Abstract
Hyperphosphatemia in chronic kidney disease (CKD) patients is a potentially life altering condition that can lead to cardiovascular calcification, metabolic bone disease (renal osteodystrophy) and the development of secondary hyperparathyroidism (SHPT). It is also associated with increased prevalence of cardiovascular diseases and mortality rates. To effectively manage hyperphosphatemia in CKD patients it is important to not only consider pharmacological and nonpharmacological treatment options but also to understand the underlying physiologic pathways involved in phosphorus homoeostasis. This review will therefore provide both a background into phosphorus homoeostasis and the management of hyperphosphatemia in CKD patients. In addition, it will cover some of the most important reasons for failure to control hyperphosphatemia with emphasis on the effect of the gastric pH on phosphate binders efficiency.
1. Introduction
Hyperphosphatemia in chronic kidney disease (CKD) patients is a potentially life altering condition that can lead to cardiovascular calcification, metabolic bone disease (renal osteodystrophy) and the development of secondary hyperparathyroidism (SHPT). To effectively manage hyperphosphatemia in CKD patients it is important to not only consider pharmacological and non-pharmacological treatment options but also to understand the underlying physiologic pathways involved in phosphorus homoeostasis. This review will therefore provide both a background into phosphorus homoeostasis and the management of hyperphosphatemia in CKD patients. In addition, it will cover some of the most important reasons for failure to control hyperphosphatemia with emphasis on the effect of the gastric pH on phosphate binders efficiency.
Phosphorus is the second most abundant element in the human body after calcium (Bellasi et al., 2006). The majority (85%) of phosphorus is found in bone and teeth as hydroxyapatite (Uribarri, 2007, Bellasi et al., 2006), 14% is located intracellularly as organic phosphate compounds; with the remaining 1% of the total body phosphate located extracellularly, mainly as inorganic phosphate (Bellasi et al., 2006, Uribarri, 2007). Of the 1% located in the vascular space, 20% is protein bound (Uribarri, 2007).
Inorganic phosphate is a component of many organic compounds and cell structures such as phospholipid cell membranes, nucleic acids, and phosphoproteins (Berndt et al., 2005). It plays significant roles in many biological processes such as cell signalling, synthesis of nucleic acid, energy metabolism, membrane functions, bone mineralisation and carbohydrate metabolism (Berndt and Kumar, 2007, Xie et al., 2000). In addition, it is essential for the normal generation of red blood cells, white blood cells and platelet function (Berndt et al., 2005). Phosphate is sourced for adenosine triphosphate synthesis, a critical energy source for physiological processes such as muscle contractility, neurological activities, electrolyte transportation, and other biological reactions (Nishi et al., 2011). Given the importance of phosphate for varied and multiple biological processes, it is not surprising that phosphate homoeostasis is a complex and highly regulated processes.
2. Phosphate homoeostasis in humans
In healthy adults, most laboratories quote a normal phosphate reference concentration range of 0.80–1.45 mmol/L (Berndt and Kumar, 2007). Overall intake and excretion is determined by the net balance of ingested and absorbed phosphate from food, and phosphate excretion through the bowels and the urinary tract (Hahn et al., 1937). In addition, the plasma phosphate concentration is also influenced by the rate of bone formation and resorption, since phosphate moves in and out of the bone (Berndt et al., 2005, Weidmann, 1956, Hahn et al., 1937).
2.1. Intestinal absorption
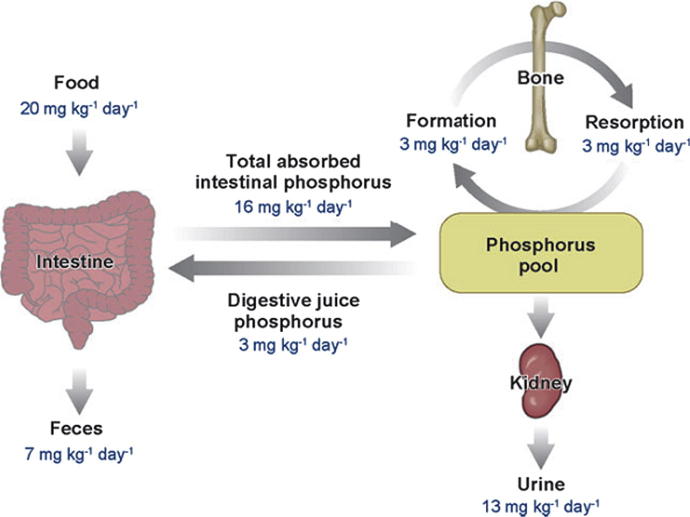
The average ingested phosphate content from dietary intake is in the order of 20 mg/kg/day (see Fig. 1) and it is typically found in foods that are rich in protein such as dairy products, meats, eggs, and cereals as well as food additives that contain phosphate (Schaefer, 1994, Berndt et al., 2005, Bellasi et al., 2006). The bioavailability of phosphate from a vegetarian diet is relatively low compared to meat dietary protein (Moe et al., 2011). Phosphorus from plant sources is mostly in the form of phytate, which is not hydrolysable by humans due the lack of phytase enzyme and hence, is not absorbable (Moe et al., 2011). Vegetarians would therefore be expected to ingest less phosphate compared to non-vegetarians.
Figure 1.
Phosphate homoeostasis in humans used with permission (Berndt and Kumar, 2007).
Of the total amount of daily ingested phosphate, 16 mg/kg/day is usually absorbed by the intestine and approximately 3 mg/kg/day is secreted back into the intestine through pancreatic and intestinal secretions (Berndt et al., 2005). The remaining 7 mg/kg/day of unabsorbed phosphate is excreted via the faecal route (Berndt et al., 2005).
The major sites for phosphate absorption are in the jejunum and ileum (Bellasi et al., 2006, Xie et al., 2000, Katai et al., 1999). The absorption process is carried out both by diffusional, non-saturable influx and through an active uptake system influenced by sodium-phosphate co-transporters (NaPi co-transporters), that account for about 60% of “normal” dietary phosphate absorption (Bellasi et al., 2006, Xie et al., 2000, Katai et al., 1999, Danisi et al., 1990, Danisi et al., 1980). This percentage can increase up to 80% under the influence of the active form of vitamin D (1α, 25(OH)2D3) and be reduced to 40% if a patient is taking phosphate binders (Bellasi et al., 2006, Cupisti et al., 2003).
Sodium-phosphate co-transporters (NaPi co-transporters) are carrier systems present in the brush border membranes of intestine and renal proximal tubular cells and are responsible for phosphate absorption from the intestine and reabsorption from proximal tubules of the kidney (Bellasi et al., 2006, Berndt et al., 2005, Moe et al., 2011). By modulating these co-transporters, many factors (discussed later) can alter intestinal phosphate absorption and influence phosphate homoeostasis.
2.2. Renal elimination
The kidney plays a major role in phosphate homoeostasis (Kay, 1926). The kidneys excrete the total net amount of absorbed phosphate (13 mg/kg/day) (Berndt et al., 2005). Under normal physiological condition, in healthy individuals phosphate is freely filtered through the glomerulus. The majority (85–90%) of filtered phosphate undergoes tubular reabsorption primarily in proximal tubules (Miyamoto et al., 1995, Miyamoto et al., 1997, Baumann et al., 1975) (Uribarri, 2007).
3. Factors affecting phosphate homoeostasis
There is significant interplay between factors that influence calcium and phosphate homoeostasis. The influences are further complicated in patients with impaired renal function. Factors that regulate phosphate homoeostasis principally alter phosphate absorption from the intestine, reabsorption from kidney and mobilisation from and/or uptake by the bone. To understand potential influences it is critical to review this interplay especially with regard to Parathyroid Hormone (PTH) and fibroblast growth factor-23 (FGF23).
3.1. Parathyroid hormone (PTH)
PTH plays a vital role in the regulation of both calcium and phosphate homoeostasis.
Parathyroid glands contain calcium-sensing receptors that sense the extracellular ionised calcium concentration and regulate PTH secretion (Garrett et al., 1995, Riccardi et al., 1995). If ionised calcium is reduced (with increased serum phosphate concentration (Patwardhan and Nhavi, 1939, McGowan, 1933)), PTH will be released and exert its phosphaturic effect directly on the kidney (Sutcliffe et al., 1973). In addition, PTH stimulates osteoclast activity in the bone to release calcium and phosphate into the extracellular pool, and also stimulate the activation of vitamin D to 1α,25(OH)2D3 via the 1α-hydroxylase enzyme (Uy et al., 1995, Garabedian et al., 1972).
PTH also reduces the number of NaPi co-transporters in the proximal tubules, leading to reduced phosphate reabsorption and therefore greater excretion of phosphate by the kidney (Goadby and Stacey, 1934, O’Donovan et al., 1993). This results in decreased serum phosphate, a reduction in calcium excretion and an increase of serum calcium concentration.
3.2. Vitamin D
Activated vitamin D (1α, 25(OH)2D3) acts on the intestine to increase the number of NaPi co-transporters thereby increasing the absorption of both phosphate and calcium (Katai et al., 1999, Danisi et al., 1980, Danisi et al., 1990). In contrast to PTH, 1α, 25(OH)2D3 increases calcium and phosphate uptake by the bones by stimulating osteoblast and inhibiting osteoclast activities (Baldock et al., 2006, Dickson and Kodicek, 1979). In addition, 1α, 25(OH)2D3 directly inhibits PTH hormone action (Friedlaender et al., 1983) and stimulates renal NaPi co-transporters to inhibit phosphate excretion (Kurnik and Hruska, 1985). As serum calcium and phosphate concentrations increase, the activity of α hydroxylase in renal tubules is reduced thus reducing the activation of vitamin D (Spanos et al., 1981).
3.3. Phosphatonins
Phosphatonins are a group of factors, originally identified in patients with tumour-induced osteomalacia. They are associated with inducing hypophosphatemia, lowering concentrations of vitamin D and increasing excretion of urinary phosphate (Cai et al., 1994). Several phosphatonins have been identified with fibroblast growth factor-23 (FGF-23), fibroblast growth factor-7, secreted frizzled-related protein and matrix extracellular phosphoglycoprotein being the most commonly cited (Berndt and Kumar, 2007).
FGF-23 reduces phosphate re-uptake by influencing renal NaPi co-transporters resulting in increased renal phosphate excretion (Inoue et al., 2005, Shimada et al., 2004b, Shimada et al., 2004a). In addition, FGF23 also reduces circulating 1α, 25(OH)2D3 through the inhibition of 1α-hydroxylase activity (Inoue et al., 2005, Shimada et al., 2004b, Shimada et al., 2004a). Actions on the intestine, involve a reduction in the number of NaPi co-transporters and consequently phosphate absorption (Shimada et al., 2004a).
FGF-23 actions are independent of PTH and are believed to be primarily influenced by phosphate concentrations (Shimada et al., 2004a).
The following factors have also been reported to alter phosphate homoeostasis by influencing NaPi co-transporters.
-
(A)Factors that stimulate renal phosphate reabsorption by NaPi co-transporters in the proximal tubules:
-
•Insulin-like growth factor 1 (Caverzasio et al., 1990).
-
•Insulin (Hammerman et al., 1984).
-
•Thyroid hormone (Ishiguro et al., 2010).
-
•Serotonin (Hafdi et al., 1996).
-
•Growth hormone (Hammerman et al., 1980).
-
•All-trans-retinoic acid (Masuda et al., 2010).
-
•
-
(B)Factors that increase phosphate excretion by inhibiting NaPi co-transporters in the proximal tubules:
-
•Dopamine (de Toledo et al., 1999).
-
•Calcitonin (Berndt and Knox, 1984).
-
•Glucocorticoids (Freiberg et al., 1982).
-
•Fasting (Kempson, 1985).
-
•Plasma volume expansion (Suki et al., 1969, Liput et al., 1989).
-
•
3.4. Dietary intake of phosphate
Evidence also suggests a regulatory influence of dietary phosphate on homoeostatic mechanisms (Martin et al., 2005, Berndt et al., 2007).
Low phosphate dietary intake stimulates intestinal and renal NaPi co-transporters expression leading to increased absorption of phosphate from the intestine and reabsorption from the proximal tubules (Danisi et al., 1990, Katai et al., 1999, Stoll et al., 1979, Jahan and Butterworth, 1988). In addition, low phosphate induces an increase in circulating 1α, 25(OH)2D3 (Danisi et al., 1990). By contrast, high dietary phosphate intake stimulates a rapid release of PTH to increase renal phosphate excretion before any changes in serum calcium or phosphate concentrations occur (Martin et al., 2005, Berndt et al., 2007).
3.5. Phosphate exchange from bone and intracellular compartment
A dynamic equilibrium exists between the bone and the extracellular phosphate pool. Effective homoeostasis of phosphate requires a balance between the uptake and release of P from bone as well as other intracellular sources (Hahn et al., 1937, Weidmann, 1956, Dickson and Kodicek, 1979).
When considering non-skeletal cellular structures several pathways are involved in the transport of phosphate from the plasma to the intracellular space. Due to the electronegativity of cellular membranes and concentration gradients, active transport systems rather than passive diffusion are required for transport (Werner and Kinne, 2001).
Changes in electronegativity gradients such as acid-base disorders can cause transcellular shifts of phosphate between intracellular and extracellular fluids (Mostellar and Tuttle, 1964, Barsotti et al., 1986). In metabolic acidosis, phosphate moves from inside the cell to extracellular space leading to increase phosphate concentration in the blood (Mostellar and Tuttle, 1964, Barsotti et al., 1986). In contrast, in case of alkalosis, phosphate moves intracellularly and serum phosphate concentration is reduced (Mostellar and Tuttle, 1964, Barsotti et al., 1986).
3.6. Diurnal variations
Diurnal fluctuations in serum phosphate concentrations occur with phosphate concentration being lowest in the early morning but increasing by 16% by midday (Havard and Reay, 1925). During an evening sleep cycle, phosphate increases by approximately 40%, remains elevated, then drops rapidly after awakening (Havard and Reay, 1925). There are also seasonal variations with phosphate concentrations being higher in the summer than in the winter (Havard and Reay, 1925).
4. Pathogenesis and consequences of hyperphosphatemia in CKD patients
Hyperphosphatemia is defined as an abnormally high serum phosphate concentration of >1.46 mmol/L (Wojcicki, 2013). It is principally observed in patients with reduced kidney function (Goadby and Stacey, 1934, Hruska et al., 2008). However, other potential causes exist. These include hypoparathyroidism, pseudohyperphosphatemia, excessive intake of phosphate, excessive cellular injury (for example rhabdomyolysis and tumour lysis syndrome), intracellular shifts (metabolic or respiratory acidosis) and vitamin D toxicity (Mostellar and Tuttle, 1964, Ettinger et al., 1978, Aydogan et al., 2006, Loh et al., 2010, Ron et al., 1984, Albright and Ellsworth, 1929, Bansal et al., 2014).
Hyperphosphatemia can occur when the kidney function is impaired to the extent that reduced renal phosphate excretion and other homoeostatic mechanisms fail to eliminate excess phosphate (Slatopolsky et al., 1968, Craver et al., 2007, Slatopolsky et al., 1966).
In CKD, the decline in the glomerular filtration rate (GFR) is compensated for by an early elevation of the FGF-23 concentration to induce decreased proximal tubule phosphate re-absorption and attempt to maintain normal phosphate concentrations (Inoue et al., 2005, Shimada et al., 2004b, Shimada et al., 2004a, Ix et al., 2010). FGF-23 also reduces the concentration of 1α, 25(OH)2D3, lowering the effects of NaPi co-transporters in the intestine and consequently reducing phosphate absorption (Inoue et al., 2005, Shimada et al., 2004b, Shimada et al., 2004a).
Phosphate however, can still be absorbed by passive diffusion resulting in an increased influence on renal mechanisms to maintain phosphate balance (Danisi et al., 1980). The reduced concentration of 1α, 25(OH)2D3 leads to a reduction of calcium absorption and stimulates PTH secretion leading to secondary hyperparathyroidism (SHPT) (Friedlaender et al., 1983). PTH increases phosphate loss through the kidney by reducing the number of NaPi co-transporters (Goadby and Stacey, 1934, O’Donovan et al., 1993).
These compensatory mechanisms attempt to normalise serum phosphate and calcium concentrations in CKD patients (Oliveira et al., 2010, Craver et al., 2007). However, as GFR continues to decline and falls below 25 ml/min, the renal phosphate excretion reaches its maximum and excess dietary phosphate accumulates leading to persistent hyperphosphatemia (Slatopolsky et al., 1968). In addition, SHPT and low circulating 1α, 25(OH)2D3 stimulate bone resorption to a greater extent than bone formation leading to the loss of bone mineral density (BMD) and further contributing to hyperphosphatemia in CKD patients (Rix et al., 1999, Huffer et al., 1975a, Huffer et al., 1975b). If the skeletal system does not accommodate the increased serum phosphate concentration, serum phosphate can interact with calcium to precipitate calcium phosphate salts (hydroxyapatite) in non-skeletal tissues (McGowan, 1933, Velentzas et al., 1978). Calcification generally occurs in the blood vessels, heart valves, myocardium, and other soft tissues (Bellasi et al., 2012, Ribeiro et al., 1998, Janigan et al., 2000, Kimura et al., 1999). Cardiovascular calcification is probably the main reason for the high prevalence of cardiovascular diseases (CVD) in CKD patients as it cannot be totally explained by the traditional cardiovascular risks in the general population (Ganesh et al., 2001, Rostand and Drueke, 1999, Foley et al., 1995, Parfrey and Foley, 1999). CVD accounts for half of all deaths among CKD population (Parfrey and Foley, 1999). Calcification risk is usually assessed by the Calcium–Phosphate product (Ca × P) (Velentzas et al., 1978). A value of 5–6 mmol2/L2 (70 mg2/dl2) is usually regarded as the ‘threshold’ value above which calcification is more likely (Levin and Hoenich, 2001, Ganesh et al., 2001). Although more likely to occur above this level, calcification can occur at (Ca × P) product values of less than the above threshold (Ribeiro et al., 1998).
Observational studies have consistently reported increased cardiovascular mortality, all-cause mortality, vascular and valvular calcifications, left ventricular hypertrophy and heart failure to be associated with increased concentrations of serum phosphate, PTH, calcium, calcium-phosphate product (Ca × P) and FGF-23 (Stevens et al., 2004, Tentori et al., 2008, Slinin et al., 2005, Gutierrez et al., 2008, Hsu and Wu, 2009, Dhingra et al., 2010, McGovern et al., 2013, Block et al., 2004, Young et al., 2004). A post hoc analysis of the Cholesterol And Recurrent Events study, found that for 4127 patients with pervious myocardial infarction, “a graded independent relation between phosphate and death” existed. The analysis also reported an association between higher serum phosphate concentrations (mostly within the normal range) and increased risk of cardiovascular diseases and the composite endpoint of death due to coronary heart diseases (Tonelli et al., 2005).
Persistent hyperphosphatemia and elevated Ca × P product can also have other negative influences on haemodynamic variables such as elevated heart rate, increased diastolic and mean blood pressures and increased cardiac stroke index (Marchais et al., 1999).
Giving the significant clinical consequences of hyperphosphatemia in CKD it is recommended to manage serum phosphate, serum calcium, Ca × P product and PTH to strict and specific targets to reduce the potential impact of such consequences, improve patients’ quality of life and probably improve survival (National Kidney Foundation, 2003, Kidney Disease: Improving Global Outcomes, 2009).
5. Management of hyperphosphatemia in CKD patients
In clinical practice, the management of hyperphosphatemia is focused on controlling factors that are responsible for the intake and removal of phosphate from the body. There are three main strategies for correcting hyperphosphatemia:
-
I.
Diet: restricting dietary phosphate intake.
-
II.
Enhancing elimination: removing phosphate with adequate dialysis.
-
III.
Minimising phosphate absorption: reducing intestinal absorption using phosphate binders.
5.1. Diet: restricting dietary phosphate intake
Reducing the daily phosphate intake in diet can be challenging as it is usually incompatible with the recommended daily protein intake of 1.0–1.2 g/kg/day (National Kidney Foundation, 2000, Kuhlmann, 2007). In addition, the required intensive patient education, the complexity of dietary tables and booklets as well as the substantial variability of phosphate contents in food from the same category are added challenges for dietary phosphate control (Kuhlmann, 2007). This makes diet control alone an insufficient and unreliable technique to keep phosphate concentrations within the recommended targets (National Kidney Foundation, 2003, Kidney Disease: Improving Global Outcomes, 2009). However, when combined with other strategies, dietary modifications can assist in management strategies to help reduce the risk of hyperphosphatemia (Kuhlmann, 2007).
5.2. Enhancing elimination: removing phosphate with adequate dialysis
Dialysis is the cornerstone of homoeostatic electrolyte management for end stage renal disease patients. However, the currently available dialysis techniques are usually ineffective in removing excess phosphate to the degree of normalising phosphate concentration since the rate of transfer of phosphate from the intracellular pool to the extracellular pool is relatively slow (DeSoi and Umans, 1993). A rapid reduction in serum phosphate is seen at the beginning of dialysis (first 60–90 min), but this slows as the concentration gradient between the plasma and dialysate fluid falls. The rate of phosphate transfer from the plasma consequently decreases and serum phosphate levels plateau (DeSoi and Umans, 1993). After dialysis, a significant rebound of phosphate can occur (DeSoi and Umans, 1993) resulting in a potential return to the pre-dialysis serum phosphate concentrations (DeSoi and Umans, 1993).
Removal of phosphate will also depend on the type and extent of dialysis performed.
For example the elimination of phosphate ranges between 300 and 1200 mg per day depending of the type of dialysis used: A standard 4 h haemodialysis treatment removes between 600 and 1200 mg/day (1800–3600 mg/week) compared to continuous ambulatory peritoneal dialysis which removes between 300 and 360 mg/day (2100–2520 mg/week) (Bellasi et al., 2006).
Modifying the dialysis frequency and length of treatment may improve efficiency. Two studies have shown a significant lowering of phosphate levels using innovative dialysis techniques (Ayus et al., 2005, Mucsi et al., 1998). One study trialled the short daily haemodialysis (six 3-h sessions per week) (Ayus et al., 2005) while the other trialled nocturnal haemodialysis (six night-sessions per week for 8–10 h each while the patient is sleeping) (Mucsi et al., 1998). Both of these dialysis techniques are still investigational and their applicability and effectiveness as alternatives to the conventional dialysis techniques in the management of hyperphosphatemia yet to be determined.
In summary, dialysis treatments even when combined with dietary phosphate restriction are usually ineffective in managing hyperphosphatemia. Most dialysis patients require phosphate binders to control their hyperphosphatemia.
5.3. Minimising phosphate absorption: reducing intestinal absorption (phosphate binders)
Using phosphate binders to assist in the management of hyperphosphatemia in patients undergoing dialysis is common with more than 95% of patients being prescribed phosphate binders (Elseviers and De Vos, 2009). Phosphate binders work by binding dietary phosphate and forming insoluble complexes that are excreted by the gut (Tonelli et al., 2010, Hutchison et al., 2011).
The most commonly utilised phosphate binders are: (Tonelli et al., 2010, Elseviers and de Vos, 2009).
-
a.
Calcium-based phosphate binders (calcium carbonate and calcium acetate).
-
b.
Non-absorbable polymers (sevelamer).
-
c.
Heavy metal salts (lanthanum carbonate and aluminium hydroxide).
5.3.1. Calcium-based phosphate binders
Calcium-based phosphate binders are the most widely prescribed phosphate binders (Elseviers and De Vos, 2009). They are more effective than placebo in reducing serum phosphate concentrations and PTH (Rudnicki et al., 1993). However, large doses of calcium-based binders (up to 17 g per day) are usually required to achieve adequate phosphate management (Chiu et al., 2009, Slatopolsky et al., 1986). This translates to high pill burden (for example a dose of 17 g of calcium carbonate would require approximately 13 tablets) to deliver the required doses (Chiu et al., 2009, Slatopolsky et al., 1986). In addition, their use is potentially associated with hypercalcaemia and calcification of vascular tissues (Delmez et al., 1992). This is of particular concern when these calcium-based phosphate binders are prescribed with vitamin D (Delmez et al., 1992).
Several studies have investigated the relationship between increased calcium load serum calcium concentration and overall mortality. A large study of 40,538 haemodialysis patients reported a direct relationship with each 0.25 mmol/l increase in serum calcium associated with a 20% increase in relative risk of death (Block et al., 2004). Other studies have also found a relationship between an increase in the calcium load and the progression of vascular calcification especially in patients with low-turnover bone diseases (Chertow et al., 2002, Chertow et al., 2004, London et al., 2008).
5.3.2. Non-absorbable polymers (sevelamer)
Sevelamer hydrochloride was the first member of its class to be marketed. It is a non-absorbable, synthetic ion-exchange polymer that binds phosphate and inhibits its absorption by the body (Hutchison et al., 2011). Polymers are considered to be as effective as the calcium-based phosphate binders (Chertow et al., 2002) but are much more expensive and have significant gastrointestinal side effects (Madan et al., 2008).
The possibility of hypercalcaemia and lowered concentrations of PTH are less likely to occur with sevelamer (Chertow et al., 2002). In addition, the ‘Treat to Goal Study’ (200 haemodialysis patients) found a significant reduction in the progression of vascular calcification in the sevelamer-treated group compared with the calcium-containing binders treated group over the 52-week study period (Chertow et al., 2002).
Proposed other benefits with sevelamer are an anti-inflammatory effect, a favourable effect on the blood lipid profile (due to an ability to bind with cholesterol in the GIT), and a reduction in uric acid (Chertow et al., 1999, Ferramosca et al., 2005, Garg et al., 2005).
Despite these advantages, the relatively high price and high tablet burden as well as the gastrointestinal side effects are the main drawbacks for the use of sevelamer in the management of hyperphosphatemia in CKD patients (Chiu et al., 2009, Madan et al., 2008).
5.3.3. Heavy metal salts (lanthanum carbonate and aluminium hydroxide)
5.3.3.1. Lanthanum carbonate (LC)
Lanthanum carbonate (LC) is a relatively new agent that has been approved as a phosphate binder in dialysis patients. Lanthanum is a “rare-earth element” that has been shown to be effective in binding phosphate and consequently managing hyperphosphatemia (Albaaj and Hutchison, 2005). The risk of accumulation in the body was shown to be minimal as only 0.00005% of lanthanum was found to be absorbed by canine intestine (Albaaj and Hutchison, 2005). In a one-year randomised study, lanthanum improved bone diseases (confirmed by bone biopsies) as compared with calcium carbonate (D’Haese et al., 2003). However, the risk of accumulation has not been adequately studied in CKD patients and there is limited data on the effect of LC on bone histology over a longer treatment period (Bellasi et al., 2006). The risk of neurological damage should be low with LC since it does not cross the blood brain barrier (Albaaj and Hutchison, 2005).
Clinical studies have found LC to be a well-tolerated phosphate binder with minimal gastrointestinal side effects (Joy et al., 2003, Hutchison et al., 2004). Doses of 375–3000 mg/day have been able to reduce phosphate, Ca × P product and PTH concentrations in a dose-dependent fashion as compared with placebo (Joy et al., 2003).
In a head to head comparison with calcium carbonate, LC (750–3000 mg/day) has been shown to be equally effective as calcium carbonate (1500–3000 mg/day), achieving optimal phosphate control in approximately 65% of participants (n = 800) (Hutchison et al., 2005). However as would expected, LC was shown to be superior in reducing the incidence of hypercalcaemia (0.4% vs. 20.2%; p < 0.001) and calcium × phosphate product (Hutchison et al., 2005). Finn et al., have reported a LC to be safe, effective and tolerable in a study conducted over a three-year period (Finn et al., 2005).
In summary LC is a promising phosphate binder. Additional studies are needed to confirm its long-term safety profile and role in preventing vascular calcification (Bellasi et al., 2006).
5.3.3.2. Aluminium salts
Aluminium containing phosphate binders are highly effective. However, they have considerable side effects that have significantly limited their use. They have been associated with anaemia, dementia, encephalopathy and bone disease (Monier-Faugere and Malluche, 1996, Alfrey et al., 1976, von Bonsdorff et al., 1990). Currently, their use is confined to short-term management of hyperphosphatemia in cases resistant to other approaches (National Kidney Foundation, 2003).
In summary, Phosphate binders are the corner stone for the management of hyperphosphatemia in patients with CKD. All phosphate binders have been found to be equally effective in terms of phosphate control if given in equivalent doses (Navaneethan et al., 2011). Calcium based binders are most widely used because of their availability and affordability. However, they increase the risk of hypercalcaemia and the progression of vascular calcification and hence, may not be suitable binders in patients with hypercalcaemia or dialysis patients with advanced calcifications. Sevelamer and lanthanum do not appear to increase the risk of hypercalcaemia but they are relatively more expensive than calcium based binders. Both are felt to improve bone diseases in CKD patients, but long term studies are lacking. Sevelamer reduces the progression of calcification but lanthanum, however, is not yet studied in this regard. Aluminium is only used for short-term hyperphosphatemia management (National Kidney Foundation, 2003, Kidney Disease: Improving Global Outcomes, 2009).
5.4. Other significant medications affecting phosphate
Active vitamin D sterols such as calcitriol and alfacalcidol and their analogues (paricalcitol, doxercalciferol, 22-oxacalcitriol and maxacalcitol) are used to treat SHPT in CKD patients (Brickman et al., 1972, Chalmers et al., 1973, Tamura et al., 2005). They directly inhibit the secretion of PTH through the activation of vitamin D receptors in parathyroid glands (Martin et al., 1998). However, they increase the absorption of both calcium and phosphate (vitamin D analogues to a lesser extent) and thus may complicate hyperphosphatemia management and promote calcifications in CKD patients (Brickman et al., 1972, Chalmers et al., 1973, Sprague et al., 2003, Tamura et al., 2005, Martin et al., 1998).
Cinacalcet is a calcimimetic agent that binds and modulates calcium-sensing receptors in parathyroid glands resulting in reduction of PTH secretion and regression of parathyroid glands proliferation (Moe et al., 2005b). It is used to treat secondary hyperparathyroidism (SHPT) in patients with CKD (Moe et al., 2005b). Unlike vitamin D sterols, its use is associated with reduced phosphate concentrations, Ca × P product and calcium concentrations in haemodialysis patients (Moe et al., 2005a, Moe et al., 2005b, Lindberg et al., 2003).
6. Reasons for failure of phosphate control
Despite an increased understanding of the physiological processes involved in phosphate homoeostasis and improved therapy for the prevention and treatment of hyperphosphotaemia in CKD patients more than 50% of dialysis patients have their serum phosphate concentrations above the recommended target range of 1.13–1.78 mmol/L (Young et al., 2004, National Kidney Foundation, 2003). Schaefer has provided ten possible reasons for this failure: (Schaefer, 1994).
-
1.
Pseudohyperphosphataemia (incorrect sample handling; analytical error).
-
2.
High dietary phosphate intake.
-
3.
High doses of active vitamin D metabolites.
-
4.
Phosphate-containing drugs (enema, infusion).
-
5.
Inadequacy of haemodialysis.
-
6.
Advanced osteitis fibrosa (efflux of P from bone independent of intestinal P uptake).
-
7.
Metabolic acidosis (phosphate shift from intracellular into extracellular space).
-
8.
Patient non-compliance with phosphate binders.
-
9.
Incorrect intake of phosphate binders (timing and dosing).
-
10.
Inefficiency of calcium carbonate because of achlorhydria (spontaneous or after medication).
Although as listed above there are many interacting and overlapping reasons for hyperphosphotaemia in CKD patients, the influence of intestinal pH on calcium and phosphate absorption as well as on the potential efficiency of phosphate binders is of special interest.
Potential influences on binding efficiency are based on the following observations:
-
(1)
The pH of intestinal tracts can affect the absorption of calcium and phosphate.
-
(2)
All phosphate binding agents, with the exception of sevelamer, act as antacids and can affect the pH.
-
(3)
CKD patients are frequent users of acid suppressing drugs such as antacids, PPIs and H2-blockers.
The theory of an influence of pH on the absorption of calcium and phosphorous from dietary sources was reported in the 1930’s (McGowan, 1933, Patwardhan and Nhavi, 1939). The rate (but not the extent) of phosphate absorption was reported to be influenced by the pH of the canine stomach contents since different phosphate species exist in the intestinal tracts that have different absorption profiles at different pH ranges (Patwardhan and Nhavi, 1939). In the presence of excess calcium, pH can be neutralised with the production of a non-absorbable insoluble calcium phosphate salt (McGowan, 1933). If the pH is acidic, and calcium and phosphate are present in moderate and equal quantities then both will be soluble and able to be absorbed together (McGowan, 1933).
Calcium carbonate, aluminium and magnesium salts have been used for many years as over-the-counter antacid preparations (Rey et al., 2004) and lanthanum carbonate was also found to have an antacid action (Damment, 2011). Calcium carbonate, based on its acid neutralising capacity, is considered to be the most potent single antacid available (Maton and Burton, 1999) and reacts with gastric acid to form calcium chloride (CaCl2) according to the following equation (Fordtran and Locklear, 1966, Sheikh et al., 1989):
This more water-soluble calcium salt form is able to bind phosphate in the gut and provide calcium for absorption (Sheikh et al., 1989). This suggests that in a non-acidic environment the insoluble calcium carbonate will not be able to dissociate into free calcium ions that will bind phosphate to form an insoluble and hence non-absorbable salt in the absence of HCl (Sheikh et al., 1989).
This issue is of particular concern in dialysis patients as they are commonly prescribed gastric acid suppressive medications to control gastrointestinal symptoms which are common in these patients (Strid et al., 2002, Strid et al., 2003, Hammer et al., 1998). Medications such as H2-blockers (e.g. cimetidine, famotidine, ranitidine and nizatidine) and proton pump inhibitors (PPIs) (e.g. esomeprazole, lansoprazole, omeprazole and pantoprazole) are widely prescribed to dialysis patients.
A study by Strid et al. reported a higher and inappropriate use of acid suppressive medications in dialysis patients as compared with other hospitalised patients (41% vs 13%; p < 0.001) (Strid et al., 2003).
In conclusion, there is a high risk for a potentially significant interaction between acid suppressant and phosphate-binding drugs given the high rates of co-administration in CKD and dialysis patients.
7. Conclusion
Hyperphosphatemia in CKD patients is an important complication of reduced kidney function. It is associated with severe clinical consequences including cardiovascular tissues calcification, bone diseases, and secondary hyperparathyroidism leading to increased cardiovascular diseases and mortality rates. Therefore, dietary phosphate restrictions, adequate dialysis and phosphate binders are usually combined for effective management of hyperphosphatemia. However, attainment of phosphate control targets is difficult and may not be achieved in many patients. The efficacy of phosphate binders, especially calcium carbonate, may be influenced by the co-administration of acid suppressant drugs and thus, clinicians should be aware of the potential of such interaction and review all CKD patients for inappropriate use of acid suppressive medications.
Footnotes
Peer review under responsibility of King Saud University.
References
- Albaaj F., Hutchison A.J. Lanthanum carbonate for the treatment of hyperphosphataemia in renal failure and dialysis patients. Expert Opin. Pharmacother. 2005;6:319–328. doi: 10.1517/14656566.6.2.319. [DOI] [PubMed] [Google Scholar]
- Albright F., Ellsworth R. Studies on the physiology of the parathyroid glands: I. calcium and phosphorus studies on a case of idiopathic hypoparathyroidism. J. Clin. Invest. 1929;7:183–201. doi: 10.1172/JCI100224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfrey A.C., Legendre G.R., Kaehny W.D. The dialysis encephalopathy syndrome. Possible aluminum intoxication. N. Engl. J. Med. 1976;294:184–188. doi: 10.1056/NEJM197601222940402. [DOI] [PubMed] [Google Scholar]
- Aydogan T., Kanbay M., Uz B., Kaya A., Isik A., Bozalan R., Erkman M., Akcay A. Fatal hyperphosphatemia secondary to a phosphosoda bowel preparation in a geriatric patient with normal renal function. J. Clin. Gastroenterol. 2006;40:177. doi: 10.1097/01.mcg.0000196408.60851.cf. [DOI] [PubMed] [Google Scholar]
- Ayus J.C., Mizani M.R., Achinger S.G., Thadhani R., Go A.S., Lee S. Effects of short daily versus conventional hemodialysis on left ventricular hypertrophy and inflammatory markers: a prospective, controlled study. J. Am. Soc. Nephrol. 2005;16:2778–2788. doi: 10.1681/ASN.2005040392. [DOI] [PubMed] [Google Scholar]
- Baldock P.A., Thomas G.P., Hodge J.M., Baker S.U., Dressel U., O’Loughlin P.D., Nicholson G.C., Briffa K.H., Eisman J.A., Gardiner E.M. Vitamin D action and regulation of bone remodeling: suppression of osteoclastogenesis by the mature osteoblast. J. Bone Miner. Res. 2006;21:1618–1626. doi: 10.1359/jbmr.060714. [DOI] [PubMed] [Google Scholar]
- Bansal R.K., Tyagi P., Sharma P., Singla V., Arora V., Bansal N., Kumar A., Arora A. Iatrogenic hypervitaminosis D as an unusual cause of persistent vomiting: a case report. J. Med. Case Rep. 2014;8:74. doi: 10.1186/1752-1947-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsotti G., Lazzeri M., Cristofano C., Cerri M., Lupetti S., Giovannetti S. The role of metabolic acidosis in causing uremic hyperphosphatemia. Miner. Electrolyte Metab. 1986;12:103–106. [PubMed] [Google Scholar]
- Baumann K., de Rouffignac C., Roinel N., Rumrich G., Ullrich K.J. Renal phosphate transport: inhomogeneity of local proximal transport rates and sodium dependence. Pflugers Arch. 1975;356:287–298. doi: 10.1007/BF00580003. [DOI] [PubMed] [Google Scholar]
- Bellasi A., Kooienga L., Block G.A. Phosphate binders: new products and challenges. Hemodial. Int. 2006;10:225–234. doi: 10.1111/j.1542-4758.2006.00100.x. [DOI] [PubMed] [Google Scholar]
- Bellasi A., Ferramosca E., Ratti C., Block G., Raggi P. Cardiac valve calcification is a marker of vascular disease in prevalent hemodialysis patients. J Nephrol. 2012;25:211–218. doi: 10.5301/JN.2011.8446. [DOI] [PubMed] [Google Scholar]
- Berndt T.J., Knox F.G. Proximal tubule site of inhibition of phosphate reabsorption by calcitonin. Am. J. Physiol. 1984;246:F927–F930. doi: 10.1152/ajprenal.1984.246.6.F927. [DOI] [PubMed] [Google Scholar]
- Berndt T., Kumar R. Phosphatonins and the regulation of phosphate homeostasis. Ann. Rev. Physiol. 2007;69:341–359. doi: 10.1146/annurev.physiol.69.040705.141729. [DOI] [PubMed] [Google Scholar]
- Berndt T., Schiavi S., Kumar R. “Phosphatonins” and the regualtion of phosphorus homeostasis. Am. J. Physiol. 2005;289:1170–1182. doi: 10.1152/ajprenal.00072.2005. [DOI] [PubMed] [Google Scholar]
- Berndt T., Thomas L.F., Craig T.A., Sommer S., Li X., Bergstralh E.J., Kumar R. Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption. Proc. Natl. Acad. Sci. USA. 2007;104:11085–11090. doi: 10.1073/pnas.0704446104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block G.A., Klassen P.S., Lazarus J.M., Ofsthun N., Lowrie E.G., Chertow G.M. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J. Am. Soc. Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- Brickman A.S., Coburn J.W., Norman A.W. Action of 1,25-dihydroxycholecalciferol, a potent, kidney-produced metabolite of vitamin D, in uremic man. N. Engl. J. Med. 1972;287:891–895. doi: 10.1056/NEJM197211022871801. [DOI] [PubMed] [Google Scholar]
- Cai Q., Hodgson S.F., Kao P.C., Lennon V.A., Klee G.G., Zinsmiester A.R., Kumar R. Brief report: inhibition of renal phosphate transport by a tumor product in a patient with oncogenic osteomalacia. N. Engl. J. Med. 1994;330:1645–1649. doi: 10.1056/NEJM199406093302304. [DOI] [PubMed] [Google Scholar]
- Caverzasio J., Montessuit C., Bonjour J.P. Stimulatory effect of insulin-like growth factor-1 on renal Pi transport and plasma 1,25-dihydroxyvitamin D3. Endocrinology. 1990;127:453–459. doi: 10.1210/endo-127-1-453. [DOI] [PubMed] [Google Scholar]
- Chalmers T.M., Hunter J.O., Davie M.W., Szaz K.F., Pelc B., Kodicek E. 1-Alpha-hydroxycholecalciferol as a substitute for the kidney hormone 1,25-dihydroxycholecalciferol in chronic renal failure. Lancet. 1973;2:696–699. doi: 10.1016/s0140-6736(73)92536-1. [DOI] [PubMed] [Google Scholar]
- Chertow G.M., Burke S.K., Dillon M.A., Slatopolsky E. Long-term effects of sevelamer hydrochloride on the calcium x phosphate product and lipid profile of haemodialysis patients. Nephrol. Dial. Transplant. 1999;14:2907–2914. doi: 10.1093/ndt/14.12.2907. [DOI] [PubMed] [Google Scholar]
- Chertow G.M., Burke S.K., Raggi P., Treat to goal working G. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- Chertow G.M., Raggi P., Chasan-Taber S., Bommer J., Holzer H., Burke S.K. Determinants of progressive vascular calcification in haemodialysis patients. Nephrol. Dial. Transplant. 2004;19:1489–1496. doi: 10.1093/ndt/gfh125. [DOI] [PubMed] [Google Scholar]
- Chiu Y.W., Teitelbaum I., Misra M., de Leon E.M., Adzize T., Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin. J. Am. Soc. Nephrol. 2009;4:1089–1096. doi: 10.2215/CJN.00290109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craver L., Marco M.P., Martinez I., Rue M., Borras M., Martin M.L., Sarro F., Valdivielso J.M., Fernandez E. Mineral metabolism parameters throughout chronic kidney disease stages 1-5-achievement of K/DOQI target ranges. Nephrol. Dial. Transplant. 2007;22:1171–1176. doi: 10.1093/ndt/gfl718. [DOI] [PubMed] [Google Scholar]
- Cupisti A., Morelli E., D’Alessandro C., Lupeti S., Barsotti G. Phosphate control in chronic uremia: don’t forget diet. J. Nephrol. 2003;16:29–33. [PubMed] [Google Scholar]
- Damment S.J. Pharmacology of the phosphate binder, lanthanum carbonate. Ren. Fail. 2011;33:217–224. doi: 10.3109/0886022X.2011.552821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danisi G., Bonjour J.P., Straub R.W. Regulation of Na-dependent phosphate influx across the mucosal border of duodenum by 1,25-dihydroxycholecalciferol. Pflugers Arch. 1980;388:227–232. doi: 10.1007/BF00658486. [DOI] [PubMed] [Google Scholar]
- Danisi G., Caverzasio J., Trechsel U., Bonjour J.P., Straub R.W. Phosphate transport adaptation in rat jejunum and plasma level of 1,25-dihydroxyvitamin D3. Scand. J. Gastroenterol. 1990;25:210–215. [PubMed] [Google Scholar]
- de Toledo F.G., Thompson M.A., Bolliger C., Tyce G.M., Dousa T.P. Gamma-l-glutamyl-l-DOPA inhibits Na(+)-phosphate cotransport across renal brush border membranes and increases renal excretion of phosphate. Kidney Int. 1999;55:1832–1842. doi: 10.1046/j.1523-1755.1999.00419.x. [DOI] [PubMed] [Google Scholar]
- Delmez J.A., Tindira C.A., Windus D.W., Norwood K.Y., Giles K.S., Nighswander T.L., Slatopolsky E. Calcium acetate as a phosphorus binder in hemodialysis patients. J. Am. Soc. Nephrol. 1992;3:96–102. doi: 10.1681/ASN.V3196. [DOI] [PubMed] [Google Scholar]
- Desoi C.A., Umans J.G. Phosphate kinetics during high-flux hemodialysis. J. Am. Soc. Nephrol. 1993;4:1214–1218. doi: 10.1681/ASN.V451214. [DOI] [PubMed] [Google Scholar]
- D’Haese P.C., Spasovski G.B., Sikole A., Hutchison A., Freemont T.J., Sulkova S., Swanepoel C., Pejanovic S., Djukanovic L., Balducci A., Coen G., Sulowicz W., Ferreira A., Torres A., Curic S., Popovic M., Dimkovic N., de Broe M.E. A multicenter study on the effects of lanthanum carbonate (Fosrenol) and calcium carbonate on renal bone disease in dialysis patients. Kidney Int. Suppl. 2003:S73–S78. doi: 10.1046/j.1523-1755.63.s85.18.x. [DOI] [PubMed] [Google Scholar]
- Dhingra R., Gona P., Benjamin E.J., Wang T.J., Aragam J., D’Agostino R.B.S.R., Kannel W.B., Vasan R.S. Relations of serum phosphorus levels to echocardiographic left ventricular mass and incidence of heart failure in the community. Eur. J. Heart Fail. 2010;12:812–818. doi: 10.1093/eurjhf/hfq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson I.R., Kodicek E. Effect of vitamin D deficiency on bone formation in the chick. Biochem. J. 1979;182:429–435. doi: 10.1042/bj1820429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elseviers M., de Vos J.Y. The use of phosphate binders: data from contributors to the European Practice Database. J. Ren. Care. 2009;35(Suppl 1):14–18. doi: 10.1111/j.1755-6686.2009.00051.x. [DOI] [PubMed] [Google Scholar]
- Ettinger D.S., Harker W.G., Gerry H.W., Sanders R.C., Saral R. Hyperphosphatemia, hypocalcemia, and transient renal failure. Results of cytotoxic treatment of acute lymphoblastic leukemia. JAMA. 1978;239:2472–2474. doi: 10.1001/jama.239.23.2472. [DOI] [PubMed] [Google Scholar]
- Ferramosca E., Burke S., Chasan-Taber S., Ratti C., Chertow G.M., Raggi P. Potential antiatherogenic and anti-inflammatory properties of sevelamer in maintenance hemodialysis patients. Am. Heart J. 2005;149:820–825. doi: 10.1016/j.ahj.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Finn W.F., Joy M.S., Group L.A.M.S. A long-term, open-label extension study on the safety of treatment with lanthanum carbonate, a new phosphate binder, in patients receiving hemodialysis. Curr. Med. Res. Opin. 2005;21:657–664. doi: 10.1185/030079905X41453. [DOI] [PubMed] [Google Scholar]
- Foley R.N., Parfrey P.S., Harnett J.D., Kent G.M., Martin C.J., Murray D.C., Barre P.E. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186–192. doi: 10.1038/ki.1995.22. [DOI] [PubMed] [Google Scholar]
- Fordtran J.S., Locklear T.W. Ionic constituents and osmolality of gastric and small-intestinal fluids after eating. Am. J. Dig. Dis. 1966;11:503–521. doi: 10.1007/BF02233563. [DOI] [PubMed] [Google Scholar]
- Freiberg J.M., Kinsella J., Sacktor B. Glucocorticoids increase the Na+–H+ exchange and decrease the Na+ gradient-dependent phosphate-uptake systems in renal brush border membrane vesicles. Proc. Natl. Acad. Sci. USA. 1982;79:4932–4936. doi: 10.1073/pnas.79.16.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlaender M.M., Kornberg Z., Wald H., Popovtzer M.M. Renal effect of vitamin D metabolites: evidence for the essential role of the 25(OH) group. Am. J. Physiol. 1983;244:F674–F678. doi: 10.1152/ajprenal.1983.244.6.F674. [DOI] [PubMed] [Google Scholar]
- Ganesh S.K., Stack A.G., Levin N.W., Hulbert-Shearon T., Port F.K. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J. Am. Soc. Nephrol. 2001;12:2131–2138. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- Garabedian M., Holick M.F., Deluca H.F., Boyle I.T. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc. Natl. Acad. Sci. USA. 1972;69:1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg J.P., Chasan-Taber S., Blair A., Plone M., Bommer J., Raggi P., Chertow G.M. Effects of sevelamer and calcium-based phosphate binders on uric acid concentrations in patients undergoing hemodialysis: a randomized clinical trial. Arthritis Rheum. 2005;52:290–295. doi: 10.1002/art.20781. [DOI] [PubMed] [Google Scholar]
- Garrett J.E., Capuano I.V., Hammerland L.G., Hung B.C., Brown E.M., Hebert S.C., Nemeth E.F., Fuller F. Molecular cloning and functional expression of human parathyroid calcium receptor cDNAs. J. Biol. Chem. 1995;270:12919–12925. doi: 10.1074/jbc.270.21.12919. [DOI] [PubMed] [Google Scholar]
- Goadby H.K., Stacey R.S. On the action of parathormone. Biochem. J. 1934;28:2092–2096. doi: 10.1042/bj0282092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez O.M., Mannstadt M., Isakova T., Rauh-Hain J.A., Tamez H., Shah A., Smith K., Lee H., Thadhani R., Juppner H., Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N. Engl. J. Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafdi Z., Couette S., Comoy E., Prie D., Amiel C., Friedlander G. Locally formed 5-hydroxytryptamine stimulates phosphate transport in cultured opossum kidney cells and in rat kidney. Biochem. J. 1996;320(Pt 2):615–621. doi: 10.1042/bj3200615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn L.A., Hevesy G.C., Lundsgaard E.C. The circulation of phosphorus in the body revealed by application of radioactive phosphorus as indicator. Biochem. J. 1937;31:1705–1709. doi: 10.1042/bj0311705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer J., Oesterreicher C., Hammer K., Koch U., Traindl O., Kovarik J. Chronic gastrointestinal symptoms in hemodialysis patients. Wien. Klin. Wochenschr. 1998;110:287–291. [PubMed] [Google Scholar]
- Hammerman M.R., Karl I.E., Hruska K.A. Regulation of canine renal vesicle Pi transport by growth hormone and parathyroid hormone. Biochim. Biophys. Acta. 1980;603:322–335. doi: 10.1016/0005-2736(80)90378-8. [DOI] [PubMed] [Google Scholar]
- Hammerman M.R., Rogers S., Hansen V.A., Gavin J.R., 3rd Insulin stimulates Pi transport in brush border vesicles from proximal tubular segments. Am. J. Physiol. 1984;247:E616–E624. doi: 10.1152/ajpendo.1984.247.5.E616. [DOI] [PubMed] [Google Scholar]
- Havard R.E., Reay G.A. Normal variations of the inorganic phosphate of blood. Biochem. J. 1925;19:882–887. doi: 10.1042/bj0190882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska K.A., Mathew S., Lund R., Qiu P., Pratt R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008;74:148–157. doi: 10.1038/ki.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H.J., Wu M.S. Fibroblast growth factor 23: a possible cause of left ventricular hypertrophy in hemodialysis patients. Am. J. Med. Sci. 2009;337:116–122. doi: 10.1097/MAJ.0b013e3181815498. [DOI] [PubMed] [Google Scholar]
- Huffer W.E., Kuzela D., Popovtzer M.M. Metabolic bone disease in chronic renal failure. I. Dialyzed uremics. Am. J. Pathol. 1975;78:365–384. [PMC free article] [PubMed] [Google Scholar]
- Huffer W.E., Kuzela D., Popovtzer M.M., Starzl T.E. Metabolic bone disease in chronic renal failure. II. Renal transplant patients. Am. J. Pathol. 1975;78:385–400. [PMC free article] [PubMed] [Google Scholar]
- Hutchison A.J., Speake M., Al-Baaj F. Reducing high phosphate levels in patients with chronic renal failure undergoing dialysis: a 4-week, dose-finding, open-label study with lanthanum carbonate. Nephrol. Dial. Transplant. 2004;19:1902–1906. doi: 10.1093/ndt/gfh282. [DOI] [PubMed] [Google Scholar]
- Hutchison A.J., Maes B., Vanwalleghem J., Asmus G., Mohamed E., Schmieder R., Backs W., Jamar R., Vosskuhler A. Efficacy, tolerability, and safety of lanthanum carbonate in hyperphosphatemia: a 6-month, randomized, comparative trial versus calcium carbonate. Nephron. Clin. Pract. 2005;100:c8–c19. doi: 10.1159/000084653. [DOI] [PubMed] [Google Scholar]
- Hutchison A.J., Smith C.P., Brenchley P.E. Pharmacology, efficacy and safety of oral phosphate binders. Nat. Rev. Nephrol. 2011;7:578–589. doi: 10.1038/nrneph.2011.112. [DOI] [PubMed] [Google Scholar]
- Inoue Y., Segawa H., Kaneko I., Yamanaka S., Kusano K., Kawakami E., Furutani J., Ito M., Kuwahata M., Saito H., Fukushima N., Kato S., Kanayama H.O., Miyamoto K. Role of the vitamin D receptor in FGF23 action on phosphate metabolism. Biochem. J. 2005;390:325–331. doi: 10.1042/BJ20041799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro M., Yamamoto H., Masuda M., Kozai M., Takei Y., Tanaka S., Sato T., Segawa H., Taketani Y., Arai H., Miyamoto K., Takeda E. Thyroid hormones regulate phosphate homoeostasis through transcriptional control of the renal type IIa sodium-dependent phosphate co-transporter (Npt2a) gene. Biochem. J. 2010;427:161–169. doi: 10.1042/BJ20090671. [DOI] [PubMed] [Google Scholar]
- Ix J.H., Shlipak M.G., Wassel C.L., Whooley M.A. Fibroblast growth factor-23 and early decrements in kidney function: the Heart and Soul Study. Nephrol. Dial. Transplant. 2010;25:993–997. doi: 10.1093/ndt/gfp699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan M., Butterworth P.J. Study of the mechanism by which the Na+-Pi co-transporter of mouse kidney proximal-tubule cells adjusts to phosphate depletion. Biochem. J. 1988;252:105–109. doi: 10.1042/bj2520105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janigan D.T., Hirsch D.J., Klassen G.A., Macdonald A.S. Calcified subcutaneous arterioles with infarcts of the subcutis and skin (“calciphylaxis”) in chronic renal failure. Am. J. Kidney Dis. 2000;35:588–597. doi: 10.1016/s0272-6386(00)70003-5. [DOI] [PubMed] [Google Scholar]
- Joy M.S., Finn W.F., Group L.A.M.S. Randomized, double-blind, placebo-controlled, dose-titration, phase III study assessing the efficacy and tolerability of lanthanum carbonate: a new phosphate binder for the treatment of hyperphosphatemia. Am. J. Kidney Dis. 2003;42:96–107. doi: 10.1016/s0272-6386(03)00554-7. [DOI] [PubMed] [Google Scholar]
- Katai K., Miyamoto K., Kishida S., Segawa H., Nii T., Tanaka H., Tani Y., Arai H., Tatsumi S., Morita K., Taketani Y., Takeda E. Regulation of intestinal Na+-dependent phosphate co-transporters by a low-phosphate diet and 1,25-dihydroxyvitamin D3. Biochem. J. 1999;343(Pt 3):705–712. [PMC free article] [PubMed] [Google Scholar]
- Kay H.D. Kidney phosphatase. Biochem. J. 1926;20:791–811. doi: 10.1042/bj0200791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempson S.A. Effects of fasting compared to low phosphorus diet on the kinetics of phosphate transport by renal brush-border membranes. Biochim. Biophys. Acta. 1985;815:85–90. doi: 10.1016/0005-2736(85)90477-8. [DOI] [PubMed] [Google Scholar]
- Kidney Disease: Improving Global Outcomes, C.K.D.M.B.D.W.G., 2009. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl., S1–130. [DOI] [PubMed]
- Kimura K., Saika Y., Otani H., Fujii R., Mune M., Yukawa S. Factors associated with calcification of the abdominal aorta in hemodialysis patients. Kidney Int. Suppl. 1999;71:S238–S241. doi: 10.1046/j.1523-1755.1999.07163.x. [DOI] [PubMed] [Google Scholar]
- Kuhlmann M.K. Practical approaches to management of hyperphosphatemia: can we improve the current situation? Blood Purif. 2007;25:120–124. doi: 10.1159/000096410. [DOI] [PubMed] [Google Scholar]
- Kurnik B.R., Hruska K.A. Mechanism of stimulation of renal phosphate transport by 1,25-dihydroxycholecalciferol. Biochim. Biophys. Acta. 1985;817:42–50. doi: 10.1016/0005-2736(85)90066-5. [DOI] [PubMed] [Google Scholar]
- Levin N.W., Hoenich N.A. Consequences of hyperphosphatemia and elevated levels of the calcium-phosphorus product in dialysis patients. Curr. Opin. Nephrol. Hypertens. 2001;10:563–568. doi: 10.1097/00041552-200109000-00003. [DOI] [PubMed] [Google Scholar]
- Lindberg J.S., Moe S.M., Goodman W.G., Coburn J.W., Sprague S.M., Liu W., Blaisdell P.W., Brenner R.M., Turner S.A., Martin K.J. The calcimimetic AMG 073 reduces parathyroid hormone and calcium x phosphorus in secondary hyperparathyroidism. Kidney Int. 2003;63:248–254. doi: 10.1046/j.1523-1755.2003.00720.x. [DOI] [PubMed] [Google Scholar]
- Liput J., Rose M., Galya C., Chen T.C., Puschett J.B. Inhibition by volume expansion of phosphate uptake by the renal proximal tubule brush border membrane. Biochem. Pharmacol. 1989;38:321–325. doi: 10.1016/0006-2952(89)90043-9. [DOI] [PubMed] [Google Scholar]
- Loh T.P., Saw S., Sethi S.K. Hyperphosphatemia in a 56-year-old man with hypochondrial pain. Clin. Chem. 2010;56:892–895. doi: 10.1373/clinchem.2009.136895. [DOI] [PubMed] [Google Scholar]
- London G.M., Marchais S.J., Guerin A.P., Boutouyrie P., Metivier F., de Vernejoul M.C. Association of bone activity, calcium load, aortic stiffness, and calcifications in ESRD. J. Am. Soc. Nephrol. 2008;19:1827–1835. doi: 10.1681/ASN.2007050622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan P., Bhayana S., Chandra P., Hughes J.I. Lower gastrointestinal bleeding: association with Sevelamer use. World J. Gastroenterol. 2008;14:2615–2616. doi: 10.3748/wjg.14.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchais S.J., Metivier F., Guerin A.P., London G.M. Association of hyperphosphataemia with haemodynamic disturbances in end-stage renal disease. Nephrol. Dial. Transplant. 1999;14:2178–2183. doi: 10.1093/ndt/14.9.2178. [DOI] [PubMed] [Google Scholar]
- Martin K.J., Gonzalez E.A., Gellens M., Hamm L.L., Abboud H., Lindberg J. 19-Nor-1-alpha-25-dihydroxyvitamin D2 (Paricalcitol) safely and effectively reduces the levels of intact parathyroid hormone in patients on hemodialysis. J. Am. Soc. Nephrol. 1998;9:1427–1432. doi: 10.1681/ASN.V981427. [DOI] [PubMed] [Google Scholar]
- Martin D.R., Ritter C.S., Slatopolsky E., Brown A.J. Acute regulation of parathyroid hormone by dietary phosphate. Am. J. Physiol. Endocrinol. Metab. 2005;289:E729–E734. doi: 10.1152/ajpendo.00065.2005. [DOI] [PubMed] [Google Scholar]
- Masuda M., Yamamoto H., Kozai M., Tanaka S., Ishiguro M., Takei Y., Nakahashi O., Ikeda S., Uebanso T., Taketani Y., Segawa H., Miyamoto K., Takeda E. Regulation of renal sodium-dependent phosphate co-transporter genes (Npt2a and Npt2c) by all-trans-retinoic acid and its receptors. Biochem. J. 2010;429:583–592. doi: 10.1042/BJ20100484. [DOI] [PubMed] [Google Scholar]
- Maton P.N., Burton M.E. Antacids revisited: a review of their clinical pharmacology and recommended therapeutic use. Drugs. 1999;57:855–870. doi: 10.2165/00003495-199957060-00003. [DOI] [PubMed] [Google Scholar]
- McGovern A.P., de Lusignan S., van Vlymen J., Liyanage H., Tomson C.R., Gallagher H., Rafiq M., Jones S. Serum phosphate as a risk factor for cardiovascular events in people with and without chronic kidney disease: a large community based cohort study. PLoS ONE. 2013;8:e74996. doi: 10.1371/journal.pone.0074996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J.P. Some considerations regarding and investigations into calcium and phosphorus metabolism. Biochem. J. 1933;27:934–942. doi: 10.1042/bj0270934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K., Tatsumi S., Sonoda T., Yamamoto H., Minami H., Taketani Y., Takeda E. Cloning and functional expression of a Na(+)-dependent phosphate co-transporter from human kidney: cDNA cloning and functional expression. Biochem. J. 1995;305(Pt 1):81–85. doi: 10.1042/bj3050081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K., Segawa H., Morita K., Nii T., Tatsumi S., Taketani Y., Takeda E. Relative contributions of Na+-dependent phosphate co-transporters to phosphate transport in mouse kidney: RNase H-mediated hybrid depletion analysis. Biochem. J. 1997;327(Pt 3):735–739. doi: 10.1042/bj3270735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe S.M., Chertow G.M., Coburn J.W., Quarles L.D., Goodman W.G., Block G.A., Drueke T.B., Cunningham J., Sherrard D.J., McCary L.C., Olson K.A., Turner S.A., Martin K.J. Achieving NKF-K/DOQI bone metabolism and disease treatment goals with cinacalcet HCl. Kidney Int. 2005;67:760–771. doi: 10.1111/j.1523-1755.2005.67139.x. [DOI] [PubMed] [Google Scholar]
- Moe S.M., Cunningham J., Bommer J., Adler S., Rosansky S.J., Urena-Torres P., Albizem M.B., Guo M.D., Zani V.J., Goodman W.G., Sprague S.M. Long-term treatment of secondary hyperparathyroidism with the calcimimetic cinacalcet HCl. Nephrol. Dial. Transplant. 2005;20:2186–2193. doi: 10.1093/ndt/gfh966. [DOI] [PubMed] [Google Scholar]
- Moe S.M., Zidehsarai M.P., Chambers M.A., Jackman L.A., Radcliffe J.S., Trevino L.L., Donahue S.E., Asplin J.R. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011;6:257–264. doi: 10.2215/CJN.05040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier-Faugere M.C., Malluche H.H. Trends in renal osteodystrophy: a survey from 1983 to 1995 in a total of 2248 patients. Nephrol. Dial. Transplant. 1996;11(Suppl 3):111–120. doi: 10.1093/ndt/11.supp3.111. [DOI] [PubMed] [Google Scholar]
- Mostellar M.E., Tuttle E.P., Jr. Effects of alkalosis on plasma concentration and urinary excretion of inorganic phosphate in man. J. Clin. Invest. 1964;43:138–149. doi: 10.1172/JCI104888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucsi I., Hercz G., Uldall R., Ouwendyk M., Francoeur R., Pierratos A. Control of serum phosphate without any phosphate binders in patients treated with nocturnal hemodialysis. Kidney Int. 1998;53:1399–1404. doi: 10.1046/j.1523-1755.1998.00875.x. [DOI] [PubMed] [Google Scholar]
- National Kidney Foundation 2000. Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am. J. Kidney Dis., 35, S1–140. [DOI] [PubMed]
- National Kidney Foundation, 2003. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am. J. Kidney Dis., 42, S1–201. [PubMed]
- Navaneethan S.D., Palmer S.C., Vecchio M., Craig J.C., Elder G.J., Strippoli G.F. Phosphate binders for preventing and treating bone disease in chronic kidney disease patients. Cochrane Database Syst. Rev. 2011:CD006023. doi: 10.1002/14651858.CD006023.pub2. [DOI] [PubMed] [Google Scholar]
- Nishi Y., Fujimoto S., Sasaki M., Mukai E., Sato H., Sato Y., Tahara Y., Nakamura Y., Inagaki N. Role of mitochondrial phosphate carrier in metabolism-secretion coupling in rat insulinoma cell line INS-1. Biochem. J. 2011;435:421–430. doi: 10.1042/BJ20101708. [DOI] [PubMed] [Google Scholar]
- O’Donovan R.M., Widnell C.C., Chen T.C., Puschett J.B. Parathyroid hormone transport effects and hormonal processing in primary cultured rat proximal tubular cells. Biochem. J. 1993;293(Pt 2):377–380. doi: 10.1042/bj2930377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira R.B., Cancela A.L., Graciolli F.G., dos Reis L.M., Draibe S.A., Cuppari L., Carvalho A.B., Jorgetti V., Canziani M.E., Moyses R.M. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin. J. Am. Soc. Nephrol. 2010;5:286–291. doi: 10.2215/CJN.05420709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfrey P.S., Foley R.N. The clinical epidemiology of cardiac disease in chronic renal failure. J. Am. Soc. Nephrol. 1999;10:1606–1615. doi: 10.1681/ASN.V1071606. [DOI] [PubMed] [Google Scholar]
- Patwardhan V.N., Nhavi N.G. The absorption of phosphates from the intestine. Biochem. J. 1939;33:663–670. doi: 10.1042/bj0330663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey E., Poves-Frances C., Sanchez G., Fueyo A., Badiola C., Diaz-Rubio M. Effects of effervescent ranitidine on gastric pH: comparison with almagate and placebo in fasting and postprandial conditions. Aliment. Pharmacol. Ther. 2004;20:683–688. doi: 10.1111/j.1365-2036.2004.02178.x. [DOI] [PubMed] [Google Scholar]
- Ribeiro S., Ramos A., Brandão A., Rebelo J.R., Guerra A., Resina C., Vila-Lobos A., Carvalho F., Remédio F., Ribeiro F. Cardiac valve calcification in haemodialysis patients: role of calcium-phosphate metabolism. Nephrol. Dial. Transplant. 1998;13:2037–2040. doi: 10.1093/ndt/13.8.2037. [DOI] [PubMed] [Google Scholar]
- Riccardi D., Park J., Lee W.S., Gamba G., Brown E.M., Hebert S.C. Cloning and functional expression of a rat kidney extracellular calcium/polyvalent cation-sensing receptor. Proc. Natl. Acad. Sci. USA. 1995;92:131–135. doi: 10.1073/pnas.92.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rix M., Andreassen H., Eskildsen P., Langdahl B., Olgaard K. Bone mineral density and biochemical markers of bone turnover in patients with predialysis chronic renal failure. Kidney Int. 1999;56:1084–1093. doi: 10.1046/j.1523-1755.1999.00617.x. [DOI] [PubMed] [Google Scholar]
- Ron D., Taitelman U., Michaelson M., Bar-Joseph G., Bursztein S., Better O.S. Prevention of acute renal failure in traumatic rhabdomyolysis. Arch. Intern. Med. 1984;144:277–280. [PubMed] [Google Scholar]
- Rostand S.G., Drueke T.B. Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int. 1999;56:383–392. doi: 10.1046/j.1523-1755.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- Rudnicki M., Hojsted J., Petersen L.J., Sorensen H.A., Hyldstrup L., Transbol I. Oral calcium effectively reduces parathyroid hormone levels in hemodialysis patients: a randomized double-blind placebo-controlled study. Nephron. 1993;65:369–374. doi: 10.1159/000187515. [DOI] [PubMed] [Google Scholar]
- Schaefer K. Unsatisfactory control of serum phosphate: why is it so common and what can be done? Nephrol. Dial. Transplant. 1994;9:1366–1367. [PubMed] [Google Scholar]
- Sheikh M.S., Maguire J.A., Emmett M., Santa ana C.A., Nicar M.J., Schiller L.R., Fordtran J.S. Reduction of dietary phosphorus absorption by phosphorus binders. A theoretical, in vitro, and in vivo study. J. Clin. Invest. 1989;83:66–73. doi: 10.1172/JCI113886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T., Hasegawa H., Yamazaki Y., Muto T., Hino R., Takeuchi Y., Fujita T., Nakahara K., Fukumoto S., Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- Shimada T., Kakitani M., Yamazaki Y., Hasegawa H., Takeuchi Y., Fujita T., Fukumoto S., Tomizuka K., Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatopolsky E., Gradowska L., Kashemsant C., Keltner R., Manley C., Bricker N.S. The control of phosphate excretion in uremia. J. Clin. Invest. 1966;45:672–677. doi: 10.1172/JCI105382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatopolsky E., Robson A.M., Elkan I., Bricker N.S. Control of phosphate excretion in uremic man. J. Clin. Invest. 1968;47:1865–1874. doi: 10.1172/JCI105877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatopolsky E., Weerts C., Lopez-Hilker S., Norwood K., Zink M., Windus D., Delmez J. Calcium carbonate as a phosphate binder in patients with chronic renal failure undergoing dialysis. N. Engl. J. Med. 1986;315:157–161. doi: 10.1056/NEJM198607173150304. [DOI] [PubMed] [Google Scholar]
- Slinin Y., Foley R.N., Collins A.J. Calcium, phosphorus, parathyroid hormone, and cardivascular disease in hemodialysis patients: the USRDS waves 1, 3, and 4 study. J. Am. Soc. Nephrol. 2005;16:1788–1793. doi: 10.1681/ASN.2004040275. [DOI] [PubMed] [Google Scholar]
- Spanos E., Freake H., Macauley S.J., Macintyre I. Regulation of vitamin D metabolism by calcium and phosphate ions in isolated renal tubules. Biochem. J. 1981;196:187–193. doi: 10.1042/bj1960187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague S.M., Llach F., Amdahl M., Taccetta C., Batlle D. Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int. 2003;63:1483–1490. doi: 10.1046/j.1523-1755.2003.00878.x. [DOI] [PubMed] [Google Scholar]
- Stevens L.A., Djurdjev O., Cardew S., Cameron E.C., Levin A. Calcium, phosphate, and parathyroid hormone levels in combination and as a function of dialysis duration predict mortality: evidence for the complexity of the association between mineral metabolism and outcomes. J. Am. Soc. Nephrol. 2004;15:770–779. doi: 10.1097/01.asn.0000113243.24155.2f. [DOI] [PubMed] [Google Scholar]
- Stoll R., Kinne R., Murer H. Effect of dietary phosphate intake on phosphate transport by isolated rat renal brush-border vesicles. Biochem. J. 1979;180:465–470. doi: 10.1042/bj1800465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strid H., Simrén M., Johansson A.-C., Svedlund J., Samuelsson O., Björnsson E.S. The prevalence of gastrointestinal symptoms in patients with chronic renal failure is increased and associated with impaired psychological general well-being. Nephrol. Dial. Transplant. 2002;17:1434–1439. doi: 10.1093/ndt/17.8.1434. [DOI] [PubMed] [Google Scholar]
- Strid H., Simrén M., Björnsson E.S. Overuse of acid suppressant drugs in patients with chronic renal failure. Nephrol. Dial. Transplant. 2003;18:570–575. doi: 10.1093/ndt/18.3.570. [DOI] [PubMed] [Google Scholar]
- Suki W.N., Martinez-Maldonado M., Rouse D., Terry A. Effect of expansion of extracellular fluid volume on renal phosphate handling. J. Clin. Invest. 1969;48:1888–1894. doi: 10.1172/JCI106155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe H.S., Martin T.J., Eisman J.A., Pilczyk R. Binding of parathyroid hormone to bovine kidney-cortex plasma membranes. Biochem. J. 1973;134:913–921. doi: 10.1042/bj1340913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura S., Ueki K., Mashimo K., Tsukada Y., Naitoh M., Abe Y., Kawai H., Tsuchida A., Wakamatsu R., Nojima Y. Comparison of the efficacy of an oral calcitriol pulse or intravenous 22-oxacalcitriol therapies in chronic hemodialysis patients. Clin. Exp. Nephrol. 2005;9:238–243. doi: 10.1007/s10157-005-0363-x. [DOI] [PubMed] [Google Scholar]
- Tentori F., Blayney M.J., Albert J.M., Gillespie B.W., Kerr P.G., Bommer J., Young E.W., Akizawa T., Akiba T., Pisoni R.L., Robinson B.M., Port F.K. Mortlaity risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The dialysis outcomes and practice patterns study (DOPPS) Am. J. Kidney Dis. 2008;52:519–530. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Tonelli M., Sacks F., Pfeffer M., Gao Z., Curhan G., Cholesterol, Recurrent events trial I. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- Tonelli M., Pannu N., Manns B. Oral phosphate binders in patients with kidney failure. N. Engl. J. Med. 2010;362:1312–1324. doi: 10.1056/NEJMra0912522. [DOI] [PubMed] [Google Scholar]
- Uribarri J. Phosphorus homeostasis in normal health and in chronic kidney disease patients with special emphasis on dietary phosphorus intake. Semin. Dial. 2007;20:295–301. doi: 10.1111/j.1525-139X.2007.00309.x. [DOI] [PubMed] [Google Scholar]
- Uy H.L., Guise T.A., de la Mata J., Taylor S.D., Story B.M., Dallas M.R., Boyce B.F., Mundy G.R., Roodman G.D. Effects of parathyroid hormone (PTH)-related protein and PTH on osteoclasts and osteoclast precursors in vivo. Endocrinology. 1995;136:3207–3212. doi: 10.1210/endo.136.8.7628353. [DOI] [PubMed] [Google Scholar]
- Velentzas C., Meindok H., Oreopoulos D.G., Meema H.E., Rabinovich S., Sutton D., Ogilvie R. Detection and pathogenesis of visceral calcification in dialysis patients and patients with malignant disease. Can. Med. Assoc. J. 1978;118:45–50. [PMC free article] [PubMed] [Google Scholar]
- von Bonsdorff M., Sipila R., Pitkanen E. Correction of haemodialysis-associated anaemia by deferoxamine. Effects on serum aluminum and iron overload. Scand. J. Urol. Nephrol. Suppl. 1990;131:49–54. [PubMed] [Google Scholar]
- Weidmann S.M. Studies on the skeletal tissues. 4. The renewal of inorganic phosphate in bones of various species of small mammal as a function of time. Biochem. J. 1956;62:593–601. doi: 10.1042/bj0620593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner A., Kinne R.K. Evolution of the Na-P(i) cotransport systems. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R301–R312. doi: 10.1152/ajpregu.2001.280.2.R301. [DOI] [PubMed] [Google Scholar]
- Wojcicki J.M. Hyperphosphatemia is associated with anemia in adults without chronic kidney disease: results from the National Health and Nutrition Examination Survey (NHANES): 2005–2010. BMC Nephrol. 2013;14:178. doi: 10.1186/1471-2369-14-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W., Tran T.L., Finegood D.T., van de Werve G. Dietary P(i) deprivation in rats affects liver cAMP, glycogen, key steps of gluconeogenesis and glucose production. Biochem. J. 2000;352(Pt 1):227–232. [PMC free article] [PubMed] [Google Scholar]
- Young E.W., Akiba T., Albert J.M., McCarthy J.T., Kerr P.G., Mendelssohn D.C., Jadoul M. Magnitude and impact of abnormal mineral metabolism in hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am. J. Kidney Dis. 2004;44:34–38. doi: 10.1053/j.ajkd.2004.08.009. [DOI] [PubMed] [Google Scholar]