Abstract
Purpose
To test the effect of three diets in their ability to sustain weight loss and improve type 2 diabetes (T2D) and cardiovascular disease (CVD) risk markers after 18-month intervention.
Methods
Following a ≥8 % weight loss, 131 healthy, overweight/obese (BMI ± SD 31.5 ± 2.6 kg/m2) men (n = 55) and women (n = 76) aged 28.2 ± 4.8 years were randomized to either 1. Moderate fat (40 E%) with 20 E% MUFA and low in glycemic index (GI) (MUFA, n = 54), 2. Low fat (25 E%) and medium in GI (LF, n = 51) or 3. Control (35 E% fat) and high in GI (CTR, n = 26) all with similar protein content, and all provided ad libitum. First 6-month intervention with 100 % food provision (previously reported) following 12 months of moderately intensive intervention with 20 % food provision now reported.
Results
Attrition rate was higher in MUFA (63 %) than in LF (37 %, P = 0.019) and CTR (42 %, P = 0.09) group. Weight regain in completers was not different between groups (mean ± SEM), MUFA 7.1 ± 2.1 % versus LF 5.6 ± 1.3 % versus CTR 7.2 ± 1.5 %, nor was body fat regain, MUFA 4.8 ± 1.0 % versus LF 4.7 ± 0.8 % versus CTR 5.7 ± 0.6 %. The MUFA group reduced LDL/HDL ratio by −0.47 ± 0.09 compared with −0.23 ± 0.11 in LF (P < 0.05) and 0.06 ± 0.14 (P < 0.005) in CTR groups.
Conclusions
Weight regain or body composition did not differ between diets over 18 months. No effects on risk markers for T2D or CVD were found, with the exception of an improvement in the LDL/HDL ratio by the MUFA diet compared to the CTR diet. The LF diet was generally more satisfactory and the MUFA diet seemed more difficult to follow.
Keywords: Weight loss, Weight maintenance, Mediterranean diet, Cardiovascular disease, Dietary intervention
Introduction
Overweight and obesity are global health problems contributing to an ever-increasing incidence of non-communicable diseases. Many current treatment programmes focus on dietary composition and are efficacious in producing short-term weight loss of moderate magnitude. However, they seem less successful in producing long-term maintenance of substantial or even modest weight loss [1, 2].
For decades the official dietary recommendations for weight loss and maintenance and prevention of cardiovascular disease (CVD) have aimed at reducing the total energy and fat content of the diet [3]. However, many popular diets with a wide variety of diet composition (e.g. low carbohydrate, high protein) have shown to be just as effective in weight management and reducing CVD risk [4–6]. A Spanish multicenter intervention trial involving more than 7000 persons at high CVD risk found a Mediterranean diet supplemented with extra-virgin olive oil or nuts reduced the incidence of major CVD events compared to a low-fat diet [7]. Furthermore, another meta-analysis concluded that Mediterranean diets appear more effective than low-fat diets in inducing clinically relevant long-term changes in CVD risk factors and inflammatory markers [8]. However, the increased total fat content of these Mediterranean diets increases energy density and may promote weight gain via a higher energy intake.
Many of these weight loss studies have methodological limitations, particularly the lack of strict long-term adherence to the diet composition which seems to be extremely difficult to achieve by dietary counselling only. We aimed at improving adherence to the diets by providing all foods free of charge from a supermarket for the first 6 months and assessing the compliance by adipose tissue fatty acid analysis and questionnaires specifically addressing dietary adherence. We conducted a randomized, dietary intervention trial comparing three ad libitum diets: LF (low in fat and medium in GI), MUFA (high in monounsaturated fat and low in GI), and CTR (high in saturated fat and high in GI). We found that the conventional low-fat diet produced a lower 6 month regain in body fat following an initial weight loss, whereas the high MUFA diet seemed to exert more beneficial effect on diabetes and CVD risk factors [9, 10]. Here we report the dietary effects on weight loss maintenance, change in diabetic and CVD risk factors, drop-out, and dietary adherence after 18-month intervention.
Materials and methods
The study was conducted at the Department of Human Nutrition, Faculty of Life Sciences (now Exercise and Sports), University of Copenhagen, Denmark. The study was approved by the Ethical Committee of the Municipalities of Copenhagen and Frederiksberg and carried out according to the Helsinki Declaration. Informed consent was obtained from all participants.
Participants and experimental diets
A total of 169 subjects were screened. Out of these, 131 achieved the required 8 % weight loss and were randomized to the dietary intervention (Fig. 1). The recruitment, inclusion criteria, weight loss period, stabilization period, and randomization to intervention have been described previously [10]. The study was a parallel, randomized, 18-month dietary intervention trial, and the allocation ratio was 2:2:1 for the MUFA/LF/CTR groups. The three prescribed ad libitum diets were (1) Moderate fat (35–45 E%), high in MonoUnsaturated Fatty Acid (>20 E%) [MUFA], (2) Low Fat (20–30 E%) [LF], and (3) Control (35 E% fat) with >15 E% saturated fatty acid [CTR]. Protein was similar (15 E%) in all three diets. The MUFA diet included more whole grain foods, nuts and legumes, whereas the CTR included more added sugar than the other diets. Foods recommended to eat plenty of, eat less of, or restrict to a minimum in the respective groups have been described [10]. Differences in both the total amount of carbohydrate and specific food components supposedly resulted in low, medium, and high glycaemic index and glycaemic load (GL) in MUFA, LF, and CTR diet, respectively. Alcohol intake was recommended at a minimum, though consumption was allowed in accordance with Danish guidelines before August 2010, i.e. <14 units/week for women and <21 units/week for men (1 unit = 12 g alcohol). Subjects were instructed to maintain their habitual physical activity level throughout the study.
Fig. 1.
Organization chart of participant flow through the study for the MUFA, LFs and CTR groups, including reasons for drop-out. LCD low-calorie diet
Supermarket model and dietary counselling
To control dietary composition, the supermarket model was used [10, 11]. During the first 6 months study participants collected all foods (100 % of their energy needs) 1–3 times/week free of charge. Food intake was permitted ad libitum and the energy content was not known by the subjects. During the following 12 months all subjects were provided freely with 20 % of their estimated calorie requirements (calculations based on body weight after weight loss, sex, age, and a moderate PAL factor of 1.4) [3]. All food items provided were characteristic of their respective diet [10], and the food items were picked up from the supermarket monthly. The subjects shopped the remaining 80 % of their foods in ordinary shops and supermarkets. To ensure high dietary compliance, all subjects received monthly individual face-to-face dietary counselling and encouragement from a dietician throughout this 12-month less strict intervention period. Additionally, fat biopsies and questionnaires were performed and used to monitor dietary compliance.
Outcome measures
Outcome measures were change in body weight and body composition, weight loss maintenance of >5 % from baseline, drop-out, dietary adherence, and changes in diabetes and CVD risk factors (fasting glucose, fasting insulin, HOMA-IR, lipid profile, and Hs-CRP) after 18-month dietary intervention. Methods for measuring body weight, body composition (by DEXA), and waist and hip circumferences, and for obtaining and analysing the blood samples have been reported previously [10]. Correlation analyses between changes in body fat and changes in fatty acids in the biopsies, and between changes in body fat or weight and self-rated dietary adherence were performed. Dietary compliance was assessed by three methods: (1) objectively, by fatty acid analyses from subcutaneous adipose tissue biopsies (<1 g) taken from the buttock before the initial LCD induced weight loss and after 18-month intervention; (2) subjectively, by participants ranking adherence to their respective diet on a scale from 1 to 5, monthly from month 6 to 18; and (3) as assessed by the dietician at the monthly meeting (Supplementary 1).
Statistics
Differences between groups at month 0 were tested by one-way ANOVA. Differences between groups in changes in outcome measures from before weight loss to month 0, month 0–6, month 0–18, and month 6–18 were tested by ANCOVA using month 0 or month 6 values as covariate, respectively. Repeated measures analyses were performed for body weight over time and for diet × time, with month 0 as covariate for completers only (n = 67) and for the whole sample attending the month 0 examination (n = 125) by intention to treat (ITT). For the ITT analysis multiple imputation (MI) via Markov chain Monte Carlo (MCMC) was conducted, assuming that the values were missing at random (MAR) [12]. The means were adjusted by the covariate. The parameter estimates of each model were combined using standard rules. The ITT analysis was conducted using SAS version 9.1. Differences in gender, the proportion of drop-out, reason for dropping out, and number of participants maintaining weight loss >5 % or achieving further weight loss were tested by Chi-squared tests. Correlations analyses are performed by using Pearson’s correlation coefficients. All results are presented as mean values with 95 % confidence intervals (CI) or mean ± SEM. The level of significance was set at P < 0.05 (two-tailed). All statistical analyses (except for the ITT) were conducted using SPSS version 13 (SPSS Inc. Chicago, IL, USA).
Results
Participant characteristics and drop-out
The groups were well matched and no significant differences among groups were found at randomization. One hundred and thirty-one participants were randomized to the dietary intervention: 81 % (106/131) completed the first 6 months, whereas 51 % (67/131) completed the 18-month intervention. The drop-out of 63 % (34/54) in the MUFA was significantly higher than the 37 % (19/51) in the LF (P = 0.019), but not statistically significant different to the 42 % (11/26) in the CTR group (P = 0.093). The most common reasons for dropping out in all groups were due to personal issues (depressed state of mind, stress, divorce, etc.) or disease (depression, other illnesses, etc.), or that the project was too demanding. No difference in reason for dropping out was seen among groups (Fig. 1).
Weight and body
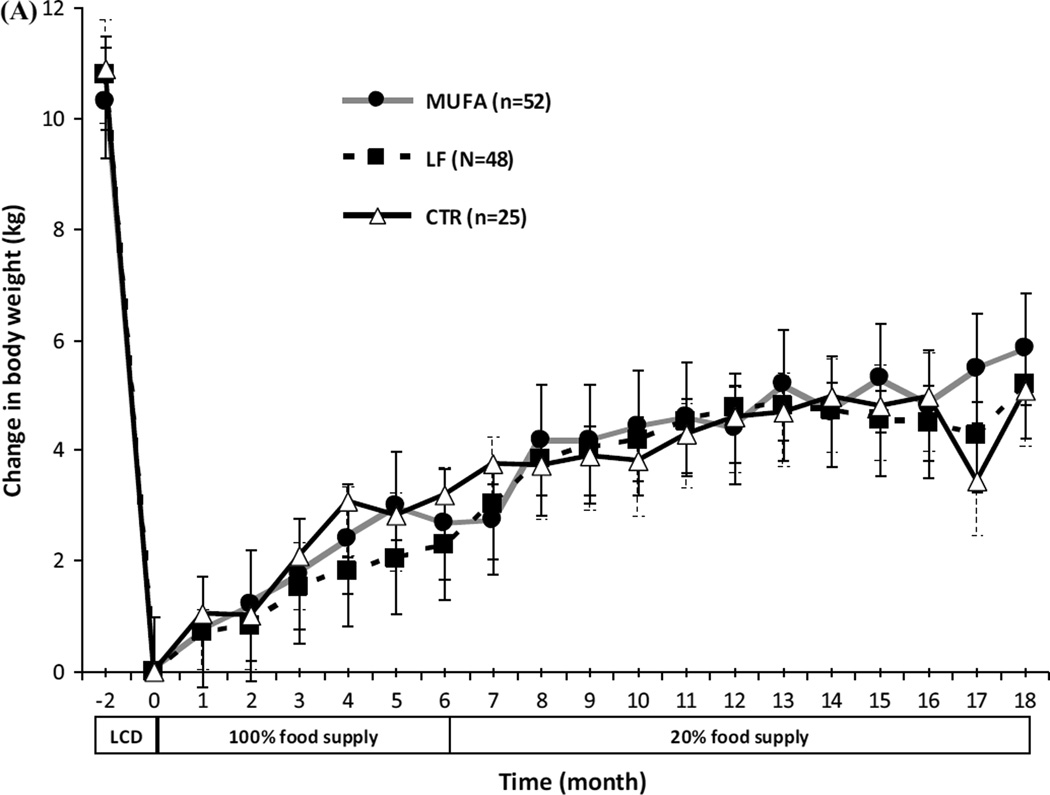
fat Body weight and body composition before weight loss, at baseline, and after 18-month dietary intervention for completers are shown in Table 1. The mean weight loss during the LCD was similar among groups: 10.7 ± 0.8 %, 12.0 ± 0.8 %, and 12.1 ± 1.1 % in the MUFA, LF, and CTR groups, respectively (P = 0.490). Following the weight loss, weight regain in completers was not different between groups, MUFA 7.1 ± 2.1 %, LF 5.6 ± 1.3 %, and CTR 7.2 ± 1.5 % (P = 0.568). Moreover, no difference was seen in body fat regain, MUFA 4.8 ± 1.0 %, LF 4.7 ± 0.8 %, and CTR 5.7 ± 0.6 % (P = 0.776) after 18 months. Twenty per cent of completing subjects (15, 31, and 13 % in the MUFA, LF, and CTR groups, respectively) maintained ≥5 % of the achieved weight loss, but no differences between groups were found (P = 0.249). The ITT analysis including all 125 subjects showed no differences among groups in changes in weight regain during the 18-month intervention (Fig. 2).
Table 1.
Body composition and cardiovascular risk factors of the 18-month completers in the MUFA, LF, and CTR groups before LCD, at baseline (month 0), and after 18-month weight maintenance
| MUFA (n = 20) | LF (n = 32) | CTR (n = 15) | P value | |
|---|---|---|---|---|
| Age (year)* | 28.9 (26.6–31.1) | 27.6 (25.9–29.3) | 26.9 (24.0–29.9) | 0.493¤ |
| Gender (M/F)* | 7/13 | 15/17 | 8/7 | 0.529¤ |
| Height (m)* | 1.72 (1.68–1.77) | 1.76 (1.72–1.79) | 1.74 (1.69–1.78) | 0.506¤ |
| Body weight* | 91.7 (84.9–98.5) | 97.6 (93.1–102.0) | 93.3 (86.0–100.5) | 0.264¤ |
| PAL* | 1.73 (1.66–1.79) | 1.68 (1.62–1.73) | 1.64 (1.56–1.72) | 0.252¤ |
| Weight loss during LCD (kg) | −9.9 (−11.6 to −8.1) | −11.9 (−13.7 to −10.1) | −11.5 (−14.4 to −8.7) | 0.302¤ |
| Body weight (kg)§ | ||||
| Month 0 | 81.9 (75.9–87.8) | 85.7 (82.1–89.3) | 81.7 (76.3–87.1) | 0.351¤ |
| Month 18 | 87.9 (79.8–96.1) | 90.4 (86.1–94.7) | 87.9 (80.4–95.3) | |
| Δ month 18–0 | 6.1 (2.2–9.9) | 4.7 (2.6–6.9) | 6.1 (3.3–9.0) | 0.612# |
| Δ month 18–0 (%) | 7.1 (2.6–11.5) | 5.6 (3.0–8.1) | 7.2 (4.1–10.3) | 0.708# |
| BMI (kg/m2) | ||||
| Month 0 | 27.3 (26.4–28.6) | 27.7 (26.9–28.6) | 27.1 (26.1–28.1) | 0.523¤ |
| Month 18 | 29.0 (27.4–30.7) | 29.4 (28.4–30.5) | 29.0 (27.6–30.5) | |
| Δ month 18–0 | 1.7 (0.7–2.8) | 1.7 (1.0–2.4) | 2.0 (1.1–2.8) | 0.903# |
| Total fat mass (kg)§ | ||||
| Month 0 | 24.5 (20.9–28.2) | 26.1 (23.1–29.2) | 23.5 (19.4–27.5) | 0.549¤ |
| Month 18 | 30.9 (25.9–35.9) | 32.0 (28.7–35.3) | 30.3 (26.2–34.4) | |
| Δ month 18–0 | 6.4 (3.7–9.1) | 5.9 (3.9–7.9) | 6.8 (5.1–8.6) | 0.816# |
| Total lean mass (kg)§ | ||||
| Month 0 | 54.2 (47.2–61.3) | 56.3 (52.2–60.4) | 55.2 (48.7–61.6) | 0.849¤ |
| Month 18 | 53.9 (46.4–61.3) | 55.1 (51.3–58.9) | 54.5 (47.4–61.5) | |
| Δ month 18–0 | −0.4 (−2.6 to 1.8) | −1.2 (−2.5 to 0.2) | −0.7 (−2.3 to 0.9) | 0.425# |
| Waist (cm) | ||||
| Month 0 | 92.3 (89.5–95.0) | 93.8 (91.1–96.6) | 91.6 (87.2–96.0) | 0.550¤ |
| Month 18 | 99.6 (94.1–105.1) | 100.5 (96.9–104.0) | 98.5 (93.1–103.9) | |
| Δ month 18–0 | 7.4 (3.5–11.2) | 6.6 (4.6–8.6) | 6.9 (2.9–10.9) | 0.847# |
| Hip (cm) | ||||
| Month 0 | 107.3 (104.9–110.6) | 108.8 (106.4–111.3) | 106.2 (102.4–110.0) | 0.454¤ |
| Month 18 | 110.8 (106.5–115.1) | 112.2 (109.2–115.2) | 110.1 (106.5–113.8) | |
| Δ month 18–0 | 3.5 (0.7–6.4) | 3.3 (1.8–4.8) | 3.9 (0.7–7.1) | 0.799# |
| Fasting glucose (mmol/L) | ||||
| Month 0 | 4.96 (4.8–5.1) | 4.79 (4.7–4.9) | 4.68 (4.4–5.0) | 0.126¤ |
| Month 18 | 5.36 (5.2–5.6) | 5.4 (5.0–5.3) | 5.16 (5.0–5.4) | |
| Δ month 18–0 | 0.40 (0.2–0.6) | 0.34 (0.2–0.4) | 0.48 (0.2–0.7) | 0.152# |
| Fasting insulin (pmol/L) | ||||
| Month 0 | 36.8 (29.4–44.1) | 42.5 (36.6–48.5) | 42.8 (30.2–55.4) | 0.471¤ |
| Month 18 | 45.7 (31.7–59.7) | 46.1 (37.5–54.8) | 47.9 (34.3–61.5) | |
| Δ month 18–0 | 8.9 (−4.4 to 22.3) | 3.8 (−4.1 to 11.6) | 8.0 (–1.9 to 17.8) | 0.363# |
| HOMA-IR | ||||
| Month 0 | 1.14 (0.9–1.4) | 1.27 (1.1–1.4) | 1.27 (0.9–1.7) | 0.707¤ |
| Month 18 | 1.56 (1.0–2.1) | 1.47 (1.2–1.8) | 1.52 (1.1–2.0) | |
| Δ month 18–0 | 0.42 (−0.1 to 0.9) | 0.22 (−0.05 to 0.5) | 0.35 (0.01–0.7) | 0.363# |
| Total cholesterol (mmol/L) | ||||
| Month 0 | 4.59 (4.3–4.9) | 4.64 (4.3–5.0) | 4.39 (4.0–4.8) | 0.614¤ |
| Month 18 | 4.66 (4.3–5.1) | 4.76 (4.4–5.1) | 4.79 (4.2–5.3) | |
| Δ month 18–0 | 0.07 (−0.3 to 0.4) | 0.11 (−0.1 to 0.3) | 0.40 (0.1–0.7) | 0.457# |
| LDL cholesterol (mmol/L) | ||||
| Month 0 | 2.86 (2.6–3.1) | 2.88 (2.6–3.2) | 2.67 (2.3–3.1) | 0.663¤ |
| Month 18 | 2.67 (2.4–3.0) | 2.84 (2.5–3.2) | 2.85 (2.3–3.4) | |
| Δ month 18–0 | −0.19 (−0.5 to 0.1) | −0.07 (−0.3 to 0.2) | 0.17 (−0.1 to 0.5) | 0.230# |
| HDL cholesterol (mmol/L) | ||||
| Month 0 | 1.24 (1.1–1.3) | 1.22 (1.1–1.3) | 1.20 (1.1–1.3) | 0.935¤ |
| Month 18 | 1.45 (1.3–1.6) | 1.36 (1.2–1.5) | 1.30 (1.1–1.5) | |
| Δ month 18–0 | 0.21 (0.1–0.3) | 0.16 (0.1–0.3) | 0.10 (−0.01 to 0.2) | 0.104# |
| LDL/HDL ratio | ||||
| Month 0 | 2.40 (2.1–2.7) | 2.50 (2.2–2.9) | 2.39 (1.8–2.9) | 0.882¤ |
| Month 18 | 1.93 (1.6–2.2) | 2.31 (1.9–2.7) | 2.45 (1.7–3.2) | |
| Δ month 18–0 | −0.47 (−0.7 to −0.3) | −0.23 (−0.5 to −0.01) | 0.06 (−0.2 to 0.3) | 0.012#a |
| Triacylglycerides (mmol/L) | ||||
| Month 0 | 1.09 (0.9–1.3) | 1.19 (1.0–1.4) | 1.13 (0.9–1.4) | 0.761¤ |
| Month 18 | 1.18 (0.9–1.5) | 1.22 (1.0–1.4) | 1.41 (1.0–1.8) | |
| Δ month 18–0 | 0.09 (−0.1 to 0.3) | 0.03 (−0.1 to 0.2) | 0.28 (0.003–0.5) | 0.526# |
| Hs-CRP (mg/L) | ||||
| Month 0 | 2.33 (1.4–3.2) | 2.88 (1.9–3.9) | 3.42 (1.8–5.0) | 0.476¤ |
| Month 18 | 2.30 (1.1–3.5) | 2.02 (1.5–2.6) | 2.14 (1.0–3.3) | |
| Δ month 18–0 | −0.08 (−1.2 to 1.0) | −0.64 (−1.5 to 0.2) | −1.28 (−2.8 to 0.3) | 0.466# |
All values are mean (95 % CI). LCD low-calorie diet, PAL physical activity level. HOMA-IR: fasting glucose (mmol/L) times fasting insulin (mU/L) divided by 22.5. LDL = Total cholesterol-HDL-(triacylglyceride/2.2)
Measurements were taken at screening before weight loss
Based on DEXA
Test for difference between groups by one-way ANOVA
Test for difference in changes (month 18–month 0) between groups by GLM univariate ANOVA with month 0 as covariates
Post hoc analyses showed differences in a changes in LDL/HDL ratio: MUFA versus LF (P = 0.041), MUFA versus CTR (P = 0.005)
Fig. 2.
Changes in body weight by intention to treat (ITT) during 18 months controlled dietary intervention with 100 % food supply (month 0–6) and 20 % food supply (month 6–18) from supermarket. No diet*time interaction was seen for either ITT (n = 125) or completer analyses (n = 67) for changes in body weight. The number of participants was 52/48/25 at month 0, 39/43/24 at month 6, and 20/32/15 at month 18 for MUFA, LF, and CTR, respectively
CVD risk factors
No differences in fasting measurements of CVD risk factors before or after the dietary intervention were seen after 18 months of intervention, except for an overall difference in the LDL/HDL ratio among groups (P = 0.012) (Table 1). Post hoc analyses showed that the reduction of −0.47 ± 0.09 in the MUFA group was significantly improved compared with −0.23 ± 0.11 in LF (P < 0.05), and an increase of 0.06 ± 0.14 (P < 0.005) in CTR group.
Dietary intake
Total dietary intake (foods from supermarket and non-supermarket) during the first 6 months strictly controlled dietary intervention period corresponded to the prescribed diets for all three groups (Table 2). During the following 12 months only 20 % of the estimated dietary needs were provided from the supermarket. For the MUFA group the primary food supply consisted of 43 % nuts (primarily almonds, hazel, peanuts, and walnuts) and legumes (red beans, chick pies, and lentils), 28 % vegetable oils (olive and rape seed), and 24 % whole grains (whole grain bread, brown rice, whole-wheat pasta). For the LF group the primary food supply consisted of 59 % red meat (beef and pork) and 22 % whole grains, whereas the primary food groups given to the CTR group consisted of 54 % fatty red meat and butter, 21 % white rice and sweets, and 14 % dairy products (Table 3).
Table 2.
Prescribed and actual dietary intake during 6-month strictly controlled dietary intervention for the 18-month completers in the MUFA, LF, and CTR groups
| Prescribed dietary composition (range) | Actual dietary composition# | P value¤ | |||||
|---|---|---|---|---|---|---|---|
| MUFA | LF | CTR | MUFA (n = 20) | LF (n = 32) | CTR (n = 15) | ||
| Energy intake pr day, MJ/d | Ad libitum | Ad libitum | Ad libitum | 10.9 (10.1–11.8) | 10.6 (9.7–11.6) | 11.4 (9.7–13.1) | 0.616 |
| Energy density, kJ/g | High | Low | High | 4.8 (4.5–5.1) | 3.7 (3.6–3.9) | 4.4 (4.1–4.8) | <0.001a |
| Total Fat, E%* | 40 (35–45) | 25 (20–30) | 35 (30–40) | 38.0 (37.2–38.8) | 23.6 (23.0–24.2) | 32.2 (31.4–33.0) | <0.001 |
| SFA, E%* | <10 | <10 | >15 | 7.2 (6.8–7.6) | 8.0 (7.6–8.4) | 14.9 (14.4–15.4) | <0.001 |
| MUFA, E%* | >20 | 10 (5–15) | 10 (5–15) | 19.9 (19.3–20.5) | 8.4 (8.1–8.7) | 10.5 (10.1–10.9) | <0.001 |
| PUFA, E%* | 5–10 | 5–10 | 0–10 | 7.7 (7.4–8.0) | 5.2 (5.0–5.3) | 4.2 (3.8–4.5) | <0.001 |
| Carbohydrate + fibre, E%* | 45 (40–50) | 60 (55–65) | 50 (45–55) | 43.7 (42.9–44.4) | 57.2 (56.5–57.9) | 49.5 (48.7–50.2) | <0.001 |
| Fibre, g/MJ | >3 | >3 | <3 | 4.2 (4.0–4.4) | 4.0 (3.7–4.2) | 2.8 (2.6–3.0) | <0.001b |
| Added sugar, E% * | <10 | <10 | 5–15 | 5.7 (4.8–6.6) | 7.5 (6.6–8.4) | 9.8 (8.7–10.9) | <0.001 |
| Protein, E%* | 15 (10–20) | 15 (10–20) | 15 (10–20) | 15.3 (14.8–15.9) | 15.9 (15.5–16.3) | 15.6 (14.9–16.3) | 0.192 |
| Alcohol, E% | <5 | <5 | <5 | 2.6 (1.7–3.5) | 2.9 (2.1–3.7) | 2.6 (1.4–3.7) | 0.840 |
| Shopping days | 180 (150–180) | 180 (150–180) | 180 (150–180) | 152 (144–159) | 154 (145–163) | 157 (144–171) | 0.793 |
All values are mean (95 % CI)
SFA saturated fatty acids, MUFA mono-unsaturated fatty acids, PUFA polyunsaturated fatty acids
Variables to be fulfilled before ending each shopping session in the 6-month total food supply from supermarket
Test for difference in actual dietary composition between groups by one-way ANOVA with diet group as fixed factor. Pairwise analyses showed differences in a energy density: LF versus MUFA (P < 0.001), LF versus CTR (P < 0.05) and MUFA versus CTR (P = 0.063), and in b fibre intake CTR versus MUFA (P < 0.001) and CTR versus LF (P < 0.001)
Table 3.
Characteristic food groups in energy percentage provided for each diet group during 6- to 18-month intervention
| MUFA (n = 20) | LF (n = 31) | CTR (n = 15) | P value¤ | |
|---|---|---|---|---|
| Total energy provided (MJ/d) | 1.9 ± 0.1 | 2.0 ± 0.1 | 2.0 ± 0.1 | 0.61 |
| Vegetable oils E% | 28.0 ± 1.7a | 2.0 ± 0.3b | 2.8 ± 0.4b | <0.001 |
| Whole grains E% | 23.7 ± 1.7b | 22.0 ± 1.7b | 6.2 ± 0.8a | <0.001 |
| Fruits E% | 0.8 ± 0.3 | 0.5 ± 0.1 | 0.7 ± 0.5 | <0.34 |
| Vegetables E% | 1.2 ± 0.2a* | 0.7 ± 0.2b* | 0.4 ± 0.1b* | <0.03 |
| Nuts & legumes E% | 42.5 ± 1.4a | 1.0 ± 2.0b | 0.4 ± 0.2b | <0.001 |
| Fish poultry and eggs E% | 0.1 ± 0.0b | 0.7 ± 0.2a | 0.1 ± 0.0b | 0.001 |
| Dairy products E% | 0.1 ± 0.0a | 6.8 ± 0.5b | 13.9 ± 0.9c | <0.001 |
| White rice, sweets etc. E%* | 1.1 ± 0.4a | 7.6 ± 0.4b | 21.0 ± 1.9c | <0.001 |
| Red meat & butter E%# | 2.7 ± 0.6a | 58.8 ± 1.9b | 54.4 ± 1.8b | <0.001 |
| Total (E%) | 100 | 100 | 100 |
All values are mean (±SEM) during study month 6–18 shopping with 20 % of total estimated energy requirements from supermarket
Only red meat for the LF group
Test for difference between groups by one-way ANOVA
Different letters indicate significant difference between groups by post hoc analyses (P < 0.001).
(P < 0.05)
Dietary compliance
Good correlations between dietary fat and changes in fatty acid composition in the buttock were found after the first 6 months controlled intervention with 100 % food supply [10]. After the subsequent 12 months of less strict intervention the MUFA diet increased the concentration of oleic acid in the buttock compared to both the LF and CTR diet and increased concentration of polyunsaturated fatty acids as compared to the CTR diet. The LF and CTR diet produced greater increases of myristic acid, stearic acid, and total saturated fat than the MUFA diet (Table 4).
Table 4.
Dietary compliance assessed by fat biopsy in percentage before LCD and after 18-month dietary intervention
| MUFA (n = 19) | LF (n = 30) | CTR (n = 14) | P value | |
|---|---|---|---|---|
| % of total g. | ||||
| Myristic 14:0 | ||||
| Before LCD | 2.5 (2.3–2.7) | 2.5 (2.3–2.6) | 2.6 (2.4–2.7) | 0.518¤ |
| Month 18 | 2.4 (2.2–2.6)a | 2.7 (2.5–2.8)b* | 2.7 (2.4–3.0)b** | 0.004# |
| Stearic 18:0 | ||||
| Before LCD | 2.7 (2.4–3.0) | 2.5 (2.2–2.7) | 2.6 (2.2–2.9) | 0.638¤ |
| Month 18 | 2.9 (2.5–3.3)b* | 3.2 (2.8–3.6)a | 2.9 (2.5–3.3)b* | 0.017# |
| Total SFA | ||||
| Before LCD | 26.8 (25.7–28.0) | 26.1 (25.2–27.0) | 26.6 (25.3–27.9) | 0.793¤ |
| Month 18 | 26.9 (25.5–28.3)a** | 28.0 (26.9–29.2)b | 27.6 (25.7–29.5) | 0.011# |
| Oleic 18:1 n−9 | ||||
| Before LCD | 45.3 (44.6–46.1) | 45.4 (44.9–46.0) | 45.3 (44.3–46.3) | 0.801¤ |
| Month 18 | 45.9 (44.6–47.3)a* | 45.1 (44.5–45.7)b | 45.0 (43.8–46.3) | 0.071# |
| Total MUFA | ||||
| Before LCD | 56.8 (55.8–57.8) | 57.5 (56.5–58.4) | 57.2 (55.8–58.5) | 0.799¤ |
| Month 18 | 56.2 (54.8–57.6)a** | 55.5 (54.3–56.7)b | 55.6 (53.7–57.5) | 0.044# |
| Linoleic 18:2 n−6 | ||||
| Before LCD | 10.8 (10.4–11.2) | 10.9 (10.5–11.2) | 10.9 (10.3–11.5) | 0.758¤ |
| Month 18 | 11.6 (10.8–12.4)a** | 11.1 (10.7–11.6) | 11.2 (9.9–12.6)b | 0.052# |
| Total PUFA | ||||
| Before LCD | 12.8 (12.4–13.2) | 12.9 (12.5–13.3) | 12.9 (12.3–13.4) | 0.943¤ |
| Month 18 | 13.7 (12.8–14.5)a** | 13.2 (12.6–13.8) | 13.2 (12.9–13.8)b | 0.029# |
All values are mean (95 % CI)
LCD low-calorie diet
Test for difference between groups before dietary intervention by one-way ANOVA
Test for difference in changes (month 18, before LCD) between groups after dietary intervention by GLM univariate ANOVA with before LCD values as covariates
Different letters indicate significant difference between groups by post hoc analyses:
(P < 0.05),
(P < 0.001)
Correlation between regain in body fat and changes in fatty acids in the fat biopsies during month 6–18 showed a positive association between fat gain and increase in saturated fatty acids (r = 0.472, P < 0.001) and a negative correlation between fat regain and monounsaturated fatty acids (r = −0.412, P = 0.002) when groups were pooled.
The overall self-rated dietary adherence level (assessed by questionnaires ranking from 1 to 5) was significantly greater in the CTR group compared to the MUFA (P = 0.001) and LF (P < 0.001) groups during study months 6–18 (Table 5). This was confirmed by the dieticians also estimating a significantly greater compliance in the CTR group compared to both the LF and MUFA groups (P = 0.002). For the MUFA group a negative correlation was seen in BW regain and the self-reported high intake of monounsaturated fat (r = −0.703, P < 0.001), low intake of sugar (r = −0.479, P < 0.033), and high intake of legumes (r = −0.587, P < 0.006), suggesting that the more they were able to adhere to these prescriptions, the lower the weight gain. Similarly for the LF group a negative correlation was seen in BF regain and the self-reported low intake of fat (r = −0.506, P < 0.004) and low intake of sugar (r = −0.361, P < 0.046), again suggesting that the more they were able to adhere to these prescriptions, the lower the weight gain. A significantly greater satisfaction with the diet was seen in the LF group, whereas the lowest satisfaction was seen in the CTR group (Table 5).
Table 5.
Self-reported adherence to the MUFA, LF, and CTR diet based on a scale from 1 (very bad) to 5 (very good) during study month 6–18
| Question | MUFA (n = 20) | LF (n = 32) | CTR (n = 15) | P value¤ |
|---|---|---|---|---|
| Overall dietary adherence (dietary principles) | 3.41 (± 0.12)b | 3.43 (± 0.11)b | 4.10 (± 0.14)a** | 0.001 |
| Satisfaction with diet allocation | 4.06 (± 0.15)a** | 4.49 (± 0.09)b** | 3.43 (± 0.24)c** | <0.001 |
| Dietary compliance assessed by dietician | 3.44 (± 0.13)b | 3.50 (± 0.10)b | 4.10 (± 0.15)a** | 0.003 |
All values are mean (± SEM)
Test for difference between groups by one-way ANOVA
Different letters indicate significant difference between groups by post hoc analyses:
(P < 0.05),
(P < 0.001)
Discussion
In this 18-month dietary intervention study, we found that all three diets were equally effective in maintaining an average weight loss of 5 % subsequent to 8 weeks LCD. The fact that the tested diets did not differ in terms of weight regains corresponds to our previous finding after 6-month strictly controlled intervention [10] and other comparable trials [13–20]. A few other studies have found favourable effects of the Mediterranean-style diet on body weight [21–23]. The trial by McManus et al. [21–23] is the most comparable to the present trial with regard to dietary composition, length of study, study population, number of subjects, and drop-out. They found a similar weight loss of 5 % in both the low-fat group (20 E%) and the moderate-fat group (35 E%) after 6-month intervention. However, after 18 months the moderate-fat group maintained a 4 kg weight loss, whereas a 3 kg increase was seen in the low-fat group, leading to a 7 kg difference in weight between groups [22]. Furthermore, greater reductions in per cent body fat and waist circumference were seen in the moderate-fat group [22]. In the study by Shai et al. [23] the Mediterranean diet caused a significantly greater weight loss of 1.5 kg after 2 years compared to the low-fat diet. The study by both McManus et al. [22] and Shai et al. [23] was hypocaloric with a daily energy restriction of 1200 and 1500 kcal for women and 1500 and 1800 kcal for men, respectively, and not ad libitum and therefore not directly comparable to the free living conditions used in the present trial.
It has been estimated that a 10 % decrease in total energy from fat can reduce body weight by 16 g/day, which can be extrapolated to weight reduction of approximately 9 kg over 18 months [24]. In the present trial twice as many participants on the LF diet maintained a weight loss of 5 % after 18-month intervention compared to the MUFA and CTR diets, however, this difference was not significant. Also, the superior effect of the LF diet in prevention of body fat regain after 6 month (10) was no longer sustained after 18 months.
One of the most desirable features of the Mediterranean diet, relative to traditional low-fat diets, is its ability to improve CVD risk [25–32]. In the present trial the MUFA diet was associated with an improvement in the LDL/HDL ratio compared to both the LF and CTR after 18 months. Despite a weight gain of 7 %, a 7 % reduction in LDL and a 17 % increase in HDL were seen in the MUFA group, suggesting that a Mediterranean-style diet has favourable effects on CVD risk factors regardless of the occurrence of a weight regain. This favourable effect could also be explained by the MUFA diet being lower in GI and GL compared to both the LF and CTR diets. Large epidemiological studies have found diets high in GL to be correlated with decreases in HDL [33] and increases in risk of CVD [34], and it is suggested that GI/GL of the diet is a very strong predictor of HDL concentration and risk of CVD [35–37]. Another 6-month dietary intervention has also found a reduction in both HDL and the LDL/HDL ratio in a diet low in GI compared to a diet high in GI in overweight type 2 diabetics [38]. In another intervention study of 8 weeks duration, a Mediterranean diet also led to an increase in HDL cholesterol and reduction in total cholesterol, LDL cholesterol, and total cholesterol/HDL cholesterol ratio compared to a westernized diet in abdominally obese subjects [39]. In a 4-week weight loss trial followed by 6-week maintenance, a LF diet leads to a significant 12 % decrease in HDL, whereas no changes were seen in the Mediterranean diet [18]. There is convincing evidence that exchanging dietary saturated fat with monounsaturated fat produces favourable effects on CVD risk factors, i.e. it lowers LDL cholesterol [40], improves postprandial lipid profile [41], and lowers blood pressure [13]. However, this was not confirmed in a German sample with coronary artery disease [16] or in the present study, probably due to the normal blood lipids of our young healthy participants and due to the antecedent weight loss that may have diluted dietary effects. Insulin resistance and type 2 diabetes are also linked to excess energy intake, particularly in the form of saturated fatty acids and simple sugars [42]. We have previously shown an improvement in fasting insulin and insulin resistance on a MUFA diet compared to both a LF and the western diets after 6 months [10]. However, this was not seen after 18 months.
The larger drop-out we found in the MUFA group remains unexplained but may be ascribed to the fact that a Mediterranean diet is less commonly eaten in a Nordic population. However, in a social and cultural acceptability study of the trial [43], all three diets were equally high in “liking of diet”, “social eating events”, and “practical matters related to eating, cooking, and shopping”. Despite this, both the MUFA and LF responders faced stronger barriers to accepting the diets when eating out compared to the CTR responders. And also the MUFA by comparison with CTR responders was more deprived of the foods that they liked and had faced more difficulties integrating the diet with the eating habits of the families [43]. Altogether these factors are likely to have impaired full adherence to the MUFA diet and leading to a greater drop-out.
Dansinger et al. [44] compared four popular diets and found that adherence to the diet was a more important factor in achieving greater weight loss and reducing CVD risk factors than the type of diet over a 1-year period. This also corresponds to a recent meta-analysis, concluding that the weight loss differences between low-carbohydrate and low-fat diets are small supporting the practice of recommending any diet that a patient will adhere to in order to lose weight [4]. In many trials dietary compliance is only assessed by 3- to 7-day weighed food records and various questionnaires and thus the accuracy of the data therefore very much depends on the reliability of the dietary reporting of the participants. The present study is unique in the use of the supermarket model and the three additional methods to assess dietary compliance. The fatty acid composition in the fat biopsies was well correlated with recent dietary fat intake monitored in the supermarket [10], and the participants’ self-reported compliance corresponded with the compliance estimated by the dieticians. In the present trial much effort was taken to give the same amount of attention and treatment to all three groups, whereas in other trials the number of visits and amount of attention (including behavioural and psychological counselling) given to the groups or study personal were different [8, 22, 23]. Whether a positive effect of one diet is attributable to the dietary intervention or the difference in attention given to the various groups can be difficult to conclude.
In conclusion, we found that based on around 50 % completers all three diets were equally effective in maintaining a weight loss and in prevention of body fat regain over 18 months. Nor were there any differences in effects on diabetes or CVD risk factors, with the exception of improvement in the LDL/HDL ratio in the MUFA diet compared to the CTR diet. Despite continuous dietary counselling, only 20 % of study participants were capable of maintaining a weight loss of >5 %. The LF diet was generally more satisfactory and this Nordic population seemed to find it more difficult to adhere to the MUFA diet. This study confirms that the success in weight maintenance very much depends on dietary adherence. Scientific evidence supporting the assumption that one diet fits all is scarce and more individually addressed dietary approaches need to be investigated. Obesity is a multifactorial disorder and it takes more than a dietary intervention to achieve successful weight maintenance. General well-being, level of stress, sleep quality, and food preferences could be other relevant factors to include in future interventions.
Supplementary Material
Acknowledgments
The authors wish to thank the project staff of the Department of Human Nutrition. Special thanks to Professor Walter Willett, Department of Epidemiology and Nutrition, Harvard School of Public Health, Boston, Massachusetts, USA, and David Ludwig MD, PhD, Department of Medicine, Children’s Hospital, Boston, Massachusetts, USA, for participating in the design of the project and Miguel Padilla, PhD, Department of Biostatistics, University of Alabama at Birmingham, USA, for the ITT analysis.
Funding This research was supported by foundations, associations, and research councils (e.g. H.A. Foundation, The Danish Heart Association, The Danish Diabetes Association, Centre for Advanced Food Research, The state Research Councils, The Danish Pork Council foundations). Effort for David B Allison was supported by P3ODK056336. Most of the foods in the supermarket (80 %) and the low-calorie diets were sponsored by the food industry. No sponsor participated in the analysis or interpretation of the data, manuscript preparation, review, or approval, or the decision to publish.
Footnotes
Author contribution Due had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Astrup, Larsen, Toubro, and Due are involved in study concept and design. Due and Larsen are involved in acquisition of data. Due, Larsen, Mu, Hermansen, Stender, Toubro, Allison, and Astrup analysed and interpreted the data. Due drafted the manuscript. Due, Larsen, Mu, Hermansen, Stender, Toubro, Allison, and Astrup contributed to the critical revision of the manuscript for important intellectual content. Due and Allison are involved in statistical analysis. Astrup, Larsen and Due obtained funding. Due, Larsen, Mu, Hermansen, Stender, and Astrup provided administrative, technical, or material support. Astrup supervised the study. All listed authors have contributed significantly to the manuscript and given their consent for publication. None of the listed authors have conflicting interests that might affect the outcome of this research.
Compliance with ethical standards
Conflict of interest On behalf of all authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material The online version of this article (doi:10.1007/s00394-015-1116-6) contains supplementary material, which is available to authorized users.
References
- 1.Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001;74:579–584. doi: 10.1093/ajcn/74.5.579. [DOI] [PubMed] [Google Scholar]
- 2.Douketis JD, Macie C, Thabane L, Williamson DF. Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes (Lond) 2005;29:1153–1167. doi: 10.1038/sj.ijo.0802982. [DOI] [PubMed] [Google Scholar]
- 3.Nordic Council oM. Nordic nutrition recommendations 2004. Integrating nutrition and physical activity, Copenhagen. 2005 [Google Scholar]
- 4.Johnston B, Kanters S, Bandayrel K, Wu P, Naji F, Siemieniuk R, Ball GDC, Busse J, Thorlund K, Guyatt G, Jansen J, Mills E. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA J Am Med Assoc. 2014;312:923–933. doi: 10.1001/jama.2014.10397. [DOI] [PubMed] [Google Scholar]
- 5.Hu T, Bazzano LA. The low-carbohydrate diet and cardiovascular risk factors: evidence from epidemiologic studies. NMCD Nutr Metab Cardiovasc Dis. 2014;24:337–343. doi: 10.1016/j.numecd.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu T, Mills K, Yao L, Demanelis K, Eloustaz M, Yancy W, Kelly T, He J, Bazzano L. Effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors: a meta-analysis of randomized controlled clinical trials. Am J Epidemiol. 2012;176(Suppl 7):S44–S54. doi: 10.1093/aje/kws264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estruch R, Ros E, Salas Salvadó J, Covas M, Corella D, Arós F, Gómez Gracia E, Ruiz Gutiérrez V, Fiol M, Lapetra J, Lamuela Raventos R, Serra Majem L, Pintó X, Basora J, Muñoz M, Sorlí J, Martínez J, Martínez González M. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 8.Nordmann A, Suter Zimmermann K, Bucher H, Shai I, Tuttle K, Estruch R, Briel M. Meta-analysis comparing Mediterranean to low-fat diets for modification of cardiovascular risk factors. Am J Med. 2011;124(841–51):e2. doi: 10.1016/j.amjmed.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 9.Due A, Larsen TM, Hermansen K, Stender S, Holst JJ, Toubro S, Martinussen T, Astrup A. Comparison of the effects on insulin resistance and glucose tolerance of 6-mo high-monounsaturated- fat, low-fat, and control diets. Am J Clin Nutr. 2008;87:855–862. doi: 10.1093/ajcn/87.4.855. [DOI] [PubMed] [Google Scholar]
- 10.Due A, Larsen TM, Mu H, Hermansen K, Stender S, Astrup A. Comparison of 3 ad libitum diets for weight-loss maintenance, risk of cardiovascular disease, and diabetes: a 6-mo randomized, controlled trial. Am J Clin Nutr. 2008;88:1232–1241. doi: 10.3945/ajcn.2007.25695. [DOI] [PubMed] [Google Scholar]
- 11.Skov AR, Toubro S, Raben A, Astrup A. A method to achieve control of dietary macronutrient composition in ad libitum diets consumed by free-living subjects. Eur J Clin Nutr. 1997;51:667–672. doi: 10.1038/sj.ejcn.1600459. [DOI] [PubMed] [Google Scholar]
- 12.Gadbury GL, Coffey CS, Allison DB. Modern statistical methods for handling missing repeated measurements in obesity trial data: beyond LOCF. Obes Rev. 2003;4:175–184. doi: 10.1046/j.1467-789x.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 13.Estruch R, Martinez-Gonzalez MA, Corella D, Salas-Salvado J, Ruiz-Gutierrez V, Covas MI, Fiol M, Gomez-Gracia E, Lopez-Sabater MC, Vinyoles E, Aros F, Conde M, Lahoz C, Lapetra J, Saez G, Ros E. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez de la Puebla RA, Fuentes F, Perez-Martinez P, Sanchez E, Paniagua JA, Lopez-Miranda J, Perez-Jimenez F. A reduction in dietary saturated fat decreases body fat content in overweight, hypercholesterolemic males. Nutr Metab Cardiovasc Dis. 2003;13:273–277. doi: 10.1016/s0939-4753(03)80031-5. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin T, Carter S, Lamendola C, Abbasi F, Schaaf P, Basina M, Reaven G. Clinical efficacy of two hypocaloric diets that vary in overweight patients with type 2 diabetes: comparison of moderate fat versus carbohydrate reductions. Diabetes Care. 2007;30:1877–1879. doi: 10.2337/dc07-0301. [DOI] [PubMed] [Google Scholar]
- 16.Michalsen A, Lehmann N, Pithan C, Knoblauch NT, Moebus S, Kannenberg F, Binder L, Budde T, Dobos GJ. Mediterranean diet has no effect on markers of inflammation and metabolic risk factors in patients with coronary artery disease. Eur J Clin Nutr. 2006;60:478–485. doi: 10.1038/sj.ejcn.1602340. [DOI] [PubMed] [Google Scholar]
- 17.Paniagua JA, Gallego DL, Romero I, Vidal-Puig A, Latre JM, Sanchez E, Perez-Martinez P, Lopez-Miranda J, Perez-Jimenez F. Monounsaturated fat-rich diet prevents central body fat distribution and decreases postprandial adiponectin expression induced by a carbohydrate-rich diet in insulin-resistant subjects. Diabetes Care. 2007;30:1717–1723. doi: 10.2337/dc06-2220. [DOI] [PubMed] [Google Scholar]
- 18.Pelkman CL, Fishell VK, Maddox DH, Pearson TA, Mauger DT, Kris-Etherton PM. Effects of moderate-fat (from monounsaturated fat) and low-fat weight-loss diets on the serum lipid profile in overweight and obese men and women. Am J Clin Nutr. 2004;79:204–212. doi: 10.1093/ajcn/79.2.204. [DOI] [PubMed] [Google Scholar]
- 19.Petersen M, Taylor MA, Saris WH, Verdich C, Toubro S, Macdonald I, Rossner S, Stich V, Guy-Grand B, Langin D, Martinez JA, Pedersen O, Holst C, Sorensen TI, Astrup A. Randomized, multi-center trial of two hypo-energetic diets in obese subjects: high- versus low-fat content. Int J Obes (Lond) 2006;30:552–560. doi: 10.1038/sj.ijo.0803186. [DOI] [PubMed] [Google Scholar]
- 20.Vincent-Baudry S, Defoort C, Gerber M, Bernard MC, Verger P, Helal O, Portugal H, Planells R, Grolier P, Miot-Carlin MJ, Vague P, Lairon D. The Medi-RIVAGE study: reduction of cardiovascular disease risk factors after a 3-mo intervention with a Mediterranean-type diet or a low-fat diet. Am J Clin Nutr. 2005;82:964–971. doi: 10.1093/ajcn/82.5.964. [DOI] [PubMed] [Google Scholar]
- 21.Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi F. Better dietary adherence and weight maintenance achieved by a long-term moderate-fat diet. Br J Nutr. 2007;97:399–404. doi: 10.1017/S0007114507328602. [DOI] [PubMed] [Google Scholar]
- 22.McManus K, Antinoro L, Sacks F. A randomized controlled trial of a moderate-fat, low-energy diet compared with a low fat, low-energy diet for weight loss in overweight adults. Int J Obes Relat Metab Disord. 2001;25:1503–1511. doi: 10.1038/sj.ijo.0801796. [DOI] [PubMed] [Google Scholar]
- 23.Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H, Tangi-Rozental O, Zuk-Ramot R, Sarusi B, Brickner D, Schwartz Z, Sheiner E, Marko R, Katorza E, Thiery J, Fiedler GM, Bluher M, Stumvoll M, Stampfer MJ. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 24.Bray GA, Popkin BM. Dietary fat intake does affect obesity. Am J Clin Nutr. 1998;68:1157–1173. doi: 10.1093/ajcn/68.6.1157. [DOI] [PubMed] [Google Scholar]
- 25.Michel de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–785. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 26.Trichopoulou A, Bamia C, Trichopoulos D. Mediterranean diet and survival among patients with coronary heart disease in Greece. Arch Intern Med. 2005;165:929–935. doi: 10.1001/archinte.165.8.929. [DOI] [PubMed] [Google Scholar]
- 27.Knoops KT, de Groot LC, Kromhout D, Perrin AE, Moreiras-Varela O, Menotti A, van Staveren WA. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2004;292:1433–1439. doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- 28.Serra-Majem L, Roman B, Estruch R. Scientific evidence of interventions using the Mediterranean diet: a systematic review. Nutr Rev. 2006;64:S27–S47. doi: 10.1111/j.1753-4887.2006.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 29.Esposito K, Marfella R, Ciotola M, Di PC, Giugliano F, Giugliano G, D’Armiento M, D’Andrea F, Giugliano D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 30.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 31.Esposito K, Maiorino MI, Ciotola M, Di PC, Scognamiglio P, Gicchino M, Petrizzo M, Saccomanno F, Beneduce F, Ceriello A, Giugliano D. Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: a randomized trial. Ann Intern Med. 2009;151:306–314. doi: 10.7326/0003-4819-151-5-200909010-00004. [DOI] [PubMed] [Google Scholar]
- 32.Tuttle K, Shuler L, Packard D, Milton J, Daratha K, Bibus D, Short R. Comparison of low-fat versus Mediterranean-style dietary intervention after first myocardial infarction (from The Heart Institute of Spokane Diet Intervention and Evaluation Trial) Am J Cardiol. 2008;101:1523–1530. doi: 10.1016/j.amjcard.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 33.Culberson A, Kafai MR, Ganji V. Glycemic load is associated with HDL cholesterol but not with the other components and prevalence of metabolic syndrome in the third National Health and Nutrition Examination Survey, 1988–1994. Int Arch Med. 2009;2:3. doi: 10.1186/1755-7682-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu S, Manson JE, Stampfer MJ, Holmes MD, Hu FB, Hankinson SE, Willett WC. Dietary glycemic load assessed by food-frequency questionnaire in relation to plasma high-density-lipoprotein cholesterol and fasting plasma triacylglycerols in postmenopausal women. Am J Clin Nutr. 2001;73:560–566. doi: 10.1093/ajcn/73.3.560. [DOI] [PubMed] [Google Scholar]
- 35.Augustin LS, Kendall CW, Jenkins DJ, Willett WC, Astrup A, Barclay AW, Bjorck I, Brand-Miller JC, Brighenti F, Buyken AE, Ceriello A, La Vecchia C, Livesey G, Liu S, Riccardi G, Rizkalla SW, Sievenpiper JL, Trichopoulou A, Wolever TM, Baer-Sinnott S, Poli A. Glycemic index, glycemic load and glycemic response: an International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC) Nutr Metab Cardiovasc Dis. 2015;25:795–815. doi: 10.1016/j.numecd.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Ford ES, Liu S. Glycemic index and serum high-density lipoprotein cholesterol concentration among us adults. Arch Intern Med. 2001;161:572–576. doi: 10.1001/archinte.161.4.572. [DOI] [PubMed] [Google Scholar]
- 37.Frost G, Leeds AA, Dore CJ, Madeiros S, Brading S, Dornhorst A. Glycaemic index as a determinant of serum HDL-cholesterol concentration. Lancet. 1999;353:1045–1048. doi: 10.1016/s0140-6736(98)07164-5. [DOI] [PubMed] [Google Scholar]
- 38.Jenkins DJ, Kendall CW, McKeown-Eyssen G, Josse RG, Silverberg J, Booth GL, Vidgen E, Josse AR, Nguyen TH, Corrigan S, Banach MS, Ares S, Mitchell S, Emam A, Augustin LS, Parker TL, Leiter LA. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: a randomized trial. JAMA. 2008;300:2742–2753. doi: 10.1001/jama.2008.808. [DOI] [PubMed] [Google Scholar]
- 39.Bos MB, de Vries JH, Feskens EJ, van Dijk SJ, Hoelen DW, Siebelink E, Heijligenberg R, de Groot LC. Effect of a high monounsaturated fatty acids diet and a Mediterranean diet on serum lipids and insulin sensitivity in adults with mild abdominal obesity. Nutr Metab Cardiovasc Dis. 2010;20:591–598. doi: 10.1016/j.numecd.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Rivellese AA, Maffettone A, Vessby B, Uusitupa M, Hermansen K, Berglund L, Louheranta A, Meyer BJ, Riccardi G. Effects of dietary saturated, monounsaturated and n-3 fatty acids on fasting lipoproteins, LDL size and post-prandial lipid metabolism in healthy subjects. Atherosclerosis. 2003;167:149–158. doi: 10.1016/s0021-9150(02)00424-0. [DOI] [PubMed] [Google Scholar]
- 41.Thomsen C, Rasmussen O, Lousen T, Holst JJ, Fenselau S, Schrezenmeir J, Hermansen K. Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Am J Clin Nutr. 1999;69:1135–1143. doi: 10.1093/ajcn/69.6.1135. [DOI] [PubMed] [Google Scholar]
- 42.Franz MJ, Bantle JP, Beebe CA, Brunzell JD, Chiasson JL, Garg A, Holzmeister LA, Hoogwerf B, Mayer-Davis E, Mooradian AD, Purnell JQ, Wheeler M. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. 2002;25:148–198. doi: 10.2337/diacare.25.1.148. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen A, Korzen S, Holm L. Inverting the food pyramid? Social and cultural acceptability of Walter Willett’s dietary recommendations among people with weight concerns. Appetite. 2008;51:178–186. doi: 10.1016/j.appet.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293:43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.