Abstract
Extraction of lipids from biological samples is a critical step in lipidomics, especially for shotgun lipidomics where lipid extracts are directly infused into a mass spectrometer. The butanol-methanol (BUME) extraction method was originally developed to extract lipids from plasma samples with 1% acetic acid. Considering some lipids are sensitive to acidic environments, we modified this protocol by replacing acetic acid with lithium chloride solution and extended the modified extraction to tissue samples. Although no significant reduction of plasmalogen levels in the acidic BUME extracts of rat heart samples was found, the modified method was established to extract various tissue samples, including rat liver, heart, and plasma. Essentially identical profiles of the majority of lipid classes were obtained from the extracts of the modified BUME and traditional Bligh-Dyer methods. However, it was found that neither the original, nor the modified BUME method was suitable for 4-hydroxyalkenal species measurement in biological samples.
Keywords: BUME, lipid extraction, lipidomics, mass spectrometry, plasmalogens, shotgun lipidomics
Introduction
Lipidomics, as an interdisciplinary field, studies cellular lipidomes including dynamic changes of lipid content and composition, lipid metabolism, and interactions with proteins and other cellular components on a large scale using analytical chemistry principles and technologies [1, 2]. Through lipidomics-based studies mechanistic insights into various diseases and pathophysiological conditions can be revealed [3–5]. Substantial advances in lipidomics have been made since its emergence a decade ago [6]. These advances are largely achieved through novel developments of mass spectrometry (MS)-based analytical methods such as nano-electrospray ionization (nESI) tandem MS [7, 8]. Moreover, shotgun lipidomics, which refers to the technology that analyzes lipid species by MS and/or tandem MS after direct infusion under constant concentration conditions, is high throughput, cost-effective, and very powerful for lipid analysis. Rapid quantitative or semi-quantitative analyses of the content and composition of many lipid classes and individual lipid species can be performed by lipidomics using a minimal amount of samples [9]. To successfully conduct lipidomics analysis, sample preparation is a key step. For example, shotgun lipidomics is highly dependent on the quality of lipid extraction since any coexisting non-lipid components such as aqueous phase contamination could lead to significant ion suppression and/or high chemical noise, resulting in reduced sensitivity and inaccurate or irreproducible MS measurements.
Traditionally, procedures based on either Folch or Bligh and Dyer [10, 11] with or without modification have been widely utilized for the extraction of lipids from biological samples. These methods are generally rapid and effective for determining total lipid content present in the majority of biological sources. However, researchers recognized that these methods possess some drawbacks in the area of lipidomics requiring high throughput. In a traditional lipid extraction, the resulting layers are composed of an upper aqueous phase and a lower organic phase that contains the majority of lipids. In order to collect the organic phase containing lipids, the aqueous phase must be penetrated and the barrier between the two layers may be disturbed. Thus, it is hard to avoid contamination or inorganic residues in lipid extracts when the bottom solvent layer is removed, regardless of how carefully an extraction procedure is performed. Moreover, it is always much more difficult to achieve extraction automatically from a bottom solvent layer than a top layer, although automated lipid extraction using the Folch method have been reported [12, 13]. Furthermore, there exist some safety concerns with both the Folch and Blight-Dyer methods since they utilize chloroform, which is a well-known carcinogen [14]. Therefore, these practical drawbacks have driven the researchers in the lipidomics field to explore and develop novel lipid extraction techniques.
A few methods have been developed to improve the traditional extraction procedures by replacing toxic chloroform. Butanol and di-isopropyl ether in a 40:60 (v/v) ratio has been reported to extract most of lipids from plasma or serum without protein precipitation [15]. However, this method requires a large sample size, which increases the cost of sample collection. The method developed by Matyash et al. employs methyl-tert-butyl ether (MTBE) in place of chloroform and possesses similar or better extraction results [16]. The resultant upper organic phase in this method can be easily collected without disturbing the lower aqueous phase and is favorable for automation. However, the MTBE organic phase contains quite a large amount of aqueous content, which not only requires a longer time to evaporate the solvent(s) from the extraction under a nitrogen stream, but also causes more sodium adducts than that from chloroform extraction along with possible ion suppression. A similar upper organic phase can be found in the method developed by Hara and Radin [17], in which hexane/isopropanol have been used. This method overcomes the issues present in chloroform extraction (i.e., toxicity) and MTBE (miscible with water) to a certain degree. However, this method requires a step of filtration and several washes, making it more costly and labor intense. Zhao and Xu have developed an extremely simple method by only utilizing a single methanol solvent and one step of centrifugation [18]. This method is easy to operate and suitable for analysis of lyso phospholipids and phospholipids. However, due to the solubility of methanol, it cannot extract more hydrophobic lipid classes from biological samples, such as TAG
Löfgren et al. have developed an automated extraction method that utilizes a butanol-methanol solution system (BUME method) in replacing chloroform-methanol, amongst other improvements [19]. The BUME method requires smaller volumes of solvents, allowing it to be automated in a 96-well robot and overcoming the safety concerns during lipid extraction. Moreover, the organic phase which contains the lipids of interest forms the upper phase, as opposed to the lower phase in chloroform extractions, which solves the problem of contamination with the aqueous phase. In comparison to the MTBE extraction method, organic phase of the BUME method contains less aqueous content although both methods show comparable extraction of lipids [19].
This BUME extraction approach is an improvement over chloroform-based methods and possesses some advantages over other extraction methods in different aspects; however, further improvements and/or tests are still needed to make this method suitable for broader applications in lipidomics. In the current study, our goal was to improve the extraction method in multiple aspects including (1) to extend the method for extraction of a variety of biological samples such as tissues and cell cultures in addition to biofluids such as plasma as originally tested; (2) to test whether lithium chloride solution could replace acetic acid as originally used to avoid any possibility of degradation of those acid-labile lipids such as plasmalogens; and (3) to expand the method for extraction of unexamined lipid classes such as 4-hydroxyalkenal (4-HA) species which could be readily recovered from the chloroform extraction.
Materials and Methods
Materials
Butanol, heptane, and ethyl acetate (EtAc) all in high performance liquid chromatography (HPLC) grade were supplied by Fisher Scientific (Waltham, MA). Methanol and chloroform were obtained from Honeywell Burdick & Jackson (Muskegon, MI). Lithium chloride and ammonium formate were purchased from Sigma-Aldrich (St. Louis, MO). Concentrated phosphate-buffered saline (PBS, 10X) was from Fisher BioReagents (Hampton, NH). Potassium chloride and ammonium acetate were supplied by MP Biomedicals, LLC (Santa Ana, CA). Disposable glass culture tubes (size of 6 mL) were purchased from Fischer Brand (Hampton, NH).
All synthetic lipid standards, including di14:1 PtdCho, di16:1 PtdEtn, di15:0 PtdGro (Na), tri17:1 TAG (Nu-Chek Prep, Inc., Waterville, MN), 14:0 lysoPtdEtn, 17:0 lysoPtdCho, N12:0 CerPCho, N17:0 Cer, di14:0 PtdSer (Na), di14:0 PtdOH (Na), tetra14:0 Ptd2Gro, and d3-4-hydroxy-2E-nonenal (d3-4-HNE) (Cayman Chemical, Ann Arbor, MI) were obtained from Avanti Polar Lipids, Inc. (Alabaster, AL), except those specified.
Animal Experiments
Female Sprague Dawley rats (around 250 gram body weight) were purchased from The Charles River Laboratory (Wilmington, Massachusetts, USA). After a 3-day acclimation period, the rats were anesthetized by isoflurane. Blood was acquired by cardiac puncture with heparin (Sagent Pharm., Schaumburg, IL) as an anticoagulant and placed on ice immediately. The rats were dissected after cervical dislocation. The liver and heart tissues were excised quickly, perfused with ice-cold PBS to remove blood, blotted with Kimwipes (Kimberly-Clark, Roswell, GA, USA) to remove excess buffer, and then immediately freeze-clamped at the temperature of liquid nitrogen. The collected whole blood was centrifuged at 2,400 rpm for 15 minutes, and the top clear plasma was pipetted into a clean plastic screw-cap vial (Corning Life Sciences, Tewksbury, MA). All tissue and plasma samples were stored at −80 °C until lipid extraction. All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy of Science, 2011) and were approved by the Institutional Animal Care and Use Committee at the Sanford Burnham Prebys Medical Discovery Institute.
Preparation of Samples for Lipid Extracts
Rat liver or heart tissue wafers were pulverized into a fine powder at the temperature of liquid nitrogen by a stainless steel Biopulverizer (BioSpec Products, Bartlesville, OK). The fine tissue powders (around 20 mg) were accurately weighed from each sample in a 2.0-ml cryogenic vial (Corning Life Sciences, Tewksbury, MA) and homogenized in 10-time-diluted PBS solution. A bicinchoninic acid protein assay kit (Thermo Scientific, Rockford, IL) was used to measure the protein content of the homogenates with bovine serum albumin as standards. Lipid internal standards were used based on the protein content of individual rat tissue samples. Rat plasma samples were pooled and aliquoted for comparison, and their lipid internal standards were added based on sample volumes.
Modification of the BUME Procedure
The original BUME procedure [18] was used, except the 300 μL of 1% acetic acid was replaced with 300 μL of 50 mM LiCl, as follows: Individual homogenate of the rat liver or heart (equal to an amount of ~ 0.5 mg protein) or rat plasma samples (10–100 μL) was accurately transferred into a disposable glass culture test tube. A certain amount of pre-mixed internal standards for quantification of lipid classes of interest was added to the test tube prior to lipid extraction, as shown in Table 1. Butanol/methanol (300 μL, 3:1, v/v) was added to the test tubes and the solution was vortexed for one minute. Heptane/EtAc (150 μL, 3:1, v/v) was added to the tubes and the solution was vortexed again for another minute. Another 150 μL Heptane/EtAc was added, and after vortexing for 1 minute, 300 μL of 50 mM LiCl was added to the test tubes to induce phase separation after the resulting solution was vigorously vortexed for another minute. Then the solution was centrifuged at 2,700 g for 10 minutes. The upper organic layer was collected and placed into a new test tube. The remaining aqueous layer was re-extracted twice, first with 320 μL of heptane/EtAc (3:1), then with 250 μL of the same solvent. After each addition of heptane/EtAc, the solution mixture was vortexed and centrifuged at 2,700 g for 10 minutes, and the resulting upper organic layers were collected and combined with the previous organic layer. The lipid extracts were flushed with nitrogen, capped, and stored at −20 °C.
Table 1.
Internal standard amounts added in biological samples prior to extraction
| Internal standard | Rat Liver (nmol/mg protein) | Rat Heart (nmol/mg protein) | Rat Plasma (nmol/mL plasma) |
|---|---|---|---|
| di14:1 PtdCho | 14.8 | 15.0 | 226.8 |
| di16:1 PtdEtn | 14.8 | 19.0 | 48.6 |
| di15:0 PtdGro (Na) | 2.1 | 2.0 | 17.0 |
| tri17:1 TAG | 15.0 | 7.5 | 486.0 |
| 14:0 lysoPtdEtn | 0.4 | 1.5 | 6.5 |
| 17:0 lysoPtdCho | 1.0 | 1.5 | 162.0 |
| N12:0 CerPCho | 0.8 | 2.0 | 81.0 |
| N17:0 Cer | 0.7 | 0.1 | 2.4 |
| di14:0 PtdSer (Na) | 1.2 | 4.0 | 6.5 |
| di14:0 PtdOH (Na) | 0.4 | 0.8 | 0.4 |
| tetra14:0 Ptd2Gro | 3.7 | 4.0 | 6.0 |
Modified Bligh and Dyer Extraction for Comparison
Individual rat liver homogenate with the same amount as the BUME extraction was accurately transferred into a disposable glass culture test tube. The same internal standard mixture (Table 1) for quantitation of lipids was added prior to lipid extraction. Lipid extraction was performed by using a modified Bligh and Dyer procedure as described previously [20]. Each lipid extract was resuspended into a volume of 200 μL of chloroform/methanol (1:1, v/v) per mg of protein and flushed with nitrogen, capped, and stored at −20 °C for lipid analysis.
Derivatization of 4-Hydroxyalkenal Species
A certain amount of lipids (equivalent to 0.1 to 0.2 mg of tissue protein) was transferred from the lipid extracts into a disposable culture glass test tube with cap. To the lipid solution, 50 μL of carnosine aqueous solution (75 mmol/L) was added to derivatize 4-HA species as previously described [21]. All of the derivatives were reconstituted with 100 μL of water/methanol (1:1, v/v) after being washed with chloroform/methanol (1:1, v/v). The final derivative solutions were flushed with nitrogen, capped, and stored at −20 °C for ESI-MS analysis.
MS Analysis of Extracted Lipids
MS analysis of the extracted lipids by the modified BUME or Bligh and Dyer procedure was performed on a QqQ mass spectrometer (Thermo TSQ Vantage, San Jose, CA) equipped with an automated nanospray device (TriVersa NanoMate, Advion Bioscience Ltd., Ithaca, NY). Ionization voltage of 1.15 kV and spray gas pressure of 0.55 psi on the NanoMate apparatus were employed for the MS analyses. The nanospray device was controlled by Chipsoft 8.3.1 software. All MS or tandem MS analyses were operated under Xcalibur software as described [20]. Typically, a 2 to 3 min period of signal averaging from 1 s/scan in the survey scan mode was used for each MS spectrum, and a 4- to 5-min period of signal averaging from 1 s/scan was employed to acquire each tandem MS spectrum. Each lipid extract was diluted to a final concentration of < 50 μM with chloroform/methanol/isopropanol (1/2/4, v/v/v) or the same solution containing 0.02–0.06% (v/v) LiOH-saturated methanol solution for MS analysis.
Results
Comparison between Acetic Acid and Lithium Chloride present in Extraction
Plasmalogen species are a type of ether phospholipids with a vinyl ether linkage present at the sn-1 position and an ester linkage at the sn-2 position of the glycerol backbone. The most common head groups for plasmalogens in mammals are ethanolamine or choline glycerophospholipids, designated as ethanolamine plasmalogen (PlsEtn) or choline plasmalogen (PlsCho). These species are found in numerous tissues and organs, especially enriched in the nervous, immune, and cardiovascular systems [22, 23]. It has been reported that the plasmalogens can protect mammalian cells from damage induced by reactive oxygen species [22–24]. Additionally, they could function as signaling molecules and modulate cell membrane dynamics [22–24]. However, due to the vinyl ether linkage in plasmalogen molecules, these lipids are acid labile, resulting in the breakage of their vinyl-ether bonds. In order to investigate if the presence of 1% acetic acid in the aqueous phase of the original protocol [19] affects the quantification of plasmalogen species, we performed the BUME extraction on rat heart samples by using 1% acetic acid and 50 mmol/L LiCl solution as the aqueous phases. LiCl solution is very weakly acidic and its ionic strength could increase the recovery of lipids in the organic phase. Moreover, lithium adducts of many lipids show more abundant structural information than proton or ammonium adducts. Thus, lithium adducts of lipids have been widely used for lipid identification and quantification [25–28].
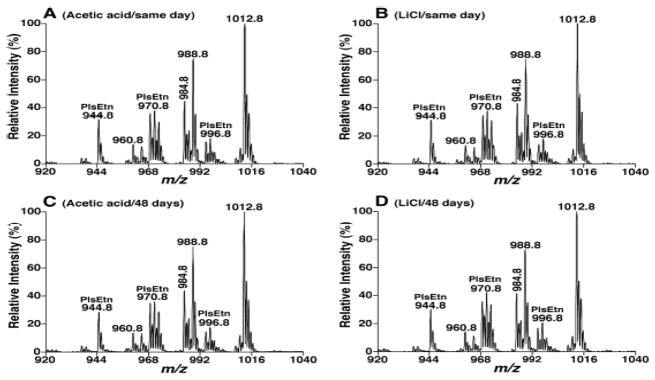
The lipid extracts from both methods were diluted and analyzed on the same day of extraction, and 48 days later as stored at −20 °C to determine the effects of acidic extraction on plasmalogen stability. The mass spectra from both extractions on different days were relatively compared with the strongest PtdEtn species (corresponding to 18:0–22:6 at m/z 1012.8 after Fmoc derivatization[29]) present in the extract. As shown in Figure 1, the relative abundance of all the EtnGpl species corresponding to plasmalogens were virtually identical for extracts obtained from both methods by using acetic acid and LiCl buffers when analyzed on the same day as extraction. Similarly, PlsEtn peaks from both samples also had no significant differences after analyses were conducted 48 days later with the trend of a decrease in relative abundance from extraction with acetic acid as much as 3% (such as PlsEtn 38:5 at m/z 970.8 after Fmoc derivatization). The acidic residues might play roles in the instabilities of PlsEtn species of rat heart in the solution.
Fig. 1.
Comparison of EtnGpl species present in rat myocardial extracts prepared with different buffer conditions and analyzed after different storage periods. Lipid extracts were prepared from rat heart with the BUME method in the presence of acetic acid or LiCl in the aqueous phase as described under the section of “Materials and Methods”. The lipid extracts were derivatized with Fmoc chloride prior to the analysis and EtnGpl species present in the derivatized lipid extracts were detected by neutral loss scan MS spectra of 222.2 Da (NLS222.2) as described under the section of “Materials and Methods”. Samples prepared with acetic acid (Panels A and C) and LiCl (Panels B and D) were analyzed on the day of extraction (Panels A and B) or after being stored for 48 days (Panels C and D). The MS spectra of NLS222.2 were acquired at collision energy of 26 eV and collision gas pressure of 1 mTorr.
Effects of Other Salt Solution on Lipid Extraction
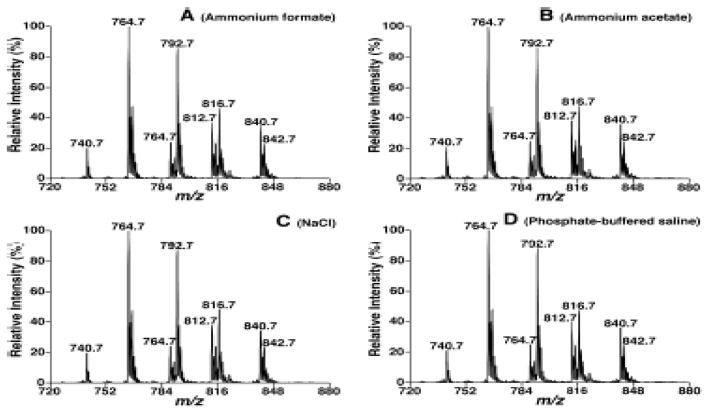
Other salt-based buffers were also used to modify the BUME method to determine if alternative salt-based buffers had the same extraction yields as the BUME method with an acetic acid or LiCl buffer. After 50 μL of rat plasma was treated with butanol/methanol (3:1 v/v) and heptane/EtAc (3:1 v/v) solution as described in the experimental section, 300 μL of either sodium chloride (50 mmol/L), ammonium acetate (50 mmol/L), ammonium formate (50 mmol/L), or PBS was added into the solution as the aqueous phase for lipid extraction. During the experiments, although all four buffers were able to induce the formation of separated aqueous and organic phases, each sample underwent centrifugation at 2,700 g for 10 minutes in order to yield the two separated layers, compared to 5 minutes of centrifugation with 1% acetic acid.
The relative extraction yields of each modified method on the rat plasma were determined by comparison of relative ion abundance. Phospholipids, sphingolipids (including Cer and CerPCho), and TAG were analyzed and compared, and the spectra of the extracts obtained from each sample treated with different salt-based buffers showed no significant differences, aside from a few variations in individual peaks. Figure 2 shows a comparison between lipid profiles of PtdCho species in each sample as an example. The results indicated high and comparable extraction yields for PC with variation ranging from 0 to 3.5%. The same small variations were also observed in other phospholipids, sphingolipid species, and TAG (data not shown).
Fig. 2.
The effects of salts used in extraction matrix on detection of ChoGpl species present in rat plasma. Tandem MS analysis of lipid extracts which were prepared by a modified BUME procedure in the presence of 50 mM ammonium formate (Panel A), 50 mM ammonium acetate (Panel B), 50 mM sodium chloride (Panel C), and diluted PBS solution (Panel D) in aqueous buffers by NLS189.2 was performed as described under the section of “Materials and Methods” at collision energy of 34 eV and collision gas pressure of 1 mTorr.
Effects of LiCl Concentrations on Extraction
The concentration of LiCl buffer was also optimized by performing BUME extractions on 50 μL of rat plasma samples using various concentrations of LiCl ranging from 25 to 50 mmol/L. The capability of each concentration to induce clear phase separation between organic and aqueous layers, and the extraction yield were compared between different LiCl concentrations. During the extraction procedure, the butanol/heptane phase could be clearly separated from 50 mmol/L of LiCl aqueous phase after centrifugation at 2,700 g for 10 minutes, whereas 25 mmol/L LiCl required centrifugation at 7,500 rpm for over 20 minutes. All other LiCl concentrations between 25 and 50 mM followed the general trend of higher concentrations of LiCl requiring slower and shorter centrifugation times than lower concentrations. Based on visual observations, it was concluded that the method with 50 mmol/L LiCl was the most efficient condition in forming distinct organic and aqueous layers. Each sample extracted with different concentrations of LiCl was analyzed by MS which showed no difference in extraction yields regardless of the concentrations of LiCl (data not shown).
Glass vs. Plastic Test Tubes
Because most of biological samples are kept in plastic tubes, such as snap-cap microcentrifuge tubes or cryogenic vials from sample collection, we investigated if the newly developed BUME method could be performed using the polypropylene vessels. Rat plasma (50 μL) was added into 2-mL plastic Eppendorf tubes and 6-mL glass tubes, respectively, and extracted with 300 μL of 50 mmol/L LiCl as buffer after mixing with 300 μL butanol/methanol (3:1, v/v) and 150 μL heptane/EtAc (3:1, v/v) solution. MS analysis showed a few very distinct anomalous peaks (such as those at m/z 536.6, 564.6, 592.6, 663.4 and 685.4) present in the full scan under the positive ion mode of the extraction carried out in the plastic Eppendorf tubes, but not in the glass tubes. However, under the negative ion scan mode, the spectra from extracts in plastic tubes showed similar ion profiles to the glass tubes. Therefore, the BUME extraction solution would still wash out some components in the plastic tubes, which would have severe ion suppression effects on lipid species, especially on the analysis in the positive-ion mode, and affect their accurate identification and quantification. Accordingly, it is advised that it would be better to perform the BUME extraction with glass test tubes.
Comparison of Lipid Recoveries between Modified BUME Method and Bligh and Dyer Extraction
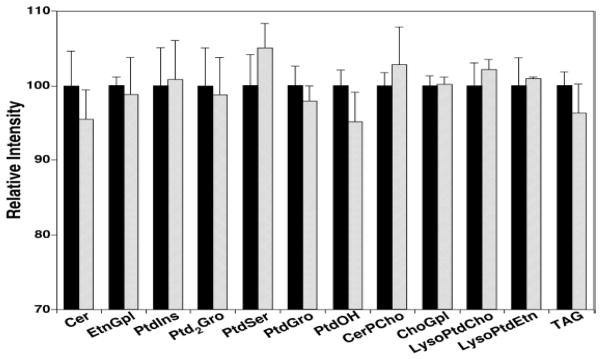
The modified BUME method with 50 mmol/L LiCl as aqueous phase instead of 1% acetic acid was compared to Bligh and Dyer extraction by relatively quantifying the endogenous lipids in rat liver samples from both extraction procedures as described in the experimental section. As seen in Figure 3, the modified BUME extraction yielded profiles statistically indistinguishable to those obtained from the commonly used Bligh and Dyer extraction for all of quantified lipid classes, including all phospholipids (ChoGpl, EtnGpl, PtdIns, PtdSer, PtdGro and PtdOH), some lyso phospholipids (lysoPtdCho and lysoPtdEtn), some sphingolipids (Cer and CerPCho), doubly charged Ptd2Gro, and nonpolar TAG. Although lipids with low abundances, like PtdOH and Cer, or nonpolar species, such as TAG, had approximate 5% reduction in the measured extraction yield when using BUME method, the differences were not statistically significant at all.
Fig. 3.
Comparison between the modified BUME and Bligh-Dyer procedures for extraction of endogenous lipids from a rat liver tissue. The total lipid amount of each class extracted by Bligh-Dyer method (Solid bars) was normalized to 100 as reference, and the amount of lipid extracted by modified BUME protocol (Open bars) was proportionally normalized.
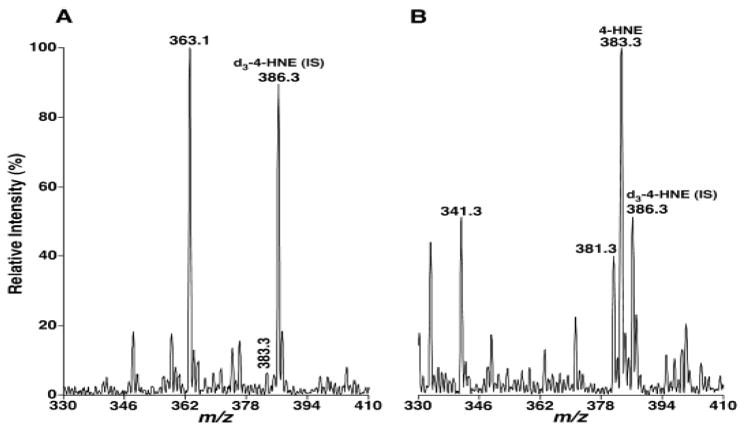
4-HA species, which serve as “toxic second messengers” in cellular systems [30], are a class of peroxidative products of polyunsaturated fatty acids. The accumulation of proteins modified by 4-HA are linked to the pathogenesis of numerous diseases, such as atherosclerosis, diabetes, muscular dystrophy, rheumatoid arthritis, actinic elastosis, cerebral ischemia, and neurodegenerative diseases (e.g., Alzheimer’s disease and Parkinson’s disease) [31–33]. We have developed a shotgun lipidomics-based method for quantification of these compounds directly from organic solvent lipid extracts of biological samples by the Bligh-Dyer method [21]. Here, the BUME method was also used to examine whether it is suitable for 4-HA extraction from biological samples. After the endogenous lipids in rat liver samples were extracted by both extraction procedures with the same amount of internal standard (i.e., d3-4-HNE, 0.7 nmol/mg protein) added, the extracts were directly reacted with a carnosine aqueous solution (75 mmol/L). As shown in Figure 4, the extract using the BUME method contained little 4-HNE species at m/z 383.3 with its intensity at around 5% of the abundance of its internal standard at m/z 386.3 (Figure 4a). However, the spectrum of Bligh-Dyer method showed a very intense 4-HNE peak, which was as much as twice that of its internal standard peak intensity (Figure 4b). Moreover, the Bligh-Dyer method also extracted other endogenous 4-HA species, such as 4-hydroxynondienal at m/z 381.3 and 4-hydroxyhexenal at m/z 341.2, from rat liver tissue, whereas the BUME procedure showed very low extraction recoveries of these 4-HA species. Therefore, these findings indicated that the BUME procedure is not suitable for 4-HA measurement, although very similar extraction recoveries from BUME and Bligh-Dyer methods were obtained for the analysis of the majority of phospholipids, sphingolipids, and non-polar TAG species.
Fig. 4.
Mass spectral comparison of 4-hydroxyalkenal measurements between the extracts from BUME and Bligh-Dyer methods. Derivatization of 4-hydroxyalkenal species was performed from lipid extracts of rat liver samples by BUME (Panel A) and Bligh-Dyer (Panel B) methods as described under the section of “Materials and Methods”. IS abbreviates internal standard.
Discussion
The BUME method significantly improves lipid extraction procedures in a number of ways. First, compared to the Bligh-Dyer method, it requires a small volume of solvents, thus decreasing the extraction cost and waste. Second, the BUME method also shortens procedure time, and makes lipid extraction more efficient; especially if it is possible to perform the extraction with an automated machine as described [19]. Modification to the BUME method by replacing acetic acid with a LiCl buffer showed similar extraction yields.
Although degradation was not significantly observed in the test samples, acid-labile lipid species, like plasmalogens, are ensured to not be affected by substituting the acidic buffer with a salt-based aqueous phase. Since 50 mmol/L of LiCl has a weaker ionic strength compared to 1% acetic acid (174 mmol/L), the modified BUME method requires centrifugation of samples to clearly separate the methanol-enriched aqueous phase from the butanol-enriched lipid phase, which makes it less possible for this procedure to be processed automatically. Löfgren et al. reported that the BUME method with an acetic acid buffer promotes gravitational separation in 5 minutes to form two clear phases [19]. However, when we extracted the rat plasma samples using the BUME method with 1% acetic acid, centrifugation was still required to avoid the contamination from the white cloudy denatured protein at the interface of butanol and aqueous phases, and to create a clear separation of the both phases with the proteins compacted in a thin layer in between. Regardless of the additional centrifugation step, the BUME method using either acidic or 50 mmol/L LiCl solution is still more time efficient than the traditional chloroform methods with similar lipid recoveries compared to the gold-standard Bligh-Dyer procedure. Although BUME extraction with alternative salt solutions showed slight variations in individual lipid peaks, the variations are negligible and do not affect the quantification of entire classes of lipids. Thus, it can be concluded that similar extraction yields can be obtained using a variety of other commonly used buffers. However, lithiated adducts of many lipid molecules yield abundant fragment ions carrying structural information, especially useful for identification of acyl chains and positional isomers [25–28], and have been widely used for lipid identification in our laboratory [25, 26]. Therefore, LiCl solution is the first choice as the aqueous component in the BUME method, even in any newly developed extraction protocol. The concentration of the salt solution is also an important consideration. While there are no significant variations in extraction yields of endogenous lipid classes with different concentrations of LiCl, the more concentrated LiCl promotes more efficient separation between butanol phase and aqueous phase. However, a lower concentration of LiCl solution makes less salt miscible into the organic phase, which can lower baseline noise in MS analysis. Therefore, it is preferable to use the minimal concentration of salt solution that promotes clear phase separation. Fifty mmol/L LiCl is sufficient and effective to form clear phase separation during BUME extraction while it produces clear mass spectra with strong signal to noise ratios.
Moreover, the anomalous contamination peaks present in the extractions carried out in plastic Eppendorf tubes using the BUME method indicate that butanol could also wash out some components in the polypropylene plastic tubes, although extraction performed in Eppendorf tubes could greatly reduce the cost of sample preparation. The very strong contamination peaks were only present in the survey scan under the positive ion mode without changes of lipid species profiles because the mass spectra under the negative ion mode or tandem mass spectra under positive mode were unaffected. However, the abundant contamination ions could still suppress other lipids for ionization and lead to narrower linear dynamic range, although the double filter function of tandem MS could get rid of the contamination ions. Therefore, the anomalous contamination peaks could still affect accurate quantification of lipid species as previously discussed [34].
Although both of the BUME and Bligh-Dyer extraction methods resulted in indistinguishable recoveries for most of lipid classes, like phospholipids, some sphingolipids, and TAG, the measurement of 4-HA species should be performed with the gold standard Bligh-Dyer extraction. Lipidomics is an emerging field that aims to comprehensively quantify as many lipid species as possible in a single sample. The endogenous lipids cover a broad array of polarity and solubility. Therefore, it is not possible to accurately extract all lipids of interest by one single method, and several extraction protocols have to be performed either in series or in parallel to achieve high-yield recoveries for lipids of interest [35–38].
In conclusion, it has been investigated that the BUME method is equally effective with the use of LiCl solution in replacing 1% acetic acid. Moreover, while this modification requires centrifugation and may not necessarily be conducted as an automated procedure, extraction time is still significantly reduced compared to the traditional chloroform-based methods, not to mention its advantages on low toxicity, which make it more environmentally friendly and less harmful to personal health. The modified BUME method is also extended to extract rat heart, liver, plasma samples, and potentially other biological tissues, culture cells, or biofluid samples with equally successful results.
Acknowledgments
This work was partially supported by National Institute of General Medical Sciences Grant R01 GM105724 and intramural institutional research funds.
Abbreviations
- 4-HA
4-hydroxyalkenal
- 4-HNE
4-hydroxy-2E-nonenal
- BUME
butanol-methanol
- Cer
ceramide
- CerPCho
sphingomyelin
- ChoGpl
choline glycerophospholipids
- ESI
electrospray ionization
- EtAc
ethyl acetate
- EtnGpl
ethanolamine glycerophospholipids
- Fmoc
Fluorenylmethyloxycarbonyl
- HPLC
high performance liquid chromatography
- lysoPtdCho
lyso phosphatidylglycerol
- lysoPtdEtn
lyso phosphatidylethanolamine
- IS
internal standard
- MS
mass spectrometry
- MTBE
methyl-tert-butyl ether
- nESI
nano-electrospray ionization
- NLS
neutral loss scan
- PlsEtn
ethanolamine plasmalogen
- PlsCho
choline plasmalogen
- PBS
phosphate-buffered saline
- Ptd2Gro
cardiolipin
- PtdCho
phosphatidylcholine
- PtdEtn
phosphatidylethanolamine
- PtdGro
phosphatidylglycerol
- PtdIns
phosphatidylinositol
- PtdOH
phosphatidic acid
- PtdSer
phosphatidylserine
- TAG
triacylglycerol(s)
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Wenk MR. The emerging field of lipidomics. Nat Rev Drug Discov. 2005;4:594–610. doi: 10.1038/nrd1776. [DOI] [PubMed] [Google Scholar]
- 2.Watson AD. Thematic review series: systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: a global approach to lipid analysis in biological systems. J Lipid Res. 2006;47:2101–2111. doi: 10.1194/jlr.R600022-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Wang M, Han X. Advanced Shotgun Lipidomics for Characterization of Altered Lipid Patterns in Neurodegenerative Diseases and Brain Injury. Methods Mol Biol. 2016;1303:405–422. doi: 10.1007/978-1-4939-2627-5_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie P, Kadegowda AK, Ma Y, Guo F, Han X, Wang M, Groban L, Xue B, Shi H, Li H, Yu L. Muscle-specific deletion of comparative gene identification-58 (CGI-58) causes muscle steatosis but improves insulin sensitivity in male mice. Endocrinology. 2015;156:1648–1658. doi: 10.1210/en.2014-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adamovich Y, Rousso-Noori L, Zwighaft Z, Neufeld-Cohen A, Golik M, Kraut-Cohen J, Wang M, Han X, Asher G. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab. 2014;19:319–330. doi: 10.1016/j.cmet.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spener F, Lagarde M, Geloen A, Record M. What is lipidomics? European Journal of Lipid Science and Technology. 2003;105:481–482. [Google Scholar]
- 7.Han X, Gross RW. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: a bridge to lipidomics. J Lipid Res. 2003;44:1071–1079. doi: 10.1194/jlr.R300004-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Roberts LD, McCombie G, Titman CM, Griffin JL. A matter of fat: an introduction to lipidomic profiling methods. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;871:174–181. doi: 10.1016/j.jchromb.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Brugger B, Erben G, Sandhoff R, Wieland FT, Lehmann WD. Quantitative analysis of biological membrane lipids at the low picomole level by nano-electrospray ionization tandem mass spectrometry. Proc Natl Acad Sci U S A. 1997;94:2339–2344. doi: 10.1073/pnas.94.6.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 11.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 12.Jung HR, Sylvanne T, Koistinen KM, Tarasov K, Kauhanen D, Ekroos K. High throughput quantitative molecular lipidomics. Biochim Biophys Acta. 2011;1811:925–934. doi: 10.1016/j.bbalip.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Heiskanen LA, Suoniemi M, Ta HX, Tarasov K, Ekroos K. Long-term performance and stability of molecular shotgun lipidomic analysis of human plasma samples. Anal Chem. 2013;85:8757–8763. doi: 10.1021/ac401857a. [DOI] [PubMed] [Google Scholar]
- 14.Schmid P, Calvert J, Steiner R. Physiol Chem Phys. 1973:5. [Google Scholar]
- 15.Cham BE, Knowles BR. A solvent system for delipidation of plasma or serum without protein precipitation. J Lipid Res. 1976;17:176–181. [PubMed] [Google Scholar]
- 16.Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J Lipid Res. 2008;49:1137–1146. doi: 10.1194/jlr.D700041-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hara A, Radin NS. Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem. 1978;90:420–426. doi: 10.1016/0003-2697(78)90046-5. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Z, Xu Y. An extremely simple method for extraction of lysophospholipids and phospholipids from blood samples. J Lipid Res. 2010;51:652–659. doi: 10.1194/jlr.D001503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lofgren L, Stahlman M, Forsberg GB, Saarinen S, Nilsson R, Hansson GI. The BUME method: a novel automated chloroform-free 96-well total lipid extraction method for blood plasma. J Lipid Res. 2012;53:1690–1700. doi: 10.1194/jlr.D023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M, Han X. Multidimensional mass spectrometry-based shotgun lipidomics. Methods Mol Biol. 2014;1198:203–220. doi: 10.1007/978-1-4939-1258-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang M, Fang H, Han X. Shotgun lipidomics analysis of 4-hydroxyalkenal species directly from lipid extracts after one-step in situ derivatization. Anal Chem. 2012;84:4580–4586. doi: 10.1021/ac300695p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagan N, Zoeller RA. Plasmalogens: biosynthesis and functions. Prog Lipid Res. 2001;40:199–229. doi: 10.1016/s0163-7827(01)00003-0. [DOI] [PubMed] [Google Scholar]
- 23.Gorgas K, Teigler A, Komljenovic D, Just WW. The ether lipid-deficient mouse: tracking down plasmalogen functions. Biochim Biophys Acta. 2006;1763:1511–1526. doi: 10.1016/j.bbamcr.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 24.Moser AB, Steinberg SJ, Watkins PA, Moser HW, Ramaswamy K, Siegmund KD, Lee DR, Ely JJ, Ryder OA, Hacia JG. Human and great ape red blood cells differ in plasmalogen levels and composition. Lipids Health Dis. 2011;10:101. doi: 10.1186/1476-511X-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Hayakawa J, Yang K, Han X. Characterization and quantification of diacylglycerol species in biological extracts after one-step derivatization: a shotgun lipidomics approach. Anal Chem. 2014;86:2146–2155. doi: 10.1021/ac403798q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang K, Zhao Z, Gross RW, Han X. Systematic analysis of choline-containing phospholipids using multi-dimensional mass spectrometry-based shotgun lipidomics. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2924–2936. doi: 10.1016/j.jchromb.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu FF, Turk J. Distinction among isomeric unsaturated fatty acids as lithiated adducts by electrospray ionization mass spectrometry using low energy collisionally activated dissociation on a triple stage quadrupole instrument. J Am Soc Mass Spectrom. 1999;10:600–612. doi: 10.1016/S1044-0305(99)00041-0. [DOI] [PubMed] [Google Scholar]
- 28.Hsu FF, Turk J. Structural characterization of triacylglycerols as lithiated adducts by electrospray ionization mass spectrometry using low-energy collisionally activated dissociation on a triple stage quadrupole instrument. J Am Soc Mass Spectrom. 1999;10:587–599. doi: 10.1016/S1044-0305(99)00035-5. [DOI] [PubMed] [Google Scholar]
- 29.Han X, Yang K, Cheng H, Fikes KN, Gross RW. Shotgun lipidomics of phosphoethanolamine-containing lipids in biological samples after one-step in situ derivatization. J Lipid Res. 2005;46:1548–1560. doi: 10.1194/jlr.D500007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uchida K, Shiraishi M, Naito Y, Torii Y, Nakamura Y, Osawa T. Activation of stress signaling pathways by the end product of lipid peroxidation. 4-hydroxy-2-nonenal is a potential inducer of intracellular peroxide production. J Biol Chem. 1999;274:2234–2242. doi: 10.1074/jbc.274.4.2234. [DOI] [PubMed] [Google Scholar]
- 31.Poli G, Schaur RJ. 4-Hydroxynonenal in the pathomechanisms of oxidative stress. IUBMB Life. 2000;50:315–321. doi: 10.1080/713803726. [DOI] [PubMed] [Google Scholar]
- 32.Uchida K. Role of reactive aldehyde in cardiovascular diseases. Free Radic Biol Med. 2000;28:1685–1696. doi: 10.1016/s0891-5849(00)00226-4. [DOI] [PubMed] [Google Scholar]
- 33.Stadtman ER. Protein oxidation in aging and age-related diseases. Ann N Y Acad Sci. 2001;928:22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- 34.Wang M, Wang C, Han X. Selection of internal standards for accurate quantification of complex lipid species in biological extracts by electrospray ionization mass spectrometry-What, how and why? Mass Spectrom Rev. 2016 doi: 10.1002/mas.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C, Wang M, Han X. Comprehensive and quantitative analysis of lysophospholipid molecular species present in obese mouse liver by shotgun lipidomics. Anal Chem. 2015;87:4879–4887. doi: 10.1021/acs.analchem.5b00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powell WS. Rapid extraction of arachidonic acid metabolites from biological samples using octadecylsilyl silica. Methods Enzymol. 1982;86:467–477. doi: 10.1016/0076-6879(82)86218-6. [DOI] [PubMed] [Google Scholar]
- 37.Jiang X, Han X. Characterization and direct quantitation of sphingoid base-1-phosphates from lipid extracts: A shotgun lipidomics approach. J Lipid Res. 2006;47:1865–1873. doi: 10.1194/jlr.D600012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai T, Shu Q, Hou J, Liu P, Niu L, Guo X, Liu CC, Yang F. Profiling and relative quantitation of phosphoinositides by multiple precursor ion scanning based on phosphate methylation and isotopic labeling. Anal Chem. 2015;87:513–521. doi: 10.1021/ac503224j. [DOI] [PubMed] [Google Scholar]