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao D-Y, Chen Y, Chen J, Shi S, Chen Z, Wang C, Danku JM, Zhao F-J, Salt DE (2014) Genome-wide association mapping identifies a new arsenate reductase enzyme critical for limiting arsenic accumulation in plants. PLoS Biol 12: e1002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhankher OP, Li Y, Rosen BP, Shi J, Salt D, Senecoff JF, Sashti NA, Meagher RB (2002) Engineering tolerance and hyperaccumulation of arsenic in plants by combining arsenate reductase and gamma-glutamylcysteine synthetase expression. Nat Biotechnol 20: 1140–1145 [DOI] [PubMed] [Google Scholar]
- Dhankher OP, Rosen BP, McKinney EC, Meagher RB (2006) Hyperaccumulation of arsenic in the shoots of Arabidopsis silenced for arsenate reductase (ACR2). Proc Natl Acad Sci USA 103: 5413–5418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Zorrilla JM, Martin AC, Solano R, Rubio V, Leyva A, Paz-Ares J (2002) Mutations at CRE1 impair cytokinin-induced repression of phosphate starvation responses in Arabidopsis. Plant J 32: 353–360 [DOI] [PubMed] [Google Scholar]
- Fu S-F, Chen P-Y, Nguyen QT, Huang L-Y, Zeng G-R, Huang T-L, Lin C-Y, Huang H-J (2014) Transcriptome profiling of genes and pathways associated with arsenic toxicity and tolerance in Arabidopsis. BMC Plant Biol 14: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden R, Goldsbrough PB, Andersen CR, Cobbett CS (1995) Cadmium-sensitive, cad1 mutants of Arabidopsis thaliana are phytochelatin deficient. Plant Physiol 107: 1059–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon J, Kim J (2013) Arabidopsis response Regulator1 and Arabidopsis histidine phosphotransfer Protein2 (AHP2), AHP3, and AHP5 function in cold signaling. Plant Physiol 161: 408–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CM, Stout PR, Broyer TC, Carlton AB (1957) Comparative chlorine requirements of different plant species. Plant Soil 8: 337–353 [Google Scholar]
- Kiba T, Takei K, Kojima M, Sakakibara H (2013) Side-chain modification of cytokinins controls shoot growth in Arabidopsis. Dev Cell 27: 452–461 [DOI] [PubMed] [Google Scholar]
- Krämer U, Cotter-Howells JD, Charnock JM, Baker AJM, Smith JAC (1996) Free histidine as a metal chelator in plants that accumulate nickel. Nature 379: 635–638 [Google Scholar]
- LeBlanc MS, McKinney EC, Meagher RB, Smith AP (2013) Hijacking membrane transporters for arsenic phytoextraction. J Biotechnol 163: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CX, Feng SL, Shao Y, Jiang LN, Lu XY, Hou XL (2007) Effects of arsenic on seed germination and physiological activities of wheat seedlings. J Environ Sci (China) 19: 725–732 [DOI] [PubMed] [Google Scholar]
- Martín AC, del Pozo JC, Iglesias J, Rubio V, Solano R, de La Peña A, Leyva A, Paz-Ares J (2000) Influence of cytokinins on the expression of phosphate starvation responsive genes in Arabidopsis. Plant J 24: 559–567 [DOI] [PubMed] [Google Scholar]
- Maruyama-Nakashita A, Nakamura Y, Yamaya T, Takahashi H (2004) A novel regulatory pathway of sulfate uptake in Arabidopsis roots: implication of CRE1/WOL/AHK4-mediated cytokinin-dependent regulation. Plant J 38: 779–789 [DOI] [PubMed] [Google Scholar]
- Meharg AA, Macnair MR (1992) Suppression of the high affinity phosphate uptake system: a mechanism of arsenate tolerance in Holcus lanatus L. J Exp Bot 43: 519–524 [Google Scholar]
- Miyawaki K, Tarkowski P, Matsumoto-Kitano M, Kato T, Sato S, Tarkowska D, Tabata S, Sandberg G, Kakimoto T (2006) Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc Natl Acad Sci USA 103: 16598–16603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narang RA, Bruene A, Altmann T (2000) Analysis of phosphate acquisition efficiency in different Arabidopsis accessions. Plant Physiol 124: 1786–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA (2013) The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect 121: 295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama R, Watanabe Y, Fujita Y, Le DT, Kojima M, Werner T, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Kakimoto T, et al. (2011) Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 23: 2169–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Villasante C, Rellán-Alvarez R, Del Campo FF, Carpena-Ruiz RO, Hernández LE (2005) Cellular damage induced by cadmium and mercury in Medicago sativa. J Exp Bot 56: 2239–2251 [DOI] [PubMed] [Google Scholar]
- Peleg Z, Blumwald E (2011) Hormone balance and abiotic stress tolerance in crop plants. Curr Opin Plant Biol 14: 290–295 [DOI] [PubMed] [Google Scholar]
- Ramireddy E, Chang L, Schmülling T (2014) Cytokinin as a mediator for regulating root system architecture in response to environmental cues. Plant Signal Behav 9: e27771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid R, Gridley K, Kawamata Y, Zhu Y (2013) Arsenite elicits anomalous sulfur starvation responses in barley. Plant Physiol 162: 401–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riefler M, Novak O, Strnad M, Schmülling T (2006) Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 18: 40–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen BP. (2002) Biochemistry of arsenic detoxification. FEBS Lett 529: 86–92 [DOI] [PubMed] [Google Scholar]
- Sánchez-Bermejo E, Castrillo G, del Llano B, Navarro C, Zarco-Fernández S, Martinez-Herrera DJ, Leo-del Puerto Y, Muñoz R, Cámara C, Paz-Ares J, et al. (2014) Natural variation in arsenate tolerance identifies an arsenate reductase in Arabidopsis thaliana. Nat Commun 5: 4617. [DOI] [PubMed] [Google Scholar]
- Schmöger ME, Oven M, Grill E (2000) Detoxification of arsenic by phytochelatins in plants. Plant Physiol 122: 793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shri M, Kumar S, Chakrabarty D, Trivedi PK, Mallick S, Misra P, Shukla D, Mishra S, Srivastava S, Tripathi RD, et al. (2009) Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol Environ Saf 72: 1102–1110 [DOI] [PubMed] [Google Scholar]
- Sobrino-Plata J, Carrasco-Gil S, Abadía J, Escobar C, Álvarez-Fernández A, Hernández LE (2014) The role of glutathione in mercury tolerance resembles its function under cadmium stress in Arabidopsis. Metallomics 6: 356–366 [DOI] [PubMed] [Google Scholar]
- Sobrino-Plata J, Ortega-Villasante C, Flores-Cáceres ML, Escobar C, Del Campo FF, Hernández LE (2009) Differential alterations of antioxidant defenses as bioindicators of mercury and cadmium toxicity in alfalfa. Chemosphere 77: 946–954 [DOI] [PubMed] [Google Scholar]
- Song W-Y, Park J, Mendoza-Cózatl DG, Suter-Grotemeyer M, Shim D, Hörtensteiner S, Geisler M, Weder B, Rea PA, Rentsch D, et al. (2010) Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc Natl Acad Sci USA 107: 21187–21192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Srivastava AK, Suprasanna P, D’Souza SF (2009) Comparative biochemical and transcriptional profiling of two contrasting varieties of Brassica juncea L. in response to arsenic exposure reveals mechanisms of stress perception and tolerance. J Exp Bot 60: 3419–3431 [DOI] [PubMed] [Google Scholar]
- Sudre D, Gutierrez-Carbonell E, Lattanzio G, Rellán-Álvarez R, Gaymard F, Wohlgemuth G, Fiehn O, Alvarez-Fernández A, Zamarreño AM, Bacaicoa E, et al. (2013) Iron-dependent modifications of the flower transcriptome, proteome, metabolome, and hormonal content in an Arabidopsis ferritin mutant. J Exp Bot 64: 2665–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung D-YY, Kim T-HH, Komives EA, Mendoza-Cózatl DG, Schroeder JI (2009) ARS5 is a component of the 26S proteasome complex, and negatively regulates thiol biosynthesis and arsenic tolerance in Arabidopsis. Plant J 59: 802–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L-S, Urao T, Qin F, Maruyama K, Kakimoto T, Shinozaki K, Yamaguchi-Shinozaki K (2007) Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc Natl Acad Sci USA 104: 20623–20628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi RD, Srivastava S, Mishra S, Singh N, Tuli R, Gupta DK, Maathuis FJ (2007) Arsenic hazards: strategies for tolerance and remediation by plants. Trends Biotechnol 25: 158–165 [DOI] [PubMed] [Google Scholar]
- Wang X, Yi K, Tao Y, Wang F, Wu Z, Jiang D, Chen X, Zhu L, Wu P (2006) Cytokinin represses phosphate-starvation response through increasing of intracellular phosphate level. Plant Cell Environ 29: 1924–1935 [DOI] [PubMed] [Google Scholar]
- Webb SM, Gaillard J-F, Ma LQ, Tu C (2003) XAS speciation of arsenic in a hyper-accumulating fern. Environ Sci Technol 37: 754–760 [DOI] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T (2003) Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15: 2532–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Nehnevajova E, Köllmer I, Novák O, Strnad M, Krämer U, Schmülling T (2010) Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 22: 3905–3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y, Lee W-MM, An Y-JJ (2015) Phytotoxicity of arsenic compounds on crop plant seedlings. Environ Sci Pollut Res Int 22: 11047–11056 [DOI] [PubMed] [Google Scholar]
- Yu LJ, Luo YF, Liao B, Xie LJ, Chen L, Xiao S, Li JT, Hu SN, Shu WS (2012) Comparative transcriptome analysis of transporters, phytohormone and lipid metabolism pathways in response to arsenic stress in rice (Oryza sativa). New Phytol 195: 97–112 [DOI] [PubMed] [Google Scholar]
- Zhu Y-G, Rosen BP (2009) Perspectives for genetic engineering for the phytoremediation of arsenic-contaminated environments: from imagination to reality? Curr Opin Biotechnol 20: 220–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwack PJ, Rashotte AM (2015) Interactions between cytokinin signalling and abiotic stress responses. J Exp Bot 66: 4863–4871 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.