Abstract
Psychosocial stress is considered to be an important mechanism underlying smoking behavior and relapse. Thus, understanding the effects of acute nicotine withdrawal on responses to stress is important to intervene to prevent stress-induced relapse. The current study investigated the neural correlates of psychosocial stress during acute nicotine withdrawal in chronic smokers. Thirty nine treatment-seeking smokers were randomized to one of two conditions (abstinent 24 hours (n=21) or smoking as usual (n=18)). They were then exposed to the Montreal Imaging Stress Task (MIST), a psychosocial stress task consisting of difficult mental arithmetic problems while receiving negative performance feedback while undergoing functional magnetic resonance imaging (fMRI). Subjective measures of stress increased following the MIST, compared to baseline. Whole brain between-group analysis identified significant activation clusters in four regions for the stress induction minus control contrast: inferior frontal gyrus (IFG), anterior/para cingulate cortex (ACC), precuneus, and supramarginal gyrus (SMG). In all regions, the deprived group showed significantly greater activation compared to the non-deprived group. No significant correlations were found between subjective stress and BOLD signal activation (ps>0.07). This study provides new evidence that brain regions previously shown to be predictive of relapse, such as the precuneus and IFG, display heightened neural responses to stress during nicotine deprivation. These data identify the brain regions that may be associated with withdrawal-related stress responses. Increased stress-related activation during nicotine withdrawal may identify those most vulnerable to relapse and represent a target for novel pharmacological intervention.
Keywords: Nicotine, withdrawal, smoking, stress, fMRI, imaging
INTRODUCTION
Stress is considered to be a primary mechanism involved in smoking behavior (Koob and Kreek 2007; Sinha 2001; 2007). Evidence from preclinical studies, laboratory studies, and clinical trials supports a critical role for stress in the initiation, maintenance, and relapse to smoking behavior (al'Absi 2006; al'Absi et al. 2005; Shiffman et al. 1996; Sinha 2001). Acute stress increases both cigarette craving (Buchmann et al. 2010) and the reinforcing properties of smoking (McKee et al. 2011). Stress also predicts the ability to resist smoking in laboratory models of smoking relapse (al'Absi et al. 2005; McKee et al. 2011) and stressful events often precede relapse to smoking in real-world quit attempts (Shiffman et al. 1996; Shiffman and Waters 2004). A greater understanding of the brain behavior relationship between psychosocial stress and smoking behavior will aid in the development of novel treatments to help more smokers quit.
Chronic smoking contributes to neuroadaptive changes in the hypothalamic-pituitary-adrenal (HPA) axis, brain dopaminergic system, and autonomic nervous system (Sinha 2007). Smokers generally show blunted cortisol responses, relative to nonsmokers (al'Absi et al. 2003; Childs and de Wit 2009; Nakajima and Al'Absi 2014), indicating that chronic nicotine exposure may reduce the body's adaptive response to stressful situations (Koob and Kreek 2007; Sinha 2008). However, studies investigating stress during nicotine withdrawal have yielded conflicting results with studies reporting both heightened and blunted responses to stress during abstinence. Nicotine withdrawal may enhance the potency of external stressors, resulting in a heightened stress response such as increased blood pressure and cortisol levels (al'Absi et al. 2002; Vanderkaay and Patterson 2006; Wardle et al. 2011). In contrast, others have found that nicotine withdrawal produces a blunted, or dampened, response to stress (al'Absi et al. 2003; Robinson and Cinciripini 2006). Variability across studies may be attributed, in part, to different underlying mechanisms assessed by various stress induction paradigms as well as methodological differences that influence the efficacy of the stress manipulation. Neural measures of stress may be more sensitive to acute stress induced in the laboratory and therefore may provide insight into the mechanisms that underlie stress responses during nicotine withdrawal.
Dysregulation of adaptive stress responses can also be observed at the neural level. Neuroimaging studies have demonstrated that acute stress alters activation in prefrontal, limbic, and midbrain regions which are involved with the HPA axis regulation (Dedovic et al. 2009a; Li and Sinha 2008; Pruessner et al. 2010). Similar to behavioral and HPA axis indices of stress, varied patterns of stress-induced brain activation across studies may be attributed to differences between stress-induction paradigms. Script-driven imagery designed to evoke emotional stress increases activation in medial prefrontal cortex (PFC), anterior cingulate cortex (ACC), striatum, thalamus, hippocampus, and posterior cingulate cortex (PCC) (Li et al. 2005; Sinha et al. 2005; Sinha et al. 2004). Mental arithmetic stress tasks, such as the Montreal Imaging Stress Task (MIST), have reported that stress reduces activation in the hippocampus, amygdala, hypothalamus, and medial orbitofrontal cortex (Dedovic et al. 2005; Dedovic et al. 2009b; Pruessner et al. 2008). Functional magnetic resonance imaging studies of the neural correlates of stress in non-deprived smokers reveal similar effects of mental arithmetic induced stress (Dagher et al. 2009).
Despite abundant evidence that stress predicts relapse to smoking, we do not yet know how nicotine withdrawal alters neural responses to stress. Examination of stress effects during early smoking withdrawal --a time when smokers are most vulnerable to relapse (Ashare et al. 2013; Hughes et al. 2004) -- may provide mechanistic insight into stress-precipitated smoking relapse. This fMRI study investigates neural responses to psychosocial stress during acute nicotine withdrawal in chronic smokers. Thirty-nine smokers were randomized to two groups: smoking as usual (non-deprived) or 24-h smoking abstinence (deprived) and underwent fMRI while being exposed to the MIST. Based on evidence that: 1) nicotine withdrawal reduces cortisol levels (Cohen et al. 2004; Wong et al. 2014), 2) blunted cortisol responses are associated with smoking relapse (al'Absi 2006; al'Absi et al. 2005), and 3) among satiated smokers (Dagher et al. 2009) and healthy individuals (Pruessner et al. 2008) stress-related deactivation in limbic regions during the MIST is associated with increased cortisol responses (Pruessner et al. 2008), we predicted deprived smokers (vs. satiated) would exhibit increased activation in brain regions associated with stress. We also explored the relationship between brain signal and subjective ratings of stress.
MATERIALS AND METHODS
Participants
Additional details regarding participants have been previously described (Falcone et al. 2014). Briefly, this sample consisted of treatment-seeking smokers between the ages of 18 and 65 who smoked at least 10 cigarettes/day for at least 6 months. In addition to a physical exam and psychiatric assessment, the Shipley Institute of Living Scale (Zachary 2000) and Fagerström Test for Nicotine Dependence (FTND; Heatherton et al. 1991) were administered. Participants with a history of DSM-IV Axis I psychiatric or substance disorders (except nicotine dependence), assessed by self-report and using the Mini International Neuropsychiatric Interview (Sheehan et al, 1998), and those taking psychotropic medications were excluded. Other exclusion criteria included: current use of chewing tobacco, snuff, or smoking cessation products; pregnancy, planned pregnancy or breastfeeding; history of brain injury; left-handedness; fMRI contraindicated material in the body; low or borderline intelligence (<90 score on Shipley's IQ test); and any impairment that would prevent task performance.
Forty-two participants completed the MIST paradigm. Three participants were excluded due to excessive motion (mean relative motion >0.3mm), resulting in a final sample of 39 smokers (deprived n=21, non-deprived n=18). Thirteen (33%) participants self-reported Caucasian race and 17 (44%) were female. On average, participants were 40 years old (SD=13.3), had an average Shipley IQ score of 102.7 (SD=8.0), smoked 15.1 cigarettes per day (SD=4.3), and were moderately nicotine dependent (mean FTND=4.8, SD=1.4).
Procedures
All procedures were approved by the University of Pennsylvania Institutional Review Board and all participants provided written informed consent. The main fMRI study (n=79) included two within-subject blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI) sessions, counterbalanced for abstinence and smoking condition (see Falcone et al., 2014). When additional scan time was available, the stress paradigm was obtained on the second scan day, resulting in a sub-sample (n=42) used for this between-subject. Counterbalancing resulted in 21 deprived and 18 non-deprived participants in the second session. Those who completed the MIST did not differ on any demographic or baseline smoking characteristics from those who did not. Those in the deprived condition were asked to refrain from smoking for 24 hours prior to the scan visit and those in the non-deprived condition were asked to smoke as usual. During the scanning session, those with a positive drug screen, a breath alcohol test >0.01, or a breath carbon monoxide (CO) test >9ppm (abstinent session only) were excluded. Participants completed the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes et al, 1984) and Questionnaire of Smoking Urges (QSU-Brief; Cox, Tiffany, and Christen, 2001) prior to the fMRI scan. Following a short practice session to allow participants to become familiar with the task and response device, participants were escorted to the radiology clinic for the fMRI scan. Those in the non-deprived group smoked a single cigarette about 60 minutes prior to completing the MIST to standardize exposure.
Montreal Imaging Stress Task
The Montreal Imaging Stress Task (MIST) is a block design fMRI paradigm that requires participants to solve difficult mental arithmetic problems presented on the visual display (Dedovic et al. 2005). There are three blocks of conditions (stress induction, control, rest) that are presented pseudo randomly. During the stress induction condition, the screen displays a virtual rotary dial for response selection, a feedback window (“correct,” “incorrect” or “timeout”), and two performance indicators (Online Supplementary Fig. 1): 1) individual subject's overall performance and 2) average performance of all subjects. In the stress induction condition, the time limit is dynamically calculated to be 10% shorter than the subject's average required time on previous trials and this limit is represented by a progress bar. Problem difficulty is increased or decreased based on the results (correct vs. incorrect) of the three previous trials. With these two variables, a range of 20% to 45% correct performance is maintained in the stress induction condition. For the control condition, mental arithmetic is presented at a comparable level of difficulty but without time restriction and neither individual nor average performance is displayed. On average, this results in an accuracy rate of 90%. During the rest condition, the response interface is displayed but no problems are presented and no response is required. The time between trials is varied as a function of the time limit imposed during the stress induction condition and total number of problems presented per condition is similar. The task is administered in three 5-min runs composed of six pseudo random 50-second blocks (2 per condition). After each run, participants are given scripted negative feedback regarding their performance on stress induction blocks (e.g., the subject must maintain a required minimum performance close to the average performance of all subjects if data are to be useful) (Dedovic et al. 2005). After the session is completed, all participants are debriefed and informed that the task was designed to be difficult to perform and was not an accurate assessment of their ability to perform mental arithmetic. They were told that negative feedback was not related to their actual performance and included only to increase their stress level.
Subjective Stress
The 11-item short form of the Profile of Mood States (POMS) was used to assess effects of the MIST on general mood disturbance or distress (Cella et al. 1987). Items were rated on Likert-type scale ranging from 0 to 4 (not at all, a little, moderately, quite a bit, extremely) and included the following items: Blue, Discouraged, Sad, Bewildered, Miserable, Gloomy, Weary, On Edge, Muddled, Uneasy, and Unhappy. A total mood disturbance score was computed by summing all items (Cella et al. 1987). The POMS was administered in the scanner immediately prior to and after the MIST.
Image Acquisition
All subject imaging sessions were acquired on the same scanner (Siemens Tim Trio 3 Tesla, Erlangen, Germany; 32 channel head coil) using a whole-brain, single-shot, multi-slice, gradient-echo (GE) echoplanar (EPI) sequence of 168 volumes with the following parameters: TR/TE=2000/30 ms, FOV=220×220 mm, matrix=64×64, flip angle=72°, slices=32, slice thickness/gap=3.4mm/0mm and effective voxel resolution=3.4 × 3.4 × 3.4. Prior to fMRI, 5-min magnetization-prepared, rapid acquisition gradient-echo (MPRAGE) T1-weighted image (TR=1810 ms, TE=3.51 ms, FOV =180×240 mm, matrix=256×192, 160 slices, TI=1100 ms, flip angle=9°, effective voxel resolution of 1 × 1 × 1mm) was acquired for anatomic overlays of functional data and to aid spatial normalization to a standard atlas space.
Image Preprocessing
BOLD time series data were preprocessed and analyzed by standard procedures using fMRI Expert Analysis Tool (FEAT version 5.98) of FSL (FMRIB's Software Library, Oxford, UK). Single subject preprocessing included skull stripping using BET (Smith 2002), slice time correction, motion correction to the median image using MCFLIRT (Jenkinson et al. 2002), high pass temporal filtering (120s), spatial smoothing using a Gaussian kernel (6 mm full-width at half-maximum, isotropic) and mean-based intensity normalization of all volumes using the same multiplicative factor. The median functional volume was coregistered to the anatomical T1-weighted structural volume and then transformed into standard anatomical space (T1 MNI template) using FLIRT (Jenkinson et al. 2002; Jenkinson and Smith 2001). Transformation parameters were later applied to all statistical contrast maps for group-level analyses.
Image Quality Assessment
Image quality assessment procedures examined the mean temporal signal-to-noise ratio (tSNR) for artifacts and poor quality. To assess excessive head motion, the root mean square (RMS) of relative volume-to-volume displacement was evaluated. Three subjects were excluded from analysis based on RMS > 0.5 and tSNR < 2SD.
Time Series Analysis
Subject-level statistical analysis was carried out voxelwise using FILM (FMRIB's Improved General Linear Model) with local autocorrelation correction (Woolrich et al. 2001). Two condition events (“experiment”, “control”) were modeled using a canonical hemodynamic response function. The rest condition acted as un-modeled baseline. Six motion correction parameters were included as nuisance covariates. Image analysis was completed for each individual in subject space, and resulting contrast maps (experiment vs. baseline, control vs. baseline, experiment vs. control) were spatially normalized as described above.
Between Group Analysis
To characterize group differences, a between-group (deprived vs. non-deprived) t-test of the stress induction minus control contrast was conducted. Resulting Z (Gaussianised F) statistic images were corrected for multiple comparisons using Gaussian Random Field Theory (voxel height of z >3.10; cluster probability of p <0.05) (Friston et al. 1994). Appropriate anatomical assignment for clusters was determined using MNI coordinates for the peak voxel in each significant cluster. Brain signal is presented as percent signal change using standardized parameter estimates generated in the voxel-wise GLM analysis described above. Mean percent signal change from each significant cluster was calculated using the featquery tool of FSL (FMRIB's Software Library, Oxford, UK). Separate linear regressions were used to predict BOLD signal from subjective measures of stress (pre- to post-MIST change score), controlling for sex and nicotine dependence.
RESULTS
Participants and Subjective Measures
Demographic and smoking characteristic by group (deprived n=21 vs. non-deprived n=18) are presented in Table 1. The groups did not differ on any demographic or baseline smoking characteristics. As expected, CO levels were higher among the non-deprived group (ps<0.001). In addition, the deprived group reported more craving and withdrawal than the non-deprived group (ps<0.001). Prior to the MIST, the groups did not differ on the POMS total mood disturbance score (p=0.4). Across all subjects, the POMS total mood disturbance score increased from 7.9 (SD=8.3) before the MIST to 15.8 (SD=12) following the MIST (p< 0.001). However, the increase in POMS mood disturbance (post-MIST minus pre-MIST change score) did not differ between groups (p=0.2)
Table 1.
Demographic and smoking characteristics by abstinence condition.
| Measure | Deprived (n=21) | Non-deprived (n=18) | p-value |
|---|---|---|---|
| Sex (n, % female) | 10 (48%) | 7 (39%) | 0.58 |
| Age (years) | 38.9 (12.7) | 41.1 (14.1) | 0.62 |
| Race (n,%) | 0.45 | ||
| Caucasian | 8, 38% | 5, 28% | |
| African-American | 13, 61% | 11, 61% | |
| Asian | 0 | 1, 5% | |
| More than one race | 0 | 1, 5% | |
| FTND score | 5.0 (1.5) | 4.7 (1.4) | 0.63 |
| Cigarettes per day | 15.3 (4.8) | 14.7 (3.6) | 0.66 |
| Shipley Institute of Living Scale | 103 (7.7) | 102.5 (8.5) | 0.88 |
| CO (ppm) | 3.3 (2.0) | 31.4 (20.2) | <0.001 |
| Craving (QSU-Brief) | 47.5 (15.4) | 20.6 (8.2) | <0.001 |
| Withdrawal (MNWS) | 11.6 (6.7) | 3.8 (5.4) | <0.001 |
| POMS Mood Disturbance | |||
| Pre-MIST | 8.9 (7.8) | 6.7 (9.1) | 0.41 |
| Post-MIST | 15.0 (12.6) | 16.8 (11.7) | 0.64 |
Note. Values are mean (standard deviation). p-values are unadjusted for multiple comparison; ppm=parts per million; FTND = Fagerström Test for Nicotine Dependence; QSU= Questionnaire on Smoking Urges; MNWS=Minnesota Nicotine Withdrawal Scale; POMS=Profile of Mood States
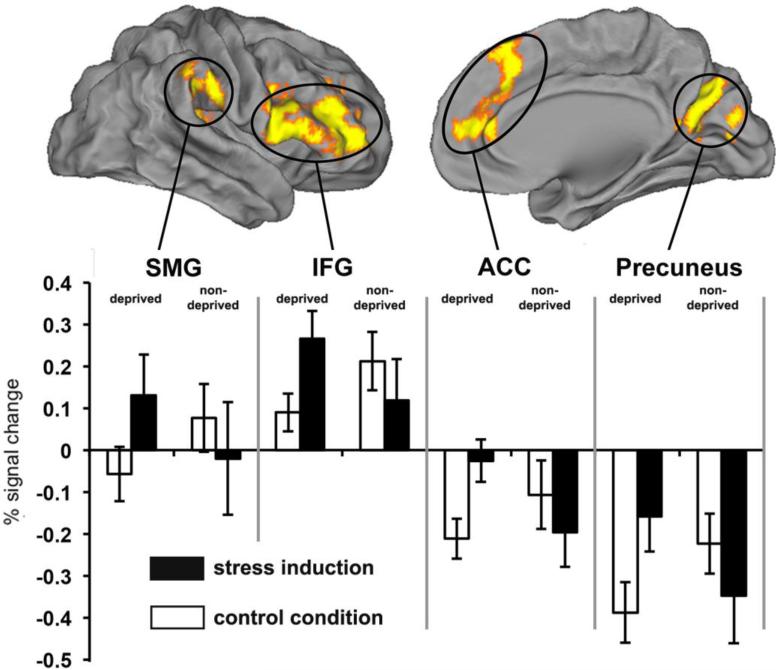
The whole brain between group analysis (deprived > non-deprived) identified significant activation clusters in several regions: inferior frontal gyrus (IFG), anterior/para cingulate cortex (ACC), precuneus, and supramarginal gyrus (SMG) between the two groups (Table 2). In all four regions, the deprived group showed greater stress-induced (experiment minus control) activation compared to the non-deprived group (Figure 1). No regions showed greater stress-induced activation for the non-deprived group. The one sample t-test showing average patterns of activation for all subjects for the experiment vs. baseline, control vs. baseline, and experiment vs. control are depicted in Supplemental Tables 1 & 2 and Supplemental Figure 2.
Table 2.
Areas of Activation Significantly Different between the Deprived and Non-deprived Groups for the Stress Induction > Control Contrast
| Regiona | BAb | Hemc | P value | voxels | Z-MAXd | X (mm)e | Y (mm) | Z (mm) |
|---|---|---|---|---|---|---|---|---|
| IFG | 9 | R | <0.001 | 2233 | 4.97 | 38 | 30 | 16 |
| ACC | 32 | L | <0.001 | 1402 | 4.54 | −2 | 38 | 28 |
| Precuneus | 31 | R | <0.001 | 1059 | 5.13 | 2 | −64 | 20 |
| SMG | 40 | R | 0.006 | 550 | 4.93 | 52 | −26 | 30 |
Significant clusters Z ≥ 3.10 and clusters probability p < 0.05
BA = Brodmann Area
HEM = cerebral hemisphere
Z-MAX values represent peak activation for cluster
MNI coordinates.
SMG=supramarginal gyrus; IFG=left inferior frontal gyrus; ACC= anterior/para cingulate cortex.
Fig. 1.
Whole-brain between-group (deprived>non-deprived) t-test of the stress induction minus control contrast. Clusters (orange/yellow) are corrected for multiple comparisons (Z>3.1 and probability of spatial extent p<0.05). In all clusters change in BOLD signal is greater in the deprived group compared to the non-deprived group. SMG=supramarginal gyrus; IFG=left inferior frontal gyrus; ACC= anterior/para cingulate cortex.
We also conducted an exploratory analysis to identify associations between BOLD signal changes in the significant clusters identified in the whole brain analysis above, and changes in POMS ratings pre- and post-MIST. The stress-induced increase in POMS total mood disturbance was not significantly associated with BOLD signal change in any region in the full sample or when each group was examined separately (ps>0.07).
DISCUSSION
This study provides new evidence that brain responses during stress differ during the early withdrawal period, compared to non-deprived. The whole brain analyses revealed four brain regions (IFG, ACC, precuneus, and SMG) that exhibited significantly greater activation changes in deprived smokers compared to those who were non-deprived. In both groups, the MIST task increased subjective ratings of distress, suggesting that the stress manipulation was successful and that manipulated smoking condition did not influence subjective stress responses (despite differences in neural responses between groups). Importantly, the brain regions identified as sensitive to acute nicotine withdrawal in the current study reveal a unique role of abstinence on the neural substrates of stress, which may shed light on the neural mechanisms that underlie stress-induced smoking relapse.
The stress response is comprised of different components (e.g., physiological, hormonal, neural) and it is possible nicotine withdrawal may have differential effects on the magnitude and direction of response for each component. For instance, some studies have found that smoking abstinence produces a blunted stress response (al'Absi et al. 2003; Robinson and Cinciripini 2006), whereas others provide evidence that abstinence produces increased stress-related cardiovascular reactivity (al'Absi et al. 2002; Tsuda et al. 1996; Vanderkaay and Patterson 2006) and cortisol levels (Wardle et al. 2011). We propose that our data support the latter view – that abstinence produced a heightened stress response, at least at the neural level. More importantly, the current findings revealed brain regions that may be particularly sensitive to stress during nicotine withdrawal. For example, a recent meta-analysis identified the IFG and SMG as brain regions activated during both psychosocial and physiological stress (Kogler et al. 2015). We observed greater stress-induced neural responses among deprived, compared to non-deprived, smokers in the IFG and SMG. In a previous study of satiated smokers, stress produced deactivation in limbic (e.g., hippocampus, amygdala, nucleus accumbens) and frontal (e.g., ventromedial PFC, anterior cingulate cortex) regions and regions within the default mode network, such as the precuneus (Dagher et al. 2009). In line with these findings, we observed stress-induced deactivation in the precuneus among non-deprived smokers. Our study also suggests that, among deprived smokers, stress may suppress deactivation in the precuneus. Although this pattern of activation must be considered relative to the non-deprived group, these novel findings highlight brain regions that exhibit differential patterns of stress-related activation which may be specific to nicotine withdrawal.
Although some brain regions may be uniquely responsive to stress, many cognitive processes share common neural substrates (Li and Sinha 2008). For instance, in addition to stress, activity in the IFG has been associated with response inhibition (Aron et al. 2004), attentional control (Hampshire et al. 2010), and suppression of intrusive thoughts (Kuhn et al. 2013). Precuneus activity is thought to be associated with self-referential thought (Cavanna and Trimble 2006) and this activity must be suppressed in order to exert effortful control over behavior (Raichle et al. 2001). Likewise, deactivation of the SMG (BA 40) may be related to inhibition of irrelevant cognitive processes (Fink et al. 2009; Wu et al. 2015). Therefore, we speculate that the stress-induced increase in BOLD signal change in the SMG, IFG, and Precuneus may be attributed to difficulty suppressing intrusive thoughts as a result of negative feedback during the stress condition. It should also be noted that these findings were specific to the deprived group. Importantly, increased activity in both the precuneus (Chua et al. 2011) and the IFG (Berkman et al. 2011) is associated with smoking relapse, highlighting the importance of our findings that deprived smokers demonstrated increased activity in these regions during stress.
In addition to the importance of suppressing task irrelevant or intrusive thoughts, stress-related decreased activity in regions important for maintaining cognitive control (e.g., ventromedial PFC/anterior cingulate cortex and medial PFC) predicts relapse to drug use (Janes et al. 2010; Seo et al. 2013). Indeed, increased working memory-related activity in the PCC, a region that is typically deactivated during goal-directed behavior, is also associated with smoking relapse (Loughead et al. 2015). As noted above, the IFG may also play an important role in inhibitory control and the execution of a planned behavior (Hampshire et al. 2010) and may be activated when salient cues are identified (Kuhn et al. 2013). Activity in the ACC has also been shown to increase in response to smoking cues (Janes et al. 2015) as well as during behavior monitoring (e.g., response conflict and error processing) (Luijten et al. 2014). Although the stress-induced increase in BOLD signal was a relative increase, the effect of the stress manipulation was specific to those in the deprived group. These patterns of activation suggest that stress may reduce the ability to exert effortful control over behavior, including drug use.
When comparing our findings to previous studies, it is important to take into consideration the type of stress manipulation employed. Although physiological and psychosocial stressors activate common regions, such as the IFG, different stress inductions may produce unique patterns of activation. For instance, physiological stress might elicit stronger activation in the dorsal striatum whereas the superior temporal gyrus may be more strongly activated during psychosocial stress (Kogler et al. 2015). Emotional stress may produce increased activation in frontal-limbic regions including the ventromedial PFC, ACC, dorsal striatum, amygdala, and hippocampus (Li and Sinha 2008; Seo et al. 2013). The current study utilized the MIST, which is often considered to be an index of psychosocial stress (Dedovic et al. 2009a; Dedovic et al. 2005; Dedovic et al. 2009b). However, it also important to note that during the MIST, subjects receive negative feedback about their performance (e.g., “...you are doing worse than an average user”) and this evaluative component may also be related to emotional stress (Dedovic et al. 2005; Lazarus 1993). Indeed, our whole brain analysis identified the ACC, which is involved with the regulation of emotion (Li and Sinha 2008), as an important region during withdrawal-related stress (Dedovic et al. 2005; Dedovic et al. 2009b). Although other regions identified in our study were not identified in previous MIST studies, our study was designed to detect brain regions that were sensitive to the effects of stress during acute nicotine withdrawal. Future studies should examine whether different stress tasks identify unique brain regions during nicotine withdrawal.
This study has several limitations. We did not assess cigarette craving following the MIST nor we did not obtain a physiological measures of stress during the scan, such as cortisol. In addition, stress-induced POMS scores did not correlate with neural activity, which may have partially been due to the timing of our assessments (i.e., there was an approximately 10 min delay between the end of the MIST and the post-MIST POMS assessment). Previous studies of the MIST have characterized individuals as “responders” and “nonresponders” according to individual differences in stress-induced changes in cortisol (Dedovic et al. 2005; Dedovic et al. 2009b; Pruessner et al. 2008). In general, only responders exhibit changes in brain activation during the MIST. We were interested in identifying brain regions that were sensitive to differences between deprived and non-deprived smokers and have no reason to suspect that group differences in smoking status would vary depending on individual cortisol responses. Although we observed subjective increases in stress during the MIST supporting the validity of the stress induction, the fact that the POMS items were all negative (e.g., Blue, Discouraged, Sad) may have produced a response bias. Thus, future work should incorporate physiological indices of stress to evaluate relationships between abstinence effects on cortisol and corresponding changes in brain activity. Second, because the smoking status condition was between-subject, our sample size was too small to evaluate whether stress-induced brain activation was related to smoking relapse. Previous studies have found sex differences in the neural substrates of stress (Potenza et al. 2012; Seo et al. 2011) and in the relationship between stress and relapse (al'Absi 2006; al'Absi et al. 2015). Here, our small sample size precluded our ability to test whether stress-induced changes in brain activity differed by sex. Thus, future studies should evaluate individual differences and employ prospective designs to evaluate whether stress-induced alterations in brain activation predict smoking relapse.
In summary, this study provides new evidence that neural responses to stress differ among deprived, compared to non-deprived, smokers. Deprived smokers demonstrated increased activation in brain regions that typically are suppressed when engaged in goal-directed behavior. Our findings suggest that nicotine withdrawal may reduce the ability to exert control over effortful behavior during stress. All smokers reported subjective increases in stress following the MIST, further supporting the sensitivity of neuroimaging tools to detect abstinence effects. The current study sheds light on the neural mechanisms that may underlie withdrawal-related stress responses – a known predictor of smoking relapse (al'Absi 2006; al'Absi et al. 2005; Shiffman et al. 1996; Sinha 2001). Our findings provide further evidence that stress-related neural responses specific to nicotine withdrawal may represent targets for attenuating stress responses during abstinence. Specifically, treatments designed to enhance the ability to suppress self-referential thought in order to maintain control over behavior may ultimately improve abstinence rates. Future work could directly test this hypothesis.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by grants from the National Cancer Institute to CL (P50 CA143187 and R35 CA197461) and National Institute on Drug Abuse to RLA (K23 DA035295). The NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
FINANCIAL DISCLOSURES
Dr. Lerman has served as a consultant to Pfizer on pharmacogenetic testing for smoking cessation treatment and has received research funding from and consulted for AstraZeneca, Targacept, Pfizer, and GlaxoSmithKline, for work unrelated to this manuscript. No other authors have any potential conflict of interests to declare.
REFERENCES
- al'Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol. 2006;59:218–27. doi: 10.1016/j.ijpsycho.2005.10.010. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Amunrud T, Wittmers LE. Psychophysiological effects of nicotine abstinence and behavioral challenges in habitual smokers. Pharmacology, biochemistry, and behavior. 2002;72:707–16. doi: 10.1016/s0091-3057(02)00739-6. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Hatsukami D, Davis G. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology. 2005;181:107–17. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Nakajima M, Allen S, Lemieux A, Hatsukami D. Sex differences in hormonal responses to stress and smoking relapse: a prospective examination. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2015;17:382–9. doi: 10.1093/ntr/ntu340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al'Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacology Biochemistry and Behavior. 2003;74:401–410. doi: 10.1016/s0091-3057(02)01011-0. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in cognitive sciences. 2004;8:170–7. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Ashare RL, Wileyto EP, Perkins KA, Schnoll RA. The first 7 days of a quit attempt predicts relapse: validation of a measure for screening medications for nicotine dependence. Journal of addiction medicine. 2013;7:249–54. doi: 10.1097/ADM.0b013e31829363e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman ET, Falk EB, Lieberman MD. In the trenches of real-world self-control: neural correlates of breaking the link between craving and smoking. Psychological science. 2011;22:498–506. doi: 10.1177/0956797611400918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann A, Laucht M, Schmid B, Wiedemann K, Mann K, Zimmermann U. Cigarette craving increases after a psychosocial stress test and is related to cortisol stress response but not to dependence scores in daily smokers. J Psychopharmacol. 2010;24:247–55. doi: 10.1177/0269881108095716. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain : a journal of neurology. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cella DF, Jacobsen PB, Orav EJ, Holland JC, Silberfarb PM, Rafla S. A brief POMS measure of distress for cancer patients. Journal of chronic diseases. 1987;40:939–42. doi: 10.1016/0021-9681(87)90143-3. [DOI] [PubMed] [Google Scholar]
- Childs E, de Wit H. Hormonal, cardiovascular, and subjective responses to acute stress in smokers. Psychopharmacology. 2009;203:1–12. doi: 10.1007/s00213-008-1359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua HF, Ho SS, Jasinska AJ, Polk TA, Welsh RC, Liberzon I, Strecher VJ. Self-related neural response to tailored smoking-cessation messages predicts quitting. Nat Neurosci. 2011;14:426–427. doi: 10.1038/nn.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LM, al'Absi M, Collins FL., Jr. Salivary cortisol concentrations are associated with acute nicotine withdrawal. Addictive behaviors. 2004;29:1673–8. doi: 10.1016/j.addbeh.2004.02.059. [DOI] [PubMed] [Google Scholar]
- Dagher A, Tannenbaum B, Hayashi T, Pruessner JC, McBride D. An acute psychosocial stress enhances the neural response to smoking cues. Brain research. 2009;1293:40–8. doi: 10.1016/j.brainres.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, D'Aguiar C, Pruessner JC. What Stress Does to Your Brain: A Review of Neuroimaging Studies. Résumé : Ce que fait le stress à votre eerveau : une revue des études de neuroimagerie. 2009a;54:6–15. doi: 10.1177/070674370905400104. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, Pruessner JC. The Montreal Imaging Stress Task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. Journal of Psychiatry and Neuroscience. 2005;30:319–25. [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, Rexroth M, Wolff E, Duchesne A, Scherling C, Beaudry T, Lue SD, Lord C, Engert V, Pruessner JC. Neural correlates of processing stressful information: an event-related fMRI study. Brain research. 2009b;1293:49–60. doi: 10.1016/j.brainres.2009.06.044. [DOI] [PubMed] [Google Scholar]
- Falcone M, Wileyto EP, Ruparel K, Gerraty RT, LaPrate L, Detre JA, Gur R, Loughead J, Lerman C. Age-related differences in working memory deficits during nicotine withdrawal. Addiction biology. 2014;19:907–17. doi: 10.1111/adb.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink A, Grabner RH, Benedek M, Reishofer G, Hauswirth V, Fally M, Neuper C, Ebner F, Neubauer AC. The creative brain: investigation of brain activity during creative problem solving by means of EEG and FMRI. Human brain mapping. 2009;30:734–48. doi: 10.1002/hbm.20538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RS, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Human brain mapping. 1994;1:210–20. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage. 2010;50:1313–9. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Janes AC, Farmer S, Peechatka AL, Frederick Bde B, Lukas SE. Insula-Dorsal Anterior Cingulate Cortex Coupling is Associated with Enhanced Brain Reactivity to Smoking Cues. Neuropsychopharmacology. 2015;40:1561–8. doi: 10.1038/npp.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biological psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical image analysis. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kogler L, Mueller VI, Chang A, Eickhoff SB, Fox PT, Gur RC, Derntl B. Psychosocial versus physiological stress - Meta-analyses on deactivations and activations of the neural correlates of stress reactions. NeuroImage. 2015 doi: 10.1016/j.neuroimage.2015.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. American Journal of Psychiatry. 2007;164:1149. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Schmiedek F, Brose A, Schott BH, Lindenberger U, Lovden M. The neural representation of intrusive thoughts. Social cognitive and affective neuroscience. 2013;8:688–93. doi: 10.1093/scan/nss047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus RS. From Psychological Stress to the Emotions: A History of Changing Outlooks. Annual Review of Psychology. 1993;44:1–22. doi: 10.1146/annurev.ps.44.020193.000245. [DOI] [PubMed] [Google Scholar]
- Li C-SR, Kosten TR, Sinha R. Sex differences in brain activation during stress imagery in abstinent cocaine users: a functional magnetic resonance imaging study. Biological psychiatry. 2005;57:487–494. doi: 10.1016/j.biopsych.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Li CS, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neuroscience and biobehavioral reviews. 2008;32:581–97. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Wileyto EP, Ruparel K, Falcone M, Hopson R, Gur R, Lerman C. Working memory-related neural activity predicts future smoking relapse. Neuropsychopharmacology. 2015;40:1311–20. doi: 10.1038/npp.2014.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M, Machielsen MW, Veltman DJ, Hester R, de Haan L, Franken IH. Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. Journal of psychiatry & neuroscience : JPN. 2014;39:149–69. doi: 10.1503/jpn.130052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee S, Sinha R, Weinberger A, Sofuoglu M, Harrison E, Lavery M, Wanzer J. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. Journal of psychopharmacology. 2011;25:490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Al'Absi M. Nicotine withdrawal and stress-induced changes in pain sensitivity: a cross-sectional investigation between abstinent smokers and nonsmokers. Psychophysiology. 2014;51:1015–22. doi: 10.1111/psyp.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. The American journal of psychiatry. 2012;169:406–14. doi: 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, Lupien S. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biological psychiatry. 2008;63:234–40. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L, Dagher A, Lupien SJ. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations - 2008 Curt Richter Award Winner. Psychoneuroendocrinology. 2010;35:179–91. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J, Cinciripini P. The effects of stress and smoking on catecholaminergic and cardiovascular response. Behavioral medicine (Washington, DC) 2006;32:13–8. doi: 10.3200/BMED.32.1.13-18. [DOI] [PubMed] [Google Scholar]
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, Sinha R. Sex differences in neural responses to stress and alcohol context cues. Human brain mapping. 2011;32:1998–2013. doi: 10.1002/hbm.21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA psychiatry. 2013;70:727–39. doi: 10.1001/jamapsychiatry.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Hickcox M, Paty JA, Gnys M, Kassel JD, Richards TJ. Progression from a smoking lapse to relapse: prediction from abstinence violation effects, nicotine dependence, and lapse characteristics. J Consult Clin Psychol. 1996;64:993–1002. doi: 10.1037//0022-006x.64.5.993. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking lapses: a prospective analysis. Journal of consulting and clinical psychology. 2004;72:192. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Curr Psychiatry Rep. 2007;9:388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology. 2005;183:171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Wexler BE. Neural circuits underlying emotional distress in humans. Annals of the New York Academy of Sciences. 2004;1032:254–257. doi: 10.1196/annals.1314.032. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human brain mapping. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda A, Steptoe A, West R, Fieldman G, Kirschbaum C. Cigarette smoking and psychophysiological stress responsiveness: effects of recent smoking and temporary abstinence. Psychopharmacology. 1996;126:226–33. doi: 10.1007/BF02246452. [DOI] [PubMed] [Google Scholar]
- Vanderkaay MM, Patterson SM. Nicotine and acute stress: effects of nicotine versus nicotine withdrawal on stress-induced hemoconcentration and cardiovascular reactivity. Biological psychology. 2006;71:191–201. doi: 10.1016/j.biopsycho.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Wardle MC, Munafo MR, de Wit H. Effect of social stress during acute nicotine abstinence. Psychopharmacology. 2011;218:39–48. doi: 10.1007/s00213-010-2150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JA, Pickworth WB, Waters AJ, al'Absi M, Leventhal AM. Cortisol levels decrease after acute tobacco abstinence in regular smokers. Human psychopharmacology. 2014;29:152–62. doi: 10.1002/hup.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14:1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Wu X, Yang W, Tong D, Sun J, Chen Q, Wei D, Zhang Q, Zhang M, Qiu J. A meta-analysis of neuroimaging studies on divergent thinking using activation likelihood estimation. Human brain mapping. 2015;36:2703–18. doi: 10.1002/hbm.22801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary RS. Shipley Institute of Living Scale - Revised Manual. Western Psychological Services; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.