Abstract
Background
Accumulating data suggest that immune effector functions mediated through the Fc portion of HIV-1-specific immunoglobulin G (IgG) are a key component of HIV-1 protective immunity, affecting both disease progression and HIV-1 acquisition. Through studying Fc gamma receptor (FcγR) variants known to alter IgG Fc-mediated immune responses, we indirectly assessed the role of FcγR-mediated effector functions in modulating perinatal HIV-1 transmission risk. In this study, genotypic data from 79 HIV-1 infected mothers and 78 HIV-1 infected infants (transmitting cases) were compared to 234 HIV-1 infected mothers and 235 HIV-1 exposed-uninfected infants (non-transmitting controls). Associations, unadjusted and adjusted for multiple comparisons, were assessed for overall transmission and according to mode of transmission—intrapartum (n = 31), in utero (n = 20), in utero-enriched (n = 48).
Results
The maternal FcγRIIIa-158V allele that confers enhanced antibody binding affinity and antibody-dependent cellular cytotoxicity capacity significantly associated with reduced HIV-1 transmission [odds ratio (OR) 0.47, 95 % confidence interval (CI) 0.28–0.79, P = 0.004; PBonf > 0.05]. In particular, the FcγRIIIa-158V allele was underrepresented in the in utero transmitting group (P = 0.048; PBonf > 0.05) and in utero-enriched transmitting groups (P = 0.0001; PBonf < 0.01). In both mother and infant, possession of an FcγRIIIb-HNA1b allotype that reduces neutrophil-mediated effector functions associated with increased transmission (OR 1.87, 95 % CI 1.08–3.21, P = 0.025; PBonf > 0.05) and acquisition (OR 1.91, 95 % CI 1.11–3.30, P = 0.020; PBonf > 0.05), respectively. Conversely, the infant FcγRIIIb-HNA1a|1a genotype was significantly protective of perinatal HIV-1 acquisition (OR 0.42, 95 % CI 0.18–0.96, P = 0.040; PBonf > 0.05).
Conclusions
The findings of this study suggest a potential role for FcγR-mediated effector functions in perinatal HIV-1 transmission. However, future studies are required to validate the findings of this study, in particular associations that did not retain significance after adjustment for multiple comparisons.
Electronic supplementary material
The online version of this article (doi:10.1186/s12977-016-0272-y) contains supplementary material, which is available to authorized users.
Keywords: HIV-1, Vertical infectious disease transmission, Risk factors, IgG receptors, Alleles, Antibody-dependent cell cytotoxicity, Phagocytosis
Background
Beyond neutralization, immunoglobulin G (IgG) has the capacity to recruit potent effector functions of the innate immune system through engagement with Fc gamma receptors (FcγRs), which are widely expressed throughout the haematopoietic system. Directly or indirectly, FcγRs mediate antiviral processes that include antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), respiratory burst, antigen display, antibody production, cell activation, and release of inflammatory mediators [1].
FcγR-mediated effector functions are increasingly recognized as a component of HIV-1 protective immunity [2]. However, the role of these effector functions in modulating perinatal HIV-1 transmission risk is currently undefined. Given the contribution of FcγR-mediated effector functions to eliminating cell-free and cell-associated virus, these processes may modify the infectiousness of an HIV-1 infected mother. In addition, transplacental transferred anti-HIV-1 IgG may recruit innate immune effector functions in the foetus/infant through engaging FcγRs expressed on foetal/infant immune cells, and in this manner modify the infant’s susceptibility to HIV-1 acquisition.
In vivo, FcγR-mediated effector functions are governed by a balance between activating and inhibitory FcγRs [3]. This balance is perturbed by functionally significant genotypic variants that modulate cellular activation and ultimately effector function capability. These include gene duplication/deletion that affects FcγR surface density [4, 5] and amino acid changes that alter the receptor’s binding affinity for antibody subclasses (FcγRIIa-H131R and FcγRIIIa-F158V) [6, 7], subcellular localization (FcγRIIb-I232T) [8], glycosylation patterns (FcγRIIIb-HNA1a|b|c) [9, 10], and the expression of a functional molecule (FcγRIIc-X57Q and c.798+1A>G) [11, 12].
Using these variants as a proxy for functional capability, this study indirectly assessed the potential role of FcγR-mediated effector functions in mother-to-child transmission of HIV-1. Due to the exploratory nature of the study, associations are reported unadjusted for multiple comparisons. However, adjusted associations were also considered. Our findings highlight a potential role for the FcγRIIIa-F158V variant in modulating maternal infectiousness, while in both mother and infant the FcγRIIIb-HNA1a|b|c variant associated with HIV-1 transmission.
Results
Cohort
A nested case–control study was undertaken to investigate FCGR variability in HIV-1 infected mothers and their infants recruited as part of four perinatal cohorts at two hospitals in Johannesburg, South Africa [13]. Overall, the four cohorts comprised 849 HIV-1 infected mothers and their infants, of whom 83 (10 %) acquired HIV-1 perinatally. In the present study, FCGR genotypic data from 79 HIV-1 infected mothers and 78 HIV-1 infected infants (transmitting cases) were compared with 234 HIV-1 infected mothers and 235 uninfected infants (non-transmitting controls). Mode of transmission was defined according to the presence/absence of detectable HIV-1 DNA in the infant at birth and 6 weeks of age. Infants that tested HIV-1 positive at 6 weeks of age, but who were negative at birth, were considered to be infected intrapartum (during labour and delivery), while infants that tested HIV-1 positive at birth were considered infected in utero. Infants that were HIV-1 positive at 6 weeks, but had no birth sample, were categorized as ‘undetermined’. Since 25/28 (89.2 %) mothers in the ‘undetermined’ category received drug interventions known to reduce intrapartum transmission [14–16], it was concluded that the majority of infants in this group were likely infected in utero and was thus combined with the in utero group to form an in utero-enriched group.
Transmitting mothers had significantly higher HIV-1 plasma viral loads and lower CD4+ T cell counts compared to non-transmitting mothers (Table 1). In addition, infants infected in utero had a significantly lower mean birth weight compared to exposed-uninfected infants. Maternal age, parity, mode of delivery, gestation, child sex, and reported breast feeding did not differ significantly between transmitting mothers (total, intrapartum or in utero) and non-transmitting mothers.
Table 1.
Demographic and clinical characteristics of mothers and infants
| Maternal viral load (log10 copies/ml) | Non-transmitting (N = 234)a | Total transmitting (N = 79) | Intrapartum transmitting (N = 31) | In utero transmitting (N = 20)b | In utero-enriched transmitting (N = 48) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nc | Nc | Nc | Nc | Nc | ||||||
| Median (IQR) | 218 | 4.08 (3.20–4.67) | 71 | 4.77 (3.77–5.34)*** | 27 | 4.77 (3.77–5.26)** | 18 | 4.89 (4.20–5.47)*** | 44 | 4.81 (3.78–5.44)*** |
| Maternal CD4+ T cell count | ||||||||||
| Mean (std) | 217 | 520 (275) | 70 | 418 (222)** | 27 | 402 (179)* | 15 | 409 (276) | 43 | 428 (247)* |
| Maternal age (years) | ||||||||||
| Mean (std) | 232 | 26.9 (5.1) | 78 | 27.6 (5.2) | 30 | 26.7 (5.0) | 20 | 27.5 (5.5) | 48 | 28.2 (5.2) |
| Parity | ||||||||||
| Mean (std) | 231 | 2.1 (1.0) | 77 | 2.3 (1.2) | 29 | 2.3 (1.2) | 20 | 2.2 (1.2) | 48 | 2.3 (1.2) |
| Mode of delivery [N (%)] | ||||||||||
| Caesarean section | 232 | 17 (7.3) | 77 | 10 (13.0) | 29 | 2 (6.9) | 20 | 3 (15.0) | 48 | 8 (16.7) |
| Gestation [N (%)] | ||||||||||
| Preterm <37 weeks | 215 | 27 (12.6) | 70 | 12 (17.1) | 25 | 7 (28.0) | 19 | 4 (21.1) | 45 | 5 (11.1) |
| Child sex [N (%)] | ||||||||||
| Male | 234 | 101 (43.1) | 79 | 39 (49.4) | 31 | 18 (58.0) | 20 | 8 (40.0) | 48 | 21 (43.8) |
| Birth weight (g) | ||||||||||
| Mean (std) | 231 | 2980 (453) | 78 | 2889 (442) | 30 | 2943 (400) | 20 | 2784 (320)* | 48 | 2856 (468) |
| Breast fed N (%) | ||||||||||
| >3 days | 233 | 34 (14.6) | 78 | 10 (12.8) | 30 | 5 (16.7) | 20 | 2 (10.0) | 48 | 5 (10.4) |
| Antiretrovirals | ||||||||||
| Nevirapine | 234 | 114 (48.7) | 79 | 47 (59.5) | 31 | 11 (35.5) | 20 | 13 (65.0) | 48 | 36 (75.0)** |
| Triple drug therapy | 234 | 6 (2.6) | 79 | 2 (2.5) | 31 | 0 | 20 | 0 | 48 | 2 (4.2) |
| Other drugsd | 234 | 11 (4.7) | 79 | 6 (7.6) | 31 | 3 (9.7) | 20 | 1 (5.0) | 48 | 3 (6.3) |
For comparisons with non-transmitting mothers: * P < 0.05; ** P < 0.01; *** P < 0.001
aFive unmatched mothers
bOne unmatched mother
cNumber of participants for whom data were available
dDifferent regimens of zidovudine (AZT) and lamivudine (3TC)
Variants not detected in the study cohort
The FcγRIIb 2B.4 promoter haplotype (c.-386C/c.-120A) and expression of functional FcγRIIc are rare to absent in Black South African individuals [17]. Accordingly, in the present cohort of Black South African mothers and infants, none possessed the FcγRIIb 2B.4 promoter haplotype. Furthermore, despite 84/313 (25.3 %) mothers and 81/313 (25.9 %) infants bearing an FcγRIIc-Q57 allele, only one non-transmitting mother expressed functional FcγRIIc as predicted by the FCGR2C c.798+1A>G splice-site variant [12].
FCGR copy number variability
The frequency of FCGR3A gene copy number variability (CNV) was low, occurring in 17/313 (5.4 %) mothers and 14/313 (4.5 %) infants (Fig. 1), and did not associate with perinatal HIV-1 transmission (P > 0.05 for all comparisons; Additional file 1: Table S1). FCGR3B gene CNV was observed more frequently in 92/313 (29.4 %) mothers and 100/313 (31.9 %) infants (Fig. 1). The overall distribution of FCGR3B gene copy number was significantly different between exposed-uninfected infants and intrapartum infected infants (P = 0.029), with the intrapartum infected group having fewer FCGR3B gene duplications and no gene deletions (Additional file 1: Table S1). Maternal FCGR3B gene CNV did not associate with HIV-1 transmission (P > 0.05 for all comparisons; Additional file 1: Table S1).
Fig. 1.
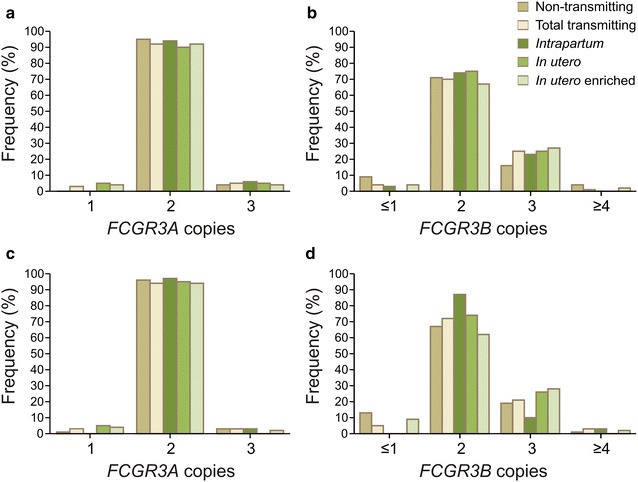
The distribution of FCGR3A and FCGR3B gene copy number in HIV-1 infected mothers (a, b, respectively) and their infants (c, d, respectively)
FcγR variants and infectiousness of the transmitter/mother
To determine if FcγR variants were associated with the infectiousness of the mother, HIV-1 transmission was assessed according to maternal genotypes and allele carriage in a univariate and multivariate model (Table 2, 3, respectively). Overall, the maternal FcγRIIIa-F158V variant significantly associated with HIV-1 transmission (P = 0.017), while a trend was observed for the FcγRIIIb-HNA1a|b|c variant (P = 0.058).
Table 2.
FcγR genotypes and allele carriage in HIV-1 non-transmitting and transmitting mothers
| Non-transmitting | Total transmitting | Intrapartum transmitting | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | OR (95 % CI) | P value | PBonf | N (%) | OR (95 % CI) | P value | PBonf | |
| FcγRIIa (rs1801274) | Overall association | P = 0.379 | ns | P = 0.688 | ns | ||||
| Genotype | |||||||||
| 131HH (ref) | 60 (25.6) | 15 (19.0) | 1 | 6 (19.4) | 1 | ||||
| 131HR | 106 (45.3) | 36 (45.6) | 1.36 (0.69–2.68) | P = 0.378 | ns | 14 (45.2) | 1.32 (0.48–3.62) | P = 0.558 | ns |
| 131RR | 68 (29.1) | 28 (35.4) | 1.65 (0.80–3.37) | P = 0.172 | ns | 11 (35.5) | 1.62 (0.56–4.64) | P = 0.371 | ns |
| Allele carriage | |||||||||
| ≥1 131H allele | 166 (70.9) | 51 (64.6) | 0.75 (0.43–1.28) | P = 0.288 | ns | 20 (64.5) | 0.74 (0.34–1.64) | P = 0.464 | ns |
| ≥1 131R allele | 174 (74.4) | 64 (81.0) | 1.47 (0.78–2.77) | P = 0.233 | ns | 25 (80.6) | 1.44 (0.56–3.67) | P = 0.449 | ns |
| FcγRIIb (rs1050501) | Overall association | P = 0.194 | ns | P = 0.397 | ns | ||||
| Genotype | |||||||||
| 232II (ref) | 113 (48.3) | 32 (40.5) | 1 | 12 (38.7) | 1 | ||||
| 232IT | 103 (44.0) | 36 (45.6) | 1.23 (0.71–2.13) | P = 0.450 | ns | 15 (48.4) | 1.37 (0.61–3.07) | P = 0.442 | ns |
| 232TT | 18 (7.7) | 11 (13.9) | 2.16 (0.93–5.03) | P = 0.075 | ns | 4 (12.9) | 2.09 (0.61–7.20) | P = 0.242 | ns |
| Allele carriage | |||||||||
| ≥1 232I allele | 216 (92.3) | 68 (86.3) | 0.52 (0.23–1.14) | P = 0.103 | ns | 27 (87.1) | 0.56 (0.18–1.79) | P = 0.239 | ns |
| ≥1 232T allele | 121 (51.7) | 47 (59.5) | 1.37 (0.82–2.30) | P = 0.231 | ns | 19 (61.3) | 1.48 (0.69–3.18) | P = 0.317 | ns |
| FcγRIIIa (rs396991) | Overall association | P = 0.017 | ns | P = 0.380 | ns | ||||
| Genotype | |||||||||
| 158F/FF/FF (ref) | 76 (32.5) | 40 (50.6) | 1 | 10 (32.3) | 1 | ||||
| 158FV/FFV/FVV | 121 (51.7) | 31 (39.2) | 0.49 (0.28–0.84) | P = 0.010 | ns | 19 (61.3) | 1.19 (0.53–2.70) | P = 0.672 | ns |
| 158V/VV | 36 (15.4) | 8 (10.1) | 0.41 (0.17–0.97) | P = 0.041 | ns | 2 (6.5) | 0.41 (0.09–1.97) | P = 0.266 | ns |
| Allele carriage | |||||||||
| ≥1 158F allele | 197 (84.2) | 71 (89.9) | 1.67 (0.74–3.75) | P = 0.217 | ns | 29 (93.5) | 2.72 (0.62–11.91) | P = 0.183 | ns |
| ≥1 158V allele | 157 (67.1) | 39 (49.4) | 0.47(0.28–0.79) | P = 0.004 | ns | 21 (67.7) | 1.01 (0.45–2.25) | P = 0.980 | ns |
| FcγRIIIb | Overall association | P = 0.058 | ns | P = 0.647 | ns | ||||
| Genotype | |||||||||
| HNA1a+/1b−/1c− | 51 (21.8) | 13 (16.5) | 0.68 (0.32–1.44) | P = 0.315 | ns | 4 (12.9) | 0.51 (0.15–1.70) | P = 0.276 | ns |
| HNA1a−/1b+/1c− | 23 (9.8) | 7 (8.9) | 0.81 (0.31–2.11) | P = 0.668 | ns | 4 (12.9) | 1.14 (0.33–3.92) | P = 0.837 | ns |
| HNA1a−/1b−/1c+ | 13 (5.6) | 0 (0) | – | 0 (0) | – | ||||
| HNA1a+/1b+/1c− (ref) | 72 (30.8) | 27 (34.2) | 1 | 11 (35.5) | 1 | ||||
| HNA1a+/1b−/1c+ | 40 (17.1) | 11 (13.9) | 0.73 (0.33–1.63) | P = 0.448 | ns | 5 (16.1) | 0.82 (0.27–2.52) | P = 0.727 | ns |
| HNA1a−/1b+/1c+ | 22 (9.4) | 17 (21.5) | 2.06 (0.95–4.46) | P = 0.066 | ns | 5 (16.1) | 1.49 (0.47–4.75) | P = 0.502 | ns |
| HNA1a+/1b+/1c+ | 12 (5.1) | 4 (5.1) | 0.89 (0.26–3.00) | P = 0.849 | ns | 2 (6.5) | 1.09 (0.21–5.54) | P = 0.916 | ns |
| Allele carriage | |||||||||
| ≥1 HNA1a allotype | 175 (74.8) | 55 (69.6) | 0.77 (0.44–1.36) | P = 0.369 | ns | 22 (71.0) | 0.82 (0.36–1.89) | P = 0.648 | ns |
| ≥1 HNA1b allotype | 129 (55.1) | 55 (69.6) | 1.87 (1.08–3.21) | P = 0.025 | ns | 22 (71.0) | 1.99 (0.88–4.50) | P = 0.099 | ns |
| ≥1 HNA1c allotype | 87 (37.2) | 32 (40.5) | 1.15 (0.68–1.94) | P = 0.599 | ns | 12 (38.7) | 1.07 (0.49–2.30) | P = 0.869 | ns |
| In utero transmitting | In utero-enriched transmitting | |||||||
|---|---|---|---|---|---|---|---|---|
| N (%) | OR (95 % CI) | P value | PBonf | N (%) | OR (95 % CI) | P value | PBonf | |
| FcγRIIa (rs1801274) | P = 0.182 | ns | P = 0.545 | ns | ||||
| Genotype | ||||||||
| 131HH (ref) | 2 (10.0) | 1 | 9 (18.8) | 1 | ||||
| 131HR | 9 (45.0) | 2.55 (0.53–12.17) | P = 0.241 | ns | 22 (45.8) | 1.38 (0.60–3.20) | P = 0.447 | ns |
| 131RR | 9 (45.0) | 3.97 (0.83–19.10) | P = 0.085 | ns | 17 (35.4) | 1.67 (0.69–4.02) | P = 0.225 | ns |
| Allele carriage | ||||||||
| ≥1 131H allele | 11 (55.0) | 0.50 (0.20–1.26) | P = 0.143 | ns | 31 (64.6) | 0.75 (0.39–1.44) | P = 0.383 | ns |
| ≥1 131R allele | 18 (90.0) | 3.10 (0.70–13.77) | P = 0.136 | ns | 39 (81.3) | 1.49 (0.68–3.27) | P = 0.314 | ns |
| FcγRIIb (rs1050501) | P = 0.125 | ns | P = 0.274 | ns | ||||
| Genotype | ||||||||
| 232II (ref) | 10 (50.0) | 1 | 20 (41.7) | 1 | ||||
| 232IT | 6 (30.0) | 0.66 (0.23–1.87) | P = 0.434 | ns | 21 (43.8) | 1.15 (0.59–2.25) | P = 0.678 | ns |
| 232TT | 4 (20.0) | 2.51 (0.71–8.87) | P = 0.153 | ns | 7 (14.6) | 2.20 (0.81–5.94) | P = 0.121 | ns |
| Allele carriage | ||||||||
| ≥1 232I allele | 16 (80.0) | 0.33 (0.10–1.10) | P = 0.072 | ns | 41 (85.4) | 0.49 (0.19–1.24) | P = 0.133 | ns |
| ≥1 232T allele | 10 (50.0) | 0.93 (0.37–2.33) | P = 0.883 | ns | 28 (58.3) | 1.31 (0.70–2.45) | P = 0.403 | ns |
| FcγRIIIa (rs396991) | P = 0.137 | ns | P = 0.0004 | 0.017 | ||||
| Genotype | ||||||||
| 158F/FF/FF (ref) | 11 (55.0) | 1 | 30 (62.5) | 1 | ||||
| 158FV/FFV/FVV | 8 (40.0) | 0.46 (0.18–1.19) | P = 0.108 | ns | 12 (25.0) | 0.25 (0.12–0.52) | P = 0.0001 | 0.004 |
| 158V/VV | 1 (5.0) | 0.19 (0.02–1.50) | P = 0.115 | ns | 6 (12.5) | 0.41 (0.16–1.07) | P = 0.069 | ns |
| Allele carriage | ||||||||
| ≥1 158F allele | 19 (95.0) | 3.57 (0.46–27.48) | P = 0.222 | ns | 42 (87.5) | 1.31 (0.52–3.31) | P = 0.562 | ns |
| ≥1 158V allele | 9 (45.0) | 0.39 (0.16–0.99) | P = 0.048 | ns | 18 (37.5) | 0.29 (0.15–0.55) | P = 0.0001 | 0.004 |
| FcγRIIIb | P = 0.320 | ns | P = 0.123 | ns | ||||
| Genotype | ||||||||
| HNA1a+/1b−/1c− | 6 (30.0) | 2.82 (0.67–11.82) | P = 0.155 | ns | 9 (18.8) | 0.79 (0.33–1.94) | P = 0.612 | ns |
| HNA1a−/1b+/1c− | 1 (5.0) | 1.04 (0.10–10.53) | P = 0.971 | ns | 3 (6.3) | 0.59 (0.16–2.20) | P = 0.429 | ns |
| HNA1a−/1b−/1c+ | 0 (0) | – | 0 (0) | – | ||||
| HNA1a+/1b+/1c− (ref) | 3 (15.0) | 1 | 16 (33.3) | 1 | ||||
| HNA1a+/1b−/1c+ | 4 (20.0) | 2.40 (0.51–11.26) | P = 0.267 | ns | 6 (12.5) | 0.68 (0.24–1.86) | P = 0.448 | ns |
| HNA1a−/1b+/1c+ | 5 (25.0) | 5.45 (1.21–24.66) | P = 0.028 | ns | 12 (25.0) | 2.45 (1.01–5.96) | P = 0.047 | ns |
| HNA1a+/1b+/1c+ | 1 (5.0) | 2.00 (0.19–20.85) | P = 0.562 | ns | 2 (4.2) | 0.75 (0.15–3.68) | P = 0.723 | ns |
| Allele carriage | ||||||||
| ≥1 HNA1a allotype | 14 (70.0) | 0.79 (0.29–2.14) | P = 0.638 | ns | 33 (68.8) | 0.74 (0.38–1.46) | P = 0.388 | ns |
| ≥1 HNA1b allotype | 10 (50.0) | 0.81 (0.33–2.03) | P = 0.659 | ns | 33 (68.8) | 1.79 (0.92–3.47) | P = 0.085 | ns |
| ≥1 HNA1c allotype | 10 (50.0) | 1.69 (0.68–4.22) | P = 0.262 | ns | 20 (41.7) | 1.21 (0.64–2.27) | P = 0.560 | ns |
P values less than 0.05 are indicated in italics
P Bonf Bonferroni corrected P value, OR odds ratio, CI confidence interval, ns not statistically significant, –, the variable of interest was not detected in any of the cases and thus could not be analysed
Table 3.
Maternal FcγR variants associated with perinatal HIV-1 transmission after adjusting for confounding variables
| Total transmitting | Intrapartum transmitting | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Adjusted for VLa | PBonf | Univariate | Adjusted for VL | PBonf | |||
| AOR (95 % CI) | P value | AOR (95 % CI) | P value | |||||
| FcγRIIa (rs1801274) | ||||||||
| Genotype | ||||||||
| 131HH (ref) | 1 | 1 | ||||||
| 131HR | P = 0.378 | 1.81 (0.82–3.99) | P = 0.141 | ns | P = 0.558 | 1.43 (0.46–4.46) | P = 0.539 | ns |
| 131RR | P = 0.172 | 2.59 (1.14–5.87) | P = 0.023 | ns | P = 0.371 | 2.57 (0.80–8.26) | P = 0.113 | ns |
| Allele carriage | ||||||||
| ≥1 131H allele | P = 0.288 | 0.58 (0.33–1.05) | P = 0.071 | ns | P = 0.464 | 0.49 (0.21–1.16) | P = 0.106 | ns |
| ≥1 131R allele | P = 0.233 | 2.11 (1.00–4.42) | P = 0.049 | ns | P = 0.449 | 1.82 (0.64–5.23) | P = 0.263 | ns |
| FcγRIIb (rs1050501) | ||||||||
| Genotype | ||||||||
| 232II (ref) | 1 | 1 | ||||||
| 232IT | P = 0.450 | 1.29 (0.71–2.35) | P = 0.408 | ns | P = 0.442 | 1.60 (0.65–3.93) | P = 0.309 | ns |
| 232TT | P = 0.075 | 2.80 (1.11–7.10) | P = 0.030 | ns | P = 0.242 | 3.25 (0.87–12.17) | P = 0.080 | ns |
| Allele carriage | ||||||||
| ≥1 232I allele | P = 0.103 | 0.41 (0.17–0.97) | P = 0.043 | ns | P = 0.239 | 0.40 (0.12–1.33) | P = 0.133 | ns |
| ≥1 232T allele | P = 0.231 | 1.49 (0.84–2.62) | P = 0.171 | ns | P = 0.317 | 1.81 (0.77–4.28) | P = 0.175 | ns |
| FcγRIIIa (rs396991) | ||||||||
| Genotype | ||||||||
| 158F/FF/FF (ref) | 1 | 1 | ||||||
| 158FV/FFV/FVV | P = 0.010 | 0.51 (0.28–0.92) | P = 0.026 | ns | P = 0.672 | 1.09 (0.45–2.64) | P = 0.850 | ns |
| 158V/VV | P = 0.041 | 0.30 (0.11–082) | P = 0.018 | ns | P = 0.266 | 0.20 (0.02–1.70) | P = 0.141 | ns |
| Allele carriage | ||||||||
| ≥1 158F allele | P = 0.217 | 2.29 (0.89–5.88) | P = 0.084 | ns | P = 0.183 | 5.22 (0.67–40.41) | P = 0.114 | ns |
| ≥1 158V allele | P = 0.004 | 0.46 (0.26–0.82) | P = 0.008 | ns | P = 0.980 | 0.89 (0.37–2.12) | P = 0.786 | ns |
| FcγRIIIb | ||||||||
| Genotype | ||||||||
| HNA1a+/1b−/1c− | P = 0.315 | 0.47 (0.20–1.10) | P = 0.083 | ns | P = 0.276 | 0.45 (0.12–1.61) | P = 0.218 | ns |
| HNA1a−/1b+/1c− | P = 0.668 | 0.90 (0.33–2.46) | P = 0.839 | ns | P = 0.837 | 1.31 (0.35–4.87) | P = 0.683 | ns |
| HNA1a−/1b−/1c+ | – | – | – | – | ||||
| HNA1a+/1b+/1c− (ref) | 1 | 1 | ||||||
| HNA1a+/1b−/1c+ | P = 0.448 | 0.63 (0.26–1.51) | P = 0.300 | ns | P = 0.727 | 0.68 (0.19–2.42) | P = 0.547 | ns |
| HNA1a−/1b+/1c+ | P = 0.066 | 1.37 (0.59–3.19) | P = 0.466 | ns | P = 0.502 | 1.20 (0.35–4.15) | P = 0.777 | ns |
| HNA1a+/1b+/1c+ | P = 0.849 | 0.42 (0.10–1.71) | P = 0.226 | ns | P = 0.916 | 0.42 (0.05–3.72) | P = 0.433 | ns |
| Allele carriage | ||||||||
| ≥1 HNA1a allotype | P = 0.369 | 0.78 (0.43–1.44) | P = 0.433 | ns | P = 0.648 | 0.73 (0.30–1.75) | P = 0.481 | ns |
| ≥1 HNA1b allotype | P = 0.025 | 2.11 (1.16–3.85) | P = 0.014 | ns | P = 0.099 | 2.18 (0.90–5.33) | P = 0.086 | ns |
| ≥1 HNA1c allotype | P = 0.599 | 0.95 (0.54–1.68) | P = 0.865 | ns | P = 0.869 | 0.88 (0.38–2.04) | P = 0.759 | ns |
| In utero transmitting | In utero-enriched transmitting | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Adjusted for VL + bwt | PBonf | Univariate | Adjusted for VL | PBonf | |||
| AOR (95 % CI) | P value | AOR (95 % CI) | P value | |||||
| FcγRIIa (rs1801274) | ||||||||
| Genotype | ||||||||
| 131HH (ref) | 1 | 1 | ||||||
| 131HR | P = 0.241 | 5.74 (0.66–49.93) | P = 0.113 | ns | P = 0.447 | 2.28 (0.84–6.17) | P = 0.105 | ns |
| 131RR | P = 0.085 | 11.46 (1.29–101.86) | P = 0.029 | ns | P = 0.225 | 2.82 (1.01–7.89) | P = 0.048 | ns |
| Allele carriage | ||||||||
| ≥1 131H allele | P = 0.143 | 0.34 (0.12–0.97) | P = 0.045 | ns | P = 0.383 | 0.63 (0.32–1.27) | P = 0.200 | ns |
| ≥1 131R allele | P = 0.136 | 7.65 (0.94–62.32) | P = 0.057 | ns | P = 0.314 | 2.50 (0.97–6.40) | P = 0.057 | ns |
| FcγRIIb (rs1050501) | ||||||||
| Genotype | ||||||||
| 232II (ref) | 1 | |||||||
| 232IT | P = 0.434 | 0.67 (0.22–2.06) | P = 0.487 | ns | P = 0.678 | 1.15 (0.56–2.35) | P = 0.707 | ns |
| 232TT | P = 0.153 | 3.38 (0.73–15.61) | P = 0.119 | ns | P = 0.121 | 2.57 (0.85–7.74) | P = 0.094 | ns |
| Allele carriage | ||||||||
| ≥1 232I allele | P = 0.072 | 0.25 (0.06–1.07) | P = 0.062 | ns | P = 0.133 | 0.42 (0.15–1.18) | P = 0.100 | ns |
| ≥1 232T allele | P = 0.883 | 0.93 (0.34–2.54) | P = 0.891 | ns | P = 0.403 | 1.33 (0.67–2.61) | P = 0.412 | ns |
| FcγRIIIa (rs396991) | ||||||||
| Genotype | ||||||||
| 158F/FF/FF (ref) | 1 | 1 | ||||||
| 158FV/FFV/FVV | P = 0.108 | 0.60 (0.21–1.71) | P = 0.341 | ns | P = 0.0001 | 0.29 (0.14–0.63) | P = 0.002 | ns |
| 158V/VV | P = 0.115 | 0.19 (0.02–1.68) | P = 0.135 | ns | P = 0.069 | 0.34 (0.11–0.98) | P = 0.046 | ns |
| Allele carriage | ||||||||
| ≥1 158F allele | P = 0.222 | 4.01 (0.48–33.16) | P = 0.198 | ns | P = 0.562 | 1.71 (0.61–4.80) | P = 0.305 | ns |
| ≥1 158V allele | P = 0.048 | 0.50 (0.18–1.36) | P = 0.174 | ns | P = 0.0001 | 0.31 (0.15–0.62) | P = 0.001 | 0.042 |
| FcγRIIIb | ||||||||
| Genotype | ||||||||
| HNA1a+/1b−/1c− | P = 0.155 | 1.44 (0.30–6.85) | P = 0.644 | ns | P = 0.612 | 0.45 (0.16–1.24) | P = 0.124 | ns |
| HNA1a−/1b+/1c− | P = 0.971 | 1.26 (0.12–13.63) | P = 0.851 | ns | P = 0.429 | 0.66 (0.17–2.56) | P = 0.544 | ns |
| HNA1a−/1b−/1c+ | – | – | – | – | ||||
| HNA1a+/1b+/1c− (ref) | 1 | 1 | ||||||
| HNA1a+/1b−/1c+ | P = 0.267 | 1.88 (0.37–9.46) | P = 0.442 | ns | P = 0.448 | 0.59 (0.20–1.68) | P = 0.321 | ns |
| HNA1a−/1b+/1c+ | P = 0.028 | 3.10 (0.60–15.95) | P = 0.177 | ns | P = 0.047 | 1.53 (0.58–4.02) | P = 0.388 | ns |
| HNA1a+/1b+/1c+ | P = 0.562 | 1.10 (0.10–12.45) | P = 0.939 | ns | P = 0.723 | 0.44 (0.08–2.28) | P = 0.326 | ns |
| Allele carriage | ||||||||
| ≥1 HNA1a allotype | P = 0.638 | 0.85 (0.28–2.63) | P = 0.783 | ns | P = 0.388 | 0.79 (0.38–1.64) | P = 0.523 | ns |
| ≥1 HNA1b allotype | P = 0.659 | 1.09 (0.39–3.02) | P = 0.868 | ns | P = 0.085 | 2.23 (1.08–4.62) | P = 0.031 | ns |
| ≥1 HNA1c allotype | P = 0.262 | 1.51 (0.55–4.14) | P = 0.420 | ns | P = 0.560 | 1.04 (0.53–2.06) | P = 0.904 | ns |
aThe multivariate analysis adjusted for demographic and clinical variables that independently associated with transmission. Due to high correlation with viral load, CD4 T cell counts were not included in the multivariate model
P values less than 0.05 are indicated in italics
P Bonf Bonferroni corrected P value, AOR adjusted odds ratio, CI confidence interval, VL viral load, bwt birth weight, ns not statistically significant, –, the variable of interest was not detected in any of the cases and thus could not be analysed
Carriage of at least one maternal FcγRIIIa-158V allele (confers enhanced antibody binding affinity) associated with a reduced odds of perinatal HIV-1 transmission (OR 0.47, 95 % CI 0.28–0.79, P = 0.004). When analysed according to mode of transmission, a similar association was observed for the in utero transmitting group (OR 0.39, 95 % CI 0.16–0.99, P = 0.048) and in utero-enriched transmitting group (OR 0.29, 95 % CI 0.15–0.55, P = 0.0001), but not for the intrapartum transmitting group (OR 1.01, 95 % CI 0.45–2.25, P = 0.980). These associations remained significant for the total transmitting group and in utero-enriched group in the multivariate analysis (P = 0.008 and P = 0.001, respectively) and for the in utero-enriched group after adjustment for multiple comparisons (univariate: PBonf = 0.004; multivariate: PBonf = 0.042).
Possession of an FcγRIIIb-HNA1b allele (modulates neutrophil function) significantly associated with an increased odds of HIV-1 transmission in both the univariate analysis (OR 1.87, 95 % CI 1.08–3.21, P = 0.025) and multivariate analysis (P = 0.014). A similar association was observed for the FcγRIIIb-HNA1b|1c genotype in the in utero transmitting group (OR 5.45, 95 % CI 1.21–24.66, P = 0.028) and in utero-enriched transmitting group (OR 2.45, 95 % CI 1.01–5.96, P = 0.047). However, these associations were not significant in the multivariate analysis.
The FcγRIIa-H131R and FcγRIIb-I232T variants did not associate with perinatal HIV-1 transmission in the univariate analysis. However, after adjustment for confounding variables, the FcγRIIa-131RR genotype (receptor has reduced affinity for IgG2) and FcγRIIb-232TT genotype (confers reduced inhibitory capacity) associated with increased odds of HIV-1 transmission (Table 3).
FcγR variants and susceptibility of the recipient/infant
In addition to an association observed in the mother, the infant FcγRIIIb-HNA1a|b|c variant also associated with susceptibility to HIV-1 acquisition in the infant (P = 0.046). In particular, carriage of least one FcγRIIIb-HNA1b allotype significantly associated with increased susceptibility to HIV-1 acquisition in the univariate analysis (OR 1.91, 95 % CI 1.11–3.30, P = 0.020; Table 4) and multivariate analysis (P = 0.019; Table 5). Conversely, homozygosity for the FcγRIIIb-HNA1a allotype associated with reduced odds of HIV-1 acquisition in the total infected group (OR 0.42, 95 % CI 0.18–0.96, P = 0.040) and intrapartum infected group (OR 0.19, 95 % CI 0.04–0.89, P = 0.035). The protective effect of FcγRIIIb-HNA1a homozygosity was also observed when compared to other allotype combinations, however not all comparisons remained significant in the multivariate analysis (Additional file 2: Table S2).
Table 4.
FcγR genotypes and allele carriage in HIV-1 exposed-uninfected and infected infants
| Exposed-uninfected | Total infected | Intrapartum infected | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | OR (95 % CI) | P value | PBonf | N (%) | OR (95 % CI) | P value | PBonf | |
| FcγRIIa (rs1801274) | Overall association | P = 0.704 | ns | P = 0.907 | ns | ||||
| Genotype | |||||||||
| 131HH (ref) | 47 (20.0) | 19 (24.4) | 1 | 7 (22.6) | 1 | ||||
| 131HR | 116 (49.4) | 36 (46.2) | 0.77 (0.40–1.47) | P = 0.426 | ns | 14 (45.2) | 0.81 (0.31–2.13) | P = 0.670 | ns |
| 131RR | 72 (30.6) | 23 (29.5) | 0.79 (0.39–1.61) | P = 0.516 | ns | 10 (32.3) | 0.93 (0.33–2.62) | P = 0.895 | ns |
| Allele carriage | |||||||||
| ≥1 131H allele | 163 (69.4) | 55 (70.5) | 1.06 (0.60–1.85) | P = 0.848 | ns | 21 (67.7) | 0.93 (0.42–2.07) | P = 0.854 | ns |
| ≥1 131R allele | 188 (80.0) | 59 (75.6) | 0.76 (0.42–1.43) | P = 0.414 | ns | 24 (77.4) | 0.86 (0.35–2.11) | P = 0.737 | ns |
| FcγRIIb (rs1050501) | Overall association | P = 0.278 | ns | P = 0.773 | ns | ||||
| Genotype | |||||||||
| 232II (ref) | 116 (49.4) | 33 (42.3) | 1 | 14 (45.2) | 1 | ||||
| 232IT | 90 (38.3) | 30 (38.5) | 1.17 (0.67–2.06) | P = 0.583 | ns | 12 (38.7) | 1.10 (0.49–2.51) | P = 0.811 | ns |
| 232TT | 29 (12.3) | 15 (19.2) | 1.82 (0.87–3.79) | P = 0.110 | ns | 5 (16.1) | 1.43 (0.48–4.29) | P = 0.525 | ns |
| Allele carriage | |||||||||
| ≥1 232I allele | 206 (86.8) | 63 (78.6) | 0.59 (0.30–1.17) | P = 0.132 | ns | 26 (83.9) | 0.73 (0.26–2.06) | P = 0.554 | ns |
| ≥1 232T allele | 119 (47.2) | 45 (55.7) | 1.33 (0.79–2.23) | P = 0.280 | ns | 17 (54.8) | 1.18 (0.56–2.51) | P = 0.660 | ns |
| FcγRIIIa (rs396991) | Overall association | P = 0.339 | ns | P = 0.964 | ns | ||||
| Genotype | |||||||||
| 158F/FF/FF (ref) | 86 (36.6) | 34 (43.6) | 1 | 12 (38.7) | 1 | ||||
| 158FV/FFV/FVV | 118 (50.2) | 38 (48.7) | 0.81 (0.47–1.40) | P = 0.456 | ns | 15 (48.4) | 0.91 (0.41–2.04) | P = 0.821 | ns |
| 158V/VV | 31 (13.2) | 6 (7.7) | 0.49 (0.19–1.28) | P = 0.145 | ns | 4 (12.9) | 0.92 (0.28–3.08) | P = 0.899 | ns |
| Allele carriage | |||||||||
| ≥1 158F allele | 194 (82.6) | 72 (92.3) | 0.75 (0.44–1.26) | P = 0.272 | ns | 27 (87.1) | 0.91 (0.42–1.97) | P = 0.819 | ns |
| ≥1 158V allele | 149 (63.4) | 44 (56.4) | 1.82 (0.73–4.55) | P = 0.198 | ns | 19 (61.3) | 1.03(0.34–3.13) | P = 0.964 | ns |
| FcγRIIIb | Overall association | P = 0.046 | ns | P = 0.023 | ns | ||||
| Genotype | |||||||||
| HNA1a+/1b−/1c− | 58 (24.7) | 9 (11.5) | 0.42 (0.18–0.96) | P = 0.040 | ns | 2 (6.5) | 0.19(0.04–0.89) | P = 0.035 | ns |
| HNA1a−/1b+/1c− | 25 (10.6) | 7 (9.0) | 0.76 (0.29–1.95) | P = 0.565 | ns | 1 (3.2) | 0.22 (0.03–1.81) | P = 0.160 | ns |
| HNA1a−/1b−/1c+ | 14 (6.0) | 4 (5.1) | 0.77 (0.23–2.55) | P = 0.672 | ns | 0 (0) | – | ||
| HNA1a+/1b+/1c− (ref) | 73 (31.2) | 27 (34.6) | 1 | 13 (41.9) | 1 | ||||
| HNA1a+/1b−/1c+ | 36 (15.3) | 11 (14.1) | 0.83 (0.37–1.85) | P = 0.643 | ns | 7 (22.6) | 1.09 (0.40–2.97) | P = 0.863 | ns |
| HNA1a−/1b+/1c+ | 22 (9.4) | 13 (16.7) | 1.60 (0.71–3.61) | P = 0.260 | ns | 7 (22.6) | 1.79 (0.63–5.03) | P = 0.272 | ns |
| HNA1a+/1b+/1c+ | 7 (3.0) | 7 (9.0) | 2.70 (0.87–8.43) | P = 0.086 | ns | 1 (3.2) | 0.80 (0.09–7.07) | P = 0.843 | ns |
| Allele carriage | |||||||||
| ≥1 HNA1a allotype | 174 (74.0) | 54 (69.2) | 0.79 (0.45–1.38) | P = 0.408 | ns | 23 (74.2) | 1.01 (0.43–2.37) | P = 0.986 | ns |
| ≥1 HNA1b allotype | 127 (54.0) | 54 (69.2) | 1.91 (1.11–3.30) | P = 0.020 | ns | 22 (71.0) | 2.08 (0.92–4.70) | P = 0.079 | ns |
| ≥1 HNA1c allotype | 79 (33.6) | 35 (44.9) | 1.61 (0.95–2.71) | P = 0.075 | ns | 15 (48.4) | 1.85 (0.87–3.94) | P = 0.110 | ns |
| In utero infected | In utero-enriched infected | |||||||
|---|---|---|---|---|---|---|---|---|
| N (%) | OR (95 % CI) | P value | PBonf | N (%) | OR (95 % CI) | P value | PBonf | |
| FcγRIIa (rs1801274) | P = 0.265 | ns | P = 0.693 | ns | ||||
| Genotype | ||||||||
| 131HH (ref) | 4 (21.1) | 1 | 12 (25.5) | 1 | ||||
| 131HR | 6 (31.6) | 0.61 (0.16–2.25) | P = 0.456 | ns | 22 (46.8) | 0.74 (0.34–1.62) | P = 0.455 | ns |
| 131RR | 9 (47.4) | 1.47 (0.43–5.04) | P = 0.541 | ns | 13 (27.7) | 0.71 (0.30–1.68) | P = 0.433 | ns |
| Allele carriage | ||||||||
| ≥1 131H allele | 10 (52.6) | 0.49 (0.19–1.26) | P = 0.139 | ns | 34 (72.3) | 1.16 (0.58–2.32) | P = 0.685 | ns |
| ≥1 131R allele | 15 (78.9) | 0.94 (0.30–2.96) | P = 0.912 | ns | 35 (74.5) | 0.73 (0.35–1.51) | P = 0.396 | ns |
| FcγRIIb (rs1050501) | P = 0.083 | ns | P = 0.218 | ns | ||||
| Genotype | ||||||||
| 232II (ref) | 7 (36.8) | 1 | 19 (40.4) | 1 | ||||
| 232IT | 6 (31.6) | 1.10 (0.36–3.40) | P = 0.862 | ns | 18 (38.3) | 1.22 (0.61–2.46) | P = 0.577 | ns |
| 232TT | 6 (31.6) | 3.43 (1.07–10.98) | P = 0.038 | ns | 10 (21.3) | 2.11 (0.88–5.01) | P = 0.092 | ns |
| Allele carriage | ||||||||
| ≥1 232I allele | 13 (68.4) | 0.31 (0.11–0.87) | P = 0.026 | ns | 37 (78.7) | 0.52 (0.23–1.16) | P = 0.110 | ns |
| ≥1 232T allele | 12 (63.2) | 1.67 (0.64–4.39) | P = 0.298 | ns | 28 (59.6) | 1.44 (0.76–2.71) | P = 0.264 | ns |
| FcγRIIIa (rs396991) | P = 0.711 | ns | P = 0.145 | ns | ||||
| Genotype | ||||||||
| 158F/FF/FF (ref) | 9 (47.4) | 1 | 22 (46.8) | 1 | ||||
| 158FV/FFV/FVV | 8 (42.1) | 0.65 (0.24–1.75) | P = 0.391 | ns | 23 (48.9) | 0.76 (0.40–1.46) | P = 0.410 | ns |
| 158V/VV | 2 (10.5) | 0.62 (0.13–3.01) | P = 0.550 | ns | 2 (4.3) | 0.25 (0.06–1.14) | P = 0.073 | ns |
| Allele carriage | ||||||||
| ≥1 158F allele | 17 (89.5) | 0.64 (0.25–1.64) | P = 0.354 | ns | 45 (95.7) | 0.66 (0.35–1.23) | P = 0.190 | ns |
| ≥1 158V allele | 10 (52.6) | 1.29 (0.28–5.87) | P = 0.740 | ns | 25 (53.2) | 3.42 (0.79–14.81) | P = 0.100 | ns |
| FcγRIIIb | P = 0.182 | ns | P = 0.079 | ns | ||||
| Genotype | ||||||||
| HNA1a+/1b−/1c− | 3 (15.8) | 0.76 (0.17–3.29) | P = 0.709 | ns | 7 (14.9) | 0.63 (0.24–1.66) | P = 0.350 | ns |
| HNA1a−/1b+/1c− | 1 (5.3) | 0.58 (0.07–5.24) | P = 0.631 | ns | 6 (12.8) | 1.25 (0.43–3.61) | P = 0.678 | ns |
| HNA1a−/1b−/1c+ | 1 (5.3) | 1.04 (0.11–9.62) | P = 0.970 | ns | 4 (8.5) | 1.49 (0.43–5.20) | P = 0.532 | ns |
| HNA1a+/1b+/1c− (ref) | 5 (26.3) | 1 | 14 (29.8) | 1 | ||||
| HNA1a+/1b−/1c+ | 2 (10.5) | 0.81 (0.15–4.39) | P = 0.808 | ns | 4 (8.5) | 0.58 (0.18–1.89) | P = 0.365 | ns |
| HNA1a−/1b+/1c+ | 5 (26.3) | 3.32 (0.88–12.52) | P = 0.077 | ns | 6 (12.8) | 1.42 (0.49–4.14) | P = 0.518 | ns |
| HNA1a+/1b+/1c+ | 2 (10.5) | 4.17 (0.68–25.59) | P = 0.123 | ns | 6 (12.8) | 4.47 (1.30–15.31) | P = 0.017 | ns |
| Allele carriage | ||||||||
| ≥1 HNA1a allotype | 12 (63.2) | 0.60 (0.23–1.60) | P = 0.307 | ns | 31 (66.0) | 0.70 (0.35–1.33) | P = 0.258 | ns |
| ≥1 HNA1b allotype | 13 (68.4) | 1.84 (0.68–5.01) | P = 0.231 | ns | 32 (68.1) | 1.81 (0.93–3.53) | P = 0.079 | ns |
| ≥1 HNA1c allotype | 10 (52.6) | 2.19 (0.86–5.62) | P = 0.101 | ns | 20 (42.6) | 1.46 (0.77–2.77) | P = 0.243 | ns |
P values less than 0.05 are indicated in italics
P Bonf Bonferroni corrected P value, OR odds ratio, CI confidence interval, ns not statistically significant, –, the variable of interest was not detected in any of the cases and thus could not be analysed
Table 5.
Infant FcγR variants associated with perinatal HIV-1 acquisition after adjusting for confounding variables
| Total infected | Intrapartum infected | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Adjusted for VLa | PBonf | Univariate | Adjusted for VL | PBonf | |||
| AOR (95 % CI) | P value | AOR (95 % CI) | P value | |||||
| FcγRIIa (rs1801274) | ||||||||
| Genotype | ||||||||
| 131HH (ref) | 1 | 1 | ||||||
| 131HR | P = 0.426 | 0.79 (0.38–1.62) | P = 0.519 | ns | P = 0.670 | 0.80 (0.27–2.32) | P = 0.685 | ns |
| 131RR | P = 0.516 | 0.84 (0.39–1.83) | P = 0.657 | ns | P = 0.895 | 0.97 (0.31–2.97) | P = 0.951 | ns |
| Allele carriage | ||||||||
| ≥1 131H allele | P = 0.848 | 1.01 (0.55–1.85) | P = 0.970 | ns | P = 0.854 | 0.89 (0.37–2.12) | P = 0.792 | ns |
| ≥1 131R allele | P = 0.414 | 0.81 (0.41–1.59) | P = 0.536 | ns | P = 0.737 | 0.87 (0.32–2.32) | P = 0.774 | ns |
| FcγRIIb (rs1050501) | ||||||||
| Genotype | ||||||||
| 232II (ref) | 1 | 1 | ||||||
| 232IT | P = 0.583 | 1.29 (0.70–2.39) | P = 0.415 | ns | P = 0.811 | 1.40 (0.57–3.44) | P = 0.469 | ns |
| 232TT | P = 0.110 | 1.97 (0.89–4.37) | P = 0.096 | ns | P = 0.525 | 1.82 (0.56–5.90) | P = 0.317 | ns |
| Allele carriage | ||||||||
| ≥1 232I allele | P = 0.132 | 0.57 (0.28–1.20) | P = 0.140 | ns | P = 0.554 | 0.65 (0.22–1.90) | P = 0.429 | ns |
| ≥1 232T allele | P = 0.280 | 1.46 (0.83–2.57) | P = 0.195 | ns | P = 0.660 | 1.50 (0.65–3.47) | P = 0.344 | ns |
| FcγRIIIa (rs396991) | ||||||||
| Genotype | ||||||||
| 158F/FF/FF (ref) | 1 | 1 | ||||||
| 158FV/FFV/FVV | P = 0.456 | 0.87 (0.49–1.56) | P = 0.647 | ns | P = 0.821 | 1.14 (0.49–2.66) | P = 0.764 | ns |
| 158V/VV | P = 0.145 | 0.28 (0.08–1.00) | P = 0.051 | ns | P = 0.899 | 0.28 (0.03–2.27) | P = 0.232 | ns |
| Allele carriage | ||||||||
| ≥1 158F allele | P = 0.272 | 3.34 (0.96–11.57) | P = 0.058 | ns | P = 0.819 | 3.89 (0.50–30.31) | P = 0.194 | ns |
| ≥1 158V allele | P = 0.198 | 0.75 (0.43–1.31) | P = 0.311 | ns | P = 0.964 | 0.95 (0.42–2.19) | P = 0.910 | ns |
| FcγRIIIb | ||||||||
| Genotype | ||||||||
| HNA1a+/1b−/1c− | P = 0.040 | 0.37 (0.15–0.92) | P = 0.033 | ns | P = 0.035 | 0.20 (0.04–0.96) | P = 0.044 | ns |
| HNA1a−/1b+/1c− | P = 0.565 | 0.69 (0.25–1.86) | P = 0.459 | ns | P = 0.160 | 0.20 (0.03–1.69) | P = 0.139 | ns |
| HNA1a−/1b−/1c+ | P = 0.672 | 0.70 (0.18–2.78) | P = 0.616 | ns | – | – | P = 0.970 | |
| HNA1a+/1b+/1c− (ref) | 1 | 1 | ||||||
| HNA1a+/1b−/1c+ | P = 0.643 | 0.73 (0.31–1.72) | P = 0.478 | ns | P = 0.863 | 0.97 (0.33–2.79) | P = 0.949 | ns |
| HNA1a−/1b+/1c+ | P = 0.260 | 1.57 (0.64–3.88) | P = 0.326 | ns | P = 0.272 | 1.80 (0.57–5.71) | P = 0.316 | ns |
| HNA1a+/1b+/1c+ | P = 0.086 | 2.36 (0.63–8.75) | P = 0.201 | ns | P = 0.843 | – | ns | P = 0.123 |
| Allele carriage | ||||||||
| ≥1 HNA1a allotype | P = 0.408 | 0.79 (0.43–1.46) | P = 0.452 | ns | P = 0.986 | 1.01 (0.40–2.56) | P = 0.981 | ns |
| ≥1 HNA1b allotype | P = 0.020 | 2.02 (1.12–3.64) | P = 0.019 | ns | P = 0.079 | 1.91 (0.81–4.53) | P = 0.140 | ns |
| ≥1 HNA1c allotype | P = 0.075 | 1.52 (0.86–2.69) | P = 0.146 | ns | P = 0.110 | 1.74 (0.77–3.96) | P = 0.185 | ns |
| In utero infected | In utero-enriched infected | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Adjusted for VL + bwt | PBonf | Univariate | Adjusted for VL | PBonf | |||
| AOR (95 % CI) | P value | AOR (95 % CI) | P value | |||||
| FcγRIIa (rs1801274) | ||||||||
| Genotype | ||||||||
| 131HH (ref) | 1 | 1 | ||||||
| 131HR | P = 0.456 | 0.71 (0.15–3.25) | P = 0.657 | ns | P = 0.455 | 0.75 (0.32–1.79) | P = 0.520 | ns |
| 131RR | P = 0.541 | 1.87 (0.45–7.79) | P = 0.390 | ns | P = 0.433 | 0.77 (0.30–1.96) | P = 0.581 | ns |
| Allele carriage | ||||||||
| ≥1 131H allele | P = 0.139 | 0.42 (0.15–1.21) | P = 0.108 | ns | P = 0.685 | 1.07 (0.51–2.22) | P = 0.858 | ns |
| ≥1 131R allele | P = 0.912 | 1.17 (0.31–4.58) | P = 0.817 | ns | P = 0.396 | 0.76 (0.34–1.70) | P = 0.503 | ns |
| FcγRIIb (rs1050501) | ||||||||
| Genotype | ||||||||
| 232II (ref) | 1 | 1 | ||||||
| 232IT | P = 0.862 | 0.80 (0.23–2.74) | P = 0.724 | ns | P = 0.577 | 1.18 (0.56–2.50) | P = 0.658 | ns |
| 232TT | P = 0.038 | 3.53 (0.95–13.14) | P = 0.060 | ns | P = 0.092 | 2.02 (079–5.16) | P = 0.144 | ns |
| Allele carriage | ||||||||
| ≥1 232I allele | P = 0.026 | 0.26 (0.08–0.86) | P = 0.028 | ns | P = 0.110 | 0.54 (0.23–1.28) | P = 0.160 | ns |
| ≥1 232T allele | P = 0.298 | 1.33 (0.47–3.77) | P = 0.593 | ns | P = 0.264 | 1.38 (0.70–2.74) | P = 0.353 | ns |
| FcγRIIIa (rs396991) | ||||||||
| Genotype | ||||||||
| 158F/FF/FF (ref) | 1 | 1 | ||||||
| 158FV/FFV/FVV | P = 0.391 | 0.61 (0.20–1.86) | P = 0.385 | ns | P = 0.410 | 0.74 (0.37–1.49) | P = 0.405 | ns |
| 158V/VV | P = 0.550 | 0.85 (0.16–4.42) | P = 0.842 | ns | P = 0.073 | 0.29 (0.06–1.36) | P = 0.117 | ns |
| Allele carriage | ||||||||
| ≥1 158F allele | P = 0.354 | 0.93 (0.19–4.53) | P = 0.931 | ns | P = 0.190 | 2.91 (0.66–12.92) | P = 0.160 | ns |
| ≥1 158V allele | P = 0.740 | 0.66 (0.23–1.85) | P = 0.425 | ns | P = 0.100 | 0.65 (0.33–1.28) | P = 0.215 | ns |
| FcγRIIIb | ||||||||
| Genotype | ||||||||
| HNA1a+/1b−/1c− | P = 0.709 | 0.77 (0.15–3.86) | P = 0.748 | ns | P = 0.350 | 0.53 (0.18–1.52) | P = 0.234 | ns |
| HNA1a−/1b+/1c− | P = 0.631 | 0.46 (0.04–4.76) | P = 0.513 | ns | P = 0.678 | 1.13 (0.37–3.42) | P = 0.827 | ns |
| HNA1a−/1b−/1c+ | P = 0.970 | 1.48 (0.14–15.83) | P = 0.744 | ns | P = 0.532 | 1.33 (0.32–5.54) | P = 0.695 | ns |
| HNA1a+/1b+/1c− (ref) | 1 | 1 | ||||||
| HNA1a+/1b−/1c+ | P = 0.808 | 0.65 (0.10–4.10) | P = 0.645 | ns | P = 0.365 | 0.50 (0.15–1.67) | P = 0.259 | ns |
| HNA1a−/1b+/1c+ | P = 0.077 | 4.47 (0.84–23.80) | P = 0.080 | ns | P = 0.518 | 1.50 (0.46–4.92) | P = 0.501 | ns |
| HNA1a+/1b+/1c+ | P = 0.123 | 3.35 (0.40–27.73) | P = 0.262 | ns | P = 0.017 | 4.44 (1.14–17.40) | P = 0.032 | ns |
| Allele carriage | ||||||||
| ≥1 HNA1a allotype | P = 0.307 | 0.58 (0.19–1.76) | P = 0.337 | ns | P = 0.258 | 0.66 (0.32–1.37) | P = 0.265 | ns |
| ≥1 HNA1b allotype | P = 0.231 | 1.82 (0.63–5.32) | P = 0.271 | ns | P = 0.079 | 2.16 (1.05–4.44) | P = 0.037 | ns |
| ≥1 HNA1c allotype | P = 0.101 | 2.16 (0.76–6.14) | P = 0.149 | ns | P = 0.243 | 1.42 (0.71–2.81) | P = 0.321 | ns |
P values less than 0.05 are indicated in italics
P Bonf Bonferroni corrected P value, AOR adjusted odds ratio, CI confidence interval, VL viral load, bwt birth weight, –, the variable of interest was not detected in any of the cases and thus could not be analysed
aThe multivariate analysis adjusted for demographic and clinical variables that independently associated with transmission. Due to high correlation with viral load, CD4 T cell counts were not included in the multivariate model
Linkage disequilibrium at the low affinity FCGR gene locus
Linkage disequilibrium (LD) between the different FcγR variants could potentially modulate associations observed for the individual FcγRs. Given the strong association of the maternal FcγRIIIa-F158V variant with perinatal HIV-1 transmission, we determined LD in the study cohort (Fig. 2) and adjusted for its possible confounding effect on the associations observed for FcγRIIIb-HNA1a|b|c, FcγRIIa-H131R and FcγRIIb-I232T in the multivariate analysis (Table 6).
Fig. 2.
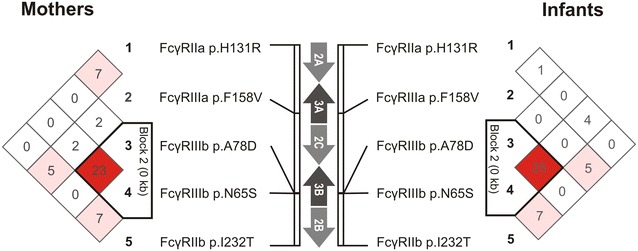
LD for FcγR variants in the study cohort comprising Black South African HIV-1 infected mothers (left) and their infants (right). Values and colours reflect r2 (× 100) and D′/LOD measures of LD, respectively. The black triangle depicts a haplotype block that is indicative of the relationship between the FcγRIIIb-HNA1b and -HNA1c allotypes. Such that HNA1b and HNA1c are identical at amino acid position 65 (p.65S) and differ only at amino acid position 78 (p.78A1b>D1c)
Table 6.
Multivariate analysis adjusted FcγRIIIa-F158V
| Multivariate, not adjusted for FcγRIIIa-F158V | PBonf | Multivariate analysis with adjustment for FcγRIIIa-F158V genotype and allele carriage | ||||||
|---|---|---|---|---|---|---|---|---|
| F158V genotype | PBonf | ≥1 158F allele | PBonf | ≥1 158V allele | PBonf | |||
| Maternal | ||||||||
| FcγRIIa (rs1801274) | ||||||||
| 131RR genotype | ||||||||
| Total transmitting | P = 0.023 | ns | 1.93 (0.82–4.57), P = 0.133 | ns | 2.25 (0.97–5.24), P = 0.133 | ns | 2.08 (0.89–4.86), P = 0.091 | ns |
| In utero transmitting | P = 0.029 | ns | 9.37 (1.01–87.22), P = 0.049 | ns | 9.59 (1.05–87.37), P = 0.045 | ns | 10.26 (1.12–94.28), P = 0.040 | ns |
| In utero-enriched transmitting | P = 0.048 | ns | 1.94 (0.66–5.70), P = 0.226 | ns | 2.60 (0.90–7.52), P = 0.077 | ns | 1.98 (0.67–5.80), P = 0.214 | ns |
| ≥1 131H allele | ||||||||
| In utero transmitting | P = 0.045 | ns | 0.42 (0.14–1.29), P = 0.132 | ns | 0.40 (0.14–1.15), P = 0.088 | ns | 0.39 (0.13–1.18), P = 0.096 | ns |
| ≥1 131R allele | ||||||||
| Total transmitting | P = 0.049 | ns | 1.80 (0.84–3.85), P = 0.128 | ns | 1.90 (0.89–4.05), P = 0.095 | ns | 1.91 (0.90–4.06), P = 0.091 | ns |
| FcγRIIb (rs1050501) | ||||||||
| 232TT genotype | ||||||||
| Total transmitting | P = 0.030 | ns | 2.06 (0.78–5.41), P = 0.144 | ns | 2.48 (0.96–9.36), P = 0.060 | ns | 2.17 (0.83–5.67), P = 0.115 | ns |
| ≥1 232I allele | ||||||||
| Total transmitting | P = 0.043 | ns | 0.49 (0.20–1.20), P = 0.118 | ns | 0.43 (0.18–1.05), P = 0.063 | ns | 0.48 (0.20–1.18), P = 0.110 | ns |
| FcγRIIIb | ||||||||
| ≥1 HNA1b allotype | ||||||||
| Total transmitting | P = 0.014 | ns | 2.26 (1.22–4.17), P = 0.009 | ns | 2.19 (1.20–4.02), P = 0.011 | ns | 2.21 (1.20–4.11), P = 0.011 | ns |
| In utero-enriched transmitting | P = 0.031 | ns | 2.43 (1.15–5.16), P = 0.020 | ns | 2.32 (1.11–4.82), P = 0.025 | ns | 2.40 (1.13–5.10), P = 0.023 | ns |
| Infant | ||||||||
| FcγRIIIb | ||||||||
| HNA1a+/1b−/1c− genotype | ||||||||
| Total infected | P = 0.033 | ns | 0.37 (0.15–0.93), P = 0.034 | ns | 0.37 (0.15–0.91), P = 0.031 | ns | 0.37 (0.15–0.93), P = 0.034 | ns |
| Intrapartum infected | P = 0.044 | ns | 0.20 (0.04–0.96), P = 0.044 | ns | 0.19 (0.04–0.95), P = 0.043 | ns | 0.20 (0.04–0.96), P = 0.044 | ns |
| HNA1a+/1b+/1c+ genotype | ||||||||
| In utero-enriched infected | P = 0.032 | ns | 5.67 (1.39–23.11), P = 0.016 | ns | 4.47 (1.13–17.64), P = 0.032 | ns | 5.74 (1.39–23.57), P = 0.015 | ns |
| ≥1 HNA1b allotype | ||||||||
| Total infected | P = 0.019 | ns | 2.11 (1.16–3.83), P = 0.014 | ns | 2.04 (1.12–3.69), P = 0.019 | ns | 2.08 (1.15–3.77), P = 0.016 | ns |
| In utero-enriched infected | P = 0.037 | ns | 2.29 (1.10–4.76), P = 0.026 | ns | 2.22 (1.07–4.58), P = 0.032 | ns | 2.26 (1.09–4.68), P = 0.028 | ns |
P values less than 0.05 are indicated in italics
P Bonf Bonferroni corrected P value, AOR adjusted odds ratio, CI confidence interval, VL viral load, bwt birth weight, ns not statistically significant
–, the variable of interest was not detected in any of the cases and thus could not be analysed
To determine LD for the FcγRIIIb-HNA1a|b|c allotypes, we used, as a tag-variant, one of four amino acid changes that differentiate HNA1a from HNA1b and HNA1c (p.Na65Sbc, rs448740) as well as the variant that differentiates HNA1c from HNA1a and HNA1b (p.Aab78Dc, rs5030738). The maternal FcγRIIIb-Na65Sbc variant was not in LD with FcγRIIIa-F158V (P = 0.057, D′ = 0.189, r2 = 0.020), while the p.Aab78Dc variant was in moderate LD with FcγRIIIa-F158V (P = 0.024, D′ = 0.471, r2 = 0.029) with the FcγRIIIa-158V allele overrepresented in individuals bearing an FcγRIIIb-78A allele (HNA1c individuals) compared to FcγRIIIb-78DD individuals (59 vs. 20 %). Following adjustment for FcγRIIIa-F158V in the multivariate analysis, the associations previously observed for the FcγRIIIb-HNA1b allotype strengthened for both the total and in utero-enriched transmitting groups (Table 6). Similarly, significance was retained in the infants with associations strengthening for the FcγRIIIb-HNA1a+|1b+|1c+ genotype in the in utero-enriched infected group and carriage of an HNA1b allotype in the total infected and in utero-enriched infected groups (Table 6). Overall, this suggests that the observed associations between the FcγRIIIb-HNA1a|b|c variant and perinatal HIV-1 transmission are not only independent of FcγRIIIa-F158V, but also potentially negatively confounded by FcγRIIIa-F158V.
Both maternal FcγRIIa-H131R and FcγRIIb-I232T was in moderate LD with FcγRIIIa-F158V (P < 0.0001, D′ = 0.351, r2 = 0.077 and P = 0.002, D′ = 0.448, r2 = 0.052, respectively), with the FcγRIIIa-158V allele overrepresented in individuals bearing an FcγRIIa-131H allele compared to FcγRIIa-131RR individuals (66 vs. 39 %) and in individuals bearing an FcγRIIb-232I allele compared to FcγRIIb-232TT individuals (59 vs. 39 %). When adjusted for FcγRIIIa-F158V in the multivariate analysis, all associations for the FcγRIIa-H131R and FcγRIIb-I232T weakened with the majority losing significance (Table 6). This suggests that the associations observed for FcγRIIa-H131R and FcγRIIb-I232T potentially resulted from LD with FcγRIIIa-F158V.
Discussion
The extent to which FcγR-mediated effector mechanisms contribute to the risk of HIV-1 transmission and acquisition is currently undefined. Through the study of FcγR functional variants we indirectly demonstrated a role for FcγR-mediated effector functions in modulating perinatal HIV-1 transmission and acquisition. Our findings indicate that the FcγRIIIa-F158V variant that alters antibody binding affinity and functional capacity is associated with infectiousness of an HIV-1 infected mother, while the FcγRIIIb-HNA1a|b|c variant that affects neutrophil effector function is associated with both maternal infectiousness and infant susceptibility.
The significance of FcγR-mediated effector functions in maintaining immune homeostasis is validated by the association of functionally significant FcγR variants with immune disorders [18]. Here we describe an association between the high binding FcγRIIIa allele and reduced maternal infectiousness in perinatal transmission of HIV-1. In particular, carriage of the FcγRIIIa-158V allele by the mother was associated with ~50 % reduction in the odds of HIV-1 transmission. The significant association in the in utero-enriched transmission group, but not in the intrapartum group, suggests that the underlying mechanism may be more pronounced at the maternofoetal interface. FcγRIIIa-bearing leukocytes, including natural killer cells, macrophages and γδ T lymphocytes, are readily recruited to the decidua where they likely contribute to eliminating cell-associated HIV-1 through ADCC [19, 20]. While decidual natural killer cells are primarily FcγRIIIa negative during a healthy pregnancy, they likely upregulate FcγRIIIa expression in the presence of HIV-1 as demonstrated for other perinatally transmitted viruses—human cytomegalovirus and hepatitis C virus [21, 22]. Since cell-associated HIV-1 is thought to be more infectious in utero compared to cell-free virus [23], ADCC-mediated killing of HIV-1 infected cells may contribute to protective immunity at the maternofoetal interface. Of consequence, the FcγRIIIa-F158V variant impacts on ADCC capacity, such that the FcγRIIIa-158V allele exhibits enhanced IgG binding and ADCC capacity compared to the FcγRIIIa-158F allele [7, 24]. The decreased in utero transmission risk associated with the FcγRIIIa-158V allele suggests that the enhanced ADCC capacity conferred by this variant may potentiate elimination of cell-associated HIV-1 and reduce the odds of HIV-1 crossing the placenta through cell–cell interactions. However, the role of ADCC and other potential FcγRIIIa-mediated immune mechanisms—systemic or localized—in perinatal HIV-1 transmission needs to be further elucidated.
In contrast to that observed for the FcγRIIIa-F158V variant, an association between the FcγRIIIb-HNA1a|b|c allotype and perinatal HIV-1 transmission was observed in both the mother and infant. The different FcγRIIIb allotypes arise from multiple amino acid substitutions that do not alter antibody binding affinity, but affect the glycosylation and tertiary structure of the receptor [9, 24–26]. Neutrophils from FcγRIIIb-HNA1a homozygous donors have an enhanced phagocytic and respiratory burst capacity compared to neutrophils from FcγRIIIb-HNA1b homozygous donors [27, 28]. In the present study, homozygosity for the FcγRIIIb-HNA1a allotype in the infant was associated with reduced odds of HIV-1 acquisition compared to other allotype combinations. In both mother and infant, carriage of at least one FcγRIIIb-HNA1b allotype was associated with increased odds of HIV-1 acquisition. Since expression of FcγRIIIb is largely restricted to neutrophils, these findings suggest a potential role for neutrophil-mediated FcγR effector functions in modulating perinatal HIV-1 transmission and acquisition. The underlying mechanism may also involve basophils as FcγRIIIb is detected at low levels on a subset of this cell population, although its function here is unknown.
To date, only the FcγRIIa-H131R variant has been studied in perinatal HIV-1 transmission, with an association reported between the FcγRIIa-131HH genotype and increased infant susceptibility [29]. This association was however not observed in the present study. The contrasting findings are likely attributable to study design. In the Brouwer et al. study, infants were considered perinatally infected if PCR positive at or before 4 months of age where in the present study infant infection status was determined up to 6 weeks of age. The implication thereof is that the number of infants that acquired HIV-1 through breastfeeding is likely higher in the Brouwer et al. study compared to the 12.8 % in the present study. If this is the case, the findings of the Brouwer et al. study may be more representative of an association with HIV-1 transmission through breastfeeding, rather than in utero or intrapartum transmission.
Perinatal HIV-1 transmission is an attractive model in which to study the role of antibodies and their effector functions in HIV-1 protective immunity. This represents a natural situation where the individual at risk is passively immunized with HIV-1-specific antibodies through transplacental transfer of IgG [30, 31]. This model also affords the opportunity to study both members of the transmitting dyad, allowing the assessment of factors contributing to the infectiousness of the transmitter (mother) as well as the susceptibility of the recipient (infant). The findings of this study therefore not only highlight additional immunological factors associated with risk of perinatal HIV-1 transmission, but further support a role for FcγR-mediated effector functions in HIV-1 protective immunity. In particular, findings underscore a potential involvement of neutrophils in protection from HIV-1 transmission and a possible role of FcγR-mediated effector functions in modulating the infectiousness of an HIV-1 infected individual. The significance of these findings in the context of sexual transmission will need to be determined.
There are a number of limitations of the current study and areas that require further investigation. Due to the small sample size and number of comparisons performed it is likely that a number of associations are due to chance. However, since the adjustment for multiple comparisons eliminate type I errors at the cost of type 2 errors, we considered it more important to identify potential factors that may play a role in perinatal HIV-1 transmission rather than dismissing these leads as chance variations brought about by multiple comparisons. Nonetheless, when a Bonferroni correction is applied (α = 0.0012), the association with the maternal FcγRIIIa-F158V variant in the in utero-enriched transmitting group remains significant.
Conclusions
The maternal and infant immune mechanisms involved in modulating the risk of perinatal HIV-1 transmission and acquisition are complex and multifactorial. Using the approach of studying FcγR genetic variants as proxy for functional capability, this study has revealed the potential importance of FcγR-mediated immune mechanisms that likely involve FcγRIIIa-bearing immune cells and neutrophils. The findings of this study need to be validated in larger cohorts, in particular associations that did not retain significance following adjustment for multiple comparisons. Moreover, understanding the role of IgG Fc-mediated mechanisms requires an appreciation for the collective contribution of multiple components in addition to FcγR genetic variants. These include factors such as the magnitude and specificity of maternal HIV-1 specific antibodies, the efficiency of antibody transfer across the placenta, immune cell phenotypes at the sites of HIV-1 exposure, and the impact of the overall immune environment and state of activation on maternal and infant immune responses.
Methods
Study populations
All study participants were Black South African individuals. Ethical clearance was obtained from the University of the Witwatersrand Human Research Ethics Committee and the Institutional Review Board of Columbia University. Written informed consent was obtained from all participants.
Cohort HIV-1 infection status
Maternal HIV-1 RNA levels were determined using the Roche Amplicor RNA Monitor assay version 1.5 (Roche Diagnostic Systems, Inc., Branchburg, New Jersey, USA). CD4+ T cell counts were determined using the FACSCount System from Becton–Dickinson (San Jose, CA, USA). Infant samples were tested for HIV-1 DNA using the Roche Amplicor Monitor version 1.5 qualitative PCR assay (Roche Diagnostic Systems).
FCGR gene copy number variability and nucleotide variant detection
Genomic DNA was extracted from EDTA anticoagulated blood samples using the QIAamp DNA Mini Kit (Qiagen, Dusseldorf, Germany). Functional FCGR variants were genotyped using the FCGR-specific multiplex ligation-dependent probe amplification (MLPA) assay (MRC Holland, Amsterdam, The Netherlands) according to manufacturer’s instructions [19, 20]. The assay detects the genomic copy number of the FCGR2C, FCGR3A and FCGR3B genes and known functional allelic variants that include FcγRIIa-H131R; FcγRIIb-I232T, FcγRIIIa-F158V, FcγRIIIb-HNA1a|b|c, FCGR2C expression variants (p.X57Q and c.798+1A>G), and the FCGR2B/C promoter variants (c.-386G>C and c.-120T>A). Genotypes assigned to study participants according to the MLPA assay were confirmed on randomly selected samples with nucleotide sequencing or TaqMan® SNP Genotyping Assays (Thermofisher, Life Technologies, Foster City, USA).
Computational and statistical analysis
Univariate analyses were used to determine the association between FcγR functional variants and perinatal HIV-1 transmission. Multivariate logistic regression was used to adjust for available confounders that were independently significantly associated with HIV-1 transmission i.e. viral load (all groups) and birth weight (in utero transmitting group) (Table 1). Due to high correlation with viral load, CD4 T+ cell count was not included in the multivariate model. The t test was used to compare normally distributed continuous variables and the Fisher’s exact test for categorical data. All analyses were performed in STATA version 10.1 (StataCorp LP, College Station, USA) and a P value of less than 0.05 was considered statistically significant. Adjustment for multiple comparisons was performed using the Bonferroni correction, which considered 42 independent tests—mothers and infants, three unrelated clinical subgroups, and seven loci (FCGR3A gene copy number, FCGR3B gene copy number, FcγRIIa-H131R, FcγRIIb-I232T, FcγRIIIa-F158V, FcγRIIIb-HNA1a|b|c, and overall FcγR variability profiles).
LD between pairs of biallelic loci was tested using an expectation–maximization likelihood-ratio test with 16 000 permutations (significance level <0.05) in Arlequin ver 3.5.2.2 [32]. LD coefficients (D′ and r2) were determined in Haploview [33]. Only individuals bearing two copies of each low affinity FCGR gene were considered. LD with FcγRIIIb-HNA1a|b|c was assessed using two loci: rs448740 (p.N65S; as tag-variant) that differentiates HNA1a (p.65 N) from HNA1b|c (p.65S) and rs5030738 (p.A78D) that differentiates HNA1a|b (p.78A) from HNA1c (p.78D).
Authors’ contributions
RL performed the researched and wrote the paper. AM and RL performed data analysis. GG recruited patients and acquired clinical data. LK contributed to the design of the study. CT designed the study and supervised the research. All co-authors critically revised the manuscript for intellectual content. All authors read and approved the manuscript.
Acknowledgements
The authors thank the study participants and Dorothy Southern for her review of the manuscript. This work is based on the research supported by grants from NICHD (HD 42402), the South African Medical Research Council and the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation. Ria Lassauniere is the recipient of bursaries from the South African National Research Foundation, the Poliomyelitis Research Foundation and a University of the Witwatersrand postgraduate merit award.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- ADCC
antibody-dependent cellular cytotoxicity
- ADCP
antibody-dependent cellular phagocytosis
- AOR
adjusted odds ratio
- CI
confidence interval
- CNV
copy number variability
- DNA
deoxyribonucleic acid
- Fc
fragment, crystallisable
- FcγR
Fc gamma receptors
- HIV
human immunodeficiency virus
- HNA
human neutrophil antigen
- IgG
immunoglobulin G
- MLPA
multiplex ligation-dependent probe amplification
- PCR
polymerase chain reaction
- RNA
ribonucleic acid
- sdNVP
single dose nevirapine
Additional files
10.1186/s12977-016-0272-y Associations of maternal and infant FCGR3A and FCGR3B gene copy number with perinatal HIV-1 transmission. Univariate and multivariate analysis of associations of maternal and infant FCGR3A and FCGR3B gene copy number with perinatal HIV-1 transmission.
10.1186/s12977-016-0272-y Association of the FcγRIIIb-HNA1a homozygous genotype with perinatal HIV-1 acquisition when compared to other combinations of FcγRIIIb-HNA allotypes. Univariate and multivariate analysis of associations of the FcγRIIIb-HNA1a homozygous genotype with perinatal HIV-1 acquisition when compared to other combinations of FcγRIIIb-HNA allotypes.
Contributor Information
Ria Lassaunière, Email: rial@nicd.ac.za.
Alfred Musekiwa, Email: ydm4@cdc.gov.
Glenda E. Gray, Email: Glenda.Gray@mrc.ac.za
Louise Kuhn, Email: lk24@cumc.columbia.edu.
Caroline T. Tiemessen, Email: carolinet@nicd.ac.za
References
- 1.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 2.Lewis GK. Role of Fc-mediated antibody function in protective immunity against HIV-1. Immunology. 2014;142(1):46–57. doi: 10.1111/imm.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- 4.Breunis WB, van Mirre E, Geissler J, Laddach N, Wolbink G, van der Schoot E, et al. Copy number variation at the FCGR locus includes FCGR3A, FCGR2C and FCGR3B but not FCGR2A and FCGR2B. Hum Mutat. 2009;30(5):E640–E650. doi: 10.1002/humu.20997. [DOI] [PubMed] [Google Scholar]
- 5.Willcocks LC, Lyons PA, Clatworthy MR, Robinson JI, Yang W, Newland SA, et al. Copy number of FCGR3B, which is associated with systemic lupus erythematosus, correlates with protein expression and immune complex uptake. J Exp Med. 2008;205(7):1573–1582. doi: 10.1084/jem.20072413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warmerdam PA, van de Winkel JG, Gosselin EJ, Capel PJ. Molecular basis for a polymorphism of human Fc gamma receptor II (CD32) J Exp Med. 1990;172(1):19–25. doi: 10.1084/jem.172.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J, Edberg JC, Redecha PB, Bansal V, Guyre PM, Coleman K, et al. A novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J Clin Invest. 1997;100(5):1059–1070. doi: 10.1172/JCI119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Floto RA, Clatworthy MR, Heilbronn KR, Rosner DR, MacAry PA, Rankin A, et al. Loss of function of a lupus-associated FcgammaRIIb polymorphism through exclusion from lipid rafts. Nat Med. 2005;11(10):1056–1058. doi: 10.1038/nm1288. [DOI] [PubMed] [Google Scholar]
- 9.Bux J, Stein EL, Bierling P, Fromont P, Clay M, Stroncek D, et al. Characterization of a new alloantigen (SH) on the human neutrophil Fc gamma receptor IIIb. Blood. 1997;89(3):1027–1034. [PubMed] [Google Scholar]
- 10.Ory PA, Goldstein IM, Kwoh EE, Clarkson SB. Characterization of polymorphic forms of Fc receptor III on human neutrophils. J Clin Invest. 1989;83(5):1676–1681. doi: 10.1172/JCI114067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metes D, Ernst LK, Chambers WH, Sulica A, Herberman RB, Morel PA. Expression of functional CD32 molecules on human NK cells is determined by an allelic polymorphism of the FcgammaRIIC gene. Blood. 1998;91(7):2369–2380. [PubMed] [Google Scholar]
- 12.van der Heijden J, Breunis WB, Geissler J, de Boer M, van den Berg TK, Kuijpers TW. Phenotypic variation in IgG receptors by nonclassical FCGR2C alleles. J Immunol. 2012;188(3):1318–1324. doi: 10.4049/jimmunol.1003945. [DOI] [PubMed] [Google Scholar]
- 13.Kuhn L, Schramm DB, Donninger S, Meddows-Taylor S, Coovadia AH, Sherman GG, et al. African infants’ CCL3 gene copies influence perinatal HIV transmission in the absence of maternal nevirapine. AIDS. 2007;21(13):1753–1761. doi: 10.1097/QAD.0b013e3282ba553a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forbes JC, Alimenti AM, Singer J, Brophy JC, Bitnun A, Samson LM, et al. A national review of vertical HIV transmission. AIDS. 2012;26(6):757–763. doi: 10.1097/QAD.0b013e328350995c. [DOI] [PubMed] [Google Scholar]
- 15.Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354(9181):795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 16.Townsend CL, Cortina-Borja M, Peckham CS, de Ruiter A, Lyall H, Tookey PA. Low rates of mother-to-child transmission of HIV following effective pregnancy interventions in the United Kingdom and Ireland, 2000–2006. AIDS. 2008;22(8):973–981. doi: 10.1097/QAD.0b013e3282f9b67a. [DOI] [PubMed] [Google Scholar]
- 17.Lassauniere R, Tiemessen CT. Variability at the FCGR locus: characterization in Black South Africans and evidence for ethnic variation in and out of Africa. Genes Immun. 2015 doi: 10.1038/gene.2015.60. [DOI] [PubMed] [Google Scholar]
- 18.Gillis C, Gouel-Cheron A, Jonsson F, Bruhns P. Contribution of human FcgammaRs to disease with evidence from human polymorphisms and transgenic animal studies. Front Immunol. 2014;5:254. doi: 10.3389/fimmu.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ditzian-Kadanoff R, Garon J, Verp MS, Zilberstein M. Gamma delta T cells in human decidua. Am J Obstet Gynecol. 1993;168(3 Pt 1):831–836. doi: 10.1016/S0002-9378(12)90829-7. [DOI] [PubMed] [Google Scholar]
- 20.Williams PJ, Searle RF, Robson SC, Innes BA, Bulmer JN. Decidual leucocyte populations in early to late gestation normal human pregnancy. J Reprod Immunol. 2009;82(1):24–31. doi: 10.1016/j.jri.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Siewiera J, El Costa H, Tabiasco J, Berrebi A, Cartron G, Le Bouteiller P, et al. Human cytomegalovirus infection elicits new decidual natural killer cell effector functions. PLoS Pathog. 2013;9(4):e1003257. doi: 10.1371/journal.ppat.1003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giugliano S, Petroff MG, Warren BD, Jasti S, Linscheid C, Ward A, et al. Hepatitis C virus sensing by human trophoblasts induces innate immune responses and recruitment of maternal NK Cells: potential implications for limiting vertical transmission. J Immunol. 2015;195(8):3737–3747. doi: 10.4049/jimmunol.1500409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milligan C, Overbaugh J. The role of cell-associated virus in mother-to-child HIV transmission. J Infect Dis. 2014;210(Suppl 3):S631–S640. doi: 10.1093/infdis/jiu344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113(16):3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 25.Ory PA, Clark MR, Kwoh EE, Clarkson SB, Goldstein IM. Sequences of complementary DNAs that encode the NA1 and NA2 forms of Fc receptor III on human neutrophils. J Clin Invest. 1989;84:1688–1691. doi: 10.1172/JCI114350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravetch JV, Perussia B. Alternative membrane forms of Fc gamma RIII(CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. J Exp Med. 1989;170(2):481–497. doi: 10.1084/jem.170.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bredius RG, Fijen CA, De Haas M, Kuijper EJ, Weening RS, Van de Winkel JG, et al. Role of neutrophil Fc gamma RIIa (CD32) and Fc gamma RIIIb (CD16) polymorphic forms in phagocytosis of human IgG1- and IgG3-opsonized bacteria and erythrocytes. Immunology. 1994;83(4):624–630. [PMC free article] [PubMed] [Google Scholar]
- 28.Salmon JE, Edberg JC, Kimberly RP. Fc gamma receptor III on human neutrophils. Allelic variants have functionally distinct capacities. J Clin Invest. 1990;85(4):1287–1295. doi: 10.1172/JCI114566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brouwer KC, Lal RB, Mirel LB, Yang C, van Eijk AM, Ayisi J, et al. Polymorphism of Fc receptor IIa for IgG in infants is associated with susceptibility to perinatal HIV-1 infection. AIDS. 2004;18(8):1187–1194. doi: 10.1097/00002030-200405210-00012. [DOI] [PubMed] [Google Scholar]
- 30.Aldrovandi GM, Kuhn L. What infants and breasts can teach us about natural protection from HIV infection. J Infect Dis. 2010;202(Suppl 3):S366–S370. doi: 10.1086/655972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braibant M, Barin F. The role of neutralizing antibodies in prevention of HIV-1 infection: what can we learn from the mother-to-child transmission context? Retrovirology. 2013;10:103. doi: 10.1186/1742-4690-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Excoffier L, Slatkin M. Incorporating genotypes of relatives into a test of linkage disequilibrium. Am J Hum Genet. 1998;62(1):171–180. doi: 10.1086/301674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]


