Abstract
Targeted delivery aims to selectively distribute drugs to targeted tumor tissue but not to healthy tissue. This can address many of clinical challenges by maximizing the efficacy but minimizing the toxicity of anti-cancer drugs. However, complex tumor microenvironment poses various barriers hindering the transport of drugs and drug delivery systems. New tumor models that allow for the systematic study of these complex environments are highly desired to provide reliable test beds to develop drug delivery systems for targeted delivery. Recently, research efforts have yielded new in vitro tumor models, the so called tumor-microenvironment-on-chip, that recapitulate certain characteristics of the tumor microenvironment. These new models show benefits over other conventional tumor models, and have the potential to accelerate drug discovery and enable precision medicines. However, further research is warranted to overcome their limitations and to properly interpret the data obtained from these models. In this article, key features of the in vivo tumor microenvironment that are relevant to drug transport processes for targeted delivery was discussed, and the current status and challenges for developing in vitro transport model systems was reviewed.
Keywords: Drug transport, Targeted delivery, Stromal Tissue, Microfluidics, Nanoparticles
1. Introduction
Many promising anti-cancer drug candidates have been identified in the last several decades. However, only a handful have exhibited therapeutic efficacy on human patients. This is largely due to the limited delivery of drugs to target tumors, which can result in unwanted accumulation of compounds to non-targeted healthy tissues and organs, and ultimately to systemic toxicity. Targeted delivery, which aims to selectively distribute drugs to targeted tumor tissue but not to healthy tissue, can address many of these difficulties. Such targeted delivery, however, is very difficult to achieve [1]. Thus, the term “targeted” is used in this article refers to the preferential delivery of drugs to the tumor site. It should be distinguished from “targeted therapy” which refers to drugs interfering with specific molecular targets in cancers.
Recent developments in the field of nanotechnology enables the synthesis of a wide variety of nanoparticles (NPs), whose size and surface properties can be designed to serve as effective vehicles for targeted delivery. These nanostructures include liposomes, polymer micelles, dendrimers, drug nanocrystals, magnetic nanoparticles, gold nanoparticles/nanoshells, nanorods, nanotubes, and drug-polymer conjugates (all of which will be collectively referred to as NPs). Research aimed at controlling the size and surface properties of these NPs to be responsive to the tumor microenvironment has been performed as reported elsewhere [2-5]. Even though improvements in the delivery efficacy have been shown, the majority of administered NPs fail to reach target tumors. One of the biggest benefits of using NP formulations is to avoid non-aqueous solvents for administering hydrophobic drugs to patients, resulting in fewer side effects, while maintaining the same efficacy. The success of Abraxane® (nanoparticle albumin-bound paclitaxel) and Doxil® (PEGylated liposome formulation), in large part, relies on delivering anticancer drugs without using organic solvents. In order to maximize the therapeutic outcomes, however, drug accumulation as well as penetration into the targeted tumors should be improved. The challenge before us is to achieve effective delivery to the cancer cells since it is significantly hindered by various barriers engendered by the complex tumor microenvironment (TME).
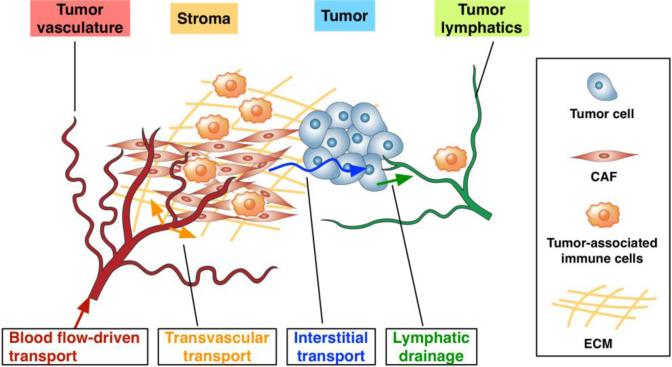
After being administered into a patient's blood stream, the drugs (for brevity, the term “drug” is used to refer both drug and drug delivery system including NPs) are thought to be subjected to complex and multi-faceted transport processes prior to reaching the cancer cells as reviewed previously [6-10]. These include – i) blood flow-driven transport to the tumor vasculature, ii) transvascular transport (i.e., extravasation), iii) interstitial transport, and iv) cellular uptake and metabolism as illustrated in Figure 1. Excess drugs often occupy the interstitial space or are transported through the lymphatic vessels. These transport phenomena are governed by diffusion and convection processes, and the significance of each process is dependent on both drugs and the biophysical conditions of TME. The drug dependent properties are the size and surface properties, and the TME dependent ones include leakiness of the blood vessel wall, interstitial fluid pressure gradient, and the extracellular matrix (ECM) microstructure within the tumor interstitium. These processes and physiological conditions are highly dynamic, interconnected and vary spatiotemporally.
Figure 1. Complexity of the tumor microenvironment.
TME poses multi-faceted barriers to drugs transport owing to the dense stromal tissue which is composed of collagens, fibronectin, and hyaluronan, an abundance of cancer-associated fibroblasts, and aberrant interactions between infiltrating tumor-associated immune cells, cancer cells, and CAFs.
Besides these biophysical barriers, the TME also poses biochemical and biological complexities. Typically, tumor tissues consist of cancerous cells as well as stromal components that consist of various stromal cells including cancer-associated fibroblasts (CAF), diverse immune and inflammatory cell types and rich extracellular matrix components, such as type I collagen [11, 12]. In addition to the highly heterogeneous cancer cell populations, i.e. intra-tumoral heterogeneity, the complex stromal tissue acts as a repository for various growth factors and cytokines that can dramatically influence tumor growth and drug response, as well as creating a hypoxic environment. Thus, it is important to understand the TME to design and develop effective targeted drug delivery systems. New tumor models that allow for the systematic study of these complex environments are highly desired and will provide reliable test beds to characterize and optimize the design of drugs.
Several tumor models are available but they do not adequately address this challenge. Conventional static in vitro systems, including cell suspensions and cell monolayers, are not sufficient to study these complex in vivo transport processes because the model systems lack dynamic interactions among the cells, ECM, interstitial fluid and NPs. Animal models can provide a TME with all of these dynamic interactions, but such models are limited to systematically studying the effects of these dynamic interactions. Recently, research efforts have yielded new in vitro tumor models, the so called tumor-microenvironment-on-chip (T-MOC), that recapitulate certain characteristics of the TME. Although various configurations have been developed, T-MOCs are basically microfluidic platforms where cancer cells are cultured within the ECM under perfusion conditions. These new models show benefits over other conventional tumor models, and have the potential to accelerate drug discovery and patient-specific personalized treatment planning. However, the TME is extremely complex and there remain significant limitations to overcome. In this article, key features of the in vivo TME that are relevant to drug transport processes for targeted delivery are reviewed, and the current status and challenges for developing transport model systems are discussed.
2. Tumor microenvironment: A complex and chaotic bed for tumor growth
The tumor microenvironment is a complex and adverse environment for drug transport and action. It comprises a highly heterogeneous mixture of tumor and stromal cells embedded in an extracellular matrix that also includes cytokines, growth factors, inflammatory cells and macrophages. Together, the TME poses multi-faceted barriers including biological, chemical and physical hindrances to drug transport and actions. These barriers are highly dynamic and often interconnected. Their interactions and relative significance with respect to drug delivery and therapeutic efficacy vary drastically depending on the cancer type, stage and organs. The current difficulty in developing new anticancer drugs and drug delivery systems partly stems from the lack of a clear understanding of the delicate interplay of these barriers at the TME [13-15]. Thus, instead of providing a generic description on these hindrances, it is more relevant to collectively discuss the interplays that are associated with one type of cancer. Here, our discussion will be focused on pancreatic cancer and its associated TME unless mentioned otherwise.
Pancreatic ductal adenocarcinoma (PDAC) is a significant clinical challenge due to its poor prognosis and extremely low (7%) five-year survival rate [16]. Its extensive TME presents many key features relevant to discussing the hindrances and resistance of drug transport and actions. One of the most notable characteristics of PDAC is its marked desmoplasia. The desmoplastic stroma of PDAC is composed of CAFs, various immune and inflammatory cell types and a dense extracellular matrix [11, 12], as illustrated in Figure 1. Moreover, PDAC is poorly vascularized and has extremely high interstitial fluid pressure (IFP) [17-19]. In this complex 3D TME, highly intricate and multifaceted interactions occur among pancreatic cancer cells (PCC), CAFs, tumor-associated macrophages (TAMs) and other immune cells. The biochemical and biophysical interactions occurring among the cells within the TME are poorly understood.
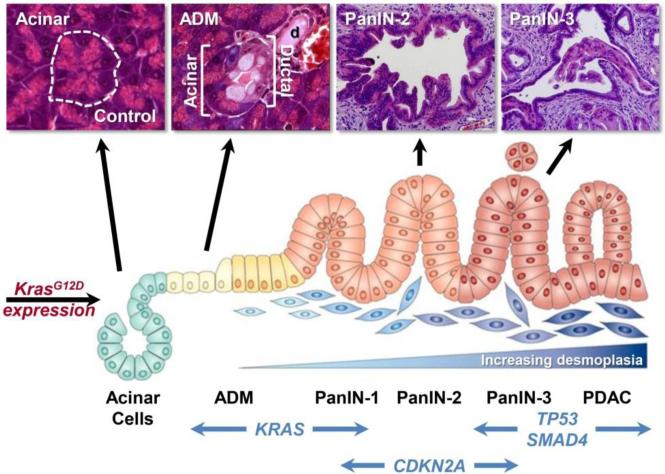
PDAC is a complex, heterogeneous and genetically unstable disease which is caused by prolonged accumulation of mutations in key oncogenes and tumor suppressor genes [13,47,113]. These include activation of the Kras2 oncogene, inactivation of the tumor suppressor gene Cdkn2A/Ink4A, and finally, inactivation of the tumor suppressor genes TP53 and Dpc4/Smad4 [25,68]. Recent studies indicate that PCCs carry an average of 63 genetic alterations per cancer, which can be grouped into 12 core signaling pathways [20]. PDAC is thought to arise from precursor lesions such as pancreatic intraepithelial neoplasia (PanIN). These lesions develop into invasive carcinoma through a multistep carcinogenic process (illustrated in Figure 2).
Figure 2. Schematic model of genetic alterations and histological examples of acinar cells, ADM (acinar-ductal metaplasia), PanIN, and PDAC progression as observed in a mouse model of PDAC.
Diagram adapted from Morris et al. [21]. Images from Zhu et al. [22] and Shi et al. [23].
The complex TME of PDAC poses multiple barriers that inhibit transport and action of drugs. First, the TME serves as a biophysical barrier that impedes effective transport of drugs to target cancer cells or associated stromal cells. After administration, drugs are subject to complex transport processes to reach the cancer cells, including blood flow-driven transport, drug-endothelium interactions, extravasation, interstitial transport and cellular uptake [6, 7]. Although the drugs are thought to preferentially extravasate more in tumors than in normal tissues via the so called enhanced permeation and retention (EPR) effect [24, 25], the hypovascularity of PDAC may limit the benefit of EPR effects [26, 27]. Nonetheless, a certain percentage of PDACs exhibit a strong angiogeneic gene signature and areas of increased microvessel density [28], raising the possibility that drug delivery may be more efficient in this patient subgroup. However, even after the drugs preferentially extravasate into the PDAC TME, the drugs encounter a very dense stroma and significantly elevated IFP. The dense stroma is attributed to activated CAF, inflammatory immune cells and an excessive deposition of a complex ECM that includes dense collagen type I and III bundles, hyaluronic acid, fibronectin and desmin [17, 18, 29], as illustrated in Figure 3. The dense ECM microstructure and cell packing of stromal tissue significantly hinders interstitial transport in conjunction with elevated IFP [18, 27, 30-33]. These features are compounded by hypoxia and infiltration of growth promoting inflammatory cells at the TME.
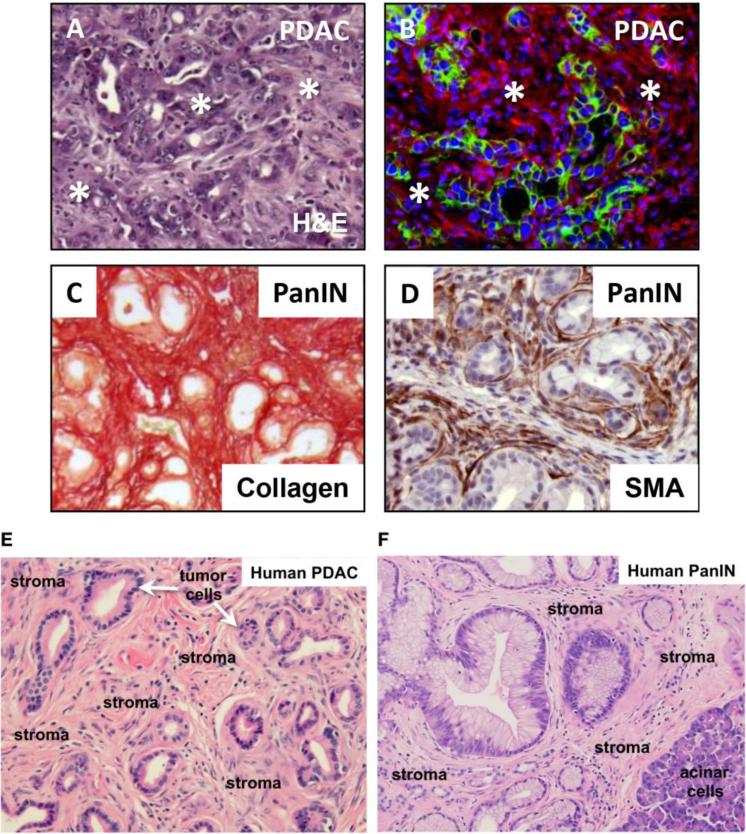
Figure 3. Stromal components in PDAC and PanIN.
(A, B) Mouse PDAC from elastase-CreERT2/LSL-KrasG12D/+/LSL-Tp53R172H/+/R26mTmG/+ mice. Upon tamoxifen treatment, CreERT2 becomes active in the acinar cell compartment, leading to activation of KRASG12D, TP53R172H and mGFP expression. In (B) membrane localized tdTomato Red (mT) labels stromal cells whereas membrane localized GFP (mG) marks the tumor epithelial cells. The majority of PDAC tumor mass consists of stromal components (indicated by asterisks in A and shown in red in B). (C, D) Mouse PanIN lesions from elastase-CreERT2/LSL-KrasG12D/+/LSL-Tp53R172H/+ mice. Sirius Red staining identifies extensive collagen deposition in (C). Similarly, anti-smooth muscle actin (SMA) (brown stain) identifies cancer associated fibroblasts (CAFs) surrounding the transformed PanIN epithelial cells in PDAC samples (D). (E, F) Human PDAC and PanIN H&E stained sections. The predominant stromal components are noted.
Second, increasing evidence has shown that various TME components, including the ECM, soluble cytokines, growth factors, the MMP family of proteases, and immunosuppressive and pro-tumorigenic immune cells, contribute to extensive tumor promoting properties. Pancreatic stellate cells are activated into CAFs, which produce and deposit fibronectin and collagens, whereas inflammatory cells and macrophages produce chemokines and cyokines. Thus, the TME of PDAC is rich in growth factors, including fibroblast growth factors (FGFs), epidermal growth factor (EGF) receptor ligands, transforming growth factor-β (TGF-β) isoforms, and connective tissue growth factor (CTGF) [19, 34, 35]. These chemical environments facilitate not only stroma production, but also enhance PCC proliferation, epithelial-mesenchymal-transition (EMT), metastatic potential, and importantly, therapeutic resistance through molecular interactions between cancer cells and CAFs. The combination of these complex biochemical environments with hypoxia and heterogeneous genetic mutations makes PDAC incredibly resistant to therapeutics.
The development of genetically engineered mouse models (GEMMs) of PDAC has greatly aided fundamental studies of the interactions between PCCs and stromal cells. For example, PDAC CAFs express vitamin D receptors, and activation of these receptors by the calcitriol analogue suppresses deleterious immunological cell infiltrates in mouse PDAC (mPDAC) [36]. Conversely, CAF depletion in mPDAC has been associated with altered immune gene expression and altered infiltrating immune cell populations, including decreased CD4+ effector T cells, increased CD4+Foxp3+ regulatory T cells (Treg), and decreased cytotoxic CD8+/Treg and CD3+/CD11b+ ratios [37]. Ctla4 expression was also increased, meaning that treatment of CAF-depleted mice with a CLTA-4 blocking antibody attenuated PDAC progression, improved overall survival, induced tumor clearance in up to 25% of the pancreas, and reprogrammed the transcriptome to a pattern that resembled control (CAF-competent) tumors. Similarly, deletion of Shh in cancer cells to suppress mPDAC stroma led to more frequent PanIN and ADM lesions at a young age, an earlier appearance of loss of differentiation of mPDAC, increased metastasis, and more rapid death [37]. There was also enhanced cancer cell proliferation and angiogenesis, increased Zeb1 and Slug expression consistent with EMT, and reduced CD45+ myeloid cells and F4/80+ monocytes infiltration. Similar results were observed when animals were treated with a Smoothened inhibitor. Genetic Shh deletion or pharmacological targeting of Shh signaling pathways attenuates stroma formation but leads to more aggressive mPDAC. Thus, depending on the strategy, stroma depletion can cause beneficial or deleterious effects in GEMMS of PDAC.
3. Transport barriers of TME and drug resistance
Pathophysiological characteristics of tumors present multiple levels of transport barriers to targeted delivery of drugs. These include leaky and chaotic vasculature of the tumor, increased IFP, less functional lymphatic vessels, dense ECM microstructure and high cell packing density [24, 25, 31-33], as illustrated in Figure 4A. These TME characteristics are highly dynamic, interconnected and vary spatiotemporally [13, 26], and the compounding effects of all of these physiological parameters on drug transport are not yet fully understood yet. In this section, the complex transport processes of how drugs reach to the targeted tumor will be discussed with relevant transport barriers posed by the TME.
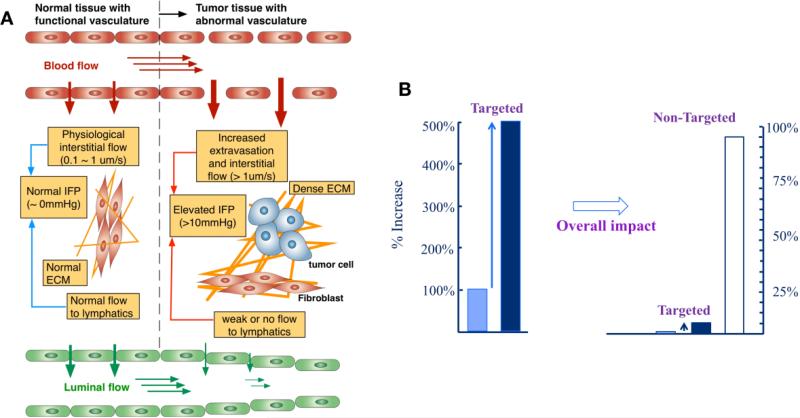
Figure 4. Transport barriers at the tumor microenvironment, and the outcome of typical targeted delivery.
(A) Schematic of vascular and tissue structure relevant to drug transport of normal and tumor tissues. In normal tissue, the endothelium is tightly packed and very low interstitial fluid flow presents. This fluid flows to the lymphatics through the normal ECM, and the IFP minimally builds up. On the contrary, the endothelium of tumor tissue is leaky and has large pores, which leads to high interstitial fluid flow and more extravasation of the NPs. In conjunction with less functional lymphatics and the dense ECM, this increased interstitial fluid flow results in elevated IFP, which adversely affects the extravasation. The compounding effects of the elevated IFP, leaky vasculature, and poor vascularization of the tumor are still not fully understood. Images from Ref [7]. (B) Relative NPs distribution at a target tumor site. Various NP-mediated drug delivery strategies have been reported to improve the drug accumulation at the intended target up to 5-fold increase. However, the majority of the administered drug ends up at non-targeted sites. Adapted from Ref [42].
Although it is not directly associated with TME, one of the most critical steps of drug transport is blood flow-driven transport. After being administered intravenously, the drugs first circulate in the blood stream consisting of complex cells and plasma proteins. During blood circulation, a significant portion of the drugs are taken up by the immune cells in the blood stream including monocytes, leukocytes, and dendritic cells; and in tissues by resident phagocytes, i.e., by the reticuloendothelial system (RES) of the spleen, liver, and lungs [38, 39]. This clearance significantly reduces the amount of the drugs available in the blood stream to reach their intended target. Thus, the surface of many drug delivery systems, particularly NPs, is PEGylated in order to decrease the uptake by the RES and prolong the circulation of the NPs [8, 38, 40]. Besides the uptake by the immune system, the NPs also interact with other components within the blood [40, 41]. These often can result in hemolysis, which refers to red blood cell destruction and degradation of NP integrity. Ultimately, this results in premature release of the encapsulated drugs.
Once the drugs reach the vicinity of their target site after escaping being cleared from the circulation, they are transported across the endothelium of the tumor vasculature to enter the tumor interstitium (i.e., extravasate). The tumor microvasculature is characterized by a highly disorganized network of blood vessels whose endothelium exhibit enlarged intercellular gaps, known as fenestrations, that are heterogeneously distributed across the tumor vasculature [43]. The size of these intercellular gaps, so called vascular pores, for different tumors have been reported to be between 300 and 700 nm, and in rare occasions can be up to 2 μm, which are significantly larger than those found within the normal tissue (typically smaller than 20 nm) [44-46]. These values should be interpreted with caution though, since many of these were measured using xenograft models and very limited information is available for human. Further research is warranted to validate whether these values are applicable to various cancer types, stages and organs in human patients.
Transvascular transport is thought to be extravasation of drugs by convection driven by the pressure gradient across this porous endothelium and diffusion by the concentration gradient. Thus, the transvascular permeability (i.e., measure of transvascular transport at a given set of pressure and concentration gradient) depends on the size of both the drugs and vascular pore. For small macromolecules with hydrodynamic diameters that are much smaller than the pore size, transvascular permeability is observed to be quite insensitive to the pore size [44]. However, for NPs that are larger in size, e.g. 50-200 nm, the effect of the pore size on transvascular permeability becomes more profound. Transvascular permeability also depends on the developmental state of the cancer. Transvascular permeability was found to be 2-fold greater in primary sites of breast cancer tumors (in the mammary fat) when compared to that at a metastatic sites, while the metastatic site was associated with a greater level of vascularization [47]. Yuan et al. [46] experimentally investigated the effect of molecular size on transvascular transport, and provided a transport property database of various molecules. Monsky et al. [48] illustrated that transvascular transport of macromolecules could be enhanced using vascular endothelial growth factor. Netti et al. [49, 50] investigated transvascular transport enhancement by modulating tumor microvascular pressure using periodic or continuous injection of angiotensin II. Recently, a strategy to normalize the tumor vasculature to achieve improved drug delivery throughout the tumor tissue has been proposed [51, 52]. This approach aims to remodel the tumor vasculature to functional vasculature of the healthy tissues using antiangiogenic factors which bind to VEGF receptors of tumor-associated endothelial cells.
After extravasation, the drug transport through the tumor interstitial space against elevated tumor IFP and abnormal ECM structure [30, 33, 53]. IFP of a solid tumor stays at an elevated level and sharply decreases at the periphery of the tumor. Due to its importance during drug delivery, physiological changes by elevated tumor IFP have been studied by many researchers [32, 50, 53]. The IFP of various tumor types varies from 4 to 50mmHg with an approximate average of 20mmHg, which is much higher than the IFP of normal tissues, approximately 2mmHg [32, 54]. Drastically higher IFPs of 75~130 mmHg are reported for PDACs [18]. This elevated IFP is thought to result from anomalous characteristics of tumor vascular structure including high vascular permeability and the lack of a well-developed lymphatic system. The elevated IFP adversely affects the transport of therapeutic agents at several different levels - i) less extravasation of the agents and ii) radially outward interstitial fluid movement at the periphery of tumor [8]. Consequently, the elevated IFP contributes to insufficient delivery of drugs to the interior of tumors. Moreover, higher collagen content and consequent dense organization of collagen fibrils results in lower diffusivity of drugs. Thus, transport of drugs is significantly limited in the tumor interstitial space [33, 55-57]. A wide variety of methods have been proposed and investigated to enhance the interstitial transport, but the main underlying strategies are either lowering tumor IFP [58-60], or modulating tumor ECM structure [33, 61]. However, due to the complex interaction involving various physiological parameters, the control or manipulation of the tumor IFP and ECM structure still warrants further research.
Once the drugs are transported through the tumor interstitial space, these should act on tumor cells, but their efficacy may also be limited due to complete or partial drug resistance [62-65]. Multidrug resistance (MDR) is thought to be caused by a group of membrane proteins that extrude cytotoxic molecules, thus maintaining the intracellular drug concentration below effective levels. These proteins belong to the ATP binding cassette (ABC) superfamily of membrane transporters [66], most of which use the energy of ATP hydrolysis for the efflux of drugs (i.e. active transport). This family includes the well-characterized P-glycoprotein (Pgp) encoded by the MDR-1 gene [67-72], the multidrug resistance protein (MRP) [73-77] and the mitoxantrone resistance protein (MXR), also known as the breast cancer resistance protein (BCRP) [78-80]. Numerous clinical data imply that MDR phenotypes in tumors are associated with the overexpression of these transporters. Since these transporters have wide recognition patterns of substrates, the overexpression of these proteins will result in multidrug resistance. In addition to the over-expression of these transporter proteins, cellular drug resistance also appears to be mediated by the binding of tumor cells to the ECM [81, 82].
The most extensively studied strategy for efficient drug delivery and efficacy is to inhibit drug efflux by modulating the activities of the MDR-associated proteins. This can be achieved by the co-application of MDR modulators with anti-cancer drugs. A wide variety of compounds have been identified as MDR modulators. For example, verapamil, cyclosporine and their derivatives have been investigated in preclinical studies and in some cases have resulted in increased intracellular drug concentration [83-85]. Besides these chemosensitizers, monoclonal antibodies have been studied as potential MDR modulators [86, 87]. In addition to the MDR-associated proteins, the membrane lipid has also been investigated as a target for manipulation, as reviewed elsewhere [88]. The alteration of membrane biophysical properties, including membrane fluidity and permeability, could increase or decrease cellular uptake of drugs [89-91]. Polymeric excipients [92] and transcriptional regulators [93] have also been studied. Although heat shock has been reported to induce MDR in some cancer cells [94, 95], an increase in cellular drug uptake and cytotoxicity by ultrasound-induced hyperthermia was reported [96, 97]. Unfortunately, delivery of these modulators to the target tumor is as challenging as the drug delivery obstacles we face.
To achieve effective targeted drug delivery, various strategies have been proposed to exploit these pathophysiological characteristics of the TME. Currently, many drug delivery systems, primarily NP-based systems, are designed based on so-called “passive” and “active” targeting strategies, which rely on increased extravasation and ligand-receptor interactions, respectively [98]. The passive targeting is based on the fenestration and prolonged circulation by PEGylation. This is often called the EPR effects since it is caused by the increased vascular permeability of tumor vasculature [24, 25]. However, it has not been shown whether such EPR effect exists in human tumor. The term “active targeting” is used to describe a strategy to attach ligands on the surface of NPs so that the NPs selectively bind to the target tumor cells or endothelium. Clearly, active targeting becomes effective only after the NPs reach the vicinity of the target tumors. These strategies can result in improved accumulation of NPs at the tumor, but the in vivo efficacy of NPs and NP-mediated drug delivery is still significantly impaired [1, 10, 38]. Only about 5% of the administered dose ends up at the target tumors. Although this may be 5-fold increase compared with drug molecules, the remaining significant portion of the NPs is still taken up by the RES of the spleen, liver, and lungs as illustrated in Figure 4B.
4. Tumor models to simulate TME
Due to the multifaceted complexities of the TME ranging from molecular, genetic, and biological, to chemical and physical parameters, it is very difficult to interpret the efficacy and resistance of drugs and drug delivery systems. All TME parameters are highly dynamic, interconnected and vary spatiotemporally, and may adversely affect the extravasation and interstitial transport of drugs and the subsequent action [13, 26]. In order to achieve effective treatment, both drug and delivery system should be designed to properly transport through, and act on, target cells in this complex environment. One critical bottleneck to developing effective targeted delivery systems is a limited quantitative understanding of the in vivo transport and action of drugs due to a lack of versatile models capable of rapid systematic study [7, 99].
Most widely used tumor models are two dimensional (2D) cell monolayers, often consisting of human cancer cell lines on a substrate. Although these cell lines are valuable tools, their 2D culture environment does not mimic the TME. Thus, the outcome from traditional 2D cell cultures often fails to be indicative of in vivo or clinical outcomes. A growing number of studies reported that the physical, chemical and mechanical microenvironment of cancer cells significantly affects cellular behaviors [100-106]. These include changes in cell morphology, gene expression and drug responses. Moreover, interactions of cancer cells with other cell types, ECM molecules, and the interstitial fluid in TME, should be properly represented. In order to address these deficiencies, three-dimensional (3D) models including spheroids and engineered tissue scaffolds have been developed [107, 108]. The 3D microenvironment and architectural structure provided by these models induce cell morphology, signaling, and gene expression similar to in vivo TME.
The simplest in vitro 3D tumor model is multicellular tumor spheroid, whereas tumor cells spontaneously aggregate and form spheroid structure [108-110]}. Spheroids can be prepared by several methods including hanging drop method, liquid-overlay cultures, and dynamic bioreactors. By handing drop method, cells are form spheroids within small droplets of cell suspensions (approximately 20 to 50 μL) [111]. Due to the surface tension, liquid can maintain droplets when the lid is inverted and cells settled at the bottom of the droplet, air-liquid interface. Liquid-overlay culture prevents cell adhesion to the culture substrates to induce cells to adhere to each other and form aggregates. To prevent adhesion to the culture vessel, the surface can be coated with agarose, poly-2-hydroxyethyl methacrylate (poly-HEMA), or Matrigel inducing cellular aggregations [110, 112]. Spheroids can also be formed by culturing in bioreactors, which can provide dynamic conditions preventing cell-substrate contact and promoting cells to form aggregates by either stirring or rotating bioreactors [113]. Nonetheless, these are convenient and relatively simple techniques to simulate in vivo solid tumor. Cells within spheroids display cell-cell interactions and produce in vivo tumor-like biochemical responses compared to 2D cell culture. However, the lack of interstitial fluid dynamics, which is highly relevant to drug transport at TME, is one of the major limitations [114, 115].
A large number of studies have historically relied on mouse xenograft and allograft (heterotopic and orthotopic) tumor models to test the efficacy of anti-tumor cytotoxic agents against established cancer cell lines. Although these studies have advanced the field and our knowledge of tumor biology, they are not ideal approaches because the tumors and TME that develop have limited similarity to the human disease. A better approach is to utilize GEMMs that initiate cancer in the correct cell type and at the correct time, to generate a tumor that more closely recapitulates the human TME. However, it is still challenging to obtain mechanistic information regarding drug and NP transport and action in GEMMs. More detailed reviews on animal models used in pre-clinical drug testing can be found elsewhere [116]. The TME of the animal models has many key features that are lacking in 2D and 3D in vitro models. However, even animal models often fail to simulate human in vivo environments and to provide a mechanistic explanation of the in vivo behavior of NPs [99]. This is because of (i) the unknown scaling factors necessary to extrapolate from animal models to human subjects [117], (ii) the mismatch between human cancer cells and mouse matrix environments [118, 119], (iii) the difficulties to simulate the heterogeneity of tumor microenvironmental parameters [120, 121], and (iv) the inability to independently control these parameters in GEMMs. Thus, a new model system is greatly desired, in which the TME parameters can be systematically and independently controlled, but at the same time the dynamic interactions among the fluids, ECM, cells and NPs are maintained.
In order to address the limitation of in vitro static cell culture and mimic a more in vivo-like environment, various new cancer cell cultures on microfluidic platforms have been developed as reviewed elsewhere [122-124]. Since this review is focused on drug transport at TME, several models relevant to study drug transport and action are presented in Figure 5. Most of them are based on microfluidic technology and often called “tumor-on-chip” or “tumor-microenvironment-on-chip.” Although not shown, first generation microfluidic cancer cell cultures were 2D cell monolayers cultured on microchannels in the presence of fluid flow exerting shear stress on the cells [125, 126]. Specifically, endothelial cells were cultured on a microfluidic channel where shear stress was applied at controlled manner to study the morphological changes in vitro [125]. Epithelial transport characteristics of multiple chemical compounds were also studied under both temporal and spatial chemical gradients [126]. The presence of cell-fluid interaction was a significant advance from the conventional 2D cell monolayer models, but the lack of 3D environment was the major limitation to be addressed since the cell physiology of 2D models is distinctively different from that of cells cultured in 3D matrices. To address this, 3D culture environments have been created by combining tumor spheroids and tissue engineering technology.
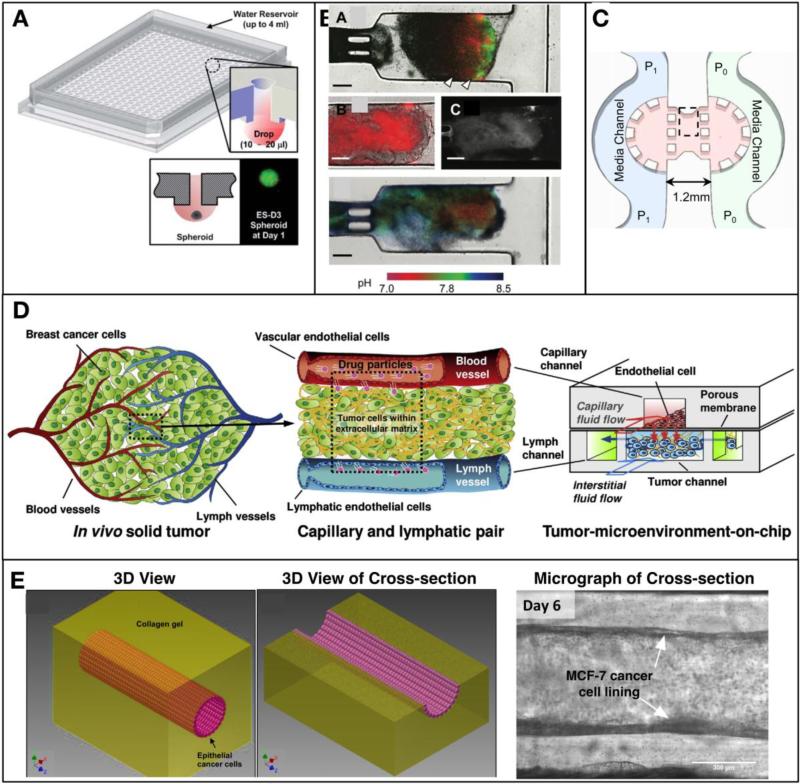
Figure 5. Recent development of in vitro tumor models.
(A) Illustration of a high throughput hanging drop spheroid culture array plate, and its cross-sectional view. (inset) Cartoon of the hanging drop formation and a spheroid [127]. (B) A microfluidic platform to culture tumor cells capable of monitoring cell growth, apoptosis, and pH [128]. (C) A tumor-on-chip platform to study the effects of interstitial fluid pressure gradient [136]. (D) A platform to mimic multiple transport processes at tumor vasculature and interstitium [145]. (E) A platform to mimic epithelial tumor growth and metastasis from circular lumen structure [146].
First, tumor spheroid models have been scaled up into a 384-well format hanging drop culture plate for high throughput assay of drug sensitivity as illustrated in Figure 5A [127]. This model significantly increased the throughout of screening of drug sensitivity for a given spheroid types, and also reported the differences in the drug response when the same types of cells were cultured in either 2D or spheroid format. In order to address the lack of interstitial fluid dynamics, recent studies tried to integrate spheroids into microfluidic platforms where spheroids were subject to interstitial fluid flow [128-130]. In these models, cancer cells or spheroids were cultured within polymeric matrices to mimic cell-matrix interactions in vivo, allowing for the generation of spatial gradients of growth factors and pH [100, 128, 131-135]. As illustrated in Figure 5B, Walsh et al. [128] reported that pH gradient of the perfused spheroid culture on a microfluidic platform, and visualized the doxorubicin diffusion through the tumor spheroids. However, this model was still limited to mimic elevated IFP, which is one of the key features of TME.
Although the interstitial fluid flow has been known to hinder drug transport as well as to affect the morphology and migration of cells, it is very difficult to recreate within in vitro tumor models, even in microfluidic platforms. Polacheck et al [136] developed a microfluidic platform to mimic stable pressure gradients and fluid flow across tumor interstitium as shown in Figure 5C. In this model, breast cancer cells (MDA-MB-231) were seeded in type I collagen matrix and cultured under the perfusion of interstitial fluid flow created by pressure difference across the matrix. By controlling the pressure of each media channel, the flow rate could be precisely controlled and its effects on cell migration behavior were studied. Cell migration relevant to metastasis and angiogenesis has also been studied using microfluidic platforms [137, 138]. In addition, a microfluidic platform has been proposed to mimic hypoxia [139].
All of these 3D tumor models show great promise for mimicking the in vivo TME, and ultimately engineered tumors [140, 141]. The most significant advantages of these microfluidic 3D models are flexibility and controllability to systematically study the effects individual TME parameters. However, microfluidics models still warrant further research to create directional cell-matrix and tissue-tissue interactions [142]. Since cells are typically seeded within polymeric scaffolds in these 3D models, their cell-matrix interactions are non-directional and this can greatly affect cell polarity differently from in vivo circumstances during cancer development [107, 143, 144]. Moreover, drug transport in vivo is greatly affected by the interfacial phenomena at the tissue-tissue interface, including endothelium-blood, endothelium-interstitium, and interstitium-lymphatics endothelium. These interactions need to be present in the model in order to properly simulate the drug transport in vivo.
As shown in Figure 5D, a T-MOC platform has been developed to recapitulate the complex and multiple transport processes in the TME [145]. Rather than mimicking a whole solid tumor, this platform was designed to recapitulate tumor tissue placed between capillary and lymphatic vessels. It had a 3D structure formed by stacking two PDMS layers of microchannels with a porous membrane inserted between the layers. Endothelial cells were cultured on the porous membrane to mimic the endothelium of the capillary. Along the capillary channel, drug-suspended medium flowed at physiologically relevant velocity and pressure. After extravasation, drugs entered the center channel of the bottom layer, which simulates the tumor interstitium. In this tumor channel, cancer cells were cultured within a 3D collagen matrix, and the interstitial fluid flowed through the matrix and exerted elevated IFP. Then, the drugs were transported through this 3D tissue structure to reach the cancer cells, and remaining drugs might be drained to two side channels mimicking the lymphatics. Using this T-MOC platform, the transport of NPs and the effects of various TME parameters on the transport were systematically studied, including the vascular pore size, IFP, and collagen content and cell packing density of tumor tissue.
Although many epithelial tumors including PDAC and ductal carcinoma in situ (DCIS) originate from round epithelial duct where malignant cells acquire invasive properties and disrupt normal epithelial duct geometry. A T-MOC to mimic this directional cell-cell and cell-matrix interactions is shown in Figure 5E [146]. A lumen structure was generated along the microfluidic channel using a fluid dynamic phenomenon called “viscous fingering” [147]. First, collagen solution was filled along the microchannel and then culture medium droplets were placed at the inlet port to initiate the viscous fingering. As the lumen formed, the microfluidic chip was incubated to polymerize the collagen and fix the structure. Once the lumen structure was produced, cancer cells were seeded along the lining of the lumen by filling with cancer cell suspension. A micrograph of the cultured tumor with epithelial ductal geometry is shown.
5. Summary and conclusion
In order to achieve targeted drug delivery, the barriers posed by various aspects of the TME must be overcome to improve the delivery and efficacy of drugs. Identifying a molecular target for delivery systems is a good starting point, but it is not enough to guarantee efficient delivery. For example, to design targeted drug delivery systems, multifaceted aspects of TME should be considered including - i) the dense stroma, hypovascularity, and high IFP of the tumor which pose biophysical barriers to drug transport; ii) hypoxia, CAF-cancer cell interactions, and genetic instability which hinder the actions of drugs; and iii) substantial immunosuppression which present within the TME. These barriers are highly dynamic, interactive, and spatiotemporally vary during development, progression and treatment. Thus, a systematic study to establish quantitative knowledge of the effects of these parameters on the transport and action of drugs is a must. To mechanistically understand this complex environment, new tumor models are needed that provide systematic control of relevant parameters and rapid/high content analysis of multifaceted drug transport and actions.
In this context, recent efforts to develop new in vitro tumor models such as T-MOC will provide a robust and convenient platform to rapidly screen various drug formulations, and to develop new targeted delivery strategies. Although these platforms have been developed to recapitulate the complex TME by culturing cancer cells with stroma cells within 3D extracellular matrices under perfusion, it is still not realistic, nor possible, to design a generic system to fully replicate every aspect of the TME. In order to maximize the benefit of these platforms, the model should be developed to test a specific hypothesis or certain aspects of TME while systematically varying biological, physical and chemical characteristics. Thus, rather than creating another step for drug discovery and screening, it can be used to obtain knowledge and insights, which can be extrapolated to drug design for animals and humans.
Highlights.
Targeted delivery aims to selectively distribute drugs to targeted tumor but not to healthy tissue.
Targeted delivery is very difficult to achieve due to transport barriers engendered by tumor microenvironment.
Tumor microenvironment comprises a heterogeneous mixture of tumor and stromal cells embedded in an extracellular matrix.
New in vitro tumor models based on microfluidic technology have been developed to recapitulate the complex transport in the tumor microenvironment.
Acknowledgements
This work was partially supported by NIH HHSN261201400021C (BH), CA129287 (KP), CA124586 (SFK), CA075059 (MK), CTR Award (BH, KP) from Indiana CTSI funded in part by UL1 TR000006 from NIH, grants from Walther Cancer Foundation (BH, MK), and Incentive Grant Program from Purdue University (BH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bae YH, Park K. Targeted drug delivery to tumors: Myths, reality and possibility. Journal of Controlled Release. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WCW. Mediating tumor targeting efficiency of nanoparticles through design. Nano Letters. 2010;9:1909–1915. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 3.Tong R, Hemmati HD, Langer R, Kohane DS. Photoswitchable nanoparticles for triggered tissue penetration and drug delivery. JACS. 2012 doi: 10.1021/ja211888a. dx.doi.org/10.1021/ja211888a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong C, Stylianopoulos T, Cui J, Martin J, Chauhan VP, Jiang W, Popovic Z, Jain RK, Bawendi MG, Fukumura D. Multistage nanoparticle delivery system for deep penetration into tumor tissue. PNAS. 2011;108:2426–2431. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bao G, Mitragotri S, Tong S. Multifunctional nanoparticles for drug delivery and molecular imaging. Annual Review of Biomedical Engineering. 2013;15:253–282. doi: 10.1146/annurev-bioeng-071812-152409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozcelikkale A, Ghosh S, Han B. Multifaceted transport characteristics of nanomedicine: Needs for characterization in dynamic environment. Molecular Pharmaceutics. 2013 doi: 10.1021/mp3005947. dx.doi.org/10.1021/mp3005947. [DOI] [PubMed] [Google Scholar]
- 7.Han B. Complex transport around tumor: Need for realistic in vitro tumor transport model. In: Bae Y, Mrsny R, Park K, editors. Cancer Targeted Drug Delivery: An Elusive Dream. Springer Sciennce+Business Media; New York: 2013. [Google Scholar]
- 8.Kwon IK, Lee SC, Han B, Park K. Analysis on the current status of targeted drug delivery to tumors. Journal of Controlled Release. 2012;164:108–114. doi: 10.1016/j.jconrel.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanapathipillai M, Brock A, Inger DE. Nanoparticle targeting of anti-cancer drugs that alter intracellular signaling or influence the tumor microenvironment. Advanced Drug Delivery Reviews. 2014;79:107–118. doi: 10.1016/j.addr.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Bae YH, Chung HJ, Park TG. Nanomaterials for cancer therapy and imaging. Molecules and Cells. 2011;31:295–302. doi: 10.1007/s10059-011-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, Friess H. The role of stroma in pancreatic cancer: diagnostic and therapeutic implication. Nature Reviews Gastroenterology and Hepatology. 2012;9:454–467. doi: 10.1038/nrgastro.2012.115. [DOI] [PubMed] [Google Scholar]
- 12.Mahadevan D, Hoff DDV. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Molecular Cancer Therapeutics. 2007;6:1186–1197. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 13.Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Current Opinion in Cell Biology. 2010;22:697–706. doi: 10.1016/j.ceb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gore J, Korc M. Pancreatic Cancer Stroma: Friend or Foe? Cancer Cell. 2014;25:711–712. doi: 10.1016/j.ccr.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nature Medicine. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 17.Kern SE. Molecular genetic alterations in ductal pancreatic adenocarcinomas. The Medical clinics of North America. 2000;84:691–695. doi: 10.1016/s0025-7125(05)70251-0. [DOI] [PubMed] [Google Scholar]
- 18.Provenzano PP, Cuevas C, Chang AE, Goel VK, Hoff DDV, Hingorani SR. Enzymatic Targeting of the Stroma Ablates Physical Barriers to Treatment of Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stromnes IM, DelGiorno KE, Greenberg PD, Hingorani SR. Stromal reengineering to treat pancreas cancer. Carcinogenesis. 2014;35:1451–1460. doi: 10.1093/carcin/bgu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones S, Zhang X, Parsons DW, Lin JC-H, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong S-M, Fu B, Lin M-T, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. Core Signaling Pathways in Human Pancreatic Cancers Revealed by Global Genomic Analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris JP, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nature Review Cancer. 2010;10:683–695. doi: 10.1038/nrc2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu L, Shi G, Schmidt CM, Hruban RH, Konieczny SF. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. American Journal of Pathology. 2007;171:263–273. doi: 10.2353/ajpath.2007.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi G, Zhu L, Sun Y, Bettencourt R, Damsz B, Hruban RH, Konieczny SF. Loss of the acinar-restricted transcription factor Mist1 accelerates Kras-induced pancreatic intraepithelial neoplasia. Gastroenterology. 2009;136:1368–1378. doi: 10.1053/j.gastro.2008.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Advanced Drug Delivery Reviews. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent SMANCS. Cancer Research. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 26.Narang AS, Varia S. Role of tumor vascular architecture in drug delivery. Advanced Drug Delivery Reviews. 2011;63:640–658. doi: 10.1016/j.addr.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Provenzano PP, Hingorani SR. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. British Journal of Cancer. 2013;108:1–8. doi: 10.1038/bjc.2012.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gore J, Craven KE, Wilson JL, Gote GA, Cheng M, Nguyen HV, Cramer HM, Sherman S, Korc M. TCGA data and patient-derived orthotopic xenografts highlight pancreatic cancer-associated angiogenesis. Oncotarget. 2015 doi: 10.18632/oncotarget.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teicher BA. Tumor models for efficacy determination. Molecular cancer therapeutics. 2006;5:2435–2443. doi: 10.1158/1535-7163.MCT-06-0391. [DOI] [PubMed] [Google Scholar]
- 30.Brown E, Mckee TD, Tomaso E.d., Pluen A, Seed B, Boucher Y, Jain RK. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nature Medicine. 2003;9:796–800. doi: 10.1038/nm879. [DOI] [PubMed] [Google Scholar]
- 31.Grantab R, Sivananthan S, Tannock IF. The penetration of anticancer drugs through tumor tissue as a function of cellular adhesion and packing density of tumor cells. Cancer Research. 2006;66:1033–1039. doi: 10.1158/0008-5472.CAN-05-3077. [DOI] [PubMed] [Google Scholar]
- 32.Heldin C-H, Rubin K, Pietras K, Östman A. High interstitial fluid pressure — an obstacle in cancer therapy. Nature Reviews Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 33.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Research. 2000;60:2497–2503. [PubMed] [Google Scholar]
- 34.Korc M. Pancreatic cancer–associated stroma production. The American Journal of Surgery. 2007;194:S84–S86. doi: 10.1016/j.amjsurg.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preis M, Korc M. Signaling pathways in pancreatic cancer. Critical Reviews in Eukarytoic Gene Expression. 2011;21:115–129. doi: 10.1615/critreveukargeneexpr.v21.i2.20. [DOI] [PubMed] [Google Scholar]
- 36.Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, Collisson EA, Connor F, Dyke TV, Kozlov S, Martin P, Tseng TW, Dawson DW, Donahue TR, Masamune A, Shimosegawa T, Apte MV, Wilson JS, Ng B, Lau SL, Gunton JE, Wahl GM, Hunter T, Drebin JA, O'Dwyer PJ, Liddle C, Tuveson DA, Downes M, Evans RM. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, Jesus-Acosta AD, Sharma P, Heidari P, Mahmood U, Chin L, Moses HL, Weaver VM, Maitra A, Allison JP, LeBleu VS, Kalluri R. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertrand N, Leroux J-C. The journey of a drug-carrier in the body: An anatomo-physiological perspective. J. Control. Release. 2012;161:152–163. doi: 10.1016/j.jconrel.2011.09.098. [DOI] [PubMed] [Google Scholar]
- 39.Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. Preclinical studies to understand nanoparticle interaction with immune system and its potential effects on nanoparticle biodistribution. Molecular Pharmaceutics. 2008;5:487–495. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bansal R, Post E, Proost JH, de Jager-Krikken A, Poelstra K, Parkash J. PEGylation improves pharmacokinetic profile, liver uptake and efficacy of interferon gamma in liver fibrosis. J. Control. Release. 2011;154:233–240. doi: 10.1016/j.jconrel.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 41.Walkey CD, Olsen JB, Song F, Rong L, Guo H, Olsen DWH, Cohen Y, Emil A, Chan WCW. Protein Corona Fingerprinting Predicts the Cellular Interaction of Gold and Silver Nanoparticles. ACS Nano. 2014;8:2439–2455. doi: 10.1021/nn406018q. [DOI] [PubMed] [Google Scholar]
- 42.Park K. Facing the Truth about Nanotechnology in Drug Delivery. ACS Nano. 2013;7:7442–7447. doi: 10.1021/nn404501g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng D, Nagy JA, Pyne K, Hammel I, Dvorak HF, Dvorak AM. Pathways of macromolecular extravasation across microvascular endothelium in response to VPF VEGF and other vasoactive mediators. Microcirculation. 1999;6:23–44. [PubMed] [Google Scholar]
- 44.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of Transport Pathways in Tumor Vessels: Role of Tumor Type and Microenvironment. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan F. Transvascular drug delivery in solid tumors. Seminars in Radiation Oncology. 1998;8:164–175. doi: 10.1016/s1053-4296(98)80042-8. [DOI] [PubMed] [Google Scholar]
- 46.Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55:3752–3756. [PubMed] [Google Scholar]
- 47.Monsky WL. Role of host microenvironment in angiogenesis and microvascular functions in human breast cancer xenografts: mammary fat pad versus cranial tumors. Clin. Cancer Res. 2002;8:1008–1013. [PubMed] [Google Scholar]
- 48.Monsky W, Yuan F, Fukumura D, Torchilin V, Jain RK. Topical superfusion of vascular endothelial growth factor increases tumor vessel endothelial pore size. Proceedings of the American Association for Cancer Research Annual Meeting. 1997;38:52. [Google Scholar]
- 49.Netti PA, Hamberg LM, babich JW, Kierstead D, Graham W, Hunter GJ, Wolf GL, Fischman A, Boucher Y, Jain RK. Enhancement of fuid filtration across tumor vessels: Implication for delivery of macromoloecules. Proc. Natl. Acad. Sci. USA. 1999;96:3137–3142. doi: 10.1073/pnas.96.6.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Netti PA, Baxter LT, Boucher Y, Skalak R, Jain RK. Time-dependent behavior of interstitial fluid pressure in solid tumors: Implications for drug delivery. Cancer Research. 1995;55:5451–5458. [PubMed] [Google Scholar]
- 51.Chauhan VP, Stylianopoulos T, Martin JD, Popovic Z, Chen O, Kamoun WS, Bawendi MG, Fukumura D, Jain RK. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nature Nanotechnology. 2012 doi: 10.1038/nnano.2012.45. (in presse) doi:10.1038/nnano2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 53.Jain RK. Transport of Molecules in the Tumor Interstitium: A review. Cancer Research. 1987;47:3039–3051. [PubMed] [Google Scholar]
- 54.Less JR, Posner MC, Boucher Y, Borochovitz D, Wolmark N, Jain RK. Interstitial hypertension in human breast and colorectoral tumors. Cancer Research. 1992;52:6371–6374. [PubMed] [Google Scholar]
- 55.Alexandrakis G, Brown E, Tong RT, Mckee TD, Campbell RB, Boucher Y, Jain RK. Two-photon fluorescence correlation microscopy reveals the two-phase nature of transport in tumors. Nature Medicine. 2004;10:203–207. doi: 10.1038/nm981. [DOI] [PubMed] [Google Scholar]
- 56.Pluen A, Boucher Y, Ramanujan S, Mckee TD, Gohongi T, Tomaso E.d., Brown EB, Izumi Y, Campbell RB, Berk DA, Jain RK. Role of tumor-host interactions in interstitial diffusion of macromolecules: cranial vs. subcutaneous tumors. Proc. Natl. Acad. Sci. 2001;98:4628–4633. doi: 10.1073/pnas.081626898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramanujan S, Pluen A, Mckee TD, Brown EB, Boucher Y, Jain RK. Diffusion and convection in collagen gels: Implications for transport in the tumor interstitium. Biophysical Journal. 2002;83:1650–1660. doi: 10.1016/S0006-3495(02)73933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Griffon-Etienne G, Boucher Y, Brekken C, Suit HD, Jain RK. Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: Clinical implications. Cancer Research. 1999;59:3776–3782. [PubMed] [Google Scholar]
- 59.Rubin K, Sjoquist M, Gustafsson A-M, Isaksson B, Salvessen G, Reed RK. Lowering of tumoral interstitial fluid pressure by prostaglandin E1 is paralleled by an increased uptake of Cr-EDTA. Int. J. Cancer. 2000;86:636–643. doi: 10.1002/(sici)1097-0215(20000601)86:5<636::aid-ijc6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 60.Salnikov AV, Iversen VV, Koisti M, Sundberg C, Johansson L, Stuhr LB, Sj√∂quist M, Ahlstr√∂m H.k., Reed RK, Rubin K. Lowering of tumor interstitial fluid pressure specifically augments efficacy of chemotherapy. The FASEB Journal. 2003;17:1756–1758. doi: 10.1096/fj.02-1201fje. [DOI] [PubMed] [Google Scholar]
- 61.Davies C.d.L., Berk DA, Pluen A, Jain RK. Comparison of IgG diffusion and extracellular matrix composition in rhabdomyosarcomas grown in mice versus in vitro as spheroids reveals the role of host stromal cells. British Journal of Cancer. 2002;86:1639–1644. doi: 10.1038/sj.bjc.6600270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clarke R, Leonessa F, Trock B. Multidrug resistance/P-glycoprotein and breast cancer: review and meta-analysis. Semin Oncol. 2005;32:S9–S15. doi: 10.1053/j.seminoncol.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 63.Hait WN, Yang JM. Clinical management of recurrent breast cancer: development of multidrug resistance (MDR) and strategies to circumvent it. Semin. Oncol. 2005;32:S16–21. doi: 10.1053/j.seminoncol.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 64.Stein U, Walther W, Lemm M, Naundorf H, Fichtner I. Development and characterization of novel human multidrug resistant mammary carcinoma lines in vitro and in vivo. Int. J. Cancer. 1997;72:885–891. doi: 10.1002/(sici)1097-0215(19970904)72:5<885::aid-ijc28>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 65.Wosikowski K, Regis JT, Robey R, Alvarez M, Buters JTM, Gudas JM, Bates SE. Normal p53 status and function despite the development of drug resistance in human breast cancer cells. Cell Growth and Differentiation. 1995;6:1395–1403. [PubMed] [Google Scholar]
- 66.Allikments R, Schrim LM, Hutchinson A, Romano-Spica V, Dean M. Characterization of the human ABC superfamily: isolation and mapping of 21 new genes using the expressed sequence tags database. Hum Mol Genet. 1966;5:1649–1655. doi: 10.1093/hmg/5.10.1649. [DOI] [PubMed] [Google Scholar]
- 67.Chekhun VF, Kulik GI, Yurchenko OV, Tryndyak VP, Todor IN, Luniv LS, Tregubova NA, Pryzimirska TV, Montgomery B, Rusetskaya NV, Pogribny IP. Role of DNA hypomethylation in the development of the resistance to doxorubicin in human MCF-7 breast adenocarcinoma cells. Cancer Letters. 2006;231:87–93. doi: 10.1016/j.canlet.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 68.Daschner PJ, Ciolino HP, Plouzek CA, Yeh GC. Increased AP-1 activity in drug resistant human breast cancer MCF-7 cells. Breast Cancer Research and Treatment. 1999;53:229–240. doi: 10.1023/a:1006138803392. [DOI] [PubMed] [Google Scholar]
- 69.Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu. Rev. Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 70.Jin J, Wang F-P, Wei H, Liu G. Reversal of multidrug resistance of cancer through inhibition of P-glycoprotein by 5-bromoterandrine. Cancer Chemother Pharmacol. 2005;55:179–188. doi: 10.1007/s00280-004-0868-0. [DOI] [PubMed] [Google Scholar]
- 71.Mechetner E, Kyshtoobayeva A, Zonis S, Kim H, Stroup R, Garcia R, Parker R, Fruehauf JP. Levels of multidrug resistance (MDR1) P-glycoprotein expression by human breast cancer correlate with in vitro resistance to taxol and doxorubicin. Clinical Cancer Research. 1998;4:389–398. [PubMed] [Google Scholar]
- 72.Vredenburg MR, Ojima I, Veith J, Pera P, Kee K, Cabral F, Sharma A, Kanter P, Greco WR, Bernacki RJ. Effects of orally active taxanes on P-glycoprotein modulation and colon and breast carcinoma drug resistance. J Natl Cancer Inst. 2001;93:1234–1245. doi: 10.1093/jnci/93.16.1234. [DOI] [PubMed] [Google Scholar]
- 73.Aszalos A, Thompson K, Yin JJ, Ross DD. Combination of P-glycoprotein blockers, verapamil, PSC833 and cremophor act differently on the multidrug resistance associated protein (MRP) and on P-glycoprogein (Pgp) Anticancer Res. 1999;19:1053–1064. [PubMed] [Google Scholar]
- 74.de Jong MC, Slootstra JW, Scheffer GL, Schroeijers AB, Puijk WC, Dinkelberg R, Kool M, Broxterman HJ, Meloen RH, Scheper RJ. Peptide transport by the multidrug resistance protein MRP1. Cancer Res. 2001;61:2552–2557. [PubMed] [Google Scholar]
- 75.Ferreira MJ, Gyemant N, Madureira AM, Tanaka M, Koos K, Didziapetris R, Molnar J. The effects of jatrophane derivatives on the reversion of MDR1- and MRP-mediated multidrug resistance in the MDA-MB-231 (HTB-26) cell line. Anticancer Res. 2005;25:4173–4178. [PubMed] [Google Scholar]
- 76.Filipits M, Suchomel RW, Dekan G, Haider K, Valdimarsson G, Depisch D, Pirker R. MRP and MDR1 gene expression in pirmary breast carcinomas. Clinical Cancer Research. 1996;2:1231–1237. [PubMed] [Google Scholar]
- 77.Muller M, Meijer C, Zaman GJR, Borst P, Scheper RJ, Mulder N, de Veris H,,EGE, Jansen PLM. Overexpression of the gene encoding the multidrug resistance-associated protein results in increased ATP-dependent glutathione S-conjugate transport. PNAS. 1994;91:13033–13037. doi: 10.1073/pnas.91.26.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Bruin M, Miyake K, Litman T, Robey R, Bates SE. Reversal of resistance by GF120918 in cell lines expressing the ABC half-transporter, MXR. Cancer Letters. 1999;146:117–126. doi: 10.1016/s0304-3835(99)00182-2. [DOI] [PubMed] [Google Scholar]
- 79.Litman T, Brangi M, Hudson E, Fetsch P, Abati A, Ross DD, Miyake K, Resau JH, Bates SE. The multidrug-resistant phenotype associated with overexpression of the new ABC half-transporter, MXR (ABCG2) Journal of Cell Science. 2000;113:2011–2021. doi: 10.1242/jcs.113.11.2011. [DOI] [PubMed] [Google Scholar]
- 80.Robey R, Medina-Perez WY, Nishiyama K, Lahusen T, Miyake K, Litman T, Senderowicz AM, Ross DD, Bates SE. Overexpression of the ATP-binding cassette half-transporter, ABCG2 (MXR/BCRP/ABCP1), in flavopiridol-resistant humen breast cancer cells. Clinical Cancer Research. 2001;7:145–152. [PubMed] [Google Scholar]
- 81.Dalton WS. The tumor microenvironment: focus on myeloma. Cancer Treatment Reviews. 2003;29:11–19. doi: 10.1016/s0305-7372(03)00077-x. [DOI] [PubMed] [Google Scholar]
- 82.Jean C, Gravelle P, Fournie J-J, Laurent G. Influence of stress on extracellular matrix and integrin biology. Oncogene. 2011;30:2697–2706. doi: 10.1038/onc.2011.27. [DOI] [PubMed] [Google Scholar]
- 83.Chambers SK, Hait WN, Kacinski BM. Enhancement of anthracycline growth inhibition in parent and multidrug-resistant Chinese hamster ovary cells by cyclosporin A and its analogues. Cancer Res. 1989;49:6275–6279. [PubMed] [Google Scholar]
- 84.Ford JM, Yang JM, Hait WN. Effect of buthionine sulfoximine on toxicitiy of verapamil and doxorubicin to multidrug resistant cells and to mice. Cancer Res. 1991;51:67–72. [PubMed] [Google Scholar]
- 85.Yang JM, Goldenberg S, Gottesman MM. Characteristics of P338/VMDRC.04, a simple, sensitive model for studying P-glycoprotein antagonists. Cancer Res. 1994;54:730–737. [PubMed] [Google Scholar]
- 86.Mano Y, Suzuki H, Terasaki T. Kinetic analysis of the diposition of MRK16, an anti-P-glycoprotein monoclonal antibody, in tumors: comparison between in vitro and in vivo disposition. J Pharmacol Exp Ther. 1997;283:391–401. [PubMed] [Google Scholar]
- 87.Naito M, Tsuge H, Kuroko C. Enhancement of cellular accumulation of cyclosporine by anit-P-glycoprotein monoclonla antibody MRK-16 and synergistic modulation of multidrug resistance. J Natl Cancer Inst. 1993;85:311–316. doi: 10.1093/jnci/85.4.311. [DOI] [PubMed] [Google Scholar]
- 88.Hendrich AB, Michalak K. Lipids as a target for drugs modulating multidrug resistance of cancer cells. Curr Drug Targets. 2003;4:23–30. doi: 10.2174/1389450033347172. [DOI] [PubMed] [Google Scholar]
- 89.Breuzard G, Piot O, Angiboust JF, Manfait M, Candeil L, Del Rio M, Millot JM. Changes in adsorption and permeability of mitoxantrone on plasma membrane of BCRP/MXR resistant cells. Biochemical and Biophysical Research Communications. 2005;329:64–70. doi: 10.1016/j.bbrc.2005.01.098. [DOI] [PubMed] [Google Scholar]
- 90.Callaghan R, Stafford A, Epand RM. Increased accumulation of drugs in a multidrug resistanct cell line by alteration of membrane biophysical properties. Biochim Biophys Acta. 1993;1175:277–282. doi: 10.1016/0167-4889(93)90217-d. [DOI] [PubMed] [Google Scholar]
- 91.Sinicrope FA, Dudeja PK, Bissonnette BM, Safa AR, Brasitus TA. Modulation of P-glycoprotein-mediated drug transport by alterations in lipid fluidity of rat liver canalicular membrane vesicles. J Biol Chem. 1992;267:24995–25002. [PubMed] [Google Scholar]
- 92.Tijerina M, Fowers KD, Kopeckova P, Kopecek J. Chronic exposure of human ovarian carcinoma cells to free or HPMA copolymer-bound mesochlorin e6 does not induce P-glycoprotein-mediated multidrug resisance. Biomaterials. 2000;21:2203–2210. doi: 10.1016/s0142-9612(00)00161-7. [DOI] [PubMed] [Google Scholar]
- 93.Orth P, Schnappinger D, Hillen W, Saenger W, Hinriches W. Structural basis of gene regulation by the retracycline inducible Tet repressor-operator system. Nat Struct Biol. 2000;7:215–219. doi: 10.1038/73324. [DOI] [PubMed] [Google Scholar]
- 94.Cheng J-Z, Sharma R, Yang Y, Singhal SS, Sharma A, Saini MK, Singh SV, Zimniak P, Awasthi S, Awasthi YC. Accelerated metabolism and exclusion of 4-hydroxynonenal through induction of RLIP76 and hGST5.8 is an early adaptive response of cells to heat and oxidative stress. Journal of Biological Chemistry. 2001;276:41213–41223. doi: 10.1074/jbc.M106838200. [DOI] [PubMed] [Google Scholar]
- 95.Szabo D, Keyzer H, Kaiser HE, Molnar J. Reversal of multidrug resistance of tumor cells. Anticancer Res. 2000;20:4261–4274. [PubMed] [Google Scholar]
- 96.Liu Y, Cho C-W, Yan X, Henthorn TK, Lillehei KO, Cobb WN, Ng K. Ultrasound-induced hyperthermia increases cellular uptake and cytotoxicity of P-glycoprotein substrates in multi-drug resistant cells. Pharmaceutical Research. 2001;18:1225–1261. doi: 10.1023/a:1013025625156. [DOI] [PubMed] [Google Scholar]
- 97.Liu Y, Lillehei KO, Cobb WN, Christians U, Ng K. Overcoming MDR by ultrasound-induced hyperthermia and P-glycoprotein modulation. Biochemical and Biophysical Research Communications. 2001;289:62–68. doi: 10.1006/bbrc.2001.5938. [DOI] [PubMed] [Google Scholar]
- 98.Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. Journal of Controlled Release. 2010;148:135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 99.Damia G, D'Incalci M. Contemporary pre-clinical development of anticancer agents - What are the optimal preclinical models? Eur. J. Cancer. 2009;45:2768–2781. doi: 10.1016/j.ejca.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 100.Loessner D, Stok KS, Lutolf MP, Hutmacher DW, Clements JA, Rizzi SC. Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials. 2010;31:8494–8506. doi: 10.1016/j.biomaterials.2010.07.064. [DOI] [PubMed] [Google Scholar]
- 101.Pampaloni F, Reynaud EG, Stelzer EHK. ” Nature Reviews, vol. 8, no. October, pp. 839–845, 2007, The third dimension bridges the gap between cell culture and live tissue. Nature Reviews Molecular Biology. 2007;8:839–845. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 102.Fournier MV, Martin KJ, Kenny PA, Xhaja K, Bosch I, Yaswen P, Bissell MJ. Gene expression signature in organized and growth-arressed mammary acini predicts good outcome in breast cancer. Cancer Res. 2006;66:7095–7102. doi: 10.1158/0008-5472.CAN-06-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, Spellman PT, Lorenz K, Lee EH, Barcellos-Hoff MH, Petersen OW, Gray JW, Bissell MJ. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol. 2007;1:84–96. doi: 10.1016/j.molonc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tanner K, Mori H, Mroue R, Bruni-Cardoso A, Bissell MJ. Coherent angular motion in the establishment of multicellular architecture of glandular tissues. PNAS. 2012;109:1973–1978. doi: 10.1073/pnas.1119578109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weigelt B, Bissell MJ. Unraveling the microenvironmental influences on the normal mammary gland and breast cancer. Semin Cancer Biol. 2008;18:311–321. doi: 10.1016/j.semcancer.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weigelt B, Lo AT, Park CC, Gray JW, Bissell MJ. HER2 signaling pathway activiation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D microenvironment. Breast Cancer Res Treat. 2010;122:35–43. doi: 10.1007/s10549-009-0502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 108.Kim JB. Three-dimensional tissue culture models in cancer biology. Seminars in Cancer Biology. 2005;15:365–377. doi: 10.1016/j.semcancer.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 109.Hamilton G. Multicellular spheroids as an in vitro tumor model. Cancer Letters. 1998;131:29–34. doi: 10.1016/s0304-3835(98)00198-0. [DOI] [PubMed] [Google Scholar]
- 110.Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA. Spheroid-based drug screen: considerations and practical approach. Nature Protocols. 2009;4:309–324. doi: 10.1038/nprot.2008.226. [DOI] [PubMed] [Google Scholar]
- 111.Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnology and Bioengineering. 2003;83:173–180. doi: 10.1002/bit.10655. [DOI] [PubMed] [Google Scholar]
- 112.Phung YT, Barbone D, Broaddus VC, Ho M. Rapid generation of in vitro multicellular spheroids for the study of monoclonal antibody therapy. Journal of Cancer. 2011;2:507–514. doi: 10.7150/jca.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nakamura K, Kuga H, Morisaki T, Baba E, Sato N, Mizumoto K, Sueishi K, Tanaka M, Katano M. Simulated microgravity culture system for a 3-D carcinoma tissue model. Biotechniques. 2002;33:1074–1076. doi: 10.2144/02335rr02. [DOI] [PubMed] [Google Scholar]
- 114.Ng CP, Swartz MA. Fibroblast alignment under interstitial fluid flow using a novel 3-D tissue culture model. Am J Physiol Heart Cric Physiol. 2003;284:H1771–H1777. doi: 10.1152/ajpheart.01008.2002. [DOI] [PubMed] [Google Scholar]
- 115.Swartz MA, Lund AW. Lymphatic and interstitial flow in the tumour microenviornment: linking mechanobiology with immunity. Nature Review Cancer. 2012;12:210–219. doi: 10.1038/nrc3186. [DOI] [PubMed] [Google Scholar]
- 116.HogenEsch H, Nikitin AY. Challenges in pre-clinical testing of anti-cancer drugs in cell culture and in animal models. Journal of Controlled Release. 2012 doi: 10.1016/j.jconrel.2012.02.031. (in press) doi:10.1016/j.jconrel.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Florence AT. Targeting nanoparticles: The constraints of physical laws and physical barriers. J. Control. Release. 2012 doi: 10.1016/j.jconrel.2012.03.022. (in press) [DOI] [PubMed] [Google Scholar]
- 118.Becher OJ, Holland EC. Genetically engineered models have advantages over xenografts for preclinical studies. Cancer Res. 2006;66:3355–3358. doi: 10.1158/0008-5472.CAN-05-3827. [DOI] [PubMed] [Google Scholar]
- 119.Frese KK, Tuveson DA. Maximizing mouse cancer models. Nature reviews. Cancer. 2007;7:645–658. doi: 10.1038/nrc2192. [DOI] [PubMed] [Google Scholar]
- 120.Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer. Nature Review Cancer. 2012;12:323–334. doi: 10.1038/nrc3261. [DOI] [PubMed] [Google Scholar]
- 121.Samuel N, Hudson TJ. The molecular and cellular heterogeneity of pancreatic ductal adenocarcinoma. Nature Review Gastroenterology & Hepatology. 2012;9:77–87. doi: 10.1038/nrgastro.2011.215. [DOI] [PubMed] [Google Scholar]
- 122.Sackmann EK, Fulton AL, Beebe DJ. The present and future role of microfluidics in biomedical research. Nature. 2014;507:181–189. doi: 10.1038/nature13118. [DOI] [PubMed] [Google Scholar]
- 123.Choudhury D, Mo X, Iliescu C, Tan LL, Tong WH, Yu H. Exploitation of physical and chemical constraints for three-dimensional microtissue construction in microfluidics. Biomicrofluidics. 2011;5:022203. doi: 10.1063/1.3593407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Valencia PM, Farokhzad OC, Karnik R, Langer R. Microfluidic technologies for accelerating the clinical translation of nanoparticles. Nature Nanotechnology. 2012;7:623–629. doi: 10.1038/nnano.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Song JW, Gu W, Futai N, Warner KA, Nor JE, Takayama S. Computer-controlled microcirculatory support system for endothelial cell culture and shearing. Anal Chem. 2005;77:3993–3999. doi: 10.1021/ac050131o. [DOI] [PubMed] [Google Scholar]
- 126.Jin B-J, Ko E-A, Namkung W, Verkman AS. Microfluidics platform for single-shot dose-response analysis of chloride channel-modulating compounds. Lab on a Chip. 2014;13:3862–3867. doi: 10.1039/c3lc50821h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tung YC, Hsiao AY, Allen SG, Torisawa Y, Ho M, Takayama S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. The Analyst. 2011;136:473–478. doi: 10.1039/c0an00609b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Walsh CL, Babin BM, Kasinskas RW, Foster JA, McGarry MJ. A multipurpose microfluidics device designed to mimic microenviornment gradients and develop targeted cancer therapeutics. Lab on a Chip. 2009;9:545–554. doi: 10.1039/b810571e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shin CS, Kwak B, Han B, Park K. Development of an in vitro 3D tumor model to study therapeutic efficacy of an anticaner drug. Molecular Pharmaceutics. 2013;10:2167–2175. doi: 10.1021/mp300595a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Albanese A, Lam AK, Sykes EA, Rocheleau JV, Chan WCW. Tumour-on-chip provides an optical window into nanoparticle tissue transport. Nature Communications. 2013;4 doi: 10.1038/ncomms3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Haessler U, Kalinin Y, Swartz MA, Wu M. An agarose-based microfluidic platform with a gradient buffer for 3D chemotaxis studies. Biomed Microdevices. 2009;11:827–835. doi: 10.1007/s10544-009-9299-3. [DOI] [PubMed] [Google Scholar]
- 132.Kim S, Kim HJ, Jeon NL. Biological applications of microfluidic gradient devices. Integr Biol. 2010;2:584–603. doi: 10.1039/c0ib00055h. [DOI] [PubMed] [Google Scholar]
- 133.Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, Polverini PJ, Mooney DJ. Engineering tumors with 3D scaffolds. Nat Methods. 2007;4:855–860. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- 134.Hsiao AY, Torisawa YS, Tung YC, Sud S, Taichman RS, Pienta KJ, Takayama S. Microfluidic system for formation of PC-3 prostate cancer co-culture spheroids. Biomaterials. 2009;30:3020–3027. doi: 10.1016/j.biomaterials.2009.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang CP, Lu J, Seon H, Lee AP, Flanagan LA, Kim HY, Putnam AJ, Jeon NL. Engineering microscale cellular niches for three-dimensional multicellular co-cultures. Lab on a Chip. 2009;9:1740–1748. doi: 10.1039/b818401a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Polacheck WJ, Charest JL, Kamm RD. Interstitial flow influences direction of tumor cell migraion through competing mechanisms. PNAS. 2011;108:11115–11120. doi: 10.1073/pnas.1103581108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, Kamm RD. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. PNAS. 2012;109:13515–13520. doi: 10.1073/pnas.1210182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bersini S, Jeon JS, Dubini G, Arrigoni C, Chung S, Charest JL, Moretti M, Kamm RD. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials. 2014;35:2454–2461. doi: 10.1016/j.biomaterials.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Funamoto K, Zervantonakis IK, Liu Y, Ochs CJ, Kim C, Kamm RD. A novel microfluidic platform for high-resolution imaging of a three-dimensional cell culture under a controlled hypoxic environment. Lab on a Chip. 2012;12:4855. doi: 10.1039/c2lc40306d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hutmacher DW, Cukierman E. Engineering of tumor microenvironments. Advanced Drug Delivery Reviews. 2014;79-80:1–2. doi: 10.1016/j.addr.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 141.Ghajar CM, Bissell MJ. Tumor engineering: The other face of tissue engineering. Tissue Engineering: Part A. 2010;16:2153–2156. doi: 10.1089/ten.tea.2010.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shen K, Luk S, Hicks DF, Elman JS, Bohr S, Iwamoto Y, Murray R, Pena K, Wang F, Seker E, Weissleder R, Yarmush ML, Toner M, Sgroi D, Parekkadan B. Resolving cancer-stroma interfacial signalling and interventions with micropatterned tumour-stromal assays. Nature Communications. 2014;5:5662. doi: 10.1038/ncomms6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lopez JI, Mouw JK, Weaver WM. Biomechanical regulation of cell orientation and fate. Oncogene. 2008;27:6981–6993. doi: 10.1038/onc.2008.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ingber DE. Can cancer be reversed by engineering the tumor microenvironment? Semin Cancer Biol. 2006;18:356–364. doi: 10.1016/j.semcancer.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kwak B, Ozcelikkale A, Shin CS, Park K, Han B. Simulation of complex transport of nanoparticles around a tumor using tumor-microenvironment-on-chip. Journal of Controlled Release. 2014;194:157–167. doi: 10.1016/j.jconrel.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Noe-Kim V, Ozcelikkale A, Han B. An in vitro microfluidic tumor model to mimic ductal carninoma in situ, Proceedings of the 2015 Summer Biomechanics. Bioengineering and BIotransport Conference. 2015:SB3C2015–673. [Google Scholar]
- 147.Bischel LL, Young EWK, Mader BR, Beebe DJ. Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels. Biomaterials. 2013;34:1471–1477. doi: 10.1016/j.biomaterials.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]