Abstract
Objective
To estimate overall survival (OS), progression-free survival (PFS), imaging responses, and toxicities of bevacizumab plus carboplatin for the treatment of recurrent malignant glioma. The secondary objective was to estimate the agreement between post-contrast T1-weighted and T2-weighted magnetic resonance imaging.
Methods
A retrospective analysis of 9 patients who received bevacizumab (10 mg/kg intravenously) and carboplatin (AUC 5 intravenously) for recurrent malignant glioma (World Health Organization grades III and IV) is presented. Eight of 9 patients received this regimen at first recurrence.
Results
The median age and Karnofsky performance score were 51 years and 70, respectively. For the 5 patients with grade III gliomas, the median PFS was 126 days, whereas median OS was not attained at 517 days of follow-up. Six-month PFS was 40%, whereas 6-month OS was 60%. For the 4 patients with grade IV gliomas, the median PFS was 216 days, whereas the median OS was not attained at 482 days of follow-up. Six-month PFS was 50%, whereas 6-month OS was 75%. The agreement between contrast-enhanced T1-weighted and T2-weighted images to determine recurrence was moderate (kappa = 0.5714). Three patients had grade 3 and 4 toxicities including hyponatremia and thrombocytopenia.
Conclusion
Patients who received the combination of bevacizumab plus carboplatin for recurrent malignant glioma had reasonable PFS, OS, and toxicities. The median OS in our series is promising at well over 1 year. Agreement between postcontrast T1- and T2-weighted images is only moderate in the context of bevacizumab therapy.
Keywords: Bevacizumab, Carboplatin, Imaging response, Recurrent malignant glioma, Toxicity
The prognosis for recurrent malignant gliomas has historically been dismal, with a median survival of 3 to 9 months.1 There is currently no standard of care for the treatment of these tumors. However, several phase 2 trials analyzing the efficacy of bevacizumab in combination with various chemotherapeutic agents including irinotecan2-5 and etoposide2,6 have shown increased 6-month progression-free survival (PFS) and 6-month overall survival (OS) with acceptable toxicity compared with patients who received chemotherapy alone.7 In a phase 2 noncomparative study, Friedman et al8 recently found that patients who received bevacizumab plus irinotecan and those who received bevacizumab alone both had improved 6-month PFS (42% and 50.3%, respectively) and OS (median 8.7 and 9.2 months, respectively) compared with historical data when used to treat recurrent glioblastoma multiforme. Other bevacizumab-containing combination regimens used to treat recurrent malignant glioma including lomustine, rapamycin, and carboplatin with variable radiographic results have also been reported.2,8 The need for other more effective chemotherapeutic agents is paramount.
Bevacizumab is a recombinant humanized monoclonal IgG1 antibody that binds to vascular endothelial growth factor and prevents the proliferation of endothelial cells and formation of new blood vessels.9 Vascular endothelial growth factor has a role in endothelial cell permeability, activation, survival proliferation, invasion, and migration, which all affect tumor progression and angiogenesis.2 Malignant gliomas have been found to express vascular endothelial growth factor receptors.10
Carboplatin has long been used to treat a variety of malignancies including ovarian cancer, breast cancer, Hodgkin's disease, and non-small cell lung cancer. Before bevacizumab was widely used, carboplatin as monotherapy was relatively effective in treating recurrent gliomas.11,12 More recently, Narayana et al13 demonstrated improved OS and PFS in patients with recurrent high-grade glioma with bevacizumab and carboplatin. Preclinical activity of bevacizumab plus carboplatin in malignant glioma has also been promising. Jahnke et al14 demonstrated significantly increased survival from the combination of bevacizumab and carboplatin compared with either bevacizumab or carboplatin alone in a malignant glioma rat model. There is currently a paucity of literature addressing survival, time to progression, imaging responses, and toxicities of bevacizumab plus carboplatin in human subjects. At our institution, bevacizumab plus irinotecan was initially used to treat patients with recurrent malignant gliomas, but bevacizumab plus carboplatin is now the preferred chemotherapeutic combination. This is a retrospective case series analyzing OS, PFS, imaging responses, and the toxicity profile of bevacizumab plus intravenous carboplatin treatment of recurrent malignant glioma.
Patients and Methods
Study Population and Patient Eligibility
All patients were treated at Oregon Health & Science University (OHSU) between 2006 and 2008, were age 18 or older, and had undergone at least 1 surgery to histologically confirm the diagnosis of a malignant glioma (anaplastic astrocytoma, anaplastic oligodendroglioma, or glioblastoma multiforme) (Table 1). All patients were identified from a confidential database of OHSU patients treated with bevacizumab maintained by Dr. Neuwelt's office. This retrospective review was approved by the OHSU Institutional Review Board. Patient consent was not obtained because all patient information was deidentified. Progression after standard chemoradiation was determined using Macdonald criteria.15 Karnofsky performance scores at the outset of bevacizumab plus carboplatin treatment ranged from 40 to 90. All patients had acceptable laboratory values (Common Terminology Criteria for Adverse Events, version 3.0) or 2 or less at the outset of bevacizumab plus carboplatin treatment.
Table 1. Patient Characteristicsa.
| Patient | Sex | Age, y | Histology | KPS |
|---|---|---|---|---|
| 1 | M | 31 | AO | 60 |
| 2 | M | 51 | AA | 40 |
| 3 | M | 47 | AO | 80 |
| 4 | F | 35 | AO | 70 |
| 5 | M | 71 | AO | 90 |
| 6 | M | 72 | GBM | 80 |
| 7 | F | 69 | GBM | 55 |
| 8 | M | 63 | GBM | 90 |
| 9 | F | 44 | GBM | 70 |
KPS, Karnofsky Performance score; AO, anaplastic oligodendroglioma; AA, anaplastic astrocytoma; GBM, glioblastoma multiforme.
Treatment Regimen
All 9 patients received bevacizumab (10 mg/kg intravenously) in addition to intravenous carboplatin (AUC 5 IV) every 4 weeks in a single setting and had been previously treated with standard temozolomide and conformal radiation before recurrence. Eight patients received only bevacizumab plus carboplatin at first recurrence. One patient had initially been briefly treated with 2 different intra-arterial adjuvant therapies including melphalan, etoposide, and carboplatin at first recurrence but was quickly switched to bevacizumab plus carboplatin secondary to compliance issues.
Response Measurement and Criteria
The starting time for each patient was the date that bevacizumab plus carboplatin therapy was initiated. All patients underwent contrast-enhanced magnetic resonance imaging (MRI) at least every 4 weeks to determine radiographic response. The patients' neurological examination and steroid use information was obtained from clinical records. A neurosurgeon and radiologist independently evaluated each patient's imaging studies. No disagreements were noted between these 2 reviewers' interpretations of the imaging. Standard Macdonald criteria were used to evaluate contrast-enhanced T1-weighted MRI.15 Macdonald criteria with T1-weighted plus gadolinium (Gd) images were used to determine PFS. Black/white contrast was maximized when evaluating both T1-weighted + Gd and T2-weighted images to most accurately determine the borders of enhancement and hyperintensity, respectively. Although Macdonald criteria do not apply to T2-weighted images, imaging responses were evaluated by Macdonald criteria using both T1-weighted + Gd and T2-weighted images to elucidate agreement between these 2 MRI sequences in the context of antiangiogenic therapy. By Macdonald criteria, complete response is the disappearance of all enhancing tumor on consecutive computed tomography or MRI scans at least 1 month apart, with the patient off steroids and neurologically stable or improved. Partial response is 50% or greater reduction in size of enhancing tumor on consecutive computed tomography or MRI scans at least 1 month apart, steroid use stable or reduced, and the patient neurologically stable or improved. Progressive disease is a 25% or greater increase in size of the enhancing tumor or any new tumor seen on computed tomography or MRI scans, a neurologically worse patient, and steroid use stable or increased. Stable disease is all other situations.
Toxicities
All patients had clinical evaluations at least every 4 weeks in addition to scheduled renal function tests, complete blood counts, and international normalized ratios performed at least every 2 weeks. All grade 3 and 4 toxicities were documented. Patients who experienced grade 3 or 4 toxicities had their chemotherapy regimen held until they no longer met this Common Terminology Criteria for Adverse Events version 3.0 criteria.16
Statistical Considerations
OS and PFS were estimated using Kaplan-Meier product-limit estimation with SAS version 9.1 for Windows (SAS Institute, Cary, North Carolina). Because of the retrospective nature of this study and its small size, hypothesis tests were not performed. Agreement between T1-weighted + Gd and T2 imaging was estimated using kappa statistics,17 which measures agreement beyond chance for categorical variables. Ranges of kappa between 0.8 and 1.0 are almost perfect, 0.6 and 0.8 are substantial, 0.4 and 0.6 moderate, 0.2 and 0.4 fair, and less than 0.2 poor. A value of zero for kappa indicates agreement is no better than chance.18
Results
Patient Characteristics
Nine patients were identified who met inclusion criteria, 5 with grade III gliomas and 4 with grade IV gliomas (Table 1). There were twice as many men as women. All patients were white. The median age and Karnofsky Performance score was 51 years and 70, respectively. The number of days between diagnosis of malignant glioma and the initiation of bevacizumab plus chemotherapy was 261 days (min-max 145-640 days) (Table 2). The minimum interval between the completion of conformal radiotherapy and the onset of bevacizumab plus chemotherapy treatment was 3 months. The median number of previous adjuvant treatment regimens and surgical interventions were 1 (min-max 1-3) and 1 (min-max 1-4), respectively. Median follow-up was 482 days.
Table 2. Patient Treatment and Responsesb.
| Patient | Time from Diagnosis to Bevacizumab Therapy, d | Clinical Response | Best Radio-graphic Response, T1 + Gd | Best Radio-graphic Response, T2 | Grade 3 or Higher Toxicity | Surgical Interventions | Steroids | Bevacizumab Doses | Time to Disease Progression, d | Survival, d |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 219 | Yes | PR | SD | No | 4 | Yes | 2 | 72 | 117 |
| 2 | 189 | Yes | SD | SD | Thrombocytopenia | 1 | Yes | 5 | 94 | 136 |
| 3 | 361 | Ye s | SD | SD | No | 2 | Yes | 18 | 614b | 614b |
| 4 | 145 | Yes | PR | PR | No | 4 | Yes | 10 | 126 | 698b |
| 5 | 199 | Ye s | PR | PR | No | 2 | Yes | 9 | 517b | 517b |
| 6 | 257 | Yes | PR | SD | Hyponatremia | 1 | No | 9 | 154 | 241 |
| 7 | 277 | Yes | PR | PR | Hyponatremia | 1 | Yes | 14 | 277 | 482b |
| 8 | 346 | Yes | SD | SD | No | 1 | No | 38 | 523b | 523b |
| 9 | 631 | No | SD | SD | No | 1 | Yes | 2 | 41 | 153 |
Gd, gadolinium; PR, partial response; SD, stable disease.
Patient alive and has not progressed at follow-up.
Survival
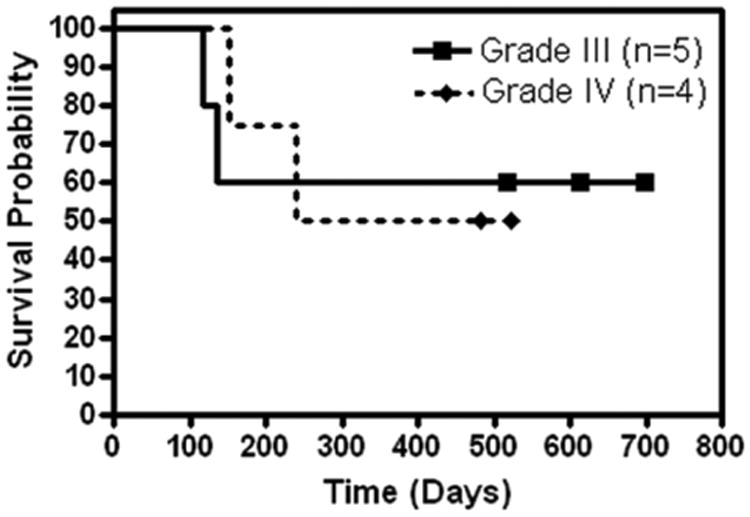
For the 5 patients with grade III gliomas, the median PFS was 126 days (95% confidence interval [CI]: 72 to not attained) with 2 patients progression free at 517 days or longer (Figure 1). The median OS was not attained (95% CI: 117 to not attained) with 3 patients surviving 517 days or longer (Figure 2). Six-month PFS was 40%, whereas 6-month OS was 60%. For the 4 patients with grade IV gliomas, the median PFS was 216 days (95% CI: 41 to not attained) with 1 patient progression free at 523 days (Figure 1). The median OS was not attained (95% CI: 153 to not attained) with 2 patients surviving 482 days or longer (Figure 2). Six-month PFS was 50%, whereas 6-month OS was 75%. For all grade III and IV patients combined, the 6-month PFS was 44.4% and the 6-month OS was 66.7%.
Figure 1. Kaplan-Meier curve of progression-free survival.
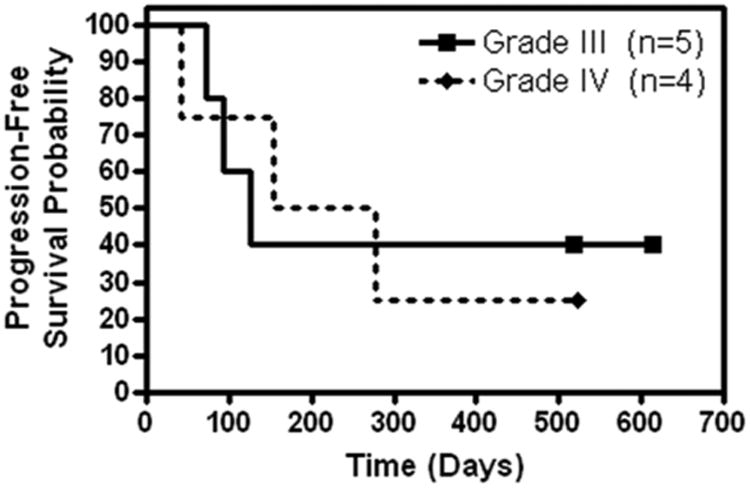
Figure 2. Kaplan-Meier curve of overall survival.
Imaging Response
All results are the patients' best imaging responses (Table 2). No patient had a complete response. Using standard Macdonald criteria with T1-weighted + Gd images, 5 patients (55.6%) had a partial response, whereas the other 4 (44.4%) had stable disease. Using Macdonald criteria with T2-weighted images, 3 patients (33.3%) had a partial response, whereas 6 (66.7%) had stable disease. Comparing best responses with T1-weighted + Gd and T2-weighted images for both groups, kappa is 0.57 (95% CI, 0.1-1.0; moderate agreement). This lack of agreement is attributable to 2 patients whose best response was only stable disease with T2-weighted images but was a partial response with T1-weighted + Gd images (Figure 3); there was agreement on the other 7 assessments.
Figure 3.
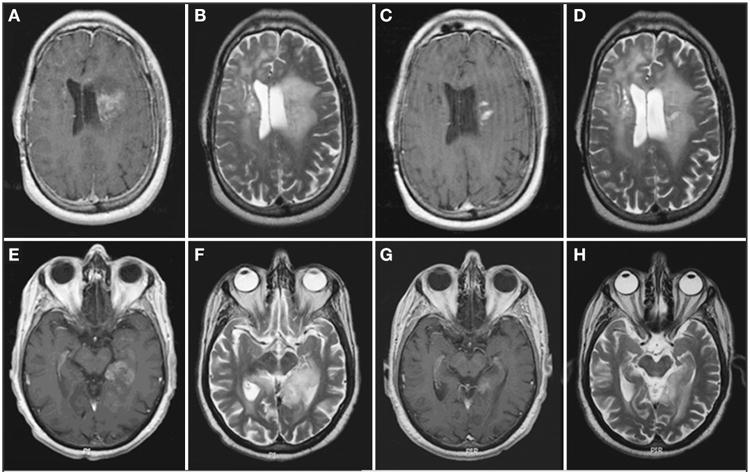
Axial magnetic resonance images of patients 1 (A-D) and 2 (E-H). A, E, pretreatment enhanced T1-weighted image. B and F, pretreatment T2-weighted image. C and G, posttreatment enhanced T1-weighted image. D and H, posttreatment T2-weighted image. After bevacizumab treatment, enhanced T1-weighted imaging demonstrates a more than 50% reduction in enhancing tumor volume, whereas the T2-weighted imaging shows an increased area of high signal in patient 1 and a less than 50% reduction in high signal in patient 2.
Toxicities
Three patients experienced grade 3 and 4 toxicities (Table 2). The grade 3 hyponatremia experienced by 2 patients was transient and did not require intervention. The patient with the grade 4 thrombocytopenia required a platelet transfusion and their bevacizumab and carboplatin dose held for 2 weeks. No patients had fatal toxicities or intracranial hemorrhages from their treatment.
Discussion
The primary goal of this retrospective study was to describe the survival, radiographic responses, and toxicities in patients who received the combination of bevacizumab and carboplatin to treat recurrent malignant glioma. Bevacizumab plus standard chemotherapy first became widely accepted for use in metastatic colon cancer in 200419 and in non-small-cell lung cancer in 200620 after demonstrating significant improvements in OS and PFS. The first published report in the neuro-oncology literature of bevacizumab plus chemotherapy by Vredenburgh et al3 in 2007 showed improved imaging responses and a moderate increase in 6-month PFS (38%) and OS (72%) in patients with recurrent malignant gliomas compared with 21% and 55%, respectively, in patients who received chemotherapy alone as described by Lamborn et al.7 Other phase 2 trials using the combination of bevacizumab and irinotecan or etoposide have supported these results.4,6 Norden et al21 conducted a retrospective review of bevacizumab plus a variety of chemotherapeutic agents that also demonstrated improved 6-month PFS and OS (39% and 65%, respectively) with rates of radiographic responses compared with the results of Lamborn et al.7 The authors used irinotecan, carboplatin, carboplatin plus erlotinib, carmustine, and temozolomide at their institution, but did not delineate between these agents in terms of outcomes and toxicity.
In a recent retrospective review by Quant et al,22 it was noted that carboplatin was the most commonly used second-line agent after progression on irinotecan plus bevacizumab. However, patients receiving bevacizumab plus irinotecan followed by carboplatin at progression had a median PFS and 6-month PFS similar to those who received different chemotherapeutic agents or in a different sequence. Narayana et al13 found a suggestive but not conclusive improvement of 6-month PFS and 6-month OS in patients who received carboplatin compared with irinotecan, but concluded that choice of agent did not affect overall outcome. To our knowledge, our series is the largest published of patients who received carboplatin plus bevacizumab at first progression after standard conformal radiotherapy and temozolomide. In this study, patients with grade III gliomas had a 6-month PFS of 40% and a 6-month OS of 60%. Patients with grade IV gliomas had a 6-month PFS of 50% and a 6-month OS of 75%. These contradictory results are likely secondary to the small numbers in this series and the progression of several patients in the grade III group from low-grade gliomas to grade III gliomas as manifested by the greater number of surgical interventions. Combining all patients in this series with grade III and IV gliomas, the 6-month PFS and OS were 44.4% and 66.7%, respectively. The combined 6-month PFS and OS in our study is similar to previously published retrospective data in a comparable, albeit larger, patient population using bevacizumab plus irinotecan that found a PFS of 46% and OS 84%.23 Because the median OS was not obtained in either the grade III or IV group at a follow-up of more than 482 days, these long-term survival results seem promising. Imaging responses using contrast-enhanced T1-weighted images in this study (55.6%) are similar to bevacizumab plus irinotecan data (63%).3
Carboplatin is an alkylating-like agent that crosslinks DNA and has predominantly renal clearance. Common toxicities related to carboplatin treatment include electrolyte imbalance, myelosuppression, peripheral neuropathy, and ototoxicity.24 In contrast, irinotecan is a topoisomerase I inhibitor and has predominantly liver clearance. Common toxicities related to irinotecan treatment include diarrhea, myelosuppression, decreased liver function, and interstitial lung disease.25 In our series, 3 patients (33.3%) experienced grade 3 and 4 toxicities, slightly higher than in another retrospective cohort who received bevacizumab plus predominantly irinotecan-based treatment (18.5%).22 Both patients who briefly experienced hyponatremia had normal kidney function (serum creatinine ≤1 mg/dL and glomerular filtration rates >60 mL/min/1.73m2). There were no intracranial hemorrhages, neurological toxicities, or deaths directly attributable to the treatment regimen. Although audiological testing was not completed, no patients in our series reported hearing loss nor was decreased hearing found on physical examination. No patients had grade 3 or 4 gastrointestinal toxicities such as profound diarrhea, which is sometimes seen with irinotecan use.5 Because carboplatin is not cleared via the liver, no dosing adjustment was necessary for patients taking enzyme-inducing antiepileptic drugs. In patients with recurrent malignant gliomas in which other treatment options have been exhausted, we find this rate of toxicity acceptable.
The secondary objective of this review was to determine the agreement between T1-weighted + Gd and T2-weighted images to evaluate radiographic response to treatment. Currently, nearly all studies analyzing the imaging response rate of regimens containing bevacizumab use Macdonald criteria or variations of the Macdonald criteria, a set of relatively arbitrary criteria used before the widespread use of antiangiogenic agents. Other groups have used novel criteria for progression to include T2-weighted or fluid-attenuated inversion recovery in combination with clinical symptoms to define progression.22 The most frequently used MRI sequences for interval changes are T1-weighted + Gd images to assess blood-brain barrier breakdown and T2-weighted or fluid-attenuated inversion recovery sequences to detect tumor-related edema and mass effect. As we demonstrated, the agreement between T1-weighted + Gd images and T2-weighted images was somewhat dubious at “moderate.” This moderate agreement suggests that enhancing tumor volume is not necessarily predictive of actual infiltrating tumor volume in the context of antiangiogenic agents such as bevacizumab. This potential disconnect in T1-weighted + Gd and T2-weighted lesion volumes was also noted by Norden et al,21 who hypothesized that bevacizumab inhibits enhancing tumor recurrence but decreases inhibition of infiltrating tumor recurrence denoted by T2.
Limited data exist on the radiological response of recurrent malignant gliomas to antiangiogenic agents such as bevacizumab, imatinib, and AZD2171. Recently, a phase 2 study that evaluated the therapeutic effect of imatinib in patients with recurrent gliomas of various histologies revealed a decrease in Gd enhancement on T1-weighted images, although the patients were clinically deteriorating.27 However, careful examination of T2-weighted images showed either unchanged or increased tumor volume. The authors hypothesized that the decreased contrast enhancement might be attributable to decreased permeability of abnormal tumor vessels or to changes in regional cerebral blood volume without a real antitumor effect. A similar finding was reported by Batchelor et al28 and Vredenburgh et al3 regarding agents that interfere with VEGF signaling pathways (AZD2171 and bevacizumab). Our results are consistent with these findings and may suggest that conventional anatomic MRI is not the most appropriate method to evaluate tumor response in patients receiving antiangiogenic agents alone or in combination with chemotherapeutics. Dynamic susceptibility contrast MRI using novel contrast agents such as ferumoxytol to assess the relative cerebral blood volume of the tumor before and after bevacizumab is a promising alternative.29 New criteria for determining the radiological response of malignant gliomas to bevacizumab plus chemotherapy is needed and is currently being addressed by several international working parties.26
Eight of 9 patients received bevacizumab plus carboplatin at first recurrence, making this patient group relatively homogeneous. However, as does any retrospective review, this study has limitations. Most importantly, the total number of patients is small and conclusions are thus limited. Because 8 of 9 patients in this group were treated at first recurrence after standard temozolomide and conformal radiotherapy, it is possible that imaging responses were falsely favorable as a result of recovery from pseudoprogression instead of response to treatment.30,31 The toxicity results may be falsely elevated because several patients who experienced grade 3 and 4 toxicities in this study had previously experienced grade 1 and 2 toxicities while taking temozolomide and thus were likely more susceptible to the toxic effects of bevacizumab and carboplatin.
Varallyay et al32 recently showed bevacizumab induced larger and more rapid vascular responses on dynamic MRI compared with high-dose steroids in a malignant glioma rat model. Because the molecular weight of most chemotherapeutic agents is similar to that of Gd, it is certainly conceivable that the reduced enhancement seen with bevacizumab treatment is indicative of reduced chemotherapeutic delivery to the tumor. Perhaps we need to rethink the timing of chemotherapy in combination with bevacizumab, possibly administering chemotherapy before bevacizumab instead of concurrently during each cycle. Bevacizumab plus intravenous carboplatin is the treatment of choice at OHSU for progressive malignant gliomas secondary to its safety and efficacy. However, we have recently started staggering the administration of each medication in 2-week intervals to minimize the potential of decreased carboplatin delivery secondary to the potentially blood-brain barrier–stabilizing characteristics of bevacizumab. A larger phase 2 clinical trial comparing carboplatin plus bevacizumab vs bevacizumab alone vs temozolomide or irinotecan plus bevacizumab for recurrent malignant glioma using more sophisticated imaging techniques is needed to validate the intriguing results of this retrospective review.
Conclusion
This is a relatively small, nonrandomized case series of patients who received bevacizumab plus carboplatin for recurrent World Health Organization grade III and IV gliomas. To our knowledge, this series of 8 of 9 patients is the largest to receive this regimen at first progression. This combination has similar 6-month OS, PFS, and imaging responses as those of a comparable bevacizumab plus irinotecan retrospective series for recurrent malignant glioma.23 However, median OS is promising with well over 1 year follow-up. In this patient population, agreement between postcontrast T1-weighted and T2 images was only moderate. Thus, in the context of antiangiogenic therapy, caution should be applied when using Macdonald criteria as the sole method for determining recurrence. A larger phase 2 clinical trial comparing carboplatin plus bevacizumab vs bevacizumab alone vs temozolomide or irinotecan plus bevacizumab for recurrent malignant glioma using more sophisticated imaging techniques is needed to validate the intriguing results of this retrospective review.
Acknowledgments
Supported by National Institutes of Health grants NS33618, NS34608, and NS44687 from the National Institute of Neurological Disorders and Stroke (E.A.N.). This work was supported in part by the Department of Veterans Affairs (E.A.N.).
Abbreviations
- CI
confidence interval
- Gd
gadolinium
- OHSU
Oregon Health & Science University
- OS
overall survival
- PFS
progression-free survival
Footnotes
Disclosure: The authors have no personal financial or institutional interest in any of the drugs or materials described in this article.
Contributor Information
Eric M. Thompson, Department of Neurological Surgery, Oregon Health & Science University, Portland, Oregon.
Edit Dosa, Department of Neurology, Oregon Health & Science University, Portland, Oregon.
Dale F. Kraemer, Department of Pharmacy Practice, Oregon Health & Science University College of Pharmacy, Oregon State University, Department of Medical Informatics, Clinical Epidemiology, and Health and Preventive Medicine, Oregon Health & Science University, Portland, Oregon.
Edward A. Neuwelt, Departments of Neurological Surgery and Neurology, Oregon Health & Science University, Portland Veterans Affairs Medical Center Portland, Oregon.
References
- 1.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17(8):2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 2.Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66(8):1258–1260. doi: 10.1212/01.wnl.0000208958.29600.87. [DOI] [PubMed] [Google Scholar]
- 3.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 4.Bokstein F, Shpigel S, Blumenthal DT. Treatment with bevacizumab and irinotecan for recurrent high-grade glial tumors. Cancer. 2008;112(10):2267–2273. doi: 10.1002/cncr.23401. [DOI] [PubMed] [Google Scholar]
- 5.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 6.Rich JN, Desjardins A, Sathornsumetee S, et al. Phase II study of bevzcizumab and etoposide in patients with recurrent malignant glioma. J Clin Oncol. 2008;26(15S):94S. [Google Scholar]
- 7.Lamborn KR, Yung WK, Chang SM, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10(2):162–170. doi: 10.1215/15228517-2007-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Fei D, Vanderlaan M, Song A. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis. 2004;7(4):335–345. doi: 10.1007/s10456-004-8272-2. [DOI] [PubMed] [Google Scholar]
- 10.Huang H, Held-Feindt J, Buhl R, Mehdorn HM, Mentlein R. Expression of VEGF and its receptors in different brain tumors. Neurol Res. 2005;27(4):371–377. doi: 10.1179/016164105X39833. [DOI] [PubMed] [Google Scholar]
- 11.Warnick RE, Prados MD, Mack EE, et al. A phase II study of intravenous carboplatin for the treatment of recurrent gliomas. J Neurooncol. 1994;19(1):69–74. doi: 10.1007/BF01051050. [DOI] [PubMed] [Google Scholar]
- 12.Yung WK, Mechtler L, Gleason MJ. Intravenous carboplatin for recurrent malignant glioma: a phase II study. J Clin Oncol. 1991;9(5):860–864. doi: 10.1200/JCO.1991.9.5.860. [DOI] [PubMed] [Google Scholar]
- 13.Narayana A, Kelly P, Golfinos J, et al. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survival. J Neurosurg. 2009;110(1):173–180. doi: 10.3171/2008.4.17492. [DOI] [PubMed] [Google Scholar]
- 14.Jahnke K, Muldoon LL, Varallyay CG, Lewin SJ, Kraemer DF, Neuwelt EA. Bevacizumab and carboplatin increase survival and asymptomatic tumor volume in a glioma model. Neuro Oncol. 2009;11(2):142–150. doi: 10.1215/15228517-2008-077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 16.CTEP. Common Terminology Criteria for Adverse Events v3.0 (CTCAE) 2006 [Google Scholar]
- 17.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20(1):37–46. [Google Scholar]
- 18.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37(5):360–363. [PubMed] [Google Scholar]
- 19.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 20.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 21.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70(10):779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 22.Quant EC, Norden AD, Drappatz J, et al. Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol. 2009;11(5):550–555. doi: 10.1215/15228517-2009-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang TY, Jin T, Elinzano H, Peereboom D. Irinotecan and bevacizumab in progressive primary brain tumors, an evaluation of efficacy and safety. J Neurooncol. 2008;89(1):113–118. doi: 10.1007/s11060-008-9599-0. [DOI] [PubMed] [Google Scholar]
- 24.Micromedix. [Accessed August 21, 2009];Carboplatin. http://www.thomsonhc.com/hcs/librarian/ND_T/HCS/ND_PR/Main/CS/619264/DUPLICATIONSHIELDSYNC/531B40/ND_PG/PRIH/ND_B/HCS/SBK/1/ND_P/Main/PFActionId/hcs.common.RetrieveDocumentCommon/DocId/107100/ContentSetId/100/SearchTerm/carboplatin/SearchOption/BeginWith.
- 25.Micromedix. [Accessed August 21, 2009];Irinotecan. http://www.thomsonhc.com/hcs/librarian/ND_T/HCS/ND_PR/Main/CS/619264/DUPLICATIONSHIELDSYNC/531B40/ND_PG/PRIH/ND_B/HCS/SBK/3/ND_P/Main/PFActionId/hcs.common.RetrieveDocumentCommon/DocId/923686/ContentSetId/100/SearchTerm/irinotecan/SearchOption/BeginWith.
- 26.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 27.Raymond E, Brandes AA, Dittrich C, et al. Phase II study of imatinib in patients with recurrent gliomas of various histologies: a European Organisation for Research and Treatment of Cancer Brain Tumor Group Study. J Clin Oncol. 2008;26(28):4659–4665. doi: 10.1200/JCO.2008.16.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinstein JS, Varallyay CG, Dosa E, et al. Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J Cereb Blood Flow Metab. 2010;30:15–35. doi: 10.1038/jcbfm.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 31.Gahramanov S, Raslan A, Muldoon LL, et al. Potential for differentiation of pseudo-progression from true tumor progression with dynamic susceptibility-weighted contrast-enhanced magnetic resonance imaging using ferumoxytol versus gadoteridol: A pilot study. Int J Radiat Oncol Biol Phys. 2010 Apr 13; doi: 10.1016/j.ijrobp.2009.10.072. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varallyay CG, Muldoon LL, Gahramanov S, et al. Dynamic MRI using iron oxide nanoparticles to assess early vascular effects of antiangiogenic versus corticosteroid treatment in a glioma model. J Cereb Blood Flow Metab. 2009;29(4):853–860. doi: 10.1038/jcbfm.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]


