Abstract
Locomotive syndrome is a condition of reduced mobility due to impairment of locomotive organs. Since upright bipedal walking involves minutely controlled movement patterns, impairment of any aspect of the locomotive organs has the potential to adversely affect it. In addition to trauma, chronic diseases of the locomotive organs, which progress with repeated bouts of acute exacerbations, are common causes of the locomotive syndrome. In Japan’s super-aging society, many people are likely to experience locomotive syndrome in the later part of their lives. Exercise intervention is effective in improving motor function, but because the subjects are elderly people with significant degenerative diseases of the locomotor organs, caution should be taken in choosing the type and intensity of exercise. The present review discusses the definition, current burden, diagnosis and interventions pertaining to the locomotive syndrome. The concept and measures are spreading throughout Japan as one of the national health policy targets.
Keywords: Locomotive syndrome, Elderly, Exercise intervention, Gait, Balance
Introduction
The average Japanese life expectancy in the year 2014 was 80.5 years for men and 86.8 years for women, higher than in the previous year. The number of Japanese people of age 65 or more in 2014 was 33 million (26.7 % of the entire population), which is the highest ever reported anywhere in the world. It is estimated that this number will reach 36.57 million (30.3 %) in 2025 [1].
This prolonged life expectancy has affected many aspects of activities of daily living among the elderly, one among which is the difficulty in locomotion. This is illustrated by a study in Kagoshima that demonstrated that issues including fear of falling (81.7 %), not being able to stand without arm support (81.1 %), not being able to ascend stairs without using rail or wall for support (81.3 %), slow gait speed (71.7 %) and refraining from going out (50 %) were common among people aged 70–74 years [2].
The locomotive system is directly responsible for mobility. The clinical practice pertaining to the locomotive systems has changed over the last 40 years owing to the higher prevalence of chronic diseases of the locomotive organs among middle-aged to elderly people [3] and markedly increased requirement for surgery for chronic diseases, in individuals over 50 years [4].
There are four key issues in clinical practice for locomotive organs, common to the geriatric population. First, acute exacerbation of diseases of the locomotive organs is often accompanied by pain, with pain in the lower extremities and back being major causes of mobility disturbance [5–9]. Second, in the presence of severe osteoporosis, procedures utilizing metal screws may not provide adequate stability and may result in specific complications [10, 11]. Third, treatment outcomes for the locomotive organ diseases in this group of patients are significantly influenced by the status of their preoperative mobility. For example, postoperative mobility following surgical operation for proximal femoral fracture is largely influenced by the patient’s preoperative mobility [12, 13], and the results of total knee arthroplasty for osteoarthritis of the knee depend on the preoperative strength of quadriceps [14, 15]. Fourth, there is an increase in the number of people whose return to their homes is delayed following orthopedic operations. This is mainly because elderly patients need a longer period of postoperative physical training to restore their mobility. Furthermore, patients who require preoperative bed rest have dramatically reduced mobility [16–18]. Difficulty in independent mobility is a risk factor for delay in discharge from the hospital [19], and motor impairments contribute to 35.1 % of cases where discharge planning is complicated. This number is much larger compared to malignant disease (16.2 %), which is the second most common cause for complicated hospital discharge [20]. These issues were not common 40 years ago.
As a part of the evolutionary process, the adaptation of vertebrates to their environment involved a change in their skeletal structure. Bipedal locomotion is a feature unique to humans [21]. Human locomotive organs have a lifespan of about 50 years, suggesting a need for additional efforts to sustain their function when used for a longer term of 80–90 years. There is evidence supporting the view that age-related movement deficits as in sit-to-stand and gait can be improved by appropriate intervention [22–31].
The Japanese Orthopaedic Association (JOA) proposed the term locomotive syndrome (locomo) in 2007, mainly to increase awareness in the society regarding this condition and its management strategies [32]. It is important that the means and purpose of management of locomotive syndrome are understood and accepted by the general population [33].
Definition and Concept of Locomotive Syndrome
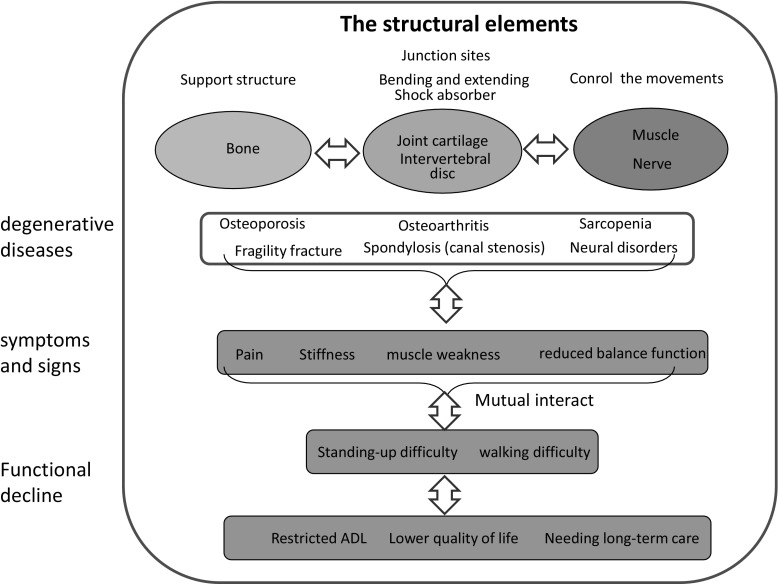
Locomotive syndrome (locomo) is a condition wherein mobility functions such as sit-to-stand or gait are declined due to locomotive organ impairment [34]. Progression of this syndrome results in limiting independence in carrying out activities of daily living (ADL) [35]. In super-aged societies, most people experience the locomotive syndrome toward the end of their lives. Therefore, intervention is required to limit this syndrome and sustain locomotive organ function. The three main components comprising the locomotive system are bones (support), joints and intervertebral disks (mobility, impact absorption) and the muscular and nervous system (drive, control) [36, 37]. Any impairment in these organs results in pain, limited range of motion at joints or at the spine, muscle weakness and balance deficits. All these impairments are inter-related and serve as multiple risk factors for disability. Progression of these impairments eventually result in limitations in ADL, reduction of quality of life (QOL) and necessity of care support [38, 39] (see conceptual scheme in Fig. 1).
Fig. 1.
The conceptual structure of locomotive syndrome
Common Locomotive Organ Diseases
A cross-sectional study was conducted by the JOA; among new outpatients (84,544 cases) in an orthopedic clinic [40], 59.8 % had non-traumatic etiology. Among them, chronic diseases, disk degeneration (lumbar spondylosis, 11.4 %; cervical spondylosis, 4.7 %; lumbar disk hernia, 3.8 %; cervical disk hernia, 1.0 %) and lower extremity cartilage degeneration [knee osteoarthritis (OA), 6.9 %; hip OA, 1.5 %] were the most common. Among the traumatic causes, fractures of the proximal femur, which too were related to osteoporosis, were the most common (1.1 %).
The Features of Locomotive Organ Diseases
High Prevalence Rate
Most of the conditions contributing to the locomotive syndrome have high prevalence rates. The prevalence rates of the different conditions are as follows: Lumbar spondylosis (Kellgren–Lawrence ≥ 2) in patients above 40 years was 81.5 (males) and 65.5 % (females); knee osteoarthritis (Kellgren–Lawrence ≥ 2), 42.6 and 62.4 %; and osteoporosis [defined as femoral neck bone mineral density below the 70th percentile of young adults on dual-energy X-ray absorptiometry scan (DXA)], 12.4 and 26.5 %, in males and females, respectively [3]. The prevalence of sarcopenia was also high with rates of 13.8 % in males and 12.4 % in females [41].
Symptoms Manifest in Subjects Over the Age of 50 Years
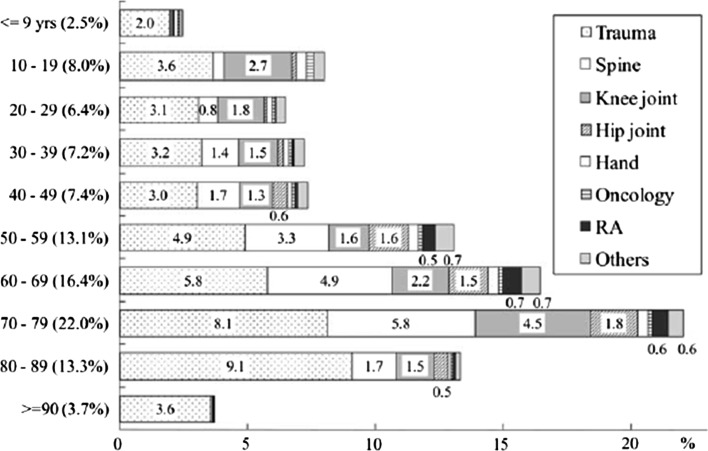
In general, although locomotive degenerative diseases present with acute exacerbations, its progression in the initial stages is largely asymptomatic. The symptoms become apparent once pathological changes of degeneration become advanced, and further interventions are necessitated. The number of orthopedic surgical treatments requiring hospitalization dramatically increases after the age of 50 years (Fig. 2). The most frequent reasons for operative interventions in chronic diseases (49.7 %) were degenerated intervertebral disk (16.6 %; spondylosis, spinal canal stenosis, disk hernia), knee OA (7.1 %) and hip OA (5.4 %). Trauma accounted for the remaining 46.3 % of all cases requiring operations, most commonly for hip fractures (18.4 %) [4].
Fig. 2.
Age distribution of orthopedic surgeries [4]
Risk of Impairments is Different for Bone/Muscle and Joint/Intervertebral Disk
The risk of impairment varies between the different tissues that are affected. Insufficient loads and extreme thinness are the risk factors for osteoporosis [42–44] and sarcopenia [45–47] affecting bones and muscles, respectively. On the other hand, excessive loading and obesity are the risk factors for deformation and impairment of joints and intervertebral disks [36, 48–50]. The load on joints tends to be concentrated on the articular cartilage and intervertebral disks since these are mobile structures that are designed to absorb impacts. Moreover, these tissues lack direct blood supply and, thus, have minimal potential to regenerate [51–53]. Therefore, joints and intervertebral disks commonly wear out over time with aging and become painful by the middle or elderly years, when they require exercise interventions [3].
Assessments
Degenerative changes in the locomotive components (bone, joint, muscle and nerve) result in decline in mobility. Although many tools have been developed to assess mobility, each method of assessment is designed for specific purposes. This variation in the purpose of the assessment makes it difficult to select an optimal tool [54]. Therefore, adequate care should be employed in the choice of an appropriate assessment tool with reference to why, where and how it is to be used [55, 56].
Early detection of symptoms and examination findings are important for early intervention and prevention of progression of the chronic diseases. Disability is defined as experienced difficulty in performing activities [57], and therefore, activities of daily living (ADL) and instrumental activities of daily living (IADL) are often used as assessment tools [23, 58].
Tobimatsu [59] used the 25-question Geriatric Locomotive Function Scale (GLFS-25) [60] as an assessment scale for difficulty and disability in daily activities related to locomotive organs and investigated the order of questionnaire items. This was done by stratifying the frequency of the people who had difficulties in accomplishing the task in each item. The results of this study suggested that people developed difficulties in IADL items earlier than in ADL items. Moreover, mild difficulties in going up- and downstairs, walking briskly and long-distance walking (more than 2–3 km), along with body pain (upper/lower extremities, back or neck), were experienced before the deficits in IADL or social functions were noted. In addition, most subjects expressed anxiety about being unable to walk in the future. These results are consistent with other previous studies that reported earlier onset of deficits with IADL items than with ADL items [61, 62]. This data highlight the importance of detecting minor changes in difficulty for IADL items [63, 64]. It is also important to recognize that restrictions and decline in life-space mobility may be early signs of increasing vulnerability to disability [65–69].
In Japan, the long-term care insurance system was started in 2000 to provide daily supports for elderly people. The reduction in the number of people requiring this service is one of the targets of the national health policy. Physical dysfunction in daily living (WOMAC function score, men ≥ 5, women ≥ 4), an ADL-related factor, was identified as a risk factor for certified need of care within a 4-year interval in community residents aged over 65 years [70]. Grip strength, knee extension torque, usual gait speed, chair stand time and muscle dysfunction (defined by the European Working Group on Sarcopenia in Older People algorithm for screening sarcopenia) were identified as factors determining physical function [71]. The results of these studies indicate the importance of sit-to-stand and gait function assessment.
To enable widespread acceptance among all subjects at risk, which comprises a large number, the assessment methods should be accessible to the population [33], feasible as self-tests [72, 73] and subject to easy and unambiguous interpretation, in addition to guiding disease management. Therefore, the JOA introduced a battery of short tests for recognizing patients with locomotive syndrome. These include “stand-up test,” “two-step test” and “25-question Geriatric Locomotive Function Scale (25-question GLFS)” [74].
Short Test Battery for Locomotive Syndrome [74]
Stand-Up Test (Fig. 3)
Fig. 3.
Stand-up test [74]
The knee extensor strength of the quadriceps femoris muscle is widely used as an assessment of lower extremity muscle strength. Weight-bearing index (WBI), as an indicator of lower extremities strength, is calculated by normalizing the knee extensor strength by the body weight [75, 76]. WBI of ≥0.4 is required for normal gait, and ≥0.6 is required for independent ADL and for performing exercises such as jogging. Muranaga [77] demonstrated that the ability to stand up from a 40-cm-high stool with single-leg stance and a 20-cm-high stool with a double-leg stance could be used as screening methods to confirm WBI of ≥0.6 and ≥0.4, respectively.
In the screening test, the ability to stand with a single- or double-leg stance from stools of heights, 40, 30, 20 and 10 cm, is evaluated. The grading of difficulty, from easy to difficulty, is in the order of double-leg stance with 40, 30, 20 and 10 cm stools, followed by single-leg stance with 40, 30, 20 and 10 cm. The test result is expressed as the minimum height of the stool that the subject was able to stand up from. The stand-up movement requires adequate range of motion at the joint, flexibility and balance, in addition to lower extremity muscle strength.
Two-Step Test (Fig. 4)
Fig. 4.
Two-step test [74]
For assessment of gait-related parameters, gait speed [78–80] and maximal step length (MSL, the ability to maximally step out and return to the initial position [81]) are used. MSL is recognized as a useful tool for evaluation of balance and can be performed within a small space [73, 82, 83]. The two-step test score is calculated by normalizing the maximal length of two steps taken by the subject, by the subject’s height. This test was developed by Muranaga for assessment of gait function [84]. This test has the ability to detect bilateral impairment, and the movement pattern assessed is similar to the actual gait of the subject [72]. The test results are easy to interpret and positively correlate with maximal gait speed [85].
25-Question GLFS
The importance of self-rated evaluation for physical function and health status is well known [26, 57, 86]. Seichi et al. [60] developed the 25-question GLFS as an assessment tool for early detection of locomotive syndrome. The scale is a self-reported comprehensive measure, consisting of 25 questions referring to the preceding month. The scale includes four questions regarding pain, 16 questions regarding activities of daily living, three questions regarding social functions, and two questions regarding mental health status. Each item is graded on a five-point scale, from no impairment (0) to severe impairment (4 points), and the total score is derived by the sum of all scores (minimum = 0, maximum = 100). The total score is assumed to represent a quantitative evaluation of the difficulties and disabilities in daily life activity related to locomotive organs. The age-specific mean values are 5.8 in the 40s, 6.0 in the 50s, 5.9 in the 60s and 8.8 in the 70s [87]. People with a score ≥16 are expected to have limitations in walking and going out [60].
Clinical Decision Limits for Assessing the Risk of Locomotive Syndrome [34, 88]
The JOA proposed clinical decision limits of these three tests as a guide to assessing the risk of locomotive syndrome. In their proposal, clinical decision limits were established in two stages.
Stage 1
The following criteria indicate a beginning of the decline of mobility function, and the subject is categorized as Stage 1 if any of the three conditions are met.
Stand-up test, difficulty in one-leg standing from a 40-cm-high seat (either leg).
Two-step test, <1.3.
25-question GLFS score, ≥7.
Subjects categorized in Stage 1 are recommended to perform exercise training (locomotion training) (vide infra).
Stage 2
The following criteria indicate a progression of the decline of mobility function, and the subject is categorized as Stage 2 if any of the three conditions are met.
Stand-up test, difficulty in standing from a 20-cm-high seat using both legs.
Two-step test, <1.1.
25-question GLFS score, ≥16.
Subjects categorized in Stage 2 need to perform exercise training. In the presence of pain, medical consultation is recommended since it may be an indicator of underlying pathological changes in locomotive organs.
Relationship Between Clinical Decision Limits and Mobility Function
Yoshimura et al. [88] evaluated the feasibility of the clinical decision limit values by analyzing their relationship with decline in mobility functions (gait speed <0.8 m/s [78–80], five times sit-to-stand test time >12 s [89]) in community residents. They demonstrated that in both Stages 1 and 2, the clinical decision limit values based on the three tests correlated with the decline in mobility functions. In addition, the odds of decline in mobility functions exponentially increased with the increase in the number of criteria fulfilled.
The Number of People with Locomotive Syndrome
The current estimated number of people above 40 years categorized as Stage 1 is 45.9 million (males, 20.2 million; females, 25.7 million) and as Stage 2 is 13.8 million (males, 4.6 million; females, 9.2 million) (unpublished data).
Locomotion Training
Physical Interventions for Mobility Function
Many studies have reported the effectiveness of physical intervention in limiting the disability and functional decline of mobility, strength, balance and gait in geriatric population [22–28, 31, 90]. In general, while physical interventions are effective in people with mild to moderate disability [23, 58], their utility is limited in people with severe disability [25], emphasizing the importance of early detection of the locomotive syndrome and early intervention. In addition, which physical interventions are the most effective remains unclear [90, 91].
Physical interventions are based on the principles of exercise [92]. First, it is known that the particular body components or skills, which are involved in a given exercise, will demonstrate improvement (principle of specificity). Second, a high load is required for any functional improvement (principle of overload). Third, it is important to gradually increase the exercise load (principle of progression) with consideration for safety since the majority of the middle- to old-aged population have chronic degeneration of intervertebral disks or lower limb cartilages such as in the knee joint [3].
Given these conditions, locomotion training, called locotra, aims to improve and sustain standing and gait functions in middle- and old-aged subjects, by recommending squatting and single-leg standing with eyes open [34, 93]. These exercises are recommended as they are directly related to standing, and gait functions [91] are safe and are feasible at home for self-management [33].
Locomotion Training (Locotra) [93]
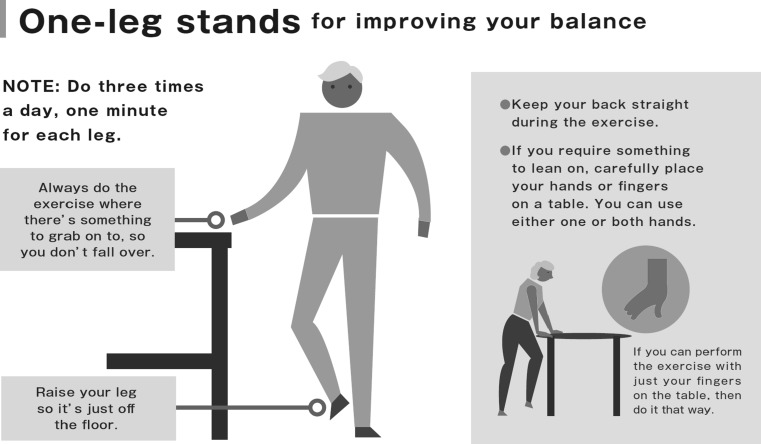
Single-Leg Standing with Eyes Open (Fig. 5)
Fig. 5.
Single-leg standing with eyes open.
Locomotive syndrome pamphlet. https://locomo-joa.jp/en/index.pdf
This balance exercise, single-leg standing with eyes open, can be done alone [94] or combined with other muscle power training (like chair-rising training) [95]. This test has been demonstrated to be effective in preventing falls.
The training involves subjects standing on one leg with their eyes open for 1 min. Subjects are instructed to perform this by standing adjacent to a stable chair or desk for arm support, to prevent from falling. The exercise performed for each leg at a time constitutes one set. Subjects are recommended to perform 3 sets each in the morning, noon and evening, every day.
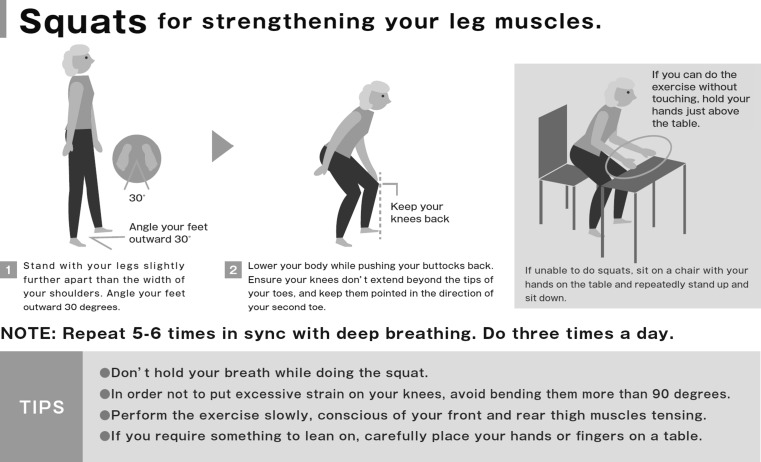
Squatting (Fig. 6)
Fig. 6.
Squatting.
Locomotive syndrome pamphlet. https://locomo-joa.jp/en/index.pdf
Previous studies have demonstrated the effectiveness of squatting in improving independence of ADL, in addition to strength and balance of lower limb and body [96, 97].
Subjects slowly move the torso down from the standing position as is done during stand–sit movement. Subjects are instructed to maintain the position of the patella (knee) over the toes in order to prevent overload on the knee. The knee flexion angle should not exceed 90°. One set comprises of 5–6 slow squats, and about three sets are to be performed each day.
Management in People with Mild Locomotive Syndrome
Walking is recommended [67, 98–100]. The number of repetition of the basic locotra is increased, and other exercises, such as heel raise and front lunges, are added.
Examples of Locotra-Intervention
In Niigata, Aoki et al. [101] recruited 97 community-dwelling adults (age, 76.8 ± 5.8 years; males, 29; females, 68) who did not participate in the government-sponsored prevention programs. The prevalence of locomotor symptoms was high among the recruited subjects: low back pain in 69.1 %, knee pain in 57.7 % and osteoporosis in 35.1 % of subjects. Participants received locomotion training (one-leg standing with eyes open and squatting) instruction and performed exercises independently for 3 months as monitored by using serial telephonic calls. Among the recruited subjects, 87 (89.7 %) completed the intervention. Scores from physical function tests (single-leg standing and five times sit-to-stand tests), and seven of eight SF-8 subscales were significantly improved. Low back pain was alleviated in 12.6 % and worsened in 2.3 %, while knee pain was alleviated in 17.2 % and worsened in 1.1 % of recruited subjects, respectively.
In Saitama, Ishibashi and Fujita recruited 151 females (age, 76.6 ± 5.6 years) who participated in a health lecture meeting [102]. Several of these women had diagnostic history of locomotive diseases: knee osteoarthritis in 61.1 %, lumbar spinal stenosis in 38.7 % and osteoporosis in 46.4 %. Participants received locomotion training (one-leg standing with eyes open and squatting) instruction and performed exercises independently for 2 months. Among the recruited subjects, 97 (64.2 %) completed the intervention. Scores from physical function tests (one-leg standing on the left side, 10 m maximal gait speed, knee extension torque) improved significantly following the intervention.
In Yamagata, 60 subjects (females, 45; males, 15; mean age, 76.3 ± 5.8 years), who did not attend the on-site preventive care programs of the long-term care insurance system, participated in an intervention program. Several of the included subjects had history of locomotor symptoms: low back pain, 56.7 %; knee pain, 73.3 %; and osteoporosis, 21.7 %. Participants received locomotion training (one-leg standing with eyes open and squatting) instruction and performed exercises independently for 3 months as monitored by using serial telephonic calls. Among the recruited subjects, 55 (91.7 %) completed the intervention. Post-intervention, there was a significant improvement in one-leg standing time. Subjects who practiced squatting more often (mean, 2.82 sets/day) were more likely to be in the highly improved group (one-leg standing time ≥9.50 s) compared to those who practiced squatting lesser (p = 0.04) [103].
Discussion
Impaired mobility is a major problem in Japan’s super-aged society. Physical performance is composed of multiple components including muscular strength, endurance, flexibility, balance, speed, reaction time and power. Therefore, the tools used for the assessment of mobility should be carefully selected after considering the purpose and utility of the results of assessment. In view of the magnitude of the problem, it should also be recognized that the feasibility of assessment methods is an important factor in preventive management of diseases with high prevalence rates [33, 55, 56].
In Japan’s super-aged society, it is common to encounter middle- and old-aged people in the community who walk or ascend/descend stairs with difficulty. This situation is more serious in clinical practice, with a high incidence of fractures in the elderly, caused mainly owing to unstable sit-to-stand or gait. In addition, refraining from going out due to knee pain contributes to social disability. From the clinical point of view, sit-to-stand and gait functions are fundamental for daily living. The motivating factors in proposing the locomotive syndrome were to enable early detection of people with declined sit-to-stand and gait functions, and early intervention as a means of improving these functions. For this to be achieved, it is important that the general population comprehends the purpose and means of management of locomotive syndrome.
The locomotive training method is multifaceted and incorporates exercises to improve balance and strengthen muscles. These include chair-rising, squats, Tai Chi, dance, walking and their combinations [22, 24, 27, 104]. However, as of now, it is unclear as to which method is the best [90, 91]. Highly effective training requires the performance of high-intensity exercises. On the other hand, safety considerations are important, especially in the middle- and old-aged population. In fact, a U-shaped correlation between exercise intensity and improvement of function in the geriatric population has been demonstrated [105, 106]. In addition, the results of the studies reviewed by us proved that locotra, comprising only low-intensity, short-duration exercises, was effective. Hashimoto et al. [103] reported that the effectiveness of training was directly proportional to the frequency of training, suggesting the importance of regular and consistent training.
Pain is an important factor contributing to impairment of the locomotor organs and is a major cause of movement disorders in humans [7, 8, 107, 108]. All the three studies discussed by us included participants with high prevalence of locomotor symptoms requiring intervention [16]. All the studies reported significant benefits with an exercise intervention program, and no adverse effects were reported [101].
The persistence rate of participants in the exercise intervention program tended to be higher when supported by serial telephonic communication [101, 103], compared to instances where there was no such support [102]. This suggests that serial telephonic support may be an effective means of ensuring compliance with the exercise program [109]. Studies have confirmed the importance of community support in ensuring the success of the exercise intervention program.
Studies documenting the benefits of locotra have certain limitations, namely none of the studies are randomized controlled trials, the duration of intervention is short and limited to a few months, and the follow-up is inadequate. In addition, analysis is based only on cross-sectional data. These limitations need to be addressed by future studies.
The concept of locomotive syndrome is gaining popularity in Japan [110]. The National Health Promotion program of Japan (2013–2022), titled “Health Japan 21 (second term),” which targets achieving an extension of healthy life expectancy, specifically aims to increase the recognition of locomotive syndrome from its present level of 17.3–80 % among the population above the age of 20 years [111]. As in April 2016, this figure has improved to 47.3 % [112]. In May 2015, a special issue was published titled, “All about Locomotive Syndrome” and distributed to doctors in all departments, which indicates that locomotive syndrome is now regarded as a theme of lifetime education for medical doctors [34]. Moreover, the concept of locomotive syndrome has been included as a part of community health promotion in Fukuoka [113] and Kagoshima prefectures [114], and Kyoto [115] and Yokohama cities [116].
In conclusion, the concept of locomotive syndrome is gaining traction in the community, and it is important to further promote awareness and to educate the population at risk as a means of extending the gains made so far.
Funding
This study was funded by Japanese Orthopaedic Association (JOA-Subsidized Science Project Research 2014-1).
Compliance with Ethical Standards
Conflict of interest
K. Nakamura and T. Ogata declare that they have no conflict of interest.
Animal/Human Studies
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s12018-016-9224-2.
References
- 1.The State of the Aging Population. In: White Paper on the aging Society (Summary) FY 2015. Cabinet Office, Government of Japan. 2016. http://www8.cao.go.jp/kourei/whitepaper/w-2015/gaiyou/27pdf_indexg.html. 2016 (in Japanese).
- 2.Plan for living a long and healthy life in Kagoshima FY 2015. In: Survey results Chap 2, 36–64. Kagoshima Prefecture. 2013. https://www.pref.kagoshima.jp/ae05/kenko-fukushi/koreisya/keikaku/documents/45011_20150423153452-1.pdf. 2016 (in Japanese).
- 3.Yoshimura N, Muraki S, Oka H, Mabuchi A, En-Yo Y, Yoshida M, et al. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J Bone Miner Metab. 2009;27(5):620–628. doi: 10.1007/s00774-009-0080-8. [DOI] [PubMed] [Google Scholar]
- 4.Kadono Y, Yasunaga H, Horiguchi H, Hashimoto H, Matsuda S, Tanaka S, et al. Statistics for orthopedic surgery 2006–2007: data from the Japanese Diagnosis Procedure Combination database. J Orthop Sci. 2010;15(2):162–170. doi: 10.1007/s00776-009-1448-2. [DOI] [PubMed] [Google Scholar]
- 5.Marley J, Tully MA, Porter-Armstrong A, Bunting B, O’Hanlon J, McDonough SM. A systematic review of interventions aimed at increasing physical activity in adults with chronic musculoskeletal pain–protocol. Syst Rev. 2014;3:106. doi: 10.1186/2046-4053-3-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee H, Hubscher M, Moseley GL, Kamper SJ, Traeger AC, Mansell G, et al. How does pain lead to disability? A systematic review and meta-analysis of mediation studies in people with back and neck pain. Pain. 2015;156(6):988–997. doi: 10.1097/j.pain.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 7.Vincent HK, Raiser SN, Vincent KR. The aging musculoskeletal system and obesity-related considerations with exercise. Ageing Res Rev. 2012;11(3):361–373. doi: 10.1016/j.arr.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamb SE, Guralnik JM, Buchner DM, Ferrucci LM, Hochberg MC, Simonsick EM, et al. Factors that modify the association between knee pain and mobility limitation in older women: the Women’s Health and Aging Study. Ann Rheum Dis. 2000;59(5):331–337. doi: 10.1136/ard.59.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muramoto A, Imagama S, Ito Z, Hirano K, Ishiguro N, Hasegawa Y. Physical performance tests are useful for evaluating and monitoring the severity of locomotive syndrome. J Orthop Sci. 2012;17(6):782–788. doi: 10.1007/s00776-012-0283-z. [DOI] [PubMed] [Google Scholar]
- 10.Goldhahn S, Kralinger F, Rikli D, Marent M, Goldhahn J. Does osteoporosis increase complication risk in surgical fracture treatment? A protocol combining new endpoints for two prospective multicentre open cohort studies. BMC Musculoskelet Disord. 2010;11:256. doi: 10.1186/1471-2474-11-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galbusera F, Volkheimer D, Reitmaier S, Berger-Roscher N, Kienle A, Wilke HJ. Pedicle screw loosening: a clinically relevant complication? Eur Spine J. 2015;24(5):1005–1016. doi: 10.1007/s00586-015-3768-6. [DOI] [PubMed] [Google Scholar]
- 12.Lyons AR. Clinical outcomes and treatment of hip fractures. Am J Med. 1997;103(2A):51S–63S. doi: 10.1016/S0002-9343(97)90027-9. [DOI] [PubMed] [Google Scholar]
- 13.Moreta J, Aguirre U, de Ugarte OS, Jauregui I, Mozos JL. Functional and radiological outcome of periprosthetic femoral fractures after hip arthroplasty. Injury. 2015;46(2):292–298. doi: 10.1016/j.injury.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Meier W, Mizner RL, Marcus RL, Dibble LE, Peters C, Lastayo PC. Total knee arthroplasty: muscle impairments, functional limitations, and recommended rehabilitation approaches. J Orthop Sports Phys Ther. 2008;38(5):246–256. doi: 10.2519/jospt.2008.2715. [DOI] [PubMed] [Google Scholar]
- 15.Stevens-Lapsley JE, Balter JE, Kohrt WM, Eckhoff DG. Quadriceps and hamstrings muscle dysfunction after total knee arthroplasty. Clin Orthop Relat Res. 2010;468(9):2460–2468. doi: 10.1007/s11999-009-1219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flanagan SR, Ragnarsson KT, Ross MK, Wong DK. Rehabilitation of the geriatric orthopaedic patient. Clin Orthop Relat Res. 1995;316:80–92. [PubMed] [Google Scholar]
- 17.Ikezoe T, Mori N, Nakamura M, Ichihashi N. Effects of age and inactivity due to prolonged bed rest on atrophy of trunk muscles. Eur J Appl Physiol. 2012;112(1):43–48. doi: 10.1007/s00421-011-1952-x. [DOI] [PubMed] [Google Scholar]
- 18.Coker RH, Hays NP, Williams RH, Wolfe RR, Evans WJ. Bed rest promotes reductions in walking speed, functional parameters, and aerobic fitness in older, healthy adults. J Gerontol A Biol Sci Med Sci. 2015;70(1):91–96. doi: 10.1093/gerona/glu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohtake M, Tashiro H, Isawa T, Sato Y, Akama A, Suzuki I, et al. A study on a screening questionnaire to support early discharge at special functioning hospitals. Yamagata Med J. 2008;26:11–23. [Google Scholar]
- 20.Ohtake M, Tashiro H, Saito A, Migita S, Kobayashi A, Yamamoto T. An examination of the roles of the Medical Information Center. A case study of particular difficulties encountered in discharge planning. Yamagata Med J. 2004;22:57–69. [Google Scholar]
- 21.Preuschoft H. Mechanisms for the acquisition of habitual bipedality: are there biomechanical reasons for the acquisition of upright bipedal posture? J Anat. 2004;204(5):363–384. doi: 10.1111/j.0021-8782.2004.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans WJ. Effects of exercise on body composition and functional capacity of the elderly. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):147–150. doi: 10.1093/gerona/50a.special_issue.147. [DOI] [PubMed] [Google Scholar]
- 23.Binder EF, Schechtman KB, Ehsani AA, Steger-May K, Brown M, Sinacore DR, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. 2002;50(12):1921–1928. doi: 10.1046/j.1532-5415.2002.50601.x. [DOI] [PubMed] [Google Scholar]
- 24.Helbostad JL, Sletvold O, Moe-Nilssen R. Effects of home exercises and group training on functional abilities in home-dwelling older persons with mobility and balance problems. A randomized study. Aging Clin Exp Res. 2004;16(2):113–121. doi: 10.1007/BF03324539. [DOI] [PubMed] [Google Scholar]
- 25.Faber MJ, Bosscher RJ, Chin APMJ, van Wieringen PC. Effects of exercise programs on falls and mobility in frail and pre-frail older adults: a multicenter randomized controlled trial. Arch Phys Med Rehabil. 2006;87(7):885–896. doi: 10.1016/j.apmr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Karinkanta S, Heinonen A, Sievanen H, Uusi-Rasi K, Pasanen M, Ojala K, et al. A multi-component exercise regimen to prevent functional decline and bone fragility in home-dwelling elderly women: randomized, controlled trial. Osteoporos Int. 2007;18(4):453–462. doi: 10.1007/s00198-006-0256-1. [DOI] [PubMed] [Google Scholar]
- 27.Chou CH, Hwang CL, Wu YT. Effect of exercise on physical function, daily living activities, and quality of life in the frail older adults: a meta-analysis. Arch Phys Med Rehabil. 2012;93(2):237–244. doi: 10.1016/j.apmr.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 28.Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;9:CD007146. doi: 10.1002/14651858.CD007146.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lihavainen K, Sipila S, Rantanen T, Kauppinen M, Sulkava R, Hartikainen S. Effects of comprehensive geriatric assessment and targeted intervention on mobility in persons aged 75 years and over: a randomized controlled trial. Clin Rehabil. 2012;26(4):314–326. doi: 10.1177/0269215511423269. [DOI] [PubMed] [Google Scholar]
- 30.Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dodds R, Sayer AA. Sarcopenia and frailty: new challenges for clinical practice. Clin Med (Lond). 2015;15(Suppl 6):s88–s91. doi: 10.7861/clinmedicine.15-6-s88. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura K. A “super-aged” society and the “locomotive syndrome”. J Orthop Sci. 2008;13(1):1–2. doi: 10.1007/s00776-007-1202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson J, Jungner G. Principles and practice of screening for disease. In: World Health Organization Relation: Public health papers. Geneva. 1968. http://apps.who.int/iris/bitstream/10665/37650/1/WHO_PHP_34.pdf. 34.
- 34.Nakamura K, Tanaka S. ed. All about Locomotive Syndrome (in Japanese). J Jpn Med Assoc vol(special issue 1). 2015.
- 35.Jackson CA, Jones M, Tooth L, Mishra GD, Byles J, Dobson A. Multimorbidity patterns are differentially associated with functional ability and decline in a longitudinal cohort of older women. Age Ageing. 2015;44(5):810–816. doi: 10.1093/ageing/afv095. [DOI] [PubMed] [Google Scholar]
- 36.Guralnik JM, Kaplan GA. Predictors of healthy aging: prospective evidence from the Alameda County study. Am J Public Health. 1989;79(6):703–708. doi: 10.2105/AJPH.79.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boult C, Kane RL, Louis TA, Boult L, McCaffrey D. Chronic conditions that lead to functional limitation in the elderly. J Gerontol. 1994;49(1):M28–M36. doi: 10.1093/geronj/49.1.M28. [DOI] [PubMed] [Google Scholar]
- 38.Ebrahim S, Wannamethee SG, Whincup P, Walker M, Shaper AG. Locomotor disability in a cohort of British men: the impact of lifestyle and disease. Int J Epidemiol. 2000;29(3):478–486. doi: 10.1093/ije/29.3.478. [DOI] [PubMed] [Google Scholar]
- 39.Muller S, Thomas E, Peat G. The effect of changes in lower limb pain on the rate of progression of locomotor disability in middle and old age: evidence from the NorStOP cohort with 6-year follow-up. Pain. 2012;153(5):952–959. doi: 10.1016/j.pain.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.JOA new patient survey 2012. Japanese Orthopaedic Association. 2012. https://www.joa.or.jp/jp/media/comment/pdf/investigation_2012.pdf. 2016 (in Japanese).
- 41.Akune T, Muraki S, Oka H, Tanaka S, Kawaguchi H, Nakamura K, et al. Exercise habits during middle age are associated with lower prevalence of sarcopenia: the ROAD study. Osteoporos Int. 2014;25(3):1081–1088. doi: 10.1007/s00198-013-2550-z. [DOI] [PubMed] [Google Scholar]
- 42.Asomaning K, Bertone-Johnson ER, Nasca PC, Hooven F, Pekow PS. The association between body mass index and osteoporosis in patients referred for a bone mineral density examination. J Womens Health (Larchmt) 2006;15(9):1028–1034. doi: 10.1089/jwh.2006.15.1028. [DOI] [PubMed] [Google Scholar]
- 43.Schnatz PF, Marakovits KA, O’Sullivan DM. Assessment of postmenopausal women and significant risk factors for osteoporosis. Obstet Gynecol Surv. 2010;65(9):591–596. doi: 10.1097/OGX.0b013e3181fc6d30. [DOI] [PubMed] [Google Scholar]
- 44.Drake MT, Murad MH, Mauck KF, Lane MA, Undavalli C, Elraiyah T, et al. Clinical review. Risk factors for low bone mass-related fractures in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97(6):1861–1870. doi: 10.1210/jc.2011-3058. [DOI] [PubMed] [Google Scholar]
- 45.Lau EM, Lynn HS, Woo JW, Kwok TC, Melton LJ., III Prevalence of and risk factors for sarcopenia in elderly Chinese men and women. J Gerontol A Biol Sci Med Sci. 2005;60(2):213–216. doi: 10.1093/gerona/60.2.213. [DOI] [PubMed] [Google Scholar]
- 46.Yu R, Wong M, Leung J, Lee J, Auyeung TW, Woo J. Incidence, reversibility, risk factors and the protective effect of high body mass index against sarcopenia in community-dwelling older Chinese adults. Geriatr Gerontol Int. 2014;14(Suppl 1):15–28. doi: 10.1111/ggi.12220. [DOI] [PubMed] [Google Scholar]
- 47.Figueiredo CP, Domiciano DS, Lopes JB, Caparbo VF, Scazufca M, Bonfa E, et al. Prevalence of sarcopenia and associated risk factors by two diagnostic criteria in community-dwelling older men: the Sao Paulo Ageing and Health Study (SPAH) Osteoporos Int. 2014;25(2):589–596. doi: 10.1007/s00198-013-2455-x. [DOI] [PubMed] [Google Scholar]
- 48.Marks R. Obesity profiles with knee osteoarthritis: correlation with pain, disability, disease progression. Obesity (Silver Spring) 2007;15(7):1867–1874. doi: 10.1038/oby.2007.221. [DOI] [PubMed] [Google Scholar]
- 49.Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008;9:132. doi: 10.1186/1471-2474-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liuke M, Solovieva S, Lamminen A, Luoma K, Leino-Arjas P, Luukkonen R, et al. Disc degeneration of the lumbar spine in relation to overweight. Int J Obes (Lond) 2005;29(8):903–908. doi: 10.1038/sj.ijo.0802974. [DOI] [PubMed] [Google Scholar]
- 51.Orth P, Rey-Rico A, Venkatesan JK, Madry H, Cucchiarini M. Current perspectives in stem cell research for knee cartilage repair. Stem Cells Cloning. 2014;7:1–17. doi: 10.2147/SCCAA.S42880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Erwin WM. Biologically based therapy for the intervertebral disk: who is the patient? Global Spine J. 2013;3(3):193–200. doi: 10.1055/s-0033-1343074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gou S, Oxentenko SC, Eldrige JS, Xiao L, Pingree MJ, Wang Z, et al. Stem cell therapy for intervertebral disk regeneration. Am J Phys Med Rehabil. 2014;93(11 Suppl 3):S122–S131. doi: 10.1097/PHM.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 54.Buurman BM, van Munster BC, Korevaar JC, de Haan RJ, de Rooij SE. Variability in measuring (instrumental) activities of daily living functioning and functional decline in hospitalized older medical patients: a systematic review. J Clin Epidemiol. 2011;64(6):619–627. doi: 10.1016/j.jclinepi.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Beaton K, Grimmer K. Tools that assess functional decline: systematic literature review update. Clin Interv Aging. 2013;8:485–494. doi: 10.2147/CIA.S42528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cawthon PM. Assessment of lean mass and physical performance in sarcopenia. J Clin Densitom. 2015;18(4):467–471. doi: 10.1016/j.jocd.2015.05.063. [DOI] [PubMed] [Google Scholar]
- 57.Ebrahim S, Adamson J, Ayis S, Beswick A, Gooberman-Hill R. Locomotor disability: meaning, causes and effects of interventions. J Health Serv Res Policy. 2008;13(Suppl 3):38–46. doi: 10.1258/jhsrp.2008.008013. [DOI] [PubMed] [Google Scholar]
- 58.Daniels R, van Rossum E, de Witte L, Kempen GI, van den Heuvel W. Interventions to prevent disability in frail community-dwelling elderly: a systematic review. BMC Health Serv Res. 2008;8:278. doi: 10.1186/1472-6963-8-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tobimatsu Y. Locomotive syndrome: its clinical features and aggravation process. Bone Joint Nerve. 2014;4:467–472. [Google Scholar]
- 60.Seichi A, Hoshino Y, Doi T, Akai M, Tobimatsu Y, Iwaya T. Development of a screening tool for risk of locomotive syndrome in the elderly: the 25-question Geriatric Locomotive Function Scale. J Orthop Sci. 2012;17(2):163–172. doi: 10.1007/s00776-011-0193-5. [DOI] [PubMed] [Google Scholar]
- 61.Hosokawa T, Tsubono Y, Tsuji I, Maesawa M, Nakamura R. Assessment of functional status with an extended ADL scale (1) a general population sample of community elderly. Jpn J Rehabil Med. 1994;31:399–408. doi: 10.2490/jjrm1963.31.399. [DOI] [Google Scholar]
- 62.Suzukawa M, Shimada H, Kobayashi K, Suzuki T. The Relationship between Going Outdoors and Physical Function of Elderly Persons Certified as in Need of Care. Rigakuryoho Kagaku. 2010;25:103–107. doi: 10.1589/rika.25.103. [DOI] [Google Scholar]
- 63.Fujiwara Y, Shinkai S, Kumagai S, Amano H, Yoshida Y, Yoshida H, et al. Longitudinal changes in higher-level functional capacity of an older population living in a Japanese urban community. Arch Gerontol Geriatr. 2003;36(2):141–153. doi: 10.1016/S0167-4943(02)00081-X. [DOI] [PubMed] [Google Scholar]
- 64.Fieo RA, Austin EJ, Starr JM, Deary IJ. Calibrating ADL–IADL scales to improve measurement accuracy and to extend the disability construct into the preclinical range: a systematic review. BMC Geriatr. 2011;11:42. doi: 10.1186/1471-2318-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Portegijs E, Rantakokko M, Viljanen A, Sipila S, Rantanen T. Identification of older people at risk of ADL disability using the life-space assessment: a longitudinal cohort study. J Am Med Dir Assoc. 2016 doi: 10.1016/j.jamda.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 66.Harada K, Shimada H, Sawyer P, Asakawa Y, Nihei K, Kaneya S, et al. Life-space of community-dwelling older adults using preventive health care services in Japan and the validity of composite scoring methods for assessment. Nihon Koshu Eisei Zasshi. 2010;57(7):526–537. [PubMed] [Google Scholar]
- 67.Strawbridge WJ, Cohen RD, Shema SJ, Kaplan GA. Successful aging: predictors and associated activities. Am J Epidemiol. 1996;144(2):135–141. doi: 10.1093/oxfordjournals.aje.a008900. [DOI] [PubMed] [Google Scholar]
- 68.Ishizaki T, Watanabe S, Suzuki T, Shibata H, Haga H. Predictors for functional decline among nondisabled older Japanese living in a community during a 3-year follow-up. J Am Geriatr Soc. 2000;48(11):1424–1429. doi: 10.1111/j.1532-5415.2000.tb02632.x. [DOI] [PubMed] [Google Scholar]
- 69.Tomioka K, Kurumatani N, Hosoi H. Social participation and the prevention of decline in effectance among community-dwelling elderly: a population-based cohort study. PLoS One. 2015;10(9):e0139065. doi: 10.1371/journal.pone.0139065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akune T, Muraki S, Oka H, Tanaka S, Kawaguchi H, Tokimura F, et al. Association of physical activities of daily living with the incidence of certified need of care in the long-term care insurance system of Japan: the ROAD study. J Orthop Sci. 2014;19(3):489–496. doi: 10.1007/s00776-014-0537-z. [DOI] [PubMed] [Google Scholar]
- 71.Akune T, Muraki S, Oka H, Tanaka S, Kawaguchi H, Tokimura F, et al. Incidence of certified need of care in the long-term care insurance system and its risk factors in the elderly of Japanese population-based cohorts: the ROAD study. Geriatr Gerontol Int. 2014;14:695–701. doi: 10.1111/ggi.12155. [DOI] [PubMed] [Google Scholar]
- 72.Demura S, Yamada T. The maximal double step length test can evaluate more adequately the decrease of physical function with age, than the maximal single step length test. Arch Gerontol Geriatr. 2011;53(1):e21–e24. doi: 10.1016/j.archger.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 73.Bongers KT, Schoon Y, Graauwmans MJ, Hoogsteen-Ossewaarde ME, Olde Rikkert MG. Safety, feasibility, and reliability of the maximal step length, gait speed, and chair test measured by seniors themselves: the senior step study. J Aging Phys Act. 2015;23(3):438–443. doi: 10.1123/japa.2013-0231. [DOI] [PubMed] [Google Scholar]
- 74.Ogata T, Muranaga S, Ishibashi H, Ohe T, Izumida R, Yoshimura N, et al. Development of a screening program to assess motor function in the adult population: a cross-sectional observational study. J Orthop Sci. 2015;20(5):888–895. doi: 10.1007/s00776-015-0737-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muraki S, Akune T, Teraguchi M, Kagotani R, Asai Y, Yoshida M, et al. Quadriceps muscle strength, radiographic knee osteoarthritis and knee pain: the ROAD study. BMC Musculoskelet Disord. 2015;16:305. doi: 10.1186/s12891-015-0737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miyatake N, Fujii M, Nishikawa H, Wada J, Shikata K, Makino H, et al. Clinical evaluation of muscle strength in 20–79-years-old obese Japanese. Diabetes Res Clin Pract. 2000;48(1):15–21. doi: 10.1016/S0168-8227(99)00132-1. [DOI] [PubMed] [Google Scholar]
- 77.Muranaga S. Evaluation of the muscular strength of the lower extremities using the standing movement and clinical application. J Showa Med Assoc. 2001;61:362–367. [Google Scholar]
- 78.Brach JS, VanSwearingen JM. Physical impairment and disability: relationship to performance of activities of daily living in community-dwelling older men. Phys Ther. 2002;82(8):752–761. [PubMed] [Google Scholar]
- 79.Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10):881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 80.Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol A Biol Sci Med Sci. 2013;68(1):39–46. doi: 10.1093/gerona/gls174. [DOI] [PubMed] [Google Scholar]
- 81.Lindemann U, Lundin-Olsson L, Hauer K, Wengert M, Becker C, Pfeiffer K. Maximum step length as a potential screening tool for falls in non-disabled older adults living in the community. Aging Clin Exp Res. 2008;20(5):394–399. doi: 10.1007/BF03325143. [DOI] [PubMed] [Google Scholar]
- 82.Cho BL, Scarpace D, Alexander NB. Tests of stepping as indicators of mobility, balance, and fall risk in balance-impaired older adults. J Am Geriatr Soc. 2004;52(7):1168–1173. doi: 10.1111/j.1532-5415.2004.52317.x. [DOI] [PubMed] [Google Scholar]
- 83.Goldberg A, Schepens S, Wallace M. Concurrent validity and reliability of the maximum step length test in older adults. J Geriatr Phys Ther. 2010;33(3):122–127. [PubMed] [Google Scholar]
- 84.Muranaga S, Hirano K. Development of a convenient way to predict ability to walk, using a two-step test. J Showa Med Assoc. 2003;63:301–303. [Google Scholar]
- 85.Muranaga S, Higashi T, Tsuchiya R, Miyamoto R. Mobility function (walking ability) and muscle strength evaluation. (in Japanse) Prog Med. 2010;30:3055–3060. [Google Scholar]
- 86.Mor V, Wilcox V, Rakowski W, Hiris J. Functional transitions among the elderly: patterns, predictors, and related hospital use. Am J Public Health. 1994;84(8):1274–1280. doi: 10.2105/AJPH.84.8.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kimura A, Seichi A, Konno S, Yabuki S, Hayashi K. Prevalence of locomotive syndrome in Japan: a nationwide, cross-sectional Internet survey. J Orthop Sci. 2014;19(5):792–797. doi: 10.1007/s00776-014-0606-3. [DOI] [PubMed] [Google Scholar]
- 88.Yoshimura N, Muraki S, Oka H, Tanaka S, Ogata T, Kawaguchi H, et al. Association between new indices in the locomotive syndrome risk test and decline in mobility: third survey of the ROAD study. J Orthop Sci. 2015;20(5):896–905. doi: 10.1007/s00776-015-0741-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tiedemann A, Shimada H, Sherrington C, Murray S, Lord S. The comparative ability of eight functional mobility tests for predicting falls in community-dwelling older people. Age Ageing. 2008;37(4):430–435. doi: 10.1093/ageing/afn100. [DOI] [PubMed] [Google Scholar]
- 90.Cumming RG. Intervention strategies and risk-factor modification for falls prevention. A review of recent intervention studies. Clin Geriatr Med. 2002;18(2):175–189. doi: 10.1016/S0749-0690(02)00004-6. [DOI] [PubMed] [Google Scholar]
- 91.Ishigaki EY, Ramos LG, Carvalho ES, Lunardi AC. Effectiveness of muscle strengthening and description of protocols for preventing falls in the elderly: a systematic review. Braz J Phys Ther. 2014;18(2):111–118. doi: 10.1590/S1413-35552012005000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ammann BC, Knols RH, Baschung P, de Bie RA, de Bruin ED. Application of principles of exercise training in sub-acute and chronic stroke survivors: a systematic review. BMC Neurol. 2014;14:167. doi: 10.1186/s12883-014-0167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Locomotive syndrome pamphlet. 2015. In: Locomotive Challenge! Council, editors. Japanese Orthopaedic Association, Tokyo. https://locomo-joa.jp/en/index.pdf. 2016.
- 94.Sakamoto K, Nakamura T, Hagino H, Endo N, Mori S, Muto Y, et al. Effects of unipedal standing balance exercise on the prevention of falls and hip fracture among clinically defined high-risk elderly individuals: a randomized controlled trial. J Orthop Sci. 2006;11(5):467–472. doi: 10.1007/s00776-006-1057-2. [DOI] [PubMed] [Google Scholar]
- 95.Iwamoto J, Suzuki H, Tanaka K, Kumakubo T, Hirabayashi H, Miyazaki Y, et al. Preventative effect of exercise against falls in the elderly: a randomized controlled trial. Osteoporos Int. 2009;20(7):1233–1240. doi: 10.1007/s00198-008-0794-9. [DOI] [PubMed] [Google Scholar]
- 96.Young CM, Weeks BK, Beck BR. Simple, novel physical activity maintains proximal femur bone mineral density, and improves muscle strength and balance in sedentary, postmenopausal Caucasian women. Osteoporos Int. 2007;18(10):1379–1387. doi: 10.1007/s00198-007-0400-6. [DOI] [PubMed] [Google Scholar]
- 97.Seitz LB, Reyes A, Tran TT, Saez de Villarreal E, Haff GG. Increases in lower-body strength transfer positively to sprint performance: a systematic review with meta-analysis. Sports Med. 2014;44(12):1693–1702. doi: 10.1007/s40279-014-0227-1. [DOI] [PubMed] [Google Scholar]
- 98.Devries MC, Breen L, Von Allmen M, MacDonald MJ, Moore DR, Offord EA, et al. Low-load resistance training during step-reduction attenuates declines in muscle mass and strength and enhances anabolic sensitivity in older men. Physiol Rep. 2015 doi: 10.14814/phy2.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Active guide: Japanese official physical activity guidelines for health promotion. 2013. Ministry of Health, Labour and Welfare. 2013. http://www0.nih.go.jp/eiken/info/pdf/active2013-e.pdf. 2016.
- 100.Smith AR, Chen C, Clarke P, Gallagher NA. Trajectories of outdoor mobility in vulnerable community-dwelling elderly: the role of individual and environmental factors. J Aging Health. 2015 doi: 10.1177/0898264315611665. [DOI] [PubMed] [Google Scholar]
- 101.Aoki K, Sakuma M, Ogisho N, Nakamura K, Chosa E, Endo N. The effects of self-directed home exercise with serial telephone contacts on physical functions and quality of life in elderly people at high risk of locomotor dysfunction. Acta Med Okayama. 2015;69(4):245–253. doi: 10.18926/AMO/53561. [DOI] [PubMed] [Google Scholar]
- 102.Ishibashi H, Fujita H. The effect of locomotion training on mobility function in the elderly female. Osteoporos Jpn. 2011;19:391–397. [Google Scholar]
- 103.Hashimoto M, Yasumura S, Nakano K, Kimura M, Nakamura K, Fujino K, et al. Feasibility study of locomotion training in a home-visit preventive care program. Nihon Ronen Igakkai Zasshi. 2012;49(4):476–482. doi: 10.3143/geriatrics.49.476. [DOI] [PubMed] [Google Scholar]
- 104.Howe TE, Rochester L, Neil F, Skelton DA, Ballinger C. Exercise for improving balance in older people. Cochrane Database Syst Rev. 2011;11:CD004963. doi: 10.1002/14651858.CD004963.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heneweer H, Vanhees L, Picavet HS. Physical activity and low back pain: a U-shaped relation? Pain. 2009;143(1–2):21–25. doi: 10.1016/j.pain.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 106.Physical activity guidelines for health promotion 2013. Ministry of Health, Labour and Welfare. 2013. http://www0.nih.go.jp/eiken/info/pdf/active2013-e.pdf. 2016 (in Japanese).
- 107.Bryant LL, Grigsby J, Swenson C, Scarbro S, Baxter J. Chronic pain increases the risk of decreasing physical performance in older adults: the San Luis Valley Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2007;62(9):989–996. doi: 10.1093/gerona/62.9.989. [DOI] [PubMed] [Google Scholar]
- 108.Weaver GD, Kuo YF, Raji MA, Al Snih S, Ray L, Torres E, et al. Pain and disability in older Mexican–American adults. J Am Geriatr Soc. 2009;57(6):992–999. doi: 10.1111/j.1532-5415.2009.02263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.King AC, Taylor CB, Haskell WL, Debusk RF. Strategies for increasing early adherence to and long-term maintenance of home-based exercise training in healthy middle-aged men and women. Am J Cardiol. 1988;61(8):628–632. doi: 10.1016/0002-9149(88)90778-3. [DOI] [PubMed] [Google Scholar]
- 110.Nakamura K. The concept and treatment of locomotive syndrome: its acceptance and spread in Japan. J Orthop Sci. 2011;16(5):489–491. doi: 10.1007/s00776-011-0108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Health of elderly people. Health Japan 21 (the second term). Ministry of Health, Labour and Welfare. 2013. http://www.mhlw.go.jp/seisakunitsuite/bunya/kenkou_iryou/kenkou/kenkounippon21/en/kenkounippon21/mokuhyou03.html. 2016 (in Japanese).
- 112.Awareness survey of Locomotive Syndrome 2016. The Bone and Joint Decade Japan. 2015. http://www.bjd-jp.org/news/doc/2016_survey_locomotivesyndrome.pdf. 2016 (in Japanese).
- 113.Training of 1200 locomo-supporters. Let’s begin measures against Locomo. Fukuoka Prefecture. http://www.pref.fukuoka.lg.jp/uploaded/life/124636_50255417_misc.pdf. 2016 (in Japanese).
- 114.Locomo prevention. Exercise habits every day. Kagoshima Prefecture. https://www.pref.kagoshima.jp/ae06/kenko-fukushi/kenko-iryo/seikatusyukan/seikatushuukanbyou/locomo-yobou.html. 2016 (in Japanese).
- 115.Exercise programs for locomotive syndrome prevention. Plus 10 min of kyo-loco-step anywhere, anytime. Kyoto City. http://www.city.kyoto.lg.jp/hokenfukushi/page/0000172026.html. 2016 (in Japanese).
- 116.Great Locomo Prevention Strategies. Yokohama City. http://www.city.yokohama.lg.jp/kenko/rokomo/leaflet9.pdf. 2016 (in Japanese).