Abstract
Xenarthrans are unique among mammals in retaining simplified teeth that are rootless and homodont, which makes it difficult to determine dental homologies. We apply computerized tomography to prenatal developmental series of extant sloths, Bradypus and Choloepus, to further elucidate the patterns of morphological variation in their dentition. We also propose new criteria based on sequences of dental mineralization, and the presence of vestigial teeth, to distinguish between caniniforms and postcaniniforms. We report for the first time the presence of vestigial incisors in Bradypus. We also show the presence of a vestigial tooth in front of the lower caniniform in both extant sloth genera and the existence of two generations for the upper caniniform in Choloepus. The study of their sequence of mineralization indicates that the lower and upper caniniform teeth are not homologous in sloths, and suggests that upper caniniforms are not homologous between the two extant sloth genera. Our results show that assessing the developmental processes and functional constraints remains crucial to understand the dental variations observed in sloths, and more generally, tooth class homology issues in mammals. Applied to the tooth row of all extinct sloths, these developmental data illuminate a potentially ancestral dental formula for sloths.
Like other xenarthrans (sloths, armadillos, and anteaters), living and extinct sloths (Folivora) depart from the rest of mammals by the simplified nature of their dentition. Teeth present in most xenarthran adults lack enamel and are usually homodont, ever-growing, tubular and primarily composed of orthodentine and vasodentine1, which makes it difficult to identify homologies with the teeth and cusps of other mammals. Both extant sloth genera are functionally monophyodont, and their dentition is generally considered to constitute a single set of permanent teeth2,3,4,5,6. The sloth dentition contrasts with the complete lack of teeth in anteaters, and the supernumerary teeth of armadillos. It mainly differs from that of other xenarthrans in showing a morphological distinction between caniniforms and molariforms, a difference based on the general morphology, occlusion, and position of their teeth.
Recent morphological and molecular phylogenetic analyses7,8,9,10 suggested that the two modern genera are only distantly related, with a divergence time that could be as long as 30 million years ago11. Despite this independent evolutionary history, both two-toed and three-toed sloths display identical dental formulae with five upper and four lower teeth, as do the majority of extinct sloth genera1,10. This apparent stability in number associated with the differentiation of the tooth row observed in extant forms masks a complex evolution of the dentition in folivorans (i.e., modern sloths end extinct gravigrade sloths). Bradypus shows a closely fit toothrow, lacking diastema with each tooth showing a peg-like morphology. Choloepus displays an enlarged, chisel-shaped caniniform at the front of the dentition and isolated from the molariforms by a diastema1,12.
While the intriguing nature of the xenarthran teeth has attracted a lot of attention, few studies have focused on the development of the whole dentition13, especially in sloths. Early workers have only described isolated foetuses of sloths or focused on the developmental sequence of their skeleton14,15. However, these do not detail the development of the teeth or provide a comparative basis upon which to analyse possible homologies with the dentition of other mammals. Using a large dataset of scanned foetuses of sloths, we provide data on xenarthran prenatal dental ontogeny, identify some developmental criteria with which to recognize homologies with other mammalian teeth, and propose a new hypothesis for the development of heterodonty in sloths.
Results
A terminology specific to sloths has been used to avoid confusion and in order to draw reliable comparisons (S1): pmx stands for premaxilla; d stands for deciduous teeth; cf and mf stand for lower caniniforms and molariforms respectively, while Cf and Mf stand for upper teeth; lower loci 1–3 and upper loci 1–4 involve functional molariform teeth; v and V stand for vestigial lower and upper teeth respectively (i.e., these loci are absent in adults).
Prenatal dental development in three-toed sloths
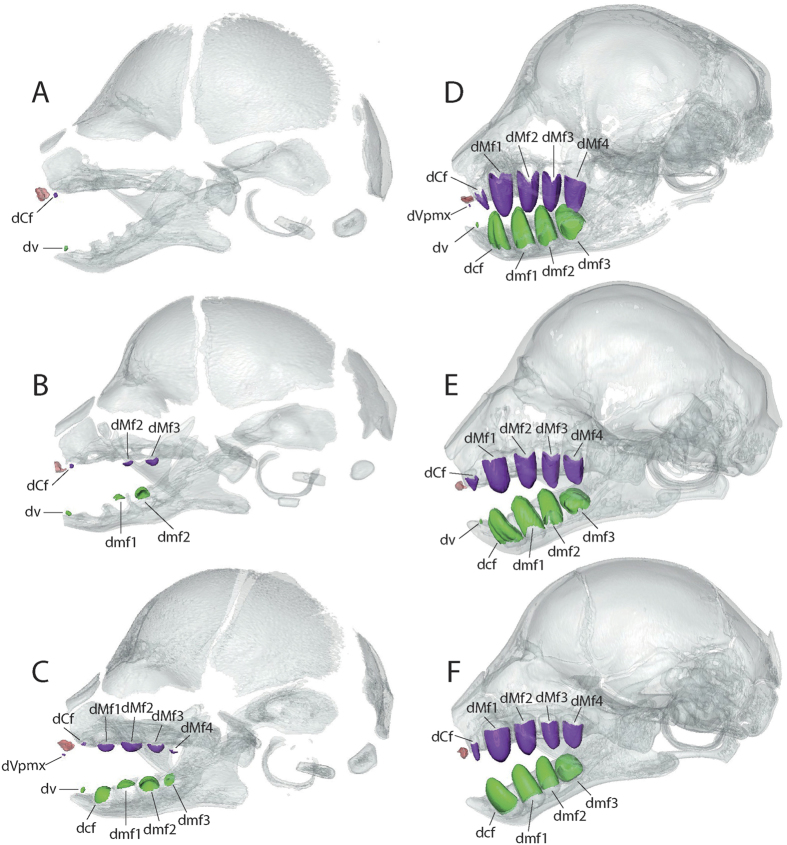
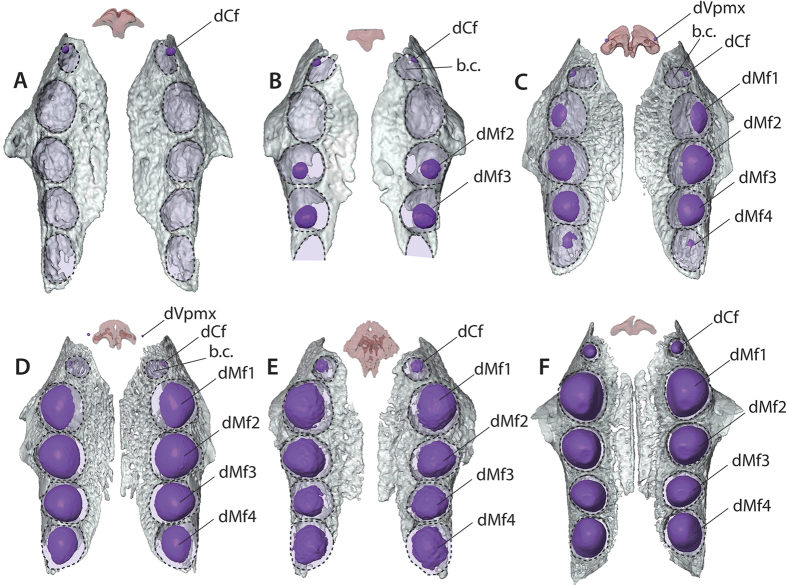
The sequence of prenatal dental eruption in Bradypus is well resolved with 18 specimens that represent a variety of developmental stages. Eleven of 18 foetuses display a number of developing teeth different from those observed in adults, which (as noted previously) are characterized by five upper and four lower teeth (i.e., 5/4). All the alveoli of the adult teeth are present early during dental development, but lack teeth. This implies that dental buds are developing but not yet mineralizing; these buds cannot be directly observed because soft tissues are difficult to detect using X-ray microtomography without soft-tissue staining. In the youngest specimen (ZMB 33812, SL = 23.7 mm, Figs 1A and 2A), only the mesialmost pairs of teeth are mineralizing on the maxillary and the dentary (dCf and dv), meaning that dentine formation has started. In contrast to other teeth, the first pair of uppers (dCf) to appear are not centered in their alveolus, but sit off-centre in the anterolateral corner of the alveolus. The dCf is the first locus to mineralize, but its size does not change drastically during the first stages. Other teeth mineralize after dCf, but grow more quickly (Figs 1A–D and 2). The second youngest specimen (ZMB 41122, SL = 25.9 mm, Figs 1B and 2B) shows mineralized dCf, dMf2, dMf3, dv, dmf1, and dmf2, with empty alveoli (i.e., teeth not yet mineralizing) at the dMf1, dMf4, dcf, and dmf3 loci. All the alveoli include mineralized teeth in subsequent stages.
Figure 1. Lateral view of three-dimensional reconstruction of CT-scans of skull in the three-toed sloth Bradypus.
(A) Bradypus variegatus (ZMB 33812), SL = 23 mm; (B) Bradypus variegatus (ZMB 41122), SL = 26 mm; (C) Bradypus variegatus (MNHN-ZM-MO-1995-326A), SL = 26 mm; (D) Bradypus variegatus (ZMB 41120), SL = 42 mm; (E) Bradypus tridactylus (BMNH 52-1173), SL = 42 mm; (F) Bradypus sp. (MNHN-ZM-MO-1995-327), SL = 38 mm. Upper teeth are in violet; lower teeth are in green; premaxillary bone is in red.
Figure 2. Palatal view of three-dimensional reconstruction of the maxillary bones in early developmental stages of the three-toed sloth Bradypus.
(A) Bradypus variegatus (ZMB 33812), SL = 23 mm; (B) Bradypus variegatus (ZMB 41122), SL = 26 mm; (C) Bradypus variegatus (MNHN-ZM-MO-1995-326A), SL = 26 mm; (D) Bradypus variegatus (MNHN-ZM-MO-1995-326B), SL = 30 mm; (E) Bradypus sp. (MNHN-ZM-MO-1902-325), SL = 30 mm; (F) Bradypus sp. (MNHN-ZM-MO-1995-327), SL = 38 mm. Upper teeth are in violet; premaxillary bone is in red. Dashed lines represent dental alveoli. Abbreviations: b.c., bony crypt.
More importantly, four of 18 specimens have six upper and five lower teeth; they show an extra pair of teeth on the premaxilla (dVpmx, Fig. 1C,D), which correspond to rudimentary incisors, absent in the adults. These incisors can be retained until relatively late in development (e.g., ZMB 41120, SL = 41.84 mm, Fig. 1D), but are resorbed before birth. Five specimens have a dental formula composed of five upper and five lower teeth. The first pair of lower teeth (dv), just mesial to the lower caniniform (dcf), is also resorbed during development, likely after the small incisors, and is absent in later stages. Both extra upper and lower teeth (dVpmx and dv) are apparent on both right and left sides, they do not have visible extension of the root, and are never associated with alveoli. We observed no major dental differences between B. variegatus and B. tridactylus, both of which exhibited similar morphology at comparable stages.
Prenatal dental development in two-toed sloths
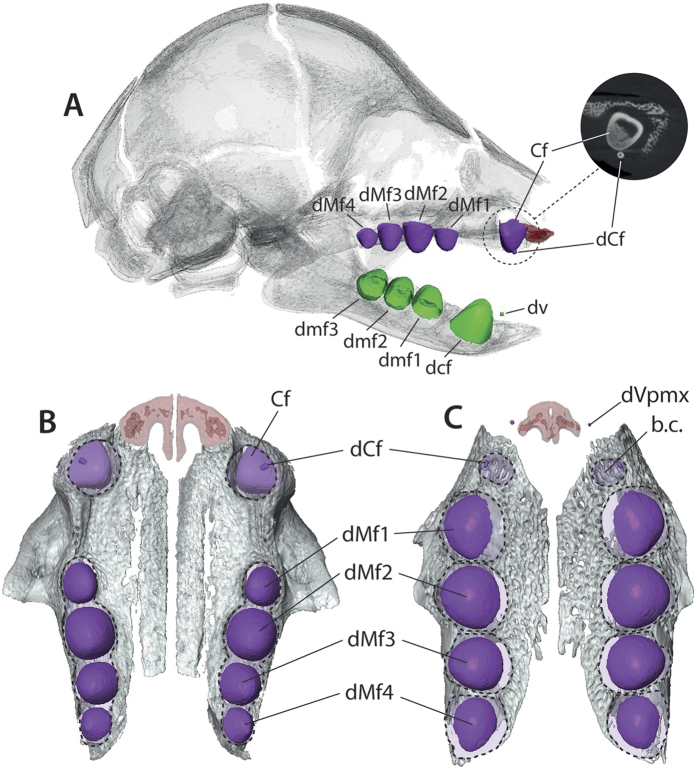
The skull length (see S2 for measurements) and number of discrete ossification centres of the cranium indicate that most of the specimens of Choloepus correspond to relatively late stages compared to Bradypus. However, as for Bradypus, the number of teeth varies greatly among our specimens and differs from the morphology observed in adult Choloepus. While the adult dental formula is 5/4, the foetuses showed either five upper and five lower teeth (60% of the cases) or six upper and five lower teeth (40% of the cases). All specimens display an extra tooth on the mandible in front of the functional adult tooth row (dv), i.e., in front of the lower caniniforms (dcf). Two younger specimens also show an extra tooth in the maxilla (dCf; Fig. 3A) in front of the upper caniniforms (Cf); these extra teeth are located in the same alveoli as Cf and are oriented mesio-buccally (Fig. 3A,B). As observed for Bradypus, all extra teeth are present on both right and left sides; however, in contrast to Bradypus, no vestigial incisor (dVpmx) was observed in the premaxilla of Choloepus, although we cannot rule out its presence in specimens younger than those in our sample. No extra teeth were detected at the level of the diastema that separates the mesialmost functional tooth from the molariforms in any of the specimens studied. As observed in Bradypus, the lower teeth (dv) do not have visible extension of the root and are never associated with alveoli, in contrast to the teeth present in adults. We observed no major dental differences between the two species of Choloepus.
Figure 3. A comparison of the tooth rows in Choloepus and Bradypus.
(A) lateral view of the skull of Choloepus. (B,C) palatal views of the tooth rows in C. didactylus ((B) MNHN-ZM-MO-1882-625, SL = 45 mm) and B. variegatus ((C) MNHN-ZM-MO-1995-326B, SL = 30 mm). Note the similar (off-centre) position of the dCf in the mesialmost alveolus.
Discussion
A history of prenatal dental development in extant sloths
For Böker16, “sloths will always remain the poor sibling of comparative anatomy, because it is neither possible to obtain complete anatomical series, nor does the possibility currently exist to gain good insights from paleontology. Only ontogeny promises good prospects to enlighten the history of sloths’ modifications. Yet this does not promise an easy path either, because not only are important developmental stages very difficult to find, but even when embryos are available, they show that development of key anatomical structures seems to occur at very early stages” (in German in the text). Comparisons of tooth development in sloths suggests that, for at least some anatomical regions, Böker is correct that ontogeny is a key source of information about homology. However, he is too pessimistic that sloths will remain poorly understood compared to other animals. Extant and extinct sloths display quite homodont teeth, which makes it difficult to determine dental homologies. Prenatal dental development in sloths shows teeth that are cone-shaped and monocuspid (see also17,18, and demonstrates that robust hypotheses of homologies cannot be drawn based on occlusal patterns alone (e.g.19,20), which simply result from rapid wear (i.e., cusp-like pattern). However, our data show that Bradypus and Choloepus display several pairs of supernumary teeth in the mesial part of their dentition during prenatal ontogeny. This is consistent with previous, anecdotal accounts of dental “anomalies” in sloths2,6,17,21,22,23. Brandts21 (cited in Röse22) reported the first record of vestigial lower teeth in Bradypus and believed them to be canines. Parker2 observed similar vestiges in Choloepus embryos, which made him recognize the lower caniniform as a premolar locus. Gervais17 seemed unaware of Brandts’ reference when he described the presence of vestigial teeth in the mandible of a foetus of Bradypus, which he considered to be incisors based on their closeness to the symphysis as well as their position compared to the caniniforms. Simon23 reached similar conclusions with two foetuses of Bradypus (CRL = 23.5 and 24.2 cm); he also proposed, probably for the first time, the non-homology between the lower and upper caniniforms based on dental eruption sequences (the upper caniniform erupting much later than the upper molariform and the lower caniniform).
Occurrence of vestigial teeth and dental anomalies in sloths
Our data show that Bradypus and Choloepus display several pairs of supernumary teeth in the mesial part of their dentition during prenatal ontogeny. This is consistent with previous, anecdotal accounts of dental “anomalies” in sloths. High-resolution X-ray computed tomography on a large number of foetuses demonstrated that these extra teeth cannot simply be explained by individual variation that occurs exceptionally within a population. Such methods also revealed that these teeth lack typical roots and do not develop in clearly individualized alveoli, unlike other teeth. More importantly, these vestigial teeth occur on both right and left sides, which is rarely the case with sloth dental anomalies12. Sloths display fewer dental anomalies compared to other mammals (i.e., 2.4% of adult specimens observed by McAfee12 exhibited any sort of anomalies), and increases in tooth number (i.e., hyperdontia) occur at a much lower rate than reductions12. Bradypus is more prone to lose teeth and all cases of tooth loss involve the dCf12, which could be logically expected since it is the most reduced tooth of the dentition24. Interestingly, nearly all of the anomalies observed in adults (unpaired hyperdontia and anodontia) affected the upper dentition in sloths12 while vestigial teeth were most consistently observed on the mandible. In fact, the only paired hyperdontia anomaly ever reported on the mandible (Fig. 1C)12 probably corresponds to a specimen that failed to resorb the mesial vestigial teeth (dcf) observed in the foetuses. This reinforces the idea that the mineralization and resorption of the vestigial teeth is an integral part of prenatal dental development in sloths. All of these teeth (dVpmx in Bradypus, dCf in Choloepus, and dv in both) correspond to the definition of vestigial structures given by Peterkova et al.25; they occur transiently during development in all members of a population, and on occasion persist into maturity. While both extant genera have similar development of the lower teeth, including the mineralization of paired mesial vestigial teeth (dv), differences are evident on the upper jaw, with Bradypus displaying premaxillary vestigial teeth and Choloepus maxillary but no premaxillary vestigial teeth.
First evidence of tooth replacement in sloths
Vestigial teeth were observed in the maxillae of Choloepus (dCf). These vestigial teeth are very close to the caniniform teeth (Cf). They are located in the same alveolus, and appear apically with respect to the Cf (Fig. 3A,B), as expected in vertical dental replacement (e.g.26,27). Determining the epithelial connections of teeth during early developmental stages provides the best criterion for defining the deciduous or permanent homologies of individual teeth28,29, but unstained CT data do not convey this information on the differentiation of the dental lamina. However, based on positional data we interpret the vestigial upper tooth (dCf) and the caniniform (Cf) in Choloepus as deciduous and permanent teeth of the same locus30. Such an occurrence of non-functional vestigial deciduous teeth, rapidly replaced by permanent teeth, has already been reported in other mammalian groups such as marsupials (e.g. Perameles29), soricids (e.g., Sorex, Suncus27,31), and mustelids (e.g., Mephitis, Enhydra32,33). The presence of two dental generations in a folivoran is here reported for the first time. Our results hint at diphyodonty for at least one locus in sloths (i.e., the caniniform in Choloepus), and are consistent with the expectation that ancestral xenarthrans possessed tooth replacement as typical for mammals. It can then be stated that the diphyodonty is a symplesiomorphy of the Xenarthra, as it is shared by the extant sloth Choloepus and the armadillo Dasypus13,34.
Dental homologies between sloths
When considering the dental mineralization sequence in Choloepus, homologies are not obvious between the upper and lower tooth rows. This is partly due to the limited resolution of our ontogenetic sequence and the lack of data on early developmental stages for this genus. The youngest specimen already shows advanced stage of mineralization for the whole dentition. This might explain why vestigial incisors (dVpmx), rapidly resorbed in Bradypus, are not observed in Choloepus.
In contrast, the different ontogenetic stages of Bradypus enable precise hypotheses of dental homologies in extant sloths. Upper caniniforms (dCf) and lower vestigial (dv) teeth are the first teeth to start their mineralization in Bradypus (Fig. 1A–C) and probably belong to the same locus since they do so simultaneously35. The similar development of the dCf in Choloepus and in Bradypus (Fig. 3B,C), both in terms of size and position in the alveolus, and the similar early stages of development between dCf and dv in Bradypus, allow us to hypothesise that upper and lower vestigial mineralized buds of Choloepus (dCf and dv) are homologous. Such an explanation would imply that the upper caniniforms are not homologous in the two extant genera of sloths, with adults Choloepus showing a permanent caniniform (Cf) for that locus while adults Bradypus retain a deciduous caniniform (dCf). Following this hypothesis, the deciduous upper teeth present at a vestigial state in Choloepus would be functional in Bradypus in concert with an absence of a permanent generation for that locus. The large bony crypt long observed during the mineralization of the dCf of Bradypus would then represent an embryological holdover when the permanent tooth primordia (Cf) was still activated for that locus. Such an assumption is supported by a case of bilateral anomaly in Bradypus12 (Fig. 3A) involving both occurrences of mesial dCf and a distally large Cf, which corresponds to the configuration observed in the foetal series of Choloepus.
Alternatively, rather than a retained deciduous caniniform in adult Bradypus, it could be proposed that succession at this locus is not represented in our ontogenetic series for that genus. Following this alternative hypothesis, the upper functional caniniforms of both genera are homologous and correspond to permanent teeth. Then, dCf observed during the ontogeny of Bradypus would correspond to a vestigial deciduous canine that would eventually be replaced later on by a permanent tooth (Cf). This would imply that we are missing several early developmental events in Bradypus, between putative mineralization of Cf and reabsorption of dCf, which appears unlikely considering that we were able to trace the evolution in shape and size of the outline of the mesialmost alveolus (Fig. 2) and that Bradypus is the best-sampled genus in terms of the number of differently sized stages. The first hypothesis is thus preferred here.
Simplified dentition vs the mammalian tooth row
Upper caniniforms (dCf) and lower vestigial (dv) teeth are the first teeth to start their mineralization in Bradypus (Figs 1A and 2A). Following the hypothesis that dCf and dv represent homologous deciduous teeth in Choloepus and Bradypus, these teeth are deciduous canines since the dC is one of the earliest tooth germs to differentiate in eutherian mammals29,35,36. The extreme mesial position of dCf on the maxillary bone (Figs 1 and 2), near the suture with the premaxilla, also provides further support in favour of this attribution.
The mineralization of dCf and dv is followed by the second and third upper molariforms (dMf2-3), which mineralize simultaneously with the first and second lower molariforms (dmf1-2). Such development is reminiscent of the initiation of distal milk premolars in mammals (e.g., dP3-428,29) although the proposed sequence is only based on two foetal Bradypus specimens. The vestigial premaxillary teeth (dVpmx) of Bradypus likely correspond to vestigial incisors that are resorbed during development and are never observed in adult specimens, not even as anomalies12. The relative timing of mineralization of the dVpmx, first upper molariform (dMf1), and lower caniniform (dcf) cannot be precisely determined based on our dataset. However, their mineralization likely occurs shortly before that of the distalmost upper and lower molariform teeth (dMf4 and dmf3) since the latter only show an incipient mineralization in the youngest specimen with evidence bearing on that locus (specimen MNHN CG 1995-326A, Figs 1C and 2C). If the general trends observed when studying the mammalian dental mineralization sequence (e.g.28,29,37) are valid for sloths, the last molariforms (dMf4 and dmf3) should be considered as first molars, preceded by three deciduous premolars (dMf1-3; dcf-dmf2) and a deciduous canine (dCf; dv). Notably, the early diverging living cingulate (Dasypus) has also been interpreted as exhibiting a single, unreplaced M1 locus in each jaw quadrant, preceded by replaced premolariforms and possibly a canine locus34. However, in contrast to Dasypus, no known folivoran shows replacement of functional milk teeth, and there is no empirical evidence to support this hypothesis since the possibility of a violation of the “normal” mammalian sequence cannot be entirely ruled out. The sloth dental formula might include supernumary teeth, as present for instance among the premolars of cingulates13,34 or in Mesozoic groups like docodonts or morganucodonts38.
In any case, these results support the assumption that the upper caniniforms present in adult Bradypus likely represent canines and that the upper and lower caniniforms (dCf, dcf) are not homologous since they mineralize at very different times during ontogeny35. The dcf can then be considered as a premolar locus, which might be homologous to dMf1 when compared their timing of mineralization. Such a hypothesis of non-homology between dCf and dcf was proposed early on and stemmed mainly from the fact that the upper caniniforms in sloths occlude with the mesial surface of the lower caniniforms, while upper canines occlude with the distal edge of the lower canines in other placentals3,24. Simon23 also noted that upper caniniforms of Bradypus erupt well after the lower caniniforms, although some studies on both extinct and extant mammals prefer developmental prenatal dental data over eruption sequences to establish dental homologies29,35.
Reconstructing the ancestral dental formula of sloths
Most fossil sloths show a very similar number of teeth compared to adult specimens of extant species with five upper and four lower teeth. Except for dental anomalies12 and for the dubious fossil sloth Entelops39,40, this number is never exceeded in extinct folivorans (S3). Some mylodontids and nothrotheriids show a reduction in tooth number with a loss of upper caniniforms10 (S3). It is therefore parsimonious to propose an ancestral dental formula of five upper and four lower teeth for Folivora10 and match our reconstruction of the sloth ancestral dental formula (S3). Our developmental data offer new evidence for the loss of teeth during the evolutionary history of sloths in showing that some loci have been retained in foetal stages of extant forms. The vestigial upper incisors found in Bradypus embryos was never reported in any other sloth, but may have been present in the earliest folivorans. The lower vestigial tooth dv was reported in both Bradypus and Choloepus. Given the phylogenetic distance between the two genera7,8,9,10, it is likely that such a vestige was also present in early ontogenetic stages of the most recent common ancestor of Folivora. This idea is corroborated by the rare occurrence of teeth at a similar position in some fossil sloths41,42,43.
We showed that the functional upper caniniforms are very different in the two genera: Choloepus shows an ephemeral and tiny mineralized bud of dCf associated with a massive caniniform Cf, whereas Bradypus shows a moderately-sized, peg-like tooth dCf. Intermediate stages between these extreme patterns may well have occurred in fossil sloths and would have shown the succession of a Cf to a well-mineralized dCf. However, evidence for such a succession (for instance an erupting Cf in juvenile or subadult stages) remains unknown in the fossil record of sloths. This absence might lie in the scarcity of well-documented ontogenetic series for fossil sloths (e.g.18), although relatively few subadult stages are needed to document a succession of dental generations (e.g.44). So far, no succession of dental generations at the Cf locus has been observed in megatherioids, one of the potential allies to Bradypus9,45. Another explanation for the absence of dental replacement at the Cf locus in the fossil record of sloths may actually lie in the potential autapomorphic condition of the pattern observed in Bradypus. This genus is thought to represent a paedomorphic lineage when compared its skull morphology to other folivorans46,47. The retention of the dCf and absence of functional Cf in adults supports the concept of a paedomorphic Bradypus and could constitute another retained juvenile feature. If the retention and subsequent growth of dCf are unique to Bradypus, it is not surprising that no intermediate stage was found in the fossil record as close fossil relatives of three-toed sloths remain virtually unknown45. A highly autapomorphic condition in Bradypus can also account for many morphological discrepancies and could have erased or modified several inherited folivoran synapomorphies in this genus; this could explain why it is retrieved as fully basal10 while it might instead be more apically nested within the folivoran clade9,12.
In Bradypus, the retention of a short rostrum associated with the development of a large dMf1 may have inhibited the development and mineralization of a large permanent caniniform (Cf, Fig. 2D). This ontogenetic pattern gives room for a complete mineralization of the deciduous caniniform (dCf), which remains reduced (Fig. 2E). The growth of dCf seems to be “reactivated” only when the mineralization of all other teeth is well underway (Fig. 2E); only then does it quickly acquire its adult size. Interestingly, such a development of vestigial teeth that recover functionality has been proposed in a few mammalian species that show a reduced and simplified dentition, explained by minor developmental modifications (e.g., frequent recovering of dP4 in the murine rodent, Rhynchomys48). This lends further credence to the findings of Simon23 who noticed the late eruption of the upper caniniforms compared to the lower caniniforms and all molariforms in Bradypus.
Following the hypothesis of an autapomorphic condition in Bradypus, the small dCf mineralized bud observed during the ontogeny of both extant genera might represent an ancestral feature for Folivora. Most of the diversity in shape of the “caniniforms” observed during the evolutionary history of sloths (i.e., caniniform, incisiform, peg-like, entirely absent; see S3) could then originate from a permanent Cf, as in Choloepus. A large caniniform is present in earliest fossil sloths like Octodontotherium49 or Pseudoglyptodon50, and contrasts with our reconstruction of the sloth ancestral dental formula (S3) that is ultimately influenced by the basal rooting position of Bradypus on sloth phylogeny. Our results illuminate a potentially different ancestral dental formula for sloths that challenges the traditional assumption that the bradypodid tooth row is primitive and megalonychid dental features derived10. Such a hypothesis also mitigates the potential weight of dental features in phylogenetical and systematic studies, especially those related to the size and shape of the caniniforms. As a matter of fact, Gaudin10 (p. 275) commented that “the family [Megalonychidae] is united largely by features associated with the caniniform first upper and lower teeth”.
In sloths, the diversification in shape of the mesialmost teeth is frequently associated with a variation in rostral length and the presence of a pre and/or post diastema (S3). Such a diastema, which is often considered as a toothless gap, could challenge the homology of the teeth between taxa. However, the intercalation of additional teeth in the diastema, as observed in armadillos13, seems unlikely because of the relative stability of the dental formula in the sloth fossil record (S3). Our observations are consistent with McAfee’s view on the development of the diastema in Choloepus12, which he proposed could result from an increase of skull length and migration of mesial teeth rather than a loss of teeth between the caniniforms (dCf/Cf-dcf) and molariforms (dMf1-dmf1). The lesser development of the diastema in the youngest stages of Choloepus and the complete absence of vestigial teeth at its level are also in line with this assertion.
In conclusion, we showed that vestigial teeth are informative in understanding dental homologies, especially in assessing the deciduous or successional nature of individual teeth. Our developmental data for extant sloths bear directly on the claim that their lower caniniform teeth are not homologous to canines of other mammals and that upper caniniforms are not homologous between the two-toed and the three-toed sloths. These results underline that defining dental homologies in extant and extinct sloths is complex and that, where possible, characters based on dental features should be augmented with developmental data to ensure proper homology assessment. Development of discrete shapes and functional domains in the tooth row is governed by developmental processes that are still poorly known in mammals and for which further investigations on non-model mammals, such as sloths, are timely and topical.
Methods
We sampled material from collections of the Museum für Naturkunde Berlin (ZMB), the Natural History Museum of London (BMNH), the Muséum National d’Histoire Naturelle in Paris (MNHN), and the Institut Royal des Sciences Naturelles de Belgique in Brussels (IRSNB). A total of 25 unsexed sloth foetuses were examined, representing four species of both extant genera: Bradypus tridactylus, Bradypus variegatus, Choloepus didactylus, and Choloepus hoffmanni51. Species identification was based on collection data (especially geographical origin) and cranial anatomy51,52 and was possible for 17 of our 25 specimens (S2). They range in size from 70 to 200 mm crown rump length (CRL), measured from the vertex of the skull to the base of the tail. Collections of such non-model organisms often include specimens collected decades ago and invariably lack data on individual age. Assignment to a relative developmental stage was based on the Skull Length (SL) and the number of discrete ossification centres throughout the skeleton15,53,54.
3-D data acquisition
Skulls were imaged using high-resolution microtomography (μCT) at the Helmholtz Zentrum (Berlin, Germany), at the Natural History Museum (London, UK), at the AST-RX platform MNHN (Paris, France), and at VISCOM SARL (Saint Ouen l’Aumône, France). This method allows 3D renderings of ossified tissues, as well as non-invasive virtual extractions of dental elements. Due to the scan resolution, we could not test for the putative presence of a small enamel cap at the tips of the forming teeth. These reconstruction and visualization were performed using stacks of digital CT images with the AVIZO 7.1 (Visualization Sciences Group) software. 3D reconstruction of the specimens were deposited in MorphoMuseum (http://www.morphomuseum.com/; M3#109 to M3#115) and Morph-D-base (https://www.morphdbase.de/).
Reconstruction of the ancestral dental morphotype (S3)
The datamatrix of Gaudin10 was downloaded from Morphobank and the following characters of interest were selected for study: character n°2: dental formula; n°6: diastema; n°13: size of Cf ; n°14: size of cf; n°19: morphology of Cf/cf; n°21: position of Cf relative to the anterior edge of the maxilla. The cladogram corresponds to the topology of the strict consensus obtained by Gaudin10: Fig.1 when all characters were weighted equally. For our analysis of character optimizations, this cladogram was pruned in order to contain only sloth taxa (Folivora) (i.e., all non-folivoran successive outgroups originally included in Gaudin’s analysis10 were excluded). Parsimonious reconstruction of the hypothetical ancestral morphotype (S3) for the selected characters was undertaken on this reduced cladogram using the software Mesquite 2.7555.
Additional Information
How to cite this article: Hautier, L. et al. The hidden teeth of sloths: evolutionary vestiges and the development of a simplified dentition. Sci. Rep. 6, 27763; doi: 10.1038/srep27763 (2016).
Supplementary Material
Acknowledgments
We are grateful to M. Herbin, C. Bens, G. Véron, A. Verguin, F. Renoult, C. Denys and J. Cuisin (Museum National d’Histoire Naturelle, Paris), Peter Giere and Frieder Mayer (Museum für Naturkunde, Berlin) Paula Jenkins and Roberto Portela Miguez (Natural History Museum, London), and their colleagues for access to comparative material. N. Karjilov (Helmholtz Zentrum Berlin), R. Abel (Natural History Museum), M. García-Sánz (AST-RX platform, Muséum national d’Histoire naturelle, Paris, France), F. Landru, C. Morlier, G. Guillemain and all the staff from Viscom SARL (St Ouen l’Aumône, France) provided generous help and advice with CT acquisition. We thank Dennyss Lelaurin and Mélanie Canas-Grosso for their help in the data acquisition. We acknowledge financial support from the Grant F/09 364/I from the Leverhulme Trust. This work has benefited from an “Investissements d’Avenir” grant managed by Agence Nationale de la Recherche, France (CEBA, ref. ANR-10-LABX-25-01). This publication is contribution No. ISEM 2016-081 of the Institut des Sciences de l’Evolution de Montpellier (UMR 5554 – UM2 + CNRS + IRD).
Footnotes
The authors declare no competing financial interests.
Author Contributions L.H. initiated the project; L.H. designed the research plan; L.H. and G.B. collected CT data; L.H. reconstructed the 3D data; L.H., H.G.R. and G.B. analyzed the data; L.H., H.G.R., G.B. and R.J.A. discussed the results and wrote the manuscript.
References
- Vizcaíno S. F. The teeth of the ‘“ toothless ”’: novelties and key innovations in the evolution of xenarthrans (Mammalia, Xenarthra). Paleobiology 35, 343–366 (2009). [Google Scholar]
- Parker W. K. On the structure and development of the skull in Mammalia Part II. Edentata. Philos. Trans. R. Soc. London 176, 1–119 (1885). [Google Scholar]
- McDonald H. G. In Morphol. Stud. Foss. extat Xenarthra (Fariña R. A., Vizcaíno S. F. & Storch G.) 5–17 (Senckenbergiana biologica, 2003). [Google Scholar]
- Naples V. L. & McAfee R. K. Chewing through the Miocene: an examination of the feeding musculature in the ground sloth Hapalops from South America (Mammalia: Pilosa). F1000Research 86, 1–22 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomes C. S. A Manual of Dental Anatomy, Human and Comparative. (Presley Blakiston, 1882). [Google Scholar]
- Leche W. Studien über die Entwicklung des Zahnsystems bei den Säugethieren. Morphol. Jahrb. 19, 502–547 (1892). [Google Scholar]
- Delsuc F., Vizcaíno S. F. & Douzery E. J. P. Influence of Tertiary paleoenvironmental changes on the diversification of South American mammals : a relaxed molecular clock study within xenarthrans. BMC Evol. Biol. 13, 1–13 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood A. D., Castresana J., Feldmaier-Fuchs G. & Pääbo S. A Molecular Phylogeny of Two Extinct Sloths. Mol. Phylogenet. Evol. 18, 94–103 (2001). [DOI] [PubMed] [Google Scholar]
- Poinar H., Kuch M., Mcdonald G., Martin P. & Pääbo S. Nuclear gene sequences from a Late Pleistocene sloth coprolite. Curr. Biol. 13, 1150–1152 (2003). [DOI] [PubMed] [Google Scholar]
- Gaudin T. J. Phylogenetic relationships among sloths (Mammalia, Xenarthra, Tardigrada): the craniodental evidence. Zool. J. Linn. Soc. 140, 255–305 (2004). [Google Scholar]
- Delsuc F., Superina M., Tilak M., Douzery E. J. P. & Hassanin A. Molecular Phylogenetics and Evolution Molecular phylogenetics unveils the ancient evolutionary origins of the enigmatic fairy armadillos. Mol. Phylogenet. Evol. 62, 673–680 (2012). [DOI] [PubMed] [Google Scholar]
- McAfee R. K. Dental anomalies within extant members of the mammalian Order Pilosa. Acta Zool. 96, 301–311 (2014). [Google Scholar]
- Martin B. E. Tooth development in Dasypus novemcinctus. J. Morphol. 27, 647–691 (1916). [Google Scholar]
- Hautier L., Weisbecker V., Sánchez-Villagra M. R., Goswami A. & Asher R. J. Skeletal development in sloths and the evolution of mammalian vertebral patterning. Proc. Natl. Acad. Sci. USA 107, 18903–18908 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautier L. et al. Skeletal ossification and sequence heterochrony in xenarthran evolution. Evol. Dev. 13, 460–476 (2011). [DOI] [PubMed] [Google Scholar]
- Böker H. Beobachtungen und Untersuchungen an Säugetieren (einschließlich südamerikanischer Edentaten) während einer biologisch-anatomischen Forschungsreise nach Brasilien. Morphol Jahrb 70, 1–66 (1932). [Google Scholar]
- Gervais P. Remarque au sujet du système dentaire de l’aï. J. Zool. 437–437 (1873). [Google Scholar]
- Cartelle C. & De Iuliis G. Eremotherium Laurillardi (Lund) (Xenarthra, Megatheriidae), the Panamerican giant ground sloth: Taxonomic aspects of the ontogeny of skull and dentition. J. Syst. Palaeontol. 4, 199–209 (2006). [Google Scholar]
- Bargo M. S., Vizcaíno S. F. & Kay R. F. Predominance or orthal masticatory movements in the early miocene Eucholaeops (Mammalia, Xenarthra, Tardigrada, Megalonychidae) and other Megatherioid sloths. J. Vertebr. Paleontol. 29, 870–880 (2009). [Google Scholar]
- Pujos F., Iuliis G. De. & Quispe B. M. Hiskatherium saintandrei, gen. et sp. nov.: an unusual sloth from the Santacrucian of Quebrada Honda (Bolivia) and an overview of middle Miocene, small megatherioids. J. Vertebr. Paleontol. 31, 1131–1149 (2011). [Google Scholar]
- Brandts P. Dissertation Inaugural de Tradigradis (Lugduni Batav, 1828). [Google Scholar]
- Röse C. Beiträge zur Zahnentwicklung der Edentaten. Anatomischer Anzeiger. 7, 495–512 (1892). [Google Scholar]
- Simon L. Beiträge Anatomie und Entwicklung der Bradypodiden. 68, 239–260 (1902). [Google Scholar]
- Naples V. L. Cranial osteology and function in the tree sloth, Bradypus and Choloepus. Am. Museum Novit. 2739, 1–41 (1982). [Google Scholar]
- Peterkova R., Lesot H. & Peterka M. Phylogenetic memory of developing mammalian dentition. J. Exp. Zool. B. Mol. Dev. Evol. 306, 234–250 (2006). [DOI] [PubMed] [Google Scholar]
- Järvinen E., Tummers M. & Thesleff I. The role of the dental lamina in mammalian tooth replacement. J Exp Zool 312, 281–291 (2009). [DOI] [PubMed] [Google Scholar]
- Järvinen E., Välimäki K., Pummila M., Thesleff I. & Jernvall J. The taming of the shrew milk teeth. Evol Dev 10, 476–485 (2008). [DOI] [PubMed] [Google Scholar]
- Luckett W. P. Ontogenetic staging of the mammalian dentition, and its value for assessment of homology and heterochrony. J. Mamm. Evol. 1, 269–282 (1993). [Google Scholar]
- Luckett W. P. In Mammal phylogeny Mesozoic Differ. multituberculates, monotremes, early therians, marsupials. (Szalay F. S., Novacek M. J. & McKenna M. C.) 182–204 (Springer, 1993). [Google Scholar]
- Moss-Salentijn L. In Dev. Funct. Evol. teeth (Butler P. M. & Joysey K. A.) 13–29 (Academic Press, 1978). [Google Scholar]
- Yamanaka A., Yasui K., Sonomura T., Iwai H. & Uemura M. Development of deciduous and permanent dentitions in the upper jaw of the house shrew (Suncus murinus). Arch Oral Biol 55, 279–287 (2010). [DOI] [PubMed] [Google Scholar]
- Verts B. J. The biology of the striped skunk. (University of Illinois Press, 1967). [Google Scholar]
- Kenyon K. The sea otter in the eastern Pacific Ocean. N Am Fauna 68, 1–352 (1969). [Google Scholar]
- Ciancio M. R., Castro M. C. & Asher R. J. Evolutionary implications of dental eruption in Dasypus (Xenarthra). J. Mamm. Evol. 19, 1–8 (2012). [Google Scholar]
- Luckett W. P. & Maier W. Development of Deciduous and Permanent Dentition in Tarsius and Its Phylogenetic Significance. Folia Primatol. 37, 1–36 (1982). [DOI] [PubMed] [Google Scholar]
- Butler P. In Dent. Anthropol. (Brothwell D.) 1–13 (Pergamon Press, 1963). [Google Scholar]
- van Nievelt A. F. H. & Smith K. K. To replace or not to replace: the significance of reduced functional tooth replacement in marsupial and placental mammals. Paleobiology 31, 324–346 (2005). [Google Scholar]
- Luo Z.-X., Kielan-Jaworowska Z. & Cifelli R. Evolution of dental replacement in mammals. Carnegie Mus Nat Hist Bull 36, 159–175 (2004). [Google Scholar]
- Pascual R. Una nueva superfamilia ‘Entelopsoidea’ descripcion de la nueva especie ‘Entelops parodii’. Acta Geol. Lilloana 3, 127–146 (1960). [Google Scholar]
- Hoffstetter R. Les Edentés Xénarthres, un groupe singulier de la faune néotropicale (origine, affinités, radiation adaptative, migrations et extinctions). In Proc. First Int. Meet. “Palaeontology, Essent. Hist. Geol. 385–443 (1982). [Google Scholar]
- Gervais P. Zoologie et Paléontologie générales: Nouvelles Recherches sur les Animaux vertébrés vivants et fossiles Vol. 1 (Libraire de la Société de Géographie, 1867). [Google Scholar]
- Burmeister H. Atlas de la description physique de la république Argentine. Mammifères (Coni, 1881). [Google Scholar]
- Lydekker R. Contribution to a knowledge of the fossil Vertebrates of Argentina 2: The extinct Edentates of Argentina. An. del Mus. La Plata, Paleontol. Argentina 32, 1–118 (1895). [Google Scholar]
- Cifelli R. L. et al.Fossil evidence for the origin of the marsupial pattern of tooth replacement. Nature 379, 715–718 (1996). [Google Scholar]
- Patterson B. & Pascual R. The fossil mammal fauna of South America. Q. Rev. Biol. 43, 409–451 (1968). [Google Scholar]
- Patterson B., Seagall W., Turnbull W. D. & Gaudin T. J. The ear region in Xenarthrans (=Edentata, Mammalia). Part II. Pilosa (slogs, anteaters), palaeanodonts, and a miscellany. Fieldiana, Geol. 24, 1–79 (1992). [Google Scholar]
- Gaudin T. J. The ear region of edentate and the phylogeny of the tardigrada (Mammalia, Xenarthra). J. Vertebr. Paleontol. 15, 672–705 (1995). [Google Scholar]
- Charles C., Solé F., Gomas Rodrigues H. & Viriot L. Under Pressure? Dental Adaptations To Termitophagy and Vermivory Among Mammals. Evolution (N. Y.). 67, 1792–1804 (2013). [DOI] [PubMed] [Google Scholar]
- Shockey B. J. & Anaya F. Grazing in a New Late Oligocene Mylodontid Sloth and a Mylodontid Radiation as a Component of the Eocene-Oligocene Faunal Turnover and the Early Spread of Grasslands/Savannas in South America. J. Mamm. Evol. 18, 101–115 (2011). [Google Scholar]
- McKenna M. C., Wyss A. R. & Flynn J. J. Paleogene Pseudoglyptodont Xenarthrans from Central Chile and Argentine Patagonia. Am. Museum Novit. 3536, 1–18 (2006). [Google Scholar]
- Wetzel R. M. In Evol. Ecol. armadillos, sloths vermilinguas (Montgomery G.) 5–21 (Smithsonian Institution Press, 1985). [Google Scholar]
- Hautier L., Billet G., Eastwood B. & Lane J. Patterns of Morphological Variation of Extant Sloth Skulls and their Implication for Future Conservation Efforts. Anat. Rec. 297, 979–1008 (2014). [DOI] [PubMed] [Google Scholar]
- Hautier L. et al. Patterns of ossification in southern versus northern placental mammals. Evolution (N. Y.). 67, 1994–2010 (2013). [DOI] [PubMed] [Google Scholar]
- Hautier L., Stansfield F. J., Allen W. R. T. & Asher R. J. Skeletal development in the African elephant and ossification timing in placental mammals. Proc. R. Soc. B Biol. Sci. 279, 2188–2195 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison W. P. & Maddison D. R. (2011). Mesquite: A Modular System for Evolutionary Analysis, Version 2.75. University of British Columbia, Vancouver, Canada. URL http://mesquiteproject.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.