ABSTRACT
Raw sausages are perishable foodstuffs; reducing their salt content raises questions about a possible increased spoilage of these products. In this study, we evaluated the influence of salt reduction (from 2.0% to 1.5% [wt/wt]), in combination with two types of packaging (modified atmosphere [50% mix of CO2-N2] and vacuum packaging), on the onset of spoilage and on the diversity of spoilage-associated bacteria. After 21 days of storage at 8°C, spoilage was easily observed, characterized by noticeable graying of the products and the production of gas and off-odors defined as rancid, sulfurous, or sour. At least one of these types of spoilage occurred in each sample, and the global spoilage intensity was more pronounced in samples stored under modified atmosphere than under vacuum packaging and in samples with the lower salt content. Metagenetic 16S rRNA pyrosequencing revealed that vacuum-packaged samples contained a higher total bacterial richness (n = 69 operational taxonomic units [OTUs]) than samples under the other packaging condition (n = 46 OTUs). The core community was composed of 6 OTUs (Lactobacillus sakei, Lactococcus piscium, Carnobacterium divergens, Carnobacterium maltaromaticum, Serratia proteamaculans, and Brochothrix thermosphacta), whereas 13 OTUs taxonomically assigned to the Enterobacteriaceae, Enterococcaceae, and Leuconostocaceae families comprised a less-abundant subpopulation. This subdominant community was significantly more abundant when 2.0% salt and vacuum packaging were used, and this correlated with a lower degree of spoilage. Our results demonstrate that salt reduction, particularly when it is combined with CO2-enriched packaging, promotes faster spoilage of raw sausages by lowering the overall bacterial diversity (both richness and evenness).
IMPORTANCE Our study takes place in the context of raw meat product manufacturing and is linked to a requirement for salt reduction. Health guidelines are calling for a reduction in dietary salt intake. However, salt has been used for a very long time as a hurdle technology, and salt reduction in meat products raises the question of spoilage and waste of food. The study was conceived to assess the role of sodium chloride reduction in meat products, both at the level of spoilage development and at the level of bacterial diversity, using 16S rRNA amplicon sequencing and raw pork sausage as a meat model.
INTRODUCTION
Traditional French raw pork sausages are made of a complex assortment of ingredients: pork meat, pork fat, spices, sugars, antioxidants, and sodium chloride, and they are stuffed in natural casing. From pork slaughtering to final packaging, their manufacture requires several steps (meat cutting, blending, and stuffing), with each a potential source of bacterial contamination (1–3). Raw pork sausages are thus very sensitive to spoilage unless specific treatments are applied to prevent their rapid sensory degradation. The preservation of this type of meat product, whose common shelf life ranges from 2 to 3 weeks, can only be ensured by a combination of several factors (the hurdle technology concept [4]). These include low storage temperature (<8°C), CO2-enriched atmosphere packaging, and the use of preservative ingredients, such as salts.
Sodium chloride (NaCl) is the most commonly used ingredient in raw pork sausages because it improves texture, enhances both flavors and taste, and lowers the water activity (aw) (5). The lowering of aw usually reduces the growth capacity of many bacterial species and consequently increases safety of the food product (6). However, excessive daily sodium intake in the diet can have harmful health effects, particularly by accentuating hypertension, the major risk factor for cardiovascular disease (7). While in 2003, a World Health Organization report (8) recommended limiting daily salt consumption to 5 g, in France, for example, the average consumer intake is still twice this suggested amount (9).
As processed meats contribute significantly to daily salt, and especially sodium, intake (10), salt reduction has become a major issue for the meat industry. In order to implement sodium reduction strategies, this industry needs to anticipate the safety and sanitary consequences of such a reduction and thus, specifically, evaluate the potential effects of sodium chloride reduction on the diversity and growth dynamics of spoilage-causing microbial ecosystems.
Meanwhile, while numerous studies have addressed the impact of salt on the growth potential of meat-borne bacteria (see reference 6) by measuring growth parameters in meat models (11) or by using mathematical modeling (12, 13), very few studies addressing the impact of salt reduction on the diversity and structure of bacterial communities are available. For example, the most recent works have focused on the reduction in growth of bacterial pathogens (14, 15) or on the numeration of spoilage bacteria using either conventional culture media or low-resolution nonculturing techniques, such as denaturing gradient gel electrophoresis (16–18). According to these studies, salt reduction is associated with faster meat spoilage, but the impact of salt reduction on the meat microbiota is not so clear. Depending on the degree of salt reduction, bacterial populations may remain quite stable, sometimes even with a decreased incidence of bacteria that cause meat spoilage (17). Furthermore, food microbiologists have recently highlighted the weaknesses of plating methods in assessing the overall diversity of potential meat spoilers (1, 2, 19–21), in that the most abundant (often psychrotrophic) species are among the hardest to cultivate using conventional media. If the effect of salt reduction on the meat microbiota is to be thoroughly assessed, it is necessary to analyze the whole bacterial community, as the meat microbiota is often divided into a core dominant population and a product-specific subdominant population (20). Variations in these complex microbial assemblages can result in different forms and degrees of spoilage (22–24).
Toward this end, two culture-independent analyses of raw or cooked pork sausages were recently published. Benson and coworkers (25) revealed complex dynamics of about 20 taxa during the storage of raw pork sausages at 4°C. This study also illustrated the role of spices as a source of spoiling bacterial species and demonstrated the important role of sodium- and acid-based preservatives (sodium lactate and sodium diacetate), first, in reducing spoilage of sausages, and second, in hindering the growth of the dominant spoilage bacteria Lactobacillus graminis and Carnobacterium divergens. More recently, Hultman and coworkers (2) showed that contamination during the initial preparation of cooked sausages is a key point that can influence spoilage dynamics during storage. However, they also underlined the importance of temperature and other conditions during distribution and storage in influencing the rate of spoilage.
Despite this progress, the following question remains open: following initial contamination, what is the real impact of salt reduction on meat bacterial diversity in the absence of acid-based preservatives, and how does this reduction influence the intensity of spoilage? We aimed to address this question using homemade raw pork sausages as a meat model. We measured the influence of a 25% sodium chloride reduction (from 2.0% [wt/wt] to 1.5% [wt/wt], according to European rule recommendations [26]), on both the diversity of spoilage bacteria (using a combination of quantitative PCR and pyrosequencing of the 16S rRNA gene) and on spoilage (using sensory analysis). This investigation was carried out using two different packaging systems commonly used on the market: (i) modified atmosphere packaging (MAP) made of 50% CO2 and 50% N2, and (ii) vacuum packaging (VPA).
MATERIALS AND METHODS
Sausage manufacturing, packaging, and storage conditions.
Fresh raw pork meat (post-rigor mortis meat) was collected from a single wholesaler and prepared in a pilot industrial laboratory. After cutting, the meat was coarsely minced and cured with a mix of spices (black and white pepper, nutmeg, garlic, and hot chili) and other ingredients (0.9% [wt/wt] glucose syrup, 0.052% [wt/wt] dextrose, 0.040% [wt/wt] ascorbic acid, and 0.015% [wt/wt] sodium ascorbate, with carmine used as red dye). Sodium chloride was added, according to the experimental conditions tested at 1.5% or 2% (wt/wt) and was confirmed by sodium assay (Larebron, Illkirch, France). Next, the mixture was stuffed (Mod 7/V; Tre spade, Turin, Italy) into desalted sheep's casings.
Around 200 g of sausages (corresponding to one unique sausage for each sample) was packed using either MAP, which was a mixture of 50% CO2 and 50% N2 (X50S Freshline; Air Products, Aubervilliers, France), or VPA (MBT-18; Multivac, Lagny-sur-Marne, France) into multilayer polyamide and polyethylene bags (product no. 10702055; Cenpac, Roissy-en-France, France) whose oxygen permeability is 35 cm3/m2 · day · 105 Pa (at 23°C and 0% relative humidity [rH]). Samples were then stored at 8°C for 21 days (usual use-by date). The control samples were immediately frozen and stored at −20°C. For each experimental condition, four samples were analyzed after storage: three for physicochemical, sensory, and microbiological analysis, and one for molecular bacterial diversity analysis by pyrosequencing and quantitative PCR. Five independent biological replicates were analyzed (named B04, B05, B06, B08, and B10), and the data set contained 80 samples (4 experimental conditions × 4 samples/condition × 5 independent replicates), with 60 samples used for sensory analysis and 20 samples used for bacterial diversity analysis.
Spoilage characterization.
The sensory characteristics of spoiled sausages were described after 21 days of storage at 8°C (time Ta) in comparison with control samples (fresh samples stored 21 days at −20°C). Visual spoilage evaluation was first based on visual color changes (confirmed by chromametric measurements), the production of exudate, and the presence of swelling in the packaging or casing (CO2 production measured by gas analysis). Immediately after the bags were opened, spoilage was smelled by a jury of four trained persons. For the smelling, all jurors choose freely the terms to describe the spoiled samples, all terms were gathered by the four members, and the jury discussed the use of the most appropriate or more general terms. The jurors then assigned a spoilage value, a unique spoilage value for one sample. The spoilage scale ranges from 1 to 5, with a value of zero being reserved for unspoiled samples. Intermediate values of 0.5 or 1.5 were attributed to the batches in case of important variations between the samples.
For each MAP sample, O2 and CO2 levels (PAK12P; Abiss, Chatillon, France) in the packaging were measured two times: 1 day after packaging, in order to estimate CO2 absorption by the meat, and once at spoilage time (Ta). For each sample, pH was measured (pH 213; Hanna Instruments, Tanneries, France) from 10 g of sausages that were homogenized in 10 ml of distilled water, according to ISO standard 2917:1999 (27). For each sample, the exudate was weighed and reported in proportion to the initial weight of the whole sausage. The color of the samples was measured by chromametric measurements (CR100; Minolta, Carrières-sur-Seine, France) performed at the surface of the spoiled sausages. The color coordinates L* (lightness), a* (red/green opponent colors), and b* (yellow/blue opponent colors) were recovered. Five measurements were performed on each of 12 samples. The results were expressed as a difference between the analyzed sample and the control fresh sample (e.g., ΔL* = L*analyzed − L*control).
Microbiological analysis.
Total viable counts were estimated from 25 g of each spoiled sausage sample after homogenization in 225 ml of peptone water (catalog no. CM 0509B; Oxoid, Dardilly, France) in a stomacher (AES Chemunex, Marcy l'Etoile, France). Dilutions were plated on tryptone soya agar (catalog no. P05012A; Oxoid) and on de Man, Rogosa, and Sharpe agar (catalog no. CM0361B; Oxoid) and incubated for 72 h at 30°C to estimate the total aerobic mesophilic population and the mesophilic lactic acid bacteria population size in CFU g−1 of meat, respectively. The psychrotrophic lactic acid bacterium population was determined on MRS agar and incubated for 7 days at 15°C.
Bacterial DNA extraction.
Ten grams of meat was homogenized in 40 ml of sterile ultrapure water with 1% Tween 80 (catalog no. 278630010; Acros Organics, Waltham, MA, USA) for 30 s in a stomacher. Then, 32 ml of the shreds was collected and centrifuged at 500 × g for 3 min at 4°C (5804R; Eppendorf, Montesson, France) to spin down the meat fibers. The clean supernatant (25 ml) was collected and centrifuged at 3,000 × g for 5 min at 4°C to spin down the bacterial cells. The cell pellet obtained was washed in 1 ml of sterile ultrapure water and collected after centrifugation at 3,000 × g for 5 min at 4°C.
Bacterial DNA was extracted with the NucleoSpin tissue kit (Macherel Nagel, Hoerdt, France), in accordance with the manufacturer's instructions, and resuspended in 100 μl of BE buffer (5 mM Tris-HCl [pH 8.5]). DNA samples were then stored at −20°C.
Barcoded-amplicon pyrosequencing.
For each sample, barcoded pyrosequencing of the 16S rRNA V1 to V3 region was performed using 27F and 534R universal primers, as described by Chaillou et al. (20). Ten nanograms of DNA was used as the PCR template, and PCR bias was minimized with the following cycling conditions: a temperature gradient (60°C to 0.5°C/cycle) and 25 cycles, as described by Barott et al. (28). Three independent PCRs were performed per sample. Each PCR product was purified using the QIAquick PCR purification kit (Qiagen, Courtaboeuf, France), according to the manufacturer's instructions. Finally, the three PCR products were pooled in equimolar quantity and checked for quality on a DNA7500 chip (Agilent Technologies, Les Ulis, France). Amplicons were pyrosequenced from the forward A-side with 454 GS FLX++ Titanium technology (Eurofins Genomics, Ebersberg, Germany) at an approximate scale of 15,000 reads per sample.
OTU-based microbial diversity analysis and bioinformatic data analysis.
The raw pyrosequencing reads were filtered using the Prinseq tool (29), with the following options: no ambiguous N; length between 350 and 650 bp; minimum quality score of 10, and an average quality score of ≥20; quality trimming at the 3′ end with a score of <25 and at position 50; and a sliding window size of 4 to calculate the quality score. Quality-filtered reads were clustered into operational taxonomic units (OTUs) using the RDP aligner tool (https://pyro.cme.msu.edu/index.jsp) (30), with a similarity value of 98%. Then, chimeric OTUs were detected using the online DECIPHER software (http://decipher.cee.wisc.edu/FindChimeras.html) (31) and subsequently excluded. An additional step of OTU removal was carried out by filtering low-abundance (<5 × 10−5) OTUs, which are likely to belong to artifact abundant species (32–34). This process removed 75% of spurious OTUs but kept 99.5% of all reads. Taxonomic assignment of the filtered OTUs was carried out by a BLASTn alignment to the nonredundant (nr99) version of the LTP-version 111 Silva (35) database (http://www.arb-silva.de), as described by Chaillou et al. (20). In parallel, a second taxonomic assignment (98% identity) was performed for OTUs with no match in the LTP-version 111 database using the SINA aligner of the SILVA database (36) and the uncultured EzTaxon database (http://www.ezbiocloud.net/eztaxon) (37) as a control. Finally, to validate the quantification of the relative abundance of the OTUs present in each sample, reads from the corresponding sample were aligned to the 78 representative OTUs resulting from the present study, as previously described by Chaillou et al. (20), and an abundance matrix was built (see Data Set S1 in the supplemental material). To compare the relative abundances of the OTUs, reads were then normalized to the median value of the total reads (as described by Chaillou et al. [20]), and the relative abundance values were subjected to a log10 transformation. The Chao1 diversity estimator (38) was calculated in an Excel spreadsheet using the abundance table. Bacterial evenness was estimated for each sample using a set of core species (species present in all samples analyzed; see Results). This evenness value was calculated using R statistical software, version 3.1.2 (39), and corresponded to the median value of all log10 differences between the most abundant OTU and all other subdominant OTUs in the set of core species (Nmax − Ni), where Nmax was the log10 number of reads from the most abundant OTU, and Ni was the log10 number of reads from each of i subdominant species. Box-and-whisker plots and bar plots were constructed with the UsingR package of R, version 2.0.5 (40), and violin plots were constructed with the R ggplot2 package, version 1.0.1 (41).
Quantitative PCR.
A set of 18 species was selected for real-time quantitative PCR. For that purpose, 23 pairs of primers were designed to target either species- or genus-specific amplification, as described in Table 4. All real-time PCR amplifications were performed as described in reference 42. Briefly, the reaction mixture contained 5 μl of total bacterial genomic DNA (diluted 10-fold to allow sufficient removal of inhibitor effects) with 15 μl of a reaction mixture containing 10 μM each primer (forward and reverse), 10 μl of SYBR Mesa green master mix reagent (Eurogentec, Liège, Belgium), and 4.2 μl of sterile water. Each PCR run included a positive-control DNA sample (isolated from type strains) and a no-template control. The initial step of quantitative PCR (qPCR) amplification was one cycle at 95°C for 2 min, followed by 40 cycles of denaturation at 95°C for 15 s and annealing at 60°C for 1 min. The step for melting curve checking was performed at 95°C for 15 s, 1 min at 60°C, and 20 min ramping from 60°C to 95°C. Each sample was quantified in duplicate, and the average threshold cycle (CT) was calculated. To estimate the population level of the target bacteria in spoiled sausages in CFU g−1 of meat, two types of equations were used, depending on whether the gene probe was based on 16S rRNA gene or on another housekeeping gene (see Fig. S1b and Table S1c in the supplemental material). These equations were obtained after three independent biological replicates of calibration curves, which were carried out as described by Chaillou et al. (42).
TABLE 4.
Descriptions of genus- or species-specific primers used for quantitative PCR detection of target species in meat
| Primer pair | Target species (target gene) | Strain used in calibration | Sequence (5′–3′) | Amplicon size (bp) | Annealing temp (°C) | Reference or source |
|---|---|---|---|---|---|---|
| Based on 16S rRNA gene | ||||||
| QSF01-ACI-F | Acinetobacter spp. (16S) | Acinetobacter calcoaceticus CIP 81.8 | GAAGCTAGAGTATGGGAGAGGA | 108 | 55 | This study |
| QSF01-ACI-R | GTCAGTATTAGGCCAGATGGCT | 55 | ||||
| QSF01-BTH-F | Brochothrix spp. (16S) | Brochothrix thermosphacta 160x8 | ACGAACGGATAAAGAGCTTGCTCTTTTG | 158 | 58 | This study |
| QSF01-BTH-R | CGAAACCGTCTTTCACTTGAACATCTTAT | 57 | ||||
| QSF01-CAR-F2 | Carnobacterium spp. (16S) | Carnobacterium divergens MFPC19D14-04 | CTGCCCATAAGAGGGGGATAACATTC | 104 | 59 | This study |
| QSF01-CAR-R2 | ATCCATAAGTGGTAGCCGAAGCCAC | 60 | ||||
| QSF01-HAL-F | Hafnia/Obesumbacterium spp. (16S) | Hafnia alvei CIP 57.31 | TAACTTGGGAACTGCATTTGAAACTGGTC | 110 | 59 | This study |
| QSF01-HAL-R | CGCCACTGGTGTTCCTCCAGATC | 61 | ||||
| QSF01-LCN-F2 | Leuconostoc spp. (16S) | Leuconostoc mesenteroides MFBP17A13-01 | GTGAAAGCCCGGAGCTCAACTC | 118 | 59 | This study |
| QSF01-LCN-R2 | CTGGTGTTCTTCCACATATCTACGCATTC | 60 | ||||
| QSF01-LIS-F | Listeria spp. (16S) | Listeria innocua CIP 80.11 | CTGCCTGTAAGTTGGGGATAACTC | 170 | 57 | This study |
| QSF01-LIS-R | GCTATGCATCGTTGCCTTGGTAG | 57 | ||||
| QSF01-PSD-F | Pseudomonas spp. (16S) | Pseudomonas fragi K1 | GGTCTTCGGATTGTAAAGCACTTTAAGTTG | 100 | 60 | This study |
| QSF01-PSD-R | GTAGTTAGCCGGTGCTTATTCTGTCG | 59 | ||||
| QEBP01-SER-F | Serratia spp. (16S) | Serratia proteamaculans CIP 103236 | CTAGCTGGTCTGAGAGGATGAC | 280 | 57 | This study |
| QEBP01-SER-R | CCGTCAATGCAATGTGCTATTAACAC | 56 | ||||
| QSF01-WVI-F | Weissella spp.(16S) | Weissella viridescens CIP102810 | TTGCTCAGATATGACGATGGACATTGCA | 170 | 58 | This study |
| QSF01-WVI -R | GACCATCTCTTAGTGATAGCAAGACCAT | 58 | ||||
| Based on single-copy gene | ||||||
| QSF03-BTH-F | B. thermosphacta (rpoC) | B. thermosphacta 160x8 | GGACCAGAGGTTATCGAAACATTAACTG | 148 | 58 | This study |
| QSF03-BTH -R | TAATACCAGCAGCAGGAATTGCTT | 54 | ||||
| QPVS05-CDI-F2 | C. divergens (rpoA) | C. divergens MFPA43A14-05 | CTTGCATAACCTCTTCCTGATTTT | 244 | 52 | This study |
| QPVS05-CDI-R2 | TGATGGCGTTGTTGAGGATGTAACT | 56 | ||||
| QPVS05-CDI-F2 | Carnobacterium maltaromaticum (rpoA) | C. maltaromaticum DSM 20722 | CTTGCATAACCTCTACCTTGTTTC | 244 | 54 | This study |
| QPVS05-CDI-R2 | CGATGGGGTTGTAGAAGATGTGACA | 58 | ||||
| QPVS-ENT-F | Enterococcus spp. (tuf) | Not used in calibration | TACTGACAAACCATTCATGATG | 112 | 56 | 58 |
| QPVS-ENT -R | AACTTCGTCACCAACGCGAAC | 61 | ||||
| QSF04-ECO-F | Escherichia coli (rpoB) | E. coli DH5α | GGTAGCTAAACGTGGTGGTGTC | 101 | 57 | 42 |
| QSF04-ECO-R | ATGTCGATACCTGCTTCACCCGGA | 59 | ||||
| QSF02-HAL-F | Hafnia alvei (fusA) | H. alvei CIP 57.31 | ATTGGCAATTGGCGCTGAAGAAGGT | 129 | 58 | This study |
| QSF02-HAL-R | GCTCAGCCATATCAGCTGGGATC | 59 | ||||
| QCMT05-LAL-F | Lactobacillus algidus (rpoA) | L. algidus CIP 106688 | TGTTGGTCAAAAAGATGATTATGACAAATTG | 140 | 55 | This study |
| QCMT05-LAL-R | AGTTCATCGGTTAAATCAACGAATAAAGT | 54 | ||||
| QPVS06-LCU-F | Lactobacillus curvatus (katA) | L. curvatus FLEC03 | CTGGTAAGAAAACACCAATGATTGCG | 123 | 56 | This study |
| QPVS06-LCU-R | TCGTAGTTGCCTTCTTCGGTATAGAAC | 58 | ||||
| QMF01-F | Lactobacillus sakei (katA) | L. sakei 23K | CTGGCTATCCCGATACATACC | 186 | 54 | 42 |
| QMF01-R | GCATATCTTGGCTACGGGCA | 54 | ||||
| QCMT05-LPI-F | Lactococcus piscium (rpoA) | L. piscium CMTALT10 | TGAAGAAAATGTCTATGGTAAATTTGTCA | 140 | 53 | This study |
| QCMT05-LPI -R | GAGTACACCGTCAATTTGGATGCTT | 56 | ||||
| QPVS05-LCA-F | Leuconostoc carnosum (rpoA) | L. carnosum MFPA29A14-05 | GATTGATTCAGATGAGGAACGTGTT | 84 | 54 | This study |
| QPVS05-LCA-R | CGCACTGCTTGTAAATCAGCT | 55 | ||||
| QPVS05-LCI-F | Leuconostoc citreum (rpoA) | L. citreum MSE2 | GTATCTTACTTTCATCGCTTCCG | 140 | 53 | This study |
| QPVS05-LCI-R | CGCCAACACAACTTTCTTAAGGTTT | 55 | ||||
| QPVS05-LGE-F | Leuconostoc gelidum (rpoA) | L. gelidum subsp. gasicomitatum MFPA44A14-01 | AGAACGAAGCCTTGAAGTTGATA | 115 | 52 | This study |
| QPVS05-LGE-R | CTGCTACGGTTGCAATATGTAG | 53 | ||||
| QEBP02-SPR-F | Serratia proteamaculans (fusA) | S. proteamaculans CIP 103236 | TGGCTATCGGCGCAGAAGAGAAA | 130 | 57 | This study |
| QEBP02-SPR-R | GTTCTTGCATATCAGCCGGGATC | 57 |
Microarray data accession numbers.
The raw FastQ data have been deposited in the ENA database (http://www.ebi.ac.uk/ena) with the accession numbers ERS937270 to ERS937289, corresponding to BioProject no. PRJEB9493.
RESULTS
Study design.
Our aim was to compare the influence of sodium chloride (NaCl) reduction, under two packaging conditions, on the degree of spoilage and the bacterial diversity of raw pork sausages. For that purpose, a square matrix of four experimental conditions was designed, which included two concentrations of NaCl (2.0% [wt/wt] and 1.5% [wt/wt]) and two packaging conditions (modified atmosphere packaging [MAP; 50% CO2 and 50% N2] and vacuum packaging [VPA]). The microbiological variability of the raw meat was assessed by analyzing five independent replicates (i.e., five different meat batches, with fresh meat for each batch, were prepared and analyzed over a period of 4 months in 2014). In all, 20 sets of meat (5 batches × 4 experimental conditions) were investigated. For each set, fresh samples (T0) were kept at −20°C and used as controls for sensory analysis (nonspoiled sausages). Samples for spoilage analysis (Ta) were stored at 8°C for 21 days (use-by date).
Salt reduction is associated with acid and exudate production.
Physicochemical parameters (pH, CO2, O2, exudate production, and chromametric measurements) of the various samples are shown in Table 1 and were used in a principal-component analysis in order to differentiate among spoiled samples (Fig. 1). The samples could be discriminated based on sodium chloride concentration but not on the type of packaging atmosphere. Samples with a lower sodium chloride concentration showed a higher degree of spoilage, which correlated with both higher exudate production (1.48% ± 0.78% versus 0.20% ± 0.23% [wt/wt] in 1.5% and 2.0% [wt/wt] NaCl, respectively) (P < 0.0006, two-tailed t test) and slightly lower pH (5.04 ± 0.35 versus 5.30 ± 0.30 in 1.5% and 2.0% [wt/wt] NaCl, respectively); these two parameters were correlated (R2 = −0.376, P = 0.102).
TABLE 1.
Physicochemical parameters of spoiled sausages
| Batch name (packaging_salta) | Sodium content (mg/100 g) | Estimation of NaCl content (%)b | Exudate (% [wt/wt]) | pH | ΔL*c | Δa*d | Δb*e | Residual O2 (%) | Residual CO2 (%) |
|---|---|---|---|---|---|---|---|---|---|
| MAP_15 | 611 ± 20 | 1.55 ± 0.05 | 0.93 ± 0.75 | 5.07 ± 0.37 | 1.7 ± 4.0 | 0.7 ± 3.6 | 0.3 ± 1.5 | 1.0 ± 0.9 | 32.7 ± 9.2 |
| MAP_20 | 829 ± 14 | 2.11 ± 0.04 | 0.08 ± 0.19 | 5.31 ± 0.33 | −0.1 ± 3.5 | 1.1 ± 3.9 | −0.3 ± 0.8 | 1.2 ± 1.5 | 28.9 ± 11.1 |
| VPA_15 | 611 ± 20 | 1.55 ± 0.05 | 2.03 ± 0.82 | 5.01 ± 0.34 | 1.2 ± 4.3 | 0.5 ± 3.4 | 0.2 ± 2.1 | NDf | ND |
| VPA_20 | 829 ± 14 | 2.11 ± 0.04 | 0.26 ± 0.41 | 5.27 ± 0.30 | −0.1 ± 3.4 | 0.6 ± 4.2 | −0.2 ± 3.0 | ND | ND |
MAP, modified atmosphere packaging; VPA, vacuum packaging. For salt concentrations: 15, 1.5% NaCl; 20, 2.0% NaCl.
NaCl = 2.54 × Na (MMNa = 23 g mol−1, MMCl = 35.5 g mol−1, MMNaCl = 58.44 g mol−1 [where MM is molecular mass]; mNaCl = 58.44/23 × mNa [where m is mass]).
ΔL*, difference between spoiled and fresh samples; ΔL* tends to be positive, indicating that spoiled samples were clearer (see Materials and Methods).
Δa*, difference between spoiled and fresh samples; Δa* tends to be negative, indicating that spoiled samples had lost red intensity.
Δb*, difference between spoiled and fresh samples; Δb* tends to be negative, indicating that spoiled samples had lost yellow intensity.
ND, not determined. Gas composition in VPA samples could not be taken.
FIG 1.
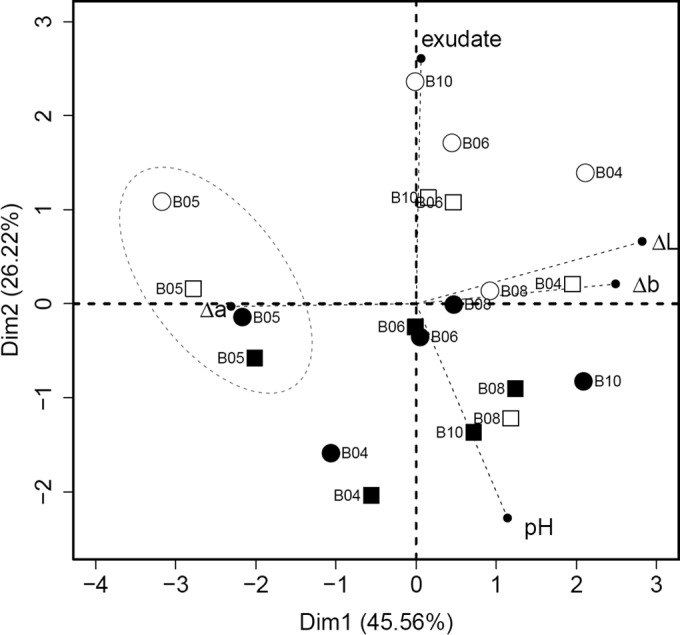
Principal-component analysis (PCA) based on physicochemical parameters (pH, production of exudate, and meat color components Δa*, Δb*, and ΔL*) of spoiled sausages at Ta (21 days). The plot shows the discrimination of samples based on salt content. MAP samples are depicted with squares, VPA with circles, 1.5% salt is in white, and 2% salt is in black. Samples from the same meat batch are marked with the same batch number (from B04 to B10). The main effect of variables is shown in the thin dashed lines. It clearly shows that the most influent variable is salt content (45.56%), with a higher production of exudate and lower pH in 1.5% samples than in 2% samples. The proportion of variance explained by each axis is shown.
Other physicochemical parameters had less discriminatory power, although noticeable graying (loss of red, negative Δa*) and bleaching (positive ΔL*) were observed in all spoiled samples (with the exception of batch B05, which was not grayed). Furthermore, in three MAP sample sets (MAP_B08_15, MAP_B10_15, and MAP_B10_20), swelling was observed; this correlated with an increase of about 80% in CO2 levels, at 44.6% ± 6% CO2 versus a normal content of 24.8% ± 4%.
Production of off-odors is packaging dependent.
Correspondence factorial analysis was performed in order to correlate off-odor type with experimental conditions (Fig. 2). This analysis revealed that the type of packaging atmosphere strongly changed the nature of spoilage odors (principal component, >90%). Rancid off-odors were mainly associated with VPA, while sour off-odors were largely linked with MAP. Furthermore, the combination of MAP and low sodium chloride concentration (1.5% NaCl) also correlated with the detection of sulfurous off-odors, whereas the combination of 2% NaCl with VPA storage gave a sensory odor analysis close to that of the control (nonspoiled) samples. In addition, during the sensory analysis, the panelists assessed the spoilage odor intensity and attributed to each sample a value ranging from 0 to 5 (Table 2 and Materials and Methods). A distribution of these values for the 20 sample sets among the four experimental conditions is shown in Fig. 3. Both packaging atmosphere conditions and sodium chloride concentration mediated changes in spoilage intensity. The general trend was a gradient, with spoilage intensity being stronger both with sodium chloride reduction and under MAP versus VPA storage conditions.
FIG 2.
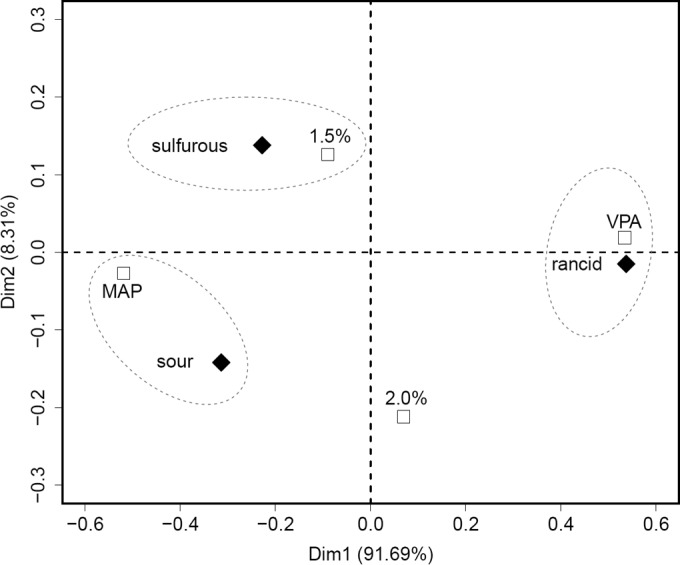
Correspondence factorial analysis (CFA) showing the relationship between off-odors (◆) identified in spoiled sausages and experimental conditions (□). This analysis distinguishes off-odors of samples, according to the experimental conditions (n = 10 per condition). Samples with 2% NaCl were not associated with an off-odor; they were close to the control samples.
TABLE 2.
Sensory parameters of spoiled sausages
| Batch name (packaging_batch no._salta) | Off-odor(s) | Spoilage sensory intensity (0 to 5)b |
|---|---|---|
| MAP_B04_15 | Sulfurous | 4 |
| MAP_B05_15 | Sulfurous, sour | 2.5 |
| MAP_B06_15 | Sulfurous | 1.5 |
| MAP_B08_15 | Sulfurous, sour | 2 |
| MAP_B10_15 | None | 1 |
| MAP_B04_20 | Sour | 1.5 |
| MAP_B05_20 | Sulfurous, sour | 1.5 |
| MAP_B06_20 | None | 0 |
| MAP_B08_20 | Sour, rancid | 1 |
| MAP_B10_20 | None | 1.5 |
| VPA_B04_15 | Sulfurous, sour | 3 |
| VPA_B05_15 | Rancid | 2 |
| VPA_B06_15 | Rancid | 0.5 |
| VPA_B08_15 | Sour, rancid | 1.5 |
| VPA_B10_15 | Sulfurous, rancid | 2 |
| VPA_B04_20 | Sulfurous | 2 |
| VPA_B05_20 | Rancid | 1 |
| VPA_B06_20 | Rancid | 1 |
| VPA_B08_20 | Rancid | 0.5 |
| VPA_B10_20 | None | 1 |
MAP, modified atmosphere packaging; VPA, vacuum packaging. For salt concentrations: 15, 1.5% NaCl; 20, 2.0% NaCl.
Spoilage sensory intensity on a scale from 0 (fresh meat) to 5 (strongly spoiled) (see Materials and Methods).
FIG 3.
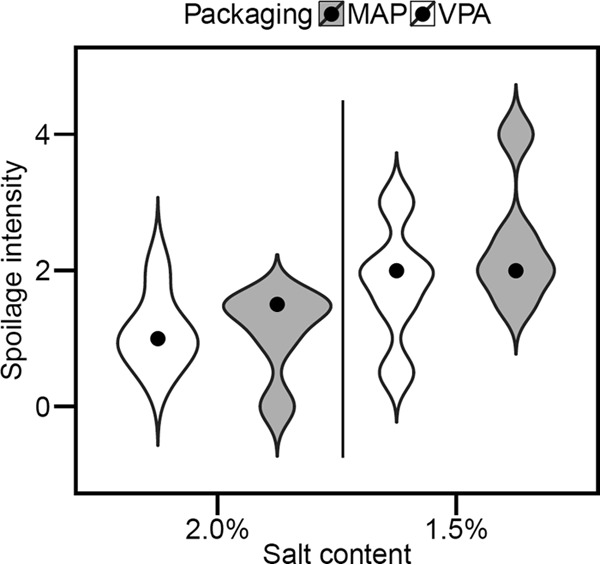
Violin plot showing the gradient of spoilage intensity (aspect and off-odors) of spoiled sausage on a scale from 0 to 5, according to the salt content (x axis) and the packaging condition (MAP samples in gray and VPA in white). The shape of each plot reflects its distribution density. The black circles represent the median value of spoilage intensity for each condition (n = 5).
Differences in types and degrees of spoilage do not correlate with concentration of culture-dependent bacteria.
The total population of cultivable microorganisms in all spoiled sausages ranged from 8.08 ± 0.31 to 9.17 ± 0.19 log10 CFU g−1 of meat, and there was no difference associated with either sodium chloride reduction or the type of packaging atmosphere (Table 3). Therefore, variations in spoilage intensity could not be correlated to the overall bacterial load after 21 days of storage. Additionally, the bacterial concentration measured in fresh T0 samples, which ranged from 3.64 ± 0.15 to 4.85 ± 0.18 log10 CFU g−1 of meat, was not linked with either of the two factors (Table 3).
TABLE 3.
Bacterial enumeration and OTU richness in spoiled sausages
| Batch name (packaging_salta) | Total viable population of cultivable microorganisms (CFU g−1 of meat) atb: |
No. of OTUs | |
|---|---|---|---|
| T0 | Ta | ||
| MAP_15 | 4.23 ± 0.51 | 8.55 ± 0.41 | 14 ± 3 |
| MAP_20 | 4.09 ± 0.38 | 8.65 ± 0.36 | 18 ± 5 |
| VPA_15 | 4.23 ± 0.51 | 8.57 ± 0.38 | 22 ± 7 |
| VPA_20 | 4.09 ± 0.38 | 8.55 ± 0.34 | 23 ± 8 |
MAP, modified atmosphere packaging; VPA, vacuum packaging. For salt concentrations: 15, 1.5% NaCl; 20, 2.0% NaCl.
Bacterial enumeration on tryptone soya agar plates (see Materials and Methods). The results on MRS agar (incubated at 30°C and 15°C) were not different, so they are not presented.
VPA storage and high salt concentration maintain higher bacterial richness.
Next, we sought to describe the bacterial diversity of spoiled sausages using a nonculturing analysis, in particular, with pyrosequencing of the 16S rRNA V1 to V3 region. Each of the 20 sample sets was sequenced at a scale of ∼15,000 reads per sample. In the whole data set, a total of 387,111 bacterial 16S rRNA sequences were analyzed (average number of reads, 17,819 ± 2,227 and average sequence length, 549 bp ± 10 bp). The rarefaction analysis is shown in Fig. S1a in the supplemental material and revealed satisfactory sequencing coverage for most of the samples. These reads were binned to a 98% identity threshold, as suggested by Kim et al. (43); after removal of chimeras and low-frequency binning clusters, we characterized 78 different species- or species/clade-level (groups of closely related species) operational taxonomic units (OTUs). The abundance table can be found in Data Set S1 in the supplemental material.
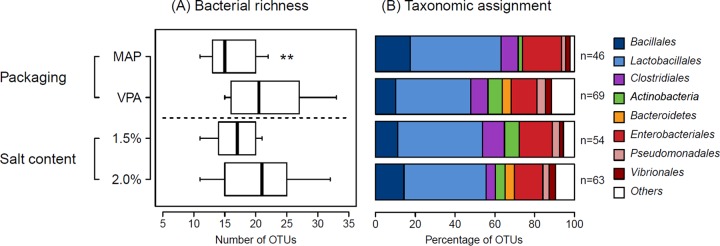
A comparative analysis of bacterial richness (total number of different OTUs), illustrated in Fig. 4A, revealed that samples that were stored under VPA conditions had significantly (P < 0.02, two-tailed t test) higher richness than those stored under MAP conditions, with an average of 22 ± 6 OTUs versus 16 ± 4 OTUs, respectively. In addition, the overall richness in all VPA samples was ∼50% higher (n = 69 versus n = 46 OTUs) than in all MAP samples. As shown in Fig. 4B, >80% of the OTUs in MAP samples belonged to only three bacterial orders (Lactobacillales, Enterobacteriales, and Bacillales), but a more diverse range of bacterial taxa was represented by the OTUs in VPA samples. This richness was, however, mostly influenced by OTUs from the subdominant population. Rarefaction curves shown in Fig. S1a in the supplemental material also indicate that higher sequencing coverage could still increase the bacterial richness in some of the VPA samples. To confirm these results, we calculated the Chao1 index (S*1), a statistical estimator that describes a predictive richness based on the number of singletons or doubletons (i.e., the subdominant population) detected per sample (38). The difference in Chao1 estimations between VPA and MAP samples, at 33.1 ± 13.1 versus 19.4 ± 6.7 OTUs, respectively, was larger than that observed between the numbers of OTUs (see above). In other words, the increased diversity of subdominant OTUs in VPA samples led to a larger difference between the Chao1 value and the number of observed OTUs in that packaging condition than was found in MAP samples, for which there was little difference between these two metrics. When a similar comparative analysis of bacterial richness was performed on samples with differing sodium chloride concentrations, we observed a higher maintained richness at the 2.0% than the 1.5% salt concentration, although it was not statistically significant.
FIG 4.
(A) Box plot showing the average number of OTUs (bacterial richness) identified in spoiled sausages according to salt content and packaging conditions (all samples combined). In the plots are marked the median (bold line) and the interquartile range (box) between the first and the third quartiles obtained from the 10 samples for each condition. Asterisks indicate that samples deviated significantly from the average (P < 0.02, two-tailed t test). (B) Bar plots representing the taxonomic assignments of OTUs according to salt content and packaging conditions. The total number of OTUs for all samples under each condition is indicated on the right of the bar plot. Only the most dominant bacterial orders obtained in this study are specifically indicated; others are grouped in “Others.”
Salt reduction modifies the subdominant population with a species-dependent effect.
We identified around 20 OTUs that constituted the conserved bacterial community of spoiled raw sausages (sum of reads, >100 across samples; prevalence, at least 30%). The highly prevalent and dominant core was composed of six OTUs (OTUs conserved in 90% of all samples), which were taxonomically assigned to Lactobacillus sakei, Lactococcus piscium, Carnobacterium divergens, Carnobacterium maltaromaticum, Serratia proteamaculans genotypic clade (including S. grimesii, S. plymuthica, and S. quinovorans) and Brochothrix thermosphacta. Considering the whole data set, L. sakei was the most abundant species. However, L. sakei was the most dominant only in two meat batches (samples from batches 04 and 05) and was slightly outcompeted either by L. piscium in samples of batch 06, Lactobacillus algidus (four samples from batch 10), or Weissella viridescens (sample MAP_B08_15, from batch 08). The high abundance of L. algidus and W. viridescens was associated with higher CO2 concentrations in the MAP samples. However, these two bacterial spoilers were present at a low frequency, as they were undetected in 10 and 13 samples out of 20, respectively. Below the core dominant group, a set of 13 subdominant OTUs was also detected with moderate frequency (>30% of samples) but with lower relative abundance. These included OTUs assigned to Lactobacillaceae (Lactobacillus curvatus, Lactobacillus fuchuensis, and Lactobacillus oligofermentans), Enterococcaceae (Enterococcus avium, Enterococcus durans genotypic clade [including E. hirae and E. lactis], and Vagococcus fluvialis), Leuconostocaceae (Leuconostoc carnosum and Leuconostoc citreum), and Enterobacteriaceae (Citrobacter youngae genotypic clade [including C. gilleni and C. braakii], Enterobacter kobei genotypic clade [including E. mori, E. ludwigii, E. cloacae subsp. cloacae, and Pantoea agglomerans], Gibbsiella quercinecans, Lelliottia amnigena, and Yersinia kristensenii).
A set of nine quantitative PCR gene probes was then chosen or designed (Table 4; see also Fig. S1b in the supplemental material) in order to quantify the bacterial concentration of most of the OTUs present in the dominant and subdominant core community. Except for Serratia proteamaculans, which was underestimated in the pyrosequencing analysis, we found a strong linear relationship between the relative abundance (number of reads) obtained from pyrosequencing and the population level, as determined by qPCR; we used linear regression in order to convert relative read counts into an extrapolated estimation of the population level in cells per gram of meat for all OTUs (see Fig. S1e in the supplemental material). The results are shown in Fig. 5.
FIG 5.
Comparative box plot analysis showing the effect of salt content (right) or packaging type (left) on the abundance of species or species groups. Abundance is expressed in estimated cells per gram (log10) (see Data Set S1 in the supplemental material). The median abundance values (red points) and interquartile range (box) obtained from the 10 samples analyzed per condition are depicted. Species or groups of species are presented in decreasing order of abundance and are separated into two groups: the dominant core and the subdominant core. The three types of classes affected by the experimental conditions are indicated by the following: A, L. carnosum/L. citreum, with increased population both upon sodium chloride reduction and when MAP packaging was used; B, B. thermosphacta and L. oligofermentans, with a decrease in population with sodium reduction and increase with MAP packaging; and C, which included OTUs belonging to Enterococcaceae and Enterobacteriaceae, in particular the species Serratia proteamaculans, showing an increase in population at the high sodium chloride concentration and under VPA. The results of several OTUs were combined, such as those for C. divergens and C. maltaromaticum, showing similar situations in terms of prevalence and abundance pattern (rank 3 and rank 4, respectively). Therefore, their abundance data were combined to lighten the plot without removing important information. Similar strategies were used to combine groups of subdominant populations. |CL| indicates a possible multiaffiliation, as the Serratia proteamaculans genotypic clade included S. grimesii, S. plymuthica, and S. quinovorans.
We observed that (i) the abundance of core dominant species was less affected by the type of packaging or by the sodium chloride concentration than that of the subdominant species, (ii) the effects of the two factors (sodium chloride and packaging) on abundance were strongly species dependent, and (iii) the reduction in sodium chloride concentration induced more changes in abundance than did differences in packaging type.
Among the species whose populations significantly changed, several effect classes could be identified. The first class (A) included only L. carnosum/L. citreum. Although present at a subdominant level (average, ∼4.5 log10 cells g−1 of meat), it had an increased population both upon sodium chloride reduction and when MAP packaging was used (this experimental condition showed the highest degree of spoilage on average; Fig. 3). The second class (B) included mainly B. thermosphacta and, to a lesser extent, L. oligofermentans; these species exhibited the opposite reaction to sodium chloride reduction (decrease in population) and to MAP packaging (increase in population). Concerning this class, we noticed that samples from batch 05 that demonstrated less graying (Fig. 1) were also characterized by a high abundance of L. oligofermentans, regardless of salt or packaging conditions. Finally, the third class (C) included OTUs belonging to Enterococcaceae and Enterobacteriaceae, in particular the species Serratia proteamaculans, which showed a significant increase in population at the high sodium chloride concentration and under VPA storage conditions.
Salt reduction induces a loss of bacterial evenness.
Together with the measure of bacterial richness (the number of taxa), the measure of bacterial evenness (distribution of abundance among taxa) is an important criterion in beta-diversity analysis. The fact that the subdominant communities observed in raw pork sausage samples showed an increase in population in the VPA-2.0% salt condition, and that this correlated with the lowest degree of spoilage on average (Fig. 3), suggests the possible involvement of bacterial evenness in decreasing the spoilage of raw pork sausages. This observation prompted us to compare, for the samples, the average differences in population found between the most dominant species and other subdominant species. However, at low levels of bacterial richness (∼20 taxa), many evenness indices (e.g., Shannon-Weaver) lack sensitivity (44). Therefore, to measure evenness, we simply calculated for each sample the mean difference in abundance between the dominant species and all subdominant species (see Materials and Methods). The results are shown in Fig. 6 and demonstrate unambiguously that packaging had no effect on bacterial evenness, whereas the high salt concentration (2.0%) was capable of maintaining a much higher degree of bacterial evenness and, in particular, more parity between OTUs belonging to the Lactobacillales order and those from the Enterobacteriaceae family.
FIG 6.
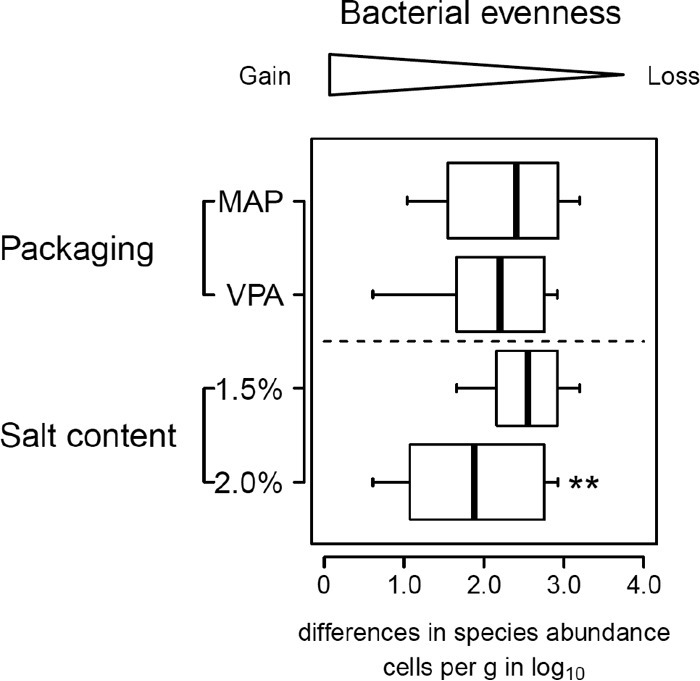
Comparative box plot analysis showing the difference in relative abundance (bacterial evenness) between the most dominant and the subdominant species. The evenness values, expressed in Δlog10 estimated cells g−1 of meat, were obtained from the mean differences in relative abundance between the dominant OTU and each of the subdominant OTUs (see text and Materials and Methods). The closer the difference is to 0, the more even the populations of OTUs are in the samples. Asterisks indicate that samples deviated significantly from the average (P < 0.05, two-tailed t test).
DISCUSSION
The aim of this study was to identify, in cold-stored raw pork sausages, the changes in spoilage intensity and bacterial community composition induced by a 25% reduction in the standard sodium chloride concentration (2% [wt/wt] NaCl). From previous research, a reduction in sodium chloride was expected to increase spoilage. We therefore combined this analysis with a comparison of two methods of packaging: modified atmosphere (50% CO2 and 50% N2) and vacuum packaging. The sausages lacked any kind of preservatives, particularly sodium-based preservatives (i.e., sodium lactate or sodium nitrite), in order to clearly distinguish the effect of sodium chloride reduction.
The absence of preservatives, with the exception of NaCl, guaranteed that following 21 days of storage at 8°C, we would have available spoilage of widely varied intensity, with some samples displaying just the onset of spoilage and others instead with strong qualitative deterioration. Our results confirmed these expectations, as the 25% sodium chloride reduction resulted in stronger spoilage, particularly when MAP packaging was used. These data corroborated those of a recent study showing higher volatile compound production under MAP than under vacuum in beef and pepper foods (45), therefore indicating that VPA would slightly but significantly help in mitigating spoilage that is due to sodium chloride reduction. In terms of the physicochemical properties of raw sausages, salt reduction had only one main effect: significantly higher exudate production due to a decrease in water-binding capacity. The other observed changes, such as the decrease in final pH and color change (graying), were largely general to all spoiled products and were similar to the results of previous studies (46, 47). Some differences were noticed for a few samples, but we could associate these with a specific change in the bacterial communities identified in these products. For example, the samples from batch 05 that demonstrated less graying were enriched in L. oligofermentans, while W. viridescens and L. algidus were abundant in the MAP-packaged samples that displayed increased CO2 production.
Sodium chloride reduction also induced the appearance of sulfurous off-odors, which, in combination with the sour smells associated with MAP packaging, resulted in strong olfactory deterioration in the 1.5% salt-MAP samples (among the highest spoilage intensity values). Our results also confirmed previous studies (22, 45) that indicated that the off-odors produced in VPA and MAP packaging are different. In our case, VPA produced rancid odors, while MAP produced sour odors.
Recent reviews on meat spoilage have highlighted the fact that spoilage phenomena are hard to decipher (22, 24, 45). Spoilage is often the complex outcome of various metabolic activities that are associated with multiple parameters, such as the type of food (and thus the nutrients available), type of packaging, storage temperature, and last but not least, the diversity of the microbial communities involved in spoilage (48). In an attempt to address this last point, we characterized changes in bacterial diversity in the raw sausages due to sodium chloride reduction. Specifically, we used non-culture-based methods (quantitative PCR and 16S amplicon sequencing) in order to evaluate both the diversity and relative abundance of each subpopulation. It is from these analyses that we obtained the most interesting and unexpected results.
Our study showed that all spoiled sausages, regardless of the experimental conditions tested and the type and intensity of spoilage, contained a core bacterial community of about 20 different species. Not surprisingly, these all belong to a group of bacteria that is highly prevalent in spoiled meat and seafood products (20). Also, the high abundance of L. sakei in many samples was expected, as this halotolerant bacterium (49, 50) is often counterselected in salted meat products, such as fermented dry sausages (49). This species has been also found to be dominant in MAP-stored poultry sausages and diced bacon (both salted products) at the end of their shelf lives (20). It should be noted that L. sakei has occasionally been associated with meat preservation (42, 51, 52) or with the spoilage of some raw meat and seafood products, leading to acidification and the production of off-odors (23, 52–55).
In our study, L. sakei was present at a scale of 5 to almost 8 log10 CFU g−1 of meat and was associated with several other species, including C. divergens, L. piscium, B. thermosphacta, S. proteamaculans, and other Enterobacteriaceae. This core bacterial community is similar to that found recently by Benson et al. (25), who studied the dynamics of microbial growth in raw pork sausage treated with various degrees (3% to 9%) of sodium- and acid-based preservatives. However, their experiments differed from ours in that they used preservatives in addition to spices and salt, and because they did not evaluate the variability of meat batches in their analysis. Nevertheless, they identified Lactobacillus graminis (phylogenetically related to L. curvatus and belonging to the L. sakei genomic clade) as the dominant species and showed that, in their situation, the bacterium originated from the spice mix used.
The purpose of our work was not to seek out the origin of the spoilage-causing bacteria but to understand how bacterial diversity (richness and evenness) correlated with spoilage. Our most surprising result came from analyses of the evenness parameter of diversity, which was higher in less-spoiled sausages and dropped in spoiled sausages. Furthermore, the loss of evenness in spoiled products was not correlated with a significant increased abundance of the dominant species but, rather, decreased abundance of the subdominant species. In other words, the subdominant bacterial populations, in particular, species of Enterobacteriaceae, were much more abundant at the higher sodium chloride concentration. Several previous studies have revealed that a total count of >7 log10 CFU g−1 of meat is the threshold for spoilage deterioration (3, 56, 57). Here, all samples contained a bacterial population that was larger than this threshold, but aspect and odor spoilage occurred at either high or low intensity, and samples with low spoilage intensity showed higher species evenness. To a lesser extent, VPA led to results similar to those found with high sodium chloride concentration, but VPA also maintained higher species richness (mainly in the subdominant population). These results are quite different from those obtained by Miller et al. (17), who found that bacterial species diversity was higher in low-sodium ready-to-eat meat products than in products with a regular sodium content. However, a comparison of the two studies should be taken with care, as this previous experiment was carried out with a wider difference in salt content (from 0.3% to 1.2%). Furthermore, they applied less-sensitive methods to assess the subdominant population (plating and denaturing gradient gel electrophoresis), in comparison to the species-specific quantitative PCR and 16S metagenetic pyrosequencing used here.
In a way, our results are quite puzzling and counterintuitive, as the role of sodium chloride, according to current prevailing wisdom, is to keep microbial growth at a low level (6). In this context, two parameters should be taken into account: the effect of salt on the growth rate of bacteria, and the global population level reached by the bacterial community after a long storage time. As a hurdle technology, the effect of salt is to lower the growth rate of many species. A possible explanation of our results is that salt reduction enables the outcompeting of many subdominant populations by few species having advantages to grow on meat. The fast growth of the very dominant species would likely curb the growth of species with lower fitness. Perhaps this is truly the case, and we would have observed this if we had included a growth kinetic study of the bacterial population in fresh products to the use-by date at 21 days. But, regardless of the growth behavior of bacteria during the early days of storage, we show clearly that less-spoiled sausages contain higher bacterial diversity by the end of their shelf life, and both VPA and a high salt concentration mediate this phenomenon. In particular, the high sodium chloride content leads to an increase in the bacterial diversity in raw sausages, which maintains bacterial evenness without decreasing the final bacterial population level to <108 CFU g−1 of meat.
From our point of view, the interpretation of our data opens an interesting question: how would the faster growth of few species upon low salt content lead to higher spoilage intensity if compared to the slower growth of a more-complex community reaching for longer storage time higher population level and evenness? One possible explanation is that high cell density is reached sooner, with few species enabling a longer period for spoilage-associated molecules to accumulate. However, this view does not fully explain why a more diverse bacterial community would lead to lower spoilage intensity. A second possible explanation is that increased diversity of the meat microbiota is associated with more diverse metabolic activity, or perhaps, with a better-equilibrated level in the bacterial trophic food chain. Such a situation may indeed correlate with a lower accumulation of spoilage molecules if some of the species identified in the sausages are organized or grouped into a commensalism relationship (metabolic activities being beneficial to each others). To test this hypothesis, a more-detailed investigation should be conducted, first to follow the dynamics of bacterial communities throughout storage and second to analyze both the microbial metabolites produced and the transcriptomic activities of the microbiota.
Finally, our results also provide evidence for the underappreciated role of the subdominant populations in food ecosystems and in spoilage phenomena. Additional studies are needed, not only to confirm our findings, but also to improve current knowledge on salt reduction in order to further evaluate guidelines about sodium chloride reduction in meat products.
Supplementary Material
ACKNOWLEDGMENTS
We thank M. Desvaux and P. Colin for their constructive advice and S. Dumoulin for her technical assistance in the manufacture of pork sausages. We are also grateful to the INRA MIGALE bioinformatics platform for providing computational resources and data storage.
Funding Statement
A part of this study was supported by the French DRAAF (Direction Régionale de l'Alimentation, de l'Agriculture et de la Forêt) of Alsace country.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00323-16.
REFERENCES
- 1.De Filippis F, La Storia A, Villani F, Ercolini D. 2013. Exploring the sources of bacterial spoilers in beefsteaks by culture-independent high-throughput sequencing. PLoS One 8:e70222. doi: 10.1371/journal.pone.0070222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hultman J, Rahkila R, Ali J, Rousu J, Björkroth KJ. 2015. Meat processing plant microbiome and contamination patterns of cold-tolerant bacteria causing food safety and spoilage risks in the manufacture of vacuum-packaged, cooked sausages. Appl Environ Microbiol 81:7088–7097. doi: 10.1128/AEM.02228-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoops J, Ruyters S, Busschaert P, Spaepen R, Verreth C, Claes J, Lievens B, Van Campenhout L. 2015. Bacterial community dynamics during cold storage of minced meat packaged under modified atmosphere and supplemented with different preservatives. Food Microbiol 48:192–199. doi: 10.1016/j.fm.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Leistner L. 2000. Basic aspects of food preservation by hurdle technology. Int J Food Microbiol 55:181–186. doi: 10.1016/S0168-1605(00)00161-6. [DOI] [PubMed] [Google Scholar]
- 5.Hutton T. 2002. Sodium technological functions of salt in the manufacturing of food and drink products. Br Food J 104:126–152. doi: 10.1108/00070700210423635. [DOI] [Google Scholar]
- 6.Taormina PJ. 2010. Implications of salt and sodium reduction on microbial food safety. Crit Rev Food Sci Nutr 50:209–227. doi: 10.1080/10408391003626207. [DOI] [PubMed] [Google Scholar]
- 7.Doyle ME, Glass KA. 2010. Sodium reduction and its effect on food safety, food quality, and human health. Compr Rev Food Sci Food Saf 9:44–56. doi: 10.1111/j.1541-4337.2009.00096.x. [DOI] [PubMed] [Google Scholar]
- 8.Nishida C, Uauy R, Kumanyika S, Shetty P. 2004. The joint WHO/FAO expert consultation on diet, nutrition and the prevention of chronic diseases: process, product and policy implications. Public Health Nutr 7:245–250. [DOI] [PubMed] [Google Scholar]
- 9.Uzan A, Delaveau P. 2009. Le sel dans l'alimentation: un problème de santé publique. Ann Pharm Fr 67:291–294. doi: 10.1016/j.pharma.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Ruusunen M, Puolanne E. 2005. Reducing sodium intake from meat products. Meat Sci 70:531–541. doi: 10.1016/j.meatsci.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 11.Samapundo S, Ampofo-Asiama J, Anthierens T, Xhaferi R, Van Bree I, Szczepaniak S, Goemaere O, Steen L, Dhooge M, Paelinck H, Dewettinck K, Devlieghere F. 2010. Influence of NaCl reduction and replacement on the growth of Lactobacillus sakei in broth, cooked ham and white sauce. Int J Food Microbiol 143:9–16. doi: 10.1016/j.ijfoodmicro.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 12.McClure PJ, Baranyi J, Boogard E, Kelly TM, Roberts TA. 1993. A predictive model for the combined effect of pH, sodium chloride and storage temperature on the growth of Brochothrix thermosphacta. Int J Food Microbiol 19:161–178. doi: 10.1016/0168-1605(93)90074-Q. [DOI] [PubMed] [Google Scholar]
- 13.Mejlholm O, Dalgaard P. 2013. Development and validation of an extensive growth and growth boundary model for psychrotolerant Lactobacillus spp. in seafood and meat products. Int J Food Microbiol 167:244–260. doi: 10.1016/j.ijfoodmicro.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Harper NM, Getty KJK. 2012. Effect of salt reduction on growth of Listeria monocytogenes in meat and poultry systems. J Food Sci 77:M669–M674. doi: 10.1111/j.1750-3841.2012.02975.x. [DOI] [PubMed] [Google Scholar]
- 15.Samapundo S, Anthierens T, Ampofo-Asiama J, Xhaferi R, Van Bree I, Szczepaniak S, Goemaere O, Steen L, Dhooge M, Paelinck H, Devlieghere F. 2013. The effect of NaCl reduction and replacement on the growth of Listeria monocytogenes in broth, cooked ham and white sauce. J Food Saf 33:59–70. doi: 10.1111/jfs.12023. [DOI] [PubMed] [Google Scholar]
- 16.Aaslyng MD, Vestergaard C, Koch AG. 2014. The effect of salt reduction on sensory quality and microbial growth in hotdog sausages, bacon, ham and salami. Meat Sci 96:47–55. doi: 10.1016/j.meatsci.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Miller P, Liu X, McMullen LM. 2015. Microbiota of regular sodium and sodium-reduced ready-to-eat meat products obtained from the retail market. Can J Microbiol 61:150–154. doi: 10.1139/cjm-2014-0630. [DOI] [PubMed] [Google Scholar]
- 18.Kovačević D, Suman K, Lenart L, Frece J, Mastanjević K, Šubarić D. 2011. Salt reduction in homemade Slavonian sausage: effect on compositional, physico-chemical, colour and texture parameters, sensory characteristics and hygienic quality. Meso 13:244–248. [Google Scholar]
- 19.Ercolini D. 2013. High-throughput sequencing and metagenomics: moving forward in the culture-independent analysis of food microbial ecology. Appl Environ Microbiol 79:3148–3155. doi: 10.1128/AEM.00256-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaillou S, Chaulot-Talmon A, Caekebeke H, Cardinal M, Christieans S, Denis C, Hélène Desmonts M, Dousset X, Feurer C, Hamon E, Joffraud J-J, La Carbona S, Leroi F, Leroy S, Lorre S, Macé S, Pilet M-F, Prévost H, Rivollier M, Roux D, Talon R, Zagorec M, Champomier-Vergès M-C. 2015. Origin and ecological selection of core and food-specific bacterial communities associated with meat and seafood spoilage. ISME J 9:1105–1118. doi: 10.1038/ismej.2014.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broekaert K, Heyndrickx M, Herman L, Devlieghere F, Vlaemynck G. 2011. Seafood quality analysis: molecular identification of dominant microbiota after ice storage on several general growth media. Food Microbiol 28:1162–1169. doi: 10.1016/j.fm.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Casaburi A, Piombino P, Nychas G-J, Villani F, Ercolini D. 2015. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol 45:83–102. doi: 10.1016/j.fm.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Doulgeraki AI, Ercolini D, Villani F, Nychas G-JE. 2012. Spoilage microbiota associated to the storage of raw meat in different conditions. Int J Food Microbiol 157:130–141. doi: 10.1016/j.ijfoodmicro.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 24.Remenant B, Jaffrès E, Dousset X, Pilet M-F, Zagorec M. 2015. Bacterial spoilers of food: behavior, fitness and functional properties. Food Microbiol 45:45–53. doi: 10.1016/j.fm.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Benson AK, David JRD, Gilbreth SE, Smith G, Nietfeldt J, Legge R, Kim J, Sinha R, Duncan CE, Ma J, Singh I. 2014. Microbial successions are associated with changes in chemical profiles of a model refrigerated fresh pork sausage during an 80-day shelf life study. Appl Environ Microbiol 80:5178–5194. doi: 10.1128/AEM.00774-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Parliament. 2006. Regulation (EC) no. 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. European Parliament, Luxembourg, France. [Google Scholar]
- 27.International Organization for Standardization. 1999. ISO 2917:1999. Meat and meat products—measurement of pH—reference method. International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- 28.Barott KL, Rodriguez-Brito B, Janouškovec J, Marhaver KL, Smith JE, Keeling P, Rohwer FL. 2011. Microbial diversity associated with four functional groups of benthic reef algae and the reef-building coral Montastraea annularis. Environ Microbiol 13:1192–1204. doi: 10.1111/j.1462-2920.2010.02419.x. [DOI] [PubMed] [Google Scholar]
- 29.Schmieder R, Edwards R. 2011. Quality control and preprocessing of metagenomic datasets. Bioinformatics 27:863–864. doi: 10.1093/bioinformatics/btr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, Brown CT, Porras-Alfaro A, Kuske CR, Tiedje JM. 2014. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res 42:D633–D642. doi: 10.1093/nar/gkt1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright ES, Yilmaz LS, Noguera DR. 2012. DECIPHER, a search-based approach to chimera identification for 16S rRNA sequences. Appl Environ Microbiol 78:717–725. doi: 10.1128/AEM.06516-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CK, Herbold CW, Polson SW, Wommack KE, Williamson SJ, McDonald IR, Cary SC. 2012. Groundtruthing next-gen sequencing for microbial ecology—biases and error in community structure estimates from PCR amplicon pyrosequencing. PLoS One 7:e44224. doi: 10.1371/journal.pone.0044224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunin V, Engelbrektson A, Ochman H, Hugenholtz P. 2010. Wrinkles in the rare biosphere: pyrosequencing errors can lead to artificial inflation of diversity estimates. Environ Microbiol 12:118–123. doi: 10.1111/j.1462-2920.2009.02051.x. [DOI] [PubMed] [Google Scholar]
- 34.Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, Mills DA, Caporaso JG. 2012. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods 10:57–59. doi: 10.1038/nmeth.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. 2013. The SILVA ribosomal RNA gene database project: improved data processing and Web-based tools. Nucleic Acids Res 41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pruesse E, Peplies J, Glöckner FO. 2012. SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics 28:1823–1829. doi: 10.1093/bioinformatics/bts252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim O-S, Cho Y-J, Lee K, Yoon S-H, Kim M, Na H, Park S-C, Jeon YS, Lee J-H, Yi H, Won S, Chun J. 2012. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62:716–721. doi: 10.1099/ijs.0.038075-0. [DOI] [PubMed] [Google Scholar]
- 38.Chao A. 1984. Nonparametric estimation of the number of classes in a population. Scand J Stat 11:265–270. [Google Scholar]
- 39.R Core Development Team. 2015. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. [Google Scholar]
- 40.Verzani J. 2015. UsingR: data sets, etc. for the text “Using R for introductory statistics,” 2nd ed. R package version 2.0.5. https://cran.r-project.org/web/packages/UsingR/index.html.
- 41.Wickham H, Chang W, Wickham MH. 2013. ggplot2: elegant graphics for data analysis, version 1.0.1. Springer-Verlag, New York, NY. [Google Scholar]
- 42.Chaillou S, Christieans S, Rivollier M, Lucquin I, Champomier-Vergès MC, Zagorec M. 2014. Quantification and efficiency of Lactobacillus sakei strain mixtures used as protective cultures in ground beef. Meat Sci 97:332–338. doi: 10.1016/j.meatsci.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 43.Kim M, Oh H-S, Park S-C, Chun J. 2014. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int J Syst Evol Microbiol 64:346–351. doi: 10.1099/ijs.0.059774-0. [DOI] [PubMed] [Google Scholar]
- 44.Beisel J-N, Usseglio-Polatera P, Bachmann V, Moreteau J-C. 2003. A comparative analysis of evenness index sensitivity. Int Rev Hydrobiol 88:3–15. doi: 10.1002/iroh.200390004. [DOI] [Google Scholar]
- 45.Pothakos V, Snauwaert C, De Vos P, Huys G, Devlieghere F. 2014. Psychrotrophic members of Leuconostoc gasicomitatum, Leuconostoc gelidum and Lactococcus piscium dominate at the end of shelf-life in packaged and chilled-stored food products in Belgium. Food Microbiol 39:61–67. doi: 10.1016/j.fm.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Sofos JN. 1983. Effects of reduced salt (NaCl) levels on the stability of frankfurters. J Food Sci 48:1684–1691. doi: 10.1111/j.1365-2621.1983.tb05061.x. [DOI] [Google Scholar]
- 47.Lee HC, Chin KB. 2011. Evaluation of various salt levels and different dairy proteins in combination with microbial transglutaminase on the quality characteristics of restructured pork ham. Int J Food Sci Technol 46:1522–1528. doi: 10.1111/j.1365-2621.2011.02654.x. [DOI] [Google Scholar]
- 48.Nychas G-JE, Skandamis PN, Tassou CC, Koutsoumanis KP. 2008. Meat spoilage during distribution. Meat Sci 78:77–89. doi: 10.1016/j.meatsci.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 49.Champomier-Vergès M-C, Chaillou S, Cornet M, Zagorec M. 2001. Lactobacillus sakei: recent developments and future prospects. Res Microbiol 152:839–848. doi: 10.1016/S0923-2508(01)01267-0. [DOI] [PubMed] [Google Scholar]
- 50.Chaillou S, Champomier-Vergès M-C, Cornet M, Crutz-Le Coq A-M, Dudez A-M, Martin V, Beaufils S, Darbon-Rongère E, Bossy R, Loux V, Zagorec M. 2005. The complete genome sequence of the meat-borne lactic acid bacterium Lactobacillus sakei 23K. Nat Biotechnol 23:1527–1533. doi: 10.1038/nbt1160. [DOI] [PubMed] [Google Scholar]
- 51.Jones RJ, Zagorec M, Brightwell G, Tagg JR. 2009. Inhibition by Lactobacillus sakei of other species in the flora of vacuum packaged raw meats during prolonged storage. Food Microbiol 26:876–881. doi: 10.1016/j.fm.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 52.Pothakos V, Devlieghere F, Villani F, Björkroth J, Ercolini D. 2015. Lactic acid bacteria and their controversial role in fresh meat spoilage. Meat Sci 109:66–74. doi: 10.1016/j.meatsci.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 53.Tsigarida E, Skandamis P, Nychas G-JE. 2000. Behaviour of Listeria monocytogenes and autochthonous flora on meat stored under aerobic, vacuum and modified atmosphere packaging conditions with or without the presence of oregano essential oil at 5°C. J Appl Microbiol 89:901–909. [DOI] [PubMed] [Google Scholar]
- 54.Ercolini D, Russo F, Torrieri E, Masi P, Villani F. 2006. Changes in the spoilage-related microbiota of beef during refrigerated storage under different packaging conditions. Appl Environ Microbiol 72:4663–4671. doi: 10.1128/AEM.00468-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ercolini D, Russo F, Nasi A, Ferranti P, Villani F. 2009. Mesophilic and psychrotrophic bacteria from meat and their spoilage potential in vitro and in beef. Appl Environ Microbiol 75:1990–2001. doi: 10.1128/AEM.02762-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koutsoumanis KP, Stamatiou AP, Drosinos EH, Nychas G-JE. 2008. Control of spoilage microorganisms in minced pork by a self-developed modified atmosphere induced by the respiratory activity of meat microflora. Food Microbiol 25:915–921. doi: 10.1016/j.fm.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 57.Degirmencioglu N, Kizilirmak Esmer O, Irkin R, Degirmencioglu A. 2012. Effects of vacuum and modified atmosphere packaging on shelf life extention of minced meat chemical and microbiological changes. J Anim Vet Adv 11:898–911. doi: 10.3923/javaa.2012.898.911. [DOI] [Google Scholar]
- 58.Ke D, Picard FJ, Martineau F, Ménard C, Roy PH, Ouellette M, Bergeron MG. 1999. Development of a PCR assay for rapid detection of enterococci. J Clin Microbiol 37:3497–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.