Abstract
Myocardial ischemia-reperfusion (I-R) injury lacks effective treatments. The miR-17-92 cluster plays important roles in regulating proliferation, apoptosis, cell cycle and other pivotal processes. However, their roles in myocardial I-R injury are largely unknown. In this study, we found that miR-19b was the only member of the miR-17-92 cluster that was downregulated in infarct area of heart samples from a murine model of I-R injury. Meanwhile, downregulation of miR-19b was also detected in H2O2-treated H9C2 cells in vitro mimicking oxidative stress occurring during myocardial I-R injury. Using flow cytometry and Western blot analysis, we found that overexpression of miR-19b decreased H2O2-induced apoptosis and improved cell survival, while downregulation of that had inverse effects. Furthermore, PTEN was negatively regulated by miR-19b at the protein level while silencing PTEN could completely block the aggravated impact of miR-19b inhibitor on H2O2-induced apoptosis in H9C2 cardiomyocytes, indicating PTEN as a downstream target of miR-19b controlling H2O2-induced apoptosis. These data indicate that miR-19b overexpression might be a novel therapy for myocardial I-R injury.
Keywords: ischemia reperfusion injury, cardiomyocytes, apoptosis, microRNA, PTEN, Pathology Section
INTRODUCTION
Acute myocardial infarction represents one of the leading causes of morbidity and mortality worldwide [1]. Although timely reperfusion of the myocardium can limit the infarct area and improve long term cardiac function and survival of the patients, reperfusion itself can also lead to myocardial injury [2, 3]. Despite the successful use of thrombolysis and coronary stenting in the treatment of myocardial ischemia, effective interventions for reducing myocardial reperfusion injury is still lacking [4].
MicroRNAs (miRNAs, miRs) are a group of small non-coding RNAs with ∼22 nucleotides in length, and have been identified as posttranscriptional negative regulators for genes mainly through degradation and/or translational inhibition of target mRNAs [5, 6]. Accumulating evidence show that miRNA dysregulation contributes to multiple cardiovascular diseases including ischemia-reperfusion (I-R) injury [7–11]. miR-1, -26, -29, -21, -24, -103, -133, and -210 have been reported to be regulators of I-R injury either in the early or in the late stage after myocardial infarction, though the underlying mechanisms are largely unclarified [12, 13]. The miR-17-92 cluster is among the best-explored miRNA clusters, including six members (miR-19a, -19b, -17, -18a, -20a, -92a). The miR-17-92 cluster plays important roles in regulating proliferation, apoptosis, cell cycle and other pivotal processes [14]. Dysregulated miR-17-92 cluster has been reported in cardiovascular, immune and neurodegenerative diseases [14]. Although the roles of miR-17-92 in cardiac development, cardiomyocyte proliferation, and cardiac ageing have been reported [14–19], it is still unclear whether, and (if so) how, the miR-17-92 cluster is involved in myocardial I-R injury.
Oxidative stress and apoptosis play fundamental roles in myocardial I-R injury [20–24]. Here we showed that miR-19b was the only member of the miR-17-92 cluster that was downregulated in infarct area of heart samples from a murine model of I-R injury. Meanwhile, decreased miR-19b was also detected in H9C2 cardiomyocytes treated with hydrogen peroxide (H2O2) mimicking myocardial IRI in vitro. Overexpression of miR-19b led to decreased apoptosis and improved survival of H2O2-treated H9C2 cardiomyocytes. Moreover, PTEN was identified as a target gene of miR-19b that was responsible for its anti-apoptosis effects. These data provide evidence that increasing miR-19b might be a novel therapeutic strategy for reducing cellular apoptosis during myocardial IRI.
RESULTS
miR-19b is downregulated in the infarct area of myocardial ischemia-reperfusion mice
Coronary artery ligation for 30 min followed by reperfusion until 24 h was conducted to induce I-R injury in mice. I-R mice displayed marked infarction compared to sham mice, as evidenced by TTC staining (Figure 1A). Using qRT-PCR, miR-19b was found to be the only one in the miR-17-92 cluster that was downregulated in infarct area of heart samples from a murine model of I-R injury (Figure 1B). However, all members of the miR-17-92 cluster remained unchanged in the border area and remote area of heart samples from a murine model of I-R injury (Figure 1C–1D).
Figure 1. miR-19b is decreased in infarct area of myocardial ischemia-reperfusion mice.
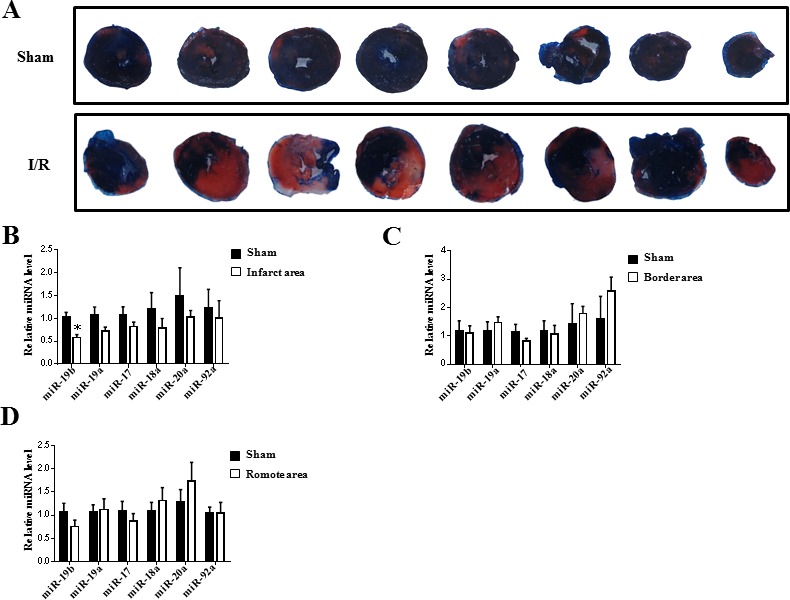
A. TTC staining for heart samples from sham (n = 5) and ischemia-reperfusion (I-R) mice (n = 5). B.-D. qRT-PCR analysis for the miR-17-92 cluster members in heart samples from infarct area, border area and remote area (n = 5). *, P < 0.05.
miR-19b is decreased in H2O2-treated H9C2 cardiomyocytes
As a common reactive oxygen species, H2O2 is usually used to mimic I-R injury in in vitro experiments. Here we found that H2O2 treatment for 2 h significantly increased apoptosis in H9C2 cardiomyocytes as analyzed by flow cytometry (Figure 2A) and western blot analysis for Bcl-2, Bax and cleaved-Caspase 3 to Caspase 3 ratio (Figure 2B). The time-course change of miR-19b was determined and miR-19b was found to be downregulated in H2O2-treated H9C2 cardiomyocytes at 2 h but remained unchanged at 5 min, 15 min, 30 min and 60 min (Figure 2C). Interestingly, hypoxia treatment for 8 h also decreased miR-19b (Figure 2D).
Figure 2. miR-19b is decreased in H2O2-treated H9C2 cardiomyocytes.
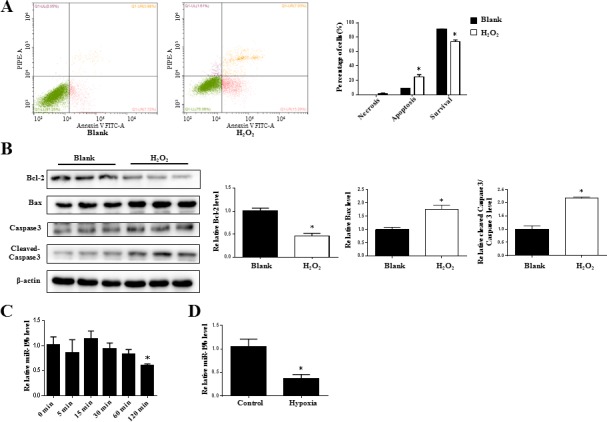
A. Flow cytometry analysis for necrosis, apoptosis, and survival of H9C2 cardiomyocytes treated with H2O2 (600 μM, 2 h) (n = 3). B. Immunoblot analysis for Bcl-2, Bax, cleaved-Caspase 3 to Caspase 3 ratio in H2O2-treated H9C2 cardiomyocytes (600 μM, 2 h) (n = 3). C. The time-course change of miR-19b as determined by qRT-PCRs in H2O2-treated H9C2 cardiomyocytes. (n = 5) D. The downregulation of miR-19b in hypoxia treatment. (n = 5) *, P < 0.05.
miR-19b reduces H2O2-induced apoptosis in H9C2 cardiomyocytes
To further examine the functional effect of miR-19b in H2O2-treated H9C2 cardiomyocytes, transfection of miR-19b mimic, inhibitor, or their negative controls, were conducted. miR-19b mimic was found to be sufficient to increase relative miR-19b level, while miR-19b inhibitor had inverse effect, confirming that miR-19b mimic and inhibitor took effects in H9C2 cardiomyocytes (Figure 3A). Flow cytometry showed that miR-19b mimic reduced H2O2-induced apoptosis in H9C2 cardiomyocytes, while miR-19b inhibitor aggravated that (Figure 3B). Meanwhile, miR-19b overexpression led to increased expression of Bcl-2, decreased expression of Bax, and reduced cleaved-Caspase 3 to Caspase 3 ratio at protein levels, while miR-19b inhibition had inverse effects (Figure 3C). These data indicate a protective effect of miR-19b against H2O2-induced apoptosis in H9C2 cardiomyocytes.
Figure 3. miR-19b reduces H2O2-induced apoptosis in H9C2 cardiomyocytes.
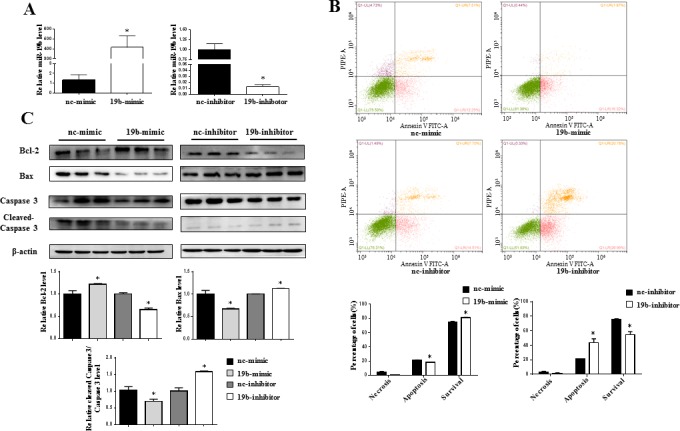
A. qRT-PCR analysis for miR-19b level in H9C2 cardiomyocytes transfected with miR-19b mimic, inhibitor, or respective negative controls (n = 4). B. Flow cytometry analysis for necrosis, apoptosis, and survival of H2O2-treated H9C2 cardiomyocytes with miR-19b overexpression or inhibition (n = 4). C. Immunoblot analysis for Bcl-2, Bax, cleaved-Caspase 3 to Caspase 3 ratio in H2O2-treated H9C2 cardiomyocytes with miR-19b overexpression or inhibition (n = 3). NC = negative control. *, P < 0.05.
PTEN is a downstream target of miR-19b controlling H2O2-induced apoptosis in H9C2 cardiomyocytes
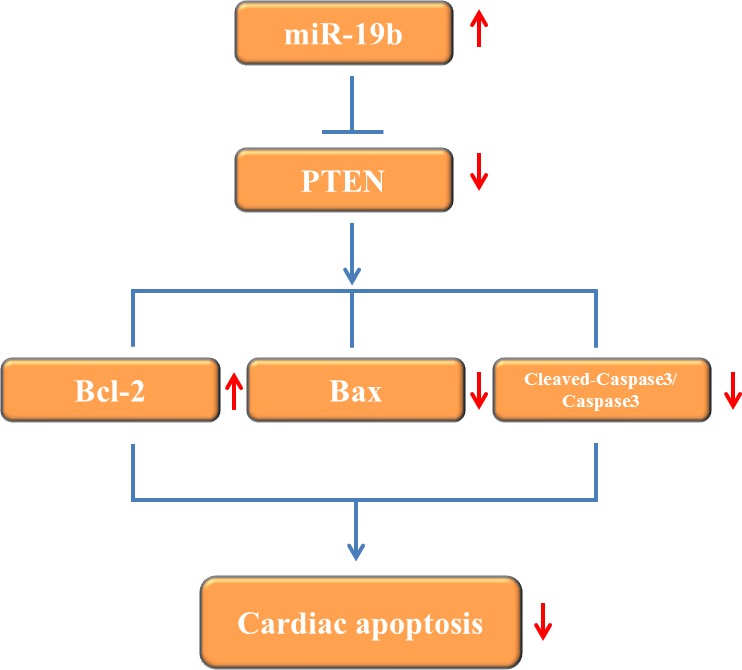
How miR-19b modulates H2O2-induced apoptosis in H9C2 cardiomyocytes was examined. PTEN is a well-known target gene of miR-19b [25–27]. In the current study, immunoblot analysis showed that PTEN was inversely regulated by miR-19b in H9C2 cells (Figure 4A). In addition, although PTEN was not changed in infarct area and border area myocardium (Figure 4B), it was significantly upregulated in H2O2-treated H9C2 cardiomyocytes (Figure 4C). Moreover, silencing PTEN alone led to reduced apoptosis and improved cell survival in H2O2-treated H9C2 cardiomyocytes, while co-transfection of PTEN-siRNA and miR-19b inhibitor could totally abolish the aggravated effect of miR-19b inhibitor on cell apoptosis in H9C2 cardiomyocytes treated with H2O2 (Figure 4D–4E). These data suggest that PTEN is responsbile for the effects of miR-19b in H2O2-induced apoptosis in H9C2 cardiomyocytes (Figure 5).
Figure 4. PTEN is a target gene of miR-19b controlling H2O2-induced apoptosis in H9C2 cardiomyocytes.
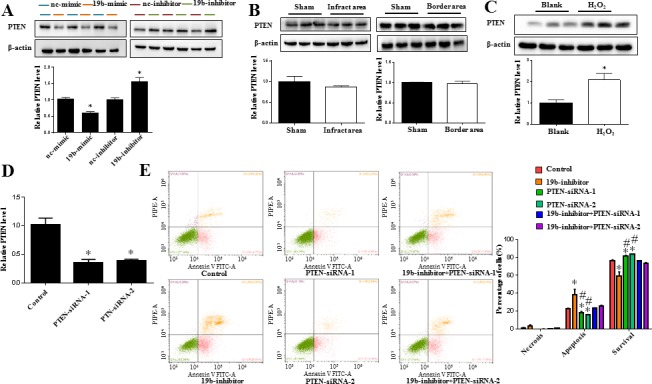
A. Immunoblot analysis for PTEN protein level in H9C2 cardiomyocytes transfected with miR-19b mimic, inhibitor, or respective negative controls (n = 3). B. Immunoblot analysis indicated that PTEN was not changed in infarct area and border area myocardium (n = 3). C. Immunoblot analysis showed that PTEN was upregulated in H2O2-treated H9C2 cardiomyocytes (n = 3). D. qRT-PCRs confirmed that siRNAs for PTEN successfully decreased PTEN at least at the mRNA level (n = 4). E. Flow cytometry analysis for necrosis, apoptosis, and survival of H2O2-treated H9C2 cardiomyocytes with miR-19b inhibition and/or PTEN silence (n = 4). NC = negative control. *, P < 0.05 vs. control; #, P < 0.05 vs. miR-19b inhibitor.
Figure 5. Proposed mechanisms by which miR-19b protects apoptosis induced by H2O2 in H9C2 cardiomyocytes.
DISCUSSION
Myocardial I-R injury is a detrimental process, usually leading to increased infarct size, impaired cardiac function, and even fibrotic hypertrophy and cardiac remodeling over time [28, 29]. However, effective interventions for myocardial I-R injury are still lacking [28, 29]. In the present study, we found that miR-19b was the only one among the miR-17-92 cluster that was decreased in infarct area of heart samples from a murine model of I-R injury. Meanwhile, miR-19b was also downregulated in H2O2-treated H9C2 cardiomyocytes, and miR-19b overexpression was sufficient to reduce H2O2-induced cardiomyocyte apoptosis. Furthermore, PTEN was identified as a downstream target of miR-19b controlling apoptosis in H2O2-treated cardiomyocytes. Therefore, our data suggest that miR-19b might be a novel therapeutic target for reducing early cellular apoptosis during myocardial I-R injury.
Many pathophysiological mechanisms are supposed to be responsible for myocardial I-R injury, including oxidative stress, cell death, calcium overload, and pressure/mechanical stress [3, 20, 21]. Actually, it has been widely accepted that oxidative stress and cell apoptosis are key contributor factors for I-R injury [22, 30]. Previously, multiple miRNAs have been reported to be involved in acute myocardial infarction and myocardial I-R injury [12, 31]. Nevertheless, the roles of miRNAs in oxidative stress-associated cardiomyocyte apoptosis are far from elucidated. Here, we found that miR-19b was markedly downregulated in infarct area heart samples from a murine model of I-R injury. As a key functional member of miR-17-92 cluster, miR-19b has emerging roles implicated in cardiac physiology and pathophysiology changes [15–19]. To the best of our knowledge, we firstly reported a downregulation of miR-19b in myocardial I-R injury, which promoted us to further determine the underlying mechanisms.
Knowing that during the early stage (first 24 h after ischemia) of myocardial I-R injury, production of reactive oxidative species triggers and further enhances cardiomyocyte apoptosis, we continued to determine the functional roles of miR-19b on H2O2-induced apoptosis in rat H9C2 cardiomyocytes. As a key by-product of I-R injury, H2O2 is commonly used to induce oxidative stress-associated apoptosis in H9C2 cardiomyocytes [32, 33]. Several lines of evidence indicates that miRNAs regulate apoptosis in H2O2-treated H9C2 cardiomyocytes. It has previously been reported that upregulating miR-21 or downregulating miR-181a reduces H2O2-induced apoptosis in H9C2 cardiomyocytes, and that overexpressing miR-210 contributes to the protective effect of insulin against apoptosis in H2O2-treated H9C2 cardiomyocytes [34–36]. Besides the reduction of miR-19b expression in infarct area of heart samples from I-R mice, we also found that miR-19b was downregulated in H2O2-treated H9C2 cardiomyocytes. Furthermore, our data demonstrated that miR-19b overexpression reduced H2O2-induced apoptosis and improved cell survival in H9C2 cardiomyocytes, accompanied with increased expression of Bcl-2 and decreased expression of Bax and cleaved-Caspase 3/Caspase 3 ratio, indicating that miR-19b overexpression might provide protective effects against oxidative stress-related cellular apoptosis.
PTEN is a well-known target gene of miR-19b, which mainly regulates cancer cell growth and proliferation [37–40]. Meanwhile, PTEN overexpression has been reported to increase cellular apoptosis [41–45]. Based on that, we proceeded to investigate whether PTEN could be a downstream effector of miR-19b mediating its effect in H2O2-induced apoptosis. As expected, PTEN was negatively regulated by miR-19b at the protein level in H9C2 cardiomyocytes. Importantly, silencing PTEN could abolish the aggravated apoptotic effect of miR-19b inhibitor in H2O2-treated H9C2 cardiomyocytes. These data suggest that PTEN is a downstream effector of miR-19b controlling cardiomyocyte apoptosis-induced by H2O2.
In conclusion, miR-19b overexpression is able to attenuate apoptosis in H2O2-treated H9C2 cardiomyocytes. PTEN is a target gene of miR-19b, responsible for the anti-apoptosis effect of miR-19b. Therefore, miR-19b overexpression might be a novel therapy for myocardial I-R injury.
MATERIALS AND METHODS
Animal model
Eight-week-old male C57BL/6 mice were purchased from Shanghai Laboratory Animal Center (SLAC, Shanghai, China) and housed in autoclaved ventilated cages with sterile food and water ad libitum. All animal experimentations were conducted under the established guidelines on the use and care of laboratory animals for biomedical research published by National Institutes of Health (No. 85-23, revised 1996). To induce myocardial I-R injury, mice were subjected to 30 min of coronary artery ligation followed by reperfusion until 24 h. In brief, after anesthetized with ketamine and sevoflurane, the ligation of left coronary artery was conducted about 2 mm under the left auricle using 7-0 silk sutures. After 30 min of ischemia, reperfusion was carried out until 24 h when mice were sacrificed. The sham mice underwent left thoracotomy only. Myocardial infarct size was analyzed using triphenyltetrazolium chloride (TTC) staining. Heart samples were collected according to samples from infarct area, border area and remote area. All samples were harvested and snap frozen in liquid nitrogen until RNA extraction.
Cell culture and H2O2 treatment
Rat cardiomyocyte H9C2 cell line was purchased from the Cell Bank of Chinese Academy of Science (Shanghai, China) and cultured in Dulbecco's modified eagle's medium (DMEM, Corning, USA) supplemented with 10% fetal bovine serum (Biolnd, Israel) and 1% streptomycin/penicillin in a humidified atmosphere containing 5% CO2 at 37°C. H9C2 cardiomyocytes were treated with 600 μM H2O2 (Sigma, USA) for 2 h to induce cell apoptosis mimicking I-R injury in vivo. In addition, to determine the time-course of miR-19b changes, H9C2 cardiomyocytes were also treated with 600 μM H2O2 from 0 min to 120 min. Moreover, hypoxia treatment (0% O2) was also conducted in H9C2 cardiomyocytes for 8 h.
Cell transfection
The miR-19b mimic, inhibitor, and their negative controls, as well as PTEN-siRNAs, were all purchased from RiboBio (Guangzhou, China). Cells were transfected after 8 h of starvation. To overexpress or inhibit miR-19b expression, miR-19b mimic (50 nM), inhibitor (100 nM), or respective negative controls was transfected to H9C2 cardiomyocytes for 48 h using lipofectamine 2000 (Invitrogen, USA). To silence PTEN expression, PTEN-siRNAs (100 nM) were transfected to H9C2 cardiomyocytes for 48 h using lipofectamine 2000 in accordance with the manufacturer's instruction.
Flow cytometry detection of cell apoptosis and necrosis
After transfected with miR-19b mimic, inhibitor, or respective controls for 48 h, cell apoptosis and necrosis were analyzed using Annexin V-FITC and propidium iodide (PI) kit (Dojindo, Japan) according to the manufacturer's instruction, followed by flow cytometry analysis (Beckman Coulter, USA).
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNAs were isolated using Trizol Reagent (Invitrogen, USA) and reverse transcribed into cDNA using iScriptTM cDNA synthesis kit (Bio-Rad, USA). For detection the mRNA level of PTEN, the RT product was subjected to 40 cycles of quantitative PCR with Takara SYBR Premix Ex TaqTM (Tli RNaseH Plus, Japan) in CFX96TM Real-Time PCR Detection System (Bio-Rad, USA). 18s was used as an internal reference. The primer sequences (forward and reverse) are as follows: PTEN, CAATGTTCAGTGGCGAACTT and GGCAATGGCTGAGGGAACT; 18s, ATTC GAACGTCTGCCCTATCAA and CGGGAGTGGGTAATTTGCG. For quantitative miRNA analysis, Bulge-Loop™ miRNA qPCR Primer Set (RiboBio, China) was used to detect miRNA expression levels with Takara SYBR Premix Ex TaqTM (Tli RNaseH Plus, Japan) by qRT-PCR in CFX96TM Real-Time PCR Detection System. 5s was used for normalization of miRNA expression levels. The relative expression levels for each mRNA and miRNAs were calculated by the 2−ΔΔCTmethod.
Western blot analysis
Samples were lysed in RIPA buffer (KeyGen, China) containing 1% phenylmethanesulfonyl fluoride (PMSF). Total proteins were quantified with the BCA protein assay kit (KeyGen, China). Lysates equivalent to 30 μg of protein were subjected to 10% SDS-PAGE gels, transferred onto PVDF membranes, and probed with primary antibodies anti-PTEN (1:1000; Epitomics, ab154812), anti-Bcl-2 (1:1000; Abclonal, A0208), anti-Bax (1:1000; Abclonal, A2211), and anti-Caspase-3 (1:1000; Abclonal, A2156) overnight at 4°C. β-actin (1:10000; Abclonal, AC004) was used as a loading control. After incubation with appropriate secondary antibodies for 2 h at room temperature, ECL System (Bio-Rad, USA) was used to visualize the protein bands with ChemiDocTM XRS Plus luminescent image analyzer (Bio-Rad, USA).
Statistical analysis
Results were presented as mean±SEM. All data were analyzed with an independent-samples T-test or one-way ANOVA test followed by Bonferroni's tests using SPSS (version 19). P < 0.05 was regarded as statistical significance.
Acknowledgments
This work was supported by the grants from National Natural Science Foundation of China (81470515 and 81270314 to JH Xu, 81570362 and 81200169 to JJ Xiao, 81472158 to L Che, 81400647 to Y Bei, 81541007 to H. Wang), Innovation Program of Shanghai Municipal Education Commission (13YZ014 to JJ Xiao), Innovation fund from Shanghai University (sdcx2012038 to JJ Xiao), Program for the integration of production, teaching and research for University Teachers supported by Shanghai Municipal Education Commission (year 2014, to JJ Xiao) and Natural Science Foundation of Shanghai (14ZR1437900 to L. Che; 14ZR1438300 to H. Wang) and Shanghai Medical Guide Project from Shanghai Science and Technology Committee (134119a3000 to J, Xu).
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
REFERENCES
- 1.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985;76:1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vander Heide RS, Steenbergen C. Cardioprotection and myocardial reperfusion: pitfalls to clinical application. Circ Res. 2013;113:464–477. doi: 10.1161/CIRCRESAHA.113.300765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibáñez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol. 2015;65:1454–1471. doi: 10.1016/j.jacc.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Khraiwesh B, Arif MA, Seumel GI, Ossowski S, Weigel D, Reski R, Frank W. Transcriptional control of gene expression by microRNAs. Cell. 2010;140:111–122. doi: 10.1016/j.cell.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Xiao J, Zhu H, Wei X, Platt C, Damilano F, Xiao C, Bezzerides V, Boström P, Che L, Zhang C, Spiegelman BM, Rosenzweig A. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015;21:584–595. doi: 10.1016/j.cmet.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kukreja RC, Yin C, Salloum FN. MicroRNAs: new players in cardiac injury and protection. Mol Pharmacol. 2011;80:558–564. doi: 10.1124/mol.111.073528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao J, Zhu X, He B, Zhang Y, Kang B, Wang Z, Ni X. MiR-204 regulates cardiomyocyte autophagy induced by ischemia-reperfusion through LC3-II. J Biomed Sci. 2011;18:35. doi: 10.1186/1423-0127-18-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu H, Fan GC. Role of microRNAs in the reperfused myocardium towards post-infarct remodelling. Cardiovasc Res. 2012;94:284–292. doi: 10.1093/cvr/cvr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Bei Y, Shi J, Xiao J, Kong X. Non-Coding RNAs in Cardiac Aging. Cell Physiol Biochem. 2015;36:1679–1687. doi: 10.1159/000430141. [DOI] [PubMed] [Google Scholar]
- 12.Song MA, Paradis AN, Gay MS, Shin J, Zhang L. Differential expression of microRNAs in ischemic heart disease. Drug Discov Today. 2015;20:223–235. doi: 10.1016/j.drudis.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang JX, Zhang XJ, Li Q, Wang K, Wang Y, Jiao JQ, Feng C, Teng S, Zhou LY, Gong Y, Zhou ZX, Liu J, Wang JL, et al. MicroRNA-103/107 Regulate Programmed Necrosis and Myocardial Ischemia/Reperfusion Injury Through Targeting FADD. Circ Res. 2015;117:352–363. doi: 10.1161/CIRCRESAHA.117.305781. [DOI] [PubMed] [Google Scholar]
- 14.Mogilyansky E, Rigoutsos I. The miR-17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013;20:1603–1614. doi: 10.1038/cdd.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao S, Liu TW, Wang Z, Jiao ZY, Cai J, Chi HJ, Yang XC. Downregulation of microRNA-19b contributes to angiotensin II-induced overexpression of connective tissue growth factor in cardiomyocytes. Cardiology. 2014;127:114–120. doi: 10.1159/000355429. [DOI] [PubMed] [Google Scholar]
- 16.Li M, Hu X, Zhu J, Zhu C, Zhu S, Liu X, Xu J, Han S, Yu Z. Overexpression of miR-19b impairs cardiac development in zebrafish by targeting ctnnb1. Cell Physiol Biochem. 2014;33:1988–2002. doi: 10.1159/000362975. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Huang Z, seok H, Ding J, Kataoka M, Zhang Z, Hu X, Wang G, Lin Z, Wang S, Pu W, Liao R, Wang D. miR-17-92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts. Circ Res. 2013;112:1557–1566. doi: 10.1161/CIRCRESAHA.112.300658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song DW, Ryu JY, Kim JO, Kwon EJ, Kim DH. The miR-19a/b family positively regulates cardiomyocyte hypertrophy by targeting atrogin-1 and MuRF-1. Biochem J. 2014;457:151–162. doi: 10.1042/BJ20130833. [DOI] [PubMed] [Google Scholar]
- 19.Qin DN, Qian L, Hu DL, Yu ZB, Han SP, Zhu C, Wang X, Hu X. Effects of miR-19b overexpression on proliferation, differentiation, apoptosis and Wnt/β-catenin signaling pathway in P19 cell model of cardiac differentiation in vitro. Cell Biochem Biophys. 2013;66:709–722. doi: 10.1007/s12013-013-9516-9. [DOI] [PubMed] [Google Scholar]
- 20.Jennings RB. Historical perspective on the pathology of myocardial ischemia/reperfusion injury. Circ Res. 2013;113:428–438. doi: 10.1161/CIRCRESAHA.113.300987. [DOI] [PubMed] [Google Scholar]
- 21.Mozaffari MS, Liu JY, Abebe W, Baban B. Mechanisms of load dependency of myocardial ischemia reperfusion injury. Am J Cardiovasc Dis. 2013;3:180–196. [PMC free article] [PubMed] [Google Scholar]
- 22.Meng G, Wang J, Xiao Y, Bai W, Xie L, Shan L, Moore PK, Ji Y. GYY4137 protects against myocardial ischemia and reperfusion injury by attenuating oxidative stress and apoptosis in rats. J Biomed Res. 2015;29:203–213. doi: 10.7555/JBR.28.20140037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho S, Cho M, Kim J, Kaeberlein M, Lee SJ, Suh Y. Syringaresinol protects against hypoxia/reoxygenation-induced cardiomyocytes injury and death by destabilization of HIF-1α in a FOXO3-dependent mechanism. Oncotarget. 2015;6:43–55. doi: 10.18632/oncotarget.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Zeng Z, Li Q, Xu Q, Xie J, Hao H, Luo G, Liao W, Bin J, Huang X, Liao Y. Inhibition of microRNA-497 ameliorates anoxia/reoxygenation injury in cardiomyocytes by suppressing cell apoptosis and enhancing autophagy. Oncotarget. 2015;6:18829–18844. doi: 10.18632/oncotarget.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu SQ, Jiang S, Li C, Zhang B, Li QJ. miR-17-92 Cluster Targets Phosphatase and Tensin Homology and Ikaros Family Zinc Finger 4 to Promote TH17-mediated Inflammation. J Biol Chem. 2014;289:12446–12456. doi: 10.1074/jbc.M114.550723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang S, Li C, Olive V, Lykken E, Feng F, Sevilla J, Wan Y, He L, Li QJ. Molecular dissection of the miR-17-92 cluster's critical dual roles in promoting Th1 responses and preventing inducible Treg differentiation. Blood. 2011;118:5487–5497. doi: 10.1182/blood-2011-05-355644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olive V, Bennett MJ, Walker JC, Ma C, Jiang I, Cordon-Cardo C, Li QJ, Lowe SW, Hannon GJ, He L. miR-19 is a key oncogenic component of mir-17-92. Genes Dev. 2009;23:2839–2849. doi: 10.1101/gad.1861409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eapen ZJ, Tang WHW, Felker GM, Hernandez AF, Mahaffey KW, Lincoff AM, Roe MT. Defining heart failure end points in ST-segment elevation myocardial infarction trials: integrating past experiences to chart a path forward. Circ Cardiovasc Qual Outcomes. 2012;5:594–600. doi: 10.1161/CIRCOUTCOMES.112.966150. [DOI] [PubMed] [Google Scholar]
- 29.O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso JE, Tracy CM, Woo YJ, Zhao DX. CF/AHA Task Force. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:529–555. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Z, Zhu J, Zhao X, Yang K, Lu L, Zhang F, Shen W, Zhang R. All-Trans Retinoic Acid Ameliorates Myocardial Ischemia/Reperfusion Injury by Reducing Cardiomyocyte Apoptosis. PloS One. 2015;10:e0133414. doi: 10.1371/journal.pone.0133414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fasanaro P, Greco S, Ivan M, Capogrossi MC, Martelli F. microRNA: emerging therapeutic targets in acute ischemic diseases. Pharmacol Ther. 2010;125:92–104. doi: 10.1016/j.pharmthera.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. Biochim Biophys Acta. 2006;1758:994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Han H, Long H, Wang H, Wang J, Zhang Y, Wang Z. Progressive apoptotic cell death triggered by transient oxidative insult in H9c2 rat ventricular cells: a novel pattern of apoptosis and the mechanisms. Am J Physiol Heart Circ Physiol. 2004;286:H2169–2182. doi: 10.1152/ajpheart.00199.2003. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Huang H, Fan Y, Kong B, Hu H, Hu K, Guo J, Mei Y, Liu WL. Effects of downregulation of microRNA-181a on H2O2-induced H9c2 cell apoptosis via the mitochondrial apoptotic pathway. Oxid Med Cell Longev. 2014;2014:960362. doi: 10.1155/2014/960362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi Y-F, Liu N, Li Y-X, Song C-L, Song X-J, Zhao Z, Liu B. Insulin protects H9c2 rat cardiomyoblast cells against hydrogen peroxide-induced injury through upregulation of microRNA-210. Free Radic Res. 2015;49:1147–1155. doi: 10.3109/10715762.2015.1050588. [DOI] [PubMed] [Google Scholar]
- 36.Cheng Y, Liu X, Zhang S, Lin Y, Yang J, Zhang C. MicroRNA-21 protects against the H(2)O(2)-induced injury on cardiac myocytes via its target gene PDCD4. J Mol Cell Cardiol. 2009;47:5–14. doi: 10.1016/j.yjmcc.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Yang S, Yan W, Yang J, Qin YJ, Lin XL, Xie RY, Wang SC, Jin W, Gao F, Shi JW, Zhao WT, Jia JS, Shen HF, Ke JR, Liu B, Zhao YQ, Huang WH, Yao KT, Li DJ, Xiao D. MicroRNA-19 triggers epithelial-mesenchymal transition of lung cancer cells accompanied by growth inhibition. Lab Investig. 2015;95:1056–1070. doi: 10.1038/labinvest.2015.76. [DOI] [PubMed] [Google Scholar]
- 38.Li X, Xie W, Xie C, Huang C, Zhu J, Liang Z, Deng F, Zhu M, Zhu W, Wu R, Wu J, Geng S, Zhong C. Curcumin modulates miR-19/PTEN/AKT/p53 axis to suppress bisphenol A-induced MCF-7 breast cancer cell proliferation. Phytother Res. 2014;28:1553–1560. doi: 10.1002/ptr.5167. [DOI] [PubMed] [Google Scholar]
- 39.Tian L, Fang Y, Xue J, Chen J. Four microRNAs promote prostate cell proliferation with regulation of PTEN and its downstream signals in vitro. PloS One. 2013;8:e75885. doi: 10.1371/journal.pone.0075885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jia Z, Wang K, Zhang A, Wang G, Kang C, Han L, Pu P. miR-19a and miR-19b overexpression in gliomas. Pathol Oncol Res. 2013;19:847–853. doi: 10.1007/s12253-013-9653-x. [DOI] [PubMed] [Google Scholar]
- 41.Miyata S, Fukuda Y, Tojima H, Matsuzaki K, Kitanaka S, Sawada H. Mechanism of the inhibition of leukemia cell growth and induction of apoptosis through the activation of ATR and PTEN by the topoisomerase inhibitor 3EZ, 20Ac-ingenol. Leuk Res. 2015;39:927–932. doi: 10.1016/j.leukres.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 42.de Araujo WM, Robbs BK, Bastos L, de Souza WF, Vidal-Cabral F, Viola JP, Morgado-Diaz JA. PTEN Overexpression Cooperates With Lithium to Reduce the Malignancy and to Increase Cell Death by Apoptosis Via PI3K/Akt Suppression in Colorectal Cancer Cells. J Cell Biochem. 2016;117:458–469. doi: 10.1002/jcb.25294. [DOI] [PubMed] [Google Scholar]
- 43.Bai H, Li H, Li W, Gui T, Yang J, Cao D, Shen K. The PI3K/AKT/mTOR pathway is a potential predictor of distinct invasive and migratory capacities in human ovarian cancer cell lines. Oncotarget. 2015;6:25520–25532. doi: 10.18632/oncotarget.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu W, Liu S, Li B, Xie Y, Adhiambo C, Yang Q, Ballard BR, Nakayama KI, Matusik RJ, Chen Z. SKP2 inactivation suppresses prostate tumorigenesis by mediating JARID1B ubiquitination. Oncotarget. 2015;6:771–788. doi: 10.18632/oncotarget.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kato S, Elkin SK, Schwaederle M, Tomson BN, Helsten T, Carter JL, Kurzrock R. Genomic landscape of salivary gland tumors. Oncotarget. 2015;6:25631–25645. doi: 10.18632/oncotarget.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]


