Abstract
The increasing ageing of our societies is accompanied by a pandemic of obesity and related cardiometabolic disorders. Progressive dysfunction of the white adipose tissue is increasingly recognized as an important hallmark of the ageing process, which in turn contributes to metabolic alterations, multi‐organ damage and a systemic pro‐inflammatory state (‘inflammageing’). On the other hand, obesity, the paradigm of adipose tissue dysfunction, shares numerous biological similarities with the normal ageing process such as chronic inflammation and multi‐system alterations. Accordingly, understanding the interplay between accelerated ageing related to obesity and adipose tissue dysfunction is critical to gain insight into the ageing process in general as well as into the pathophysiology of obesity and other related conditions. Here we postulate the concept of ‘adipaging’ to illustrate the common links between ageing and obesity and the fact that, to a great extent, obese adults are prematurely aged individuals.
Abbreviations
- ASC
adipose‐derived mesenchymal stem cell
- BAT
brown adipose tissue
- BDNF
brain‐derived neurotrophic factor
- BMI
body mass index
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- ER
endoplasmic reticulum
- IGF
insulin‐like growth factor
- IL
interleukin
- MHO
metabolically healthy obese
- NF‐κB
nuclear factor κ‐light‐chain‐enhancer of activated B cells
- p53
tumour protein 53
- PPAR‐γ
peroxisome proliferator activated receptor‐γ
- PVAT
perivascular adipose tissue
- RAAS
renin–angiotensin–aldosterone system
- ROS
reactive oxygen species
- SIRT1
sirtuin 1
- SNS
sympathetic nervous system
- TNF
tumour necrosis factor
- Wnt
wingless‐type MMTV integration site
Introduction
White adipose tissue (here referred to as ‘adipose tissue’) plays a key role in energy storage as well as in other vital functions such as metabolic regulation, immunity, response to injury, and production of hormones, inflammatory cytokines and chemokines (Ouchi et al. 2011). This tissue is divided into two main depots that differ in gene expression profile (Gerhard et al. 2014) and embryonic origin (Berry et al. 2013; Chau et al. 2014): visceral (also termed ‘internal’) and subcutaneous fat. The subcutaneous reservoir has mainly beneficial roles, including storage of lipids, and secretion of adipokines, e.g. leptin and adiponectin (see the section ‘Endocrine dysfunction in ageing and obesity’ for more information) with beneficial metabolic effects such as lipid oxidation, increased insulin action and anti‐inflammatory functions (Ma et al. 2015). In contrast, visceral fat is associated with metabolic syndrome, insulin resistance and related cardiometabolic complications (Mathieu et al. 2010). The beneficial vs. detrimental effects of subcutaneous and visceral fat, respectively, are exemplified by the features of the human immunodeficiency virus (HIV)‐associated lipodystrophy, a syndrome that was originally described as loss of fat wasting of the face, limbs and upper trunk, as well as hyperlipidaemia and insulin resistance in patients receiving highly active antiretroviral therapy (Carr et al. 1998). This syndrome, which is usually characterized by loss of subcutaneous fact (‘lipoatrophy’) with increased visceral fat (‘lipohypertrophy’) (Alves et al. 2014) is associated with increased risk of cardiovascular disease (CVD) and diabetes (Bevilacqua et al. 2009; Finkelstein et al. 2015). Of note, the beneficial function of subcutaneous fat is typically disrupted in obese people, in whom excessive subcutaneous fat mass occurs together with tissue dysfunction, adipocyte hypertrophy, and decreased adipogenesis and angiogenesis (Patel & Abate, 2013).
The adipose tissue is mainly composed of adipocytes and, to a lesser extent, a stromal vascular fraction that includes preadipocytes, pericytes or multipotent stem cells, vascular wall and endothelial cells, macrophages (Eto et al. 2013), lymphocytes (Lolmede et al. 2011), eosinophils (Schipper et al. 2012), neutrophils (Elgazar‐Carmon et al. 2008), mast cells (Anderson et al. 2010), and haematopoietic progenitor cells (De Toni et al. 2011). The capacity of the adipose tissue to expand or shrink relies mostly on adipocytes, preadipocytes and stem cells with a regenerative capacity (Baptista et al. 2015). Importantly, the macrophage population can switch phenotypes between non‐inflammatory and inflammatory states (Lumeng et al. 2007).
Other types of fat, such as the brown adipose tissue (BAT), are also involved in maintaining metabolic homeostasis. Particularly abundant in newborns, the BAT is a highly vascularized tissue rich in mitochondria with a high content of uncoupling protein‐1 (UCP‐1), a molecule that produces heat by uncoupling the respiratory chain (Shimizu et al. 2015). Besides its thermogenic function, BAT contributes to systemic metabolism by virtue of its high‐energy expenditure ratio (Shimizu et al. 2015). Thus, BAT has basically antagonistic functions to white adipose tissue, i.e. it is specialized in the production of heat (thermogenesis) whilst white adipose tissue stores excess energy as triglycerides (Saely et al. 2012). High amounts of BAT are related to lower body weight and are present in a considerable proportion of adults, whereas ageing decreases BAT and increases body weight (Saely et al. 2012). Importantly, the functional activity of BAT is decreased not only in ageing, but also in obesity as well as in certain cardiometabolic conditions (Peng et al. 2015).
The ageing adipose tissue
In general, as we age, adiposity and especially percentage body fat increase whereas lean mass and bone mineral density decrease. Another major change is that fat mass tends to be preferentially distributed in the abdominal region, a phenomenon that has been reported in both sexes (Enzi et al. 1986) and has been associated with insulin resistance (Kohrt & Holloszy, 1995; Barzilai & Gupta, 1999 a), and higher risk of CVD, diabetes (St‐Onge, 2005) and cancer (Sanchis‐Gomar et al. 2015). Ageing also promotes fat redistribution outside normal adipose tissue reservoirs, with ectopic lipid accumulation occurring not only in visceral depots but also in bone marrow or muscle, among other tissues (Tchkonia et al. 2013). This phenomenon is linked to higher risk of cardiometabolic disorders (Shimizu et al. 2015). The ageing process is associated with an increased accumulation of senescent cells in the adipose tissue, with causative factors being cytokines, metabolic stress, and reduced removal of these cells, which lose the ability to respond efficiently to chemokine signalling (see ‘Stem cell populations’ section). The aged adipose tissue is also characterized by reduced adipocyte size, tissue fibrosis, endothelial dysfunction, and reduced vascularization and angiogenic capacity (Donato et al. 2014). In addition, major metabolic alterations in this tissue occur with age, notably increased insulin resistance or altered lipolysis (Das et al. 2004). In fact, alterations in fatty acid metabolism cause an excessive free fatty acid release into plasma with subsequent lipotoxicity (Yang & Li, 2012) and insulin resistance (Basu et al. 2003).
Adipose tissue mass is determined by the energetic balance between net fat storage in adipocytes (of lipids originating from dietary (exogenous) or from non‐lipid precursors, mainly carbohydrates) on one hand, and total fat oxidation on the other (Schutz, 2004). Of note, the measurement of fat balance (fat input minus fat output) involves the accurate estimation of both metabolizable fat intake and total fat oxidation, which is possible mostly under laboratory conditions; in free living conditions, the fat retention/mobilization ratio can be estimated with accurate sequential body composition measurements (Schutz, 2004). The balance between fat storage and fat oxidation is progressively disrupted during ageing, with the capacity of tissues to oxidize fat gradually decreasing. Increases in adiposity with age may also be due, at least partly, to a chronic positive energy balance throughout life, associated with decreased physical activity and basal metabolic rate that are not accompanied by proportional decreases in energy intake (Enzi et al. 1986). Decline with age of sirtuin 1 (SIRT1), a nicotinamide adenine dinucleotide (NAD+)‐dependent protein deacetylase that is highly evolutionarily conserved in mammals (Schwer & Verdin, 2008), might play a pivotal role in the dysfunction of the adipose tissue as well as in other chronic conditions associated with the normal ageing process.
SIRT1 and metabolic dysfunction
By virtue of deacetylation of numerous substrates such as peroxisome proliferator‐activated receptor‐1α (PGC‐1α), forkhead box O3 (FOXO3), tumour protein p53 (p53) or the nuclear factor κ‐light‐chain‐enhancer of activated B cells (NF‐κB), SIRT1 modulates mitochondrial function, apoptosis and inflammation (Canto et al. 2009; Poulose & Raju, 2015). It also modulates epigenetic changes (Vaquero et al. 2007), regulates circadian rhythm at the peripheral and central nervous system level (Chang & Guarente, 2013), and acts as a key regulatory sensor. SIRT1 is increased by caloric restriction and reduced by overfeeding, and in turn it increases leptin and insulin sensitivity (Sasaki, 2015). It also plays an important role in adipocyte metabolism. In white adipocytes, SIRT1 increases fat mobilization by repressing the transcriptional activity of peroxisome proliferator activated receptor‐γ (PPAR‐γ; Picard et al. 2004) and protects cells from tumour necrosis factor (TNF)‐α‐induced insulin resistance (Yoshizaki et al. 2009). SIRT1 also acts as a nutrient‐dependent modulator of obesity‐associated inflammation in the adipose tissue (Kotas et al. 2013). A recent report showed an inverse relationship between SIRT1 levels in adipose tissue and inflammation in this tissue (Gillum et al. 2011), so that suppression of SIRT1 led to inflammation and macrophage infiltration, whilst overexpression of SIRT1 prevented these changes, thereby suggesting that SIRT1 is a key regulator of adipose tissue macrophage content in conditions of obesity and overnutrition; further, genetic ablation of SIRT1 specifically from adipose tissue resulted in increased adiposity and predisposition to metabolic dysfunction, with gene expression studies showing that SIRT1 activity is necessary to protect adipose tissue from transcriptional changes that lead to obesity and insulin resistance (Gillum et al. 2011). In addition, a high‐fat diet induces the cleavage of SIRT1 in adipose tissue by the inflammation‐activated caspase‐1, providing a link between excess nutrient intake and predisposition to metabolic dysfunction (Chalkiadaki & Guarente, 2012).
On the other hand, ageing is characterized by a pseudo‐hypoxic state leading to declining NAD+ and low SIRT1 activity (Poulose & Raju, 2015), particularly at the hypothalamic level, which in turn promotes leptin resistance and increased adiposity (Sasaki, 2015), whereas caloric restriction and exercise stimulate SIRT1 activity (Warolin et al. 2014). Importantly, SIRT1 activators improve the health and extend the lifespan of mice fed either a high‐calorie (Baur et al. 2006; Minor et al. 2011) or normal diet (Mitchell et al. 2014).
Ageing and obesity share numerous disease phenotypes
It is well established that the risk of obesity increases with age (Villareal et al. 2005; Canning et al. 2014). In turn, obesity and obesity‐related metabolic disturbances can accelerate the rate of ageing and lead to early mortality (Ahima et al. 2000; Tzanetakou et al. 2012). Both conditions, obesity and ageing, are associated with increased risk of CVD, diabetes, dyslipidaemia, hypertension and mortality (North & Sinclair, 2012; Chen & Tseng, 2013). They also share an association with low‐grade inflammation, insulin resistance, increased levels of chemotactic and pro‐coagulant proteins at the local‐tissue and systemic level, as well as the abovementioned ectopic lipid deposition with subsequent lipotoxicity (Xu et al. 2003). Although the underlying mechanism(s) remains to be elucidated, progressive BAT dysfunction or ‘whitening’ is also linked to both ageing and obesity/insulin resistance (Shimizu et al. 2015).
Several reports have indicated a link between central obesity or high body mass index (BMI, weight (kg)/height2 (m2)) at mid‐ or late‐life, and higher risk of dementia (Gustafson et al. 2003, 2009; Kivipelto et al. 2005; Whitmer et al. 2005 a, 2007, 2008; Hayden et al. 2006; Fitzpatrick et al. 2009; Emmerzaal et al. 2015) –see also the ‘Central nervous system’ section. Recent provocative data have, however, indicated a negative association between higher BMI and risk of dementia in an impressive cohort of ∼2 million adults (median age at baseline of 55 years) followed for a median of 9 years (Qizilbash et al. 2015), supporting the notion that higher BMI might actually play a certain protective role in late life (Emmerzaal et al. 2015). Controversy in the field might be due to the fact that BMI is not necessarily a surrogate of regional adiposity, which would also explain, at least partly, the so‐called ‘obesity paradox’, i.e. the fact that a high BMI at late life might be associated with lower mortality compared with normal weight (Dorner & Rieder, 2012; Hainer & Aldhoon‐Hainerova, 2013): because BMI is not necessarily a good proxy of regional adiposity and individuals with CVD and abdominal obesity die earlier, a relatively high proportion of old people with high BMI due to lower‐body obesity might survive. Further, many elders show late‐onset obesity, with the short duration of this condition precluding manifestation of other related cardiometabolic comorbidities (Hainer & Aldhoon‐Hainerova, 2013).
The abovementioned associations suggest that a physiological condition, ageing, and a pathological state, obesity, might share several common causative mechanisms that, in turn, might be largely linked to a dysfunctional adipose tissue, including (i) metabolic dysfunction, (ii) multi‐organ damage, (iii) endocrine disruption, (iv) impaired immune function, and (v) chronic inflammation. Thus, understanding the interplay between accelerated ageing related to obesity and adipose tissue dysfunction is critical to gain insight into the ageing process in general as well as into the pathophysiology of obesity and other related conditions.
A striking phenomenon: the metabolically healthy obese phenotype
Although there is not a standard definition, metabolically healthy obese (MHO) people are individuals who, despite their excess adiposity, are insulin sensitive, normotensive, have a favourable lipid profile and have less visceral fat than the typical individual with obesity‐related comorbidities (Karelis, 2008; Wildman et al. 2008; Kuk & Ardern, 2009 a; Camhi & Katzmarzyk, 2014). However, a true MHO phenotype, i.e. absence of clinical as well as subclinical metabolic risk factors, is rare, possibly representing ≤6% of all obese adults or ∼1.3% of the US population (Kuk & Ardern, 2009 a). The underlying mechanisms of the MHO phenotype remain to be clearly elucidated (Brown & Kuk, 2015). Although there is no unanimity (Brown & Kuk, 2015), one factor that might potentially differentiate MHO from unhealthy obese people, together with preserved insulin sensitivity, is higher levels of physical activity and physical fitness (Hayes et al. 2010; Ortega et al. 2013; Poelkens et al. 2014). Indeed, regular physical activity has a ‘polypill‐like’ effect that confers a powerful, independent protective effect against cardiometabolic conditions across the human lifespan; it attenuates not only ‘traditional’ CVD risk factors but also age‐ and obesity‐related alterations such as hyperactivity of the sympathetic nervous system – see the ‘Cardiovascular system’ section and Fiuza‐Luces et al. (2013) for an in depth‐review.
Some authors have found that MHO adults are not at an elevated risk for CVD (Calori et al. 2011; Ogorodnikova et al. 2012) or myocardial infarction (Morkedal et al. 2014) and do not have excess mortality risk compared with metabolically healthy normal weight adults (Calori et al. 2011; Kuk et al. 2011; Hamer & Stamatakis, 2012). In contrast, others have reported that MHO individuals are still at a higher risk for premature mortality (Kuk & Ardern, 2009 a; Kramer et al. 2013) as well as type 2 diabetes (Bell et al. 2014; Hinnouho et al. 2015), heart failure (Morkedal et al. 2014) and subclinical atherosclerosis (Chang et al. 2014), suggesting that being an MHO is not really a harmless condition.
On the other hand, several studies have reported that following weight loss, MHO individuals significantly improved body composition and cardiometabolic risk factors (Janiszewski & Ross, 2010; Sesti et al. 2011), as well as physical fitness (when the weight loss intervention was combined with intense exercise training) (Dalzill et al. 2014). In obese women with no other pre‐existing illness (n = 28,388), intentional weight loss of ≥ 9.1 kg that occurred within the previous year was associated with a reduction of ∼25% in all‐cause mortality (Williamson et al. 1995). These findings are in contrast to those observed by other authors who, despite a significant loss of body weight in HMO adults, failed to show significant benefits on metabolic risk factors (Shin et al. 2006; Kantartzis et al. 2011) or cardiovascular mortality (Williamson et al. 1999), and in fact reported increases in diabetes‐associated mortality (Williamson et al. 1999), decreases in insulin sensitivity (Karelis et al. 2008), or a higher mortality risk compared with those who remained weight stable (Sorensen et al. 2005).
In summary, although there is evidence of an MHO phenotype, it may represent a minor proportion of obese individuals. More research is undoubtedly needed to elucidate the mechanisms underlying the MHO phenotype as well as the effect of weight loss on cardiometabolic health and mortality in this population segment.
An overlapping biological hallmark in ageing and obesity: inflammation
Ageing and obesity share numerous alterations from the organ to the molecular level. First, ageing is characterized by a progressive organ dysfunction that complicates the maintenance of homeostatic processes (Barzilai et al. 2012), with obesity inducing a comparable effect (Shapiro et al. 2011). A major deleterious effect of ageing, linked to adipose tissue dysfunction, is the insulin resistance syndrome, whose main complications include diabetes mellitus, hypertension and CVD. Two common contributors to both ageing and obesity are oxidative stress due to the reactive oxygen species (ROS) generated by biological oxidations and chronic inflammation. Besides activating the p53 tumour suppressor gene, ROS cause telomere damage (Jurk et al. 2014) and produce cumulative oxidative damage to macromolecules, thereby inducing cellular dysfunction and eventually cell death (Lee & Wei, 2007). Obesity accelerates the ageing of adipose tissue, a process only now beginning to come to light at the molecular level, with experiments in mice suggesting that obesity increases the formation of ROS in adipocytes, shortens telomeres, and ultimately results in the activation of the tumour suppressor p53, inflammation and promotion of insulin resistance (Ahima, 2009). Remarkably, a recent study showed that excessive calorie intake led to the accumulation of oxidative stress in the adipose tissue of mice with type 2 diabetes‐like disease and promoted senescence‐like changes, along with increased expression of p53 and increased production of pro‐inflammatory cytokines (Minamino et al. 2009). Conversely, inhibition of p53 activity in adipose tissue markedly ameliorated these senescence‐like changes, whereas upregulating p53 levels caused an inflammatory response that led to insulin resistance.
Obesity is not only caused by lipid accumulation, but is highly linked to an inflammatory state characterized by increased concentrations of inflammatory cytokines and macrophage infiltration in subcutaneous adipose tissue (Hotamisligil et al. 1993; Weisberg et al. 2003; Xu et al. 2003; Lasselin et al. 2014). In obese old adults, higher levels of adiposity are associated with higher blood levels of inflammatory markers such as interleukin (IL)‐1 receptor antagonist (IL‐1RA), IL‐6, TNF‐α and the acute phase reactant, C‐reactive protein (Lisko et al. 2012; Aguirre et al. 2014). Although the role of ROS in ageing is under reconsideration (Ristow & Schmeisser, 2011; Lopez‐Otin et al. 2013), a major hallmark of this natural process is an altered intercellular communication or ‘inflammageing’, i.e. a pro‐inflammatory phenotype that accompanies ageing in mammals (Salminen et al. 2012) and may result from multiple causes, including accumulation of pro‐inflammatory tissue damage, a dysfunctional immune system unable to effectively combat pathogens, the propensity of senescent cells to secrete pro‐inflammatory cytokines, increased NF‐κB activation and decreased autophagy (Salminen et al. 2012; Lopez‐Otin et al. 2013).
Further research might elucidate other common biological alterations linking ageing and obesity. Notably, recent data connect mitochondrial dynamics and architecture with the balance between energy demand and nutrient supply, with excess nutrient intake and obesity leading to the progressive mitochondrial alterations that are common to major age‐related diseases (Liesa & Shirihai, 2013).
Multi‐organ common damage in ageing and obesity
Although the pattern of organ‐specific deterioration associated with obesity differs from that induced by the normal ageing process, the actual decline in organ function induced by both conditions is remarkably similar (Barness et al. 2007; Tzanetakou et al. 2012; Lopez‐Otin et al. 2013; Romacho et al. 2014). To a certain extent, being obese also implies being prematurely aged (as explained below and summarized in Fig. 1).
Figure 1. Main multi‐organ alterations common to obesity and ageing .
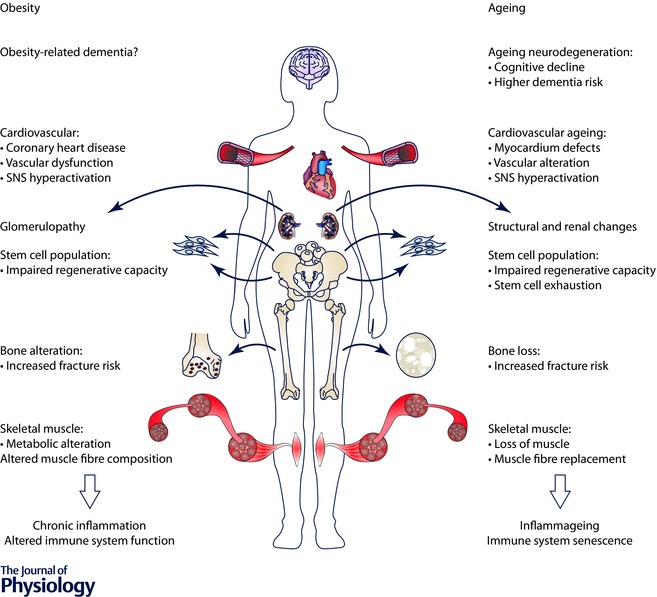
Abbreviation: SNS, sympathetic nervous system.
Cardiovascular system
Ageing and obesity might share important similarities in the way they alter the cardiovascular system. The age decline in cardiac function is associated with decreases in cardiomyocyte number, left‐ventricular hypertrophy, and cardiac fibrosis and accumulation of collagen (Olivetti et al. 1991). Ageing structural changes involve the myocardium as well as the cardiac conduction system and endocardium. There is also a progressive tissue degeneration, including a loss of elasticity, together with fibrotic changes and calcification of cardiac valves (Hinton & Yutzey, 2011), and amyloid infiltration (Maurer, 2015), with subsequent impairment of the cardiac pumping capacity. The elasticity, and thus the functionality of arterial vessels, declines with ageing, owing to a wall thickening and stiffening due to increased collagen and reduced elastin, together with vessel wall calcification.
Paradoxically, a protective mechanism to prevent excessive adiposity during ageing, that is, tonic activation of the sympathetic nervous system (SNS) to stimulate thermogenesis, has several deleterious consequences on the cardiovascular system that, in turn, increase CVD risk, i.e. reduced leg blood flow, increased arterial blood pressure, impaired baroreflex function and hypertrophy of large arteries (Seals & Dinenno, 2004). Chronic reductions in peripheral blood flow due to such increased SNS activity also contribute to the aetiology of the metabolic syndrome, by increasing glucose intolerance and insulin resistance (Baron et al. 1990; Lind & Lithell, 1993). Further, excessive lipolysis associated with high SNS activity increases ROS production and activates p53 signalling in the adipose tissue, potentially leading to inflammation of this tissue (Shimizu et al. 2015). Indeed, although p53 is a transcriptional factor involved in preservation of genomic stability and inhibition of tumorigenesis, it also has some deleterious effects related to age‐associated cardiovascular disorders, e.g. activation of p53 signalling is found in aged vessels or failing hearts (Minamino & Komuro, 2007; Sano et al. 2007; Minamino & Komuro, 2008). In contrast, inhibition of lipolysis by sympathetic denervation or through a treatment with a lipase inhibitor significantly down‐regulates adipose tissue p53 expression and inflammation, thereby improving not only insulin resistance but also cardiac function in conditions of chronic pressure overload (Shimizu et al. 2012).
The SNS is also exceedingly active in obese adults and plays a key role in the development of insulin resistance (Thorp & Schlaich, 2015). Obesity increases the risk of coronary heart disease, atrial fibrillation and heart failure through a variety of mechanisms, including the aforementioned SNS hyperactivity, systemic inflammation, hypercoagulability, and activation of the renin–angiotensin–aldosterone system (RAAS) (Zalesin et al. 2011). In lean individuals, perivascular adipose tissue (PVAT) has beneficial vasodilatory and anti‐inflammatory functions; however, obesity results in PVAT dysfunction and inflammation, characterized by an imbalance between anti‐ and pro‐inflammatory cells as well as pro‐inflammatory adipocytokines (see the section ‘Endocrine dysfunction in ageing and disease’ for further information), leading to impaired vasodilatation and vascular remodelling (Gu & Xu, 2013; Lastra & Manrique, 2015). In fact, both ageing and obesity may affect PVAT in a comparable manner, causing inflammatory infiltrate, inducing imbalance of PVAT‐derived growth factors and inhibitors, and leading to the development of proliferative vascular diseases such as atherosclerosis, restenosis and hypertension (Miao & Li, 2012). General and central adiposity in later midlife are strong independent predictors of aortic stiffening (Brunner et al. 2015). Excess weight gain, especially when associated with high visceral adiposity, is indeed a major cause of hypertension (Hall et al. 2015) and obesity. Moreover, it is associated with a markedly increased prevalence of vascular fibrosis and stiffness due to RAAS activation, reduced bioavailable nitric oxide, increased vascular extracellular matrix and extracellular matrix remodelling (Jia et al. 2015), as well as with renovascular disease (Zhang & Lerman, 2015) –see also ‘Kidney’ section.
Central nervous system
Cognitive dysfunction is a natural consequence of ageing. Although cognitive dysfunction is a diffuse concept, it can be described as a significant decline in the cognitive function compared with the previous mental performance that primarily affects learning, memory, perception and problem solving (Petersen, 2011). The next stage in the cognitive dysfunction process is ‘mild cognitive impairment’ (MCI), which is considered as an intermediate step between the expected cognitive decline of normal ageing and the more aggravated decline of dementia (Petersen, 2011). Dementia can be caused by both neurodegenerative (Alzheimer's disease, frontotemporal dementia and dementia with Lewy body) and non‐neurodegenerative conditions (vascular dementia and abnormal pressure hydrocephalus) (Burns & Iliffe, 2009). Alzheimer's disease is considered the most prevalent chronic neurodegenerative disease reaching 35 million people worldwide and 5.5 million in the United States (Querfurth & LaFerla, 2010). It accounts for 50–56% of all dementias with 13–17% of all cases of Alzheimer's disease characterized by the presence of other cerebrovascular disorders (i.e. ‘mixed dementia’) (Querfurth & LaFerla, 2010). Age is the main risk factor for Alzheimer's disease, with the incidence of this disorder doubling every 5 years in people aged 65+ years (Hirtz et al. 2007).
There are several factors related to overweight and obesity that increase dementia risk, i.e. physical inactivity, high fat diet, hypertension, diabetes, hypercholesterolaemia and metabolic syndrome (Hirtz et al. 2007). Prospective studies suggest a U‐shaped relationship between body weight and the risk of cognitive impairment and Alzheimer's disease (Razay & Vreugdenhil, 2005; Stewart et al. 2005; Gustafson et al. 2009) that is dependent on age (Whitmer et al. 2008). Different factors intrinsic to central adiposity increase risk of dementia including the lifetime exposure to an altered metabolic and inflammatory state induced by high visceral adiposity (Whitmer et al. 2008). The visceral adipose is a metabolically active endocrine tissue secreting several inflammatory cytokines and hormones collectively known as ‘adipokines’ (see ‘Endocrine dysfunction in ageing and obesity’ section for more details). Some adipokines such as leptin and IL‐6 are associated with greater cognitive decline (Yaffe et al. 2003). High amounts of adipokines and pro‐inflammatory factors released by adipocytes, e.g. IL‐6 and TNF‐α, are advocated in the potential link between obesity and dementia, through a toxic effect at the brain level, i.e. impairments in neurogenesis, synaptic plasticity, memory and learning processes (Gustafson, 2010; Arnoldussen et al. 2014; Kiliaan et al. 2014). Leptin crosses the blood–brain barrier and plays a role in neurodegeneration (Funahashi et al. 2003; Harvey, 2003) and could be implicated in the amyloid‐β (Aβ) deposition (Fewlass et al. 2004), the main component of the senile plaques, not only in Alzheimer's disease but also in the cognitive decline that is commonly associated with ageing. Evidence has shown that obese middle‐aged adults have decreased brain volume compared with normal weight individuals (Ward et al. 2005), whereas high central obesity in elderly is associated with decreased hippocampal brain volume and greater brain atrophy (Jagust et al. 2005).
Other obesity‐related alterations, especially hypertension and type 2 diabetes, promote cognitive dysfunction (Klein & Waxman, 2003; Craft & Watson, 2004; Stranahan, 2015). Although there is controversy, blood pressure in late life has been related to cognitive decline and dementia (Kivipelto et al. 2001; Whitmer et al. 2005 b). Hypertension increases the risk of Alzheimer's disease through an effect on the vascular integrity of the blood–brain barrier (Kalaria, 2010). The resultant protein extravasation into the brain tissue may produce cell damage, impaired neuronal or synaptic function, apoptosis and an increase in Aβ deposition leading to cognitive alterations (Deane et al. 2004). Type 2 diabetes has been found to double the risk of Alzheimer's disease (Leibson et al. 1997; Luchsinger et al. 2001). Although the biological mechanisms are unclear, dyslipidaemia and hyperinsulinaemia can be also associated with higher risk of dementia. Insulin in the brain increases Aβ accumulation and tau protein hyperphosphorylation (Park, 2001). In effect, peripheral insulin infusion in elderly has been demonstrated to increase Aβ levels in the cerebrospinal fluid (Watson et al. 2003). Finally, a decrease in the brain‐derived neurotrophic factor (BDNF) levels has been extensively associated with cognitive dysfunction and dementia (Phillips et al. 1991; Holsinger et al. 2000; Yamada et al. 2002; Binder & Scharfman, 2004; Komulainen et al. 2008; Cunha et al. 2010; Autry & Monteggia, 2012; Weinstein et al. 2014). In this regard, as reviewed by Vaynman & Gomez‐Pinilla (2006), disorders of energy metabolism such as obesity, hyperglycaemia and insulin insensitivity are associated with diminished BDNF levels in animal models (Lyons et al. 1999; Kernie et al. 2000; Rios et al. 2001). In humans, impaired glucose metabolism is also associated with low levels of BDNF (Krabbe et al. 2007). In addition, a functional loss of one copy of the BDNF gene is associated with severe obesity and impaired cognitive function (Gray et al. 2006).
Skeletal muscle
One of the major problems associated with ageing is sarcopenia (from Greek σάρξ sarx, ‘flesh’ and πενία penia, ‘poverty’), or the loss of muscle mass and function that occurs as we age (Morley et al. 2001). Sarcopenia is characterized by a reduction in the number and size of muscle fibres, and is caused by progressive muscular denervation, reduced quantities and functions of satellite cells, reduced protein synthesis, decline in anabolic hormone levels, increased levels of pro‐inflammatory cytokines, oxidative stress, and physical inactivity (Garatachea et al. 2015). Altered mitochondrial activity is also involved in the ageing decline of muscle function (Johannsen et al. 2012; Peterson et al. 2012; Sanchis‐Gomar & Derbre, 2014; Sanchis‐Gomar et al. 2014) with oxidative damage to mitochondrial DNA increasing with age and affecting its replication and transcription machinery, which in turn, impairs respiratory chain complex proteins (Lopez‐Otin et al. 2013).
There is also accumulating data supporting that the maintenance of muscle mitochondrial function is impaired in obesity and related conditions, i.e. insulin resistance and type 2 diabetes (Jheng et al. 2015). (Of note, ‘deficiency’ of mitochondria in muscle does not cause insulin resistance per se in this tissue; Holloszy, 2013). Data from human (Stuart et al. 2013) and animal research show that increased adipose tissue levels drive fundamental changes in muscle fibre composition, towards a less oxidative phenotype leading to impaired metabolic function (Denies et al. 2014).
Bone tissue
Bone is a heterogeneous tissue made up of various components, whose proportions vary with age, sex and disease states (Boskey & Coleman, 2010). Bone remodelling occurs constantly and simultaneously in several parts of the skeleton and thus the physiological energy demands of the skeleton are notable (Confavreux et al. 2009). Several energy‐associated hormones (notably insulin, leptin, adiponectin and adrenaline/noradrenaline) are involved in the fine regulation of bone turnover in response to energy availability or needs (Lombardi et al. 2016). In turn, bone regulates energy metabolism by communicating its energetic needs based on loading by releasing osteocalcin (in both its carboxylated and undercarboxylated forms), which, among other functions, acts as a true hormone modulating glucose and energy metabolism (Lombardi et al. 2015). The relationship between bone and energy metabolisms is reflected by the fact that metabolic dysfunctions, including metabolic syndrome, diabetes and obesity, are frequently associated with osteoporosis (Confavreux et al. 2009).
Since adipocytes and osteoblasts are derived from a common mesenchymal stem cell precursor, molecules that lead to osteoblastogenesis inhibit adipogenesis and vice versa. Two examples of molecules that regulate adipocyte and osteoblast differentiation are PPAR‐γ and the wingless‐type MMTV integration site (Wnt) (Colaianni et al. 2014). In turn, sclerostin, the product of the SOST gene, is a secreted glycoprotein antagonist of Wnt through blockage of Wnt/β‐catenin signalling that is responsible for osteoprogenitor expansion and reduced apoptosis rate in mature osteoblasts. Inactivating mutations of the SOST gene cause disorders associated with high bone mass (Li et al. 2008) whereas sclerostin concentrations directly correlate with age, BMI and bone mineral content and negatively with bone formation markers (Schwab & Scalapino, 2011; Cheung & Giangregorio, 2012).
Overall, both men and women lose bone mass as they age, a process called osteoporosis, due to reductions in the levels of several hormones such as mainly sex hormones (androgens, oestrogens) and insulin‐like growth factor (IGF)‐1, as well as to an imbalance between proteins involved in bone turnover like osteoprotegerin and receptor activated NF‐κB ligand (RANK) (Banu, 2013). With ageing, the composition of bone marrow shifts to favour the presence of adipocytes, which further increases the risk of fracture in the aged population (Wehrli et al. 2000). In addition, osteoclast activity increases while osteoblast function declines, resulting in osteoporosis (Rosen & Bouxsein, 2006).
Although obesity has been traditionally thought to be beneficial to bone health thereby protecting against osteoporosis owing to the positive effect on bone formation conferred by mechanical loading imposed by weight bearing (Cao, 2011), this belief has recently been questioned (Migliaccio et al. 2014). A high proportion of fractures among postmenopausal women occur in those who are obese (Compston, 2015), and high‐fat mass might be a risk factor for osteoporosis and fragility fractures (Migliaccio et al. 2014). There is growing evidence of a cross‐talk between adipose tissue, muscle and bone, with different components such as myokines and adipocytokines released by muscle and fat tissue, respectively, regulating skeletal health and thus being involved in the risk of developing osteoporosis (Migliaccio et al. 2014). Further, several cardiometabolic phenotypes as well as body fat are correlated with bone turnover markers and bone mineral density (Nava‐Gonzalez et al. 2014).
Inflammation might be a main link explaining loss of bone mass in both conditions, ageing and obesity. Indeed, there seems to exist a vicious cycle in which inflammation induces adipogenesis and increased adiposity induces inflammation: the net result is bone loss (osteopenia) and, possibly, muscle loss (sarcopenia) (Tchkonia et al. 2010). In this view, osteopenia, sarcopenia and obesity, either combined or alone, appear as different presentations of the same pathological condition, i.e. a pro‐inflammatory state (Ilich et al. 2014; Ormsbee et al. 2014).
Kidney
Ageing is associated with structural and functional renal changes (Zhou et al. 2008). The normal kidney loses ∼20–25% of its mass during ageing (McLachlan & Wasserman, 1981), with this phenomenon affecting glomerular, tubular and endocrine functions. In turn, there is a rapidly increasing prevalence of overweight/obese patients with chronic kidney disease (CKD) (Flegal et al. 2002), and obesity is emerging as an independent risk factor for CKD, starting in childhood (Ding et al. 2015). Obesity is associated with glomerular hyperfiltration and hypertension (Ding et al. 2015) and obesity‐related glomerulopathy is characterized by moderate proteinuria, minimal oedema, lower serum cholesterol and higher serum albumin (Srivastava, 2006). In brief, the main pathways involved in the association between obesity/metabolic syndrome and increased progression of CKD are proteinuria due to obesity‐related glomerulopathy, hypertension due to decreased nitric oxide production, albuminuria and renal cytotoxicity caused by insulin resistance, increased levels of pro‐inflammatory cytokines, and higher RAAS activity (Ding et al. 2015).
Stem cell populations
The decline in the regenerative potential of tissues due to functional attrition of stem cells is one of the major hallmarks of ageing (Lopez‐Otin et al. 2013). Stem cell exhaustion affects virtually all adult stem cell compartments (Lopez‐Otin et al. 2013), including adipose‐derived mesenchymal stem cells (ASCs) (Beane et al. 2014). Mechanisms involved in age‐related stem cell loss and dysfunction entail factors inside stem cells, such as accumulation of ROS, aggregates of damaged proteins, mitochondrial dysfunction, epigenetic alterations (Oh et al. 2014), DNA damage (Rossi et al. 2008) and overexpression of cell‐cycle inhibitory proteins such as p16INK4a (also known as cyclin‐dependent kinase inhibitor 2A, multiple tumour suppressor 1) (Janzen et al. 2006), e.g. telomere shortening in haematopoietic stem cells (Flores et al. 2005; Sharpless & DePinho, 2007); or factors that affect the interaction between stem cells and their niche such as exhaustion of supportive cells, certain circulatory factors (Wnt, chemokine (C‐C motif) ligand 1 (CCL11), oxytocin), chronic inflammation (Oh et al. 2014) and increase in fibroblast growth factor 2 (FGF2) (Chakkalakal et al. 2012).
Obesity also has a negative impact on adult stem cell properties, particularly ASCs (Perez et al. 2015). Oñate et al. showed a reduced ASC reservoir, impaired adipogenic and angiogenic differentiation, and up‐regulated inflammatory genes in ASCs of obese subjects (Onate et al. 2012, 2013). Metabolic analysis has demonstrated that both mitochondrial content and function are also impaired in obese‐derived ASCs (Perez et al. 2015). Of note, ASCs, particularly in the subcutaneous adipose tissue, play an important homeostatic/defensive role aiming to reduce tissue damage, particularly when exposed to an inflammatory milieu, by increasing their cytokine secretion and increasing dedifferentiation (in an attempt to create a new ACS reservoir) and migration processes (Shoshani & Zipori, 2015). In contrast, obesity‐induced inflammatory cytokine secretion by non‐healthy ASCs reflects a failure to evade stress (Baptista et al. 2015). Thus, another common feature of obesity and ageing is the shift of ASCs in the subcutaneous adipose tissue towards a non‐healthy pro‐inflammatory phenotype, which ultimately exacerbates the systemic chronic inflammation that characterizes both conditions.
Endocrine dysfunction in ageing and obesity
The adipose tissue acts as an endocrine organ, by virtue of releasing a variety of bioactive peptides, the so‐called ‘adipocytokines’ (or ‘adipokines’), which act at local and systemic levels (Kershaw & Flier, 2004). It produces, among others, adiponectin (the most abundant adipokine, which has an anti‐inflammatory function and increases insulin sensitivity, fatty acid oxidation and energy expenditure, whilst it reduces the production of glucose by the liver), leptin (which regulates whole‐body metabolism by stimulating energy expenditure, restraining food intake and maintaining normal glycaemia), complement components, plasminogen activator inhibitor‐1, proteins of the RAAS and resistin, and also activates other hormones secreted elsewhere such as glucocorticoids or sex steroids (Tilg & Moschen, 2006; Ouchi et al. 2011).
Progressive deregulation of the endocrine nutrient‐sensing system, which comprises the growth hormone, and the IGF‐1 and insulin signalling pathway, is a major characteristic of the normal ageing process in mammals (Lopez‐Otin et al. 2013) and is also associated with leptin resistance (Sasaki, 2015). Increased adiponectin levels can be a distinctive feature of some of the most long‐lived individuals (centenarians) but are also associated with mortality in younger old people and CVD patients (Bik & Baranowska, 2009; Gulcelik et al. 2013). Less controversial are the data obtained in obese people, with adiponectin likely to be the only adipokine whose production really decreases with obesity (Letra et al. 2014; Poonpet & Honsawek, 2014). Although leptin increases with adiposity, its biological effects are limited by leptin resistance in the vast majority of obesity cases (Galic et al. 2010). The effects of ageing or obesity on other adipokines like resistin or retinol binding protein‐4 are less described, but their expression levels seem to be positively correlated with adiposity, being implicated in insulin resistance processes (Galic et al. 2010).
Immune dysfunction and its link with inflammation
An important element for the secretory function of adipose tissue is macrophages (Galic et al. 2010). These cells are a major source of inflammatory cytokines, such as TNF‐α, IL‐6 and IL‐1β, which contribute to the chronic low‐grade inflammatory state that is associated with both ageing and obesity (Galic et al. 2010). Ageing is linked with immune senescence (Gruver et al. 2007), notably with T‐lymphocyte dysfunction (Salam et al. 2013), a phenomenon that also leads to systemic increases in TNF‐α and IL‐6, and acute phase proteins such as C‐reactive protein and serum amyloid A (Bruunsgaard & Pedersen, 2003). In addition, multiple complex mechanisms contribute to the interplay between age‐related inflammation and immune senescence. The so‐called ‘redox stress hypothesis’ postulates that the functional losses associated with ageing are mainly caused by a cellular pro‐oxidizing status, which leads to disruption of the redox‐regulated signalling mechanisms (Sohal & Orr, 2012). Hence, the age‐related redox imbalance would activate numerous pro‐inflammatory signalling pathways, including those dependent on NF‐κB, thereby leading to major ageing conditions such as ‘inflammageing’ of tissues, including the adipose tissue, and immune deregulation (Chung et al. 2009).
Obesity is also linked with conditions associated with immune dysfunction, such as increased susceptibility to infection or bacteraemia (Matarese et al. 2005). Similarly to ageing, T‐lymphocyte subpopulations and their functions are impaired in obese people (Tanaka et al. 2001). Yet these immune abnormalities are reversed with energy restriction (with subsequent decreases in leptin levels) in both humans and animals (Lamas et al. 2004). The low‐grade chronic inflammation of the adipose tissue that characterizes excess fat storage leads to ‘stress reactions’ within its adipocytes and immune cells, with a subsequent release of pro‐inflammatory factors from both sources (Ghigliotti et al. 2014). Stress reactions are mainly oxidative stress, and cellular and organelle hypertrophy (Monteiro & Azevedo, 2010). Regarding the latter, the metabolic stress to which adipose tissue is subjected in obesity results in organelle dysfunction, particularly of the endoplasmic reticulum (ER), which is the organelle where triacylglycerol droplet formation takes places and which participates in the regulation of lipid, glucose, cholesterol and protein metabolism (Gregor & Hotamisligil, 2007). The adipocyte may be particularly challenged by excess nutrient intake, because it is forced to secrete large amounts of substances and synthesize lipids. Under such conditions, the ER function may be impaired, leading to the accumulation of misfolded or unfolded proteins in its lumen (Monteiro & Azevedo, 2010). In order to cope with it, the stressed ER engages the unfolded protein response which, if not relieved, may induce cell death via apoptosis (Mori, 2000; Zhao & Ackerman, 2006; Monteiro & Azevedo, 2010). As in other metabolically active tissues undergoing increased demand, there is usually relative hypoxia together with the increased need for nutrient oxidation, which results in unusually high amounts of ROS, activating in turn kinases like JUN N‐terminal kinase 1 (JNK1), JNK, p38 mitogen‐activated protein kinase (MAPK) or inhibitor of NFκB kinase (IKK), thereby interfering with insulin signalling either directly or indirectly (via induction of NFκB and increased cytokine production) (Qatanani & Lazar, 2007).
Obesity also induces accumulation of macrophages in the adipose tissue, which further increases the secretion of pro‐inflammatory mediators (Weisberg et al. 2003; Xu et al. 2003), with insulin resistance promoting macrophage activation through NF‐κB or activator protein‐1 (AP‐1) signalling (Olefsky & Glass, 2010). Finally, the pro‐inflammatory cytokines (TNF‐α, IL‐1β and IL‐6) secreted by the macrophages accumulated in the ‘obese’ adipose tissue also stimulate adipocytes to further secrete leptin and pro‐inflammatory cytokines such as TNF‐α (Mantzoros et al. 1997; Papathanassoglou et al. 2001). Of note, although IL‐6 has been traditionally considered as a pro‐inflammatory cytokine, its link with obesity‐associated inflammation is more controversial, with recent mechanistic research actually indicating an unexpected anti‐inflammatory role of IL‐6, which limits pro‐inflammatory gene expression and augments IL‐4 responsiveness in macrophages, thereby attenuating the typical shift of macrophage populations towards a pro‐inflammatory (M1) phenotype (Mauer et al. 2014).
Epigenetic alterations in ageing and obesity
Epigenetic modifications are heritable changes, such as DNA methylation, post‐translational modification of histones, chromatin remodelling or noncoding RNA expression that occur over life and affect gene expression without actually changing the DNA sequence (Holliday & Pugh, 1975; Wolffe & Guschin, 2000). Many of these epigenetic changes are necessary for normal cellular development and differentiation, involving stem cells, but abnormalities may also occur due to inappropriate epigenetic signalling (Tollervey & Lunyak, 2011, 2012). Epigenetic changes are induced by physiological and pathological conditions as well as environmental (Aguilera et al. 2010; Ling & Ronn, 2014; Pareja‐Galeano et al. 2014) or nutritional‐related factors, e.g. the phytochemicals resveratrol and curcumin act as epigenetic modifiers that can potentially delay ageing (Huffman, 2012; Martin et al. 2013). The systems in charge of generation and maintenance of epigenetic patterns include DNA methyltransferases, histone acetylases, deacetylases, methylases and demethylases, and the protein complex involved in chromatin remodelling (Lopez‐Otin et al. 2013; Sanchis‐Gomar et al. 2014).
Major age‐induced epigenetic marks are increased histone H4K16 acetylation, H4K20/H3K4/H3K27 trimethylation and decreased H3K9 methylation (Fraga & Esteller, 2007; Han & Brunet, 2012). Ageing is also accompanied by a dramatic change in the distribution of 5‐methylcytosine across the genome, resulting in a decrease in global DNA methylation (Li et al. 2011). Epigenetic deregulation with age is tissue dependent, e.g. animal research suggests significant differences in DNA methylation with age in the liver and visceral adipose tissue (Thompson et al. 2010). In the adipose tissue, senescence is associated with chromatin dysregulation (Stransky et al. 2012). Other epigenetic alterations in the aged adipose tissue involve RNA splicing, mRNA metabolism, and plasma membrane and mitochondrial metabolism, and differ between adipocytes and stromal vascular fractions (Stransky et al. 2012).
Epigenetic alterations are also involved in obesity. A high BMI relates to accelerated DNA methylation in a tissue‐specific manner; thus, obese individuals show increased epigenetic age of liver (Horvath et al. 2014). The subcutaneous adipose tissue of obese women is characterized by changes in DNA methylation and expression of genes linked to generation, distribution and metabolic function of fat cells (Arner et al. 2015). Differential methylation of the gene (LPL) encoding lipoprotein lipase (which hydrolyses circulating triglyceride‐rich lipoproteins with subsequent fatty acid uptake into the adipose tissue) might be linked to obesity and regional fat distribution (Drogan et al. 2015). Finally, obesity‐induced inflammation induces a specific miRNA pattern in adipocytes and macrophages (Ortega et al. 2015). Further research might elucidate common epigenetic signatures of ageing and obesity, especially in genes modulating adipose tissue function.
Additional mechanistic evidence in support of the link between ageing and obesity: implications of caloric restriction and loss of adiposity in the ageing process
Cigarette smoking was the major risk for environmentally related death in the United States at the end of the 20th century whereas it is now the epidemic of obesity, suggesting that calorie intake contributes to human ageing and lifespan (Barzilai & Bartke, 2009). Indeed, obesity leads to reduced lifespan and clinical consequences similar to those common in ageing (Ahima, 2009; Tchkonia et al. 2010), whilst caloric restriction has an opposite effect, reducing ageing and improving glucose homeostasis. As reviewed by Barzilai & Bartke (2009), (i) caloric restriction experiments in rodents have proven reliable in showing an overall dose–response benefit on lifespan; (ii) such an effect has been corroborated in other mammalian species including dogs and rabbits, and preliminary results in rhesus monkeys would also indicate that this intervention can increase longevity; and (iii) calorie‐restricted animals seem robust until a late age, that is, they have not only a longer lifespan but also a longer ‘health span’, and the most consistent physiological effects of caloric restriction are reduced body weight and temperature. Importantly, a main effect of a lifespan‐extending intervention such as caloric restriction is reduction of visceral fat (Barzilai & Gupta, 1999 b; Masoro, 2006). Further, lifespan is also extended: (i) in fat cell insulin receptor, insulin receptor substrate‐1 and S6 kinase‐1 deficient (knockout) mice (each of which has limited fat development; Bluher et al. 2003; Um et al. 2004; Selman et al. 2008; Selman et al. 2009); (ii) in growth hormone receptor knockout (GHRKO) mice (which have delayed increase in the ratio of visceral to subcutaneous and reduced fat cell progenitor turnover; Berryman et al. 2008); (iii) with rapamycin treatment (which limits fat tissue development; Chang et al. 2009; Harrison et al. 2009); and (iv) after surgical removal of visceral fat in rats (Muzumdar et al. 2008).
In humans, overall and abdominal obesity are associated with greater mortality risk in adults aged < 65 years and the association seems stronger with measures of abdominal obesity than with measures of overall obesity or fat‐free mass (Kuk & Ardern, 2009 b). In 116,564 middle‐aged women (30–55 years) free of known CVD and cancer, even a modest weight gain during adulthood, independent of physical activity, was associated with a higher risk of death (Hu et al. 2004). Finally, in obese adults, intentional weight loss may be associated with an ∼15% reduction in all‐cause mortality as shown in a recent meta‐analysis of 15 randomized controlled trials that included a total of 17,186 participants (53% female) with an average age of 52 years at baseline (Kritchevsky et al. 2015).
Conclusions
The last decades have been exciting for clinicians and researchers interested in understanding the broader health consequences of excess adiposity. There is now consistent evidence that obesity, a worldwide health concern, may be linked not only to a number of age‐related disorders, but also to ageing itself. However, our knowledge of the molecular mechanisms through which obesity may promote accelerated senescence remains only partial. Evidence indicates that accumulation of a dysfunctional adipose tissue promotes SIRT1 hypo‐expression, inflammation and epigenetic patterns, among other alterations, which might explain a mechanistic link between ageing and obesity (see Fig. 2 for a summary of the potential mechanistic links between obesity and ageing). From the point of view of basic research, the development of high‐throughput technologies will allow the collection of large amounts of data concerning the commonalities and dissimilarities between the pathophysiological underpinnings of obesity and ageing. From an epidemiological perspective, the potential links between obesity and ageing under the new ‘adipaging’ framework (postulating a common soil for the two conditions) should prompt future studies aimed at investigating whether interventions that may reduce the burden of obesity may also promote ‘well‐ageing’ at the population level.
Figure 2. Schematic representation of potential mechanisms explaining the link between obesity and ageing .
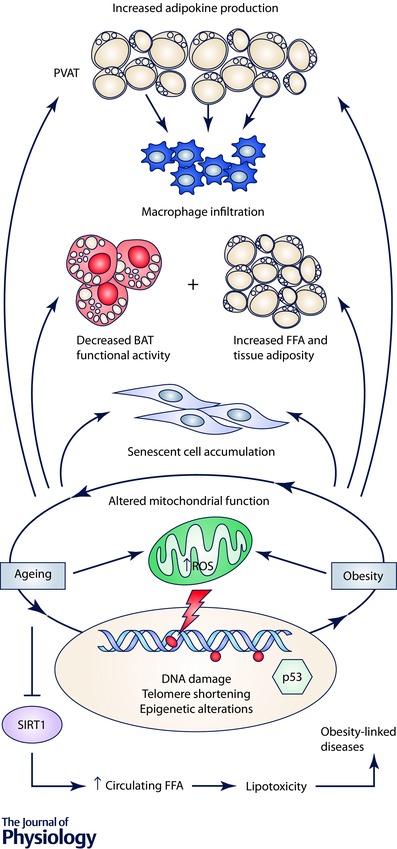
Abbreviations: BAT, brown adipose tissue; FFA, free fatty acids; PVAT, perivascular adipose tissue; ROS, reactive oxygen species; SIRT1, sirtuin 1. Symbols: →, stimulation; ⊥, inhibition.
Additional information
Conflict of interest
The authors declare no conflict of interest.
Author contributions
All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This study was supported by grants from the Spanish Ministry of Science and Innovation (SAF 2010–15239 and SAF 2015–67911) to BGG. The CNIC is supported by the Spanish Ministry of Science and Innovation and the Pro‐CNIC Foundation. L.M.P. is supported by FPI fellowships from the Spanish Ministry of Science and Innovation. Research in the field of ageing by Alejandro Lucia is funded by Fondo de Investigaciones Sanitarias (grant number PI12/00914) and Fondos Feder.
Biographies
Laura M. Pérez, PhD, is a postdoctoral researcher at the Spanish National Center for Cardiovascular Research (‘CNIC’, Madrid). Her research addresses the obesity effects in adipose‐derived mesenchymal stem cells (ASCs), and the metabolic responses and therapeutic properties of these cells.
![]()
Helios Pareja‐Galeano, PhD, is an assistant professor and postdoctoral researcher at the European University and Research Institute of the ‘Hospital 12 de Octubre’ (‘i+12’) (Madrid, Spain). His research field is focused on the molecular pathways by which physical exercise impacts on different conditions.
Fabián Sanchis Gomar, MD, PhD, is a post‐doctoral researcher at the ‘i+12’ Research Institute who is interested and actively involved in several biomedical research topics. His current main interest is cardiovascular research, particularly with regard to long‐term exercise.
Enzo Emanuele, MD, PhD, is the CEO at Bioenx srl, an Italy‐based biotechnology company. He is also the owner and scientific director of 2E Science, a private scientific consulting firm. His main research interests are biological markers of ageing and disease conditions, genetics, and clinical trials.
Alejandro Lucia, MD, PhD, is a professor in Exercise Physiology and senior researcher at the European University and ‘i+12’. His main research interests are exercise effects in disease conditions, as well as human longevity.
Beatriz G. Gálvez, PhD, is the Director for the Research Center of Health, Sports and Life Science at the the European University (Madrid, Spain). She is a researcher in the field of cell therapies in the area of muscular and cardiac regeneration as well as in obesity and metabolic diseases.
References
- Aguilera O, Fernandez AF, Munoz A & Fraga MF (2010). Epigenetics and environment: a complex relationship. J Appl Physiol (1985) 109, 243–251. [DOI] [PubMed] [Google Scholar]
- Aguirre L, Napoli N, Waters D, Qualls C, Villareal DT & Armamento‐Villareal R (2014). Increasing adiposity is associated with higher adipokine levels and lower bone mineral density in obese older adults. J Clin Endocrinol Metab 99, 3290–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS (2009). Connecting obesity, aging and diabetes. Nat Med 15, 996–997. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Saper CB, Flier JS & Elmquist JK (2000). Leptin regulation of neuroendocrine systems. Front Neuroendocrinol 21, 263–307. [DOI] [PubMed] [Google Scholar]
- Alves MD, Brites C & Sprinz E (2014). HIV‐associated lipodystrophy: a review from a Brazilian perspective. Ther Clin Risk Manag 10, 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EK, Gutierrez DA & Hasty AH (2010). Adipose tissue recruitment of leukocytes. Curr Opin Lipidol 21, 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner P, Sinha I, Thorell A, Ryden M, Dahlman‐Wright K & Dahlman I (2015). The epigenetic signature of subcutaneous fat cells is linked to altered expression of genes implicated in lipid metabolism in obese women. Clin Epigenetics 7, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoldussen IA, Kiliaan AJ & Gustafson DR (2014). Obesity and dementia: adipokines interact with the brain. Eur Neuropsychopharmacol 24, 1982–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE & Monteggia LM (2012). Brain‐derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev 64, 238–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banu J (2013). Causes, consequences, and treatment of osteoporosis in men. Drug Des Devel Ther 7, 849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista LS, Silva KR & Borojevic R (2015). Obesity and weight loss could alter the properties of adipose stem cells? World J Stem Cells 7, 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barness LA, Opitz JM & Gilbert‐Barness E (2007). Obesity: genetic, molecular, and environmental aspects. Am J Med Genet A 143A, 3016–3034. [DOI] [PubMed] [Google Scholar]
- Baron AD, Laakso M, Brechtel G, Hoit B, Watt C & Edelman SV (1990). Reduced postprandial skeletal muscle blood flow contributes to glucose intolerance in human obesity. J Clin Endocrinol Metab 70, 1525–1533. [DOI] [PubMed] [Google Scholar]
- Barzilai N & Bartke A (2009). Biological approaches to mechanistically understand the healthy life span extension achieved by calorie restriction and modulation of hormones. J Gerontol A Biol Sci Med Sci 64, 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N & Gupta G (1999. a). Interaction between aging and syndrome X: new insights on the pathophysiology of fat distribution. Ann N Y Acad Sci 892, 58–72. [DOI] [PubMed] [Google Scholar]
- Barzilai N & Gupta G (1999. b). Revisiting the role of fat mass in the life extension induced by caloric restriction. J Gerontol A Biol Sci Med Sci 54, B89–B96; discussion B97–B98. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Huffman DM, Muzumdar RH & Bartke A (2012). The critical role of metabolic pathways in aging. Diabetes 61, 1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Breda E, Oberg AL, Powell CC, Dalla Man C, Basu A, Vittone JL, Klee GG, Arora P, Jensen MD, Toffolo G, Cobelli C & Rizza RA (2003). Mechanisms of the age‐associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes 52, 1738–1748. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez‐Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R & Sinclair DA (2006). Resveratrol improves health and survival of mice on a high‐calorie diet. Nature 444, 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beane OS, Fonseca VC, Cooper LL, Koren G & Darling EM (2014). Impact of aging on the regenerative properties of bone marrow‐, muscle‐, and adipose‐derived mesenchymal stem/stromal cells. PLoS One 9, e115963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JA, Kivimaki M & Hamer M (2014). Metabolically healthy obesity and risk of incident type 2 diabetes: a meta‐analysis of prospective cohort studies. Obes Rev 15, 504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DC, Stenesen D, Zeve D & Graff JM (2013). The developmental origins of adipose tissue. Development 140, 3939–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryman DE, Christiansen JS, Johannsson G, Thorner MO & Kopchick JJ (2008). Role of the GH/IGF‐1 axis in lifespan and healthspan: lessons from animal models. Growth Horm IGF Res 18, 455–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M, Dominguez LJ & Barbagallo M (2009). Insulin resistance and the cardiometabolic syndrome in HIV infection. J Cardiometab Syndr 4, 40–43. [DOI] [PubMed] [Google Scholar]
- Bik W & Baranowska B (2009). Adiponectin – a predictor of higher mortality in cardiovascular disease or a factor contributing to longer life? Neuro Endocrinol Lett 30, 180–184. [PubMed] [Google Scholar]
- Binder DK & Scharfman HE (2004). Brain‐derived neurotrophic factor. Growth Factors 22, 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluher M, Kahn BB & Kahn CR (2003). Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 299, 572–574. [DOI] [PubMed] [Google Scholar]
- Boskey AL & Coleman R (2010). Aging and bone. J Dent Res 89, 1333–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE & Kuk JL (2015). Consequences of obesity and weight loss: a devil's advocate position. Obes Rev 16, 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner EJ, Shipley MJ, Ahmadi‐Abhari S, Tabak AG, McEniery CM, Wilkinson IB, Marmot MG, Singh‐Manoux A & Kivimaki M (2015). Adiposity, obesity, and arterial aging: longitudinal study of aortic stiffness in the Whitehall II cohort. Hypertension 66, 294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruunsgaard H & Pedersen BK (2003). Age‐related inflammatory cytokines and disease. Immunol Allergy Clin North Am 23, 15–39. [DOI] [PubMed] [Google Scholar]
- Burns A & Iliffe S (2009). Dementia. BMJ 338, b75. [DOI] [PubMed] [Google Scholar]
- Calori G, Lattuada G, Piemonti L, Garancini MP, Ragogna F, Villa M, Mannino S, Crosignani P, Bosi E, Luzi L, Ruotolo G & Perseghin G (2011). Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: the Cremona Study. Diabetes Care 34, 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camhi SM & Katzmarzyk PT (2014). Differences in body composition between metabolically healthy obese and metabolically abnormal obese adults. Int J Obes (Lond) 38, 1142–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning KL, Brown RE, Jamnik VK & Kuk JL (2014). Relationship between obesity and obesity‐related morbidities weakens with aging. J Gerontol A Biol Sci Med Sci 69, 87–92. [DOI] [PubMed] [Google Scholar]
- Canto C, Gerhart‐Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P & Auwerx J (2009). AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458, 1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JJ (2011). Effects of obesity on bone metabolism. J Orthop Surg Res 6, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ & Cooper DA (1998). A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. Aids 12, F51–F58. [DOI] [PubMed] [Google Scholar]
- Chakkalakal JV, Jones KM, Basson MA & Brack AS (2012). The aged niche disrupts muscle stem cell quiescence. Nature 490, 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkiadaki A & Guarente L (2012). High‐fat diet triggers inflammation‐induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab 16, 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GR, Chiu YS, Wu YY, Chen WY, Liao JW, Chao TH & Mao FC (2009). Rapamycin protects against high fat diet‐induced obesity in C57BL/6 J mice. J Pharmacol Sci 109, 496–503. [DOI] [PubMed] [Google Scholar]
- Chang HC & Guarente L (2013). SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 153, 1448–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Kim BK, Yun KE, Cho J, Zhang Y, Rampal S, Zhao D, Jung HS, Choi Y, Ahn J, Lima JA, Shin H, Guallar E & Ryu S (2014). Metabolically‐healthy obesity and coronary artery calcification. J Am Coll Cardiol 63, 2679–2686. [DOI] [PubMed] [Google Scholar]
- Chau YY, Bandiera R, Serrels A, Martinez‐Estrada OM, Qing W, Lee M, Slight J, Thornburn A, Berry R, McHaffie S, Stimson RH, Walker BR, Chapuli RM, Schedl A & Hastie N (2014). Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol 16, 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SC & Tseng CH (2013). Dyslipidemia, kidney disease, and cardiovascular disease in diabetic patients. Rev Diabet Stud 10, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AM & Giangregorio L (2012). Mechanical stimuli and bone health: what is the evidence? Curr Opin Rheumatol 24, 561–566. [DOI] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP & Leeuwenburgh C (2009). Molecular inflammation: underpinnings of aging and age‐related diseases. Ageing Res Rev 8, 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaianni G, Brunetti G, Faienza MF, Colucci S & Grano M (2014). Osteoporosis and obesity: Role of Wnt pathway in human and murine models. World J Orthop 5, 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston J (2015). Obesity and fractures in postmenopausal women. Curr Opin Rheumatol 27, 414–419. [DOI] [PubMed] [Google Scholar]
- Confavreux CB, Levine RL & Karsenty G (2009). A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Mol Cell Endocrinol 310, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S & Watson GS (2004). Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol 3, 169–178. [DOI] [PubMed] [Google Scholar]
- Cunha C, Brambilla R & Thomas KL (2010). A simple role for BDNF in learning and memory? Front Mol Neurosci 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalzill C, Nigam A, Juneau M, Guilbeault V, Latour E, Mauriege P & Gayda M (2014). Intensive lifestyle intervention improves cardiometabolic and exercise parameters in metabolically healthy obese and metabolically unhealthy obese individuals. Can J Cardiol 30, 434–440. [DOI] [PubMed] [Google Scholar]
- Das M, Gabriely I & Barzilai N (2004). Caloric restriction, body fat and ageing in experimental models. Obes Rev 5, 13–19. [DOI] [PubMed] [Google Scholar]
- Deane R, Wu Z & Zlokovic BV (2004). RAGE (yin) versus LRP (yang) balance regulates alzheimer amyloid beta‐peptide clearance through transport across the blood‐brain barrier. Stroke 35, 2628–2631. [DOI] [PubMed] [Google Scholar]
- Denies MS, Johnson J, Maliphol AB, Bruno M, Kim A, Rizvi A, Rustici K & Medler S (2014). Diet‐induced obesity alters skeletal muscle fiber types of male but not female mice. Physiol Rep 2, e00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Toni F, Poglio S, Youcef AB, Cousin B, Pflumio F, Bourin P, Casteilla L & Laharrague P (2011). Human adipose‐derived stromal cells efficiently support hematopoiesis in vitro and in vivo: a key step for therapeutic studies. Stem Cells Dev 20, 2127–2138. [DOI] [PubMed] [Google Scholar]
- Ding W, Cheung WW & Mak RH (2015). Impact of obesity on kidney function and blood pressure in children. World J Nephrol 4, 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Henson GD, Hart CR, Layec G, Trinity JD, Bramwell RC, Enz RA, Morgan RG, Reihl KD, Hazra S, Walker AE, Richardson RS & Lesniewski LA (2014). The impact of ageing on adipose structure, function and vasculature in the B6D2F1 mouse: evidence of significant multisystem dysfunction. J Physiol 592, 4083–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner TE & Rieder A (2012). Obesity paradox in elderly patients with cardiovascular diseases. Int J Cardiol 155, 56–65. [DOI] [PubMed] [Google Scholar]
- Drogan D, Boeing H, Janke J, Schmitt B, Zhou Y, Walter J, Pischon T & Tierling S (2015). Regional distribution of body fat in relation to DNA methylation within the LPL, ADIPOQ and PPARgamma promoters in subcutaneous adipose tissue. Nutr Diabetes 5, e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgazar‐Carmon V, Rudich A, Hadad N & Levy R (2008). Neutrophils transiently infiltrate intra‐abdominal fat early in the course of high‐fat feeding. J Lipid Res 49, 1894–1903. [DOI] [PubMed] [Google Scholar]
- Emmerzaal TL, Kiliaan AJ & Gustafson DR (2015). 2003–2013: a decade of body mass index, Alzheimer's disease, and dementia. J Alzheimers Dis 43, 739–755. [DOI] [PubMed] [Google Scholar]
- Enzi G, Gasparo M, Biondetti PR, Fiore D, Semisa M & Zurlo F (1986). Subcutaneous and visceral fat distribution according to sex, age, and overweight, evaluated by computed tomography. Am J Clin Nutr 44, 739–746. [DOI] [PubMed] [Google Scholar]
- Eto H, Ishimine H, Kinoshita K, Watanabe‐Susaki K, Kato H, Doi K, Kuno S, Kurisaki A & Yoshimura K (2013). Characterization of human adipose tissue‐resident hematopoietic cell populations reveals a novel macrophage subpopulation with CD34 expression and mesenchymal multipotency. Stem Cells Dev 22, 985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewlass DC, Noboa K, Pi‐Sunyer FX, Johnston JM, Yan SD & Tezapsidis N (2004). Obesity‐related leptin regulates Alzheimer's Aβ. FASEB J 18, 1870–1878. [DOI] [PubMed] [Google Scholar]
- Finkelstein JL, Gala P, Rochford R, Glesby MJ & Mehta S (2015). HIV/AIDS and lipodystrophy: implications for clinical management in resource‐limited settings. J Int AIDS Soc 18, 19033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O'Meara ES, Longstreth WT Jr & Luchsinger JA (2009). Midlife and late‐life obesity and the risk of dementia: cardiovascular health study. Arch Neurol 66, 336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiuza‐Luces C, Garatachea N, Berger NA & Lucia A (2013). Exercise is the real polypill. Physiology (Bethesda) 28, 330–358. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL & Johnson CL (2002). Prevalence and trends in obesity among US adults, 1999–2000. JAMA 288, 1723–1727. [DOI] [PubMed] [Google Scholar]
- Flores I, Cayuela ML & Blasco MA (2005). Effects of telomerase and telomere length on epidermal stem cell behavior. Science 309, 1253–1256. [DOI] [PubMed] [Google Scholar]
- Fraga MF & Esteller M (2007). Epigenetics and aging: the targets and the marks. Trends Genet 23, 413–418. [DOI] [PubMed] [Google Scholar]
- Funahashi H, Yada T, Suzuki R & Shioda S (2003). Distribution, function, and properties of leptin receptors in the brain. Int Rev Cytol 224, 1–27. [DOI] [PubMed] [Google Scholar]
- Galic S, Oakhill JS & Steinberg GR (2010). Adipose tissue as an endocrine organ. Mol Cell Endocrinol 316, 129–139. [DOI] [PubMed] [Google Scholar]
- Garatachea N, Pareja‐Galeano H, Sanchis‐Gomar F, Santos‐Lozano A, Fiuza‐Luces C, Moran M, Emanuele E, Joyner MJ & Lucia A (2015). Exercise attenuates the major hallmarks of aging. Rejuvenation Res 18, 57–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard GS, Styer AM, Strodel WE, Roesch SL, Yavorek A, Carey DJ, Wood GC, Petrick AT, Gabrielsen J, Ibele A, Benotti P, Rolston DD, Still CD & Argyropoulos G (2014). Gene expression profiling in subcutaneous, visceral and epigastric adipose tissues of patients with extreme obesity. Int J Obes (Lond) 38, 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghigliotti G, Barisione C, Garibaldi S, Fabbi P, Brunelli C, Spallarossa P, Altieri P, Rosa G, Spinella G, Palombo D, Arsenescu R & Arsenescu V (2014). Adipose tissue immune response: novel triggers and consequences for chronic inflammatory conditions. Inflammation 37, 1337–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum MP, Kotas ME, Erion DM, Kursawe R, Chatterjee P, Nead KT, Muise ES, Hsiao JJ, Frederick DW, Yonemitsu S, Banks AS, Qiang L, Bhanot S, Olefsky JM, Sears DD, Caprio S & Shulman GI (2011). SirT1 regulates adipose tissue inflammation. Diabetes 60, 3235–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J, Yeo GS, Cox JJ, Morton J, Adlam AL, Keogh JM, Yanovski JA, El Gharbawy A, Han JC, Tung YC, Hodges JR, Raymond FL, O'Rahilly S & Farooqi IS (2006). Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain‐derived neurotrophic factor (BDNF) gene. Diabetes 55, 3366–3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor MF & Hotamisligil GS (2007). Thematic review series: Adipocyte Biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res 48, 1905–1914. [DOI] [PubMed] [Google Scholar]
- Gruver AL, Hudson LL & Sempowski GD (2007). Immunosenescence of ageing. J Pathol 211, 144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu P & Xu A (2013). Interplay between adipose tissue and blood vessels in obesity and vascular dysfunction. Rev Endocr Metab Disord 14, 49–58. [DOI] [PubMed] [Google Scholar]
- Gulcelik NE, Halil M, Ariogul S & Usman A (2013). Adipocytokines and aging: adiponectin and leptin. Minerva Endocrinol 38, 203–210. [PubMed] [Google Scholar]
- Gustafson D, Rothenberg E, Blennow K, Steen B & Skoog I (2003). An 18‐year follow‐up of overweight and risk of Alzheimer disease. Arch Intern Med 163, 1524–1528. [DOI] [PubMed] [Google Scholar]
- Gustafson DR (2010). Adiposity hormones and dementia. J Neurol Sci 299, 30–34. [DOI] [PubMed] [Google Scholar]
- Gustafson DR, Backman K, Waern M, Ostling S, Guo X, Zandi P, Mielke MM, Bengtsson C & Skoog I (2009). Adiposity indicators and dementia over 32 years in Sweden. Neurology 73, 1559–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainer V & Aldhoon‐Hainerova I (2013). Obesity paradox does exist. Diabetes Care 36 Suppl 2, S276–S281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JE, do Carmo JM, da Silva AA, Wang Z & Hall ME (2015). Obesity‐induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res 116, 991–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M & Stamatakis E (2012). Metabolically healthy obesity and risk of all‐cause and cardiovascular disease mortality. J Clin Endocrinol Metab 97, 2482–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S & Brunet A (2012). Histone methylation makes its mark on longevity. Trends Cell Biol 22, 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E & Miller RA (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J (2003). Novel actions of leptin in the hippocampus. Ann Med 35, 197–206. [DOI] [PubMed] [Google Scholar]
- Hayden KM, Zandi PP, Lyketsos CG, Khachaturian AS, Bastian LA, Charoonruk G, Tschanz JT, Norton MC, Pieper CF, Munger RG, Breitner JC & Welsh‐Bohmer KA (2006). Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Dis Assoc Disord 20, 93–100. [DOI] [PubMed] [Google Scholar]
- Hayes L, Pearce MS, Firbank MJ, Walker M, Taylor R & Unwin NC (2010). Do obese but metabolically normal women differ in intra‐abdominal fat and physical activity levels from those with the expected metabolic abnormalities? A cross‐sectional study. BMC Public Health 10, 723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnouho GM, Czernichow S, Dugravot A, Nabi H, Brunner EJ, Kivimaki M & Singh‐Manoux A (2015). Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: the Whitehall II cohort study. Eur Heart J 36, 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton RB & Yutzey KE (2011). Heart valve structure and function in development and disease. Annu Rev Physiol 73, 29–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirtz D, Thurman DJ, Gwinn‐Hardy K, Mohamed M, Chaudhuri AR & Zalutsky R (2007). How common are the “common” neurologic disorders? Neurology 68, 326–337. [DOI] [PubMed] [Google Scholar]
- Holliday R & Pugh JE (1975). DNA modification mechanisms and gene activity during development. Science 187, 226–232. [PubMed] [Google Scholar]
- Holloszy JO (2013). “Deficiency” of mitochondria in muscle does not cause insulin resistance. Diabetes 62, 1036–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsinger RM, Schnarr J, Henry P, Castelo VT & Fahnestock M (2000). Quantitation of BDNF mRNA in human parietal cortex by competitive reverse transcription‐polymerase chain reaction: decreased levels in Alzheimer's disease. Brain Res Mol Brain Res 76, 347–354. [DOI] [PubMed] [Google Scholar]
- Horvath S, Erhart W, Brosch M, Ammerpohl O, von Schonfels W, Ahrens M, Heits N, Bell JT, Tsai PC, Spector TD, Deloukas P, Siebert R, Sipos B, Becker T, Rocken C, Schafmayer C & Hampe J (2014). Obesity accelerates epigenetic aging of human liver. Proc Natl Acad Sci USA 111, 15538–15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS & Spiegelman BM (1993). Adipose expression of tumor necrosis factor‐alpha: direct role in obesity‐linked insulin resistance. Science 259, 87–91. [DOI] [PubMed] [Google Scholar]
- Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA & Manson JE (2004). Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med 351, 2694–2703. [DOI] [PubMed] [Google Scholar]
- Huffman K (2012). The developing, aging neocortex: how genetics and epigenetics influence early developmental patterning and age‐related change. Front Genet 3, 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilich JZ, Kelly OJ, Inglis JE, Panton LB, Duque G & Ormsbee MJ (2014). Interrelationship among muscle, fat, and bone: connecting the dots on cellular, hormonal, and whole body levels. Ageing Res Rev 15, 51–60. [DOI] [PubMed] [Google Scholar]
- Jagust W, Harvey D, Mungas D & Haan M (2005). Central obesity and the aging brain. Arch Neurol 62, 1545–1548. [DOI] [PubMed] [Google Scholar]
- Janiszewski PM & Ross R (2010). Effects of weight loss among metabolically healthy obese men and women. Diabetes Care 33, 1957–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE & Scadden DT (2006). Stem‐cell ageing modified by the cyclin‐dependent kinase inhibitor p16INK4a. Nature 443, 421–426. [DOI] [PubMed] [Google Scholar]
- Jheng HF, Huang SH, Kuo HM, Hughes MW & Tsai YS (2015). Molecular insight and pharmacological approaches targeting mitochondrial dynamics in skeletal muscle during obesity. Ann N Y Acad Sci 1350, 82–94. [DOI] [PubMed] [Google Scholar]
- Jia G, Aroor AR, DeMarco VG, Martinez‐Lemus LA, Meininger GA & Sowers JR (2015). Vascular stiffness in insulin resistance and obesity. Front Physiol 6, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen DL, Conley KE, Bajpeyi S, Punyanitya M, Gallagher D, Zhang Z, Covington J, Smith SR & Ravussin E (2012). Ectopic lipid accumulation and reduced glucose tolerance in elderly adults are accompanied by altered skeletal muscle mitochondrial activity. J Clin Endocrinol Metab 97, 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurk D, Wilson C, Passos JF, Oakley F, Correia‐Melo C, Greaves L, Saretzki G, Fox C, Lawless C, Anderson R, Hewitt G, Pender SLF, Fullard N, Nelson G, Mann J, van de Sluis B, Mann DA & von Zglinicki T (2014). Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun 2, 4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaria RN (2010). Vascular basis for brain degeneration: faltering controls and risk factors for dementia. Nutr Rev 68 Suppl 2, S74–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantartzis K, Machann J, Schick F, Rittig K, Machicao F, Fritsche A, Haring HU & Stefan N (2011). Effects of a lifestyle intervention in metabolically benign and malign obesity. Diabetologia 54, 864–868. [DOI] [PubMed] [Google Scholar]
- Karelis AD (2008). Metabolically healthy but obese individuals. Lancet 372, 1281–1283. [DOI] [PubMed] [Google Scholar]
- Karelis AD, Messier V, Brochu M & Rabasa‐Lhoret R (2008). Metabolically healthy but obese women: effect of an energy‐restricted diet. Diabetologia 51, 1752–1754. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Liebl DJ & Parada LF (2000). BDNF regulates eating behavior and locomotor activity in mice. EMBO J 19, 1290–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw EE & Flier JS (2004). Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89, 2548–2556. [DOI] [PubMed] [Google Scholar]
- Kiliaan AJ, Arnoldussen IA & Gustafson DR (2014). Adipokines: a link between obesity and dementia? Lancet Neurol 13, 913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J & Nissinen A (2001). Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ 322, 1447–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H & Nissinen A (2005). Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 62, 1556–1560. [DOI] [PubMed] [Google Scholar]
- Klein JP & Waxman SG (2003). The brain in diabetes: molecular changes in neurons and their implications for end‐organ damage. Lancet Neurol 2, 548–554. [DOI] [PubMed] [Google Scholar]
- Kohrt WM & Holloszy JO (1995). Loss of skeletal muscle mass with aging: effect on glucose tolerance. J Gerontol A Biol Sci Med Sci 50 Spec No, 68–72. [DOI] [PubMed] [Google Scholar]
- Komulainen P, Pedersen M, Hanninen T, Bruunsgaard H, Lakka TA, Kivipelto M, Hassinen M, Rauramaa TH, Pedersen BK & Rauramaa R (2008). BDNF is a novel marker of cognitive function in ageing women: the DR's EXTRA Study. Neurobiol Learn Mem 90, 596–603. [DOI] [PubMed] [Google Scholar]
- Kotas ME, Gorecki MC & Gillum MP (2013). Sirtuin‐1 is a nutrient‐dependent modulator of inflammation. Adipocyte 2, 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbe KS, Nielsen AR, Krogh‐Madsen R, Plomgaard P, Rasmussen P, Erikstrup C, Fischer CP, Lindegaard B, Petersen AM, Taudorf S, Secher NH, Pilegaard H, Bruunsgaard H & Pedersen BK (2007). Brain‐derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 50, 431–438. [DOI] [PubMed] [Google Scholar]
- Kramer CK, Zinman B & Retnakaran R (2013). Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta‐analysis. Ann Intern Med 159, 758–769. [DOI] [PubMed] [Google Scholar]
- Kritchevsky SB, Beavers KM, Miller ME, Shea MK, Houston DK, Kitzman DW & Nicklas BJ (2015). Intentional weight loss and all‐cause mortality: a meta‐analysis of randomized clinical trials. PLoS One 10, e0121993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuk JL & Ardern CI (2009. a). Are metabolically normal but obese individuals at lower risk for all‐cause mortality? Diabetes Care 32, 2297–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuk JL & Ardern CI (2009. b). Influence of age on the association between various measures of obesity and all‐cause mortality. J Am Geriatr Soc 57, 2077–2084. [DOI] [PubMed] [Google Scholar]
- Kuk JL, Ardern CI, Church TS, Sharma AM, Padwal R, Sui X & Blair SN (2011). Edmonton Obesity Staging System: association with weight history and mortality risk. Appl Physiol Nutr Metab 36, 570–576. [DOI] [PubMed] [Google Scholar]
- Lamas O, Martinez JA & Marti A (2004). Energy restriction restores the impaired immune response in overweight (cafeteria) rats. J Nutr Biochem 15, 418–425. [DOI] [PubMed] [Google Scholar]
- Lasselin J, Magne E, Beau C, Ledaguenel P, Dexpert S, Aubert A, Laye S & Capuron L (2014). Adipose inflammation in obesity: relationship with circulating levels of inflammatory markers and association with surgery‐induced weight loss. J Clin Endocrinol Metab 99, E53–E61. [DOI] [PubMed] [Google Scholar]
- Lastra G & Manrique C (2015). Perivascular adipose tissue, inflammation and insulin resistance: link to vascular dysfunction and cardiovascular disease. Horm Mol Biol Clin Investig 22, 19–26. [DOI] [PubMed] [Google Scholar]
- Lee HC & Wei YH (2007). Oxidative stress, mitochondrial DNA mutation, and apoptosis in aging. Exp Biol Med (Maywood) 232, 592–606. [PubMed] [Google Scholar]
- Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O'Brien PC & Palumbo PJ (1997). The risk of dementia among persons with diabetes mellitus: a population‐based cohort study. Ann N Y Acad Sci 826, 422–427. [DOI] [PubMed] [Google Scholar]
- Letra L, Santana I & Seica R (2014). Obesity as a risk factor for Alzheimer's disease: the role of adipocytokines. Metab Brain Dis 29, 563–568. [DOI] [PubMed] [Google Scholar]
- Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D'Agostin D, Kurahara C, Gao Y, Cao J, Gong J, Asuncion F, Barrero M, Warmington K, Dwyer D, Stolina M, Morony S, Sarosi I, Kostenuik PJ, Lacey DL, Simonet WS, Ke HZ & Paszty C (2008). Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23, 860–869. [DOI] [PubMed] [Google Scholar]
- Li Y, Daniel M & Tollefsbol TO (2011). Epigenetic regulation of caloric restriction in aging. BMC Med 9, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesa M & Shirihai OS (2013). Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab 17, 491–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind L & Lithell H (1993). Decreased peripheral blood flow in the pathogenesis of the metabolic syndrome comprising hypertension, hyperlipidemia, and hyperinsulinemia. Am Heart J 125, 1494–1497. [DOI] [PubMed] [Google Scholar]
- Ling C & Ronn T (2014). Epigenetic adaptation to regular exercise in humans. Drug Discov Today 19, 1015–1018. [DOI] [PubMed] [Google Scholar]
- Lisko I, Tiainen K, Stenholm S, Luukkaala T, Hurme M, Lehtimaki T, Hervonen A & Jylha M (2012). Inflammation, adiposity, and mortality in the oldest old. Rejuvenation Res 15, 445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolmede K, Duffaut C, Zakaroff‐Girard A & Bouloumie A (2011). Immune cells in adipose tissue: key players in metabolic disorders. Diabetes Metab 37, 283–290. [DOI] [PubMed] [Google Scholar]
- Lombardi G, Perego S, Luzi L & Banfi G (2015). A four‐season molecule: osteocalcin. Updates in its physiological roles. Endocrine 48, 394–404. [DOI] [PubMed] [Google Scholar]
- Lombardi G, Sanchis‐Gomar F, Perego S, Sansoni V & Banfi B (2016). Implications of exercise‐induced adipo‐myokines in bone metabolism. Endocrine Doi: 10.1007/s12020‐015‐0834‐0. [DOI] [PubMed] [Google Scholar]
- Lopez‐Otin C, Blasco MA, Partridge L, Serrano M & Kroemer G (2013). The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Tang MX, Stern Y, Shea S & Mayeux R (2001). Diabetes mellitus and risk of Alzheimer's disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol 154, 635–641. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL & Saltiel AR (2007). Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE & Tessarollo L (1999). Brain‐derived neurotrophic factor‐deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci USA 96, 15239–15244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Lee P, Chisholm DJ & James DE (2015). Control of adipocyte differentiation in different fat depots; implications for pathophysiology or therapy. Front Endocrinol 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzoros CS, Moschos S, Avramopoulos I, Kaklamani V, Liolios A, Doulgerakis DE, Griveas I, Katsilambros N & Flier JS (1997). Leptin concentrations in relation to body mass index and the tumor necrosis factor‐alpha system in humans. J Clin Endocrinol Metab 82, 3408–3413. [DOI] [PubMed] [Google Scholar]
- Martin SL, Hardy TM & Tollefsbol TO (2013). Medicinal chemistry of the epigenetic diet and caloric restriction. Curr Med Chem 20, 4050–4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ (2006). Caloric restriction and aging: controversial issues. J Gerontol A Biol Sci Med Sci 61, 14–19. [DOI] [PubMed] [Google Scholar]
- Matarese G, Moschos S & Mantzoros CS (2005). Leptin in immunology. J Immunol 174, 3137–3142. [DOI] [PubMed] [Google Scholar]
- Mathieu P, Lemieux I & Despres JP (2010). Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther 87, 407–416. [DOI] [PubMed] [Google Scholar]
- Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, Theurich S, Hausen AC, Schmitz J, Bronneke HS, Estevez E, Allen TL, Mesaros A, Partridge L, Febbraio MA, Chawla A, Wunderlich FT & Bruning JC (2014). Signaling by IL‐6 promotes alternative activation of macrophages to limit endotoxemia and obesity‐associated resistance to insulin. Nat Immunol 15, 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer MS (2015). Noninvasive identification of ATTRwt cardiac amyloid: the re‐emergence of nuclear cardiology. Am J Med 128, 1275–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan M & Wasserman P (1981). Changes in sizes and distensibility of the aging kidney. Br J Radiol 54, 488–491. [DOI] [PubMed] [Google Scholar]
- Miao CY & Li ZY (2012). The role of perivascular adipose tissue in vascular smooth muscle cell growth. Br J Pharmacol 165, 643–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio S, Greco EA, Wannenes F, Donini LM & Lenzi A (2014). Adipose, bone and muscle tissues as new endocrine organs: role of reciprocal regulation for osteoporosis and obesity development. Horm Mol Biol Clin Investig 17, 39–51. [DOI] [PubMed] [Google Scholar]
- Minamino T & Komuro I (2007). Vascular cell senescence: contribution to atherosclerosis. Circ Res 100, 15–26. [DOI] [PubMed] [Google Scholar]
- Minamino T & Komuro I (2008). Vascular aging: insights from studies on cellular senescence, stem cell aging, and progeroid syndromes. Nat Clin Pract Cardiovasc Med 5, 637–648. [DOI] [PubMed] [Google Scholar]
- Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, Nojima A, Nabetani A, Oike Y, Matsubara H, Ishikawa F & Komuro I (2009). A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med 15, 1082–1087. [DOI] [PubMed] [Google Scholar]
- Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, Abdelmohsen K, Shin YK, Canto C, Scheibye‐Knudsen M, Krawczyk M, Irusta PM, Martin‐Montalvo A, Hubbard BP, Zhang Y, Lehrmann E, White AA, Price NL, Swindell WR, Pearson KJ, Becker KG, Bohr VA, Gorospe M, Egan JM, Talan MI, Auwerx J, Westphal CH, Ellis JL, Ungvari Z, Vlasuk GP, Elliott PJ, Sinclair DA & de Cabo R (2011). SRT1720 improves survival and healthspan of obese mice. Sci Rep 1, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Martin‐Montalvo A, Mercken EM, Palacios HH, Ward TM, Abulwerdi G, Minor RK, Vlasuk GP, Ellis JL, Sinclair DA, Dawson J, Allison DB, Zhang Y, Becker KG, Bernier M & de Cabo R (2014). The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep 6, 836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro R & Azevedo I (2010). Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm 2010, 289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K (2000). Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell 101, 451–454. [DOI] [PubMed] [Google Scholar]
- Morkedal B, Vatten LJ, Romundstad PR, Laugsand LE & Janszky I (2014). Risk of myocardial infarction and heart failure among metabolically healthy but obese individuals: HUNT (Nord‐Trondelag Health Study), Norway. J Am Coll Cardiol 63, 1071–1078. [DOI] [PubMed] [Google Scholar]
- Morley JE, Baumgartner RN, Roubenoff R, Mayer J & Nair KS (2001). Sarcopenia. J Lab Clin Med 137, 231–243. [DOI] [PubMed] [Google Scholar]
- Muzumdar R, Allison DB, Huffman DM, Ma X, Atzmon G, Einstein FH, Fishman S, Poduval AD, McVei T, Keith SW & Barzilai N (2008). Visceral adipose tissue modulates mammalian longevity. Aging Cell 7, 438–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava‐Gonzalez EJ, Gallegos‐Cabriales EC & Bastarrachea RA (2014). [Phenotypes of bone and adipose tissue metabolism. A systematic review of their relationship]. Revista medica del Instituto Mexicano del Seguro Social 52, 644–650. [PubMed] [Google Scholar]
- North BJ & Sinclair DA (2012). The intersection between aging and cardiovascular disease. Circ Res 110, 1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogorodnikova AD, Kim M, McGinn AP, Muntner P, Khan U & Wildman RP (2012). Incident cardiovascular disease events in metabolically benign obese individuals. Obesity (Silver Spring) 20, 651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Lee YD & Wagers AJ (2014). Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med 20, 870–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky JM & Glass CK (2010). Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 72, 219–246. [DOI] [PubMed] [Google Scholar]
- Olivetti G, Melissari M, Capasso JM & Anversa P (1991). Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res 68, 1560–1568. [DOI] [PubMed] [Google Scholar]
- Onate B, Vilahur G, Camino‐Lopez S, Diez‐Caballero A, Ballesta‐Lopez C, Ybarra J, Moscatiello F, Herrero J & Badimon L (2013). Stem cells isolated from adipose tissue of obese patients show changes in their transcriptomic profile that indicate loss in stemcellness and increased commitment to an adipocyte‐like phenotype. BMC Genomics 14, 625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onate B, Vilahur G, Ferrer‐Lorente R, Ybarra J, Diez‐Caballero A, Ballesta‐Lopez C, Moscatiello F, Herrero J & Badimon L (2012). The subcutaneous adipose tissue reservoir of functionally active stem cells is reduced in obese patients. FASEB J 26, 4327–4336. [DOI] [PubMed] [Google Scholar]
- Ormsbee MJ, Prado CM, Ilich JZ, Purcell S, Siervo M, Folsom A & Panton L (2014). Osteosarcopenic obesity: the role of bone, muscle, and fat on health. J Cachexia Sarcopenia Muscle 5, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega FB, Lee DC, Katzmarzyk PT, Ruiz JR, Sui X, Church TS & Blair SN (2013). The intriguing metabolically healthy but obese phenotype: cardiovascular prognosis and role of fitness. Eur Heart J 34, 389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega FJ, Moreno M, Mercader JM, Moreno‐Navarrete JM, Fuentes‐Batllevell N, Sabater M, Ricart W & Fernandez‐Real JM (2015). Inflammation triggers specific microRNA profiles in human adipocytes and macrophages and in their supernatants. Clin Epigenetics 7, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Parker JL, Lugus JJ & Walsh K (2011). Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papathanassoglou ED, Moynihan JA, Ackerman MH & Mantzoros CS (2001). Serum leptin levels are higher but are not independently associated with severity or mortality in the multiple organ dysfunction/systemic inflammatory response syndrome: a matched case control and a longitudinal study. Clin Endocrinol (Oxf) 54, 225–233. [DOI] [PubMed] [Google Scholar]
- Pareja‐Galeano H, Sanchis‐Gomar F & Garcia‐Gimenez JL (2014). Physical exercise and epigenetic modulation: elucidating intricate mechanisms. Sports Med 44, 429–436. [DOI] [PubMed] [Google Scholar]
- Park CR (2001). Cognitive effects of insulin in the central nervous system. Neurosci Biobehav Rev 25, 311–323. [DOI] [PubMed] [Google Scholar]
- Patel P & Abate N (2013). Body fat distribution and insulin resistance. Nutrients 5, 2019–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng XR, Gennemark P, O'Mahony G & Bartesaghi S (2015). Unlock the thermogenic potential of adipose tissue: pharmacological modulation and implications for treatment of diabetes and obesity. Front Endocrinol 6, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez LM, Bernal A, De Lucas B, San Martin N, Mastrangelo A, García A, Barbas C & Galvez BG (2015). Altered metabolic and stemness capacity of adipose tissue‐derived stem cells from obese mouse and human. PLoS One 10, e123397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC (2011). Clinical practice. Mild cognitive impairment. N Engl J Med 364, 2227–2234. [DOI] [PubMed] [Google Scholar]
- Peterson CM, Johannsen DL & Ravussin E (2012). Skeletal muscle mitochondria and aging: a review. J Aging Res 2012, 194821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA & Winslow JW (1991). BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron 7, 695–702. [DOI] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark‐Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW & Guarente L (2004). Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR‐gamma. Nature 429, 771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelkens F, Eijsvogels TM, Brussee P, Verheggen RJ, Tack CJ & Hopman MT (2014). Physical fitness can partly explain the metabolically healthy obese phenotype in women. Exp Clin Endocrinol Diabetes 122, 87–91. [DOI] [PubMed] [Google Scholar]
- Poonpet T & Honsawek S (2014). Adipokines: Biomarkers for osteoarthritis? World J Orthop 5, 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulose N & Raju R (2015). Sirtuin regulation in aging and injury. Biochim Biophys Acta 1852, 2442–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qatanani M & Lazar MA (2007). Mechanisms of obesity‐associated insulin resistance: many choices on the menu. Genes Dev 21, 1443–1455. [DOI] [PubMed] [Google Scholar]
- Qizilbash N, Gregson J, Johnson ME, Pearce N, Douglas I, Wing K, Evans SJ & Pocock SJ (2015). BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diabetes Endocrinol 3, 431–436. [DOI] [PubMed] [Google Scholar]
- Querfurth HW & LaFerla FM (2010). Alzheimer's disease. N Engl J Med 362, 329–344. [DOI] [PubMed] [Google Scholar]
- Razay G & Vreugdenhil A (2005). Obesity in middle age and future risk of dementia: midlife obesity increases risk of future dementia. BMJ 331, 455; author reply 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan RM & Jaenisch R (2001). Conditional deletion of brain‐derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol 15, 1748–1757. [DOI] [PubMed] [Google Scholar]
- Ristow M & Schmeisser S (2011). Extending life span by increasing oxidative stress. Free Radic Biol Med 51, 327–336. [DOI] [PubMed] [Google Scholar]
- Romacho T, Elsen M, Rohrborn D & Eckel J (2014). Adipose tissue and its role in organ crosstalk. Acta Physiol (Oxf) 210, 733–753. [DOI] [PubMed] [Google Scholar]
- Rosen CJ & Bouxsein ML (2006). Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol 2, 35–43. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Jamieson CH & Weissman IL (2008). Stems cells and the pathways to aging and cancer. Cell 132, 681–696. [DOI] [PubMed] [Google Scholar]
- Saely CH, Geiger K & Drexel H (2012). Brown versus white adipose tissue: a mini‐review. Gerontology 58, 15–23. [DOI] [PubMed] [Google Scholar]
- Salam N, Rane S, Das R, Faulkner M, Gund R, Kandpal U, Lewis V, Mattoo H, Prabhu S, Ranganathan V, Durdik J, George A, Rath S & Bal V (2013). T cell ageing: effects of age on development, survival & function. Indian J Med Res 138, 595–608. [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K & Kauppinen A (2012). Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging (Albany NY) 4, 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis‐Gomar F & Derbre F (2014). Mitochondrial fission and fusion in human diseases. N Engl J Med 370, 1073–1074. [DOI] [PubMed] [Google Scholar]
- Sanchis‐Gomar F, Garcia‐Gimenez JL, Gomez‐Cabrera MC & Pallardo FV (2014). Mitochondrial biogenesis in health and disease. Molecular and therapeutic approaches. Curr Pharm Des 20, 5619–5633. [DOI] [PubMed] [Google Scholar]
- Sanchis‐Gomar F, Lucia A, Yvert T, Ruiz‐Casado A, Pareja‐Galeano H, Santos‐Lozano A, Fiuza‐Luces C, Garatachea N, Lippi G, Bouchard C & Berger NA (2015). Physical inactivity and low fitness deserve more attention to alter cancer risk and prognosis. Cancer Prev Res (Phila) 8, 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y & Komuro I (2007). p53‐induced inhibition of Hif‐1 causes cardiac dysfunction during pressure overload. Nature 446, 444–448. [DOI] [PubMed] [Google Scholar]
- Sasaki T (2015). Age‐associated weight gain, leptin, and SIRT1: A possible role for hypothalamic SIRT1 in the prevention of weight gain and aging through modulation of leptin sensitivity. Front Endocrinol 6, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipper HS, Prakken B, Kalkhoven E & Boes M (2012). Adipose tissue‐resident immune cells: key players in immunometabolism. Trends Endocrinol Metab 23, 407–415. [DOI] [PubMed] [Google Scholar]
- Schutz Y (2004). Concept of fat balance in human obesity revisited with particular reference to de novo lipogenesis. Int J Obes Relat Metab Disord 28 Suppl 4, S3–S11. [DOI] [PubMed] [Google Scholar]
- Schwab P & Scalapino K (2011). Exercise for bone health: rationale and prescription. Curr Opin Rheumatol 23, 137–141. [DOI] [PubMed] [Google Scholar]
- Schwer B & Verdin E (2008). Conserved metabolic regulatory functions of sirtuins. Cell Metab 7, 104–112. [DOI] [PubMed] [Google Scholar]
- Seals DR & Dinenno FA (2004). Collateral damage: cardiovascular consequences of chronic sympathetic activation with human aging. Am J Physiol Heart Circ Physiol 287, H1895–H1905. [DOI] [PubMed] [Google Scholar]
- Selman C, Lingard S, Choudhury AI, Batterham RL, Claret M, Clements M, Ramadani F, Okkenhaug K, Schuster E, Blanc E, Piper MD, Al‐Qassab H, Speakman JR, Carmignac D, Robinson IC, Thornton JM, Gems D, Partridge L & Withers DJ (2008). Evidence for lifespan extension and delayed age‐related biomarkers in insulin receptor substrate 1 null mice. FASEB J 22, 807–818. [DOI] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al‐Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D & Withers DJ (2009). Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326, 140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesti G, Folli F, Perego L, Hribal ML & Pontiroli AE (2011). Effects of weight loss in metabolically healthy obese subjects after laparoscopic adjustable gastric banding and hypocaloric diet. PLoS One 6, e17737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro H, Lutaty A & Ariel A (2011). Macrophages, meta‐inflammation, and immuno‐metabolism. TheScientificWorldJournal 11, 2509–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless NE & DePinho RA (2007). How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol 8, 703–713. [DOI] [PubMed] [Google Scholar]
- Shimizu I, Yoshida Y, Katsuno T, Tateno K, Okada S, Moriya J, Yokoyama M, Nojima A, Ito T, Zechner R, Komuro I, Kobayashi Y & Minamino T (2012). p53‐induced adipose tissue inflammation is critically involved in the development of insulin resistance in heart failure. Cell Metab 15, 51–64. [DOI] [PubMed] [Google Scholar]
- Shimizu I, Yoshida Y & Minamino T (2015). Pathological role of adipose tissue dysfunction in cardio‐metabolic disorders. Int Heart J 56, 255–259. [DOI] [PubMed] [Google Scholar]
- Shin MJ, Hyun YJ, Kim OY, Kim JY, Jang Y & Lee JH (2006). Weight loss effect on inflammation and LDL oxidation in metabolically healthy but obese (MHO) individuals: low inflammation and LDL oxidation in MHO women. Int J Obes (Lond) 30, 1529–1534. [DOI] [PubMed] [Google Scholar]
- Shoshani O & Zipori D (2015). Stress as a fundamental theme in cell plasticity. Biochim Biophys Acta 1849, 371–377. [DOI] [PubMed] [Google Scholar]
- Sohal RS & Orr WC (2012). The redox stress hypothesis of aging. Free Radic Biol Med 52, 539–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen TI, Rissanen A, Korkeila M & Kaprio J (2005). Intention to lose weight, weight changes, and 18‐y mortality in overweight individuals without co‐morbidities. PLoS Med 2, e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava T (2006). Nondiabetic consequences of obesity on kidney. Pediatr Nephrol 21, 463–470. [DOI] [PubMed] [Google Scholar]
- St‐Onge MP (2005). Relationship between body composition changes and changes in physical function and metabolic risk factors in aging. Curr Opin Clin Nutr Metab Care 8, 523–528. [PubMed] [Google Scholar]
- Stewart R, Masaki K, Xue QL, Peila R, Petrovitch H, White LR & Launer LJ (2005). A 32‐year prospective study of change in body weight and incident dementia: the Honolulu‐Asia Aging Study. Arch Neurol 62, 55–60. [DOI] [PubMed] [Google Scholar]
- Stranahan AM (2015). Models and mechanisms for hippocampal dysfunction in obesity and diabetes. Neuroscience 309, 125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stransky CA, Hsu VM, Dierov R, Hoover WJ, Donahue G, Bucky LP & Percec I (2012). Beyond fat grafting: what adipose tissue can teach us about the molecular mechanisms of human aging. Ann Plast Surg 69, 489–492. [DOI] [PubMed] [Google Scholar]
- Stuart CA, McCurry MP, Marino A, South MA, Howell ME, Layne AS, Ramsey MW & Stone MH (2013). Slow‐twitch fiber proportion in skeletal muscle correlates with insulin responsiveness. J Clin Endocrinol Metab 98, 2027–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Isoda F, Ishihara Y, Kimura M & Yamakawa T (2001). T lymphopaenia in relation to body mass index and TNF‐α in human obesity: adequate weight reduction can be corrective. Clin Endocrinol (Oxf) 54, 347–354. [PubMed] [Google Scholar]
- Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD & Kirkland JL (2010). Fat tissue, aging, and cellular senescence. Aging Cell 9, 667–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD & Kirkland JL (2013). Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab 17, 644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF, Atzmon G, Gheorghe C, Liang HQ, Lowes C, Greally JM & Barzilai N (2010). Tissue‐specific dysregulation of DNA methylation in aging. Aging Cell 9, 506–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp AA & Schlaich MP (2015). Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. J Diabetes Res 2015, 341583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H & Moschen AR (2006). Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6, 772–783. [DOI] [PubMed] [Google Scholar]
- Tollervey JR & Lunyak VV (2011). Adult stem cells: simply a tool for regenerative medicine or an additional piece in the puzzle of human aging? Cell Cycle 10, 4173–4176. [DOI] [PubMed] [Google Scholar]
- Tollervey JR & Lunyak VV (2012). Epigenetics: judge, jury and executioner of stem cell fate. Epigenetics 7, 823–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzanetakou IP, Katsilambros NL, Benetos A, Mikhailidis DP & Perrea DN (2012). “Is obesity linked to aging?”: adipose tissue and the role of telomeres. Ageing Res Rev 11, 220–229. [DOI] [PubMed] [Google Scholar]
- Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J & Thomas G (2004). Absence of S6K1 protects against age‐ and diet‐induced obesity while enhancing insulin sensitivity. Nature 431, 200–205. [DOI] [PubMed] [Google Scholar]
- Vaquero A, Scher M, Erdjument‐Bromage H, Tempst P, Serrano L & Reinberg D (2007). SIRT1 regulates the histone methyl‐transferase SUV39H1 during heterochromatin formation. Nature 450, 440–444. [DOI] [PubMed] [Google Scholar]
- Vaynman S & Gomez‐Pinilla F (2006). Revenge of the “sit”: how lifestyle impacts neuronal and cognitive health through molecular systems that interface energy metabolism with neuronal plasticity. J Neurosci Res 84, 699–715. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Apovian CM, Kushner RF, Klein S; American Society for Nutrition; NAASO, The Obesity Society (2005). Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res 13, 1849–1863. [DOI] [PubMed] [Google Scholar]
- Ward MA, Carlsson CM, Trivedi MA, Sager MA & Johnson SC (2005). The effect of body mass index on global brain volume in middle‐aged adults: a cross sectional study. BMC Neurol 5, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warolin J, Coenen KR, Kantor JL, Whitaker LE, Wang L, Acra SA, Roberts LJ 2nd & Buchowski MS (2014). The relationship of oxidative stress, adiposity and metabolic risk factors in healthy Black and White American youth. Pediatric obesity 9, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson GS, Peskind ER, Asthana S, Purganan K, Wait C, Chapman D, Schwartz MW, Plymate S & Craft S (2003). Insulin increases CSF Aβ42 levels in normal older adults. Neurology 60, 1899–1903. [DOI] [PubMed] [Google Scholar]
- Wehrli FW, Hopkins JA, Hwang SN, Song HK, Snyder PJ & Haddad JG (2000). Cross‐sectional study of osteopenia with quantitative MR imaging and bone densitometry. Radiology 217, 527–538. [DOI] [PubMed] [Google Scholar]
- Weinstein G, Beiser AS, Choi SH, Preis SR, Chen TC, Vorgas D, Au R, Pikula A, Wolf PA, DeStefano AL, Vasan RS & Seshadri S (2014). Serum brain‐derived neurotrophic factor and the risk for dementia: the Framingham Heart Study. JAMA Neurol 71, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL & Ferrante AW Jr (2003). Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112, 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, Gunderson EP, Barrett‐Connor E, Quesenberry CP Jr & Yaffe K (2005. a). Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ 330, 1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, Gunderson EP, Quesenberry CP Jr, Zhou J & Yaffe K (2007). Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res 4, 103–109. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Gustafson DR, Barrett‐Connor E, Haan MN, Gunderson EP & Yaffe K (2008). Central obesity and increased risk of dementia more than three decades later. Neurology 71, 1057–1064. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Sidney S, Selby J, Johnston SC & Yaffe K (2005. b). Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64, 277–281. [DOI] [PubMed] [Google Scholar]
- Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie‐Rosett J & Sowers MR (2008). The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med 168, 1617–1624. [DOI] [PubMed] [Google Scholar]
- Williamson DF, Pamuk E, Thun M, Flanders D, Byers T & Heath C (1995). Prospective study of intentional weight loss and mortality in never‐smoking overweight US white women aged 40–64 years. Am J Epidemiol 141, 1128–1141. [DOI] [PubMed] [Google Scholar]
- Williamson DF, Pamuk E, Thun M, Flanders D, Byers T & Heath C (1999). Prospective study of intentional weight loss and mortality in overweight white men aged 40–64 years. Am J Epidemiol 149, 491–503. [DOI] [PubMed] [Google Scholar]
- Wolffe AP & Guschin D (2000). Review: chromatin structural features and targets that regulate transcription. J Struct Biol 129, 102–122. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA & Chen H (2003). Chronic inflammation in fat plays a crucial role in the development of obesity‐related insulin resistance. J Clin Invest 112, 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S & Harris T (2003). Inflammatory markers and cognition in well‐functioning African‐American and white elders. Neurology 61, 76–80. [DOI] [PubMed] [Google Scholar]
- Yamada K, Mizuno M & Nabeshima T (2002). Role for brain‐derived neurotrophic factor in learning and memory. Life Sci 70, 735–744. [DOI] [PubMed] [Google Scholar]
- Yang H & Li X (2012). The role of fatty acid metabolism and lipotoxicity in pancreatic β‐cell injury: Identification of potential therapeutic targets. Acta Pharm Sin B 2, 396–402. [Google Scholar]
- Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, Lu JC, Smith JJ, Jirousek MR & Olefsky JM (2009). SIRT1 exerts anti‐inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol 29, 1363–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesin KC, Franklin BA, Miller WM, Peterson ED & McCullough PA (2011). Impact of obesity on cardiovascular disease. Med Clin North Am 95, 919–937. [DOI] [PubMed] [Google Scholar]
- Zhang X & Lerman LO (2015). Obesity and renovascular disease. Am J Physiol Renal Physiol 309, F273–F279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L & Ackerman SL (2006). Endoplasmic reticulum stress in health and disease. Curr Opin Cell Biol 18, 444–452. [DOI] [PubMed] [Google Scholar]
- Zhou XJ, Rakheja D, Yu X, Saxena R, Vaziri ND & Silva FG (2008). The aging kidney. Kidney Int 74, 710–720. [DOI] [PubMed] [Google Scholar]


