Abstract
Understanding the processes governing the ability of the heart to repair and regenerate after injury is crucial for developing translational medical solutions. New avenues of exploration include cardiac cell therapy and cellular reprogramming targeting cell death and regeneration. An attractive possibility is the exploitation of cytoprotective genes that exist solely for self-preservation processes and serve to promote and support cell survival. While the antioxidant and heat shock proteins are included in this category, one enzyme that has received a great deal of attention as a master protective sentinel is heme oxygenase-1 (HO-1), the rate-limiting step in the catabolism of heme into the bioactive signaling molecules carbon monoxide, biliverdin and iron. The remarkable cardioprotective effects ascribed to HO-1 are best evidenced by its ability to regulate inflammatory processes, cellular signaling and mitochondrial function ultimately mitigating myocardial tissue injury and the progression of vascular-proliferative disease. We discuss here new insights into the role of HO-1 and heme on cardiovascular health, and importantly, how they might be leveraged to promote heart repair after injury.
Keywords: heme oxygenase-1, carbon monoxide, cardiac ischemia, mitochondria, inflammation
INTRODUCTION
Self-preservation is a fundamental tenet exhibited by all organisms and is perhaps most apparent when the organism is confronted by various threats to survival. This concept also holds true at the most basic cellular level where the cell coordinates a series of responses evolved to ensure the best chance of defense and survival. The ability of cells and tissues to mount an adaptive response to stress, which is ultimately responsible for protecting against damage and restoring homeostasis, is a powerful intrinsic strategy that depends on the induction of several beneficial defensive systems. Among these, the stress protein heme oxygenase-1 (HO-1, encoded by the Hmox1 gene) plays a prominent role, which has been recognized in different organs and tissues as well as different pathological scenarios.1-4 The main function of HO-1 is to degrade heme and generate carbon monoxide (CO) and biliverdin while simultaneously releasing iron, which is stored within the iron-binding protein ferritin.5;6 These products exert signaling and cytoprotective activities that mitigate apoptosis and inflammation, regulate vasomotor tone, and exert antioxidant and immunomodulatory functions.2 It is remarkable that such a wide range and significant set of salutary effects can be associated with one single enzymatic reaction and that these properties have gradually been uncovered over the last two decades starting from the erroneous assumption that heme oxygenase metabolites were essentially waste products. The discovery of the first case of human HO-1 deficiency and the creation of the HO-1 deficient mouse has significantly substantiated the importance of HO-1 in health and disease states. In addition to HO-1-derived products, the role of this enzyme is to counteract oxidative tissue injury triggered by free heme. This is particularly important when there is cellular damage and cells suddenly release large amounts of heme, which is likely amplified in injured tissues containing high amounts of heme such as the heart and muscle. The dichotomous nature of heme stems from its function as an essential prosthetic group of enzymes and proteins such as guanylate cyclase and hemoglobin, and its propensity to do harm once liberated from these same proteins upon damage.7;8 Interestingly, a new facet of heme biology has emerged and is dependent on the activation of immune cells through its binding to Toll-like receptor-4 (TLR4).9 TLR4 and other pattern recognition receptors in this family distinguish specific exogenous pathogenic stimuli known as Pathogen Associated Molecular Patterns (PAMPs) that include bacterial endotoxin peptidoglycan (PGN), and double-stranded RNA. Similarly, there are intracellular molecules known as Danger Associated Molecular Patterns (DAMPs) that when released can initiate and perpetuate a sterile, non-infectious immune response. They include mitochondrial DNA, ATP, formyl peptides, HMGB1, and the serum amyloid protein family.10;11 Recent evidence suggests that also heme could be considered a DAMP or an alarmin.9;12;13 One common factor is that many of these molecular pattern molecules have been shown to increase the activity of HO-1. Thus, HO-1 is positioned as a crucial arbiter of oxidative stress and inflammatory responses. This review will discuss the participation of HO-1 and its products in protection and modulation of function in cardiac and vascular tissues. We will begin by recalling the most salient discoveries that have shaped our understanding of the HO-1 system in physiology and disease.
ACTIVITY AND FUNCTION OF HEME OXYGENASES: A CLOSER LOOK AT THE HISTORY OF HO-1
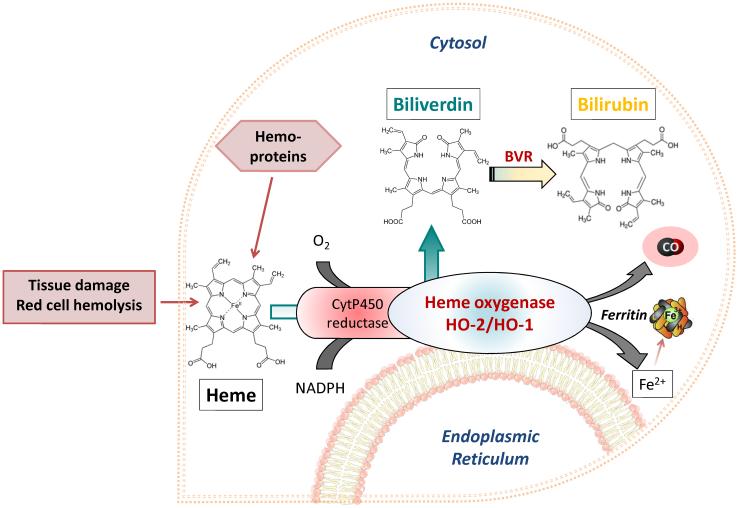
Heme oxygenases are ubiquitous and evolutionary conserved proteins found in both the plant and animal kingdoms.14 In mammals, they are endoplasmic reticulum-anchored enzymes that catalyze the rate limiting step in the degradation of heme. The oxidation of heme by heme oxygenases involves a series of redox reactions and the participation of cytochrome P450 reductase that ultimately generate stoichiometric amounts of CO, iron and biliverdin, which is then converted to bilirubin by the cytosolic biliverdin reductase (BVR).15-17 The characterization of this enzymatic activity was carried out in 1969 by Tenhunen who demonstrated that the system has an absolute requirement for molecular oxygen and NADPH reducing equivalents18 (Figure 1).
Figure 1. Schematic representation of the heme oxygenase pathway.
Heme, either derived from intracellular sources, such as hemoproteins and mitochondria, or from damaged tissues and red blood cell hemolysis (extracellular sources) is utilized by heme oxygenase enzymes (HO-1 and HO-2) to generate carbon monoxide (CO), biliverdin and iron. Biliverdin is converted to bilirubin by biliverdin reductase (BVR), while iron is stored in the ferritin protein. Although heme oxygenase enzymes were initially localized in the endoplasmic reticulum, recent reports suggest that HO-1 can be found under certain conditions in other cellular compartments such as the nucleus.19
Interestingly, and prior to the discovery of heme oxygenase, Sjostrand observed twenty years earlier that CO was produced constantly in the human body and was considerably increased in conditions accompanied by abnormal red blood cell decomposition.20 These data were confirmed in 1966 by Coburn and colleagues, who demonstrated augmented endogenous CO production in patients with haemolytic anemia.21 For the ensuing fifteen years, the degradation of heme was viewed primarily as a catabolic pathway performed by specialized tissues such as the spleen and the liver to regulate heme levels and iron recycling from senescent erythrocytes or oxidant-damaged hemoglobin.22 However, the true biological role for the heme oxygenases started to emerge during the mid 1980s, when Maines and colleagues demonstrated the existence of two major isoforms of heme oxygenase: a constitutively expressed HO-2 present primarily in liver, spleen and testes and an HO-1 protein that could be highly induced in various tissues by a host of chemicals including the substrate heme and heavy metals.1;23;24 It was also found that HO-2 was refractory to all these potential inducers and that HO-1 was encoded by a distinct gene, thus indicating a different regulation and function for the two proteins.1;25 In 1989, Keyse and Tyrrell reported that human skin fibroblasts exposed to UVA radiation, hydrogen peroxide and arsenite displayed high levels of a 32-kd stress protein.26 The same group proposed later on that induction of this protein, identified as HO-1, represented a general and adaptive response to oxidative stress in mammalian cells.27;28 These studies were a turning point in the field of heme oxygenase as they inspired other laboratories to investigate the role of HO-1 as an endogenous defense mechanism against cellular injury. But how could a protein fundamental in heme catabolism contribute to protect tissues under conditions of cellular stress?
Some answers to this question emerged when scientists began to analyze the intrinsic biochemical and bioactive properties of the substrate and the products of HO-1/HO-2 enzymatic activities. The findings by Stocker and colleagues showing that low micromolar concentrations of biliverdin and bilirubin in vitro efficiently scavenge peroxyl radicals and decrease peroxidation of low density lipoproteins provided the first clue that these bile pigments act as endogenous chain-breaking antioxidants.29-31 Snyder and co-workers also proposed that heme oxygenase-derived CO could function as a neurotransmitter, despite the renowned poisonous effect of this gas at high concentrations. The authors described co-localization in the brain of a heme-dependent guanylate cyclase and HO-2 and that inhibition of HO-2 with zinc-protoporphyrin abrogated the production of the second messenger cGMP by guanylate cyclase.32 Therefore, they concluded that, similar to nitric oxide (NO), CO was functioning as a signaling molecule and neurotransmitter. Although the proof that endogenous CO activated cGMP in neurons emerged later,33 application of exogenous CO gas in vitro was already known to exert a series of cGMP-dependent vascular effects including inhibition of platelet aggregation, smooth muscle cell proliferation and vasodilatation.34;35 Importantly, in 1995 Suematsu and co-workers demonstrated that blockade of HO-2 activity in the perfused liver reduced CO flux in the venous effluent thus increasing hepatic vascular resistance and reducing sinusoidal flow rate.36 It was then confirmed that induction of HO-1 in vascular tissues under stress conditions also resulted in increased endogenous CO,37 which in the absence of NO activity acted as a major regulator of vasomotor tone and blood pressure.38;39 The different pharmacological actions of CO, including its ability to modulate the immune response, are highlighted in recent investigations by the groups of Motterlini, Otterbein, Soares, Choi and Pinsky. In fact, CO possesses the ability to modulate inflammation and apoptosis to the point that non-toxic doses of CO gas or CO releasing molecules (CO-RMs) provide therapeutic benefit in a variety of disease models.40-45 Importantly, the effects of exogenously applied CO mimicked that observed with HO-1 induction. These studies raised the concrete possibility of using pharmacological approaches to deliver controlled amounts of CO for the treatment of vascular and ischemic heart disease as well as inflammatory disorders.45-47
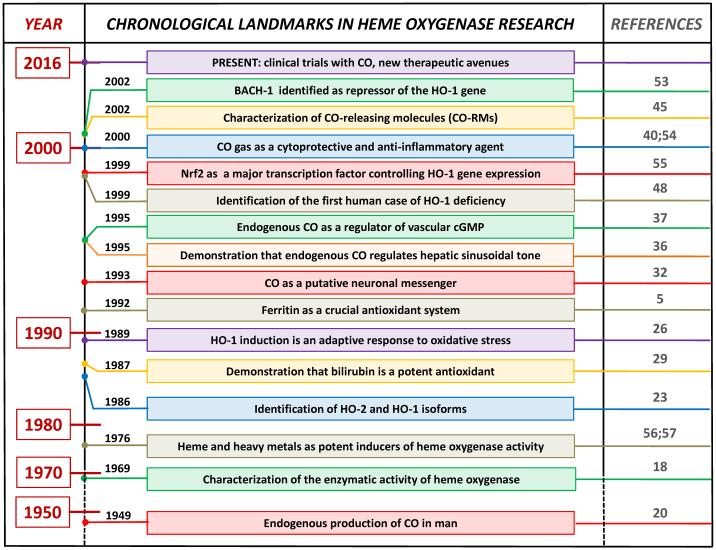
Within this chronological account (Figure 2), the identification of the first human case of HO-1 deficiency by Yachie and colleagues underscored the importance of this stress inducible enzyme in cellular homeostasis.48 The patient suffered from severe growth retardation and persistent hemolytic anemia characterized by marked intravascular hemolysis, which was associated with low levels of bilirubin, an abnormal coagulation/fibrinolysis system, and persistent endothelial damage. Growth retardation, anemia, iron deposition, and vulnerability to stressful injury are all characteristics observed in HO-1 deficient mice.49 Thus, lack of HO-1 is clearly detrimental to mammalian tissues. Application of biliverdin/bilirubin or CO can compensate for the absence of HO-1 in several disease models2;41;50;51 indicating that increased levels of HO-1 products during stress conditions is a pre-requisite for cytoprotection.
Figure 2. Landmarks in the history of heme oxygenase research.
This is not an exhaustive list as additional important findings have been published over the years by scientists working in the field and are not reported here due to space limitation.
From a historical perspective the production of bile was observed centuries ago by the ancient Egyptians and Greeks who both considered it as one of four humors that reflect human health and allowed diagnoses to be made. This is not surprising since the process of heme degradation is probably the only example of enzymatic reactions that can be visibly observed in the human body. Essentially, the evolution of a bruise in the skin is a “real-time spectrophotometric assay” of heme oxygenase and biliverdin reductase at work as the rupture of capillaries and consequent red blood cell lysis results in the development of a red-brown color (hemoglobin/met-hemoglobin), which then turns green (biliverdin) and subsequently into a yellow pigment (bilirubin). Although CO is also produced, it is colorless and thus cannot be seen. However, in the 19th century John Haldane already realized that CO was physiologically important in respiration and affected the oxygen carrying capacity of hemoglobin in man.52 The questions remained, however, as to why the body generates CO and the bile pigments, which at that time were perceived as waste products and detrimental to survival.
HO-1 AND CARDIOVASCULAR PROTECTION
HO-1 in myocardial ischemia-reperfusion
During its early characterization HO-1 was defined as heat shock protein-32 (HSP-32) and was shown to be expressed in primary cultures of rat cardiomyocytes and rat hearts under conditions of oxidative stress.58;59 The findings on the isolated rat hearts are interesting in that induction of HO-1 occurred only during the reperfusion phase but not during the preceding period of global ischemia. Furthermore, application of catalase or superoxide dismutase at reperfusion nearly abolished the increase in HO-1.59 Therefore, HO-1 was responding to oxidative signals produced by pathological events such as ischemia-reperfusion (IR) or other stimuli, which we now suggest could have been heme released into the environment as a result of tissue injury. We note that HO-1 was up-regulated only at reperfusion but it is possible that the period of ischemic time (5 or 20 minutes) was insufficient to induce HO-1. Indeed, prolonged exposure to hypoxia increases HO-1 expression in primary cultures of cardiomyocytes and cardiac cell lines.60;61 The explanation for hypoxia-induced HO-1 could be related to effects on cellular bioenergetics involving mitochondria, where complex I is in a low activity state during cardiac ischemia and is reactivated by reperfusion, generating superoxide and hydrogen peroxide which cause cell damage and death.62 HO-1 induction during IR suggested either a role for the protein as a marker of oxidative stress or its active participation in the tissue adaptation to stress. Several additional studies have since confirmed the cytoprotective action of HO-1 in the heart. Over-expression of HO-1 prior to IR reduces cardiac ischemic damage in the isolated rat heart while inhibition of HO-1 activity abrogated the protective effects and in many instances enhanced IR damage.63;64 Transgenic mice with cardiac-specific over-expression of HO-1 or enhanced cardiac HO-1 levels following gene therapy or pharmacological approaches exhibit markedly lower infarct size and improved cardiac function after IR both in the short term65-70 and even after one year from the ischemic event.71 Remarkably, cardioprotection was observed also when HO-1 gene transfer with an adenoviral vector was performed one year prior to myocardial infarction in mice,72 indicating the long-term potential of this strategy as a sort of ‘immunization against infarction‘. In contrast, HO-1 null mice displayed increased myocardial damage after IR.73 In another interesting approach, Ma and colleagues generated a recombinant HO-1 protein that crossed cell membranes resulting in prolonged preservation of heart grafts and reduced IR after cardiac transplant.74 The enzymatic activity of HO-1 was crucial for this beneficial outcome since treatment with a mutant HO-1 protein lacking enzymatic function did not afford protection.74 Consistent with this finding, the products of heme metabolism were as effective as HO-1 induction in decreasing IR injury. Bilirubin given in the low nanomolar range prior to IR was initially established as a candidate for cardioprotection and hearts from heme-treated animals contained higher levels of bilirubin.63 However, a role for CO was also documented by demonstrating that CORM-3, one of the first CO-releasing molecules synthesized in our laboratory, prevented the damage induced by IR in ex-vivo isolated rat heart preparations and in vivo models of myocardial infarction.46;75-77 The reduction in infarct size in vivo was comparable to that observed with ischemic preconditioning, the most powerful intervention that reproducibly and consistently diminishes cardiac injury after IR.76
Besides acute ischemic events, HO-1 is also able to counteract cardiac dysfunction caused by chronic heart failure. In this case, enhanced HO-1 expression in cardiomyocytes of transgenic mice or mice deficient in Bach1, a repressor of HO-1 gene transcription, prevented the left ventricular remodeling and hypertrophy caused by coronary artery ligation.68;78 In cardiomyocytes in vitro HO-1 significantly diminished hypertrophy induced by endothelin-1.79 Diverse mechanisms appear to mediate the protective activities of HO-1 in the heart, including reduction in oxidative stress and inhibition of apoptotic cell death due to decreased pro-apoptotic proteins such as p53 and increased anti-apoptotic factors like Bcl-2 and inhibition of NF-kB and AP-1.66;67;73;78;80;81 In addition, changes in the function of mitochondria, which are known to be critical during IR injury and control signaling, metabolic and cell death pathways involved in cellular adaptation to stress, have been associated with HO-1 over-expression and prevention of damage.63;78;82-84 The production of inflammatory mediators and the inflammatory response are also modulated by HO-1. Considering the latest emerging findings in the field and the paramount importance of inflammation in the repair phase of cardiac tissue after damage, we will analyze this subject in a separate section below.
HO-1 in heart preservation and transplantation
The penultimate tissue injury is one in which the entire organ becomes devoid of oxygen compared to a focal injury such as an infarcted region resulting from sudden stenosis of a blood vessel. Solid organ transplantation has no solution for depriving an organ of oxygen as it is transported and surgically implanted into a recipient. Harvesting an organ from a donor in most instances necessitates a period of warm ischemia followed by a period of cold ischemia. Restoration of blood flow to that organ in the recipient results in massive amounts of additional tissue injury driven in large part by oxidative stress. The successful functioning of the organ post-transplantation is believed to be directly related to the severity of the IR injury. In seminal studies by Soares et al in models of rat to mouse cardiac xenografts where complement and T cell responses are inactivated, induction of HO-1 with protoporphyrins led to long-term graft survival driven by less inflammatory infiltrates, thrombosis and transplant vascular stenosis (TVS).85 Transplantation of hearts from Hmox1−/− or Hmox1+/− mice led to rapid rejection in 7 days or less compared to Hmox1+/+ mice which survived >60 days. In the same year Hancock et al showed in mouse cardiac allografts that HO-1 was required to prevent transplant arteriosclerosis.86 Interestingly, exclusive cardiac HO-1 over-expression increased the time of graft survival after transplantation but this effect was less pronounced compared to that obtained when hearts were transplanted in recipients with systemic HO-1 over-expression.87 Grafts exhibited diminished T cell and infiltrates early after transplantation with minimal expression of CD25 as an immune cell activation marker, in addition to protecting cardiac tissue from injury. Subsequent studies validated the importance of HO-1 expression in endothelial (EC) and vascular smooth muscle cells (VSMC) where HO-1 induction prevented apoptosis of EC and hyperproliferation of VSMC to inhibit neointimal proliferation that leads to TVS through pathways involving NF-kB and p38 MAP kinase signaling, respectively.42;88 The protective nature of HO-1 described in these early models has been extensively expanded to show that over-expression of HO-1 using viral vectors or direct administration of HO-1 protein74;89;90 protect heart allografts. Moreover, other agents known to prevent heart allograft rejection such as IL-10 or IL-13 are ineffective in grafts where HO-1 is absent.91 Importantly, the effects of HO-1 have now been described in both small and large animal models supporting translation to human heart transplantation. Furthermore, the use of CO and biliverdin or bilirubin mimics the positive effects observed with HO-1. Treatment with CO by inhaled gas or administration of a CO-RM essentially recapitulates that observed with HO-1 induction.92 CO treatment of the donor, graft and/or recipient has been extensively studied and there is no doubt that CO imparts remarkable beneficial effects in preventing TVS, thrombosis while promoting cardiac graft function. Addition of CORM-3 to the preservation solution resulted in significant improvements in systolic and diastolic function and coronary flow when compared with hearts treated with an inactive CORM-3. This improved cardiac function correlated with lower cardiac enzyme levels of creatine kinase and lactate dehydrogenase.92 In a model of arterial thrombosis, Hmox1−/− mice exhibited a pro-thrombotic phenotype that could be ameliorated with administration of either CO or biliverdin. The mechanisms involved specific effects on the regulation of cell cycle, coagulation, thrombosis, and reactive oxygen species (ROS).93 Both biliverdin and bilirubin as well as iron chelation with ferritin have also been studied and show protective effects in preventing allograft dysfunction.94-96 The mechanisms have been dissected and include a number of signaling cascades involved in the effects including modulation of the mitogen-activated protein kinases (MAPKs), NF-κB, NO, cGMP and likely changes in bioenergetics that lead to increased cell survival led by mitochondrial biogenesis.97-99 What remains unclear is whether the molecular target is the same regardless of the cell type. In addition, it is still unclear whether CO simply modulates the function of existing hemoproteins or additional effects may be exerted by CO on gene expression patterns directly via heme-containing transcription factors such as NPAS2 or Bach1 or indirectly through generation of ROS that trigger redox activation of Nrf2 and Maf proteins. Regardless of direct or indirect mechanisms of action, CO has evolved into a novel therapeutic in transplantation that resulted in the first clinical trial for inhaled CO in transplantation (www.clinicaltrials.gov).
HO-1 in pulmonary hypertension and right heart failure
Pulmonary arterial hypertension (PAH) is a disease with an unknown etiology that results from progressive increases in pulmonary vascular resistance that leads to right heart failure. PAH is characterized as a disease of small pulmonary arteries that exhibit uncontrolled smooth muscle proliferation, a narrowing of vessel lumen, and a thickening of right heart musculature necessary to counter the continuous rise in resistance as the heart strives to deliver adequate blood supply to the lungs. HO-1 is induced in models of PAH likely as a stress-dependent compensatory mechanism in attempts to maintain vascular homeostasis after inflammatory insults that include monocrotaline, platelet activation and hypoxia.100 When HO-1 is blocked the beneficial effects of known therapeutic agents including simvastatin, IL-10 or hydrogen sulphide (H2S) are lost.101-103 Indeed, there is a clear interrelationship between the gasotransmitters H2S, CO and NO where each contribute to appropriate vasomotor activity. Induction of HO-1 with hemin, injection of mesenchymal stromal cells over-expressing HO-1, or administration of exogenous CO can not only prevent hypoxia-induced PAH, but also reverse established disease.100;104;105 Interestingly, studies in dogs and sheep show that both endogenous and exogenous CO reduce pulmonary artery vasoconstriction likely involving increased cGMP and blockade of endothelin-1.106-108 This offers explanations and insight into potential mechanisms of action even with the knowledge that CO is known as a poor vasodilator compared to NO. Mechanistically, HO-1 and CO have been shown to exhibit multiple mechanisms of action in the vasculature including early anti-inflammatory effects with reduced endothelial cell activation, thrombosis, leukocyte infiltration and cytokine production all of which reduce arterial injury that otherwise contributes to vessel remodelling. From a therapeutic standpoint, however, the above events are already ongoing and established at the time the patients present with symptoms, making therapeutic interventions challenging. There are currently no therapeutic options for patients suffering with PAH. Administration of inhaled CO, however, at the time of peak right heart hypertrophy, targets the endothelium to generate increased NO via eNOS that in turn activates cell death programs in the hyperproliferative smooth muscle cells as measured by TUNEL positivity.100 The reversal of the intimal thickening restored normal arterial pressures and retro-remodelling of the right heart to normal size.100 A similar effect can be observed in a model of carotid artery intimal hyperplasia where treatment with CO mediates regression of intimal lesions.109 Additionally, treatment with CO activates large-conductance voltage and Ca++-activated K+ channels that attenuate development of PAH.110 One tantalizing explanation for the differences in molecular mechanisms could be differential effects of CO in the conducting vessels versus those comprising the microcirculation. Intravital microscopy of hepatic tissue clearly shows vasodilatory effects in capillary beds without affecting central pressures.111 Whether a similar phenomenon occurs in the lung and heart remains to be studied. In all likelihood all are valid explanations towards understanding how HO-1/CO influences vessel remodeling and that collectively each contributes towards the maintenance and restoration of normal pulmonary artery and right heart function.
HO-1 and post-ischemic inflammation
Following IR injury the immune system has a fundamental role in clearing the damaged tissue and in the coordination of the processes underlying tissue regeneration. When cardiomyocytes die or are severely injured by IR, endogenous cellular material will be released and detected by the immune system as DAMPs. By binding to specific cognate receptors, DAMPs activate tissue macrophages that in turn produce a battery of cytokines and chemokines to recruit additional immune cells including neutrophils to the site of injury. One recent perspective on this response is that the necessary involvement of immune cells in re-establishing tissue homeostasis after tissue injury comes with unwanted inflammatory damage.112 Therefore, while the clearance of cellular debris by neutrophils and monocytes/macrophages is required for protection and maintenance of the healthy cardiac tissue and stimulation of the reparative phase in the injured area, the inflammatory mediators and enzymes activities that are stimulated in immune cells can exert cytotoxic effects on healthy tissue. To balance the beneficial and detrimental actions of the immune response, timely suppression and containment of inflammation are critical processes for effective repair.
The observations that HO-1 is induced by cellular stress and injury and has the capacity to modulate inflammatory processes argue for the idea that this protein has evolved to participate in the crucial events that govern the transition between tissue damage and initiation of repair processes. Although never intensely explored, it was already observed in the first HO-1 over-expression animal studies that the protection against IR damage correlated with a decreased infiltration of neutrophils and macrophages in the heart.65;80 A recent and elegant article by Hinkel and colleagues examined in detail the influx of post-ischemic inflammatory cells in mice and pigs over-expressing human HO-1 in cardiac tissue after treatment with a recombinant adenovirus.69 They showed that the number of neutrophils and pro-inflammatory monocytes recruited within the first 24 h after reperfusion is significantly lower in transgenic animals compared to controls. This inhibited inflammation was associated with decreased infarct size and better functional recovery and, very interestingly, was similar whether IR was performed in ubiquitously transgenic or regional (intracardiac) HO-1 over-expressing animals. Hence, it appears that local HO-1 is sufficient to provide protection against IR injury, possibly by modulating intracellular events that cause the consequent recruitment of inflammatory cells and induce damage. If heme is a DAMP that initiates and propagates inflammation, we suggest that HO-1 over-expressing cardiomyocytes are better equipped at the onset of IR to deal locally with excessive heme released during cell death and can therefore dampen in the early reperfusion phase heme-mediated pro-inflammatory cell recruitment and damage. This makes sense because the first few minutes after reperfusion will determine the response of the tissue to the insult that causes long-term damage and dysfunction. The metabolic activity of HO-1 that provides CO, biliverdin and bilirubin would then amplify these protective and anti-inflammatory functions in multiple ways. For example, HO-1 induction, biliverdin/bilirubin and CO have been shown to inhibit the expression of adhesion molecules in the endothelium88;113-117 and reduce leukocyte rolling and adhesion as early control of inflammation. In addition, CO liberated by CORM-3 inhibits myeloperoxidase activity, which produces in leukocytes strong oxidizing compounds like hypochlorous acid which cause endothelial oxidative stress and dysfunction.118 HO-1, biliverdin and CO also inhibit pro-inflammatory molecules (such as TNF-α)99 and stimulate the production of interleukin-10 (IL-10),119 the anti-inflammatory molecule that is produced by macrophages exhibiting a pro-healing phenotype.120 Viable cardiomyocytes surrounding the infarcted area could be directly responsive to heme released by injured cells and play a fundamental role both in regulating inflammation and for initiating the reparative response that is dependent in part on tissue macrophages.121 In fact, cardiomyocytes situated in the border zone of the damaged cardiac area have been recently described to actively modulate macrophage trafficking that is essential for heart healing.122 It is likely that other DAMPs liberated over the course of reperfusion will synergize with heme to induce HO-1, even though only heme can be used as a substrate by the enzyme. In general, the mechanisms underlying the regulation of the innate immune response and acute inflammation by HO-1 as well as how DAMPs are implicated in the activation of the stress response are still poorly defined and necessitate focused investigations.
The vascular endothelium and smooth muscle are also compromised by IR injury in the heart and the reparative processes to restore myocardial function involve angiogenesis and endothelial progenitor cells. HO-1 and its products have not been directly examined in the regeneration of heart vessels after IR but can confer protection against vascular injury and inflammation in models of atherosclerosis and vessel injury113;123 and significantly contribute to neoangiogenesis and neovascularisation124;125 (see below). Therefore, we can foresee a multifunctional implication of the HO-1 system in IR: 1) as a sensor of cardiac injury and DAMPs; 2) as a modulator of inflammation and the immune response and; 3) as a mediator that aids the repair and reconstruction of cardiac tissue.
HO-1 and vascular dysfunction
Circulating heme leads to vascular dysfunction in part by damaging the endothelium, which likely involves its ability to increase the levels of oxidized low-density lipoproteins that contribute to endothelial cell death.126 In the setting of elevated hemoglobin and iron such as after vessel trauma or intravascular hemolysis there is induction of HO-1 in both endothelial and smooth muscle cells. The importance of this regulation is evidenced in the Hmox1−/− mice that exhibit exaggerated injury under similar conditions and likely reflect an inability to modulate inflammation and subsequent repair processes.93;127-129 Indeed animals lacking HO-1 show elevated mean arterial pressures basally.130 In contrast, in mice in which HO-1 was selectively over-expressed in the heart, there is improved cardiac function, a reduced number of myocardial infarctions, and an overall reduction in inflammatory and oxidative injury after coronary artery ligation and reperfusion.130 This phenotypic outcome has been recently confirmed both in mice and in population-based cohort where length polymorphisms of the Hmox1 promoter region were assessed.131 The authors found that Hmox1−/− mice infused with angiotensin II, treated with streptozotocin to induce diabetes or during aging, exhibited increased vascular dysfunction which was inversely correlated with heme oxygenase activity. Endothelial inflammation and infiltration of pro-inflammatory monocytes and neutrophils were also exacerbated in Hmox1−/− mice after angiotensin II treatment. Likewise, in hypertensive subjects, the expression of Hmox1 mRNA in monocytes was positively correlated with flow-mediated vasodilation and inversely with pro-inflammatory monocytes. The authors also found that an unfavorable Hmox1 length polymorphism (>30 GT(n) repeats in the promoter region, which results in lower HO-1 expression) potentially increases the risk of arterial hypertension.131
HO-1 expression and activity when induced can impact the same cell type differently depending on the environmental conditions, the type of stressor, and likely even the location, e.g. conducting vessels versus capillaries or sterile vs. pathogen-mediated immune responses. In general, most cellular stressors, which include changes in oxygen tension, cytokines/chemokines, shear stress, DAMPs as well as PAMPs, all increase HO-1 in vascular endothelial and smooth muscle cells as well as tissue leukocytes and fibroblasts.132 One of the earliest reports demonstrating the role of HO-1 showed that its induction prior to TNF-α induced apoptosis prevented cell death of endothelial cells via specific activation of the p38 MAP kinase signaling pathway.133;134 This was one of the seminal papers to define HO-1 as an anti-apoptotic gene. Similarly, HO-1 over-expression in VSMC was first defined as anti-proliferative and critical in preventing intimal expansion and vascular stenosis after angioplasty. Again, the Hmox1−/− mice supported these findings showing enhanced stenosis and VSMC proliferation in response to vessel trauma or TVS leading to cardiac allograft rejection.42;88;123;135 Further studies demonstrated that the effects of HO-1 can be attributed to one or more of the products which, in certain instances, could rescue tissue function in the absence of HO-1.50;91 In recent years it has become apparent that while HO-1 is induced under numerous conditions, it would be inaccurate to conclude that HO-1 is only anti-apoptotic and/or only anti-proliferative. Studies by Grochot-Przeczek, Deshane and Wegiel clearly show that HO-1 and CO act to promote endothelial cell growth in models of wound healing, peripheral artery disease, and vessel repair136-138 and can also promote dysregulated smooth muscle cell death100;109 to remove unwanted and unnecessary cell mass found in stenotic or overly muscularized vessels as in intimal hyperplasia and pulmonary artery hypertension respectively. In prostate and lung cancer models the role of HO-1 is complicated and heavily debated. On the one hand there is strong evidence that HO-1 prevents endothelial proliferation and tumor angiogenesis resulting in inhibition in tumor growth.139-141 Importantly, there are numerous reports that blockade of HO-1 results in anti-cancer and anti-angiogenic effects strongly arguing a pro-cancer role for HO-1.142-145 Whether the differences are cancer-specific is unclear as the mechanisms that have been described range from changes in intracellular HO-1 protein localization that influences proliferation, to an angiogenic switch with Sp1-dependent increases in VEGF, to inhibition in apoptosis and immune cell function.141;146-148 CO, unlike NO is a poor vasodilator. This only holds true however in large vessels that control central pressure. In the microcirculation HO-1 and CO do exert effects on vasomotor tone, acting to vasodilate and encourage small capillary recruitment. These data suggest different regulatory mechanisms at work that are cGMP independent.111 The reports described above would suggest that HO-1 and CO act in a manner that befits the needs of the tissue and restricting their function to one cellular process or another such as anti-apoptotic or anti-inflammatory does not fully and accurately define their role in pathological settings. Induction of HO-1 and CO generation provides the cell, and organism with the optimal chance at maintaining function to ensure survival. It is important to note in the context of cardiovascular physiology the complex interaction among the bioactive gases that include NO, H2S and even CO2 and oxygen. The interplay of these gases as well as the cellular targets they are known to modulate e.g. cyclooxygenase, HO-1, NOS and arachidonic acid metabolites must be considered as we seek to understand how best to interfere and treat cardiovascular disease.
INTERACTION OF CO WITH CELLULAR TARGETS
CO and cytosolic hemoproteins
Unlike other gasotransmitters including oxygen, CO does not undergo any physical or chemical changes in the cell or body. Its high diffusivity permits it to traverse into essentially all cellular compartments where it binds principally to iron-containing heme moieties in proteins. The CO molecule carries a negative charge on the carbon but is neutral due to oxygen having a positive charge. However, the C is quite electropositive, and seeks to let go of this stress of carrying the negative charge, which is why it is attracted to positively charged iron atoms. Under basal conditions, CO is continuously generated during heme turnover and occupies approximately 1% of the heme sites in hemoglobin and myoglobin. So, how does CO impart such a diverse set of effects spanning ever-increasing and diverse areas of biology and medicine? The answer is complex and continues to be actively explored although it is unlikely that one target is solely responsible for the multifaceted set of effects of CO. An important question to raise is whether the effects of exogenous CO are similar to that generated endogenously by HO-1 (and HO-2). Based on theoretical algorithms, Levitt and colleagues suggested in a recent review that CO production by tissue HO must be sufficiently rapid to at least temporarily maintain a concentration of 0.1 μM in the presence of diffusion into the local blood sink.149 While interesting, what is not considered in detail here is how much CO is truly present at the cell surface when in proximity to HO-1 activity, what amount enters the cell, and perhaps more importantly how much CO is necessary at the cell surface and within the cell to elicit a response. One might argue that this is a fail-safe mechanism to control the amount of CO present in and around the cell. CO primarily diffuses away, drawn into the “blood sink” by partial pressure differences and is therefore unavailable to cause toxic effects on the cell and tissue until homeostasis is achieved and HO-1 is turned off.
In quiescent cells, any CO produced will target hemoproteins that are necessary for basal function such as soluble guanylate cyclase (sGC), oxidases, NO synthases as well as the heme-containing transcription factors including Bach1 and NPAS2.53;150;151 In the cardiovascular tissues, these heme-based proteins sensitive to gaseous molecules differ in prominence between cell types. For instance, guanylate cyclase is highly prevalent in smooth muscles cells while virtually absent in macrophages. Endothelial NO synthase (eNOS) is constitutively active in the endothelium while in most other cells is absent. Mitochondria, are perhaps the only constant among cell types, but differ in terms of number per cell type. Cardiac myocytes contain many more mitochondria per cell then smooth muscle and endothelial cells. In each cell type, the function of the hemoproteins can be increased or blocked by binding CO. When CO binds sGC or eNOS it activates the enzyme generating more cGMP or NO respectively.37;42;100;152;153 As described below, when bound to cytochrome c oxidase in mitochondria, CO inhibits their activity resulting in increased superoxide ions154-156 that rapidly provoke signaling cascades as ROS leading to changes in gene regulation and ultimately influence cellular behavior. The consequences that have been observed include the modulation of a number of non-heme proteins including the p38 and ERK MAP kinases,134;157 PPARγ,98 Nrf2,83 heat shock proteins,158 adenosine receptors,159 and HIF-1α.155;160;161 By increasing ROS there is activation of PPARγ and HIF1-α that in turn regulates gene expression towards a more tolerant anti-inflammatory phenotype that prevents TLR4 expression, MAP kinase activation, ion channel activation/inhibition, NADPH oxidase complex formation. In the vasculature the sGC present in VSMC is a constant target for CO and thus influences vasomotor tone albeit to a lesser extent then NO, and involves sGC-induced activation of protein kinase G that controls VSMC relaxation and protein kinase B that regulates Ca+2 flux. Some of the cellular targets described above are likely affected by CO indirectly as they do not contain transition metals to which CO would bind and modulate their activity. Proteins such as guanylate cyclase, NO synthase, ion channels, and NADPH oxidase all bind CO that results in altered activity. Others are activated by ROS such as PPARγ while a third set of targets relates to the oxygen sensors that result in a pseudohypoxia resulting in stabilization of HIF-1α.
However, the literature is comprised of alternative accounts of CO effects in the body particularly in the heart as it relates to ion channel status. How CO interacts with specific ion channels is unclear, but the speculation is that it is a combination of the channel itself and associated cellular heme.162 The myocardium possesses three primary ion channels. Two channels, the L-type Ca2+ and the voltage gated sodium channels (Nav1.5), are located primarily in sinoatrial and atrioventricular nodes of the heart, as well as in blood vessels (carotid body). CO inhibits the L-type Ca2+ channels, reducing Ca2+ influx into the cell.163 Inhibition of these channels decreases heart rate, atrioventricular node conduction, and ultimately reduces myocyte contractility resulting in cardiac vasodilation. Such an effect on the heart would be important in patients with unstable angina; however, it should be noted that there are no clear controlled studies demonstrating this effect.
In contrast to cells or animals that are in a basal state of activity, exposure of cells or animals to CO in the presence of an on-going stress now present a different set of heme-based sensors. Inducible proteins such as iNOS, NADPH oxidase, or other heme-dependent proteins may become targets for CO to bind to and modulate their function. If the purpose of the research is clinical relevance, there are very few indications that would warrant prophylactic therapy. Balloon angioplasty of a stenotic vessel and organ transplantation are among the only scenarios where administering CO prior to tissue manipulation would be clinically relevant. In each model, CO is effective at blocking the ensuing insult essentially via preconditioning the tissue that limits the pro-inflammatory response. In each setting CO prevents up-regulation of cytokines/chemokines, adhesion molecules, and hyperproliferative signals such as growth factors that lead to cytoskeletal reorganization. The result is little to no activation of the cell in response to the ensuing stimuli. In contrast and perhaps more a rarity is that CO is also effective if started after a stress such as bacterial infection, PAH or myocardial infarction. In these settings CO is pro-proliferative and induces tissue repair including stem cell function (see below), tissue remodelling (described above) and enhancing bacterial killing by augmenting the host immune response.12;164 Work by Lin et al showed that treatment with CO gas or CORM-2 promoted neovascularization and myocardial repair after coronary artery ligation.125 CO increased the activation of SDF-1 via an Akt-dependent AP-2α expression. Blockade of AP-2α abrogated the beneficial effects of CO treatment. Collectively, whether CO is initiated before or after the insult, the result is salutary and befits the needs of the tissue. As such, perhaps the best definition for CO is homeo’dynamic’ in that its effects on the cell are dependent on the state of the cell at the time of exposure. HO-1 is similar in many ways. If HO-1 is absent the ability to respond appropriately to any of the insults described above is exaggerated with increased morbidity and in most cases increased mortality.
CO and mitochondria
One intriguing mechanism of action that has been proposed to explain the cytoprotective effects of CO in myocardial tissue is its ability to modulate mitochondrial activity and function. This may appear at first counterintuitive because CO gas is known to bind to hemoglobin with a 200 times greater affinity then oxygen. Additionally, CO inhibits tissue cytochrome c oxidase, which is fundamental for sustaining electron transport, oxygen consumption and energy production in mitochondria.165 However, many of the studies showing the effects of CO on mitochondrial function were performed in isolated mitochondria, a model that is not fully representative of the cellular milieu. As observed for other signaling gases (such as NO and H2S166), modulation of mitochondrial function by CO is multifaceted and will depend on the concentration and status of mitochondrial targets. Controlled delivery of CO by CORM-3 to mice undergoing peritonitis-induced sepsis was shown to reduce inflammation by eliciting a mild oxidative stress response that leads to improved mitochondrial energetics and increased biogenesis in the heart.167 These data confirm previous results by Piantadosi and co-workers showing that CO gas administered to mice in controlled amounts for limited periods of time act as a stimulus of retrograde signaling for cardiac mitochondrial biogenesis by triggering the production of mitochondrial hydrogen peroxide.98 The effect of CO was associated with a significant increase in mtDNA and the coordinated expression of both mitochondrial and nuclear transcription factors (TFAM and PGC-1α, respectively) that activate genes for mitochondrial proteins. It is important to emphasize that the transcription of these cardiac genes by CO were stimulated by mechanisms that were independent of tissue hypoxia and did not involve the NO pathway. The response to CO was confirmed in cardiomyocytes demonstrating that in addition to an increased expression of transcription factors regulating mitochondria biogenesis, CO stimulated cGMP production and activated the phosphatidylinositol-3 (PI3)-kinase/Akt pathway, which are both involved in pro-survival activities.98
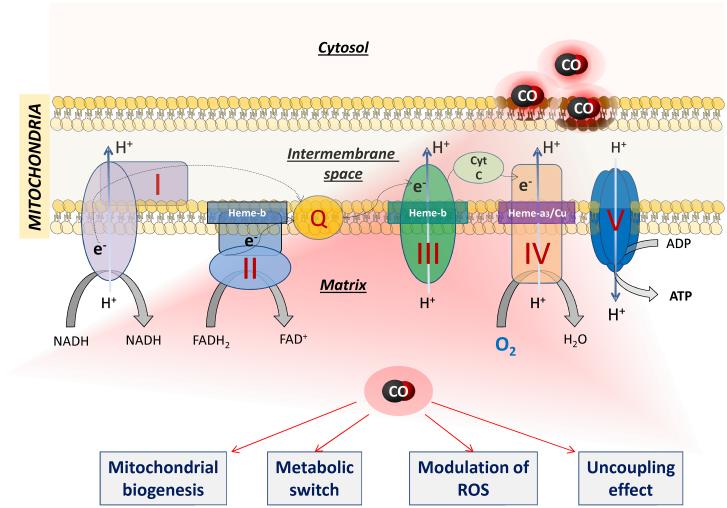
In a second series of studies, Piantadosi and colleagues reported that the HO-1/CO pathway plays a crucial role in restoring the impairment in mitochondrial function induced by heart failure.82 Using a model of doxorubicin-induced cardiomyopathy in mice, it was observed that hearts damaged by doxorubicin lacked the ability to engage a nuclear program for mitochondrial biogenesis leading to severe depletion of mtDNA, sarcomere destruction and cardiac fibrosis. Notably, periodic exposures to CO gas protected the heart from doxorubicin toxicity by mitigating the loss of mtDNA, reducing oxidative stress and maintaining a normal structure of the left ventricular wall. These results were confirmed in isolated cardiomyocytes demonstrating that over-expression of HO-1 prevented, whereas HO-1 gene-silencing exacerbated, doxorubicin-induced mitochondrial disruption and apoptosis. The possibility that an increase in ROS production by CO occurs via transient inhibition of cytochrome c oxidase is plausible156 and is in line with emerging evidence on the obligatory role of mitochondrial ROS in resistance to cardiomyopathy and cardiac failure, an adaptive response known as “mitohormesis”.168;169 Cardioprotective actions by HO-1 linked to modulation of mitochondrial function have been corroborated more recently in chronic heart failure induced by coronary ligation. In this model, transgenic mice over-expressing myocyte-specific HO-1 exhibited significantly improved cardiac ejection fraction and post-infarction survival in association with reduced hypertrophy, interstitial fibrosis and oxidative stress.78 Moreover, mitochondria isolated from HO-1 transgenic hearts displayed markedly reduced oxygen consumption rates, an effect recapitulated in mitochondria of non-transgenic mice treated with CORM-3. Although the overall respiration was decreased, mitochondria treated with CORM-3 showed an increase in both state 3 (ATP-linked) and state 4 (ADP-independent) respirations, the latter being indicative of an uncoupling effect.78 Indeed, CORM-3 at low micromolar concentrations significantly uncouples mitochondrial respiration in heart isolated mitochondria, an effect accompanied by a mild decrease in membrane potential and reversed by the CO scavenger myoglobin.154;170 It is also interesting that the basal production of ROS in heart mitochondria fed with pyruvate through complex I is increased by CORM-3, while excessive production of hydrogen peroxide in succinate-driven respiration through complex II is markedly attenuated by CO.154 Thus, on one side CO may temporarily inhibit cytochrome c oxidase to trigger production of ROS that in turn serves as signal for mitochondrial biogenesis, while on the other hand CO can transiently increase oxygen consumption and reduce ROS production by uncoupling respiration at the expense of oxidative phosphorylation (Figure 3).
Figure 3. Interaction of CO with mitochondria.
CO at high concentrations is known to inhibit mitochondrial respiration by competing with oxygen for the binding to cytochrome c oxidase (complex IV). In contrast, controlled delivery of CO gas and CO-RMs at non-toxic concentrations can protect cardiac tissue by promoting mitochondrial biogenesis, uncoupling activity and metabolic switch (see text for details). The molecular mechanism(s) underlying these effects remains to be defined. However, the interaction of CO with mitochondrial targets different from cytochrome c oxidase is likely as the electron transport chain contains other heme-complexes that may display distinct sensitivities to CO.
Although the exact molecular target(s) and the sequence of events by which CO regulates uncoupling and energy metabolism remain to be characterized, the direct involvement of CO on cardiac energetics is supported by in vivo evidence. For instance, in pigs undergoing cardiopulmonary by-pass, pre-treatment for 2 h with CO gas leading to 12% blood HbCO resulted in significantly higher cardiac ATP and phosphocreatine levels and reduced interstitial edema, heart fibrillation and apoptosis.171 Similarly, pigs exposed to CO gas inhalation for 3 h (5% HbCO) and subsequently subjected to coronary occlusion showed a much lower concentration of lactate in blood, less utilization of glucose and increased pyruvate levels during ischemia compared to untreated animals, while cardiac ATP and energy charge were unchanged between the two groups.172 It is known that pyruvate dehydrogenase is inhibited in the heart by increased fatty acid β-oxidation173 and it is tempting to suggest that reduced lactate in association with increased pyruvate are due to a CO-mediated switch in substrate utilization for energy. Altogether, these findings indicate that the HO-1/CO pathway exerts some of its cardiac protective effects by dynamically regulating aerobic and anabolic metabolisms and thus counteracting the metabolic dysfunction occurring during stress conditions.
HEME AND HO-1-DERIVED PRODUCTS: THE BALANCE BETWEEN CELL INJURY AND PROTECTION
One outstanding issue that remains unsolved in the field of heme oxygenase is the source of heme to support its enzymatic activity and generate these essential cytoprotective products. It is known that the intracellular heme pool is tightly regulated by a precise balance between the rates of heme biosynthesis, catabolism and export.174 However, no studies have been conducted to determine the source of heme used by heme oxygenase either during physiological conditions or when HO-1 is induced in disease states. What is plausible and is intrinsically accepted by all scientists working in this area is that under normal conditions heme is reclaimed from the protein turnover that occurs during typical wear and tear of the cell. But is this an indiscriminate process where heme-containing proteins in the cytosol or in organelles such as the mitochondria or nucleus provide the heme substrate for heme oxygenase activity? Alternatively, might there be tighter regulation of heme catabolism? If under pathophysiologic conditions heme suddenly becomes available, as would occur after cell rupture in sickle cell anemia, trauma or infection, is it the endothelial cells and leukocytes that recognize and uptake the excess heme that would escape the prototypical heme scavengers haptoglobin and hemopexin? Can the internalized heme be used by the endothelial cell or leukocyte? Or heme is simply a DAMP arising from dying cells and much like other debris is internalized and removed from the damaged tissue? Given that heme has a cognate receptor in TLR4, is heme a signalling molecule that activates endothelial cells and leukocytes via a specific MyD88 cascade? Could there be an increase in heme synthesis, which starts and ends in the mitochondria and is an energy consuming process, simply to support increased enzymatic activity after HO-1 induction? It is clear that novel experimental approaches need to be developed to answer these fundamental questions. It has also become evident that the cytoprotective effects of HO-1-derived products may be stronger or better exploited where abundant heme sources are available. Thus, HO-1 and its products may have more influential roles in immune cells that are principally responsible for TLR4-mediated heme uptake. In such scenario, the products would then serve as additional signalling molecules, diffusing endogenously and exogenously to further respond, defend and repair the environs. The fact that cardiac muscle contains high amounts of myoglobin and a high mitochondrial number suggests that the heart may benefit more than other tissues from the salutary effects of heme recognition and HO-1 induction.
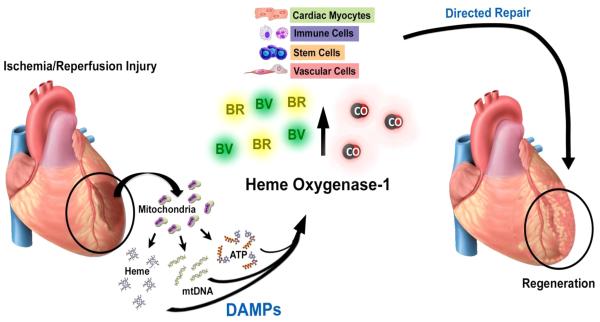
Heme is essential to all living organisms. Its formation and degradation are highly regulated and depend on the needs of the cell and tissue. Heme, in and of itself, is characterized as a potent agonist of oxidative stress and highly detrimental to cells and tissues. Structurally, heme is an iron–protoporphyrin complex comprising four pyrrole rings. When the iron atom is in the ferrous state, the complex is called heme, versus when the iron atom is in the ferric state, where it is called hemin. The toxic effects of heme are extremely diverse, but a commonality is that heme increases the generation of iron-derived ROS. Hemin causes DNA damage as well as oxidation of lipids and proteins.175 Many of these conclusions are based in large part on data demonstrating that exogenous administration of heme in the presence of an additional cellular stressor such as cytokines, endotoxin or hypoxia exacerbates tissue stress and damage.13 This is best demonstrated in sickle cell anemia or malaria where heme is released in large amounts (>20 µM) due to hemolysis resulting in induction of a pro-inflammatory state with increased cell death.176 In the heart, heme production rises with injury due to activation of the heme synthesis enzyme δ-aminolevulinic acid synthase 2 (ALAS2) in cardiomyocytes.177 Blockade of ALAS2 and heme synthesis abrogates cell death. It is clear that heme will be released in the presence of any tissue injury given the large heme pool inside cells and likely reflects why HO-1 induction occurs in the absence of extracellular heme administration such as in instances of UV irradiation, cytokines signaling, and agents that increase oxidative stress. Heme is a deadly molecule that needs to be eliminated when it is released from cells, it has recently been suggested to act as an alarmin and a DAMP13;178 and is recognized by its cognate receptor TLR4 in activation of inflammasome pathways.9 Heme is however actively secreted during erythropoiesis via the feline leukemia virus, subgroup C, receptor FLVCR. Absence of this receptor results in midgestational lethality with severe deformities.179 Recent reports suggest that FLVCR is also critical in T cell development and survival.180 While these effects may validate that excess heme is toxic, it may also speak to the necessity of heme secretory pathways in cell and tissue development and ultimately innate immune functionality. Does heme serve a purpose when released from cells, much like HMGB1 and other mediators including cytokines/chemokines or even growth factors? We would propose that heme is actually a signaling molecule like other DAMPs such as ATP, mitochondrial DNA and formyl peptides, which akin to other cellular mediators can be actively or passively released from cells and recognized by neighboring cells to elicit appropriate responses (Figure 4). Heme released as a result of tissue damage up-regulates HO-1, which as presented throughout this review is absolutely critical in heart repair and survival. HO-1 in myocytes, vascular cells, stem cells and immune cells each contribute to restoration of function.
Figure 4. Heme release and cardiac repair.
IR injury leads to the sudden release of cellular contents including heme, mitochondrial DNA and ATP. These cellular DAMPs have each been shown to induce HO-1. HO-1 expression and the subsequent generation CO, biliverdin (BV) and bilirubin (BR) target a variety of cell types that impact cellular repair and tissue regeneration.
There is no doubt that CO, at appropriate concentrations, binds to mitochondrial oxidases in mammals. There is a plethora of literature that concludes that by binding to these hemoproteins CO increases ROS such as H2O2 and O2− that function as potent signalling molecules and activators of transcription and modification of proteins such as IKK kinases or protein kinase A leading to expression of antioxidant genes (e.g. MnSOD, thioredoxins) as well as HO-1 and Nrf2.83;98;100;181;182 Increased expression of HO-1 via ROS and the antioxidant responsive element (ARE) has been well described. In this context, it is intriguing to speculate a feed-forward system where ROS indirectly increase HO-1 that results in generation of CO and CO in turns targets many of the ROS generating systems like the mitochondria oxidases, catalase, NADPH oxidase, and xanthine oxidase. It is interesting to speculate that the powerful antioxidant bilirubin generated as the final product of heme catalysis serves to ultimately resolve the oxidative burden and return the cell to quiescence. These derivative or secondary effects of HO-1/CO must be considered in the context of the cell and tissue responses under both physiologic and pathophysiologic circumstances.
FUTURE EXPLOITATION OF HO-1/CO AS PROTECTIVE THERAPIES IN THE HEART
Implications of HO-1/CO in cell therapy
There is strong interest in the possibility of using stem cells for repairing and regenerating lost cardiac tissue during myocardial infarction since the post-ischemic heart has limited capacity for self-renewal and undergoes remodeling that inevitably impairs left ventricular function.183 Since the reparative potential of stem cells is severely compromised by their poor survival after transplantation into the infarcted heart, induction of cytoprotective genes such as HO-1 is a plausible stratagem to increase their viability and therapeutic efficacy. As a consequence, scientists have started to explore whether HO-1 has any active role in the repairing abilities of stem cells. Initial studies published in 2005 reported that mesenchymal stem cells (MSC) transfected with an HO-1 vector become more resistant to apoptosis induced by hypoxia-reoxygenation in vitro; in addition, HO-1 transfection increased the tolerance of MSC once engrafted in vivo, which promoted significantly less ventricular remodelling and enhanced functional recovery of the infarcted heart.184;185 These protective effects were associated with a marked increase in VEGF expression, reduced expression of cardiac pro-inflammatory mediators (TNF-α, IL-1β and IL-6) and augmented capillary density.185 Injection of human bone marrow MSC into the post-ischemic myocardium has also been shown to increase HO-1 expression both in MSC and cardiomyocytes resulting in less ischemic damage and improved cardiac contractility.186 Interestingly, HO-1 gene transfer in cardiac tissue during myocardial infarction correlates with enhanced neovascularisation via the production of angiogenic factors (VEGF and SDF-1) and an increase in the number of c-kit stem cells recruited to the injured area several days after coronary artery ligation.187;188 These data indicate that increased HO-1 leads to a dual protective effect: 1) it promotes the paracrine activities of exogenously administered MSC, which helps in preventing myocardial cell injury and apoptosis and stimulates the formation of new vessels; 2) it triggers the recruitment of circulating progenitor stem cells thus enhancing the endogenous regenerative capacities of the heart.
The ability of HO-1 to enhance cell therapy approaches has been confirmed in human late outgrowth endothelial progenitor cells which, following transduction ex-vivo with HO-1 and the pro-survival gene Akt, improve their ability to adhere to extracellular matrix and migrate towards human cardiomyocytes showing a more robust paracrine profile under stress.189 Even in this instance, injection of human endothelial progenitor cells in the infarcted area of nude mice in vivo markedly attenuated inflammation, enhanced neovascularisation and reduced the negative remodelling ultimately ameliorating cardiac performance. A more recent study conducted in pigs showed, however, that while intracoronary delivery of allogeneic bone marrow-derived stem cells reduced infarct size after myocardial reperfusion injury, injection of stem cells over-expressing HO-1 did not further limit post-ischemic damage despite the hearts displaying an improved left ventricular ejection fraction.190 It is possible that the type of cells used as well as the mode and timing of intervention are crucial for a successful outcome and more systematic procedures are required to fully exploit the potential of cell therapy as efficacious interventions for cardiac tissue repair. Nevertheless, manipulating stem cells to increase their therapeutic action by transferring HO-1 or other cytoprotective genes appears to be a promising approach.191 Consequently, increasing endogenous expression of these genes in stem cells could also be attainable by pharmacological means. Along this line, recent studies revealed that the HO-1 inducer cobalt protoporphyrin IX (CoPPIX) can render stem cells more resistant to oxidative injury and consequently increase their beneficial effects following transplantation into the infarcted myocardium.192;193 Treatment with C-kit+ human cardiac stem cells (hCSC), which are known to differentiate in vivo in cardiomyocytes, smooth muscle and endothelial cells, has been shown to improve heart function in different models of myocardial infarction as well as in patients with ischemic cardiomyopathy.194;195 Bolli and co-workers demonstrated the following: 1) induction of HO-1 by CoPPIX increases the resistance of hCSC to hydrogen peroxide-mediated oxidative stress and apoptosis, while silencing HO-1 abrogated the cytoprotective effects mediated by CoPPIX; 2) treatment of hCSC with CoPPIX stimulated the release of important growth factors into the media and the same media applied to naïve cardiac stem cells conferred remarkable resistance against cellular damage and apoptosis; 3) CoPPIX in hCSC induces the expression of the transcription factor Nrf2 and increases phosphorylation of ERK1/2, which are key players in the modulation of several detoxifying and pro-survival genes;192 4) compared to untreated cells, hCSC “preconditioned” with CoPPIX survived much longer once injected in the infarcted myocardium of immunodeficient mice, at the same time the treatment markedly increased cardiac performance, promoted greater proliferation of cardiac cells and reduced left ventricular remodelling.193 These data were then confirmed by Luo and colleagues showing that human embryonic stem cell-derived cardiomyocytes pre-treated with CoPPIX exhibited a greater resistance to hypoxia-reoxygenation injury, increased VEGF production and reduced ROS-mediated apoptosis.196;197 Moreover, when CoPPIX-treated stem cells were injected immediately after myocardial infarction in rats, human cardiomyocyte graft size increased up to 12% of the total ventricular area and was associated with increased human-derived capillaries and thus improved vascularisation of the damaged tissue.197 Similar results were obtained more recently with adipose-derived stem cells pre-treated with curcumin, another well-established and potent inducer of the Nrf2/HO-1 axis.198;199 Specifically, adipose stem cells preconditioned with curcumin displayed increased resistance to oxidative stress and significantly ameliorated their efficacy in a model of rat myocardial IR injury by improving cardiac contractility, decreasing tissue damage and fibrosis and enhancing both capillary density and the formation of new vessels.200 Whether the products of HO-1 (biliverdin and CO) have an impact on the intrinsic capacities of stem cells to promote the repair of injured cardiac tissues remains to be fully established. A recent report supporting this possibility showed that rats treated with a compound that generates CO (methylene chloride) prior to coronary artery ligation displayed increased accumulation of c-kit+ stem progenitor cells in the infarcted cardiac tissue and this effect was associated with formation of new coronary arteries and involved the expression of angiogenic factors such as HIF-1α, SDF-1α and VEGF-B.201 In addition, a new report by Suliman et al revealed that HO-1 and CORM-2 can control the differentiation of embryonic stem cells into cardiomyocytes. This study demonstrated that CO-mediated mitochondria biogenesis is a crucial step in the maturation of embryonic stem cells into energetically efficient cardiomyocytes,202 providing additional evidence on the emerging and perhaps crucial role of the HO-1 pathway in the control of energetic metabolism not only in cardiac tissue but also during stem cell differentiation.203
Leveraging HO-1 and its products as innovative cardiotherapeutics
As demonstrated in the previous sections, substantial amounts of data have emerged indicating that HO-1 and its products can modulate the outcome of several cardiovascular diseases, and hence are obvious therapeutic targets for these conditions. There are now intense efforts underway by groups worldwide that are attempting to leverage and translate the benefits of these molecules for human use. There have been efforts towards inducing HO-1 by administering heme arginate, gene therapy strategies and those targeting the regulation of HO-1 through transcriptional control of Nrf2. While effective in inducing HO-1, this approach is still in the early phases but we note that HO-1 is induced by a number of approved agents such as statins, aspirin, NO, and even steroids. In many instances mechanistic studies have shown that these agents act through and even require HO-1 in order to exert their effects.204 In addition, dimethylfumarate, which is approved by the Food and Drug Administration for the treatment of multiple sclerosis (Tecfidera), activates Nrf2 and induces HO-1, suggesting again the possibility that HO-1 may contribute to the pharmacological action of the drug.205 Targeting enzymatic activity, either endogenous or genetically, is fraught with uphill battles related to sufficient activity and potency of the induction. The goal is to generate sufficient and therapeutic amounts of the HO-1 products. Although a detailed discussion of the transcriptional regulation of HO-1 is beyond the scope of this review, it should be noted that Nrf2 is not the only factor that regulates expression of HO-1 and indeed there are instances where the Hmox1 gene is induced despite a lack of Nrf2.124 The repressor Bach1, HIF-1α, AP1 and other factors can control HO-1 expression depending on the tissue and pathological condition considered.206 Thus, future investigations that will elucidate important mechanisms of HO-1 transcriptional regulation may identify novel pharmacological targets for drug design based on HO-1.
The most advanced of these efforts is in the use of inhaled CO, which has undergone substantial evaluation in numerous clinical trials (www.clincaltrials.gov). To date there are ongoing trials administering CO by mask in lung disease including pulmonary fibrosis, hypertension and adult respiratory distress syndrome. Phase II studies in kidney transplant showed a trend toward improved renal function after transplant akin to what was observed in large animal studies in swine.207 The challenges with inhaled CO include precise delivery and dosing, non-patient exposure to individuals administering the gas, cumbersome gas cylinder transport storage, and the necessity for hospital delivery due to safety concerns. In parallel, there has been the development of CO-RMs followed by extensive studies, showing their beneficial pharmacological activities in models of acute injury and inflammation. The first-generation of CO-RMs was based on small metal-containing CO carriers that could release their CO over time in vitro and in vivo. These early molecules showed a spectrum of kinetics of CO release and in most instances recapitulated the salutary effects promoted by HO-1 induction. The challenge with the development of metal-based CO-RMs revolvs around improving their solubility, stability and potency while limiting toxicity related to the metal cores, even though so far only few studies have been published that objectively address in vivo the toxicity of CO-RMs arising from the metal.208 Likewise, no studies have characterized the effect of the inactive CO-RMs, that is, the chemical entities that remain in the circulation after CO has been liberated. Chemists have just began to look into the design of non-metal based CO-RMs or compounds engineered to release CO in the presence of ROS, or light or even changes in pH as would occur in the stomach. However, the vast majority of these new compounds are still metal-based and therefore the progress is somewhat limited if the elimination of the metal is the goal of this development. In addition, all transition metal carbonyls are intrinsically light sensitive and therefore all metal CO-RMs are by definition “photo-CO-RMs”. In essence, the high affinity of CO for transition metals is what allows its physiological targeting and activity and is the property of CO that is exploited in CO-RMs. Importantly, one may see in the binding of CO with different metal-containing proteins of the organism the formation of ‘temporary’ endogenous CO-RMs. The human body contains a most straightforward metal carbonyl CO-RM in the form of COHb209. This has driven the creation of carbonmonoxy hemoglobins using both human (CO-MP4) and bovine pegylated hemoglobins that are being tested in human trials for a variety of disease indications.210 The major problem with the use of hemoglobin-based carriers and blood substitutes in general is related to their vasoconstrictive effects, alterations in fluid balances and, as mentioned above, an increase in heme-based toxicity. However, administration of MP4-CO did not show increases in mean arterial pressure and protected against myocardial infarction in rats and sickle cell disease to prevent vascular stasis.210 Additional approaches include organic-based ‘click and release’ pro-drugs that rely upon chemical reactions for the generation of CO instead of simple release211 and the efficacy of these molecules in ongoing models of inflammation are showing promising results (personal communication), but have not been tested in the setting of cardiac injury.
To circumvent toxicity of many of the above compounds, Steiger et al have developed an innovative approach defined as Therapeutic Gas Releasing Systems (TGRS) which allows for controlled release of gases from sealed containers that permit generation and exposure to CO without the concern of toxic byproducts, solubility issues and complex metabolites to identify, track, and characterize.212 Finally, and perhaps most intriguing is a simple concept that CO can be saturated within an oral formulation that can be well-controlled and delivered with clear preclinical bioavailability, which would be amenable to both acute and chronic use, obviate concerns surrounding dosing, safety and toxicity and take advantage of the ability of CO to rapidly diffuse into the bloodstream from the stomach. One commonality among each of the approaches described above, is that CO enters the body as CO, circulates, meets its targets and is eventually eliminated (t1/2 in humans of 4 h) from the body as CO exhaled through the lungs having undergone nearly no metabolism. Very small amounts are converted to CO2. A recent report by Yuan et al delineate how CO influences carotid body signaling in response to changes in oxygen sensing as an elegant signaling cascade dependent on the delicate balance of hemoprotein sensor activation.213 This signaling also involves H2S and perhaps links peripheral and central neural activity related to breathing. Even though CO and CO-RMs exert pharmacological actions by interacting with different cellular targets, in some instances they also induce Nrf2 and HO-1 as part of a pre-conditioning effect or as a stress response to supra-physiological levels of CO.77;83;214 Thus, the initial protective activity obtained with exogenous CO can be amplified by the induction of intrinsic defensive mechanisms.
More recent strategies include the synthesis of hybrid molecules, termed HYCOs, designed to both deliver CO and induce HO-1 by Nrf2 activation.215;216 The premise is that HYCOs, containing an Nrf2 inducer bound to a CO-RM, will provide greater tissue protection by first limiting damage through CO delivery and subsequently promoting the endogenous up-regulation of Nrf2-dependent defensive genes and proteins, a process that takes several hours due to transcription and translation processes. Thus, it is postulated that these molecules may offer a therapeutic advantage compared to Nrf2/HO-1 inducers or CO-RMs alone. While the characterization of several HYCOs is ongoing in models of inflammation with promising results, no data are yet available on their potential beneficial effects in cardiac pathologies.
The bile pigments have also been proposed to possess protective properties similar to HO-1 with preclinical data supporting their use to prevent IR injury after a heart transplant95;99 or intimal hyperplasia after balloon angioplasty217 driven in large part by modulation of inflammation and smooth muscle cell proliferation, respectively. The limitations with this approach relates to obtaining a reliable and safe source of bilirubin, which can then be converted back to biliverdin. Of note, Asian cultures have been consuming bilirubin for centuries for medicinal purposes by eating the gall stones of various animal species. Biliverdin would however be the pigment of choice due to it being easier to solubilize and that it is converted to bilirubin by biliverdin reductase to also provide bilirubin. The relative impact of biliverdin versus bilirubin in terms of importance and efficacy has yet to be determined. Efforts are ongoing to utilize bacteria and yeast systems to generate biliverdin. While proof of concept shows feasibility, generating large enough amounts for further preclinical and eventual clinical testing is still being explored. That a biliverdin/bilirubin-based therapy could be useful in the prevention of several human pathologies, and specifically heart disease, is supported by epidemiological studies. The interesting findings of these reports highlight that subjects exhibiting modestly increased plasma bilirubin levels, such as those observed in Gilbert’s syndrome, display a reduced risk of developing cardiovascular disease and decreased mortality,218-220 and this is applicable also to diabetic patients221 as well as in cancer.222
CONCLUSION
Since the identification of heme oxygenases as metabolic enzymes designed to catabolize heme, there has been a remarkable increase in the number of laboratories evaluating and characterizing its ability beyond simple heme metabolism. The protective role of HO-1 and its products in the heart and cardiovascular system is unequivocal. The creation of the Hmox1−/− mice, the identification of the HO-1 deficient human and the characterization of HO-1 polymorphisms showing that specific short GT repeats are associated with lower risk of heart disease, cardiac allograft vasculopathy, and susceptibility to restenosis following coronary stenting support this concept. We have provided in this review a comprehensive perspective of the role of the HO-1 pathway in cardiovascular disease. Importantly, we have delineated how HO-1 has evolved from a straightforward catabolic protein into a critical enzyme necessary for normal heart and vascular function and finally to identifying its products as potential therapeutics for the treatment of a variety of heart, lung and blood disorders. Heme is undeniably part of the foundation of cellular resources necessary for life of complex multicellular organisms. Without it, erythrocytes would be unable to deliver life-sustaining oxygen using hemoglobin, and tissues would be incapable of maintaining sufficient amounts of ATP without the heme-containing mitochondrial oxidases. But does this seemingly benign, iron-laden porphyrin ring carry the potential to wreak havoc if left unchecked, or does it serve a greater purpose? Is it a dangerous molecule and a signaling molecule, or is it perhaps a simple ring that holds great power within its bonds? Bonds that when broken by HO-1 set free a bioactive gas and bile pigments that provide the cell the defense and healing it demands.
Acknowledgments
Sources of Funding
Drs. Foresti and Motterlini are supported by funding from Agence Nationale de la Recherche (MITO-CO and CO-HEAL), SATT Ile-de-France INNOV, AREMCAR Foundation, INSERM and University Paris Est Creteil. Dr. Otterbein is supported by the NIH, the Julie Henry Foundation and the BIDMC Department of Surgery.
Non Standard Abbreviations and Acronyms
- ALAS2
δ-aminolevulinic acid synthase 2
- AP-2α
activating enhancer binding protein 2 alpha
- Bach1
BTB And CNC Homology 1 transcription factor
- BVR
biliverdin reductase
- cGMP
cyclic guanosine monophosphate
- CO
carbon monoxide
- CoPPIX
cobalt proporphyrin IX
- CO-RMs
carbon monoxide-releasing molecules
- DAMPs
Danger Associated Molecular Patterns
- EC
endothelial cells
- ERK
extracellular signal-regulated kinases
- hCSC
human cardiac stem cells
- HIF-1α
hypoxia inducible factor -1 alpha
- Hmox1
heme oxygenase-1 gene
- HO-1
heme oxygenase-1 protein
- HO-2
heme oxygenase-2 protein
- H2S
hydrogen sulfide
- IL-10
interleukin 10
- IR
ischemia-reperfusion
- MAPKs
mitogen-activated protein kinases
- MSC
mesenchymal stem cells
- mtDNA
mitochondrial DNA
- NADPH
nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
- NOS
nitric oxide synthase
- Nrf2
nuclear factor erythroid 2 (NFE2)-related factor 2 transcription factor
- PAH
pulmonary arterial hypertension
- PGC-1α
peroxisome proliferator-activated receptor gamma, coactivator 1 alpha
- PAMPs
Pathogen Associated Molecular Patterns
- PPARγ
peroxisome proliferator-activator receptor gamma
- ROS
reactive oxygen species
- sGC
soluble guanylate cyclase
- SDF-1
stromal cell-derived factor 1
- TFAM
mitochondrial transcription factor A
- TLR4
toll-like receptor-4
- TNF-α
tumor necrosis factor alpha
- VEGF
vascular endothelial growth factor
- VSMC
vascular smooth muscle cells
Footnotes
Disclosures:
none
REFERENCES
- 1.Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- 2.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 3.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 4.Motterlini R, Foresti R. Heme oxygenase-1 as a target for drug discovery. Antioxid Redox Signal. 2014;20:1810–1826. doi: 10.1089/ars.2013.5658. [DOI] [PubMed] [Google Scholar]
- 5.Balla G, Jacob HS, Balla J, Rosenberg M, Nath KA, Apple F, Eaton JW, Vercellotti GM. Ferritin: a cytoprotective antioxidant stratagem of endothelium. J Biol Chem. 1992;267:18148–18153. [PubMed] [Google Scholar]
- 6.Balla J, Jacob HS, Balla G, Nath KA, Eaton JW, Vercellotti GM. Endothelial-cell heme uptake from heme proteins: induction of sensitization and desensitization to oxidant damage. Proc Natl Acad Sci USA. 1993;90:9285–9289. doi: 10.1073/pnas.90.20.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun SR, Weiss FR, Keller AI, Ciccone JR, Preuss HG. Evaluation of the renal toxicity of heme proteins and their derivatives: a role in the genesis of acute tubule necrosis. J Exp Med. 1970;131:443–460. doi: 10.1084/jem.131.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent SH. Oxidative effects of heme and porphyrins on proteins and lipids. Semin Hematol. 1989;26:105–113. [PubMed] [Google Scholar]
- 9.Dutra FF, Alves LS, Rodrigues D, Fernandez PL, de Oliveira RB, Golenbock DT, Zamboni DS, Bozza MT. Hemolysis-induced lethality involves inflammasome activation by heme. Proc Natl Acad Sci U S A. 2014;111:E4110–E4118. doi: 10.1073/pnas.1405023111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wenceslau CF, McCarthy CG, Szasz T, Spitler K, Goulopoulou S, Webb RC. Mitochondrial damage-associated molecular patterns and vascular function. Eur Heart J. 2014;35:1172–1177. doi: 10.1093/eurheartj/ehu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wegiel B, Hauser CJ, Otterbein LE. Heme as a danger molecule in pathogen recognition. Free Radic Biol Med. 2015;89:651–661. doi: 10.1016/j.freeradbiomed.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Soares MP, Bozza MT. Red alert: labile heme is an alarmin. Curr Opin Immunol. 2016;38:94–100. doi: 10.1016/j.coi.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Wilks A. Heme oxygenase: evolution, structure, and mechanism. Antioxid Redox Signal. 2002;4:603–614. doi: 10.1089/15230860260220102. [DOI] [PubMed] [Google Scholar]
- 15.Ortiz de Montellano PR. The mechanism of heme oxygenase. Curr Opin Chem Biol. 2000;4:221–227. doi: 10.1016/s1367-5931(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 16.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottlieb Y, Truman M, Cohen LA, Leichtmann-Bardoogo Y, Meyron-Holtz EG. Endoplasmic reticulum anchored heme-oxygenase-1 faces the cytosol. Haematologica. 2012 doi: 10.3324/haematol.2012.063651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tenhunen R, Marver HS, Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969;244:6388–6394. [PubMed] [Google Scholar]
- 19.Lin Q, Weis S, Yang G, Weng YH, Helston R, Rish K, Smith A, Bordner J, Polte T, Gaunitz F, Dennery PA. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem. 2007;282:20621–20633. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]
- 20.Sjostrand T. Endogenous formation of carbon monoxide in man. Nature. 1949;164:580. doi: 10.1038/164580a0. [DOI] [PubMed] [Google Scholar]
- 21.Coburn RF, Williams WJ, Kahn SB. Endogenous carbon monoxide production in patients with hemolytic anemia. J Clin Invest. 1966;45:460–468. doi: 10.1172/JCI105360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kappas A, Simionatto CS, Drummond GS, Sassa S, Anderson KE. The liver excretes large amounts of heme into bile when heme oxygenase is inhibited competitively by Sn-protoporphyrin. Proc Natl Acad Sci U S A. 1985;82:896–900. doi: 10.1073/pnas.82.3.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maines MD, Trakshel GM, Kutty RK. Characterization of two constitutive forms of rat liver microsomal heme oxygenase: only one molecular species of the enzyme is inducible. J Biol Chem. 1986;261:411–419. [PubMed] [Google Scholar]
- 24.Trakshel GM, Maines MD. Multiplicity of heme oxygenase isozymes: HO-1 and HO-2 are different molecular species in rat and rabbit. J Biol Chem. 1989;264:1323–1328. [PubMed] [Google Scholar]
- 25.Cruse I, Maines MD. Evidence suggesting that the two forms of heme oxygenase are products of different genes. J Biol Chem. 1988;263:3348–3353. [PubMed] [Google Scholar]
- 26.Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci USA. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Applegate LA, Luscher P, Tyrrell RM. Induction of heme oxygenase: A general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991;51:974–978. [PubMed] [Google Scholar]
- 28.Vile GF, Basu-Modak S, Waltner C, Tyrrell RM. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci USA. 1994;91:2607–2610. doi: 10.1073/pnas.91.7.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 30.Stocker R, Glazer AN, Ames BN. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci USA. 1987;84:5918–5922. doi: 10.1073/pnas.84.16.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuzil J, Stocker R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J Biol Chem. 1994;269:16712–16719. [PubMed] [Google Scholar]
- 32.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 33.Ingi T, Cheng J, Ronnett GV. Carbon-monoxide: an endogenous modulator of the nitric oxide-cyclic GMP signaling system. Neuron. 1996;16:835–842. doi: 10.1016/s0896-6273(00)80103-8. [DOI] [PubMed] [Google Scholar]
- 34.Furchgott RF, Jothianandan D. Endothelium-dependent and -independent vasodilation involving cGMP: relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels. 1991;28:52–61. doi: 10.1159/000158843. [DOI] [PubMed] [Google Scholar]
- 35.Brune B, Ullrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol Pharmacol. 1987;32:497–504. [PubMed] [Google Scholar]
- 36.Suematsu M, Goda N, Sano T, Kashiwagi S, Egawa T, Shinoda Y, Ishimura Y. Carbon monoxide: an endogenous modulator of sinusoidal tone in the perfused rat liver. J Clin Invest. 1995;96:2431–2437. doi: 10.1172/JCI118300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morita T, Perrella MA, Lee ME, Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci USA. 1995;92:1475–1479. doi: 10.1073/pnas.92.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Motterlini R, Gonzales A, Foresti R, Clark JE, Green CJ, Winslow RM. Heme oxygenase-1-derived carbon monoxide contributes to the suppression of acute hypertensive responses in vivo. Circ Res. 1998;83:568–577. doi: 10.1161/01.res.83.5.568. [DOI] [PubMed] [Google Scholar]
- 39.Sammut IA, Foresti R, Clark JE, Exon DJ, Vesely MJJ, Sarathchandra P, Green CJ, Motterlini R. Carbon monoxide is a major contributor to the regulation of vascular tone in aortas expressing high levels of haeme oxygenase-1. Br J Pharmacol. 1998;125:1437–1444. doi: 10.1038/sj.bjp.0702212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nature Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 41.Sato K, Balla J, Otterbein L, Smith RN, Brouard S, Lin Y, Csizmadia E, Sevigny J, Robson SC, Vercellotti G, Choi AM, Bach FH, Soares MP. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J Immunol. 2001;166:4185–4194. doi: 10.4049/jimmunol.166.6.4185. [DOI] [PubMed] [Google Scholar]
- 42.Otterbein LE, Zuckerbraun BS, Haga M, Liu F, Song R, Usheva A, Stachulak C, Bodyak N, Smith RN, Csizmadia E, Tyagi S, Akamatsu Y, Flavell RJ, Billiar TR, Tzeng E, Bach FH, Choi AM, Soares MP. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nature Med. 2003;9:183–190. doi: 10.1038/nm817. [DOI] [PubMed] [Google Scholar]
- 43.Dolinay T, Szilasi M, Liu M, Choi AM. Inhaled carbon monoxide confers antiinflammatory effects against ventilator-induced lung injury. Am J Respir Crit Care Med. 2004;170:613–620. doi: 10.1164/rccm.200401-023OC. [DOI] [PubMed] [Google Scholar]
- 44.Fujita T, Toda K, Karimova A, Yan SF, Naka Y, Yet SF, Pinsky DJ. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat Med. 2001;7:598–604. doi: 10.1038/87929. [DOI] [PubMed] [Google Scholar]
- 45.Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res. 2002;90:e17–e24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- 46.Clark JE, Naughton P, Shurey S, Green CJ, Johnson TR, Mann BE, Foresti R, Motterlini R. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ Res. 2003;93:e2–e8. doi: 10.1161/01.RES.0000084381.86567.08. [DOI] [PubMed] [Google Scholar]
- 47.Motterlini R, Sawle P, Bains S, Hammad J, Alberto R, Foresti R, Green CJ. CORM-A1: a new pharmacologically active carbon monoxide-releasing molecule. FASEB J. 2005;19:284–286. doi: 10.1096/fj.04-2169fje. [DOI] [PubMed] [Google Scholar]
- 48.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci USA. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen B, Guo L, Fan C, Bolisetty S, Joseph R, Wright MM, Agarwal A, George JF. Carbon monoxide rescues heme oxygenase-1-deficient mice from arterial thrombosis in allogeneic aortic transplantation. Am J Pathol. 2009;175:422–429. doi: 10.2353/ajpath.2009.081033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takamiya R, Hung CC, Hall SR, Fukunaga K, Nagaishi T, Maeno T, Owen C, Macias AA, Fredenburgh LE, Ishizaka A, Blumberg RS, Baron RM, Perrella MA. High mobility group Box 1 contributes to lethality of endotoxemia in heme oxygenase-1 deficient mice. Am J Respir Cell Mol Biol. 2008;41:129–135. doi: 10.1165/rcmb.2008-0331OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haldane JB. Carbon monoxide as a tissue poison. Biochem J. 1927;21:1068–1075. doi: 10.1042/bj0211068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun J, Hoshino H, Takaku K, Nakajima O, Muto A, Suzuki H, Tashiro S, Takahashi S, Shibahara S, Alam J, Taketo MM, Yamamoto M, Igarashi K. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. Embo J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Otterbein LE, Mantell LL, Choi AMK. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol. 1999;276:L688–L694. doi: 10.1152/ajplung.1999.276.4.L688. [DOI] [PubMed] [Google Scholar]
- 55.Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 56.Kappas A, Maines MD. Tin: a potent inducer of heme oxygenase in kidney. Science. 1976;192:60–62. doi: 10.1126/science.1257757. [DOI] [PubMed] [Google Scholar]
- 57.Maines MD, Kappas A. Metals as regulators of heme metabolism. Science. 1977;198:1215–1221. doi: 10.1126/science.337492. [DOI] [PubMed] [Google Scholar]
- 58.Hoshida S, Nishida M, Yamashita N, Igarashi J, Aoki K, Hori M, Kuzuya T, Tada M. Heme oxygenase-1 expression and its relation to oxidative stress during primary culture of cardiomyocytes. J Mol Cell Cardiol. 1996;28:1845–1855. doi: 10.1006/jmcc.1996.0177. [DOI] [PubMed] [Google Scholar]
- 59.Maulik N, Sharma HS, Das DK. Induction of the heme oxygenase gene-expression during the reperfusion of ischemic rat myocardium. J Mol Cell Cardiol. 1996;28:1261–1270. doi: 10.1006/jmcc.1996.0116. [DOI] [PubMed] [Google Scholar]
- 60.Borger DR, Essig DA. Induction of HSP32 gene in hypoxic cardiomyocytes is attenuated by treatment with N-acetyl-L-cysteine. Am J Physiol. 1998;274:H965–H973. doi: 10.1152/ajpheart.1998.274.3.H965. [DOI] [PubMed] [Google Scholar]
- 61.Foresti R, Goatly H, Green CJ, Motterlini R. Role of heme oxygenase-1 in hypoxia-reoxygenation: requirement of substrate heme to promote cardioprotection. Am J Physiol Heart Circ Physiol. 2001;281:H1976–H1984. doi: 10.1152/ajpheart.2001.281.5.H1976. [DOI] [PubMed] [Google Scholar]
- 62.Chouchani ET, Methner C, Nadtochiy SM, Logan A, Pell VR, Ding S, James AM, Cocheme HM, Reinhold J, Lilley KS, Partridge L, Fearnley IM, Robinson AJ, Hartley RC, Smith RA, Krieg T, Brookes PS, Murphy MP. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med. 2013;19:753–759. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clark JE, Foresti R, Sarathchandra P, Kaur H, Green CJ, Motterlini R. Heme oxygenase-1-derived bilirubin ameliorates post-ischemic myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2000;278:H643–H651. doi: 10.1152/ajpheart.2000.278.2.H643. [DOI] [PubMed] [Google Scholar]
- 64.Akamatsu Y, Haga M, Tyagi S, Yamashita K, Graca-Souza AV, Ollinger R, Czismadia E, May GA, Ifedigbo E, Otterbein LE, Bach FH, Soares MP. Heme oxygenase-1-derived carbon monoxide protects hearts from transplant associated ischemia reperfusion injury. FASEB J. 2004;18:771–772. doi: 10.1096/fj.03-0921fje. [DOI] [PubMed] [Google Scholar]
- 65.Yet SF, Tian R, Layne MD, Wang ZY, Maemura K, Solovyeva M, Ith B, Melo LG, Zhang L, Ingwall JS, Dzau VJ, Lee ME, Perrella MA. Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res. 2001;89:168–173. doi: 10.1161/hh1401.093314. [DOI] [PubMed] [Google Scholar]
- 66.Melo LG, Agrawal R, Zhang L, Rezvani M, Mangi AA, Ehsan A, Griese DP, Dell'Acqua G, Mann MJ, Oyama J, Yet SF, Layne MD, Perrella MA, Dzau VJ. Gene therapy strategy for long-term myocardial protection using adeno-associated virus-mediated delivery of heme oxygenase gene. Circulation. 2002;105:602–607. doi: 10.1161/hc0502.103363. [DOI] [PubMed] [Google Scholar]
- 67.Vulapalli SR, Chen Z, Chua BH, Wang T, Liang CS. Cardioselective overexpression of HO-1 prevents I/R-induced cardiac dysfunction and apoptosis. Am J Physiol Heart Circ Physiol. 2002;283:H688–H694. doi: 10.1152/ajpheart.00133.2002. [DOI] [PubMed] [Google Scholar]
- 68.Mito S, Ozono R, Oshima T, Yano Y, Watari Y, Yamamoto Y, Brydun A, Igarashi K, Yoshizumi M. Myocardial protection against pressure overload in mice lacking Bach1, a transcriptional repressor of heme oxygenase-1. Hypertension. 2008;51:1570–1577. doi: 10.1161/HYPERTENSIONAHA.107.102566. [DOI] [PubMed] [Google Scholar]
- 69.Hinkel R, Lange P, Petersen B, Gottlieb E, Ng JK, Finger S, Horstkotte J, Lee S, Thormann M, Knorr M, El Aouni C, Boekstegers P, Reichart B, Wenzel P, Niemann H, Kupatt C. Heme oxygenase-1 gene therapy provides cardioprotection via control of post-ischemic inflammation: an experimental study in a pre-clinical pig model. J Am Coll Cardiol. 2015;66:154–165. doi: 10.1016/j.jacc.2015.04.064. [DOI] [PubMed] [Google Scholar]
- 70.Kusmic C, Barsanti C, Matteucci M, Vesentini N, Pelosi G, Abraham NG, L'Abbate A. Up-regulation of heme oxygenase-1 after infarct initiation reduces mortality, infarct size and left ventricular remodeling: experimental evidence and proof of concept. J Transl Med. 2014;12:89. doi: 10.1186/1479-5876-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu X, Simpson JA, Brunt KR, Ward CA, Hall SR, Kinobe RT, Barrette V, Tse MY, Pang SC, Pachori AS, Dzau VJ, Ogunyankin KO, Melo LG. Preemptive heme oxygenase-1 gene delivery reveals reduced mortality and preservation of left ventricular function 1 yr after acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;293:H48–H59. doi: 10.1152/ajpheart.00741.2006. [DOI] [PubMed] [Google Scholar]
- 72.Li Q, Guo Y, Ou Q, Wu WJ, Chen N, Zhu X, Tan W, Yuan F, Dawn B, Luo L, Hunt GN, Bolli R. Gene transfer as a strategy to achieve permanent cardioprotection II: rAAV-mediated gene therapy with heme oxygenase-1 limits infarct size 1 year later without adverse functional consequences. Basic Res Cardiol. 2011;106:1367–1377. doi: 10.1007/s00395-011-0208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu X, Wei J, Peng DH, Layne MD, Yet SF. Absence of heme oxygenase-1 exacerbates myocardial ischemia/reperfusion injury in diabetic mice. Diabetes. 2005;54:778–784. doi: 10.2337/diabetes.54.3.778. [DOI] [PubMed] [Google Scholar]
- 74.Ma J, Lau CK, Obed A, Dada A, Doenecke A, Fan ST, Schlitt HJ, Tsui TY. A cell penetrating heme oxygenase protein protects heart graft against ischemia/reperfusion injury. Gene Ther. 2009;16:320–328. doi: 10.1038/gt.2008.162. [DOI] [PubMed] [Google Scholar]
- 75.Musameh MD, Fuller BJ, Mann BE, Green CJ, Motterlini R. Positive inotropic effects of carbon monoxide-releasing molecules (CO-RMs) in the isolated perfused rat heart. Br J Pharmacol. 2006;149:1104–1112. doi: 10.1038/sj.bjp.0706939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guo Y, Stein AB, Wu WJ, Tan W, Zhu X, Li QH, Dawn B, Motterlini R, Bolli R. Administration of a CO-releasing molecule at the time of reperfusion reduces infarct size in vivo. Am J Physiol Heart Circ Physiol. 2004;286:H1649–H1653. doi: 10.1152/ajpheart.00971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stein AB, Bolli R, Dawn B, Sanganalmath SK, Zhu Y, Wang OL, Guo Y, Motterlini R, Xuan YT. Carbon monoxide induces a late preconditioning-mimetic cardioprotective and antiapoptotic milieu in the myocardium. J Mol Cell Cardiol. 2012;52:228–236. doi: 10.1016/j.yjmcc.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang G, Hamid T, Keith RJ, Zhou G, Partridge CR, Xiang X, Kingery JR, Lewis RK, Li Q, Rokosh DG, Ford R, Spinale FG, Riggs DW, Srivastava S, Bhatnagar A, Bolli R, Prabhu SD. Cardioprotective and antiapoptotic effects of heme oxygenase-1 in the failing heart. Circulation. 2010;121:1912–1925. doi: 10.1161/CIRCULATIONAHA.109.905471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tongers J, Fiedler B, Konig D, Kempf T, Klein G, Heineke J, Kraft T, Gambaryan S, Lohmann SM, Drexler H, Wollert KC. Heme oxygenase-1 inhibition of MAP kinases, calcineurin/NFAT signaling, and hypertrophy in cardiac myocytes. Cardiovasc Res. 2004;63:545–552. doi: 10.1016/j.cardiores.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 80.Tang YL, Qian K, Zhang YC, Shen L, Phillips MI. A vigilant, hypoxia-regulated heme oxygenase-1 gene vector in the heart limits cardiac injury after ischemia-reperfusion in vivo. J Cardiovasc Pharmacol Ther. 2005;10:251–263. doi: 10.1177/107424840501000405. [DOI] [PubMed] [Google Scholar]
- 81.Yeh CH, Chen TP, Wang YC, Lin YM, Lin PJ. HO-1 activation can attenuate cardiomyocytic apoptosis via inhibition of NF-kappaB and AP-1 translocation following cardiac global ischemia and reperfusion. J Surg Res. 2009;155:147–156. doi: 10.1016/j.jss.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 82.Suliman HB, Carraway MS, Ali AS, Reynolds CM, Welty-Wolf KE, Piantadosi CA. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest. 2007;117:3730–3741. doi: 10.1172/JCI32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res. 2008;103:1232–1240. doi: 10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rhodes MA, Carraway MS, Piantadosi CA, Reynolds CM, Cherry AD, Wester TE, Natoli MJ, Massey EW, Moon RE, Suliman HB. Carbon monoxide, skeletal muscle oxidative stress, and mitochondrial biogenesis in humans. Am J Physiol Heart Circ Physiol. 2009;297:H392–H399. doi: 10.1152/ajpheart.00164.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Soares MP, Lin Y, Anrather J, Csizmadia E, Takigami K, Sato K, Grey ST, Colvin RP, Choi AM, Poss KD, Bach FH. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nature Med. 1998;4:1073–1077. doi: 10.1038/2063. [DOI] [PubMed] [Google Scholar]
- 86.Hancock WW, Buelow R, Sayegh MH, Turka LA. Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nature Med. 1998;4:1392–1396. doi: 10.1038/3982. [DOI] [PubMed] [Google Scholar]
- 87.Araujo JA, Meng L, Tward AD, Hancock WW, Zhai Y, Lee A, Ishikawa K, Iyer S, Buelow R, Busuttil RW, Shih DM, Lusis AJ, Kupiec-Weglinski JW. Systemic rather than local heme oxygenase-1 overexpression improves cardiac allograft outcomes in a new transgenic mouse. J Immunol. 2003;171:1572–1580. doi: 10.4049/jimmunol.171.3.1572. [DOI] [PubMed] [Google Scholar]
- 88.Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, Yu J, Tsui TY, Bach FH. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol. 2004;172:3553–3563. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- 89.Tsui TY, Wu X, Lau CK, Ho DW, Xu T, Siu YT, Fan ST. Prevention of chronic deterioration of heart allograft by recombinant adeno-associated virus-mediated heme oxygenase-1 gene transfer. Circulation. 2003;107:2623–2629. doi: 10.1161/01.CIR.0000066911.03770.8D. [DOI] [PubMed] [Google Scholar]
- 90.Chauveau C, Bouchet D, Roussel JC, Mathieu P, Braudeau C, Renaudin K, Tesson L, Soulillou JP, Iyer S, Buelow R, Anegon I. Gene transfer of heme oxygenase-1 and carbon monoxide delivery inhibit chronic rejection. Am J Transplant. 2002;2:581–592. doi: 10.1034/j.1600-6143.2002.20702.x. [DOI] [PubMed] [Google Scholar]
- 91.Chen S, Kapturczak MH, Wasserfall C, Glushakova OY, Campbell-Thompson M, Deshane JS, Joseph R, Cruz PE, Hauswirth WW, Madsen KM, Croker BP, Berns KI, Atkinson MA, Flotte TR, Tisher CC, Agarwal A. Interleukin 10 attenuates neointimal proliferation and inflammation in aortic allografts by a heme oxygenase-dependent pathway. Proc Natl Acad Sci U S A. 2005 doi: 10.1073/pnas.0502407102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Musameh MD, Green CJ, Mann BE, Fuller BJ, Motterlini R. Improved myocardial function after cold storage with preservation solution supplemented with a carbon monoxide-releasing molecule (CORM-3) J Heart Lung Transplant. 2007;26:1192–1198. doi: 10.1016/j.healun.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 93.True AL, Olive M, Boehm M, San H, Westrick RJ, Raghavachari N, Xu X, Lynn EG, Sack MN, Munson PJ, Gladwin MT, Nabel EG. Heme oxygenase-1 deficiency accelerates formation of arterial thrombosis through oxidative damage to the endothelium, which is rescued by inhaled carbon monoxide. Circ Res. 2007;101:893–901. doi: 10.1161/CIRCRESAHA.107.158998. [DOI] [PubMed] [Google Scholar]
- 94.Chevion M, Leibowitz S, Aye NN, Novogrodsky O, Singer A, Avizemer O, Bulvik B, Konijn AM, Berenshtein E. Heart protection by ischemic preconditioning: a novel pathway initiated by iron and mediated by ferritin. J Mol Cell Cardiol. 2008;45:839–845. doi: 10.1016/j.yjmcc.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 95.Yamashita K, McDaid J, Ollinger R, Tsui TY, Berberat PO, Usheva A, Csizmadia E, Smith RN, Soares MP, Bach FH. Biliverdin, a natural product of heme catabolism, induces tolerance to cardiac allografts. FASEB J. 2004;18:765–767. doi: 10.1096/fj.03-0839fje. [DOI] [PubMed] [Google Scholar]
- 96.Ollinger R, Wang H, Yamashita K, Wegiel B, Thomas M, Margreiter R, Bach FH. Therapeutic applications of bilirubin and biliverdin in transplantation. Antioxid Redox Signal. 2007;9:2175–2185. doi: 10.1089/ars.2007.1807. [DOI] [PubMed] [Google Scholar]
- 97.Nakao A, Toyokawa H, Abe M, Kiyomoto T, Nakahira K, Choi AM, Nalesnik MA, Thomson AW, Murase N. Heart allograft protection with low-dose carbon monoxide inhalation: effects on inflammatory mediators and alloreactive T-cell responses. Transplantation. 2006;81:220–230. doi: 10.1097/01.tp.0000188637.80695.7f. [DOI] [PubMed] [Google Scholar]
- 98.Suliman HB, Carraway MS, Tatro LG, Piantadosi CA. A new activating role for CO in cardiac mitochondrial biogenesis. J Cell Sci. 2006;120:299–308. doi: 10.1242/jcs.03318. [DOI] [PubMed] [Google Scholar]
- 99.Nakao A, Neto JS, Kanno S, Stolz DB, Kimizuka K, Liu F, Bach FH, Billiar TR, Choi AM, Otterbein LE, Murase N. Protection against ischemia/reperfusion injury in cardiac and renal transplantation with carbon monoxide, biliverdin and both. Am J Transplant. 2005;5:282–291. doi: 10.1111/j.1600-6143.2004.00695.x. [DOI] [PubMed] [Google Scholar]
- 100.Zuckerbraun BS, Chin BY, Wegiel B, Billiar TR, Czsimadia E, Rao J, Shimoda L, Ifedigbo E, Kanno S, Otterbein LE. Carbon monoxide reverses established pulmonary hypertension. J Exp Med. 2006;203:2109–2119. doi: 10.1084/jem.20052267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hsu HH, Ko WJ, Hsu JY, Chen JS, Lee YC, Lai IR, Chen CF. Simvastatin ameliorates established pulmonary hypertension through a heme oxygenase-1 dependent pathway in rats. Respir Res. 2009;10:32. doi: 10.1186/1465-9921-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ito T, Okada T, Miyashita H, Nomoto T, Nonaka-Sarukawa M, Uchibori R, Maeda Y, Urabe M, Mizukami H, Kume A, Takahashi M, Ikeda U, Shimada K, Ozawa K. Interleukin-10 expression mediated by an adeno-associated virus vector prevents monocrotaline-induced pulmonary arterial hypertension in rats. Circ Res. 2007;101:734–741. doi: 10.1161/CIRCRESAHA.107.153023. [DOI] [PubMed] [Google Scholar]
- 103.Qingyou Z, Junbao D, Weijin Z, Hui Y, Chaoshu T, Chunyu Z. Impact of hydrogen sulfide on carbon monoxide/heme oxygenase pathway in the pathogenesis of hypoxic pulmonary hypertension. Biochem Biophys Res Commun. 2004;317:30–37. doi: 10.1016/j.bbrc.2004.02.176. [DOI] [PubMed] [Google Scholar]
- 104.Liang OD, Mitsialis SA, Chang MS, Vergadi E, Lee C, Aslam M, Fernandez-Gonzalez A, Liu X, Baveja R, Kourembanas S. Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells. 2011;29:99–107. doi: 10.1002/stem.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shimzu K, Takahashi T, Iwasaki T, Shimizu H, Inoue K, Morimatsu H, Omori E, Matsumi M, Akagi R, Morita K. Hemin treatment abrogates monocrotaline-induced pulmonary hypertension. Med Chem. 2008;4:572–576. doi: 10.2174/157340608786241972. [DOI] [PubMed] [Google Scholar]
- 106.Vassalli F, Pierre S, Julien V, Bouckaert Y, Brimioulle S, Naeije R. Inhibition of hypoxic pulmonary vasoconstriction by carbon monoxide in dogs. Crit Care Med. 2001;29:359–366. doi: 10.1097/00003246-200102000-00026. [DOI] [PubMed] [Google Scholar]
- 107.Stanford SJ, Walters MJ, Mitchell JA. Carbon monoxide inhibits endothelin-1 release by human pulmonary artery smooth muscle cells. Eur J Pharmacol. 2004;486:349–352. doi: 10.1016/j.ejphar.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 108.Nachar RA, Pastene CM, Herrera EA, Riquelme RA, Sanhueza EM, Troncoso S, Llanos AJ. Low-dose inhaled carbon monoxide reduces pulmonary vascular resistance during acute hypoxemia in adult sheep. High Alt Med Biol. 2001;2:377–385. doi: 10.1089/15270290152608552. [DOI] [PubMed] [Google Scholar]
- 109.Madigan M, Entabi F, Zuckerbraun B, Loughran P, Tzeng E. Delayed inhaled carbon monoxide mediates the regression of established neointimal lesions. J Vasc Surg. 2015;61:1026–1033. doi: 10.1016/j.jvs.2013.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dubuis E, Potier M, Wang R, Vandier C. Continuous inhalation of carbon monoxide attenuates hypoxic pulmonary hypertension development presumably through activation of BKCa channels. Cardiovasc Res. 2005;65:751–761. doi: 10.1016/j.cardiores.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 111.Nassour I, Kautza B, Rubin M, Escobar D, Luciano J, Loughran P, Gomez H, Scott J, Gallo D, Brumfield J, Otterbein LE, Zuckerbraun BS. Carbon monoxide protects against hemorrhagic shock and resuscitation-induced microcirculatory injury and tissue injury. Shock. 2015;43:166–171. doi: 10.1097/SHK.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol. 2015;15:117–129. doi: 10.1038/nri3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Calay D, Mason JC. The multifunctional role and therapeutic potential of HO-1 in the vascular endothelium. Antioxid Redox Signal. 2014;20:1789–1809. doi: 10.1089/ars.2013.5659. [DOI] [PubMed] [Google Scholar]
- 114.Belcher JD, Mahaseth H, Welch TE, Otterbein LE, Hebbel RP, Vercellotti GM. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J Clin Invest. 2006 doi: 10.1172/JCI26857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Urquhart P, Rosignoli G, Cooper D, Motterlini R, Perretti M. Carbon monoxide-releasing molecules modulate leukocyte-endothelial interactions under flow. J Pharmacol Exp Ther. 2007;321:656–662. doi: 10.1124/jpet.106.117218. [DOI] [PubMed] [Google Scholar]
- 116.Vachharajani TJ, Work J, Issekutz AC, Granger DN. Heme oxygenase modulates selectin expression in different regional vascular beds. Am J Physiol Heart Circ Physiol. 2000;278:H1613–7. doi: 10.1152/ajpheart.2000.278.5.H1613. [DOI] [PubMed] [Google Scholar]
- 117.Serizawa F, Patterson E, Potter RF, Fraser DD, Cepinskas G. Pretreatment of human cerebrovascular endothelial cells with CO-releasing molecule-3 interferes with JNK/AP-1 signaling and suppresses LPS-induced proadhesive phenotype. Microcirculation. 2015;22:28–36. doi: 10.1111/micc.12161. [DOI] [PubMed] [Google Scholar]
- 118.Patterson EK, Fraser DD, Capretta A, Potter RF, Cepinskas G. Carbon monoxide-releasing molecule 3 inhibits myeloperoxidase (MPO) and protects against MPO-induced vascular endothelial cell activation/dysfunction. Free Radic Biol Med. 2014;70:167–173. doi: 10.1016/j.freeradbiomed.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 119.Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 120.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110:159–173. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Frangogiannis NG. The reparative function of cardiomyocytes in the infarcted myocardium. Cell Metab. 2015;21:797–798. doi: 10.1016/j.cmet.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 122.Lorchner H, Poling J, Gajawada P, Hou Y, Polyakova V, Kostin S, Adrian-Segarra JM, Boettger T, Wietelmann A, Warnecke H, Richter M, Kubin T, Braun T. Myocardial healing requires Reg3β-dependent accumulation of macrophages in the ischemic heart. Nat Med. 2015;21:353–362. doi: 10.1038/nm.3816. [DOI] [PubMed] [Google Scholar]
- 123.Duckers HJ, Boehm M, True AL, Yet SF, San H, Park JL, Clinton WR, Lee ME, Nabel GJ, Nabel EG. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med. 2001;7:693–698. doi: 10.1038/89068. [DOI] [PubMed] [Google Scholar]
- 124.Florczyk U, Jazwa A, Maleszewska M, Mendel M, Szade K, Kozakowska M, Grochot-Przeczek A, Viscardi M, Czauderna S, Bukowska-Strakova K, Kotlinowski J, Jozkowicz A, Loboda A, Dulak J. Nrf2 regulates angiogenesis: effect on endothelial cells, bone marrow-derived proangiogenic cells and hind limb ischemia. Antioxid Redox Signal. 2014;20:1693–1708. doi: 10.1089/ars.2013.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lin HH, Chen YH, Chiang MT, Huang PL, Chau LY. Activator protein-2alpha mediates carbon monoxide-induced stromal cell-derived factor-1alpha expression and vascularization in ischemic heart. Arterioscler Thromb Vasc Biol. 2013;33:785–794. doi: 10.1161/ATVBAHA.112.301143. [DOI] [PubMed] [Google Scholar]
- 126.Jeney V, Balla J, Yachie A, Varga Z, Vercellotti GM, Eaton JW, Balla G. Pro-oxidant and cytotoxic effects of circulating heme. Blood. 2002;100:879–887. doi: 10.1182/blood.v100.3.879. [DOI] [PubMed] [Google Scholar]
- 127.Ujhelyi L, Balla G, Jeney V, Varga Z, Nagy E, Vercellotti GM, Agarwal A, Eaton JW, Balla J. Hemodialysis reduces inhibitory effect of plasma ultrafiltrate on LDL oxidation and subsequent endothelial reactions. Kidney Int. 2006;69:144–151. doi: 10.1038/sj.ki.5000007. [DOI] [PubMed] [Google Scholar]
- 128.Nagy E, Jeney V, Yachie A, Szabo RP, Wagner O, Vercellotti GM, Eaton JW, Balla G, Balla J. Oxidation of hemoglobin by lipid hydroperoxide associated with low-density lipoprotein (LDL) and increased cytotoxic effect by LDL oxidation in heme oxygenase-1 (HO-1) deficiency. Cell Mol Biol (Noisy -le-grand) 2005;51:377–385. [PubMed] [Google Scholar]
- 129.Anwar AA, Li FY, Leake DS, Ishii T, Mann GE, Siow RC. Induction of heme oxygenase 1 by moderately oxidized low-density lipoproteins in human vascular smooth muscle cells: role of mitogen-activated protein kinases and Nrf2. Free Radic Biol Med. 2005;39:227–236. doi: 10.1016/j.freeradbiomed.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 130.Chen YH, Yet SF, Perrella MA. Role of heme oxygenase-1 in the regulation of blood pressure and cardiac function. Exp Biol Med (Maywood ) 2003;228:447–453. doi: 10.1177/15353702-0322805-03. [DOI] [PubMed] [Google Scholar]
- 131.Wenzel P, Rossmann H, Muller C, Kossmann S, Oelze M, Schulz A, Arnold N, Simsek C, Lagrange J, Klemz R, Schonfelder T, Brandt M, Karbach SH, Knorr M, Finger S, Neukirch C, Hauser F, Beutel ME, Kroller-Schon S, Schulz E, Schnabel RB, Lackner K, Wild PS, Zeller T, Daiber A, Blankenberg S, Munzel T. Heme oxygenase-1 suppresses a pro-inflammatory phenotype in monocytes and determines endothelial function and arterial hypertension in mice and humans. Eur Heart J. 2015;36:3437–3446. doi: 10.1093/eurheartj/ehv544. [DOI] [PubMed] [Google Scholar]
- 132.Su JB. Vascular endothelial dysfunction and pharmacological treatment. World J Cardiol. 2015;7:719–741. doi: 10.4330/wjc.v7.i11.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Soares MP, Usheva A, Brouard S, Berberat PO, Gunther L, Tobiasch E, Bach FH. Modulation of endothelial cell apoptosis by heme oxygenase-1-derived carbon monoxide. Antioxid Redox Signal. 2002;4:321–329. doi: 10.1089/152308602753666370. [DOI] [PubMed] [Google Scholar]
- 134.Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Christou H, Morita T, Hsieh CM, Koike H, Arkonac B, Perrella MA, Kourembanas S. Prevention of hypoxia-induced pulmonary hypertension by enhancement of endogenous heme oxygenase-1 in the rat. Circ Res. 2000;86:1224–1229. doi: 10.1161/01.res.86.12.1224. [DOI] [PubMed] [Google Scholar]
- 136.Grochot-Przeczek A, Lach R, Mis J, Skrzypek K, Gozdecka M, Sroczynska P, Dubiel M, Rutkowski A, Kozakowska M, Zagorska A, Walczynski J, Was H, Kotlinowski J, Drukala J, Kurowski K, Kieda C, Herault Y, Dulak J, Jozkowicz A. Heme oxygenase-1 accelerates cutaneous wound healing in mice. PLoS One. 2009;4:e5803. doi: 10.1371/journal.pone.0005803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Deshane J, Chen S, Caballero S, Grochot-Przeczek A, Was H, Li CS, Lach R, Hock TD, Chen B, Hill-Kapturczak N, Siegal GP, Dulak J, Jozkowicz A, Grant MB, Agarwal A. Stromal cell-derived factor 1 promotes angiogenesis via a heme oxygenase 1-dependent mechanism. J Exp Med. 2007;204:605–618. doi: 10.1084/jem.20061609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Grochot-Przeczek A, Dulak J, Jozkowicz A. Therapeutic angiogenesis for revascularization in peripheral artery disease. Gene. 2013;525:220–228. doi: 10.1016/j.gene.2013.03.097. [DOI] [PubMed] [Google Scholar]
- 139.Wegiel B, Gallo D, Csizmadia E, Harris C, Belcher J, Vercellotti GM, Penacho N, Seth P, Sukhatme V, Ahmed A, Pandolfi PP, Helczynski L, Bjartell A, Persson JL, Otterbein LE. Carbon monoxide expedites metabolic exhaustion to inhibit tumor growth. Cancer Res. 2013;73:7009–7021. doi: 10.1158/0008-5472.CAN-13-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Skrzypek K, Tertil M, Golda S, Ciesla M, Weglarczyk K, Collet G, Guichard A, Kozakowska M, Boczkowski J, Was H, Gil T, Kuzdzal J, Muchova L, Vitek L, Loboda A, Jozkowicz A, Kieda C, Dulak J. Interplay between heme oxygenase-1 and miR-378 affects non-small cell lung carcinoma growth, vascularization, and metastasis. Antioxid Redox Signal. 2013;19:644–660. doi: 10.1089/ars.2013.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ferrando M, Gueron G, Elguero B, Giudice J, Salles A, Leskow FC, Jares-Erijman EA, Colombo L, Meiss R, Navone N, De Siervi A, Vazquez E. Heme oxygenase 1 (HO-1) challenges the angiogenic switch in prostate cancer. Angiogenesis. 2011;14:467–479. doi: 10.1007/s10456-011-9230-4. [DOI] [PubMed] [Google Scholar]
- 142.Loboda A, Jozkowicz A, Dulak J. HO-1/CO system in tumor growth, angiogenesis and metabolism - Targeting HO-1 as an anti-tumor therapy. Vascul Pharmacol. 2015;74:11–22. doi: 10.1016/j.vph.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 143.Cheng CC, Guan SS, Yang HJ, Chang CC, Luo TY, Chang J, Ho AS. Blocking heme oxygenase-1 by zinc protoporphyrin reduces tumor hypoxia-mediated VEGF release and inhibits tumor angiogenesis as a potential therapeutic agent against colorectal cancer. J Biomed Sci. 2016;23:18. doi: 10.1186/s12929-016-0219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Was H, Sokolowska M, Sierpniowska A, Dominik P, Skrzypek K, Lackowska B, Pratnicki A, Grochot-Przeczek A, Taha H, Kotlinowski J, Kozakowska M, Mazan A, Nowak W, Muchova L, Vitek L, Ratajska A, Dulak J, Jozkowicz A. Effects of heme oxygenase-1 on induction and development of chemically induced squamous cell carcinoma in mice. Free Radic Biol Med. 2011;51:1717–1726. doi: 10.1016/j.freeradbiomed.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sacca P, Meiss R, Casas G, Mazza O, Calvo JC, Navone N, Vazquez E. Nuclear translocation of haeme oxygenase-1 is associated to prostate cancer. Br J Cancer. 2007;97:1683–1689. doi: 10.1038/sj.bjc.6604081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lin HH, Lai SC, Chau LY. Heme oxygenase-1/carbon monoxide induces vascular endothelial growth factor expression via p38 kinase-dependent activation of Sp1. J Biol Chem. 2011;286:3829–3838. doi: 10.1074/jbc.M110.168831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gandini NA, Fermento ME, Salomon DG, Blasco J, Patel V, Gutkind JS, Molinolo AA, Facchinetti MM, Curino AC. Nuclear localization of heme oxygenase-1 is associated with tumor progression of head and neck squamous cell carcinomas. Exp Mol Pathol. 2012;93:237–245. doi: 10.1016/j.yexmp.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 148.Nemeth Z, Li M, Csizmadia E, Dome B, Johansson M, Persson JL, Seth P, Otterbein L, Wegiel B. Heme oxygenase-1 in macrophages controls prostate cancer progression. Oncotarget. 2015;6:33675–33688. doi: 10.18632/oncotarget.5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Levitt DG, Levitt MD. Carbon monoxide: a critical quantitative analysis and review of the extent and limitations of its second messenger function. Clin Pharmacol. 2015;7:37–56. doi: 10.2147/CPAA.S79626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gilles-Gonzalez MA, Gonzalez G. Heme-based sensors: defining characteristics, recent developments, and regulatory hypotheses. J Inorg Biochem. 2005;99:1–22. doi: 10.1016/j.jinorgbio.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 151.Igarashi K, Sun J. The heme-Bach1 pathway in the regulation of oxidative stress response and erythroid differentiation. Antioxid Redox Signal. 2006;8:107–118. doi: 10.1089/ars.2006.8.107. [DOI] [PubMed] [Google Scholar]
- 152.Sarady JK, Zuckerbraun BS, Bilban M, Wagner O, Usheva A, Liu F, Ifedigbo E, Zamora R, Choi AM, Otterbein LE. Carbon monoxide protection against endotoxic shock involves reciprocal effects on iNOS in the lung and liver. FASEB J. 2004;18:854–856. doi: 10.1096/fj.03-0643fje. [DOI] [PubMed] [Google Scholar]
- 153.Zuckerbraun BS, Billiar TR, Otterbein SL, Kim PKM, Liu F, Choi AMK, Bach FH, Otterbein LE. Carbon monoxide protects against liver failure through nitric oxide-induced heme oxygenase 1. J Exp Med. 2003;198:1707–1716. doi: 10.1084/jem.20031003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lo Iacono L, Boczkowski J, Zini R, Salouage I, Berdeaux A, Motterlini R, Morin D. A carbon monoxide-releasing molecule (CORM-3) uncouples mitochondrial respiration and modulates the production of reactive oxygen species. Free Rad Biol Med. 2011;50:1556–1564. doi: 10.1016/j.freeradbiomed.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 155.Chin BY, Jiang G, Wegiel B, Wang HJ, MacDonald T, Zhang XC, Gallo D, Cszimadia E, Bach FH, Lee PJ, Otterbein LE. Hypoxia-inducible factor 1α stabilization by carbon monoxide results in cytoprotective preconditioning. Proc Natl Acad Sci U S A. 2007;104:5109–5114. doi: 10.1073/pnas.0609611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zuckerbraun BS, Chin BY, Bilban M, de Costa dJ, Rao J, Billiar TR, Otterbein LE. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J. 2007;21:1099–1106. doi: 10.1096/fj.06-6644com. [DOI] [PubMed] [Google Scholar]
- 157.Peers C, Steele DS. Carbon monoxide: a vital signalling molecule and potent toxin in the myocardium. J Mol Cell Cardiol. 2012;52:359–365. doi: 10.1016/j.yjmcc.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 158.Kim HP, Wang X, Zhang J, Suh GY, Benjamin IJ, Ryter SW, Choi AM. Heat shock protein-70 mediates the cytoprotective effect of carbon Monoxide: involvement of p38β MAPK and Heat Shock Factor-1. J Immunol. 2005;175:2622–2629. doi: 10.4049/jimmunol.175.4.2622. [DOI] [PubMed] [Google Scholar]
- 159.Haschemi A, Wagner O, Marculescu R, Wegiel B, Robson SC, Gagliani N, Gallo D, Chen JF, Bach FH, Otterbein LE. Cross-regulation of carbon monoxide and the adenosine A2a receptor in macrophages. J Immunol. 2007;178:5921–5929. doi: 10.4049/jimmunol.178.9.5921. [DOI] [PubMed] [Google Scholar]
- 160.Nakao A, Huang CS, Stolz DB, Wang Y, Franks JM, Tochigi N, Billiar TR, Toyoda Y, Tzeng E, McCurry KR. Ex vivo carbon monoxide delivery inhibits intimal hyperplasia in arterialized vein grafts. Cardiovasc Res. 2011;89:457–463. doi: 10.1093/cvr/cvq298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Choi YK, Kim CK, Lee H, Jeoung D, Ha KS, Kwon YG, Kim KW, Kim YM. Carbon monoxide promotes VEGF expression by increasing HIF-1α protein level via two distinct mechanisms, translational activation and stabilization of HIF-1α protein. J Biol Chem. 2010;285:32116–32125. doi: 10.1074/jbc.M110.131284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hou S, Xu R, Heinemann SH, Hoshi T. The RCK1 high-affinity Ca2+ sensor confers carbon monoxide sensitivity to Slo1 BK channels. Proc Natl Acad Sci U S A. 2008;105:4039–4043. doi: 10.1073/pnas.0800304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Scragg JL, Dallas ML, Wilkinson JA, Varadi G, Peers C. Carbon monoxide inhibits L-type Ca2+ channels via redox modulation of key cysteine residues by mitochondrial reactive oxygen species. J Biol Chem. 2008;283:24412–24419. doi: 10.1074/jbc.M803037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wegiel B, Larsen R, Gallo D, Chin BY, Harris C, Mannam P, Kaczmarek E, Lee PJ, Zuckerbraun BS, Flavell R, Soares MP, Otterbein LE. Macrophages sense and kill bacteria through carbon monoxide-dependent inflammasome activation. J Clin Invest. 2014;124:4926–4940. doi: 10.1172/JCI72853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Piantadosi CA. Biological chemistry of carbon monoxide. Antioxid Redox Signal. 2002;4:259–270. doi: 10.1089/152308602753666316. [DOI] [PubMed] [Google Scholar]
- 166.Szabo C. Gaseotransmitters: new frontiers for translational science. Sci Transl Med. 2010;2:59ps54. doi: 10.1126/scitranslmed.3000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lancel S, Hassoun SM, Favory R, Decoster B, Motterlini R, Neviere R. Carbon monoxide rescues mice from lethal sepsis by supporting mitochondrial energetic metabolism and activating mitochondrial biogenesis. J Pharmacol Exp Ther. 2009;1329:641–648. doi: 10.1124/jpet.108.148049. [DOI] [PubMed] [Google Scholar]
- 168.Ristow M, Schmeisser K. Mitohormesis: Promoting Health and Lifespan by Increased Levels of Reactive Oxygen Species (ROS) Dose Response. 2014;12:288–341. doi: 10.2203/dose-response.13-035.Ristow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schulz TJ, Westermann D, Isken F, Voigt A, Laube B, Thierbach R, Kuhlow D, Zarse K, Schomburg L, Pfeiffer AF, Tschope C, Ristow M. Activation of mitochondrial energy metabolism protects against cardiac failure. Aging (Albany NY) 2010;2:843–853. doi: 10.18632/aging.100234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sandouka A, Balogun E, Foresti R, Mann BE, Johnson TR, Tayem Y, Green CJ, Fuller B, Motterlini R. Carbon monoxide-releasing molecules (CO-RMs) modulate respiration in isolated mitochondria. Cell Mol Biol. 2005;51:425–432. [PubMed] [Google Scholar]
- 171.Lavitrano M, Smolenski RT, Musumeci A, Maccherini M, Slominska E, Di Florio E, Bracco A, Mancini A, Stassi G, Patti M, Giovannoni R, Froio A, Simeone F, Forni M, Bacci ML, D'Alise G, Cozzi E, Otterbein LE, Yacoub MH, Bach FH, Calise F. Carbon monoxide improves cardiac energetics and safeguards the heart during reperfusion after cardiopulmonary bypass in pigs. FASEB J. 2004;18:1093–1095. doi: 10.1096/fj.03-0996fje. [DOI] [PubMed] [Google Scholar]
- 172.Ahlstrom K, Biber B, Aberg A, Waldenstrom A, Ronquist G, Abrahamsson P, Stranden P, Johansson G, Haney MF. Metabolic responses in ischemic myocardium after inhalation of carbon monoxide. Acta Anaesthesiol Scand. 2009 doi: 10.1111/j.1399-6576.2009.01992.x. [DOI] [PubMed] [Google Scholar]
- 173.Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD. Targeting fatty acid and carbohydrate oxidation--a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta. 2011;1813:1333–1350. doi: 10.1016/j.bbamcr.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 174.Bonkovsky HL, Guo JT, Hou W, Li T, Narang T, Thapar M. Porphyrin and heme metabolism and the porphyrias. Compr Physiol. 2013;3:365–401. doi: 10.1002/cphy.c120006. [DOI] [PubMed] [Google Scholar]
- 175.Kumar S, Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol Lett. 2005;157:175–188. doi: 10.1016/j.toxlet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 176.Vinchi F, Costa dS, Ingoglia G, Petrillo S, Brinkman N, Zuercher A, Cerwenka A, Tolosano E, Muckenthaler MU. Hemopexin therapy reverts heme-induced proinflammatory phenotypic switching of macrophages in a mouse model of sickle cell disease. Blood. 2016;127:473–486. doi: 10.1182/blood-2015-08-663245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sawicki KT, Shang M, Wu R, Chang HC, Khechaduri A, Sato T, Kamide C, Liu T, Naga Prasad SV, Ardehali H. Increased heme levels in the heart lead to exacerbated ischemic injury. J Am Heart Assoc. 2015;4:e002272. doi: 10.1161/JAHA.115.002272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wegiel B, Hauser CJ, Otterbein LE. Heme as a danger molecule in pathogen recognition. Free Radic Biol Med. 2015;89:651–661. doi: 10.1016/j.freeradbiomed.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 179.Keel SB, Doty RT, Yang Z, Quigley JG, Chen J, Knoblaugh S, Kingsley PD, De D, Vaughn MB, Kaplan J, Palis J, Abkowitz JL. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science. 2008;319:825–828. doi: 10.1126/science.1151133. I. [DOI] [PubMed] [Google Scholar]
- 180.Philip M, Funkhouser SA, Chiu EY, Phelps SR, Delrow JJ, Cox J, Fink PJ, Abkowitz JL. Heme exporter FLVCR is required for T cell development and peripheral survival. J Immunol. 2015;194:1677–1685. doi: 10.4049/jimmunol.1402172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.MacGarvey NC, Suliman HB, Bartz RR, Fu P, Withers CM, Welty-Wolf KE, Piantadosi CA. Activation of mitochondrial biogenesis by heme oxygenase-1-mediated NF-E2-related factor-2 induction rescues mice from lethal Staphylococcus aureus sepsis. Am J Respir Crit Care Med. 2012;185:851–861. doi: 10.1164/rccm.201106-1152OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ejima K, Layne MD, Carvajal IM, Nanri H, Ith B, Yet SF, Perrella MA. Modulation of the thioredoxin system during inflammatory responses and its effect on heme oxygenase-1 expression. Antioxid Redox Signal. 2002;4:569–575. doi: 10.1089/15230860260220067. [DOI] [PubMed] [Google Scholar]
- 183.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 185.Zeng B, Chen H, Zhu C, Ren X, Lin G, Cao F. Effects of combined mesenchymal stem cells and heme oxygenase-1 therapy on cardiac performance. Eur J Cardiothorac Surg. 2008;34:850–856. doi: 10.1016/j.ejcts.2008.05.049. [DOI] [PubMed] [Google Scholar]
- 186.Zhang S, Lu S, Ge J, Guo J, Chen P, Li T, Zhang P, Jia Z, Ma K, Liu Y, Zhou C, Li L. Increased heme oxygenase-1 expression in infarcted rat hearts following human bone marrow mesenchymal cell transplantation. Microvasc Res. 2005;69:64–70. doi: 10.1016/j.mvr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 187.Lin HH, Chen YH, Chang PF, Lee YT, Yet SF, Chau LY. Heme oxygenase-1 promotes neovascularization in ischemic heart by coinduction of VEGF and SDF-1. J Mol Cell Cardiol. 2008;45:44–55. doi: 10.1016/j.yjmcc.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 188.Zeng B, Ren X, Lin G, Zhu C, Chen H, Yin J, Jiang H, Yang B, Ding D. Paracrine action of HO-1-modified mesenchymal stem cells mediates cardiac protection and functional improvement. Cell Biol Int. 2008;32:1256–1264. doi: 10.1016/j.cellbi.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 189.Brunt KR, Wu J, Chen Z, Poeckel D, Dercho RA, Melo LG, Funk CD, Ward CA, Li RK. Ex vivo Akt/HO-1 gene therapy to human endothelial progenitor cells enhances myocardial infarction recovery. Cell Transplant. 2012;21:1443–1461. doi: 10.3727/096368912X653002. [DOI] [PubMed] [Google Scholar]
- 190.Wojakowski W, Tendera M, Cybulski W, Zuba-Surma EK, Szade K, Florczyk U, Kozakowska M, Szymula A, Krzych L, Paslawska U, Paslawski R, Milewski K, Buszman PP, Nabialek E, Kuczmik W, Janiszewski A, Dziegiel P, Buszman PE, Jozkowicz A, Dulak J. Effects of intracoronary delivery of allogenic bone marrow-derived stem cells expressing heme oxygenase-1 on myocardial reperfusion injury. Thromb Haemost. 2012;108:464–475. doi: 10.1160/TH12-05-0303. [DOI] [PubMed] [Google Scholar]
- 191.Jiang S, Haider HK, Idris NM, Salim A, Ashraf M. Supportive interaction between cell survival signaling and angiocompetent factors enhances donor cell survival and promotes angiomyogenesis for cardiac repair. Circ Res. 2006;99:776–784. doi: 10.1161/01.RES.0000244687.97719.4f. [DOI] [PubMed] [Google Scholar]
- 192.Cai C, Teng L, Vu D, He JQ, Guo Y, Li Q, Tang XL, Rokosh G, Bhatnagar A, Bolli R. The heme oxygenase 1 inducer (CoPP) protects human cardiac stem cells against apoptosis through activation of the extracellular signal-regulated kinase (ERK)/NRF2 signaling pathway and cytokine release. J Biol Chem. 2012;287:33720–33732. doi: 10.1074/jbc.M112.385542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cai C, Guo Y, Teng L, Nong Y, Tan M, Book MJ, Zhu X, Wang XL, Du J, Wu WJ, Xie W, Hong KU, Li Q, Bolli R. Preconditioning human cardiac stem cells with an HO-1 inducer exerts beneficial effects after cell transplantation in the infarcted murine heart. Stem Cells. 2015;33:3596–3607. doi: 10.1002/stem.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–S64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Luo J, Weaver MS, Dennis JE, Whalen E, Laflamme MA, Allen MD. Targeting survival pathways to create infarct-spanning bridges of human embryonic stem cell-derived cardiomyocytes. J Thorac Cardiovasc Surg. 2014;148:3180–3188. doi: 10.1016/j.jtcvs.2014.06.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Luo J, Weaver MS, Cao B, Dennis JE, Van Biber B, Laflamme MA, Allen MD. Cobalt protoporphyrin pretreatment protects human embryonic stem cell-derived cardiomyocytes from hypoxia/reoxygenation injury in vitro and increases graft size and vascularization in vivo. Stem Cells Transl Med. 2014;3:734–744. doi: 10.5966/sctm.2013-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Rad Biol Med. 2000;28:1303–1312. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- 199.Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the heme oxygenase-1 gene via regulation of Nrf2 and the antioxidant responsive element. Biochem J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Liu J, Zhu P, Song P, Xiong W, Chen H, Peng W, Wang S, Li S, Fu Z, Wang Y, Wang H. Pretreatment of Adipose Derived Stem Cells with Curcumin Facilitates Myocardial Recovery via Antiapoptosis and Angiogenesis. Stem Cells Int. 2015;2015:638153. doi: 10.1155/2015/638153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lakkisto P, Kyto V, Forsten H, Siren JM, Segersvard H, Voipio-Pulkki LM, Laine M, Pulkki K, Tikkanen I. Heme oxygenase-1 and carbon monoxide promote neovascularization after myocardial infarction by modulating the expression of HIF-1alpha, SDF-1alpha and VEGF-B. Eur J Pharmacol. 2010;635:156–164. doi: 10.1016/j.ejphar.2010.02.050. [DOI] [PubMed] [Google Scholar]
- 202.Suliman HB, Zobi F, Piantadosi CA. HO-1/CO system and embryonic stem cell differentiation and maturation into cardiomyocytes. Antioxid Redox Signal. 2016 doi: 10.1089/ars.2015.6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kozakowska M, Szade K, Dulak J, Jozkowicz A. Role of heme oxygenase-1 in postnatal differentiation of stem cells: a possible cross-talk with microRNAs. Antioxid Redox Signal. 2014;20:1827–1850. doi: 10.1089/ars.2013.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bach FH. Heme oxygenase-1: a therapeutic amplification funnel. FASEB J. 2005;19:1216–1219. doi: 10.1096/fj.04-3485cmt. [DOI] [PubMed] [Google Scholar]
- 205.Burness CB, Deeks ED. Dimethyl fumarate: a review of its use in patients with relapsing-remitting multiple sclerosis. CNS Drugs. 2014;28:373–387. doi: 10.1007/s40263-014-0155-5. [DOI] [PubMed] [Google Scholar]
- 206.Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem Pharmacol. 2010;80:1895–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 207.Hanto DW, Maki T, Yoon MH, Csizmadia E, Chin BY, Gallo D, Konduru B, Kuramitsu K, Smith NR, Berssenbrugge A, Attanasio C, Thomas M, Wegiel B, Otterbein LE. Intraoperative administration of inhaled carbon monoxide reduces delayed graft function in kidney allografts in swine. Am J Transplant. 2010;10:2421–2430. doi: 10.1111/j.1600-6143.2010.03289.x. [DOI] [PubMed] [Google Scholar]
- 208.Wang P, Liu H, Zhao Q, Chen Y, Liu B, Zhang B, Zheng Q. Syntheses and evaluation of drug-like properties of CO-releasing molecules containing ruthenium and group 6 metal. Eur J Med Chem. 2014;74:199–215. doi: 10.1016/j.ejmech.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 209.Foresti R, Motterlini R. Interaction of carbon monoxide with transition metals: evolutionary insights into drug target discovery. Curr Drug Targets. 2010;11:1595–1604. doi: 10.2174/1389450111009011595. [DOI] [PubMed] [Google Scholar]
- 210.Vandegriff KD, Young MA, Lohman J, Bellelli A, Samaja M, Malavalli A, Winslow RM. CO-MP4, a polyethylene glycol-conjugated haemoglobin derivative and carbon monoxide carrier that reduces myocardial infarct size in rats. Br J Pharmacol. 2008;154:1649–1661. doi: 10.1038/bjp.2008.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wang D, Viennois E, Ji K, Damera K, Draganov A, Zheng Y, Dai C, Merlin D, Wang B. A click-and-release approach to CO prodrugs. Chem Commun (Camb ) 2014;50:15890–15893. doi: 10.1039/c4cc07748b. [DOI] [PubMed] [Google Scholar]
- 212.Steiger C, Wollborn J, Gutmann M, Zehe M, Wunder C, Meinel L. Controlled therapeutic gas delivery systems for quality-improved transplants. Eur J Pharm Biopharm. 2015;97:96–106. doi: 10.1016/j.ejpb.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 213.Yuan G, Vasavda C, Peng YJ, Makarenko VV, Raghuraman G, Nanduri J, Gadalla MM, Semenza GL, Kumar GK, Snyder SH, Prabhakar NR. Protein kinase G-regulated production of H2S governs oxygen sensing. Sci Signal. 2015;8:ra37. doi: 10.1126/scisignal.2005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fayad-Kobeissi S, Ratovonantenaina J, Dabire H, Wilson JL, Rodriguez AM, Berdeaux A, Dubois-Rande JL, Mann BE, Motterlini R, Foresti R. Vascular and angiogenic activities of CORM-401, an oxidant-sensitive CO-releasing molecule. Biochem Pharmacol. 2016;102:64–77. doi: 10.1016/j.bcp.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 215.Wilson JL, Fayad-Kobeissi S, Oudir S, Haas B, Michel BW, Dubois-Rande JL, Ollivier A, Martens T, Rivard M, Motterlini R, Foresti R. Design and synthesis of novel hybrid molecules that activate the transcription factor Nrf2 and simultaneously release carbon monoxide. Chemistry. 2014;20:14698–14704. doi: 10.1002/chem.201403901. [DOI] [PubMed] [Google Scholar]
- 216.Nikam A, Ollivier A, Rivard M, Wilson JL, Mebarki K, Martens T, Dubois-Rande JL, Motterlini R, Foresti R. Diverse Nrf2 activators coordinated to cobalt carbonyls induce heme oxygenase-1 and release carbon monoxide in vitro and in vivo. J Med Chem. 2016;59:756–762. doi: 10.1021/acs.jmedchem.5b01509. [DOI] [PubMed] [Google Scholar]
- 217.Nakao A, Murase N, Ho C, Toyokawa H, Billiar TR, Kanno S. Biliverdin administration prevents the formation of intimal hyperplasia induced by vascular injury. Circulation. 2005;112:587–591. doi: 10.1161/CIRCULATIONAHA.104.509778. [DOI] [PubMed] [Google Scholar]
- 218.Schwertner HA, Vitek L. Gilbert syndrome, UGT1A1*28 allele, and cardiovascular disease risk: possible protective effects and therapeutic applications of bilirubin. Atherosclerosis. 2008;198:1–11. doi: 10.1016/j.atherosclerosis.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 219.Bulmer AC, Verkade HJ, Wagner KH. Bilirubin and beyond: A review of lipid status in Gilbert's syndrome and its relevance to cardiovascular disease protection. Prog Lipid Res. 2012;52:193–205. doi: 10.1016/j.plipres.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 220.Vitek L, Jirsa M, Brodanova M, Kala'b M, Marecvek Z, Danzig V, Novotny L, Kotal P. Gilbert syndrome and ischemic heart disease: a protective effect of elevated bilirubin levels. Atherosclerosis. 2002;160:449–456. doi: 10.1016/s0021-9150(01)00601-3. [DOI] [PubMed] [Google Scholar]
- 221.Inoguchi T, Sasaki S, Kobayashi K, Takayanagi R, Yamada T. Relationship between Gilbert syndrome and prevalence of vascular complications in patients with diabetes. JAMA. 2007;298:1398–1400. doi: 10.1001/jama.298.12.1398-b. [DOI] [PubMed] [Google Scholar]
- 222.Temme EH, Zhang J, Schouten EG, Kesteloot H. Serum bilirubin and 10-year mortality risk in a Belgian population. Cancer Causes Control. 2001;12:887–894. doi: 10.1023/a:1013794407325. [DOI] [PubMed] [Google Scholar]