Abstract
The E2F1 transcription factor is a recognized regulator of the cell cycle as well as a potent mediator of DNA damage-induced apoptosis and the checkpoint response. Understanding the diverse and seemingly dichotomous functions of E2F1 activity has been the focus of extensive ongoing research. Although the E2F pathway is frequently deregulated in cancer, the contributions of E2F1 itself to tumorigenesis, as a promoter of proliferation or cell death, are far from understood. In this review we aim to provide an update on our current understanding of E2F1, with particular insight into its novel interaction partners and post-translational modifications, as a means to explaining its diverse functional complexity.
Keywords: apoptosis, cancer, cell cycle, DNA damage response, E2F1, epigenetics
Abbreviations
- APAF
apoptotic protease activating factor
- ARID
AT-rich interaction domain
- ATM
ataxia telangiectasia mutated protein
- ATR
ataxia telangiectasia and Rad3-related protein
- ANCCA
AAA nuclear coregulator cancer-associated protein
- BAK
BCL2-antagonist/killer 1
- BARD
BRCA1 associated RING domain
- BASC
BRCA1-associated genome surveillance complex
- BAX
BCL2-associated X protein
- BCL2
B-cell CLL/lymphoma 2
- BCL-xL
B-cell lymphoma-extra large
- BH
Bcl-2 homology domain
- BIM
BCL2-like 11
- BRCA
breast cancer n, early onset
- BRCT
BRCA1 C-terminus domain
- BRG
Brahma-related gene 1 protein (ATPase)
- BRM
homolog of Drosophila melanogaster Brahma protein
- CBP
CREB binding protein
- CDC
cell division control protein
- CDK
cyclin-dependent kinase
- CHD
chromodomain helicase DNA binding protein
- CycA
cyclin A binding site
- DBD
DNA binding domain
- DP
E2F dimerization partner
- E2F
E2 promoter binding factor
- ERK
ELK-related tyrosine kinase
- FAK
focal adhesion kinase
- HAT
histone acetyl transferase
- HCF
host cell factor
- HDAC
histone deacetylase
- HPV
human papilloma virus
- IAP
inhibitors of apoptosis
- MB
marked box
- MCL1
myeloid cell leukemia 1
- MCM
minichromosome maintenance complex
- miRNA/miR
microRNA
- MLL
mixed lineage leukemia protein
- MPF
maturation-promoting factor
- MYB
myeloblastosis oncogene
- MYC
myelocytomatosis oncogene
- NEDD
neural precursor cell expressed, developmentally down-regulated
- NLS
nuclear localization signal
- PCAF
p300/CBP-associated factor
- PCNA
proliferating cell nuclear antigen
- PI3K
phosphatidylinositol-4,5-bisphosphate 3-kinase
- PRKCD
protein kinase C, delta
- PRMT
protein arginine methyltransferase
- PTP-PEST, protein tyrosine phosphatase with PEST motif (rich in P, E
S and T residues)
- PUMA
p53 upregulated modulator of apoptosis
- RAS
rat sarcoma viral oncogene homolog
- pRB
retinoblastoma protein
- RING
really interesting new gene
- SHP
Src homology 2 (SH2) domain-containing protein tyrosine phosphatase
- SKP
S-phase kinase-associated protein
- SMAC/DIABLO
second mitochondrial-derived activator of caspase/direct IAP-binding protein with low pI
- SWI/SNF
switch/sucrose nonfermenting
- TAD
transactivation domain
- TBP
TATA binding protein
- TIP48/49
TBP/transcription activating interacting protein
- TIP60
TAT interacting protein 60kDa
- TSN
translin
- WDR
WD repeat domain containing
Introduction
The E2F family is a group of 8 transcription factors, loosely assigned as activators (E2F1–3) or repressors (E2F4–8) of transcription. E2F subunits collectively play a crucial role in control of the cell cycle and cellular proliferation, in addition to numerous other functions, including differentiation, DNA damage checkpoints, and metabolism. Although the activator E2F family members have been shown to exhibit extensive functional redundancy and overlap in vivo, there is nonetheless a certain degree of target gene specificity and tissue dependency in their effects. In particular, among all the subunits explored, E2F1 has been associated with apoptosis and the checkpoint response.1,2
E2F1 shows structural homology with other E2F activators. It has a single DNA binding domain and heterodimerizes with a dimerization partner (DP1/DP2) in order to effectively bind DNA and recruit other transcription factors and co-activators to target gene promoters. A third DP protein has recently been discovered, which, unlike DP1 and DP2, is an inhibitory binding partner and reduces E2F1-dependent gene transcription.3 E2F1–DP1/2 heterodimers are inhibited by a family of pocket proteins, pRB, p109, and p130, that are named for a groove between their conserved A and B domains. The pocket proteins exhibit varying degrees of binding specificity for E2F subunits, with E2F1 preferentially binding to pRB. pRB-mediated suppression of E2F1 activity occurs at 2 levels: passively, in which pRB binds and hinders the transcriptional activation domain of E2F1, and actively, in which pRB recruits histone deacetylases (HDACs) and SWI/SNF complexes to alter the local chromatin structure of E2F target genes.4,5
Here, we provide an overview of the diverse functional roles of E2F1, the large host of target genes, the extensive network of interactions, and the plethora of post-translational modifications that define the current status of research addressing this crucial transcription factor. We propose that the levels of E2F1 protein, its precise constellation of post-translational modifications, and resulting interaction partners define the unique biological functions of E2F1 (Fig. 1).
Figure 1 (See previous page).
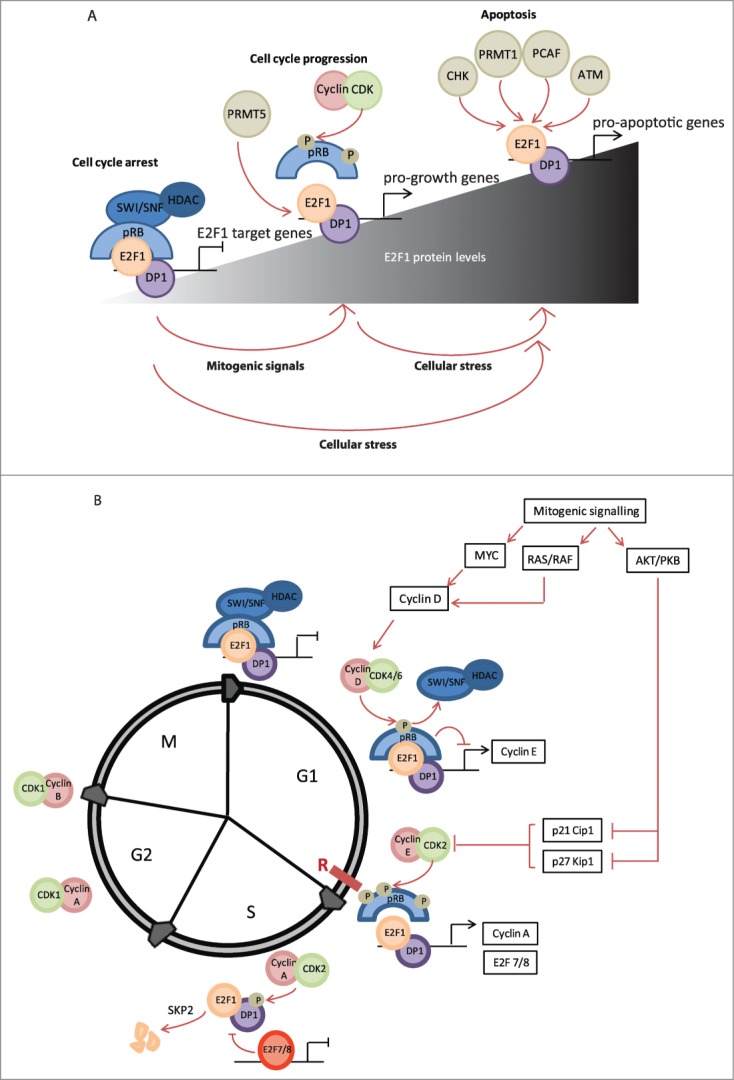
Cell cycle-dependent regulation of E2F1. (A) It is hypothesized that the E2F1–DP1 heterodimer can mediate proliferation versus apoptosis depending on the cellular levels of E2F1 and its post-translational modifications. Upon mitogenic stimulation binding of pRB is reduced and E2F1 can enhance transcription of cell cycle target genes. However, after DNA damage E2F1 is stabilized by various post-translational modifications and this results in transcription of pro-apoptotic target genes. (B) During early G1 phase E2F1 is maintained in an inactive state via interaction with pRB; this blocks the transcriptional activation domain of E2F1 and furthermore recruits HDACs and the SWI/SNF complex to actively suppress transcription from E2F1 target genes. Upon mitogenic stimulation, cyclin D levels are induced; cyclin D forms a complex with CDK4–6 that phosphorylates pRB and relieves the inhibition from HDACs and the SWI/SNF complex. This allows transcription of several E2F1 target genes, including cyclin E, to proceed. As cyclin E levels accumulate and cells progress toward late G1, the CDK2-cyclin E complex is formed, which hyperphosphorylates pRB to completely dissociate it from E2F1. Subsequently, transcription of several other E2F1 target genes is induced, including cyclin A and DNA replication genes. During late S phase E2F1 is inactivated, the DP1 binding partner is targeted for phosphorylation by CDK2–cyclin A, and E2F1 targeted for degradation by SKP2 and transcriptional repression by E2F7/8.
Regulation of E2F1 in the Cell Cycle
The restriction point (R-point) is a position in G1 after which cells become committed to completing the cell cycle in a mitogen-independent manner. The all-or-nothing nature of this response is stimulated by a variety of mitogenic signals that become converted into a binary switch. As E2F1 upregulates the transcription of genes required for cells to progress into late G1/S phase and advance the cell cycle, it has been suggested to be a mediator of this switch. Since prolonged or excessive E2F1 expression results in G1 checkpoint activation and apoptosis, in order to prevent inappropriate reinitiation of DNA synthesis, E2F1 activity is subject to complex regulatory cues during cell cycle progression (Fig. 1).6
Signal amplification contributes to cell cycle progression
Mitogenic signaling culminates in MYC activation and upregulation of target genes including E2F1, -2, and -3 as well as cyclin D, CDK4, and CDC25. Subsequently, pRB undergoes sequential phosphorylation by cyclin D–CDK4/6 and cyclin E–CDK2 at multiple serine (S) and threonine (T) residues, causing dissociation of E2F1 from the pocket of pRB. One study demonstrated that phosphorylation at T356/373 and S788/795 causes a 10-fold reduction in binding of E2F1 to the pocket.7 It is believed that rapid positive feedback amplifies these effects at late G1 through accumulation of active E2F1 because E2F1 upregulates the transcription of E2F1–3, cyclin E, and MYC to both increase E2F1 protein levels and further inhibit pRB (Fig. 1 and Table 1).8
Table 1.
E2F1 target genes
| Gene | Function | Ref | |
|---|---|---|---|
| Cell cycle | E2F1 | Transcription factor with roles in many cellular processes | 38 |
| E2F2 | Similar to E2F1, it shares many E2F1 target genes | 28 | |
| E2F3 | Similar to E2F1, it shares many E2F1 target genes | 28 | |
| POLA1 | Catalytic subunit of DNA polymerase required for DNA synthesis | 26,28 | |
| CDK1 | Catalytic subunit of MPF complex required for both G1/S and G2/M transitions | 26,29 | |
| CCNB1 | Forms part of the MPF with CDK1 to regulate mitosis | 29 | |
| CCNA2 | Cyclin A2 binds to CDK2 to drive DNA synthesis from pre-formed replication complexes | 28,29 | |
| CCNE | Cyclin E binds to CDK2 to stimulate formation of the DNA replication complex | 26,29 | |
| CDK2 | Catalytic subunit that binds cyclins E and A to facilitate G1/S transition | 28 | |
| MCM3 | Part of the MCM pre-replication complex required for DNA replication | 27,28 | |
| MCM6 | Another constituent of the MCM complex required for DNA replication | 27,28 | |
| CDC6 | Part of the prereplication complex, stimulates DNA replication | 27,28 | |
| TYMS | Thymidylate synthase; catalyzes the reaction of deoxyuridylate to deoxythymidylate to generate dTMP | 29 | |
| FEN1 | Processes Okazaki fragments during DNA replication | 26,28,29 | |
| TOP2A | Topoisomerase II breaks and repairs DNA to relieve supercoiling during DNA replication and transcription | 26,28,29 | |
| DNA damage response | RAD52 | Binds single-stranded DNA ends to stimulate double-strand break repair and homologous recombination | 51 |
| PCNA | Cofactor of DNA polymerase delta, also plays a role in DNA repair. | 27,28 | |
| MLH1 | Promotes mismatch repair | 28 | |
| RAD54L | Involved in homologous recombination and DNA repair | 28 | |
| LIG1 | DNA ligase with roles in DNA replication and base excision repair | 29 | |
| BRCA1 | Part of the BASC complex, which has roles in DNA recombination and repair of double-strand breaks | 26 | |
| MSH2 | Homolog of E.coli mismatch repair gene | 26 | |
| Apoptosis | TP73 | Encodes p73, a transcription factor that targets proapoptotic and DNA repair genes | 51 |
| CASP3 | Caspase-3, an effector caspase that drives apoptosis by proteolytically processing a range of proteins | 53 | |
| APAF1 | Becomes active after cytochrome c release from the mitochondria and drives caspase-9 activation | 53 | |
| CASP7 | Caspase-7, another effector caspase involved in apoptosis | 53 | |
| BAD | Encodes a BH3 family member that inhibits antiapoptotic BCL-2 and BCL-xL | 51 | |
| BAK1 | Forms homo-oligomers at the mitochondrial membrane to facilitate outer membrane permeabilization and release of cytochrome c | 51 | |
| TP53 | Encodes the p53 transcription factor, implicated in DNA damage checkpoints | 28,51 | |
| Metabolism | COX7C | Cytochrome c oxidase subunit 7C, part of cytochrome c oxidase, the final part of the electron transport chain required for oxidative metabolism | 26,27 |
| COX4 | Cytochrome c oxidase subunit 4, another component of cytochrome c oxidase | 26 | |
| SLC25A10 | Mitochondrial dicarboxylate carrier, transports malate and succinate across the inner mitochondrial membrane. Implicated in the TCA cycle and fatty acid synthesis | 26 | |
| PDK1 | Pyruvate dehydrogenase complex, catalyzes the first step of the conversion of pyruvate to acetyl-coA in glycolysis | 26,27 | |
| VARS | tRNA valine synthetase links valine with its corresponding tRNA | 26,27 | |
| FH | Fumarate hydratase, catalyzes the formation of malate from fumarate in the TCA cycle | 26,27 | |
| MAP1LC3 | Encodes LC3, which binds to the phagophore membrane during elongation to form the autophagosome | 39 | |
| ATG1/ULK1 | Autophagy regulated 1 protein forms part of the ULK1 complex that associates with membranes to form the autophagosome | 39 | |
| DRAM1 | DNA-damage regulated autophagy modulator 1, enhances lysosyme acidification and fusion with autophagosomes | 39 | |
| Other | VEGFB | Binds to VEGFR1 at the cell surface to stimulate angiogenesis | 51 |
| MMP16 | Metalloproteinase, breaks down components of the extracellular matrix | 51 | |
| SLC3A2 | Transmembrane protein that carries both calcium and amino acids across the plasma membrane | 28 | |
| ABCG2 | ATP-binding cassette sub-family G member 2, multidrug transporter involved in removing toxins from cells. Can also lead to drug resistance in cancer cells. | 46 | |
| TRAF2 | Part of the TNF receptor signaling pathway resulting in NFκB production, also recruits IAPs to inhibit apoptosis | 28 |
Delayed negative feedback inhibits E2F1 in S phase
The mechanism for switching off E2F1 is likely through negative feedback responses from the products of E2F1 target genes (Fig. 1B). However, these genes have a different pattern of expression; cyclin E peaks at late G1/S whereas cyclin A starts to be expressed in S phase and peaks at G2. Thus, the proteins involved in negative feedback loops have a delayed expression. This lag is crucial to ensure that the cell passes into S phase. The mechanism of this delay has been described for cyclin A expression, although it remains unclear whether this mechanism is shared by the other delayed targets.9,10
Like other E2F1 targets, the cyclin A promoter is repressed by E2F1–pRB complexes, which inhibit expression until cyclin E is present at high enough levels (at late G1/S) to cause phosphorylation of pRB and subsequent gene activation. However, the cyclin A promoter has atypical E2F1 binding sites that may be less sensitive to gene activation than the ‘early’ genes, thereby creating a delay in gene expression. It has also been suggested that whereas the cyclin E promoter is repressed by a HDAC–pRB–hSWI/SNF complex that dissociates after pRB phosphorylation and allows gene expression, the cyclin A promoter is repressed by a pRB-hSWI/SNF complex that is more stable (i.e., less effected by pRB phosphorylation). Instead, the latter complex requires phosphorylation of BRG1 (a component of the SWI/SNF complex) at S phase, which results in derepression of the cyclin A promoter. This ensures that cyclin E is always expressed before cyclin A, which is absolutely necessary for correct initiation of DNA replication. Cyclin A–CDK2 then forms complexes with E2F1 that enable phosphorylation of DP1, resulting in reduced affinity for DNA and repression of transcription. Additionally, cyclins D and E are downregulated at both a transcriptional level (as E2F1 becomes inactive) and through degradation, resulting in reduced pRB inhibition and therefore greater E2F1 repression.9-11
Other E2F target genes with delayed expression include the repressor E2F7 and 8 subunits that can bind to E2F target genes; however, because they lack a transcriptional activation domain this does not result in active transcription but rather acts to prevent the expression of E2F target genes.12 SKP2 is another E2F delayed target gene that peaks during S phase; subsequent ubiquitination of E2F1 by the SKP2 ubiquitin ligase enhances its degradation.13
E2F1 Plays a Complex Role in Cancer
As the functions of a large host of E2F1 target genes are tied to promoting cell cycle progression, such as DNA replication and G1/S phase transition, E2F1 has been considered to be tumor promoting, with its deregulation contributing to unrestrained cell cycle progression. However, mutations in E2F1 itself are rare; the COSMIC database lists 43 somatic mutations in E2F1, compared to 1,143 found in pRB and 7,716 in p53.14 As well as undergoing loss-of-function mutation, the pRB gene is subject to chromosomal deletion in some cancers.15 Additionally, adenovirus, human papilloma virus (HPV), and simian virus 40 (SV40) all encode proteins that contain a pRB-binding LXCXE motif, which causes E2F1 displacement from pRB and deregulation of E2F1. Not surprisingly, overexpression or gain-of-function mutations of CDK4/6 can also result in E2F1 pathway activation since enhanced CDK phosphorylation of pRB promotes release of E2F1. Interestingly, many gain-of-function mutations in CDK4 are in the p16INK4A binding site. p16INK4A binding inhibits CDK4 activity, therefore inactivation of p16INK4a is also seen frequently in tumors through mutation or gene silencing, for example through promoter methylation.15-17
Accordingly, overexpression of E2F1 (under the keratin 5 promoter) in mouse models predisposes the animals to cancers, predominantly of the vaginal, skin, and fore stomach epithelium, as the cells lose normal control of the cell cycle. However, as increased E2F1 activity can result in transcription of p14ARF and therefore activation of p53, among other proapoptotic targets aberrations in the p53 pathway are especially common in tumors that have pRB inactivation. Thus, tumor formation is enhanced when functional p53 is also absent.18-20
Conversely, E2F1−/− mice also show spontaneous tumor development that appears to be tissue specific. The most common tumors are histiosarcomas, hemiangiosarcomas, hepatocarcinomas, and lung tumors. This tumor disposition is possibly due to loss of an E2F1 checkpoint and perhaps reduced expression of proapoptotic target genes, thus supporting a tumor suppressor-like role of E2F1.21,22 Accordingly, somatic mutations of DP1, which reduce E2F1–DP1 transcriptional activity, can decrease the levels of apoptosis after DNA damage.23 Overall, these findings suggest that the loss of E2F1 activity facilitates tumor progression by reducing cellular apoptosis. Indeed, it may be appropriate to consider E2F1 as a protein involved in checkpoint control given its diverse functional roles in cell cycle progression, DNA repair, and apoptosis.
A plethora of E2F1 target genes are required for cell cycle progression, many of which are directly involved in DNA synthesis. Microarray-based studies have allowed genome-wide identification of E2F1 target genes, although a thorough consideration of even a subset of these is beyond the scope of this review. Consequently we have highlighted select groups of E2F targets that reflect the diverse roles taken on by E2F1 (Table 1).
In addition to cyclins, E2F1 augments the expression of MCM2–7 proteins that form the preinitiation complex required for subsequent DNA replication. Cyclin E–CDK2 phosphorylates CDC6 and stabilizes it, enabling MCM2 complex assembly at late G1. Cyclin A–CDK2 upregulation at S phase activates these complexes to initiate DNA synthesis, but also inhibits (via downregulation of cyclin E) the assembly of new complexes to ensure the DNA is replicated once, and only once, per cycle.24,25 Other components of the DNA replication machinery shown to be targets of E2F1 include proliferating cell nuclear antigen (PCNA, which tethers DNA polymerase to the DNA), DNA primase (which creates an RNA primer to enable subsequent elongation as DNA replication starts), flap endonuclease (for Okazaki fragment maturation), and topoisomerase II (which relieves supercoiling and replication stress). Additionally, thymidylate synthase and dihydrofolate reductase are both enzymes whose nucleotide precursor products are essential for DNA replication (Table 1).26-28
E2F1 also upregulates a host of other transcription factors including MYB, MYC, and TATA box binding protein (TBP), creating a second wave of transcription required for progression through the cell cycle. Interestingly, some E2F1 targets are required for the G2/M phase of the cell cycle despite the fact that E2F1 is largely inactive at this point. It has been suggested that early remodeling of the chromatin by E2F1 might facilitate subsequent upregulation of these genes later in the cell cycle, thus signifying an indirect role for E2F1 in expression of these target genes.28,29
E2F1 and Epigenetics
The epigenetic landscape of a cell is integrated with the cell cycle, with various histone modifications peaking and declining at particular phases. As these phases are largely controlled by the E2F transcription factors, it is not surprising that E2F1 can be found associated with several epigenetic regulators, coinciding with particular histone modifications and gene expression outcomes (Table 2).30
Table 2.
E2F1 interaction partners
| Interactor | Function | Reference |
|---|---|---|
| ACTR | Co-activator for nuclear hormone receptors; enhances estrogen-induced cell proliferation; interacts with N-terminal domain of E2F1 to augment transcription of cell cycle-associated target genes | 75 |
| ANCCA | Bromodomain-containing member of the ATPase family; interacts with E2F1 and recruits MLL1 and MLL2 to E2F1 target promoters | 76 |
| ARID1B | Component of SWI/SNF remodeling complex; interacts with E2F1 and recruits SWI/SNF complex to cause their transcriptional activation | 37 |
| BRD2 | Transcriptional regulator of the BET family; interacts with E2F1 and augments transcription of cell cycle associated genes; recruits the transcriptional activator TBP | 77,78 |
| CHD8 | ATP-dependent chromatin remodeling protein of the SNF2 family; recruits MLL methyltransferases to E2F1 target promoters | 36 |
| DP | A family of 3 transcription factors (DP1, 2 and 3) that heterodimerize with E2F members to cooperatively bind DNA | 3 |
| HCF-1 | Member of the Host cell factor family, involved in control of the cell cycle; interacts with E2F1 and recruits the MLL and SET1 methyltransferases to E2F1 target promoters | 34 |
| JAB1 | A subunit of the COP9 signalosome, involved in multiple signaling pathways; interacts with the marked box of E2F1 and serves as a proapoptotic coactivator | 79 |
| MDM2 | Oncoprotein and main negative regulator of p53; interacts with the transactivation of E2F1 and increases activity of the E2F1/DP1 heterodimer | 80 |
| MDMX | Binds E2F1 close to the DNA binding domain; reduces DNA binding and hence transcriptional activity without altering the levels of E2F1 | 81 |
| NPDC1 | Neural factor involved in the control of cell proliferation and differentiation; interacts with E2F1 to reduce DNA binding and transcriptional activity; influences neural differentiation through association with E2F1 | 82 |
| PARP | Chromatin-associated enzyme that modifies nuclear proteins by ADP-ribosylation; associates with E2F1 and promotes S-phase entry | 83 |
| pRB | A member of the pocket proteins, which interact with E2F1 to suppress its transcriptional activity | 5 |
| TopBP1 | Interacts with E2F1 in a DNA damage and ATM-mediated phosphorylation-dependent manner; inhibits E2F1-dependent apoptosis | 54 |
| TRIM28 | Transcriptional regulator of tripartite motif family; binds E2F1 in a pRB-independent fashion to inhibit its transcriptional activity | 84 |
| TRRAP | Component of several acetyltransferases; interacts with E2F1 and helps recruit the TIP60 HAT to E2F1 target promoters | 32 |
| TSN | Tudor domain containing protein, interacts with methylated E2F1 to suppress its apoptotic activity | 66 |
Histone acetylation
As cells progress through G1 and approach S phase there is an increase in global acetylation levels. This is associated with gene activation, as it disrupts the DNA–histone interaction to open up chromatin and thus facilitates binding of transcription factors and RNA polymerase to the underlying DNA sequence.31 Interestingly, the cell cycle-dependent recruitment of E2F1–3 to chromatin during G1-S has also been shown to coincide with the acquisition of activating histone marks on the local chromatin, particularly acetylation on H3 and H4. Indeed, overexpression of a dominant-negative E2F1 mutant, which abolishes E2F1 DNA binding, can eliminate the accumulation of H4 acetylation during late G1 and reduce H3 acetylation, suggesting a relationship between the recruitment of E2F1 and acquisition of histone acetylation.32
In line with this, E2F1 has been reported to interact with the histone acetyl transferase (HAT) adaptor protein, TRRAP, which is a component of several acetyl transferases and is involved in transcription and DNA repair. In one study this interaction was shown to be important for the E2F1-dependent recruitment of all subunits of the TIP60 HAT (TIP60, TRRAP, p400, TIP48, and TIP49) to target promoters in vivo. Critically, it was shown that following mitogenic stimulation at late G1, the E2F1-dependent recruitment of TIP60 to chromatin could directly promote hyperacetylation of H3 and H4 and thus facilitate the expression of proliferation-associated genes as well as facilitating DNA replication.32
Histone methylation
Histone methylation on lysine residues has differential impacts on gene expression: methylation of H3K9, H3K27, and H4K20 is associated with gene repression, whereas methylation of H3K4, H3K36 and H3K79 is associated with gene activation. The levels of these methylation events fluctuate in a cell cycle-dependent manner. For example, ChIP studies have shown that active marks occupy the active promoters of PCNA during G1-S transition, but are absent from the inactive promoter of cyclin B.30,33
The host cell factor HCF-1 is implicated in control of the cell cycle and associates with both repressive and activator E2F members. Importantly, during G1-S transition HCF-1 interacts with E2F1 and recruits the H3K4 methyl transferases MLL and SET1 to E2F target promoters to induce their transcriptional activation. siRNA-mediated suppression of HCF-1 levels has been shown to reduce H3K4 trimethylation on E2F1 target genes.34 In another study, the bromodomain protein ANCCA was found to interact with E2F1 and subsequently recruit MLL1 and MLL2 to target promoters. The occupation of ANCCA on E2F target genes is cell cycle regulated, and its knockdown impairs expression of cell cycle-associated genes and inhibits proliferation.35 Similarly, CHD8, an ATP-dependent chromatin remodeling protein of the SNF2 family, interacts with WDR5, a common component of MLL1, MLL2, and SET1, and is required for its recruitment to E2F target genes. Accordingly, depletion of CHD8 was shown to reduce H3K4 trimethylation on E2F target promoters, reduce expression levels of cell cycle regulated genes, and suppress cell cycle progression.36
Nucleosome remodeling
The SWI/SNF nucleosome remodeling complex alters the chromatin structure in an ATP-dependent manner, which can play either a positive or negative role in transcription. The complex has a core ATPase (either BRG1 or BRM), plus 7 non-catalytic subunits. Two variants of one of the major subunits of the complex, ARID1A and ARID1B, influence the subsequent association of SWI/SNF with other activator or repressor factors and therefore direct this complex to different cellular outcomes.37
Although repressor E2F subunits associate with both ARID1 subunits, E2F1 can only associate with ARID1B. This in turn has been shown to coincide with recruitment of the SWI/SNF complex to cell cycle gene promoters and their transcriptional activation. Indeed, depleting cells of ARID1B and serum results in cell cycle arrest, yet delayed cell cycle entry occurs when cells are replenished with serum.37
The SWI/SNF complex also negatively regulates gene transcription, as illustrated by a study on TOPBP1-mediated E2F1 repression. TOPBP1 is a BRCT domain containing protein that is capable of interacting with the SWI/SNF remodeling complex through its core subunits BRG1/BRM. It is induced by E2F1 and interacts with it during G1-S transition, thus suppressing its transcriptional activity. This repression of E2F1 by TOPBP1 is critically dependent on BRG1/BRM, and is important for dampening E2F1-mediated apoptosis during normal cell cycle progression.38
Overall, many lines of investigation suggest a role for epigenetic modifiers in modulating the activity of E2F1 and its impact on the cell cycle.
DNA Damage Response and E2F1
E2F1 is stabilized under conditions of DNA damage with kinetics similar to those of p53, and can prompt DNA repair or apoptosis. The accumulation of E2F1 under these conditions, and its selectivity toward apoptotic genes, is partly influenced by post-translational modifications (Figs. 1A and 2B) as these alter the stability, target gene specificity, cellular localization, and interaction of E2F1 with other binding partners. Accumulation of E2F1 as a result of post-translational modifications is believed to drive E2F1-mediated apoptosis because a large subset of E2F1 target genes, including p73, APAF1, and caspase genes, are directly implicated in the apoptotic response and thus might help mediate the tumor suppressive effects of E2F1 (Fig. 2A). However, E2F1 can also stimulate upregulation of DNA repair target genes such as RAD51 and BARD1. Additionally, E2F1 has also been shown to upregulate genes involved in autophagy (ATG1, DRAM1, and LC3) in response to DNA damage,39 although the contribution of these to cell death or cell survival is a matter of debate.
Figure 2.
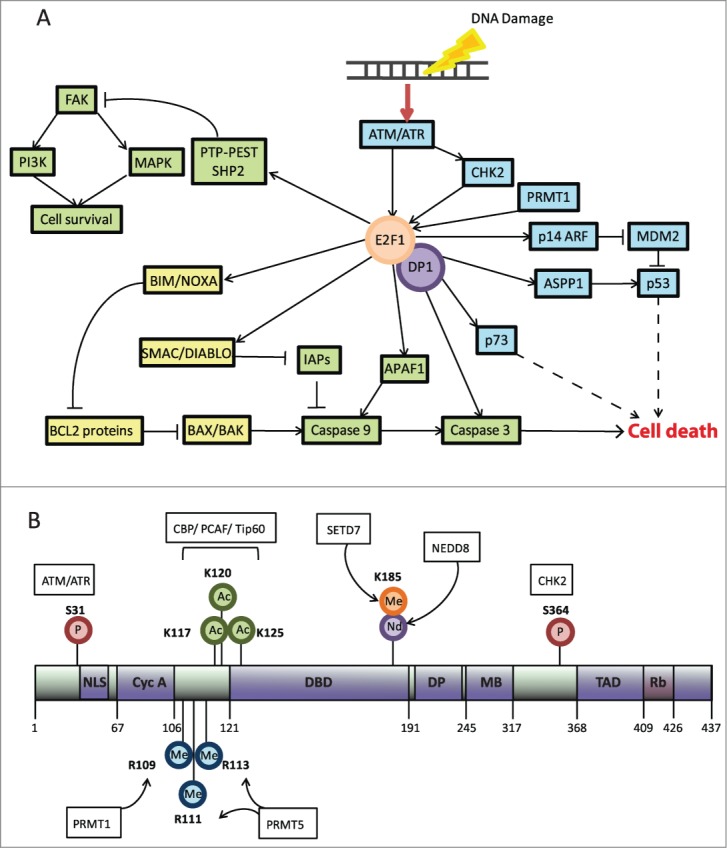
DNA damage-dependent regulation of E2F1. (A) E2F1 is modified following DNA damage resulting in cellular accumulation and upregulation of apoptotic E2F1 target genes and repression of survival pathways. This results in transcription of proapoptotic target genes, which largely affect the intrinsic apoptotic signaling pathway at the mitochondria or, in the case of p53 and p73, increase apoptotic gene transcription to amplify these effects. The intrinsic p53-dependent and -independent pathways that culminate in caspase-3 activation and cell death are shown. Blue describes nuclear events, yellow portrays events at the mitochondrial outer membrane, and green depicts cytoplasmic events. Dashed lines represent steps that have been omitted. (B) The main domains of E2F1 include the nuclear localization signal (NLS), cyclin A binding domain (CycA), the DNA binding domain (DBD), the DP binding domain (DP), the marked box (MB), and the transactivation domain (TAD), which also contains the pRB binding domain (RB). The main modifications of E2F1, the respective residues, and the responsible enzyme are outlined in the figure. Encircled (P) signifies phosphorylation, (Me) methylation, (Ac) acetylation, and (Nd) NEDDylation.
E2F1 and Apoptosis
E2F1 acts through several pathways and mechanisms to induce apoptosis following DNA damage, including the death receptor pathway, the p53 pathway, and p73 induction, and also through the intrinsic apoptotic pathway.40,41 A recent study also suggested that E2F1 may act through PTP-PEST and SHP2 to inhibit FAK, a player in the prosurvival PI3K and RAS/ERK pathways (Fig. 2A).42 Some of these pathways are considered below.
p73, a protein that is closely related to p53, is a key transcription target of E2F1 and an important mediator of E2F1-induced apoptosis. Through a number of target genes that are shared with p53, it can promote enhanced transcription of several apoptotic, cell cycle arrest, and DNA repair target genes. However, p73 can also act independently of p53, making it a major tumor suppressor in cells with no functional p53.43 In line with this, it has been demonstrated that loss of p73 as a result of formation of a repressive E2F1–pRB–HDAC complex formation at the p73 promoter can contribute to multidrug resistance in cultured cells with non-functional p53.44 Interestingly, E2F1 can also upregulate a dominant negative N-terminally truncated p73 isoform that hinders the DNA binding activity of WTp73. In scenarios where this happens, the loss of functional p73 has also been shown to contribute to multidrug resistance.45 These examples demonstrate the importance of the E2F1–p73 axis in promoting apoptosis. On a similar note, a recent study has demonstrated that the multidrug transporter ABCG2 is under E2F1 transcriptional control, which further contributes to the observed chemoresistance.46
The intrinsic apoptotic pathway is mediated through proteins located at the mitochondria. BCL-2 proteins including BCL-2, BCL-XL, and MCL-1 bind and sequester the proapoptotic proteins BAX and BAK to ensure cell survival. However, the BH3-only proteins (such as BIM, NOXA, and PUMA) are upregulated upon cell stress and displace BAX/BAK. The latter then form oligomers, causing mitochondrial outer membrane permeabilization and release of cytochrome c into the cytosol. Cytochrome c in turn activates APAF1 and triggers formation of the apoptosome, leading to caspase-9 autoactivation and initiation of the caspase cascade. This series of events ultimately mediates cellular apoptosis.47
E2F1 and p73 upregulate several proteins in this pathway, including BIM and NOXA. This dual upregulation of BH3-only proteins by E2F1 and p73 is likely required for cells to commit fully to apoptosis by disrupting the balance of proapoptotic to antiapoptotic proteins at the mitochondria, marking the ‘point-of-no-return’. In order to survive this death signal some cancer cells with aberrant E2F1 activation often upregulate BCL-2 protein expression.48,49 Additionally, E2F1 plays a role in the maintenance of mitochondrial function via other gene targets (including those involved in metabolism and oxidative phosphorylation), which is required for effective apoptosis independent of proapoptotic gene transcription levels.50 E2F1 also increases transcription of APAF1, caspases 3 and 7, and SMAC/DIABLO, which is also released from the mitochondria after outer membrane permeabilization and targets the IAPs (inhibitors of apoptosis) for proteasomal degradation (Fig. 2A).51-53
E2F1 and DNA repair
Despite the role of E2F1 in cell death following DNA damage, there is increasing evidence suggesting that it can also block apoptotic signaling to enable repair. For example, E2F1 can be relocalized after DNA damage, which may result in reduced transcription of canonical E2F1 target genes such as p14ARF and p73. Additionally, TOPBP1 associates with E2F1 and can recruit BRG1/BRM components of the SWI/SNF chromatin remodeling complex to E2F1 target genes, which may prevent apoptotic signaling during DNA repair (Table 2).38,54
Despite evidence that acetylation of E2F1 triggers apoptosis (see below), other studies suggest that it is also involved in DNA damage repair as acetylated E2F1 has a reduced affinity for pRB and instead increases its interaction with TOPBP1. It has been suggested that this complex may localize to the BRCA1 repair complex at sites of damage or stalled replication forks.54 E2F1 may also upregulate transcription of p19INK4D in an ATM/ATR-dependent manner after UV damage, which has been shown to stimulate DNA repair independently of p53.55,56 Finally, E2F1 can also enhance transcription of target genes involved in DNA repair (Table 2), including RAD51 (which repairs double-strand breaks), BARD1 (which stabilizes BRCA1), and PRKCD (required for non-homologous end joining).2,26,28
Post-Translational Modifications of E2F1
Under conditions of DNA damage E2F1 becomes rapidly phosphorylated on S31 by the DNA damage responsive kinases ATM and/or ATR (reflecting the nature of damage in the cell) and on S364 by CHK2 (Fig. 2B).57 Interestingly, CHK2-mediated phosphorylation increases the affinity of E2F1 for pRB, with the resulting Rb–E2F1 complex enhancing transcription of apoptotic target genes. Furthermore, it has been shown that pRB is cleaved by caspases and that its p68 product binds E2F1 at both NOXA and p73 promoters to enhance their expression.58,59 These studies therefore highlight a complex interplay between E2F1 and pRB under DNA damage conditions, which deviates from the simplistic cell cycle-regulated pRB-mediated repression of E2F1.
Phosphorylation of E2F1 is followed by its acetylation on residues K117, 120, and 125, which lie close to the DNA binding domain (Fig. 2B). These acetylation events are mediated by several acetyltransferases including p300/CBP, PCAF, and TIP60. Crucially, acetylation of E2F1 not only increases its stability, but also increases its DNA binding for the proapoptotic gene promoter p73. Therefore, acetylation and phosphorylation of E2F1 have been predominantly associated with its proapoptotic activity. Interestingly, some studies suggest that despite the increased stability of E2F1 under DNA damage conditions its ubiquitination levels increase, especially in the form of K11-linked ubiquitin chains. Furthermore, this ubiquitination seems to be dependent on the acetylation of E2F1 and is augmented in the presence of p300 acetyl transferase, although the relevance of ubiquitination to E2F1 activity remains unexplored.32,60-62
E2F1 can also be methylated at K185 by SET9/SETD7 (Fig. 2B). According to one report, methylation coincides with reduced apoptotic activity, particularly as it hinders acetylation and phosphorylation of E2F1 at distal sites. In contrast, demethylation of E2F1 by LSD1 after DNA damage allows for its stabilization.63 Interestingly, in another study E2F1 was shown to become NEDDylated by NEDD8 in a manner that was influenced by the methylation status of K185. It appeared that methylation of this residue could augment the NEDDylation of E2F1, and hence target it for degradation. Accordingly, DNA damage can significantly reduce the levels of E2F1 NEDDylation.64
The interplay between various modifications on E2F1 and their relevance to the regulation of E2F1 activity and biology has been further illustrated by a recent study describing arginine methylation. In this study E2F1 was shown to undergo asymmetric arginine methylation on R109 by PRMT1, and symmetric methylation on R111 and R113 by PRMT5 (Fig. 2B). Interestingly, not only do these modifications target E2F1 to distinctly different functional outcomes but, furthermore, they are mutually exclusive. Whereas asymmetrically methylated E2F1 accumulated under DNA damage to augment apoptosis, symmetrically methylated E2F1 was maintained in the cell progression mode, with its apoptotic potential significantly hindered. Furthermore, the modifications exhibited differential interplay with cyclin A binding, with asymmetric arginine methylation hindering the interaction between E2F1 and cyclin A and symmetric methylation augmenting this interaction. Crucially, the study identified a novel methyl-reader of E2F1; the tudor domain protein p100-TSN interacts with the symmetric methyl mark of E2F1 to suppress the apoptotic activity of E2F1.65,66
In conclusion, there have been a multitude of studies investigating the post-translational modifications of E2F1 and readers of these marks. These studies have provided a better understanding of E2F1 activity and regulation, particularly with regard to the opposing roles of this transcription factor.
The E2F1: miRNA regulatory network
MicroRNAs have emerged as important post-transcriptional regulators of gene expression. Mature miRNAs are 20–30 nucleotides in length and base pair with a 7-nucleotide seed sequence in the 3´ UTR of their target mRNAs to attenuate their translation.67 The E2F1 transcription factor has been reported to control the expression of several miRNA clusters, and is itself targeted by many miRNAs. Given the dual role of E2F1 in cell cycle control and apoptosis, the miRNAs that it interacts with have also been considered to mediate oncogenic apoptotic effects, depending on which aspect of E2F1 biology they most strongly influence. Not surprisingly, many of these miRNAs have been shown to be deregulated in cancer.68
The miR-17–92 cluster has been reported to be under the transcriptional control of E2F and MYC. Two miRNAs in this cluster, miR-17–5p and miR-20a, have in turn been shown to negatively regulate the levels of E2F1, thereby promoting cell survival and proliferation by protecting against excessive apoptotic activity of E2F1.69 Similar findings have been reported for miR-106b and miR-93 of the miR-106b–25 cluster, a family whose expression is activated by E2F1 in parallel with the host gene MCM7.70 E2F1 is also targeted by other miRNAs, which exhibit an overall antiproliferative effect by prohibiting E2F-mediated cell cycle progression. Such an effect has been reported for miR-330–3p in prostate cancer cells, miR-205 in melanoma cells, and miR-223 in myeloid cells. The p53 transcription factor target miR-34a has also been shown to regulate E2F1 levels under DNA damage conditions and to mediate potent antiproliferative effects.68 Similarly miR-449, miR-15, and miR-16, which are all direct transcriptional targets of E2F1, have been reported to exert proapoptotic effects through downregulation of several cell cycle genes.71,72
Overall, there is a complex network of interactions between E2F1 and miRNAs. The resultant crosstalk and regulatory loops help us understand how E2F1 activity can be controlled at different levels to facilitate cell cycle progression versus cell death.
Conclusion and Perspectives
E2F1 belongs to a family of transcription factors that have earned a reputation as master regulators of cell cycle control. Indeed, there are a multitude of studies relating E2F1 activity to the control of cell proliferation. However, it is also clear that E2F1 has a much greater functional diversity, being involved in many other processes including apoptosis, DNA damage repair, stress response, differentiation, and metabolism.73 It may therefore be more accurate to consider E2F1 as a master controller of cell fate, involved in balancing diverse cellular outcomes. In support of such complexity, chromatin immunoprecipitation analysis has shown that E2F1 is actually present on a much larger proportion of active genes in cells than anticipated, and only a small fraction of these contain the canonical E2F sequence motif.27 Furthermore, microarray analysis of E2F1 null mice has shown that while the absence of E2F1 does not affect expression levels of typical E2F target genes, perhaps due to redundancy with other E2F members, there is nonetheless a small set of genes that are uniquely regulated by E2F1 and whose functions fall outside the typical proliferative and apoptotic role.74 Such studies therefore suggest a much wider role for E2F1 in the control of cell behavior and further reinforce the need to understand and unravel E2F1 biology.
Almost 30 years on from the discovery of E2F1, we still have much to learn. The contribution of the different arms of E2F1 activity to cancer development is the subject of extensive ongoing research. In this respect, deciphering the extensive transcriptional and non-transcriptional networks that E2F1 is either implicated in or controls, its range of post-translational modifications and their associated readers, and its interaction partners will help us better understand its complex biology, particularly in the context of cancer. We believe that future research will focus on clarifying how the switch from growth promotion to growth inhibition is achieved and, importantly, whether E2F-1 contributes to a wider repertoire of functional pathways than is currently understood. Ultimately, such knowledge may provide us with the means of manipulating E2F1 levels and activity as a therapeutic strategy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Medical Research Council (G1000807) and Cancer Research UK (Program Award 300/A13058).
References
- 1. DeGregori J, Leone G, Miron A, Jakoi L, Nevins JR. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc Natl Acad Sci 1997; 94:7245-50; PMID:9207076; http://dx.doi.org/ 10.1073/pnas.94.14.7245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Black EP, Hallstrom T, Dressman HK, West M, Nevins JR. Distinctions in the specificity of E2F function revealed by gene expression signatures. Proc Natl Acad Sci 2005; 102:15948-53; PMID:16249342; http://dx.doi.org/ 10.1073/pnas.0504300102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ingram L, Munro S, Coutts AS, La Thangue NB. E2F-1 regulation by an unusual DNA damage-responsive DP partner subunit. Cell Death Differ 2011; 18:122-32; PMID:20559320; http://dx.doi.org/ 10.1038/cdd.2010.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Narita M, Nunez S, Heard E, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 2003; 113:703-16; PMID:12809602; http://dx.doi.org/ 10.1016/S0092-8674(03)00401-X [DOI] [PubMed] [Google Scholar]
- 5. Lees JA, Saito M, Vidal M, Valentine M, Look T, Harlow E, Dyson N, Helin K. The retinoblastoma protein binds to a family of E2F transcription factors. Mol Cell Biol 1993; 13:7813-25; PMID:8246996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yao G, Lee TJ, Mori S, Nevins JR, You L. A bistable Rb-E2F switch underlies the restriction point. Nat Cell Biol 2008; 10:476-82; PMID:18364697; http://dx.doi.org/ 10.1038/ncb1711 [DOI] [PubMed] [Google Scholar]
- 7. Burke JR, Liban TJ, Restrepo T, Lee H-W, Rubin SM. Multiple mechanisms for E2F binding inhibition by phosphorylation of the retinoblastoma protein C-terminal domain. J Mol Biol 2014; 426:245-55; PMID:24103329; http://dx.doi.org/ 10.1016/j.jmb.2013.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Araki K, Nakajima Y, Eto K, Ikeda M-A. Distinct recruitment of E2F family members to specific E2F-binding sites mediates activation and repression of the E2F1 promoter. Oncogene 2003; 22:7632-41; PMID:14576826; http://dx.doi.org/ 10.1038/sj.onc.1206840 [DOI] [PubMed] [Google Scholar]
- 9. Schulze A, Zerfass K, Spitkovsky D, Middendorp S, Berges J, Helin K, Jansen-Durr P, Henglein B. Cell cycle regulation of the cyclin A gene promoter is mediated by a variant E2F site. Proc Natl Acad Sci 1995; 92:11264-8; PMID:7479977; http://dx.doi.org/ 10.1073/pnas.92.24.11264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang HS, Gavin M, Dahiya A, Postigo AA, Ma D, Luo RX, Harbour JW, Dean DC. Exit from G1 and S phase of the cell cycle is regulated by repressor complexes containing HDAC-Rb-hSWI/SNF and Rb-hSWI/SNF. Cell 2000; 101:79-89; PMID:10778858; http://dx.doi.org/ 10.1016/S0092-8674(00)80625-X [DOI] [PubMed] [Google Scholar]
- 11. Xu M, Sheppard KA, Peng CY, Yee AS, Piwnica-Worms H. Cyclin A/CDK2 binds directly to E2F-1 and inhibits the DNA-binding activity of E2F-1/DP-1 by phosphorylation. Mol Cell Biol 1994; 14:8420-31; PMID:7969176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Logan N, Graham A, Zhao X, Fisher R, Maiti B, Leone G, La Thangue NB. E2F-8: an E2F family member with a similar organization of DNA-binding domains to E2F-7. Oncogene 2005; 24:5000-4; PMID:15897886; http://dx.doi.org/ 10.1038/sj.onc.1208703 [DOI] [PubMed] [Google Scholar]
- 13. Zhang L, Wang C. F-box protein Skp2: a novel transcriptional target of E2F. Oncogene 2006; 25:2615-27; PMID:16331253; http://dx.doi.org/ 10.1038/sj.onc.1209286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bamford S, Dawson E, Forbes S, Clements J, Pettett R, Dogan A, Flanagan A, Teague J, Futreal PA, Stratton MR, et al. . The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Brit J Cancer 2004; 91:355-8. Accessed 08/06/2014; PMID:15188009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Di Fiore R, D’Anneo A, Tesoriere G, Vento R. RB1 in cancer: different mechanisms of RB1 inactivation and alterations of pRb pathway in tumorigenesis. J Cell Physiol 2013; 228:1676-87; PMID:23359405; http://dx.doi.org/ 10.1002/jcp.24329 [DOI] [PubMed] [Google Scholar]
- 16. Romagosa C, Simonetti S, Lopez-Vicente L, Mazo A, Lleonart ME, Castellvi J, Ramon y Cajal S. p16(Ink4a) overexpression in cancer: a tumor suppressor gene associated with senescence and high-grade tumors. Oncogene 2011; 30:2087-97; PMID:21297668; http://dx.doi.org/ 10.1038/onc.2010.614 [DOI] [PubMed] [Google Scholar]
- 17. Harbour JW, Dean DC. The Rb/E2F pathway: expanding roles and emerging paradigms. Gene Dev 2000; 14:2393-409; PMID:11018009; http://dx.doi.org/ 10.1101/gad.813200 [DOI] [PubMed] [Google Scholar]
- 18. Laurie NA, Donovan SL, Shih C-S, Zhang J, Mills N, Fuller C, Teunisse A, Lam S, Ramos Y, Mohan A, et al. . Inactivation of the p53 pathway in retinoblastoma. Nature 2006; 444:61-6; PMID:17080083; http://dx.doi.org/ 10.1038/nature05194 [DOI] [PubMed] [Google Scholar]
- 19. Pierce AM, Gimenez-Conti IB, Schneider-Broussard R, Martinez LA, Conti CJ, Johnson DG. Increased E2F1 activity induces skin tumors in mice heterozygous and nullizygous for p53. Proc Natl Acad Sci 1998; 95:8858-63; PMID:9671769; http://dx.doi.org/ 10.1073/pnas.95.15.8858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pierce AM, Schneider-Broussard R, Gimenez-Conti IB, Russell JL, Conti CJ, Johnson DG. E2F1 has both oncogenic and tumor-suppressive properties in a transgenic model. Mol Cell Biol 1999; 19:6408-14; PMID:10454586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamasaki L, Jacks T, Bronson R, Goillot E, Harlow E, Dyson NJ. Tumor induction and tissue atrophy in mice lacking E2F-1. Cell 1996; 85:537-48; PMID:8653789; http://dx.doi.org/ 10.1016/S0092-8674(00)81254-4 [DOI] [PubMed] [Google Scholar]
- 22. Yamasaki L, Bronson R, Williams BO, Dyson NJ, Harlow E, Jacks T. Loss of E2F-1 reduces tumorigenesis and extends the lifespan of Rb1(+/-)mice. Nat Genet 1998; 18:360-4; PMID:9537419; http://dx.doi.org/ 10.1038/ng0498-360 [DOI] [PubMed] [Google Scholar]
- 23. Munro S, Oppermann U, La Thangue NB. Pleiotropic effect of somatic mutations in the E2F subunit DP-1 gene in human cancer. Oncogene 2014; 33:3594-3603; PMID:23934193; http://dx.doi.org/ 10.1038/onc.2013.316 [DOI] [PubMed] [Google Scholar]
- 24. Coverley D, Laman H, Laskey RA. Distinct roles for cyclins E and A during DNA replication complex assembly and activation. Nat Cell Biol 2002; 4:523-8; PMID:12080347; http://dx.doi.org/ 10.1038/ncb813 [DOI] [PubMed] [Google Scholar]
- 25. Mailand N, Diffley JFX. CDKs promote DNA replication origin licensing in human cells by protecting Cdc6 from APC/C-dependent proteolysis. Cell 2005; 122:915-26; PMID:16153703; http://dx.doi.org/ 10.1016/j.cell.2005.08.013 [DOI] [PubMed] [Google Scholar]
- 26. Polager S, Kalama Y, Berkovich E, Ginsberg D. E2Fs up-regulate expression of genes involved in DNA replication, DNA repair and mitosis. Oncogene 2002; 21:437-46; PMID:11821956; http://dx.doi.org/ 10.1038/sj.onc.1205102 [DOI] [PubMed] [Google Scholar]
- 27. Bieda M, Xu X, Singer MA, Green R, Farnham PJ. Unbiased location analysis of E2F1-binding sites suggests a widespread role for E2F1 in the human genome. Genome Res 2006; 16:595-605; PMID:16606705; http://dx.doi.org/ 10.1101/gr.4887606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ren B, Cam H, Takahashi Y, Volkert T, Terragni J, Young RA, Dynlacht BD. E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Gene Dev 2002; 16:245-56; PMID:11799067; http://dx.doi.org/ 10.1101/gad.949802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ishida S, Huang E, Zuzan H, Spang R, Leone G, West M, Nevins JR. Role for E2F in control of both DNA replication and mitotic functions as revealed from DNA microarray analysis. Mol Cell Biol 2001; 21:4684-99; PMID:11416145; http://dx.doi.org/ 10.1128/MCB.21.14.4684-4699.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bou Kheir T, Lund AH. Epigenetic dynamics across the cell cycle. Essays Biochem 2010; 48:107-20; PMID:20822490; http://dx.doi.org/ 10.1042/bse0480107 [DOI] [PubMed] [Google Scholar]
- 31. Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell 2012; 150:12-27; PMID:22770212; http://dx.doi.org/ 10.1016/j.cell.2012.06.013 [DOI] [PubMed] [Google Scholar]
- 32. Taubert S, Gorrini C, Frank SR, Parisi T, Fuchs M, Chan HM, Livingston DM, Amati B. E2F-dependent histone acetylation and recruitment of the Tip60 acetyltransferase complex to chromatin in late G1. Mol Cell Biol 2004; 24:4546-56; PMID:15121871; http://dx.doi.org/ 10.1128/MCB.24.10.4546-4556.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Probst AV, Dunleavy E, Almouzni G. Epigenetic inheritance during the cell cycle. Nat Rev Mol Cell Biol 2009; 10:192-206; PMID:19234478; http://dx.doi.org/ 10.1038/nrm2640 [DOI] [PubMed] [Google Scholar]
- 34. Tyagi S, Chabes AL, Wysocka J, Herr W. E2F activation of S phase promoters via association with HCF-1 and the MLL family of histone H3K4 methyltransferases. Mol Cell 2007; 27:107-19; PMID:17612494; http://dx.doi.org/ 10.1016/j.molcel.2007.05.030 [DOI] [PubMed] [Google Scholar]
- 35. Revenko AS, Kalashnikova EV, Gemo AT, Zou JX, Chen HW. Chromatin loading of E2F-MLL complex by cancer-associated coregulator ANCCA via reading a specific histone mark. Mol Cell Biol 2010; 30:5260-72; PMID:20855524; http://dx.doi.org/ 10.1128/MCB.00484-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Subtil-Rodríguez A, Vázquez-Chávez E, Ceballos-Chávez M, Rodríguez-Paredes M, Martín-Subero JI, Esteller M, Reyes JC. The chromatin remodeller CHD8 is required for E2F-dependent transcription activation of S-phase genes. Nucleic Acids Res 2014; 42:2185-96; http://dx.doi.org/ 10.1093/nar/gkt1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nagl NG, Wang X, Patsialou A, Van Scoy M, Moran E. Distinct mammalian SWI/SNF chromatin remodeling complexes with opposing roles in cell-cycle control. EMBO J 2007; 26:752-63; PMID:17255939; http://dx.doi.org/ 10.1038/sj.emboj.7601541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu K, Luo Y, Lin FT, Lin WC. TopBP1 recruits Brg1/Brm to repress E2F1-induced apoptosis, a novel pRb-independent and E2F1-specific control for cell survival. Gene Dev 2004; 18:673-86; PMID:15075294; http://dx.doi.org/ 10.1101/gad.1180204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Polager S, Ofir M, Ginsberg D. E2F1 regulates autophagy and the transcription of autophagy genes. Oncogene 2008; 27:4860-4; PMID:18408756; http://dx.doi.org/ 10.1038/onc.2008.117 [DOI] [PubMed] [Google Scholar]
- 40. Bates S, Phillips AC, Clark PA, Stott F, Peters G, Ludwig RL, Vousden KH. p14ARF links the tumour suppressors RB and p53. Nature 1998; 395:124-5; PMID:9744267; http://dx.doi.org/ 10.1038/25867 [DOI] [PubMed] [Google Scholar]
- 41. Phillips AC, Ernst MK, Bates S, Rice NR, Vousden KH. E2F-1 Potentiates Cell Death by Blocking Antiapoptotic Signaling Pathways. Mol Cell 1999; 4:771-81; PMID:10619024; http://dx.doi.org/ 10.1016/S1097-2765(00)80387-1 [DOI] [PubMed] [Google Scholar]
- 42. Morales LD, Pena K, Kim DJ, Lieman JH. SHP-2 and PTP-pest induction during Rb-E2F associated apoptosis. Cell Mol Biol Lett 2012; 17:422-32; PMID:22644489; http://dx.doi.org/ 10.2478/s11658-012-0020-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Irwin M, Marin MC, Phillips AC, Seelan RS, Smith DI, Liu W, Flores ER, Tsai KY, Jacks T, Vousden KH, et al. . Role for the p53 homologue p73 in E2F-1-induced apoptosis. Nature 2000; 407:645-8; PMID:11034215; http://dx.doi.org/ 10.1038/35036614 [DOI] [PubMed] [Google Scholar]
- 44. La Sala D, Macaluso M, Trimarchi C, Giordano A, Cinti C. Triggering of p73-dependent apoptosis in osteosarcoma is under the control of E2Fs-pRb2/p130 complexes. Oncogene 2003; 22:3518-29; PMID:12789260; http://dx.doi.org/ 10.1038/sj.onc.1206487 [DOI] [PubMed] [Google Scholar]
- 45. Alla V, Kowtharapu BS, Engelmann D, Emmrich S, Schmitz U, Steder M, Putzer BM. E2F1 confers anticancer drug resistance by targeting ABC transporter family members and Bcl-2 via the p73/DNp73-miR-205 circuitry. Cell Cycle 2012; 11:3067-78; PMID:22871739; http://dx.doi.org/ 10.4161/cc.21476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rosenfeldt MT, Bell LA, Long JS, O’Prey J, Nixon C, Roberts F, Dufes C, Ryan KM. E2F1 drives chemotherapeutic drug resistance via ABCG2. Oncogene 2014; 33:4164-72; PMID:24276245; http://dx.doi.org/ 10.1038/onc.2013.470 [DOI] [PubMed] [Google Scholar]
- 47. Tait SWG, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 2010; 11:621-32; PMID:20683470; http://dx.doi.org/ 10.1038/nrm2952 [DOI] [PubMed] [Google Scholar]
- 48. Ruhul Amin ARM, Thakur VS, Gupta K, Agarwal MK, Wald DN, Shin DM, Agarwal ML. N-(phosphonacetyl)-L-aspartate induces TAp73-dependent apoptosis by modulating multiple Bcl-2 proteins: potential for cancer therapy. Oncogene 2013; 32:920-9; PMID:22430213; http://dx.doi.org/ 10.1038/onc.2012.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhao Y, Tan J, Zhuang L, Jiang X, Liu ET, Yu Q. Inhibitors of histone deacetylases target the Rb-E2F1 pathway for apoptosis induction through activation of proapoptotic protein Bim. Proc Natl Acad Sci 2005; 102:16090-5; PMID:16243973; http://dx.doi.org/ 10.1073/pnas.0505585102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ambrus AM, Islam ABMMK, Holmes KB, Moon NS, Lopez-Bigas N, Benevolenskaya EV, Frolov MV. Loss of dE2F compromises mitochondrial function. Dev Cell 2013; 27:438-51; PMID:24286825; http://dx.doi.org/ 10.1016/j.devcel.2013.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stanelle J, Stiewe T, Theseling CC, Peter M, Pützer BM. Gene expression changes in response to E2F1 activation. Nucleic Acids Res 2002; 30:1859-67; PMID:11937641; http://dx.doi.org/ 10.1093/nar/30.8.1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xie W, Jiang P, Miao L, Zhao Y, Zhimin Z, Qing L, Zhu W-g, Wu M. Novel link between E2F1 and Smac/DIABLO: proapoptotic Smac/DIABLO is transcriptionally upregulated by E2F1. Nucleic Acids Res 2006; 34:2046-55; PMID:16617145; http://dx.doi.org/ 10.1093/nar/gkl150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Müller H, Bracken AP, Vernell R, Moroni MC, Christians F, Grassilli E, Prosperini E, Vigo E, Oliner JD, Helin K. E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Gene Dev 2001; 15:267-85; PMID:11159908; http://dx.doi.org/ 10.1101/gad.864201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu K, Lin F-T, Ruppert JM, Lin W-C. Regulation of E2F1 by BRCT domain-containing protein TopBP1. Mol Cell Biol 2003; 23:3287-304; PMID:12697828; http://dx.doi.org/ 10.1128/MCB.23.9.3287-3304.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ogara MF, Sirkin PF, Carcagno AL, Marazita MC, Sonzogni SV, Ceruti JM, Cánepa ET. Chromatin relaxation-mediated induction of p19INK4d increases the ability of cells to repair damaged DNA. PLoS ONE 2013; 8:e61143-e; PMID:23593412; http://dx.doi.org/ 10.1371/journal.pone.0061143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ceruti JM, Scassa ME, Flo JM, Varone CL, Canepa ET. Induction of p19INK4d in response to ultraviolet light improves DNA repair and confers resistance to apoptosis in neuroblastoma cells. Oncogene 2005; 24:4065-80; PMID:15750620; http://dx.doi.org/ 10.1038/sj.onc.1208570 [DOI] [PubMed] [Google Scholar]
- 57. Stevens C, Smith L, La Thangue NB. Chk2 activates E2F-1 in response to DNA damage. Nat Cell Biol 2003; 5:401-9; PMID:12717439; http://dx.doi.org/ 10.1038/ncb974 [DOI] [PubMed] [Google Scholar]
- 58. Carnevale J, Palander O, Seifried LA, Dick FA. DNA damage signals through differentially modified E2F1 molecules to induce apoptosis. Mol Cell Biol 2012; 32:900-12; PMID:22184068; http://dx.doi.org/ 10.1128/MCB.06286-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bertin-Ciftci J, Barré B, Le Pen J, Maillet L, Couriaud C, Juin P, Braun F. pRb/E2F-1-mediated caspase-dependent induction of Noxa amplifies the apoptotic effects of the Bcl-2/Bcl-xL inhibitor ABT-737. Cell Death Differ 2013; 20:755-64; PMID:23429261; http://dx.doi.org/ 10.1038/cdd.2013.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Galbiati L, Mendoza-Maldonado R, Gutierrez MI, Giacca M. Regulation of E2F-1 after DNA damage by p300-mediated acetylation and ubiquitination. Cell Cycle 2005; 4:930-9; PMID:15917652; http://dx.doi.org/ 10.4161/cc.4.7.1784 [DOI] [PubMed] [Google Scholar]
- 61. Nagy Z, Tora L. Distinct GCN5/PCAF-containing complexes function as co-activators and are involved in transcription factor and global histone acetylation. Oncogene 2007; 26:5341-57; PMID:17694077; http://dx.doi.org/ 10.1038/sj.onc.1210604 [DOI] [PubMed] [Google Scholar]
- 62. Budhavarapu VN, White ED, Mahanic CS, Chen L, Lin FT, Lin WC. Regulation of E2F1 by APC/C Cdh1 via K11 linkage-specific ubiquitin chain formation. Cell Cycle 2012; 11:2030-8; PMID:22580462; http://dx.doi.org/ 10.4161/cc.20643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kontaki H, Talianidis I. Lysine methylation regulates E2F1-induced cell death. Mol Cell 2010; 39:152-60; PMID:20603083; http://dx.doi.org/ 10.1016/j.molcel.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 64. Loftus SJ, Liu G, Carr SM, Munro S, La Thangue NB. NEDDylation regulates E2F-1-dependent transcription. EMBO Rep 2012; 13:811-8; PMID:22836579; http://dx.doi.org/ 10.1038/embor.2012.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cho EC, Zheng S, Munro S, Liu G, Carr SM, Moehlenbrink J, Lu YC, Stimson L, Khan O, Konietzny R, et al. . Arginine methylation controls growth regulation by E2F-1. EMBO J 2012; 31:1785-97; PMID:22327218; http://dx.doi.org/ 10.1038/emboj.2012.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zheng S, Moehlenbrink J, Lu YC, Zalmas LP, Sagum CA, Carr S, McGouran JF, Alexander L, Fedorov O, Munro S, et al. . Arginine methylation-dependent reader-writer interplay governs growth control by E2F-1. Mol Cell 2013; 52:37-51; PMID:24076217; http://dx.doi.org/ 10.1016/j.molcel.2013.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009; 136:642-55; PMID:19239886; http://dx.doi.org/ 10.1016/j.cell.2009.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Knoll S, Emmrich S, Pützer BM. The E2F1-miRNA cancer progression network. Adv Exp Med Biol 2013; 774:135-47; PMID:23377972; http://dx.doi.org/ 10.1007/978-94-007-5590-1_8 [DOI] [PubMed] [Google Scholar]
- 69. Pickering MT, Stadler BM, Kowalik TF. miR-17 and miR-20a temper an E2F1-induced G1 checkpoint to regulate cell cycle progression. Oncogene 2009; 28:140-5; PMID:18836483; http://dx.doi.org/ 10.1038/onc.2008.372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li Y, Tan W, Neo TW, Aung MO, Wasser S, Lim SG, Tan TM. Role of the miR-106b-25 microRNA cluster in hepatocellular carcinoma. Cancer Sci 2009; 100:1234-42; PMID:19486339; http://dx.doi.org/ 10.1111/j.1349-7006.2009.01164.x [DOI] [PubMed] [Google Scholar]
- 71. Ofir M, Hacohen D, Ginsberg D. MiR-15 and miR-16 are direct transcriptional targets of E2F1 that limit E2F-induced proliferation by targeting cyclin E. Mol Cancer Res 2011; 9:440-7; PMID:21454377; http://dx.doi.org/ 10.1158/1541-7786.MCR-10-0344 [DOI] [PubMed] [Google Scholar]
- 72. Lizé M, Klimke A, Dobbelstein M. MicroRNA-449 in cell fate determination. Cell Cycle 2011; 10:2874-82; http://dx.doi.org/ 10.4161/cc.10.17.17181 [DOI] [PubMed] [Google Scholar]
- 73. Chen HZ, Tsai SY, Leone G. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev Cancer 2009; 9:785-97; PMID:19851314; http://dx.doi.org/ 10.1038/nrc2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wells J, Graveel CR, Bartley SM, Madore SJ, Farnham PJ. The identification of E2F1-specific target genes. Proc Natl Acad Sci 2002; 99:3890-5; PMID:11904439; http://dx.doi.org/ 10.1073/pnas.062047499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Louie MC, Zou JX, Rabinovich A, Chen HW. ACTR/AIB1 functions as an E2F1 coactivator to promote breast cancer cell proliferation and antiestrogen resistance. Mol Cell Biol 2004; 24:5157-71; PMID:15169882; http://dx.doi.org/ 10.1128/MCB.24.12.5157-5171.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Revenko AS, Kalashnikova EV, Gemo AT, Zou JX, Chen HW. Chromatin loading of E2F-MLL complex by cancer-associated coregulator ANCCA via reading a specific histone mark. Mol Cell Biol 2010; 30:5260-72; PMID:20855524; http://dx.doi.org/ 10.1128/MCB.00484-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Denis GV, Vaziri C, Guo N, Faller DV. RING3 kinase transactivates promoters of cell cycle regulatory genes through E2F. Cell Growth Differ 2000; 11:417-24; PMID:10965846 [PMC free article] [PubMed] [Google Scholar]
- 78. Peng J, Dong W, Chen L, Zou T, Qi Y, Liu Y. Brd2 is a TBP-associated protein and recruits TBP into E2F-1 transcriptional complex in response to serum stimulation. Mol Cell Biochem 2007; 294:45-54; PMID:17111193; http://dx.doi.org/ 10.1007/s11010-006-9223-6 [DOI] [PubMed] [Google Scholar]
- 79. Hallstrom TC, Nevins JR. Jab1 is a specificity factor for E2F1-induced apoptosis. Genes Dev 2006; 20:613-23; PMID:16481464; http://dx.doi.org/ 10.1101/gad.1345006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Martin K, Trouche D, Hagemeier C, Sorensen TS, La Thangue NB, Kouzarides T. Stimulation of E2F1/DP1 transcriptional activity by MDM2 oncoprotein. Nature 1995; 375:691-4; PMID:7791903; http://dx.doi.org/ 10.1038/375691a0 [DOI] [PubMed] [Google Scholar]
- 81. Strachan GD, Jordan-Sciutto KL, Rallapalli R, Tuan RS, Hall DJ. The E2F-1 transcription factor is negatively regulated by its interaction with the MDMX protein. J Cell Biochem 2003; 88:557-68; PMID:12532331; http://dx.doi.org/ 10.1002/jcb.10318 [DOI] [PubMed] [Google Scholar]
- 82. Sansal I, Dupont E, Toru D, Evrard C, Rouget P. NPDC-1, a regulator of neural cell proliferation and differentiation, interacts with E2F-1, reduces its binding to DNA and modulates its transcriptional activity. Oncogene 2000; 19:5000-9; PMID:11042687; http://dx.doi.org/ 10.1038/sj.onc.1203843 [DOI] [PubMed] [Google Scholar]
- 83. Simbulan-Rosenthal CM, Rosenthal DS, Luo R, Samara R, Espinoza LA, Hassa PO, Hottiger MO, Smulson ME. PARP-1 binds E2F-1 independently of its DNA binding and catalytic domains, and acts as a novel coactivator of E2F-1-mediated transcription during re-entry of quiescent cells into S phase. Oncogene 2003; 22:8460-71; PMID:14627987; http://dx.doi.org/ 10.1038/sj.onc.1206897 [DOI] [PubMed] [Google Scholar]
- 84. Wang C, Rauscher FJ, 3rd, Cress WD, Chen J. Regulation of E2F1 function by the nuclear corepressor KAP1. J Biol Chem 2007; 282:29902-9; PMID:17704056; http://dx.doi.org/ 10.1074/jbc.M704757200 [DOI] [PubMed] [Google Scholar]


