Abstract
Background
Pesticides are associated with poorer neurodevelopmental outcomes, but little is known about the effects on sensory functioning.
Methods
Auditory brainstem response (ABR) and pesticide data were available for 27 healthy, full-term 9-month-old infants participating in a larger study of early iron deficiency and neurodevelopment. Cord blood was analyzed by gas chromatography-mass spectrometry for levels of 20 common pesticides. The ABR forward-masking condition consisted of a click stimulus (masker) delivered via ear canal transducers followed by an identical stimulus delayed by 8, 16, or 64 milliseconds (ms). ABR peak latencies were evaluated as a function of masker-stimulus time interval. Shorter wave latencies reflect faster neural conduction, more mature auditory pathways, and greater degree of myelination. Linear regression models were used to evaluate associations between total number of pesticides detected and ABR outcomes. We considered an additive or synergistic effect of poor iron status by stratifying our analysis by newborn ferritin (based on median split).
Results
Infants in the sample were highly exposed to pesticides; a mean of 4.1 pesticides were detected (range 0-9). ABR Wave V latency and central conduction time (CCT) were associated with the number of pesticides detected in cord blood for the 64ms and non-masker conditions. A similar pattern seen for CCT from the 8ms and 16ms conditions, although statistical significance was not reached. Increased pesticide exposure was associated with longer latency. The relation between number of pesticides detected in cord blood and CCT depended on the infant’s cord blood ferritin level. Specifically, the relation was present in the lower cord blood ferritin group but not the higher cord blood ferritin group.
Conclusions
ABR processing was slower in infants with greater prenatal pesticide exposure, indicating impaired neuromaturation. Infants with lower cord blood ferritin appeared to be more sensitive to the effects of prenatal pesticide exposure on ABR latency delay, suggesting an additive or multiplicative effect.
Keywords: Pesticides, Infancy, Auditory Brainstem Response, Sensory Function
Introduction
Billions of pounds of synthetic pesticides are applied globally each year for crop protection and pest management in agricultural and residential settings [1]. The highest usage occurs in the agricultural sector, with over 4.6 million tons applied each year [2, 3]. China is one of the world’s largest consumers of agricultural pesticides [2-4], applying 2.5-5 fold more per field unit than the global average [5]. In Zhejiang province, the site of this study, agricultural applications are some of the highest in China, at nearly double the national rate [6]. Due to their heavy use in agriculture, non-occupational pesticide exposure is most likely to occur via consumption of contaminated food. Additional exposure may also occur via contaminated drinking water, dust, and spray drift, especially in rural, farming communities, as well as from the use of residential pesticides in the home or yard [6].
Synthetic pesticides are toxic to biological systems by design. Many act by disrupting signaling mechanisms in the central nervous system (CNS) thereby inhibiting neurological function. Evidence from animal studies and adult occupational poisonings has demonstrated that these insecticides act via similar neurotoxic mechanisms in mammals following high-dose exposure [7, 8]. Less is known about the mechanisms of neurotoxicity at low-level exposures that are relevant to the general population. Low-level pesticide exposures are an important concern in pregnant women and young children. Fetal and infant brains are rapidly developing, leaving them highly vulnerable to potentially long-lasting effects of pesticide exposure, such as disruption of brain architecture or circuitry [9]. Adding to concerns for fetal exposure, pesticides are able to cross the placenta [10] and fetuses tend to have lower levels of detoxifying enzymes [11], both of which are thought to increase fetal susceptibility. Low-level pesticide exposures during pregnancy or childhood have been found to be associated with neurodevelopmental deficits such as lower IQ [12-15] and disorders such as autism [16, 17], attention deficit-hyperactivity disorder [18-20], and pervasive developmental disorder [14, 20].
Very little is known about the effects of early-life pesticide exposure on the auditory pathways of the brain or other sensory systems. There have been some reports of hearing loss and ototoxicity following pesticide exposure, but most of the evidence comes from animal or occupational case studies where high-level exposures are the norm [21]. One recent study found deficits in cochlear status in children exposed to organochlorine pesticides.[22] Regarding visual sensory function, a recent study in an Arctic population with high DDE exposure found that both pre- and postnatal DDE exposure were associated with visual processing impairment at school age.[23]
Auditory system development in infancy helps to provide the foundation for subsequent communication and language acquisition in childhood. [24, 25] Therefore, early life deficits in auditory function, potentially as a result of prenatal pesticide exposure, could contribute to detrimental long-term effects on learning or other cognitive functions later in childhood.[24, 26-28]
Nutrient-toxicant interactions are an important area of research given that concurrent exposure to toxicants and early-life nutrient deficiencies are common in many parts of the world. Iron is an important nutrient for early neurodevelopment [29, 30] and an essential factor in myelination and oligodendrocyte biology. [31, 32] Previous studies that found longer ABR latencies among iron-deficient infants are consistent with impaired myelination of the auditory pathways [25, 33]. Because iron and pesticides both appear to have impacts on neurodevelopment and myelination, it is relevant to consider them jointly, specifically in regard to auditory system development.
The principle aim of this pilot study was to explore the effects of environmental exposures to multiple pesticides on infant auditory function at nine months, as measured by auditory brainstem response (ABR). A secondary aim was to explore the pesticide-iron interaction as it relates to ABR in infants.
Materials and Methods
Ethics statement
Signed written informed consent was obtained from infants’ parents. All study protocols were approved by the Institutional Review Boards and ethics committees at both the University of Michigan and Children’s Hospital of Zhejiang University.
Participants
The study population was a subset of Chinese infants participating in a study of early iron deficiency and neurodevelopment jointly conducted by the University of Michigan and the Children’s Hospital of Zhejiang University. Women/infant pairs meeting the following criteria were invited to participate: singleton full-term birth (37-41 weeks completed gestation); birth weight greater than 2,500 grams; no perinatal complications or congenital malformations; no general undernutrition (<10th percentile for weight or length); no acute or chronic illness; no multiple or prolonged hospitalizations (>5 days). Consecutive participants in the study with due dates between April and June 2009 (n = 116) were included in this pilot study of pesticide exposure; 27 of the infants had ABR data.
Cord blood pesticides
Cord blood collection and pesticide analysis methods have been described elsewhere [34]. Briefly, a 30 mL umbilical cord blood sample was collected, separated, and serum was stored at -80C. Serum samples underwent solid-phase extraction and analysis using isotope dilution gas chromatography-mass spectrometry (GC-MS) at Nanjing Medical University in Nanjing, China. Pesticides were selected based on usage data, availability of analytical standards, method compatibility, and preliminary data. The final list included: organophosphate insecticides (chlorpyrifos, diazionon, fonofos, malathion, parathion-ethyl, parathion-methyl, profenofos, terbufos), carbamate insecticides (carbofuranphenol, propoxur), herbicides (acetochlor, alachlor, atrazine, linuron, metolachlor, trifluralin), fungicides (dicloran, metalaxyl, vinclozolin), and repellant (diethyltoluamide). Pesticide concentrations were analyzed by Thermo Trace GC and DSQ Mass Spectrometer (Thermo, USA). Limits of detection (LOD) ranged from 0.05 ng/mL to 0.50 ng/mL (Table 1). Quality control samples were analyzed in parallel with unknown samples in each analytical series.
Table 1.
Distribution of pesticide concentrations in umbilical cord serum (ng/ml) at delivery, Zhejiang Province, China 2009 (n=27).
| Pesticide | Limit of detection (LOD) (ng/ml) |
Number of infants with levels > LOD (%) |
Selected percentiles | ||||
|---|---|---|---|---|---|---|---|
| 50 | 75 | 90 | 95 | max | |||
| Organophosphates | |||||||
| Chlorpyrifos | 0.05 | 5 (19%) | ND | ND | 0.16 | 0.17 | 0.17 |
| Diazinon | 0.05 | 2 (7%) | ND | ND | ND | 0.45 | 0.87 |
| Fonofos | 0.05 | 3 (11%) | ND | ND | 0.26 | 0.56 | 1.06 |
| Malathion | 0.50 | 5 (19%) | ND | ND | 2.87 | 2.88 | 3.06 |
| Parathion-ethyl | 0.05 | 0 (0%) | ND | ND | ND | ND | ND |
| Parathion-methyl | 0.05 | 6 (22%) | ND | ND | 1.83 | 2.14 | 2.53 |
| Profenofos | 0.50 | 10 (37%) | ND | 0.68 | 0.74 | 0.84 | 0.96 |
| Terbufos | 0.05 | 7 (26%) | ND | 0.14 | 0.34 | 0.39 | 0.39 |
| Carbamates | |||||||
| Carbofuranphenol | 0.05 | 9 (33%) | ND | 20.60 | 31.22 | 32.34 | 46.10 |
| Propoxur | 0.05 | 0 (0%) | ND | ND | ND | ND | ND |
| Herbicides | |||||||
| Acetochlor | 0.50 | 5 (19%) | ND | ND | 0.61 | 0.82 | 0.99 |
| Alachlor | 0.05 | 8 (30%) | ND | 0.08 | 3.26 | 3.82 | 5.26 |
| Atrazine | 0.25 | 7 (26%) | ND | 0.67 | 1.53 | 1.68 | 1.83 |
| Linuron | 0.50 | 1 (4%) | ND | ND | ND | ND | 1.10 |
| Metolachlor | 0.05 | 0 (0%) | ND | ND | ND | ND | ND |
| Trifluralin | 0.05 | 0 (0%) | ND | ND | ND | ND | ND |
| Fungicides | |||||||
| Dicloran | 0.05 | 8 (30%) | ND | 1.22 | 7.87 | 9.06 | 11.46 |
| Metalaxyl | 0.05 | 0 (0%) | ND | ND | ND | ND | ND |
| Vinclozolin | 0.05 | 16 (59%) | 0.36 | 0.60 | 1.31 | 1.61 | 1.85 |
| Repellent | |||||||
| Diethyltoluamide | 0.05 | 20 (74%) | 0.29 | 0.52 | 0.78 | 0.98 | 1.08 |
* ND = non-detect
Binary exposure variables (≥LOD=1, <LOD=0) were created for each of the pesticides of interest and summed to determine the total number of pesticides detected in cord blood; this was our primary independent variable. In this sense, we hoped to estimate the additive or synergistic impact of exposure to multiple pesticides. [35] [34] We also calculated the total number of detected pesticides in the following four categories: organophosphates, carbamates, herbicides, and fungicides. Given the small sample size of this pilot study and the relatively high LODs for the pesticides, we felt this method of exposure classification was most appropriate.
Auditory Brainstem Response (ABR) recording
ABR is a highly reliable, noninvasive way of measuring the maturation of the auditory pathway, from the periphery to the brainstem, as well as overall central nervous system maturation in infants.[36-38] A series of clicks are transmitted via ear canal transducers, and electroencephalogram (EEG) electrodes on the scalp record the neural activity at auditory centers along the brainstem. ABRs typically consist of three well-defined peaks in infants, corresponding to nerve activation in the cochlear nerve (wave I), the cochlear nuclei (wave III), and the lateral lemniscus (wave V)[39, 40] . ABR peak latencies decline throughout infancy, indicating faster signal transmission, as the auditory pathways mature and become increasingly myelinated.
For this study, ABRs were obtained from nine-month-old infants during unsedated sleep following an initial test for basic hearing function [41]. ABR testing was carried out using a Biologic Navigator (Bio-Logic Systems Corp., Mundeleiu, IL)/Traveler evoked potential system. We assessed temporal processing abilities (rapid acoustic processing) by using a forward masking paradigm.[42] The use of the forward-masking paradigm in this sample is described in detail elsewhere.[24] Briefly, the forward-masking paradigm has been used to investigate the temporal processing and frequency discrimination of the auditory system. [24, 43, 44] It differs from standard ABR because as long as the signal and the masker are closely spaced (70ms or less), both signals activate auditory nerve fibers, and nerve fibers responding to the first stimulus have not recovered to become available to respond to the second stimulus.[24] The closer the masker is to the stimulus, the longer it takes for the auditory nerve fibers to recover and become available to respond.[45]
ABR recordings used clicks presented at 80 decibels above normal adult hearing level (dB nHL) (the signal), which were preceded by another click (the masker) at different time intervals: 8, 16, and 64 milliseconds (ms). Each averaged response consisted of 1,300 accepted sweeps, which was then replicated, yielding a waveform representing 2600 responses. The data acquisition program automatically rejected any traces contaminated by high-amplitude artifacts (voltage exceeding ±23.80 μV). The latency and amplitude values obtained for the right and left ears were averaged. ABR waveforms were analyzed for latencies for component peaks I, III, and V. We focused on wave V latency and central conduction time (CCT), which is the interpeak latency from wave I to wave V. These measures have been suggested as useful measures of auditory processing because they are easily identifiable and reproducible.[46] Wave V is frequently used as an indicator of the neurological integrity of the auditory system. [47]
Statistical analysis
Data analysis was conducted with SAS 9.4 (SAS Institute Inc., Cary, NC). Descriptive statistics, frequencies, and correlations were examined for variables of interest. To address our primary research question, linear regression models were used to evaluate associations between total number of all pesticides detected and ABR outcomes (wave V latencies and CCT at the 8, 16, and 64 ms conditions, as well as the non-masker condition) at nine months of age. As a secondary analysis, we then repeated these models while replacing total number of detected pesticides with total number of detected organophosphates, carbamates, herbicides, or fungicides. To examine the dose-response relationship of pesticides which were detected in greater than 50% of the samples, we used linear regression models where vinclozolin and diethyltoluamide predicted ABR outcomes. Samples that fell below the LOD were imputed as LOD divided by the square root of two. In addition to the main effects of the primary pesticide exposure variable, we considered an additive or synergistic effect of poor iron status by stratifying our analysis by newborn ferritin (based on median split of 146 ng/mL).
We examined bivariate associations between potential covariates and the various ABR outcomes of interest using t-tests and Pearson correlation coefficients, as well as the bivariate associations between potential covariates and our pesticide exposure index variable. The small sample size with ABR data in this pilot study limited power to include covariates in the pesticide-iron interaction analysis. Small sample size also makes results sensitive to the impact of outliers. One child had ABR CCT and wave V latency values more than 2.5 standard deviation units from the mean. These values were removed before final analysis.
Results
The mean number of pesticides detected was 4.1, with a standard deviation of 2.3 and a range of 0-9. Only one infant had no pesticides detected in cord blood. A full description of the sample is shown in Table 1 and Table 2. Child sex was the only background variable that showed consistent statistically significant associations with the various ABR wave V latencies and CCTs. However, child sex was not statistically significantly associated with the total number of detected pesticides, eliminating concern about potential confounding. We therefore did not include child sex as a covariate in our final models.
Table 2.
Characteristics of the study population
| Variable | Sample with ABR data (n = 27) |
|---|---|
| Child male, n (%) | 15 (55.6%) |
| Total number of pesticides detected (M and SD) | 4.1 (2.3) |
| Total number of organophosphate pesticides detected (M and SD) | 1.48 (1.16) |
| Total number of carbamate pesticides detected (M and SD) | 0.33 (0.48) |
| Total number of herbicides detected (M and SD) | 0.74 (0.65) |
| Total number of fungicides detected (M and SD) | 0.96 (0.76) |
| Birth weight (grams, M and SD) | 3393 (485) |
| Gestational age (weeks, M and SD) | 39.4 (1.2) |
| Cord blood ferritin (ng/mL) | 162.9 (81.1) |
| Weight-for-age z-score at 9 month ABR testing (M and SD) | 0.73 (0.93) |
| Age at 9 month ABR testing (months, M and SD) | 9.6 (0.5) |
| Head circumference-for-age z-score at 9 month ABR testing (M and SD) | 0.08 (0.95) |
| Father smokes, n (%) | 15 (55.6%) |
| Cord blood lead (μg/dL, M and SD) | 3.3 (1.3) |
Given the small sample size, we closely examined the distributions of the dependent variables before pursuing linear regression to avoid violating model assumptions. We used SAS Proc Univariate to display histograms to allow for visual inspection of data distributions, as well as to perform tests for normality (Shapiro-Wilk, Kolmogorov-Smirnov, Cramer-von Mises, Anderson-Darling). All tests indicated that all dependent variables were normally distributed. Based on linear regression models of ABR wave V latencies and CCT (Table 3), the total number of pesticides detected was significantly associated with the wave V latency for the 64ms condition (p = 0.03) and approached statistical significance for the non-masker condition (p = 0.05). Interpeak latencies from wave I to V (CCT) were significantly associated with the total number of detected pesticides for the 64ms and non-masker condition (p = 0.008 and p = 0.01, respectively). This pattern was also seen for the 8ms and 16ms conditions, although statistical significance was not reached (p = 0.06 and p = 0.09, respectively). In all models, increased pesticide exposure was associated with longer latency.
Table 3.
Parameter estimates for models predicting ABR Wave V and CCT from total number of detected pesticides and number of pesticides detected in each category.
| Non-masker | 8ms masker | 16ms masker | 64ms masker | |||||
|---|---|---|---|---|---|---|---|---|
| Wave V | CCT | Wave V | CCT | Wave V | CCT | Wave V | CCT | |
| N = 27 | N = 27 | N = 20 | N = 19 | N = 21 | N = 21 | N = 27 | N =26 | |
| Total pesticides detected (95% CI) |
0.03 (0.00- 0.06)† |
0.04 (0.01- 0.07)* |
0.02 (− 0.02 – 0.05) |
0.03 (0.00 – 0.07)† |
0.02 (− 0.02 – 0.07) |
0.03 (− 0.01 – 0.08)† |
0.04 (0.00 – 0.08)* |
0.04 (0.01 – 0.07)** |
| Organophosphates | 0.04 (− 0.03 – 0.11) |
0.05 (− 0.01 – 0.12) |
0.01 (− 0.06 – 0.08) |
0.05 (− 0.02 – 0.13) |
0.03 (− 0.06 – 0.12) |
0.03 (− 0.06 – 0.12) |
0.04 (− 0.04 – 0.12) |
0.05 (− 0.02 – 0.12) |
| Carbamates | 0.09 (− 0.08 – 0.25) |
0.05 (− 0.13 – 0.23) |
0.02 (− 0.16 – 0.20) |
0.00 (− 0.20 – 0.20) |
0.07 (− 0.15 – 0.28) |
0.09 (− 0.13 – 0.31) |
0.20 (0.02 – 0.38)* |
0.09 (− 0.08 – 0.26) |
| Herbicides | 0.07 (− 0.04 – 0.18) |
0.10 (− 0.02 – 0.21)† |
−0.01 (− 0.14 – 0.13) |
0.06 (− 0.08 – 0.19) |
0.01 (− 0.14 – 0.17) |
0.05 (− 0.11 – 0.21) |
0.09 (− 0.05 – 0.23) |
0.11 (− 0.01 – 0.22)† |
| Fungicides | 0.07 (− 0.02 – 0.17) |
0.08 (− 0.02 – 0.18)† |
0.06 (− 0.05 – 0.18) |
0.07 (− 0.05 – 0.19) |
0.06 (− 0.08 – 0.21) |
0.09 (− 0.05 – 0.24) |
0.11 (− 0.01 – 0.22)† |
0.11 (0.01 – 0.20)* |
p < 0.01,
p < 0.05,
p < 0.10
N’s vary based on availability of useable ABR data.
The small sample size was a concern, especially for the 8ms and 16ms condition where data availability limited the analytic sample more than it did for the 64ms and non-masker condition. In our primary models where total detected pesticides predicted CCT, the beta coefficients were similar in magnitude for all four conditions, yet only significant for the non-masker and 64ms conditions (Table 3, first row). Statistical power to detect the differences found in the 8ms and 16ms condition models was 0.35 and 0.25, respectively, leading us to believe that the lack of significant findings in these models may be due, at least partially, to the smaller sample size rather than a lack of a true association.
The results for the secondary models with number of detects in the four pesticide classes (Table 3) showed only two significant findings; the number of detected carbamates predicted longer wave V latency on the 64ms condition and the number of fungicides detected predicted longer CCT on the 64ms condition (p = 0.03 for both models).
Results of the dose-response analyses for vinclozolin and diethyltoluamide are shown in table 4. There was a statistically significant linear association between vinclozolin concentration and wave V and CCT from the 8ms condition. There was a statistically significant association between diethyltoluamide and wave V and CCT for the 16ms and 64ms conditions. In all cases, a higher cord blood concentration of the pesticide was associated with longer latencies providing evidence of a linear dose-response relation.
Table 4.
Parameter estimates for models predicting ABR Wave V and CCT from vinclozolin and diethyltoluamide concentrations
| Non-masker | 8ms masker | 16ms masker | 64ms masker | |||||
|---|---|---|---|---|---|---|---|---|
| Wave V | CCT | Wave V | CCT | Wave V | CCT | Wave V | CCT | |
| N = 27 | N = 27 | N = 20 | N = 19 | N = 21 | N = 21 | N = 27 | N =26 | |
| Vinclozolin (ng/mL) |
0.14 (− 0.01 – 0.28)† |
0.12 (− 0.03 – 0.28) |
0.20 (0.05 – 0.36)* |
0.18 (0.01 – 0.35)* |
0.14 (− 0.07 – 0.36) |
0.12 (− 0.10 – 0.35) |
0.10 (− 0.08 – 0.29) |
0.15 (0.00 – 0.30)† |
| Diethyltoluamide (ng/mL) |
0.23 (− 0.01 – 0.48)† |
0.24 (− 0.02 – 0.49)† |
0.24 (0.00 – 0.48)† |
0.21 (− 0.05 – 0.48) |
0.35 (0.04 – 0.67)* |
0.42 (0.11 – 0.73)** |
0.34 (0.04 – 0.63)* |
0.26 (0.01 – 0.51)* |
p < 0.01,
p < 0.05,
p < 0.10
N’s vary based on availability of useable ABR data.
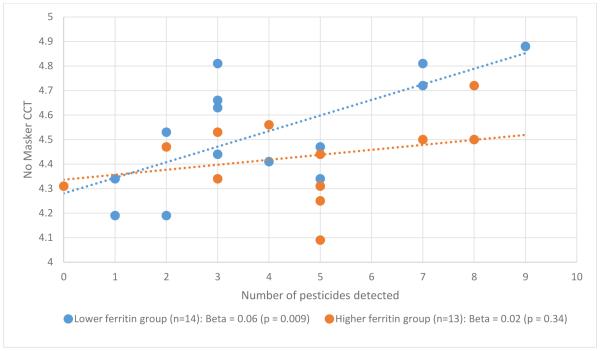
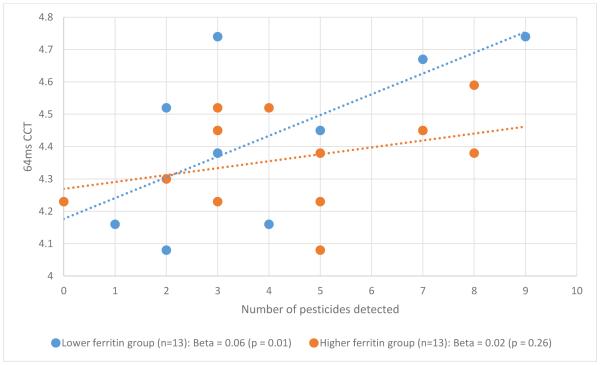
In our secondary subgroup analyses, the relation between number of pesticides detected in cord blood and CCT depended on the infant’s cord blood ferritin level (Figures 1 and 2). Specifically, the relation was present in the lower cord blood ferritin group (p = 0.009 for non-masker condition; p = 0.01 for 64ms condition) but not the higher cord blood ferritin group (p= 0.34 for non-masker condition; p = 0.26 for 64ms condition).
Figure 1.
Pesticide – CCT relation by newborn ferritin group, non-masker condition
Figure 2.
Pesticide-CCT relation by newborn ferritin group, 64ms condition
Discussion
This study demonstrated an association between prenatal exposure to multiple pesticides (as measured in cord blood) and infant auditory function at 9 months of age. Infants who were exposed to higher numbers of pesticides prenatally had longer wave 5 latencies and CCT intervals on ABR testing. These associations seemed to be strongest in infants with lower levels of cord ferritin. The fact that the findings were strongest for the 64ms forward-masking condition was important given that performance on this condition was associated with language acquisition at 9 months in the same sample.[24]
Testing the association between ABR function and each pesticide class individually showed limited statistically significant results, but echoed the findings of the total pesticide models with trends toward longer latencies with more pesticide exposure and significant results concentrated in the 64ms condition. It did not allow us to pinpoint which specific class or classes are most important when considering the link between prenatal pesticide exposure and auditory processing in infancy. Dose-response models for vinclozolin and diethyltoluamide suggested that ABR latencies increase linearly with increasing cord blood concentrations, at least for these highly-detected pesticides.
To our knowledge, this is only the second study to examine pesticide exposures occurring prenatally and auditory function in human infants.[22] Considering how a nutritional deficiency, such as limited iron stores, might affect relations between pesticide exposure and infant auditory function is another novel aspect of this pilot study.
Occupational and laboratory studies provide some limited evidence of hearing loss and deficits in auditory-related function and morphology following high exposures to some pesticides [21]. Studies of farm workers, agricultural crop sprayers, and insecticide sprayers for mosquito control programs have reported hearing loss/ poorer scores on audiometric tests [48-51], hearing dysfunction [52], and self-reported reduced hearing capacity [53]. Relationships between pesticide exposure and hearing loss in occupational settings may be confounded by co-exposure to noise, however, making it difficult to study the effects of pesticides alone [21]. Laboratory studies using animals have also reported evidence of hearing loss [54], increased thresholds [54, 55], increased ABR inter-peak latencies [55], altered cochlear morphology [56], and decreased numbers of outer and inner hair cells [57, 58], though results vary largely depending on type of pesticide, and route and duration of exposure.
The mechanism of ototoxicity following high levels of pesticide exposure is believed to target the sensitive outer hair cells of the corti organ in the inner ear [21]. Free radical and reactive oxygen species generation are thought to lead to increased apoptosis in this region of the ear because of its low levels of glutathione peroxidase, making it more vulnerable to the effects of oxidative stress [59]. The mechanism for low-dose developmental toxicity is less clear. However, a recent study of environmental exposure to organochlorine pesticides in children found evidence of deficits in cochlear status.[22]
One potential mechanism for the developmental neurotoxicity of low-dose pesticide exposure is the disruption of oligodendrocyte development and function in the brain. Several laboratory studies show that oligodendrocytes may actually be more sensitive to low-level pesticide exposure than neurons [60-62]. Oligodendrocytes are responsible for the synthesis and maintenance of the myelin sheaths that surround neuronal axons. Myelin insulates, protects, and enhances the speed and quality of the transmission of action potentials that move along neurons [63]. Premyelinating oligodendrocytes populate the developing cortex during gestation and may be particularly sensitive to the effects of prenatal pesticide exposure. Disruption of oligodendrocytes early in life could potentially lead to deficits in myelination and predispose infants to poorer cognitive and neurodevelopmental outcomes later in childhood [63]. Myelination of the human brain begins in the third trimester, with sensory tracts being myelinated first [63, 64].
Previous research on the effects of pesticide exposure on oligodendrocyte function and brain myelination has been in rats and has focused largely or primarily on organophosphate (OP) insecticides, specifically chlorpyrifos (CPF) and diazinon (DZN), or the broad-leaf herbicide, 2,4-dichlorophenoxyacetic acid (2,4-D). Pups born to pregnant rats treated with CPF during gestation or postnatally, corresponding to the onset of myelination in rats, showed immediate and long-term deficits in an oligodendrocyte-specific marker, myelin basic protein (MBP)[61, 62]. Another study exposed neonatal rats to CPF or DZN and found that CPF evoked a decrease in overall expression of myelin-related genes, as well as eliciting statistically significant deficits in the expression of specific myelin-related genes. Treatment with DZN did not have an overall effect on myelin gene expression, but elicited a statistically significant reduction in individual myelin-related gene expression [65]. Similarly, neonatal exposure to 2,4-D resulted in myelin deficits in the rat pup brain, as measured by protein expression [66, 67] and electron microscopy [67, 68], and alterations in behavior [68].
Taken together, these studies indicate that exposure to CPF, DZN, or 2,4-D during the developmental period corresponding to the onset of myelination can induce negative effects on myelin-related gene expression and function and myelination in the brain. Though much of the work to this point has focused on a few select pesticides, the results may be pertinent to other pesticides. Despite their differing modes of action at high doses, these different classes of pesticides appear to elicit similar effects on myelination in in vivo laboratory studies.
While we were unable to examine the effects of CPF or DZN alone due to low levels of detection, our ABR results support the hypothesis that pesticide exposure inhibits myelination. Here we observed that exposure to a higher number of pesticides during pregnancy was associated with longer CCT and wave V latencies. Longer CCT intervals and wave V latencies indicate slower auditory transmission speeds. Since myelination occurs centripetally, from the more central to the more distal parts of the pathway, the wave V latency is expected to increase with a disorder of myelination [69]. Additionally, myelination of the auditory pathways begins late in gestation in the human. Thus, pesticide levels in umbilical cord blood may adequately reflect fetal exposure during the onset of myelination.
We chose to examine a nutrient-toxicant interaction, the pesticide-iron effect, because iron is important for early neurodevelopment and concurrent exposure to pesticides and early-life iron deficiency is common in many parts of the world. Iron is essential for oligodendrocyte function and myelination. [31, 32] Iron deficiency alters myelin-related genes and proteins in the short- and long-term in developing rats and primate infants.[70-73] Previous findings of longer ABR latencies in iron-deficient human infants are consistent with impaired myelination of the auditory pathways [25, 33]. Our sub-analyses of the pesticide effect in infants with lower vs. higher ferritin at birth are consistent with these previous findings. We observed slower auditory transmission (longer CCTs) in infants with more pesticide exposures and lower cord blood ferritin levels compared with similarly exposed infants with higher ferritin levels at birth, suggesting that there may be important ferritin-pesticide interactions.
Our small sample size limits both our statistical power for detecting subtle associations and our ability to adjust for possible confounding variables. The study is further restricted by the methods of pesticide detection. The relatively high LODs limited our ability to assess relations between individual pesticides or mixtures and ABR outcomes more quantitatively. Exposure measurement at only one time point (delivery) means that we cannot characterize pesticide exposure throughout pregnancy and may miss other potentially sensitive windows of exposure. Finally, the results may not adequately account for the effects of other pesticides that may be present, since only a selection of non-persistent pesticides were measured in cord blood. We anticipate that many of these concerns will be ameliorated when this pilot study is expanded to a larger sample.
Despite its limitations, this is only the second study to examine the effects of prenatal pesticide exposures and human auditory function. By examining a relatively large number and variety of pesticides we could begin to explore the effects of the multiple exposures, unlike many other studies that focus on only one pesticide or metabolite. Also, measuring pesticide levels in cord blood provided stronger evidence of exposure than non-specific urinary metabolites [74, 75]. Finally, the use of ABR to assess of auditory function provides a noninvasive indication of infant auditory system myelination, giving insight into a possible mechanism for low-level pesticide neurotoxicity.
Conclusions
This work provides preliminary evidence that auditory system maturation may be delayed in infants with multiple prenatal pesticide exposures and that this effect is strongest in infants with lower iron stores at birth. The auditory system starts myelinating in late gestation and matures rapidly in infancy. Longer CCT and wave V latencies suggest that exposure to multiple pesticides prenatally has negative effects on auditory system myelination early in life. Auditory system development in infancy provides the foundation for many subsequent learning processes, such as communication and language development [24, 25]. Therefore, delays or altered timing of auditory systems myelination, related to prenatal pesticide exposures, may contribute to detrimental long-term effects on learning or other cognitive functions in childhood and possibly beyond. Larger studies of pesticide exposure, iron deficiency, and auditory system development are needed to verify the findings of this pilot study.
Highlights.
A sample of 9 month old Chinese infants had high levels of pesticide exposure.
Auditory processing was slower in infants with greater prenatal pesticide exposure.
Pesticides interacted with lower ferritin to cause slower auditory processing.
Acknowledgements
We are grateful to the families who participated in the study, the nurses and colleagues at Children’s Hospital of Zhejiang University School of Medicine for their dedicated assistance, and Paul R. Kileny, University of Michigan, for help with training in ABR data recording and processing. This study was supported by grants from the National Natural Science Foundation of China (#81273085), the US National Institutes of Health (P01 HD039386), the US National Institute for Environmental Health Sciences (R01 ES021465), and the Michigan Institute for Clinical and Health Research (UL 1RR024986).
List of abbreviations
- ABR
auditory brainstem response
- CNS
central nervous system
- GC-MS
gas chromatography-mass spectrometry
- LOD
limit of detection
- EEG
electroencephalogram
- dB nHL
decibel above normal adult hearing level
- ms
milliseconds
- CCT
central conduction time
- ng/mL
nanogram per milliliter
- μV
microvolt
- SD
standard deviation
- SE
standard error
- ND
non-detect
- OP
organophosphate
- CPF
chlorpyrifos
- DZN
diazinon
- 2,4-D
2,4-dichlorophenoxyacetic acid
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JSturza drafted and revised the paper, conducted data analysis, and approved the final manuscript as submitted.
MKS drafted and revised the paper, and approved the final manuscript as submitted.
LX collected data, critically revised the manuscript, and approved the final manuscript as submitted.
ML collected data, critically revised the manuscript, and approved the final manuscript as submitted
XM processed ABR data, critically revised the manuscript, and approved the final manuscript as submitted.
YX processed cord blood samples, critically revised the manuscript, and approved the final manuscript as submitted.
JShao contributed to study conception and design, conducted the research, critically revised the manuscript, and approved the final manuscript as submitted.
BL conceptualized and designed the study, interpreted data, critically revised the paper, and approved the final manuscript as submitted.
JM conceptualized and designed the study, interpreted data, critically revised the paper, and approved the final manuscript as submitted.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burns CJ, et al. Pesticide exposure and neurodevelopmental outcomes: review of the epidemiologic and animal studies. J Toxicol Environ Health B Crit Rev. 2013;16(3-4):127–283. doi: 10.1080/10937404.2013.783383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang W, Jiang F, JF. O. Global pesticide consumption and pollution: with China as a focus. Proceedings of the International Academy of Ecology and Environmental Sciences. 2011;1:125–144. [Google Scholar]
- 3.U.S.EPA . Pesticide industry sales and usage: 2006 and 2007 market estimates. US Environmental Protection Agency; Washington, DC: 2011. [Google Scholar]
- 4.Ding G, Bao Y. Revisiting pesticide exposure and children's health: Focus on China. Sci Total Environ. 2013;472C:289–295. doi: 10.1016/j.scitotenv.2013.11.067. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, et al. Prenatal exposure to organophosphate pesticides and neurobehavioral development of neonates: a birth cohort study in shenyang, china. PLoS One. 2014;9(2):e88491. doi: 10.1371/journal.pone.0088491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J, et al. Farm pesticide, rice production, and human health. Economy and Environment Program for Southeast Asia; Singapore: 2001. [Google Scholar]
- 7.Abdollahi M, Karami-Mohajeri S. A comprehensive review on experimental and clinical findings in intermediate syndrome caused by organophosphate poisoning. Toxicol Appl Pharmacol. 2012;258(3):309–14. doi: 10.1016/j.taap.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Yang CC, Deng JF. Intermediate syndrome following organophosphate insecticide poisoning. J Chin Med Assoc. 2007;70(11):467–72. doi: 10.1016/S1726-4901(08)70043-1. [DOI] [PubMed] [Google Scholar]
- 9.Garcia SJ, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: targeting glial cells. Environ Toxicol Pharmacol. 2005;19(3):455–61. doi: 10.1016/j.etap.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Bradman A, et al. Measurement of pesticides and other toxicants in amniotic fluid as a potential biomarker of prenatal exposure: a validation study. Environ Health Perspect. 2003;111(14):1779–82. doi: 10.1289/ehp.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eskenazi B, et al. Pesticide toxicity and the developing brain. Basic Clin Pharmacol Toxicol. 2008;102(2):228–36. doi: 10.1111/j.1742-7843.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- 12.Bouchard MF, et al. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ Health Perspect. 2011;119(8):1189–95. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rauh V, et al. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ Health Perspect. 2011;119(8):1196–201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eskenazi B, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115(5):792–8. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engel SM, et al. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect. 2011;119(8):1182–8. doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts EM, et al. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environ Health Perspect. 2007;115(10):1482–9. doi: 10.1289/ehp.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shelton JF, et al. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ Health Perspect. 2014;122(10):1103–9. doi: 10.1289/ehp.1307044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marks AR, et al. Organophosphate pesticide exposure and attention in young Mexican-American children: the CHAMACOS study. Environ Health Perspect. 2010;118(12):1768–74. doi: 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouchard MF, et al. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics. 2010;125(6):e1270–7. doi: 10.1542/peds.2009-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rauh VA, et al. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118(6):1845–59. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gatto MP, et al. Effects of potential neurotoxic pesticides on hearing loss: a review. Neurotoxicology. 2014;42:24–32. doi: 10.1016/j.neuro.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Sisto R, et al. Environmental exposure to organochlorine pesticides and deficits in cochlear status in children. Environ Sci Pollut Res Int. 2015;22(19):14570–8. doi: 10.1007/s11356-015-4690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cartier C, et al. Prenatal and 5-year p,p'-DDE exposures are associated with altered sensory processing in school-aged children in Nunavik: a visual evoked potential study. Neurotoxicology. 2014;44:8–16. doi: 10.1016/j.neuro.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Chonchaiya W, et al. Developmental trends in auditory processing can provide early predictions of language acquisition in young infants. Dev Sci. 2013;16(2):159–72. doi: 10.1111/desc.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Algarin C, et al. Iron deficiency anemia in infancy: long-lasting effects on auditory and visual system functioning. Pediatr Res. 2003;53(2):217–23. doi: 10.1203/01.PDR.0000047657.23156.55. [DOI] [PubMed] [Google Scholar]
- 26.Molfese DL. The use of auditory evoked responses recorded from newborn infants to predict later language skills. Birth Defects Orig Artic Ser. 1989;25(6):47–62. [PubMed] [Google Scholar]
- 27.Molfese DL. Predicting dyslexia at 8 years of age using neonatal brain responses. Brain Lang. 2000;72(3):238–45. doi: 10.1006/brln.2000.2287. [DOI] [PubMed] [Google Scholar]
- 28.Benasich AA, Tallal P. Infant discrimination of rapid auditory cues predicts later language impairment. Behav Brain Res. 2002;136(1):31–49. doi: 10.1016/s0166-4328(02)00098-0. [DOI] [PubMed] [Google Scholar]
- 29.Lozoff B. Iron deficiency and child development. Food Nutr Bull. 2007;28(4 Suppl):S560–71. doi: 10.1177/15648265070284S409. [DOI] [PubMed] [Google Scholar]
- 30.Georgieff MK, Brunette KE, Tran PV. Early life nutrition and neural plasticity. Dev Psychopathol. 2015;27(2):411–23. doi: 10.1017/S0954579415000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Badaracco ME, Siri MV, Pasquini JM. Oligodendrogenesis: the role of iron. Biofactors. 2010;36(2):98–102. doi: 10.1002/biof.90. [DOI] [PubMed] [Google Scholar]
- 32.Todorich B, et al. Oligodendrocytes and myelination: the role of iron. Glia. 2009;57(5):467–78. doi: 10.1002/glia.20784. [DOI] [PubMed] [Google Scholar]
- 33.Roncagliolo M, et al. Evidence of altered central nervous system development in infants with iron deficiency anemia at 6 mo: delayed maturation of auditory brainstem responses. Am J Clin Nutr. 1998;68(3):683–90. doi: 10.1093/ajcn/68.3.683. [DOI] [PubMed] [Google Scholar]
- 34.Wickerham EL, et al. Reduced birth weight in relation to pesticide mixtures detected in cord blood of full-term infants. Environ Int. 2012;47:80–5. doi: 10.1016/j.envint.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rider CV, et al. Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity. Int J Androl. 2010;33(2):443–62. doi: 10.1111/j.1365-2605.2009.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Despland PA, Galambos R. The auditory brainstem response (ABR) is a useful diagnostic tool in the intensive care nursery. Pediatr Res. 1980;14(2):154–8. doi: 10.1203/00006450-198002000-00018. [DOI] [PubMed] [Google Scholar]
- 37.Jacobson JT. Normative aspects of the pediatric auditory brainstem response. J Otolaryngol Suppl. 1985;14:7–11. [PubMed] [Google Scholar]
- 38.Song JH, Nicol T, Kraus N. Test-retest reliability of the speech-evoked auditory brainstem response. Clin Neurophysiol. 2011;122(2):346–55. doi: 10.1016/j.clinph.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall JI. The new handbook of auditory evoked responses. Allyn & Bacon; Boston: 2007. [Google Scholar]
- 40.DeBonis DA, Donohue CL. Survey of Audiology: Fundamentals for Audiologists and Health Professionals. 2nd Boston: Allyn & Bacon: 2008. [Google Scholar]
- 41.Despland PA, Galambos R. The auditory brainstem response (ABR) is a useful diagnostic tool in the intensive care nursery. Pediatric research. 1980;14(2):154–8. doi: 10.1203/00006450-198002000-00018. [DOI] [PubMed] [Google Scholar]
- 42.Mai X, et al. Temporal processing in the auditory brainstem response by full-term 6-week- and 9-month-old infants. Scientific Reports. 2015 doi: 10.1038/srep12647. (in progress) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lasky RE. The effects of rate and forward masking on human adult and newborn auditory evoked brainstem response thresholds. Dev Psychobiol. 1991;24(1):51–64. doi: 10.1002/dev.420240105. [DOI] [PubMed] [Google Scholar]
- 44.Walton J, Orlando M, Burkard R. Auditory brainstem response forward-masking recovery functions in older humans with normal hearing. Hearing Research. 1999;127:86–94. doi: 10.1016/s0378-5955(98)00175-0. [DOI] [PubMed] [Google Scholar]
- 45.Abbas P, Gorga M. AP responses in forward-masking paradigms and their relationship to response of auditory-nerve fibers. Journal of the Acoustical Society of America. 1981;69(2):492–499. doi: 10.1121/1.385477. [DOI] [PubMed] [Google Scholar]
- 46.Berglund SK, et al. Effects of iron supplementation on auditory brainstem response in marginally LBW infants. Pediatr Res. 2011;70(6):601–6. doi: 10.1203/PDR.0b013e3182320cd0. [DOI] [PubMed] [Google Scholar]
- 47.Hecox K, Galambos R. Brain stem auditory evoked responses in human infants and adults. Arch Otolaryngol. 1974;99(1):30–3. doi: 10.1001/archotol.1974.00780030034006. [DOI] [PubMed] [Google Scholar]
- 48.Beckett WS, et al. Hearing conservation for farmers: source apportionment of occupational and environmental factors contributing to hearing loss. J Occup Environ Med. 2000;42(8):806–13. doi: 10.1097/00043764-200008000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Guida HL, Morini RG, Cardoso AC. Audiological evaluation in workers exposed to noise and pesticide. Braz J Otorhinolaryngol. 2010;76(4):423–7. doi: 10.1590/S1808-86942010000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoshino AC, et al. Ototoxicity study in workers exposed to organophosphate. Braz J Otorhinolaryngol. 2008;74(6):912–8. doi: 10.1016/S1808-8694(15)30153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harell M, Shea JJ, Emmett JR. Bilateral sudden deafness following combined insecticide poisoning. Laryngoscope. 1978;88(8):1348–51. doi: 10.1288/00005537-197808000-00018. Pt 1. [DOI] [PubMed] [Google Scholar]
- 52.Teixeira CF, Augusto LG, Morata TC. [Hearing health of workers exposed to noise and insecticides] Rev Saude Publica. 2003;37(4):417–23. doi: 10.1590/s0034-89102003000400005. [DOI] [PubMed] [Google Scholar]
- 53.Crawford JM, et al. Hearing loss among licensed pesticide applicators in the agricultural health study. J Occup Environ Med. 2008;50(7):817–26. doi: 10.1097/JOM.0b013e31816a8caf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hadjab S, et al. Hexachlorobenzene, a dioxin-like compound, disrupts auditory function in rat. Hear Res. 2004;191(1-2):125–34. doi: 10.1016/j.heares.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 55.Bergler W, et al. Effect of organophosphorus compound intoxication on auditory brainstem response in mini pigs. ORL J Otorhinolaryngol Relat Spec. 1996;58(4):219–23. doi: 10.1159/000276840. [DOI] [PubMed] [Google Scholar]
- 56.Korbes D, et al. Organophosphate-related ototoxicity: Description of the vestibulocochlear system ultrastructural aspects of guinea pigs. Braz J Otorhinolaryngol. 2010;76(2):238–44. doi: 10.1590/S1808-86942010000200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bielefeld EC, et al. Damage and threshold shift resulting from cochlear exposure to paraquat-generated superoxide. Hear Res. 2005;207(1-2):35–42. doi: 10.1016/j.heares.2005.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nicotera TM, et al. Paraquat-induced hair cell damage and protection with the superoxide dismutase mimetic m40403. Audiol Neurootol. 2004;9(6):353–62. doi: 10.1159/000081284. [DOI] [PubMed] [Google Scholar]
- 59.Cardinaal RM, et al. Cisplatin-induced ototoxicity: morphological evidence of spontaneous outer hair cell recovery in albino guinea pigs? Hear Res. 2000;144(1-2):147–56. doi: 10.1016/s0378-5955(00)00060-5. [DOI] [PubMed] [Google Scholar]
- 60.Garcia SJ, et al. Does the developmental neurotoxicity of chlorpyrifos involve glial targets? Macromolecule synthesis, adenylyl cyclase signaling, nuclear transcription factors, and formation of reactive oxygen in C6 glioma cells. Brain Res. 2001;891(1-2):54–68. doi: 10.1016/s0006-8993(00)03189-9. [DOI] [PubMed] [Google Scholar]
- 61.Garcia SJ, et al. Chlorpyrifos targets developing glia: effects on glial fibrillary acidic protein. Brain Res Dev Brain Res. 2002;133(2):151–61. doi: 10.1016/s0165-3806(02)00283-3. [DOI] [PubMed] [Google Scholar]
- 62.Garcia SJ, Seidler FJ, Slotkin TA. Developmental neurotoxicity elicited by prenatal or postnatal chlorpyrifos exposure: effects on neurospecific proteins indicate changing vulnerabilities. Environ Health Perspect. 2003;111(3):297–303. doi: 10.1289/ehp.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35(1):147–68. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carlson BM. Human Embryology and Developmental Biology. 5th Elsevier; 2014. [Google Scholar]
- 65.Slotkin TA, Seidler FJ. Comparative developmental neurotoxicity of organophosphates in vivo: transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Brain Res Bull. 2007;72(4-6):232–74. doi: 10.1016/j.brainresbull.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duffard R, et al. Central nervous system myelin deficit in rats exposed to 2,4-dichlorophenoxyacetic acid throughout lactation. Neurotoxicol Teratol. 1996;18(6):691–6. doi: 10.1016/s0892-0362(96)00087-6. [DOI] [PubMed] [Google Scholar]
- 67.Konjuh C, et al. Neonatal hypomyelination by the herbicide 2,4-dichlorophenoxyacetic acid. Chemical and ultrastructural studies in rats. Toxicol Sci. 2008;104(2):332–40. doi: 10.1093/toxsci/kfn085. [DOI] [PubMed] [Google Scholar]
- 68.Rosso SB, et al. 2,4-Dichlorophenoxyacetic acid in developing rats alters behaviour, myelination and regions brain gangliosides pattern. Neurotoxicology. 2000;21(1-2):155–63. [PubMed] [Google Scholar]
- 69.Jiang ZD. Maturation of the auditory brainstem in low risk-preterm infants: a comparison with age-matched full term infants up to 6 years. Early Hum Dev. 1995;42(1):49–65. doi: 10.1016/0378-3782(95)01639-k. [DOI] [PubMed] [Google Scholar]
- 70.Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- 71.Beard J. Recent evidence from human and animal studies regarding iron status and infant development. J Nutr. 2007;137(2):524S–530S. doi: 10.1093/jn/137.2.524S. [DOI] [PubMed] [Google Scholar]
- 72.Siddappa AJ, et al. Iron deficiency alters iron regulatory protein and iron transport protein expression in the perinatal rat brain. Pediatr Res. 2003;53(5):800–7. doi: 10.1203/01.PDR.0000058922.67035.D5. [DOI] [PubMed] [Google Scholar]
- 73.Clardy SL, et al. Acute and chronic effects of developmental iron deficiency on mRNA expression patterns in the brain. J Neural Transm Suppl. 2006;(71):173–96. doi: 10.1007/978-3-211-33328-0_19. [DOI] [PubMed] [Google Scholar]
- 74.Barr DB, et al. Strategies for biological monitoring of exposure for contemporary-use pesticides. Toxicol Ind Health. 1999;15(1-2):168–79. doi: 10.1191/074823399678846556. [DOI] [PubMed] [Google Scholar]
- 75.Munoz-Quezada MT, et al. Neurodevelopmental effects in children associated with exposure to organophosphate pesticides: a systematic review. Neurotoxicology. 2013;39:158–68. doi: 10.1016/j.neuro.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]