Abstract
Reproductive endosymbionts have been shown to have wide-ranging effects on many aspects of their hosts’ biology. A first step to understanding how these endosymbionts interact with their hosts is to determine their incidences. Here, we screened for four reproductive endosymbionts (Wolbachia, Cardinium, Spiroplasma and Rickettsia) in 28 populations of spider mites (Acari: Tetranychidae) representing 12 species. Each of the four endosymbionts were identified in at least some of the tested specimens, and their infection patterns showed variations at the species-level and population-level, suggesting their distributions can be correlated with both the phylogeny and ecology of the hosts. Co-infections of unrelated bacteria, especially double infections of Wolbachia and Cardinium within the same individuals were common. Spiroplasma and Rickettsia infections were specific to particular host species, respectively. Further, the evolutionary histories of these endosymbionts were inferred by comparing the phylogenies of them and their hosts. These findings can help to clarify the interactions between endosymbionts and arthropods.
Symbiotic bacteria are ubiquitous and have profound impacts on their host’s biology1,2,3,4. The interest in the field has come largely from the discovery of Wolbachia, a bacterium that manipulates hosts’ reproduction through cytoplasmic incompatibility (CI), parthenogenesis, male-killing, feminization5 and oogenesis6. Within the last decade, a second symbiotic bacterium, Cardinium, has been found to have reproductive effects, including cytoplasmic incompatibility7,8, parthenogenesis9 and feminization10. Other reproductive endosymbionts have been recorded in the genera Spiroplasma and Rickettsia. Spiroplasma induces male-killing in Drosophila11, butterflies12, ladybird beetles13 and planthoppers14. Rickettsia has been shown to manipulate the reproductive biology of wasps15 and beetles16,17. In addition, many of them also may provide direct fitness benefits to infected individuals, such as protection from pathogens18 or increasing fecundity19 under certain circumstances. These endosymbionts should thus be recognized as important components of arthropod biology.
Wolbachia is widespread in arthropods20 and its distribution is related to host ecology and host biology21,22. Some lineages of Wolbachia (termed supergroups A and B) have spread ubiquitously, while others (e.g., supergroups C and D) are taxon-specific. Cardinium infections are rarer than Wolbachia, and are restricted to Hymenoptera, Hemiptera, Diptera and Acari23,24,25. Double infections of Wolbachia and Cardinium within the same host species have been found8,26,27. The distributions of Spiroplasma and Rickettsia and other reproductive bacteria have been widely investigated in some arthropods28,29,30.
In view of the wide distribution of endosymbionts in arthropods and their potential influences on hosts, much remains to be learned about host-bacteria interactions. Spider mites (Acari: Tetranychidae) represent a distinctive evolutionary group that is comprised of about 1200 species, including many closely related species31. They are so named because some species utilize silk in constructing webbing on leaves or pads for oviposition and also for dispersal via ballooning much in the manner of some spiders. Spider mites have two reproductive strategies (bisexual and parthenogenetic). Many species of them have a wide host range, whereas others are highly host-specific. For example, Tetranychus urticae, Tetranychus truncatus, Tetranychus kanzawai and Panonychus citri are polyphagous and are serious pests of agricultural and horticultural crops. However, these genera also include oligophagous species, such as Tetranychus bambusae and Oligonychus orthius which inhabit only Poaceae plants. Previous studies have revealed that Wolbachia is widespread in spider mites. For example, Wolbachia has been detected in the genus Tetranychus32,33, Oligonychus33, Panonychus33, Schizotetranychus33, Bryobia34 and Amphitetranychus35, Furthermore, it was found associated with CI phenotypes in several species8,26,27,32,33,36 Cardinium was present in 15 species of family Tetranychidae, and induced CI in Tetranychus piercei8, Tetranychus phaselus26, T. truncatus27 and Eotetranychus suginamensis37. Unlike the widespread distribution of Wolbachia and Cardinium, Rickettsia and Spiroplasma were less common, they were only found in T. urticae38,39. As yet, comparative studies that focus on these endosymbionts in a group of spider mites species are very limited.
Here, we surveyed for the first time incidences of the four endosymbionts in economically important species of spider mites. Double infections of more than one endosymbiont were frequent within the same species, we then evaluated the levels of co-infection. We further clarified the phylogenetic relationships of these detected endosymbionts to infer their evolutionary histories. Our data provide insights into the evolution and distribution of endosymbionts in spider mites and may thus be regarded as a basis for future studies on spider mites and endosymbionts interactions.
Results
Incidences of tested endosymbionts in spider mites
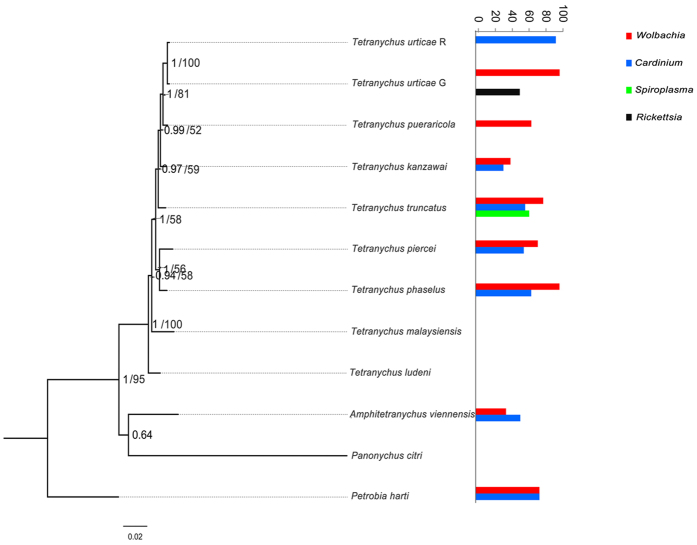
Of the 12 spider mite species examined, Wolbachia was found to infect 8 species with prevalence ranging from 16.7 to 100%. Cardinium was found to infect 7 species and their infection frequencies ranging from 4.3 to 100% (Table 1). Among them, Cardinium infections in T. kanzawai and Amphitetranychus viennensis are new reports. Wolbachia infections were more frequent in T. truncatus than in A. viennensis (80.4% vs 36.5%, P < 0.05), while Cardinium infections showed no difference between them (58.9% vs 53.1%, P = 0.18). Other endosymbionts infections showed some host species-specificity, as Spiroplasma was found in T. truncatus and Rickettsia was detected in T. urticae G (Table 1, Fig. 1).
Table 1. Prevalence of investigated endosymbionts in different lines of spider mites.
| Population code | Species | Host plant | Location | Collection date | Collection source | Endosymbionts infection* |
|||
|---|---|---|---|---|---|---|---|---|---|
| Wolbachia% infected | Cardinium% infected | Spiroplasma% infected | Rickettsia% infected | ||||||
| 1 | Tetranychus truncatus | Cotton | Harbin, Heilongjiang | Aug-2011 | Field collected | 100 (24/24) wTtru5 | 100 (24/24) cTtru | 83.3 (20/24) Spiroplasma sp. | – |
| 2 | Eggplant | Changchun, Jilin | Jul-2014 | Field collected | – | 100 (12/12) cTtru | – | – | |
| 3 | Japan Caryatia | Yanji, Jilin | Aug-2011 | Field collected | 100 (24/24) wTtru1 | 12.5 (3/24) cTtru | 37.5 (9/24) Spiroplasma sp | – | |
| 4 | Mung bean | Shenyang, Liaoning | Aug-2011 | Field collected | 69.6 (16/23) wTtru5 | 4.3 (1/23) cTtru | 82.6 (19/23) Spiroplasma sp | – | |
| 5 | Bean | Hohhot, Inner Mongolia | Aug-2014 | Field collected | 100 (12/12) wTtru1 | 100 (12/12) cTtru | 100 (12/12) Spiroplasma sp | – | |
| 6 | Eggplant | Jiuquan, Gansu | Sep-2012 | Field collected | 75 (9/12) wTtru5 | – | – | – | |
| 7 | Snake gourd | Cangzhou, Hebei | Aug-2014 | Field collected | 100 (24/24) wTtru1 | 100 (24/24) cTtru | 100 (24/24) Spiroplasma sp | – | |
| 8 | Eggplant | Changzhi, Shanxi | Aug-2014 | Field collected | 100 (20/20) wTtru1 | 100 (20/20) cTtru | 100 (20/20) Spiroplasma sp | – | |
| 9 | Corn | Chuzhou, Anhui | Sep-2010 | Field collected | 16.7 (2/12) wTtru1 | – | – | – | |
| 10 | Tetranychus kanzawai | Chinese rose | Qingdao, Shandong | Jun-2011 | Field collected | 41.7 (5/12) wTkan | 33.3 (4/12) cTkan | – | – |
| 12 | Tetranychus urticae (Green form) | Apple | Taian, Shandong | Aug-2014 | Lab reared | 100 (12/12) wTurt | – | – | 75 (9/12) Rickettsia sp |
| 13 | Bean | Hohhot, Inner Mongolia | Aug-2014 | Lab reared | 100 (12/12) wTurt | – | – | 100 (10/12) Rickettsia sp | |
| 14 | Apple | Missouri, USA | Jul-2012 | Lab reared | 100 (12/12) wTurt | – | – | – | |
| 15 | Tetranychus urticae (Red form) | Willow | Kunming, Yunnan | Jul-2012 | Lab reared | − | 95.8 (23/24) cTurt | – | – |
| 16 | Bean | Ibaraki, Japan | Lab reared | – | – | – | – | ||
| 17 | Tetranychus pueraricola | Purple yam | Yongfu, Guangxi | Aug-2014 | Field collected | 66.7 (8/12) wTpue | – | – | – |
| 18 | Tetranychus phaselus | Bean | Quanzhou, Fujian | Jun-2014 | Field collected | 100 (12/12) wTpha | 66.7 (8/12) cTpha | – | – |
| 19 | Tetranychus malaysiensis | Lingshui, Hainan | Jun-2014 | Field collected | – | – | – | – | |
| 20 | Tetranychus piercei | Kidney bean | Mayang, Hunan | Jul-2014 | Field collected | 75 (9/12) wTpie | 58.3 (7/12) cTpie | – | – |
| 21 | Tetranychus ludeni | Melon | Shantou, Guangdong | Jul-2014 | Field collected | – | – | – | – |
| 22 | Amphetetranychus viennensis | Plum | Daqing, Heilongjiang | Aug-2012 | Field collected | – | – | – | – |
| 23 | Cherry | Yanji, Jilin | Aug-2012 | Field collected | 58.3 (14/24) wAvie | 66.7 (16/24) cAvie | – | – | |
| 24 | Purple-leaf plum | Zhengzhou, Henan | Jul-2012 | Field collected | 16.7 (4/24) wAvie | – | – | – | |
| 25 | Purple-leaf plum | Sanmenxia, Henan | Jun-2012 | Field collected | – | 83,3 (20/24) cAvie | – | – | |
| 26 | Peach | Nanjing, Jiangsu | Aug-2014 | Field collected | 70.8 (17/24) wAvie | 62.5 (15/24) cAvie | – | – | |
| 27 | Petrobia harti | Clover | Nanjing, Jiangsu | Oct-2010 | Field collected | 75 (9/12) wPhar | 75 (9/12) cPhar | – | – |
| 28 | Panonychus citri | Citrus | Suzhou, Jiangsu | Sep-2010 | Field collected | – | – | – | – |
aThe infection rate of each endosymbiont was presented. Data in the bracket indicates the number of infected individuals and the number of test individuals, respectively. – indicates endosymbionts were not detected.
Figure 1. Phylogeny of spider mites based on COI, 18S rRNA, 28S rRNA gene sequences (left) and endosymbionts infection (right).
Bayesian posterior (left numbers) and ML bootstrap values (right numbers, values >50% are indicated) are given in the tree. Prevalence of each endosymbiont is given.
Correlated infections with multiple endosymbionts
Of note, co-infections of unrelated endosymbionts were observed in several species. For instance, Wolbachia and Cardinium usually co-infect T. truncatus, T. kanzawai, T. phaselus, T. piercei, A. viennensis and Petrobia harti. Similarly, T. urticae G was infected by Wolbachia and Rickettsia, and T. truncatus showed triple infections (Table 1, Fig. 1). Furthermore, Spearman correlation analyses of the presence/absence of each endosymbiont within spider mite individuals against the presence/absence of other endosymbionts revealed that infections with Wolbachia and Cardinium were significantly correlated to each other (r = 0.4344, P < 0.01). Similarly, infections with Wolbachia and Spiroplasma were significantly correlated to each other (r = 0.4737, P < 0.01) in T. truncatus.
Phylogeny of spider mites hosts and detected endosymbionts
Bayesian and maximum likelihood phylogenies of spider mites based on COI, 18SrRNA and 28SrRNA were identical. Species of the genus Tetranychus appeared to be monophyletic with strong support of posterior probabilities (>0.9) and moderate support of maximum likelihood bootstrap values (>50), other branches were not be well resolved (Fig. 1).
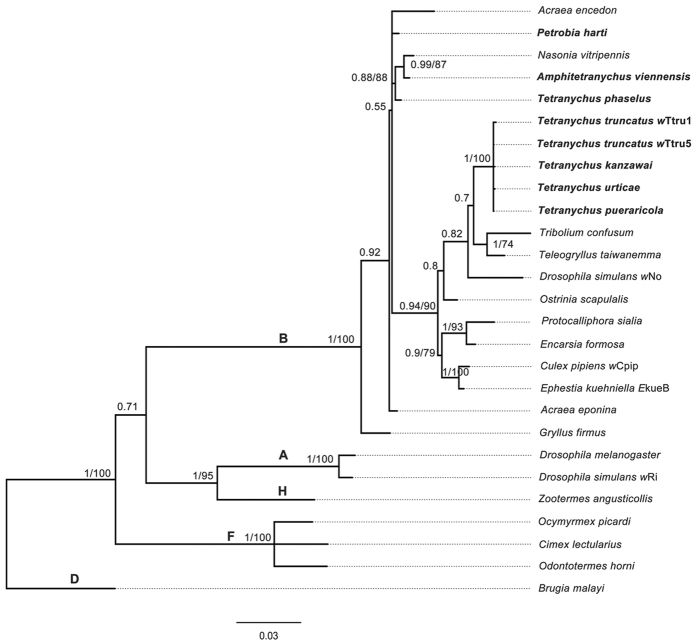
Analyses of the wsp gene sequences in the seven positive species revealed nine Wolbachia strains, which were designated as wPhar, wAvie, wTpha, wTurt, wTpue, wTkan, wTpie, wTtru1 and wTtru5 (Table 1). These strains, except for wTpie, were characterized by MLST. Wolbachia phylogenies based on MLST genes were largely identical for both Bayesian and maximum likelihood analyses, and most splits were highly supported. All of the Wolbachia strains from spider mites were assigned to supergroup B. Wolbachia strains from T. truncatus, T. kanzawai, T. urticae and T. pueraricola showed little divergence, and formed a monophyletic group (Fig. 2). While the Wolbachia strains obtained from T. phaselus, A. viennensis and P. harti respectively exhibited a distinct node in the phylogenies (Fig. 2).
Figure 2. Bayesian inference phylogeny of Wolbachia based on the concatenated MLST data.
The topology resulting from the Maximum Likelihood method was similar. The Wolbachia strains obtained from in this study are indicated in bold letters. Strains are characterized by the names of their host species. Bayesian posterior (left numbers) and ML bootstrap values (right numbers) are given (only values >50% are indicated).
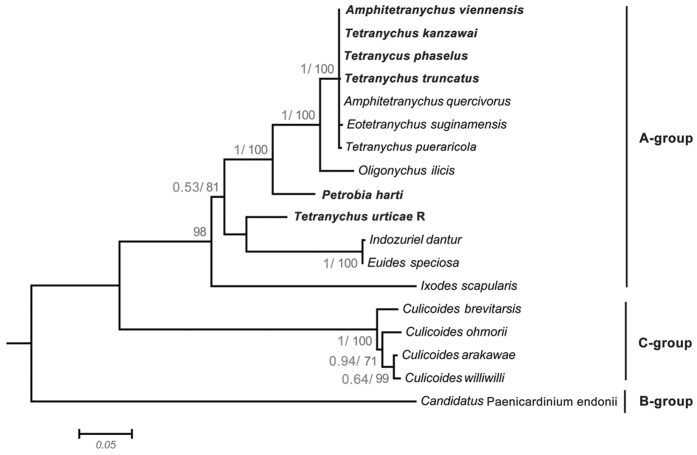
Based on the 16S rRNA sequences, a total of seven Cardinium strains (cTpie, cPhar, cAvie, cTpha, cTurt, cTkan and cTtru) were found. Owing to the 16S rRNA’s low discriminating ability, we performed phylogenetic analyses using the gyrB sequences. The gyrB gene of cTpie was not successfully sequenced, which was therefore not represented in the Cardinium phylogeny. The remaining strains all belonged to group A-clade, and strains derived from A. viennensis, T. kanzawai, T. phaselus and T. truncatus plus with Cardinium from other spider mites species formed a monophyletic group (Fig. 3).
Figure 3. Phylogenetic analyses of Cardinium based on the gyrB gene sequences from this study (highlighted) and others downloaded from GenBank.
Cardinium group names are used in accordance to the reference25. Bayesian posterior (left numbers) and ML bootstrap values (right numbers, values >50% are indicated) are given in the trees.
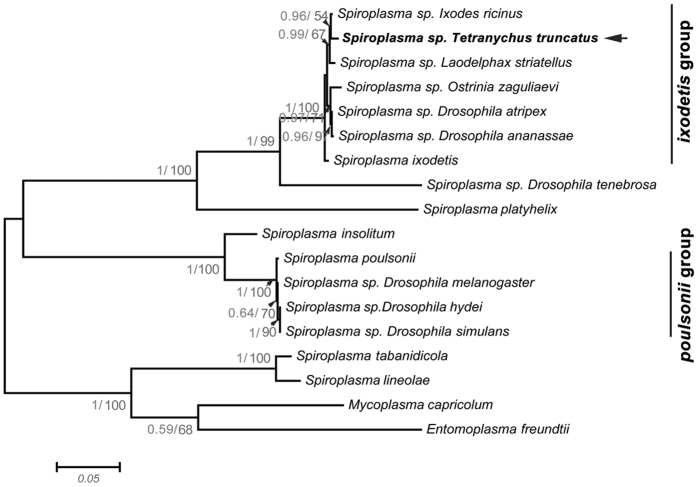
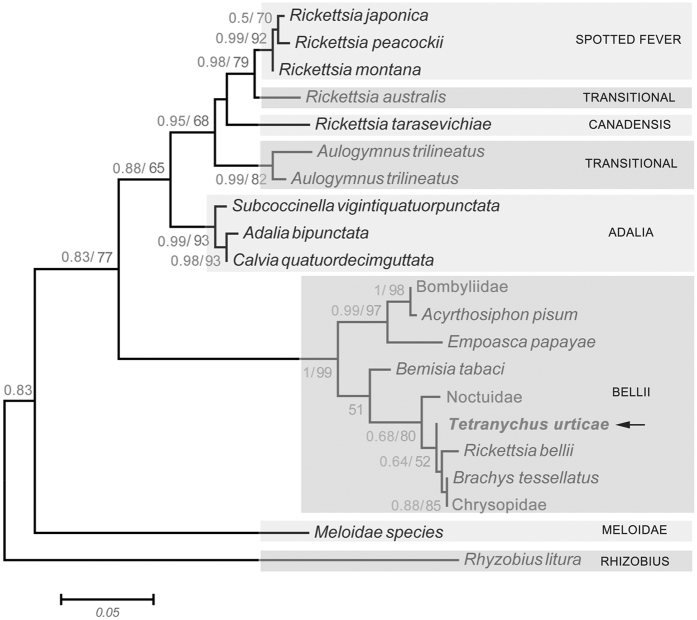
Sequencing of the Spiroplasma’s 16S rRNA, rpoB gene and the Rickettsia’s gltA gene identified one Spiroplasma strain from T. truncatus and one Rickettsia strain from T. urticae G, respectively (Table 1, Table S2). Phylogenetic tree based on rpoB gene demonstrated that the Spiroplasma of T. truncatus fell into the ixodetis group, which includes Spiroplasma ixodetis and Spiroplasma infecting tick, planthopper, moth and flies (Fig. 4). The Rickettsia detected from T. urticae G fell within the bellii group (Fig. 5).
Figure 4. Phylogenetic analyses of Spiroplama based on the rpoB gene sequences.
Spiroplama infecting T. truncatus is indicated in bold letters. Bayesian posterior (left numbers) and ML bootstrap values (right numbers, values >50% are indicated) are given in the trees.
Figure 5. ML inference of Rickettsia strains from T. urticae and other arthropod hosts based on the gltA gene sequences.
Rickettsia strain obtained from this study is indicated in bold letters. Rickettsia group names are used in accordance to the reference39. Bayesian posterior (left numbers) and ML bootstrap values (right numbers, values >50% are indicated) are given in the trees.
Correlation between Wolbachia, Cardinium and hosts genetic distances
The pattern of association and genetic divergence between Wolbachia, Cardinium and hosts were examined. Pairwise genetic distances of hosts and associated Wolbachia were significantly correlated (r = 0.4828, P = 0.005). While there was no significant correlation between Cardinium and hosts genetic distances (r = 0.1939, P = 0.29).
Discussion
Studies of endosymbiont’ incidences in a wide variety of arthropods suggest that Wolbachia is the most common bacterial symbiont20. Acari is assumed to be a hotspot for Wolbachia infections25,29. The finding of about 67% Wolbachia-positive species in our study is in line with estimations of a general Wolbachia prevalence among arthropods (40–60%). Cardinium infections were identified in 7 out 12 species (58.3%), suggesting that spider mites are prone to be infected with Cardinium, which is consistent with previous estimates23,24,40. Whereas Spiroplasma and Rickettsia showed host species-specificity, they were only detected in T. truncatus and T. urticae, respectively. Ecological traits of host can affect the infection dynamics of endosymbionts in them41,42. Although the infection frequencies of these endosymbionts varied among geographical populations, our survey data did not detect a clear correlation between their distribution and host ecological traits (Table 1). It is worth noting that three lab reared lines of T. urticae G were completely infected with Wolbachia, indicating fixation of infection has been reached in rearing. In addition, three species T. malaysiensis, T. ludeni, P. citri did not carry any of the tested endosymbionts, raising the possibility that there have been repeated losses of infection during post-speciation from an infected ancestor or due to limited samples. Wolbachia infections were more frequent in T. truncatus than in A. viennensis. The two species are phylogenetically divergent and have different habitats, thus it remains possible that host phylogeny combined with host ecology shapes the distribution of endosymbionts in spider mites.
Manipulating reproduction and providing fitness advantages in their hosts are thought to be two important determinants of endosymbionts infection frequencies43. The high infection frequencies of Wolbachia and Cardinium in spider mites may be due to their reproductive manipulations or fitness advantages. Nine Wolbachia strains were detected from the positive specimens, and several of these strains have previously been studied in detail. For example, there is evidence that Wolbachia induces CI in several spider mites, including T. urticae32,33,43, T. phaselus26, T. truncatus27, T. piercei8 and A. viennensis35. Regarding Cardinium, it was found to induce CI in T. piercei8, T. phaselus26 and T. truncatus27. Furthermore, Weinert et al.40 have speculated that high Cardinium incidences in spider mites might reflect evolutionary changes in arthropod immunity, as spider mites lack components of the immune deficiency (IMD) pathway, and IMD is activated by diaminopimelic acid-type (DAP-type) peptidoglycan, which is produced by Cardinium44.
Spider mites showed co-infections with more than one endosymbiont. Statistical analyses revealed that infections with Wolbachia and Cardinium, Wolbachia and Spiroplasma were significantly correlated to each other within the same individuals. There are a number of possible mechanisms that can facilitate such endosymbiont co-infections45. For example, the co-infecting endosymbionts may additively or synergistically confer fitness advantages on their host. Another mechanism is that when one of the co-infecting endosymbionts causes a reproductive manipulation, the manipulation may facilitate not only its own prevalence but also spread of another co-infecting endosymbiont via a hitchhiking effect46. As mentioned previously, both Wolbachia and Cardinium can induce CI in doubly-infected spider mites T. piercei8, T. phaselus26, T. truncatus27 and A. viennensis35, which would increase the prevalence of both endosymbionts. Also, the CI phenotypes induced by Wolbachia in T. truncatus and T. urticae G would theoretically facilitate the spread of co-infecting Spiroplasma and Rickettsia, respectively.
Spiroplasma and Rickettsia act as reproductive mediators in some arthropods11,12,13,14,15,16,17. They are much less common in spider mites than Wolbachia and Cardinium. Here, Spiroplasma and Rickettsia were identified only in T. truncatus and T. urticae G, respectively. Phylogenetic analyses confirmed that the two endosymbionts have horizontally transferred among different hosts, and there is experimental evidence for horizontal transmission of these symbionts via host plants or host invertebrates47,48, thus Spiroplasma and Rickettsia occurrences in spider mites may be the result of an individual horizontal transmission event, respectively. Furthermore, Spiroplasma of T. truncatus fell into the ixodetis group, which includes Spiroplasma ixodetis and Spiroplasma infecting tick, planthopper, moth and flies. Unlike the male-killing Spiroplasmas infecting the small brown planthopper, Laodelphax striatellus14, the Spiroplasma strain found in T. truncatus is not a male killer because it was found in both males and females. While it seemed increase its host development speed (unpublished data), and we are currently testing the underlying reasons. Rickettsia in T. urticae clustered within the bellii group, in which there are Rickettsia strains found in sap sucking arthropods and predatory insect hosts. Previously, Hoy and Jeyaprakash49 also found that four North American populations of T. urticae were infected with Rickettsia, as well as Wolbachia and Caulobacter. However, what role the Rickettsia play in the biology of spider mites is unknown.
Theory suggested that maternally inherited symbiontes’ strict mutualistic associations with their hosts will result in co-cladogenesis phylogenetic patterns50,51. Multiple strains of Wolbachia and Cardinium were detected in this study, and their infection histories could be inferred. Mapping the Wolbachia phylogeny to spider mites’ phylogeny revealed some degree of congruence, as similar strains are found in closely related hosts. Meanwhile, pairwise genetic distances of hosts and associated Wolbachia were significantly correlated. Two scenarios might explain this finding. First, the common ancestor of spider mite hosts could have originally harbored Wolbachia and that the host and Wolbachia have co-speciated. Second, horizontal transmission can explain the sharing of Wolbachia strains among different hosts. By contrast, there was no significant association between the phylogeny of Cardinium and its host, and the Mantel test confirmed this result. Whilst we cannot completely exclude the possibility that there has been repeated loss of infection during post-speciation from an infected ancestor, this difference between the two phylogeneies indicated that Cardinium was not solely acquired vertically and points to the likelihood of horizontal transmission. In addition, the phylogeny based on a single gene will fail to reflect the accurate phylogeny of Cardinium, thus more rapidly evolving and phylogenetically informative Cardinium genes are required in further study.
In summary, four endosymbionts were identified in the tested specimens, and their distributions were found to be shaped by both host phylogeny and host ecology. The levels of co-infections within the same individuals were significantly higher than would be expected by chance. Comparison between the phylogenies of hosts and associated endosymbionts allowed us explore the evolutionary histories of these endosymbionts’ infections. Together with these endosymbionts’ reproductive effects on spider mites, these findings are helpful for understanding the interaction between endosymbionts and spider mites.
Methods
All experimental protocols were approved by Chinese Academy of Sciences and Nanjing Agricultural University. Methods were carried out in accordance with relevant guidelines and regulations.
Spider mites samples
This study was based on specimens from 28 populations of spider mites representing 12 species that were collected from field or lab reared lines (Table 1). All samples were stored in 100% ethanol and frozen at −20 C until DNA extraction.
PCR screening and sequencing
Spider mite DNA was extracted as previously described52. The DNA quality was tested by amplifying a fragment of the cytochrome oxidase, subunit I (COI) gene of spider mites53. Then, the presence of Wolbachia, Cardinium, Spiroplasma and Rickettsia was assessed by PCR amplification using specific primers and annealing temperatures listed in Table S1. PCRs were carried out on a Veriti machine (ABI Biosystems, USA) in 25 μl volume containing 12.5 μl 2 × Taq Master Mix (Vazyme Biotech, China), 0.5 μl primer (20 μM each), 1 μl of DNA extract. Positive and negative controls were included in PCR reactions. PCR products (5 μl) were visualized on a 1.5% agarose gel stained with ethidium bromide54. The positive products were purified using AxyPrep DNA Gel Extraction kit (AxyGEN, USA) and then directly sequenced (Majorbio Company, Shanghai, China). For Wolbachia, single-infection status was confirmed during wsp sequencing. Then, the MLST gene sequences of single infected Wolbachia from different individuals were amplified using standard primers and PCR protocols (http://www.pubmlst.org/wolbachia/). The COI, 18SrRNA and 28SrRNA sequences of spider mites were amplified and sequenced to construct host phylogeny. The obtained sequences have been deposited in GenBank (Table S2).
Phylogenetic analysis
All sequences were aligned and manually corrected using BioEdit55. Prior to phylogenetic analysis, the best-fitting nucleotide models were determined by jModeltest version 256. Wolbachia phylogeny was determined by reconstructing Bayesian Inference (BI) and Maximum-Likelihood (ML) trees of the concatenated data set of MLST genes from this study and PubMLST database (http://www.pubmlst.org/wolbachia/). Bayesian analyses were performed in MrBayes 3.157 under the GTR + I + G model. Four Markov chains were run for 15,000,000 generations with sampling every 100 generations, and the first 37,500 generations were discarded. Convergence of runs was assumed when split frequencies reached <0.01. ML analysis was also conducted for the concatenated data set of MLST genes in MEGA 5.058 under the GTR + I + G model, bootstrap pseudoreplicates were calculated 1,000 times.
The phylogenetic analyses of Cardinium, Spiroplasma and Rickettsia were performed using Bayesian Inference (BI) and Maximum-Likelihood (ML) estimation for the sequences of gyrB gene, rpoB gene and gltA gene, respectively. The evolutionary models used were as follows: gyrB- GTR + I + G, rpoB-GTR + I + G, and gltA-HKY+G. For each Bayesian analysis, four Markov chains were run 1,000,000 generations with sampling every 100 generations, and the first 25% of samples were discarded as burn-in. Convergence of runs was assumed when split frequencies reached <0.01. ML analyses were conducted in MEGA 5.058, bootstrap pseudoreplicates were calculated 1,000 times.
In addition, Bayesian and ML phylogenetic trees of spider mite were constructed using a supermatrix that consisting of the COI, 18SrRNA and 28SrRNA sequences. Analyses were performed in MrBayes 3.1 and MEGA 5.0 under the GTR+G+I model, respectively.
Statistical analysis
Endosymbiont infection patterns were first compared on the level of genera and species of spider mites. Two extensively sampled species, T. truncatus and A. viennensis were selected to test the differences of Wolbachia and Cardinium infections. Because co-infection was common in spider mites, co-infection levels were evaluated with a Spearman correlation analysis of the presence/absence of each endosymbiont within spider mite individuals against the presence/absence of other endosymbionts. The analysis was performed with Stata 11.059. In addition, we tested for correlation between host genetic distance and the corresponding Wolbachia, Cardinium strains genetic divergence to infer their infection patterns. Correlation analyses were performed using a Mantel test in Arlequin 3.160 with 1000 permutations. Genetic distance matrices of the concatenated MLST dataset for Wolbachia, gyrB genes for Cardinium and the concatenated nuclear and mitochondrial loci for spider mites were calculated with the Kimura 2-parameter model in MEGA 5.0.
Additional Information
How to cite this article: Zhang, Y.-K. et al. Screening of spider mites (Acari: Tetranychidae) for reproductive endosymbionts reveals links between co-infection and evolutionary history. Sci. Rep. 6, 27900; doi: 10.1038/srep27900 (2016).
Supplementary Material
Acknowledgments
We thank Xiao-Lin Li, Peng-Yu Jin and Jia Zhang of the Department of Entomology, Nanjing Agricultural University, for their help with the experiments. This study was supported in part by a grant-in-aid from the Science and Technology Program of the National Public Welfare Professional Fund (201103020) from the Ministry of Agriculture of China.
Footnotes
Author Contributions Conceived and designed the experiments: Y.-K.Z., G.-X.Q. and X.-Y.H. Performed the experiments: Y.-K.Z., K.Y. and Y.-T.C. Wrote the paper: Y.-K.Z., G.-X.Q. and X.-Y.H.
References
- Tsuchida T. et al. Symbiotic bacterium modifies aphid body color. Science 330, 1102–1104 (2010). [DOI] [PubMed] [Google Scholar]
- Douglas A. E. The microbial dimension in insect nutritional ecology. Funct. Ecol. 23, 38–47 (2009). [Google Scholar]
- Sharon G. et al. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 107, 20051–20056 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I. I. & Littman D. R. Modulation of immune homeostasis by commensal bacteria. Curr. Opin. Microbiol. 14, 106–114 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren J. H., Baldo L. & Clark M. E. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 6, 741–751 (2008). [DOI] [PubMed] [Google Scholar]
- Dedeine F., Bouletreau M. & Vavre F. Wolbachia requirement for oogenesis: occurrence within the genus Asobara (Hymenoptera, Braconidae) and evidence for intraspecific variation in A. tabida. Heredity 95, 394–400 (2005). [DOI] [PubMed] [Google Scholar]
- Hunter M. S., Perlman S. J. & Kelly S. E. A bacterial symbiont in the bacteroidetes induces cytoplasmic incompatibility in the parasitoid wasp Encarsia pergandiella. Proc. R. Soc. B Biol. Sci. 270, 2185–2190 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L. Y. et al. Wolbachia strengthens Cardinium-induced cytoplasmic incompatibility in the spider mite Tetranychus piercei McGregor. Curr. Microbiol. 65, 516–523 (2012). [DOI] [PubMed] [Google Scholar]
- Zchori-Fein E. et al. A newly discovered bacterium associated with parthenogenesis and a change in host selection behavior in parasitoid wasps. Proc. Natl. Acad. Sci. USA 98, 12555–12560 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks A., Marec F. & Breeuwer J. A. J. A mite species that consists entirely of haploid females. Science 29, 2479–2482 (2001). [DOI] [PubMed] [Google Scholar]
- Pool J. E., Wong A. & Aquadro C. F. Finding of male-killing Spiroplasma infecting Drosophila melanogaster in Africa implies transatlantic migration of this endosymbiont. Heredity 97, 27–32 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiggins F. M., Hurst. G. D. D., Jiggins. C. D. & Majerus M. The butterfly Danaus chrysippus is infected by a male-killing Spiroplasma bacterium. Parasitol. 120, 439–446 (2000). [DOI] [PubMed] [Google Scholar]
- Tinsley M. C. & Majerus M. E. N. A new male-killing parasitism: Spiroplasma bacteria infect the ladybird beetle Anisosticta novemdecimpunctata (Coleoptera: Coccinellidae). Parasitol. 132, 757–765 (2006). [DOI] [PubMed] [Google Scholar]
- Sanada-Morimura S., Matsumura M. & Noda H. Male killing caused by a Spiroplasma symbiont in the small brown planthopper, Laodelphax striatellus. J. Hered. 104, 821–829 (2013). [DOI] [PubMed] [Google Scholar]
- Giorgini M., Bernardo U., Monti M. M., Nappo A. G. & Gebiola M. Rickettsia symbionts cause parthenogenetic reproduction in the parasitoid wasp Pnigalio soemius (Hymenoptera: Eulophidae). Appl. Environ. Microbiol. 76, 2589–2599 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson E. T., Mousseau T. A., Klaper R., Hunter M. D. & Werren J. H. Rickettsia associated with male-killing in a buprestid beetle. Heredity 86, 497–505 (2001). [DOI] [PubMed] [Google Scholar]
- von der Schulenburg, J. H. G. et al. Incidence of male-killing Rickettsiaspp (α-Proteobacteria) in the ten-spot ladybird beetle Adalia decempunctata L. (Coleoptera: Coccinellidae). Appl. Environ. Microbiol. 67, 270–277 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira L., Ferreira Á. & Ashburner M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 6, e1000002 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himler A. G. et al. Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science 332, 254–256 (2011). [DOI] [PubMed] [Google Scholar]
- Zug R. & Hammerstein P. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE 7, e38544 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker D. D. et al. The distribution of Wolbachia in fig wasps: correlation with host phylogeny, ecology and population structure. Proc. R. Soc. B Biol. Sci. 269, 2257–2267 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachowska D. Kajtoch, Ł. & Knutelski, S. Occurrence of Wolbachia in central European weevils: correlations with host systematics, ecology, and biology. Entomol. Exp. Appl. 135, 105–118 (2010). [Google Scholar]
- Weeks A. R., Velten R. & Stouthamer R. Incidence of a new sex-ratio-distorting endosymbiotic bacterium among arthropods. Proc. R. Soc. B Biol. Sci. 270, 1857–1865 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zchori-Fein E. & Perlman S. J. Distribution of the bacterial symbiont Cardiniumin in arthropods. Mol. Ecol. 13, 2009–2016 (2004). [DOI] [PubMed] [Google Scholar]
- Nakamura Y. et al. Prevalence of Cardinium bacteria in planthoppers and spider mites and taxonomic revision of “Candidatus Cardinium hertigii” based on detection of a new Cardinium group from biting midges. Appl. Environ. Microbiol. 75, 6757–6763 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D. X., Chen D. S., Ge C., Gotoh T. & Hong X. Y. Multiple infections with Cardinium and two strains of Wolbachia in the spider mite Tetranychus phaselus Ehara: revealing new forces driving the spread of Wolbachia. PLoS ONE 8, e54964 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D. X., Zhang X. F. & Hong X. Y. Host-symbionts interactions in spider mite Tetranychus truncatus doubly infected with Wolbachia and Cardinium. Enviro. Entomol. 42, 445–452 (2013). [DOI] [PubMed] [Google Scholar]
- Goodacer S. L., Martin O. Y., Thomas F. G. & Hewitt G. M. Wolbachia and other endosymbiont infections in spiders. Mol. Ecol. 15, 517–527 (2006). [DOI] [PubMed] [Google Scholar]
- Duron O. et al. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 6, 27 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toju H. & Fukatsu T. Diversity and infection prevalence of endosymbionts in natural populations of the chestnut weevil: relevance of local climate and host plants. Mol. Ecol. 20, 853–868 (2011). [DOI] [PubMed] [Google Scholar]
- Migeon A., Nouguier E. & Dorkeld F. Spider Mites Web: a comprehensive database for the Tetranychidae. In Trends in Acarology (pp. 557–560). Springer: Netherlands, 2011. [Google Scholar]
- Breeuwer J. A. J. Wolbachia and cytoplasmic incompatibility in the spider mites Tetranychus urticae and T. turkestani. Heredity 79, 41–47 (1997). [Google Scholar]
- Gotoh T., Noda H. & Hong X. Y. Wolbachia distribution and cytoplasmic incompatibility based on a survey of 42 spider mite species (Acari: Tetranychidae) in Japan. Heredity 91, 208–216 (2003). [DOI] [PubMed] [Google Scholar]
- Ros V. I., Fleming V. M., Feil E. J. & Breeuwer J. A. How diverse is the genus Wolbachia? Multiple-gene sequencing reveals a putatively new Wolbachia supergroup recovered from spider mites (Acari: Tetranychidae). Appl. Environ. Microbiol. 75, 1036–1043 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. K., Sun B. & Hong X. Y. Infection and reproductive effects of Wolbachia in the hawthorn spider mite, Amphitetranychus viennensis (Acarina: Tetranychidae). Acta Entomol. Sinica 57, 914–920 (2014). [Google Scholar]
- Gotoh T. et al. Wolbachia and nuclear-nuclear interactions contribute to reproductive incompatibility in the spider mite Panonychus mori (Acari: Tetranychidae). Heredity 94, 237–246 (2005). [DOI] [PubMed] [Google Scholar]
- Gotoh T., Noda H. & Ito S. Cardinium symbionts cause cytoplasmic incompatibility in spider mites. Heredity 98, 13–20 (2007). [DOI] [PubMed] [Google Scholar]
- Enigl M. & Schausberger P. Incidence of the endosymbionts Wolbachia, Cardinium and Spiroplasma in phytoseiid mites and associated prey. Exp. Appl. Acarol. 42, 75–85 (2007). [DOI] [PubMed] [Google Scholar]
- Weinert L. A., Werren J. H., Aebi A., Stone G. N. & Jiggins F. M. Evolution and diversity of Rickettsia bacteria. BMC Biol. 7, 6 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert L. A., Araujo-Jnr E. V., Ahmed M. Z. & Welch J. J. The incidence of bacterial endosymbionts in terrestrial arthropods. Proc. R. Soc. B Biol. Sci. 282, 20150249 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontowski R., Bernhard D., Bleidorn C., Schlegel M. & Gerth M. Wolbachia distribution in selected beetle taxa characterized by PCR screens and MLST data. Ecol. Evol. 5, 4345–4353 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerth M., Saeed A., White J. A. & Bleidorn C. Extensive screen for bacterial endosymbionts reveals taxon-specific distribution patterns among bees (Hymenoptera, Anthophila). FEMS Microbiol. Ecol. 91, fiv047 (2015). [DOI] [PubMed] [Google Scholar]
- Zhao D. X., Zhang X. F., Chen D. S., Zhang Y. K. & Hong X. Y. Wolbachia-host interactions: host mating patterns affect Wolbachia density dynamics. PLoS ONE 8, e66373 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penz T. et al. Comparative genomics suggests an independent origin of cytoplasmic incompatibility in Cardinium hertigii. PLoS Genet. 8, e1003012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vautrin E. & Vavre F. Interactions between vertically transmitted symbionts: cooperation or conflict? Trends Microbiol. 17, 95–99 (2009). [DOI] [PubMed] [Google Scholar]
- Engelstädter J., Telschow A. & Hammerstein P. Infection dynamics of different Wolbachia-types within one host population. J. Theor. Biol. 231, 345–355 (2004). [DOI] [PubMed] [Google Scholar]
- Jaenike J., Polak M., Fiskin A., Helou M. & Minhas M. Interspecific transmission of endosymbiotic Spiroplasma by mites. Biol. Lett. 3, 23–25 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi-Fluger A. et al. Horizontal transmission of the insect symbiont Rickettsia is plant-mediated. Proc. R. Soc. B. Biol. Sci. 279, 1791–1796 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy, M. A. & Jeyaprakash A. Microbial diversity in the predatory mite Metaseiulus occidentalis (Acari: Phytoseiidae) and its prey, Tetranychus urticae (Acari:Tetranychidae). Biol. Control 32, 427–441 (2005). [Google Scholar]
- Moran N. & Baumann P. Phylogenetics of cytoplasmically inherited microorganisms of arthropods. Trends Ecol. Evol. 9, 15–20 (1994). [DOI] [PubMed] [Google Scholar]
- Lo N., Bandi C., Watanabe H., Nalepa C. & Beninati T. Evidence for cocladogenesis between diverse dictyopteran lineages and their intracellular endosymbionts. Mol. Biol. Evol. 20, 907–913 (2003). [DOI] [PubMed] [Google Scholar]
- Zhang Y. K. et al. Diversity of Wolbachia in natural populations of spider mites (genus Tetranychus): evidence for complex infection history and disequilibrium distribution. Microb. Ecol. 65, 731–739 (2013). [DOI] [PubMed] [Google Scholar]
- Navajas M., Gutierrez J., Lagnel J. & Boursot J. Mitochondrial cytochrome oxidase I in tetranychid mites: a comparison between molecular phylogeny and changes of morphological and life history traits. Bull. Entomol. Res. 86, 407–417 (1996). [Google Scholar]
- LePecq J. B. & Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids: physical-chemical characterization. J. Mol. Biol. 27, 87–106 (1967). [DOI] [PubMed] [Google Scholar]
- Hall T. A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids. Symp. Ser. 41, 95–98 (1999). [Google Scholar]
- Darriba D., Taboada G. L., Doallo R. & Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9, 772 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F. & Huelsenbeck J. P. MrBayes3: Bayesian phylogenetic inference under mixed models. Bioinform. 19, 1572–1574 (2003). [DOI] [PubMed] [Google Scholar]
- Tamura K. et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stata Corporation STATA statistical software: Version 11. College Station, Texas, USA http://www.stata.com/ (2009).
- Excoffier L., Laval G. & Schneider S. Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol. Bioinform. Online 1, 47–50 (2005). [PMC free article] [PubMed] [Google Scholar]
- Baldo L. et al. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl. Environ. Microbiol. 72, 7098–7110 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselkorn T. S., Markow T. A. & Moran N. A. Multiple introductions of the Spiroplasma bacterial endosymbiont into Drosophila. Mol. Ecol. 18, 1294–1305 (2009). [DOI] [PubMed] [Google Scholar]
- Davis M. J., Ying Z., Brunner B. R., Pantoja A. & Ferwerda F. H. Rickettsial relative associated with papaya bunchy top disease. Curr. Microbiol. 26, 80–84 (1998). [DOI] [PubMed] [Google Scholar]
- Matsuda T., Morishita M., Hinomoto N. & Gotoh T. Phylogenetic analysis of the spider mite sub-family Tetranychinae (Acari: Tetranychidae) based on the mitochondrial COI gene and the 18S and the 5′ end of the 28S rRNA genes indicates that several genera are polyphyletic. PLoS ONE. 9, e108672 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.