Abstract
MYC regulates tumorigenesis by coordinating the expression of thousands of genes. We found that MYC appears to regulate the decisions between cell survival versus death and self-renewal versus senescence through the microRNA miR-17–92 cluster. Addiction to the MYC oncogene may therefore in fact be an addiction to miR-17–92.
Keywords: lymphoma, microRNA miR-17-92, MYC, oncogene addiction
Abbreviations
- Bcl2l11
Bcl2-like 11
- Btg1
B cell translocation gene 1
- Hbp1
high mobility group box transcription factor 1
- HDACs
histone deacetylases
- Prmt1
protein arginine methyltransferase 1
- Sin3b
Sin3 transcription regulator family member B
- Suv420h1
suppressor of variegation 4–20 homolog 1
Although cancer cells contain multiple genetic and epigenetic abnormalities, they are often dependent on specific oncogenes for the maintenance of their malignant phenotype. The inactivation of a single driver oncogene can result in rapid and sustained tumor regression.1,2 The term “oncogene addiction” has been coined to describe this phenomenon, which has been supported by multiple animal cancer models and is best exemplified clinically by the targeted therapy of chronic myelogenous leukemia with imatinib.2
The MYC oncogene has been implicated in the pathogenesis of most types of human tumors.3 In animal models, activation of MYC in lymphocyte precursors drives lymphomagenesis whereas its inactivation results in rapid, complete, and sustained tumor regression.2 MYC inactivation is associated with the loss of many of the hallmark features of tumorigenesis and results in proliferative arrest, apoptosis, differentiation, and senescence.2 An overarching question is: how does MYC maintain a neoplastic state?
Recent work suggests that MYC may not specifically regulate gene expression but may instead serve as a general transcriptional amplifier to boost the production of already existing transcripts.4,5 However, the effects of transcriptional amplification are generally similar to the effects attributed to MYC's influence on ribosomal biogenesis, which globally regulates protein production.3 Both processes are probably critical for MYC's biological function, and particularly its function as an oncogene, by serving as rate-limiting constraints on tumor cell growth and proliferation.4,5 However, transcriptional amplification may not explain major decisions on cell fate, such as those between survival versus apoptosis and self-renewal versus senescence, which appear to be dictated by levels of MYC expression. Hence, they may not explain how MYC maintains the neoplastic state.
While examining the mechanism by which MYC inactivation induces apoptosis in tumor cells we identified the pro-apoptotic protein Bcl2-like 11 (Bcl2l11, best known as Bim) as a key mediator of this process. Further investigation of how Bim expression is regulated led to the conclusion that it is suppressed by the microRNA cluster miR-17–92, a known MYC target. The miR-17–92 cluster has previously been reported to regulate proliferation, survival, and angiogenesis, several of the key phenotypes associated with MYC oncogene addiction.6 The similarity of biological functions between MYC and miR-17–92 evoked the hypothesis that miR-17–92 may mediate MYC oncogene addiction. Indeed, we found that downregulation of miR-17–92 was responsible not only for apoptosis upon MYC suppression but also for the loss of proliferation, and even self-renewal.7 Hence, the expression of miR-17–92 was required for MYC to make the cell fate decisions between survival versus apoptosis and self-renewal versus senescence (Fig. 1).
Figure 1.
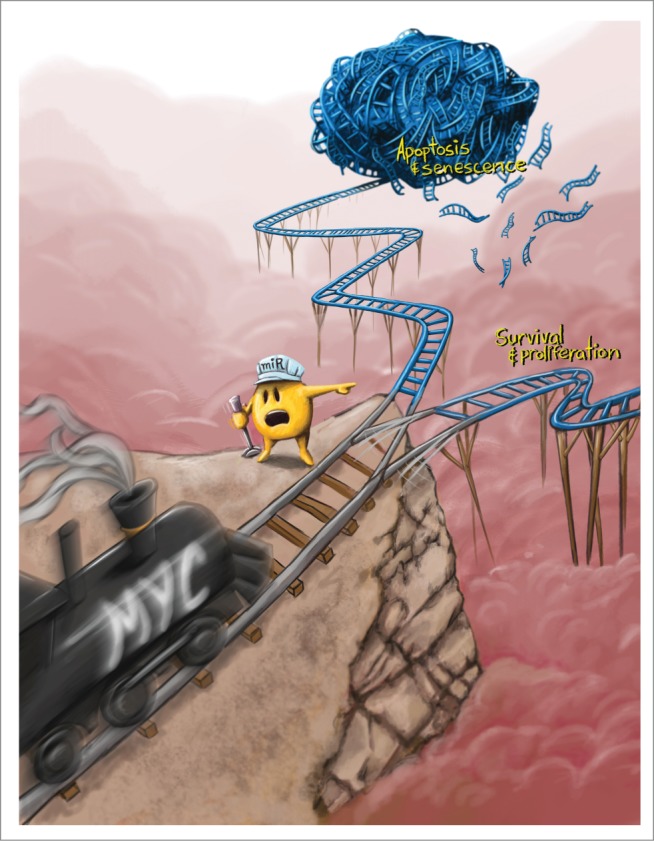
miR-17–92 controls a neoplastic switch for MYC-driven tumors. Expression of miR-17–92 can determine the cellular fate between senescence and apoptosis and between proliferation and survival and is essential for the MYC oncogene to maintain a neoplastic state. (Artwork by Nick Harper).
By comparative microarray analysis we identified genes that are regulated by both MYC and miR-17–92. Among genes that had multiple miR-17–92 binding sites, we identified histone modifiers, such as Sin3 transcription regulator family member B (Sin3b), high mobility group box transcription factor 1 (Hbp1), suppressor of variegation 4–20 homolog 1 (Suv420h1), and B cell translocation gene 1 (Btg1), as well as the apoptosis regulator Bim.7 Functional analysis by shRNA knockdown confirmed that knockdown of all 5 genes impeded proliferation arrest and blocked apoptosis and senescence upon MYC inactivation, and thus largely phenocopied the effects of miR-17–92 expression.7
These chromatin regulators had been previously implicated in the regulation of cellular senescence. Sin3b interacts with Hbp1 and recruits histone deacetylases (HDACs) to specifically deacetylate proliferation-related genes and mediate cell cycle exit and senescence. Suv420h1 trimethylates H4K20, which is known to direct chromatin compaction and is a marker of heterochromatin formation and senescence.8 Btg1, a biomarker of chemotherapy-induced cellular senescence, activates protein arginine methyltransferase 1 (Prmt1) to dimethylate histone H4 arginine 3 and regulate proliferation and differentiation.8 Thus, the coordinated action of these chromatin modifiers may explain how MYC activation through miR-17–92 maintains a neoplastic state, and why even brief MYC inactivation can result in sustained tumor remission.2
Hence, the regulation of miR-17–92, and thereby of specific chromatin and apoptosis regulatory genes, by MYC may explain oncogene addiction. Other reports are consistent with this hypothesis. miR-17–92 is the only microRNA known to be upregulated by MYC and its expression can cooperate with MYC in lymphomagenesis.6 Furthermore, overexpression of miR-17–92 alone can initiate lymphomagenesis.9 Deletion of miR-17–92 significantly impedes the ability of MYC to maintain tumors even in the presence of miR-106a-363 and miR-106b-25, which provide some functional redundancy.10 Thus, many observations suggest that this single microRNA cluster, miR-17–92, may be the key nodal point in the ability of the MYC oncogene to maintain a neoplastic state.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Weinstein IB. Cancer. Addiction to oncogenes–the Achilles heal of cancer. Science 2002; 297:63-64; PMID:12098689; http://dx.doi.org/ 10.1126/science.1073096 [DOI] [PubMed] [Google Scholar]
- 2. Li Y, Casey SC, Felsher DW. Inactivation of MYC reverses tumorigenesis. J Int Med 2014; 276:52-60; PMID:24645771; doi: 10.1111/joim.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dang CV. MYC on the path to cancer. Cell 2012; 149:22-35; PMID:22464321; http://dx.doi.org/ 10.1016/j.cell.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin CY, Lovén J, Rahl PB, Paranal RM, Burge CB, Bradner JE, Lee TI, Young RA. Transcriptional amplification in tumor cells with elevated c-Myc. Cell 2012; 151:56-67; PMID:23021215; http://dx.doi.org/ 10.1016/j.cell.2012.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nie Z, Hu G, Wei G, Cui K, Yamane A, Resch W, Wang R, Green DR, Tessarollo L, Casellas R, Zhao K, et al. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell 2012; 151:68-79; PMID:23021216; http://dx.doi.org/ 10.1016/j.cell.2012.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Olive V, Jiang I, He L. mir-17-92, a cluster of miRNAs in the midst of the cancer network. Int J Biochem Cell Biol 2010; 42:1348-54; PMID:20227518; http://dx.doi.org/ 10.1016/j.biocel.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Y, Choi PS, Casey SC, Dill DL, Felsher DW. MYC through miR-17-92 suppresses specific target genes to maintain survival, autonomous proliferation, and a neoplastic state. Cancer Cell 2014; 26:262-72; PMID:25117713; http://dx.doi.org/ 10.1016/j.ccr.2014.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet 2012; 13:343-57; PMID:22473383; http://dx.doi.org/ 10.1038/nrg3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sandhu SK, Fassan M, Volinia S, Lovat F, Balatti V, Pekarsky Y, Croce CM. B-cell malignancies in microRNA Emu-miR-17∼92 transgenic mice. Proc Natl Acad Sci U S A 2013; 110:18208-13; PMID:24145403; http://dx.doi.org/ 10.1073/pnas.1315365110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mu P, Han YC, Betel D, Yao E, Squatrito M, Ogrodowski P, de Stanchina E, D'Andrea A, Sander C, Ventura A. Genetic dissection of the miR-17∼92 cluster of microRNAs in Myc-induced B-cell lymphomas. Genes Dev 2009; 23:2806-11; PMID:20008931; http://dx.doi.org/ 10.1101/gad.1872909 [DOI] [PMC free article] [PubMed] [Google Scholar]


