Abstract
Glutamatergic neurotransmission governs excitatory signaling in the mammalian brain, and abnormalities of glutamate signaling have been shown to contribute to both epilepsy and hyperkinetic movement disorders. The etiology of many severe childhood movement disorders and epilepsies remains uncharacterized. We describe a neurological disorder with epilepsy and prominent choreoathetosis caused by biallelic pathogenic variants in FRRS1L, which encodes an AMPA receptor outer-core protein. Loss of FRRS1L function attenuates AMPA-mediated currents, implicating chronic abnormalities of glutamatergic neurotransmission in this monogenic neurological disease of childhood.
Main Text
Pediatric movement disorders encompass a heterogeneous group of neurodevelopmental and neurodegenerative disorders affecting movement and limiting activities of daily living.1 The etiology of many pediatric movement disorders remains uncharacterized at the current time. Epileptic-dyskinetic encephalopathies2 (MIM: 308350) are a recently described class of disorders. Although these were originally described in association with ARX (MIM: 300382) mutations,2 mutations in SCN8A3 (MIM: 600702), STXBP14 (MIM: 602926), and FOXG15 (MIM: 164874) have also been found to cause a similar phenotype characterized by dystonia and/or choreoathetosis, epilepsy, and encephalopathy.
The pathophysiology of choreoathetosis is poorly understood, although a loss of striatal inhibition appears to be a common mechanism.6 Prior work has implicated abnormalities of glutamatergic neurotransmission in individuals with Huntington disease (MIM: 143100), the most common cause of chorea. This includes a loss of putaminal7 (NMDA [N-methyl-d-aspartate]) and fronto-cortical8 (AMPA [α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid]) glutamatergic receptors in postmortem brain tissue in individuals with Huntington disease.
AMPA receptors (AMPARs) represent the most common receptor subtype in the brain and mediate most fast excitatory post-synaptic potentials. AMPARs also play important roles in learning and memory via their role in long-term potentiation. In the case of long-term potentiation, repetitive presynaptic glutamate release leads to enhanced intracellular sodium concentrations through AMPAR conduction. This in turn allows for NMDA receptor activation, which itself conducts inwardly directed calcium currents. Increased levels of intracellular calcium activate CAMKII (calcium-calmodulin kinase II) activity and lead to enhanced AMPAR function by both increasing cell-surface AMPAR tracking and phosphorylating AMPARs.
AMPARs are composed of four subunits, designated GluR1–GluR4. Functional AMPARs are composed of a dimer of dimers containing two GluR2 subunits plus two of GluR1, GluR2, or GluR3. A variety of post-transcriptional mechanisms contribute to the finely orchestrated regulation of AMPAR function. Alternative splicing mediates the choice of isoforms (designed flip or flop), whereas RNA editing effectively limits Ca2+ permeability. In addition, a host of diverse auxiliary subunits and interacting proteins also modulate AMPAR properties.
A recent study identified FRRS1L (ferric chelate reductase 1-like) as an important component of the outer core of AMPAR accessory proteins,9 suggesting that loss of FRRS1L would affect AMPAR constituency and thus AMPAR function. Our studies suggest that this indeed is the case and that pathogenic variants in FRRS1L (aka C9orf4 [MIM: 604574]) lead to an epileptic-dyskinetic encephalopathy.
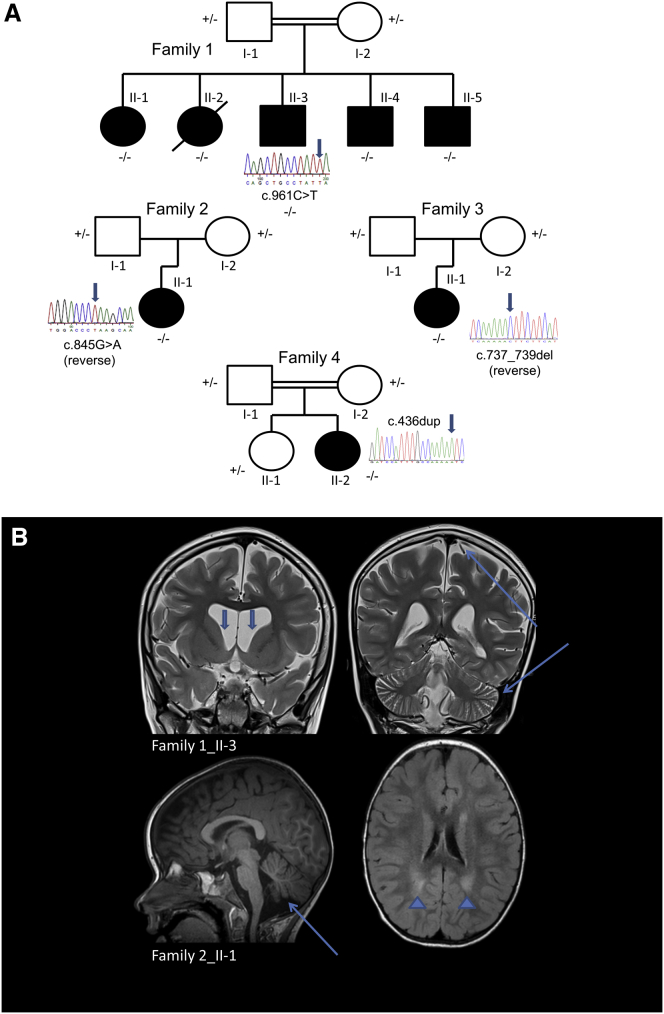
In our study, the index family hails from southern Saudi Arabia. The parents are distant cousins (Figure 1A). All five of the couple’s children exhibit encephalopathy, epilepsy, and progressive choreoathetosis (Table 1). One child (individual 1_II-2) had already succumbed to the disease at the time of ascertainment. All children were born at term without complications. All had normal early development but began to regress between the ages of 18 and 22 months, when they ceased to walk and lost expressive language. This coincided with the onset of hemiclonic and tonic-clonic seizures, which showed some response to carbamazepine and/or valproate. Examinations disclosed no dysmorphic features, normal fundi, global developmental delay, diffuse hypotonia, and generalized chorea with a paucity of volitional movement. Routine laboratory screening was unremarkable, as was triplet repeat analysis of HTT (MIM: 613004). Brain MRI was initially normal, but repeat neuroimaging demonstrated diffuse cortical and cerebellar volume loss and flattening of the caudate heads (Figure 1B). All of the affected children became less responsive to their environment over time, and their hyperkinetic movement disorder gradually gave way to a rigid, akinetic state in late adolescence.
Figure 1.
Families Affected by Choreoathetosis, Epilepsy, and Cerebral Volume Loss
(A) A total of eight individuals (one deceased) from four unrelated families are affected by this progressive neurological disease.
(B) Brain MRI shows diffuse cortical and cerebellar volume loss (long arrows), ex vacuo ventriculomegaly and flattening of the heads of the caudate nuclei (short arrows), and periventricular FLAIR (fluid attenuation inversion recovery) hyperintense signal (arrowheads).
Table 1.
Clinical Features of Individuals with FRRS1L Mutations
| Individual | Mutation | Age at Onset | Regression | Intellectual Impairment | Movement Disorder | Epilepsy | Neuromotor Impairment | Other |
|---|---|---|---|---|---|---|---|---|
| 1_II-1 | c.961C>T (p.Gln321∗) | 18 months | yes | severe; no expressive speech | chorea; later rigidity, hypokinesia | generalized tonic-clonic seizures | spasticity; impaired volitional movement | – |
| 1_II-2 | c.961C>T (p.Gln321∗) | 22 months | yes | severe; no expressive speech | chorea; later rigidity, hypokinesia | hemiclonic seizures; later generalized | spasticity; impaired volitional movement | died at 16 years of age |
| 1_II-3 | c.961C>T (p.Gln321∗) | 22 months | yes | severe; no expressive speech | chorea; later rigidity, hypokinesia | hemiclonic seizures | spasticity; impaired volitional movement | – |
| 1_II-4 | c.961C>T (p.Gln321∗) | 22 months | yes | severe; no expressive speech | chorea; later rigidity, hypokinesia | generalized seizures; hemiclonic seizures | spasticity; impaired volitional movement | – |
| 1_II-5 | c.961C>T (p.Gln321∗) | 24 months | no | severe; no expressive speech | chorea | hemiclonic seizures | – | – |
| 2_II-1 | c.845G>A (p.Trp282∗) | 4 months | no | severe; no expressive speech | chorea; ballism | multifocal; intractable | hypotonia; impaired volitional movement | – |
| 3_II-1 | c.737_739del (p.Gly246del) | 24 months | yes | severe; no expressive speech | chorea; cogwheel rigidity | juvenile spasms evolved to Lennox-Gastaut syndrome | hypotonia; impaired volitional movement | horizontal and vertical nystagmus |
| 4_II-2 | c.436dup (p.Ile146Asnfs∗10) | 6 months | yes | severe; no expressive speech | chorea; myoclonus | multifocal; clonic | hypotonia; no volitional movement | – |
Given the family structure and putative autosomal-recessive nature of the disorder, we then applied tandem homozygosity mapping and whole-exome sequencing. Family members were enrolled in our institutional-review-board-approved protocol after written informed consent was obtained. Genomic DNA was extracted from whole blood, and homozygous regions shared by affected individuals but absent in unaffected individuals in family 1 were identified with Affymetrix Whole-Genome CytoScan HD SNP arrays according to the manufacturer’s instructions. We identified six >3 Mb homozygous regions shared by affected family members alone (Table S1).
We performed whole-exome sequencing on individuals 1_II-1 to 1_II-4 and focused our analysis on homozygous stop-gain, stop-loss, or frameshift and splice-site or missense variants predicted to be deleterious. Exonic targets were enriched with the SeqCap EZ Human Exome Library v.2.0 (Nimblegen), and sequencing was performed on an Illumina HiSeq 2500 according to the manufacturer’s instructions for the generation of paired-end reads. Average coverage depth was >70× within the targeted exome. Sequencing reads were aligned to the NCBI human reference genome (GRCh37) with the Burrow-Wheeler Aligner (0.7.10). Picard Tools (1.111) was subsequently used for sorting and marking duplicates. Local realignment, base-quality-score recalibration, and variant calling were performed with the Genome Analysis Toolkit (GATK 3.3.-0), and variant-call files were evaluated with NextCode or Cartagenia Bench Lab next-generation sequencing software. Filtering included removing low-quality calls (Illumina threshold < 20) and variants with a minor allele frequency > 0.01 in the Exome Aggregation Consortium (ExAC) Browser and NHLBI Exome Sequencing Project (ESP) Exome Variant Server while prioritizing homozygous variants predicted to be deleterious by SIFT, PolyPhen-2, MutationTaster, and CADD.
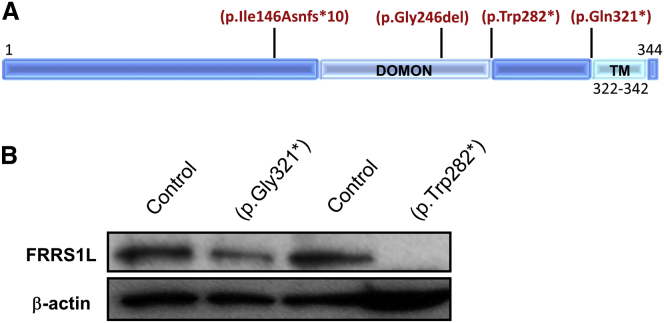
Using this strategy, we identified a homozygous c.961C>T (p.Gln321∗) variant (GenBank: NM_014334.2) in FRRS1L within a prominent block of homozygosity (chr9: 104,622,396–119,149,440; UCSC Genome Browser hg19). We confirmed segregation within affected family members by Sanger sequencing (Figure 1A). This variant has not been reported in variant repositories and is predicted to lead to loss of a C-terminal hydrophobic motif that might contribute to FRRS1L’s membrane localization (Figure 2A).
Figure 2.
Variants in Human FRRS1L and Effects on Protein Abundance
(A) The three variants leading to premature truncation codons are all predicted to lead to loss of the FRRS1L transmembrane (TM) domain, whereas p.Ile146Asnfs∗10 and p.Gly246del are predicted to disrupt the predicted transmembrane and extracellular DOMON (dopamine beta-monooxygenase N-terminal) domain (InterPro: IPR005018).
(B) Immunoblot in affected fibroblasts demonstrates lower amounts of FRRS1L in individuals with premature stop variants than in age- and sex-matched control individuals (representative image); anti-FRRS1L (1:1,000, Atlas, Sigma-Aldrich) and anti-β-actin (1:1,000, Santa Cruz) served as loading controls.
We subsequently identified three additional, unrelated individuals with similar phenotypes and biallelic FRRS1L variants by whole-exome sequencing (Table 1) and confirmed sequence changes by Sanger sequencing (Figure 1A). None of these variants has been identified in public repositories. Individual 4_II-2 was the most severely affected.
Individual 2_II-1, an unrelated girl, was born to non-consanguineous parents of mixed European descent after an unremarkable pregnancy at 31 weeks of gestation. At 4 months of age, she started having choreoathetotic movements of her hands, opisthotonic posturing, and occasional hand tremors. She reached developmental motor and language milestones until the age of 13 months, when she began to have generalized tonic-clonic seizures. During periods of illness, her seizures were accompanied by chaotic eye movements and forced gaze deviation, frequent episodes of tone loss, fluctuating levels of alertness, and truncal hypotonia. Between 14 and 27 months of age, her seizures remained refractory to levetiracetam, phenobarbital, clobazam, and the ketogenic diet. This was associated with developmental regression, and she remained nonverbal with diminished volitional movement. Her examination was significant for generalized chorea and ballism (Movie S1). Whole-exome sequencing identified a homozygous c.845G>A (p.Trp282∗) change in FRRS1L.
Individual 3_II-1, a Puerto Rican girl with no recognized consanguinity, was born at an estimated 37 weeks of gestation. She began to cruise with an uncoordinated gait at 11 months and used several words appropriately before experiencing a neurodevelopmental regression at the age of 24 months. She developed juvenile spasms and was later diagnosed with Lennox-Gastaut syndrome. She initially benefited from the ketogenic diet, but her seizures were never fully controlled. Examination revealed horizontal and vertical nystagmus with esotropia, generalized chorea and cogwheel rigidity of her upper limbs, hyperreflexia, hypotonia, and diminished volitional movement (she did not reach purposefully or have a means of locomotion). No expressive language was evident. A homozygous in-frame deletion (c.737_739del [p.Gly246del]) was identified by whole-exome sequencing. This variant was predicted to be deleterious by PROVEAN (score = −7.386).
Individual 4_II-1, an unrelated girl, was born at term by caesarean section to consanguineous Moroccan parents after a pregnancy complicated by gestational diabetes. Poor muscle tone was noted from early infancy, and she never attained good head control. She was delayed in reaching early motor milestones. At the age of 10 months, she could fix and follow objects, albeit with saccadic pursuit, and could grasp nearby objects and bring them to her mouth. She demonstrated choreoathetosis of her arms and hands. Her alertness fluctuated, putatively related to hypomotor seizures. These proved resistant to levetiracetam, valproic acid, and vigabatrin, and her eye contact and muscle tone deteriorated. At the age of 17 months, she developed status epilepticus. Phenobarbital led to satisfactory seizure control, and now she has mainly short, multifocal clonic seizures and intermittent myoclonus. She is currently fed via nasogastric tube. Expressive speech is absent. A homozygous variant in FRRS1L (c.436dup [p.Ile146Asnfs∗10]) was identified by whole-exome sequencing.
Targeted sequencing of FRRS1L in a cohort of 52 pediatric and adult individuals with early-onset Huntington-like disease and lacking triplet repeat expansions in HTT did not identify any predicted pathogenic alleles. Expanding our analysis to >500 in-house exomes of individuals with neurodegenerative disease of unknown etiology revealed four alleles predicted to be deleterious in the heterozygous form (Table S2). No biallelic FRRS1L variants predicted to be deleterious were detected in datasets from the Epi4K and EuroEPINOMICS-RES consortia.
Although FRRS1L is predominantly expressed in the brain, it is expressed at a lower level in fibroblasts (Figure 2B), as are AMPARs.10 Using affected fibroblasts and age- and sex-matched controls, we next determined that FRRS1L amounts were markedly reduced in both p.Gln321∗- and p.Trp282∗-affected cells (Figure 2B). Given that the c.961C>T variant is found within the final exon of the transcript, we sought to determine whether this mRNA escapes nonsense-mediated decay. We found comparable levels of both control and mutant transcripts (Figure S1), indicating that the decreased abundance of FRRS1L in c.961C>T-affected cells is most likely related to post-transcriptional mechanisms.
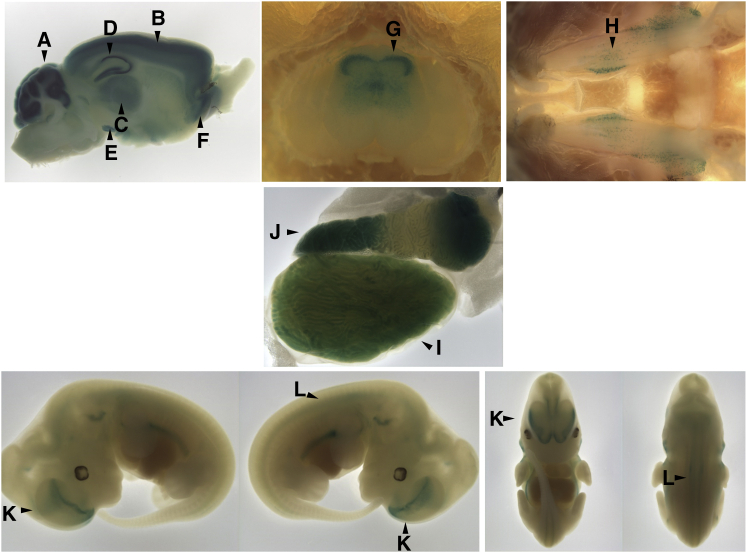
Given the severe neurological phenotype in the affected individuals, we next evaluated Frrs1l expression in embryos and adult mice by using a lacZ reporter. In embryonic day 12.5 (E12.5) embryos, Frrs1l expression was evident in the ventral forebrain, but a lower level of expression was seen in the remainder of the embryos. Frrs1l expression was highest in the adult brain in the cortex, cerebellum, hippocampus, and basal ganglia (Figure 3). This is consistent with prior reports of robust expression in multiple brain regions, including the striatum, thalamus, and cortex.7, 8 Developmentally, FRRS1L might play a role in neuronal maturation, given that AMPARs promote the formation and maturation of synapses during development.11
Figure 3.
Frrs1l Expression Pattern
Murine Frrs1l expression detected by lacZ reporter. Frrs1l is expressed throughout the adult brain, predominantly in the cerebellum (A) and differentially throughout layers of the cortex (B), thalamus (C), hippocampus (D), substantia nigra (E), and anterior olfactory nucleus (F). There is expression in the dorsal horn of the lumbar spinal cord (G) and the trigeminal ganglion (H). Outside of the nervous system, Frrs1l is expressed in the epididymis (I) and seminiferous tubules (J) of the testis. During development (E12.5), Frrs1l is expressed in the ventral forebrain (K) and weakly in the spinal cord (L).
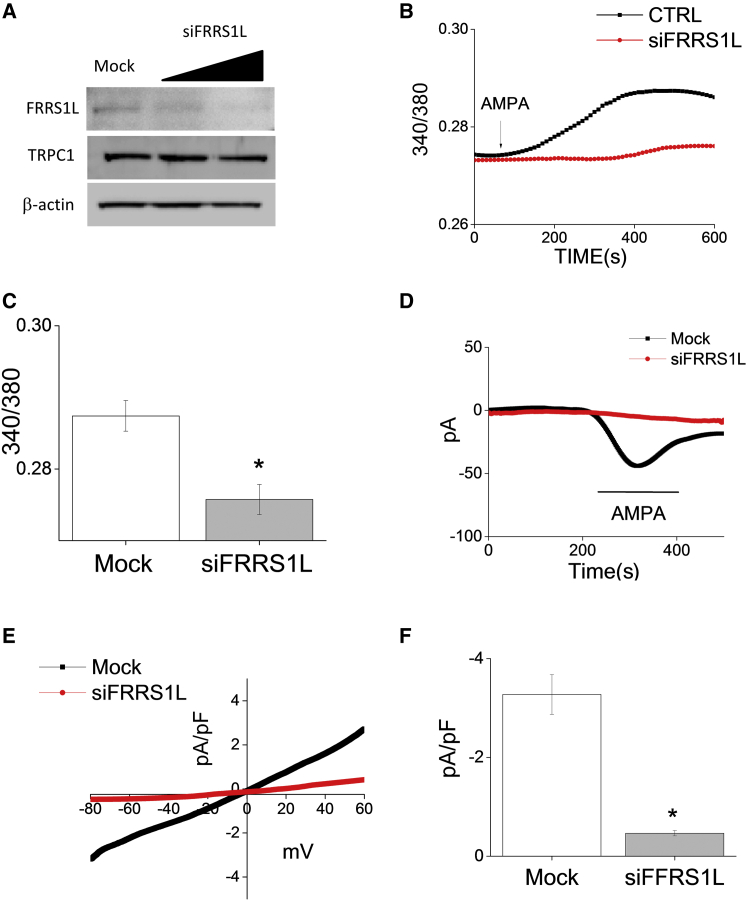
Little is known about the biological function of FRRS1L. Despite its name (ferric chelate reductase 1-like), it lacks a ferrochelatase domain and so is unlikely to function in iron storage and processing. In a prior study, FRRS1L co-immunoprecipitated with GluR1, suggesting a direct interaction between the two proteins.9 We thus sought to determine whether the loss of FRRS1L in affected individuals could affect AMPAR function. The human sympathoadrenal cell line SH-SY5Y can be differentiated to a neuronal phenotype with the use of retinoic acid; these cell lines express subunits GluR1–GluR4 of AMPAR.12 Using neuronally differentiated SH-SY5Y cells and siRNA, we knocked down FRRS1L, leading to reduced FRRS1L amounts analogous to those seen in affected cells (Figure 4A). We next performed a series of patch-clamp experiments with a focus on AMPA-mediated currents. We found that loss of FRRS1L significantly attenuated calcium influx (Figures 4B and 4C) and diminished AMPA-induced inward currents (Figures 4D–4F).
Figure 4.
Effects of FRRS1L Loss on Electrophysiology
(A) siRNA knockdown of FRRS1L in neuronally differentiated SH-SY5Y cells led to FRRS1L amounts comparable to those seen in affected fibroblasts (n = 3); transient receptor potential channel 1 (1:500, Alomone) and β-actin served as loading controls.
(B) siFRRS1L-treated cells showed decreased Ca2+ influx after stimulation with 100 μM AMPA (representative tracing).
(C) Mean Ca2+ influx was significantly decreased by FRRS1L knockdown (mean ± SEM; n = 30–50 cells per condition; ∗p < 0.05).
(D) A bath applied 100 μM AMPA-induced inward currents to control and siFRRS1L cells at a holding potential of −80 mV (representative tracings).
(E) Current-voltage (I-V) curves under the conditions in (D).
(F) Mean current intensity at −80 mV (mean ± SEM; n = 8–10 recordings per condition; ∗p < 0.05).
Our findings implicate FRRS1L as an important modulator of glutamate signaling with substantial implications in health and disease. AMPAR complexity is only now beginning to be appreciated, given that dozens of auxiliary subunits govern ion-channel gating properties, and dynamic changes in AMPAR composition are likely to affect localization and abundance within the synapse.13, 14, 15 FRRS1L is an outer-core protein that is thought to function within the AMPAR and directly interact with inner-core components.9 The significant diminution of AMPA-mediated currents we observed with FRRS1L knockdown points to an important role for the protein in normal AMPAR function, and its relevance has not previously been appreciated. Additional studies are needed to determine whether FRRS1L influences AMPAR function by modulating trafficking or by influencing channel gating properties more directly. FRRS1L could also have other roles outside of its interaction with glutamate receptors.
Our findings suggest that loss-of-function pathogenic variants might impair normal glutamatergic neurotransmission and thus lead to choreoathetosis, epilepsy, and persistent encephalopathy related to chronic disruption of normal neuronal-circuit function. Interestingly, pathogenic STXBP1 variants, which also lead to an epileptic-dyskinetic encephalopathy, putatively impair normal SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor)-mediated neurotransmitter release, including glutamate exocytosis into the synaptic cleft.16 Pathogenic variants in another AMPAR outer-core component, PRRT2 (MIM: 614386), have been shown to lead to seizures and choreoathetosis in affected individuals.17 Individuals with mutations in PRRT2 generally have paroxysmal symptoms rather than a progressive course, but such cases typically involve heterozygous pathogenic variants rather than the homozygous pathogenic variants we observed in our individuals. The recent identification of individuals with biallelic PRRT2 mutations suggests a more severe phenotype in such individuals.18 The identification of additional individuals with pathogenic variants in FRRS1L or related AMPAR core proteins will help shed light on the role of these accessory proteins in modulating glutamatergic signaling in the CNS, whose complexities are now beginning to be unraveled.19
Acknowledgments
We thank the families for their gracious participation in these studies. M.C.K. has served as a grant reviewer for the Department of Defense and an advisory committee member for Lundbeck Inc. and receives grant support from Retrophin Inc. Medical Research Council Harwell is a member of the International Mouse Phenotyping Consortium and has received funding from the NIH for generating (U42OD011174) and/or phenotyping (U54HG006348) the Frrs1l-tm1b(EUCOMM)Hmgu mice. The research reported in this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. These studies were also supported by the Deanship of Scientific Research at King Saud University (RGP-VPP-301 to M.A.S.), by a Sanford Seed Grant (to A.M., P.L.C., and M.C.K.), by the NIH Centers for Mendelian Genomics (5U54HG006504 to R.L., K.B., and S.M.), by the NIH National Institute of Neurological Disorders and Stroke (NS083739 to M.C.K.), by the National Institute of Dental and Craniofacial Research (DE017102 to B.B.S.), and by a Doris Duke Charitable Foundation Clinical Scientist Development Award (CSDA2014112 to M.C.K.).
Published: May 26, 2016
Footnotes
Supplemental Data include one figure, two tables, and one movie and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2016.04.008.
Accession Numbers
The accession numbers for the pathogenic FRRS1L variants reported in this paper are ClinVar: SCV000257326 (c.961C>T), SCV000257327 (c.845G>A), SCV000257328 (c.737_739del), and SCV000257329 (c.436dup).
Web Resources
1000 Genomes Project, http://www.1000genomes.org/
Burrow-Wheeler Aligner, http://bio-bwa.sourceforge.net
ClinVar, http://www.ncbi.nlm.nih.gov/clinvar/
Combined Annotation Dependent Depletion, http://cadd.gs.washington.edu
Ensembl, http://www.ensembl.org/index.html
Exome Aggregation Consortium (ExAC) Browser, http://exac.broadinstitute.org/
Genome Analysis Toolkit, https://www.broadinstitute.org/gatk
HGVS, http://www.hgvs.org/
International Mouse Phenotyping Consortium, http://www.mousephenotype.org/
Mouse Genome Informatics, http://www.informatics.jax.org/
MutationTaster, http://www.mutationtaster.org/
NCBI GRCh37 Assembly, http://www.ncbi.nlm.nih.gov/assembly/GCF_000001405.13/
NHLBI Exome Sequencing Project (ESP) Exome Variant Server, http://evs.gs.washington.edu/EVS/
PICARD, http://picard.sourceforge.net/
Phosphosite Plus, http://www.phosphosite.org/
PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/
PROVEAN, http://provean.jcvi.org
SIFT, http://sift.jcvi.org/
UCSC Human Genome Browser (hg19), http://genome.ucsc.edu/cgi-bin/hgGateway
UniProt, http://www.uniprot.org/
Supplemental Data
References
- 1.Kruer M.C. Pediatric movement disorders. Pediatr. Rev. 2015;36:104–115. doi: 10.1542/pir.36.3.104. quiz 116, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guerrini R., Moro F., Kato M., Barkovich A.J., Shiihara T., McShane M.A., Hurst J., Loi M., Tohyama J., Norci V. Expansion of the first PolyA tract of ARX causes infantile spasms and status dystonicus. Neurology. 2007;69:427–433. doi: 10.1212/01.wnl.0000266594.16202.c1. [DOI] [PubMed] [Google Scholar]
- 3.Gardella E., Becker F., Møller R.S., Schubert J., Lemke J.R., Larsen L.H., Eiberg H., Nothnagel M., Thiele H., Altmüller J. Benign infantile seizures and paroxysmal dyskinesia caused by an SCN8A mutation. Ann. Neurol. 2016;79:428–436. doi: 10.1002/ana.24580. [DOI] [PubMed] [Google Scholar]
- 4.Deprez L., Weckhuysen S., Holmgren P., Suls A., Van Dyck T., Goossens D., Del-Favero J., Jansen A., Verhaert K., Lagae L. Clinical spectrum of early-onset epileptic encephalopathies associated with STXBP1 mutations. Neurology. 2010;75:1159–1165. doi: 10.1212/WNL.0b013e3181f4d7bf. [DOI] [PubMed] [Google Scholar]
- 5.Cellini E., Vignoli A., Pisano T., Falchi M., Molinaro A., Accorsi P., Bontacchio A., Pinelli L., Giordano L., Guerrini R., FOXG1 Syndrome Study Group The hyperkinetic movement disorder of FOXG1-related epileptic-dyskinetic encephalopathy. Dev. Med. Child Neurol. 2016;58:93–97. doi: 10.1111/dmcn.12894. [DOI] [PubMed] [Google Scholar]
- 6.Mink J.W. The Basal Ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch. Neurol. 2003;60:1365–1368. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- 7.Young A.B., Greenamyre J.T., Hollingsworth Z., Albin R., D’Amato C., Shoulson I., Penney J.B. NMDA receptor losses in putamen from patients with Huntington’s disease. Science. 1988;241:981–983. doi: 10.1126/science.2841762. [DOI] [PubMed] [Google Scholar]
- 8.Wagster M.V., Hedreen J.C., Peyser C.E., Folstein S.E., Ross C.A. Selective loss of [3H]kainic acid and [3H]AMPA binding in layer VI of frontal cortex in Huntington’s disease. Exp. Neurol. 1994;127:70–75. doi: 10.1006/exnr.1994.1081. [DOI] [PubMed] [Google Scholar]
- 9.Schwenk J., Harmel N., Brechet A., Zolles G., Berkefeld H., Müller C.S., Bildl W., Baehrens D., Hüber B., Kulik A. High-resolution proteomics unravel architecture and molecular diversity of native AMPA receptor complexes. Neuron. 2012;74:621–633. doi: 10.1016/j.neuron.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 10.Demêmes D., Lleixa A., Dechesne C.J. Cellular and subcellular localization of AMPA-selective glutamate receptors in the mammalian peripheral vestibular system. Brain Res. 1995;671:83–94. doi: 10.1016/0006-8993(94)01322-9. [DOI] [PubMed] [Google Scholar]
- 11.McKinney R.A. Excitatory amino acid involvement in dendritic spine formation, maintenance and remodelling. J. Physiol. 2010;588:107–116. doi: 10.1113/jphysiol.2009.178905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christnacher A., Sommer B. Alternative splicing of AMPA receptor subunits: regulation in clonal cell lines. FEBS Lett. 1995;373:93–96. doi: 10.1016/0014-5793(95)01016-8. [DOI] [PubMed] [Google Scholar]
- 13.Soto D., Coombs I.D., Renzi M., Zonouzi M., Farrant M., Cull-Candy S.G. Selective regulation of long-form calcium-permeable AMPA receptors by an atypical TARP, gamma-5. Nat. Neurosci. 2009;12:277–285. doi: 10.1038/nn.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomita S., Adesnik H., Sekiguchi M., Zhang W., Wada K., Howe J.R., Nicoll R.A., Bredt D.S. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature. 2005;435:1052–1058. doi: 10.1038/nature03624. [DOI] [PubMed] [Google Scholar]
- 15.von Engelhardt J., Mack V., Sprengel R., Kavenstock N., Li K.W., Stern-Bach Y., Smit A.B., Seeburg P.H., Monyer H. CKAMP44: a brain-specific protein attenuating short-term synaptic plasticity in the dentate gyrus. Science. 2010;327:1518–1522. doi: 10.1126/science.1184178. [DOI] [PubMed] [Google Scholar]
- 16.Patzke C., Han Y., Covy J., Yi F., Maxeiner S., Wernig M., Südhof T.C. Analysis of conditional heterozygous STXBP1 mutations in human neurons. J. Clin. Invest. 2015;125:3560–3571. doi: 10.1172/JCI78612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heron S.E., Grinton B.E., Kivity S., Afawi Z., Zuberi S.M., Hughes J.N., Pridmore C., Hodgson B.L., Iona X., Sadleir L.G. PRRT2 mutations cause benign familial infantile epilepsy and infantile convulsions with choreoathetosis syndrome. Am. J. Hum. Genet. 2012;90:152–160. doi: 10.1016/j.ajhg.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delcourt M., Riant F., Mancini J., Milh M., Navarro V., Roze E., Humbertclaude V., Korff C., Des Portes V., Szepetowski P. Severe phenotypic spectrum of biallelic mutations in PRRT2 gene. J. Neurol. Neurosurg. Psychiatry. 2015;86:782–785. doi: 10.1136/jnnp-2014-309025. [DOI] [PubMed] [Google Scholar]
- 19.Aoto J., Martinelli D.C., Malenka R.C., Tabuchi K., Südhof T.C. Presynaptic neurexin-3 alternative splicing trans-synaptically controls postsynaptic AMPA receptor trafficking. Cell. 2013;154:75–88. doi: 10.1016/j.cell.2013.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.