Abstract
Congenital disorders of glycosylation (CDG) arise from pathogenic mutations in over one hundred genes leading to impaired protein or lipid glycosylation. ALG1 encodes a β1,4 mannosyltransferase that catalyzes the addition of the first of nine mannose moieties to form a dolichol-lipid linked oligosaccharide intermediate (DLO) required for proper N-linked glycosylation. ALG1 mutations cause a rare autosomal recessive disorder termed ALG1-CDG. To date thirteen mutations in eighteen patients from fourteen families have been described with varying degrees of clinical severity. We identified and characterized thirty-nine previously unreported cases of ALG1-CDG from thirty-two families and add twenty-six new mutations. Pathogenicity of each mutation was confirmed based on its inability to rescue impaired growth or hypoglycosylation of a standard biomarker in an alg1-deficient yeast strain. Using this approach we could not establish a rank order comparison of biomarker glycosylation and patient phenotype, but we identified mutations with a lethal outcome in the first two years of life. The recently identified protein-linked xeno-tetrasaccharide biomarker, NeuAc-Gal-GlcNAc2, was seen in all twenty-seven patients tested. Our study triples the number of known patients and expands the molecular and clinical correlates of this disorder.
Keywords: CDG, Asparagine-linked glycosylation protein 1, Carbohydrate-deficient transferrin, Xeno-tetrasaccharide
INTRODUCTION
Congenital disorders of glycosylation (CDG) are a group of predominantly autosomal recessive diseases characterized by broad multisystem defects; nearly all have neurological involvement [Freeze et al., 2012; Freeze et al., 2015]. Disruption of the N-linked glycosylation pathway can cause more than fifty genetic disorders, but screening can be easily accomplished by analyzing the glycosylation status of the abundant serum glycoprotein, transferrin (Tf; MIM# 190000) [Freeze et al., 2014; Freeze et al., 2015]. An abnormal serum sialotransferrin pattern provides clues as to which portion of the pathway is affected, but it typically cannot pinpoint the specific gene defect. Type I CDG’s involve defects in either the synthesis or transfer of a dolichol lipid-linked oligosaccharide (DLO) [Dolichol-P-P GlcNAc2Man9Glc3 (2 N-acetylglucosamine (GlcNAc), 9 mannose (Man) and 3 glucose (Glc)] to nascent proteins in the endoplasmic reticulum (ER) [Freeze and Schachter, 2009]. An inability to efficiently synthesize or transfer full-sized DLO results in under-occupied glycosylation sites [Freeze and Schachter, 2009]. Defects in Golgi-dependent processing of the protein bound oligosaccharide or in Golgi-associated multifunctional protein complexes can result in type II CDG’s [Freeze and Schachter, 2009]. Abnormal glycosylation of serum Tf is the most convenient indicator of deficient N-glycosylation in CDG patients, but some with confirmed CDG can have normal Tf [Freeze et al., 2012; Freeze et al., 2015].
By far the most common CDG type involving the N-linked pathway is PMM2-CDG (MIM# 212065) with >800 (reported and unreported) affected individuals [Freeze et al., 2012]. However, in the vast majority of N-linked disorders, fewer than a dozen cases have been identified [Freeze et al., 2012].
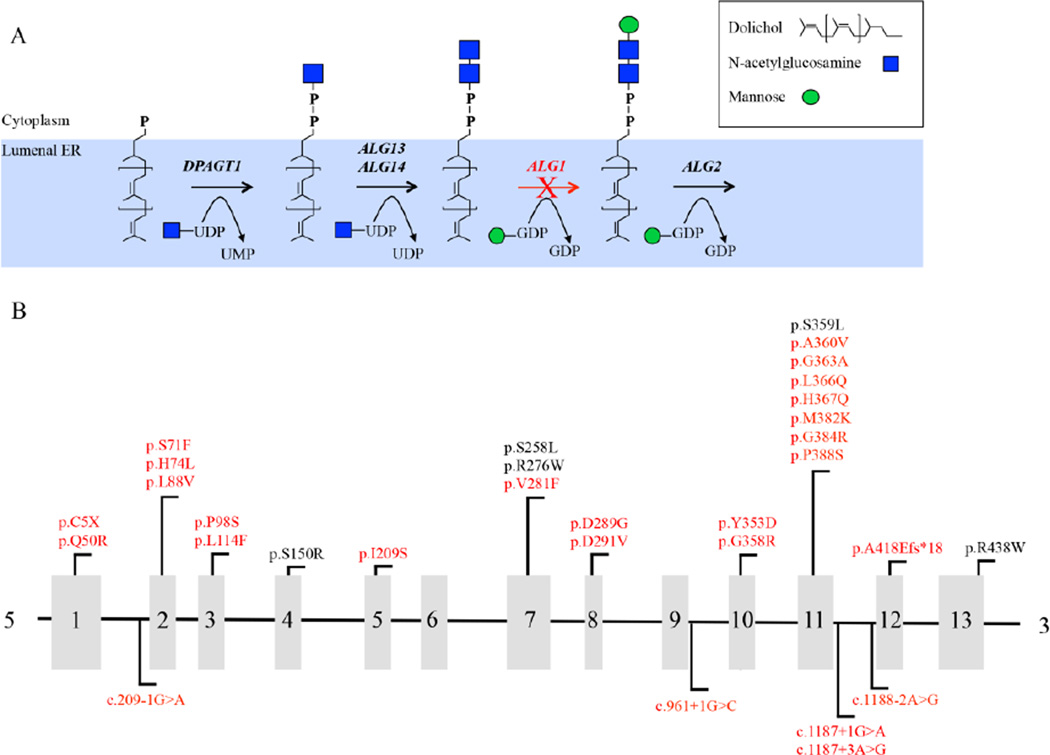
Asparagine-linked glycosylation protein 1 homolog (ALG1; MIM# 605907) encodes an ER localized β1,4 mannosyltransferase that catalyzes the transfer of the first of nine Man moieties onto the growing DLO [Huffaker and Robbins, 1982] (Figure 1a). Mutations in ALG1 cause a rare autosomal recessive disorder termed ALG1-CDG (formerly CDG-Ik; MIM# 608540) originally described by three independent groups who characterized four unrelated individuals [Grubenmann et al., 2004; Kranz et al., 2004; Schwarz et al., 2004]. Since then, an additional fourteen cases in ten families have been described with a broad clinical spectrum ranging from mild intellectual disability to death within the first few weeks of life [Dupre et al., 2010; Morava et al., 2012; Snow et al., 2012; Rohlfing et al., 2014]. Here we present an additional thirty-nine previously unreported cases of ALG1-CDG from thirty-two families highlighting twenty-six new pathogenic mutations.
Figure 1.
Pathway showing the enzymatic step requiring ALG1 mannosyltransferase activity and schematic showing mutations identified within ALG1. (A) Schematic showing only the early N-linked glycosylation pathway on the cytoplasmic facing side of the ER with the ALG1-dependent step highlighted with a red (X) over the arrow. (B) Schematic showing exon location of all the ALG1 mutations identified in this study. The mutations identified in this study are highlighted in red. For splicing mutations, nucleotide numbering for cDNA uses +1 as the A of the ATG translation initiation codon in the reference sequence.
MATERIALS and METHODS
Subjects and clinical information
Families included in this research study provided written consent under an approved Sanford Burnham Prebys Medical Discovery Institute IRB protocol. Inclusion criteria for this study required at least one of the following: (1) the occurrence of at least one abnormal carbohydrate deficient transferrin (CDT) test result indicating a type I CDG, or (2) molecular findings showing the presence of homozygous or compound heterozygous variants in ALG1. Several individuals appeared to have similar facial characteristics and consent was provided for including pictures from these and other affected individuals (Supp. Figure S1).
Molecular analysis of ALG1
Mutation analysis for ALG1 (NM_019109.4, ENST00000262374) was performed at multiple centers by either direct gene sequencing of all thirteen coding exons, including the exon-intron boundaries or in the instances where NGS panels or exome sequencing identified potential variants, only those exons were Sanger-confirmed. All primers were designed to exclude ALG1 pseudogenes. All variants have been deposited in the LOVD3.0 (https://grenada.lumc.nl/LOVD2/mendelian_genes/home.php?select_db=ALG1).
Yeast Complementation Assay
Human ALG1 cDNA (RC206343 - Origene) was cloned into the yeast expression construct pYES2.1 (Thermo Fisher) to generate pYES2.1-hALG1. Before proceeding with complementation studies, all non-synonymous SNPs within the expression construct were removed so that only the human reference sequence (NM_019109.4) was used. Subsequently, all patient-relevant missense mutations were generated using QuickChange Lightening (Agilent Technologies). Growth complementation and CPY glycosylation analysis of the alg1 deficient yeast strain was previously described [Grubenmann et al, 2004; Schwarz et al., 2004]. All yeast assays were replicated independently on three different occasions.
Analysis of Xeno-tetrasaccharide
As previously described [Bengtson et al., 2015; Zhang et al., 2015]. Biological samples tested for the Xeno-tetrasaccharide included serum and/or plasma and primary fibroblast.
RESULTS
Mutation analysis
We utilized a combination of exome sequencing, targeted gene panels, and Sanger sequencing to identify a total of thirty-one potential mutations in thirty-nine affected individuals of which 26/31 (84%) were not previously reported to cause ALG1-CDG (Figure 1b and Table 1). The five known mutations were p.Ser150Arg, p.Ser258Leu, p.Arg276Trp, p.Ser359Leu and p.Arg438Trp. We searched the Exome Aggregation Consortium (ExAC) database (http://exac.broadinstitute.org) [v0.3 accessed 10.11.2015] of 60,706 unrelated individuals and found that 9/31 (35%) of these variants occurred at very low frequencies in heterozygous carriers, but 22/31 (65%) had not been reported previously (Supp. Table S1). The most frequently observed mutation, in both the homozygous and compound heterozygous state (17/39, 44%) (Table 1) was c.773C>T, which encodes a known pathogenic mutation p.Ser258Leu (rs28939378) and has been suggested to be a possible founder mutation [Dupre et al., 2010]. All six individuals homozygous for the p.Ser258Leu died before six months of age.
Table 1.
Genotypes from 39 ALG1-CDG cases
| Mutation 1 | Mutation 2 | Detection method | |||
|---|---|---|---|---|---|
| cDNA | Protein | cDNA | Protein | ||
| Patient 1 | c.866A>G | p.Asp289Gly | c.1312C>T | p.Arg438Trp | Sanger |
| Patient 2 | c.841G>T | p.Val281Phe | c.1101C>G | p.His367Gln | Sanger |
| Patient 3 | c.773C>T | p.Ser258Leu | c.773C>T | p.Ser258Leu | Sanger |
| Patient 4 | c.773C>T | p.Ser258Leu | c.773C>T | p.Ser258Leu | Sanger |
| Patient 5 | c.293C>T | p.Pro98Leu | c.773C>T | p.Ser258Leu | Sanger |
| Patient 6 | c.293C>T | p.Pro98Leu | c.773C>T | p.Ser258Leu | Sanger |
| Patient 7 | c.773C>T | p.Ser258Leu | c.773C>T | p.Ser258Leu | Sanger |
| Patient 8 | c.773C>T | p.Ser258Leu | c.1162C>T | p.Pro388Ser | Sanger |
| Patient 9 | c.841G>T | p.Val281Phe | c.1057T>G | p.Tyr353Asp | WES |
| Patient 10 | c.866A>G | p.Asp289Gly | c.866A>G | p.Asp289Gly | Sanger |
| Patient 11 | c.212C>T, c.221A>T | p.Ser71Phe, p.His74Leu | c.1079C>T | p.Ala360Val | WES |
| Patient 12 | c.149A>G | p.Gln50Arg | c.773C>T | p.Ser258Leu | Sanger |
| Patient 13 | c.149A>G | p.Gln50Arg | c.773C>T | p.Ser258Leu | Sanger |
| Patient 14 | c.773C>T | p.Ser258Leu | c.773C>T | p.Ser258Leu | Sanger |
| Patient 15 | c.1079C>T | p.Ala360Val | c.1187+1G>A | NA | Sanger |
| Patient 16 | c.1079C>T | p.Ala360Val | c.1187+1G>A | NA | Sanger |
| Patient 17 | c.149A>G | p.Gln50Arg | c.626T>G | p.Ile209Ser | WES |
| Patient 18 | c.773C>T | p.Ser258Leu | c.773C>T | p.Ser258Leu | WES |
| Patient 19 | c.342G>C | p.Leu114Phe | c.773C>T | p.Ser258Leu | WES |
| Patient 20 | c.262T>G | p.Leu88Val | c.1188-2A>G | NA | Sanger |
| Patient 21 | c.15C>A | p.Cys5Ter | c.149A>G | p.Gln50Arg | Sanger |
| Patient 22 | c.773C>T | p.Ser258Leu | c.1187+3A>G | NA | Sanger |
| Patient 23 | c.149A>G | p.Gln50Arg | c.1072G>C | p.Gly358Arg | Sanger |
| Patient 24 | c.773C>T | p.Ser258Leu | c.1079C>T | p.Ala360Val | WES |
| Patient 25 | c.773C>T | p.Ser258Leu | c.773C>T | p.Ser258Leu | Capture |
| Patient 26 | c.1076C>T | p.Ser359Leu | c.1250_1251insTG | p.Ala418Glufs*18 | Capture |
| Patient 27 | c.872A>T | p.Asp291Val | c.872A>T | p.Asp291Val | Capture |
| Patient 28 | c.1076C>T | p.Ser359Leu | c.1076C>T | p.Ser359Leu | Capture |
| Patient 29 | c.626T>G | p.Ile209Ser | c.773C>T | p.Ser258Leu | Capture |
| Patient 30 | c.1097T>A | p.Leu366Gln | c.1097T>A | p.Leu366Gln | Capture |
| Patient 31 | c.1097T>A | p.Leu366Gln | c.1097T>A | p.Leu366Gln | Sanger |
| Patient 32 | c.450C>A | p.Ser150Arg | c.1312C>T | p.Arg438Trp | WES |
| Patient 33 | c.450C>A | p.Ser150Arg | c.1312C>T | p.Arg438Trp | WES |
| Patient 34 | c.209-1G>C | NA | c.1079C>T | p.Ala360Val | WES |
| Patient 35 | c.209-1G>C | NA | c.1079C>T | p.Ala360Val | WES |
| Patient 36 | c.1088G>C | p.Gly363Ala | c.1150G>A | p.Gly384Arg | WES |
| Patient 37 | c.773C>T | p.Ser258Leu | c.826C>T | p.Arg276Trp | Sanger |
| Patient 38 | c.773C>T | p.Ser258Leu | c.1145T>A | p.Met382Lys | Sanger |
| Patient 39 | c.961+1G>C | NA | c.961+1G>C | NA | Sanger |
Genotypes identified in thirty-nine ALG1-CDG cases. Only the variants believed to be pathogenic mutations are listed. However, in addition to the listed mutations, patients 11 and 20 were carriers of the p.Thr64Asn variant and patients 15 and 16 were carriers of a p.Pro346Arg variant. Detecting method is noted as well. Nucleotide numbering for cDNA uses +1 as the A of the ATG translation initiation codon in the reference sequence, with the initiation codon as codon 1. ALG1 NCBI Accession (NM_019109.4) and ENSEMBL (ENST00000262374).
Complementation of the Δ alg1 growth and CPY glycosylation defects by hALG1 mutations
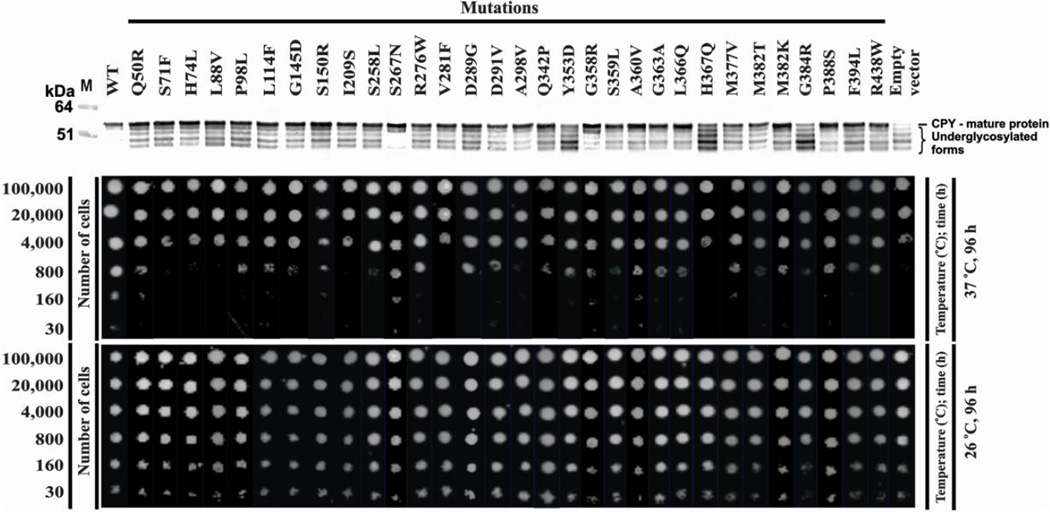
We determined the pathogenicity of unreported mutations using a temperature-sensitive (TS) alg1-deficient yeast strain [Grubenmann et al, 2004]. When grown at 26°C, this strain exhibits normal growth and appropriate glycosylation of an endogenous yeast glycoprotein, carboxypeptidase Y (CPY) [Grubenmann et al, 2004]. However, when grown at 37°C, this mutant expresses a p.Trp434X mutation leading to a C-terminal truncation of sixteen amino acids. This results in growth suppression and CPY hypoglycosylation lacking between 1–4 of its N-glycan chains [Couto et al., 1984; Gao et al., 2004; Grubenmann et al., 2004]. In total, we tested thirty missense variants, including eleven reported disease-causing missense mutations and an additional nineteen unreported variants. In silico pathogenicity prediction programs, Polyphen2 (http://genetics.bwh.harvard.edu/pph2/) and SIFT (http://sift.jcvi.org) gave mixed results for both novel and known mutations, despite nearly all our mutations occurring at highly conserved amino acids (Supp. Table S1). We also applied a third prediction method to score the impact of our variants: Combined Annotation Dependent Depletion (CADD) (http://cadd.gs.washington.edu/) integrates multiple annotations into a single metric to predict and prioritize disease causing variants [Kircher et al., 2014]. These three programs were used as a guide for prioritizing which variants should be tested in our yeast model. However, since Polyphen2 and SIFT gave a number of conflicting results, we opted to test all the identified variants. Transformation of the mutant strain with each of the thirty individual missense variants followed by plating serial dilutions of the initial inoculum showed that none was able to fully rescue growth at the restrictive temperature when compared to wild-type ALG1, suggesting that each variant was, in fact, a pathogenic mutation (Figure 2). At low dilutions on agar plates all mutants supported some growth, but differences were evident when 800 cells were spotted. (Figure 2). The visible growth suggests that either the mutant ALG1 protein retained residual activity or perhaps there was still endogenous yeast alg1 residual activity remaining in a small subset of cells. However, at lower cell densities (160 and 30 cells) most showed no growth. A small set of mutations (H74L, G145D) were listed as benign or tolerated yet didn’t support growth, where as one mutant (L114F) was listed as probably damaging/damaging but only had a mild growth defect. Based on these results, CADD was superior to to Polyphen2 or SIFT in predicting the impact of ALG1 variants
Figure 2.
Yeast complementation assay using the Δ alg1 deficient yeast strain. The alg1 deficient yeast strain was transformed with the indicated pYES2.1 construct expressing either human wild-type ALG1 or the indicated missense variants and allowed to grow for 96 hours at both permissive (26°C) and restrictive temperatures (37°C). Western blot analysis of carboxypeptidase Y (CPY) glycosylation was performed as previously described [Grubenmann et al., 2004]. Both CPY glycosylation and growth complementation were performed in triplicate with representative data presented.
CPY is used to monitor hypoglycosylation since it has 4 occupied glycosylation sites. Hypoglycosylation can result in the absence of 1–4 glycans and each glycan-deficient band is clearly visible in western blots. alg1-deficient complemented with different hALG1 variants were initially grown at 26°C and transferred to 37°C. before analysis by western blotting. None of the mutants fully corrected CPY hypoglycosylation (Figure 2), while wild-type hALG1 cDNA fully restored both normal growth and full CPY glycosylation (Figure 2). We included a common polymorphism (p.Ser267Asn) (Figure 2) to show our assay can differentiate pathogenic and benign variants. The hypoglycosylation patterns varied among the different mutants and we tried to rank order the degree of CPY hypoglycosylation using a weighted scale based on the proportion of molecules lacking the most N-glycans, e.g., Y353D would be most severe, P388S least. Neither growth nor weighted CPY hypoglycosylation analysis allowed a rank order of which mutations are most damaging.
There was also no correlation between patient clinical severity and the degree of CPY hypoglycosylation (data not shown). Furthermore, we know that in humans, homozygosity for p.Ser258Leu is always lethal within the first several months of life, yet it was not the most severe mutation in either yeast assays. For these reasons we feel the yeast model should be used as a tool for determining pathogenicity, not clinical severity of potential mutants.
Clinical Summary
In total, we studied thirty-nine patients (17 male / 22 female) from thirty-two unrelated families. Each of the thirty-nine patients had at least one confirmed abnormal carbohydrate deficient transferrin (CDT) test result. CDT is a general term used to describe a number of methods for analyzing serum Tf glycosylation status; methods used in this study included electrospray ionization mass spectrometry (ESI-MS) and isoelectric focusing (IEF).
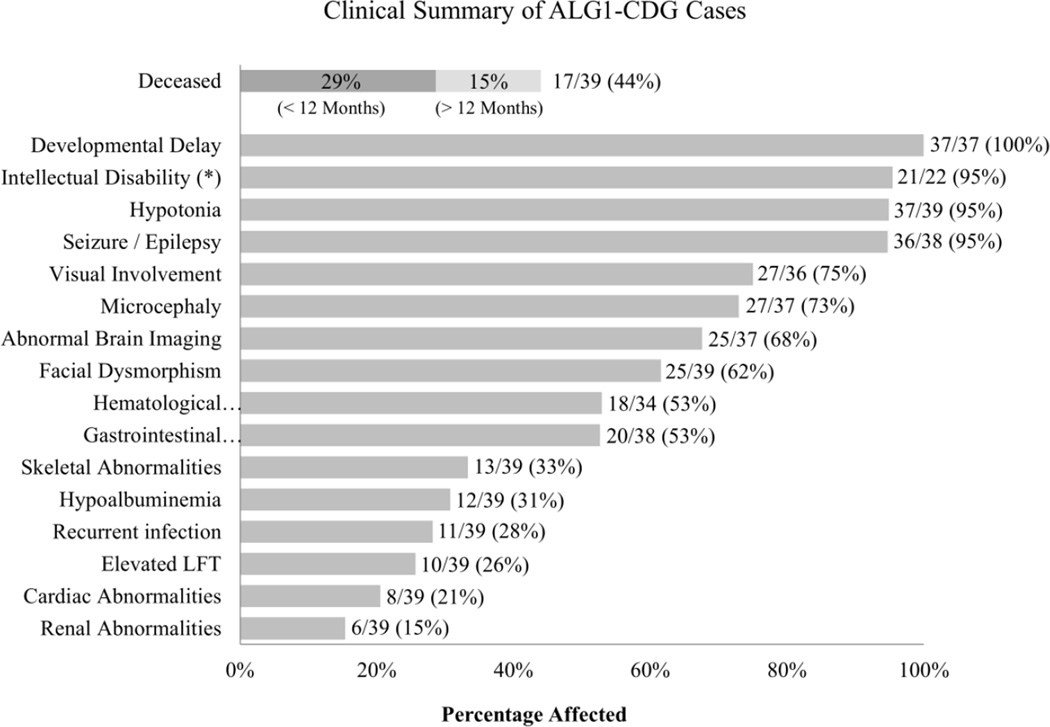
Patients had variable degree of neurodevelopmental deficiencies including developmental delay 37/37 (100%), hypotonia 37/39 (95%), seizures /epilepsy 36/38 (95%), microcephaly 27/37 (73%), abnormal brain imaging 25/37 (68%) which consisted primarily of cerebral or cerebellar atrophy 11/25 (44%) and for those who could be evaluated, intellectual disability 21/22 (95%) (Figure 3, Supp. Table S2). These findings are consistent with previously reported cases. Ocular abnormalities that mainly involved strabismus 10/27 (37%) and nystagmus 6/27 (22%) were found in 27/36 (75%) (Figure 3, Supp. Table S2). We also report a substantial number of patients with dysmorphic facial features 24/39 (62%), hematological defects 18/34 (53%), gastrointestinal problems 20/38 (53%), skeletal abnormalities 13/39 (33%) and hypoalbuminemia 12/39 (31%) (Figure 3, Supp. Table S2). Hypoalbuminemia is noteworthy because all twelve of these individuals died at an average age of 6.75 months. Within this group protein losing enteropathy (PLE) was documented in two individuals while three had renal disease. Furthermore, 6/12 individuals presenting with hypoalbuminemia were homozygous for the p.Ser258Leu. Specifically, gastrointestinal manifestations were most often chronic diarrhea (7/20) and/or PLE (5/20) (Supp. Table S2). Skeletal abnormalities consisted of scoliosis (5/13), kyphosis (2/13) or joint contractures (3/13) (Supp. Table S2). The phenotypes seen in ALG1-CDG individuals is not unique to this CDG type. Nearly all can be found in PMM2-CDG cases; whereas other characteristics such as PLE are more commonly seen in MPI-CDG, ALG6-CDG and ALG8-CDG (Freeze et al., 2014).
Figure 3.
Phenotype summary from the thirty-nine ALG1-CDG cases. Phenotypic data were provided for each individual and summarized as a percentage of patients affected. The number of deceased individuals is further broken down by the age at which the individual passed away. (*) Indicates that not all individuals could be assessed for intellectual disability, due to early death or young age.
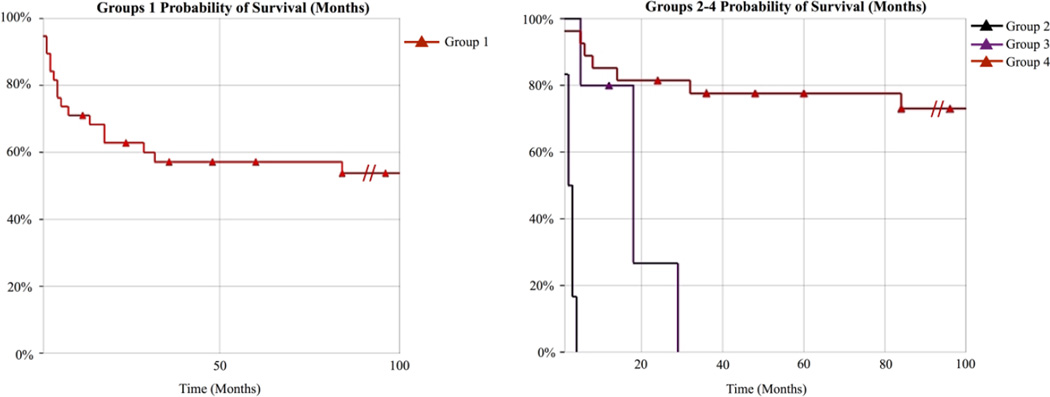
Premature death occurred in 17/39 (44%) cases (Figure 3) for a number of reasons including respiratory or renal failure and in several instances various infections leading to sepsis. The age distribution for those individuals who died ranged from < 12 months of age in 11/17 (65%) to >12 months of age in 6/17 (35%) (Figure 3, Supp. Table S2). Kaplan-Meier estimator curves show that individuals homozygous for p.Ser258Leu, or compound heterozygous for the p.Gln50Arg are very likely to have lethal outcomes (Figure 4). Premature death was previously reported in 8/18 (44%) carrying seven different mutations [Grubenmann et al., 2004; Kranz et al., 2004; Schwarz et al., 2004; Dupre et al., 2010; Morava et al., 2012; Snow et al., 2012; Rohlfing et al., 2014].
Figure 4. Kaplan-Meier survival curves for ALG1-CDG patients.
(A) Probability of survival for all thirty-nine ALG1-CDG cases “Group 1”. (B) Probability of survival for all thirty-nine ALG1-CDG cases separated by genotype. Group 2 are individuals homozygous for the p.Ser258Leu (n=6), Group 3 are individuals compound heterozygous for the p.Gln50Arg (n=5) and Group 4 comprises the remaining individuals (n=28). In order to see the effect of the p.Ser258Leu and p.Gln50Arg we made the time scale limit 100 months, however it should be noted that several individuals survived pass 100 months and this is denoted using ( ).
).
All six individuals homozygous for the p.Ser258Leu mutation died within the first five months of life. Two previously reported cases homozygous for this mutation died at two and eleven weeks of life [Kranz et al., 2004; Schwarz et al., 2004]. These individuals had characteristic dysmorphic facial features (Supp. Figure S1). Four of the five individuals who were compound heterozygous for the p.Gln50Arg allele died between 5–28 months of age, irrespective of the second mutation. These four individuals also had similar facial features compared to other ALG1-CDG individuals with different genotypes (Supp. Figure S1). One individual who is compound heterozygous for the p.Gln50Arg remains alive at nine months of age.
DISCUSSION
We report thirty-nine individuals from thirty-two unrelated families with ALG1-CDG, more than tripling the number of known patients. To better characterize this disorder, we attempted to create an ALG1-deficient human cell line utilizing CRISPR/CAS9 technology to test pathogenicity of the variants. However, we were unable to generate a cell line carrying a homozygous INDEL despite targeting four different exons (data not shown). This suggests that this gene is essential for viability.
Because we were unable to generate a null human cell line for complementation studies, we used a temperature-sensitive alg1 mutant yeast strain. At the restrictive temperature, this mutant not only accumulates DLO containing dolichol-PP-GlcNAc2, but the GlcNAc2 can be transferred to yeast glycoproteins such as exoglucanase [Cueva et al., 1998]. Various human cell lines grown under glucose-deprived conditions or siRNA knockdown of ALG1, as well as the yeast alg1 mutant, all accumulated proteins with GlcNAc2 (Isono, 2011). This suggests that severely truncated oligosaccharides from ALG1-deficient cells can still be transferred to nascent proteins. It remains unclear if the majority of glycoproteins from ALG1-deficient cells bear this unusual glycan or if it only affects selected glycoproteins.
These observations in a model system have become more important because ALG1-CDG patients are known to accumulate a novel N-linked xeno-tetrasaccharide NeuAc-Gal-GlcNAc2 [2 N-acetylglucosamine (GlcNAc), 1 galactose (Gal), 1 sialic acid (NeuAc)] on both total serum glycoproteins and on ~2–8% of purified serum Tf [Bengtson et al., 2015; Zhang et al., 2015]. The presence of this tetrasaccharide shows that Dol-PP-GlcNAc2 is flipped from the cytoplasmic face into the luminal face of the ER and subsequently transferred to proteins via the oligosaccharyltransferase complex (OST). Once transferred, modified proteins enter the Golgi where GlcNAc2 serves as an acceptor substrate for β1,4 galactosyltransferase (MIM# 137060). Finally, Gal-GlcNAc2 is capped with a NeuAc added by 2,6 sialyltransferase to form the final product NeuAc2,6-Gal β1,4GlcNAc β1,4GlcNAcβ [Bengtson et al., 2015; Zhang et al., 2015] (Supp. Figure S2). Importantly, since this tetrasaccharide does not normally occur in mammals and is primarily detected in ALG1-CDG cases (trace amounts are detected in PMM2-CDG and MPI-CDG cases), it serves as a biomarker for either detecting or confirming a diagnosis of ALG1-CDG [Bengtson et al., 2015; Zhang et al., 2015]. We tested a limited number of type I CDG samples and cannot exclude the possibility that other type I’s could have this tetrasaccharide. Yet, mechanistically it seems unlikely subsequent steps to ALG1 would have this specific glycan. In fact, of the thirty-nine affected individuals enrolled into our study, twenty-seven were tested and all had this novel tetrasaccharide present on either serum or fibroblast glycoproteins (Supp. Table S3). Of the twelve not tested, three were homozygous for the lethal p.Ser258Leu, four had previously reported mutations, and three were affected sibling pairs of tested patients. Two patients with novel mutations were not tested for the tetrasaccharide. We can confidently claim that this marker is associated with ALG1-CDG.
As seen with many glycosylation-related disorders; the clinical phenotype of ALG1-CDG varies dramatically between affected individuals (Figure 3, Supp. Table S2). While all ALG1-CDG patients do have substantial neurological involvement, other systems are variably affected (Figure 3, Supp. Table S2). This may be attributed to several factors including how ALG1 mutations affect residual mannosyltransferase activity or GDP-Man binding efficiency, complex formations with other proteins, tissue specific expression or even protein stability. For example, several ALG1 mutations including the p.Ser258Leu are highly unstable, resulting in near absence of protein expression in patient fibroblasts, while other mutations have no effect on stability (data not shown).
ALG1 is thought to form multiple protein complexes in yeast. Gao et al. show that ALG1 forms homo-oligomer structures and also interacts with subsequent α-mannosyltransferases, ALG2 (MIM# 607905) and ALG11 (MIM# 613666). Together they add all four mannosyl-residues to the DLO on the cytoplasmic face of the ER [Gao et al., 2004]. With the discovery that mutations in DPAGT1 (MIM#s 608093, 191350) and ALG2 (MIM#s 607906, 616228) [Belaya et al., 2012; Cossins et al., 2013] can cause a CDG phenotype as well as a congenital myasthenic syndrome (CMS), it is conceivable that specific ALG1 mutations could also cause a mild CMS-like phenotype. Furthermore, traditional CDT testing could potentially miss such ALG1 cases given individuals with DPAGT1-CMS or ALG2-CMS had normal CDT testing [Belaya et al, 2012; Cossins et al., 2013].
We identified one case suspected of having ALG-CDG because of developmental delay, intellectual disability, hypotonia, epilepsy and skeletal abnormalities. This individual was homozygous for a variant of unknown significance (VUS) (p.Thr64Asn) in ALG1 which failed to rescue yeast growth or CPY glycosylation, suggesting it was deleterious. However, Tf glycosylation was normal and no tetrasaccharide was detected. Thus, she was excluded from our study. This finding shows the limitations of the yeast model and highlights the importance of having multiple methods to assess the pathogenicity of questionable variants.
In conclusion, we present molecular, clinical and biochemical findings in the largest collection of ALG1-CDG cases ever reported at a single time with thirty-nine cases, bringing the total number of known ALG1-CDG to fifty-seven. This ranks it the third most common CDG type behind PMM2-CDG and ALG6-CDG [Freeze et al., 2012]. In addition, we identify highly lethal genotypes and confirm the presence of a unique xeno-tetrasaccharide in ALG1-CDG patients, thus showing it to be a biomarker for this disorder.
Supplementary Material
Acknowledgments
We thank the families for their continued support. Dr. Markus Aebi for providing yeast strains and Dr. Claude Sansaricq for his contribution.
FUNDING
This work is supported by The Rocket Fund, National Institutes of Health (NIH) grants R01DK099551, R01DK55615 (to H.H.F). The University of Washington Center for Mendelian Genomics is funded by the National Human Genome Research Institute and the National Heart Lung Blood Institute (1U54HG006493) to D.N., J.S., and M.B. NIGMS grant GM102129 to C. J. W. The research was also funded by the grant ERARE11-135 of the ERA-Net for Research Programs on Rare Diseases Joint Transnational Call 2011 (EURO-CDG).
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to disclose.
REFERENCES
- Belaya K, Finlayson S, Slater CR, Cossins J, Liu WW, Maxwell S, McGowan SJ, Maslau S, Twigg SR, Walls TJ, Pascual Pascual SI, Palace J, et al. Mutations in DPAGT1 cause a limb-girdle congenital myasthenic syndrome with tubular aggregates. Am J Hum Genet. 2012;91:193–201. doi: 10.1016/j.ajhg.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtson P, Ng BG, Jaeken J, Matthijs G, Freeze HH, Eklund EA. Serum transferrin carrying the xeno-tetrasaccharide NeuAc-Gal-GlcNAc2 is a biomarker of ALG1-CDG. J Inherit Metab Dis. 2015 doi: 10.1007/s10545-015-9884-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossins J, Belaya K, Hicks D, Salih MA, Finlayson S, Carboni N, Liu WW, Maxwell S, Zoltowska K, Farsani GT, Laval S, Seidhamed MZ, et al. Congenital myasthenic syndromes due to mutations in ALG2 and ALG14. Brain. 2013;136:944–956. doi: 10.1093/brain/awt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto JR, Huffaker TC, Robbins PW. Cloning and expression in Escherichia coli of a yeast mannosyltransferase from the asparagine-linked glycosylation pathway. J Biol Chem. 1984;259:378–382. [PubMed] [Google Scholar]
- Cueva R, Cotano C, Larriba G. N-glycosylation by transfer of GlcNAc2 from dolichol-PP-GlcNAc2 to the protein moiety of the major yeast exoglucanase. Yeast. 1998;14:773–781. doi: 10.1002/(SICI)1097-0061(19980615)14:8<773::AID-YEA284>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Dupre T, Vuillaumier-Barrot S, Chantret I, Sadou Yaye H, Le Bizec C, Afenjar A, Altuzarra C, Barnerias C, Burglen L, de Lonlay P, Feillet F, Napuri S, et al. Guanosine diphosphate-mannose:GlcNAc2-PP-dolichol mannosyltransferase deficiency (congenital disorders of glycosylation type Ik): five new patients and seven novel mutations. J Med Genet. 2010;47:729–735. doi: 10.1136/jmg.2009.072504. [DOI] [PubMed] [Google Scholar]
- Freeze HH, Schachter H. Genetic Disorders of Glycosylation. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. Chapter 42. Cold Spring Harbor (NY): 2009. [PubMed] [Google Scholar]
- Freeze HH, Eklund EA, Ng BG, Patterson MC. Neurology of inherited glycosylation disorders. Lancet Neurol. 2012;11:453–466. doi: 10.1016/S1474-4422(12)70040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze HH, Chong JX, Bamshad MJ, Ng BG. Solving glycosylation disorders: fundamental approaches reveal complicated pathways. Am J Hum Genet. 2014;94:161–175. doi: 10.1016/j.ajhg.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze HH, Eklund EA, Ng BG, Patterson MC. Neurological aspects of human glycosylation disorders. Annu Rev Neurosci. 2015;38:105–125. doi: 10.1146/annurev-neuro-071714-034019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XD, Nishikawa A, Dean N. Physical interactions between the Alg1, Alg2, and Alg11 mannosyltransferases of the endoplasmic reticulum. Glycobiology. 2004;14:559–570. doi: 10.1093/glycob/cwh072. [DOI] [PubMed] [Google Scholar]
- Grubenmann CE, Frank CG, Hulsmeier AJ, Schollen E, Matthijs G, Mayatepek E, Berger EG, Aebi M, Hennet T. Deficiency of the first mannosylation step in the N-glycosylation pathway causes congenital disorder of glycosylation type Ik. Hum Mol Genet. 2004;13:535–542. doi: 10.1093/hmg/ddh050. [DOI] [PubMed] [Google Scholar]
- Huffaker TC, Robbins PW. Temperature-sensitive yeast mutants deficient in asparagine-linked glycosylation. J Biol Chem. 1982;257:3203–3210. [PubMed] [Google Scholar]
- Isono T. O-GlcNAc-specific antibody CTD110.6 cross-reacts with N-GlcNAc2-modified proteins induced under glucose deprivation. PLoS One. 2011;6:e18959. doi: 10.1371/journal.pone.0018959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz C, Denecke J, Lehle L, Sohlbach K, Jeske S, Meinhardt F, Rossi R, Gudowius S, Marquardt T. Congenital disorder of glycosylation type Ik (CDG-Ik): a defect of mannosyltransferase I. Am J Hum Genet. 2004;74:545–551. doi: 10.1086/382493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher M, Witten DM, Jain P, O'Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morava E, Vodopiutz J, Lefeber DJ, Janecke AR, Schmidt WM, Lechner S, Item CB, Sykut-Cegielska J, Adamowicz M, Wierzba J, Zhang ZH, Mihalek I, et al. Defining the phenotype in congenital disorder of glycosylation due to ALG1 mutations. Pediatrics. 2012;130:e1034–e1039. doi: 10.1542/peds.2011-2711. [DOI] [PubMed] [Google Scholar]
- Rohlfing AK, Rust S, Reunert J, Tirre M, Du Chesne I, Wemhoff S, Meinhardt F, Hartmann H, Das AM, Marquardt T. ALG1-CDG: a new case with early fatal outcome. Gene. 2014;534:345–351. doi: 10.1016/j.gene.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Thiel C, Lubbehusen J, Dorland B, de Koning T, von Figura K, Lehle L, Korner C. Deficiency of GDP-Man:GlcNAc2-PP-dolichol mannosyltransferase causes congenital disorder of glycosylation type Ik. Am J Hum Genet. 2004;74:472–481. doi: 10.1086/382492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow TM, Woods CW, Woods AG. Congenital disorder of glycosylation: a case presentation. Adv Neonatal Care. 2012;12:96–100. doi: 10.1097/ANC.0b013e318241bc1b. [DOI] [PubMed] [Google Scholar]
- Zhang W, James PM, Ng BG, Li X, Xia B, Rong J, Asif G, Raymond K, Jones MA, Hegde M, Ju T, Cummings RD, et al. A Novel N-Tetrasaccharide in Patients with Congenital Disorders of Glycosylation Including Asparagine-Linked Glycosylation Protein 1, Phosphomannomutase 2, and Phosphomannose Isomerase Deficiencies. Clin Chem. 2015 doi: 10.1373/clinchem.2015.243279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.