Abstract
This review compares the biological and physiological function of Sigma receptors [σRs] and their potential therapeutic roles. Sigma receptors are widespread in the central nervous system and across multiple peripheral tissues. σRs consist of sigma receptor one (σ1R) and sigma receptor two (σ2R) and are expressed in numerous regions of the brain. The sigma receptor was originally proposed as a subtype of opioid receptors and was suggested to contribute to the delusions and psychoses induced by benzomorphans such as SKF-10047 and pentazocine. Later studies confirmed that σRs are non-opioid receptors (not an µ opioid receptor) and play a more diverse role in intracellular signaling, apoptosis and metabolic regulation. σ1Rs are intracellular receptors acting as chaperone proteins that modulate Ca2+ signaling through the IP3 receptor. They dynamically translocate inside cells, hence are transmembrane proteins. The σ1R receptor, at the mitochondrial-associated endoplasmic reticulum membrane, is responsible for mitochondrial metabolic regulation and promotes mitochondrial energy depletion and apoptosis. Studies have demonstrated that they play a role as a modulator of ion channels (K+ channels; N-methyl-d-aspartate receptors [NMDAR]; inositol 1,3,5 triphosphate receptors) and regulate lipid transport and metabolism, neuritogenesis, cellular differentiation and myelination in the brain. σ1R modulation of Ca2+ release, modulation of cardiac myocyte contractility and may have links to G-proteins. It has been proposed that σ1Rs are intracellular signal transduction amplifiers. This review of the literature examines the mechanism of action of the σRs, their interaction with neurotransmitters, pharmacology, location and adverse effects mediated through them.
Keywords: Apoptosis, cannabinoids, central nervous system, glutamate, neoplasia, non-opioid receptors
Introduction
Sigma receptors [σRs] are a relatively novel group of receptors originally discovered in the central nervous system [CNS] of mammals in 1976 (1). They represent a ubiquitously expressed unique binding site in the CNS and other peripheral tissues (2–6). σRs are a member of the orphan receptor class for which no endogenous ligand was known until recently – dimethyltryptamine [DMT] (7–9). They also bind with high affinity to several classes of chemically unrelated ligands such as neurosteroids (10), neuroleptics, dextrobenzomorphans [DEX] and several psychostimulants such as cocaine (11), methamphetamine [METH] (12,13) methylenedioxymethamphetamine [MDMA] (14) and methacathinone (15,16). Consequently, it is thought that the σR may mediate the immunosuppressant, antipsychotic and neuroprotective effects of many drugs (17).
Historically, the σR was identified as one of the subtypes of opiate receptors, differentiated using a chronic spinal pain model in the dog, the unique psychomimetic effects induced by N-allylnormetazocine [SKF-10,047] (18) (σ-syndrome), from the effects induced by morphine (µ-syndrome) and ketocyclazocine (κ-syndrome) (1). However, subsequent studies established that σR sites possess negligible affinity for naloxone or naltrexone (19,20); thus, establishing a complete distinction between the non-opiate σ binding sites and the classical µ-, δ- and µ-opiate receptors (21,22). It has recently been suggested that σ1R antagonism be used with opioids to increase pain control without increasing the adverse effects of the opioids (23).
Two subtypes of σRs were found originally: sigma-1 [σ1R] and sigma-2 [σ2R] (24–27). Although another subtype, sigma-3 [σ3R], has been suggested, it has not been defined adequately (28,29). σ1Rs have been cloned (2), assayed (30) and their biological and physiological roles have been examined more intensively than σ2Rs, as until now σ2Rs have not been cloned (31).
σ1Rs regulate a number of neurotransmitter systems, including the glutamatergic [Glu], dopaminergic [DA], serotonergic [5HT], noradrenergic [NE] and cholinergic [Ch] systems. As these transmitters, which interact with the σ1Rs, are involved in many neuropsychiatric disorders their role has been evaluated in a number of these disorders (32). In fact, several lines of evidence have demonstrated that σ1R play a role in the pathophysiology of neuropsychiatric disorders such as mood (33), anxiety disorders (34,35) and schizophrenia (9).
Hence, σR ligands are potential therapeutic agents for several neuropsychiatric disorders (36,37). σ1R has also been suggested as a target for the treatment of neuropathic pain (38,39) and a treatment for dementia, such as seen associated with Alzheimers disease [AD] (40). In addition, σ1R mutations have been implicated in frontotemporal lobar degeneration and motor neuron disease [MND] (41), diseases in which they have been shown to have a low density (42). It appears that there is an association between a variant of the σ1R gene and AD (43) where genetic polymorphisms in σ1R and apolipoprotein E interact to influence the severity of AD (44).
Many psychostimulant drugs, including cocaine (45) and METH (46,47), interact with σRs in the brain and heart, offering a logical target for medication development efforts (48). σR antagonists and antisense oligonucleotides ameliorate cocaine-induced convulsions, lethality and locomotor activity (49,50), as well as sensitization, and conditioned place-preference in rodents (51). They also reduce alcohol consumption in alcohol-drinking rats (52,53) and Swiss mice (54). Interestingly, the interaction of fluvoxamine [Luvox], a selective serotonin reuptake inhibitor [SSRI], and the σRs may account for its potential amelioration of psychotic depression (55,40), where increased glutamate [Glu] release occurs through activation of serotonin [5-HT3] mediated by σ1Rs (56), and in patients with schizophrenia (57,40). These findings are supported by research on a depressive phenotype in σ1R knockout mice (53). In contrast, the SSRI sertraline worsens the symptoms (58). Not all SSRIs induce their antidepressant activity via the σ1R, e.g. paroxetine (59). This detailed review explores the σRs in normal homeostatic and diseased states. First, the structure and function of these receptors are described. Next, sites of σRs, disease states and their relationship to σRs are discussed.
Molecular biology of σRs
Due to their CNS pharmacological action, most work has been focused on evaluation of σRs in the CNS; however, considerable current research has also been directed toward neoplasia, its treatment and imaging (σ2R) (60). σRs are highly expressed in all parts of the brain (25,61,62), where they are predominantly localized in the cell plasma membrane and at the endoplasmic reticulum [ER] of both neurons and oligodendrocytes (63). They are dynamically translocated upon ligand binding into cells from the cell membrane (64–66). σ1Rs agonists provide protection of the ER from oxidative stress (67).
More recently, a σ1R receptor knockout mouse has been developed that displays a depressive-like phenotype, supporting the receptors importance in this psychiatric disorder (53). The database concerning the molecular biology of σRs is large.
Sigma-1 receptors [σ1Rs]
The two subclasses of σR sites (σ1R and σ2R), distinguished based on their different drug selectivity patterns and molecular weights (21) have no homology to any other mammalian protein (2,68). However, several biochemical features have been observed for σ1Rs, such as an allosteric modulation by phenytoin (69) and sensitivity to pertussis toxin or G-protein modulators (70–73), probably though potentiation of opioid transduction independent from receptor binding (74). The σ1R site also shows a stereo selectivity with high affinity for the dextro isomers of benzomorphans [BZM], whereas σ2R sites show the reverse stereo selectivity with a lower affinity range. 1,3,Di-O- tolylguanidin [DTG], 3-(3-Hydroxyphenyl)-N-n-propyliperidin (+) 3-PPP [preclamol] and haloperidol [Haladol®] are non-discriminating ligands with high affinity for both σ1R and σ2R subtypes (75).
The σ1R is a 29 kDa single polypeptide that has been cloned in mice, rats and humans (2,3,6,76,77), the ligand binding profile of which is similar to those described in brain homogenates studies (78,79). The σ1R gene, located on chromosome 9, band p13, in human and chromosome 2 in rodents, is approximately 7 kbp long and contains four exons, interrupted by three introns, where exon 3 is the shortest (93 bp) and exon 4 is the longest (1132 bp) (68). Exon 2 encodes 25 kDa membrane proteins for the single transmembrane domain, identified at present, but two other hydrophobic regions exist and one of them may putatively constitute a second transmembrane domain (80).
The σ1R sequence contains a 22 amino acid [AA] retention signal for the ER at its N-terminal region and two short C-terminal hydrophobic AA sequences that are probably involved in sterol binding (2). The 223 amino acid sequence of the purified protein is highly preserved, with 87–92% identity and 90–93% homology among tissues and animal species (81). This protein is identical in peripheral tissues and brain, and probably is similar in other tissues as well. It shares a similarity, 33% identity and 66% homology, with a sterol C8–C7 isomerase (82), but nevertheless is different from any other mammalian protein identified (2,68), outlining the uniqueness of the σ1R as compared with any other known receptor.
Hydropathic analysis of the σ1R indicates three hydrophobic regions, with some evidence for two transmembrane segments. A crystal structure of the σ1R was unavailable at the time of writing, but a 3D model has recently been validated showing agreement of the in vitro and the in silico model (83).
The σ1R gene also has been isolated from human, guinea pig, mouse and rat (2,6,76). AA substitutions in transmembrane domains do not alter the expression levels of the protein but suppresses ligand binding activity (80), suggesting that these AAs belong to the binding site pharmacophore located within the transmembrane domain. In addition, anionic AA residues have been identified that also appear critical for ligand binding (68,77).
Exon-2 codes for a single transmembrane domain present in the σR (68). The fact that the gene for the σ1R is located on chromosome 9p13, a region associated with psychiatric disorders (68), helps explain the psychiatric effects of σ1R agonists and antagonists.
A splice variant of the σ1R has been found in Jurkat cells, an immortalized line of T-lymphocyte cells (84) and in mice (85). Interestingly, σ1R-splicing variants have been reported to display σ2R characteristics (86,87).
The σ1R has been cloned from guinea pig and mouse liver, human placental cell line, and human, mouse and rat brain (2–6). The protein cloned is a 223 AA, 1 transmembrane protein with potent (+)-pentazocine [PTZ], haloperidol, ditolylguanidine (1,3,di-O-tolylguanidin) [DTG] and (+)-3-PPP binding, but does not couple with G-proteins (5,76).
At this point, it is not completely clear whether the cloned σ1R is the ligand binding subunit of a multi-subunit complex or represents one subtype of the σ1R. A study investigating putative transmembrane segments based on homology identified two putative transmembrane segments for the σ1R (88). Thus, as research investigates the σRs further, subtypes of the σ1R, σ2R and possibly the σ3R might be found.
Regardless, cloning has led to an important focus on the molecular biology and signal transduction mechanisms of σ1R, e.g. inhibition of Ca2+ entry into epithelial cells (89). This is discussed in more detail in Sections “σ1R ligands” and “Neoplasia”. However, given the one-transmembrane segment cloned, it is most likely that it does not represent the complete functional receptor. More experiments using techniques such as the use of selective σ1R gene antisense will elucidate the exact structure of the functional σR in the future (63).
Sigma-2 receptors [σ2Rs]
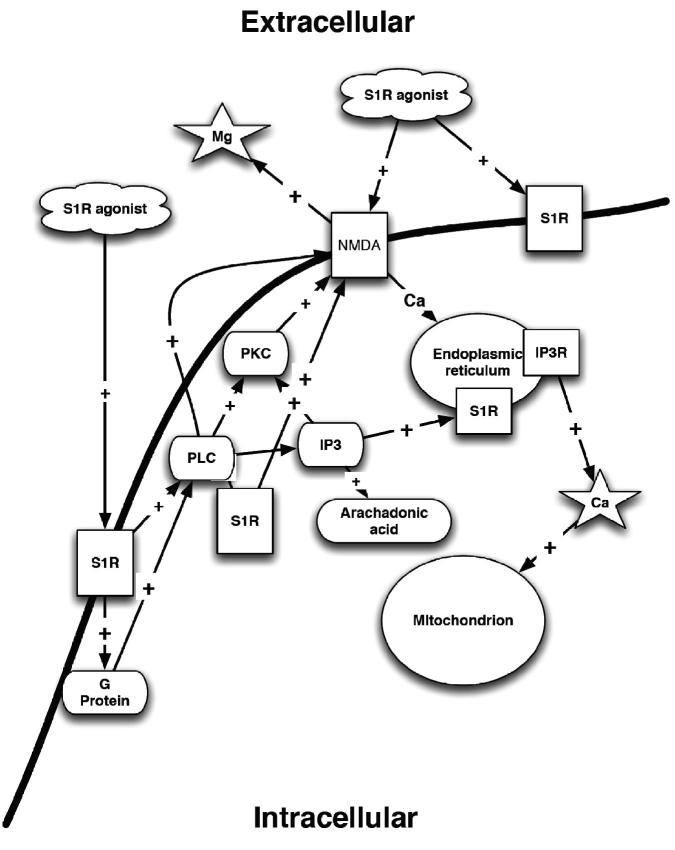
The σ2R site has not been cloned as of yet, but a comprehensive ligand based mapping of the receptor binding pocket has been done (90). The σ2R site was first characterized in pheochromocytoma PC12 cells (91), and has a low affinity for (+)-BZM and has an apparent molecular weight of 18 to 21 kDa (92). Some selective and high affinity σ2R site ligands are now available such as 1′-(4-(1-(4-fluorophenyl))-1H-indol-3-yl)-1-butyl)spiro (isobenzofuran-1(3H),4′piperidine [Lu 28-179] (93), N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl) ethylamine [BD1008] (92), and ibogaine (94). The site also appears to be important in the modulation of cellular Ca2+ concentrations (Figure 1) (95).
Figure 1.
σRs and their effect on intracellular calcium concentrations. PLC – phospholipases C; PKC – protein kinase C; S1R – sigma1; IP3 – inositol triphosphate; IP3R – inositol triphosphate receptor; NMDA – N-methyl-D-aspartate receptor; Mg – magnesium; Ca – calcium.
Several attributes have been proposed for σ2R sites: stem cell differentiation (96); regulation of motor functions (97–99), induction of dystonia after in situ administration in the red nucleus (97), regulation of ileal function (100). The sites are also important in the blockade of tonic K+ channels (101), potentiation of the neuronal response to N-methyl-d-aspartate [NMDA] in the CA3 region of the rat dorsal hippocampus (102), or activation of a novel p53- and caspase-independent apoptotic pathway. The mechanism of the induction of apoptosis is distinct from other apoptotic stimuli (103).
The σ2R is an σR that preferentially binds to siramesine® (26), selective σ2R agonist and also PB28 (104). Activation of the σ2R causes apoptosis (104) via triggering of cancer selective cell death signaling (105) by multiple pathways (106). This finding is an important observation for potential antineoplastic drug development. The mechanism by which σ2R stimulation induces apoptosis may result from its modulation of intracellular Ca2+ stores in some tumors (95). This is of particular importance in those tumors that induce hypercalcemia, e.g. some lymphomas.
The molecular nature of the σ2R is still to be fully characterized; however, a structure-affinity and comparative molecular field analysis of σ2R receptor ligands has been reported (107). A photo affinity labeling study, using DTG, revealed the existence of two protein bands of MW 25 000 and 21 500 (92). Because the σ1R has been cloned (6,77) and shown to be a protein of MW 25 300, it has been presumed that the σ2R gene encodes a protein of MW 21 500.
Despite efforts to define the gene for the σ2R, it remains unidentified. It has been suggested that the σ2R characteristics are, in fact, a consequence of σ1 gene alternative splicing (108). However, in the σ1R knockout mouse, although σ1R-specific drug binding is significantly reduced, binding of nonspecific σR drugs, such as DTG, is not affected, suggesting that the σ2R is unaffected (63).
Recently, a novel iodinated σ2R ligand (a conformationally-flexible benzamide derivative, 5-bromo-2,3-dimethoxy-N-[2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-butyl]-benzamide, which has 1000-fold selectivity for σ2R) has been evaluated as a cell proliferation marker (109).
σ2Rs have been implicated in a number of neoplasms, e.g. pancreatic carcinoma (110), urinary bladder tumors (111,112) and breast tumor cell lines (103); therefore, they have been primarily investigated for possible use as cancer chemotherapy targets (113). A more detailed discussion regarding the σ2Rs and neoplasia can be found later in the Section “Neoplasia”.
Sigma3 receptors [σ3Rs] have been proposed (28,29) and were suggested to be linked to the conversion of tyrosine to dopamine [DA] and the activation of protein kinase C [PKC] (114). Here, the proposed σ3R agonists may increase the rate of DA synthesis. In addition, putative σ3Rs have been imaged in the mammalian brain, and appear to have histamine receptor [H1R] properties (115,116). Regardless of these findings, the molecular basis for this diversity is not clear, and the limited amount of literature regarding the subject questions whether the σ3Rs really exist, or whether they are a subtype of σ1Rs or σ2Rs.
Mechanism of action
σ1Rs are intracellular receptors acting as chaperone proteins (46,117). Chaperone proteins assist in the correct folding of other proteins, either during their synthesis or function (118). More specifically, σ1Rs modulate Ca2+ signaling through the inositol triphosphate [IP3] receptor. They dynamically translocate inside cells, hence are transmembrane proteins (118). In fact, it has been suggested that the σ1R receptor at the mitochondrial-associated endoplasmic reticulum membrane is responsible for mitochondrial metabolic regulation (119). σ1R also promotes mitochondrial energy depletion, Ca2+ influx and apoptosis (120). The σ1R chaperone protein can be activated or deactivated by specific ligands (121).
These σ1R chaperones act at the functional inositol triphosphate receptor [IP3R] to the ER and mitochondrion interface to ensure proper Ca2+ signaling from ER into mitochondrion. However, under pathological conditions where cells encounter excess stress that results in the ER losing its global Ca2+ homeostasis, the σ1R translocates and counteracts the potential apoptosis. Thus, the σ1R is a receptor chaperone essential for the metabotropic receptor signaling and for the survival against cellular stress (46). σ2R is now thought to be a histone binding protein (111).
Although the precise mechanism of the biological response of σRs is still uncertain, it is accepted that σR can modulate a number of neurotransmitter systems, including neurosteroids (49), glutamatergic [Glu] (56), noradrenergic [NA] (122) and dopaminergic [DAergic] ones (26,98) thought to be especially important functional modulators of Glu activity at this site (123–128).
Neurochemical and electrophysiological studies have been crucial in revealing that the σRs regulate the NMDA receptor-mediated glutamatergic, cholinergic and catecholaminergic neuronal responses (26,129,130). σ1Rs, at least in part, are intracellular amplifiers creating a super sensitized state for signal transduction (82,131).
Signal transduction by σRs
The cloning of a one transmembrane domain σ1R, which does not correspond to a G-protein-coupled receptor, reactivated the debate over whether or not σRs act through G-protein-dependent signaling cascades (132). Manipulation of G-proteins alters σR-mediated effects on K+ currents (133), acid sensing ion channels (134) and NMDA-evokes release of [3H]norepinephrine [NE] (135–137). Yet this manipulation has no effect on K+ currents in other models, or on the NMDA response with other σR ligands (138,139). Contrasting evidence exists for the effects of G-proteins on σ1R ligand binding (140–143). Therefore, the data concerning the mechanism by which σRs act at the cell membrane level is often conflicting, if not controversial. Given the presumed heterogeneity of the σ1R subgroup, it is likely that one subtype of the σR interacts with G-proteins, while another subtype relies on G-protein-independent signal transduction mechanisms, probably via NMDAR.
G-proteins
Studies on the modulation of ion channels by σ1Rs have made advances in deducing the nature of the signal transduction mechanism (144). It has been suggested, despite the lack of homology between the σ1R and classic G-protein-coupled receptors, that σ1Rs use G-proteins (74,133,145,146). Accordingly, the σ1R could interact functionally with G-proteins through a mechanism that differs from that of classical G-protein-coupled receptors (147). However, many physiological experiments suggest that σ2R signal transduction does not involve any G-protein. Experiments on rat neurohypophysis also produced negative results for secondary messenger or G-protein mediation of σ1R signaling (138). This finding may be a result of the dose response curve previously described.
In support of σRs’ association with G-proteins, manipulating GTP and 5 guanylylimidodiphosphate [Gpp(NH)p] alters the binding of σR some ligands (70,71,148,149). Contrasting results have also been found for the effects of G-proteins on σ1R ligand binding (142,143). Chronic treatments with haloperidol [Haladol®] in rats cause decrease responsiveness to guanine nucleotides following repeated exposure (72). Some selective σR agonists stimulate GTPase activity (132).
The mechanisms of these σR effects are not well understood, even though σ1Rs have been linked circumstantially to a wide variety of signal transduction pathways (150). Links between σ1Rs and G-proteins have been suggested, but there is also some evidence against this hypothesis (142). Regardless of their involvement of G-proteins, it is more likely that σ1Rs act through the NMDAR rather than through these G-proteins (138,139,151).
Ion channels and cations
In support of the majority of effects of σ1R stimulation being mediated by the ionotropic glutamate receptors [iGluRs], such as the NMDAR, the σ1R has been shown to appear in a complex with voltage-gated K+ channels, leading to the suggestion that these receptors are auxiliary subunits of the voltage-gated channels (88,138). For example, K+ conductance is the prominent target of σ1R in rat cortical synaptosomes, C6 glioma cells (101), NCB-20 cells (152), rat neurohypophysis (139) and frog melanotropic cells (133,145).
Calcium
An interaction between σRs and Ca2+ channels is probable, as (+)-PTZ inhibits the rise in Ca2+ levels induced by depolarization of cell membranes and σR ligands decrease basal intracellular Ca2+ concentration ([Ca2+]i). This finding supports the hypothesis that the σR activation alone affects [Ca2+]i (2,153,154) and that the σ1R is likely coupled to the nicotine-receptor-associated Ca2+ ionophore (155).
σR-induced increases in Ca2+ currents, which develop progressively following relatively long lasting applications of σR ligands, suggest a direct intracellular coupling of σR to Ca2+ channels, through which σR ligands can stimulate voltage-activated Ca2+ conductance, independent of the K+ channel pathway (156). It is possible that an atypical σ1R subtype might also interfere with [Ca2+]i homoeostasis (153,154,157).
In rat sympathetic and parasympathetic neurons, σRs have been shown to modulate high-voltage-activated Ca2+ channels including N-, L-, P/Q- and R-type Ca2+ channels (158). Although σ2R -selective σR ligands were not used, the rank order potency observed, which was haloperidol > ibogaine (an indole alkaloid (159) > (+)-PTZ > DTG, would suggest that this effect may be mediated by σ2Rs. In addition to reducing the peak amplitude of the Ca2+ current, σRs altered the kinetic properties of these channels.
Several lines of evidence have added further arguments for the involvement of σ1R in Ca2+ signaling (160). Specifically, the σ1R ligands (+)-PTZ and PRE-084 modulate Ca2+ signaling in NG108 cells via σ1Rs by two different modes of action. Firstly, intracellularly, perhaps on the ER, σ1R ligands potentiate bradykinin-induced increase in cytosolic free Ca2+ in a biphasic manner, which can be blocked by σ1R antisense oligodeoxynucleotide (161), and a second mode of action at the plasma membrane (153,161).
However, the NMDA receptor is probably involved, as such an interaction explains the potentiating action of σ1R drugs on NMDA receptor-mediated responses (137,162,163). Further support for this notion is provided by the parallel between their effect on [Ca2+]i mobilization and on the neuronal response to NMDA (135,163,164). It is possible that the major physiological function of the σ1R in the CNS is to regulate both types of intracellular Ca2+ equilibrium (165).
The changes reported above may cause the reported amplification of Glu, acetylcholine [ACh] and DA responses via the σ2R (82,157,164). For example, DTG decreases, whereas reduced haloperidol increases, [Ca2+]i mobilization in colon and mammary adenocarcinoma cells independently of any effect on Ca2+ entry through the plasma membrane (153,166). These observations suggest that the biological effect of σ1R drugs may be more complex in the regulation of the [Ca2+]i equilibrium; regardless, these results give support to the suggestion that σ2R also impacts [Ca2+]i homoeostasis (95,135,153,167).
It has been proposed that the modulation of Ca2+ signaling mediated by σ1Rs involves the formation of a multiprotein complex, or σ1Rs that form multiunit complexes responsible for the modulation of these ion channels (163,165). Specifically, σ1Rs have recently been found to anchor ankyrin, a cytoskeletal adaptor protein, to the ER membrane and modulate the function of ankyrin and IP3 on the ER (82,164). In this model, the presence of the σR agonist (+)-PTZ leads to the σ1R-ankyrin complex dissociating from the IP3 (168). This dissociation leads to an increased binding of IP3, which in turn increases Ca2+ efflux. On the other hand, in the presence of the σ1R antagonist NE-100 (156), the σ1R dissociates from ankyrin, which remains coupled to IP3 on the ER (164).
According to the heterogeneity of the σR subtypes, it has been proposed that in the guinea-pig brain, which expresses mainly the σ2R protein, bivalent cations zinc [Zn2+], nickel [Ni2+], sodium [Na+], strontium [Sr2+], magnesium [Mg2+] and Ca2+ inhibit [3H]DTG binding in a monophasic manner within a micromolar concentration range (169). However, [3H](+)-PTZ binds in a biphasic manner within an mM concentration range, thereby supporting a hypothesis of preferential involvement of the σ2R subtype as modulator of Ca2+ entry (170). Subsequent dissociation experiments performed with [3H]DTG show that verapamil and amidirone, but not nifedipine, BAY-K8644 or amiloride, enhanced the dissociation of [3H]DTG from σR-binding sites further supporting the involvement of σ2R in the modulation of Ca2+ channels.
Potassium
K+ conductance is the prominent target of σ1R in rat cortical synaptosomes, C6 glioma cells (101), NCB-20 cells (152) rat neurohypophysis (139), or frog melanotropic cells (101,133,145,146). An observation has been made that there is interaction between σRs and K+ channels. Here the σR ligands DTG and (+)-PTZ inhibit K+ currents (133,138,139).
The inhibition of K+ channels by σR agonists and antagonists in NCB-20 cells is not affected by pretreatment with A23187, forskolin, phorbol-12,13-dibutyrate, cholera toxin, or pertussis toxin has been shown (152). These results are consistent with the well-known intracellular secondary messenger systems not being essential for the modulation of voltage-gated K+ channels by σ1R.
Further investigations of this modulation suggest that a protein-protein interaction is the likely mechanism of signal transduction by σRs, as σR ligands do not interact directly with K+ channels (88,138), although this effect is enhanced in the presence of σR ligands (138). Therefore, σRs may serve as auxiliary subunits to voltage-gated K+ channels in the plasma membrane (88), which also may involve other proteins such as ankyrin and IP3R.
Studies on σ1R modulation of K+ channels, to date, have led to the conclusion that the signal transduction mechanism of σ1Rs is membrane independent of G-protein coupling and protein phosphorylation (158) reconstructable in a heterologous system, not requiring cytoplasmic factors, and necessitating the σ1R and the K+ channel to be in close proximity (138), probably to form a stable macro-molecular complex (88).
Additional studies are required to determine whether the σ1R modulation of K+ channels is through a direct protein–protein interaction or through intermediate signaling molecules. Given the wide variety of functions that the σ1Rs are reported to serve, the most likely explanation is a σ1R signaling mechanism involving one or more intermediate signaling molecules, which are localized at or in the plasma membrane, rather than a direct interaction.
σ1R as an intracellular amplifier
Acute activation of the σ1R results in a direct modulation of ([Ca2+]i) mobilization (161,163), and prevents intracellular Ca2+ dysregulation in neurons follow an ischemic event. After depletion of intracellular Ca2+ from ER stores, the depolarization-induced increase in [Ca2+]i in the cells is modulated by σ1R agonists. Both effects are blocked by an antisense oligodeoxynucleotide targeting the σ1R (161). Therefore, activation of the σ1R results in a complex, bipolar modulation of Ca2+ homeostasis.
At the ER level, the σ1R activation facilitates the mobilization of IP3R-gated intracellular Ca2+ pools. This change also occurs at the plasma membrane level. A co-immunoprecipitation study further revealed that the σ1R could regulate the coupling of the IP3R with the cytoskeleton via an ankyrin B anchor protein, a cytoskeletal protein originally attached to ER membranes (164).
As stated previously, activation of the σ1R dissociates ankyrin B from IP3R in NG-108 cells, and this dissociation correlate with the efficacy of each ligand in potentiating the Ca2+ efflux induced by bradykinin. These results, in conjunction with the σ1R subcellular localization (171,165), show that the σ1R might act as a sensor or modulator for the neuronal intracellular Ca2+ mobilizations and consecutively for extracellular Ca2+ influx.
Stimulation of the σ1R results in its translocation from the ER (64,163,164), via lipid droplets, to plasma membranes when stimulated by agonists (65,172,173). Thus the translocation of σ1Rs at the plasma membrane, associated with the ankyrin B protein consequently affects Ca2+ mobilization at the ER (174).
Lipid droplets are formed by coalescence of neutral lipids within the ER membrane bilayer when the coalesced lipids reach a critical size they bud off to form cytosolic lipid droplets, serving as a new transport pathway of lipids between the ER and Golgi apparatus or plasma membrane (65,172,173). Therefore, σ1R on the ER may play a role in the compartmentalization of lipids into the ER lipid storage sites and in the export of lipids to peripheries of cells (64).
Lipid rafts play a role in a variety of cellular functions including vesicle transport, receptor clustering and internalization, and coupling of receptors with proteins involved signal transduction (175). Over-expression of functional σ1R increases cholesterol contents and alters glycosphingolipid components in lipid rafts of NG108 or PC-12 cells (65,176,177), suggesting that up-regulation of σ1Rs potentiates lipid raft formation. Since glycosylated moieties of gangliosides have been proposed to play a role in regulating the localization of growth factor receptors in lipid rafts (175), chronic activation of σ1R may present substantial consequences in cell viability and differentiation.
Potential endogenous ligand
It has been demonstrated that alterations in endogenous hormonal levels, via adrenalectomy [ADX], castration [CX] (178), ovariectomy [OVX], or pregnancy, affect σR ligands activity when these have been evaluated in the electrophysiological model of the modulation of the NMDA response in the hippocampus (179,180). Similar findings have been seen when investigating the “antidepressant-like” effects of σR ligands in behavioral models of depression (181). Moreover, radioligand binding studies show a 30–40% decrease in [3H]SKF-10,047 binding during pregnancy, while ADX/CX enhances [3H]SKF-10,047 binding. Subsequent treatment with finasteride, which increases progesterone [PROG] levels, produces decreased [3H]SKF-10,047 binding (178,182–184).
Steroid hormones had been original proposed as endogenous ligands of σ1Rs, and more recently DMT, a natural tryptamine alkaloid, has been defined as the σ1Rs endogenous ligand (7,8). DMT is a hallucinogen found endogenously in human brain. It is commonly recognized to target the 5-hydroxytryptamine 2A receptor [5HT2AR] or the trace amine-associated receptor to exert its psychedelic effect. DMT has been recently shown to bind the σ1R molecular chaperones, whose function includes inhibiting various voltage-sensitive ion channels (9). Thus, it is possible that the psychedelic action of DMT might be mediated in part through σ1Rs.
Cell development and plasticity
σR drugs and neurosteroids, acting at the level of the σ1R protein, may act in cell development and cell trophic actions (82,185). For example, they have been shown to suppress multiple aspects of microglial activation (186), probably increasing intracellular Ca2+. These morphological changes have been previously ascribed to the prominent role of Ca2+ in cellular plasticity. This plasticity, which is associated both with the same prerequisite enhancement of NMDA-mediated glutamatergic neurotransmission and protein dephosphorylation that occur downstream from the massive entry of Ca2+ into the cell cytoplasm, as well as [Ca2+]i mobilization from the ER and the mitochondria. These events often occur synergistically (187,188).
The amplitude and reliability of both induction and maintenance of long-term potentiation [LTP] in neurons represent an effective model for memory acquisition and consolidation (189). The blockade of LTP and of several learning processes in mice, including spatial learning or passive avoidance, by Ca2+ depletion further supports the notion that Ca2+ influx and Ca2+ compartments are mandatory for memory (187,188). Additional evidence is provided by the poor capacity for acquisition and storage of spatial memory, combined with the lack of hippocampal LTP in transgenic strains of mice lacking subtypes of ryanodine receptors and IP3 kinase. This receptor-mediated postsynaptic Ca2+ accumulation (Ca2+ influx plus massive Ca2+ release from internal stores) is reinforced by subsequent activation of kinases such as Ca2+/calmodulin-dependent protein kinase II [CaMKII] and PKC (17). Thus, σ1R are probably involved in LTP via altering Ca2+ influx.
The initial statement that drugs acting via σRs may affect the regulation of [Ca2+]i equilibrium and likely the Ca2+ entry through the plasma membrane emerged from in vitro binding studies (163,190). The binding studies showed that inorganic Ca2+ channel blockers, such as cadmium [Cd2+], nickel [Ni2+] and Ca2+, and the non-selective Na+ and Ca2+ channel blockers phenylamine, cinnarizine, amidirone and amiloride, reduced the labeling of [3H]dextromethorphan (191) and [3H]DTG to σR sites (192,193) (Figure 2).
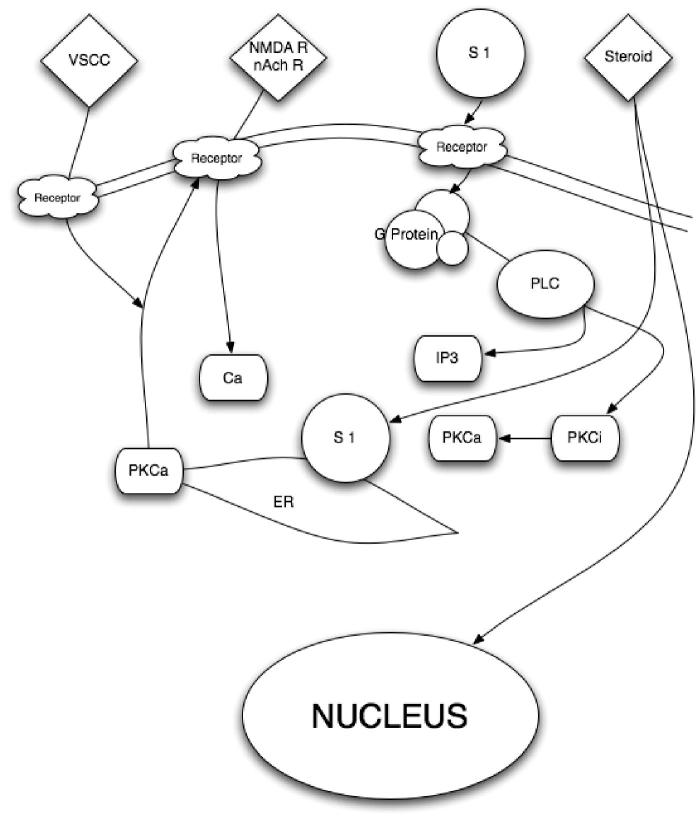
Figure 2.
Putative biological action of the σ1R on neuronal function. PLC – phospholipases C; PKCa – protein kinase C alpha; PKCi – protein kinase C inhibitor; S 1 – sigma1 receptor; IP3 – inositol triphosphate; nAch – nicotinic acetylcholine; nAchR – nicotinic acetylcholine receptor; NMDAR – N-methyl-d-aspartate receptors; Ca – calcium; VSCC – voltage-sensitive calcium channels. Once a neuron has been activated, e.g. via Glu or acetylcholine, a concomitant influx of Ca2+ and [Ca2+]i mobilization occur, facilitated by the activation of the endoplasmic-reticulum-bound σ1R, which is also triggered by numerous xenobiotics and steroids. The subsequent activation of PLC and the recruitment of the PKCs from its inactive form [PKCi] to its active form [PKCa], which is translocated to the plasma membrane, result in the activation of various enzymatic processes, as well as the phosphorylation of membrane-bound neurotransmitter receptors. In turn, the σ1R translocates to the plasma membrane where it decreases the excitatory neurotransmitter-induced Ca2+ influx.
An interesting feature of σRs is that they do not follow the classical pharmacology of a more or less linear dose-response curve followed by a plateau effect. A biphasic bell-shaped dose response curve has been observed for σR ligands in various behavioral, biochemical and electrophysiological paradigms (135,161,182,194). For example, because of the bell-shaped dose response curves, in the electrophysiological paradigm of the modulation of the NMDA response, low doses of σR agonists induce a potentiation of the NMDA response (162,195). At higher doses, the effects of σR agonists such as DTG and JO-1784 progressively decrease and disappear and these molecules act as antagonists by preventing the potentiation induced by low doses of other σR agonists (194).
A similar shaped dose response curve has also been described with σR ligands in other models such as in release experiments (135) and in behavioral models (182,183). The exact reason for such dose response curves obtained in so many models have not been well established. It has been proposed that they may be due to the fact that low doses of σR ligands activate one subtype of σRs for which they have high affinity, whereas higher doses may activate another subtype(s) of the σR for which they have a lower affinity. Such activity would counteract the effects observed at lower doses (194,196,197). Nonetheless, it is important to note that the different, and sometimes opposite, results obtained with low and high doses of σR ligands could constitute a very important factor to explain much of the controversy seen in the literature regarding σRs (Figure 3). The importance of the curves seen in these and other experiments will be discussed further on.
Figure 3.
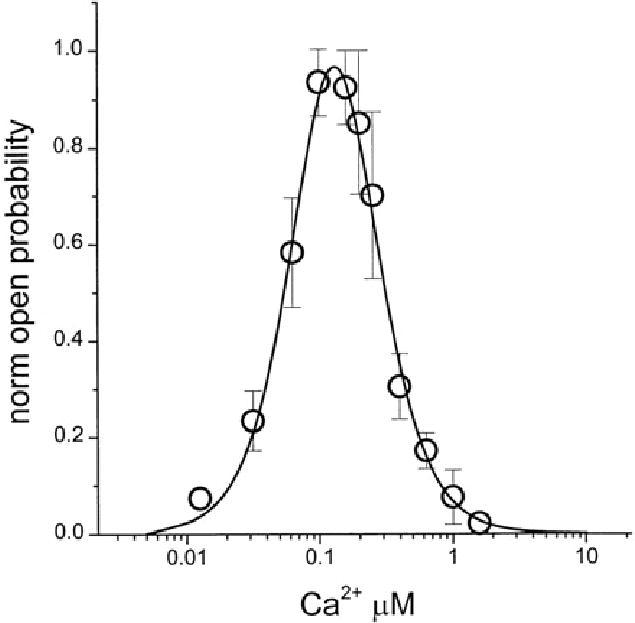
Bell curve dose response. Bell-shaped Ca2+ dependence of recombinant IP3R. Recombinant IP3R activity was measured in bilayers in the presence of 2 µM InsP3 and 1 mM Na2ATP at cis (cytosolic) Ca2+ concentrations in the range between 10 nM and 5 µM Ca2+. Ca2+ concentration in the cis chamber was adjusted by using calibrated 20 mM CaCl2 stock solution and 1 mM mixture of HEDTA and EGTA. Po in each experiment was normalized to maximum Po observed in the same experiment, and then data from three independent experiments were averaged together at each Ca2+ concentration (○) (477).
Throughout adulthood, differences in the motor changes elicited by drugs affecting σRs are correlated with the number of receptors in the P2, and not the P3, cellular fraction (198), which supports the hypothesis that translocation of the σ1R from the ER to the cell membrane occurs (190). This change decreases with age in motor structures as has been observed in the aged monkey brain where an increase of σ1Rs has been found (199).
σR agonists enhance memory performance in young rodents and in rodent models of cognitive impairment (200–205). For this reason, it has been suggested that age-related memory deficits may be responsive to up regulation of the σRs, implying that σ1R agonists may have therapeutic potential in dementia (204). In fact, such ability to alleviate memory deficits during aging has also been confirmed in humans for the selective σ1R agonist Igmesine® [(+)-N-cyclopropylmethyl-N-methyl-1,4-diphenyl-1-ethyl-but-3-en-1-ylamine hydrochloride], which appears more efficient among the elderly (206).
Conversely, the σ2R subtype exhibits no stereo selectivity and only low affinities for the (+)-BZM (91). It does not appear to be modulated by pertussis toxin-sensitive Gi /o proteins (207), and is predominantly located in the motor system and periphery (21). Clinically, the σ2R subtype may be preferentially involved in the motor and anxiolytic effects of σR ligands, as well as in diseases affecting motor and postural control (208). Interestingly, brainstem motor function, which is profoundly sensitive to σR drugs, decreases with age, during which the accuracy and consistency of fine and complex motor performance decrease (208).
The modulatory role of neurosteroids on neuronal function is typified by dihydroepiandrosterone (sulfate) [DHEA(S)] and its effect on σRs (209). NE release induced by NMDA via the stimulation of the σR is significantly enhanced by the addition of DHEAS (210). These findings have been replicated (123,183,210) and the overall data are consistent with the activity of DHEAS as a σ1R agonist; hence, neurosteroids potentiate NMDA-induced neuronal excitability (180).
It now appears as though DHEA(S) has an ability to modulate neurotransmitter receptors in the CNS that are primarily involved in learning and memory (209). σR agonists (205) enhance memory performance in young rodents and in rodent models of cognitive impairment (183,200,203,211,212), probably via the NMDAR which is involved in the development of LTP (213–216), an essential element of neural plasticity.
Activity through neurotransmitters
Neurotransmitters rarely act alone. The delicate balance of the major neurotransmitters, receptors and other methods of transmission control are central to normal homeostasis. These interactions make a reductionist approach to determining the effect of one specific neurotransmitter difficult (217). In fact, experiments that address only one major neurotransmitter may be misleading due to the lack of evaluation of other neurotransmitters and associated receptors.
As σRs are central to a number of CNS and other actions, it is not surprising that they interact with many other concurrent events within and outside the cell membranes on cells of many types. A functional interaction between σR ligands and neurosteroids, such as PROG (218), GluR and opioids, DA and 5-HT exists (98,126–128,183,194,195,219–222).
Neuroactive steroids (neurosteroids)
Neurosteroids (223–225), such as PROG, pregnenolone [PREG], dihydroepiandrosterone [DHEA(S)] and their respective sulfate esters PREGS or DHEAS, are involved in regulating the imbalance between excitation and inhibition in the CNS (226); hence, they have been suggested as a treatment for anxiety (227).
The initial proposition that steroids behave like endogenous σ1R ligands emerged from binding studies (222) and pharmacological experiments (210) leading to the hypothesis that neurosteroids may constitute endogenous ligands for the σ1R (2). A functional interaction between σR ligands and neurosteroids, such as PROG (218), GluRs and neurotransmitters exists (Figure 4) (98,194,195,220). Early studies found that neurosteroids bind to σ1R (183,228–230), but not to σ2R (231). For example, the neurosteroids PROG and DHEA(S) dose-dependently inhibit the in vivo binding of [3H]-SKF-10,047, an σR agonist, PROG being the most potent (228, 230). These binding data led to the hypothesis that PROG might be the endogenous ligand for σ1Rs, which is controversial, as the affinity of PROG for σ1R does not appear very high for an endogenous ligand (232). DMT, a natural tryptamine alkaloid, is now recognized as the σ1Rs endogenous ligand (8).
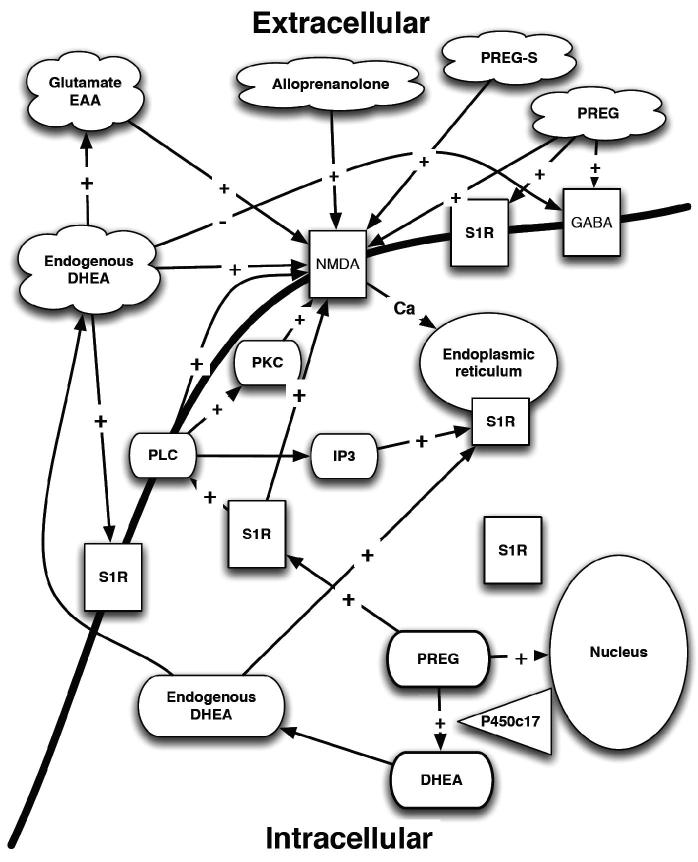
Figure 4.
Neurosteroids and their interactions with σRs. PLC – phospholipases C; PKC – protein kinase C; PKCi – protein kinase C inhibitor; S1R – sigma1 receptor; IP3 – inositol triphosphate; EAA – excitotoxic amino acid; GABA – γ-aminobutyric acid; NMDA – N-methyl-d-aspartate receptor; Ca – calcium; DHEA – dihydroepiandrosterone; PREG – pregnenolone; PREG-S – pregnenolone sulfate ester; P450c17 – cytochrome P450 C17.
The non-neuronal physiological actions of the neurosteroids, demonstrated from embryogenesis through adult life, are mediated secondarily by steroid receptors translocating into the nucleus, and non-genomic neuromodulatory actions affecting directly several ion channels, neurotransmitter receptors and second messenger systems (32). Neurosteroids activate transcription factors; hence, they regulate gene expression and stimulate protein synthesis (233–238). Only the human σ1R gene contains a steroid-binding component (239). These neurosteroids are found in the cortex, hippocampus and brainstem, areas of the brain containing high densities of σ1R (98).
The neurosteroids 3α-hydroxy-5α-pregnan-20-one (allopregnanolone) [ALLO], allotetrahydrodeoxy-corticosterone, PREGS and DHEAS possess anti-stress, anxiolytic and antiamnesic properties in experimental animal models (212,240–246), and have a possible neuroprotective effect in AD (247). In AD, decreased levels of PREG(S), DHEA(S) and PROG have been identified in the hippocampus (248), cortex and cerebellum, compared to the control animals (249–251). Their actions are mediated via the σ1R (Figure 4).
DHEAS and PREGS may also play an important role in depression (252), as decreased levels of DHEA, DHEAS and PREGS have been associated with clinical depression (253), cognitive dysfunction (254,255), dementia (253,256,257) and other neurological conditions (190,258–260). Although there is still controversy as to whether and how the steroidogenic enzymes are involved in the physiology of nervous system (261) and the pathophysiology of neuropsychiatric disorders (262), σRs are critical to their cellular effects.
Clinical investigations in humans have produced evidence for an involvement of neurosteroids in conditions such as fatigue during pregnancy, premenstrual syndrome, postpartum depression, catamenial epilepsy, depressive disorders (252). They possibly alter the expression of conditioned fear stress response in mice (263). However, the exact mechanism underlying the beneficial effects of neurosteroids is not yet fully elucidated (82,263–267).
Modulation of GABAA, NMDA, nicotinic, muscarinic, serotonin [5-HT3], kainate [Ka], glycine [Gly] and σRs, plus neuroprotection and induction of neurite outgrowth, dendritic spine development, and synaptogenesis are properties of specific neurosteroids (268,269). However, only the human σ1R gene contains a steroid-binding component (239), which exerts effects on the genome via individual intracellular steroid receptors (270). Neurosteroids rapidly alter neuronal excitability through interaction with neurotransmitter-gated ion channels, e.g. NMDA.
The 3α-hydroxy ring A-reduced pregnane steroids, ALLO and tetrahydrodeoxycorticosterone, enhance γ-aminobutyric acid [GABA]-mediated chloride [Cl−] currents, whereas PREG sulfate and DHEAS display functional antagonistic properties at GABAARs (271–275). At physiologically relevant concentrations, that is, below 100 nM, these steroids directly activated the GABAAR–channel complex (276,277) and exerted a GABAmimetic effect sufficient to suppress excitatory neurotransmission (277).
Certain steroids, including PREG, DHEA, PROG, ALLO and their S (sulfate) esters, rapidly affect neuronal excitability through the modulation of voltage-gated ion channels (278), e.g. voltage-sensitive Ca2+ channels [VSCC]s (226,279–283), and neurotransmitter-gated ion channels, such as at the NMDAR level (210,226,284–288). These steroids act at specific extracellular sites that are distinct from one another and from the spermine, redox, Gly Mg2+, phencyclidine [PCP] and arachidonic acid sites (289,290). In addition, DHEA(S), but not PREG(S), potentiates the NMDA-evoked catecholaminergic release (210) and firing activity of CA3 hippocampal neurons (123). Moreover, the NMDA-stimulates NE release is inhibited by PREGS (210).
It remains unclear whether σ1R and neurosteroids exert a common action via the regulation of Ca2+ influx and [Ca2+]i regarding amnesic and age-dependent cognitive abilities (163,291). In humans, plasma levels of DHEAS decline with age (258,259), PREG and PREG(S), DHEA and DHEA(S), or PROG decrease in aged mice (292–295) and PREGS decreases in aged Sprague Dawley rats correlating with impaired memory functions (260). However, attenuating effects of DHEAS and PREGS on the conditioned fear stress response are mediated via σ1Rs and that PROG has a σ1R antagonistic property (263).
It should be noted that σR sites are different from high affinity PCP binding sites, located within the ion channel associated with NMDAR (21). PCP receptor is dependent on the presence of l-Glu; this has led to the suggestion that there may exist an NMDA/PCP receptor complex (296). The lack of selectivity between the σ1Rs and PCP binding sites seen following exposure to several compounds, including BZM or PCP derivatives, has led to confusion that was eventually clarified by the availability of new highly selective drugs (212). These compounds are now reference compounds in terms of selectivity between σR and PCPRs.
Opioids
Opioids have subtle differences in binding to the µ, κ and σRs; however, σRs are a receptor class in their own right (297,298). Although σRs now have been classified as a separate group of receptors from the opioid receptors, the outcome of σR binding is not necessarily different from that when these receptors are bound to opiates (Figure 5) (299), especially since interactions between κRs and σRs have been reported (278,300).
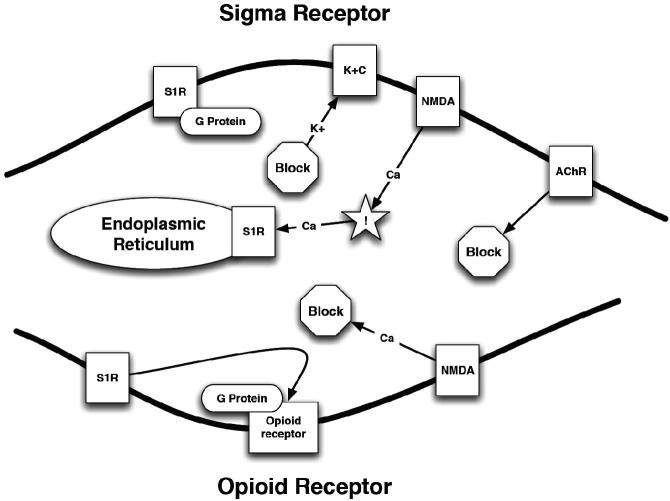
Figure 5.
A schematic representation of the opioid receptor and σ1Rs. NMDA – N-methyl-d-aspartate receptor; K+C – potassium channel; ! – increased concentration; AChR – acetylcholine receptor; S1R – σ1R; Ca2+ – calcium; K+ – potassium.
σ1Rs have been implicated in the modulation of opioid analgesia. It has been shown that coadministration of a σ1R agonist decrease the analgesic power of morphine, whereas the use of an antagonist, DEX, increase analgesia (301), thus illustrating the pharmacological importance of σ1R in the brainstem modulation of opioid analgesia (302). Interestingly, the dysphoric and psychomimetic side effects of σRs reside in the levorotatory (L) or (−) and not in the dextrorotatory (D) or (+)-isomer (303) as exemplified by nalorphine, levallorphan, (−)-PTZ, (−)-3-hydroxy-N-propargylmorphinan and MR 2034. All L opiates, produce dysphoria and psychomimetic effects, whereas the D isomers of PTZ and MR 2034 do not. Despite this selective response, both (+) and (−) PTZ improve memory via the σRs rather than via the µ and κ opioid receptors per se (304–306).
Serotonin [5-HT]
There is controversial evidence regarding possible interactions between σR and the 5-HT system (Figure 6). The distribution of 5-HT binding sites in the CNS has been well described (307). These sites include σ1Rs. 5-HT and tryptophan (308) play a key role in depression and the mechanism of action of many antidepressants (88,309), probably via a decrease in the firing activity of 5-HT neurons (310–313).
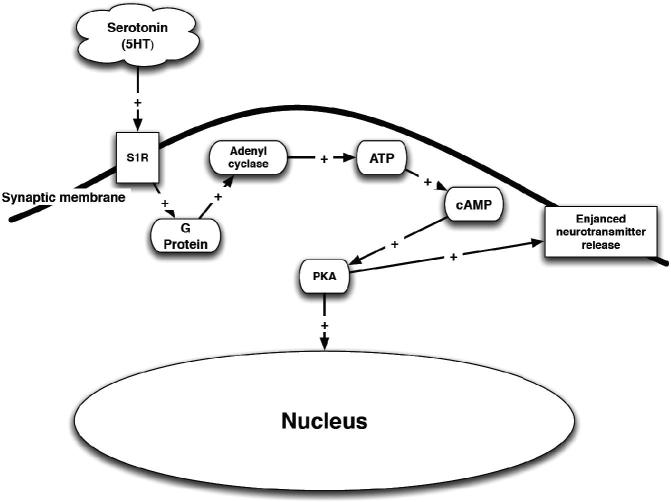
Figure 6.
Serotonin (5HT) stimulation of the σ1R. PKA – phosphokinase A; ATP – adenosine triphosphate; cAMP – cyclic adenosine monophosphate; S1R – σ1R.
Peripheral 5-HT-σR interactions have been proposed, as DTG, haloperidol and BMY-14802 inhibit the 5-HT evoked contractions of the guinea pig ileum longitudinal muscle and myenteric plexus preparations, showing high correlation with their potency to compete with DTG binding (314). However, the σ1R agonist ligand EMD 57445 does not affect 5-HT-related parameters such as 8-OH-DPAT induced behavioral syndrome, m-chlorophenylpiperazine-induced hypothermia or l-5-hydroxytryptophaninduced head twitches (130). In addition, EMD 57445 and the σ1R ligand PD144418 do not induce any change in 5-HT or 5-hydroxyindoleacetic acid [5-HIAA] levels in various brain regions, suggesting that these ligands exert no effect on 5-HTR populations or 5-HT metabolism (130,264). Interestingly, EMD 57445 and PhmD 144415 have been suggested to be σ1R antagonists.
Whether or not σR ligands can modulate 5-HT neuronal activity in vivo, the effects of short- and long-term administration of various σR ligands on 5-HT basal neuronal activity in the dorsal raphe nucleus [DRN], have shown that acute treatments with SSRIs and MAOIs induce a decrease in the firing activity of DRN 5-HT neurons (310,312,313,315). There is an eventual restoration of the firing activity of these neurons (312,313,316,317) due to the desensitization of the 5-HT1A autoreceptors in the CNS (311,318–321).
In contrast to what has been observed in the dorsal hippocampus, acute iv administration of (+)-PTZ has no effect in the DRN. Interestingly, however, the σR ligands, 4-IBP, (+)-PTZ and DTG, after either two or 21 days of treatment induce a 50% increase in the firing activity of 5-HT neurons of the DRN (322). These findings suggest modulation of 5-HT neurotransmission by σR ligands in vivo, a novel finding with respect to σR research, again supporting a role for σR in depression, probably mediated by σ1Rs via the 5-HT1A receptor (323–325).
Interestingly, OPC-14523, a σ1R agonist, decreases the responsiveness of the 5-HT1AR after two days of treatment (326,327). This is particularly significant given that classical antidepressant medications require chronic treatment for this decreased receptor response to occur (313,319,320). If this effect is shown to be a general effect, present with other σ1R agonists, the rapid desensitization of the 5-HT1A autoreceptor, in addition to the observed rapid increase in the firing activity of 5-HT neurons after only two days of treatment with σ1R agonists, would constitute another argument to suggest that σR agonists have potential to produce a fast onset of antidepressant effect.
The neurosteroid σ1R agonist PROG does not have any effect by itself on 5-HT neuronal activity in the DRN, but several of its metabolites, such as ALLO or DHEA, increase the firing activity of DRN 5-HT neurons. Interestingly, at least part of the effects of neurosteroids is mediated through an activation of σRs as they are reversed by NE-100 (328).
The precise mechanism by which σR ligands increase the firing activity of DRN 5-HT neurons has not been established. One possibility is that the effect is mediated locally, in the DRN, as a consequence of the modulation of the Glu neurotransmission, since AMPA and NMDA GluRs have been shown to mediate glutamatergic excitatory input in the DRN (329).
The σ1R-mediated effect on firing could also be an indirect one, as σR ligands rapidly modulate NMDAR-mediated transmission in the hippocampus, which leads to a modulation of 5-HT neurotransmission in the DRN via feedback loops to DRN 5-HT neurons. In fact, an afferent connection has been identified that projects from the hippocampus to the DRN via the lateral habenula (330–336), and the long feedback loop that projects from the DRN to the prefrontal cortex [PFC] and back to the DRN (329,333,335,337–340).
Therefore, the activity of σRs on the DRN neurons is dependent on the balance between the excitatory input (the Glu system) from various brain regions (e.g. lateral habenula and mPFC) and inhibitory input from GABAergic interneurons in distal areas (e.g. periaqueductal gray area) and local GABAergic interneurons situated in the DRN (341,336).
Another factor likely contributing to the requirement of a sustained treatment of σR agonists to observe an antidepressant effect is based on the density of σRs at the plasma membrane, which is progressively altered by the presence of σR ligands. σR agonists induce an increase in the σR density at the plasma membrane following a minimum of two days of treatment (185) exerting effects on NMDAR-mediated signaling.
Dopamine
The σ1R subtype is involved in the facilitation of cortical Dopamine [DA] transmission in the rat brain (342). σ1Rs are located in limbic areas, including nucleus accumbens [NAC] (343) and PFC, both of which are thought to be involved in schizophrenia (344). Many antipsychotics, including haloperidol (345), bind with high affinity to σ1Rs, where the DAergic hyperactivity in the NAC is thought to underlie positive symptoms of schizophrenia (including delusions, disordered thoughts and speech, and tactile, auditory, visual, olfactory and gustatory hallucinations, typically regarded as manifestations of psychosis), while DAergic hypoactivity in PFC the negative symptoms (including deficits of normal emotional responses or of other thought processes). σ1R ligand agonists increase extracellular DA levels in rats (346) whereas their antagonism inhibits DA-induced abnormal involuntary movements (347).
σRs regulate NMDA-[3H]DA release in caudate-putamen [CP], the neuroanatomical substrate for extrapyramidal side effects resulting from chronic 2-amino-7-phosphonoheptanoic acid [AP-7] treatment (348). In that study, in the NAC, regulation of DA release by the prototypical σR agonist (+)PTZ mediated predominantly by the σ1R, whereas in the PFC a portion of the (+)PTZ effect is likely mediated by the σ2R.
In both the NAC and PFC, regulation of DA release by the σR agonist BD737 is mediated primarily by the σ1R, not via the opioid receptors, the NMDAR-operated cation channel, or by σR effects upon [3H]DA accumulated by noradrenergic terminals in PFC (349). In fact, the action of NMDA in primary cortical neurons is regulated differently by ligands with differential affinities at DA D2 and σRs (291).
The effects of different selective σR ligands on DA and Glu-NMDA neurotransmissions, both in origin (A10 and A9 areas) and terminal NAC and CP regions of the rat mesolimbic and nigrostriatal DA-ergic systems, have been evaluated. The selective σ1R ligands 2-[4-(4-methoxy-benzyl)piperazin-1-yl-methyl]4-oxo[4H]-benzo-thiazolin-2-one [S-21377] and 2[(4-benzyl piperazin-1-yl) methyl] naphthalene, dichlorydrate [S-21378] slightly increase the spontaneous firing rate and potentiate the NMDA-induced neuronal activation of DA-ergic neurons in the A9 and A10 regions. (+)N-cyclopropylmethyl-N-methyl-1,4-diphenyl-1-ethyl-butyl-2-N [JO-1784], another selective σ1R ligand, has produced no or little effect in these areas (350).
A selective σ2R ligand 1,4-bis-spiro[isobenzofuran-1(3H), 4′-piperidin-1′yl]butane [Lu 29–252] significantly potentiates the NMDA-induced increase in firing activity of A10 DA neurons. Functional interaction between σ2R and NMDARs in the A10 region has been reported (350); thus, DA release in the striatum may be modulated by multiple σR subtypes. In such a situation, NMDARs may mediate the stimulatory effect of σR ligands on DA release in the striatum (351).
In addition, σR may regulate the release of DA along with an action at the NMDAR, e.g. the pharmacological effects of amantadine on DAergic transmission are proposed to result from an uncompetitive antagonism at this receptor (352). These data demonstrate that aminoadamantanes behave as σ1R agonists, and confirm an involvement of this receptor in modulating DA receptors exerted by therapeutically relevant concentrations of amantadine (352,353).
The regulation of DA release is much more complicated than has been alluded above. Regardless, work has showed that activation of σ2R results in the regulation of dopamine transporter [DAT] activity via a Ca2+- and PKC-dependent signaling mechanism (354).
Nicotine and acetyl choline [ACh]
σ1R ligands noncompetitively inhibit nicotine-stimulated catecholamine release from bovine adrenal chromaffin cells in a concentration-dependent and reversible manner (355). The rank order of potency of ligands to inhibit nicotine stimulated catecholamine release is correlated with that observed in radioligand binding assays selective for the σ1R subtype. This naltrexone-insensitive effect is paralleled by an inhibition of nicotine-stimulated increases in [Ca2+]i. σR ligands are without effect on catecholamine release or [Ca2+]i in the absence of nicotine (155), although the inhibitory effect of σR ligands on the nicotine-evoked Ca2+ uptake is not directly coupled with either the σ1R or σ2R sites (356).
Nicotine accelerates the association of the receptor selective radioligand, [3H](+)PTZ, to adrenal medullary homogenates while having no effect on the rate of ligand dissociation, consistent with a σR ligand binding site closely associated with and allosterically modulated by the nicotinic acetylcholine receptor [AChR] (155). Thus, the actions of agonists at the nicotinic AChR are modulated by σ1R selective ligands (160). In addition, the increased ACh level seen in rat frontal cortex induced by (+)N-allylnormetazocine supports the activity of σRs in ACh regulation (357–359).
Nitric oxide
It has been shown in vitro that σR ligands prevent Glu-induced activation of nitric oxide synthetase [NOS] (360). Nitric oxide [NO] is an important mediator in ischemic brain injury (361–363), and in many other disease states. Specifically, NO derived from constitutively expressed NOS in neurons [nNOS] and the inducible isoform expressed by many cells [iNOS] are important in excitotoxic injury cascades (363,364), such as can be seen following exposure to EAAs. Pharmacologically selective inhibitors of nNOS and iNOS, such as the σ1R (365), attenuate infarction volume after focal cerebral ischemia (362,366,367).
A potent σ1R infusion into normal striatum by microdialysis attenuates basal, and NMDA-evoked, striatal NO production in situ (368); therefore, it is not surprising that systemic σR ligand treatment reduces stroke damage by preventing ischemia-induced NO production (369) with reduced infarct volume (370). These findings have been reproduced more recently (187). For this reason it has been suggested that σ1R agonists should be considered as neuroprotective drugs, where some of the protection offered occurs through inhibition of inducible NOS (365).
Glutamate [Glu]
Although many AAs play a role in neurotransmission, Glu, Gly and GABA are among the more common and better-understood neurotransmitters (371–375). Glu mediates an estimated 50% of all the synaptic transmissions in the CNS. Glu, glycine and GABA are metabolic intermediates and neurotransmitters, where Glu is the major excitatory neurotransmitter, and Gly and GABA are the major inhibitory neurotransmitters (326,350,376–380). Glu is involved in nearly all aspects of normal brain function including learning, memory, movement, cognition and development (381–390).
Glu is synthesized, stored and released from the presynaptic terminal, has specific neurotransmitter receptors are localized on the postsynaptic cells, and is eliminated from the synaptic cleft by specific transporters. In addition to Glu, aspartate [Asp] also acts as a major excitatory neurotransmitter (382,391–395) by stimulating or exciting postsynaptic neurons.
From Glu labeling studies, the average concentration of Glu in ganglion cells is 5 mM (396). Physiological studies using isolated cells indicate that only µM levels of Glu are required to activate GluRs (397–399). Thus, the amount of Glu released into the synaptic cleft is several orders of magnitude higher than the concentration required to activate most postsynaptic receptors. As σRs seem to mediate a number of processes through the Glu system, a more detailed discussion of the Glu system is provided (Figure 7).
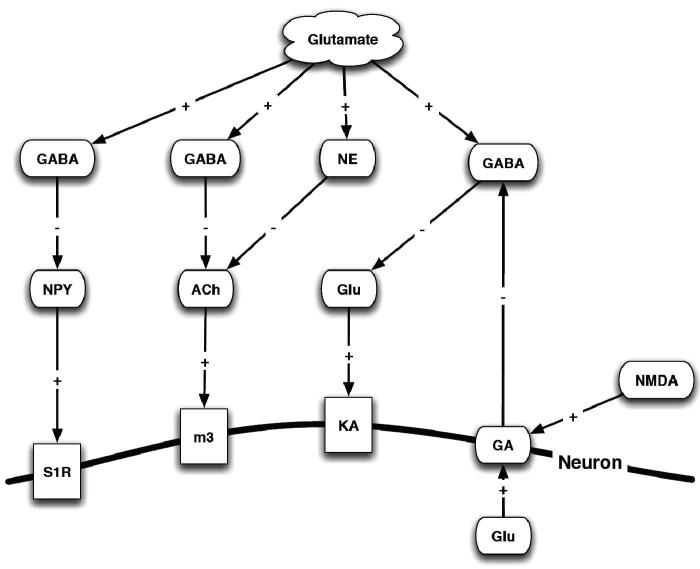
Figure 7.
Interaction of glutamate, neurotransmitters and the σR. NMDA – N-methyl-d-aspartate receptor; NE – norepinephrine; NPY – neuropeptide Y; ACh – acetylcholine; M3 – rat muscarinic acetyl choline receptor; GABA – γ-aminobutyric acid; GA – Ga-binding protein α-chain; Ka – kainate; Glu – glutamate; S1R – σ1R.
N-Acetyl-aspartyl-glutamate [NAAG] is abundant in the mammalian CNS, which has led to the hypothesis that this dipeptide is the storage form of Glu (400,401). Brain tissue has a remarkable ability to accumulate Glu, an ability resulting from Glu transporter [GluT] proteins present in the plasma membranes of both glial cells and neurons (402). Glu is at the center of other metabolic events, e.g. Glu serves as substrate for the synthesis of N-acetyl Glu, an essential allosteric activator of carbamyl phosphate synthetase I, a key regulatory enzyme in the urea cycle (403). It has a well-described transdeamination system involving aminotransferases and Glu dehydrogenase, where Glu plays a key catalytic role in the removal of α-amino nitrogen from AAs. Finally, the “Glu family” of AAs (arginine, ornithine, proline, histidine and glutamine) requires the conversion of these AAs to Glu for their metabolic disposal. The Glu system is probably the mediator of excitatory effects seen following σRs stimulation (404) by σR agonists such as phencyclidine [PCP] (201).
At toxic concentrations, Glu acts as a neurotoxin (excitatory amino acid [EAA]) capable of inducing severe neuronal damage and necrosis by causing over excitation of neurons through receptor-mediated depolarization and Ca2+ influx (373,405–411). However, Glu is not the only EAA that can cause excitotoxicity and cell death in the CNS (382,391,392,394,412,413). The σ1R ligand PRE-084 protects against excitotoxic perinatal brain injury in newborn mice (414), indicating a central role for the σRs in modulating the excitatory effects of Glu.
Other EAAs access the brain tissue of the circumventricular organs located outside the blood brain barrier [BBB] (415–420). An array of GluRs are known to be present on pre- and postsynaptic membranes that are used to transduce integrated signals using an increased ion flux and second messenger pathways (382,389–392,421,422,). It is the excessive activation of these receptors that leads to neurotoxicity, often referred to as “excitotoxicity”.
There are five main factors necessary for the transition of Glu and Asp from neurotransmitters to excitotoxins, including inadequate neuronal ATP levels, inadequate neuronal levels of Mg2+; high concentrations of inflammatory prostaglandins; excessive free radical formation (423,424) and inadequate removal of synaptic Glu (296,373,408,425,426). It has been postulated that excitotoxicity is involved in the pathogenesis of many types of acute and chronic insults to the CNS (416) and peripheral tissues (418), and interestingly, excitotoxicity has also been suggested as a central mechanism in fluoride neurotoxicity (427).
Glu and its structural analogues may enter the food supply during preparation or processing as contaminants or additives in its free form or bound to peptides and proteins (428–437). These analogues include monosodium Glu [MSG], l-aspartate, l-cysteine, related sulfur AAs, B-N -oxalyamino-l-alanine [BOAA or ODAP], B-N-methyl-amino-l-alanine [BMAA] and the seafood toxin domoic acid [DomA] (429,432,435,438–440). Structurally similar environmental dietary excitotoxins (441), such as DomA, one of the most potent neurotoxins in seafood can enter our food supply (439). Contamination of mussels by sea diatoms producing DomA (429–431,442), results in neuronal excitation resulting in severe seizures (429–431,433,434,439,442). Survivors of severe cases suffered permanent loss of short-term memory, a phenomenon that lead to the term amnesic shellfish poisoning (415,418,431,434,437,439).
It now is clear that the σRs are important in modulating Glu-mediated seizures (443), and protects neurons against Glu toxicity in vitro (444), although direct interaction with NMDARs should not be forgotten as a crucial element in the neuroprotective effects of σRs ligands with affinity for NMDARs (445,446).
Although excitotoxic effects can be pronounced during acute events such as ischemic stroke and trauma, they can occur in prolonged chronic neurodegenerative diseases such as AD (425), Parkinsons disease [PD] (447), Huntingtons disease [HD] (448) and Amyotrophic Lateral Sclerosis [ALS] (373,449) schizophrenia, anxiety, depression (425,450,451). These are likely associated with σR stimulation. Recently, a mutation in the σ1R has been associated with juvenile ALS (452); therefore, it is not surprising that σ1R agonists improve motor function and motor neuron survival in ALS mice (453). In fact, loss of σ1R has been associated with defective autophagy and lipid raft disturbances (454).
In contrast to the effects of σR stimulation, antagonism of the σRs blocks compulsive-like eating behavior (455), enhances brain plasticity (456) and exacerbates other addictions (457). In addition, glutamatergic dysfunction has been postulated as being part of the development of disorders associated with long-term plastic changes in the CNS such as chronic pain (458), drug tolerance, dependence, addiction, partial complex seizures and tardive dyskinesia (373,459).
l-Glu acts through both ligand-gated ion channels at the iGluR and at G-protein-coupled metabotropic glutamate receptors [mGluR] (Figure 8). Activation of these receptors is responsible for basal excitatory synaptic transmission and many forms of synaptic plasticity such as LTP and long-term depression [LTD], which are thought to underlie learning and memory (216,371,460–473).
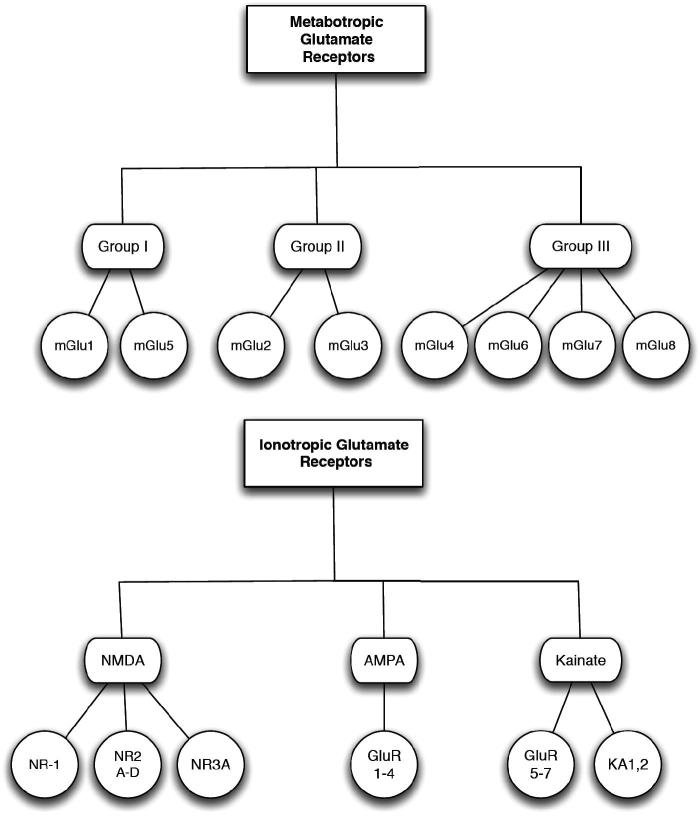
Figure 8.
Glutamate receptors types. NMDA – N-methyl-d-aspartate receptor; AMPA – α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; KA – kainate; GluR – glutamate receptor; NR – NMDA receptor subtype.
Transporter proteins (Glutamate transporter [GluT]) represent the only significant mechanism for removal of Glu from the extracellular fluid and are important for the long-term maintenance of low and non-toxic concentrations of Glu and appear to have more sophisticated functions in the modulation of neurotransmission (402). A number of soluble compounds, including Glu, cytokines and growth factors, influence the GluT expression and activities (474). It is not known as to whether the σRs are involved in regulation of this transport.
The genes encoding GluT proteins have been cloned both from rats and humans (475–480). They are found in astroglia and microglia widely distributed throughout the CNS (481,482) and provide Glu for synthesis of GABA, glutathione and protein (402,483). They rapidly remove Glu from the synaptic cleft to prevent cell death (484).
Many tissues demonstrate Glu, GluR and GluTs, (396,418,432,434,485–526). mGluRs and l-Glu, l-aspartate and d-aspartate are substrates for the transporters (217,495,521,527), whereas GluR agonists (528) and antagonists (495,529) are not.
GluTs incorporate Glu into cells along with the co-transport of three Na+ ions (527,530) and the antiport of one K+ ion (529,531) and either one OH− or one 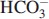 ion. The excess Na+ ions generate a net positive inward current, which drives the GluT (527). In addition, a Glu-elicited Cl− current is also associated with some GluT (475,532). In contrast, the vesicular transporter selectively concentrates Glu into synaptic vesicles in a Na+-independent, ATP-dependent manner (533–535) that requires Cl− (374,375,533,536–542). Given the complexity of the Glu system and the limited information regarding the interaction of the multiple components with σRs, further research is necessary to fully elucidate interaction of σR and the system components.
ion. The excess Na+ ions generate a net positive inward current, which drives the GluT (527). In addition, a Glu-elicited Cl− current is also associated with some GluT (475,532). In contrast, the vesicular transporter selectively concentrates Glu into synaptic vesicles in a Na+-independent, ATP-dependent manner (533–535) that requires Cl− (374,375,533,536–542). Given the complexity of the Glu system and the limited information regarding the interaction of the multiple components with σRs, further research is necessary to fully elucidate interaction of σR and the system components.
Glu receptors [GluR]
Two classes of GluRs have been characterized based on studies in the CNS: iGluRs and mGluRs (382,388,389,391,392,421,422,518,543,544). The iGluRs are ligand-gated ion channels that mediate the vast majority of excitatory neurotransmission in the brain. They are classified into three major subtypes: NMDARs, AMPARs and KaRs (296,373,381,382,392–394,449,543,545–554). These receptors exhibit varied pharmacological and electrophysiological properties, including ionic channel selectivity to Na+, K+ and Ca2+ (389,543).
NMDA receptors [NMDAR]
The NMDAR is perhaps the best characterized of the iGlu, in part due to the existence of selective agonists and antagonists that can be used to study its physiology. These receptors are modulated by σ1R. NMDAR are ubiquitous (555). NMDARs are composed of assemblies of NR1 subunits and NR2 subunits, which can be one of four separate gene products [NR2A-D]. Expression of both subunits is required to form functional channels (556).
NMDARs are structurally complex, with separate binding sites for Glu, Gly, Mg2+ (557,558), Zn2+ and a polyamine recognition site, where Mg2+ ions provide a voltage-dependent block of NMDA-gated channels (394).
NMDAR are highly permeable for Ca2+. They show slower gating kinetics with the channel blocked in a voltage- and use-dependent manner by physiological concentrations of Mg2+ ions (371,372,458,459,462,559). It is this property of the NMDAR that enables σRs to trigger cell death via Ca2+ overload.
AMPA receptors [AMPAR]
AMPAR are involved in mediating most forms of fast glutamatergic neurotransmission, which corresponds to a Ca2+ influx, a function controlled by the GluR2 subunit (560). There are four known subunits GluR1 to GluR4, sometimes referred to as GluRA to GluRD, are widely, but differentially, distributed throughout the CNS (392). AMPARs play an important role in memory function. They are localized in the hippocampus and striatum and also may play a role in the generation of seizures (560–562).
Kainate receptors [KaR]
Kainate receptors [KaR] constitute a separate group from the NMDAR and AMPAR, although they share many of the same structural characteristics. KaRs and AMPARs are localized in the hippocampus and striatum and also may play a role in the generation of seizures (563–566). Also they are involved post-synaptically in neurotransmission in some pathways (566–569).
Metabotropic Glu receptors [mGluR]
mGluRs form a family of currently eight subtypes (mGluR1–8), subdivided into three groups (I–III) (570–572). Activation of group-II (mGluR2,3) or group-III mGluRs (mGluR4,6–8) has been established to be neuroprotective in vitro and in vivo (572), and for the NMDA iGluR (573). In contrast, group-I mGluRs (mGluR1,5) need to be antagonized in order to evoke protection (448) antagonists, and drugs acting on 5-HT2A, α2-adrenergic, adenosine (A2A) and cannabinoid [CB1] receptors may be helpful (574).
Members of this family of mGluR exert their effects either on the second messengers or ion channels via the activation of the GTP-binding proteins and regulate the synthesis of different intracellular second messengers such as IP3, cAMP or cGMP, as do σRs (382,422). They function to modulate the presynaptic release of Glu and the post-synaptic sensitivity of the cell to Glu excitation (382,389,390,392,422).
mGluRs have both chemical and electrical signaling properties (575). Glu binding onto an mGluR opens non-selective cation channels more permeable to Na+ and K+ ions than Ca2+ (548,576). mGlu binding elicits a rapidly activating inward and outward current and Ka, quisqualate and AMPA are the specific agonists at these receptors (399,577–583).
As with iGluRs, the mGluRs are classified into 4 groups (Group I–IV) based on AA sequence similarities, agonist pharmacology and the signal transduction pathways to which they are coupled (584). Each receptor is formed from the co-assembly of several subunits (584–587). To date, eight subunits (named GluR1 through GluR8) have been cloned (393,576,586,588–591).
σRs and Glu neurotransmission
Numerous studies have shown interactions between σRs and NMDAR-mediated responses. For example, σR ligands, including haloperidol, (+)-PTZ, 4-IBP (592), (+)-3-PPP, (+)-SKF-10,047 (593) and DTG (594), antagonize NMDAR currents in Xenopus oocytes (595). The effects of σR ligands on NMDARs in are thought to be indirect; however, high doses (µM) and nonselective σR ligands have been used in past studies. Furthermore, there was no correlation between the potency of NMDAR inhibition and the affinity or stereo selectivity for σR sites (595–597). Thus, it is difficult to assess whether these observations have been based on σR mediated actions rather than on non-specific effects. In vitro radioligand binding studies have shown that haloperidol, (+)-PTZ, DTG, (+)-SKF-10,047 and (+)-3-PPP inhibited [3H]TCP binding to NMDARs in neuronal cells, with a potency correlated with the affinity for DTG binding sites (64,598).
In a model of modulation of the NMDA response in dorsal hippocampal pyramidal neurons of the CA3 region, it was found that low doses of the σR ligands DTG, JO-1784, (+)-PTZ and l-687,384 selectively potentiated the response of these neurons to microiontophoretic applications of NMDA (137,194,195,197). Other σR ligands such as BD-737, 4-IBP and OPC-14523 were less selective (196,376).
Interestingly, it was also found that depending on the initial level of excitatory response to QUIS and NMDA, σR agonists could increase or decrease NMDA-induced responses, thus suggesting a real modulatory role of σR ligands on the Glu response (377). Antagonists including SA4503 (593), BMY-14802, (+)-3-PPP and NE-100, suppress the potentiation induced by σR agonists (162,195,197).
The effects of all σ1R agonists on the NMDA response produce a biphasic dose response curve, which will be discussed later (194,376,377). As stated above, this particular pharmacological profile could explain the discrepancies observed for the effects of σR ligands with respect to inhibition versus potentiation on NMDAR-mediated responses, as most in vitro studies may have used high doses, at which the σR ligands were acting as antagonists.
In contrast, the antidepressants paroxetine and tranylcypromine, which have a low affinity for σRs, have no effect on the NMDA response despite their similar monoaminergic profiles to sertraline and clorgyline. Moreover, the effects of sertraline and clorgyline are suppressed by the σR antagonist haloperidol but not by spiperone, suggesting that their effects are likely mediated by σRs (197). The σ2R ligands Lu 28-179 (599) and BD-1008 (600) have also been shown to modulate NMDA mediated responses.
Despite their high affinity for σ2Rs, the doses required for antidepressant activity are 5–10 times higher than σ1R ligands (102). The effects of the specific σ2R ligand Lu 28–179, are not blocked by the σ1R antagonists NE-100, PROG, or haloperidol, suggesting that these effects are mediated through σ2R (102).
In vitro models have also suggested a modulatory role for σR agonists on NMDA-mediated responses. For example, JO-1784, BD-737, (+)-PTZ and (+)-3-PPP potentiates in a concentration-dependent manner NMDA-induced [3H]NE release from preloaded rat hippocampal slices (135,162,210), whereas DTG and BD-737 act as inverse agonists, by concentration dependently inhibiting the overflow of [3H]NE evoked by NMDA. Haloperidol and BD-1063 (208) alone do not modify [3H]NE release, but completely prevent the effects of JO-1784, BD-737, (+)-PTZ, DTG and (+)-3-PPP (162), whereas DuP734 inhibits that of BD-737 (122).
Neurosteroids, acting as σR agonists, have also been shown to modulate NMDAR-mediated effects (601), as DHEA at low doses potentiates the NMDA response in extracellular recordings from the dorsal hippocampus. The effect of DHEA is blocked by NE-100 and haloperidol (123,179). In this model, neither PREG nor PREGS modifies the NMDA response or act as antagonists (602), which may be due to their lower affinity for the σ1R (82,283).
Endogenous hormone levels also affect the σRs modulatory effect on NMDA-mediated responses. For example, two weeks following OVX, the potentiation of the NMDA response induced by DTG was significantly greater than in control female rats, suggesting that σRs may be tonically inhibited by endogenous PROG (123,180). In agreement, 10 times higher doses of (+)-PTZ and DHEA are required in pregnant females to potentiate the NMDA response. This reduction of effect of σR agonists in late pregnancy may be due to occupation of σR by high concentrations of PROG and the apparent super sensitivity of σR observed during the post-partum period that might be due to the rapid drop of PROG levels after parturition (603–605). Overall, many σ1R ligands have demonstrated the ability to modulate NMDA-mediated Glu neurotransmission.
γ-Aminobutyric acid (GABA)
Glutamic acid decarboxylase [GAD] in mouse brain is capable of decarboxylating Glu to GABA but requires pyridoxal 5-phosphate as a cofactor (606–611). The role of GABA as a neurotransmitter is that of inhibitory neurotransmission, although this property has been questioned recently (612). Following the purification of GAD and the generation of GAD antisera, immunohistochemical studies reveal that many GABAergic neurons in brain are interneurons and are, therefore, uniquely able to alter the excitability of local circuits within a given brain region (611,613). From these and other studies it has been confirmed that 30–40% of all CNS neurons utilize GABA as their primary neurotransmitter.
GABA is formed in vivo via a metabolic pathway called the “GABA shunt.” The initial step in this pathway utilizes α-ketoglutarate formed from glucose metabolism via the Krebs cycle. α-Ketoglutarate is then transaminated by α-oxoglutarate transaminase (GABA-T) to form Glu, the immediate precursor of GABA. Finally, Glu is decarboxylated to form GABA by the GAD (607,608,614). GAD is expressed only in GABAergic neurons and in certain peripheral tissues, which are also known to synthesize GABA (615).
The principal neuronal GABA transporter is a 70–80 kDa glycoprotein that contains 12 hydrophobic membrane-spanning domains (616,617). Specific inhibitors of GABA uptake that directly bind to the transporter have anticonvulsant and antinociceptive properties in laboratory animals (500). The role of σR interaction with GABA and GABA transporters has yet to be elucidated, but given their role in NMDARs, a role for them could be postulated.
Conformationally-restricted analogues of GABA have been used to help identify three major GABARs, termed GABAA(618–620), GABAB and GABAC receptors (621,622) GABAA and GABAC receptors are members of a superfamily of transmitter-gated ion channels that include nACh (623), strychnine-sensitive Gly and 5-HT3 receptors (618,619). On the other hand, GABABRs are seven transmembrane receptors that are coupled to G-proteins and activate second messenger systems and Ca2+ and K+ ion channels, resembling the activity of mGluRs (624).
The large numbers of drug recognition sites associated with GABAARs, suggested that there may be an endogenous receptor ligand including two natural reduced steroid metabolites of PROG and deoxycorticosterone: ALLO and allotetrahydro-DOC (619,625,626). However there is little compelling evidence at present that any interact with GABAARs in vivo (627). More recently, N,N-dimethyltryptamine [DMT] has been shown to be the endogenous ligand of σRs (7,8), not the neurosteroids as previously thought.
To date, five distinct classes of polypeptide subunits (α, β, γ, δ and ρ) have been cloned (618,620) and multiple isoforms of each have been shown to (628). There is approximately 70% sequence identity between the polypeptide subunits within a given class, but only approximately 30% between classes (629,630–632).
Glycine [Gly]
Gly is the major inhibitory neurotransmitter in the brainstem and spinal cord and functions as a co-agonist at the NMDA subtype of GluR, finely modulated by local expression of specific Gly transporters such as GLYT1 (633) in the forebrain, where it promotes the actions of Glu, the major excitatory neurotransmitter (449). Thus, Gly serves both inhibitory and excitatory functions within the CNS.
The actions of Gly are terminated primarily by reuptake via Na+–Cl−-dependent, high-affinity Gly transporters [GlyT]. Like GABA, this increase in Cl− ion conductance results in a hyperpolarization of the neuronal membrane and an antagonism of other depolarizing stimuli (634). Given their impact on NMDARs, σ1Rs and their activation are probably potentiating factors for glycine transmission.
Cannabinoids
Cannabis and cannabinoids exert most of their biological functions through receptor-mediated mechanisms. Two types of cannabinoid receptors [CB] have been identified – namely CB1 and CB2 – both coupled to a G protein (635). CB1 receptors have been detected and quantified in the CNS (636). They are responsible for the characteristic effects of cannabis, including catalepsy, depression of motor activity, analgesia and feelings of relaxation/well being. Cannabis also affects peripheral neurons; activation of CBs produces suppression in neurotransmitter release in the heart, bladder, intestine and vas deferens (637,638).
CB1 cannabinoid receptors appear to mediate most, if not all of the psychoactive effects of δ-9-tetrahydrocannabinol [δTHC] and related cannabinoid compounds. This G protein-coupled receptor has a characteristic distribution in the nervous system: It is particularly enriched in cortex, hippocampus, amygdala, basal ganglia outflow tracts and cerebellum, a distribution that corresponds to the most prominent behavioral effects of cannabis (637).
Cannabinoid CB2 receptors have only been detected outside the central nervous system, mostly in cells of the immune system, presumably mediating cannabinoid-induced immunosuppression and anti-inflammatory effects (639). With the discovery of cannabinoid receptors for exogenous cannabinoids, endogenous cannabinoids (anandamide, 2-arachidonylglycerol [2-AG]) have been described subsequently (638,640).
Endocannabinoids not only act at cannabinoid receptors, but potentially also at vanilloid and 5-HT3 receptors, both of which are expressed in the gastrointestinal tract. The interactions between endocannabinoids and these other important receptor systems have not been extensively investigated (641). Additionally, experimental evidence also suggests that endocannabinoids mediate neuron-astrocyte communication (642).
The relationship of cannabinoids to the σRs has received little attention, although the interaction of CB1 with the classical opiate receptors has been investigated (635). Decades ago, it was been shown that the morphine-induced dopamine release in the nucleus accumbens requires the CB1 activation (643). Although studies seldom include investigation of the σRs, the effects on other neurotransmitter systems suggest a possibility of interaction of the CB and σRs. For example, both the serotonergic (644) and endocannabinoid systems modulate frontocortical Glu release (645). Cannabinoid CB1 receptor antagonists rimonabant (SR141716) and AM251 directly potentiate GABAA receptors (646), inferring that CB1 receptor agonists may do the reverse; thus damping the excitatory effects of Glu. In fact, endocannabinoids control GABA effects (647), mediates inhibition Glu transmission in the hippocampus (648) leading to the neuroprotective role on the cannabinoids (649) via negative signaling through the G-protein-coupled cannabinoid receptors (650). Although specific, direct data are absent for the role that σRs play in the cannabinoid modulation, the role that σRs play in Glu modulation suggests that they are probably involved in the modulation of Glu produced by the endocannabinoids. To support this hypothesis, the endocannabinoid system plays a central role in the phenomenon of addiction (651), as do the σRs. Hence, some of the changes in σR signaling seen in addictions probably occur in concert with the endocannabinoid system. In fact, it has recently been suggested that σ1R dysfunction might increase vulnerability to cannabis-induced psychosis (652).
Summary
Summarizing the interactions of σR with neurotransmitters is difficult. Data are scarce and incomplete. In addition, the dose-response of stimulation of the σR to an agonist can show stimulatory effects at a low dose and inhibitory effects at high doses, when used experimentally using greater concentrations than physiological levels. As most work is done in in vitro, doses are often excessive and may reflect an overexposure that would not be seen in the in vivo situation. Nonetheless, it seems clear that the σRs have a core and only partly defined role in regulation of neurotransmission.
Pharmacology
A diverse class of psychotropic drugs bind to σ1Rs (653), including antipsychotics, e.g. haloperidol [Haldol®], which have the highest affinity for σ1R (176,177,654), SSRIs, which have medium to high affinities for σ1Rs, and tricyclic antidepressants [TCAs], which have less (176,655). Other compounds that bind to the receptor include morphinans (e.g. DEX) (69), guanidines (e.g. DTG) (193), phenothiazines (e.g. chlorpromazine) (656), butyrophenones (e.g. haloperidol) (657), TCAs (e.g. imipramine) (658), monoamine oxidase inhibitors [MAOI] (e.g. clorgyline) (659), SSRIs (e.g. sertraline) (660), cytochrome P450 inhibitors (e.g. proadifen) (192), anticonvulsants (e.g. phenytoin) (141), addictive drugs (e.g. cocaine, METH) (661), polyamines (e.g. ifenprodil) (662) and certain steroids (e.g. progesterone [PROG] and testosterone) (98). The effects of cocaine occur through direct involvement of σ1R and the DA1 receptor (663). In addition, the anticonvulsant drug phenytoin allosterically modulates σ1Rs (141). These receptors also exhibit a high affinity for (+)-isomers and are proposed to be associated with both pertussis toxin-sensitive Gi /o and cholera toxin-sensitive Gs proteins, PLC and PKC (165).
The σ1Rs have an affinity for a number of specific stereoisomers of these drugs (e.g. (+) PTZ and (+) cyclazocine) (65,79,179,252,653,664). The lack of selectivity between the σ and PCP binding sites seen following exposure to several compounds, including BZM or PCP derivatives, led to a confusion resolved by the availability of new highly selective drugs. Among them, the reference PCP non-competitive antagonist (+)MK-801 maleate [dizocilpine] failed to displace radioligands labeling the σR sites. Selective σR agonists like 1,3-di-O-tolylguanidine [DTG], (+)N-cyclopropylmethyl-N-methyl-1,4-diphenyl-1-ethyl-but-3-en-1-ylamine hydrochloride [JO-1784, igmesine], 2-(4-morpholino)ethyl-1-phenylcyclohexane-1-carboxylate hydrochloride [PRE-084] and 1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl)piperazine dihydrochloride [SA4503], do not bind to the NMDAR-associated PCP site (212). These compounds are now reference compounds in terms of selectivity between σ and PCP receptors.
Neurochemical and electrophysiological studies have then been crucial in revealing the function of the σR (26,129,130). These studies have demonstrated that σRs play a role as modulators of Ca2+ release (164,665) and inhibitors of voltage-gated potassium K+ channels (138,139), NMDARs, tyrosine kinase [TK]-related processes (666), IP3R activation (139,161,164,165), other iGluR and mGluR functions (548), neurosteroids (667) and other neurotransmitter activities (211).
σ1Rs also regulate compartmentalization of lipids in the ER (64,65,164,668,669), and have antitumor activity in vitro and in vivo (670). Studies also have suggested that σ1Rs regulate lipid transport and metabolism, neurogenesis (671), cellular differentiation and myelination in the brain (672); the latter has implications for diseases such as multiple sclerosis [MS].
The actions mediated by σ1Rs at the cellular level can be considered either as acute or chronic. The acute actions include the modulation of ion channels (e.g. K+ channel), NMDARs, IP3R and σ1R translocation. Chronic actions of σ1Rs are basically considered to be the result of an up- or down regulation of the σ1R itself. For example, the up regulation of σ1R per se, even without exogenous ligands, promotes cellular differentiation and reconstitution of lipid “micro domains” in cultured cells (65,673). Recent in vitro and in vivo studies strongly point to the possibility that σ1Rs participate in membrane remodeling and cellular differentiation in the nervous system (65).
Metabolic studies support the view that σRs have functional significance in brain glucose metabolism as glucose utilization is affected by ligands in areas of brain that show high densities of σRs (78). The findings of up and down regulation, suggest that σ1Rs might possess a constitutive biological activity, and that σ1R ligands might merely work as modulators of the innate activity of this protein.
σ1Rs are present throughout vertebrate evolution, with conserved pharmacologic properties (674), in sea anemones, planaria, earthworm, crayfish, cricket, hadfish, shark, goldfish, frog, turtle, chicken, guinea pig and monkey. There does not appear to be a family-related trend in quantity, e.g. monkey has only 20% of the σRs seen in the guinea pig (675). Also, σRs differ from classical neurotransmitter receptors in that they show no postnatal ontogeny in the rat and no age-dependent change in the receptor density. The lack of postnatal development of receptors in the CNS, as compared with postnatal changes in other classical neurotransmitter receptors, and the fact that σR sites are much denser in peripheral organs, such as the liver (418), immune and endocrine tissues (676, 677), suggest a universal role for σRs in cellular function. Because of their widespread modulatory role, σ1R ligands have been proposed to be useful in several therapeutic fields such as amnesic and cognitive deficits, depression and anxiety, schizophrenia, analgesia and against some effects of drugs of abuse (such as cocaine and METH) (32,678).
σ 1R ligands
σR agonists and antagonists are common in easily available drugs, e.g. DEX in cough medications. These antagonists show a GTP-sensitive high affinity binding to the σ1R (679). A complete list of σR ligands is difficult to obtain, as many compounds only have been used in research (680) and are not available, due to the proprietary nature of drug development. A short list of σ1R and σ2R ligands can be seen in Table 1.
Table 1. Some σR ligands in order of their potency.
| σR ligands in rough order of potency | |
|---|---|
| Sigma1 ligands | Sigma2 ligands |
| (+)-pentazocine [PTZ] | 1,3 di-o-tolyl-guanidine [DTG] |
| Haloperidol [Haldol®] | Haloperidol [Haldol®] |
| 1,3di-o-tolyl-guanidine [DTG] | (+)-3-PPP [preclamol] |
| (+)-3-PPP | (−)-pentazocine [PTZ] |
| Dextromethorphan [DEX] | Phencyclidine |
| (+)-SKF 10,047 | (+)-pentazocine [PTZ] |
| (+)-cyclazocine | (−)-SKF 10,047 |
| (−)-pentazocine [PTZ] | BD1047 |
| Phencyclidine | BD1063 |
| (−)-SKF 10,047 | |
Each of the above acts as either an agonist or antagonists often depending on the dose. This biphasic dose-response makes evaluation of studies difficult, but does help to explain conflicting findings (681). Bearing in mind the biphasic dose-response of agonists and antagonists, a summary of some ligands and their agonistic or antagonistic activities can be seen in Table 2.
Table 2. σR agonists and antagonists.
| σ1R and σ2R ligands as agonists or antagonists | |
|---|---|
| σR ligands – agonists | σR ligands – antagonists |
| (+)-N-allylnormetazocine [(+)-SKF 10,047] | (1-[2-(3,4-dichlorophenyl)ethyl]-methylpiperazine [BD1063] |
| 2-(4-morpholino)ethyl-1-phenylcyclohexane-1-carboxylate [PRE-084] | (N-(3,4-dichloropheny)ethyl]—4-methylpiperazine [BD 1008] |
| 1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl)piperazine [SA 4503] | [1-cyclopropylmethyl)-4-(2′(4″-flurophenyl)-2′-oxoethyl)piperidine [DuP 734] |
| 1′-[4-[1-(4-fluorophenyl)-1H-indol-3-yl]-1-butyl]- spiro[isobenzofuran-1(3H), 4′piperidine] [Lu 28-179] | 2-amino-7phosphonoheptanoic acid [AP-7] |
| Fluoxamine | NE-100 |
| Pregenolone-S | E-5842 |
| DHEA-S | BD1139 |
| Donepezil | BIMU-8 |
| PPBP | BMY 14802 |
| Amitriptyline | Cabetapentane |
| BD 737 | Dextromethorphan [DEX] |
| Ibogane | Eliprodil [SL 82.0715] |
| Haloperidol (σ2R) [Haldol®] | Fenpropimorph |
| BD 737 | Haloperidol (σ1R) [Haldol®] |
| 4-(N-benzylpiperidin-4-yl)-4-iodobenzamide [4-IBP] | Ifenprodil tartrate |
| 3,4-methylenedioxymethamphetamine [MDMA] | N,N-dipropyl-2-[4-mrthoxy-3-(2-phenylethoxy)phenyl]ethylamine monohydrochloride [NE-100]) |
| Dehyroepiandrosterone sulfate [DHEA-S] [suggested as the endogenous σR agonist] | N-2-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino) ethylamine [BD 1047] |
| (+)Cyclozocine | N-methyl-d-aspartate [NMDA] |
| Siramesine | N-phenthylpiperidine |
| Igmesine | Opipramole |
| Fluvoxamine | Panamasine |
| Dextromethorphan [DEX] | Pregnalone |
| OPC-14523 | PROG (suggested as the endogenous σR antagonist) |
| CB-64D | Rimcazole |
| Ditolylguanidine [1,3-di-O-tolyguanidin] [DTG] | Sabeluzole |
| Memantine | Testosterone |
| Certain steroids (agonist plus steroidal effect) | Tiopirone |
| Phencyclidine [PCP] | Verapamil |
| Donepezil | WAY 100635 |
| Igmesine [J01783] | Sertraline |
| Interleukin 10 [I l-10] | |
| (+)3H-3-3(3-Hydroxyphenyl)-N-(1-propyl)-piperidine [SA4503] | |
| Methamphetamine | |
| Phenothazines | |
| (+)-pentazocine [PTZ] | |
| Heroin | |
| Cocaine | |
| 2-(4-morholinethyl)1-phenycyclohexanecarboxylate | |
| Amantadine | |
| CB-184 | |
| Dimemorfan | |
Dose response
As previously mentioned, σRs do not show a linear dose-response curve, but show a biphasic dose response curve in various behavioral, biochemical and electrophysiological paradigms (135,137,161,182,194,195). For example, the σR agonist, SA4503, both attenuates and enhances the effects of methamphetamine depending on the dose (682).
A similar dose response curve has also been described with σR ligands in other models such as in release experiments (135) and in behavioral models (182,184). It has been proposed that the different dose response may be due to low doses of σR ligands activating one subtype of σRs for which they have high affinity, whereas higher doses may activate another subtype(s) of the σR for which they have a lower affinity). Such activity would counteract the effects observed at lower doses (194,196,197). Nonetheless, it is important to note that the different, and sometimes opposite, results obtained with low and high doses of σR ligands may explain much of the controversy seen in the literature on σRs.
The fact that low dose effects do not follow the classical dose-response curve has been known for many years and has only been recently revived under the title of hormesis (683–709). The Arndt-Schulz rule or Schulz’ “law” is a basically a hypothesis concerning the observed effects of many chemicals in low concentrations (710–713). According to the Arndt-Schulz rule, highly diluted chemicals enhance life processes, while strong concentrations of the same chemical may inhibit these processes and even terminate these processes (714).
Depending on the process affected, this interplay results in either a J-shaped or inverted J-shaped dose response, which are sometimes called “bell-shaped”, “U-shaped,” “inverted U-shaped,” “biphasic” or “β-curve” (685,686,710,715–732). The point at which the hormetic curve crosses the reference level of response (i.e. the threshold) is the zero equivalent point [ZEP]; in other words, the point at which there is no toxic or stimulatory effect.
At low doses of an σR agonist induction and potentiation of the NMDA response is seen (162,195). In contrast, at higher doses the effects of σR agonists such as DEX, and Igmesine (733) progressively decrease and disappear (194). A similar dose response curve has also been described with σR ligands in other models such as in neurotransmitter release experiments (135) and in behavioral models (182).
It has been proposed that the biphasic dose response curves may be explained by low doses of an σR ligand activating one subtype of σR for which they have high affinity, whereas higher doses activate another or other subtype(s) of the σRs for which they have a lower affinity.
Such a mechanism would counteract the effects observed at lower doses (194,196,197). Nonetheless, it is important to note that the different, and sometimes opposite, results obtained with low and high doses of σR ligands.
Models
σR ligands have been proposed for tumor imaging studies (734), particularly in the detection of pulmonary and abdominal tumors (735), despite irreversible binding in some cases, e.g. (11C)-SA5845 (736). In fact, selective σ2R ligands preferentially bind to pancreatic adenocarcinomas; thus, expanding the possibility of σ2R-based applications in diagnostic imaging, in addition to therapy (110) or drug development (239). A haloperidol challenge has shown that [123I]TPCNE is a novel is a single photon emission-coupled tomography [SPECT] tracer for the σ1R (737).
A σ1R knockout mouse has been developed. The mice demonstrated no overt abnormal phenotype when compared to the wild type. The activity of σ2Rs seems to be unaffected in σ1R-mutant mice. (63). As expected, however, they do lack the locomotor response to the σR ligand (+)-SKF100047 and display reduced response to pain via the σ1R (39).
Cell development and plasticity
σR drugs and neurosteroids, acting at the level of the σ1R protein, are important for plasticity, cell development and trophic actions. These are probably mediated by Ca2+ (82,185,190,210). This observed plasticity, which is both associated with the same prerequisite enhancement of NMDA-mediated glutamatergic neurotransmission and protein dephosphorylation that occur downstream from the massive entry of Ca2+, and [Ca2+]i mobilization from the endoplasmic reticulum and the mitochondria, often occur synergistically (188,738).
Intracellular chaperones, reside specifically at the endoplasmic reticulum (ER)-mitochondrial interface, referred to as the mitochondrial-associated ER membrane [MAM]. Here, σ1Rs is an inter-organelle signaling moderator (665) and regulates ER-mitochondrion Ca2+ signaling (739).
As previously mentioned (88,133,139,145,146,740), K+ channels, which control the fine tuning of Ca2+ entry through both VSCCs and SOCs (store-operated channels), are also prominent targets of the σ1R agonist and antagonist drugs.
Age changes in σRs
Throughout adulthood, differences in the motor changes elicited by drugs affecting σR are correlated with the number of receptors in the P2, and not the P3, cellular fraction (198). Thus, translocation of the σ1R from the ER to the cell membrane (190) decreases with age in motor neuron regions. An increase in density of σ1Rs found in the aged monkey brain supports this hypothesis (199), as they are not as readily translocated and, therefore, increase in density.
For this reason it has been suggested that age-related memory deficits associated with advancing age may be responsive to up regulation of the σRs. In fact, such ability to alleviate memory deficits during aging has been confirmed in humans for the selective σ1R agonist (+)-N-cyclopropylmethyl-N-methyl-1,4-diphenyl-1-ethyl-but-3-en-1-ylamine hydrochloride [Igmesine®], which appears more efficient among the elderly (206).
Conversely, the σ2R subtype exhibits no stereo selectivity and only low affinities for the (+)-BZM. It does not appear to be modulated by pertussis toxin-sensitive Gi /o proteins (207), and is predominantly located in the motor system and periphery (21). Interestingly, brainstem motor function, which is profoundly sensitive to σR drugs, decreases with age, resulting in the reduced accuracy and consistency of fine and complex motor performance (208).
In addition to other neurosteroids, which change with age, the discussion above reflects the effects of DHEA(S) on σ1Rs. Therefore, the age-related changes in neurosteroids probably affect the σRs. Alteration of age-related changes in memory probably relates to the balance of excitatory and inhibitory effects on the CNS, in which σRs play a role. Several studies in rodents show that GABAA agonists impair learning and memory while GABAA antagonists enhance memory (213,214).
In humans benzodiazepines [BZD] (σR agonist) may impair cognition (741–743). On the other hand, σR agonists enhance memory performance in young rodents and in rodent models of cognitive impairment (182,200,201,203,744). In addition, the NMDAR is involved in the development of long-term potentiation [LTP] (215,216), an essential element of neural plasticity. In addition, it now appears as though DHEA(S) has an ability to modulate neurotransmitter receptors in the CNS that are primarily involved in learning and memory (209). Thus, σRs appear to be essential for maintaining neural health and protecting against age-related mammary defects.
Sigma ligands
Numerous σR agonists and antagonists have been previously described. Some are common and easily available drugs, e.g. DEX in cough medications. A short list of σ1R and σ2R ligands can be seen in Table 3.
Table 3. Some σR ligands in order of their potency.
| σR ligands in rough order of potency | |
|---|---|
| σ1R ligands | σ2R ligands |
| (+)-pentazocine [PTZ] | 1,3 di-o-tolyl-guanidine [DTG] |
| Haloperidol | Haloperidol |
| 1,3 di-o-tolyl-guanidine [DTG] | (+)-3-PPP [preclamol) |
| (+)-3-PPP | (−)-pentazocine |
| Dextromethorphan [DEX] | Phencyclidine |
| (+)-SKF 10,047 | (+)-pentazocine |
| (+)-cyclazocine | (−)-SKF 10,047 |
| (−)-pentazocine | BD1047 |
| Phencyclidine | BD1063 |
| (−)-SKF 10,047 | |
Each of the above acts as either an agonist or antagonists. A list of agonists and antagonists can be seen in Table 4.
Table 4. σR agonists and antagonists.
| σ1R and σ2R ligands as agonists or antagonists | |
|---|---|
| σR ligands – agonists | σR ligands antagonists |
| (+)-N-allylnormetazocine [(+)-SKF 10,047] | (1-[2-(3,4-dichlorophenyl)ethyl]-methylpiperazine [BD1063] |
| 2-(4-morpholino)ethyl-1-phenylcyclohexane-1-carboxylate [PRE-084] | (N-(3,4-dichloropheny)ethyl]—4-methylpiperazine [BD 1008] |
| 1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl)piperazine [SA 4503] | [1-cyclopropylmethyl)-4-(2′(4″-flurophenyl)-2′-oxoethyl)piperidine [DuP 734] |
| 1′-[4-[1-(4-fluorophenyl)-1H-indol-3-yl]-1-butyl]-spiro[isobenzofuran-1(3H), 4′piperidine] [Lu 28-179] | 2-amino-7phosphonoheptanoic acid [AP-7] |
| Amitriptyline | E-5842 |
| BD 737 | BD1139 |
| Ibogane | BIMU-8 |
| Haloperidol (σ2R) | BMY 14802 |
| BD 737 | Cabetapentane |
| 4-(N-benzylpiperidin-4-yl)-4-iodobenzamide [4-IBP] | Dextromethorphan [DEX] |
| 3,4-methylenedioxymethamphetamine [MDMA] | Eliprodil [SL 82.0715] |
| Dehyroepiandrosterone sulfate [DHEA-S] [suggested as the endogenous σR agonist] | Fenpropimorph |
| (+)Cyclozocine | Haloperidol (σ1R) |
| Siramesine | Ifenprodil tartrate |
| Igmesine | N,N-dipropyl-2-[4-mrthoxy-3-(2-phenylethoxy)phenyl] ethylamine monohydrochloride [NE-100]) |
| Fluvoxamine | N-2-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2- (dimethylamino)ethylamine [BD 1047] |
| Dextromethorphan [DEX] | N-methyl-d-aspartate [NMDA] |
| OPC-14523 | N-phenthylpiperidine |
| CB-64D | Opipramole |
| Ditolylguanidine [1,3-di-O-tolyguanidin] [DTG] | Panamasine |
| Memantine | Pregnalone |
| Certain steroids (agonist plus steroidal effect) | PROG (suggested as the endogenous σR antagonist) |
| Phencyclidine [PCP] | Rimcazole |
| Donepezil | Sabeluzole |
| Igmesine [J01783] | Testosterone |
| Interleukin 10 [I l-10] | Tiopirone |
| (+)3H-3-3(3-Hydroxyphenyl)-N-(1-propyl)-piperidine [SA4503] | Verapamil |
| Methamphetamine | WAY 100635 |
| Phenothazines | |
| (+)-pentazocine | |
| Heroin | |
| Cocaine | |
| 2-(4-morholinethyl)1-phenycyclohexanecarboxylate | |
| Amantadine | |
| CB-184 | |
| 3-(4-(4-cyclohexylpiperazin-1-yl)butyl)benzo[d]thiazole-2(3H)-thione (CM156) | |
| Dimemorfan | |
Known σRs – location and effects
Steroids binding to σRs has suggested that σRs serve as a link among endocrine, nervous, heart, lung, kidney, liver, intestines, and sexual (745) and immune systems (221); hence, evaluation of σRs in one organ in isolation can miss a significant lesion that is only apparent when viewed in conjunction with other σR-containing organs showing similar, but subtler, lesions.
The tissue density of σRs is not uniform and is different for each subtype (376). The highest concentration of receptors is seen in the CNS, followed by the periphery (liver, spleen, endocrine, GIT lung) (171,676,746–749).
Links between σ1Rs and G-proteins and mGlu (133,138,139,142,750) implies that the σ1R mediates a large number of its effects via the Glu system and as such Glu-related diseases probably have an σR component to them.
The σ1R has been implicated in myriad of disease phenomena, including cardiovascular arrhythmias (751,167), schizophrenia (15), clinical depression [DEP] (752), Parkinsons disease [PD] (574), Alzheimers disease [AD] (753), the effects of cocaine abuse (754) and cancer (95,377,755,756). σ1Rs are distributed throughout the brain in normal subjects, but decreased in the frontal, temporal and occipital lobes, cerebellum and thalamus in patients with early AD and in the putamen in patients with PD (757). Compromising σ1Rs at the endoplasmic reticulum results in cytotoxicity in a dose response manner at physiologically relevant concentrations of dopamine (758). In fact, the cytotoxicity of σR agonists is associated with major changes in cellular metabolism when there is occupancy of the σ2R (759). More recently, the pharmacological stimulation of the σ1R has shown some neuro-restorative effects in experimental PD (760).
Central nervous system
As previously outlined, the CNS appears to be the primary site of σR activity and effects. Specific regions that have been shown to have concentrations of σ1R include, but are not limited to, corpus striatum, nucleus accumbens (61), substantia nigra, pars compacta (656), hippocampal pyramidal cell layer (761), hypothalamus, central grey and red nucleus, pontine and cranial nerve nuclei, pontine nuclei, pons – medulla (761), amygdala (762) and cerebellum (763). σ1Rs have also been seen in the spinal cord, particularly the ventral and dorsal route ganglia (761), a site that is important for σR agonist induction of neck dystonia in rats (764). More specifically, the regional distribution of σR binding within the brain has shown densities at sites as follows: medulla-pons > midbrain > cerebellum > thalamus > striatum > cortex > hippocampus (78,79).
Studies comparing σ1R versus σ2R distributions have established that σ1R sites are most abundant in the dentate gyrus of the hippocampus, facial nucleus, thalamic and hypothalamic nuclei, with moderate densities found in the striatum, cerebellum, dorsal raphe nucleus and locus coeruleus (171,302,765,766). In agreement, studies of σ1R mRNA levels have found high levels of expression in all layers of the cerebral cortex, striatum, hippocampus and cerebellum (767). In comparison, σ2R sites are prominent in the substantia nigra, central gray matter, oculomotor nuclei, cerebellum, nucleus accumbens, amygdala, olfactory bulb, hippocampus and motor cortex (766,768).
The σRs are probably essential for Glu regulation. Glu neurons, also containing GluRs, make up an extensive network throughout the cortex, hippocampus, striatum, thalamus, hypothalamus, cerebellum, and visual and auditory centers in the CNS (386,417,769,770).
At the cellular level, in the CNS, the σ1R is expressed in neurons, ependymocytes, oligodendrocytes and in the peripheral nervous system [PNS], Schwann cells (171,672,771–773). GluRs are similarly expressed in these regions (485), which may mediate the excitatory influence of the σRs.
The highest levels of σ1R immunostaining can be observed in the neurons of the granular layer of the olfactory bulb, hypothalamic nuclei and pyramidal layers of the hippocampus (171). Among other areas that exhibit intense to moderate σ1R immunostaining are the superficial cortical layers, striatal areas including the CP and nucleus accumbens (core and shell), the midbrain, the motor nuclei of the hindbrain, cerebellar Purkinje cells in the cerebellum and the dorsal horn of the spinal cord. At the subcellular level, the σ1R is mostly present within neuronal perikarya and dendrites, where it is associated with microsomal, plasmic, nuclear, or ER membranes (171).
There is adequate direct and circumstantial evidence for abnormal Glu and Glu analogue neurotransmission, suggesting altered σR activity, in the etiology and pathophysiology of many neurological and psychiatric disorders such as epilepsy, schizophrenia, addiction, DEP, anxiety, AD, HD, PD and ALS. In fact, it has been suggested that the lack, or dysfunction, of σ1R exacerbates ALS (774) and AD (42).
σRs probably dampen the excitotoxic effect Glu. Excessive Glu effects can be pronounced during acute events such as ischemic stroke and trauma, or milder but prolonged in chronic neurodegenerative diseases such as AD, PD, HD and ALS (425,447,450,451,775,). In addition there appears to be a role for Glu system, and hence σRs, in regulation of manganese [Mn2+], mercury [Hg2+] and lead [Pb2+] neurotoxicity (379) (Table 5).
Table 5. Summary of σ1R and associated psychiatric diseases.
| Disorder | Substances tested | Study type | Events (121) |
|---|---|---|---|
| Schizophrenia | Haloperidol | Nonclinical | σ1R ligands modulate NMDA receptors effecting dopamine regulation |
| Eliprodil | |||
| Fluoxamine | Clinical | Reduction of σ1R receptors in the postmortem schizophrenic brain Adjunctive medication of σ1R ligands effective for cognitive deficits of schizophrenia | |
| Major depressive disorder | Fluoxamine SA4503 | Nonclinical | σ1R ligands show antidepressive effects in the forced swimming test Neurosteroids, considered as endogenous σ1R ligands, show antidepressive effects |
| Igmesine | |||
| Neurosteroids | |||
| Clinical | Psychotic major depression is improved by fluvoxamine monotherapy | ||
| Obsessive-compulsive | Fluoxamine | Nonclinical | Fluvoxamine improves marble-burying behavior in mice through σ1R activity |
| disorder | Clinical | Fluvoxamine effective for obsessive-compulsive disorder | |
| Fluvoxamine enhances the effect of cognitive behavioral therapy | |||
| Alzheimers disease | Donepezil | Nonclinical | Donepezil show neuroprotective properties against Aβ25-35 peptide-induced toxicity |
| Donepezil show anti-amnesic effects which are antagonized by σ1R antagonists | |||
| Clinical | Decrease of σ1R receptors in the Alzheimers disease brain |
Memory loss
At the behavioral level, σ1R agonists are antiamnesic (602,776–779) and improve the cognitive abilities in experimental animals via the cholinergic system (777,780). In studies using amnesic rodents, the animals’ amnesia seemed to be alleviated by σ1R agonist ligands (781). Examples include PCP-induced cognitive dysfunctions, and amnesias induced by scopolamine (782), the Ca2+ channel blocker nimodipine or carbon monoxide (212). In addition, σ1R agonists show an enhanced efficacy in animal models of AD-related learning impairments or DEP responses (783,784).
The cognition-improving action of neurosteroids has been shown to be mediated via σ1R (181,212). Indeed, σ1R ligands and related neurosteroids interfere with the cocaine-induced state of memory loss (785) mediated through inhibition of iNOS (365). σ1R agonists also have a similar effect (376).
Neuroprotection
The detailed mechanism by which σRs protect the nervous system is not clear (786). The basic mechanism of neuroprotection can be seen in (Figure 9). At least two subtypes of σ1R may affect differentially the Glu-medicated NMDA neurotransmission in the terminal and origin regions of the mesolimbic and nigrostriatal DA-ergic systems. There also probably exists a functional interaction between σ2R and NMDARs in the hippocampus (360). However, there is some question as to whether the interaction is direct or indirect. Even so, administration of a σ1R agonist delays middle cerebral artery occlusion induced neurodegeneration and white matter injury (787), thus confirming the neuroprotective effect of the σ1R. Similarly, σR agonists have been show to attenuate brain injury after experimental focal cerebral ischemia in several species (788). The current hypothesis is that σ1R agonists protect neurons by a mechanism involving the anti-apoptotic protein bcl-2 (27).
Figure 9.
The basic mechanism of neuroprotection by σ1R agonists.
σRs and neurogenesis
Recent evidence has shown hippocampal atrophy can persist long after CNS damage is resolved resulting in major depression (789–792). This atrophy could be due to a regression of dendritic processes, an inhibition of neurogeneration or the loss of hippocampal neurons (793). It has also been shown that hippocampal atrophy can be reversed by successful antidepressant treatments and that in vitro, classical antidepressants promote neurogenesis (794). σ1R has a role in cell morphological changes, specifically in the initiation of neurite outgrowth and sprouting (164,185). In fact, in addition to noted neuronal regeneration, functional recovery has been described following SA-4503 administration (32). Additional support for the neuroprotective effects of σ1R is the finding that mutations of the receptor are associated with frontotemporal lobe degeneration and MND (41).
σ1R and ankyrins are highly concentrated in the growth cone of NG-108 cells, a region related to neurite sprouting, extension and guidance (164). The σ1R agonist (+)- PTZ has no effect by itself on neurite sprouting, but potentiates the neurite-sprouting, induced by nerve growth factor [NGF] (185). In contrast, neurite sprouting, induced by cAMP in PC12 cells, is not affected by (+)-PTZ. The σ1R antagonist NE-100, regardless of the presence of NGF, does not affect neurite sprouting, but antagonizes the potentiation induced by (+)-PTZ, thus clearly indicating mediation via σ1R (185).
Interestingly, similar to σR agonists, the antidepressants imipramine and fluvoxamine potentiate the effects of NGF induce neurite sprouting in PC12 cells (185). These effects of imipramine and fluvoxamine were antagonized by NE-100, while no concentration of 5-HT tested affected neurite sprouting induced by NGF (185), suggesting that the effect on NGF-induced neurite outgrowth of both σ1R agonists and classical antidepressants are mediated by σ1R. Moreover, cell treatments with NGF, even in the absence of σ1R agonists, increased the level of σ1Rs in a dose-dependent manner, and the effects of (+)-PTZ and NGF were additive (185).
Interestingly, treatment with imipramine and fluvoxamine also increased σ1R. In another in vitro model, MT40 cells expressing high levels of σ1R, NGF was found to be more potent in inducing neurite sprouting, whereas treatments with σ1R antisense DNA significantly reduced the degree of neurite sprouting (176,177,185). Together, these data suggest a primary role for σ1R ligands in enhancing NGF-induced neurite growth.
One member of the neurotrophin family, brain derived neurotrophic factor [BDNF], has been heavily implicated in the actions of antidepressants, and perhaps σR agonists, since chronic treatments with a variety of antidepressant therapies induce an increase in BDNF expression (795–798). Moreover, BDNF administration itself has been shown to produce antidepressant effects in behavioral models of depression (799–801).
The effects of σ1R ligands on BDNF expression need to be defined further. Thus far, this has only been studied with the σR ligand E-5842, which showed no effects on BDNF or NGF levels following chronic treatments (802). However, E-5842 presents an σR antagonist profile, so its lack of efficacy cannot be considered as an indication of what might be the effects generated by σ1R agonists. It is still conceivable that σ1R agonists would potentiate the effects of BDNF, similarly to that observed previously with NGF.
Another growth factor of importance is epithelial growth factor [EGF] (803). EGF is present in the CNS and known to stimulate cell proliferation in PC12 cells. A recent report indicated that in PC12 cells, the overexpression of σ1R induces a three-fold increase in neurite sprouting. This effect is suppressed by the σ1R antagonist NE-100 (176,177). The overexpression of the σ1R in squamous cell carcinomas has shown a strong positive correlation with tumor node metastases [TNM], indicating a potential prognostic tool based on pathology assessment and σ1R expression (804). In the context of this review, these data are even more interesting if one considers that EGF has been shown to enhance NMDA-induced modulation of intracellular Ca2+.
More research will be required to elucidate the exact basis for the observed potentiation of neurotrophic effects by σR agonists and whether σR ligands always affect neuronal survival and neurogenesis (805).
Depression, antidepressants and stress
There are a number of possible mechanisms of action for σR ligands to act as antidepressants, including σR, Glu, 5-HT neurotransmission and Ca2+ regulation (26,806). σ1R ligands may present a novel mechanism of antidepressant action with potential for a faster onset of action than classical antidepressants (805,807) and SSRI drugs (55,808).
Depression often coexists with cardiovascular diseases, such as hypertension and heart failure, in which sympathetic hyperactivation is critically involved. Reduced σ1R brain function in depression decreases heart rate via neuronal activity modulation (809). Reduced brain σ1R exacerbates heart failure, especially when combined with pressure overload via sympathetic hyperactivation and worsening depression (810).
The first interest in σR ligands as antidepressants originated from the observation that the antidepressants fluvoxamine (811,812), fluoxetine (813), citalopram, sertraline, clorgyline and imipramine all possess moderate to high affinity (Ki 36–343 nM) for σ1R sites (655,659,660,814). Antidepressant treatments, or other modifications of the 5-HT system, induce changes in σR binding properties. For example, repeated treatments with the TCA imipramine (14 days) causes a decrease in the total number of σ1R binding sites without affecting the affinity of [3H]DTG binding to σR sites in the striatum, hippocampus and cortex of the rat (800,815). Therefore, certain differences in the clinical effects of various antidepressants may, in part, be explained by their distinct influence on cerebral σRs (800,815).
More direct evidence of the potential antidepressant properties of σR ligands was obtained from behavioral experiments. SA 4503 (359), (+)- PTZ, DTG, JO-1784 and SKF-10,047 agonists decrease in a dose dependent fashion the immobility in the FST, whereas the σR antagonists NE-100 and BD1047 blocked these effects (181,208,816). In addition, SA 4503 and (+)-PTZ also decreased immobility time in the Tail Suspension Test [TST], an effect also antagonized by NE-100 (817). Interestingly the antidepressant-like effect of SA 4503 in the FST, a test of a rodents behavioral response to the threat of drowning, was potentiated by the non-competitive NMDA antagonist amantadine (129).
OPC-14523, a combined σ1R and 5-HT1A receptor ligand (323), decreases immobility time where the effect of OPC-14523 can be enhanced by its daily administration for 7 days using the FST as a behavioral biomarker (324). Both the σ1R antagonist NE-100 and the selective 5-HT1A antagonist, WAY 100635 (818) antagonized the behavioral effects of a single dose of OPC-14523 in the FST (324).
Moreover, a one-week pretreatment with para(4)-chloroamphetamine [p-CPA] depletion of brain 5-HT, failed to diminish the antidepressant effects of OPC-14523 in the FST (819), suggesting that σRs alone can mediate the antidepressant effects produced by OPC-14523 and that the combination of the σR and 5-HT1A-receptor activity could induce a more potent or rapid “antidepressant-like” effect.
In keeping with this hypothesis, a potentiation of the “antidepressant-like” effects in the rodent FST has been observed following the combined administration of σR and 5-HT1A-receptor agonists compared with their separate administration (820). In the chronic mild stress behavioral [CMS] model (chronic stress is believed to be involved in the etiology of affective psychiatric disorders), the σR ligands SKF-10,047 (821) and DEX reversed the motor suppression induced by stress (767,805).
Most of the data regarding σR and depression have focused on the σ1R; however, the σ2R ligand Lu 28-179 also has shown “antidepressant like” activity in the CMS model of depression. Specifically, three-week treatments with antidepressants led to a normalized sucrose intake in rats, which reversed the decreased intake caused by the stress. Lu 28-179 did not affect sucrose intake in non-stressed controls, but produced a significant increase in sucrose intake in rats exposed to CMS (822,823). However, even if Lu 28-179 has a higher affinity for σ2R, it also has affinity for σ1R (822); therefore, a role of the σ1R in these “antidepressant-like” effects of Lu 28-179 cannot be excluded.
In animal models, neurosteroids with affinity for σRs have also been shown to exert “antidepressant-like” effects that are dependent on the endogenous neurosteroidal systems. For example, the effect of JO-1784 σR agonist on the FST was enhanced in ADX/CX mice compared to control animals, whereas another σR agonist, PRE-084 (127), demonstrated a significant antidepressant effect only in ADX/CX mice (181); however, this effect has been reported more recently in C57BL/6J and to a lesser degree in Albino Swiss mice (824).
The σ1R-antagonist BD 10047 (208) blocked all these effects (181). Furthermore, treatments with finasteride, which lead to the accumulation of PROG, also blocked σ1R-mediated antidepressant effects. Thus, as discussed previously, circulating steroids appear to exert a tonic modulatory effect on the σ1R and therefore on σ1R-mediated “antidepressant-like” effects (181). It follows that the potency of σ1R agonists as antidepressants is highly dependent on the endogenous PROG levels. Depressed patients such as the elderly with decreased levels of neurosteroids, which would be tonically inhibiting σR to a lesser degree, might be particularly sensitive to such treatments (181).
Only a few controlled clinical trial data are available regarding the effect of σR ligands in depressed patients (825). The results from a double-blind placebo controlled study, obtained from an interim analysis, showed that a dose of 20 mg/day of JO-1784 was superior to placebo and to 20 mg/day of fluoxetine. However, at 100 mg/day, JO-1784 was not different from the placebo (206), which is in keeping with the dose response curves mentioned above (206). A phase II study of SA4503 (cutamesine) in patients with major depression is currently underway (121).
Therefore, even if very limited, the clinical data support the hypothesis that σR agonists could be effective antidepressant medications. However, the mechanisms of action through which σR ligands could exert their antidepressant effects have not been clearly identified. Recent work points to the involvement of the executive function of the PFC (826). As attention deficit disorder [ADD] responds to the stimulant methylphenidate operating via the σ1R (827), investigation of these stimulant effects might help elucidate the mechanism of action of σR ligands in depression.
Attempts to identify the mechanisms by which σR ligands exert their effects have brought to light the role of σRs in the regulation of Ca2+ (153,161,164,165,376) or K+ signaling (88,133,138,139). The effects of JO-1784 in the FST were demonstrated to be Ca2+-dependent, since the extracellular Ca2+ chelator ethylenediamine tetraacetic acid [EDTA] prevented the effect of JO-1784 in a dose-dependent manner. In addition, a lower dose of JO-1784 had no effect by itself, but co-administered with the l-type voltage-dependent Ca2+ channel [VDCC] positive modulator (−)-Bay K8644, it significantly reduced immobility time in the TST.
In agreement with the hypothesis that σRs affect Ca2+ regulation, the l-type VDCC antagonist, verapamil and the N-type VDCC antagonist, α-conotoxin, blocks the effects of JO-1784 (181,249–251). Therefore, σ1R may be interacting with pre- or postsynaptic VDCCs to exert antidepressant-like effects in the FST (181).
Bradykinin, which increases IP3 levels, enhances the effect of JO-1784 (249), whereas the IP3R antagonist, xestospongin C, blocks the effect of JO-1784. Thus the mobilization of intracellular Ca2+ from IP3R-sensitive pools appears to participate initially in the behavioral effects mediated by σ1Rs located on the ER membranes (249). The σ1R then putatively moves to the plasma membrane and interacts with the VDCCs (154,161).
It is likely that σR ligands’ ability to modulate both Glu and 5-HT transmissions also contribute to the antidepressant-like effect observed in behavioral models. The molecular mechanism underlying σRs’ ability to modulate 5-HT and Glu-ergic transmissions may involve σRs’ ability to modulate Ca2+. This modulation could represent a secondary target involved in the effects of σR ligands on both the Glu and the 5-HT systems, thus leading to one primary target (377,828). Recently, practical treatment efforts have shown that σ1Rs are also one of the major pharmacological therapeutic targets of selective serotonin reuptake inhibitors [SSRIs] (829).
Schizophrenia and psychosis
Schizophrenia is one of the most devastating diseases for both the affected patient and those close to him or her. In the search for medications the antipsychotic effect of stimulation σ1Rs has been investigated (830). Experimentation with a number of typical and atypical antipsychotics has been investigated, but the SIGMAR1 gene (σ1R gene) does not confer susceptibility to schizophrenia (831). Interestingly, σ1R polymorphism is associated with an increased risk of schizophrenia and differential activation of the PFC (344) and the severity of AD (44).
The effect of chronic administration of the atypical antipsychotic E-5842, a preferential σ1R ligand, on iGluR subunit levels of mRNA and protein reveals differentially regulated levels of the NMDA2A and of GluR2 subunits in a regionally specific manner. Concentrations of immunoreactivity for the NMDA2A subunit are unregulated in the medial PFC, the frontoparietal cortex, the cingulate cortex and in the dorsal striatum, while they are down regulated in the nucleus accumbens. Concentrations of the GluR2 subunit of the AMPAR are up regulated in the medial PFC and the nucleus accumbens and down-regulation is observed in the dorso-lateral striatum, indicating that E-5842 is able to modify levels of several GluR subunits (15,380,802).
Psychosis
Psychosis occurs in 10% to 37.1% of patients with mood disorders (832,833). Psychotic depression is a clinical subtype of major depressive disorder and is characterized by psychosis accompanied by greater severity of depressive symptoms that include psychomotor impairment (retardation or agitation), morbid cognition (involving guilt and a sense of deserving punishment), suicidal ideation and neuropsychological impairment (834,835). Psychotic depression has been shown to have poor prognosis when compared to nonpsychotic depression (i.e. higher rates of recurrence, greater incapacitation, more frequent hospitalization, longer episodes and greater mortality) (836–839). Although several reports suggest abnormalities of endocrine, DA-ergic and serotonergic systems in psychotic depression (840,841), pathophysiology of psychotic depression is still unclear.
Psychotic depression has traditionally been treated with electroconvulsive therapy and classical antipsychotics, such as respiridone (842), in conjunction with tricyclic antidepressants, such as desipramine (843), although tardive dyskinesia may occur following protracted exposure (844). More recent studies have demonstrated the efficacy of atypical antipsychotics and SSRIs in treating psychotic depression (845,846).
Interestingly, SSRI monotherapy, especially fluvoxamine (Luvox), has been shown effective against both the psychotic and depressive symptoms of this disorder (845,847–851). Based on these findings, it has been recently proposed that SSRIs might have multiple action sites in the brain, in addition to serotonin transporters: perhaps σ1Rs might play a role in the therapeutic action of SSRIs (752).
Some studies have demonstrated the possible link of psychotic depression to dysregulation of neurotransmitters, such as DA and 5HT; abnormality of brain lipid ganglioside; or hyperactivation of the neuroendocrine and the DA system (840,852). In addition, some studies have suggested that the abnormality of cortisol responses to the dexamethasone suppression test is more prevalent in psychotic depression than in nonpsychotic depression (837,838,841); hence it is possible that psychotic symptoms in depression could be due to increased DA activity secondary to hypothalamic-pituitary-adrenal [HPA] axis over activity (853,854). The observation that psychotic depression frequently appears in patients with neuroendocrine diseases such as Cushings syndrome supports the involvement of the abnormalities of the endocrine system in psychotic depression (855–858).
As previously stated σ1R antagonists show antipsychotic effects in vivo. Although some σ1R antagonists have been shown to inhibit apomorphine- or amphetamine-induced behavioral alterations (859), other studies clearly show that selective σ1R antagonists more specifically inhibit the PCP-induced behaviors (156,771).
σ1R antagonists rimcazole and BMY-14802 have been tested in clinical trials of acute psychotic symptoms of schizophrenia, but the antipsychotic actions of these compounds have not been confirmed (860,861). The synthesized σ1R ligands SL82.0715 and EMD 57445 (panamesine) have been shown to improve negative symptoms in open clinical trials (862–864).
Fluvoxamine (Luvox), showing the utmost potent effectiveness in the treatment of psychotic depression, has the highest affinity for σ1R (Ki = 36 nM) among SSRIs (655). Indeed, the efficacy of SSRIs in psychotic depression appears to correlate better with their affinities for σ1R than with those for 5HT transporters (655,849,865). Chronic fluvoxamine exposure in vitro causes an up regulation of σ1R and potentiates the neuritogenesis in a σ1R-dependent, but 5HT-independent, manner (185). Studies have also demonstrated that chronic fluvoxamine increases, in a 5HT-independent manner, ALLO in rat brains and in the cerebrospinal fluid [CSF] of patients with depression (866,867). One study demonstrated a statistically significant correlation between symptomatology improvement and the increase in ALLO following fluoxetine (Prozac) or fluvoxamine treatment (867).
Selective σ1R ligands potently stimulate adrenocorticotropic hormone release after central or peripheral administrations in rats (868,869). Therefore, it is possible that one of the action sites of fluvoxamine may involve σ1R that regulate the neuroendocrine system in the brain (870).
Seizures
Seizures associated with cocaine intoxication are a serious clinical problem requiring immediate and adequate treatment. The seizures appear to arise from the interaction of cocaine with GABAergic and Glu systems (871). Accordingly, pharmacological studies have demonstrated that GABAAR agonists and NMDAR antagonists can efficiently inhibit cocaine-induced seizures, whereas Na+ and Ca2+ channel blockers were ineffective (457). The likely interactions are extremely complex; hence, looking at one component in isolation could be misleading.
An involvement of 5-HT2, DA and σRs in cocaine-induced seizures has also been proposed (872). Some of these changes, such as expression of immediate early genes and increase in neuropeptide biosynthesis may play a compensatory anticonvulsive role; however, other alterations e.g. up-regulation of NMDARs may increase susceptibility to seizures (538). Stimulation of σRs down-regulate electro-acupuncture induced seizures (873). In fact, sigma receptor-mediated events may play some role in seizure processes in the central nervous system and can modulate the protective activity of some conventional antiepileptic drugs (874).
Pain
Although no specific σR ligand has reached the market for the treatment of pain, different pharmacological approaches to the alleviation and treatment of pain have been investigated using σ1R agonists and antagonists (15,38,875–879), particularly regarding potential interaction with opioid analgesics and the effect on analgesia (302,880). Activation of σ1Rs antagonizes opioid analgesia (881,882), where antagonists potentiate opioid analgesia (681,883). σ1Rs differentially modulate acute vs. chronic pain (884–887) and possibly migraine headaches (888): they are also involved in visceral pain (889). In fact, the σRs have been proposed as a modulatory system influencing the analgesic activity of opioid drugs (39). The most promising effects of the σRs lie in the potentiation or modification of the action of other analgesics such as acetaminophen (890) and morphine (891). Such observations may provide a starting point for the development of novel analgesics.
Work has been carried out to identify the mechanism of action of σRs in pain (892). The findings that EAAs have actions on σRs indicated that the EAAs might act via the Glu system in the transmission of nociceptive information (483). In fact, NMDARs receptors play an important role in the potentiation of morphine antinociception (893), and it has been shown that activation of spinal σ1R enhances NMDA-induced pain via PKC- and PKA-dependent phosphorylation of the NMDA receptor NR1 subunit (458,894).
Addiction
Many drugs of abuse, including cocaine and METH, produce effects that can be mitigated through σRs, particularly the σ1R subtype (872) including neurotoxicity (12); hence, it has been suggested that σ1Rs should be considered as potential compound for substance abuse (895). More specifically, agonists at σ1R and σ2R inhibit NMDA-stimulated DA release from motor and limbic areas of rat brain (896). Both cocaine and METH exhibit significant affinities for σRs, and about a 10- to 20-fold preference for the σ1R subtype (897,898). Because of these effects, it has been suggested that σR antagonists are an obvious potential medications for the treatment of drug abuse (872), and CM156 has been shown to attenuate the neurotoxic effects of METH (13).
These interactions appear physiologically relevant because treatment of animals with selective σR antagonists significantly attenuates cocaine-induced locomotor activity, conditioned place preference, behavioral sensitization, convulsions, lethality and changes in gene expression (897,899–902). The importance of the σ1R subtype is supported by the ability of antisense oligonucleotides against them to prevent a number of cocaine-induced behaviors including locomotor hyperactivity, conditioned place preference and convulsions (897,902).
Under normal conditions the brain maintains a delicate balance between inputs of reward seeking controlled by neurons having the D1-like family of dopamine receptors and inputs of aversion coming from neurons having the D2-like ones (663). Cocaine is able to subvert these balanced inputs by altering the cell signaling of these two pathways such that D1 reward seeking pathway dominates. D2 receptors (the long isoforms of the D2 receptor) can complex with σ1Rs, a result that is specific to D2 receptors; thus, signaling via D2 receptor containing neurons, destabilizes the delicate signaling balance influencing drug seeking that emanates from the D1 and D2 receptor containing neurons in the brain (663).
Antagonism of σRs, using either putative antagonists or antisense oligonucleotides, also reduces METH-induced locomotor activity and behavioral sensitization (754,898). In addition, σR proteins levels become up regulated in the brains of rodents who self-administer or are repeatedly injected with METH (71,903). Despite the known interactions between σRs and psycho stimulants such as cocaine and METH, other than an early abstract reporting the binding of 3,4-methylenedioxymethamphetamine [MDMA] to σRs, no other studies to investigate this interaction have been conducted (14). METH and MDMA are structurally similar so the question of whether the interaction between σR and MDMA is similar. Experimental evidence has now shown that indeed the interaction is similar (14).
σ1Rs are critically involved in the rewarding effect of cocaine (900,904). Cocaines mechanism of action involves initial inhibition of neuronal monoamine transporters primarily in the DA reuptake systems located on mesolimbic neurons. Cocaine rapidly increases the DA neurotransmission and triggers adaptive changes in numerous neuronal circuits underlying reinforcement, reward, sensitization and the high addictive potential of cocaine (122,784).
There appears to be regional differences as to the up-regulation of the σ1R. At present the major up-regulation has been recorded in the regions involved in addiction and reward (899). The observation that repeated administration of cocaine rapidly provokes over expression of the σ1R outlines its major role in these first psychological steps of addictive processes (905). Indeed, there is little question that the behavioral effects of cocaine can be related to the σ1R (897).
In utero cocaine [IUC] exposure results in offspring rats with complex neurochemical and behavioral alterations, particularly affecting learning and memory processes (871). However an investigation into the impact of IUC exposure on memory functions in male and female offspring rats revealed that the activation of the σ1R neuromodulatory receptor in utero allows a complete behavioral recovery of the memory functions in prenatally cocaine-exposed rats (779).
Neurodegeneration
Seizure activity, by overstimulation of the σR is mediated via iGlu, particularly NMDAR; this has been associated with neurodegenerative processes such as status epilepticus (906,907), cerebral ischemia (908), perinatal asphyxia and traumatic brain injury (407).
Because the iGluRs are ion-gated channels selective to Na+, K+ and Ca2+, any sustained stimulation of the GluRs results in osmotic damage due to the entry of excessive ions, in particular Ca2+ and water. The entry of this material results in apoptosis and necrosis in neurons (382,384–386,394,412,438,511).
This increase in the intracellular Ca2+ concentration in neurons is crucial to the determinant of injury that occurs following activation of several enzyme pathways and signaling cascades including as phospholipases, PKC, proteases, protein phosphatases, nitric acid synthases, oxidative stress (423) and the generation of oxygen-based free radicals [ROS] (382,384,386,387,394,412,415,428,486,489,490,491,543,909,910,911). Activation of the σ1R inhibits glutamate-induced death of neuron by reducing ROS (912,913).
Neurons are not the only cell type in the nervous system to be damaged by high concentrations of Glu (914). Functional NMDAR recently have been reported in brain glia (915), astrocytes (247,916) and oligodendroglia (409,559,917,). Glial and neuronal NMDARs are functionally and structurally different from the neuronal NMDR; however, the structure of σRs in these cell types is not known at present, and it can only be speculated that the alteration of σRs in these cell types would ameliorate damage caused by overstimulation of NMDARs.
Activation upon ischemia triggers Ca2+-dependent damage of oligodendrocytes and myelin (559,918), a finding that has implications for many central nervous disorders such as MS. Because of the association between σR and Glu, it is likely that σRs are involved in exogenous and endogenous Glu toxicity in oligodendrocytes.
Heart and vessels
σRs have been implicated in the regulation of the cardiovascular system [CV], and σ1R transcripts have been found in parasympathetic intracardiac neurons (756) and the human ether-a-go-go-related gene [hERG] channel (919). These structures are central to cardiac excitation and rhythmic control (920–922).
Both σ1R and σ2R interact with the human ether-à-gogo-related gene (hERG). hERG encodes a cardiac channel that is also abnormally expressed in many primary human cancers, potentiating tumor progression through the modulation of extracellular matrix adhesive interactions. σ1R potentiates hERG current by stimulating channel subunit biosynthesis and σ1R silencing does not modify hERG mRNA contents but reduces hERG mature form densities. A physical association has been shown in HEK cells expressing hERG and σ1R: both proteins co-immunoprecipitate. σ1R expression enhances both channel protein maturation and stability (919).
In rabbits, all σ2R agonists have been shown to reduce phenylephrine-induced cardiac arrhythmias. They prolonged action potential duration in rabbit Purkinje fibers and reduced human ether-a-go-go-related gene (HERG) K(+) currents. It has been suggested that σ2R-receptor ligands block I(Kr) and that this effect could explain part of the antiarrhythmic properties of this ligands family. Nevertheless, an interaction with HERG channels not involving σ2R seems to share this pharmacological property. The repolarization prolongation and the early-after depolarization can be responsible for “torsades de pointe” and sudden cardiac death. It is for this reason that particular caution has to be taken using ligands with affinity for σ2R with respect to abnormal cardiac function (923,924).
The relationship between depression and heart failure is known, but the mechanism has not been fully elucidated. Depression is associated with a substantial increase in the risk of developing heart failure and is independently associated with increased cardiovascular morbidity and mortality. Reduced σ1Rs density in depression decreases heart rate via the sympathetic stimulation in the autonomic nervous system [ANS] (809) and exacerbates heart failure, especially when combined with pressure overload and worsening depression (810). Conversely, cardiovascular disease can lead to severe depression. Thus, therapy with SSRIs used for treatment of depression, has been recommended to reduce cardiovascular disease morbidity and mortality (829,925).
Similarly, GluR have been found in cardiac intramural nerve fibers and ganglia cells as the main structures expressing GluRs in the conducting system (926) and similar findings have been seen in human hearts (500).
These effects of the σ1Rs are probably mediated via PKC- and PKA-dependent phosphorylation of the NMDA receptor (458); however, the exact cellular function of σ1R in these cells remains to be determined. Regardless, a reduction of brain σ1Rs also contribute to sympathetic hyperactivation of the heart (810,927), probably via altered Na+ channels (150,928,929). In the reverse, stimulation of brain σ1Rs ameliorates hypertrophy in mice (925) and cardiac function following myocardial infarction (927).
σR ligands have been shown to modulate contractility, Ca2+ influx and cardiac rate in vitro (930,931), where σR stimulation causes changes in beating frequencies, followed by irregular contractions. In this case, changes in Ca2+ are not mediated by sarcoplasmic reticulum Ca2+ transport systems (923,930).
Experimentally, pre-treatment with an σR agonist improves the reperfusion recovery of cardiac pump function in rat hearts (932) and is cardioprotective (925,933). Activation of the cardiac σR prompts an augmentation of tolerance to the reperfusion damage; however, this effect decreases with time, indicating a possible desensitization of the receptor (934).
In any case, σR activation prevents reperfusion contracture, increases pressure in the left ventricle, and improves survival of cardiac myocytes after ischemia and reperfusion. Conversely, pre-treatment with an σR antagonist augments the reperfusion systolic dysfunction of the myocardium and prevents post-ischemic contractures and cardiac cell lesions (932). Interestingly, the electrical stability in the rat model of post-infarction cardiac sclerosis and stress, activation of either µ- or κ1-opioid receptors or blockade of σ1R reverses the decrease in ventricular fibrillation threshold (935) increasing the probability of sudden death.
By contrast, l-Glu increases the frequency of Ca2+ oscillations in cardiac excitation and rhythmic control (436), which has been positively correlated with increased contraction frequency in myocardial cells. Such an increase may reduce cardiac filling, hypoxia and angina-like chest pains (936,937). It would appear that in the case of the heart, GluR and σRs might have opposing actions.
Activation of σR reversibly blocks the delay in outwardly rectifying K+ channels, large conductance Ca2+ sensitive K+ channels and the M-current. This blockade is dose-dependent suggesting the effect is mediated by σ1R activation (930,931). Thus, activation of σ1R depresses the excitability of intracardiac neurons and is likely to block parasympathetic input to the heart. σR stimulation has been shown to cause changes in beating frequencies, which are followed by irregular contractions (923), probably mediated through NMDARs. It also has been suggested that in the heart the signal transduction pathway does not involve a diffusible cytosolic second messenger or a G protein (158,756), a finding that is supported (207) and refuted by others (174). Therefore, the activation of σ1R is most likely mediated via iGluRs rather than mGluRs.
In addition to myocardial contraction modulation, σRs also are involved in the regulation of coronary and peripheral arterial vascular tension (923). Experiments have shown that the changes in Ca2+ induced by σ1R stimulation are not mediated by sarcoplasmic reticulum Ca2+ transport systems and do not affect the apparent sensitivity of the myofilaments to Ca2+ (930). In fact, σR agonists increase the intracellular Ca2+ levels by stimulating IP3 production and, thus, modulate contractility (167).
Muscle and bones
Many neuroleptic drugs reported to play a role in the control of movement bind with high affinity to σ2R. The high affinity of some neuroleptics for these sites suggests their possible involvement in some σ2R-mediated side effects, such as drug-induced dystonia (938). A correlation between the clinical incidence of neuroleptic-induced acute dystonia and binding affinity of drugs at the σR, indicate that the σR might be involved in neuroleptic-induced acute dystonia, which has been confirmed by σR agonist induced neck dystonia of rats (764).
As bone has been shown to express many of the molecules associated with Glu- mediated signaling (939–941), it is probably that σRs are involved in normal bone function as well as in disease states, although there is some debate regarding the role of Glu in controlling bone growth (942–944). Nonetheless, all osteoblasts (943,944), osteocytes and osteoclasts express one or more of the GluR subunits, including NMDARs (492,497,945–948). The Glu Asp transporter [GLAST] has also been identified in bone (492,949,950). As activation of σRs is an integral part of Glu system, it is likely that they act in conjunction with GluRs to affect cellular changes.
Lung
Considerable data are available for the presence of σRs in lung tissue and a role for them with respect to cancer and chemotherapy (951). In fact, σRs are expressed in a wide variety of tumour cell lines (755,952,953), including non-small-cell lung carcinoma, large-cell-carcinoma (NCI-H1299 and NCI-H838), lung cancer cell line (NCI-H727) (755,953,954) and small-cell lung cancer (NCI-H209/N417) (955). More recently, in material obtained from patients with lung tumors elevated PROG receptor membrane component was seen associated with increased σ2Rs levels in the tumor mass and blood plasma (956).
The anatomical sites of σRs in normal lung are likely to be associated with pulmonary nerves (951). However, expression of the σR has been used to visualize cancerous cells in the lung (86,108,676).
The presence σRs in the airway structures such as the larynx, esophagus and mast cells also implicate the GluRs (and probably the σRs) in the mediation of asthmatic episodes (516,957,958). Thus the excitation of GluRs in the air passages may be important in airway inflammation (959) and hyper reactivity observed in bronchial asthma (440,960). Their presence also could explain the enhancement of acute asthmatic attacks by Glu-containing foods (957).
Current antitussive medications have limited efficacy and often contain the opiate-like agent DEX, which is an σR agonist, or antagonist, depending on the dose administered (194,733). The mechanism whereby DEX inhibits cough is ill defined; however, DEX displays affinity at both NMDARs and σRs, suggesting that the antitussive activity may involve central or peripheral activity at either of these receptors. Experimental findings in guinea pigs support the argument that antitussive effects of DEX may be mediated via σR, since both systemic and aerosol administration of σ1R agonists experimentally inhibit citric-acid-induced cough (961).
Endocrine system
Visualization using autoradiography with σR radioligands has revealed these receptors in the rat pituitary, adrenal, testis and ovary (676). The highest density of σRs is present in the ovary, with progressively lower densities present in the testis, pituitary, adrenal and cerebellum, respectively (676). This distribution is not surprising given that studies have found that PROG and DHEAS bind to σ1Rs (183,228–230).
σRs are believed to be responsible for important regulatory functions in the endocrine system (222,962,963). However, the role of σRs in endocrine cells remains unclear, particularly given the plethora of possible neurotransmitter interactions in the HPA (964). It has been suggested that endogenous σR ligand(s) would contribute, together with other endocrine factors such as DA, neuropeptide Y, or GABA, to the control of pituitary functions (868).
Because steroids have been shown to interact with σ1R (123,210,222) and because they exhibit a significant physiological relevance in the modulation of the electrical activity of frog melanotrope cells (965,966), it can be hypothesized that they represent a very interesting class of endogenous σR modulators in endocrine cells.
Because of the many effects of the endocrine system are related to homeostasis, it is not surprising to note that manipulation of the σRs has numerous potential effects (967). For example, long-term administration of neuroleptic agents, such as haloperidol, has been associated with the development of a drug induced syndrome of inappropriate antidiuretic hormone release, which occurs in the absence of other abnormalities in endocrine function (968). Furthermore, it has now been hypothesized that interaction with some neuroleptic agents and the posterior pituitary σR ligands can inhibit K+-channel function (138,139).
There are minimal data concerning role of σRs and the involvement of GluRs in diabetes mellitus and associated dysfunctional islet cells (418,435,486–488,512,523,524,669,969–973), and abnormalities of HPA function (507,974,975). It is likely that σRs also play a role in these related disorders.
Reproduction
σ1Rs are expressed in the placenta (976), in spermatozoa (977) and other parts of the reproductive system. As stated previously, the highest density of σRs is present in the ovary, with lower densities present in the testis (676). In the ovary, σRs are seen in highest density in the maturing follicles, and lower densities in resting follicles. In the testis, they are present in highest concentrations in the ductuli efferentes and ductus epididymis. Lower densities of binding sites are present in the seminiferous tubules, but none in the interstitial tissue (676). This pattern is mirrored by the distribution of GluRs (978). In addition, σRs (977) and GluRs (418) are abundant in spermatozoa and may affect they signaling pathways (977) in conjunction with PROG or prostaglandin E1 [PGE1].
The developing fetus may be indirectly affected by PROG levels, which have been shown to decrease brain σR function (179). Here PROG acts as an antagonist ligand for the σR during pregnancy. At parturition, Glu output from the fetal liver reduces, leading to a fall in fetal arterial Glu concentrations, which correlate with a marked decrease in PROG output from the pregnant uterus (979), with probable up regulation of σRs (179).
Modulation of ion channels in Xenopus oocytes was observed in the presence or absence of σ1R ligands, suggesting that the σ1R may form a functional complex with the expressed ion channels (88). In fact, these authors went on to show that the σ2R forms an immunoprecipitating complex with ion channels both in rat neurohypophysis and when co expressed in Xenopus oocytes (88).
Liver and kidney
Liver contains high densities of σ1R and σ2R (92,980), and these receptors are specifically localized to lipid rafts in rat liver phospholipid membranes (981,982), particularly mitochondria (113,983).
iGluRs and mGluRs also have been demonstrated in the liver (502,520) and mGluRs are involved in the hydrolysis of IP3 and reduction of viable hepatocytes (984). In fact, it has been suggested that GluR is activated by the Glu present in the portal blood and may contribute to toxic liver damage. At present the relationship of σRs and liver disease is still to be elucidated. As a number of σ1R agonists are being developed for cancer treatment, especially those with EGFR activity, and as the liver is endodermal in origin, it is likely that a lot more findings will result from further hepatic cancer research (981).
Similarly, the kidney contains high densities of σ1R and σ2R as determined by using selective σR probes and photo affinity labeling (92). Interestingly, this work, using kidney tissue in vitro shows that the 25 and 21.5 kDa proteins represent σ1R and σ2Rs, respectively. The role of these σRs in renal disease has yet to be determined.
Eye
Loss of retinal ganglion cells [RGC] is a hallmark of many ophthalmic diseases including glaucoma, diabetes retinopathy, retinal ischemia due to central artery occlusion, anterior ischemic optic neuropathy and may be significant in optic neuritis, optic nerve trauma and AIDS (985). The expression of σ1R mRNA in the mammalian retina is greatest in ganglion cells (986), as determined via mRNA expression, cells of the inner nuclear layer, inner segments of the photoreceptor cells and retinal pigment epithelial cells (987). As Glu toxicity is seen mainly in the ganglion cells, a possibility of neuroprotection by σR ligands against ganglion cell Glu toxicity has been suggested (987). In fact, the σR ligand (+)-PTZ prevents Glu-induced apoptosis in retinal ganglion cells (988).
Expression, subcellular localization and regulation of σR experiments have been undertaken in retinal Mueller cells (989). Mueller cells express σ1R and demonstrate robust σ1R binding activity, which is inhibited by σ1R ligands and is stimulated during oxidative stress (913,990). A similar response is seen for σ2Rs (991). Additionally, late-onset inner retinal dysfunction in mice lacking σ1R has been reported, confirming the importance of σ1R in retinal health (992,993). In adult Mueller cells, σ1Rs are bound and stimulated under the conditions of oxidative stress, an effect that is amplified when cells were incubated with NO and reactive oxygen species [ROS] (989).
Exposure of lens cells to σR antagonists has been shown to lead to growth inhibition and pigment granule production (994,995), implying that σRs are important during lens development.
Gastrointestinal system
σR binding sites have been shown to be present in the myenteric plexus of the guinea pig ileum (747) and are important in the regulation of ileal contractions (314), as are the GluRs (64,390,513,518,996,971,997). As Glu and Asp are both involved in regulating acid secretion in the stomach (998,999), it is likely that σRs are also involved.
DTG and its σR-active congeners inhibit electrically or 5-HT-evoked contractions of the longitudinal muscle and myenteric plexus [LMMP] preparation by a neuronal mechanism (314), and as such σR agonists might be possible novel targets for antisecretory therapy in diarrhea (1000). In fact, the importance of σR manipulation in a number of diseases is highlighted by the recent patent applications (1001), where a method of stimulation of salivary secretion using oral administration of certain σR ligands which may be generally described as N,N-disubstituted phenylalkylamine (US Patent 5387614).
σRs induce emesis in a number of species, probably mediated centrally via the vagus as has been shown for Glu, GluR and GLUTs (1002–1004). It is not surprising to note that emesis and nausea are often associated with the use of σR agonists and antagonists (1005).
Unfortunately, nausea is a difficult endpoint to measure in animal studies; hence, most endpoints used with respect to the gastrointestinal system have been limited to vomiting. Nonetheless, symptoms reported by human subjects include nausea following chemical manipulation of σR in vivo, but their quality of life scores improved (1006). Nausea and vomiting is probably a common endpoint for EAA poisoning mediated via the Glu, and most likely, σR systems in such poisonings, such as is seen in DomA toxicity (415,418,434,437,439,442).
σRs stimulate physiological motility and inhibit experimentally induced colonic hypermotility. They stimulate the postprandial colonic motility in dogs by acting selectively on sigma receptors located peripherally and probably by affecting the release of cholecystokinin octapeptide through a central adrenergic mechanism (1007). Other findings indicate that σR ligand igmesine, blocks the corticotropin releasing factor and emotional stress-induced colonic hypermotility also via an interaction with central cholecystokinin octapaptide mechanisms (1008,1009).
Immune system
Pharmacological studies initially identified high-affinity σRs on human peripheral blood mononuclear cells using DTG and haloperidol (677). Subsequent studies employing the σR selective radioligand, [3H] (+)-PTZ (600) then identified a lower affinity-binding site on murine B- and T-enriched lymphocytes (1010), human and rat lymphocytes (1011). Here, high concentrations of PCP (gM) compete for binding to σRs on the lymphocytes (98). σ2Rs also inhibit T lymphocyte activation (1012).
Evaluation of the effects of σR and the immune system has helped solidify the understanding of the link between the endocrine, nervous and immune systems, although there is still an enormous amount of work required to sort out this relationship (222). σR ligands have potent immunoregulatory properties, including the induction of I l-10 (1013) and the suppression of IFN-γ and granulocyte colony stimulating factor [GM-CSF] (1014).
In murine studies, treatment with σR ligands prevents both graft versus host reactions and delayed-type hypersensitivity granuloma formation (1014). These studies indicate that σR-dependent signaling plays a role in immune-mediated responses. Cocaine, a σ1R ligand, is also known to modulate immune function in vivo and in vitro (1015,1016). σ1R has been shown to regulate early steps of viral RNA replication at the onset of hepatitis C virus infection (1017) and a reovirus nonstructural protein σ1R is required for establishment of viremia and systemic dissemination (1018).
Because immunocompetent animal models of tumorigenicity and tumor progression can serve as sensitive indicators of immune dysfunction, it has been found that σR ligands do impact host antitumor immunity, probably through a σR-dependent cytokine modulation (1013). Sigma ligands, especially σ2R agonists, can inhibit proliferation and induce apoptosis by a mechanism involving changes in cytosolic Ca2+, ceramide and sphingolipid concentrations (1019).
Specific cell types that assist in immunoregulation have been investigated σR activity. Studies initially identified high-affinity σRs on human peripheral blood mononuclear cells using DTG and haloperidol (677). Subsequent studies employing the σR selective radioligand, [3H] (+)-PTZ (600) have identified a lower affinity-binding site on murine B- and T-enriched lymphocytes (1010).
Previous work has shown high that concentrations (pM) of PCP suppressed lymphocyte proliferation, mitogen-induced IgG and IgM production, and LPS-induced IL-1 production (1020). Another report indicates pM concentrations of PCP and PCP analogues inhibit IL-2 production by concanavalin A [Con A]-stimulated murine splenocytes (1021). Similarly, DTG, haloperidol, (+)-PTZ and (−)-PTZ have been shown to enhance LPS-stimulated murine splenocyte proliferation while PCP was without effect (1010). Lymphocytes do not possess PCP-selective receptors as determined in radio receptor assays using the PCP-selective ligand, [3H]N-[1-(2-thienyl)cyclohexyl]-piperidine (677), but a high concentration of PCP (gM) competes for binding to σRs on splenocytes (98).
Regardless of the literature available, it is possible that current research into the effects of manipulation of σRs is not widely known to the public due to proprietary efforts to develop new treatment regimens using stimulation or antagonism of σRs on the immune system and the cells thereof. Due to the many body systems, cell types and substances involved in immunoregulation, tissues that also contain σRs, the manipulation of σRs holds promise for increasing our understanding of immune mediated diseases, cancer and “difficult” infections in which immune dysregulation is an essential part of the pathogenesis of the disease, e.g. HIV and AIDS.
Neoplasia
Pharmaceutical agents acting at the σR have been used in the treatment of cancer and are receiving considerable attention (1022). A large number of drugs are known to bind with high affinity to σ2Rs and these receptors are overexpressed in many cancer tissues, suggesting potential applications for σR ligands in cancer diagnosis and therapy (1023). The potential and specific signal transduction pathways and mechanisms involved in the actions of σR ligands in cancer biology include modulations of the plasma membrane and lipid raft components, intracellular Ca2+ levels, cytoskeletal protein functions and ER stress (1022).
Expression of σRs in neoplastic cell lines and tissues
Both σR subtypes, σ1R and σ1R, are highly expressed in tumor cell lines from various human cancer tissues, including, but not limited to, small- and non-small-cell lung carcinoma (755,953–955), large-cell carcinoma (954), renal carcinoma (952), colon carcinoma (952), sarcoma (952), brain tumors (1024), breast cancer (103,755,953), melanoma (755,953), glioblastoma (755,953), neuroblastoma (755,953) and prostate cancer (755,953).
Comparable findings available from rat cancer cell lines, such as C6 glioma (755), N1E-115 neuroblastoma (94,953) and NG108–15 neuroblastoma X glioma hybrid (755), which generally agree with the human data. Many of these observations are based on the binding of labeled σR ligands that are σ1R- or σ2R- non-specific. In some cases, σ1R sites are masked with DEX so as to determine the relative amounts of σ1R and σ2R sites in the cell preparations. However, these results await confirmation by Western blotting and reverse transcription PCR [RT-PCR] studies (Table 6).
Table 6. σR drug binding in tumor tissues and cell lines.
| Cell line or tumor tissue | σR ligands tested | Reference |
|---|---|---|
| Non-small-cell lung carcinoma | IPAB, haloperidol, DTG | (954) |
| Large-cell-carcinoma (NCI-H1299 and NCI-H838) | IPAB, haloperidol, DTG | (954) |
| Lung cancer cell line (NCI-H727) | IPAB, haloperidol, PTZ, DTG, (+/−) dextrallorphan | (755,953) |
| Breast ductal carcinoma (T47D) | PTZ, DTG, (+/−) dextrallorphan | (755,953) |
| Renal carcinoma | DTG | (952) |
| Colon carcinoma | DTG | (952) |
| Sarcoma | DTG | (952) |
| Brain tumor tissue | DTG | (1024) |
| (Nude mouse) neuroblastoma and glioma | DTG | (1024) |
| Rat neuroblastoma(NIE-115), rat glioma (c6) | PTZ, DTG, (+/−) dextrallorphan | (755,953) |
| U-138MG glioblastomas | PTZ, DTG, (+/−) dextrallorphan | (755,953) |
| Breast cancer cell line (MCF-7; T47D; SKBr3) | Haloperidol, CB-64D, CB-184, IPAB | (103,1025) |
| Small-cell lung cancer (NCI-H209/N417) | IBP, haloperidol | (955) |
| Neuroblastoma [BE(2)]; SK-N-SH) | PTZ, DTG, (+/−) dextrallorphan | (755,953,1026) |
| Prostate tumor cells (DU-145) (LnCap) | IPAB, PTZ, DTG, (+/−) dextrallorphan | (755,953,1025) |
| Mammary adenocarcinoma (line 66) | DTG | (1027) |
| Melanoma (A375) | PTZ, DTG, (+/−) dextrallorphan | (955) |
| C6 glioma cells | 11C-SA4503 | (1028) |
A comparative study on mouse mammary adenocarcinoma revealed that proliferative cells possessed 10 times more σ2R than did quiescent cells (1027); hence, the development of pharmaceuticals to block these receptors is a field of endeavor. The density of σR2 sites have been evaluated after the stimulation of mitosis and progression through the cell cycle in the human mammary tumor cell lines T47D and MCF-7 as well as in the prostate tumor cell line DU-145 (1025). The results suggest that there is a direct correlation between the binding of the σR drug [N-[1α(2-piperidinyl)ethyl]-4-[I125]iodobenzamide [125I-PAB], moderately selective for σ1Rs and proliferative status; and an up-regulation of σR binding sites occurred before mitosis.
Using N-[2-(1′-piperidinyl)- ethyl]-3-123I-iodo-4 methoxybenzamide, also moderately selective for σ1Rs, another study also found that σ1Rs and σ2Rs were present at high density on human breast tumor biopsies but virtually absent in normal tissues (980). Expression of the σ1R, monitored immunocytochemically, has been suggested as a possible marker for predicting the aggressiveness of breast tumors, in particular, where there was a significant correlation between σ1R expression and PROG receptor status (1028,1029). The age-related decrease in PROG may be important in the binding of σ1R agonists in tumor cells (759).
σR ligands as tumor imaging agents
The high densities of σ1R and σ2R binding sites in tumor cell lines and tissues are indicative of their involvement in the cellular pathophysiology of cancer, and as such could have diagnostic potential in tumor imaging. In fact, previous work has developed probes for imaging σ2R both in vitro and in vivo (1030). Most of what is known about σ2R has been obtained using either radiolabeled or fluorescent probes, or biochemical analysis of the effect of σ2R selective ligands on cells growing under tissue culture conditions. Now it has been shown that the PGRMC1 protein complex is the putative σ2R binding site (31).
Numerous nonclinical studies have evaluated the usefulness of radiolabeled σR ligands (1031,1032), as tumor imaging agents in melanoma (734,1033–1036,), breast cancer (954,980,1030,1032,1037–1039), prostate cancer (954,1038,1040) and non-small-cell lung cancer in mouse tumor models (1035). These observations suggested that σ2R ligands could be effective for tumor imaging, including radiotracers (1041), coupled with techniques such as positron emission tomography [PET] (757) or single-photon emission computerized tomography [SPECT] (737,1025,1027,1031,1040) and two-photon confocal microscopy (1042). Recently, development of these tracers (σ2R ligands) has allowed differentiation of tumors from inflammation (1043), especially T cell lymphocytes (1012), or mast cells (1044). Most of these σR ligands are nonselective for the σ1Rs and σ2Rs, but, more recently, σ2R-selective agents have shown the most promise in this regard (1037,1039).
Physiology and pathophysiology of σRs in neoplasia
Effects of σR ligands on cancer cell proliferation and death
Several studies have tested the potential effectiveness of σR ligands on proliferation of tumor cells in vitro. The effects of various σR ligands (e.g. haloperidol, DTG, SKF10047, PTZ and Rimcazole) on the in vitro growth of human mammary adenocarcinoma, colon carcinomas and melanomas show promise (1045).
Cellular proliferation is inhibited, and cell detachment and rounding subsequent to cell death are observed by light microscopy. Of the σR ligands tested, the σ1R- and σ2R-nonspecific rimcazole, and reduced haloperidol, which is the main metabolite of haloperidol in humans (657), were the most potent inhibitors of cell proliferation (1045). Similar inhibitory effects of σR ligands [e.g. N-[2-(piperidino) ethyl]-2-iodobenzamide [2-IBP], haloperidol and N-(2-piperidinoethyl)4-iodobenzamide [IPAB] were observed on small-cell lung cancer (NCI-H209 and NCI-N417) cells (955). IPAB or 2-IBP also inhibited the in vivo xenograft proliferation of NCI-N417 cells (955).
The question of the mechanism(s) underlying the inhibitory effect of σR ligands on tumor cell proliferation is an important one. The morphological effect of treating C6 glioma cells with various σR ligands (generally σ2R- and σ2R-nonspecific) has been examined (755,953). These compounds cause loss of cellular processes, assumption of spherical shape and cessation of cell division, and the time course and magnitude of these effects are dependent on the concentration of the various σR ligands used. Continued exposure to σR ligands for 3–24 h results in cell death, although the morphological effects are reversible if the drug is removed shortly after rounding (755,953). Reduced haloperidol also potently inhibited proliferation of WIDr colon and MCF-7 breast adenocarcinoma cell lines, where in these cells, the intracellular Ca2+ levels were raised, and apoptosis was observed (166), although a direct link between them has not been shown.
The ability of σ2R ligands to induce cell death in the human breast tumor cell lines MCF-7, MCF-7/Adr−, T47D and SKBr3 also has been demonstrated (103). Both σ2R subtype-specific and σ2R non-selective σR ligands result in cell death by a mechanism that involves apoptosis. This has been suggested to be a novel p53- and caspase-independent apoptotic pathway (103).
The effects of σR ligands on cell growth and apoptosis are thought to occur via the sphingolipid pathway. Therefore, it is not surprising to note that σ2R ligands applied to MCF-7/Adr− and T47D breast tumor cells induce a dose-dependent increase in ceramide and concomitant decreases in sphingomyelin (103).
Progress is being made in the development of potential treatment modalities. Nanoparticles have been extensively used as carriers to deliver molecules into tumors through the enhanced permeation and retention effect, and to regulate the release of a chemical or biological effector in response to environmental stimuli such as temperature or pH change. In these cases, cell uptake of nanoparticles has been studied to maximize their delivery into the target cells (1046). Recently, the surface of gold nanocages was functionalized with SV119, a synthetic small molecule specific to σ2R, and then was shown to be effective in for targeting cancer cells (1047).
Possible mechanisms of σR signal transduction and relevance to cancer cell biology
Although there is considerable evidence for the involvement of σRs in cancer cell biology, the mechanism(s) through which these effects occur has not fully been discerned. As has been discussed previously, σRs have been implicated in a wide range of functions, and formulating a unifying hypothesis for the molecular physiology of σRs to account for all of the varied functions will be a great challenge. Few reports exist that deal directly with the mode of action of σRs.
The homology between the σ2R and the sterol isomerase, ERG2, of yeast is interesting, given that both the σ1R and the sterol isomerase have high affinity for σ1R ligands (1048). However, the σ2R has never been demonstrated to possess sterol isomerase activity. On the other hand, emopamil-binding protein, which also binds σR ligands, was found to complement a yeast strain containing a deletion of the ERG2 gene and is a sterol isomerase like ERG2 (147).
Modulation of ion channels
Ion channels are expressed in cell lines derived from several different cancer types and can play an important role in metastasis, an integral aspect of which is the control of cell growth and proliferation (1049–1052). The dual observation that σR expression is increased in tumor cell lines or tissues, and that σ1Rs act as secondary subunits for some ion channels including Cl− channels (1053) might be of importance, given the accumulating evidence for the involvement of different types of ion channels in proliferation (1049,1050,1052) and metastatic activities of cancer cells (1050,1054–1056). Because down-regulation of K+ channel amplitude has been associated with the metastatic phenotype in human prostate and breast cancer (1049,1057), such an effect could underlie the proposed association between cancer progression and σR ligands.
In addition to roles such as proliferation (1057–1059), there are a number of ways in which ion channel activity may contribute to the cancer cell behavior, including migration (1060), apoptosis (1061), adhesion and cytoskeletal organization (1013,1062,1063) and secretion (1064). It remains to be determined whether ion channels, such as the voltage-gated Na+ channel (1050,1064–1067) are also modulated by σR ligands in the cancer process.
Modulation of Ankyrin
σ1R may play a role in controlling the functioning of cytoskeletal proteins (46,66,164). Using immunocytochemical techniques, σ1R, ankyrin B and IP3R have been co-localized in perinuclear areas and areas of cell-to-cell communication. It has been proposed that this trimeric complex may regulate Ca2+ signaling (164). Although the exact underlying molecular mechanism has not yet been described, it is well known that adhesion and cytoskeletal organization are important factors in cancer cell biology (1068,1069).
Modulation of Intracellular Ca2+
Evidence suggest that σR in neuroblastoma cells may use Ca2+ signals to produce cellular effects (95). By using σR-inactive (but structurally similar) ligands, σ2R-selective agents such as CB-64D, and σ1R-selective agents have shown that a fast and transient release of Ca2+ from the ER is induced specifically by the action of the σ1R and σ2Rs (1070). In turn, intracellular Ca2+ modulation can affect PKC activity. Indeed, in rat brain synaptosomes, DA transporter activity is modulated by σ2R ligands via activation of PKC (354). Because intracellular Ca2+ signaling is broadly important for many cellular processes, this may be an important mechanism through which σ2R ligands produce their documented effects on cancer cells.
Modulation of sphingolipid levels
Sphingolipid levels in MCF-7/Adr− and T47D breast tumor cell lines have been investigated following application of σ2R specific agonists in order to understand further the molecular mechanism by which σ2R ligands could cause their observed morphological and apoptotic effects in various cancer cell lines (103). CB-184 causes a dose-dependent increase in ceramide levels and concomitant decrease in sphingomyelin within the MCF-7/Adr− and T47D breast tumor cell lines.
These effects can be attenuated by N-phenethylpiperidine, a nonspecific σR antagonist. These results suggest that σ2Rs may use sphingolipid products to affect Ca2+ signaling, cell proliferation and survival (86,103). In fact, imaging of σ1R in the human brain using SPECT radioligands has started to investigate whether σRs can be used as prognostic indicators (808), even though it is already known that σ2R are potentially useful tumor imaging ligands.
Immunological changes
The mechanism by which σRs affect tumor cells has been more recently investigated with respect to immunological alterations (1071). σR agonists in mice promote the in vivo growth of a syngeneic lung cancer cell lines, which was accompanied by an increase in IL-10 and a decrease in interferon production in spleen cells and at the tumor site. The tumor-promoting effects produced were abrogated by administration of specific antibodies to IL-10, or by administration of a σ1R antagonist, indicating that σ1R agonist ligands augment tumor growth via a cytokine-dependent, receptor-mediated mechanism that involves regulation of T helper1/T helper2 cytokine balance (1071). Most likely, the alteration of immune cells and function will impact the process of carcinogenesis.
Vascular effects
As tumors progress to increased malignancy, cells within them develop the ability to invade into surrounding normal tissues and through tissue boundaries to form metastases at sites distinct from the primary tumor. The molecular mechanisms involved in this process are incompletely understood but those associated with cell-cell and cell-matrix adhesion, with the degradation of extracellular matrix, and with the initiation and maintenance of early growth at the new site are generally accepted to be critical (1068). σR ligands have also been shown to inhibit stem cell differentiation (96), and modulate endothelial cell proliferation and can control angiogenesis, which makes them a promising target for oncology applications (239).
Apoptosis
Apoptosis is a key process in cancer development and progression. The ability of cancer cells to avoid apoptosis and continue to proliferate is one of the fundamental hallmarks of cancer and is a major target of cancer therapy development (110). Apoptosis is the most common mechanism by which the body eliminates damaged or unneeded cells without local inflammation from leakage of cell contents. As the σRs have known apoptotic effects on tumors (86), a more detailed review on their anticancer effects follows.
σ1R
σ1R ligands cause a cell cycle arrest underlined by p27 accumulation. Studies indicate σ1Rs modulate cell regulating volume processes in physiological conditions, indicating that σ1Rs protect cancer cells from apoptosis. It appears that the σ1Rs modulate differentiation (1053). However, other findings suggest that the σ2Rs play a very significant role in σR associated toxicity (1072).
4-(N-benzylpiperidin-4-yl)-4-iodobenzamide [4-IBP], a selective σ1R agonist, has been used to investigate whether this compound modifies the migration and proliferation of human cancer cells. 4-IBP has weak antiproliferative effects on human U373-MG glioblastoma and C32 melanoma cells but induces marked concentration-dependent decreases in the growth of human A549 NSCLC and PC3 prostate cancer cells by eliciting apoptosis. The compound was also significantly antimigratory in all four cancer cell lines (1073). These results indicate that up regulation of the σ1R decrease growth and migration of malignant human cells in vitro, a finding that has been supported by investigations using cells from other species and non-malignant cell types (1074). These authors investigated the expression of σ1R in various human cancer cell lines in comparison to non-cancerous cell lines, using real time RT-PCR and by western blotting with a σ1R specific antibody. Also investigated were the effect of σ1R and σ2R drugs and a σ1R silencing construct. The results suggest σ1R plays a role in proliferation and adhesion of breast cancer cells (1074).
σ2R
Over expression of σ2R induces apoptosis (740). σ2R proteins are over expressed in several tumor cell lines, but the bimolecular mechanism of this over expression still needs further clarification, although two-photon confocal has shown σ2Rs are present in mitochondria, lysozomes, endoplasmic reticulum and plasma membranes (1075). There is a possibility that this over expression can be used with a radioligand to visualize in human bladder cancer specimens, then if a possible correlation could be established between σ2R over expression and tumor tissue stage and grade. In studies done so far, results demonstrate that σ2R protein is normally expressed in human bladder and over expressed in the case of high-grade transitional cell carcinomas (112), indicating this technique shows promise for staging of some cancers (111).
σ2R agonists induce apoptosis in drug-resistant cancer cells (1038), enhance the potency of DNA damaging agents, and down-regulates expression of p-glycoprotein mRNA (1076). σ2R agonists increase lysosomal membrane permeability in the early stages of σ2R-induced cell death (1077). Thus, σ2R agonists may be useful in treatment of drug-resistant cancers and the σ2R may serve as a novel signaling pathway to apoptosis (15,981). Further work has demonstrated that the σ2Rs are located in lipid rafts in the cell membrane, and these lipid rafts may play an important role in the mechanism of σ2R -induced apoptosis (982).
Several σ2Rs ligands have been shown to trigger apoptosis in pancreatic cancer cells. More importantly, σ2Rs ligands are internalized rapidly by the cancer cells and are capable of delivering other small-molecule therapeutics (1077).
A summary of references related to the expression of σRs are outlined in Table 7. A summary of the references that describe the molecular action of σRs are outlined in Table 8. A summary of the references describing σR binding are outlined in Table 9. A summary of the references describing the role of σRs in pathophysiology are outlined in Table 10.
Table 7. Reference Summary: Expression of sigma R (σR -regions/tissues).
| Location of σR in tissues | Function(s) | Reference(s) |
|---|---|---|
| CNS: corpus striatum, nucleus accumbens | (61) | |
| Brain: substantia nigra, pars compacta | (656) | |
| CNS: dentate gyrus of hippocampus, facial nucleus, thalamic, hypothalamic nucei, straitum, cerebellum dorsal raphe nucleus and locus coeruleus | (171,765,766,302) | |
| Hippocampal pyramidal cell layer, hypothalamus, central grey and red nucleus, pontine, cranial nerve nuclei, pontine nuclei, Pons – medulla, spinal cord – ventral and dorsal route ganglia | (761) | |
| Brain: cortex limbic area amygdala | (762) | |
| Brain: cerebellum | (763) | |
| Brain: Medulla – pons, midbrain, cerebellum, thalamus, straitum, cortex, hippocampus | (78,79) | |
| Brain: cerebral cortex, straitum, hippocampus, cerebellum | (767) | |
| Brain: substantia nigra, central grey matter, oculomotor nuclei, cerebellum, nucleus accumbens, amygdale, olfactory bulb, hippocampus, motor cortex | (766,768) | |
| CNS | Glu regulation, regulates excitotoxic effect of Glu | (386,417,425,447,450,451, 769,770,775,) |
| CNS | Regulation of Mn2+, Hg2+ and Pb2+ neurotoxicity. | (379) |
| Neurons: ependymocytes, oligodendrocytes and peripheral nervous system Schwann cells | (171,672,771–773) | |
| CNS | σ1R agonist are antiamnesic, improve cognitive abilities | (27,181,212,365,376,602,753, 776–781,783,785) |
| CNS | Neuroprotection – two subtypes of σ1R may affect differentially the Glu-mediated NMDA neurotransmission in the terminal and origin regions of the mesolimbic and nigrostriatal DA-ergic systems. Also, functional interaction between σ2R and NMDARs in the hippocampus. σ1R agonist may protect neurons by mechanism involving anti-apototic protein bcl-2. | (360,786–788, ) |
| CNS | σ1R initiates neurite outgrowth and sprouting. σ1R agonist potentiates neurite-sprouting by nerve growth factor. σ1R agonist may potentiate effects of BDNF and EGF | (32,41,164,176,177,185,803) |
| CNS | Mechanism of action of σR ligands in depression – regulation of Ca2+ or K+ signaling. Interacts with VDCC’s. Modulate Glu and 5-HT transmissions. May be a target for serotonin reuptake inhibitors. | (88,133,139,153,161,164–166,181,249–251, 376,377,664,828, 829) |
| Brain: frontoparietal cortex, cingulated cortex, dorsal striatum, nucleus accumbens | σ1R ligands affect iGluR subunit levels of mRNA and protein, differentially regulating levels of NMDA2A and GluR2 in a regionally specific manner. | (15,380,802) |
| CNS | Activation of σ1Rs antagonize opioid analgesia whereas antagonists potentiate opioid analgesia. Excitatory amino acids have actions on σRs indicating action via the Glu system. Activation of spinal σ1R enhances NMDA-induced pain via PKC- and PKA-dependent phosphorylation of the NMDA receptor NR1 subunit | (458,483,681,881–883,894) |
| CNS | Agonist of σ1R and σ2R inhibit NMDA-stimulated DA release from motor and limbic areas of rat brain. | (896) |
| ANS: Parasympathetic intracardiac neurons | Cardiac excitation and rhythmic control | (756,920–922) |
| Ion Channel: hERG channel | Cardiac excitation and rhythmic control | (919–922) |
| Heart and vessels | Effects of σ1R mediated via PKC- and PKA dependent phosphorylation of the NMDA receptor, altered Na+ channels | (150,458,928,929) |
| Heart | σR ligands modulate contractility, Ca2+ influx and cardiac rate. σR activation prevent reperfusion contracture, increases pressure in left ventricle and improves survival of cardiac myocytes after ischemia and reperfusion. Activation of σR reversibly blocks the delay in outwardly rectifying K+ channels, conductance Ca2+ sensitive K+ channels and the M-current. | (923,930–932) |
| Peripheral arteries | σR agonist increase intracellular Ca2+ levels by stimulating IP3 production, modulating contractility. | (167) |
| Muscle | Dystonia | (764,938) |
| Osteoblasts | Act in conjunction with GluRs to affect cellular changes | (943,944) |
| Osteocytes, osteoclasts | Act in conjunction with GluRs to affect cellular changes | (492,497,765,945–947) |
| Lung: pulmonary nerves | (951) | |
| Larynx, esophagus, mast cells | (516,957,958) | |
| Airway passage | Excitation of GluRs may be important in airway inflammation and hyper reactivity observed in bronchial asthma | (440,959,960) |
| Airways | Antitussive | (194,733,961) |
| Pituitary, adrenal, testis and ovary | (676) | |
| Endocrine system | Regulatory functions | (127,222,962) |
| Pituitary | Control of pituitary functions | (868) |
| Endocrine system | Antidiuretic hormone release | (968) |
| Posterior pituitary | Inhibit K+ channel function | (138,139) |
| Placenta | (976) | |
| Spermatozoa | May affect signaling pathways in conjunction with PROG or prostaglandin E1. | (976,977) |
| Ovary – follicles | (676) | |
| Testis: ductuli efferentes, ductus epididymis, seminiferous tubules | (676) | |
| Xenopus oocytes, neurohypophysis | Modulation of ion channels. Forms a immunoprecipitating complex with ion channels | (88) |
| Liver, localized to lipid rafts in rat liver phospholipid membranes, mitochondria | (92,113,980–983) | |
| Kidney | (92) | |
| Eye: retinal ganglion cells, inner nuclear membrane, inner segments of the photoreceptors, retinal pigment epithelial cells, retinal Mueller cells, | Neuroprotection against ganglion Glu toxicity, apoptosis, σ1R and σ2R binding activity stimulated during oxidative stress, important during lens development | (74,783,913,987,989–991,994,995) |
| Myenteric plexus of the guinea pig ileum | Regulation of ileal contractions, may be involved in regulating acid secretion in stomach | (314,747,998,999) |
| Gastrointestinal longitudinal muscle and myenteric plexus | Inhibit electrically or 5-HT-evoked contractions, stimulation of salivary secretion | (314,1001) |
| Vagus | Induce emesis | (1002–1005) |
| Human peripheral blood mononuclear cells, lymphocytes | σ2Rs inhibit lymphocyte activation. Potent immunoregulatory properties including induction of IL-10, suppression of IFN-γ and suppression of granulocyte colony stimulating factor. | (98,677,1010–1014) |
| Splenocytes | Lymphocyte proliferation, mitogen-induced IgG and IgM production, LPS-induced IL-I production | (98,1020) |
| Viral RNA | Regulate early steps in viral RNA replication | (1017,1018) |
| Host antitumor immunity | σR-dependent cytokine modulation. Ligand can induce apoptosis by changes in cytosolic Ca2+, ceramide and sphingolipid concentrations. | (1019,1013) |
| Neoplasia | Receptors overexpressed in many cancer tissues | (1022,1023) |
Table 8. Reference Summary: Molecular Action of σRs.
| Molecular action of σR | Tissue | Reference(s) |
|---|---|---|
| Glu regulation: regulates excitotoxic effect of Glu. two subtypes of σ1R may affect differentially the Glu-mediated NMDA neurotransmission in the terminal and origin regions of the mesolimbic and nigrostriatal DA-ergic systems. Functional interaction between σ2R and NMDARs in the hippocampus. σ1R agonist may protect neurons by mechanism involving anti-apototic protein bcl-2 | CNS | (27,360,386,417,425,447,450,451, 769,770,775,786–788) |
| Regulation of Mn2+, Hg2+ and Pb2+ neurotoxicity | CNS | (379) |
| σ1R initiates neurite outgrowth and sprouting. σ1R agonist potentiates nerite-sprouting by nerve growth factor. σ1R agonist may potentiate effects of BDNF and EGF | CNS | (32,41,164,176,177,185,803) |
| Regulation of Ca2+ or K+ signaling. Interacts with VDCC’s. Modulate Glu and 5-HT transmissions. | CNS | (88,133,138,139,153,154,161,164--166, 181,249–251,377,821,829) |
| σ1R ligands affect iGluR subunit levels of mRNA and protein, differentially regulating levels of NMDA2A and GluR2 in a regionally specific manner. | Frontoparietal cortex, cingulated cortex, dorsal striatum, nucleus accumbens | (15,380,802) |
| Activation of spinal σ1R enhances NMDA-induced pain via PKC- and PKA-dependent phosphorylation of the NMDA receptor NR1 subunit | CNS | (458,483,681,881–883,894) |
| Agonist of σ1R and σ2R inhibit NMDA-stimulated DA release from motor and limbic areas of rat brain. | CNS | (896) |
| Cardiac excitation and rhythmic control | Parasympathetic intracardiac neurons, hERG channel | (756,919–922) |
| Effects of σ1R mediated via PKC- and PKA dependent phosphorylation of the NMDA receptor, altered Na+ channels | Heart and vessels | (150,458,928,929) |
| σR ligands modulate contractility, Ca2+ influx and cardiac rate. σR activation prevent reperfusion contracture, increases pressure in left ventricle and improves survival of cardiac myocytes after ischemia and reperfusion. Activation of σR reversibly blocks the delay in outwardly rectifying K+ channels, conductance Ca2+ sensitive K+ channels and the M-current. | Heart | (923,930–932) |
| σR agonist increase intracellular Ca2+ levels by stimulating IP3 production, modulating contractility | Peripheral arteries | (167) |
| Ca2+ influx | Muscle | (764,938) |
| Act in conjunction with GluRs to affect cellular changes | Osteoblasts, osteocytes, osteoclasts | (492,497,943–948) |
| Modulate ion channels | Lungs | (194,440,733,959–961) |
| Regulatory functions. Control of pituitary functions. Antidiuretic hormone release. Inhibit K+ channel function. | Endocrine system, pituitary | (127,222,868,962,968) |
| May affect their signaling pathways in conjunction with PROG or prostaglandin E1. | Spermatozoa | (976,977) |
| Modulation of ion channels. Forms a immunoprecipitating complex with ion channels | Xenopus oocytes, neurohypophysis | (88) |
| Neuroprotection against ganglion Glu toxicity, apoptosis, σ1R and σ2R binding activity stimulated during oxidative stress, important during lens development | Eye – retinal ganglion cells, inner nuclear membrane, inner segments of the photoreceptors, retinal pigment epithelial cells, retinal Mueller cells | (74,783,913,987,989–991,994,995) |
| Regulation of ileal contractions, may be involved in regulating acid secretion in stomach | Myenteric plexus of the guinea pig ileum | (314,747,998,999) |
| Inhibit electrically or 5-HT-evoked contractions, stimulation of salivary secretion | Gastrointestinal longitudinal muscle and myenteric plexus | (314,1001) |
| 5-HT transmissions. | Vagus | (1002–1005) |
| σ2Rs inhibit lymphocyte activation. Potent immunoregulatory properties including induction of IL-10, suppression if IFN-γ and suppression of granulocyte colony stimulating factor. | Human peripheral blood mononuclear cells, lymphocytes | (98,677,1010–1014) |
| Lymphocyte proliferation, miogen-induced IgG and IgM production, LPS-induced IL-I production | Splenocytes | (98,1020) |
Table 9. Reference Summary: σR Binding (679, 680).
| Drug | Target tissue |
|---|---|
| (+)-pentazocine [PTZ] | Sigma1 Sigma2 ligands |
| Haloperidol [Haldol®] | Sigma1 Sigma2 ligands |
| 1,3 di-o-tolyl-guanidine [DTG] | Sigma1 Sigma2 ligands |
| (+)-3-PPP [preclamol] | Sigma1 Sigma2 ligands |
| (+)-SKF 10,047 | Sigma1 Sigma2 ligands |
| (+)-pentazocine [PTZ] | Sigma1 Sigma2 ligands |
| Phencyclidine | Sigma1 Sigma2 ligands |
| Dextromethorphan [DEX] | Sigma1 ligands |
| (+)-cyclazocine | Sigma1 ligands |
| BD1047 | Sigma2 ligands |
| BD1063 | Sigma2 ligands |
Table 10. Reference Summary: Role of σRs in Pathophysiology.
| Tissue type – disorder | Function or role in pathology | Reference(s) | |
|---|---|---|---|
| CNS | Memory loss | σR ligands may be antiamnesic, improve cognitive abilities | (181,212,365,376,602,753,776–783,785) |
| CNS | Neurodegeneration | Delays cerebral artery occlusion-induced neurodegeneration and white matter injury. σ1R agonist protect neurons by a mechanism involving the anti-apoptotic protein bcl-2. Initiation of neurite outgrowth and sprouting. Overstimulation of σR is mediated via Glu, particularly NMDAR leading to osmotic damage, apoptosis and necrosis. | (27,32,41,164,176,177,185,382,384–386,394,412,438,511,787,788) |
| CNS | Schizophrenia | σ1R polymorphism is associated with increased rick of schizophrenia and differential activation of PFC and the severity of AD. | (44,344) |
| CNS | Depression, stress | σR, Glu, 5-HT neurotransmission and Ca2+ regulation. Reduced brain σ1R exacerbates heart failure. Regulation of Ca2+ or K+ in neurotransmission. | (26,57,88,133,138,139,153,154,161,164,165,181,249–251,326,376,805–810,828,829) |
| CNS | Psychosis | Selective σ1R ligans potentially stimulate adrenocorticotropic hormone release, regulation of neuroendocrine system in brain. | (868–870) |
| CNS | Seizures | Complex involvement of 5-HT2, DA and σRs. Increase in neuropeptide biosynthesis may play a compensatory anticonvulsive role. Seizure activity by overstimulation of σR is mediated via Glu, particularly NMDAR. | (407,538,872,873,906–908) |
| CNS | Pain | Activation of σ1Rs antagonize opioid analgesia whereas antagonists potentiate opioid analgesia. Excitatory amino acids have actions on σRs indicating action via the Glu system. Activation of spinal σ1R enhances NMDA-induced pain via PKC- and PKA-dependent phosphorylation of the NMDA receptor NR1 subunit. | (483,458,681,881–883,894) |
| CNS | Addiction | Both cocaine and METH exhibit a significant affinities for σRs. Agonist of σ1R and σ2R inhibit NMDA-stimulated DA release from motor and limbic areas of rat brain. | (872,896–898) |
| Heart and blood vessels | Heart failure | Reduced σ1Rs density in depression decreases heart rate via the sympathetic stimulation in the autonomic nervous system. Reduction of brain σ1Rs also contribute to sympathetic hyperactivation of the heart via altered Na+ channels. Activation of σ1R depresses the excitability of intracardiac neurons causing changes in beating frequencies, which are followed by irregular contractions. σRs are involved in the regulation of coronary and peripheral arterial vascular tension. | (150,751,809,810,923,927–933) |
| Muscle | drug-induced dystonia | High affinity of some neuroleptics for these sites suggests their possible involvement in some σ2R-mediated side effects. | (764,938) |
| Bone | Bone | All osteoblasts, osteocytes and osteoclasts express one or more of the GluR subunits, including NMDARs. Possible that σRs are involved in normal bone function as well as in disease states. | (946,943,944,947,492,945,497,948) |
| Lung | Asthma | The presence σRs in the airway structures such as the larynx, esophagus and mast cells also implicate the GluRs (and probably the σRs) in the mediation of asthmatic episodes. | (440,516,957–960) |
| Endocrine | Drug induced syndrome of inappropriate antidiuretic hormone release. | Interaction with some neuroleptic agents and the posterior pituitary σR ligands can inhibit K+-channel function. | (138,139,968) |
| Endocrine | Diabetes mellitus | Dysfunctional islet cells | (435,486–488,499,523,524,669,969,970,971,512,972,973) |
| Eye | Glaucoma, diabetes retinopathy, retinal ischemia due to central artery occlusion, anterior ischemic optic neuropathy, optic neuritis, optic nerve trauma | Possible neuroprotection by σR ligands against ganglion cell Glu toxicity. Late-onset inner retinal dysfunction in mice lacking σ1R. Exposure of lens cells to σR antagonists has been shown to lead to growth inhibition and pigment granule production implying importance during lens development. Gene silencing of the σ1R induces cell death. | (987,988,992–995) |
| Gastrointestinal | Emesis | σRs induce emesis in a number of species mediated centrally via the vagus. | (1002–1004) |
| Immune system | Graft versus host reactions and delayed-type hypersensitivity granuloma formation. Immune dysregulation | σ2Rs inhibit T lymphocyte activation. σR ligands have potent immunoregulatory properties, including the induction of IL-10 and the suppression of IFN-γ and granulocyte colony stimulating factor [GM-CSF]. | (98,1012–1014) |
Conclusions
The neuropharmacological properties of σ1R ligands relate to the neuron modulatory role of σ1R. σ1Rs act as intracellular amplifiers for signal transductions involving IP3R and modulate neurotransmitter systems (mainly through NMDA receptors). σ1Rs and ion channels may play an important role in neuroplasticity processes. σ1R ligands are highly active when a pharmacological or pathological imbalanced state arises.
The combined administration of σ1R receptor ligands and medications with a known therapeutic effect has been shown to improve these effects due to the modulatory role of σ1R receptors resulting in the need for lower doses to reach therapeutic concentrations. Of particular interest is the non-linear dose response curve of σ1R agonists in in vitro experiments, in which σ1R agonists are active, e.g. learning and memory processes, depression (1078) and anxiety. These findings imply that researchers should take hormesis into account in order to design informative experiments or clinical trials with σ1R agonists. For example, the σ1R SA4503 agonist attenuates or enhances the effects of methamphetamine depending on the dose (682).
The most promising therapeutic targets for σ1R antagonism are nociception and some deleterious effects of certain drugs of abuse such as cocaine, methamphetamine and ethanol (1079). Many drugs used routinely show affinity for σ1R receptors and exert the same effects as other more selective σ1R ligands in many behavioral tests and in vitro assays. Therefore, the therapeutic properties of these drugs might be due, at least in part, to their interaction with σ1R receptors.
The involvement of σRs in the cellular pathophysiology of cancer is apparent from the high density of σ1R and σ2R-binding sites found in various tumor cell lines and tissues. Consequently, σR drugs have been suggested to be potentially useful tumor imaging agents.
The ability of σ2R drugs to inhibit tumor cell proliferation through mechanisms that may involve apoptosis, intracellular Ca2+ and sphingolipids have been investigated, and such findings may lead to the development of σ drugs as cancer therapeutic agents. It is possible that an increase in σ2R expression is a significant event in transition from normal to malignant cells. Further research would be interesting to determine whether σR are involved in other metastatic cell behaviors such as adhesion, secretion, motility and invasion.
The interaction of σRs and other neurotransmitters is complex. As has been discussed in this review, σRs are intimately involved with the glutamate system, and are probably an essential part of the expression of excitotoxicity (1080). Other interactions with opiates, neurosteroids, serotonin, dopamine and cannabinoids have been difficult to fully elucidate due to the biphasic nature of dose response curves and the large combination of potential effects. In addition, as most work is done in in vitro, doses are often excessive and may reflect an overexposure that would not be seen in the in vivo situation. Even though the interaction of σRs with various tissues is complex, it is apparent that σRs play a central role in neurotransmission and apoptosis. The development of new knockout mice and transgenic initiatives will be important to further research.
Acknowledgements
The authors gratefully acknowledge the critical review and comments of Dr. M.A. Wallig of the University of Illinois.
Declaration of interest
This work was supported by a grant from MannKind Corporation, USA.
References
- Martin WR, Eades CG, Thompson JA, et al. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharm Exp Therap. 1976;197:517–32. [PubMed] [Google Scholar]
- Hanner M, Moebius F, Flandorfer A, et al. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci USA. 1996;93:8072–7. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YX, Mei J, Xu J, et al. Cloning and characterization of a mouse sigma1 receptor. J Neurochem. 1998;70:2279–85. doi: 10.1046/j.1471-4159.1998.70062279.x. [DOI] [PubMed] [Google Scholar]
- Prasad PD, Srinivas SR, Wang H, et al. Electrogenic nature of rat sodium-dependent multivitamin transport. Biochem Biophys Res Commun. 2000;270:836–40. doi: 10.1006/bbrc.2000.2498. [DOI] [PubMed] [Google Scholar]
- Seth P, Fei YJ, Li HW, et al. Cloning and functional characterization of a sigma receptor from rat brain. J Neurochem. 1998;70:922–31. doi: 10.1046/j.1471-4159.1998.70030922.x. [DOI] [PubMed] [Google Scholar]
- Seth P, Leibach FH, Ganapathy V. Cloning and structural analysis of the cDNA and the gene encoding the murine type 1 sigma receptor. Biochem Biophys Res Commun. 1997;241:535–40. doi: 10.1006/bbrc.1997.7840. [DOI] [PubMed] [Google Scholar]
- Cozzi N, Gopalakrishnan A, Anderson L, et al. Dimethyltryptamine and other hallucinogenic tryptamines exhibit substrate behavior at the serotonin uptake transporter and the vesicle monoamine transporter. J Neural transmission (Vienna, Austria: 1996) 2009;116:1591–9. doi: 10.1007/s00702-009-0308-8. [DOI] [PubMed] [Google Scholar]
- Fontanilla D, Johannessen M, Hajipour A, et al. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science (NY) 2009;323:934–7. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP, Hayashi T, Vaupel DB. When the endogenous hallucinogenic trace amine N,N-dimethyltryptamine meets the sigma-1 receptor. Sci Signal. 2009;2:pe12. doi: 10.1126/scisignal.261pe12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastianetto S, Ramassamy C, Poirier J, Quirion R. Dehydroepiandrosterone (DHEA) protects hippocampal cells from oxidative stress-induced damage. Brain Res Mol Brain Res. 1999;66:35–41. doi: 10.1016/s0169-328x(99)00002-9. [DOI] [PubMed] [Google Scholar]
- Menkel M, Terry P, Pontecorvo M, et al. Selective sigma ligands block stimulant effects of cocaine. Eur J Pharmacol. 1991;201:251–2. doi: 10.1016/0014-2999(91)90355-t. [DOI] [PubMed] [Google Scholar]
- Kaushal N, Matsumoto RR. Role of sigma receptors in methamphetamine-induced neurotoxicity. Curr Neuropharmacol. 2011;9:54–7. doi: 10.2174/157015911795016930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal N, Seminerio MJ, Shaikh J, et al. CM156, a high affinity sigma ligand, attenuates the stimulant and neurotoxic effects of methamphetamine in mice. Neuropharmacology. 2011;61:992–1000. doi: 10.1016/j.neuropharm.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brammer M, Gilmore D, Matsumoto R. Interactions between 3,4-methylenedioxymethamphetamine and sigma1 receptors. Eur J Pharmacol. 2006;553:141–5. doi: 10.1016/j.ejphar.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guitart X, Codony X, Monroy X. Sigma receptors: biology and therapeutic potential. Psychopharmacology. 2004;174:301–19. doi: 10.1007/s00213-004-1920-9. [DOI] [PubMed] [Google Scholar]
- Meririnne E, Kankaanpaa A, Lillsunde P, Seppala T. The effects of diazepam and zolpidem on cocaine- and amphetamine-induced place preference. Pharmacol Biochem Behav. 1999;62:159–64. doi: 10.1016/s0091-3057(98)00139-7. [DOI] [PubMed] [Google Scholar]
- Cormaci G, Mori T, Hayashi T, Su TP. Protein kinase A activation down-regulates, whereas extracellular signal-regulated kinase activation up-regulates sigma-1 receptors in B-104 cells: implication for neuroplasticity. J Pharm Exp Therap. 2007;320:202–10. doi: 10.1124/jpet.106.108415. [DOI] [PubMed] [Google Scholar]
- Freeman A, Bunney B. The effects of phencyclidine and N-allylnormetazocine on midbrain dopamine neuronal activity. Eur J Pharmacol. 1984;104:287–93. doi: 10.1016/0014-2999(84)90404-7. [DOI] [PubMed] [Google Scholar]
- Ohno M, Watanabe S. Intrahippocampal administration of (+)-SKF 10,047, a sigma ligand, reverses MK-801-induced impairment of working memory in rats. Brain Res. 1995;684:237–42. doi: 10.1016/0006-8993(95)00489-d. [DOI] [PubMed] [Google Scholar]
- Vaupel DB. Naltrexone fails to antagonize the sigma effects of PCP and SKF 10,047 in the dog. Eur J Pharmacol. 1983;92:269–74. doi: 10.1016/0014-2999(83)90297-2. [DOI] [PubMed] [Google Scholar]
- Quirion R, Bowen WD, Itzhak Y, et al. A proposal for the classification of sigma binding sites. Trends Pharmacol Sci. 1992;12:85–6. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- Su TP. Evidence for sigma opioid receptor: binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J Pharm Exp Therap. 1982;223:284–90. [PubMed] [Google Scholar]
- Vidal-Torres A, de la Puente B, Rocasalbas M, et al. Sigma-1 receptor antagonism as opioid adjuvant strategy: enhancement of opioid antinociception without increasing adverse effects. Eur J Pharmacol. 2013;711:63–72. doi: 10.1016/j.ejphar.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Bowen W, Hellewell S, McGarry K. Evidence for a multi-site model of the rat brain sigma receptor. Eur J Pharmacol. 1989;163:309–18. doi: 10.1016/0014-2999(89)90200-8. [DOI] [PubMed] [Google Scholar]
- Kitaichi K, Chabot JG, Moebius FF, et al. Expression of the purported sigma(1) (sigma(1)) receptor in the mammalian brain and its possible relevance in deficits induced by antagonism of the NMDA receptor complex as revealed using an antisense strategy. J Chem Neuroanat. 2000;20:375–87. doi: 10.1016/s0891-0618(00)00106-x. [DOI] [PubMed] [Google Scholar]
- Skuza G, Rogoz Z. The synergistic effect of selective sigma receptor agonists and uncompetitive NMDA receptor antagonists in the forced swim test in rats. J Physiol Pharmacol. 2006;57:217–29. [PubMed] [Google Scholar]
- Yang S, Bhardwaj A, Cheng J, et al. Sigma receptor agonists provide neuroprotection in vitro by preserving bcl-2. Anesth Analg. 2007;104:1179–84. doi: 10.1213/01.ane.0000260267.71185.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JH, Lee S, Wittenberg GF, et al. Neurosteroid regulation of inhibitory synaptic transmission in the rat hippocampus in vitro. Neuroscience. 1999;90:1177–83. doi: 10.1016/s0306-4522(98)00543-0. [DOI] [PubMed] [Google Scholar]
- Myers AM, Charifson PS, Owens CE, et al. Conformational analysis, pharmacophore identification, and comparative molecular field analysis of ligands for the neuromodulatory sigma 3 receptor. J Med Chem. 1994;37:4109–17. doi: 10.1021/jm00050a008. [DOI] [PubMed] [Google Scholar]
- Lee IT, Chen S, Schetz JA. An unambiguous assay for the cloned human sigma1 receptor reveals high affinity interactions with dopamine D4 receptor selective compounds and a distinct structure-affinity relationship for butyrophenones. Eur J Pharmacol. 2008;578:123–36. doi: 10.1016/j.ejphar.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zeng C, Chu W, Pan F, et al. Identification of the PGRMC1 protein complex as the putative sigma-2 receptor binding site. Nature Commun. 2011;2:380–94. doi: 10.1038/ncomms1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos E, Entrena J, Nieto F, Cendan C, Del Pozo E. Pharmacology and therapeutic potential of sigma(1) receptor ligands. Current neuropharmacology. 2008;6:344–66. doi: 10.2174/157015908787386113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschos KA, Veletza S, Chatzaki E. Neuropeptide and sigma receptors as novel therapeutic targets for the pharmacotherapy of depression. CNS Drugs. 2009;23:755–72. doi: 10.2165/11310830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, Dhir A. sigma-1 receptors in major depression and anxiety. Expert review of neurotherapeutics. 2009;9:1021–34. doi: 10.1586/ern.09.40. [DOI] [PubMed] [Google Scholar]
- Navarro JF, Beltran D, Cavas M. Effects of (+) SKF 10,047, a sigma-1 receptor agonist, on anxiety,tested in two laboratory models in mice. Psicothema. 2012;24:427–30. [PubMed] [Google Scholar]
- Hashimoto K. Comments on “An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-d-aspartate antagonist, CP-101,606 in patients with treatment-refractory major depressive disorder”. J Clin Psychopharmacol. 2009;29:411–12. doi: 10.1097/JCP.0b013e3181ace848. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Nguyen Q, Rotaru D, et al. Protracted developmental trajectories of GABAA receptor alpha1 and alpha2 subunit expression in primate prefrontal cortex. Biol Psychiatry. 2009;65:1015–23. doi: 10.1016/j.biopsych.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J, Zamanillo D, Corbera J, et al. Selective sigma-1 (sigma1) receptor antagonists: emerging target for the treatment of neuropathic pain. Central Nerv Syst Agents Med Chem. 2009;9:172–83. doi: 10.2174/1871524910909030172. [DOI] [PubMed] [Google Scholar]
- Zamanillo D, Romero L, Merlos M, Vela JM. Sigma 1 receptor: a new therapeutic target for pain. Eur J Pharmacol. 2013;716:78–93. doi: 10.1016/j.ejphar.2013.01.068. [DOI] [PubMed] [Google Scholar]
- Furuse T, Hashimoto K. Sigma-1 receptor agonist fluvoxamine for delirium in patients with Alzheimer's disease. Ann Gen Psychiatry. 2010;9:6. doi: 10.1186/1744-859X-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luty AA, Kwok JB, Dobson-Stone C, Loy CT, et al. Sigma nonopioid intracellular receptor 1 mutations cause frontotemporal lobar degeneration-motor neuron disease. Ann Neurol. 2010;68:639–49. doi: 10.1002/ana.22274. [DOI] [PubMed] [Google Scholar]
- Mishina M, Ohyama M, Ishii K, et al. Low density of sigma1 receptors in early Alzheimer's disease. Ann Nuclear Med. 2008;22:151–6. doi: 10.1007/s12149-007-0094-z. [DOI] [PubMed] [Google Scholar]
- Feher A, Juhasz A, Laszlo A, et al. Association between a variant of the sigma-1 receptor gene and Alzheimer's disease. Neurosci Lett. 2012;517:136–9. doi: 10.1016/j.neulet.2012.04.046. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zheng L, Halliday G, et al. Genetic polymorphisms in sigma-1 receptor and apolipoprotein E interact to influence the severity of Alzheimer's disease. Current Alzheimer research. 2011;8:765–70. doi: 10.2174/156720511797633232. [DOI] [PubMed] [Google Scholar]
- Mesangeau C, Narayanan S, Green AM, et al. Conversion of a highly selective sigma-1 receptor-ligand to sigma-2 receptor preferring ligands with anticocaine activity. J Med Chem. 2008;51:1482–6. doi: 10.1021/jm701357m. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Justinova Z, Hayashi E, et al. Regulation of sigma-1 receptors and endoplasmic reticulum chaperones in the brain of methamphetamine self-administering rats. J Pharm Exp Therap. 2010;332:1054–63. doi: 10.1124/jpet.109.159244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Butler TR, Prendergast MA. Inhibition of sigma-1 receptor reduces N-methyl-d-aspartate induced neuronal injury in methamphetamine-exposed and -naive hippocampi. Neurosci Lett. 2010;481:144–8. doi: 10.1016/j.neulet.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su T. An update on the development of drugs for neuropsychiatric disorders: focusing on the sigma 1 receptor ligand. Expert Opin Therap Targets. 2008;12:45–58. doi: 10.1517/14728222.12.1.45. [DOI] [PubMed] [Google Scholar]
- Dhir A, Kulkarni S. Possible involvement of sigma-1 receptors in the anti-immobility action of bupropion, a dopamine reuptake inhibitor. Fundam Clin Pharmacol. 2008;22:387–94. doi: 10.1111/j.1472-8206.2008.00605.x. [DOI] [PubMed] [Google Scholar]
- Lever JR, Miller DK, Fergason-Cantrell EA, et al. Relationship between cerebral sigma-1 receptor occupancy and attenuation of cocaine's motor stimulatory effects in mice by PD144418. J Pharm Exp Therap. 2014;351:153–63. doi: 10.1124/jpet.114.216671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto RR, Shaikh J, Wilson LL, et al. Attenuation of methamphetamine-induced effects through the antagonism of sigma (sigma) receptors: evidence from in vivo and in vitro studies. Eur Neuropsychopharmacol. 2008;18:871–81. doi: 10.1016/j.euroneuro.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Blasio A, et al. Activation of sigma-receptors induces binge-like drinking in Sardinian alcohol-preferring rats. Neuropsychopharmacology. 2011;36:1207–18. doi: 10.1038/npp.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Parylak SL, et al. Sigma-1 receptor knockout mice display a depressive-like phenotype. Behav Brain Res. 2009;198:472–6. doi: 10.1016/j.bbr.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutada P, Mundhada Y, Ghodki Y, et al. Influence of sigma-1 receptor modulators on ethanol-induced conditioned place preference in the extinction-reinstatement model. Behav Pharmacol. 2012;23:25–33. doi: 10.1097/FBP.0b013e32834eafe6. [DOI] [PubMed] [Google Scholar]
- Furuse T, Hashimoto K. Fluvoxamine monotherapy for psychotic depression: the potential role of sigma-1 receptors. Ann Gen Psychiatry. 2009;8:26–9. doi: 10.1186/1744-859X-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Yu S, Guo X, et al. Fluvoxamine increased glutamate release by activating both 5-HT(3) and sigma-1 receptors in prelimbic cortex of chronic restraint stress C57BL/6 mice. Biochim Biophys Acta. 2012;1823:826–37. doi: 10.1016/j.bbamcr.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Furuse T, Hashimoto K. Fluvoxamine for aripiprazole-associated akathisia in patients with schizophrenia: a potential role of sigma-1 receptors. Ann Gen Psychiatry. 2010;9:11–14. doi: 10.1186/1744-859X-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto Y, Terada S, Sato S, et al. Repetitive questioning behavior in Alzheimer's disease: relationship to regional cerebral blood flow. Psychiatry Res. 2010;184:151–6. doi: 10.1016/j.pscychresns.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Tagawa N, Kobayashi Y, et al. Involvement of the sigma1 receptor in the antidepressant-like effects of fluvoxamine in the forced swimming test in comparison with the effects elicited by paroxetine. Eur J Pharmacol. 2012;696:96–100. doi: 10.1016/j.ejphar.2012.09.030. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Shiba K, Akhter N, et al. Evaluation of radioiodinated vesamicol analogs for sigma receptor imaging in tumor and radionuclide receptor therapy. Cancer Sci. 2009;100:2188–92. doi: 10.1111/j.1349-7006.2009.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo J, McEwen B. Molecular aspects of neuropeptide regulation and function in the corpus striatum and nucleus accumbens. Brain Res Brain Res Rev. 1994;19:1–28. doi: 10.1016/0165-0173(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Heroux J, Tam S, De Souza E. Autoradiographic identification and characterization of sigma receptors in guinea pig brain using [3H]1(cyclopropylmethyl)-4-(2′-(4″-fluorophenyl)-2′-oxoethyl) piperidine ([3H]DuP 734): a novel sigma receptor ligand. Brain Res. 1992;598:76–86. doi: 10.1016/0006-8993(92)90170-e. [DOI] [PubMed] [Google Scholar]
- Langa F, Codony X, Tovar V, et al. Generation and phenotypic analysis of sigma receptor type I (sigma 1) knockout mice. Eur J Neurosci. 2003;18:2188–96. doi: 10.1046/j.1460-9568.2003.02950.x. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su T. Intracellular dynamics of sigma-1 receptors (sigma(1) binding sites) in NG108-15 cells. J Pharm Exp Therap. 2003;306:726–33. doi: 10.1124/jpet.103.051292. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su T. The potential role of sigma-1 receptors in lipid transport and lipid raft reconstitution in the brain: implication for drug abuse. Life Sci. 2005;77:1612–24. doi: 10.1016/j.lfs.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su T. Sigma-1 receptors (sigma(1) binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: roles in endoplasmic reticulum lipid compartmentalization and export. J Pharm Exp Therap. 2003;306:718–25. doi: 10.1124/jpet.103.051284. [DOI] [PubMed] [Google Scholar]
- Mitsuda T, Omi T, Tanimukai H, et al. Sigma-1Rs are upregulated via PERK/eIF2alpha/ATF4 pathway and execute protective function in ER stress. Biochem Biophys Res Commun. 2011;415:519–25. doi: 10.1016/j.bbrc.2011.10.113. [DOI] [PubMed] [Google Scholar]
- Prasad PD, Li HW, Fei YJ, et al. Exon-intron structure, analysis of promoter region, and chromosomal localization of the human type 1 sigma receptor gene. J Neurochem. 1998;70:443–51. doi: 10.1046/j.1471-4159.1998.70020443.x. [DOI] [PubMed] [Google Scholar]
- Musacchio JM, Klein M, Santiago LJ. High affinity dextromethorphan binding sites in guinea pig brain: further characterization and allosteric interactions. J Pharm Exp Therap. 1988;247:424–31. [PubMed] [Google Scholar]
- Itzhak Y. Different modulation of the binding to two phencyclidine (PCP) receptor subtypes: effects of N-methyl-d-aspartate agonists and antagonists. Neurosci Lett. 1989;104:314–19. doi: 10.1016/0304-3940(89)90595-8. [DOI] [PubMed] [Google Scholar]
- Itzhak Y. Multiple affinity binding states of the sigma receptor: effect of GTP-binding protein-modifying agents. Mol Pharmacol. 1989;36:512–17. [PubMed] [Google Scholar]
- Itzhak Y, Stein I. Regulation of sigma receptors and responsiveness to guanine nucleotides following repeated exposure of rats to haloperidol: further evidence for multiple sigma binding sites. Brain Res. 1991;666:166–72. doi: 10.1016/0006-8993(91)91695-w. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Stein I. Sigma binding sites in the brain; an emerging concept for multiple sites and their relevance for psychiatric disorders. Life Sci. 1990;47:1073–81. doi: 10.1016/0024-3205(90)90165-n. [DOI] [PubMed] [Google Scholar]
- Kim FJ, Kovalyshyn I, Burgman M, et al. Sigma 1 receptor modulation of G-protein-coupled receptor signaling: potentiation of opioid transduction independent from receptor binding. Mol Pharmacol. 2010;77:695–703. doi: 10.1124/mol.109.057083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Steele TD, Sharpe LG. Effects of strychnine-insensitive glycine receptor ligands in rats discriminating dizocilpine or phencyclidine from saline. J Pharm Exp Therap. 1997;280:46–52. [PubMed] [Google Scholar]
- Kekuda R, Prasad PD, Fei YJ, et al. Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1) Biochem Biophys Res Commun. 1996;229:553–8. doi: 10.1006/bbrc.1996.1842. [DOI] [PubMed] [Google Scholar]
- Seth P, Ganapathy ME, Conway SJ, et al. Expression pattern of the type 1 sigma receptor in the brain and identity of critical anionic amino acid residues in the ligand-binding domain of the receptor. Biochim Biophys Acta. 2001;1540:59–67. doi: 10.1016/s0167-4889(01)00117-3. [DOI] [PubMed] [Google Scholar]
- Weissman AD, Dam M, London ED. Alterations in local cerebral glucose utilization induced by phencyclidine. Brain Res. 1987;435:29–40. doi: 10.1016/0006-8993(87)91583-6. [DOI] [PubMed] [Google Scholar]
- Weissman AD, Su TP, Hedreen JC, London ED. Sigma receptors in post-mortem human brains. J Pharm Exp Therap. 1988;247:29–33. [PubMed] [Google Scholar]
- Yamamoto H, Miura R, Yamamoto T, et al. Amino acid residues in the transmembrane domain of the type 1 sigma receptor critical for ligand binding. FEBS Lett. 1999;445:19–22. doi: 10.1016/s0014-5793(99)00084-8. [DOI] [PubMed] [Google Scholar]
- Kavanaugh MP, Tester BC, Scherz MW, et al. Identification of the binding subunit of the sigma-type opiate receptor by photoaffinity labeling with 1-(4-azido-2-methyl[6-3H]phenyl)-3-(2-methyl[4,6-3H]phenyl)guanidine. Proc Natl Acad Sci USA. 1988;85:2844–8. doi: 10.1073/pnas.85.8.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP, Hayashi T. Understanding the molecular mechanism of sigma-1 receptors: towards a hypothesis that sigma-1 receptors are intracellular amplifiers for signal transduction. Curr Med Chem. 2003;10:2073–80. doi: 10.2174/0929867033456783. [DOI] [PubMed] [Google Scholar]
- Laurini E, Marson D, Dal Col V, et al. Another brick in the wall. Validation of the sigma1 receptor 3D model by computer-assisted design, synthesis, and activity of new sigma1 ligands. Mol Pharm. 2012;9:3107–26. doi: 10.1021/mp300233y. [DOI] [PubMed] [Google Scholar]
- Ganapathy M, Prasad P, Huang W, et al. Molecular and ligand-binding characterization of the sigma-receptor in the Jurkat human T lymphocyte cell line. J Pharm Exp Therap. 1999;289:251–60. [PubMed] [Google Scholar]
- Wang LM, Shelness GS, Childers SR, et al. Cloning and expression of an alternative splice variant of the mouse sigma-1 receptor. Proc Am Assoc Cancer Res. 2000;41:205–6. [Google Scholar]
- Aydar E, Palmer C, Djamgoz M. Sigma receptors and cancer: possible involvement of ion channels. Cancer Res. 2004;64:5029–35. doi: 10.1158/0008-5472.CAN-03-2329. [DOI] [PubMed] [Google Scholar]
- Wang K, Hackett JT, Cox ME, et al. Regulation of the neuronal nicotinic acetylcholine receptor by SRC family tyrosine kinases. J Biol Chem. 2004;279:8779–86. doi: 10.1074/jbc.M309652200. [DOI] [PubMed] [Google Scholar]
- Aydar E, Palmer C, Klyachko V, Jackson M. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Amer M, McKeown L, Tumova S, et al. Inhibition of endothelial cell Ca(2)(+) entry and transient receptor potential channels by Sigma-1 receptor ligands. Br J Pharmacol. 2013;68:1445–55. doi: 10.1111/bph.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades DJ, Kinder DH, Mahfouz TM. A comprehensive ligand based mapping of the sigma2 receptor binding pocket. Med Chem (Shariqah (UAE)) 2014;10:98–121. doi: 10.2174/1573406409999131119103621. [DOI] [PubMed] [Google Scholar]
- Hellewell S, Bowen W. A sigma-like binding site in rat pheochromocytoma (PC12) cells: decreased affinity for (+)-benzomorphans and lower molecular weight suggest a different sigma receptor form from that of guinea pig brain. Brain Res. 1990;527:244–53. doi: 10.1016/0006-8993(90)91143-5. [DOI] [PubMed] [Google Scholar]
- Hellewell S, Bruce A, Feinstein G, et al. Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur J Pharmacol. 1994;268:9–18. doi: 10.1016/0922-4106(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Moltzen EK, Perregaard J, Meier E. Sigma ligands with subnanomolar affinity and preference for the sigma 2 binding site. 2: spiro-joined benzofuran, isobenzofuran, and benzopyran piperidines. J Med Chem. 1995;38:2009–17. doi: 10.1021/jm00011a020. [DOI] [PubMed] [Google Scholar]
- Bowen W, Vilner B, Williams W, et al. Ibogaine and its congeners are sigma 2 receptor-selective ligands with moderate affinity. Eur J Pharmacol. 1995;279:R1–3. doi: 10.1016/0014-2999(95)00247-i. [DOI] [PubMed] [Google Scholar]
- Vilner BJ, Bowen WD. Modulation of cellular calcium by sigma-2 receptors: release from intracellular stores in human SK-N-SH neuroblastoma cells. J Pharm Exp Therap. 2000;292:900–11. [PubMed] [Google Scholar]
- Haller J, Panyutin I, Chaudhry A, et al. Sigma-2 receptor as potential indicator of stem cell differentiation. Molecular imaging and biology: MIB. 2012;14:325–35. doi: 10.1007/s11307-011-0493-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JM, Bowen WD, Patrick SL, et al. A comparison of (−)-deoxybenzomorphans devoid of opiate activity with their dextrorotatory phenolic counterparts suggests role of sigma 2 receptors in motor function. Eur J Pharmacol. 1993;231:61–8. doi: 10.1016/0014-2999(93)90684-a. [DOI] [PubMed] [Google Scholar]
- Walker JM, Bowen WD, Walker FO, et al. Sigma receptors: biology and function. Pharmacol Rev. 1990;42:355–402. [PubMed] [Google Scholar]
- Walker R. The significance of excursions above the ADI. Case study: monosodium glutamate. Regul Toxicol Pharmacol. 1999;30:S119–21. doi: 10.1006/rtph.1999.1337. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Harris EW, Ray R, Hudzik TJ. sigma2 Site-mediated inhibition of electrically evoked guinea pig ileum longitudinal muscle/myenteric plexus contractions. Eur J Pharmacol. 1995;294:547–53. doi: 10.1016/0014-2999(95)00584-6. [DOI] [PubMed] [Google Scholar]
- Jeanjean AP, Mestre M, Maloteaux JM, Laduron PM. Is the sigma 2 receptor in rat brain related to the K+ channel of class III antiarrhythmic drugs? Eur J Pharmacol. 1993;241:111–16. doi: 10.1016/0014-2999(93)90940-j. [DOI] [PubMed] [Google Scholar]
- Couture S, Debonnel G. Modulation of the neuronal response to N-methyl-d-aspartate by selective sigma2 ligands. Synapse (NY) 1998;29:62–71. doi: 10.1002/(SICI)1098-2396(199805)29:1<62::AID-SYN5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Crawford K, Bowen W. Sigma-2 receptor agonists activate a novel apoptotic pathway and potentiate antineoplastic drugs in breast tumor cell lines. Cancer Res. 2002;62:313–22. [PubMed] [Google Scholar]
- Cassano G, Gasparre G, Contino M, et al. The sigma-2 receptor agonist PB28 inhibits calcium release from the endoplasmic reticulum of SK-N-SH neuroblastoma cells. Cell Calcium. 2006;40:23–8. doi: 10.1016/j.ceca.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Spitzer D, Simon PO, Jr, Kashiwagi H, et al. Use of multifunctional sigma-2 receptor ligand conjugates to trigger cancer-selective cell death signaling. Cancer Res. 2012;72:201–9. doi: 10.1158/0008-5472.CAN-11-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Rothfuss J, Zhang J, et al. Sigma-2 ligands induce tumour cell death by multiple signalling pathways. Br J Cancer. 2012;106:693–701. doi: 10.1038/bjc.2011.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abate C, Mosier P, Berardi F, Glennon R. A structure-affinity and comparative molecular field analysis of sigma-2 (sigma2) receptor ligands. Central Nerv Syst Agents Med Chem. 2009;9:246–57. doi: 10.2174/1871524910909030246. [DOI] [PubMed] [Google Scholar]
- Wang B, Rouzier R, Albarracin CT, et al. Expression of sigma 1 receptor in human breast cancer. Breast Cancer Res Treat. 2004;87:205–14. doi: 10.1007/s10549-004-6590-0. [DOI] [PubMed] [Google Scholar]
- Hou X, Hui YN, Han QH, et al. Effects of magnetic field on MAPK signaling pathways of human retinal pigment epithelial cells bound with beads in vitro. [Zhonghua yan ke za zhi] Chin J Opthamol. 2006;42:1103–8. [PubMed] [Google Scholar]
- Kashiwagi H, McDunn JE, Simon PO, Jr, et al. Selective sigma-2 ligands preferentially bind to pancreatic adenocarcinomas: applications in diagnostic imaging and therapy. Mol Cancer. 2007;6:48–60. doi: 10.1186/1476-4598-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colabufo N, Berardi F, Abate C, et al. Is the sigma2 receptor a histone binding protein? J Med Chem. 2006;49:4153–8. doi: 10.1021/jm0600592. [DOI] [PubMed] [Google Scholar]
- Schepmann D, Lehmkuhl K, Brune S, Wunsch B. Expression of sigma receptors of human urinary bladder tumor cells (RT-4 cells) and development of a competitive receptor binding assay for the determination of ligand affinity to human sigma(2) receptors. J Pharm Biomed Anal. 2011;55:1136–41. doi: 10.1016/j.jpba.2011.03.044. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Prasad TK, Rao NM, Banerjee R. Haloperidol-associated stealth liposomes: a potent carrier for delivering genes to human breast cancer cells. J Biol Chem. 2005;280:15619–27. doi: 10.1074/jbc.M409723200. [DOI] [PubMed] [Google Scholar]
- Fu Y, Zhao Y, Luan W, et al. Sigma-1 receptors amplify dopamine D1 receptor signaling at presynaptic sites in the prelimbic cortex. Biochim Biophys Acta. 2010;1803:1396–408. doi: 10.1016/j.bbamcr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Booth R, Owens C, Brown R, et al. Putative sigma(3) sites in mammalian brain have histamine H(1) receptor properties: evidence from ligand binding and distribution studies with the novel H(1) radioligand [(3)H]-(−)-trans-1-phenyl-3-aminotetralin. Brain Res. 1999;837:95–105. doi: 10.1016/s0006-8993(99)01602-9. [DOI] [PubMed] [Google Scholar]
- Lathe R, Seckl JR. Neurosteroids and brain steroids. Boca Raton: CRX Press; 2002. [Google Scholar]
- Tsai SY, Rothman RK, Su TP. Insights into the Sigma-1 receptor chaperone's cellular functions: a microarray report. Synapse (NY) 2012;66:42–51. doi: 10.1002/syn.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SY, Hayashi T, Mori T, Su TP. Sigma-1 receptor chaperones and diseases. Central Nerv Syst Agents Med Chem. 2009;9:184–9. doi: 10.2174/1871524910909030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott KS, Prasad M, Thapliyal V, Bose HS. sigma-1 receptor at the mitochondrial-associated endoplasmic reticulum membrane is responsible for mitochondrial metabolic regulation. J Pharm Exp Therap. 2012;343:578–86. doi: 10.1124/jpet.112.198168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda N, Ishikawa K, Tagashira H, et al. Expression of a truncated form of the endoplasmic reticulum chaperone protein, sigma1 receptor, promotes mitochondrial energy depletion and apoptosis. J Biol Chem. 2012;287:23318–31. doi: 10.1074/jbc.M112.349142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Hashimoto K. The role of sigma-1 receptors in the pathophysiology of neuropsychiatric diseases. J Receptor Ligand Channel Res. 2009;33:25–36. [Google Scholar]
- Gonzalez-Alvear G, Werling L. Sigma receptor regulation of norepinephrine release from rat hippocampal slices. Brain Res. 1995;673:61–9. doi: 10.1016/0006-8993(94)01394-w. [DOI] [PubMed] [Google Scholar]
- Bergeron R, de Montigny C, Debonnel G. Potentiation of neuronal NMDA response induced by dehydroepiandrosterone and its suppression by progesterone: effects mediated via sigma receptors. J Neurosci. 1996;16:1193–202. doi: 10.1523/JNEUROSCI.16-03-01193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debonnel G. Current hypotheses on sigma receptors and their physiological role: possible implications in psychiatry. J Psychiatry Neurosci. 1993;18:157–72. [PMC free article] [PubMed] [Google Scholar]
- Debonnel G, de Montigny C. Modulation of NMDA and dopaminergic neurotransmissions by sigma ligands: possible implications for the treatment of psychiatric disorders. Life Sci. 1996;58:721–34. doi: 10.1016/0024-3205(95)02248-1. [DOI] [PubMed] [Google Scholar]
- Su TP. Delineating biochemical and functional properties of sigma receptors: emerging concepts. Crit Rev Neurobiol. 1993;7:187–203. [PubMed] [Google Scholar]
- Su TP. Sigma receptors: putative links between nervous, endocrine and immune systems. Eur J Biochem. 1991;200:633–42. doi: 10.1111/j.1432-1033.1991.tb16226.x. [DOI] [PubMed] [Google Scholar]
- Su TP, Shukla K, Gund T. Steroid binding at sigma receptors: CNS and immunological implications. Ciba Found Symp. 1990;153:107–13. doi: 10.1002/9780470513989.ch6. discussion 113–106. [DOI] [PubMed] [Google Scholar]
- Skuza G, Rogoz Z. A potential antidepressant activity of SA4503, a selective sigma 1 receptor agonist. Behav Pharmacol. 2002;13:537–43. doi: 10.1097/00008877-200211000-00003. [DOI] [PubMed] [Google Scholar]
- Skuza G, Rogoz Z, Golembiowska K. EMD 57445, the selective sigma receptor ligand, has no effect on the 5-hydroxytryptamine system. Pol J Pharmacol. 1997;49:489–93. [PubMed] [Google Scholar]
- Maurice T, Privat A. SA4503, a novel cognitive enhancer with sigma1 receptor agonist properties, facilitates NMDA receptor-dependent learning in mice. Eur J Pharmacol. 1997;328:9–18. doi: 10.1016/s0014-2999(97)83020-8. [DOI] [PubMed] [Google Scholar]
- Tokuyama S, Hirata K, Ide A, Ueda H. Sigma ligands stimulate GTPase activity in mouse prefrontal membranes: evidence for the existence of metabotropic sigma receptor. Neurosci Lett. 1997;233:141–4. doi: 10.1016/s0304-3940(97)00657-5. [DOI] [PubMed] [Google Scholar]
- Soriani O, Vaudry H, Mei YA, et al. Sigma ligands stimulate the electrical activity of frog pituitary melanotrope cells through a G-protein-dependent inhibition of potassium conductances. J Pharm Exp Therap. 1998;286:163–71. [PubMed] [Google Scholar]
- Carnally S, Johannessen M, Henderson R, et al. Demonstration of a direct interaction between sigma-1 receptors and acid-sensing ion channels. Biophys J. 2010;98:1182–91. doi: 10.1016/j.bpj.2009.12.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnet FP, de Costa BR, Bowen WD. Differentiation of sigma ligand-activated receptor subtypes that modulate NMDA-evoked [3H]-noradrenaline release in rat hippocampal slices. Br J Pharmacol. 1996;119:65–72. doi: 10.1111/j.1476-5381.1996.tb15678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnet FP, Debonnel G, Bergeron R, et al. The effects of sigma ligands and of neuropeptide Y on N-methyl-d-aspartate-induced neuronal activation of CA3 dorsal hippocampus neurones are differentially affected by pertussin toxin. Br J Pharmacol. 1994;112:709–15. doi: 10.1111/j.1476-5381.1994.tb13134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnet FP, Debonnel G, de Montigny C. In vivo electrophysiological evidence for a selective modulation of N-methyl-d-aspartate-induced neuronal activation in rat CA3 dorsal hippocampus by sigma ligands. J Pharm Exp Therap. 1992;261:123–30. [PubMed] [Google Scholar]
- Lupardus PJ, Wilke RA, Aydar E, et al. Membrane-delimited coupling between sigma receptors and K+ channels in rat neurohypophysial terminals requires neither G-protein nor ATP. J Physiol. 2000;526:527–39. doi: 10.1111/j.1469-7793.2000.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke RA, Lupardus PJ, Grandy DK, et al. K+ channel modulation in rodent neurohypophysial nerve terminals by sigma receptors and not by dopamine receptors. J Physiol. 1999;517:391–406. doi: 10.1111/j.1469-7793.1999.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHaven-Hudkins D, Fleissner L, Ford-Rice F. Characterization of the binding of [3H](+)-pentazocine to sigma recognition sites in guinea pig brain. Eur J Pharmacol. 1992;227:371–8. doi: 10.1016/0922-4106(92)90153-m. [DOI] [PubMed] [Google Scholar]
- DeHaven-Hudkins D, Ford-Rice F, Allen J, Hudkins R. Allosteric modulation of ligand binding to [3H](+)pentazocine-defined sigma recognition sites by phenytoin. Life Sci. 1993;53:41–8. doi: 10.1016/0024-3205(93)90609-7. [DOI] [PubMed] [Google Scholar]
- Hong W, Werling LL. Evidence that the sigma(1) receptor is not directly coupled to G proteins. Eur J Pharmacol. 2000;408:117–25. doi: 10.1016/s0014-2999(00)00774-3. [DOI] [PubMed] [Google Scholar]
- McCann DJ, Weissman AD, Su TP. Sigma-1 and sigma-2 sites in rat brain: comparison of regional, ontogenetic, and subcellular patterns. Synapse (NY) 1994;17:182–9. doi: 10.1002/syn.890170307. [DOI] [PubMed] [Google Scholar]
- Palmer CP, Aydar E, Jackson MB. Sigma receptor modulation of ion channels. New York: Kluwer Academic Publishers; 2004. [Google Scholar]
- Soriani O, Foll FL, Roman F, et al. A current down-modulated by sigma receptor in frog pituitary melanotrope cells through a G protein-dependent pathway. J Pharm Exp Therap. 1999;289:321–8. [PubMed] [Google Scholar]
- Soriani O, Le Foll F, Galas L, et al. The sigma-ligand (+)-pentazocine depresses M current and enhances calcium conductances in frog melanotrophs. Am J Physiol. 1999;277:E73–80. doi: 10.1152/ajpendo.1999.277.1.E73. [DOI] [PubMed] [Google Scholar]
- Jbilo O, Vidal H, Paul R, et al. Purification and characterization of the human SR 31747A-binding protein. A nuclear membrane protein related to yeast sterol isomerase. J Biol Chem. 1997;272:27107–15. doi: 10.1074/jbc.272.43.27107. [DOI] [PubMed] [Google Scholar]
- Beart P, O'Shea R, Manallack D. Regulation of sigma-receptors: high- and low-affinity agonist states, GTP shifts, and up-regulation by rimcazole and 1,3-Di(2-tolyl)guanidine. J Neurochem. 1989;53:779–88. doi: 10.1111/j.1471-4159.1989.tb11773.x. [DOI] [PubMed] [Google Scholar]
- Connick J, Hanlon G, Roberts J, et al. Multiple sigma binding sites in guinea-pig and rat brain membranes: G-protein interactions. Br J Pharmacol. 1992;107:726–31. doi: 10.1111/j.1476-5381.1992.tb14514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen M, Ramachandran S, Riemer L, et al. Voltage-gated sodium channel modulation by sigma-receptors in cardiac myocytes and heterologous systems. Am J Physiol Cell Physiol. 2009;296:C1049–57. doi: 10.1152/ajpcell.00431.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp JA, Foster AC, Wong EH, Middlemiss DN. A comment on the classification and nomenclature of phencyclidine and sigma receptor sites. Trends Neurosci. 1998;11:388–9. doi: 10.1016/0166-2236(88)90073-2. [DOI] [PubMed] [Google Scholar]
- Morio Y, Tanimoto H, Yakushiji T, Morimoto Y. Characterization of the currents induced by sigma ligands in NCB20 neuroblastoma cells. Brain Res. 1994;637:190–6. doi: 10.1016/0006-8993(94)91232-7. [DOI] [PubMed] [Google Scholar]
- Brent P, Herd L, Saunders H, et al. Protein phosphorylation and calcium uptake into rat forebrain synaptosomes: modulation by the sigma ligand, 1,3-ditolylguanidine. J Neurochem. 1997;68:2201–11. doi: 10.1046/j.1471-4159.1997.68052201.x. [DOI] [PubMed] [Google Scholar]
- Brent P, Saunders H, Dunkley P. Intrasynaptosomal free calcium levels in rat forebrain synaptosomes: modulation by sigma (sigma) receptor ligands. Neurosci Lett. 1996;211:138–42. doi: 10.1016/0304-3940(96)12711-7. [DOI] [PubMed] [Google Scholar]
- Paul IA, Basile AS, Rojas E, et al. Sigma receptors modulate nicotinic receptor function in adrenal chromaffin cells. FASEB J. 1993;7:1171–8. doi: 10.1096/fasebj.7.12.8375616. [DOI] [PubMed] [Google Scholar]
- Okuyama S, Imagawa Y, Ogawa S, et al. NE-100, a novel sigma receptor ligand: in vivo tests. Life Sci. 1993;53:Pl285–90. doi: 10.1016/0024-3205(93)90588-t. [DOI] [PubMed] [Google Scholar]
- Church J, Fletcher E. Blockade by sigma site ligands of high voltage-activated Ca2+ channels in rat and mouse cultured hippocampal pyramidal neurones. Br J Pharmacol. 1995;116:2801–10. doi: 10.1111/j.1476-5381.1995.tb15929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Cuevas J. Sigma receptors inhibit high-voltage-activated calcium channels in rat sympathetic and parasympathetic neurons. J Neurophysiol. 2002;87:2867–79. doi: 10.1152/jn.2002.87.6.2867. [DOI] [PubMed] [Google Scholar]
- Bowen W. Sigma receptors and iboga alkaloids. Alkaloids Chem Biol. 2001;56:173–91. doi: 10.1016/s0099-9598(01)56013-7. [DOI] [PubMed] [Google Scholar]
- Paul IA, Layer RT, Skolnick P, Nowak G. Adaptation of the NMDA receptor in rat cortex following chronic electroconvulsive shock or imipramine. Eur J Pharmacol. 1993;247:305–11. doi: 10.1016/0922-4106(93)90199-j. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Maurice T, Su T. Ca(2+) signaling via sigma(1)-receptors: novel regulatory mechanism affecting intracellular Ca(2+) concentration. J Pharm Exp Therap. 2000;293:788–98. [PubMed] [Google Scholar]
- Monnet FP, Blier P, Debonnel G, de Montigny C. Modulation by sigma ligands of N-methyl-d-aspartate-induced [3H]noradrenaline release in the rat hippocampus: G-protein dependency. Naunyn–Schmiedeberg's Arch Pharmacol. 1992;346:32–9. doi: 10.1007/BF00167567. [DOI] [PubMed] [Google Scholar]
- Monnet FP, Morin-Surun MP, Leger J, Combettes L. Protein kinase C-dependent potentiation of intracellular calcium influx by sigma1 receptor agonists in rat hippocampal neurons. J Pharm Exp Therap. 2003;307:705–12. doi: 10.1124/jpet.103.053447. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su T. Regulating ankyrin dynamics: roles of sigma-1 receptors. Proc Natl Acad Sci USA. 2001;98:491–6. doi: 10.1073/pnas.98.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin-Surun MP, Collin T, Denavit-Saubie M, et al. Intracellular sigma1 receptor modulates phospholipase C and protein kinase C activities in the brainstem. Proc Natl Acad Sci USA. 1999;96:8196–9. doi: 10.1073/pnas.96.14.8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent P, Pang G, Little G, et al. The sigma receptor ligand, reduced haloperidol, induces apoptosis and increases intracellular-free calcium levels [Ca2+]i in colon and mammary adenocarcinoma cells. Biochem Biophys Res Commun. 1996;219:219–26. doi: 10.1006/bbrc.1996.0208. [DOI] [PubMed] [Google Scholar]
- Novakova M, Ela C, Bowen WD, et al. Highly selective sigma receptor ligands elevate inositol 1,4,5-trisphosphate production in rat cardiac myocytes. Eur J Pharmacol. 1998;353:315–27. doi: 10.1016/s0014-2999(98)00398-7. [DOI] [PubMed] [Google Scholar]
- Kostyuk P, Akaike N, Osipchuk Y, et al. Gating and permeation of different types of Ca channels. Ann NY Acad Sci. 1989;560:63–79. doi: 10.1111/j.1749-6632.1989.tb24081.x. [DOI] [PubMed] [Google Scholar]
- Morin D, Sapena R, Elimadi A, et al. [3H]-Trimetazidine mitochondrial binding sites: regulation by cations, effect of trimetazidine derivatives and other agents and interaction with an endogenous substance. Br J Pharmacol. 2000;130:655–63. doi: 10.1038/sj.bjp.0703348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile A, Paul I, Mirchevich A, et al. Modulation of (+)-[3H]pentazocine binding to guinea pig cerebellum by divalent cations. Mol Pharmacol. 1992;42:882–9. [PubMed] [Google Scholar]
- Alonso G, Phan V, Guillemain I, et al. Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience. 2000;97:155–70. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Murphy DJ, Vance J. Mechanisms of lipid-body formation. Trends Biochem Sci. 1999;24:109–15. doi: 10.1016/s0968-0004(98)01349-8. [DOI] [PubMed] [Google Scholar]
- Ohashi M, Mizushima N, Kabeya Y, Yoshimori T. Localization of mammalian NAD(P)H steroid dehydrogenase-like protein on lipid droplets. J Biol Chem. 2003;278:36819–29. doi: 10.1074/jbc.M301408200. [DOI] [PubMed] [Google Scholar]
- Monnet FP. Sigma-1 receptor as regulator of neuronal intracellular Ca2+: clinical and therapeutic relevance. Biol Cell. 2005;97:873–83. doi: 10.1042/BC20040149. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–72. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Takebayashi M, Hayashi T, Su TP. A perspective on the new mechanism of antidepressants: neuritogenesis through sigma-1 receptors. Pharmacopsychiatry. 2004;37:S208–13. doi: 10.1055/s-2004-832679. [DOI] [PubMed] [Google Scholar]
- Takebayashi M, Hayashi T, Su TP. Sigma-1 receptors potentiate epidermal growth factor signaling towards neuritogenesis in PC12 cells: potential relation to lipid raft reconstitution. Synapse (NY) 2004;53:90–103. doi: 10.1002/syn.20041. [DOI] [PubMed] [Google Scholar]
- Maurice T, Su TP, Privat A. Sigma1 (sigma 1) receptor agonists and neurosteroids attenuate B25-35-amyloid peptide-induced amnesia in mice through a common mechanism. Neuroscience. 1998;83:413–28. doi: 10.1016/s0306-4522(97)00405-3. [DOI] [PubMed] [Google Scholar]
- Bergeron R, de Montigny C, Debonnel G. Pregnancy reduces brain sigma receptor function. Br J Pharmacol. 1999;127:1769–76. doi: 10.1038/sj.bjp.0702724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debonnel G, Bergeron R, de Montigny C. Potentiation by dehydroepiandrosterone of the neuronal response to N-methyl-d-aspartate in the CA3 region of the rat dorsal hippocampus: an effect mediated via sigma receptors. J Endocrinol. 1996;150:S33–42. [PubMed] [Google Scholar]
- Urani A, Roman FJ, Phan VL, et al. The antidepressant-like effect induced by sigma(1)-receptor agonists and neuroactive steroids in mice submitted to the forced swimming test. J Pharm Exp Therap. 2001;298:1269–79. [PubMed] [Google Scholar]
- Maurice T, Hiramatsu M, Itoh J, et al. Behavioral evidence for a modulating role of sigma ligands in memory processes. I: attenuation of dizocilpine (MK-801)-induced amnesia. Brain Res. 2994;647:44–56. doi: 10.1016/0006-8993(94)91397-8. [DOI] [PubMed] [Google Scholar]
- Maurice T, Roman FJ, Privat A. Modulation by neurosteroids of the in vivo (+)-[3H]SKF-10,047 binding to sigma 1 receptors in the mouse forebrain. J Neurosci Res. 1996;46:734–43. doi: 10.1002/(SICI)1097-4547(19961215)46:6<734::AID-JNR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Maurice T, Su TP, Parish DW, et al. PRE-084, a sigma selective PCP derivative, attenuates MK-801-induced impairment of learning in mice. Pharmacol Biochem Behav. 1994;49:859–69. doi: 10.1016/0091-3057(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Takebayashi M, Hayashi T, Su TP. Nerve growth factor-induced neurite sprouting in PC12 cells involves sigma-1 receptors: implications for antidepressants. J Pharm Exp Therap. 2002;303:1227–37. doi: 10.1124/jpet.102.041970. [DOI] [PubMed] [Google Scholar]
- Hall A, Herrera Y, Ajmo C, Jr, et al. Sigma receptors suppress multiple aspects of microglial activation. Glia. 2009;57:744–54. doi: 10.1002/glia.20802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajmo C, Jr, Vernon D, Collier L, et al. Sigma receptor activation reduces infarct size at 24 hours after permanent middle cerebral artery occlusion in rats. Curr Neurovasc Res. 2006;3:89–98. doi: 10.2174/156720206776875849. [DOI] [PubMed] [Google Scholar]
- Luscher C, Nicoll RA, Malenka RC, Muller D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nature Neurosci. 2000;3:545–50. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- Moschovos C, Kostopoulos G, Papatheodoropoulos C. Long-term potentiation of high-frequency oscillation and synaptic transmission characterize in vitro NMDA receptor-dependent epileptogenesis in the hippocampus. Neurobiol Dis. 2008;29:368–80. doi: 10.1016/j.nbd.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Monnet FP, Maurice T. The sigma1 protein as a target for the non-genomic effects of neuro(active)steroids: molecular, physiological, and behavioral aspects. J Pharmacol Sci. 2006;100:93–118. doi: 10.1254/jphs.cr0050032. [DOI] [PubMed] [Google Scholar]
- Carpenter C, Marks S, Watson D, Greenberg D. Dextromethorphan and dextrorphan as calcium channel antagonists. Brain Res. 1988;439:372–5. doi: 10.1016/0006-8993(88)91497-7. [DOI] [PubMed] [Google Scholar]
- Klein M, Canoll PD, Musacchio JM. SKF 525-A and cytochrome P-450 ligands inhibit with high affinity the binding of [3H]dextromethorphan and sigma ligands to guinea pig brain. Life Sci. 1991;48:543–50. doi: 10.1016/0024-3205(91)90469-r. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Reid A, Mahboubi A, et al. Labeling by [3H]1,3-di(2-tolyl)guanidine of two high affinity binding sites in guinea pig brain: evidence for allosteric regulation by calcium channel antagonists and pseudoallosteric modulation by sigma ligands. Mol Pharmacol. 1991;39:222–32. [PubMed] [Google Scholar]
- Bergeron R, de Montigny C, Debonnel G. Biphasic effects of sigma ligands on the neuronal response to N-methyl-d-aspartate. Naunyn–Schmiedeberg's Arch Pharmacol. 1995;351:252–60. doi: 10.1007/BF00233244. [DOI] [PubMed] [Google Scholar]
- Monnet FP, Debonnel G, Junien JL, De Montigny C. N-methyl-d-aspartate-induced neuronal activation is selectively modulated by sigma receptors. Eur J Pharmacol. 1990;179:441–5. doi: 10.1016/0014-2999(90)90186-a. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Debonnel G. Effects of low and high doses of selective sigma ligands: further evidence suggesting the existence of different subtypes of sigma receptors. Psychopharmacology. 1997;129:215–24. doi: 10.1007/s002130050183. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Debonnel G, De Montigny C. Modification of the N-methyl-d-aspartate response by antidepressant sigma receptor ligands. Eur J Pharmacol. 1993;240:319–23. doi: 10.1016/0014-2999(93)90918-8. [DOI] [PubMed] [Google Scholar]
- Hemstreet M, Matsumoto R, Bowen W, Walker J. Sigma binding parameters in developing rats predict behavioral efficacy of a sigma ligand. Brain Res. 1993;627:291–8. doi: 10.1016/0006-8993(93)90333-i. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Kimura Y, Tsukada H, et al. An increase of sigma receptors in the aged monkey brain. Neurobiol Aging. 2003;24:745–52. doi: 10.1016/s0197-4580(02)00152-5. [DOI] [PubMed] [Google Scholar]
- Matsuno K, Senda T, Matsunaga K, Mita S. Ameliorating effects of sigma receptor ligands on the impairment of passive avoidance tasks in mice: involvement in the central acetylcholinergic system. Eur J Pharmacol. 1994;261:43–51. doi: 10.1016/0014-2999(94)90298-4. [DOI] [PubMed] [Google Scholar]
- Maurice T, Lockhart BP. Neuroprotective and anti-amnesic potentials of sigma (sigma) receptor ligands. Progr Neuro-psychopharmacology Biol Psychiatry. 1997;21:69–102. doi: 10.1016/s0278-5846(96)00160-1. [DOI] [PubMed] [Google Scholar]
- Maurice T, Phan VL, Noda Y, et al. The attenuation of learning impairments induced after exposure to CO or trimethyltin in mice by sigma (sigma) receptor ligands involves both sigma1 and sigma2 sites. Br J Pharmacol. 1999;127:335–42. doi: 10.1038/sj.bjp.0702553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo W, Dellu F, Robel P, et al. Infusion of neurosteroids into the nucleus basalis magnocellularis affects cognitive processes in the rat. Brain Res. 1993;607:324–8. doi: 10.1016/0006-8993(93)91524-v. [DOI] [PubMed] [Google Scholar]
- Niitsu T, Iyo M, Hashimoto K. Sigma-1 receptor agonists as therapeutic drugs for cognitive impairment in neuropsychiatric diseases. Current Pharm Design. 2012;18:875–83. doi: 10.2174/138161212799436476. [DOI] [PubMed] [Google Scholar]
- Senda T, Matsuno K, Okamoto K, et al. Ameliorating effect of SA4503, a novel sigma 1 receptor agonist, on memory impairments induced by cholinergic dysfunction in rats. Eur J Pharmacol. 1996;315:1–10. doi: 10.1016/s0014-2999(96)00572-9. [DOI] [PubMed] [Google Scholar]
- Pande AC, Geneve J, Scherrer B. Igmesine, a novel sigma ligand, has antidepressant properties. Int J Neuropsychopharmacol. 1998;1:S8–S9. [Google Scholar]
- Odagaki Y, Toyoshima R, Yamauchi T. Lack of G protein-coupled sigma receptors in rat brain membranes: receptor-mediated high-affinity GTPase activity and [35S]GTPgammaS binding studies. J Neural Transmission (Vienna, Austria: 1996) 2005;112:873–83. doi: 10.1007/s00702-004-0237-5. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Bowen WD, Tom MA, et al. Characterization of two novel sigma receptor ligands: antidystonic effects in rats suggest sigma receptor antagonism. Eur J Pharmacol. 1995;280:301–10. doi: 10.1016/0014-2999(95)00208-3. [DOI] [PubMed] [Google Scholar]
- Racchi M, Balduzzi C, Corsini E. Dehydroepiandrosterone (DHEA) and the aging brain: flipping a coin in the "fountain of youth". CNS Drug Rev. 2003;9:21–40. doi: 10.1111/j.1527-3458.2003.tb00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnet FP, Mahe V, Robel P, Baulieu EE. Neurosteroids, via sigma receptors, modulate the [3H]norepinephrine release evoked by N-methyl-d-aspartate in the rat hippocampus. Proc Natl Acad Sci USA. 1995;92:3774–8. doi: 10.1073/pnas.92.9.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno K, Senda T, Kobayashi T, Mita S. Involvement of sigma 1 receptor in (+)-N-allylnormetazocine-stimulated hippocampal cholinergic functions in rats. Brain Res. 1995;690:200–6. doi: 10.1016/0006-8993(95)00618-z. [DOI] [PubMed] [Google Scholar]
- Maurice T, Junien JL, Privat A. Dehydroepiandrosterone sulfate attenuates dizocilpine-induced learning impairment in mice via sigma 1-receptors. Behav Brain Res. 1997;83:159–64. doi: 10.1016/s0166-4328(97)86061-5. [DOI] [PubMed] [Google Scholar]
- Castellano C, McGaugh J. Effects of post-training bicuculline and muscimol on retention: lack of state dependency. Behav Neural Biol. 1990;54:156–64. doi: 10.1016/0163-1047(90)91352-c. [DOI] [PubMed] [Google Scholar]
- Introini-Collison IB, Castellano C, McGaugh JL. Interaction of GABAergic and beta-noradrenergic drugs in the regulation of memory storage. Behav Neural Biol. 1994;61:150–5. doi: 10.1016/s0163-1047(05)80068-8. [DOI] [PubMed] [Google Scholar]
- Izquierdo I. Pharmacological evidence for a role of long-term potentiation in memory. FASEB J. 1994;8:1139–45. [PubMed] [Google Scholar]
- Rison RA, Stanton PK. Long-term potentiation and N-methyl-d-aspartate receptors: foundations of memory and neurologic disease? Neurosci Biobehav Rev. 1995;19:533–52. doi: 10.1016/0149-7634(95)00017-8. [DOI] [PubMed] [Google Scholar]
- Sabbatini R. Neurons and synapses. Brain Mind. 2003;17:1–6. [Google Scholar]
- Falkenstein E, Schmieding K, Lange A, et al. Localization of a putative progesterone membrane binding protein in porcine hepatocytes. Cell Mol Biol (Noisy-le-Grand, France) 1998;44:571–8. [PubMed] [Google Scholar]
- McCann DJ, Su TP. Haloperidol-sensitive (+)[3H]SKF-10,047 binding sites (sigma sites) exhibit a unique distribution in rat brain subcellular fractions. Eur J Pharmacol. 1990;188:211–18. doi: 10.1016/0922-4106(90)90004-h. [DOI] [PubMed] [Google Scholar]
- Nabeshima T, Okuyama S. [Physiological function of sigma receptors: central pharmacological effects of sigma ligands] Nihon Shinkei Seishin Yakurigaku Zasshi. 1994;14:51–76. [PubMed] [Google Scholar]
- Su TP, London ED, Jaffe JH. Response: steroid binding at sgr-"opioid" receptors. Science (NY) 1989;246:1637–8. doi: 10.1126/science.246.4937.1637. [DOI] [PubMed] [Google Scholar]
- Su TP, London ED, Jaffe JH. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science (NY) 1988;240:219–21. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- Baulieu E, Robel P. Neurosteroids: a new brain function? J Steroid Biochem Mol Biol. 1990;37:395–403. doi: 10.1016/0960-0760(90)90490-c. [DOI] [PubMed] [Google Scholar]
- Corpechot C, Robel P, Axelson M, et al. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc Natl Acad Sci USA. 1981;78:4704–7. doi: 10.1073/pnas.78.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpechot C, Synguelakis M, Talha S, et al. Pregnenolone and its sulfate ester in the rat brain. Brain Res. 1983;270:119–25. doi: 10.1016/0006-8993(83)90797-7. [DOI] [PubMed] [Google Scholar]
- Wu FS, Gibbs TT, Farb DH. Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-d-aspartate receptor. Mol Pharmacol. 1991;40:333–6. [PubMed] [Google Scholar]
- Longone P, di Michele F, D'Agati E, et al. Neurosteroids as neuromodulators in the treatment of anxiety disorders. Front Endocrinol. 2011;2:55–64. doi: 10.3389/fendo.2011.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy JD, Ramamoorthy S, Mahesh VB, et al. Cocaine-sensitive sigma-receptor and its interaction with steroid hormones in the human placental syncytiotrophoblast and in choriocarcinoma cells. Endocrinology. 1995;136:924–32. doi: 10.1210/endo.136.3.7867601. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nabeshima T. Stress-induced behavioral responses and multiple opioid systems in the brain. Behav Brain Res. 1995;67:133–45. doi: 10.1016/0166-4328(94)00150-e. [DOI] [PubMed] [Google Scholar]
- Yamada M, Nishigami T, Nakasho K, et al. Relationship between sigma-like site and progesterone-binding site of adult male rat liver microsomes. Hepatology (Baltimore, MD) 1994;20:1271–80. [PubMed] [Google Scholar]
- Elfverson M, Johansson T, Zhou Q, et al. Chronic administration of the anabolic androgenic steroid nandrolone alters neurosteroid action at the sigma-1 receptor but not at the sigma-2 or NMDA receptors. Neuropharmacology. 2011;61:1172–81. doi: 10.1016/j.neuropharm.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Schwarz S, Pohl P, Zhou GZ. Steroid binding at sigma-“opioid” receptors. Science (NY) 1989;246:1635–8. doi: 10.1126/science.2556797. [DOI] [PubMed] [Google Scholar]
- Cheney D, Uzunov D, Guidotti A. Pregnenolone sulfate antagonizes dizocilpine amnesia: role for allopregnanolone. Neuroreport. 1995;6:1697–700. doi: 10.1097/00001756-199508000-00025. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Hauser CA, Trapp T, Holsboer F. Neurosteroids: molecular mechanisms of action and psychopharmacological significance. J Steroid Biochem Mol Biol. 1996;56:163–8. doi: 10.1016/0960-0760(95)00233-2. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Reul JM, Trapp T, et al. Progesterone receptor-mediated effects of neuroactive steroids. Neuron. 1993;11:523–30. doi: 10.1016/0896-6273(93)90156-l. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Akwa Y, Guennoun R, et al. Steroid synthesis and metabolism in the nervous system: trophic and protective effects. J Neurocytol. 2000;29:307–26. doi: 10.1023/a:1007152904926. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Weill-Engerer S, Liere P, et al. Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Progr Neurobiol. 2003;71:3–29. doi: 10.1016/j.pneurobio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Coirini H, Westlind-Danielsson A, et al. Steroid hormones as mediators of neural plasticity. J Steroid Biochem Mol Biol. 1991;39:223–32. doi: 10.1016/0960-0760(91)90067-f. [DOI] [PubMed] [Google Scholar]
- Collier T, Waterhouse R, Kassiou M. Imaging sigma receptors: applications in drug development. Current Pharm Design. 2007;13:51–72. doi: 10.2174/138161207779313740. [DOI] [PubMed] [Google Scholar]
- Bitran D, Hilvers R, Kellogg C. Anxiolytic effects of 3 alpha-hydroxy-5 alpha[beta]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991;561:157–61. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Brot M, Akwa Y, Purdy R, et al. The anxiolytic-like effects of the neurosteroid allopregnanolone: interactions with GABA(A) receptors. Eur J Pharmacol. 1997;325:1–7. doi: 10.1016/s0014-2999(97)00096-4. [DOI] [PubMed] [Google Scholar]
- Maurice T, Phan VL, Urani A, et al. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn J Pharmacol. 1999;81:125–55. doi: 10.1254/jjp.81.125. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kaur G, Kulkarni SK. Sigma (sigma1) receptor mediated anti-depressant-like effects of neurosteroids in the Porsolt forced swim test. Neuroreport. 1998;9:3069–73. doi: 10.1097/00001756-199809140-00028. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. The effects of neurosteroids on acquisition and retention of a modified passive-avoidance learning task in mice. Brain Res. 1998;791:108–16. doi: 10.1016/s0006-8993(98)00085-7. [DOI] [PubMed] [Google Scholar]
- Urani A, Privat A, Maurice T. The modulation by neurosteroids of the scopolamine-induced learning impairment in mice involves an interaction with sigma1 (sigma1) receptors. Brain Res. 1998;799:64–77. doi: 10.1016/s0006-8993(98)00469-7. [DOI] [PubMed] [Google Scholar]
- Wieland S, Lan NC, Mirasedeghi S, Gee KW. Anxiolytic activity of the progesterone metabolite 5 alpha-pregnan-3 alpha-o1-20-one. Brain Res. 1991;565:263–8. doi: 10.1016/0006-8993(91)91658-n. [DOI] [PubMed] [Google Scholar]
- Brown D. Neurons depend on astrocytes in a coculture system for protection from glutamate toxicity. Mol Cell Neurosci. 1999;13:379–89. doi: 10.1006/mcne.1999.0751. [DOI] [PubMed] [Google Scholar]
- Weill-Engerer S, David JP, Sazdovitch V, et al. Neurosteroid quantification in human brain regions: comparison between Alzheimer's and nondemented patients. J Clin Endocrinol Metab. 2002;87:5138–43. doi: 10.1210/jc.2002-020878. [DOI] [PubMed] [Google Scholar]
- Urani A, Romieu P, Portales-Casamar E, et al. The antidepressant-like effect induced by the sigma(1) (sigma(1)) receptor agonist igmesine involves modulation of intracellular calcium mobilization. Psychopharmacology. 2002;163:26–35. doi: 10.1007/s00213-002-1150-y. [DOI] [PubMed] [Google Scholar]
- Urani A, Romieu P, Roman FJ, Maurice T. Enhanced antidepressant effect of sigma(1) (sigma(1)) receptor agonists in beta(25–35)-amyloid peptide-treated mice. Behav Brain Res. 2002;134:239–47. doi: 10.1016/s0166-4328(02)00033-5. [DOI] [PubMed] [Google Scholar]
- Urani A, Romieu P, Roman FJ, et al. Enhanced antidepressant efficacy of sigma1 receptor agonists in rats after chronic intracerebroventricular infusion of beta-amyloid-(1–40) protein. Eur J Pharmacol. 2004;486:151–61. doi: 10.1016/j.ejphar.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B. Neurosteroid biosynthesis in the human brain and its clinical implications. Ann NY Acad Sci. 2003;1007:64–78. doi: 10.1196/annals.1286.007. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI, Roberts E, et al. Dehydroepiandrosterone (DHEA) treatment of depression. Biol Psychiatry. 1997;41:311–18. doi: 10.1016/s0006-3223(96)00043-1. [DOI] [PubMed] [Google Scholar]
- Flood J, Morley J, Roberts E. Memory-enhancing effects in male mice of pregnenolone and steroids metabolically derived from it. Proc Natl Acad Sci USA. 1992;89:1567–71. doi: 10.1073/pnas.89.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood J, Morley J, Roberts E. Pregnenolone sulfate enhances post-training memory processes when injected in very low doses into limbic system structures: the amygdala is by far the most sensitive. Proc Natl Acad Sci USA. 1995;92:10806–10. doi: 10.1073/pnas.92.23.10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet K, Brown R. Cognitive effects of DHEA replacement therapy. New York: Walter de Gruyter; 1990. [Google Scholar]
- Flood J, Roberts E. Dehydroepiandrosterone sulfate improves memory in aging mice. Brain Res. 1988;448:178–81. doi: 10.1016/0006-8993(88)91116-x. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J clin Endocrinol Metab. 1984;59:551–5. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Brind JL, Vogelman JH, et al. Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J clin Endocrinol Metab. 1992;75:1002–4. doi: 10.1210/jcem.75.4.1400863. [DOI] [PubMed] [Google Scholar]
- Vallee M, Mayo W, Darnaudery M, et al. Neurosteroids: deficient cognitive performance in aged rats depends on low pregnenolone sulfate levels in the hippocampus. Proc Natl Acad Sci USA. 1997;94:14865–70. doi: 10.1073/pnas.94.26.14865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata K, Takebayashi M, Morinobu S, Yamawaki S. beta-estradiol, dehydroepiandrosterone, and dehydroepiandrosterone sulfate protect against N-methyl-d-aspartate-induced neurotoxicity in rat hippocampal neurons by different mechanisms. J Pharm Exp Therap. 2004;311:237–45. doi: 10.1124/jpet.104.067629. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Tsai SY, Mori T, et al. Targeting ligand-operated chaperone sigma-1 receptors in the treatment of neuropsychiatric disorders. Expert Opin Therap Targets. 2011;15:557–77. doi: 10.1517/14728222.2011.560837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda Y, Kamei H, Kamei Y, et al. Neurosteroids ameliorate conditioned fear stress: an association with sigma receptors. Neuropsychopharmacology. 2000;23:276–84. doi: 10.1016/S0893-133X(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Akunne H, Whetzel S, Wiley J, et al. The pharmacology of the novel and selective sigma ligand, PD 144418. Neuropharmacology. 1997;36:51–62. doi: 10.1016/s0028-3908(96)00161-x. [DOI] [PubMed] [Google Scholar]
- Farb D, Gibbs T, Wu F, Gyenes M, et al. Steroid modulation of amino acid neurotransmitter receptors. Adv Biochem Psychopharmacol. 1992;47:119–31. [PubMed] [Google Scholar]
- Gasior M, Carter R, Witkin J. Neuroactive steroids: potential therapeutic use in neurological and psychiatric disorders. Trends Pharmacol Sci. 1999;20:107–12. doi: 10.1016/s0165-6147(99)01318-8. [DOI] [PubMed] [Google Scholar]
- Takebayashi M, Kagaya A, Uchitomi Y, et al. Differential regulation by pregnenolone sulfate of intracellular Ca2+ increase by amino acids in primary cultured rat cortical neurons. Neurochem Int. 1998;32:205–11. doi: 10.1016/s0197-0186(97)00070-3. [DOI] [PubMed] [Google Scholar]
- Martins RA, Linden R, Dyer MA. Glutamate regulates retinal progenitors cells proliferation during development. Eur J Neurosci. 2006;24:969–80. doi: 10.1111/j.1460-9568.2006.04966.x. [DOI] [PubMed] [Google Scholar]
- Martins RA, Pearson RA. Control of cell proliferation by neurotransmitters in the developing vertebrate retina. Brain Res. 2008;1192:37–60. doi: 10.1016/j.brainres.2007.04.076. [DOI] [PubMed] [Google Scholar]
- Smith SS. Progesterone administration attenuates excitatory amino acid responses of cerebellar Purkinje cells. Neuroscience. 1991;42:309–20. doi: 10.1016/0306-4522(91)90377-z. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert J. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–75. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Harrison N, Simmonds M. Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res. 1984;323:287–92. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Hill-Venning C, et al. Neurosteroid modulation of native and recombinant GABAA receptors. Cell Mol Neurobiol. 1996;16:155–74. doi: 10.1007/BF02088174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis C, Paul SM, Crawley JN. The neurosteroid pregnenolone sulfate blocks NMDA antagonist-induced deficits in a passive avoidance memory task. Psychopharmacology. 1994;116:201–6. doi: 10.1007/BF02245063. [DOI] [PubMed] [Google Scholar]
- Mathis C, Vogel E, Cagniard B, et al. The neurosteroid pregnenolone sulfate blocks deficits induced by a competitive NMDA antagonist in active avoidance and lever-press learning tasks in mice. Neuropharmacology. 1996;35:1057–64. doi: 10.1016/s0028-3908(96)00041-x. [DOI] [PubMed] [Google Scholar]
- Callachan H, Cottrell G, Hather N, et al. Modulation of the GABAA receptor by progesterone metabolites. Proc R Soc Lond ser B. 1987;231:359–69. doi: 10.1098/rspb.1987.0049. [DOI] [PubMed] [Google Scholar]
- Shu HJ, Eisenman LN, Jinadasa D, et al. Slow actions of neuroactive steroids at GABAA receptors. J Neurosci. 2004;24:6667–75. doi: 10.1523/JNEUROSCI.1399-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W, Nuwayhid SJ, Werling LL. Modulation of bradykinin-induced calcium changes in SH-SY5Y cells by neurosteroids and sigma receptor ligands via a shared mechanism. Synapse. 2004;54:102–10. doi: 10.1002/syn.20069. [DOI] [PubMed] [Google Scholar]
- Bukusoglu C, Sarlak F. Pregnenolone sulfate increases intracellular Ca2+ levels in a pituitary cell line. Eur J Pharmacol. 1996;298:79–85. doi: 10.1016/0014-2999(95)00772-5. [DOI] [PubMed] [Google Scholar]
- Fahey J, Lindquist D, Pritchard G, Miller L. Pregnenolone sulfate potentiation of NMDA-mediated increases in intracellular calcium in cultured chick cortical neurons. Brain Res. 1995;669:183–8. doi: 10.1016/0006-8993(94)01223-5. [DOI] [PubMed] [Google Scholar]
- French-Mullen J, Danks P, Spence K. Neurosteroids modulate calcium currents in hippocampal CA1 neurons via a pertussis toxin-sensitive G-protein-coupled mechanism. J Neurosci. 1994;14:1963–77. doi: 10.1523/JNEUROSCI.14-04-01963.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French-Mullen J, Spence K. Neurosteroids block Ca2+ channel current in freshly isolated hippocampal CA1 neurons. Eur J Pharmacol. 1991;202:269–72. doi: 10.1016/0014-2999(91)90303-8. [DOI] [PubMed] [Google Scholar]
- Maurice T. Neurosteroids and sigma1 receptors, biochemical and behavioral relevance. Pharmacopsychiatry. 2004;37:S171–82. doi: 10.1055/s-2004-832675. [DOI] [PubMed] [Google Scholar]
- Compagnone N, Mellon S. Dehydroepiandrosterone: a potential signalling molecule for neocortical organization during development. Proc Natl Acad Sci USA. 1998;95:4678–83. doi: 10.1073/pnas.95.8.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky B. Neurosteroids, neuroactive steroids, and symptoms of affective disorders. Pharmacol Biochem Behav. 2006;84:644–55. doi: 10.1016/j.pbb.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Irwin RP, Lin SZ, Rogawski MA, et al. Steroid potentiation and inhibition of N-methyl-d-aspartate receptor-mediated intracellular Ca++ responses: structure-activity studies. J Pharm Exp Therap. 1994;271:677–82. [PubMed] [Google Scholar]
- Irwin RP, Maragakis NJ, Rogawski MA, et al. Pregnenolone sulfate augments NMDA receptor mediated increases in intracellular Ca2+ in cultured rat hippocampal neurons. Neurosci Lett. 1992;141:30–4. doi: 10.1016/0304-3940(92)90327-4. [DOI] [PubMed] [Google Scholar]
- Wakerley JB, Richardson CM. The neurosteroid pregnenolone sulphate enhances NMDA-induced phasic firing of vasopressin neurones in the rat supraoptic nucleus. Neurosci Lett. 1997;226:123–6. doi: 10.1016/s0304-3940(97)00260-7. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Wu FS, Farb DH. 3 alpha-Hydroxy-5 beta-pregnan-20-one sulfate: a negative modulator of the NMDA-induced current in cultured neurons. Mol Pharmacol. 1994;46:146–50. [PubMed] [Google Scholar]
- Park-Chung M, Wu FS, Purdy RH, et al. Distinct sites for inverse modulation of N-methyl-d-aspartate receptors by sulfated steroids. Mol Pharmacol. 1997;52:1113–23. doi: 10.1124/mol.52.6.1113. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Kagaya A, Takebayashi M, et al. Modulation by sigma ligands of intracellular free Ca++ mobilization by N-methyl-d-aspartate in primary culture of rat frontal cortical neurons. J Pharm Exp Therap. 1995;275:207–14. [PubMed] [Google Scholar]
- Phan VL, Miyamoto Y, Nabeshima T, Maurice T. Age-related expression of sigma1 receptors and antidepressant efficacy of a selective agonist in the senescence-accelerated (SAM) mouse. J Neurosci Res. 2005;79:561–72. doi: 10.1002/jnr.20390. [DOI] [PubMed] [Google Scholar]
- Phan VL, Su TP, Privat A, Maurice T. Modulation of steroidal levels by adrenalectomy/castration and inhibition of neurosteroid synthesis enzymes affect sigma1 receptor-mediated behaviour in mice. Eur J Neurosci. 1999;11:2385–96. doi: 10.1046/j.1460-9568.1999.00656.x. [DOI] [PubMed] [Google Scholar]
- Phan VL, Urani A, Romieu P, Maurice T. Strain differences in sigma(1) receptor-mediated behaviours are related to neurosteroid levels. Eur J Neurosci. 2002;15:1523–34. doi: 10.1046/j.1460-9568.2002.01989.x. [DOI] [PubMed] [Google Scholar]
- Phan VL, Urani A, Sandillon F, et al. Preserved sigma1 (sigma1) receptor expression and behavioral efficacy in the aged C57BL/6 mouse. Neurobiol Aging. 2003;24:865–81. doi: 10.1016/s0197-4580(02)00231-2. [DOI] [PubMed] [Google Scholar]
- Danysz W, Wroblewski J, Brooker G, Costa E. Modulation of glutamate receptors by phencyclidine and glycine in the rat cerebellum: cGMP increase in vivo. Brain Res. 1989;479:270–6. doi: 10.1016/0006-8993(89)91628-4. [DOI] [PubMed] [Google Scholar]
- Swegle JM, Logemann C. Management of common opioid-induced adverse effects. Am Fam Phys. 2006;74:1347–54. [PubMed] [Google Scholar]
- Tam SW. Naloxone-inaccessible sigma receptor in rat central nervous system. Proc Natl Acad Sci USA. 1983;80:6703–7. doi: 10.1073/pnas.80.21.6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May EL, Aceto MD, Bowman ER, et al. Antipodal alpha-N-(methyl through decyl)-N-normetazocines (5,9 alpha-dimethyl-2′-hydroxy-6,7-benzomorphans): in vitro and in vivo properties. J Med Chem. 1994;37:3408–18. doi: 10.1021/jm00046a026. [DOI] [PubMed] [Google Scholar]
- Young GA, Hudson GM, Stamidis H, Steinfels GF. Interactions between U-50,488H and sigma receptor antagonists: EEG, EEG power spectral and behavioral correlates. Eur J Pharmacol. 1993;231:473–6. doi: 10.1016/0014-2999(93)90127-4. [DOI] [PubMed] [Google Scholar]
- Grisel J, Allen S, Nemmani K, et al. The influence of dextromethorphan on morphine analgesia in Swiss Webster mice is sex-specific. Pharmacol Biochem Behav. 2005;81:131–8. doi: 10.1016/j.pbb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Mei J, Pasternak GW. Modulation of brainstem opiate analgesia in the rat by sigma 1 receptors: a microinjection study. J Pharm Exp Therap. 2007;322:1278–85. doi: 10.1124/jpet.107.121137. [DOI] [PubMed] [Google Scholar]
- Musacchio JM. The psychotomimetic effects of opiates and the sigma receptor. Neuropsychopharmacology. 1990;3:191–200. [PubMed] [Google Scholar]
- Hiramatsu M, Hoshino T. Improvement of memory impairment by (+)- and (−)-pentazocine via sigma, but not kappa opioid receptors. Brain Res. 2005;1057:72–80. doi: 10.1016/j.brainres.2005.07.028. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M, Hoshino T, Kanematsu K. Pharmacological characterization of the ameliorating effect on short-term memory impairment and antinociceptive effect of KT-90 in mice. Behav Brain Res. 2005;160:374–81. doi: 10.1016/j.bbr.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M, Mizuno N, Kanematsu K. Pharmacological characterization of the ameliorating effect on learning and memory impairment and antinociceptive effect of KT-95 in mice. Behav Brain Res. 2006;167:219–25. doi: 10.1016/j.bbr.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kohler C. The distribution of serotonin binding sites in the hippocampal region of the rat brain: an autoradiographic study. Neuroscience. 1984;13:667–80. doi: 10.1016/0306-4522(84)90087-3. [DOI] [PubMed] [Google Scholar]
- Heninger G, Delgado P, Charney D, et al. Tryptophan-deficient diet and amino acid drink deplete plasma tryptophan and induce a relapse of depression in susceptible patients. J Chem Neuroanat. 1992;5:347–8. doi: 10.1016/0891-0618(92)90025-l. [DOI] [PubMed] [Google Scholar]
- Andrade R, Nicoll R. Pharmacologically distinct actions of serotonin on single pyramidal neurones of the rat hippocampus recorded in vitro. J Physiol. 1987;394:99–124. doi: 10.1113/jphysiol.1987.sp016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, De Montigny C. Electrophysiological investigations on the effect of repeated zimelidine administration on serotonergic neurotransmission in the rat. J Neurosci. 1983;3:1270–8. doi: 10.1523/JNEUROSCI.03-06-01270.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Serotoninergic but not noradrenergic neurons in rat central nervous system adapt to long-term treatment with monoamine oxidase inhibitors. Neuroscience. 1985;16:949–55. doi: 10.1016/0306-4522(85)90107-1. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C, Tardif D. Effects of the two antidepressant drugs mianserin and indalpine on the serotonergic system: single-cell studies in the rat. Psychopharmacology. 1984;84:242–9. doi: 10.1007/BF00427453. [DOI] [PubMed] [Google Scholar]
- Chaput Y, de Montigny C, Blier P. Effects of a selective 5-HT reuptake blocker, citalopram, on the sensitivity of 5-HT autoreceptors: electrophysiological studies in the rat brain. Naunyn–Schmiedeberg's Arch Pharmacol. 1986;333:342–8. doi: 10.1007/BF00500007. [DOI] [PubMed] [Google Scholar]
- Campbell B, Scherz M, Keana J, Weber E. Sigma receptors regulate contractions of the guinea pig ileum longitudinal muscle/myenteric plexus preparation elicited by both electrical stimulation and exogenous serotonin. J Neurosci. 1989;9:3380–91. doi: 10.1523/JNEUROSCI.09-10-03380.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermack J, Haddjeri N, Debonnel G. Effects of the potential antidepressant OPC-14523 [1-[3-[4-(3-chlorophenyl)-1-piperazinyl]propyl]-5-methoxy-3,4-dihydro-2-quinolino ne monomethanesulfonate] a combined sigma and 5-HT1A ligand: modulation of neuronal activity in the dorsal raphe nucleus. J Pharm Exp Therap. 2004;310:578–83. doi: 10.1124/jpet.104.066472. [DOI] [PubMed] [Google Scholar]
- Blier P, Chaput Y, de Montigny C. Long-term 5-HT reuptake blockade, but not monoamine oxidase inhibition, decreases the function of terminal 5-HT autoreceptors: an electrophysiological study in the rat brain. Naunyn–Schmiedeberg's Arch Pharmacol. 1998;337:246–54. doi: 10.1007/BF00168834. [DOI] [PubMed] [Google Scholar]
- Chaput Y, de Montigny C, Blier P. Presynaptic and postsynaptic modifications of the serotonin system by long-term administration of antidepressant treatments. An in vivo electrophysiologic study in the rat. Neuropsychopharmacology. 1991;5:219–29. [PubMed] [Google Scholar]
- Kia HK, Miquel MC, Brisorgueil MJ, et al. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J Compar Neurobiol. 1996;365:289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Kreiss DS, Lucki I. Effects of acute and repeated administration of antidepressant drugs on extracellular levels of 5-hydroxytryptamine measured in vivo. J Pharm Exp Therap. 1995;274:866–76. [PubMed] [Google Scholar]
- Le Poul E, Boni C, Hanoun N, et al. Differential adaptation of brain 5-HT1A and 5-HT1B receptors and 5-HT transporter in rats treated chronically with fluoxetine. Neuropharmacology. 2000;39:110–22. doi: 10.1016/s0028-3908(99)00088-x. [DOI] [PubMed] [Google Scholar]
- Le Poul E, Laaris N, Doucet E, et al. Early desensitization of somato-dendritic 5-HT1A autoreceptors in rats treated with fluoxetine or paroxetine. Naunyn–Schmiedeberg's Arch Pharmacol. 1995;352:141–8. doi: 10.1007/BF00176767. [DOI] [PubMed] [Google Scholar]
- Finn D, Phillips T, Okorn D, et al. Rewarding effect of the neuroactive steroid 3 alpha-hydroxy-5 alpha-pregnan-20-one in mice. Pharmacol Biochem Behav. 1997;56:261–4. doi: 10.1016/s0091-3057(96)00218-3. [DOI] [PubMed] [Google Scholar]
- Oshiro Y, Sakurai Y, Sato S, et al. 3,4-dihydro-2(1H)-quinolinone as a novel antidepressant drug: synthesis and pharmacology of 1-[3-[4-(3-chlorophenyl)-1-piperazinyl]propyl]-3,4- dihydro-5-methoxy-2(1H)-quinolinone and its derivatives. J Med Chem. 2000;43:177–89. doi: 10.1021/jm980333v. [DOI] [PubMed] [Google Scholar]
- Tottori K, Miwa T, Uwahodo Y, et al. Antidepressant-like responses to the combined sigma and 5-HT1A receptor agonist OPC-14523. Neuropharmacology. 2001;41:976–88. doi: 10.1016/s0028-3908(01)00147-2. [DOI] [PubMed] [Google Scholar]
- Tottori K, Nakai M, Uwahodo Y, et al. Attenuation of scopolamine-induced and age-associated memory impairments by the sigma and 5-hydroxytryptamine(1A) receptor agonist OPC-14523 (1-[3-[4-(3-chlorophenyl)-1-piperazinyl]propyl]-5-methoxy-3,4-dihydro-2[1H]-quino linone monomethanesulfonate) J Pharm Exp Therap. 2002;301:249–57. doi: 10.1124/jpet.301.1.249. [DOI] [PubMed] [Google Scholar]
- Bermack J, Debonnel G. Modulation of serotonergic neurotransmission by short- and long-term treatments with sigma ligands. Br J Pharmacol. 2001;134:691–9. doi: 10.1038/sj.bjp.0704294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewicz M, Verge D, Gozlan H, et al. Autoradiographic evidence for the heterogeneity of 5-HT1 sites in the rat brain. Brain Res. 1984;291:159–63. doi: 10.1016/0006-8993(84)90664-4. [DOI] [PubMed] [Google Scholar]
- Robichaud M, Beauchemin V, Lavoie N, et al. Effects of bilateral olfactory bulbectomy on N-methyl-d-aspartate receptor function: autoradiographic and behavioral studies in the rat. Synapse (NY) 2001;42:95–103. doi: 10.1002/syn.1105. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig M, Casanovas J, et al. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABA(A), and glutamate receptors. J Neurosci. 2001;21:9917–29. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanian G, Wang R. Habenular and other midbrain raphe afferents demonstrated by a modified retrograde tracing technique. Brain Res. 1997;122:229–42. doi: 10.1016/0006-8993(77)90291-8. [DOI] [PubMed] [Google Scholar]
- Aghajanian G, Wang R, Baraban J. Serotonergic and non-serotonergic neurons of the dorsal raphe: reciprocal changes in firing induced by peripheral nerve stimulation. Brain Res. 1978;153:169–75. doi: 10.1016/0006-8993(78)91140-x. [DOI] [PubMed] [Google Scholar]
- Ferraro G, Montalbano M, Sardo P, La Grutta V. Lateral habenular influence on dorsal raphe neurons. Brain Res Bull. 1996;41:47–52. doi: 10.1016/0361-9230(96)00170-0. [DOI] [PubMed] [Google Scholar]
- Hajos M, Gartside S, Varga V, Sharp T. In vivo inhibition of neuronal activity in the rat ventromedial prefrontal cortex by midbrain-raphe nuclei: role of 5-HT1A receptors. Neuropharmacology. 2003;45:72–81. doi: 10.1016/s0028-3908(03)00139-4. [DOI] [PubMed] [Google Scholar]
- Kalen P, Karlson M, Wiklund L. Possible excitatory amino acid afferents to nucleus raphe dorsalis of the rat investigated with retrograde wheat germ agglutinin and D-[3H]aspartate tracing. Brain Res. 1985;360:285–97. doi: 10.1016/0006-8993(85)91244-2. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, et al. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–68. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Varga V, Kocsis B, Sharp T. Electrophysiological evidence for convergence of inputs from the medial prefrontal cortex and lateral habenula on single neurons in the dorsal raphe nucleus. Eur J Neurosci. 2003;17:280–6. doi: 10.1046/j.1460-9568.2003.02465.x. [DOI] [PubMed] [Google Scholar]
- Ceci A, Baschirotto A, Borsini F. The inhibitory effect of 8-OH-DPAT on the firing activity of dorsal raphe serotoninergic neurons in rats is attenuated by lesion of the frontal cortex. Neuropharmacology. 1994;33:709–13. doi: 10.1016/0028-3908(94)90177-5. [DOI] [PubMed] [Google Scholar]
- Hajos M, Richards C, Szekely A, Sharp T. An electrophysiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the rat. Neuroscience. 1998;87:95–108. doi: 10.1016/s0306-4522(98)00157-2. [DOI] [PubMed] [Google Scholar]
- O'Hearn E, Molliver ME. Organization of raphe-cortical projections in rat: a quantitative retrograde study. Brain Res Bull. 1984;13:709–26. doi: 10.1016/0361-9230(84)90232-6. [DOI] [PubMed] [Google Scholar]
- Varga V, Szekely AD, Csillag A, et al. Evidence for a role of GABA interneurones in the cortical modulation of midbrain 5-hydroxytryptamine neurones. Neuroscience. 2001;106:783–92. doi: 10.1016/s0306-4522(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Varga EV, Rubenzik MK, Stropova D, et al. Converging protein kinase pathways mediate adenylyl cyclase superactivation upon chronic delta-opioid agonist treatment. J Pharm Exp Therap. 2003;306:109–15. doi: 10.1124/jpet.103.049643. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Matsuno K, Murai M, Mita S. Sigma 1 receptor subtype is involved in the facilitation of cortical dopaminergic transmission in the rat brain. Neurochem Res. 1997;22:1105–9. doi: 10.1023/a:1027361101419. [DOI] [PubMed] [Google Scholar]
- Barrot M, Vallee M, Gingras M, et al. The neurosteroid pregnenolone sulphate increases dopamine release and the dopaminergic response to morphine in the rat nucleus accumbens. Eur J Neurosci. 1999;11:3757–60. doi: 10.1046/j.1460-9568.1999.00816.x. [DOI] [PubMed] [Google Scholar]
- Ohi K, Hashimoto R, Yasuda Y, et al. The SIGMAR1 gene is associated with a risk of schizophrenia and activation of the prefrontal cortex. Progr Neuro-psychopharmacol Biol Psychiatry. 2011;35:1309–15. doi: 10.1016/j.pnpbp.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Bonomo V, Fogliani A. Citalopram and haloperidol for psychotic depression. Am J Psychiatry. 2000;157:1706–7. doi: 10.1176/appi.ajp.157.10.1706-a. [DOI] [PubMed] [Google Scholar]
- Patrick SL, Walker JM, Perkel JM, et al. Increases in rat striatal extracellular dopamine and vacuous chewing produced by two sigma receptor ligands. Eur J Pharmacol. 1993;231:243–9. doi: 10.1016/0014-2999(93)90456-r. [DOI] [PubMed] [Google Scholar]
- Paquette MA, Foley K, Brudney EG, et al. The sigma-1 antagonist BMY-14802 inhibits l-DOPA-induced abnormal involuntary movements by a WAY-100635-sensitive mechanism. Psychopharmacology. 2009;204:743–54. doi: 10.1007/s00213-009-1505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfels GF, Tam SW. Selective sigma receptor agonist and antagonist affect dopamine neuronal activity. Eur J Pharmacol. 1989;163:167–70. doi: 10.1016/0014-2999(89)90413-5. [DOI] [PubMed] [Google Scholar]
- Weatherspoon JK, Gonzalez-Alvear GM, Frank AR, Werling LL. Regulation of [3H] dopamine release from mesolimbic and mesocortical areas of guinea pig brain by sigma receptors. Schizophr Res. 1996;21:51–62. doi: 10.1016/0920-9964(96)00030-8. [DOI] [PubMed] [Google Scholar]
- Gronier B, Debonnel G. Involvement of sigma receptors in the modulation of the glutamatergic/NMDA neurotransmission in the dopaminergic systems. Eur J Pharmacol. 1999;368:183–96. doi: 10.1016/s0014-2999(99)00025-4. [DOI] [PubMed] [Google Scholar]
- Gudelsky G. Biphasic effect of sigma receptor ligands on the extracellular concentration of dopamine in the striatum of the rat. J Neural Transmission (Vienna, Austria: 1996) 1999;106:849–56. doi: 10.1007/s007020050205. [DOI] [PubMed] [Google Scholar]
- Peeters M, Romieu P, Maurice T, et al. Involvement of the sigma 1 receptor in the modulation of dopaminergic transmission by amantadine. Eur J Neurosci. 2004;19:2212–20. doi: 10.1111/j.0953-816X.2004.03297.x. [DOI] [PubMed] [Google Scholar]
- Sershen H, Hashim A, Lajtha A. The effect of ibogaine on sigma- and NMDA-receptor-mediated release of [3H]dopamine. Brain Res Bull. 1996;40:63–7. doi: 10.1016/0361-9230(96)00039-1. [DOI] [PubMed] [Google Scholar]
- Derbez A, Mody R, Werling L. Sigma(2)-receptor regulation of dopamine transporter via activation of protein kinase C. J Pharm Exp Therap. 2002;301:306–14. doi: 10.1124/jpet.301.1.306. [DOI] [PubMed] [Google Scholar]
- von Ruden L, Neher E. A Ca-dependent early step in the release of catecholamines from adrenal chromaffin cells. Science (NY) 1993;262:1061–5. doi: 10.1126/science.8235626. [DOI] [PubMed] [Google Scholar]
- Sagi N, Yamamoto H, Yamamoto T, et al. Possible expression of a sigma 1 site in rat pheochromocytoma (PC12) cells. Eur J Pharmacol. 1996;304:185–90. doi: 10.1016/0014-2999(96)00115-x. [DOI] [PubMed] [Google Scholar]
- Matsuno K, Matsunaga K, Mita S. Increase of extracellular acetylcholine level in rat frontal cortex induced by (+)N-allylnormetazocine as measured by brain microdialysis. Brain Res. 1992;575:315–19. doi: 10.1016/0006-8993(92)90096-r. [DOI] [PubMed] [Google Scholar]
- Matsuno K, Matsunaga K, Senda T, Mita S. Increase in extracellular acetylcholine level by sigma ligands in rat frontal cortex. J Pharm Exp Therap. 1993;265:851–9. [PubMed] [Google Scholar]
- Matsuno K, Senda T, Kobayashi T, et al. SA4503, a novel cognitive enhancer, with sigma 1 receptor agonistic properties. Behav Brain Res. 1997;83:221–4. doi: 10.1016/s0166-4328(97)86074-3. [DOI] [PubMed] [Google Scholar]
- Lesage AS, De Loore KL, Peeters L, Leysen JE. Neuroprotective sigma ligands interfere with the glutamate-activated NOS pathway in hippocampal cell culture. Synapse (NY) 1995;20:156–64. doi: 10.1002/syn.890200210. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz M. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–7. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Casey R, et al. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J Neurosci. 1997;17:9157–64. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samdani AF, Dawson TM, Dawson VL. Nitric oxide synthase in models of focal ischemia. Stroke. 1997;28:1283–8. doi: 10.1161/01.str.28.6.1283. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997;20:132–9. doi: 10.1016/s0166-2236(96)10074-6. [DOI] [PubMed] [Google Scholar]
- Vagnerova K, Hurn PD, Bhardwaj A, Kirsch JR. Sigma 1 receptor agonists act as neuroprotective drugs through inhibition of inducible nitric oxide synthase. Anesth Analg. 2006;103:430–4. doi: 10.1213/01.ane.0000226133.85114.91. [DOI] [PubMed] [Google Scholar]
- Jiang MH, Kaku T, Hada J, Hayashi Y. 7-Nitroindazole reduces nitric oxide concentration in rat hippocampus after transient forebrain ischemia. Eur J Pharmacol. 1999;380:117–21. doi: 10.1016/s0014-2999(99)00555-5. [DOI] [PubMed] [Google Scholar]
- Nanri K, Montecot C, Springhetti V, et al. The selective inhibitor of neuronal nitric oxide synthase, 7-nitroindazole, reduces the delayed neuronal damage due to forebrain ischemia in rats. Stroke. 1998;29:1248–53. doi: 10.1161/01.str.29.6.1248. discussion 1253–1244. [DOI] [PubMed] [Google Scholar]
- Bhardwaj A, Sawada M, London E, et al. Potent sigma1-receptor ligand 4-phenyl-1-(4-phenylbutyl) piperidine modulates basal and N-methyl-d-aspartate-evoked nitric oxide production in vivo. Stroke. 1998;29:2404–10. discussion 2411. [PubMed] [Google Scholar]
- Goyagi T, Goto S, Bhardwaj A, et al. Neuroprotective effect of sigma(1)-receptor ligand 4-phenyl-1-(4-phenylbutyl) piperidine (PPBP) is linked to reduced neuronal nitric oxide production. Stroke. 2001;32:1613–20. doi: 10.1161/01.str.32.7.1613. [DOI] [PubMed] [Google Scholar]
- Allahtavakoli M, Jarrott B. Sigma-1 receptor ligand PRE-084 reduced infarct volume, neurological deficits, pro-inflammatory cytokines and enhanced anti-inflammatory cytokines after embolic stroke in rats. Brain Res Bull. 2011;85:219–24. doi: 10.1016/j.brainresbull.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Collingridge G, Bliss T. Memories of NMDA receptors and LTP. Trends Neurosci. 1995;18:54–6. [PubMed] [Google Scholar]
- Collingridge G, Singer W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol Sci. 1990;11:290–6. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G. Glutamate in CNS disorders as a target for drug development: an update. Drug News Perspect. 1998;11:523–69. doi: 10.1358/dnp.1998.11.9.863689. [DOI] [PubMed] [Google Scholar]
- Rousseaux CG. A review of Glutamate receptors I: current understanding of their biology. Japan J Toxicol Pathol. 2008;21:21–51. [Google Scholar]
- Rousseaux CG. A review of Glutamate receptors II: Pathophysiology and pathology. Japan J Toxicol Pathol. 2008;21:133–73. [Google Scholar]
- Bermack J, Debonnel G. Distinct modulatory roles of sigma receptor subtypes on glutamatergic responses in the dorsal hippocampus. Synapse (NY) 2005;55:37–44. doi: 10.1002/syn.20085. [DOI] [PubMed] [Google Scholar]
- Bermack J, Debonnel G. The role of sigma receptors in depression. J Pharmacol Sci. 2005;97:317–36. doi: 10.1254/jphs.crj04005x. [DOI] [PubMed] [Google Scholar]
- Dong H, Hayar A, Ennis M. Activation of group I metabotropic glutamate receptors on main olfactory bulb granule cells and periglomerular cells enhances synaptic inhibition of mitral cells. J Neurosci. 2007;27:5654–63. doi: 10.1523/JNEUROSCI.5495-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitsanakis V, Aschner M. The importance of glutamate, glycine, and gamma-aminobutyric acid transport and regulation in manganese, mercury and lead neurotoxicity. Toxicol Appl Pharmacol. 2005;204:343–54. doi: 10.1016/j.taap.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Guitart X, Mendez R, Ovalle S, et al. Regulation of ionotropic glutamate receptor subunits in different rat brain areas by a preferential sigma(1) receptor ligand and potential atypical antipsychotic. Neuropsychopharmacology. 2000;23:539–46. doi: 10.1016/S0893-133X(00)00142-1. [DOI] [PubMed] [Google Scholar]
- Asztely F, Erdemli G, Kullmann D. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron. 1997;18:281–93. doi: 10.1016/s0896-6273(00)80268-8. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis S. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Frye C, Sturgis J. Neurosteroids affect spatial/reference, working, and long-term memory of female rats. Neurobiol Learn Memory. 1995;64:83–96. doi: 10.1006/nlme.1995.1046. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Prospects for clinically tolerated NMDA antagonists: open-channel blockers and alternative redox states of nitric oxide. Trends Neurosci. 1993;16:527–32. doi: 10.1016/0166-2236(93)90198-u. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Gendelman HE. Seminars in medicine of the Beth Israel Hospital, Boston. Dementia associated with the acquired immunodeficiency syndrome. N Eng J Med. 1995;332:934–40. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Eng J Med. 1994;330:613–22. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- Meldrum BS. The role of glutamate in epilepsy and other CNS disorders. Neurology. 1994;44:S14–23. [PubMed] [Google Scholar]
- Miller S, Kesslak JP, Romano C, Cotman CW. Roles of metabotropic glutamate receptors in brain plasticity and pathology. Ann NY Acad Sci. 1995;757:460–74. doi: 10.1111/j.1749-6632.1995.tb17506.x. [DOI] [PubMed] [Google Scholar]
- Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Progr Neurobiol. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Schoepp DD. Novel functions for subtypes of metabotropic glutamate receptors. Neurochem Int. 1994;24:439–49. doi: 10.1016/0197-0186(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Gasic G, Hollmann M. Mol Neurobiol of glutamate receptors. Annu Rev Physiol. 1992;54:507–36. doi: 10.1146/annurev.ph.54.030192.002451. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hollmann M, O'Shea-Greenfield A, Rogers S, Heinemann S. Cloning by functional expression of a member of the glutamate receptor family. Nature. 1989;342:643–8. doi: 10.1038/342643a0. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Mayer ML. N-methyl-d-aspartic acid receptor structure and function. Physiol Rev. 1994;74:723–60. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- Young VR, Ajami AM. Glutamate: an amino acid of particular distinction. J Nutr. 2000;130:892s–900s. doi: 10.1093/jn/130.4.892S. [DOI] [PubMed] [Google Scholar]
- Marc RE, Liu WL, Kalloniatis M, et al. Patterns of glutamate immunoreactivity in the goldfish retina. J Neurosci. 1990;10:4006–34. doi: 10.1523/JNEUROSCI.10-12-04006.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenman E, Frosch M, Lipton S. Responses mediated by excitatory amino acid receptors in solitary retinal ganglion cells from rat. J Physiol. 1988;396:75–91. doi: 10.1113/jphysiol.1988.sp016951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Kaneko A. l-Glutamate-induced responses in OFF-type bipolar cells of the cat retina. Vis Res. 1996;36:787–95. doi: 10.1016/0042-6989(95)00176-x. [DOI] [PubMed] [Google Scholar]
- Zhou ZJ, Fain GL, Dowling JE. The excitatory and inhibitory amino acid receptors on horizontal cells isolated from the white perch retina. J Neurophysiol. 1993;70:8–19. doi: 10.1152/jn.1993.70.1.8. [DOI] [PubMed] [Google Scholar]
- Jackson PF, Slusher BS. Design of NAALADase inhibitors: a novel neuroprotective strategy. Curr Med Chem. 2001;8:949–57. doi: 10.2174/0929867013372797. [DOI] [PubMed] [Google Scholar]
- Neale JH, Bzdega T, Wroblewska B. N-Acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. J Neurochem. 2000;75:443–52. doi: 10.1046/j.1471-4159.2000.0750443.x. [DOI] [PubMed] [Google Scholar]
- Danbolt N. Glutamate uptake. Progr Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Brosnan J. Glutamate, at the interface between amino acid and carbohydrate metabolism. J Nutr. 2000;130:988s–90s. doi: 10.1093/jn/130.4.988S. [DOI] [PubMed] [Google Scholar]
- Skuza G. Pharmacology of sigma (sigma) receptor ligands from a behavioral perspective. Current Pharm Design. 2012;18:863–74. doi: 10.2174/138161212799436458. [DOI] [PubMed] [Google Scholar]
- Ankarcrona M, Dypbukt J, Bonfoco E, et al. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–73. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Nicotera P. Calcium, free radicals and excitotoxins in neuronal apoptosis. Cell Calcium. 1998;23:165–71. doi: 10.1016/s0143-4160(98)90115-4. [DOI] [PubMed] [Google Scholar]
- Nicotera P, Lipton SA. Excitotoxins in neuronal apoptosis and necrosis. J Cerebral Blood Flow Metab. 1999;19:583–91. doi: 10.1097/00004647-199906000-00001. [DOI] [PubMed] [Google Scholar]
- Obrenovitch TP, Urenjak J. Altered glutamatergic transmission in neurological disorders: from high extracellular glutamate to excessive synaptic efficacy. Progr Neurobiol. 1997;51:39–87. doi: 10.1016/s0301-0082(96)00049-4. [DOI] [PubMed] [Google Scholar]
- Rosin C, Bates TE, Skaper SD. Excitatory amino acid induced oligodendrocyte cell death in vitro: receptor-dependent and -independent mechanisms. J Neurochem. 2004;90:1173–85. doi: 10.1111/j.1471-4159.2004.02584.x. [DOI] [PubMed] [Google Scholar]
- Truong DD, Bhidayasiri R, Wolters E. Management of non-motor symptoms in advanced Parkinson disease. J Neurol Sci. 2008;266:216–28. doi: 10.1016/j.jns.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Underhill SM, Goldberg MP. Hypoxic injury of isolated axons is independent of ionotropic glutamate receptors. Neurobiol Dis. 2007;25:284–90. doi: 10.1016/j.nbd.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. Excitotoxic cell death. J Neurobiol. 1992;23:1261–76. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Ozono R, O'Connell DP, Vaughan C, et al. Expression of the subtype 1A dopamine receptor in the rat heart. Hypertension. 1996;27:693–703. doi: 10.1161/01.hyp.27.3.693. [DOI] [PubMed] [Google Scholar]
- Griesmaier E, Posod A, Gross M, et al. Neuroprotective effects of the sigma-1 receptor ligand PRE-084 against excitotoxic perinatal brain injury in newborn mice. Exp Neurol. 2012;237:388–95. doi: 10.1016/j.expneurol.2012.06.030. [DOI] [PubMed] [Google Scholar]
- Bruni J, Bose R, Pinsky C, Glavin G. Circumventricular organ origin of domoic acid-induced neuropathology and toxicology. Brain Res Bull. 1991;26:419–24. doi: 10.1016/0361-9230(91)90016-d. [DOI] [PubMed] [Google Scholar]
- Choi D. Calcium: still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci. 1995;18:58–60. [PubMed] [Google Scholar]
- Choi D. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–34. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Gill S, Pulido O. Glutamate receptors in peripheral tissues: current knowledge, future research, and implications for toxicology. Toxicol Pathol. 2001;29:208–23. doi: 10.1080/019262301317052486. [DOI] [PubMed] [Google Scholar]
- Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130:1007s–15s. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- Price MT, Olney JW, Cicero TJ. Acute elevations of serum luteinizing hormone induced by kainic acid, N-methyl aspartic acid or homocysteic acid. Neuroendocrinology. 1978;26:352–8. doi: 10.1159/000122790. [DOI] [PubMed] [Google Scholar]
- Conn P, Pin J. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–7. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Cunningham M, Ferkany J, Enna S. Excitatory amino acid receptors: a gallery of new targets for pharmacological intervention. Life Sci. 1994;54:135–48. doi: 10.1016/0024-3205(94)00583-4. [DOI] [PubMed] [Google Scholar]
- Boldyrev A, Bulygina E, Makhro A. Glutamate receptors modulate oxidative stress in neuronal cells: a mini-review. Neurotoxic Res. 2004;6:581–7. doi: 10.1007/BF03033454. [DOI] [PubMed] [Google Scholar]
- Singh P, Mann KA, Mangat HK, Kaur G. Prolonged glutamate excitotoxicity: effects on mitochondrial antioxidants and antioxidant enzymes. Mol Cell Biochem. 2003;243:139–45. doi: 10.1023/a:1021668314070. [DOI] [PubMed] [Google Scholar]
- Beal M. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol. 1995;38:357–66. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- Li S, Stys PK. Mechanisms of ionotropic glutamate receptor-mediated excitotoxicity in isolated spinal cord white matter. J Neurosci. 2000;20:1190–8. doi: 10.1523/JNEUROSCI.20-03-01190.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaylock R. Excitotoxicity: a possible central mechanism in fluoride neurotoxicity. Fluoride. 2004;37:301–14. [Google Scholar]
- Butcher S, Sandberg M, Hagberg H, Hamberger A. Cellular origins of endogenous amino acids released into the extracellular fluid of the rat striatum during severe insulin-induced hypoglycemia. J Neurochem. 1987;48:722–8. doi: 10.1111/j.1471-4159.1987.tb05576.x. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P, Hansen JJ. Naturally-occurring excitatory amino acids as neurotoxins and leads in drug design. Toxicol Lett. 1992;64:409–16. doi: 10.1016/0378-4274(92)90214-5. [DOI] [PubMed] [Google Scholar]
- Peng YG, Taylor TB, Finch RE, et al. Neuroexcitatory and neurotoxic actions of the amnesic shellfish poison, domoic acid. Neuroreport. 1994;5:981–5. doi: 10.1097/00001756-199404000-00032. [DOI] [PubMed] [Google Scholar]
- Perl TM, Bedard L, Kosatsky T, et al. Amnesic shellfish poisoning: a new clinical syndrome due to domoic acid. Can Dis Week Rep. 1990;16:7–8. [PubMed] [Google Scholar]
- Rockhold RW, Acuff CG, Clower BR. Excitotoxin-induced myocardial necrosis. Eur J Pharmacol. 1989;166:571–6. doi: 10.1016/0014-2999(89)90379-8. [DOI] [PubMed] [Google Scholar]
- Teitelbaum JS, Zatorre RJ, Carpenter S, et al. Neurologic sequelae of domoic acid intoxication due to the ingestion of contaminated mussels. N Eng J Med. 1990;322:1781–7. doi: 10.1056/NEJM199006213222505. [DOI] [PubMed] [Google Scholar]
- Tryphonas L, Truelove J, Iverson F, et al. Neuropathology of experimental domoic acid poisoning in non-human primates and rats. Can Dis Week Rep. 1990;16:75–81. [PubMed] [Google Scholar]
- Watters MR. Organic neurotoxins in seafoods. Clin Neurol Neurosurg. 1995;97:119–24. doi: 10.1016/0303-8467(95)00015-c. [DOI] [PubMed] [Google Scholar]
- Winter CR, Baker RC. l-glutamate-induced changes in intracellular calcium oscillation frequency through non-classical glutamate receptor binding in cultured rat myocardial cells. Life Sci. 1995;57:1925–34. doi: 10.1016/0024-3205(95)02179-m. [DOI] [PubMed] [Google Scholar]
- Zautcke JL, Schwartz JA, Mueller EJ. Chinese restaurant syndrome: a review. Ann Emerg Med. 1986;15:1210–13. doi: 10.1016/s0196-0644(86)80869-1. [DOI] [PubMed] [Google Scholar]
- Erdo S. Excitatory amino acid receptors in the mammalian periphery. Trends Pharmacol Sci. 1991;12:426–9. doi: 10.1016/0165-6147(91)90622-y. [DOI] [PubMed] [Google Scholar]
- Iverson F, Truelove J, Tryphonas L, Nera EA. The toxicology of domoic acid administered systemically to rodents and primates. Can Dis Week Rep. 1990;16:15–8. discussion 18–19. [PubMed] [Google Scholar]
- Olney JW. Glutamate: a neurotoxic transmitter. J Child Neurol. 1989;4:218–26. doi: 10.1177/088307388900400315. [DOI] [PubMed] [Google Scholar]
- Meldrum B. Amino acids as dietary excitotoxins: a contribution to understanding neurodegenerative disorders. Brain Res Brain Res Rev. 1993;18:293–314. doi: 10.1016/0165-0173(93)90014-q. [DOI] [PubMed] [Google Scholar]
- Truelove J, Mueller R, Pulido O, Iverson F. Subchronic toxicity study of domoic acid in the rat. Food Chem Toxicol. 1996;34:525–9. doi: 10.1016/0278-6915(96)81814-x. [DOI] [PubMed] [Google Scholar]
- Kim HC, Jhoo WK, Kim WK, et al. Carbetapentane attenuates kainate-induced seizures via sigma-1 receptor modulation. Life Sci. 2001;69:915–22. doi: 10.1016/s0024-3205(01)01181-x. [DOI] [PubMed] [Google Scholar]
- DeCoster M, Klette K, Knight E, Tortella F. Sigma receptor-mediated neuroprotection against glutamate toxicity in primary rat neuronal cultures. Brain Res. 1995;671:45–53. doi: 10.1016/0006-8993(94)01294-r. [DOI] [PubMed] [Google Scholar]
- Farber N, Jiang X, Heinkel C, Nemmers B. Antiepileptic drugs and agents that inhibit voltage-gated sodium channels prevent NMDA antagonist neurotoxicity. Mol Psychiatry. 2002;7:726–33. doi: 10.1038/sj.mp.4001087. [DOI] [PubMed] [Google Scholar]
- Nishikawa J, Saito K, Sasaki M, et al. Molecular cloning and functional characterization of a novel nuclear receptor similar to an embryonic benzoate receptor BXR. Biochem Biophys Res Commun. 2000;277:209–15. doi: 10.1006/bbrc.2000.3649. [DOI] [PubMed] [Google Scholar]
- Beal M. Excitotoxicity and nitric oxide in Parkinson's disease pathogenesis. Ann Neurol. 1998;44:S110–14. doi: 10.1002/ana.410440716. [DOI] [PubMed] [Google Scholar]
- Flor P, Battaglia G, Nicoletti F, et al. Neuroprotective activity of metabotropic glutamate receptor ligands. Adv Exp Med Biol. 2002;513:197–23. doi: 10.1007/978-1-4615-0123-7_7. [DOI] [PubMed] [Google Scholar]
- Danysz W, Parsons C. Glycine and N-methyl-d-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol Rev. 1998;50:597–664. [PubMed] [Google Scholar]
- Shaw PJ, Ince PG. Glutamate, excitotoxicity and amyotrophic lateral sclerosis. J Neurol. 1997;244:S3–14. doi: 10.1007/BF03160574. [DOI] [PubMed] [Google Scholar]
- Starr MS. Antiparkinsonian actions of glutamate antagonists–alone and with l-DOPA: a review of evidence and suggestions for possible mechanisms. J Neural Transm Parkinson's Dis Demen Sect. 1995;10:141–85. doi: 10.1007/BF02251229. [DOI] [PubMed] [Google Scholar]
- Al-Saif A, Al-Mohanna F, Bohlega S. A mutation in sigma-1 receptor causes juvenile amyotrophic lateral sclerosis. Ann Neurol. 2011;70:913–19. doi: 10.1002/ana.22534. [DOI] [PubMed] [Google Scholar]
- Mancuso R, Olivan S, Rando A, et al. Sigma-1R agonist improves motor function and motoneuron survival in ALS mice. Neurotherapeutics. 2012;9:814–26. doi: 10.1007/s13311-012-0140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath JT, Sechi A, Dreser A, et al. Loss of function of the ALS protein SigR1 leads to ER pathology associated with defective autophagy and lipid raft disturbances. Cell Death Dis. 2014;5:e1290. doi: 10.1038/cddis.2014.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Wang X, Park J, et al. Antagonism of sigma-1 receptors blocks compulsive-like eating. Neuropsychopharmacology. 2012;37:2593–604. doi: 10.1038/npp.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscher K, Shamloo M, Rickhag M, et al. The sigma-1 receptor enhances brain plasticity and functional recovery after experimental stroke. Brain. 2011;134:732–46. doi: 10.1093/brain/awq367. [DOI] [PubMed] [Google Scholar]
- Katz JL, Su TP, Hiranita T, et al. A role for sigma receptors in stimulant self administration and addiction. Pharmaceuticals (Basel, Switzerland) 2011;4:880–914. doi: 10.3390/ph4060880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HW, Roh DH, Yoon SY, et al. Activation of the spinal sigma-1 receptor enhances NMDA-induced pain via PKC- and PKA-dependent phosphorylation of the NR1 subunit in mice. Br J Pharmacol. 2008;154:1125–34. doi: 10.1038/bjp.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo KA, Akil H. Excitatory amino acids and drugs of abuse: a role for N-methyl-d-aspartate receptors in drug tolerance, sensitization and physical dependence. Drug Alcohol Depend. 1995;38:139–54. doi: 10.1016/0376-8716(95)01119-j. [DOI] [PubMed] [Google Scholar]
- Artola A, Hensch T, Singer W. Calcium-induced long-term depression in the visual cortex of the rat in vitro. J Neurophysiol. 1996;76:984–94. doi: 10.1152/jn.1996.76.2.984. [DOI] [PubMed] [Google Scholar]
- Artola A, Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 1993;16:480–7. doi: 10.1016/0166-2236(93)90081-v. [DOI] [PubMed] [Google Scholar]
- Bear M, Malenka R. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–99. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Christie B, Kerr D, Abraham W. Flip side of synaptic plasticity: long-term depression mechanisms in the hippocampus. Hippocampus. 1994;4:127–35. doi: 10.1002/hipo.450040203. [DOI] [PubMed] [Google Scholar]
- Christie B, Magee J, Johnston D. Dendritic calcium channels and hippocampal long-term depression. Hippocampus. 1996;6:17–23. doi: 10.1002/(SICI)1098-1063(1996)6:1<17::AID-HIPO4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Cummings J, Mulkey R, Nicoll R, Malenka R. Ca2+ signaling requirements for long-term depression in the hippocampus. Neuron. 1996;16:825–33. doi: 10.1016/s0896-6273(00)80102-6. [DOI] [PubMed] [Google Scholar]
- Derrick B, Martinez J., Jr Associative, bidirectional modifications at the hippocampal mossy fibre-CA3 synapse. Nature. 1996;381:429–34. doi: 10.1038/381429a0. [DOI] [PubMed] [Google Scholar]
- Edwards F. LTP – a structural model to explain the inconsistencies. Trends Neurosci. 1995;18:250–5. doi: 10.1016/0166-2236(95)80003-k. [DOI] [PubMed] [Google Scholar]
- Hansel C, Artola A, Singer W. Different threshold levels of postsynaptic [Ca2+]i have to be reached to induce LTP and LTD in neocortical pyramidal cells. J Physiol. 1996;90:317–19. doi: 10.1016/s0928-4257(97)87906-5. [DOI] [PubMed] [Google Scholar]
- Jeffery KJ. LTP and spatial learning – where to next? Hippocampus. 1997;7:95–110. doi: 10.1002/(SICI)1098-1063(1997)7:1<95::AID-HIPO10>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Rioult MC, Bear MF. Experience-dependent modification of synaptic plasticity in visual cortex. Nature. 1996;381:526–8. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- Malenka RC. Synaptic plasticity in the hippocampus: LTP and LTD. Cell. 1994;78:535–8. doi: 10.1016/0092-8674(94)90517-7. [DOI] [PubMed] [Google Scholar]
- Maren S, Tocco G, Standley S, et al. Postsynaptic factors in the expression of long-term potentiation (LTP): increased glutamate receptor binding following LTP induction in vivo. Proc Natl Acad Sci USA. 1993;90:9654–8. doi: 10.1073/pnas.90.20.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumoto T, Yasuda H, Fukuda M, Akaneya Y. Postsynaptic calcium and calcium-dependent processes in synaptic plasticity in the developing visual cortex. J Physiol (Paris) 1996;90:151–6. doi: 10.1016/s0928-4257(97)81414-3. [DOI] [PubMed] [Google Scholar]
- Bode B. Recent molecular advances in mammalian glutamine transport. J Nutr. 2001;131:2475S–85S. doi: 10.1093/jn/131.9.2475S. discussion 2486S–7S. [DOI] [PubMed] [Google Scholar]
- Arriza J, Fairman W, Wadiche J, et al. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci. 1994;14:5559–69. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairman W, Vandenberg R, Arriza J, et al. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360:467–71. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- Malandro MS, Kilberg MS. Molecular biology of mammalian amino acid transporters. Annu Rev Biochem. 1996;65:305–36. doi: 10.1146/annurev.bi.65.070196.001513. [DOI] [PubMed] [Google Scholar]
- Pines G, Danbolt NC, Bjoras M, et al. Cloning and expression of a rat brain l-glutamate transporter. Nature. 1992;360:464–7. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- Schultz K, Stell WK. Immunocytochemical localization of the high-affinity glutamate transporter, EAAC1, in the retina of representative vertebrate species. Neurosci Lett. 1996;211:191–4. doi: 10.1016/0304-3940(96)12762-2. [DOI] [PubMed] [Google Scholar]
- Bai L, Xu H, Collins J, Ghishan F. Molecular and functional analysis of a novel neuronal vesicular glutamate transporter. J Biol Chem. 2001;276:36764–9. doi: 10.1074/jbc.M104578200. [DOI] [PubMed] [Google Scholar]
- Moriyama Y, Yamamoto A. Glutamatergic chemical transmission: look! Here, there, and anywhere. J Biochem. 2004;135:155–63. doi: 10.1093/jb/mvh018. [DOI] [PubMed] [Google Scholar]
- Aanonsen L, Wilcox G. Nociceptive action of excitatory amino acids in the mouse: effects of spinally administered opioids, phencyclidine and sigma agonists. J Pharm Exp Therap. 1987;243:9–19. [PubMed] [Google Scholar]
- Kanai Y, Bhide PG, DiFiglia M, Hediger MA. Neuronal high-affinity glutamate transport in the rat central nervous system. Neuroreport. 1995;6:2357–62. doi: 10.1097/00001756-199511270-00020. [DOI] [PubMed] [Google Scholar]
- Aas P, Tanso R, Fonnum F. Stimulation of peripheral cholinergic nerves by glutamate indicates a new peripheral glutamate receptor. Eur J Pharmacol. 1989;164:93–102. doi: 10.1016/0014-2999(89)90235-5. [DOI] [PubMed] [Google Scholar]
- Barb C, Campbell R, Armstrong J, Cox N. Aspartate and glutamate modulation of growth hormone secretion in the pig: possible site of action. Domestic Anim Endocrinol. 1996;13:81–90. doi: 10.1016/0739-7240(95)00047-x. [DOI] [PubMed] [Google Scholar]
- Bertrand G, Gross R, Puech R, et al. Evidence for a glutamate receptor of the AMPA subtype which mediates insulin release from rat perfused pancreas. Br J Pharmacol. 1992;106:354–9. doi: 10.1111/j.1476-5381.1992.tb14340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand G, Gross R, Puech R, et al. Glutamate stimulates glucagon secretion via an excitatory amino acid receptor of the AMPA subtype in rat pancreas. Eur J Pharmacol. 1993;237:45–50. doi: 10.1016/0014-2999(93)90091-u. [DOI] [PubMed] [Google Scholar]
- Burns G, Stephens K, Benson J. Expression of mRNA for the N-methyl-d-aspartate (NMDAR1) receptor by the enteric neurons of the rat. Neurosci Lett. 1994;170:87–90. doi: 10.1016/0304-3940(94)90245-3. [DOI] [PubMed] [Google Scholar]
- Carlton S, Hargett G, Coggeshall R. Localization and activation of glutamate receptors in unmyelinated axons of rat glabrous skin. Neurosci Lett. 1995;197:25–8. doi: 10.1016/0304-3940(95)11889-5. [DOI] [PubMed] [Google Scholar]
- Chaudhari N, Yang H, Lamp C, et al. The taste of monosodium glutamate: membrane receptors in taste buds. J Neurosci. 1996;16:3817–26. doi: 10.1523/JNEUROSCI.16-12-03817.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenu C, Serre C, Raynal C, et al. Glutamate receptors are expressed by bone cells and are involved in bone resorption. Bone. 1998;22:295–9. doi: 10.1016/s8756-3282(97)00295-0. [DOI] [PubMed] [Google Scholar]
- Coggeshall R, Carlton S. Ultrastructural analysis of NMDA, AMPA, and kainate receptors on unmyelinated and myelinated axons in the periphery. J Compar Neurobiol. 1998;391:78–86. doi: 10.1002/(sici)1096-9861(19980202)391:1<78::aid-cne7>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- Dememes D, Lleixa A, Dechesne C. Cellular and subcellular localization of AMPA-selective glutamate receptors in the mammalian peripheral vestibular system. Brain Res. 1995;671:83–94. doi: 10.1016/0006-8993(94)01322-9. [DOI] [PubMed] [Google Scholar]
- Eliasof S, Werblin F. Characterization of the glutamate transporter in retinal cones of the tiger salamander. J Neurosci. 1993;13:402–11. doi: 10.1523/JNEUROSCI.13-01-00402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genever P, Maxfield S, Kennovin G, et al. Evidence for a novel glutamate-mediated signaling pathway in keratinocytes. J Invest Dermatol. 1999;112:337–42. doi: 10.1046/j.1523-1747.1999.00509.x. [DOI] [PubMed] [Google Scholar]
- Genever P, Skerry T. Regulation of spontaneous glutamate release activity in osteoblastic cells and its role in differentiation and survival: evidence for intrinsic glutamatergic signaling in bone. FASEB J. 2001;15:1586–8. doi: 10.1096/fj.00-0594fje. [DOI] [PubMed] [Google Scholar]
- Genever P, Wilkinson D, Patton A, et al. Expression of a functional N-methyl-d-aspartate-type glutamate receptor by bone marrow megakaryocytes. Blood. 1999;93:2876–83. [PubMed] [Google Scholar]
- Gill S, Barker M, Pulido O. Neuroexcitatory targets in the female reproductive system of the nonhuman primate (Macaca fascicularis) Toxicol Pathol. 2008;36:478–84. doi: 10.1177/0192623308315663. [DOI] [PubMed] [Google Scholar]
- Gill S, Veinot J, Kavanagh M, Pulido O. Human heart glutamate receptors – implications for toxicology, food safety, and drug discovery. Toxicol Pathol. 2007;35:411–17. doi: 10.1080/01926230701230361. [DOI] [PubMed] [Google Scholar]
- Gill S, Pulido O, Mueller R, McGuire P. Immunochemical localization of the metabotropic glutamate receptors in the rat heart. Brain Res Bull. 1999;48:143–6. doi: 10.1016/s0361-9230(98)00154-3. [DOI] [PubMed] [Google Scholar]
- Gill S, Pulido O, Mueller R, McGuire P. Molecular and immunochemical characterization of the ionotropic glutamate receptors in the rat heart. Brain Res Bull. 1998;46:429–34. doi: 10.1016/s0361-9230(98)00012-4. [DOI] [PubMed] [Google Scholar]
- Gonoi T, Mizuno N, Inagaki N, et al. Functional neuronal ionotropic glutamate receptors are expressed in the non-neuronal cell line MIN6. J Biol Chem. 1994;269:16989–92. [PubMed] [Google Scholar]
- Hardy M, Younkin D, Tang C, et al. Expression of non-NMDA glutamate receptor channel genes by clonal human neurons. J Neurochem. 1994;63:482–9. doi: 10.1046/j.1471-4159.1994.63020482.x. [DOI] [PubMed] [Google Scholar]
- Haxhiu M, Erokwu B, Dreshaj I. The role of excitatory amino acids in airway reflex responses in anesthetized dogs. J automat Nerv Syst. 1997;67:192–9. doi: 10.1016/s0165-1838(97)00110-0. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Zviman M, Brand J, et al. Measurement of membrane potential and [Ca2+]i in cell ensembles: application to the study of glutamate taste in mice. Biophys J. 1996;71:1057–70. doi: 10.1016/S0006-3495(96)79306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyama H, Sato K, Tohyama M. Characteristic localization of non-NMDA type glutamate receptor subunits in the rat pituitary gland. Brain Res. 1993;19:262–8. doi: 10.1016/0169-328x(93)90039-r. [DOI] [PubMed] [Google Scholar]
- Lindstrom P, Ohlsson L. Effect of N-methyl-D, l-aspartate on isolated rat somatotrophs. Endocrinology. 1992;131:1903–7. doi: 10.1210/endo.131.4.1396334. [DOI] [PubMed] [Google Scholar]
- Liu HP, Tay SS, Leong SK. Localization of glutamate receptor subunits of the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) type in the pancreas of newborn guinea pigs. Pancreas. 1997;14:360–8. doi: 10.1097/00006676-199705000-00006. [DOI] [PubMed] [Google Scholar]
- Marc RE, Lam DM. Uptake of aspartic and glutamic acid by photoreceptors in goldfish retina. Proc Natl Acad Sci USA. 1981;78:7185–9. doi: 10.1073/pnas.78.11.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick G. Non-N-methyl-d-aspartate glutamate receptors in glial cells and neurons of the pineal gland in a higher primate. Neuroendocrinology. 1995;61:256–64. doi: 10.1159/000126847. [DOI] [PubMed] [Google Scholar]
- Molnar E, Varadi A, McIlhinney RA, Ashcroft SJ. Identification of functional ionotropic glutamate receptor proteins in pancreatic beta-cells and in islets of Langerhans. FEBS Lett. 1995;371:253–7. doi: 10.1016/0014-5793(95)00890-l. [DOI] [PubMed] [Google Scholar]
- Moroni F, Luzzi S, Franchi-Micheli S, Zilletti L. The presence of N-methyl-d-aspartate-type receptors for glutamic acid in the guinea pig myenteric plexus. Neurosci Lett. 1986;68:57–62. doi: 10.1016/0304-3940(86)90229-6. [DOI] [PubMed] [Google Scholar]
- O'Connell DP, Aherne AM, Lane E, et al. Detection of dopamine receptor D1A subtype-specific mRNA in rat kidney by in situ amplification. Am J Physiol. 1998;274:F232–41. doi: 10.1152/ajprenal.1998.274.1.F232. [DOI] [PubMed] [Google Scholar]
- Pulido O, Veinot J, Mueller R, et al. Toxicol pathol of glutamate receptors (GluRs) – an opportunity for pharmaceutical development. Part I – human heart. Washington (DC): STP Annual General Meeting, 2005
- Said SI. Glutamate receptors and asthmatic airway disease. Trends Pharmacol Sci. 1999;20:132–4. doi: 10.1016/s0165-6147(98)01275-9. [DOI] [PubMed] [Google Scholar]
- Sasa M, Takeshita S, Amano T, Kurisu K. Primary neurotransmitters and regulatory substances onto vestibular nucleus neurons. Uchu Seibutsu Kagaku. 2001;15:371–4. doi: 10.2187/bss.15.371. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Sawyer BD. Glutamate receptors of the N-methyl-d-aspartate subtype in the myenteric plexus of the guinea pig ileum. J Pharm Exp Therap. 1989;251:518–23. [PubMed] [Google Scholar]
- Stuhmer T, Amar M, Harvey RJ, et al. Structure and pharmacological properties of a molluscan glutamate-gated cation channel and its likely role in feeding behavior. J Neurosci. 1996;16:2869–80. doi: 10.1523/JNEUROSCI.16-09-02869.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureda F, Copani A, Bruno V, et al. Metabotropic glutamate receptor agonists stimulate polyphosphoinositide hydrolysis in primary cultures of rat hepatocytes. Eur J Pharmacol. 1997;338:R1–2. doi: 10.1016/s0014-2999(97)81950-4. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Kaneko A. l-glutamate-induced depolarization in solitary photoreceptors: a process that may contribute to the interaction between photoreceptors in situ. Proc Natl Acad Sci USA. 1988;85:5315–19. doi: 10.1073/pnas.85.14.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Mishina M, Inoue Y. Distinct gene expression of the N-methyl-d-aspartate receptor channel subunit in peripheral neurons of the mouse sensory ganglia and adrenal gland. Neurosci Lett. 1994;165:183–6. doi: 10.1016/0304-3940(94)90740-4. [DOI] [PubMed] [Google Scholar]
- Weaver CD, Gundersen V, Verdoorn TA. A high affinity glutamate/aspartate transport system in pancreatic islets of Langerhans modulates glucose-stimulated insulin secretion. J Biol Chem. 1998;273:1647–53. doi: 10.1074/jbc.273.3.1647. [DOI] [PubMed] [Google Scholar]
- Weaver CD, Yao TL, Powers AC, Verdoorn TA. Differential expression of glutamate receptor subtypes in rat pancreatic islets. J Biol Chem. 1996;271:12977–84. doi: 10.1074/jbc.271.22.12977. [DOI] [PubMed] [Google Scholar]
- Yamaguchi I, Jose PA, Mouradian MM, et al. Expression of dopamine D1A receptor gene in proximal tubule of rat kidneys. Am J Physiol. 1993;264:F280–5. doi: 10.1152/ajprenal.1993.264.2.F280. [DOI] [PubMed] [Google Scholar]
- Yang JH, Wu SM. Characterization of glutamate transporter function in the tiger salamander retina. Vis Res. 1997;37:827–38. doi: 10.1016/s0042-6989(96)00231-3. [DOI] [PubMed] [Google Scholar]
- Brew H, Attwell D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. Nature. 1987;327:707–9. doi: 10.1038/327707a0. [DOI] [PubMed] [Google Scholar]
- Schwartz EA, Tachibana M. Electrophysiology of glutamate and sodium co-transport in a glial cell of the salamander retina. J Physiol. 1990;426:43–80. doi: 10.1113/jphysiol.1990.sp018126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour B, Brew H, Attwell D. Electrogenic uptake of glutamate and aspartate into glial cells isolated from the salamander (Ambystoma) retina. J Physiol. 1991;436:169–93. doi: 10.1113/jphysiol.1991.sp018545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour B, Brew H, Attwell D. Electrogenic glutamate uptake in glial cells is activated by intracellular potassium. Nature. 1998;335:433–5. doi: 10.1038/335433a0. [DOI] [PubMed] [Google Scholar]
- Bouvier M, Szatkowski M, Amato A, Attwell D. The glial cell glutamate uptake carrier countertransports pH-changing anions. Nature. 1992;360:471–4. doi: 10.1038/360471a0. [DOI] [PubMed] [Google Scholar]
- Eliasof S, Jahr C. Retinal glial cell glutamate transporter is coupled to an anionic conductance. Proc Natl Acad Sci USA. 1996;93:4153–8. doi: 10.1073/pnas.93.9.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fykse E, Fonnum F. Amino acid neurotransmission: dynamics of vesicular uptake. Neurochem Res. 1996;21:1053–60. doi: 10.1007/BF02532415. [DOI] [PubMed] [Google Scholar]
- Naito S, Ueda T. Adenosine triphosphate-dependent uptake of glutamate into protein I-associated synaptic vesicles. J Biol Chem. 1983;258:696–9. [PubMed] [Google Scholar]
- Tabb JS, Ueda T. Phylogenetic studies on the synaptic vesicle glutamate transport system. J Neurosci. 1991;11:1822–8. doi: 10.1523/JNEUROSCI.11-06-01822.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant G, Dowling J. On bipolar cell responses in the teleost retina are generated by two distinct mechanisms. J Neurophysiol. 1996;76:3842–9. doi: 10.1152/jn.1996.76.6.3842. [DOI] [PubMed] [Google Scholar]
- Grant G, Werblin F. A glutamate-elicited chloride current with transporter-like properties in rod photoreceptors of the tiger salamander. Vis Neurosci. 1996;13:135–44. doi: 10.1017/s0952523800007185. [DOI] [PubMed] [Google Scholar]
- Harrington E, Moddel G, Najm I, Baraban S. Altered glutamate receptor – transporter expression and spontaneous seizures in rats exposed to methylazoxymethanol in utero. Epilepsia. 2007;48:158–68. doi: 10.1111/j.1528-1167.2006.00838.x. [DOI] [PubMed] [Google Scholar]
- Kew JN, Kemp JA. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology. 2005;179:4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- Picaud S, Larsson HP, Wellis DP, et al. Cone photoreceptors respond to their own glutamate release in the tiger salamander. Proc Natl Acad Sci USA. 1995;92:9417–21. doi: 10.1073/pnas.92.20.9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picaud SA, Larsson HP, Grant GB, et al. Glutamate-gated chloride channel with glutamate-transporter-like properties in cone photoreceptors of the tiger salamander. J Neurophysiol. 1995;74:1760–71. doi: 10.1152/jn.1995.74.4.1760. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–94. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Asztely F, Gustafsson B. Ionotropic glutamate receptors: their possible role in the expression of hippocampal synaptic plasticity. Mol Neurobiol. 1996;12:1–11. doi: 10.1007/BF02740744. [DOI] [PubMed] [Google Scholar]
- Seeburg PH. The TINS/TiPS lecture: the molecular biology of mammalian glutamate receptor channels. Trends Neurosci. 1993;16:359–65. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- Cancela J, Churchill G, Galione A. Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature. 1999;398:74–6. doi: 10.1038/18032. [DOI] [PubMed] [Google Scholar]
- Laube B, Hirai H, Sturgess M, et al. Molecular determinants of agonist discrimination by NMDA receptor subunits: analysis of the glutamate binding site on the NR2B subunit. Neuron. 1997;18:493–503. doi: 10.1016/s0896-6273(00)81249-0. [DOI] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Betz H. Evidence for a tetrameric structure of recombinant NMDA receptors. J Neurosci. 1998;18:2954–61. doi: 10.1523/JNEUROSCI.18-08-02954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Armstrong N. Structure and function of glutamate receptor ion channels. Annu Rev Physiol. 2004;66:161–81. doi: 10.1146/annurev.physiol.66.050802.084104. [DOI] [PubMed] [Google Scholar]
- McFeeters RL, Oswald RE. Emerging structural explanations of ionotropic glutamate receptor function. FASEB J. 2004;18:428–38. doi: 10.1096/fj.03-0873rev. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stern-Bach Y, Stevens CF. The tetrameric structure of a glutamate receptor channel. Science (New York, NY) 1998;280:1596–9. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- Wisden W, Seeburg PH. Mammalian ionotropic glutamate receptors. Curr Opin Neurobiol. 1993;3:291–8. doi: 10.1016/0959-4388(93)90120-n. [DOI] [PubMed] [Google Scholar]
- Yuzaki M. New insights into the structure and function of glutamate receptors: the orphan receptor delta2 reveals its family's secrets. Keio J Med. 2003;52:92–9. doi: 10.2302/kjm.52.92. [DOI] [PubMed] [Google Scholar]
- Mansour M, Nagarajan N, Nehring RB, et al. Heteromeric AMPA receptors assemble with a preferred subunit stoichiometry and spatial arrangement. Neuron. 2001;32:841–53. doi: 10.1016/s0896-6273(01)00520-7. [DOI] [PubMed] [Google Scholar]
- Yoneda Y, Kuramoto N, Kitayama T, Hinoi E. Consolidation of transient ionotropic glutamate signals through nuclear transcription factors in the brain. Progr Neurobiol. 2001;63:697–719. doi: 10.1016/s0301-0082(00)00036-8. [DOI] [PubMed] [Google Scholar]
- Sharp CD, Hines I, Houghton J, et al. Glutamate causes a loss in human cerebral endothelial barrier integrity through activation of NMDA receptor. Am J Physiol Heart Circulatory Physiol. 2003;285:H2592–8. doi: 10.1152/ajpheart.00520.2003. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. Permeation and block of N-methyl-d-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987;394:501–27. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–31. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science (NY) 1988;241:835–7. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Kirchhoff F. NMDA Receptors in glia. Neuroscientist. 2007;13:28–37. doi: 10.1177/1073858406294270. [DOI] [PubMed] [Google Scholar]
- Chavez A, Singer J, Diamond J. Fast neurotransmitter release triggered by Ca influx through AMPA-type glutamate receptors. Nature. 2006;443:705–8. doi: 10.1038/nature05123. [DOI] [PubMed] [Google Scholar]
- Monyer H, Seeburg PH, Wisden W. Glutamate-operated channels: developmentally early and mature forms arise by alternative splicing. Neuron. 1991;6:799–810. doi: 10.1016/0896-6273(91)90176-z. [DOI] [PubMed] [Google Scholar]
- Sommer B, Keinanen K, Verdoorn TA, et al. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science (NY) 1990;249:1580–5. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Braithwaite S, Clarke V, Henley J. Kainate receptors: subunits, synaptic localization and function. Trends Pharmacol Sci. 1999;20:26–35. doi: 10.1016/s0165-6147(98)01286-3. [DOI] [PubMed] [Google Scholar]
- Contractor A, Swanson G, Heinemann S. Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus. Neuron. 2001;29:209–16. doi: 10.1016/s0896-6273(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Mellor J, Nicoll RA. Presynaptic kainate receptor mediation of frequency facilitation at hippocampal mossy fiber synapses. Science (NY) 2001;291:1972–6. doi: 10.1126/science.1057105. [DOI] [PubMed] [Google Scholar]
- Vignes M, Collingridge GL. The synaptic activation of kainate receptors. Nature. 1997;388:179–82. doi: 10.1038/40639. [DOI] [PubMed] [Google Scholar]
- Abram S, Olson E. Systemic opioids do not suppress spinal sensitization after subcutaneous formalin in rats. Anesthesiology. 1994;80:1114–19. doi: 10.1097/00000542-199405000-00020. [DOI] [PubMed] [Google Scholar]
- Lerma J, Morales M, Vicente MA, Herreras O. Glutamate receptors of the kainate type and synaptic transmission. Trends Neurosci. 1997;20:9–12. doi: 10.1016/S0166-2236(96)20055-4. [DOI] [PubMed] [Google Scholar]
- Wilding TJ, Huettner JE. Activation and desensitization of hippocampal kainate receptors. J Neurosci. 1997;17:2713–21. doi: 10.1523/JNEUROSCI.17-08-02713.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunishima N, Shimada Y, Tsuji Y, et al. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature. 2000;407:971–7. doi: 10.1038/35039564. [DOI] [PubMed] [Google Scholar]
- Marin YE, Chen S. Involvement of metabotropic glutamate receptor 1, a G protein coupled receptor, in melanoma development. J Mol Med (Berlin, Germany) 2004;82:735–49. doi: 10.1007/s00109-004-0566-8. [DOI] [PubMed] [Google Scholar]
- Senkowska A, Ossowska K. Role of metabotropic glutamate receptors in animal models of Parkinson's disease. Pol J Pharmacol. 2003;55:935–50. [PubMed] [Google Scholar]
- Hallett P, Standaert D. Rationale for and use of NMDA receptor antagonists in Parkinson's disease. Pharmacol Therap. 2004;102:155–74. doi: 10.1016/j.pharmthera.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Konitsiotis S. Novel pharmacological strategies for motor complications in Parkinson's disease. Expert Opinion Invest Drugs. 2005;14:377–92. doi: 10.1517/13543784.14.4.377. [DOI] [PubMed] [Google Scholar]
- Coutinho V, Knopfel T. Metabotropic glutamate receptors: electrical and chemical signaling properties. Neuroscientist. 2002;8:551–61. doi: 10.1177/1073858402238514. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Schoepp D. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Boos R, Schneider H, Wassle H. Voltage- and transmitter-gated currents of all-amacrine cells in a slice preparation of the rat retina. J Neurosci. 1993;13:2874–88. doi: 10.1523/JNEUROSCI.13-07-02874.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Miller R. The role of NMDA and non-NMDA excitatory amino acid receptors in the functional organization of primate retinal ganglion cells. Vis Neurosci. 1994;11:317–32. doi: 10.1017/s0952523800001668. [DOI] [PubMed] [Google Scholar]
- Gilbertson T, Scobey R, Wilson M. Permeation of calcium ions through non-NMDA glutamate channels in retinal bipolar cells. Science (NY) 1991;251:1613–15. doi: 10.1126/science.1849316. [DOI] [PubMed] [Google Scholar]
- Kessler M, Arai A, Quan A, Lynch G. Effect of cyclothiazide on binding properties of AMPA-type glutamate receptors: lack of competition between cyclothiazide and GYKI 52466. Mol Pharmacol. 1996;49:123–31. [PubMed] [Google Scholar]
- Kessler M, Arai A, Vanderklish P, Lynch G. Failure to detect changes in AMPA receptor binding after long-term potentiation. Brain Res. 1991;560:337–41. doi: 10.1016/0006-8993(91)91255-y. [DOI] [PubMed] [Google Scholar]
- Yamada KA, Tang CM. Benzothiadiazides inhibit rapid glutamate receptor desensitization and enhance glutamatergic synaptic currents. J Neurosci. 1993;13:3904–15. doi: 10.1523/JNEUROSCI.13-09-03904.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Miller RF. NBQX, an improved non-NMDA antagonist studied in retinal ganglion cells. Brain Res. 1995;692:190–4. doi: 10.1016/0006-8993(95)00665-d. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science (NY) 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Boulter J, Hollmann M, O'Shea-Greenfield A, et al. Molecular cloning and functional expression of glutamate receptor subunit genes. Science (NY) 1990;249:1033–7. doi: 10.1126/science.2168579. [DOI] [PubMed] [Google Scholar]
- Nakanishi N, Shneider NA, Axel R. A family of glutamate receptor genes: evidence for the formation of heteromultimeric receptors with distinct channel properties. Neuron. 1990;5:569–81. doi: 10.1016/0896-6273(90)90212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S. Metabotropic glutamate receptors: synaptic transmission, modulation, and plasticity. Neuron. 1994;13:1031–7. doi: 10.1016/0896-6273(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Bettler B, Boulter J, Hermans-Borgmeyer I, et al. Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron. 1990;5:583–95. doi: 10.1016/0896-6273(90)90213-y. [DOI] [PubMed] [Google Scholar]
- Bettler B, Egebjerg J, Sharma G, et al. Cloning of a putative glutamate receptor: a low affinity kainate-binding subunit. Neuron. 1992;8:257–65. doi: 10.1016/0896-6273(92)90292-l. [DOI] [PubMed] [Google Scholar]
- Egebjerg J, Bettler B, Hermans-Borgmeyer I, Heinemann S. Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature. 1991;351:745–8. doi: 10.1038/351745a0. [DOI] [PubMed] [Google Scholar]
- Keinanen K, Wisden W, Sommer B, et al. A family of AMPA-selective glutamate receptors. Science (NY) 1990;249:556–60. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- John CS, Vilner BJ, Bowen WD. Synthesis and characterization of [125I]-N-(N-benzylpiperidin-4-yl)-4-iodobenzamide: a new sigma receptor radiopharmaceutical: high-affinity binding to MCF-7 breast tumor cells. J Med Chem. 1994;37:1737–9. doi: 10.1021/jm00038a002. [DOI] [PubMed] [Google Scholar]
- Zou LB, Yamada K, Nabeshima T. Sigma receptor ligands (+)-SKF10,047 and SA4503 improve dizocilpine-induced spatial memory deficits in rats. Eur J Pharmacol. 1998;355:1–10. doi: 10.1016/s0014-2999(98)00464-6. [DOI] [PubMed] [Google Scholar]
- Wallace DR, Mactutus CF, Booze RM. Sigma binding sites identified by [(3)H] DTG are elevated in aged Fischer-344 × Brown Norway (F1) rats. Synapse (NY) 2000;35:311–13. doi: 10.1002/(SICI)1098-2396(20000315)35:4<311::AID-SYN9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Whittemore ER, Ilyin VI, Woodward RM. Antagonism of N-methyl-d-aspartate receptors by sigma site ligands: potency, subtype-selectivity and mechanisms of inhibition. J Pharm Exp Therap. 1997;282:326–8. [PubMed] [Google Scholar]
- Fletcher E, Church J, Abdel-Hamid K, MacDonald J. Blockade by sigma site ligands of N-methyl-d-aspartate-evoked responses in rat and mouse cultured hippocampal pyramidal neurones. Br J Pharmacol. 1995;116:2791–800. doi: 10.1111/j.1476-5381.1995.tb15928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontecorvo MJ, Karbon EW, Goode S, et al. Possible cerebroprotective and in vivo NMDA antagonist activities of sigma agents. Brain Res Bull. 1991;26:461–5. doi: 10.1016/0361-9230(91)90025-f. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Yamamoto T, Sagi N, et al. Sigma ligands indirectly modulate the NMDA receptor-ion channel complex on intact neuronal cells via sigma 1 site. J Neurosci. 1995;15:731–6. doi: 10.1523/JNEUROSCI.15-01-00731.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perregaard J, Moltzen EK, Meier E, Sanchez C. Sigma ligands with subnanomolar affinity and preference for the sigma 2 binding site. 1: 3-(omega-aminoalkyl)-1H-indoles. J Med Chem. 1995;38:1998–2008. doi: 10.1021/jm00011a019. [DOI] [PubMed] [Google Scholar]
- de Costa B, Bowen W, Hellewell S, et al. Synthesis and evaluation of optically pure [3H]-(+)-pentazocine, a highly potent and selective radioligand for sigma receptors. FEBS Lett. 1989;251:53–8. doi: 10.1016/0014-5793(89)81427-9. [DOI] [PubMed] [Google Scholar]
- Meyer DA, Carta M, Partridge LD, et al. Neurosteroids enhance spontaneous glutamate release in hippocampal neurons: possible role of metabotropic sigma1-like receptors. J Biol Chem. 2002;277:28725–32. doi: 10.1074/jbc.M202592200. [DOI] [PubMed] [Google Scholar]
- Earley B, Burke M, Leonard B, et al. Evidence for an anti-amnesic effect of JO 1784 in the rat: a potent and selective ligand for the sigma receptor. Brain Res. 1991;546:282–6. doi: 10.1016/0006-8993(91)91492-j. [DOI] [PubMed] [Google Scholar]
- Klink R, Robichaud M, Debonnel G. Gender and gonadal status modulation of dorsal raphe nucleus serotonergic neurons. Part I: effects of gender and pregnancy. Neuropharmacology. 2002;43:1119–28. doi: 10.1016/s0028-3908(02)00219-8. [DOI] [PubMed] [Google Scholar]
- Klink R, Robichaud M, Debonnel G. Gender and gonadal status modulation of dorsal raphe nucleus serotonergic neurons. Part II: regulatory mechanisms. Neuropharmacol. 2002;43:1129–38. doi: 10.1016/s0028-3908(02)00218-6. [DOI] [PubMed] [Google Scholar]
- Pepe GJ, Rothchild I. A comparative study of serum progesterone levels in pregnancy and in various types of pseudopregnancy in the rat. Endocrinology. 1974;95:275–9. doi: 10.1210/endo-95-1-275. [DOI] [PubMed] [Google Scholar]
- Awapara J, Landua A, Fuerst R, Seale B. Free gamma-aminobutyric acid in brain. J Biol Chem. 1950;187:35–9. [PubMed] [Google Scholar]
- Erlander M, Tillakaratne N, Feldblum S, et al. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Erlander M, Tobin A. The structural and functional heterogeneity of glutamic acid decarboxylase: a review. Neurochem Res. 1991;16:215–26. doi: 10.1007/BF00966084. [DOI] [PubMed] [Google Scholar]
- Krnjevic K. Chemical nature of synaptic transmission in vertebrates. Physiol Rev. 1974;54:418–540. [Google Scholar]
- Otsuka M, Iversen LL, Hall ZW, Kravitz EA. Release of gamma-aminobutyric acid from inhibitory nerves of lobster. Proc Natl Acad Sci USA. 1966;56:1110–15. doi: 10.1073/pnas.56.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E, Frankel S. gamma-Aminobutyric acid in brain: its formation from glutamic acid. J Biol Chem. 1950;187:55–63. [PubMed] [Google Scholar]
- Walters RJ. Excitation and adrenaline: GABA – the biopolar neurotransmitter. Cell Sci. 2004;1:20–22. [Google Scholar]
- Roberts E. Pregneolone – from Selye to Alzheimer and a model of the pregnenolone sulfate binding site on the GABAA receptor. Biochem Pharmacol. 1995;49:1–16. doi: 10.1016/0006-2952(94)00258-n. [DOI] [PubMed] [Google Scholar]
- Roberts E. GABA: the road to neurotransmitter status. New York: Alan R Liss; 1986. [Google Scholar]
- Hosie AM, Buckingham SD, Hamon A, Sattelle DB. Replacement of asparagine with arginine at the extracellular end of the second transmembrane (M2) region of insect GABA receptors increases sensitivity to penicillin G. Invertebrate Neurosci. 2006;6:75–9. doi: 10.1007/s10158-006-0020-4. [DOI] [PubMed] [Google Scholar]
- Borden L, Smith K, Hartig P, et al. Molecular heterogeneity of the gamma-aminobutyric acid (GABA) transport system: cloning of two novel high affinity GABA transporters from rat brain. J Biol Chem. 1992;267:21098–104. [PubMed] [Google Scholar]
- Guastella J, Nelson N, Nelson H, et al. Cloning and expression of a rat brain GABA transporter. Science (NY) 1990;249:1303–6. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- Barnard E, Darlison M, Fujita N, et al. Molecular biology of the GABAA receptor. Adv Exp Med Biol. 1988;236:31–45. doi: 10.1007/978-1-4757-5971-6_3. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Tobin AJ. Molecular biology of GABAA receptors. FASEB J. 1990;4:1469–80. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- Schofield PR, Darlison MG, Fujita N, et al. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987;328:221–7. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Bormann J. Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci. 1998;11:112–16. doi: 10.1016/0166-2236(88)90156-7. [DOI] [PubMed] [Google Scholar]
- Chebib M, Johnston G. The ‘ABC' of GABA receptors: a brief review. Clin Exp Pharmacol Physiol. 1999;26:937–40. doi: 10.1046/j.1440-1681.1999.03151.x. [DOI] [PubMed] [Google Scholar]
- Darnaudery M, Koehl M, Piazza P, et al. Pregnenolone sulfate increases hippocampal acetylcholine release and spatial recognition. Brain Res. 2000;852:173–9. doi: 10.1016/s0006-8993(99)01964-2. [DOI] [PubMed] [Google Scholar]
- Chen G, Trombley P, van den Pol A. GABA receptors precede glutamate receptors in hypothalamic development; differential regulation by astrocytes. J Neurophysiol. 1995;74:1473–84. doi: 10.1152/jn.1995.74.4.1473. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, et al. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science (NY) 1986;232:1004–7. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–22. [PubMed] [Google Scholar]
- Invernizzi R, Pozzi L, Samanin R. Release of dopamine is reduced by diazepam more in the nucleus accumbens than in the caudate nucleus of conscious rats. Neuropharmacology. 1991;30:575–8. doi: 10.1016/0028-3908(91)90075-m. [DOI] [PubMed] [Google Scholar]
- Burt D, Kamatchi G. GABAA receptor subtypes: from pharmacology to molecular biology. FASEB J. 1991;5:2916–23. doi: 10.1096/fasebj.5.14.1661244. [DOI] [PubMed] [Google Scholar]
- Luddens H, Wisden W. Function and pharmacology of multiple GABAA receptor subunits. Trends Pharmacol Sci. 1991;12:49–51. doi: 10.1016/0165-6147(91)90495-e. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Luddens H, Seeburg PH. Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science (NY) 1989;245:1389–92. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Sontheimer H, Gorman CM, et al. Transient expression shows ligand gating and allosteric potentiation of GABAA receptor subunits. Science (NY) 1988;242:1306–8. doi: 10.1126/science.2848320. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Sontheimer H, Shivers BD, et al. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–5. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Supplisson S, Bergman C. Control of NMDA receptor activation by a glycine transporter co-expressed in Xenopus oocytes. J Neurosci. 1997;17:4580–90. doi: 10.1523/JNEUROSCI.17-12-04580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz H. Structure and function of inhibitory glycine receptors. Q Rev Biophys. 1992;25:381–94. doi: 10.1017/s0033583500004340. [DOI] [PubMed] [Google Scholar]
- Childers S, Pacheco M, Bennett B, et al. Cannabinoid receptors: G-protein-mediated signal transduction mechanisms. Biochem Soc symp. 1993;59:27–50. [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull R. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors: where they are and what they do. J Neuroendocrinol. 2008;20:10–14. doi: 10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. HEP. 2005;168:299–325. doi: 10.1007/3-540-26573-2_10. [DOI] [PubMed] [Google Scholar]
- Patel KD, Davison JS, Pittman QJ, Sharkey KA. Cannabinoid CB(2) receptors in health and disease. Curr Med Chem. 2010;17:1393–410. doi: 10.2174/092986710790980041. [DOI] [PubMed] [Google Scholar]
- Nocerino E, Amato M, Izzo AA. Cannabis and cannabinoid receptors. Fitoterapia. 2000;71:S6–12. doi: 10.1016/s0367-326x(00)00171-4. [DOI] [PubMed] [Google Scholar]
- Duncan M, Davison J, Sharkey K. Review article: endocannabinoids and their receptors in the enteric nervous system. Aliment Pharmacol Therap. 2005;22:667–83. doi: 10.1111/j.1365-2036.2005.02648.x. [DOI] [PubMed] [Google Scholar]
- Navarrete M, Araque A. Endocannabinoids mediate neuron-astrocyte communication. Neuron. 2008;57:883–93. doi: 10.1016/j.neuron.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Mascia MS, Obinu MC, Ledent C, et al. Lack of morphine-induced dopamine release in the nucleus accumbens of cannabinoid CB(1) receptor knockout mice. Eur J Pharmacol. 1999;383:R1–2. doi: 10.1016/s0014-2999(99)00656-1. [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, Shen R. Modulation of the serotonin system by endocannabinoid signaling. Neuropharmacology. 2011;61:414–20. doi: 10.1016/j.neuropharm.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira S, Teixeira F, Garcao P, et al. Presynaptic CB(1) cannabinoid receptors control frontocortical serotonin and glutamate release – species differences. Neurochem Int. 2012;61:219–26. doi: 10.1016/j.neuint.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur R, Gertsch J, Sigel E. The cannabinoid CB1 receptor antagonists rimonabant (SR141716) and AM251 directly potentiate GABA(A) receptors. Br J Pharmacol. 2012;165:2479–84. doi: 10.1111/j.1476-5381.2011.01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyas F, Yanovsky Y, Mackie K, et al. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience. 2006;137:337–61. doi: 10.1016/j.neuroscience.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Takahashi KA, Castillo PE. The CB1 cannabinoid receptor mediates glutamatergic synaptic suppression in the hippocampus. Neuroscience. 2006;139:795–802. doi: 10.1016/j.neuroscience.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Garcia C, Sagredo O, et al. The endocannabinoid system as a target for the treatment of neuronal damage. Expert Opin Therap Targets. 2010;14:387–404. doi: 10.1517/14728221003709792. [DOI] [PubMed] [Google Scholar]
- Croxford J. Therapeutic potential of cannabinoids in CNS disease. CNS drugs. 2003;17:179–202. doi: 10.2165/00023210-200317030-00004. [DOI] [PubMed] [Google Scholar]
- Lopez-Moreno JA, Gonzalez-Cuevas G, Moreno G, Navarro M. The pharmacology of the endocannabinoid system: functional and structural interactions with other neurotransmitter systems and their repercussions in behavioral addiction. Addiction Biol. 2008;13:160–87. doi: 10.1111/j.1369-1600.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- Arnold J. Why sigma-1 receptor dysfunction might confer vulnerability to cannabis-induced psychosis. Int J Neuropsychopharmacol. 2014;17:1911–13. doi: 10.1017/S1461145714000960. [DOI] [PubMed] [Google Scholar]
- Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol Therap. 2009;124:195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Mack AL, Coop A, Matsumoto RR. Novel sigma (sigma) receptor agonists produce antidepressant-like effects in mice. Eur Neuropsychopharmacol. 2007;17:708–16. doi: 10.1016/j.euroneuro.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita N, Hashimoto K, Tomitaka S, Minabe Y. Interactions of selective serotonin reuptake inhibitors with subtypes of sigma receptors in rat brain. Eur J Pharmacol. 1996;307:117–19. doi: 10.1016/0014-2999(96)00254-3. [DOI] [PubMed] [Google Scholar]
- Graybiel A, Besson M, Weber E. Neuroleptic-sensitive binding sites in the nigrostriatal system: evidence for differential distribution of sigma sites in the substantia nigra, pars compacta of the cat. J Neurosci. 1989;9:326–38. doi: 10.1523/JNEUROSCI.09-01-00326.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles D, Stedman T, Pond S. Nonlinear relationship between circulating concentrations of reduced haloperidol and haloperidol: evaluation of possible mechanisms. Psychopharmacology. 1994;116:161–6. doi: 10.1007/BF02245058. [DOI] [PubMed] [Google Scholar]
- Villard V, Meunier J, Chevallier N, Maurice T. Pharmacological interaction with the sigma1 (sigma1)-receptor in the acute behavioral effects of antidepressants. J Pharmacol Sci. 2011;115:279–92. doi: 10.1254/jphs.10191fp. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Kassim CO. Clorgyline displays high affinity for sigma binding sites in C57BL/6 mouse brain. Eur J Pharmacol. 1990;176:107–8. doi: 10.1016/0014-2999(90)90139-w. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Lebel L, Koe BK, et al. Sertraline potently displaces (+)-[3H]3-PPP binding to sigma sites in rat brain. Eur J Pharmacol. 1989;165:335–6. doi: 10.1016/0014-2999(89)90734-6. [DOI] [PubMed] [Google Scholar]
- Fennell A, Swant J, Goodwin S, Khoshbouei H. Involvement of striatal and hippocampal sigma receptors in methamphetamine-induced maladaptation. [Abstract] FASEB J. 2011;25:619.12. [Google Scholar]
- Johnson TD. Modulation of channel function by polyamines. Trends Pharmacol Sci. 1996;17:22–7. doi: 10.1016/0165-6147(96)81566-5. [DOI] [PubMed] [Google Scholar]
- Navarro G, Moreno E, Bonaventura J, et al. Cocaine inhibits dopamine D2 receptor signaling via sigma-1-D2 receptor heteromers. PLoS One. 2013;8:e61245. doi: 10.1371/journal.pone.0061245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meziane H, Mathis C, Paul SM, Ungerer A. The neurosteroid pregnenolone sulfate reduces learning deficits induced by scopolamine and has promnestic effects in mice performing an appetitive learning task. Psychopharmacology. 1996;126:323–30. doi: 10.1007/BF02247383. [DOI] [PubMed] [Google Scholar]
- Su TP, Hayashi T, Maurice T, et al. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol Sci. 2010;31:557–66. doi: 10.1016/j.tips.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellado M, Rodriguez-Frade JM, Manes S, Martinez AC. Chemokine signaling and functional responses: the role of receptor dimerization and TK pathway activation. Annu Rev Immunol. 2001;19:397–421. doi: 10.1146/annurev.immunol.19.1.397. [DOI] [PubMed] [Google Scholar]
- Maurice T, Phan VL, Urani A, Guillemain I. Differential involvement of the sigma(1) (sigma(1)) receptor in the anti-amnesic effect of neuroactive steroids, as demonstrated using an in vivo antisense strategy in the mouse. Br J Pharmacol. 2001;134:1731–41. doi: 10.1038/sj.bjp.0704355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Morimoto R, Yamamoto A, Moriyama Y. Expression and localization of vesicular glutamate transporters in pancreatic islets, upper gastrointestinal tract, and testis. J Histochem Cytochem. 2003;51:1375–90. doi: 10.1177/002215540305101014. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Yamada H, Uehara S, et al. Secretory granule-mediated co-secretion of l-glutamate and glucagon triggers glutamatergic signal transmission in islets of Langerhans. J Biol Chem. 2003;278:1966–74. doi: 10.1074/jbc.M206758200. [DOI] [PubMed] [Google Scholar]
- Berthois Y, Bourrie B, Galiegue S, et al. SR31747A is a sigma receptor ligand exhibiting antitumoural activity both in vitro and in vivo. Br J Cancer. 2003;88:438–46. doi: 10.1038/sj.bjc.6600709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi S, Shinoda Y, Yamamoto Y, et al. Stimulation of the sigma-1 receptor by DHEA enhances synaptic efficacy and neurogenesis in the hippocampal dentate gyrus of olfactory bulbectomized mice. PLoS One. 2013;8:e60863. doi: 10.1371/journal.pone.0060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su T. Sigma-1 receptors at galactosylceramide-enriched lipid microdomains regulate oligodendrocyte differentiation. Proc Natl Acad Sci USA. 2004;101:14949–54. doi: 10.1073/pnas.0402890101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stantchev TS, Markovic I, Telford WG, et al. The tyrosine kinase inhibitor genistein blocks HIV-1 infection in primary human macrophages. Virus Res. 2007;123:178–89. doi: 10.1016/j.virusres.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TH, Weissman AD, London ED. Pharmacological characteristics and distributions of sigma- and phencyclidine receptors in the animal kingdom. J Neurochem. 1990;54:598–604. doi: 10.1111/j.1471-4159.1990.tb01913.x. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Parameswaran S, Vu T, London ED. Divergent ontogeny of sigma and phencyclidine binding sites in the rat brain. Brain Res Dev Brain Res. 1989;47:13–18. doi: 10.1016/0165-3806(89)90103-x. [DOI] [PubMed] [Google Scholar]
- Wolfe SA, Jr, Culp SG, De Souza EB. Sigma-receptors in endocrine organs: identification, characterization, and autoradiographic localization in rat pituitary, adrenal, testis, and ovary. Endocrinology. 1989;124:1160–72. doi: 10.1210/endo-124-3-1160. [DOI] [PubMed] [Google Scholar]
- Wolfe SA, Jr, Kulsakdinun C, Battaglia G, et al. Initial identification and characterization of sigma receptors on human peripheral blood leukocytes. J Pharm Exp Therap. 1988;247:1114–19. [PubMed] [Google Scholar]
- Narayanan S, Mesangeau C, Poupaert JH, McCurdy CR. Sigma receptors and cocaine abuse. Curr Topics Med Chem. 2011;11:1128–50. doi: 10.2174/156802611795371323. [DOI] [PubMed] [Google Scholar]
- Brimson J, Brown C, Safrany S. Antagonists show GTP-sensitive high-affinity binding to the sigma-1 receptor. Br J Pharmacol. 2011;164:772–80. doi: 10.1111/j.1476-5381.2011.01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holl R, Schepmann D, Frohlich R, et al. Dancing of the second aromatic residue around the 6,8-diazabicyclo[3.2.2]nonane framework: influence on sigma receptor affinity and cytotoxicity. J Med Chem. 2009;52:2126–37. doi: 10.1021/jm801522j. [DOI] [PubMed] [Google Scholar]
- Chien C, Pasternak G. (−)-Pentazocine analgesia in mice: interactions with a sigma receptor system. Eur J Pharmacol. 1995;294:303–8. doi: 10.1016/0014-2999(95)00552-8. [DOI] [PubMed] [Google Scholar]
- Rodvelt KR, Miller DK. Could sigma receptor ligands be a treatment for methamphetamine addiction? Curr Dug Abuse Rev. 2010;3:156–62. doi: 10.2174/1874473711003030156. [DOI] [PubMed] [Google Scholar]
- Brandes L. Hormetic effects of hormones, antihormones, and antidepressants on cancer cell growth in culture: in vivo correlates. Crit Rev Toxicol. 2005;35:587–92. doi: 10.1080/10408440500246801. [DOI] [PubMed] [Google Scholar]
- Brugmann WB, Firmani MA. Low concentrations of nitric oxide exert a hormetic effect on Mycobacterium tuberculosis in vitro. J clin Microbiol. 2005;43:4844–6. doi: 10.1128/JCM.43.9.4844-4846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese E. Paradigm lost, paradigm found: the re-emergence of hormesis as a fundamental dose response model in the Toxicol sci. Environ Pollut (Barking, Essex: 1987) 2005;138:379–411. doi: 10.1016/j.envpol.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Calabrese E, Blain R. The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: an overview. Toxicol Appl Pharmacol. 2005;202:289–301. doi: 10.1016/j.taap.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Calabrese E. Defining hormesis. Hum Exp Toxicol. 2002;21:91–7. doi: 10.1191/0960327102ht217oa. [DOI] [PubMed] [Google Scholar]
- Cedergreen N, Ritz C, Streibig J. Improved empirical models describing hormesis. Environ Toxicol Chem. 2005;24:3166–172. doi: 10.1897/05-014r.1. [DOI] [PubMed] [Google Scholar]
- Celik I, Surucu O, Dietz C, et al. Therapeutic efficacy of endostatin exhibits a biphasic dose-response curve. Cancer Res. 2005;65:11044–50. doi: 10.1158/0008-5472.CAN-05-2617. [DOI] [PubMed] [Google Scholar]
- Chiueh C, Andoh T, Chock P. Induction of thioredoxin and mitochondrial survival proteins mediates preconditioning-induced cardioprotection and neuroprotection. Ann NY Acad Sci. 2005;1042:403–18. doi: 10.1196/annals.1338.034. [DOI] [PubMed] [Google Scholar]
- Dietert R. Commentary on hormetic dose-response relationships in immunology: occurrence, quantitative features of the dose response, mechanistic foundations, and clinical implications. Crit Rev Toxicol. 2005;35:305–6. doi: 10.1080/10408440590917080. [DOI] [PubMed] [Google Scholar]
- Fukushima S, Kinoshita A, Puatanachokchai R, et al. Hormesis and dose-response-mediated mechanisms in carcinogenesis: evidence for a threshold in carcinogenicity of non-genotoxic carcinogens. Carcinogenesis. 2005;26:1835–45. doi: 10.1093/carcin/bgi160. [DOI] [PubMed] [Google Scholar]
- Kaiser J. Hormesis. Sipping from a poisoned chalice. Science (NY) 2003;302:376–9. doi: 10.1126/science.302.5644.376. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Wood JG, Sinclair DA. Small molecules that regulate lifespan: evidence for xenohormesis. Mol Microbiol. 2004;53:1003–9. doi: 10.1111/j.1365-2958.2004.04209.x. [DOI] [PubMed] [Google Scholar]
- Laughlin RB, Jr, Ng J, Guard HE. Hormesis: a response to low environmental concentrations of petroleum hydrocarbons. Science (NY) 1981;211:705–7. doi: 10.1126/science.211.4483.705. [DOI] [PubMed] [Google Scholar]
- Lindsay DG. Nutrition, hormetic stress and health. Nutrition Res Rev. 2005;18:249–58. doi: 10.1079/NRR2005110. [DOI] [PubMed] [Google Scholar]
- Liu SZ. Nonlinear dose-response relationship in the immune system following exposure to ionizing radiation: mechanisms and implications. Nonlinear Biol Toxicol Med. 2003;1:71–92. doi: 10.1080/15401420390844483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puatanachokchai R, Morimura K, Wanibuchi H, et al. Alpha-benzene hexachloride exerts hormesis in preneoplastic lesion formation of rat hepatocarcinogenesis with the possible role for hepatic detoxifying enzymes. Cancer Lett. 2006;240:102–13. doi: 10.1016/j.canlet.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Goto S. Exercise and hormesis: oxidative stress-related adaptation for successful aging. Biogerontology. 2005;6:71–5. doi: 10.1007/s10522-004-7386-7. [DOI] [PubMed] [Google Scholar]
- Randic M, Estrada E. Order from chaos: observing hormesis at the proteome level. J Proteome Res. 2005;4:2133–6. doi: 10.1021/pr050229j. [DOI] [PubMed] [Google Scholar]
- Rattan SI. Aging intervention, prevention, and therapy through hormesis. J Gerontol Ser A. 2004;59:705–9. doi: 10.1093/gerona/59.7.b705. [DOI] [PubMed] [Google Scholar]
- Rattan SI. Hormetic mechanisms of anti-aging and rejuvenating effects of repeated mild heat stress on human fibroblasts in vitro. Rejuvenat Res. 2004;7:40–8. doi: 10.1089/154916804323105071. [DOI] [PubMed] [Google Scholar]
- Rattan SI. Mechanisms of hormesis through mild heat stress on human cells. Ann NY Acad Sci. 2004;1019:554–8. doi: 10.1196/annals.1297.103. [DOI] [PubMed] [Google Scholar]
- Renner R. Hormesis. Nietzsche's toxicology. Sci Am. 2003;389:28–30. doi: 10.1038/scientificamerican0903-28. [DOI] [PubMed] [Google Scholar]
- Shama G, Alderson P. UV hormesis in fruits: a concept ripe for commercialization. Trends Food Sci Technol. 2005;16:128–36. [Google Scholar]
- Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Age Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Stebbing AR. Hormesis: interpreting the beta-curve using control theory. J Appl Toxicol. 2000;20:93–101. doi: 10.1002/(sici)1099-1263(200003/04)20:2<93::aid-jat640>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Low Dose Irradiation and Biological Defense Mechanisms [Google Scholar]
- Tubiana M. Dose-effect relationship and estimation of the carcinogenic effects of low doses of ionizing radiation: the joint report of the Academie des Sciences (Paris) and of the Academie Nationale de Medecine. Int J Radiat Oncol Biol Phys. 2005;63:317–19. doi: 10.1016/j.ijrobp.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Calabrese E. Historical blunders: how toxicology got the dose-response relationship half right. Cell Mol Biol (Noisy-le-Grand, France) 2005;51:643–54. [PubMed] [Google Scholar]
- Hueppe F. Principles of bcteriology. Chicago, IL: The Open Court Publishing Company; 1896. [Google Scholar]
- Schulz H. Uber Hefegifte. Pflugers Archiv Gesamte Physiologie Menschen Tiere. 1888;42:517–41. [Google Scholar]
- Schulz H. Zur Lehre von der Arzneiwirkung. [Virchows] Arch Pathol Anat Physiol Klin Med. 1887;108:423–45. [Google Scholar]
- Cook R, Calabrese E. The importance of hormesis to public health. Ciencia Saude Coletiva. 2007;12:955–63. doi: 10.1590/s1413-81232007000400017. [DOI] [PubMed] [Google Scholar]
- Calabrese E. 5-Hydroxytryptamine (serotonin): biphasic dose responses. Crit Rev Toxicol. 2001;31:553–61. doi: 10.1080/20014091111820. [DOI] [PubMed] [Google Scholar]
- Calabrese E. Adenosine: biphasic dose responses. Crit Rev Toxicol. 2001;31:539–51. doi: 10.1080/20014091111811. [DOI] [PubMed] [Google Scholar]
- Calabrese E. Adrenergic receptors: biphasic dose responses. Crit Rev Toxicol. 2001;31:523–38. doi: 10.1080/20014091111802. [DOI] [PubMed] [Google Scholar]
- Calabrese E. Amyloid beta-peptide: biphasic dose responses. Crit Rev Toxicol. 2001;31:605–6. doi: 10.1080/20014091111857. [DOI] [PubMed] [Google Scholar]
- Calabrese E. Androgens: biphasic dose responses. Crit Rev Toxicol. 2001;31:517–22. doi: 10.1080/20014091111794. [DOI] [PubMed] [Google Scholar]
- Calabrese E. Apoptosis: biphasic dose responses. Crit Rev Toxicol. 2001;31:607–13. doi: 10.1080/20014091111866. [DOI] [PubMed] [Google Scholar]
- Calabrese E. Dopamine: biphasic dose responses. Crit Rev Toxicol. 2001;31:563–83. doi: 10.1080/20014091111839. [DOI] [PubMed] [Google Scholar]
- Calabrese E. Estrogen and related compounds: biphasic dose responses. Crit Rev Toxicol. 2001;31:503–15. doi: 10.1080/20014091111785. [DOI] [PubMed] [Google Scholar]
- Calabrese E. Hormetic dose-response relationships in immunology: occurrence, quantitative features of the dose response, mechanistic foundations, and clinical implications. Crit Rev Toxicol. 2005;35:89–95. doi: 10.1080/10408440590917044. [DOI] [PubMed] [Google Scholar]
- Calabrese E. Nitric oxide: biphasic dose responses. Crit Rev Toxicol. 2001;31:489–501. doi: 10.1080/20014091111776. [DOI] [PubMed] [Google Scholar]
- Calabrese E. Opiates: biphasic dose responses. Crit Rev Toxicol. 2001;31:585–604. doi: 10.1080/20014091111848. [DOI] [PubMed] [Google Scholar]
- Calabrese E. Overcompensation stimulation: a mechanism for hormetic effects. Crit Rev Toxicol. 2001;31:425–70. doi: 10.1080/20014091111749. [DOI] [PubMed] [Google Scholar]
- Calabrese E. Prostaglandins: biphasic dose responses. Crit Rev Toxicol. 2001;31:475–87. doi: 10.1080/20014091111767. [DOI] [PubMed] [Google Scholar]
- Calabrese E, Baldwin L. Agonist concentration gradients as a generalizable regulatory implementation strategy. Crit Rev Toxicol. 2001;31:471–3. doi: 10.1080/20014091111758. [DOI] [PubMed] [Google Scholar]
- Calabrese E, Baldwin L. The frequency of U-shaped dose responses in the toxicological literature. Toxicol Sci. 2001;62:330–8. doi: 10.1093/toxsci/62.2.330. [DOI] [PubMed] [Google Scholar]
- Calabrese E, Baldwin L. Hormesis: a generalizable and unifying hypothesis. Crit Rev Toxicol. 2001;31:353–424. doi: 10.1080/20014091111730. [DOI] [PubMed] [Google Scholar]
- Calabrese E, Baldwin L. Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharmacol Sci. 2001;22:285–91. doi: 10.1016/s0165-6147(00)01719-3. [DOI] [PubMed] [Google Scholar]
- Calabrese E, Staudenmayer J, Stanek E, III, Hoffmann G. Hormesis outperforms threshold model in National Cancer Institute antitumor drug screening database. Toxicol Sci. 2006;94:368–78. doi: 10.1093/toxsci/kfl098. [DOI] [PubMed] [Google Scholar]
- Junien JL, Roman FJ, Brunelle G, Pascaud X. JO1784, a novel sigma ligand, potentiates [3H]acetylcholine release from rat hippocampal slices. Eur J Pharmacol. 1991;200:343–5. doi: 10.1016/0014-2999(91)90593-f. [DOI] [PubMed] [Google Scholar]
- Waterhouse RN, Chapman J, Izard B, et al. Examination of four 123I-labeled piperidine-based sigma receptor ligands as potential melanoma imaging agents: initial studies in mouse tumor models. Nuclear Med Biol. 1996;24:587–93. doi: 10.1016/s0969-8051(97)00030-9. [DOI] [PubMed] [Google Scholar]
- van Waarde A, Buursma AR, Hospers GA, et al. Tumor imaging with 2 sigma-receptor ligands, 18F-FE-SA5845 and 11C-SA4503: a feasibility study. J Neuclear Med. 2004;45:1939–45. [PubMed] [Google Scholar]
- Kortekaas R, Maguire RP, van Waarde A, et al. Despite irreversible binding, PET tracer [11C]-SA5845 is suitable for imaging of drug competition at sigma receptors-the cases of ketamine and haloperidol. Neurochem Int. 2008;53:45–50. doi: 10.1016/j.neuint.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Stone JM, Arstad E, Erlandsson K, et al. [123I]TPCNE – a novel SPET tracer for the sigma-1 receptor: first human studies and in vivo haloperidol challenge. Synapse (NY) 2006;60:109–17. doi: 10.1002/syn.20281. [DOI] [PubMed] [Google Scholar]
- Abel T, Lattal K. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;1:180–7. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Su TP, Fujimoto M, Bonci A. The sigma-1 receptor: roles in neuronal plasticity and disease. Trends Neurosci. 2012;35:762–71. doi: 10.1016/j.tins.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudo A, Watry V, Chassot AA, et al. Inhibition of tumor cell proliferation by sigma ligands is associated with K+ Channel inhibition and p27kip1 accumulation. J Pharm Exp Therap. 2004;311:1105–14. doi: 10.1124/jpet.104.072413. [DOI] [PubMed] [Google Scholar]
- Engelhardt W, Friess K, Hartung E, et al. EEG and auditory evoked potential P300 compared with psychometric tests in assessing vigilance after benzodiazepine sedation and antagonism. Br J Anaesth. 1992;69:75–80. doi: 10.1093/bja/69.1.75. [DOI] [PubMed] [Google Scholar]
- Kulikowski JJ, McGlone FF, Kranda K, Ott H. Are the amplitudes of visual evoked potentials sensitive indices of hangover effects after repeated doses of benzodiazepines? Psychopharmacol Supplement. 1984;1:154–64. doi: 10.1007/978-3-642-69659-6_13. [DOI] [PubMed] [Google Scholar]
- Martin F, Siddle DA, Gourley M, et al. P300 and traffic scenes: the effect of temazepam. Biol Psychol. 1992;33:225–40. doi: 10.1016/0301-0511(92)90034-r. [DOI] [PubMed] [Google Scholar]
- Senda T, Mita S, Kaneda K, et al. Effect of SA4503, a novel sigma1 receptor agonist, against glutamate neurotoxicity in cultured rat retinal neurons. Eur J Pharmacol. 1998;342:105–11. doi: 10.1016/s0014-2999(97)01450-7. [DOI] [PubMed] [Google Scholar]
- Nam Y, Shin EJ, Yang BK, et al. Dextromethorphan-induced psychotoxic behaviors cause sexual dysfunction in male mice via stimulation of sigma-1 receptors. Neurochem Int. 2012;61:913–22. doi: 10.1016/j.neuint.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Kamei H, Noda Y, Nabeshima T. The psychological stress model using motor suppression. Nihon Yakurigaku Zasshi Folia Pharmacol Japonica. 1999;113:113–20. doi: 10.1254/fpj.113.113. [DOI] [PubMed] [Google Scholar]
- Roman F, Pascaud X, Vauche D, Junien JL. Evidence for a non-opioid sigma binding site in the guinea-pig myenteric plexus. Life Sci. 1988;42:2217–22. doi: 10.1016/0024-3205(88)90373-6. [DOI] [PubMed] [Google Scholar]
- Whitlock BB, Liu Y, Chang S, et al. Initial characterization and autoradiographic localization of a novel sigma/opioid binding site in immune tissues. J Neuroimmunol. 1996;67:83–96. doi: 10.1016/0165-5728(96)00041-0. [DOI] [PubMed] [Google Scholar]
- Wolfe SA, Jr, Ha BK, Whitlock BB, Saini P. Differential localization of three distinct binding sites for sigma receptor ligands in rat spleen. J Neuroimmunol. 1997;72:45–58. doi: 10.1016/s0165-5728(96)00140-3. [DOI] [PubMed] [Google Scholar]
- Chorawala M, Oza P, Shah G. Cellular and molecular pharmacology of sigma receptors. Intl J Pharm Sci Rev Res. 2011;10:45–53. [Google Scholar]
- Novakova M, Bruderova V, Sulova Z, et al. Modulation of expression of the sigma receptors in the heart of rat and mouse in normal and pathological conditions. Gen Physiol Biophys. 2007;26:110–17. [PubMed] [Google Scholar]
- Stahl SM. Antidepressant treatment of psychotic major depression: potential role of the sigma receptor. CNS Spectr. 2005;10:319–23. doi: 10.1017/s1092852900022641. [DOI] [PubMed] [Google Scholar]
- Maurice T. Improving Alzheimer's disease-related cognitive deficits with sigma1 receptor agonists. Drug News Perspect. 2002;15:617–25. doi: 10.1358/dnp.2002.15.10.740241. [DOI] [PubMed] [Google Scholar]
- Ujike H, Kuroda S, Otsuki S. sigma Receptor antagonists block the development of sensitization to cocaine. Eur J Pharmacol. 1996;296:123–8. doi: 10.1016/0014-2999(95)00693-1. [DOI] [PubMed] [Google Scholar]
- Vilner BJ, John CS, Bowen WD. Sigma-1 and sigma-2 receptors are expressed in a wide variety of human and rodent tumor cell lines. Cancer Res. 1995;55:408–13. [PubMed] [Google Scholar]
- Zhang H, Cuevas J. Sigma receptor activation blocks potassium channels and depresses neuroexcitability in rat intracardiac neurons. J Pharm Exp Therap. 2005;313:1387–96. doi: 10.1124/jpet.105.084152. [DOI] [PubMed] [Google Scholar]
- Toyohara J, Sakata M, Ishiwata K. Imaging of sigma1 receptors in the human brain using PET and [11C]SA4503. Central Nerv Syst Agents Med Chem. 2009;9:190–6. doi: 10.2174/1871524910909030190. [DOI] [PubMed] [Google Scholar]
- Mori T, Hayashi T, Su TP. Compromising sigma-1 receptors at the endoplasmic reticulum render cytotoxicity to physiologically relevant concentrations of dopamine in a nuclear factor-kappaB/Bcl-2-dependent mechanism: potential relevance to Parkinson's disease. J Pharm Exp Therap. 2012;341:663–71. doi: 10.1124/jpet.111.190868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybczynska AA, Elsinga PH, Sijbesma JW, et al. Steroid hormones affect binding of the sigma ligand 11C-SA4503 in tumour cells and tumour-bearing rats. Eur J Nuclear Med Mol Imaging. 2009;36:1167–75. doi: 10.1007/s00259-009-1076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francardo V, Bez F, Wieloch T, et al. Pharmacological stimulation of sigma-1 receptors has neurorestorative effects in experimental parkinsonism. Brain. 2014;137:1998–2014. doi: 10.1093/brain/awu107. [DOI] [PubMed] [Google Scholar]
- Gundlach A, Largent B, Snyder S. Autoradiographic localization of sigma receptor binding sites in guinea pig and rat central nervous system with (+)3H-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. J Neurosci. 1986;6:1757–70. doi: 10.1523/JNEUROSCI.06-06-01757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash DC, Zabetian CP. Sigma receptors are associated with cortical limbic areas in the primate brain. Synapse (NY) 1992;12:195–205. doi: 10.1002/syn.890120304. [DOI] [PubMed] [Google Scholar]
- Largent BL, Gundlach AL, Snyder SH. Pharmacological and autoradiographic discrimination of sigma and phencyclidine receptor binding sites in brain with (+)-[3H]SKF 10,047, (+)-[3H]-3-[3-hydroxyphenyl]-N-(1-propyl)piperidine and [3H]-1-[1-(2-thienyl)cyclohexyl]piperidine. J Pharm Exp Therap. 1986;238:739–48. [PubMed] [Google Scholar]
- Nakazawa M, Kobayashi T, Matsuno K, Mita S. Possible involvement of a sigma receptor subtype in the neck dystonia in rats. Pharmacol Biochem Behav. 1999;62:123–6. doi: 10.1016/s0091-3057(98)00146-4. [DOI] [PubMed] [Google Scholar]
- Bouchard P, Quirion R. [3H]1,3-di(2-tolyl)guanidine and [3H](+)pentazocine binding sites in the rat brain: autoradiographic visualization of the putative sigma1 and sigma2 receptor subtypes. Neuroscience. 1997;76:467–77. doi: 10.1016/s0306-4522(96)00221-7. [DOI] [PubMed] [Google Scholar]
- Inoue A, Sugita S, Shoji H, et al. Repeated haloperidol treatment decreases sigma(1) receptor binding but does not affect its mRNA levels in the guinea pig or rat brain. Eur J Pharmacol. 2000;401:307–16. doi: 10.1016/s0014-2999(00)00455-6. [DOI] [PubMed] [Google Scholar]
- Kamei H, Noda Y, Kameyama T, Nabeshima T. Role of (+)-SKF-10,047-sensitive sub-population of sigma 1 receptors in amelioration of conditioned fear stress in rats: association with mesolimbic dopaminergic systems. Eur J Pharmacol. 1997;319:165–72. doi: 10.1016/s0014-2999(96)00851-5. [DOI] [PubMed] [Google Scholar]
- Soby KK, Mikkelsen JD, Meier E, Thomsen C. Lu 28-179 labels a sigma(2)-site in rat and human brain. Neuropharmacology. 2002;43:95–100. doi: 10.1016/s0028-3908(02)00071-0. [DOI] [PubMed] [Google Scholar]
- Blaylock R. Excitotoxins. Santa Fe: Health Press; 1997. [Google Scholar]
- Greenamyre J, Porter R. Anatomy and physiology of glutamate in the CNS. Neurology. 1994;44:S7–13. [PubMed] [Google Scholar]
- Hayashi T, Su T. Sigma-1 receptor ligands: potential in the treatment of neuropsychiatric disorders. CNS drugs. 2004;18:269–84. doi: 10.2165/00023210-200418050-00001. [DOI] [PubMed] [Google Scholar]
- Palacios G, Muro A, Vela JM, et al. Immunohistochemical localization of the sigma1-receptor in oligodendrocytes in the rat central nervous system. Brain Res. 2003;961:92–9. doi: 10.1016/s0006-8993(02)03892-1. [DOI] [PubMed] [Google Scholar]
- Palacios G, Muro A, Verdu E, et al. Immunohistochemical localization of the sigma1 receptor in Schwann cells of rat sciatic nerve. Brain Res. 2004;1007:65–70. doi: 10.1016/j.brainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Mavlyutov TA, Epstein ML, Verbny YI, et al. Lack of sigma-1 receptor exacerbates ALS progression in mice. Neuroscience. 2013;240:129–34. doi: 10.1016/j.neuroscience.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banister S, Kassiou M. The therapeutic potential of sigma (sigma) receptors for the treatment of central nervous system diseases: evaluation of the evidence. Current Pharm Design. 2012;18:884–901. doi: 10.2174/138161212799436539. [DOI] [PubMed] [Google Scholar]
- Antonini V, Marrazzo A, Kleiner G, et al. Anti-amnesic and neuroprotective actions of the sigma-1 receptor agonist (−)-MR22 in rats with selective cholinergic lesion and amyloid infusion. J Alzheime Dis. 2011;24:569–86. doi: 10.3233/JAD-2011-101794. [DOI] [PubMed] [Google Scholar]
- Antonini V, Prezzavento O, Coradazzi M, et al. Anti-amnesic properties of (+/−)-PPCC, a novel sigma receptor ligand, on cognitive dysfunction induced by selective cholinergic lesion in rats. J Neurochem. 2009;109:744–54. doi: 10.1111/j.1471-4159.2009.06000.x. [DOI] [PubMed] [Google Scholar]
- Meunier J, Demeilliers B, Celerier A, Maurice T. Compensatory effect by sigma1 (sigma1) receptor stimulation during alcohol withdrawal in mice performing an object recognition task. Behav Brain Res. 2006;166:166–76. doi: 10.1016/j.bbr.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Meunier J, Maurice T. Beneficial effects of the sigma1 receptor agonists igmesine and dehydroepiandrosterone against learning impairments in rats prenatally exposed to cocaine. Neurotoxicol Teratol. 2004;26:783–97. doi: 10.1016/j.ntt.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Yang R, Chen L, Wang H, et al. Anti-amnesic effect of neurosteroid PREGS in Abeta25-35-injected mice through sigma1 receptor- and alpha7nAChR-mediated neuroprotection. Neuropharmacology. 2012;63:1042–50. doi: 10.1016/j.neuropharm.2012.07.035. [DOI] [PubMed] [Google Scholar]
- Matsuno K. Anti-amnesic effects of sigma (sigma)-receptor agonists. Nihon Yakurigaku Zasshi Folia Pharmacol Japonica. 1999;114:25–33. doi: 10.1254/fpj.114.25. [DOI] [PubMed] [Google Scholar]
- Li PK, Rhodes ME, Jagannathan S, Johnson DA. Reversal of scopolamine induced amnesia in rats by the steroid sulfatase inhibitor estrone-3-O-sulfamate. Brain Res Cognitive Brain Res. 1995;2:251–4. doi: 10.1016/0926-6410(95)90016-0. [DOI] [PubMed] [Google Scholar]
- Marrazzo A, Caraci F, Salinaro ET, et al. Neuroprotective effects of sigma-1 receptor agonists against beta-amyloid-induced toxicity. Neuroreport. 2005;16:1223–6. doi: 10.1097/00001756-200508010-00018. [DOI] [PubMed] [Google Scholar]
- Maurice T, Martin-Fardon R, Romieu P, Matsumoto RR. Sigma(1) (sigma(1)) receptor antagonists represent a new strategy against cocaine addiction and toxicity. Neurosci Biobehav Rev. 2002;26:499–527. doi: 10.1016/s0149-7634(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Romieu P, Lucas M, Maurice T. Sigma1 receptor ligands and related neuroactive steroids interfere with the cocaine-induced state of memory. Neuropsychopharmacology. 2006;31:1431–43. doi: 10.1038/sj.npp.1300885. [DOI] [PubMed] [Google Scholar]
- Tan F, Guio-Aguilar PL, Downes C, et al. The sigma 1 receptor agonist 4-PPBP elicits ERK1/2 phosphorylation in primary neurons: a possible mechanism of neuroprotective action. Neuropharmacology. 2010;59:416–24. doi: 10.1016/j.neuropharm.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Leonardo CC, Hall AA, Collier LA, et al. Administration of a Sigma Receptor Agonist Delays MCAO-Induced Neurodegeneration and White Matter Injury. Translational Stroke Research. 2010;1:135–45. doi: 10.1007/s12975-009-0005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schetz JA, Perez E, Liu R, et al. A prototypical Sigma-1 receptor antagonist protects against brain ischemia. Brain Res. 2007;1181:1–9. doi: 10.1016/j.brainres.2007.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J. Does stress damage the brain? Biol Psychiatry. 1999;45:797–805. doi: 10.1016/s0006-3223(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–18. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–43. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, et al. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA. 1996;93:3908–13. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol Psychiatry. 2000;48:755–65. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–10. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar C. Neurotrophins and depression. Trends Pharmacol Sci. 1999;20:59–61. doi: 10.1016/s0165-6147(99)01309-7. [DOI] [PubMed] [Google Scholar]
- Altar C, Whitehead R, Chen R, et al. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol Psychiatry. 2003;54:703–9. doi: 10.1016/s0006-3223(03)00073-8. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–47. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterstrom TS, Pei Q, Grahame-Smith DG. Repeated electroconvulsive shock extends the duration of enhanced gene expression for BDNF in rat brain compared with a single administration. Brain Res Molecular Brain Res. 1998;57:106–10. doi: 10.1016/s0169-328x(98)00077-1. [DOI] [PubMed] [Google Scholar]
- Colino A, Halliwell J. Differential modulation of three separate K-conductances in hippocampal CA1 neurons by serotonin. Nature. 1987;328:73–7. doi: 10.1038/328073a0. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, et al. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–61. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacol Biochem Behav. 1997;56:131–7. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- Ovalle S, Andreu F, Perez MP, et al. Effect of the novel sigma1 receptor ligand and putative atypical antipsychotic E-5842 on BDNF mRNA expression in the rat brain. Neuroreport. 2002;13:2345–8. doi: 10.1097/00001756-200212030-00035. [DOI] [PubMed] [Google Scholar]
- Abe K, Saito H. Epidermal growth factor selectively enhances NMDA receptor-mediated increase of intracellular Ca2+ concentration in rat hippocampal neurons. Brain Res. 1992;587:102–8. doi: 10.1016/0006-8993(92)91433-f. [DOI] [PubMed] [Google Scholar]
- Xu QX, Li EM, Zhang YF, et al. Overexpression of sigma1 receptor and its positive associations with pathologic TNM classification in esophageal squamous cell carcinoma. J Histochem Cytochem. 2012;60:457–66. doi: 10.1369/0022155412443542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda Y, Kamei H, Nabeshima T. Sigma-receptor ligands and anti-stress actions. Nihon Yakurigaku Zasshi Folia Pharmacol Japonica. 1999;114:43–9. doi: 10.1254/fpj.114.43. [DOI] [PubMed] [Google Scholar]
- Skuza G. Potential antidepressant activity of sigma ligands. Pol J Pharmacol. 2003;55:923–34. [PubMed] [Google Scholar]
- Connolly K, Thase M. Emerging drugs for major depressive disorder. Expert Opinionemerging drugs. 2012;17:105–26. doi: 10.1517/14728214.2012.660146. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Ishiwata K. Sigma receptor ligands: possible application as therapeutic drugs and as radiopharmaceuticals. Current Pharm Design. 2006;12:3857–76. doi: 10.2174/138161206778559614. [DOI] [PubMed] [Google Scholar]
- Delaunois A, De Ron P, Detrait E, Guyaux M. Inhibitory effects of sigma-1 ligands on handling-induced tachycardia in conscious tethered rats. Fundam Clin Pharmacol. 2013;27:354–63. doi: 10.1111/j.1472-8206.2012.01042.x. [DOI] [PubMed] [Google Scholar]
- Ito K, Hirooka Y, Matsukawa R, et al. Decreased brain sigma-1 receptor contributes to the relationship between heart failure and depression. Cardiovasc Res. 2012;93:33–40. doi: 10.1093/cvr/cvr255. [DOI] [PubMed] [Google Scholar]
- Fond G. Using second-generation antidepressants (SGA) in daily practice. Ann Med Psychol. 2013;171:198–203. [Google Scholar]
- Hindmarch I, Hashimoto K. Cognition and depression: the effects of fluvoxamine, a sigma-1 receptor agonist, reconsidered. Human Psychopharmacol. 2010;25:193–200. doi: 10.1002/hup.1106. [DOI] [PubMed] [Google Scholar]
- Matthews JD, Bottonari KA, Polania LM, et al. An open study of olanzapine and fluoxetine for psychotic major depressive disorder: interim analyses. J Clin Psychiatry. 2002;63:1164–70. doi: 10.4088/jcp.v63n1212. [DOI] [PubMed] [Google Scholar]
- Beck S, Halloran P. Imipramine alters beta-adrenergic, but not serotonergic, mediated responses in rat hippocampal pyramidal cells. Brain Res. 1989;504:72–81. doi: 10.1016/0006-8993(89)91599-0. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Nishikawa T, Umino A, Takahashi K. p-chlorophenylalanine-reversible reduction of sigma binding sites by chronic imipramine treatment in rat brain. Eur J Pharmacol. 1993;237:117–26. doi: 10.1016/0014-2999(93)90100-v. [DOI] [PubMed] [Google Scholar]
- Kinsora JJJ, Corbin AE, Snider B, et al. Effects of igmesine in preclinical antidepressant tests [abstract] Soc Neurocsi. 1998;24:744. [Google Scholar]
- Ukai M, Maeda H, Nanya Y, et al. Beneficial effects of acute and repeated administrations of sigma receptor agonists on behavioral despair in mice exposed to tail suspension. Pharmacol Biochem Behav. 1998;61:247–52. doi: 10.1016/s0091-3057(98)00093-8. [DOI] [PubMed] [Google Scholar]
- Craven R, Grahame-Smith D, Newberry N. WAY-100635 and GR127935: effects on 5-hydroxytryptamine-containing neurones. Eur J Pharmacol. 1994;271:R1–3. doi: 10.1016/0014-2999(94)90289-5. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Yamada S, Nakai M, et al. The rapid antidepressant-like effect of OPC-14523 in the forced swimming test is independent of action on 5-HT nerve terminals [abstract] Soc Neurocsi. 2000;26:387.16. [Google Scholar]
- Yamada S, Uwahodo Y, Tottori K, et al. Role of sigma and 5-HT1A receptors in the forced swimming test: supporting the mechanism of action of OPC-14523 [abstract] Soc Neurocsi. 2000;26:2326. [Google Scholar]
- Tsao LI, Su TP. Naloxone-sensitive, haloperidol-sensitive, [3H](+)SKF-10047-binding protein partially purified from rat liver and rat brain membranes: an opioid/sigma receptor? Synapse (NY) 1997;25:117–24. doi: 10.1002/(SICI)1098-2396(199702)25:2<117::AID-SYN2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Arnt J, Costall B, et al. The selective sigma2-ligand Lu 28-179 has potent anxiolytic-like effects in rodents. J Pharm Exp Therap. 1997;283:1323–32. [PubMed] [Google Scholar]
- Sanchez C, Papp M. The selective sigma2 ligand Lu 28-179 has an antidepressant-like profile in the rat chronic mild stress model of depression. Behav Pharmacol. 2000;11:117–24. doi: 10.1097/00008877-200004000-00003. [DOI] [PubMed] [Google Scholar]
- Skuza G, Rogoz Z. Antidepressant-like effect of PRE-084, a selective sigma1 receptor agonist, in Albino Swiss and C57BL/6J mice. Pharmacol Rep. 2009;61:1179–83. doi: 10.1016/s1734-1140(09)70181-1. [DOI] [PubMed] [Google Scholar]
- Manji HK, Quiroz JA, Sporn J, et al. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol Psychiatry. 2003;53:707–2. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- Saeki T, Nakamura M, Hirai N, et al. Localized potentiation of sleep slow-wave activity induced by prefrontal repetitive transcranial magnetic stimulation in patients with a major depressive episode. Brain Stimulation. 2013;6:390–6. doi: 10.1016/j.brs.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Zhang CL, Feng ZJ, Liu Y, et al. Methylphenidate enhances NMDA-receptor response in medial prefrontal cortex via sigma-1 receptor: a novel mechanism for methylphenidate action. PLoS One. 2012;7:e51910. doi: 10.1371/journal.pone.0051910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes N, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bhuiyan M, Tagashira H, Fukunaga K. Crucial interactions between selective serotonin uptake inhibitors and sigma-1 receptor in heart failure. J Pharmacol Sci. 2013;121:177–84. doi: 10.1254/jphs.12r13cp. [DOI] [PubMed] [Google Scholar]
- Lopez-Munoz F, Alamo C. Neurobiological background for the development of new drugs in schizophrenia. Clinl Neuropharmacol. 2011;34:111–26. doi: 10.1097/WNF.0b013e318215c2f7. [DOI] [PubMed] [Google Scholar]
- Uchida N, Ujike H, Nakata K, et al. No association between the sigma receptor type 1 gene and schizophrenia: results of analysis and meta-analysis of case-control studies. BMC Psychiatry. 2003;3:13. doi: 10.1186/1471-244X-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Horwath E, Weissman MM. The validity of major depression with psychotic features based on a community study. Arch Gen Psychiatry. 1991;48:1075–81. doi: 10.1001/archpsyc.1991.01810360039006. [DOI] [PubMed] [Google Scholar]
- Thakur M, Hays J, Krishnan KR. Clinical, demographic and social characteristics of psychotic depression. Psychiatry Res. 1999;86:99–106. doi: 10.1016/s0165-1781(99)00030-x. [DOI] [PubMed] [Google Scholar]
- Flint A, Rifat S. The treatment of psychotic depression in later life: a comparison of pharmacotherapy and ECT. Int J Geriatr Psychiatry. 1998;13:23–8. [PubMed] [Google Scholar]
- Jeste DV, Heaton SC, Paulsen JS, et al. Clinical and neuropsychological comparison of psychotic depression with nonpsychotic depression and schizophrenia. Am J Psychiatry. 1996;153:490–6. doi: 10.1176/ajp.153.4.490. [DOI] [PubMed] [Google Scholar]
- Iwanami A, Oyamada S, Shirayama Y, Kamijima K. Algorithms for the pharmacotherapy psychotic depression. Psychiatry Clin Neurosci. 1999;53:S45–8. [PubMed] [Google Scholar]
- Schatzberg AF. New approaches to managing psychotic depression. J Clin Psychiatry. 2003;64:19–23. [PubMed] [Google Scholar]
- Schatzberg AF, Rothschild AJ, Bond TC, Cole JO. The DST in psychotic depression: diagnostic and pathophysiologic implications. Psychopharmacol Bull. 1984;20:362–4. [PubMed] [Google Scholar]
- Vythilingam M, Chen J, Bremner JD, et al. Psychotic depression and mortality. Am J Psychiatry. 2003;160:574–6. doi: 10.1176/appi.ajp.160.3.574. [DOI] [PubMed] [Google Scholar]
- Hamner M, Gold P. Plasma dopamine beta-hydroxylase activity in psychotic and non-psychotic post-traumatic stress disorder. Psychiatry Res. 1998;77:175–81. doi: 10.1016/s0165-1781(98)00002-x. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Davis JM. DST studies in psychotic depression: a meta-analysis. Am J Psychiatry. 1997;154:1497–503. doi: 10.1176/ajp.154.11.1497. [DOI] [PubMed] [Google Scholar]
- Miodownik C, Lerner V. Risperidone in the treatment of psychotic depression. Clin Neuropharmacol. 2000;23:335–7. doi: 10.1097/00002826-200011000-00007. [DOI] [PubMed] [Google Scholar]
- Mjellem N, Lund A, Hole K. Reduction of NMDA-induced behaviour after acute and chronic administration of desipramine in mice. Neuropharmacology. 1993;32:591–5. doi: 10.1016/0028-3908(93)90055-8. [DOI] [PubMed] [Google Scholar]
- Keck PE, Jr, McElroy SL, Strakowski SM, Soutullo CA. Antipsychotics in the treatment of mood disorders and risk of tardive dyskinesia. J Clin Psychiatry. 2000;61:33–8. [PubMed] [Google Scholar]
- Hirschfeld R. Efficacy of SSRIs and newer antidepressants in severe depression: comparison with TCAs. J Clin Psychiatry. 1999;60:326–35. doi: 10.4088/jcp.v60n0511. [DOI] [PubMed] [Google Scholar]
- Masan PS. Atypical antipsychotics in the treatment of affective symptoms: a review. Ann Clin Psychiatry. 2004;16:3–13. doi: 10.1080/10401230490281410. [DOI] [PubMed] [Google Scholar]
- Bel N, Artigas F. Chronic treatment with fluvoxamine increases extracellular serotonin in frontal cortex but not in raphe nuclei. Synapse (NY) 1993;15:243–45. doi: 10.1002/syn.890150310. [DOI] [PubMed] [Google Scholar]
- Gatti F, Bellini L, Gasperini M, Perez J, et al. Fluvoxamine alone in the treatment of delusional depression. Am J Psychiatry. 1996;153:414–16. doi: 10.1176/ajp.153.3.414. [DOI] [PubMed] [Google Scholar]
- Zanardi R, Franchini L, Gasperini M, et al. Double-blind controlled trial of sertraline versus paroxetine in the treatment of delusional depression. Am J Psychiatry. 1996;153:1631–3. doi: 10.1176/ajp.153.12.1631. [DOI] [PubMed] [Google Scholar]
- Zanardi R, Franchini L, Gasperini M, et al. Long-term treatment of psychotic (delusional) depression with fluvoxamine: an open pilot study. Int Clin Psychopharmacol. 1997;12:195–7. doi: 10.1097/00004850-199707000-00002. [DOI] [PubMed] [Google Scholar]
- Zanardi R, Franchini L, Serretti A, et al. Venlafaxine versus fluvoxamine in the treatment of delusional depression: a pilot double-blind controlled study. J Clin Psychiatry. 2000;61:26–9. doi: 10.4088/jcp.v61n0107. [DOI] [PubMed] [Google Scholar]
- Meyers BS, Alexopoulos GS, Kakuma T, et al. Decreased dopamine beta-hydroxylase activity in unipolar geriatric delusional depression. Biol Psychiatry. 1999;45:448–52. doi: 10.1016/s0006-3223(98)00085-7. [DOI] [PubMed] [Google Scholar]
- Duval F, Mokrani M, Crocq M, et al. Dopaminergic function and the cortisol response to dexamethasone in psychotic depression. Progr Neuro-psychopharmacol Biol Psychiatry. 2000;24:207–25. doi: 10.1016/s0278-5846(99)00098-6. [DOI] [PubMed] [Google Scholar]
- Lykouras L, Markianos M, Hatzimanolis J, et al. Prolactin secretion in response to haloperidol challenge in delusional (psychotic) and non-delusional depression. Eur Psychiatry. 2000;15:480–2. doi: 10.1016/s0924-9338(00)00516-2. [DOI] [PubMed] [Google Scholar]
- Belanoff J, Flores B, Kalezhan M, et al. Rapid reversal of psychotic depression using mifepristone. J Clin Psychopharmacol. 2001;21:516–21. doi: 10.1097/00004714-200110000-00009. [DOI] [PubMed] [Google Scholar]
- Chu J, Matthias D, Belanoff J, et al. Successful long-term treatment of refractory Cushing's disease with high-dose mifepristone (RU 486) J clin Endocrinol Metab. 2001;86:3568–73. doi: 10.1210/jcem.86.8.7740. [DOI] [PubMed] [Google Scholar]
- Simpson GM, El Sheshai A, Loza N, et al. An 8-week open-label trial of a 6-day course of mifepristone for the treatment of psychotic depression. J Clin Psychiatry. 2005;66:598–602. doi: 10.4088/jcp.v66n0509. [DOI] [PubMed] [Google Scholar]
- Simpson GM, El Sheshai A, Rady A, et al. Sertraline as monotherapy in the treatment of psychotic and nonpsychotic depression. J Clin Psychiatry. 2003;64:959–65. doi: 10.4088/jcp.v64n0817. [DOI] [PubMed] [Google Scholar]
- Kameyama T, Nagasaka M. Effects of apomorphine and diazepam on a quickly learned conditioned suppression in rats. Pharmacol Biochem Behav. 1982;17:59–63. doi: 10.1016/0091-3057(82)90263-5. [DOI] [PubMed] [Google Scholar]
- Borison R, Diamond B, Dren A. Does sigma receptor antagonism predict clinical antipsychotic efficacy? Psychopharmacol Bull. 1991;27:103–6. [PubMed] [Google Scholar]
- Gewirtz G, Gorman J, Volavka J, et al. BMY 14802, a sigma receptor ligand for the treatment of schizophrenia. Neuropsychopharmacology. 1994;10:37–40. doi: 10.1038/npp.1994.5. [DOI] [PubMed] [Google Scholar]
- Frieboes R, Murck H, Wiedemann K, et al. Open clinical trial on the sigma ligand panamesine in patients with schizophrenia. Psychopharmacology. 1997;132:82–8. doi: 10.1007/s002130050323. [DOI] [PubMed] [Google Scholar]
- Modell S, Naber D, Holzbach R. Efficacy and safety of an opiate sigma-receptor antagonist (SL 82.0715) in schizophrenic patients with negative symptoms: an open dose-range study. Pharmacopsychiatry. 1996;29:63–6. doi: 10.1055/s-2007-979546. [DOI] [PubMed] [Google Scholar]
- Muller MJ, Grunder G, Wetzel H, et al. Antipsychotic effects and tolerability of the sigma ligand EMD 57445 (panamesine) and its metabolites in acute schizophrenia: an open clinical trial. Psychiatry Res. 1999;89:275–80. doi: 10.1016/s0165-1781(99)00100-6. [DOI] [PubMed] [Google Scholar]
- Hirano K, Kimura R, Sugimoto Y, et al. Relationship between brain serotonin transporter binding, plasma concentration and behavioural effect of selective serotonin reuptake inhibitors. Br J Pharmacol. 2005;144:695–702. doi: 10.1038/sj.bjp.0706108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin L, Mellon S. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci USA. 1999;96:13512–17. doi: 10.1073/pnas.96.23.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, et al. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci USA. 1998;95:3239–44. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S, Mick S, Dilworth V, et al. Sigma receptors modulate the hypothalamic-pituitary-adrenal (HPA) axis centrally: evidence for a functional interaction with NMDA receptors, in vivo. Neuropharmacology. 1990;29:299–303. doi: 10.1016/0028-3908(90)90017-l. [DOI] [PubMed] [Google Scholar]
- Iyengar S, Wood PL, Mick SJ, et al. +) 3-[3-hydroxyphenyl-N-(1-propyl) piperidine] selectively differentiates effects of sigma ligands on neurochemical pathways modulated by sigma receptors: evidence for subtypes, in vivo. Neuropharmacology. 1991;30:915–22. doi: 10.1016/0028-3908(91)90127-w. [DOI] [PubMed] [Google Scholar]
- Zamanillo D, Andreu F, Ovalle S, et al. Up-regulation of sigma(1) receptor mRNA in rat brain by a putative atypical antipsychotic and sigma receptor ligand. Neurosci Lett. 2000;282:169–72. doi: 10.1016/s0304-3940(00)00884-3. [DOI] [PubMed] [Google Scholar]
- Cervo L, Samanin R. Effects of dopaminergic and glutamatergic receptor antagonists on the acquisition and expression of cocaine conditioning place preference. Brain Res. 1995;673:242–50. doi: 10.1016/0006-8993(94)01420-m. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR. Targeting sigma receptors: novel medication development for drug abuse and addiction. Expert review of clinical pharmacology. 2009;2:351–8. doi: 10.1586/ecp.09.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Wang C. Role of intrahippocampal sigma receptor in inhibiting seizure by electroacupuncture in rats. Med Acupunct. 2010;22:19–24. [Google Scholar]
- Borowicz K, Kleinrok Z, Czuczwar S. Influence of 3-PPP, a sigma receptor ligand, on the anticonvulsive action of conventional antiepileptic drugs. Psychopharmacol Res. 1999;40:509–16. doi: 10.1006/phrs.1999.0548. [DOI] [PubMed] [Google Scholar]
- Almansa C, Vela J. Selective sigma-1 receptor antagonists for the treatment of pain. Future Med Chem. 2014;6:1179–99. doi: 10.4155/fmc.14.54. [DOI] [PubMed] [Google Scholar]
- Corbera J, Vano D, Martinez D, et al. A medicinal-chemistry-guided approach to selective and druglike sigma 1 ligands. ChemMedChem. 2006;1:140–54. doi: 10.1002/cmdc.200500034. [DOI] [PubMed] [Google Scholar]
- Gilmore D, Liu Y, Matsumoto R. Review of the pharmacological and clinical profile of rimcazole. CNS Drug Rev. 2004;10:1–22. doi: 10.1111/j.1527-3458.2004.tb00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggi AS, Singh N. Therapeutic targets for the management of peripheral nerve injury-induced neuropathic pain. CNS Neurol Disorders Drug Targets. 2011;10:589–609. doi: 10.2174/187152711796235041. [DOI] [PubMed] [Google Scholar]
- Kwon YB, Jeong YC, Kwon JK, et al. The Antinociceptive Effect of Sigma-1 Receptor Antagonist, BD1047, in a Capsaicin Induced Headache Model in Rats. Korean J Physiol Pharmacol. 2009;13:425–9. doi: 10.4196/kjpp.2009.13.6.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezzavento O, Parenti C, Marrazzo A, et al. A new sigma ligand, (+/−)-PPCC, antagonizes kappa opioid receptor-mediated antinociceptive effect. Life Sci. 2008;82:549–53. doi: 10.1016/j.lfs.2007.11.032. [DOI] [PubMed] [Google Scholar]
- Sugai N, Yajima C, Chinzei M, et al. Postoperative pain relief by patient controlled analgesia using intravenous pentazocine. (Masui) Jpn J Anesthesiol. 1995;44:216–20. [PubMed] [Google Scholar]
- Wu HE, Hong JS, Tseng LF. Stereoselective action of (+)-morphine over (−)-morphine in attenuating the (−)-morphine-produced antinociception via the naloxone-sensitive sigma receptor in the mouse. Eur J Pharmacol. 1007;571:145–51. doi: 10.1016/j.ejphar.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MA, Bradshaw S, Chang AH, et al. Potentiation of opioid analgesia in dopamine2 receptor knock-out mice: evidence for a tonically active anti-opioid system. J Neurosci. 2001;21:7788–92. doi: 10.1523/JNEUROSCI.21-19-07788.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cendan C, Pujalte J, Portillo-Salido E, et al. Formalin-induced pain is reduced in sigma(1) receptor knockout mice. Eur J Pharmacol. 2005;511:73–4. doi: 10.1016/j.ejphar.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Kest B, Mogil JS, Sternberg WF, et al. 1,3-Di-o-tolylguanidine (DTG) differentially affects acute and tonic formalin pain: antagonism by rimcazole. Pharmacol Biochem Behav. 1995;52:175–8. doi: 10.1016/0091-3057(95)00085-b. [DOI] [PubMed] [Google Scholar]
- Kest B, Mogil JS, Sternberg WF, et al. Antinociception following 1,3,-di-o-tolylguanidine, a selective sigma receptor ligand. Pharmacol Biochem Behav. 1995;50:587–92. doi: 10.1016/0091-3057(94)00346-7. [DOI] [PubMed] [Google Scholar]
- Pyun K, Son JS, Kwon YB. Chronic activation of sigma-1 receptor evokes nociceptive activation of trigeminal nucleus caudalis in rats. Pharmacol Biochem Behav. 2014;124c:278–83. doi: 10.1016/j.pbb.2014.06.023. [DOI] [PubMed] [Google Scholar]
- Anderson T, Andrew R. Spreading depression: imaging and blockade in the rat neocortical brain slice. J Neurophysiol. 2002;88:2713–25. doi: 10.1152/jn.00321.2002. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cano R, Merlos M, Baeyens J, Cendan C. Sigma1 receptors are involved in the visceral pain induced by intracolonic administration of capsaicin in mice. Anesthesiology. 2013;118:691–700. doi: 10.1097/ALN.0b013e318280a60a. [DOI] [PubMed] [Google Scholar]
- Bujalska M. Effect of nonselective and selective opioid receptors antagonists on antinociceptive action of acetaminophen [part III] Pol J Pharmacol. 2004;56:539–45. [PubMed] [Google Scholar]
- Terashvili M, Wu HE, Moore RM, et al. +)-Morphine and (−)-morphine stereoselectively attenuate the (−)-morphine-produced tail-flick inhibition via the naloxone-sensitive sigma receptor in the ventral periaqueductal gray of the rat. Eur J Pharmacol. 2007;571:1–7. doi: 10.1016/j.ejphar.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung AS, Yaksh TL. In vivo evidence for multiple opiate receptors mediating analgesia in the rat spinal cord. Brain Res. 1982;247:75–83. doi: 10.1016/0006-8993(82)91029-0. [DOI] [PubMed] [Google Scholar]
- Chow L, Huang E, Ho S, Lee T, Tao P. Dextromethorphan potentiates morphine antinociception at the spinal level in rats. Can J Anaest. 2004;51:905–10. doi: 10.1007/BF03018888. [DOI] [PubMed] [Google Scholar]
- Yoon SY, Roh DH, Seo HS, et al. An increase in spinal dehydroepiandrosterone sulfate (DHEAS) enhances NMDA-induced pain via phosphorylation of the NR1 subunit in mice: involvement of the sigma-1 receptor. Neuropharmacology. 2010;59:460–7. doi: 10.1016/j.neuropharm.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Robson MJ, Noorbakhsh B, Seminerio MJ, Matsumoto RR. Sigma-1 receptors: potential targets for the treatment of substance abuse. Current Pharm Design. 2012;18:902–19. doi: 10.2174/138161212799436601. [DOI] [PubMed] [Google Scholar]
- Nuwayhid SJ, Werling LL. Sigma2 (sigma2) receptors as a target for cocaine action in the rat striatum. Eur J Pharmacol. 2006;535:98–103. doi: 10.1016/j.ejphar.2005.12.077. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Liu Y, Lerner M, et al. Sigma receptors: potential medications development target for anti-cocaine agents. Eur J Pharmacol. 2003;469:1–12. doi: 10.1016/s0014-2999(03)01723-0. [DOI] [PubMed] [Google Scholar]
- Nguyen EC, McCracken KA, Liu Y, et al. Involvement of sigma (sigma) receptors in the acute actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacology. 2005;49:638–45. doi: 10.1016/j.neuropharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen GD, Lerner MR, et al. Cocaine up-regulates Fra-2 and sigma-1 receptor gene and protein expression in brain regions involved in addiction and reward. J Pharm Exp Therap. 2005;314:770–9. doi: 10.1124/jpet.105.084525. [DOI] [PubMed] [Google Scholar]
- Romieu P, Martin-Fardon R, Bowen WD, Maurice T. Sigma 1 receptor-related neuroactive steroids modulate cocaine-induced reward. J Neurosci. 2003;23:3572–6. doi: 10.1523/JNEUROSCI.23-09-03572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu P, Martin-Fardon R, Maurice T. Involvement of the sigma1 receptor in the cocaine-induced conditioned place preference. Neuroreport. 2000;11:2885–8. doi: 10.1097/00001756-200009110-00011. [DOI] [PubMed] [Google Scholar]
- Romieu P, Phan VL, Martin-Fardon R, Maurice T. Involvement of the sigma(1) receptor in cocaine-induced conditioned place preference: possible dependence on dopamine uptake blockade. Neuropsychopharmacology. 2002;26:444–55. doi: 10.1016/S0893-133X(01)00391-8. [DOI] [PubMed] [Google Scholar]
- Stefanski R, Justinova Z, Hayashi T, et al. Sigma1 receptor upregulation after chronic methamphetamine self-administration in rats: a study with yoked controls. Psychopharmacology. 2004;175:68–75. doi: 10.1007/s00213-004-1779-9. [DOI] [PubMed] [Google Scholar]
- McCracken KA, Bowen WD, Matsumoto RR. Novel sigma receptor ligands attenuate the locomotor stimulatory effects of cocaine. Eur J Pharmacol. 1999;365:35–8. doi: 10.1016/s0014-2999(98)00876-0. [DOI] [PubMed] [Google Scholar]
- Maurice T, Romieu P. Involvement of the sigma1 receptor in the appetitive effects of cocaine. Pharmacopsychiatry. 2001;37:S198–207. doi: 10.1055/s-2004-832678. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Schoepp DD. The metabotropic excitatory amino acid receptor agonist 1S,3R-ACPD selectively potentiates N-methyl-d-aspartate-induced brain injury. Eur J Pharmacol. 1992;215:353–4. doi: 10.1016/0014-2999(92)90058-c. [DOI] [PubMed] [Google Scholar]
- Sacaan AI, Schoepp DD. Activation of hippocampal metabotropic excitatory amino acid receptors leads to seizures and neuronal damage. Neurosci Lett. 1992;139:77–82. doi: 10.1016/0304-3940(92)90862-2. [DOI] [PubMed] [Google Scholar]
- Meldrum B. Protection against ischaemic neuronal damage by drugs acting on excitatory neurotransmission. Cerebrovasc Brain Metab Rev. 1990;2:27–57. [PubMed] [Google Scholar]
- Castelli M, Ingianni A, Stefanini E, Gessa G. Distribution of GABA(B) receptor mRNAs in the rat brain and peripheral organs. Life Sci. 1999;64:1321–8. doi: 10.1016/s0024-3205(99)00067-3. [DOI] [PubMed] [Google Scholar]
- Dierkes P, Hochstrate P, Schlue W. Distribution and functional properties of glutamate receptors in the leech central nervous system. J Neurophysiol. 1996;75:2312–21. doi: 10.1152/jn.1996.75.6.2312. [DOI] [PubMed] [Google Scholar]
- Spillson AB, Russell JW. Metabotropic glutamate receptor regulation of neuronal cell death. Exp Neurol. 2003;184:S97–105. doi: 10.1016/j.expneurol.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Yamamoto H, Atta M, et al. Chloroquine inhibits glutamate-induced death of a neuronal cell line by reducing reactive oxygen species through sigma-1 receptor. J Neurochem. 2011;119:839–47. doi: 10.1111/j.1471-4159.2011.07464.x. [DOI] [PubMed] [Google Scholar]
- Pal A, Fontanilla D, Gopalakrishnan A, et al. The sigma-1 receptor protects against cellular oxidative stress and activates antioxidant response elements. Eur J Pharmacol. 2012;682:12–20. doi: 10.1016/j.ejphar.2012.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichberg VI. Glial glutamate receptors: likely actors in brain signaling. FASEB J. 1991;5:3086–91. doi: 10.1096/fasebj.5.15.1660422. [DOI] [PubMed] [Google Scholar]
- Lai AY, Dhami KS, Todd KG. Moving past the “neuroncentric” perspective: a role for glia in neuropsychiatric disorders. J Psychiatry Neurosci. 2009;34:173–4. [PMC free article] [PubMed] [Google Scholar]
- Rosenberg PA, Aizenman E. Hundred-fold increase in neuronal vulnerability to glutamate toxicity in astrocyte-poor cultures of rat cerebral cortex. Neurosci Lett. 1989;103:162–8. doi: 10.1016/0304-3940(89)90569-7. [DOI] [PubMed] [Google Scholar]
- Lopez-Bayghen E, Cruz-Solis I, Corona M, et al. Glutamate-induced octamer DNA binding and transcriptional control in cultured radial glia cells. J Neurochem. 2006;98:851–9. doi: 10.1111/j.1471-4159.2006.03929.x. [DOI] [PubMed] [Google Scholar]
- Kaminska B, Kaczmarek L, Zangenehpour S, Chaudhuri A. Rapid phosphorylation of Elk-1 transcription factor and activation of MAP kinase signal transduction pathways in response to visual stimulation. Mol Cell Neurosci. 1999;13:405–14. doi: 10.1006/mcne.1999.0757. [DOI] [PubMed] [Google Scholar]
- Crottes D, Martial S, Rapetti-Mauss R, et al. Sig1R protein regulates hERG channel expression through a post-translational mechanism in leukemic cells. J Biol Chem. 2011;286:27947–58. doi: 10.1074/jbc.M111.226738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzynski H, Nikolski V, Sambelashvili A, Greener I, Yamamoto M, Boyett M, Efimov I. Site of origin and molecular substrate of atrioventricular junctional rhythm in the rabbit heart. Circulation Res. 2003;93:1102–10. doi: 10.1161/01.RES.0000101913.95604.B9. [DOI] [PubMed] [Google Scholar]
- Gorza L, Vitadello M. Distribution of conduction system fibers in the developing and adult rabbit heart revealed by an antineurofilament antibody. Circulation Res. 1989;65:360–9. doi: 10.1161/01.res.65.2.360. [DOI] [PubMed] [Google Scholar]
- Widran J, Lev M. The dissection of the atrioventricular node, bundle and bundle branches in the human heart. Circulation. 1951;4:863–7. doi: 10.1161/01.cir.4.6.863. [DOI] [PubMed] [Google Scholar]
- Monassier L, Bousquet P. Sigma receptors: from discovery to highlights of their implications in the cardiovascular system. Fundam Clin Pharmacol. 2002;16:1–8. doi: 10.1046/j.1472-8206.2002.00063.x. [DOI] [PubMed] [Google Scholar]
- Monassier L, Manoury B, Bellocq C, et al. sigma(2)-receptor ligand-mediated inhibition of inwardly rectifying K(+) channels in the heart. J Pharm Exp Therap. 2007;322:341–50. doi: 10.1124/jpet.107.122044. [DOI] [PubMed] [Google Scholar]
- Bhuiyan M, Tagashira H, Shioda N, Fukunaga K. Targeting sigma-1 receptor with fluvoxamine ameliorates pressure-overload-induced hypertrophy and dysfunctions. Expert Opin Therap Targets. 2010;14:1009–22. doi: 10.1517/14728222.2010.509348. [DOI] [PubMed] [Google Scholar]
- Mueller RW, Gill SS, Pulido OM. The monkey (Macaca fascicularis) heart neural structures and conducting system: an immunochemical study of selected neural biomarkers and glutamate receptors. Toxicol Pathol. 2003;31:227–34. doi: 10.1080/01926230390183724. [DOI] [PubMed] [Google Scholar]
- Ito K, Hirooka Y, Sunagawa K. Brain sigma-1 receptor stimulation improves mental disorder and cardiac function in mice with myocardial infarction. J Cardiovasc Pharmacol. 2013;62:222–8. doi: 10.1097/FJC.0b013e3182970b15. [DOI] [PubMed] [Google Scholar]
- Gao X, Yao J, He Y, et al. Sigma-1 receptor agonists directly inhibit Nav1.2/1.4 channels. PLoS One. 2012;7:e49384. doi: 10.1371/journal.pone.0049384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen M, Fontanilla D, Mavlyutov T, et al. Antagonist action of progesterone at sigma-receptors in the modulation of voltage-gated sodium channels. Am J Physiol Cell Physiol. 2011;300:C328–37. doi: 10.1152/ajpcell.00383.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ela C, Barg J, Vogel Z, et al. Sigma receptor ligands modulate contractility, Ca++ influx and beating rate in cultured cardiac myocytes. J Pharm Exp Therap. 1994;269:1300–9. [PubMed] [Google Scholar]
- Ela C, Hasin Y, Eilam Y. Apparent desensitization of a sigma receptor sub-population in neonatal rat cardiac myocytes by pre-treatment with sigma receptor ligands. Eur J Pharmacol. 1996;295:275–80. doi: 10.1016/0014-2999(95)00750-4. [DOI] [PubMed] [Google Scholar]
- Maslov LN, Lishmanov Iu B, Bogomaz SA, et al. [Dependence of the pump function of the isolated rat heart on the functional activity of sigma receptors during reperfusion] Rossiiskii Fiziologicheskii Zhurnal Imeni IM Sechenova. 1999;85:1396–408. [PubMed] [Google Scholar]
- Bhuiyan M, Fukunaga K. Targeting sigma-1 receptor signaling by endogenous ligands for cardioprotection. Expert Opin Therap Targets. 2011;15:145–55. doi: 10.1517/14728222.2011.546350. [DOI] [PubMed] [Google Scholar]
- Fialova K. Acute and chronic effects of sigma receptor ligands in mammalian myocardium. [PhD Thesis] Czech Republic: Department of Physiology, Masaryk University; 2010. 1 pp.154 pp. [Google Scholar]
- Lishmanov Yu B, Maslov LN, Naryzhnaya NV, Tam SW. Ligands for opioid and sigma-receptors improve cardiac electrical stability in rat models of post-infarction cardiosclerosis and stress. Life Sci. 1999;65:Pl13–17. doi: 10.1016/s0024-3205(99)00226-x. [DOI] [PubMed] [Google Scholar]
- Li DP, Averill DB, Pan HL. Differential roles for glutamate receptor subtypes within commissural NTS in cardiac-sympathetic reflex. Am J Physiol Regulatory Integrative Comparative Physiol. 2001;281:R935–43. doi: 10.1152/ajpregu.2001.281.3.R935. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Ahmed A, Khogali SE, et al. Glutamine metabolism and transport in skeletal muscle and heart and their clinical relevance. J Nutr. 1996;126:1142s–9s. doi: 10.1093/jn/126.suppl_4.1142S. [DOI] [PubMed] [Google Scholar]
- Jeanjean AP, Laterre EC, Maloteaux JM. Neuroleptic binding to sigma receptors: possible involvement in neuroleptic-induced acute dystonia. Biol Psychiatry. 1997;41:1010–19. doi: 10.1016/s0006-3223(96)00264-8. [DOI] [PubMed] [Google Scholar]
- Hinoi E, Fujimori S, Nakamura Y, Yoneda Y. Group III metabotropic glutamate receptors in rat cultured calvarial osteoblasts. Biochem Biophys Res Commun. 2001;281:341–6. doi: 10.1006/bbrc.2001.4355. [DOI] [PubMed] [Google Scholar]
- Laketic-Ljubojevic I, Suva LJ, Maathuis FJ, et al. Functional characterization of N-methyl-d-aspartic acid-gated channels in bone cells. Bone. 1999;25:631–7. doi: 10.1016/s8756-3282(99)00224-0. [DOI] [PubMed] [Google Scholar]
- Patton AJ, Genever PG, Birch MA, et al. Expression of an N-methyl-d-aspartate-type receptor by human and rat osteoblasts and osteoclasts suggests a novel glutamate signaling pathway in bone. Bone. 1998;22:645–9. doi: 10.1016/s8756-3282(98)00061-1. [DOI] [PubMed] [Google Scholar]
- Gray C, Marie H, Arora M, et al. Glutamate does not play a major role in controlling bone growth. J Bone Mineral Res. 2001;16:742–9. doi: 10.1359/jbmr.2001.16.4.742. [DOI] [PubMed] [Google Scholar]
- Gu Y, Publicover S. Expression of functional metabotropic glutamate receptors in primary cultured rat osteoblasts. Cross-talk with N-methyl-d-aspartate receptors. J Biol Chem. 2000;275:34252–9. doi: 10.1074/jbc.M004520200. [DOI] [PubMed] [Google Scholar]
- Hinoi E, Fujimori S, Takemori A, et al. Demonstration of expression of mRNA for particular AMPA and kainate receptor subunits in immature and mature cultured rat calvarial osteoblasts. Brain Res. 2002;943:112–16. doi: 10.1016/s0006-8993(02)02726-9. [DOI] [PubMed] [Google Scholar]
- Espinosa L, Itzstein C, Cheynel H, et al. Active NMDA glutamate receptors are expressed by mammalian osteoclasts. J Physiol. 1999;518:47–53. doi: 10.1111/j.1469-7793.1999.0047r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Genever P, Skerry T, Publicover S. The NMDA type glutamate receptors expressed by primary rat osteoblasts have the same electrophysiological characteristics as neuronal receptors. Calcified Tissue Int. 2002;70:194–203. doi: 10.1007/s00223-001-2004-z. [DOI] [PubMed] [Google Scholar]
- Hinoi E, Fujimori S, Yoneda Y. Modulation of cellular differentiation by N-methyl-d-aspartate receptors in osteoblasts. FASEB J. 2003;17:1532–34. doi: 10.1096/fj.02-0820fje. [DOI] [PubMed] [Google Scholar]
- Peet NM, Grabowski PS, Laketic-Ljubojevic I, Skerry TM. The glutamate receptor antagonist MK801 modulates bone resorption in vitro by a mechanism predominantly involving osteoclast differentiation. FASEB J. 1999;13:2179–85. doi: 10.1096/fasebj.13.15.2179. [DOI] [PubMed] [Google Scholar]
- Bhangu P, Genever P, Spencer G, et al. Evidence for targeted vesicular glutamate exocytosis in osteoblasts. Bone. 2001;29:16–23. doi: 10.1016/s8756-3282(01)00482-3. [DOI] [PubMed] [Google Scholar]
- Mason DJ, Suva LJ, Genever PG, et al. Mechanically regulated expression of a neural glutamate transporter in bone: a role for excitatory amino acids as osteotropic agents? Bone. 1997;20:199–205. doi: 10.1016/s8756-3282(96)00386-9. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Kubota K, Kobayashi T, et al. Evaluation of [11C]SA5845 and [11C]SA4503 for imaging of sigma receptors in tumors by animal PET. Ann Nuclear Med. 2005;19:701–9. doi: 10.1007/BF02985120. [DOI] [PubMed] [Google Scholar]
- Bem W, Thomas G, Mamone J, et al. Overexpression of sigma receptors in nonneural human tumors. Cancer Res. 1991;51:6558–62. [PubMed] [Google Scholar]
- Vilner BJ, de Costa BR, Bowen WD. Cytotoxic effects of sigma ligands: sigma receptor-mediated alterations in cellular morphology and viability. J Neurosci. 1995;15:117–34. doi: 10.1523/JNEUROSCI.15-01-00117.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CS, Bowen WD, Varma VM, et al. Sigma receptors are expressed in human non-small cell lung carcinoma. Life Sci. 1995;56:2385–92. doi: 10.1016/0024-3205(95)00232-u. [DOI] [PubMed] [Google Scholar]
- Moody TW, Leyton J, John C. Sigma ligands inhibit the growth of small cell lung cancer cells. Life Sci. 2000;66:1979–86. doi: 10.1016/s0024-3205(00)00523-3. [DOI] [PubMed] [Google Scholar]
- Mir SU, Ahmed IS, Arnold S, Craven RJ. Elevated progesterone receptor membrane component 1/sigma-2 receptor levels in lung tumors and plasma from lung cancer patients. Int J Cancer. 2012;131:E1–9. doi: 10.1002/ijc.26432. [DOI] [PubMed] [Google Scholar]
- Allen D, Delohery J, Baker G. Monosodium l-glutamate-induced asthma. J Allergy Clin Immunol. 1987;80:530–7. doi: 10.1016/0091-6749(87)90003-0. [DOI] [PubMed] [Google Scholar]
- Said SI, Berisha HI, Pakbaz H. Excitotoxicity in the lung: N-methyl-d-aspartate-induced, nitric oxide-dependent, pulmonary edema is attenuated by vasoactive intestinal peptide and by inhibitors of poly(ADP-ribose) polymerase. Proc Natl Acad Sci USA. 1996;93:4688–92. doi: 10.1073/pnas.93.10.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell WM, Doyle KM, Westgate C, Atterwill CK. Characterisation of a functional polyamine site on rat mast cells: association with a NMDA receptor macrocomplex. J Neuroimmunol. 1996;65:49–53. doi: 10.1016/0165-5728(95)00182-4. [DOI] [PubMed] [Google Scholar]
- Olney JW. Excitotoxins in foods. Neurotoxicology. 1994;15:535–44. [PubMed] [Google Scholar]
- Brown C, Fezoui M, Selig W, et al. Antitussive activity of sigma-1 receptor agonists in the guinea-pig. Br J Pharmacol. 2004;141:233–40. doi: 10.1038/sj.bjp.0705605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton M, Lookingland K, Moore K. The sigma receptor ligand rimcazole alters secretion of prolactin and alpha-melanocyte stimulating hormone by dopaminergic and non-dopaminergic mechanisms. Eur J Pharmacol. 1996;299:171–7. doi: 10.1016/0014-2999(95)00841-1. [DOI] [PubMed] [Google Scholar]
- Su TP, Wu XZ, Cone EJ, et al. Sigma compounds derived from phencyclidine: identification of PRE-084: a new, selective sigma ligand. J Pharm Exp Therap. 1991;259:543–50. [PubMed] [Google Scholar]
- Eaton M, Lookingland K, Moore K. The sigma ligand rimcazole activates noradrenergic neurons projecting to the paraventricular nucleus and increases corticosterone secretion in rats. Brain Res. 1996;733:162–6. doi: 10.1016/0006-8993(96)00290-9. [DOI] [PubMed] [Google Scholar]
- Le Foll F, Castel H, Soriani O, et al. Gramicidin-perforated patch revealed depolarizing effect of GABA in cultured frog melanotrophs. J Physiol. 1998;507:55–69. doi: 10.1111/j.1469-7793.1998.055bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll F, Louiset E, Castel H, et al. Electrophysiological effects of various neuroactive steroids on the GABA(A) receptor in pituitary melanotrope cells. Eur J Pharmacol. 1997;331:303–11. doi: 10.1016/s0014-2999(97)01042-x. [DOI] [PubMed] [Google Scholar]
- Pechnick RN. Effects of opioids on the hypothalamo-pituitary-adrenal axis. Annu Rev Pharmacol Toxicol. 1993;33:353–82. doi: 10.1146/annurev.pa.33.040193.002033. [DOI] [PubMed] [Google Scholar]
- Peck V, Shenkman L. Haloperidol-induced syndrome of inappropriate secretion of antidiuretic hormone. Clin Pharmacol Therap. 1979;26:442–4. doi: 10.1002/cpt1979264442. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Kawasaki H, Nagai H. Characterization of purification-associated reduction in IgE-dependent histamine release from rat peritoneal mast cells. Inflamm Res. 1995;44:541–7. doi: 10.1007/BF01757359. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Kuromi H, Gonoi T, et al. Expression and role of ionotropic glutamate receptors in pancreatic islet cells. FASEB J. 1995;5:686–91. [PubMed] [Google Scholar]
- Liu MT, Rothstein JD, Gershon MD, Kirchgessner AL. Glutamatergic enteric neurons. J Neurosci. 1997;17:4764–84. doi: 10.1523/JNEUROSCI.17-12-04764.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Ouedraogo R, Kirchgessner AL. Localization and function of group III metabotropic glutamate receptors in rat pancreatic islets. Am J Physio Endocrinol Metab. 2002;282:E1324–33. doi: 10.1152/ajpendo.00460.2001. [DOI] [PubMed] [Google Scholar]
- Trudeau F, Gagnon S, Massicotte G. Hippocampal synaptic plasticity and glutamate receptor regulation: influences of diabetes mellitus. Eur J Pharmacol. 2004;490:177–86. doi: 10.1016/j.ejphar.2004.02.055. [DOI] [PubMed] [Google Scholar]
- Caruso C, Bottino M, Pampillo M, et al. Glutamate induces apoptosis in anterior pituitary cells through group II metabotropic glutamate receptor activation. Endocrinology. 2004;145:4677–84. doi: 10.1210/en.2004-0550. [DOI] [PubMed] [Google Scholar]
- Yoneda Y, Ogita K. Localization of [3H]glutamate binding sites in rat adrenal medulla. Brain Res. 1986;383:387–91. doi: 10.1016/0006-8993(86)90046-6. [DOI] [PubMed] [Google Scholar]
- Ronsisvalle G, Marrazzo A, Pasquinucci L, et al. Specific kappa opioid receptor agonists. Farmaco (Societa chimica italiana: 1989) 2001;56:121–5. doi: 10.1016/s0014-827x(01)01022-9. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Habenicht UF, Brautigam M, Gudermann T. Steroidal sigma receptor ligands affect signaling pathways in human spermatozoa. Biol Reprod. 2000;63:57–63. doi: 10.1095/biolreprod63.1.57. [DOI] [PubMed] [Google Scholar]
- Lara H, Bastos-Ramos W. Glutamate and kainate effects on the noradrenergic neurons innervating the rat vas deferens. J Neurosci Res. 1988;19:239–44. doi: 10.1002/jnr.490190209. [DOI] [PubMed] [Google Scholar]
- Battaglia F. Glutamine and glutamate exchange between the fetal liver and the placenta. J Nutr. 2000;130:974s–7s. doi: 10.1093/jn/130.4.974S. [DOI] [PubMed] [Google Scholar]
- Caveliers V, Everaert H, John C, Lahoutte T, Bossuyt A. Sigma receptor scintigraphy with N-[2-(1′-piperidinyl)ethyl]-3-(123)I-iodo-4-methoxybenzamide of patients with suspected primary breast cancer: first clinical results. J Neuclear Med. 2002;43:1647–9. [PubMed] [Google Scholar]
- Bowen W. Sigma receptors: recent advances and new clinical potentials. Pharm Acta Helvet. 2000;74:211–18. doi: 10.1016/s0031-6865(99)00034-5. [DOI] [PubMed] [Google Scholar]
- Gebreselassie D, Bowen W. Sigma-2 receptors are specifically localized to lipid rafts in rat liver membranes. Eur J Pharmacol. 2004;493:19–28. doi: 10.1016/j.ejphar.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Klouz A, Sapena R, Liu J, et al. Evidence for sigma-1-like receptors in isolated rat liver mitochondrial membranes. Br J Pharmacol. 2002;135:1607–15. doi: 10.1038/sj.bjp.0704626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe T, Sugihara H, Nawa H, et al. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J Biol Chem. 1992;267:13361–8. [PubMed] [Google Scholar]
- Lipton SA, Tauck DL. Voltage-dependent conductances of solitary ganglion cells dissociated from the rat retina. J Physiol. 1987;385:361–91. doi: 10.1113/jphysiol.1987.sp016497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LL, Wang L, Zhong YM, Yang XL. Expression of sigma receptor 1 mRNA and protein in rat retina. Neuroscience. 2010;167:1151–9. doi: 10.1016/j.neuroscience.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Ola MS, Moore P, El-Sherbeny A, et al. Expression pattern of sigma receptor 1 mRNA and protein in mammalian retina. Brain Res Mol Brain Res. 2001;95:86–95. doi: 10.1016/s0169-328x(01)00249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PM, Roon P, Van Ells TK, et al. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Opthamol Vis Sci. 2004;45:3330–6. doi: 10.1167/iovs.04-0247. [DOI] [PubMed] [Google Scholar]
- Jiang G, Mysona B, Dun Y, et al. Expression, subcellular localization, and regulation of sigma receptor in retinal muller cells. Invest Opthamol Vis Sci. 2006;47:5576–82. doi: 10.1167/iovs.06-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuerxun T, Numakawa T, Adachi N, et al. SA4503, a sigma-1 receptor agonist, prevents cultured cortical neurons from oxidative stress-induced cell death via suppression of MAPK pathway activation and glutamate receptor expression. Neurosci Lett. 2010;469:303–8. doi: 10.1016/j.neulet.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Jonhede S, Petersen A, Zetterberg M, Karlsson JO. Acute effects of the sigma-2 receptor agonist siramesine on lysosomal and extra-lysosomal proteolytic systems in lens epithelial cells. Mol Vis. 2010;16:819–27. [PMC free article] [PubMed] [Google Scholar]
- Ha Y, Dun Y, Thangaraju M, Duplantier J, et al. Sigma receptor 1 modulates endoplasmic reticulum stress in retinal neurons. Invest Ophthalmol Vis Sci. 2011;52:527–40. doi: 10.1167/iovs.10-5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha Y, Saul A, Tawfik A, et al. Late-onset inner retinal dysfunction in mice lacking sigma receptor 1 (sigmaR1) Invest Ophthalmol Vis Sci. 2011;52:7749–60. doi: 10.1167/iovs.11-8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Duncan G. Silencing of sigma-1 receptor induces cell death in human lens cells. Exp Cell Res. 2006;312:1439–46. doi: 10.1016/j.yexcr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Wang L, Prescott AR, Spruce BA, et al. Sigma receptor antagonists inhibit human lens cell growth and induce pigmentation. Invest Opthamol Vis Sci. 2005;46:1403–8. doi: 10.1167/iovs.04-1209. [DOI] [PubMed] [Google Scholar]
- Li T, Ghishan FK, Bai L. Molecular physiology of vesicular glutamate transporters in the digestive system. World J Gastroenterol. 2005;11:1731–6. doi: 10.3748/wjg.v11.i12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai LH. Function of GABAergic and glutamatergic neurons in the stomach. J Biomed Sci. 2005;12:255–66. doi: 10.1007/s11373-005-1357-0. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Lee YJ, Wu J. Effect of excitatory amino acid neurotransmitters on acid secretion in the rat stomach. J Biomed Sci. 1999;6:36–44. doi: 10.1007/BF02256422. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Tsai W, Wu JY. Effect of l-glutamic acid on acid secretion and immunohistochemical localization of glutamatergic neurons in the rat stomach. J Neurosci Res. 1994;38:188–95. doi: 10.1002/jnr.490380209. [DOI] [PubMed] [Google Scholar]
- Farthing M. Novel agents for the control of secretory diarrhoea. Expert Opin Invest Drugs. 2004;13:777–85. doi: 10.1517/13543784.13.7.777. [DOI] [PubMed] [Google Scholar]
- Collina S, Gaggeri R, Marra A, et al. Sigma receptor modulators: a patent review. Expert Opin Therap patents. 2013;23:597–613. doi: 10.1517/13543776.2013.769522. [DOI] [PubMed] [Google Scholar]
- Niijima A. Reflex effects of oral, gastrointestinal and hepatoportal glutamate sensors on vagal nerve activity. J Nutr. 2000;130:971s–3s. doi: 10.1093/jn/130.4.971S. [DOI] [PubMed] [Google Scholar]
- Raab M, Neuhuber WL. Glutamatergic functions of primary afferent neurons with special emphasis on vagal afferents. Int Rev Cytol. 2007;256:223–75. doi: 10.1016/S0074-7696(07)56007-9. [DOI] [PubMed] [Google Scholar]
- Seagard JL, Dean C, Hopp FA. Role of glutamate receptors in transmission of vagal cardiac input to neurones in the nucleus tractus solitarii in dogs. J Physiol. 1999;520:243–53. doi: 10.1111/j.1469-7793.1999.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudzik TJ, De Costa BR, McMillan DE. Sigma receptor-mediated emetic response in pigeons: agonists, antagonists and modifiers. Eur J Pharmacol. 1993;236:279–87. doi: 10.1016/0014-2999(93)90599-d. [DOI] [PubMed] [Google Scholar]
- Brooks B, Thisted R, Appel S, et al. Treatment of pseudobulbar affect in ALS with dextromethorphan/quinidine: a randomized trial. Neurology. 2004;63:1364–70. doi: 10.1212/01.wnl.0000142042.50528.2f. [DOI] [PubMed] [Google Scholar]
- Junien JL, Gue M, Pascaud X, et al. Selective stimulation of colonic motor response to a meal by sigma ligands in dogs. Gastroenterology. 1990;99:684–9. doi: 10.1016/0016-5085(90)90955-z. [DOI] [PubMed] [Google Scholar]
- Gue M, Gleizes-Escala C, Del Rio-Lacheze C, Junien J, Bueno L. Reversal of CRF- and dopamine-induced stimulation of colonic motility by CCK and igmesine (JO 1784) in the rat. Br J Pharmacol. 1994;111:930–4. doi: 10.1111/j.1476-5381.1994.tb14828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gue M, Junien J, Del Rio C, Bueno L. Neuropeptide Y and sigma ligand (JO 1784) suppress stress-induced colonic motor disturbances in rats through sigma and cholecystokinin receptors. J Pharm Exp Therap. 1992;261:850–5. [PubMed] [Google Scholar]
- Carr D, De Costa B, Radesca L, Blalock J. Functional assessment and partial characterization of [3H](+)-pentazocine binding sites on cells of the immune system. J Neuroimmunol. 1991;35:153–66. doi: 10.1016/0165-5728(91)90170-c. [DOI] [PubMed] [Google Scholar]
- Coccini T, Manzo L, Costa L. 3H-spiperone labels sigma receptors, not dopamine D2 receptors, in rat and human lymphocytes. Immunopharmacology. 1991;22:93–105. doi: 10.1016/0162-3109(91)90034-v. [DOI] [PubMed] [Google Scholar]
- Iniguez MA, Punzon C, Nieto R, et al. Inhibitory effects of sigma-2 receptor agonists on T lymphocyte activation. Front Pharmacol. 2013;4:1–12. doi: 10.3389/fphar.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Kwan HY, Chan HC, et al. Blockage of voltage-gated K+ channels inhibits adhesion and proliferation of hepatocarcinoma cells. International J Mol Med. 2003;11:261–6. [PubMed] [Google Scholar]
- Carayon P, Bouaboula M, Loubet J, et al. The sigma ligand SR 31747 prevents the development of acute graft-versus-host disease in mice by blocking IFN-gamma and GM-CSF mRNA expression. Int J Immunopharmacol. 1995;17:753–61. doi: 10.1016/0192-0561(95)00066-b. [DOI] [PubMed] [Google Scholar]
- Pellegrino T, Bayer BM. In vivo effects of cocaine on immune cell function. J Neuroimmunol. 1998;83:139–47. doi: 10.1016/s0165-5728(97)00230-0. [DOI] [PubMed] [Google Scholar]
- Xu W, Flick T, Mitchel J, et al. Cocaine effects on immunocompetent cells: an observation of in vitro cocaine exposure. Int J Immunopharmacol. 1999;21:463–72. doi: 10.1016/s0192-0561(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Friesland M, Mingorance L, Chung J, et al. Sigma-1 receptor regulates early steps of viral RNA replication at the onset of hepatitis C virus infection. J Virol. 2013;87:6377–90. doi: 10.1128/JVI.03557-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme K, Guglielmi K, Dermody T. Reovirus nonstructural protein sigma1s is required for establishment of viremia and systemic dissemination. Proc Natl Acad Sci USA. 2009;106:19986–91. doi: 10.1073/pnas.0907412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Waarde A, Rybczynska AA, Ramakrishnan N, et al. Sigma receptors in oncology: therapeutic and diagnostic applications of sigma ligands. Current Pharm Design. 2010;16:3519–37. doi: 10.2174/138161210793563365. [DOI] [PubMed] [Google Scholar]
- Khansari N, Whitten HD, Chou YK, Fudenberg HH. Effect of polyvinyl pyrrolidone derivative compound on production of interleukin-1 by monocytes. Biomedi Pharmacotherap. 1984;38:308–11. [PubMed] [Google Scholar]
- Dornand J, Kamenka J, Bartegi A, Mani J. PCP and analogs prevent the proliferative response of T lymphocytes by lowering IL2 production. An effect related to the blockade of mitogen-triggered enhancement of free cytosolic calcium concentration. Biochem Pharmacol. 1987;36:3929–36. doi: 10.1016/0006-2952(87)90460-6. [DOI] [PubMed] [Google Scholar]
- Megalizzi V, Le Mercier M, Decaestecker C. Sigma receptors and their ligands in cancer biology: overview and new perspectives for cancer therapy. Med Res Rev. 2012;32:410–27. doi: 10.1002/med.20218. [DOI] [PubMed] [Google Scholar]
- Tucci M, Quatraro C, Dammacco F, Silvestris F. Role of active drug transporters in refractory multiple myeloma. Curr Topics Med Chem. 2009;9:218–24. doi: 10.2174/156802609787521625. [DOI] [PubMed] [Google Scholar]
- Thomas GE, Szucs M, Mamone JY, et al. Sigma and opioid receptors in human brain tumors. Life Sci. 1990;46:1279–86. doi: 10.1016/0024-3205(90)90360-4. [DOI] [PubMed] [Google Scholar]
- Zamora PO, Moody TW, John CS. Increased binding to sigma sites of N-[1′(2-piperidinyl)ethyl)-4-[I-125]-iodobenzamide (I-125-PAB) with onset of tumor cell proliferation. Life Sci. 1998;63:1611–18. doi: 10.1016/s0024-3205(98)00430-5. [DOI] [PubMed] [Google Scholar]
- Ryan-Moro J, Chien CC, Standifer KM, Pasternak GW. Sigma binding in a human neuroblastoma cell line. Neurochem Res. 1996;21:1309–14. doi: 10.1007/BF02532372. [DOI] [PubMed] [Google Scholar]
- Wheeler KT, Wang LM, Wallen CA, et al. Sigma-2 receptors as a biomarker of proliferation in solid tumours. Br J Cancer. 2000;82:1223–32. doi: 10.1054/bjoc.1999.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybczynska AA, Dierckx RA, Ishiwata K, et al. Cytotoxicity of sigma-receptor ligands is associated with major changes of cellular metabolism and complete occupancy of the sigma-2 subpopulation. J Neuclear Med. 2008;49:2049–56. doi: 10.2967/jnumed.108.053876. [DOI] [PubMed] [Google Scholar]
- Simony-Lafontaine J, Esslimani M, Bribes E, et al. Immunocytochemical assessment of sigma-1 receptor and human sterol isomerase in breast cancer and their relationship with a series of prognostic factors. Br J Cancer. 2000;82:1958–66. doi: 10.1054/bjoc.2000.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach RH, Smith CR, Al-Nabulsi I, et al. Sigma 2 receptors as potential biomarkers of proliferation in breast cancer. Cancer Res. 1997;57:156–61. [PubMed] [Google Scholar]
- John CS, Gulden ME, Vilner BJ, Bowen WD. Synthesis, in vitro validation and in vivo pharmacokinetics of [125I]N-[2-(4-iodophenyl)ethyl]-N-methyl-2-(1-piperidinyl) ethylamine: a high-affinity ligand for imaging sigma receptor positive tumors. Nuclear Med Biol. 1996;23:761–6. doi: 10.1016/0969-8051(96)00070-4. [DOI] [PubMed] [Google Scholar]
- John CS, Lim BB, Geyer BC, et al. 99mTc-labeled sigma-receptor-binding complex: synthesis, characterization, and specific binding to human ductal breast carcinoma (T47D) cells. Bioconj Chem. 1997;8:304–9. doi: 10.1021/bc9700087. [DOI] [PubMed] [Google Scholar]
- Dittmann H, Coenen H, Zolzer F, et al. In vitro studies on the cellular uptake of melanoma imaging aminoalkyl-iodobenzamide derivatives (ABA) Nuclear Med Biol. 1999;26:51–6. doi: 10.1016/s0969-8051(98)00046-8. [DOI] [PubMed] [Google Scholar]
- Eisenhut M, Hull W, Mohammed A, et al. Radioiodinated N-(2-diethylaminoethyl)benzamide derivatives with high melanoma uptake: structure-affinity relationships, metabolic fate, and intracellular localization. J Med Chem. 2000;43:3913–22. doi: 10.1021/jm991079p. [DOI] [PubMed] [Google Scholar]
- Everaert H, Flamen P, Franken P, et al. Sigma-receptor imaging by means of I123-IDAB scintigraphy: clinical application in melanoma and non-small cell lung cancer. AntiCancer Res. 1997;17:1577–82. [PubMed] [Google Scholar]
- Friebe M, Mahmood A, Bolzati C, et al. [99mTc]oxotechnetium(V) complexes amine-amide-dithiol chelates with dialkylaminoalkyl substituents as potential diagnostic probes for malignant melanoma. J Med Chem. 2001;44:3132–40. doi: 10.1021/jm0005407. [DOI] [PubMed] [Google Scholar]
- Choi S, Yang B, Plossl K, et al. Development of a Tc-99m labeled sigma-2 receptor-specific ligand as a potential breast tumor imaging agent. Nuclear Med Biol. 2001;28:657–66. doi: 10.1016/s0969-8051(01)00234-7. [DOI] [PubMed] [Google Scholar]
- John CS, Vilner BJ, Geyer BC, et al. Targeting sigma receptor-binding benzamides as in vivo diagnostic and therapeutic agents for human prostate tumors. Cancer Res. 1999;59:4578–83. [PubMed] [Google Scholar]
- Shiue C, Shiue GG, Benard F, et al. N-(n-Benzylpiperidin-4-yl)-2-[18F]fluorobenzamide: a potential ligand for PET imaging of breast cancer. Nuclear Med Biol. 2000;27:763–7. doi: 10.1016/s0969-8051(00)00161-x. [DOI] [PubMed] [Google Scholar]
- John CS, Gulden ME, Li J, et al. Synthesis, in vitro binding, and tissue distribution of radioiodinated 2-[125I]N-(N-benzylpiperidin-4-yl)-2-iodo benzamide, 2-[125I]BP: a potential sigma receptor marker for human prostate tumors. Nuclear Med Biol. 1998;25:189–94. doi: 10.1016/s0969-8051(97)00168-6. [DOI] [PubMed] [Google Scholar]
- Abate C, Ferorelli S, Contino M, et al. Arylamides hybrids of two high-affinity sigma2 receptor ligands as tools for the development of PET radiotracers. European J Med Chem. 2011;46:4733–41. doi: 10.1016/j.ejmech.2011.05.057. [DOI] [PubMed] [Google Scholar]
- Zeng C, Vangveravong S, Xu J, et al. Subcellular localization of sigma-2 receptors in breast cancer cells using two-photon and confocal microscopy. Cancer Res. 2007;67:6708–16. doi: 10.1158/0008-5472.CAN-06-3803. [DOI] [PubMed] [Google Scholar]
- van Waarde A, Jager PL, Ishiwata K, et al. Comparison of sigma-ligands and metabolic PET tracers for differentiating tumor from inflammation. J Neuclear Med. 2006;47:150–4. [PubMed] [Google Scholar]
- Spirkoski J, Melo FR, Grujic M, et al. Mast cell apoptosis induced by siramesine, a sigma-2 receptor agonist. Biochem Pharmacol. 2012;84:1671–80. doi: 10.1016/j.bcp.2012.09.028. [DOI] [PubMed] [Google Scholar]
- Brent P, Pang G. Sigma binding site ligands inhibit cell proliferation in mammary and colon carcinoma cell lines and melanoma cells in culture. Eur J Pharmacol. 1995;278:151–60. doi: 10.1016/0014-2999(95)00115-2. [DOI] [PubMed] [Google Scholar]
- Cho E, Zhang Y, Cai X, Moran C, Wang L, Xia Y. Quantitative analysis of the fate of gold nanocages in vitro and in vivo after uptake by U87-MG tumor cells. Angew Chem. 2013;52:1152–5. doi: 10.1002/anie.201208096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu J, Xia X, et al. SV119-gold nanocage conjugates: a new platform for targeting cancer cells via sigma-2 receptors. Nanoscale. 2012;4:421–4. doi: 10.1039/c1nr11469g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moebius FF, Reiter RJ, Hanner M, Glossmann H. High affinity of sigma 1-binding sites for sterol isomerization inhibitors: evidence for a pharmacological relationship with the yeast sterol C8-C7 isomerase. Br J Pharmacol. 1997;121:1–6. doi: 10.1038/sj.bjp.0701079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser S, Grimes J, Diss J, et al. Predominant expression of Kv1.3 voltage-gated K+ channel subunit in rat prostate cancer cell lines: electrophysiological, pharmacological and molecular characterisation. Pflugers Archiv. 2003;446:559–71. doi: 10.1007/s00424-003-1077-0. [DOI] [PubMed] [Google Scholar]
- Fraser S, Grimes J, Djamgoz M. Effects of voltage-gated ion channel modulators on rat prostatic cancer cell proliferation: comparison of strongly and weakly metastatic cell lines. The Prostate. 2000;44:61–76. doi: 10.1002/1097-0045(20000615)44:1<61::aid-pros9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Fraser S, Koyuturk M, Djamgoz M. Ion channel activity and cancer cell proliferation: a short review with particular reference to prostate cancer. Trivendrum, India: Research Signpost; 2002. [Google Scholar]
- Fraser S, Salvador V, Manning E, et al. Contribution of functional voltage-gated Na+ channel expression to cell behaviors involved in the metastatic cascade in rat prostate cancer: I. Lateral motility. J Cell Physiol. 2003;195:479–87. doi: 10.1002/jcp.10312. [DOI] [PubMed] [Google Scholar]
- Renaudo A, L'Hoste S, Guizouarn H, et al. Cancer cell cycle modulated by a functional coupling between sigma-1 receptors and Cl-channels. J Biol Chem. 2007;282:2259–67. doi: 10.1074/jbc.M607915200. [DOI] [PubMed] [Google Scholar]
- Ouadid-Ahidouch H, Chaussade F, Roudbaraki M, et al. KV1.1 K(+) channels identification in human breast carcinoma cells: involvement in cell proliferation. Biochem Biophys Res Commun. 2000;278:272–7. doi: 10.1006/bbrc.2000.3790. [DOI] [PubMed] [Google Scholar]
- Woodfork KA, Wonderlin WF, Peterson VA, Strobl JS. Inhibition of ATP-sensitive potassium channels causes reversible cell-cycle arrest of human breast cancer cells in tissue culture. J Cell Physiol. 1995;162:163–71. doi: 10.1002/jcp.1041620202. [DOI] [PubMed] [Google Scholar]
- Yao X, Kwan HY. Activity of voltage-gated K+ channels is associated with cell proliferation and Ca2+ influx in carcinoma cells of colon cancer. Life Sci. 1999;65:55–62. doi: 10.1016/s0024-3205(99)00218-0. [DOI] [PubMed] [Google Scholar]
- Wonderlin WF, Strobl JS. Potassium channels, proliferation and G1 progression. J Membrane Biol. 1996;154:91–107. doi: 10.1007/s002329900135. [DOI] [PubMed] [Google Scholar]
- Klimatcheva E, Wonderlin WF. An ATP-sensitive K(+) current that regulates progression through early G1 phase of the cell cycle in MCF-7 human breast cancer cells. J Membrane Biol. 1999;171:35–46. doi: 10.1007/s002329900556. [DOI] [PubMed] [Google Scholar]
- Nilius B, Wohlrab W. Potassium channels and regulation of proliferation of human melanoma cells. J Physiol. 1992;445:537–48. doi: 10.1113/jphysiol.1992.sp018938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab A, Reinhardt J, Schneider SW, et al. K(+) channel-dependent migration of fibroblasts and human melanoma cells. Cell Physiol Biochem. 1999;9:126–32. doi: 10.1159/000016309. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang Y, Cao L, et al. HERG K+ channel, a regulator of tumor cell apoptosis and proliferation. Cancer Res. 2002;62:4843–8. [PubMed] [Google Scholar]
- Cantiello H, Prat A, Bonventre J, et al. Actin-binding protein contributes to cell volume regulatory ion channel activation in melanoma cells. J Biol Chem. 1993;268:4596–9. [PubMed] [Google Scholar]
- Shen MR, Chou CY, Hsu KF, et al. Modulation of volume-sensitive Cl – channels and cell volume by actin filaments and microtubules in human cervical cancer HT-3 cells. Acta Physiol Scand. 1999;167:215–25. doi: 10.1046/j.1365-201x.1999.00611.x. [DOI] [PubMed] [Google Scholar]
- Mycielska ME, Fraser SP, Szatkowski M, Djamgoz MB. Contribution of functional voltage-gated Na+ channel expression to cell behaviors involved in the metastatic cascade in rat prostate cancer: II. Secretory membrane activity. J Cell Physiol. 2003;195:461–9. doi: 10.1002/jcp.10265. [DOI] [PubMed] [Google Scholar]
- Diss J, Archer S, Hirano J, et al. Expression profiles of voltage-gated Na(+) channel alpha-subunit genes in rat and human prostate cancer cell lines. Prostate. 2001;48:165–78. doi: 10.1002/pros.1095. [DOI] [PubMed] [Google Scholar]
- Grimes J, Fraser S, Stephens G, et al. Differential expression of voltage-activated Na+ currents in two prostatic tumour cell lines: contribution to invasiveness in vitro. FEBS Lett. 1995;369:290–4. doi: 10.1016/0014-5793(95)00772-2. [DOI] [PubMed] [Google Scholar]
- Smith P, Rhodes NP, Shortland AP, et al. Sodium channel protein expression enhances the invasiveness of rat and human prostate cancer cells. FEBS Lett. 1998;423:19–24. doi: 10.1016/s0014-5793(98)00050-7. [DOI] [PubMed] [Google Scholar]
- Cairns R, Khokha R, Hill R. Molecular mechanisms of tumor invasion and metastasis: an integrated view. Curr Mol Med. 2003;3:659–71. doi: 10.2174/1566524033479447. [DOI] [PubMed] [Google Scholar]
- Pawlak G, Helfman DM. Cytoskeletal changes in cell transformation and tumorigenesis. Curr Opin Genet Dev. 2001;11:41–7. doi: 10.1016/s0959-437x(00)00154-4. [DOI] [PubMed] [Google Scholar]
- Cassano G, Gasparre G, Niso M, et al. F281, synthetic agonist of the sigma-2 receptor, induces Ca2+ efflux from the endoplasmic reticulum and mitochondria in SK-N-SH cells. Cell Calcium. 2009;45:340–5. doi: 10.1016/j.ceca.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Gardner B, Zhu L, Roth M, et al. Cocaine modulates cytokine and enhances tumor growth through sigma receptors. J Neuroimmunol. 2004;147:95–8. doi: 10.1016/j.jneuroim.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Kedjouar B, Daunes S, Vilner BJ, et al. Structural similitudes between cytotoxic antiestrogen-binding site (AEBS) ligands and cytotoxic sigma receptor ligands. Evidence for a relationship between cytotoxicity and affinity for AEBS or sigma-2 receptor but not for sigma-1 receptor. Biochem Pharmacol. 1999;58:1927–39. doi: 10.1016/s0006-2952(99)00285-3. [DOI] [PubMed] [Google Scholar]
- Megalizzi V, Mathieu V, Mijatovic T, et al. 4-IBP, a sigma1 receptor agonist, decreases the migration of human cancer cells, including glioblastoma cells, in vitro and sensitizes them in vitro and in vivo to cytotoxic insults of proapoptotic and proautophagic drugs. Neoplasia (NY) 2007;9:358–69. doi: 10.1593/neo.07130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydar E, Onganer P, Perrett R, et al. The expression and functional characterization of sigma (sigma) 1 receptors in breast cancer cell lines. Cancer Lett. 2006;242:245–57. doi: 10.1016/j.canlet.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Zheng K, Scimemi A, Rusakov DA. Receptor actions of synaptically released glutamate: the role of transporters on the scale from nanometers to microns. Biophys J. 2008;95:4584–96. doi: 10.1529/biophysj.108.129874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordenberg J, Perlmutter I, Lavie G, et al. Anti-proliferative activity of haloperidol in B16 mouse and human SK-ME l-28 melanoma cell lines. Int J Oncol. 2005;27:1097–103. [PubMed] [Google Scholar]
- Hornick JR, Spitzer D, Goedegebuure P, et al. Therapeutic targeting of pancreatic cancer utilizing sigma-2 ligands. Surgery. 2012;152:S152–6. doi: 10.1016/j.surg.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Yoshimura R, Okochi T, et al. Association analysis of SIGMAR1 with major depressive disorder and SSRI response. Neuropharmacology. 2010;58:1168–73. doi: 10.1016/j.neuropharm.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Strong EM, Weiss F. Effect of sigma(1) receptor antagonism on ethanol and natural reward seeking. Neuroreport. 2012;23:809–13. doi: 10.1097/WNR.0b013e32835717c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegleiter K, Hermann M, Posod A, et al. The sigma-1 receptor agonist 4-phenyl-1-(4-phenylbutyl) piperidine (PPBP) protects against newborn excitotoxic brain injury by stabilizing the mitochondrial membrane potential in vitro and inhibiting microglial activation in vivo. Exp Neurol. 2014;261c:501–9. doi: 10.1016/j.expneurol.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Delgado P, Charney D, Price L, et al. Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry. 1990;47:411–18. doi: 10.1001/archpsyc.1990.01810170011002. [DOI] [PubMed] [Google Scholar]