Abstract
Background
Glycans, complex oligosaccharides, are directly involved in almost every biological process, have a fundamental role in the immune system and are probably involved in nearly every human disease. However, glycosylation has been greatly ignored in the area of allogeneic hematopoietic stem cell transplantation (alloHSCT) and graft versus host disease (GVHD). Both acute and chronic GVHD are multisystemic debilitating immunological disturbances arising after alloHSCT.
Scope of Review
In this paper we review the glycosylation research already done in the field of alloHSCT and GVHD, and evaluate further potential of glycan analysis in GVHD by looking into resembling inflammatory and autoimmune conditions.
Major Conclusions
Glycan research could bring significant improvement in alloHSCT procedure with reduction in following complications, such as GVHD. Identifying glycan patterns that induce self-tolerance and the ones that cause the auto- and allo-immune response could lead to innovative and tissue specific immunomodulative therapy instead of the current immunosuppressive treatment, enabling preservation of the graft-versus-tumor effect. Moreover, improved glycan pattern analyses could offer a more complete assessment and greatly needed dynamic biomarkers for GVHD.
General Significance
This review is written with a goal to encourage glycan research in the field of alloHSCT and GVHD as a perspective tool leading to improved engraftment, discovery of much needed biomarkers for GVHD, enabling an appropriate therapy and improved monitoring of therapeutic response.
Keywords: glycan, glycosylation, graft versus host disease, hematopoietic stem cell transplantation
1. Introduction
Glycans – complex oligosaccharides – are a major component of the cell: they exist on the surface of every cell and are a part of almost all membranes (attached to lipids and proteins) and secreted proteins [1]. Numerous cells, protein receptors and soluble mediators of the immune system, such as class I and class II major histocompatibility complex proteins, T and B cell receptors, chemokine and cytokine receptors, and antibodies, contain significant amounts of covalently attached glycans [2]. Glycans play important roles in major biological events (such as cell–cell interaction, protein folding and receptor binding) and are directly involved in almost every biological process [1]. They have already been associated to a number of inflammatory conditions [3], autoimmune diseases and hematological cancers [4,5]. In addition, it is suggested that glycans have a major role in nearly every human disease [1,6,7].
Recent advances in the field of glycan pattern analyses hold great promise for understanding various diseases mechanisms and for biomarkers research. In this review, we present current knowledge of glycosylation characteristics and its possible role and research potential in the field of graft versus host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (alloHSCT).
2. Glycans: biology and analysis
Glycans are non-linear branched oligosaccharides, structurally extremely complex. Because of their molecular complexity, the absence of a direct genetic template and methodological difficulties, glycan research lagged behind genomics and proteomics. Recent studies showed that glycans are a product of both genetic and environmental factors [8], and that glycosylation is a tightly regulated process where different glycan attachments are of great biochemical importance [9,10].
Ten commonly found monosaccharide building blocks compose numerous diverse combinations of human oligosaccharides which vary in type, number, order and spatial relation of monosaccharide units. Number of possible variations grows even further since hydroxyl groups of different monosaccharides can become subject to phosphorylation, sulfation, methylation, O-acetylation, or fatty acylation.
Glycoproteins are glycoconjugates in which glycans are covalently linked to a polypeptide backbone, usually via N- or O-linkages. Addition of complex oligosaccharides to polypeptide backbone is the most abundant and the most structurally diverse posttranslational modification of proteins. It greatly affects protein conformation and leads to changes in the protein behavior affecting its biological functions.
Recognition of glycans and transfer of information consisted in the sugar moiety is being done via glycan-binding proteins, which can be sorted in two classes: lectins and glycosaminoglycan-binding proteins. Lectins recognize N-glycans, O-glycans and glycosphingo-lipids, while glycosaminoglycan-binding proteins tend to bind different types of sulfated glycosaminoglycans [11]. Review of basic glycobiology terminology is given in Table 1.
Table 1.
Glossary and review of glycobiology terminology mentioned in the article (according to Varki et al. [11]).
| ADCC | Antibody dependent cell-mediated cytotoxicity. |
| Alarmins | Pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), glycoproteins also known as alarmins. |
| DAMPs | See Alarmins. |
| Fab | The fragment antigen binding region, see Immunoglobulin. |
| Fc | The fragment crystallisable region, see Immunoglobulin. |
| FcR | Fc receptor, see Immunoglobulin. |
| Galectin | S-type (sulphydryl-dependent) β-galactoside-binding lectins, usually occurring in a soluble form, expressed by a wide variety of animal cell types and distinguishable by the amino acid sequence of their carbohydrate-recognition domains. |
| Glycan | A generic term for any sugar or assembly of sugars, in free form or attached to another molecule, used interchangeably with saccharide or carbohydrate. |
| Glycan-binding proteins | Proteins that recognize and bind to specific glycans and mediate their biological function. Sorted in two classes: lectins and glycosaminoglycan-binding protein. |
| Glycoconjugate | A molecule in which one or more glycan units are covalently linked to a noncarbohydrate entity. |
| Glycoform | Different molecular forms of a glycoprotein, resulting from variable glycan structure and/or glycan attachment site occupancy. |
| Glycoprotein | A protein with one or more covalently bound glycans. |
| Glycosaminoglycans | Polysaccharide side chains of proteoglycans or free complex polysaccharides composed of linear disaccharide repeating units, each composed of a hexosamine and a hexose or a hexuronic acid. |
| Glycosylation | The enzyme-catalyzed covalent attachment of a carbohydrate to a polypeptide, lipid, polynucleotide, carbohydrate, or other organic compound, generally catalyzed by glycosyltransferases, utilizing specific sugar nucleotide donor substrates. |
| Glycosyltransferase | Enzyme that catalyzes transfer of a sugar from a sugar nucleotide donor to a substrate. |
| Immunoglobulin (Ig) | Part of the adaptive immune system, produced by B cells and plasma cells. It consists of two heavy and two light chains. Five types of heavy chains (γ, α, μ, δ and ε) exist in mammals and the type of the heavy chain defines the class of the Ig molecule. The ‘arms’ of the Ig consisting of both the heavy and the light chains, contain the Fab region that recognizes and binds to the antigen. The Fc region binds to the corresponding FcR of immune cells and participates in the regulation of their activity. |
| Lectin | A protein that specifically recognizes and binds to glycans without catalyzing a modification of the glycan. |
| Monosaccharide | Carbohydrate that cannot be hydrolyzed into a simpler carbohydrate. It is the building block of oligosaccharides and polysaccharides. Commonly found monosaccharide building blocks: D-glucose (Glc), D-galactose (Gal), D-mannose (Man), D-glucuronic acid (GlcA), N-acetyl-D-glucosamine (GlcNAc), N-acetyl-D-galactosamine (GalNAc), L-fucose (Fuc), D-xylose (Xyl), D-ribose (Rib) and N-acetylneuraminic acid (Neu5Ac or NeuAc). |
| Mucin | Large glycoprotein with a high content of serine, threonine, and proline residues and numerous O-GalNAc-linked saccharides, often occurring in clusters on the polypeptide. Secreted by epithelial surfaces such as gastrointestinal, genitourinary and respiratory tracts. |
| N-(linked)glycan | Glycan covalently linked to an asparagine residue of a polypeptide chain in the consensus sequence: -Asn-X-Ser/Thr. |
| O-(linked)glycan | A glycan glycosidically linked to the hydroxyl group of the amino acids serine, threonine, tyrosine, or hydroxylysine. Glycoproteins rich in O-glycans are often called mucins. |
| Oligosaccharide | Linear or branched chain of monosaccharides attached to one another via glycosidic linkages. The number of monosaccharide units can vary. |
| PAMPs | See Alarmins. |
| Polysaccharide | Glycan composed of repeating monosaccharides, generally greater than ten monosaccharide units in length. |
| Saccharide | A generic term for any carbohydrate or assembly of carbohydrates, in free form or attached to another molecule, used interchangeably with carbohydrate and glycan. |
| Selectin | A C-type (Ca++-dependent) lectin expressed by cells in the vasculature and bloodstream. The three known selectins are L-selectin/CD62L (expressed by most leukocytes), E-selectin/CD62E (expressed by cytokine-activated endothelial cells), and P-selectin/CD62P (expressed by activated endothelial cells and platelets). |
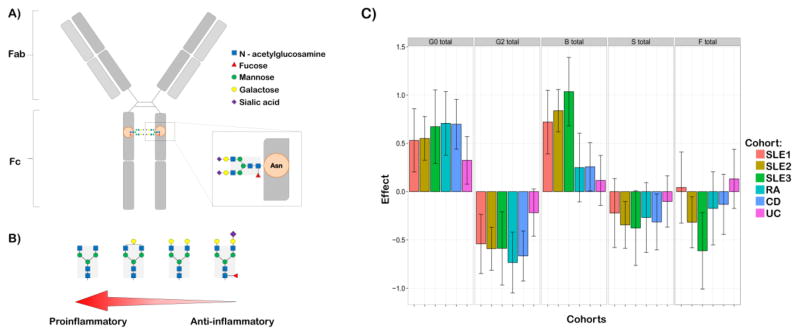
Glycosylation analysis can be performed on whole serum N-glycan profile as well as on specific glycoproteins, such as immunoglobulin (Ig) G and A. One of the most analyzed glycoproteins is IgG, the most abundant class of antibody in the human plasma (around three quarter of serum Igs) [12]. IgG carries N-linked glycans at constant domain 2 (CH2, Asn 297) of its Fc region, most of which are of a complex type with a biantennary heptameric core (three mannose and four N-acetyl-glucosamine residues) and possible additions of N-acetylglucosamine, fucose, galactose and sialic acid residues (Figure 1.A). The attached sugar shows great variability with more than 30 identified different IgG glycosylation variants for any of the four different human IgG subclasses [13]. The IgG glycome composition is rather stable in healthy individuals, but inter-individual differences are very large [14,15], with both genetic, epigenetic and environmental factors contributing to these differences [4]. Despite significant heritability of the steady-state composition [16], IgG glycome composition can change rapidly in the state of proinflammatory response [17] (Figure 1.B.). The composition of the IgG N-glycan affects the protein conformation and subsequently its ability to bind to the FcγRs which can modulate ADCC [18,19], complement activation [20] and other immune responses. Minute changes in the IgG N-glycan composition influence its FcR affinity. For example, lack of core fucose increases affinity for FcγRIIIa receptor leading to an improved effector function [21]. The addition of terminal sialic acid changes the conformation of the protein and initiates an anti-inflammatory cascade by binding to an alternative class of receptors [22]. Differences in abundances of IgG glycan traits in patients with different autoimmune diseases sets them as a leading candidate for new biomarkers for various autoimmune diseases [23,24] (Figure 1.C.).
Figure 1.
A) Three mannose and four N-acetyl-glucosamine residues comprise a biantennary heptameric core (highlighted in grey) of IgG glycans. Possible additions of N-acetylglucosamine, fucose, galactose and sialic acid residues make up to 30 possible glycosylation variants. The sugar moiety is attached to the Asn 297 of constant Fc region of IgG. B) Minute changes in structure of the IgG N-glycan modulate the inflammatory activity of IgG (depiction according to Maverakis et al., 2015(7)). C) Differences in abundances of IgG glycan traits in patients with different autoimmune diseases (depiction according to data published in I. Trbojevic Akmacic et al., 2015 and F. Vuckovic et al., 2015(23,24)).
G0 total – total agalactosylated glycans; G2 total – total digalactosylated glycans; B total – total glycans with bisecting GlcNAc; S total – total sialylated glycans; F total – total fucosylated glycans; SLE1-3 – Latin America, Trinidad and Chinese cohorts of patients with systemic lupus erythematosus; RA – Chinese cohort of patients with rheumatoid arthritis; CD – UK cohort of patients with Chron’s disease; UC – UK cohort of patients with ulcerative colitis.
3. Glycans in alloHSCT
Allogeneic HSCT is a potentially lifesaving procedure for a variety of hematological malignant and non-malignant disorders. Although there are areas of alloHSCT that could benefit from better understanding of glycosylation mechanisms involved, glycosylation analysis has been greatly ignored in alloHSCT.
3.1. Engraftment and glycan research
A successful alloHSCT depends upon the ability of infused hematopoietic stem cells (HSCs) to home from blood to the bone marrow (BM) cavity to reestablish productive hematopoiesis. Such homing is a nonrandom process regulated by adhesive interactions between HSCs and BM endothelium. Marrow endothelial cells constitutively express E-selectin [25], a member of selectin family of adhesion molecules that binds to sialofucosylated glycans expressed on glycoproteins or glycolipids of circulating cells. Engagement of E-selectin promotes homing of circulating HSCs to BM [26]. E-selectin on the BM vasculature is also known to directly induce HSC proliferation at the expense of HSC self renewal [27].
Integral membrane glycoprotein hematopoietic cell E-/L-selectin ligand (HCELL), a specialized glycoform of CD44, has been identified as the predominant E-selectin ligand on circulating human HSC [28]. Glycans of BM hematopoietic stem and progenitor cells are suggested to be remodeled by a remotely produced glycosyltransferase which mediates the formation of cell surface α2,6-linked sialic acids important for cell interaction with other cells and its surroundings [29]. Glycosylation manipulation of naïve CD44 on a cell unable to achieve tissue-specific migration could be custom modified into HCELL glycoform without affecting cell viability or native phenotype. The method is called ‘glycosyltransferase-programmed stereosubstitution’ [30] and could potentially improve delivery of HSC into BM with a purpose of a more successful reconstitution of hematopoiesis. Glycoengineering is also considered as a solution to poorer engraftment results of cord blood transplantation, often limited by a low number of HSC contained in the graft. Ex vivo fucosylation of cord blood HSC using recombinant human fucosyltransferases is currently being tested as a promising method to improve the rate and magnitude of engraftment [31].
Glycans could also help to clarify some of the complex interactions between donor’s and host’s immunological systems after alloHSCT, including one of the more severe consequences–graft versus host disease. This post-transplant complication, occurrs in two distinct, clinically well characterized forms: acute and chronic.
3.2. Acute graft-versus-host disease (aGVHD)
There are two subcategories of aGVHD: (1) classic (presenting itself with typical aGVHD symptoms within 100 days of alloHSCT or donor lymphocyte infusion (DLI)) and (2) persistent, recurrent, or late-onset aGVHD with features of classic aGVHD occurring beyond 100 days after alloHSCT or DLI in a patient not meeting criteria for the diagnosis of chronic GVHD (cGVHD)[32,33]. Acute GVHD affects skin, gastrointestinal system and liver. Typical presentations include erythema and/or maculopapular rash on the palms and soles, secretory diarrhoea and cholestatic liver disease [34]. Incidence reaches up to 50% of patients receiving HLA-matched transplants from a related donor and up to 70% of those receiving transplants from an unrelated donor [35].
Studies emphasize the role of innate immunity in the initiation of aGVHD. Total body irradiation and chemotheraphy cause an injury of gastrointestinal mucosa allowing the transit of commensal bacteria from the lumen. Host’s pattern recognition receptors recognize PAMPs and DAMPs, glycoproteins also known as alarmins, following the conditioning regimen and activate the innate immune system. This reaction is supported by inflammatory cytokines secreted by the damaged tissue which leads to recruitment of effector cells and enhances the recognition of host alloantigens by donor-derived T-cells [36,37]. The described process initiates a complex cascade of tissue destruction creating a pro-inflammatory milieu, which amplifies and perpetuates aGVHD. Since aGVHD mostly manifests itself in three locations – skin, liver and gut – it is obvious that immune cells are being navigated towards those organs. Glycans are crucial in cell communication and recognition, and highly involved in trafficking of immune cells. Hence, manipulation of immune cell trafficking and regional immunity seems like a promising area of research. Glycans are also well known as markers of acute systemic inflammation and could hopefully aid in clarifying GVHD pathogenesis. For example, it has been demonstrated that in a whole-body inflammatory reaction (such as a cardiovascular surgery) certain glycan groups of plasma proteins show rapid and uniform increase. Results from that research indicated that it is possible to predict the severity of acute inflammatory response and identify individuals with a higher mortality risk prior to an invasive procedure based on the level of fucosylation of IgG N-glycans [38]. Changes of serum glycans have also been described in other inflammatory conditions, such as sepsis and acute pancreatitis, early in the acute phase response [38,39]. Since glycan profiles in healthy serum are more or less constant, it is suggested they could have a valuable prognostic potential [17], which might also be exploitable in GVHD.
Another candidate in demystifying GVHD could be galectin-9 (Gal-9). This molecule is a member of the galectin family of carbohydrate-binding proteins (lectins), and it is often associated with modulation and homeostasis of T cells. Elevated expression of endogenous Gal-9 was found in the process of rejection of allografted solid organs, correlating with the progression of the process [40]. It has been found to promote differentiation of naïve T cells into regulatory T cells [41]. It also represses differentiation into T-helper 17 cells and induces apoptosis in mature CD4+ T cells, and CD8+ [42]. Recombinant Gal-9 has already been tested on a mouse model of aGVHD with promising results [43].
3.3. Chronic graft-versus-host disease (cGVHD)
Chronic GVHD is the major late complication following alloHSCT, associated with increased mortality, impaired physical and functional status and decreased quality of life [44]. It is a systemic alloimmune and autoimmune disease characterized by immune deregulation, immunodeficiency, and development of signs and symptoms of various autoimmune disorders targeting multiple organ systems (oral, digestive and genital mucosa, glandular tissue, skin, eyes, lungs, liver, and joints). Reported incidence rates of cGVHD range from 30–70% [45,46] according to recipient age, donor type, stem cell source and use of posttransplantation DLIs.
In 2005, the National Institutes of Health (NIH), USA, convened a cGVHD Consensus Conference defining the new conceptual understanding of cGVHD, with the new scoring system based on number of organs involved, severity, and functional disability [32,47–51]. In June 2014, the second cGVHD consensus conference was held at the National Cancer Institute, NIH, USA. Updated recommendations about cGVHD diagnosis, staging, histopathology, biomarkers, response criteria, design of clinical trials, ancillary and supportive care resulted from that second cGVHD conference [33,52–56].
In spite of huge effort and progress in cGVHD after two NIH consensus meetings, there are still many unresolved questions regarding understanding of the pathophysiology and predicting occurrence of cGVHD, improving diagnosis and staging, measuring short-term responses to treatment and predicting long-term clinical benefit. Moreover, there is still no USA Food and Drug Administration or European Medicines Agency approved agent for cGVHD treatment or prevention, and the first line treatment with steroids has 50% failure rate with significant steroid toxicity. There is no standard second and subsequent line of therapy, and preventive and preemptive strategies to decrease incidence and severity of cGVHD are not standardized.
As we know today, cGVHD is a result of complex series of immune interactions that occur as the donor immune system develops in antigenically disparate recipient environment. Although it has been demonstrated that donor T cells transferred along with the allograft are the primary immunocompetent cells that induce GVHD [57], increased attention in this systemic immunological disturbance is being directed towards additional cell populations [58], especially B cells [59–62]. It is known that B-cell reconstitution in patients with cGVHD is delayed, and these patients have elevated plasma B-cell activating factor (BAFF)/naïve B-cell ratio [63,64]. The unique post-alloHSCT surrounding with high levels of BAFF is known to promote the differentiation and survival of allo- and auto-reactive B cells [65]. Successful treatment with high-dose prednisone was associated with reduced BAFF levels in patients with active cGVHD, whereas lower concentration of BAFF were measured in patients who never developed cGVHD [66]. Involvement of B cells would also explain the partial success of rituximab (anti-CD20 monoclonal antibody), drug used both in prophylaxis [67,68] and as a second line of steroid refractory cGVHD [69]. New insights into the rituximab mechanism of action suggest that the partial response to the drug could be due to the polymorphism of FcγR. It has been reported that follicular lymphoma patients with higher affinity allelic variants of receptor FcγRIIIa have a better response than patients with low-affinity polymorphisms [70], which could be applicable to cGVHD. One of the possible resolutions of the incomplete response to rituximab may be Fc-glycoengineering in order to enhance ADCC either by reducing N-glycan core fucosylation or incorporation of bisecting sugar residue [71]. Although a recent study described that patients with long-lasting cGVHD have a trend towards higher incidence of allo- and auto-antibodies (AAbs) [72], it has not been linked to the severity or activity of neither the disease, nor it has been elucidated whether these are pathogenic or represent a consequence of disturbed B-cell homeostasis.
Distortion of B cell homeostasis and production of AAbs common for cGVHD can be compared to autoimmune diseases, where the pathogenic role of AAbs has been confirmed [73–77]. Clinical observations also support the autoimmune nature of the disease, since cGVHD is well known for mimicking autoimmune disorders such as Sjögren syndrome (SS), systemic lupus erythematosus (SLE), myositis, immune cytopenia, and others (Table 2.).
Table 2.
Glycosylation research in autoimmune diseases mimicked by cGVHD.
| Author reference/year | Disease* | Key findings of the study |
|---|---|---|
| Dry mouth and eye disorders | ||
| P. Youinou et al80/1992 | Sjögren’s syndrome | Elevated levels of asialylated IgG. |
| I. Castro et al81/2013 | Sjögren’s syndrome | Altered salivary mucins. |
| Gastrointestinal disorders | ||
| J. M. H. Larsson et al85/2011 | IBD | Reduced glycosylation of gastrointestinal mucins. |
| A. M. Dias et al87/2013 | IBD | Aberrant N-glycosylation of T cell receptor. |
| I. Trbojevic Akmacic et al23/2015 | IBD | Reduced immunosuppressive potential of IgG in IBD. |
| Hematological disorders | ||
| T. Bakchoul et al92/2013 | ITP | N-glycosylation of AAbs suggested to be prerequisite for platelet phagocytosis in vitro and in vivo. |
| Neurological and muscular disorders | ||
| M. H. J. Selman et al94/2011 | Myasthenia gravis | Lower levels of IgG2 galactosylation. |
| I. Perdivara et al95/2011 | Myositis | Lower levels of IgG galactosylation. |
| Other | ||
| C. Panzironi et al83/1997 | SLE | ‘Incresead carbohydrate moiety’ and higher concentration of galactose in α2-macroglobulin. |
| X.-X. Chen et al84/2015 | SLE | Reduced IgG sialylation. |
| F. Vuckovic et al24/2015 | SLE | Reduced immunosuppressive potential of IgG. |
IBD=inflammatory bowel disease; ITP= immune thrombocytopenia; SLE= systemic lupus erythematosus
4. Glycosylation patterns in various autoimmune diseases resembling cGVHD
Autoimmune-like features of cGVHD have already been reviewed on several occasions [78,79] and here are described in the light of glycosylation research potential.
Some of the more common symptoms of cGVHD are xerostomia and dry eyes, resembling SS, an autoimmune disease causing a functional impairment of salivary and lacrimal glands. It is considered that B cells are over-stimulated and produce excessive amounts of Igs and various AAbs in SS [74]. Elevated levels of asialylated IgG were detected in SS patients [80], and recent publications suggested altered glycosylation of salivary mucins which reduces lubricating properties and quality of the saliva [81]. This mechanism could explain the sensation of dry mouth even if the saliva volume and the glands remain intact and might be tested in cGVHD.
Development of AAbs and breakdown in B cell tolerance is characteristic of SLE [82], and glycosylation is also disturbed in this comprehensive immunological disorder. Increased glycan moiety in α2-macroglobulin and the significantly higher content of galactose in the glycan of the same protein was reported [83]. Recent extensive study of three independent cohorts of SLE patients also observed significant changes in glycome composition, which correlate to the symptom severity [24]. Changes of IgG sialylation have also been suggested as a novel biomarker for distinguishing patients with SLE from patients with other autoimmune diseases [84].
Gastrointestinal GVHD resembles inflammatory bowel disease (IBD), another autoimmune disorder. Inflammation in IBD is believed to be triggered by an aberrant immune response to gut microbiota in genetically susceptible individuals. Intestinal mucus of IBD patients shows decreased glycosylation which gives rise to increased bacterial contact with the epithelium and potentially triggers inflammation [85]. A recent research indicated a significantly increased inflammatory potential of IgG in IBD due to changes in its glycosylation. These changes are considered to contribute to the disease pathogenesis, and have been suggested as the biomarker of the disease onset and severity [23,86]. Dysregulation of T cell receptor N-glycosylation is also believed to be a part of the mechanism of ulcerative colitis, a type of IBD. Patients with severe degree of the disease showed a defect in N-glycan branching in T cell receptor [87], which might be tested in GVHD patients.
Autoimmune hematological diseases are frequently reported to occur following HSCT [79,88]. Thrombocytopenia in cGVHD could be autoimmune mediated but may as well have multifactorial etiology, and it is one of the risk factors for poorer survival in cGVHD patients [89–91]. AAbs which are likely to contribute to the increased rate of platelet destruction may develop, as in immune thrombocytopenia (ITP) [33]. It has been shown that IgG N-glycans are involved in the pathophysiology of ITP [92]. In vitro experiments demonstrated decreased phagocytic activity of monocytes mediated by deglycosylated AAbs compared to native AAbs from ITP patiens. Cleavage of carbohydrates also interfered with Fc-mediated phagocytosis and complement activation and prolonged platelet survival in vivo.
Neurological manifestations, such as myasthenia gravis, myositis and immune-mediated neuropathies [93], have also been described as a part of the cGVHD clinical picture. Clinicians are often challenged in discriminating the damage caused by cGVHD from drug-induced toxicities, long-term immunosuppression or opportunistic infections and glycans could represent a tool to resolve that dilemma. Myasthenia gravis is an antibody-mediated autoimmune disorder of the neuromuscular junction. N-glycosylation analysis of total IgG showed lower levels of IgG2 galactosylation in myasthenia gravis patients compared to controls, while there were no notable differences in the IgG core-fucosylation and the overall degree of sialylation remained similar [94]. Myositis patients had overall elevated amounts of IgG glycoforms lacking terminal galactose [95].
5. Conclusions and future considerations
Recent advances in technology of glycan analysis give hope of promising future research in alloHSCT and GVHD to better understand consequences of merging two immunological systems. Identifying glycan patterns that induce self-tolerance and the ones that cause the auto- and allo-immune response could lead to improved alloHSCT procedure. Some of the potential therapy ideas include remodeling of glycosylated proteins in vivo. Possibility of HSC manipulation and successful control of the engraftment process could result in further reducement of conditioning intensity, improved delivery of HSCs to their niche, cut down in number of graft rejections and enable transplatations with grafts limited with low HSC number, such is cord blood. This kind of innovative and tissue specific immunomodulative therapy could help us move past current immunosuppressive treatment with its toxic side-effects, enabling preservation of the graft-versus-tumor effect. This technology could also enable us to interfere with lymphocyte homing to the site of inflammation or restructure glycoforms contributing to the rise of acute and/or chronic GHVD [96]. In addition to that, neglected glycans could possibly represent candidate biomarkers for GVHD, much needed to diagnose and monitor the disease, prognose its course and outcome, and characterize its activity. For example, anti-glycan antibodies profiling is a new and promising tool and such antibodies have already been suggested as biomarkers for multiple sclerosis and IBD [97]. Systematic screening of blood could lead to a timely discovery of glycoforms indicative of developing acute or chronic GVHD or biomarkers specific for the organ system endangered by GVHD - enabling an appropriate therapy and monitoring of therapeutic response. To conclude, it is likely to expect that future research of glycans in the field of alloHSCT and GVHD would improve outcome of those patients.
Highlights.
Glycans are involved in almost every biological process
Glycans are associated with immunological disturbances and autoimmune diseases
GVHD is alloimmune and autoimmune disorder following alloHSCT
Glycans have a great research potential in clarifying events after alloHSCT and GVHD
Glycan research could lead to improved therapy and discovery of biomarkers
Acknowledgments
This work is supported in part by the Unity Through Knowledge Fund, Croatia (project ‘Clinical and biological factors determining severity and activity of chronic Graft-versus-Host Disease after allogeneic hematopoietic stem cell transplantion’), European Commission HighGlycan (contract #278535), MIMOmics (contract #305280), HTP-GlycoMet (contract #324400) and IntegraLife (contract #315997) grants and in part by the intramural program of the National Cancer Institute, Center for Cancer Research, National Institutes of Health, USA.
Footnotes
CONFLICT OF INTEREST: The authors declare no conflict of interest.
Disclaimer: The opinions expressed here are those of the authors and do not represent the official position of the National Institutes of Health or the US Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hart GW, Copeland RJ. Glycomics Hits the Big Time. Cell. 2010;143:672–676. doi: 10.1016/j.cell.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabinovich GA, van Kooyk Y, Cobb BA. Glycobiology of immune responses. Ann N Y Acad Sci. 2012;1253 doi: 10.1111/j.1749-6632.2012.06492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gornik O, Lauc G. Glycosylation of Serum Proteins in Inflammatory Diseases. Disease Markers. 2008;25:267–278. doi: 10.1155/2008/493289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauc G, Huffman JE, Pucic M, Zgaga L, Adamczyk B, Muzinic A, Novokmet M, Polasek O, Gornik O, Kristic J, Keser T, Vitart V, Scheijen B, Uh H-W, Molokhia M, Patrick AL, McKeigue P, Kolcic I, Lukic IK, Swann O, van Leeuwen FN, Ruhaak LR, Houwing-Duistermaat JJ, Slagboom PE, Beekman M, de Craen AJM, Deelder AM, Zeng Q, Wang W, Hastie ND, Gyllensten U, Wilson JF, Wuhrer M, Wright AF, Rudd PM, Hayward C, Aulchenko Y, Campbell H, Rudan I. Loci Associated with N-Glycosylation of Human Immunoglobulin G Show Pleiotropy with Autoimmune Diseases and Haematological Cancers. PLoS Genet. 2013;9:e1003225. doi: 10.1371/journal.pgen.1003225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aurer I, Lauc G, Dumic J, Rendic D, Matisic D, Milos M, Heffer-Lauc M, Flogel M, Labar B. Aberrant glycosylation of Igg heavy chain in multiple myeloma. Coll Antropol. 2007;31:247–251. [PubMed] [Google Scholar]
- 6.Lauc G, Pezer M, Rudan I, Campbell H. Mechanisms of disease: The human N-glycome. Biochim Biophys Acta. 2015 doi: 10.1016/j.bbagen.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Maverakis E, Kim K, Shimoda M, Gershwin ME, Patel F, Wilken R, Raychaudhuri S, Ruhaak LR, Lebrilla CB. Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: A critical review. J Autoimmun. 2015;57:1–13. doi: 10.1016/j.jaut.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lauc G, Rudan I, Campbell H, Rudd PM. Complex genetic regulation of protein glycosylation. Mol BioSyst. 2010;6:329–335. doi: 10.1039/B910377E. [DOI] [PubMed] [Google Scholar]
- 9.Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol. 2012;13:448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gornik O, Pavic T, Lauc G. Alternative glycosylation modulates function of IgG and other proteins—Implications on evolution and disease. Biochim Biophys Acta. 2012;1820:1318–1326. doi: 10.1016/j.bbagen.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. 2. Cold Spring Harbor Laboratory Press; Cold Spring Harbor (NY): 2009. [Accessed October 21, 2015]. Available at http://www.ncbi.nlm.nih.gov/books/NBK1908/ [PubMed] [Google Scholar]
- 12.Aschermann S, Lux A, Baerenwaldt A, Biburger M, Nimmerjahn F. The other side of immunoglobulin G: suppressor of inflammation. Clin Exp Immunol. 2010;160:161–167. doi: 10.1111/j.1365-2249.2009.04081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Böhm S, Schwab I, Lux A, Nimmerjahn F. The role of sialic acid as a modulator of the anti-inflammatory activity of IgG. Semin Immunopathol. 2012;34:443–453. doi: 10.1007/s00281-012-0308-x. [DOI] [PubMed] [Google Scholar]
- 14.Pucic M, Knezevic A, Vidic J, Adamczyk B, Novokmet M, Polasek O, Gornik O, Supraha-Goreta S, Wormald MR, Redzic I, Campbell H, Wright A, Hastie ND, Wilson JF, Rudan I, Wuhrer M, Rudd PM, Josic D, Lauc G. High Throughput Isolation and Glycosylation Analysis of IgG–Variability and Heritability of the IgG Glycome in Three Isolated Human Populations. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M111.010090. M111.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kristic J, Vuckovic F, Menni C, Klaric L, Keser T, Beceheli I, Pucic-Bakovic M, Novokmet M, Mangino M, Thaqi K, Rudan P, Novokmet N, Sarac J, Missoni S, Kolcic I, Polasek O, Rudan I, Campbell H, Hayward C, Aulchenko Y, Valdes A, Wilson JF, Gornik O, Primorac D, Zoldos V, Spector T, Lauc G. Glycans Are a Novel Biomarker of Chronological and Biological Ages. J Gerontol A Biol Sci Med Sci. 2014;69:779–789. doi: 10.1093/gerona/glt190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menni C, Keser T, Mangino M, Bell JT, Erte I, Akmacic I, Vuckovic F, Pucic Bakovic M, Gornik O, McCarthy MI, Zoldos V, Spector TD, Lauc G, Valdes AM. Glycosylation of Immunoglobulin G: Role of Genetic and Epigenetic Influences. PLoS One. 2013;8 doi: 10.1371/journal.pone.0082558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gornik O, Wagner J, Pucic M, Knezevic A, Redzic I, Lauc G. Stability of N-glycan profiles in human plasma. Glycobiology. 2009;19:1547–1553. doi: 10.1093/glycob/cwp134. [DOI] [PubMed] [Google Scholar]
- 18.Hanson QM, Barb AW. A Perspective on the Structure and Receptor Binding Properties of Immunoglobulin G Fc. Biochemistry. 2015;54:2931–2942. doi: 10.1021/acs.biochem.5b00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subedi GP, Barb AW. The Structural Role of Antibody N-Glycosylation in Receptor Interactions. Structure. 2015;23:1573–1583. doi: 10.1016/j.str.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quast I, Keller CW, Maurer MA, Giddens JP, Tackenberg B, Wang L-X, Münz C, Nimmerjahn F, Dalakas MC, Lünemann JD. Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity. J Clin Invest. 2015;125:4160–4170. doi: 10.1172/JCI82695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrara C, Grau S, Jäger C, Sondermann P, Brünker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, Umaña P, Benz J. Unique carbohydrate–carbohydrate interactions are required for high affinity binding between FcγRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A. 2011;108:12669–12674. doi: 10.1073/pnas.1108455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sondermann P, Pincetic A, Maamary J, Lammens K, Ravetch JV. General mechanism for modulating immunoglobulin effector function. Proc Natl Acad Sci U S A. 2013;110:9868–9872. doi: 10.1073/pnas.1307864110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trbojevic Akmacic I, Ventham NT, Theodoratou E, Vuckovic F, Kennedy NA, Kristic J, Nimmo ER, Kalla R, Drummond H, Stambuk J, Dunlop MG, Novokmet M, Aulchenko Y, Gornik O, Campbell H, Pucic Bakovic M, Satsangi J, Lauc G. Inflammatory Bowel Disease Associates with Proinflammatory Potential of the Immunoglobulin G Glycome. Inflamm Bowel Dis. 2015;21:1237–1247. doi: 10.1097/MIB.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vuckovic F, Kristic J, Gudelj I, Teruel M, Keser T, Pezer M, Pucic-Bakovic M, Stambuk J, Trbojevic-Akmacic I, Barrios C, Pavic T, Menni C, Wang Y, Zhou Y, Cui L, Song H, Zeng Q, Guo X, Pons-Estel BA, McKeigue P, Leslie Patrick A, Gornik O, Spector TD, Harjacek M, Alarcon-Riquelme M, Molokhia M, Wang W, Lauc G. Association of Systemic Lupus Erythematosus With Decreased Immunosuppressive Potential of the IgG Glycome. Arthritis Rheumatol. 2015;67:2978–2989. doi: 10.1002/art.39273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schweitzer KM, Dräger AM, van der Valk P, Thijsen SF, Zevenbergen A, Theijsmeijer AP, van der Schoot CE, Langenhuijsen MM. Constitutive expression of E-selectin and vascular cell adhesion molecule-1 on endothelial cells of hematopoietic tissues. Am J Pathol. 1996;148:165–175. [PMC free article] [PubMed] [Google Scholar]
- 26.Nabors LK, Wang LD, Wagers AJ, Kansas GS. Overlapping roles for endothelial selectins in murine hematopoietic stem/progenitor cell homing to bone marrow. Exp Hematol. 2013;41:588–596. doi: 10.1016/j.exphem.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winkler IG, Barbier V, Nowlan B, Jacobsen RN, Forristal CE, Patton JT, Magnani JL, Lévesque J-P. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med. 2012;18:1651–1657. doi: 10.1038/nm.2969. [DOI] [PubMed] [Google Scholar]
- 28.Sackstein R. The Bone Marrow Is Akin to Skin: HCELL and the Biology of Hematopoietic Stem Cell Homing. J Investig Dermatol. 2004;122:1061–1069. doi: 10.1111/j.0022-202X.2004.09301.x. [DOI] [PubMed] [Google Scholar]
- 29.Nasirikenari M, Veillon L, Collins CC, Azadi P, Lau JTY. Remodeling of Marrow Hematopoietic Stem and Progenitor Cells by Non-self ST6Gal-1 Sialyltransferase. J Biol Chem. 2014;289:7178–7189. doi: 10.1074/jbc.M113.508457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sackstein R. Glycoengineering of HCELL, the Human Bone Marrow Homing Receptor: Sweetly Programming Cell Migration. Ann Biomed Eng. 2011;40:766–776. doi: 10.1007/s10439-011-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson SN, Thomas MW, Simmons PJ, Lu J, Yang H, Parmar S, Liu X, Shah N, Martín-Antonio B, Bollard C, Dotti G, Savoldo B, Cooper LJ, Najjar A, Rezvani K, Kaur I, McNiece IK, Champlin RE, Miller LP, Zweidler-McKay PA, Shpall EJ. Fucosylation with Fucosyltransferase (FT)-VI or FT-VII Improves Cord Blood Engraftment. Cytotherapy. 2014;16:84–9. doi: 10.1016/j.jcyt.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, Couriel D, Cowen EW, Dinndorf P, Farrell A, Hartzman R, Henslee-Downey J, Jacobsohn D, McDonald G, Mittleman B, Rizzo JD, Robinson M, Schubert M, Schultz K, Shulman H, Turner M, Vogelsang G, Flowers MED. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J, Weisdorf D, Treister NS, Cheng G-S, Kerr H, Stratton P, Duarte RF, McDonald GB, Inamoto Y, Vigorito A, Arai S, Datiles MB, Jacobsohn D, Heller T, Kitko CL, Mitchell SA, Martin PJ, Shulman H, Wu RS, Cutler CS, Vogelsang GB, Lee SJ, Pavletic SZ, Flowers MED. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2015;21:389–401.e1. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dignan FL, Clark A, Amrolia P, Cornish J, Jackson G, Mahendra P, Scarisbrick JJ, Taylor PC, Hadzic N, Shaw BE, Potter MN on behalf of the Haemato-oncology Task Force of the British Committee for Standards in Haematology and the British Society for Blood and Marrow Transplantation. Diagnosis and management of acute graft-versus-host disease. Br J Haematol. 2012;158:30–45. doi: 10.1111/j.1365-2141.2012.09129.x. [DOI] [PubMed] [Google Scholar]
- 35.Johnston L. Acute graft-versus-host disease: differing risk with differing graft sources and conditioning intensity. Best Pract Res Clin Haematol. 2008;21:177–192. doi: 10.1016/j.beha.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Maeda Y. Pathogenesis of graft-versus-host disease: innate immunity amplifying acute alloimmune responses. Int J Hematol. 2013;98:293–299. doi: 10.1007/s12185-013-1421-x. [DOI] [PubMed] [Google Scholar]
- 37.Ramadan A, Paczesny S. Various Forms of Tissue Damage and Danger Signals Following Hematopoietic Stem-Cell Transplantation. Front Immunol. 2015;6 doi: 10.3389/fimmu.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novokmet M, Lukic E, Vuckovic F, Duric Z, Keser T, Rajsl K, Remondini D, Castellani G, Gasparovic H, Gornik O, Lauc G. Changes in IgG and total plasma protein glycomes in acute systemic inflammation. Sci Rep. 2014;4 doi: 10.1038/srep04347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gornik O, Royle L, Harvey DJ, Radcliffe CM, Saldova R, Dwek RA, Rudd P, Lauc G. Changes of Serum Glycans During Sepsis and Acute Pancreatitis. Glycobiology. 2007;17:1321–1332. doi: 10.1093/glycob/cwm106. [DOI] [PubMed] [Google Scholar]
- 40.Naka EL, Ponciano VC, Cenedeze MA, Pacheco-Silva A, Saraiva Câmara NO. Detection of the Tim-3 ligand, galectin-9, inside the allograft during a rejection episode. Int Immunopharmacol. 2009;9:658–662. doi: 10.1016/j.intimp.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Wu C, Thalhamer T, Franca RF, Xiao S, Wang C, Hotta C, Zhu C, Hirashima M, Anderson AC, Kuchroo VK. Galectin-9-CD44 Interaction Enhances Stability and Function of Adaptive Regulatory T Cells. Immunity. 2014;41:270–282. doi: 10.1016/j.immuni.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiersma VR, de Bruyn M, Helfrich W, Bremer E. Therapeutic potential of Galectin-9 in human disease. Med Res Rev. 2013;33:E102–E126. doi: 10.1002/med.20249. [DOI] [PubMed] [Google Scholar]
- 43.Sakai K, Kawata E, Ashihara E, Nakagawa Y, Yamauchi A, Yao H, Nagao R, Tanaka R, Yokota A, Takeuchi M, Hirai H, Kimura S, Hirashima M, Yoshimura N, Maekawa T. Galectin-9 ameliorates acute GVH disease through the induction of T-cell apoptosis. Eur J Immunol. 2011;41:67–75. doi: 10.1002/eji.200939931. [DOI] [PubMed] [Google Scholar]
- 44.Choi SW, Levine JE, Ferrara JLM. Pathogenesis and Management of Graft versus Host Disease. Immunol Allergy Clin North Am. 2010;30:75–101. doi: 10.1016/j.iac.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SJ, Flowers MED. Recognizing and Managing Chronic Graft-Versus-Host Disease. Hematology. 2008;2008:134–141. doi: 10.1182/asheducation-2008.1.134. [DOI] [PubMed] [Google Scholar]
- 46.Arai S, Arora M, Wang T, Spellman SR, He W, Couriel DR, Urbano-Ispizua A, Cutler CS, Bacigalupo AA, Battiwalla M, Flowers ME, Juckett MB, Lee SJ, Loren AW, Klumpp TR, Prockup SE, Ringdén OTH, Savani BN, Socié G, Schultz KR, Spitzer T, Teshima T, Bredeson CN, Jacobsohn DA, Hayashi RJ, Drobyski WR, Frangoul HA, Akpek G, Ho VT, Lewis VA, Gale RP, Koreth J, Chao NJ, Aljurf MD, Cooper BW, Laughlin MJ, Hsu JW, Hematti P, Verdonck LF, Solh MM, Norkin M, Reddy V, Martino R, Gadalla S, Goldberg JD, McCarthy PL, Pérez-Simón JA, Khera N, Lewis ID, Atsuta Y, Olsson RF, Saber W, Waller EK, Blaise D, Pidala JA, Martin PJ, Satwani P, Bornhäuser M, Inamoto Y, Weisdorf DJ, Horowitz MM, Pavletic SZ. Increasing Incidence of Chronic Graft-versus-Host Disease in Allogeneic Transplantation: A Report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2015;21:266–274. doi: 10.1016/j.bbmt.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin PJ, Weisdorf D, Przepiorka D, Hirschfeld S, Farrell A, Rizzo JD, Foley R, Socie G, Carter S, Couriel D, Schultz KR, Flowers MED, Filipovich AH, Saliba R, Vogelsang GB, Pavletic SZ, Lee SJ. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: VI. Design of Clinical Trials Working Group Report. Biol Blood Marrow Transplant. 2006;12:491–505. doi: 10.1016/j.bbmt.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Shulman HM, Kleiner D, Lee SJ, Morton T, Pavletic SZ, Farmer E, Moresi JM, Greenson J, Janin A, Martin PJ, McDonald G, Flowers MED, Turner M, Atkinson J, Lefkowitch J, Washington MK, Prieto VG, Kim SK, Argenyi Z, Diwan AH, Rashid A, Hiatt K, Couriel D, Schultz K, Hymes S, Vogelsang GB. Histopathologic Diagnosis of Chronic Graft-versus-Host Disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant. 2006;12:31–47. doi: 10.1016/j.bbmt.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 49.Schultz KR, Miklos DB, Fowler D, Cooke K, Shizuru J, Zorn E, Holler E, Ferrara J, Shulman H, Lee SJ, Martin P, Filipovich AH, Flowers MED, Weisdorf D, Couriel D, Lachenbruch PA, Mittleman B, Vogelsang GB, Pavletic SZ. Toward Biomarkers for Chronic Graft-versus-Host Disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: III. Biomarker Working Group Report. Biol Blood Marrow Transplant. 2006;12:126–137. doi: 10.1016/j.bbmt.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 50.Pavletic SZ, Martin P, Lee SJ, Mitchell S, Jacobsohn D, Cowen EW, Turner ML, Akpek G, Gilman A, McDonald G, Schubert M, Berger A, Bross P, Chien JW, Couriel D, Dunn JP, Fall-Dickson J, Farrell A, Flowers MED, Greinix H, Hirschfeld S, Gerber L, Kim S, Knobler R, Lachenbruch PA, Miller FW, Mittleman B, Papadopoulos E, Parsons SK, Przepiorka D, Robinson M, Ward M, Reeve B, Rider LG, Shulman H, Schultz KR, Weisdorf D, Vogelsang GB. Measuring Therapeutic Response in Chronic Graft-versus-Host Disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group Report. Biol Blood Marrow Transplant. 2006;12:252–266. doi: 10.1016/j.bbmt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 51.Couriel D, Carpenter PA, Cutler C, Bolaños-Meade J, Treister NS, Gea-Banacloche J, Shaughnessy P, Hymes S, Kim S, Wayne AS, Chien JW, Neumann J, Mitchell S, Syrjala K, Moravec CK, Abramovitz L, Liebermann J, Berger A, Gerber L, Schubert M, Filipovich AH, Weisdorf D, Schubert MM, Shulman H, Schultz K, Mittelman B, Pavletic S, Vogelsang GB, Martin PJ, Lee SJ, Flowers MED. Ancillary Therapy and Supportive Care of Chronic Graft-versus-Host Disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2006;12:375–396. doi: 10.1016/j.bbmt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Shulman HM, Cardona DM, Greenson JK, Hingorani S, Horn T, Huber E, Kreft A, Longerich T, Morton T, Myerson D, Prieto VG, Rosenberg A, Treister N, Washington K, Ziemer M, Pavletic SZ, Lee SJ, Flowers MED, Schultz KR, Jagasia M, Martin PJ, Vogelsang GB, Kleiner DE. NIH Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. The 2014 Pathology Working Group Report. Biol Blood Marrow Transplant. 2015;21:589–603. doi: 10.1016/j.bbmt.2014.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paczesny S, Hakim FT, Pidala J, Cooke K, Lathrop J, Griffith LM, Hansen J, Jagasia M, Miklos D, Pavletic S, Parkman R, Russek-Cohen E, Flowers MED, Lee S, Martin P, Vogelsang G, Walton M, Schultz KR. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: III. The 2014 Biomarker Working Group Report. Biol Blood Marrow Transplant. 2015;21:780–92. doi: 10.1016/j.bbmt.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee SJ, Wolff D, Kitko C, Koreth J, Inamoto Y, Jagasia M, Pidala J, Olivieri A, Martin PJ, Przepiorka D, Pusic I, Dignan F, Mitchell SA, Lawitschka A, Jacobsohn D, Hall AM, Flowers MED, Schultz KR, Vogelsang G, Pavletic S. Measuring Therapeutic Response in Chronic Graft-versus-Host Disease. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. The 2014 Response Criteria Working Group Report. Biol Blood Marrow Transplant. 2015;21:984–999. doi: 10.1016/j.bbmt.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carpenter PA, Kitko CL, Elad S, Flowers MED, Gea-Banacloche JC, Halter JP, Hoodin F, Johnston L, Lawitschka A, McDonald GB, Opipari AW, Savani BN, Schultz KR, Smith SR, Syrjala KL, Treister N, Vogelsang GB, Williams KM, Pavletic SZ, Martin PJ, Lee SJ, Couriel DR. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: V. The 2014 Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2015;21:1167–1187. doi: 10.1016/j.bbmt.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin PJ, Lee SJ, Przepiorka D, Horowitz MM, Koreth J, Vogelsang GB, Walker I, Carpenter PA, Griffith LM, Akpek G, Mohty M, Wolff D, Pavletic SZ, Cutler CS. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: VI. The 2014 Clinical Trial Design Working Group Report. Biol Blood Marrow Transplant. 2015;21:1343–1359. doi: 10.1016/j.bbmt.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coghill JM, Sarantopoulos S, Moran TP, Murphy WJ, Blazar BR, Serody JS. Effector CD4+ T cells, the cytokines they generate, and GVHD: something old and something new. Blood. 2011;117:3268–3276. doi: 10.1182/blood-2010-12-290403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12:443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shimabukuro-Vornhagen A, Hallek MJ, Storb RF, von Bergwelt-Baildon MS. The role of B cells in the pathogenesis of graft-versus-host disease. Blood. 2009;114:4919–4927. doi: 10.1182/blood-2008-10-161638. [DOI] [PubMed] [Google Scholar]
- 60.Young JS, Wu T, Chen Y, Zhao D, Liu H, Yi T, Johnston H, Racine J, Li X, Wang A, Todorov I, Zeng D. Donor B cells in Transplants Augment Clonal Expansion and Survival of Pathogenic CD4+ T cells That Mediate Autoimmune-like Chronic GVHD. J Immunol. 2012;189:222–233. doi: 10.4049/jimmunol.1200677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sarantopoulos S, Ritz J. Aberrant B cell homeostasis in chronic GVHD. Blood. 2015;125:1703–7. doi: 10.1182/blood-2014-12-567834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allen JL, Fore MS, Wooten J, Roehrs PA, Bhuiya NS, Hoffert T, Sharf A, Deal AM, Armistead P, Coghill J, Gabriel DA, Irons R, Essenmacher A, Shea TC, Richards K, Cutler C, Ritz J, Serody J, Baldwin AS, Sarantopoulos S. B cells from patients with chronic GVHD are activated and primed for survival via BAFF-mediated pathways. Blood. 2012;120:2529–2536. doi: 10.1182/blood-2012-06-438911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sarantopoulos S, Stevenson KE, Kim HT, Cutler CS, Bhuiya NS, Schowalter M, Ho VT, Alyea EP, Koreth J, Blazar BR, Soiffer RJ, Antin JH, Ritz J. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113:3865–3874. doi: 10.1182/blood-2008-09-177840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacobson CA, Sun L, Kim HT, McDonough SM, Reynolds CG, Schowalter M, Koreth J, Cutler CS, Ho VT, Alyea EP, Armand P, Blazar BR, Soiffer RJ, Antin JH, Ritz J, Sarantopoulos S. Post-Transplantation B Cell Activating Factor and B Cell Recovery before Onset of Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2014;20:668–675. doi: 10.1016/j.bbmt.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brink R. Regulation of B cell self-tolerance by BAFF. Semin Immunol. 2006;18:276–283. doi: 10.1016/j.smim.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 66.Sarantopoulos S, Stevenson KE, Kim HT, Bhuiya NS, Cutler CS, Soiffer RJ, Antin JH, Ritz J. High Levels of B-Cell Activating Factor in Patients with Active Chronic Graft-Versus-Host Disease. Clin Cancer Res. 2007;13:6107–6114. doi: 10.1158/1078-0432.CCR-07-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cutler C, Kim HT, Bindra B, Sarantopoulos S, Ho VT, Chen Y-B, Rosenblatt J, McDonough S, Watanaboonyongcharoen P, Armand P, Koreth J, Glotzbecker B, Alyea E, Blazar BR, Soiffer RJ, Ritz J, Antin JH. Rituximab prophylaxis prevents corticosteroid-requiring chronic GVHD after allogeneic peripheral blood stem cell transplantation: results of a phase 2 trial. Blood. 2013;122:1510–1517. doi: 10.1182/blood-2013-04-495895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arai S, Sahaf B, Narasimhan B, Chen GL, Jones CD, Lowsky R, Shizuru JA, Johnston LJ, Laport GG, Weng W-K, Benjamin JE, Schaenman J, Brown J, Ramirez J, Zehnder JL, Negrin RS, Miklos DB. Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence. Blood. 2012;119:6145–6154. doi: 10.1182/blood-2011-12-395970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sarantopoulos S, Stevenson KE, Kim HT, Washel WS, Bhuiya NS, Cutler CS, Alyea EP, Ho VT, Soiffer RJ, Antin JH, Ritz J. Recovery of B-cell homeostasis after rituximab in chronic graft-versus-host disease. Blood. 2011;117:2275–2283. doi: 10.1182/blood-2010-10-307819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alduaij W, Illidge TM. The future of anti-CD20 monoclonal antibodies: are we making progress? Blood. 2011;117:2993–3001. doi: 10.1182/blood-2010-07-298356. [DOI] [PubMed] [Google Scholar]
- 71.Kellner C, Derer S, Valerius T, Peipp M. Boosting ADCC and CDC activity by Fc engineering and evaluation of antibody effector functions. Methods. 2014;65:105–113. doi: 10.1016/j.ymeth.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 72.Kuzmina Z, Gounden V, Curtis L, Avila D, Rnp TT, Baruffaldi J, Cowen EW, Naik HB, Hasni SA, Mays JW, Mitchell S, Baird K, Steinberg SM, Pavletic SZ. Clinical significance of autoantibodies in a large cohort of patients with chronic graft-versus-host disease defined by NIH criteria. Am J Hematol. 2015;90:114–119. doi: 10.1002/ajh.23885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dörner T, Giesecke C, Lipsky PE. Mechanisms of B cell autoimmunity in SLE. Arthritis Res Ther. 2011;13:243. doi: 10.1186/ar3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cornec D, Devauchelle-Pensec V, Tobón GJ, Pers J-O, Jousse-Joulin S, Saraux A. B cells in Sjögren’s syndrome: From pathophysiology to diagnosis and treatment. J Autoimmun. 2012;39:161–167. doi: 10.1016/j.jaut.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 75.François A, Chatelus E, Wachsmann D, Sibilia J, Bahram S, Alsaleh G, Gottenberg J-E. B lymphocytes and B-cell activating factor promote collagen and profibrotic markers expression by dermal fibroblasts in systemic sclerosis. Arthritis Res Ther. 2013;15:R168. doi: 10.1186/ar4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pers J-O, Daridon C, Devauchelle V, Jousse S, Saraux A, Jamin C, Youinou P. BAFF Overexpression Is Associated with Autoantibody Production in Autoimmune Diseases. Ann N Y Acad Sci. 2005;1050:34–39. doi: 10.1196/annals.1313.004. [DOI] [PubMed] [Google Scholar]
- 77.Eggert M, Zettl UK, Neeck G. Autoantibodies in Autoimmune Diseases. Curr Pharm Des. 2010;16:1634–1643. doi: 10.2174/138161210791164144. [DOI] [PubMed] [Google Scholar]
- 78.Tyndall A, Dazzi F. Chronic GVHD as an autoimmune disease. Best Pract Res Clin Haematol. 2008;21:281–289. doi: 10.1016/j.beha.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 79.Daikeler T, Tyndall A. Autoimmunity following haematopoietic stem-cell transplantation. Best Pract Res Clin Haematol. 2007;20:349–360. doi: 10.1016/j.beha.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 80.Youinou P, Pennec Y-L, Casburn-Budd R, Dueymes M, Letoux G, Lamour A. Galactose terminating oligosaccharides of IgG in patients with primary Sjögren’s syndrome. J Autoimmun. 1992;5:393–400. doi: 10.1016/0896-8411(92)90151-F. [DOI] [PubMed] [Google Scholar]
- 81.Castro I, Sepúlveda D, Cortés J, Quest AFG, Barrera MJ, Bahamondes V, Aguilera S, Urzúa U, Alliende C, Molina C, González S, Hermoso MA, Leyton C, González MJ. Oral dryness in Sjögren’s syndrome patients. Not just a question of water. Autoimmun Rev. 2013;12:567–574. doi: 10.1016/j.autrev.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 82.Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Panzironi C, Mo M, Mruk D, Cheng CY, Silvestrini B, Lahita R. An increase in the carbohydrate moiety of α2-macroglobulin is associated with systemic lupus erythematosus (SLE) Biochem Mol Biol Int. 1997;43:1305–1322. doi: 10.1080/15216549700205131. [DOI] [PubMed] [Google Scholar]
- 84.Chen X-X, Chen Y-Q, Ye S. Measuring decreased serum IgG sialylation: a novel clinical biomarker of lupus. Lupus. 2015 doi: 10.1177/0961203315570686. 0961203315570686. [DOI] [PubMed] [Google Scholar]
- 85.Larsson JMH, Karlsson H, Crespo JG, Johansson MEV, Eklund L, Sjövall H, Hansson GC. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm Bowel Dis. 2011;17:2299–2307. doi: 10.1002/ibd.21625. [DOI] [PubMed] [Google Scholar]
- 86.Theodoratou E, Campbell H, Ventham NT, Kolarich D, Pucic-Bakovic M, Zoldos V, Fernandes D, Pemberton IK, Rudan I, Kennedy NA, Wuhrer M, Nimmo E, Annese V, McGovern DPB, Satsangi J, Lauc G. The role of glycosylation in IBD. Nat Rev Gastroenterol Hepatol. 2014;11:588–600. doi: 10.1038/nrgastro.2014.78. [DOI] [PubMed] [Google Scholar]
- 87.Dias AM, Dourado J, Lago P, Cabral J, Marcos-Pinto R, Salgueiro P, Almeida CR, Carvalho S, Fonseca S, Lima M, Vilanova M, Dinis-Ribeiro M, Reis CA, Pinho SS. Dysregulation of T cell receptor N-glycosylation: a molecular mechanism involved in ulcerative colitis. Hum Mol Genet. 2013;23:2416–27. doi: 10.1093/hmg/ddt632. [DOI] [PubMed] [Google Scholar]
- 88.Faraci M, Zecca M, Pillon M, Rovelli A, Menconi MC, Ripaldi M, Fagioli F, Rabusin M, Ziino O, Lanino E, Locatelli F, Daikeler T, Prete A. Autoimmune Hematological Diseases after Allogeneic Hematopoietic Stem Cell Transplantation in Children: An Italian Multicenter Experience. Biol Blood Marrow Transplant. 2014;20:272–278. doi: 10.1016/j.bbmt.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 89.Jacobsohn DA, Arora M, Klein JP, Hassebroek A, Flowers ME, Cutler CS, Urbano-Ispizua A, Bolwell BJ, Antin JH, Boyiadzis M, Cahn J-Y, Cairo MS, Herzig RH, Isola LM, Klumpp TR, Lee SJ, Petersdorf EW, Santarone S, Gale RP, Schouten HC, Spellman SR, Weisdorf DJ, Wingard JR, Horowitz MM, Pavletic SZ. Risk factors associated with increased nonrelapse mortality and with poor overall survival in children with chronic graft-versus-host disease. Blood. 2011;118:4472–4479. doi: 10.1182/blood-2011-04-349068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pavletic SZ, Smith LM, Bishop MR, Lynch JC, Tarantolo SR, Vose JM, Bierman PJ, Hadi A, Armitage JO, Kessinger A. Prognostic factors of chronic graft-versus-host disease after allogeneic blood stem-cell transplantation. Am J Hematol. 2005;78:265–274. doi: 10.1002/ajh.20275. [DOI] [PubMed] [Google Scholar]
- 91.Pulanic D, Lozier JN, Pavletic SZ. Thrombocytopenia and hemostatic disorders in chronic graft versus host disease. Bone Marrow Transplant. 2009;44:393–403. doi: 10.1038/bmt.2009.196. [DOI] [PubMed] [Google Scholar]
- 92.Bakchoul T, Walek K, Krautwurst A, Rummel M, Bein G, Santoso S, Sachs UJ. Glycosylation of autoantibodies: Insights into the mechanisms of immune thrombocytopenia. Thromb Haemost. 2013;110:1259–1266. doi: 10.1160/TH13-04-0294. [DOI] [PubMed] [Google Scholar]
- 93.Grauer O, Wolff D, Bertz H, Greinix H, Kühl J-S, Lawitschka A, Lee SJ, Pavletic SZ, Holler E, Kleiter I. Neurological manifestations of chronic graft-versus-host disease after allogeneic haematopoietic stem cell transplantation: report from the Consensus Conference on Clinical Practice in chronic graft-versus-host disease. Brain. 2010;133:2852–2865. doi: 10.1093/brain/awq245. [DOI] [PubMed] [Google Scholar]
- 94.Selman MHJ, Niks EH, Titulaer MJ, Verschuuren JJGM, Wuhrer M, Deelder AM. IgG Fc N-Glycosylation Changes in Lambert-Eaton Myasthenic Syndrome and Myasthenia Gravis. J Proteome Res. 2011;10:143–152. doi: 10.1021/pr1004373. [DOI] [PubMed] [Google Scholar]
- 95.Perdivara I, Peddada SD, Miller FW, Tomer KB, Deterding LJ. Mass Spectrometric Determination of IgG Subclass-Specific Glycosylation Profiles in Siblings Discordant for Myositis Syndromes. J Proteome Res. 2011;10:2969–2978. doi: 10.1021/pr200397h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dalziel M, Crispin M, Scanlan CN, Zitzmann N, Dwek RA. Emerging Principles for the Therapeutic Exploitation of Glycosylation. Science. 2014;343:1235681. doi: 10.1126/science.1235681. [DOI] [PubMed] [Google Scholar]
- 97.Seow CH, Stempak JM, Xu W, Lan H, Griffiths AM, Greenberg GR, Steinhart AH, Dotan N, Silverberg MS. Novel Anti-Glycan Antibodies Related to Inflammatory Bowel Disease Diagnosis and Phenotype. Am J Gastroenterol. 2009;104:1426–1434. doi: 10.1038/ajg.2009.79. [DOI] [PubMed] [Google Scholar]