Abstract
Neuropathic pain is a debilitating consequence of spinal cord injury (SCI) that correlates with sensory fiber sprouting. Recent data indicates that exercise initiated early after SCI prevents the development of allodynia and modulated nociceptive afferent plasticity. This study determined if delaying exercise intervention until pain is detected would similarly ameliorate established SCI-induced pain. Adult, female Sprague-Dawley rats with a C5 unilateral contusion were separated into SCI allodynic and SCI non-allodynic cohorts at 14 or 28 dpi when half of each group began exercising on automated running wheels. Allodynia, assessed by von Frey testing, was not ameliorated by exercise. Furthermore, rats that began exercise with no allodynia developed paw hypersensitivity within 2 weeks. At the initiation of exercise, the SCI Allodynia group displayed marked overlap of peptidergic and non-peptidergic nociceptive afferents in the C7 and L5 dorsal horn, while the SCI No Allodynia group had scant overlap. At the end of 5 weeks of exercise both the SCI Allodynia and SCI No Allodynia group had extensive overlap of the 2 c fiber types. Our findings show that exercise therapy initiated at early stages of allodynia is ineffective at attenuating neuropathic pain, but rather that it induces allodynia aberrant afferent plasticity in previously pain-free rats. These data, combined with our previous results suggest that there is a critical therapeutic window when exercise therapy may be effective at treating SCI-induced allodynia and that there are post-injury periods when exercise can be deleterious.
Keywords: mechanical allodynia, central pain, spinal cord injury, nociceptor, neuroplasticity, rehabilitation
INTRODUCTION
Spinal cord injury (SCI) impairs sensation causing chronic, debilitating neuropathic pain in over two-thirds of people with SCI. A staggering $100 billion is spent annually in the United States on health care, lost income and lost productivity due to chronic pain. Clinical hallmarks of neuropathic pain are the development of allodynia, where normally innocuous stimuli elicit a painful response and hyperalgesia, where noxious stimuli elicit an amplified pain response (1). Neuropathic pain is a complex phenomenon that is often unresolved with pharmacological treatment.
The effect of SCI on nociceptive primary sensory neurons and their centrally directed afferent fibers is a major contributor to the development of SCI-induced pain (2-4). After SCI, the amplification of nocifensive or neuropathic signaling begins in the primary sensory neuron (5). It is well-established that the afferent fibers of these nociceptors exhibit dramatic maladaptive arborization into the deep dorsal horn (laminas III-V) above, at and below the lesion epicenter in clinical (6, 7) as well as experimental SCI (8-11).
Exercise is a non-invasive, clinically used treatment that promotes long lasting plasticity of primary afferents and local spinal cord circuitry after SCI (12-16). Importantly, exercise that is initiated within the first week post injury can also modulate the development of allodynia in experimental SCI (11, 17, 18) and other experimental models of neuropathic pain (19-23). Our laboratory has recently shown that exercise beginning within the first week after SCI is sufficient to prevent aberrant plasticity of nociceptive primary afferent fibers and to prevent onset of tactile allodynia (11).
In the present study, we evaluated the effect of daily exercise therapy that was initiated at the onset of SCI-induced neuropathic pain or after it was established in the fore- and hindpaws. The results show that delaying exercise until allodynia develops or is more fully established neither reversed or attenuated allodynic behavior nor aberrant changes in the topographic distribution of nociceptive afferents in the dorsal horn. These data indicate that the therapeutic window where exercise rehabilitation is most beneficial for the retention of normal sensation after SCI in rats closes before 14 days post injury (dpi).
METHODS
Subjects
One hundred and sixty-two adult, female Sprague-Dawley rats (225-250 g; Charles River Laboratories) were housed 2-3 per cage in a controlled environment (12 h light-dark cycles) with food and water ad libitum. All experimental procedures were approved by the Drexel University Institutional Animal Care and Use Committee. Two experiments were conducted in this study—the first determined the effect of exercise on established SCI pain and the second examined the effect of SCI on the topographic distribution of primary afferent fibers over time. See Tables 1 and 2 for group assignment details.
Table 1.
Experimental Design for Experiment 1. Effect of Exercise on Established SCI Pain
| Group | n | Initiation of Exercise (dpi) |
Survival Time (dpi) |
|---|---|---|---|
| Naïve | 5 | 42 | |
| SCI No Allodynia | 13 | 42 | |
| SCI Allodynia | 7 | 42 | |
| SCI No Allodynia +Exercise | 9 | 14 | 42 |
| SCI Allodynia + Exercise | 9 | 14 | 42 |
| SCI No Allodynia | 12 | 56 | |
| SCI Allodynia | 8 | 56 | |
| SCI No Allodynia +Exercise | 9 | 28 | 56 |
| SCI Allodynia + Exercise | 9 | 28 | 56 |
Table 2.
Experimental Design for Experiment 2. Distribution of Primary Afferents Over Time
| Group | n |
|---|---|
| Naïve | 5 |
| 3 d SCI | 8 |
| 7 d SCI | 8 |
| 14 d SCI No Allodynia | 12 |
| 14 d SCI Allodynia | 8 |
| 28 d SCI No Allodynia | 13 |
| 28 d SCI Allodynia | 7 |
| 42 d SCI No Allodynia | 13 |
| 42 d SCI Allodynia | 7 |
As we have previously shown, at 14 dpi, SCI rats can be partitioned into 2 groups based on their paw withdrawal threshold in response to mechanical stimuli during von Frey testing (24). For a rat to be considered in the SCI Allodynia group the animal must exhibit a 50% reduction in paw withdrawal threshold in the affected forepaw that is maintained from 14 dpi to the duration of the experiment (42 or 56 dpi). At 14 dpi, tactile sensory thresholds were determined for all SCI rats. For Experiment 1, rats from the SCI Allodynia and the SCI No Allodynia groups (n=9/group) were randomly assigned to begin exercise at 14 or 28 dpi. The remaining rats were not exercised (as shown in Table 1).
For Experiment 2, SCI rats were randomly designated for sacrifice at specific dates post injury (3, 7, 14, 28, 42 dpi). SCI rats that were sacrificed at 14, 28, or 42 dpi were partitioned into SCI Allodynia and SCI No Allodynia groups in the same manner as in Experiment 1, with a minimum of 7/group. Group assignments for SCI rats in Experiment 2 are shown in Table 2.
Surgical Procedures
C5 unilateral spinal cord contusion
We utilized a clinically-relevant and accepted model of SCI pain (24). Rats were anesthetized with xylazine (6 mg/kg), ketamine (60 mg/kg) and acepromazine (6 mg/kg; XAK) and given antibiotics (ampicillin, s.c., 100 mg/kg or cefazolin, s.c., 160 mg/kg, daily for 7d). After C5 hemilaminectomy, the spinal cord was rapidly contused at a force of 200 kilodynes (no dwell time) using the Infinite Horizon Impact Device (Precision Systems and Instrumentation, Fairfax, VA (25)). The incision was closed in layers and 5 cc of lactated Ringer’s solution (LRS) was administered subcutaneously to prevent dehydration.
Three days prior to being euthanized rats in Experiment 2 were anesthetized with XAK, and an axial incision to the ipsilesional (right) forelimb exposed the median and ulnar nerves (26), and the perineurium was removed. A 5-µL Hamilton syringe fitted with a custom 30 gauge needle (#7803-07, Hamilton Company, Reno, NV) was inserted into each nerve, and 2.0 µL of cholera toxin β subunit (CTB;10 µg/µL; Product #104, List Biological Laboratories, Inc., Campbell, CA) was injected to label myelinated sensory afferents. Rats received 5 cc of LRS and cefazolin subcutaneously following surgery.
Forced Exercise Paradigm
Rats in the SCI + Exercise groups began exercise in the forced exercise wheel walking system (Lafayette Instruments, Lafayette, IN) at 14 or 28 dpi at an initial speed of 6 m/min with speed increasing by 1 m/min at 30 second intervals according to the forelimb capabilities of the rats or until a maximum speed of 14.0 m/min was achieved (11). Rats underwent this exercise session for 20 min/day, 5 days/week and continued for 5 weeks. All rats reached the maximum speed by 1 week after exercise was initiated.
Behavioral Measures
Rats were acclimated to the individual testing environments for at least 7 days (20 min/day) prior to preoperative testing. Behavioral testing was conducted preoperatively to establish baseline responses and weekly after SCI by blinded experimenters. Due to the unilateral nature of the injury model, we evaluated the ipsilesional (right) and contralesional (left) forepaws separately.
Tactile allodynia
The up-down method for von Frey hair monofilaments (VFH, Stoelting Co., Wood Dale, Il) was used to measure the degree of tactile sensory changes in the forepaws and hindpaws after SCI (27-29). Rats were enclosed in a chamber, and ten VFH stimulus applications were collected for each paw on each day of testing, beginning with the 5.18 g VFH. The paw withdrawal threshold was determined as the lowest force (g) that produced a forepaw withdrawal and supraspinal behaviors in at least 50% of the applications. Additionally, the rat must exhibit one or more signs of supraspinal awareness such as, vocalizing, licking, looking at, or guarding of the paw, or moving away from the stimulus after the application of the von Frey monofilament that elicits a paw withdrawal response. The responses are recorded, and represented as a percent of supraspinal responses. Paw testing order was performed randomly to minimize an order effect.
Histology
Rats were euthanized at prescribed time points post SCI (Euthasol; 390 mg/kg sodium pentobarbital and 50 mg/kg phenytoin, i.p.) and perfused transcardially with 250 ml of 0.9% saline followed by 350 ml of 4% paraformaldehyde. Blocks of C4-C6 cord spanning the lesion, C7-8 cord and L3-5 cord were dissected, post-fixed in paraformaldehyde at 4°C overnight and then submersed in 30% sucrose for cryoprotection. Series of 25-30 micron thick transverse sections 250 µm apart through the extent of these tissue blocks were mounted on subbed slides or kept floating in 0.1M phosphate buffered saline (PBS).
Nissl-Myelin staining and analysis of the lesion site
Sections from a single series of C4-C6 spinal cord were stained with cresyl violet (Sigma, St. Louis, MO) for Nissl substance and euriochrome cyanine (Sigma) for myelin, dehydrated, and coverslipped with DPX mounting medium (Fisher Scientific, Pittsburgh, PA).
To determine the amount of spared tissue at the lesion epicenter, the area of spared grey and white matter in the ipsilesional and contralesional cord and the lesion cavity were measured separately at lesion epicenter using the Cavalieri estimator method (Stereo Investigator, MicroBrightfield, Burlington, VT) by an evaluator blinded to experimental groups. The proportion of the spared tissue area on the ipsilesional side of the spinal cord to the tissue area on the contralesional (uninjured) spinal cord was determined (32).
Spinal Cord Immunohistochemistry
For all immunohistochemical staining, vendor information, catalog number and dilution for each antibody are provided in Table 3. In Experiment 1, spinal cord sections mounted on slides were double-labeled for calcitonin gene-regulated peptide (CGRP) and isolectin-B4 (IB-4) immunofluorescence to identify peptidergic and non-peptidergic nociceptive afferent distribution respectively. Slides were incubated in 10% normal goat serum (NGS) and 0.2% Triton-X100 in PBS for 1 h. The CGRP antibody and biotin-conjugated IB-4 were applied for 48 h at room temperature in a humid chamber and were followed by goat-anti-rabbit Alexa Fluor 488 and streptavidin conjugated Alexa Fluor 594, respectively, and coverslipped with FluorSave Reagent (Calbiochem, Bedford, MA).
Table 3.
Antibody Information
| Primary Antibodies | ||
|---|---|---|
| Name | Source | Dilution Information |
| Rabbit α CGRPα | Peninsula #T-4032 | Floating: 1:1000 Slides: 1:500 |
| Goat α Cholera toxin-β | List Biological Laboratories #703 | Floating: 1:2000 |
| Isolectin-B4 | Sigma #L2140 | Floating: 1:2000 Slides: 1:2000 |
| Secondary Antibodies | ||
| Name | Source | Dilution Information |
| Goat α rabbit IgG to Alexa Fluor 488 |
Life Technologies #A11008 | Floating: 1:400 |
| Donkey α goat, Rhodamine Red | Jackson ImmunoResearch Laboratories #705-295-003 |
Floating: 1:400 |
| Streptavidin-conjugated to Alexa Fluor 594 |
Jackson ImmunoResearch Laboratories #016-580-084 |
Floating: 1:400 |
| Dylight 405-conjugated Streptavidin |
Jackson ImmunoResearch Laboratories #016-470-084 |
Floating: 1:200 |
In Experiment 2, floating sections were blocked in 10% normal donkey serum, 0.2% Trition-X100 in 0.1M PBS for 1 hr, incubated overnight with goat-anti-CTB and then incubated in donkey-anti-goat secondary antibody for 2 h in the dark. Sections were rinsed in PBS, blocked in 10% NGS, 0.2% Trition-X100 in 0.1M PBS for 1 h, and incubated with rabbit-anti-CGRP and biotin-conjugated IB-4 for 48 h at room temperature and visualized by incubating with goat-anti-rabbit Alexa Fluor 488 and streptavidin conjugated Dylight 305 for 2 h. All procedures were conducted at room temperature on a rotary shaker. Sections were mounted on gelatin coated slides, dried overnight and coverslipped with Fluorsave Reagent.
Separate images of CGRP and IB4 labeling were taken of five ipsilesional and contralesional dorsal horn sections per rat (250 microns apart in Experiment 1 and 180 microns apart in Experiment 2) were taken using a Leica DM5500 B microscope. at the same magnification, lens aperture and exposure time. Images included the cap of the dorsal horn, excluded Lissauer’s tract and dorsal column white matter. Each image was then opened in ImageJ and a threshold was applied to include the positive labeling with in the dorsal horn. All images with similar staining were examined equally by applying a constant optical density threshold. Thresholded area was then quantified and is represented as a proportion to total area of the image. For each rat, the proportional area of positively labeled tissue was averaged, generating one value for CGRP and one value for IB4 for the ipsilesional dorsal horn and one value for CGRP and one for IB4 for the contralesional dorsal horn. These values were used to generate group means.
To quantify the proportional area of overlap, the area of CGRP+ and IB4+ tissue were outlined separately in the ipsilesional and contralesional dorsal horn using StereoInvestigator for 5 sections/rat. The area of overlap between CGRP and IB4 fibers was quantified and is represented as a proportion of the combined area of CGRP+ and IB4+ in the dorsal horn relative to the area of laminae I-III of the dorsal horn. For each rat, the proportional area of overlap was averaged, generating one value for the ipsilesional dorsal horn and one value for the contralesional dorsal horn. These values were used to generate group means.
Statistical Analysis
Two way repeated measure ANOVAs were performed for von Frey and incidence of supraspinal responses data (group x time) followed by Tukey’s post-hoc comparisons using the harmonic mean to correct for unequal group sizes. Epicenter sparing and proportional area measurements for IB-4, CGRP and CTB immunoreactivity were analyzed via one-way ANOVA with Bonferroni’s post-hoc analysis. Significance was determined if p<.05. Means and standard error of the mean (SEM) are reported throughout.
RESULTS
Exercise therapy does not ameliorate acute or established stages of tactile allodynia
At 14 dpi, tactile allodynia can be determined in all four paws of the rat when defined as a >50% reduction in the paw withdrawal threshold. The unilateral cervical SCI elicits two significantly different cohorts of SCI rats—those that demonstrate persistent tactile allodynia in all four paws (SCI Allodynia, ~40% of SCI rats) and those that maintain normal sensation (SCI No Allodynia, ~60% of SCI rats).
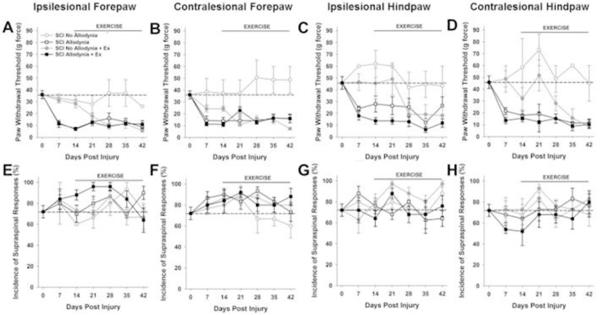
Prior to SCI the mean mechanical threshold to tactile stimuli was approximately 35 g for either forepaw (Figure 1A, B, Figure 2A, B) and approximately 45 g for either hindpaw (Figure 1C, D, Figure 2C, D). In the SCI Allodynia group, at 7-14 dpi there was a significant reduction in the paw withdrawal threshold of all four paws compared to naïve or SCI No Allodynia groups (#p<.05). Rats that began forced exercise closer to the onset of tactile allodynia (14dpi), mirrored the SCI Allodynia group as there was no change of tactile thresholds in any paw at any timepoint (Figure 1A-D). When forced exercise was initiated after allodynia was firmly established (28 dpi), paw withdrawal thresholds did not change and tactile allodynia persisted throughout the duration of the study (Figure 2A-D).
Figure 1. Exercise therapy initiated at 14 dpi is ineffective.
The paw withdrawal threshold for the ipsilesional (A) and contralesional (B) forepaw as well as ipsilesional (C) and contralesional (D) hindpaw was recorded prior to and after spinal cord injury over a period of 42 days. Two behavioral cohorts emerge after SCI, No Allodynia that maintain tactile thresholds at or near normal values (dashed line) and those that exhibit a significant reduction in paw withdrawal threshold (SCI Allodynia). These responses occurred by 14 dpi and persisted through the end of the study. SCI Allodynia rats that began exercising at 14 dpi did not show any attenuation of tactile hypersensitivity and maintained allodynic paw withdrawal thresholds (SCI Allodynia + Ex). SCI No Allodynia rats that began exercising at 14 dpi developed paw hypersensitivity by 28 dpi that persisted (SCI No Allodynia + Ex) All rats produced supraspinal behaviors in conjunction with a positive response to tactile stimulation of the forepaws (E, F) or hindpaws (G,H), regardless of the presence or absence of tactile allodynia. The normal incidence of supraspinal responses is denoted by the dashed line. Even after injury, the incidence of supraspinal responses was not significantly different than a normal rat (p>.05). (#p<.05 for SCI Allodynia, SCI Allodynia + Ex vs. Naïve, SCI No Allodynia, SCI No Allodynia + Ex; $p<.05 for SCI Allodynia, SCI Allodynia + Ex vs. Naïve, SCI No Allodynia; *p<.05 for SCI Allodynia, SCI Allodynia + Ex, SCI No Allodynia + Ex vs. Naïve, SCI No Allodynia)
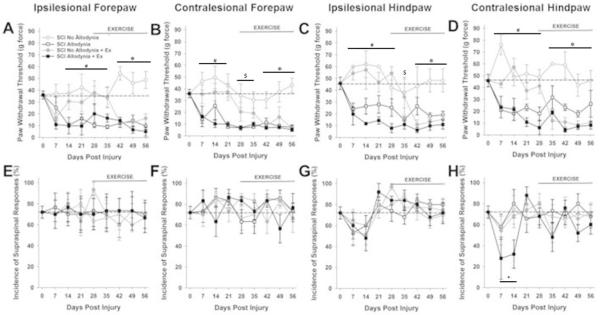
Figure 2. Exercise therapy initiated at 28 dpi is ineffective.
The paw withdrawal threshold for the ipsilesional (A) and contralesional (B) forepaw as well as ipsilesional (C) and contralesional (D) hindpaw was recorded prior to spinal cord injury over a period of 56 days. SCI Allodynia rats that began exercising at 28 dpi did not show any attenuation of tactile hypersensitivity and maintained allodynic paw withdrawal thresholds (SCI Allodynia + Ex). Interestingly, SCI No Allodynia rats receiving exercise maintained normal sensation for 1-2 weeks after exercise was initiated, but then exhibited a significant reduction in tactile paw withdrawal threshold (SCI No Allodynia + Ex). All rats produced supraspinal behaviors in conjunction with a positive response to tactile stimulation of the forepaws (E, F) or hindpaws (G,H), regardless of the presence or absence of tactile allodynia. The normal incidence of supraspinal responses is denoted by the dashed line. Even after injury, supraspinal responses occurred in all rats. At 7 and 14 dpi, the SCI Allodynia + Ex exhibited a significant decrease in the incidence of supraspinal responses (#p<.05 for SCI Allodynia, SCI Allodynia + Ex vs. Naïve, SCI No Allodynia, SCI No Allodynia + Ex; $p<.05 for SCI Allodynia, SCI Allodynia + Ex vs. Naïve, SCI No Allodynia; *p<.05 for SCI Allodynia, SCI Allodynia + Ex, SCI No Allodynia + Ex vs. Naïve, SCI No Allodynia;+p<.05 SCI Allodynia + Ex vs. Naïve).
In SCI No Allodynia rats that began exercise at 14 dpi paw withdrawal thresholds were significantly decreased in all four paws after two weeks of exercise therapy compared to Naïve and SCI No Allodynia groups (*p<.05). SCI No Allodynia + Ex rats exhibited similar paw withdrawal thresholds to SCI Allodynia and SCI Allodynia + Ex (Figure 1A-D).
SCI No Allodynia rats that began exercise at 28 dpi had significantly decreased paw withdrawal thresholds compared to Naïve and SCI No Allodynia groups (Figure 2A-D). Importantly, the SCI No Allodynia + Ex group exhibited paw withdrawal thresholds similar to SCI Allodynia and SCI Allodynia + Ex groups.
Positive responses to nocifensive tactile stimuli in all rats were accompanied by hypervigilant, supraspinally initiated behaviors that included licking the stimulated paw, turning and looking at the stimulated paw, and occasionally moving away from the stimulus. No rats vocalized in response to von Frey stimulus application. The incidence of supraspinal awareness to noxious tactile stimulation is plotted over time for all four paws (Figures 1E-H, 2E-H). Regardless of the presence or absence of tactile allodynia, all SCI rats exhibited aversive responses to the tactile stimuli. Exercise did not alter the incidence of supraspinal responses.
Importantly, von Frey testing requires both sensory and motor function and these functions are both affected by contusive SCI. Importantly, rarely saw evidence of spasticity when observing SCI rats throughout the course of this study in their home cages, in the open field, or during von Frey testing. During von Frey testing, we rarely observed flutter-like withdrawal responses to monofilament application. This type of spastic response occurred in fewer than one-quarter of their post SCI testing sessions of only 6 out of 152 SCI rats (1 rat in the SCI Allodynia, 2 rats in SCI No Allodynia, 1 rat in the SCI Allodynia + Ex, and 2 rats in the SCI No Allodynia + Ex), suggesting that the increased responsiveness to tactile stimuli is likely due to sensitization of the sensory systems as opposed to a change in spasticity.
Delayed exercise therapy does not affect tissue sparing
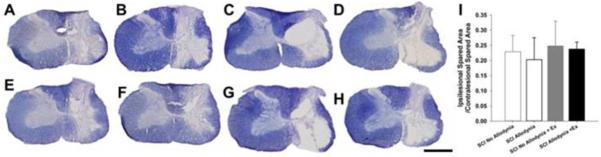
Lateralized, contusive SCI produced a core lesion consisting of a cystic cavity surrounded by a rim of white matter that was confined to the injured side of the spinal cord (Figure 3). For all groups, nearly all grey matter was eliminated and only a small outer rim of densely stained white matter remained, in the lateral and ventral funiculi. There was no evidence of tissue damage in the contralesional spinal cord for any group (Figure 3A-H). Quantitatively, the proportion of spared tissue of the ipsilesional versus contralesional side of the spinal cord had no relationship to the presence of allodynic behavior and exercise had no effect on tissue sparing (Figure 3I).
Figure 3. Anatomical assessments in SCI rats with and without tactile allodynia and exercise.
Representative transverse sections of lesion epicenter sacrificed at 42 dpi stained with euriochrome cyanine for myelin SCI No Allodynia (A), SCI Allodynia (B), SCI No Allodynia +Delayed Ex (E), SCI Allodynia + Delayed Ex (F). Sections from SCI rats sacrificed at 56 dpi in groups SCI No Allodynia (C), SCI Allodynia (D), SCI No Allodynia +Delayed Ex (G), SCI Allodynia + Delayed Ex (H) are also presented. The 200 kdyn impact produced a moderate, lateralized lesion with near complete degeneration of grey matter and a spared rim of white matter on the right side of the spinal cord. Importantly, the grey and white matter of the contralesional spinal cord does not exhibit any overt loss of grey or white matter. (Scale bar = 1.0 mm). There were no significant differences in tissue sparing at the lesion epicenter between any groups (I).
Effects of delayed exercise on nociceptive afferent plasticity
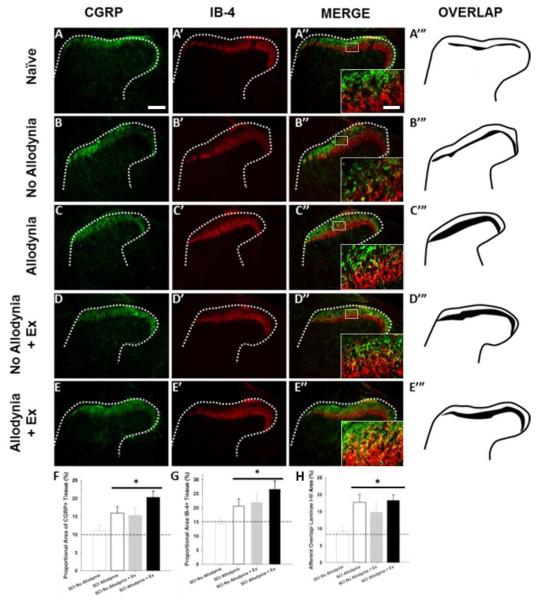
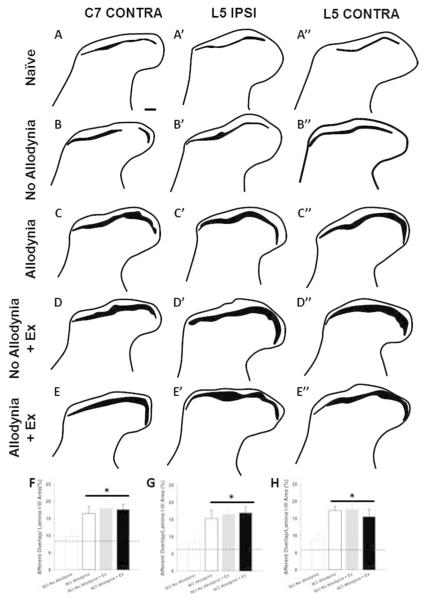
The C7-C8 and L3-5 dorsal horn corresponds to the dermatomes of the fore- and hindpaw, respectively. In the naïve dorsal horn, peptidergic, CGRP+ nociceptive afferents terminate in lamina I and the outer layer of lamina II, while non-peptidergic, IB-4+ nociceptive afferents terminate in the inner layer of lamina II (Figure 4A-A’’). The laminar distributions of these two fiber types are anatomically distinct, with little overlap in the uninjured dorsal horn for both ipsilateral and contralateral C7 and L5 spinal cord (Figure 4A-A’’’, 5A-A’’). At 42 dpi, the SCI Allodynia group showed a marked increase in the dorsal horn distribution of both c fiber types as the bands of CGRP+ and IB-4+ nociceptive afferents widened, increasing the area of overlap (Figure 4C-C’’’, 5C-C’’), in contrast to only modest overlap in the SCI No Allodynia group (Figure 4B-B’’’, 5B-B’’). Significant overlap of nociceptive afferents occurred in SCI No Allodynia rats that were exercised compared to their unexercised counterparts (Figure 4D-D’’’, 5D-D’’). The degree of overlap was similar between SCI Allodynia and SCI Allodynia+Exercise (Figure 4E-E’’’, 5E-E’’). Delayed Exercise beginning at 14 or 28 dpi leads to c-fiber overlap in SCI No Allodynia group and no change in the SCI Allodynia group. The proportional area of the ipsilesional C7 dorsal horn that was positively labeled for CGRP+ or IB-4+ c fibers was significantly increased in the SCI Allodynia, SCI No Allodynia + Ex, and SCI Allodynia + Ex groups compared with Naïve and SCI No Allodynia groups (Figure 4F-G). The area of overlap of nociceptive afferents in the contralesional C7 and ipsi- and contralesional L5 dorsal horn at 42 dpi are depicted in Figure 5. Visually, a larger overlapping area of nociceptive afferents in the cervical or lumbar dorsal horn correlated with the presence of tactile allodynia in the corresponding paw at the end of the experiment. The area of nociceptive afferent overlap was significantly greater in SCI Allodynia, SCI No Allodynia + Ex and SCI Allodynia + Ex groups compared to naïve and SCI No Allodynia groups (Figure 4H, Figure 5F-H).
Figure 4. Immunolabeling and quantification of peptidergic and non-peptidergic primary afferent fibers in the C7-8 dorsal horn.
Representative sections of the ipsilesional C7 dorsal horn of unexercised naïve (A), SCI No Allodynia (B), SCI Allodynia (C), and groups that received 5 weeks of exercise beginning at 14 dpi: SCI No Allodynia + Ex (D) and SCI Allodynia + Ex (E) reacted for calcitonin gene-regulated peptide (CGRP) and Isolectin-B4 (IB-4) at 42 dpi. In normal rats, these two types of nociceptive primary afferent fibers show very little overlap. In SCI rats that develop allodynia, there is marked overlap between these two pain fiber types (C’’’, D’’’, E’’’) compared to those that do not develop pain after SCI (B’’’). Proportional area of positively labeled tissue within the cap of the dorsal horn was determined across the C7 and C8 spinal cord. Proportional area within the superficial dorsal horn showed that SCI Allodyina, SCI No Allodynia + Ex, and SCI Allodynia +Ex groups had greater area fraction of both CGRP (F) and IB-4 (G) compared to Naïve and SCI No Allodynia (*p<.05, dashed line indicates naïve levels). The proportional area of nociceptive afferent overlap in laminae I-III of the dorsal horn was significantly greater in SCI Allodynia, SCI No Allodynia + Ex, and SCI Allodynia + Ex groups compared to Naïve, and SCI No Allodynia groups (H; *p<.05). Scale bar = 200 microns, inset scale bar = 50 microns.
Figure 5. Overlap of nociceptive afferents in the cervical and lumbar dorsal horn.
Representative drawings of the contralesional C7 and ipsi- and contralesional L5 dorsal horn of unexercised naïve (A), SCI No Allodynia (B), SCI Allodynia (C), and groups that received 5 weeks of exercise beginning at 14 dpi: SCI No Allodynia + Ex (D) and SCI Allodynia + Ex (E) reacted for calcitonin gene-regulated peptide (CGRP) and Isolectin-B4 (IB-4) at 42 dpi. In normal rats, these two types of nociceptive primary afferent fibers show very little overlap. In SCI rats that develop allodynia, there is marked overlap between these two types of nociceptive afferents in the cervical and lumbar dorsal horn (C, D, E) compared to those that do not develop pain after SCI (B). Scale bar = 100 microns.
Temporal relationship between afferent plasticity and the development of allodynia
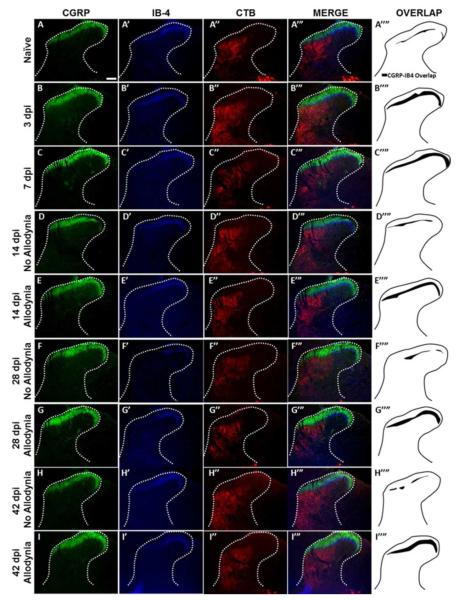
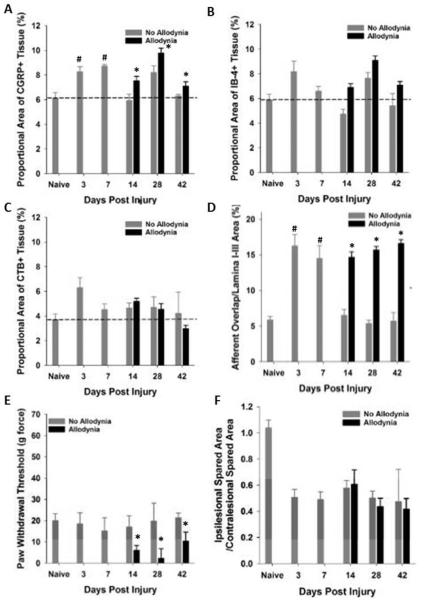
We evaluated the distribution of primary afferent fibers in the dorsal horn at time points prior to allodynic development (3 and 7 dpi), at its earliest detection (14 dpi) and after it is established (28 and 42 dpi) (Figure 6). As in Experiment 1, paw withdrawal thresholds to tactile stimuli were recorded for the ipsilesional forepaw over time, and two groups emerged: SCI No Allodynia and SCI Allodynia (Figure 7D). When we examined the topographic distribution of nociceptive and non-nociceptive afferents in the dorsal horn, we found that both peptidergic and non-peptidergic nociceptive afferents (CGRP+ and IB-4+) but not non-nociceptive CTB-traced afferents exhibited an increase in the distribution in the dorsal horn at 3 and 7 dpi (compared to the naïve condition), time points which are prior to the detection of SCI-induced tactile allodynia (Figure 6A-A’’’’, B-B’’’’, C-C’’’’). At 14 and 28 dpi, the two timepoints when forced exercise was initiated, SCI Allodynia group had a larger band of overlap in the superficial dorsal horn compared to the SCI No Allodynia group (Figure 6D’’’’-G’’’’). The SCI Allodynia groups exhibit a greater overlap of nociceptive afferents compared to the SCI No Allodynia groups (Figure 6F-I). Importantly, at no timepoint did the distribution of non-nociceptive CTB-traced fibers significantly overlap with either peptidergic or non-peptidergic afferents in the dorsal horn (Figure 6 A’’’-I’’’). The increased distribution of nociceptive afferent fibers in the dorsal horn coincides with a significant increase in the area fraction as early as 3 dpi that is maintained for at least 42 dpi in SCI Allodynia rats (Figure 7A-B). The SCI No Allodynia group exhibited near normal area fraction measurements of CGRP+ and IB-4+ afferents in the dorsal horn at 14-42 dpi (Figure 7A-B). The area fraction of CTB+ afferents in the dorsal horn was unchanged at any timepoint after injury for SCI No Allodynia and SCI Allodynia groups (Figure 7C). The area of nociceptive afferent overlap increased significantly at 3 and 7 dpi and in SCI animals with allodynia at 14, 28, and 42 dpi (Figure 7D). The increased immunoreactivity of nociceptive afferents was not associated with overt differences in the amount of tissue sparing at the lesion epicenter (Figure 7F).
Figure 6. Immunolabeling and topographic distribution of primary afferent fibers in the C7 dorsal horn over time after SCI.
Representative section of the ipsilesional C7 dorsal horn of naïve rats (A), SCI sacrificed before the onset of tactile allodynia (3 and 7 dpi; B, C), SCI rats that do not develop tactile allodynia (14, 28 and 42 dpi; D, F, H) and SCI rats that develop tactile allodynia (14, 28, and 42 dpi; E, G, I). labeled with antibodies against calcitonin gene-regulated peptide (CGRP), isolectin-B4 (IB-4), and the tract tracer cholera toxin subunit b (CTB). In normal rats, the nociceptive (CGRP, IB-4) and non-nociceptive (CTB) primary afferent fibers show little overlap. As early as 3 days after SCI, nociceptive afferents the area of overlap increased (B’’’’). This increased overlap is maintained at 7 dpi (C’’’’). Importantly, at later timepoints when tactile allodynia can be reliably assessed, the two behavioral cohorts (No Allodynia and Allodynia) exhibit different degree of overlap. Rats with no allodynia at 14, 28 or 42 dpi exhibit only modest overlap of their nociceptive primary afferent fibers (D’’’’, F’’’’, H’’’’). However, rats that develop tactile allodynia exhibit a greater area of overlap that persists over time (E’’’’, G’’’’, I’’’’). There was almost no overlap between either nociceptive afferent fiber type and CTB traced non-nociceptive afferent fibers. Scale bar = 200 microns.
Figure 7. Quantitative analysis of primary afferents in the C7-8 dorsal horn.
Proportional area within the ipsilesional C7-8 dorsal horn was determined. Proportional area of positively labeled afferents within the dorsal horn revealed significant increases in CGRP (A) and IB-4 (B) but not CTB (C) immunoreactivity within the dorsal horn at 3 and 7 days post SCI (#p<.05 vs. naïve), as well as in the cohort of SCI rats that exhibit allodynia at 14, 28 and 42 dpi (*p<.05 vs naïve, SCI No allodynia at 14 dpi, dashed line indicates naïve levels). The area of nociceptive afferent overlap was significantly greater at 3 and 7dpi, and in SCI rats that have SCI-induced allodynia at 14, 28 and 42 dpi. A subset of SCI rats exhibited tactile allodynia at 14, 28 and 42 dpi (E, black bars, p<.05 vs naïve, 3 and 7 dpi, and No Allodynia 14, 28 and 42 dpi) that correlated with the increase in proportional area of CGRP+ and IB-4+ nociceptive afferent fibers in the dorsal horn. The increased immunoreactivity of nociceptive primary afferents was not associated with changes in the amount of tissue sparing at the lesion epicenter (F).
DISCUSSION
The current work demonstrates that initiating exercise when allodynia is first detectable or after it is fully established does not ameliorate, reverse or even reduce the degree of paw hypersensitivity after SCI. SCI-induced allodynia corresponds with an increase in the topographic distribution of nociceptive afferent fibers in the dorsal horn of the spinal cord that is present as early as 3 dpi and persists chronically. The delayed exercise paradigm was unable to modify this aberrant plasticity in rats with SCI-induced allodynia. Alarmingly, we found that executing these same exercise paradigms in SCI rats that exhibit normal tactile sensation led to the development of allodynia within 2 weeks of the start of therapy. In the case of SCI No Allodynia animals, exercise appears to have contributed to the alterations seen in the density and distribution of nociceptive afferents in the cervical and lumbar spinal cord, although we have no direct evidence that this increase in afferent distribution is the root of observed allodynia after SCI. Taken together, these data suggest that exercise dependent-plasticity is not always beneficial.
Exercise is a non-invasive rehabilitative therapy that has profound potential to improve the functional recovery of people who have sustained a SCI. Several laboratories have shown that exercise is effective at attenuating tactile hypersensitivity in several models of peripheral nerve injuries (nerve ligations, chronic constriction and crush) as well as in models of diabetes (19-23, 33-36). Recently, clinical and experimental studies indicated that exercise may be an effective therapy for the prevention or reduction of SCI-induced chronic pain (11, 17, 18, 37). Previous findings from our laboratory showed that the same exercise paradigm implemented within the first week of SCI prevented allodynia from developing (11) and reversed injury induced overlap in nociceptive afferent distribution in the dorsal horn. That a 9 day difference (day 5 post injury vs. day 14 post injury) in the initiation of a rehabilitative therapy can have such profound effects on sensory function and afferent plasticity indicates a limited window for effective exercise therapy though administration too soon after SCI also can be detrimental to recovery (38-40). Changing variables of exercise such as the duration, intensity or frequency may extend the period efficacy for reducing or preventing neuropathic pain.
An additional contributor to the effectiveness of a rehabilitative therapy on sensory recovery is the state of the local environment within the spinal cord and DRG. Physical injury to the spinal cord damages grey matter and disrupts ascending and descending white matter tracts at the injury epicenter, but SCI is an evolving condition where the environment of the injury site and other remote regions of the spinal cord are modulated by a persisting secondary injury cascade that involves apoptosis, blood spinal cord barrier permeability and a potent innate and adaptive immune response that changes over time (41-3). It is likely that the relative balance of each of these processes contributes to the concurrent sprouting of nociceptive primary afferent fibers thereby changing their terminal distribution within the dorsal horn along the neuroaxis (10, 11, 44). Exercise (cycling, treadmill training or automated wheel running) is a potent modulator of many processes that occur secondary to the primary injury including apoptosis (45), blood spinal cord barrier function (40), inflammatory response and extravasation of immune cells into the cord (40), increased levels of neuroplasticity-associated proteins like neurotrophic factors and their receptors (11, 46-48) as well as afferent plasticity (11, 49). While exercise has many beneficial effects after SCI, in the current experiment it did not modulate aberrant afferent sprouting in SCI rats with allodynia.
Perhaps more intriguing is the observation that exercise triggered robust alteration in the topographic distribution of nociceptive afferents in SCI rats that did not exhibit allodynia prior to the initiation of exercise. Early after SCI (3d and 7d), prior to our ability to reliably assess changes in tactile sensation in animals, nociceptive afferents had an increased presence in the superficial dorsal horn. By 14 dpi this distribution of nociceptive afferents in the C7 dorsal horn in SCI Allodynia and SCI No Allodynia groups was different. SCI Allodynia rats maintained the increased afferent distribution in the dorsal horn as 3 and 7 day time points, while the SCI No Allodynia group had a more normal distribution of nociceptive afferents. What is responsible for the persistence of this overlap in some animals but not in others is unknown. After exercise, the distribution of nociceptive afferents in the 14d or 28d SCI No Allodynia groups exhibited a similar degree of overlap in the superficial dorsal horn as the SCI Allodynia group and animals were allodynic. Why exercise failed to modulate nociceptive afferents in the case of SCI Allodynia or elicited nociceptive afferent sprouting in the case of SCI No Allodynia is unclear. We postulate that the mechanisms underlying this exercise-induced aberrant afferent plasticity may involve changes in neurotrophic factor expression and/or the heightened inflammatory response in the injured cord and DRG, which could signal changes in nociceptive DRG neurons that affect excitability and possible amplification of nociceptive signaling.
Many studies have examined sprouting or regeneration of sensory afferents after peripheral nerve and spinal cord injury (9, 50-61) however, the findings have ignited some controversy regarding the interpretation of the data (62). The data presented in these previous studies may represent true sprouting and change in the terminal arbors of nociceptive afferents, or it may represent alterations in the phenotypic composition of normally cutaneous primary sensory neurons to a nociceptive phenotype. Indeed Aβ nociceptors exist, and neuropathic pain has been linked with Aβ activation (63, 64). We do not believe this to be the case here as there was no expansion of myelinated afferents in the dorsal horn of allodynic animals. Additionally, we counted the number of nociceptive neurons in normal, SCI animals with and without allodynia as well as SCI animals that received exercise. We did not observe a change in the number of peptidergic or non-peptidergic neurons within the DRG suggesting that the redistribution of nociceptive afferents within the dorsal horn is indeed sprouting, and not a consequence of gene modification in non-nociceptive DRG neurons. The nociceptive neurons of the DRG must not be discounted in their ability to generate aberrant sensory behavior, as these nociceptors exhibit increased spontaneous activity, likely amplifying neuropathic signaling(5). Nociceptor spontaneous activity may be amplified is through an increased macrophage presence and elevated proinflammatory cytokine levels in the DRG after injury (65-69). After SCI macrophages recruited to DRGs within a week of injury persist chronically (70). DRG neurons cultured with conditioned medium from activated bone-marrow derived macrophages exhibited increased axonal growth (71). Moreover, in vivo, activation of macrophages and microglia near the dorsal root entry zone increased axonal growth of rhizotomized nociceptive afferents (72). Thus, macrophage infiltration into the DRG and dorsal horn after injury could sensitize nociceptive neurons, initiate sprouting, and ultimately result in topographic changes in the distribution of nociceptive primary afferents in the dorsal horn after SCI without actually triggering a change in the functional phenotype of primary sensory neurons in the DRG.
Detailed and extensive characterization of the lesion site as well as the cervical and lumbar spinal cords associated with paw dermatomes is necessary to better understand the mechanism by which aberrant nociceptive afferent plasticity is occurring. Ongoing and future experiments are specifically focusing on the effect of inflammation and gliosis on nociceptor plasticity and allodynic behavior.
In summary, our findings provide strong evidence that exercise therapy initiated at early or later stages of allodynia is ineffective at attenuating neuropathic pain. More disconcerting is the observation that exercise can induce maladaptive changes in the distribution of nociceptive afferents in the dorsal horn. These results, combined with the previous results from our laboratory, highlight the likelihood of a “therapeutic window” during which exercise may be an effective treatment before the onset of SCI-induced allodynia. Here exercise therapy must be initiated at some time earlier than 14dpi in order to be a successful therapy for allodynia. A challenge for future research and for clinical translation is the need to balance the effects of exercise-induced plasticity by developing an exercise paradigm that maximizes afferent plasticity associated with improvements in locomotor recovery while minimizing the maladaptive nociceptive afferent plasticity associated with the development of chronic neuropathic pain. As we move toward personalized medicine in clinical SCI, it may be that individual injury situations will require a unique combination of intensity or duration of exercise in order to see beneficial anti-allodynic effects.
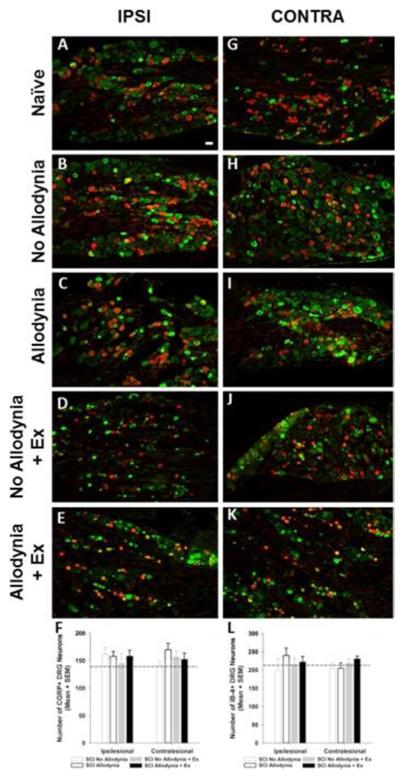
Figure 8. Immunolabeling and quantification of peptidergic and non-peptidergic primary sensory neurons in the C7 dorsal root ganglia.
Representative section of the ipsilesional and contralesional C7 DRGs of naïve rats (A, G), SCI No Allodynia (B, H), SCI Allodynia (C, I), SCI No Allodynia + Ex (D, J), and SCI Allodynia + Ex (E, K) reacted for calcitonin gene-regulated peptide (CGRP) and Isolectin-B4 (IB-4) at 56 dpi. All groups exhibit similar distribution of CGRP+ and IB-4+ DRG neurons in the ipsilesional and contralesional C7 DRG (F, L; p>.05, dashed line indicates naïve levels). Scale bar = 30 microns.
Acknowledgements
This study was supported by Paralyzed Veterans of America Fritz Krauth Memorial Fellowship #2707 (MRD), Craig H. Neilsen Foundation Fellowship #220798 (MRD), NIH Grant NS 55976 (JDH) and Drexel University College of Medicine Summer Medical Student Research Fellowships (ASJ, LD, ADN, AN, VN).
References
- 1.Christensen MD, Everhart AW, Pickelman JT, Hulsebosch CE. Mechanical and thermal allodynia in chronic central pain following spinal cord injury. Pain. 1996;68(1):97–107. doi: 10.1016/S0304-3959(96)03224-1. [DOI] [PubMed] [Google Scholar]
- 2.Ramer LM, van Stolk AP, Inskip JA, Ramer MS, Krassioukov AV. Plasticity of TRPV1-Expressing Sensory Neurons Mediating Autonomic Dysreflexia Following Spinal Cord Injury. Front Physiol. 2012;3:257. doi: 10.3389/fphys.2012.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walters ET. Nociceptors as chronic drivers of pain and hyperreflexia after spinal cord injury: an adaptive-maladaptive hyperfunctional state hypothesis. Front Physiol. 2012;3:309. doi: 10.3389/fphys.2012.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang WL, Robson D, Liu MC, King VR, Averill S, Shortland PJ, et al. Spinal cord compression and dorsal root injury cause up-regulation of activating transcription factor-3 in large-diameter dorsal root ganglion neurons. Eur J Neurosci. 2006;23(1):273–8. doi: 10.1111/j.1460-9568.2005.04530.x. [DOI] [PubMed] [Google Scholar]
- 5.Bedi SS, Yang Q, Crook RJ, Du J, Wu Z, Fishman HM, et al. Chronic spontaneous activity generated in the somata of primary nociceptors is associated with pain-related behavior after spinal cord injury. J Neurosci. 2010;30(44):14870–82. doi: 10.1523/JNEUROSCI.2428-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calancie B, Molano MR, Broton JG. Epidemiology and demography of acute spinal cord injury in a large urban setting. J Spinal Cord Med. 2005;28(2):92–6. doi: 10.1080/10790268.2005.11753804. [DOI] [PubMed] [Google Scholar]
- 7.Kakulas BA. Neuropathology: the foundation for new treatments in spinal cord injury. Spinal Cord. 2004;42(10):549–63. doi: 10.1038/sj.sc.3101670. [DOI] [PubMed] [Google Scholar]
- 8.Krenz NR, Weaver LC. Changes in the morphology of sympathetic preganglionic neurons parallel the development of autonomic dysreflexia after spinal cord injury in rats. Neurosci Lett. 1998;243(1-3):61–4. doi: 10.1016/s0304-3940(98)00101-3. [DOI] [PubMed] [Google Scholar]
- 9.Weaver LC, Verghese P, Bruce JC, Fehlings MG, Krenz NR, Marsh DR. Autonomic dysreflexia and primary afferent sprouting after clip-compression injury of the rat spinal cord. J Neurotrauma. 2001;18(10):1107–19. doi: 10.1089/08977150152693782. [DOI] [PubMed] [Google Scholar]
- 10.Ondarza AB, Ye Z, Hulsebosch CE. Direct evidence of primary afferent sprouting in distant segments following spinal cord injury in the rat: colocalization of GAP-43 and CGRP. Exp Neurol. 2003;184(1):373–80. doi: 10.1016/j.expneurol.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Detloff MR, Smith EJ, Quiros Molina D, Ganzer PD, Houlé JD. Acute exercise prevents the development of neuropathic pain and the sprouting of non-peptidergic (GDNF- and artemin-responsive) c-fibers after spinal cord injury. Exp Neurol. 2014;255:38–48. doi: 10.1016/j.expneurol.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petruska JC, Ichiyama RM, Jindrich DL, Crown ED, Tansey KE, Roy RR, et al. Changes in motoneuron properties and synaptic inputs related to step training after spinal cord transection in rats. J Neurosci. 2007;27(16):4460–71. doi: 10.1523/JNEUROSCI.2302-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cote MP, Gossard JP. Step training-dependent plasticity in spinal cutaneous pathways. J Neurosci. 2004;24(50):11317–27. doi: 10.1523/JNEUROSCI.1486-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson AR, Huie JR, Crown ED, Baumbauer KM, Hook MA, Garraway SM, et al. Maladaptive spinal plasticity opposes spinal learning and recovery in spinal cord injury. Front Physiol. 2012;3:399. doi: 10.3389/fphys.2012.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grau JW, Huie JR, Garraway SM, Hook MA, Crown ED, Baumbauer KM, et al. Impact of behavioral control on the processing of nociceptive stimulation. Front Physiol. 2012;3:262. doi: 10.3389/fphys.2012.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Leon RD, Roy RR, Edgerton VR. Is the recovery of stepping following spinal cord injury mediated by modifying existing neural pathways or by generating new pathways? A perspective. Phys Ther. 2001;81(12):1904–11. [PubMed] [Google Scholar]
- 17.Hutchinson KJ, Gomez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–14. doi: 10.1093/brain/awh160. Pt 6. [DOI] [PubMed] [Google Scholar]
- 18.Dugan E, Sagen J. An Intensive Locomotor Training Paradigm Improves Neuropathic Pain following Spinal Cord Compression Injury in Rats. J Neurotrauma. 2014 doi: 10.1089/neu.2014.3692. [DOI] [PubMed] [Google Scholar]
- 19.Cobianchi S, Marinelli S, Florenzano F, Pavone F, Luvisetto S. Short- but not long-lasting treadmill running reduces allodynia and improves functional recovery after peripheral nerve injury. Neuroscience. 2010;168(1):273–87. doi: 10.1016/j.neuroscience.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 20.Udina E, Cobianchi S, Allodi I, Navarro X. Effects of activity-dependent strategies on regeneration and plasticity after peripheral nerve injuries. Annals of anatomy = Anatomischer Anzeiger : official organ of the Anatomische Gesellschaft. 2011;193(4):347–53. doi: 10.1016/j.aanat.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Shankarappa SA, Piedras-Renteria ES, Stubbs EB., Jr Forced-exercise delays neuropathic pain in experimental diabetes: effects on voltage-activated calcium channels. J Neurochem. 2011;118(2):224–36. doi: 10.1111/j.1471-4159.2011.07302.x. [DOI] [PubMed] [Google Scholar]
- 22.Shen J, Fox LE, Cheng J. Swim therapy reduces mechanical allodynia and thermal hyperalgesia induced by chronic constriction nerve injury in rats. Pain Med. 2013;14(4):516–25. doi: 10.1111/pme.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groover AL, Ryals JM, Guilford BL, Wilson NM, Christianson JA, Wright DE. Exercise-mediated improvements in painful neuropathy associated with prediabetes in mice. Pain. 2013;154(12):2658–67. doi: 10.1016/j.pain.2013.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Detloff MR, Wade RE, Jr., Houle JD. Chronic at- and below-level pain after moderate unilateral cervical spinal cord contusion in rats. J Neurotrauma. 2013;30(10):884–90. doi: 10.1089/neu.2012.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE., Jr Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003;20(2):179–93. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- 26.Papalia I, Tos P, Scevola A, Raimondo S, Geuna S. The ulnar test: a method for the quantitative functional assessment of posttraumatic ulnar nerve recovery in the rat. J Neurosci Methods. 2006;154(1-2):198–203. doi: 10.1016/j.jneumeth.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 28.Detloff MR, Clark LM, Hutchinson KJ, Kloos AD, Fisher LC, Basso DM. Validity of acute and chronic tactile sensory testing after spinal cord injury in rats. Exp Neurol. 2010;225(2):366–76. doi: 10.1016/j.expneurol.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Detloff MR, Fisher LC, Deibert RJ, Basso DM. Acute and chronic tactile sensory testing after spinal cord injury in rats. J Vis Exp. 2012;62:e3247. doi: 10.3791/3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh A, Krisa L, Frederick KL, Sandrow-Feinberg H, Balasubramanian S, Stackhouse SK, et al. Forelimb locomotor rating scale for behavioral assessment of recovery after unilateral cervical spinal cord injury in rats. J Neurosci Methods. 2014;226:124–31. doi: 10.1016/j.jneumeth.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandrow HR, Shumsky JS, Amin A, Houle JD. Aspiration of a cervical spinal contusion injury in preparation for delayed peripheral nerve grafting does not impair forelimb behavior or axon regeneration. Exp Neurol. 2008;210(2):489–500. doi: 10.1016/j.expneurol.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cote MP, Detloff MR, Wade RE, Jr., Lemay MA, Houle JD. Plasticity in ascending long propriospinal and descending supraspinal pathways in chronic cervical spinal cord injured rats. Front Physiol. 2012;3:330. doi: 10.3389/fphys.2012.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuphal KE, Fibuch EE, Taylor BK. Extended swimming exercise reduces inflammatory and peripheral neuropathic pain in rodents. J Pain. 2007;8(12):989–97. doi: 10.1016/j.jpain.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, et al. Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology. 2011;114(4):940–8. doi: 10.1097/ALN.0b013e318210f880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Meeteren NL, Brakkee JH, Helders PJ, Gispen WH. The effect of exercise training on functional recovery after sciatic nerve crush in the rat. J Peripher Nerv Syst. 1998;3(4):277–82. [PubMed] [Google Scholar]
- 36.Dobson JL, McMillan J, Li L. Benefits of exercise intervention in reducing neuropathic pain. Frontiers in cellular neuroscience. 2014;8:102. doi: 10.3389/fncel.2014.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heutink M, Post MW, Wollaars MM, van Asbeck FW. Chronic spinal cord injury pain: pharmacological and non-pharmacological treatments and treatment effectiveness. Disability and rehabilitation. 2011;33(5):433–40. doi: 10.3109/09638288.2010.498557. [DOI] [PubMed] [Google Scholar]
- 38.Griesbach GS, Gomez-Pinilla F, Hovda DA. Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. J Neurotrauma. 2007;24(7):1161–71. doi: 10.1089/neu.2006.0255. [DOI] [PubMed] [Google Scholar]
- 39.Maldonado MA, Allred RP, Felthauser EL, Jones TA. Motor skill training, but not voluntary exercise, improves skilled reaching after unilateral ischemic lesions of the sensorimotor cortex in rats. Neurorehabil Neural Repair. 2008;22(3):250–61. doi: 10.1177/1545968307308551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen CN, Fisher LC, Deibert RJ, Jakeman LB, Zhang H, Noble-Haeusslein L, et al. Elevated MMP-9 in the lumbar cord early after thoracic spinal cord injury impedes motor relearning in mice. J Neurosci. 2013;33(32):13101–11. doi: 10.1523/JNEUROSCI.1576-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houle JD, Tessler A. Repair of chronic spinal cord injury. Exp Neurol. 2003;182(2):247–60. doi: 10.1016/s0014-4886(03)00029-3. [DOI] [PubMed] [Google Scholar]
- 42.Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209(2):378–88. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beattie MS. Inflammation and apoptosis: linked therapeutic targets in spinal cord injury. Trends in molecular medicine. 2004;10(12):580–3. doi: 10.1016/j.molmed.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Christensen MD, Hulsebosch CE. Chronic central pain after spinal cord injury. J Neurotrauma. 1997;14(8):517–37. doi: 10.1089/neu.1997.14.517. [DOI] [PubMed] [Google Scholar]
- 45.Liu G, Keeler BE, Zhukareva V, Houle JD. Cycling exercise affects the expression of apoptosis-associated microRNAs after spinal cord injury in rats. Exp Neurol. 2010 doi: 10.1016/j.expneurol.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cote MP, Azzam GA, Lemay MA, Zhukareva V, Houle JD. Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J Neurotrauma. 2011;28(2):299–309. doi: 10.1089/neu.2010.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keeler BE, Liu G, Siegfried RN, Zhukareva V, Murray M, Houle JD. Acute and prolonged hindlimb exercise elicits different gene expression in motoneurons than sensory neurons after spinal cord injury. Brain Res. 2012;1438:8–21. doi: 10.1016/j.brainres.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu G, Detloff MR, Miller KN, Santi L, Houle JD. Exercise modulates microRNAs that affect the PTEN/mTOR pathway in rats after spinal cord injury. Exp Neurol. 2012;233(1):447–56. doi: 10.1016/j.expneurol.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petruska JC, Hubscher CH, Rabchevsky AG. Challenges and opportunities of sensory plasticity after SCI. Front Physiol. 2013;4:231. doi: 10.3389/fphys.2013.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;355(6355):75–8. doi: 10.1038/355075a0. [DOI] [PubMed] [Google Scholar]
- 51.Woolf CJ. Generation of acute pain: central mechanisms. Br Med Bull. 1991;47(3):523–33. doi: 10.1093/oxfordjournals.bmb.a072490. [DOI] [PubMed] [Google Scholar]
- 52.Shortland P, Woolf CJ. Chronic peripheral nerve section results in a rearrangement of the central axonal arborizations of axotomized A beta primary afferent neurons in the rat spinal cord. J Comp Neurol. 1993;330(1):65–82. doi: 10.1002/cne.903300106. [DOI] [PubMed] [Google Scholar]
- 53.Koerber HR, Mirnics K, Brown PB, Mendell LM. Central sprouting and functional plasticity of regenerated primary afferents. J Neurosci. 1994;14(6):3655–71. doi: 10.1523/JNEUROSCI.14-06-03655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nacimiento W, Mautes A, Topper R, Oestreicher AB, Gispen WH, Nacimiento AC, et al. B-50 (GAP-43) in the spinal cord caudal to hemisection: indication for lack of intraspinal sprouting in dorsal root axons. J Neurosci Res. 1993;35(6):603–17. doi: 10.1002/jnr.490350604. [DOI] [PubMed] [Google Scholar]
- 55.Murray M, Goldberger ME. Replacement of synaptic terminals in lamina II and Clarke's nucleus after unilateral lumbosacral dorsal rhizotomy in adult cats. J Neurosci. 1986;6(11):3205–17. doi: 10.1523/JNEUROSCI.06-11-03205.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodin BE, Sampogna SL, Kruger L. An examination of intraspinal sprouting in dorsal root axons with the tracer horseradish peroxidase. J Comp Neurol. 1983;215(2):187–98. doi: 10.1002/cne.902150206. [DOI] [PubMed] [Google Scholar]
- 57.Hulsebosch CE, Coggeshall RE. Sprouting of dorsal root axons. Brain Res. 1981;224(1):170–4. doi: 10.1016/0006-8993(81)91128-8. [DOI] [PubMed] [Google Scholar]
- 58.Hulsebosch CE, Coggeshall RE. Quantitation of sprouting of dorsal root axons. Science. 1981;213(4511):1020–1. doi: 10.1126/science.7268404. [DOI] [PubMed] [Google Scholar]
- 59.Tessler A, Himes BT, Soper K, Murray M, Goldberger ME, Reichlin S. Recovery of substance P but not somatostatin in the cat spinal cord after unilateral lumbosacral dorsal rhizotomy: a quantitative study. Brain Res. 1984;305(1):95–102. doi: 10.1016/0006-8993(84)91123-5. [DOI] [PubMed] [Google Scholar]
- 60.Tessler A, Himes BT, Krieger NR, Murray M, Goldberger ME. Sciatic nerve transection produces death of dorsal root ganglion cells and reversible loss of substance P in spinal cord. Brain Res. 1985;332(2):209–18. doi: 10.1016/0006-8993(85)90590-6. [DOI] [PubMed] [Google Scholar]
- 61.Polistina DC, Murray M, Goldberger ME. Plasticity of dorsal root and descending serotoninergic projections after partial deafferentation of the adult rat spinal cord. J Comp Neurol. 1990;299(3):349–63. doi: 10.1002/cne.902990307. [DOI] [PubMed] [Google Scholar]
- 62.Hughes DI, Scott DT, Todd AJ, Riddell JS. Lack of evidence for sprouting of Abeta afferents into the superficial laminas of the spinal cord dorsal horn after nerve section. J Neurosci. 2003;23(29):9491–9. doi: 10.1523/JNEUROSCI.23-29-09491.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Devor M. Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp Brain Res. 2009;196(1):115–28. doi: 10.1007/s00221-009-1724-6. [DOI] [PubMed] [Google Scholar]
- 64.Djouhri L, Lawson SN. Abeta-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Brain Res Rev. 2004;46(2):131–45. doi: 10.1016/j.brainresrev.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 65.Hu P, McLachlan EM. Macrophage and lymphocyte invasion of dorsal root ganglia after peripheral nerve lesions in the rat. Neuroscience. 2002;112(1):23–38. doi: 10.1016/s0306-4522(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 66.Morin N, Owolabi SA, Harty MW, Papa EF, Tracy TF, Jr., Shaw SK, et al. Neutrophils invade lumbar dorsal root ganglia after chronic constriction injury of the sciatic nerve. J Neuroimmunol. 2007;184(1-2):164–71. doi: 10.1016/j.jneuroim.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 67.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6(7):521–32. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 68.Wagner R, Myers RR. Schwann cells produce tumor necrosis factor alpha: expression in injured and non-injured nerves. Neuroscience. 1996;73(3):625–9. doi: 10.1016/0306-4522(96)00127-3. [DOI] [PubMed] [Google Scholar]
- 69.Zhao R, Pei GX, Cong R, Zhang H, Zang CW, Tian T. PKC-NF-kappaB are involved in CCL2-induced Nav1.8 expression and channel function in dorsal root ganglion neurons. Biosci Rep. 2014;34(3) doi: 10.1042/BSR20140005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McKay SM, McLachlan EM. Inflammation of rat dorsal root ganglia below a mid-thoracic spinal transection. Neuroreport. 2004;15(11):1783–6. doi: 10.1097/01.wnr.0000135700.52904.77. [DOI] [PubMed] [Google Scholar]
- 71.Gensel JC, Nakamura S, Guan Z, van Rooijen N, Ankeny DP, Popovich PG. Macrophages promote axon regeneration with concurrent neurotoxicity. J Neurosci. 2009;29(12):3956–68. doi: 10.1523/JNEUROSCI.3992-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prewitt CM, Niesman IR, Kane CJ, Houle JD. Activated macrophage/microglial cells can promote the regeneration of sensory axons into the injured spinal cord. Exp Neurol. 1997;148(2):433–43. doi: 10.1006/exnr.1997.6694. [DOI] [PubMed] [Google Scholar]