Abstract
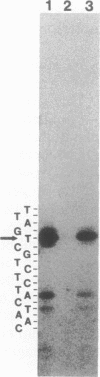
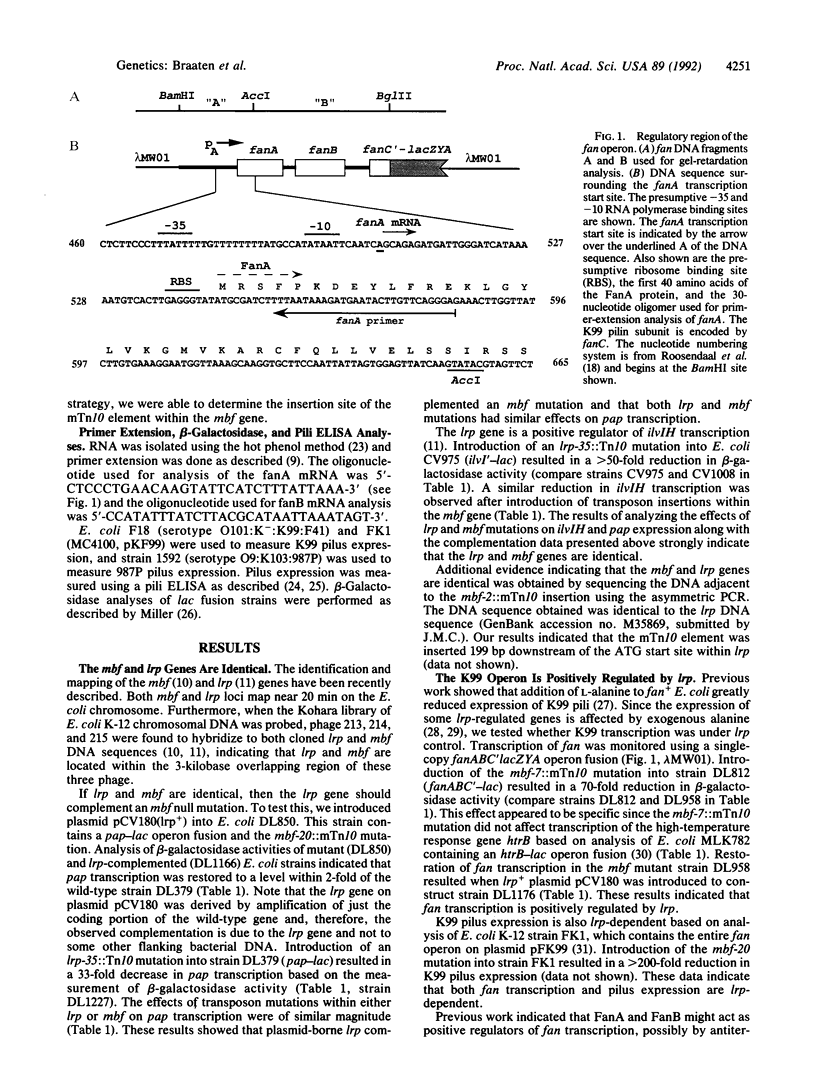
The methylation blocking factor gene (mbf) in Escherichia coli is required for specific methylation inhibition of two DNA GATC sites upstream of the papBA pilin promoter and transcriptional activation of pap. Complementation and mutational analysis using pap-lac and ilvIH-lac operon fusions indicates that the mbf gene is identical to a recently described global regulatory gene lrp (leucine-responsive regulatory protein) that acts as a positive regulator of some genes and a negative regulator of others in E. coli. DNA sequence analysis of an mbf::mTn10 insertion showed that the mbfDNA sequence was identical to lrp. Thus Lrp inhibits DNA methylation at specific GATC sites. We also show that Lrp positively regulates transcription of the fan operon, which encodes K99 pili of diarrheagenic E. coli. Purified Lrp was found to bind to DNA fragments encompassing the pap and fan promoters, which is consistent with previous results indicating that Lrp controls gene expression by binding to regulatory DNA sites. Exogenous leucine significantly reduced fan transcription and K99 pili expression, similar to results obtained with the ilvIH operon. However, pap gene expression was unresponsive to leucine, which distinguishes pap from other lrp-regulated genes whose expression is modulated by leucine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews J. C., Short S. A. opp-lac Operon fusions and transcriptional regulation of the Escherichia coli trp-linked oligopeptide permease. J Bacteriol. 1986 Feb;165(2):434–442. doi: 10.1128/jb.165.2.434-442.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyn L. B., Braaten B. A., Low D. A. Regulation of pap pilin phase variation by a mechanism involving differential dam methylation states. EMBO J. 1990 Dec;9(12):4045–4054. doi: 10.1002/j.1460-2075.1990.tb07626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyn L. B., Braaten B. A., White-Ziegler C. A., Rolfson D. H., Low D. A. Phase-variation of pyelonephritis-associated pili in Escherichia coli: evidence for transcriptional regulation. EMBO J. 1989 Feb;8(2):613–620. doi: 10.1002/j.1460-2075.1989.tb03416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braaten B. A., Blyn L. B., Skinner B. S., Low D. A. Evidence for a methylation-blocking factor (mbf) locus involved in pap pilus expression and phase variation in Escherichia coli. J Bacteriol. 1991 Mar;173(5):1789–1800. doi: 10.1128/jb.173.5.1789-1800.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Båga M., Göransson M., Normark S., Uhlin B. E. Transcriptional activation of a pap pilus virulence operon from uropathogenic Escherichia coli. EMBO J. 1985 Dec 30;4(13B):3887–3893. doi: 10.1002/j.1460-2075.1985.tb04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Båga M., Norgren M., Normark S. Biogenesis of E. coli Pap pili: papH, a minor pilin subunit involved in cell anchoring and length modulation. Cell. 1987 Apr 24;49(2):241–251. doi: 10.1016/0092-8674(87)90565-4. [DOI] [PubMed] [Google Scholar]
- Clark R. L., Neidhardt F. C. Roles of the two lysyl-tRNA synthetases of Escherichia coli: analysis of nucleotide sequences and mutant behavior. J Bacteriol. 1990 Jun;172(6):3237–3243. doi: 10.1128/jb.172.6.3237-3243.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinée P. A., Jansen W. H., Agterberg C. M. Detection of the K99 antigen by means of agglutination and immunoelectrophoresis in Escherichia coli isolates from calves and its correlation with entertoxigenicity. Infect Immun. 1976 May;13(5):1369–1377. doi: 10.1128/iai.13.5.1369-1377.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Hull R., Hull S., Falkow S., Freter R., Svanborg Edén C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983 Apr;40(1):265–272. doi: 10.1128/iai.40.1.265-272.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney S. A., Platko J. V., Oxender D. L., Calvo J. M. Lrp, a leucine-responsive protein, regulates branched-chain amino acid transport genes in Escherichia coli. J Bacteriol. 1992 Jan;174(1):108–115. doi: 10.1128/jb.174.1.108-115.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull S., Clegg S., Sanborg Eden C., Hull R. Multiple forms of genes in pyelonephritogenic Escherichia coli encoding adhesins binding globoseries glycolipid receptors. Infect Immun. 1985 Jan;47(1):80–83. doi: 10.1128/iai.47.1.80-83.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karow M., Fayet O., Cegielska A., Ziegelhoffer T., Georgopoulos C. Isolation and characterization of the Escherichia coli htrB gene, whose product is essential for bacterial viability above 33 degrees C in rich media. J Bacteriol. 1991 Jan;173(2):741–750. doi: 10.1128/jb.173.2.741-750.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P., Orskov I., Orskov F. F7 and type 1-like fimbriae from three Escherichia coli strains isolated from urinary tract infections: protein chemical and immunological aspects. Infect Immun. 1982 May;36(2):462–468. doi: 10.1128/iai.36.2.462-468.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler H., Svanborg-Edén C. Glycolipid receptors for uropathogenic Escherichia coli on human erythrocytes and uroepithelial cells. Infect Immun. 1981 Dec;34(3):920–929. doi: 10.1128/iai.34.3.920-929.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low D., Robinson E. N., Jr, McGee Z. A., Falkow S. The frequency of expression of pyelonephritis-associated pili is under regulatory control. Mol Microbiol. 1987 Nov;1(3):335–346. doi: 10.1111/j.1365-2958.1987.tb01940.x. [DOI] [PubMed] [Google Scholar]
- Mooi F. R., de Graaf F. K., van Embden J. D. Cloning, mapping and expression of the genetic determinant that encodes for the K88ab antigen. Nucleic Acids Res. 1979 Mar;6(3):849–865. doi: 10.1093/nar/6.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W. Colonization factor antigens of enterotoxigenic Escherichia coli in animals. Curr Top Microbiol Immunol. 1990;151:147–165. doi: 10.1007/978-3-642-74703-8_8. [DOI] [PubMed] [Google Scholar]
- Norgren M., Normark S., Lark D., O'Hanley P., Schoolnik G., Falkow S., Svanborg-Edén C., Båga M., Uhlin B. E. Mutations in E coli cistrons affecting adhesion to human cells do not abolish Pap pili fiber formation. EMBO J. 1984 May;3(5):1159–1165. doi: 10.1002/j.1460-2075.1984.tb01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Lark D., Hull R., Norgren M., Båga M., O'Hanley P., Schoolnik G., Falkow S. Genetics of digalactoside-binding adhesin from a uropathogenic Escherichia coli strain. Infect Immun. 1983 Sep;41(3):942–949. doi: 10.1128/iai.41.3.942-949.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanley P., Low D., Romero I., Lark D., Vosti K., Falkow S., Schoolnik G. Gal-Gal binding and hemolysin phenotypes and genotypes associated with uropathogenic Escherichia coli. N Engl J Med. 1985 Aug 15;313(7):414–420. doi: 10.1056/NEJM198508153130704. [DOI] [PubMed] [Google Scholar]
- Platko J. V., Willins D. A., Calvo J. M. The ilvIH operon of Escherichia coli is positively regulated. J Bacteriol. 1990 Aug;172(8):4563–4570. doi: 10.1128/jb.172.8.4563-4570.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex J. H., Aronson B. D., Somerville R. L. The tdh and serA operons of Escherichia coli: mutational analysis of the regulatory elements of leucine-responsive genes. J Bacteriol. 1991 Oct;173(19):5944–5953. doi: 10.1128/jb.173.19.5944-5953.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen M., Väisänen-Rhen V., Pere A., Korhonen T. K. Complementation and regulatory interaction between two cloned fimbrial gene clusters of Escherichia coli strain KS71. Mol Gen Genet. 1985;200(1):60–64. doi: 10.1007/BF00383312. [DOI] [PubMed] [Google Scholar]
- Ricca E., Aker D. A., Calvo J. M. A protein that binds to the regulatory region of the Escherichia coli ilvIH operon. J Bacteriol. 1989 Mar;171(3):1658–1664. doi: 10.1128/jb.171.3.1658-1664.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. A., Suarez G. M., Kaack B., Kallenius G., Svenson S. B. Experimental pyelonephritis in the monkey. VII. Ascending pyelonephritis in the absence of vesicoureteral reflux. J Urol. 1985 Jun;133(6):1068–1075. doi: 10.1016/s0022-5347(17)49382-7. [DOI] [PubMed] [Google Scholar]
- Roosendaal B., Damoiseaux J., Jordi W., de Graaf F. K. Transcriptional organization of the DNA region controlling expression of the K99 gene cluster. Mol Gen Genet. 1989 Jan;215(2):250–256. doi: 10.1007/BF00339725. [DOI] [PubMed] [Google Scholar]
- Roosendaal E., Boots M., de Graaf F. K. Two novel genes, fanA and fanB, involved in the biogenesis of K99 fimbriae. Nucleic Acids Res. 1987 Aug 11;15(15):5973–5984. doi: 10.1093/nar/15.15.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels P. L., Moseley S. L., Moon H. W. F41 pili as protective antigens of enterotoxigenic Escherichia coli that produce F41, K99, or both pilus antigens. Infect Immun. 1987 Mar;55(3):555–558. doi: 10.1128/iai.55.3.555-558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Houman F., Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53(1):85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Tuan L. R., D'Ari R., Newman E. B. The leucine regulon of Escherichia coli K-12: a mutation in rblA alters expression of L-leucine-dependent metabolic operons. J Bacteriol. 1990 Aug;172(8):4529–4535. doi: 10.1128/jb.172.8.4529-4535.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willins D. A., Ryan C. W., Platko J. V., Calvo J. M. Characterization of Lrp, and Escherichia coli regulatory protein that mediates a global response to leucine. J Biol Chem. 1991 Jun 15;266(17):10768–10774. [PubMed] [Google Scholar]
- de Graaf F. K., Klaasen-Boor P., van Hees J. E. Biosynthesis of the K99 surface antigen is repressed by alanine. Infect Immun. 1980 Oct;30(1):125–128. doi: 10.1128/iai.30.1.125-128.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf F. K., Krenn B. E., Klaasen P. Organization and expression of genes involved in the biosynthesis of K99 fimbriae. Infect Immun. 1984 Feb;43(2):508–514. doi: 10.1128/iai.43.2.508-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gabain A., Belasco J. G., Schottel J. L., Chang A. C., Cohen S. N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci U S A. 1983 Feb;80(3):653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]