Abstract
Therapeutic hypothermia has emerged as the first empirically supported therapy for neuroprotection in neonates with hypoxic-ischemic encephalopathy (HIE). We used magnetic resonance spectroscopy (1H-MRS) to characterize the effects of hypothermia on energy metabolites, neurotransmitters, and antioxidants. Thirty-one neonates with HIE were studied during hypothermia and after rewarming. Metabolite concentrations (mmol/kg) were determined from the thalamus, basal ganglia, cortical grey matter, and cerebral white matter. In the thalamus, phosphocreatine concentrations were increased by 20% during hypothermia when compared to after rewarming (3.49 ± 0.88 vs. 2.90 ± 0.65, p < 0.001) while free creatine concentrations were reduced to a similar degree (3.00 ± 0.50 vs. 3.74 ± 0.85, p < 0.001). Glutamate (5.33 ± 0.82 vs. 6.32 ± 1.12, p < 0.001), aspartate (3.39 ± 0.66 vs. 3.87 ± 1.19, p < 0.05), and GABA (0.92 ± 0.36 vs. 1.19 ± 0.41, p < 0.05) were also reduced, while taurine (1.39 ± 0.52 vs. 0.79 ± 0.61, p < 0.001) and glutathione (2.23 ± 0.41 vs. 2.09 ± 0.33, p < 0.05) were increased. Similar patterns were observed in other brain regions. These findings support that hypothermia improves energy homeostasis by decreasing the availability of excitatory neurotransmitters, and thereby, cellular energy demand.
Keywords: Energy metabolism, glutamate, hypothermia, neonatal ischemia, neuroprotection, neurotransmitters
Introduction
Hypoxic-ischemic encephalopathy (HIE) is a leading cause of neonatal death and neurodevelopmental disability, including motor, sensorineural, and cognitive-behavioral impairments.1 Following multiple randomized clinical trials2–7 and endorsements from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (United States)8 and the National Institute for Health and Clinical Excellence (United Kingdom),9 therapeutic hypothermia (TH) is now standard of care for neuroprotection for neonates with HIE. Still, treatment effects are modest, and efforts to improve treatment efficacy would be improved with the availability of in vivo data detailing the effects of hypothermia in a clinical population.
Three decades ago, magnetic resonance spectroscopy (MRS) demonstrated that brain injury in neonatal HIE is an evolving process.10 Acutely, hypoxia-ischemia disrupts mitochondrial metabolism, leading to a rapid depletion of adenosine triphosphate (ATP) and phosphocreatine, high energy phosphates necessary for vital cellular functions, and a concomitant build-up of lactic acid and other anaerobic metabolites.11–14 Clinically, resuscitation restores cerebral blood flow and oxygenation, and in most cases, allows for the recovery of ATP and phosphocreatine to baseline.10 However, in neonates with HIE, this latent period is followed ∼8–24 h later by a period of secondary energy failure stemming from an accumulation of excitatory amino acids, intracellular calcium, cytotoxic edema, free radicals and reactive oxygen species, and, at the core, a progressive failure in mitochondrial metabolism (for a review, see Johnston et al.15).
Laboratory research has shown that hypothermia has significant effects on energy metabolism,16,17 mitochondrial membrane potential,18,19 and the availability of excitatory amino acids20 and reactive oxygen species.21 However, most of these effects have been documented in animal models whereby hypothermia is applied concurrent with or immediately following hypoxia-ischemia—i.e. acutely, when the neuroprotective effects of hypothermia are maximized. Far less is known about the effects of hypothermia during the latent period or early phase of secondary energy failure, ∼6–78 h after birth—the window for hypothermia in clinical practice. Yet, such data would not only allow for a more tailored approach to the delivery of hypothermia in neonates with HIE, but also foster the development of early biomarkers that could be used to direct adjuvant neuroprotective therapies toward the neonates who are most likely to benefit.
The aim of this study was to interrogate the effects of hypothermia on cerebral metabolism in human neonates with HIE. Using 1H-MRS, we quantitated the concentrations of key metabolites related to energy metabolism, neurotransmission, and antioxidant mechanisms during hypothermia and after rewarming. We hypothesized that hypothermia would exert broad effects on metabolites known to be involved in energy utilization, neurotransmission, and oxidative stress.
Methods
All procedures were approved by the Children’s Hospital Los Angeles (CHLA) Institutional Review Board and written consent was provided by a parent prior to their child’s participation in the research study.
Patient selection and recruitment
Between April 2012 and January 2015, 40 term neonates (gestational age ≥36 weeks) undergoing TH for HIE were prospectively recruited from the Newborn and Infant Critical Care Unit (NICCU) at CHLA for this observational research study. Per clinical protocol, whole-body hypothermia was initiated within 6 h after birth and maintained using a blanket cooling device (Blanketrol III, Cincinnati Subzero Medical) programmed to maintain rectal temperature at 33.5 ± 0.5℃. Patients were excluded from the research study if they had major congenital anomalies or required high frequency oscillatory ventilation, extra-corporeal membrane oxygenation support, or had other clinical conditions deemed unsafe for in-house transport to the magnetic resonance imaging (MRI) suite. Demographic and clinical outcome data were coded from the electronic medical record.
Of the 40 infants prospectively enrolled, seven were ultimately too unstable to undergo MRI during hypothermia, and thus, were excluded from the study. Additionally, two infants were retrospectively excluded, because they were later confirmed to be suffering from a congenital infection (n = 1) or be an infant of a substance abusing mother (n = 1). Data presented below represent the final sample (n = 31).
MR acquisition
Neonates underwent MRI once during hypothermia (median: 61.0 h after birth; interquartile range: 46.0–68.8 h) and again at least 24 h after rewarming (median: 139.3 h after birth; interquartile range: 116.5–187.0 h). During the first MRI, whole-body hypothermia was maintained using the same blanket-cooling device as above, modified with extension tubing.22,23 Rectal temperature was continuously monitored throughout both examinations and recorded via an MR compatible temperature probe (Invivo Corporation, Gainesville, FL).
MR data were acquired on a 3 T MR system (Philips Medical, Best, The Netherlands, Achieva, Ver. 3.2.1.1) using either a neonatal SENSE coil or a standard 8-channel SENSE head coil. To minimize movement during imaging, infants were secured in Med-Vac Immobilization Bag (CFI Medical, Fenton, MI) with multiple levels of ear protection, including earplugs, MiniMuffs (Natus Medical Incorporated, Pleasanton, CA), and standard head phones. Per research protocol, sedation was not administered for the MR examination; however, most neonates were receiving sedation as part of their intensive care, which was maintained during the MR examination (see Table 1, for further details).
Table 1.
Clinical and demographic characteristics.
| Gestational age (mean ± SD) | 39.0 ± 1.49 |
|---|---|
| Sex | 13 M; 18 F |
| Birth weight (g) | 3309 ± 540 |
| Apgar scores (median) | 11 45 510 |
| Cord blooda | |
| pH | 6.9 ± 0.1 |
| Base deficit | −18.7 ± 7.2 |
| Arterial blood gas at ≤ 1 hb | |
| pH | 7.15 ± 0.2 |
| Base deficit | −15.4 ± 6.3 |
| Sarnat stage (mild/moderate/severe) | 2/25/4 |
| Inotropes no. (%) | 11 (35%) |
| Antiepileptic medications no. (%) | 12 (39%) |
| Fentanyl sedation no. (%) | 25 (81%) |
| Morphine prn no. (%) | 9 (29%) |
| Rectal temperature during MRI | |
| During hypothermia | 33.3 ± 0.4℃ |
| After hypothermia | 36.5 ± 0.7℃ |
n = 25.
n = 26.
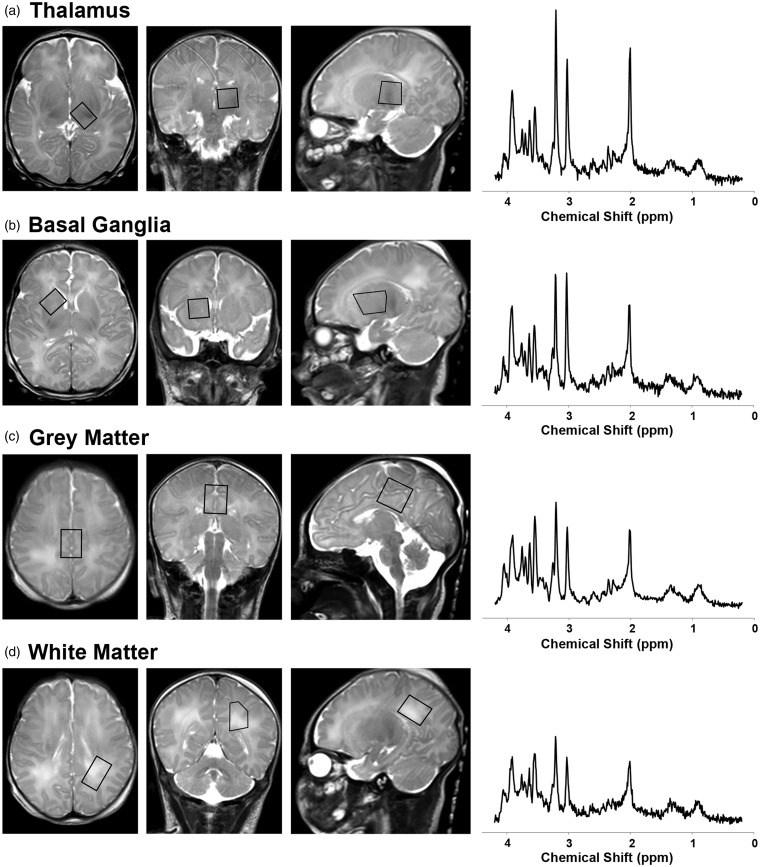
1H-MRS data were acquired using a single-voxel point resolved spectroscopy sequence (PRESS; TR = 2 s; TE = 35 ms; 128 signal averages; voxel size: ∼3 cm3) localized to the left thalamus, right basal ganglia, medial cortical grey matter and left parietal white matter on axial, sagittal and coronal T2-weighed images (Figure 1). (Note that the left parietal white matter region of interest was added to the imaging protocol after the tenth neonate.) These regions were selected a priori because they are known areas of vulnerability to hypoxia-ischemia in neonates and corresponded to the central (thalamus/basal ganglia/perirolandic cortex) and peripheral/watershed (white matter) patterns of brain injury in neonatal HIE.24 Finally, conventional T1-weighted, T2*-weighted, and diffusion-weighted images were acquired and reviewed by a neuroradiologist, together with the T2-weighted images, for evidence of acute infarction, hemorrhage or other brain injury. The total examination time was approximately 1 h.
Figure 1.
Overview of 1H-MRS acquisition. 1H-MRS spectra were acquired using a short-echo (TE 35 ms) PRESS sequence as shown here on a single neonate during hypothermia (note the high signal posterior to the occiput on the sagittal T2-weighted images, which arises from the water in the cooling blanket). MRS voxels (black boxes) were positioned in the (a) left thalamus, (b) right basal ganglia, (c) medial frontal-parietal cortical grey matter, and (d) left parietal white matter on axial, coronal and sagittal T2-weighted images. The acquired MRS spectra (raw, unsmoothed) are shown to the right of the localizer voxels. Metabolite concentrations were quantitated from these spectra in LCModel.25 (For reference, the prominent metabolites associated with each peak are labeled in Figure 5).
MRS data processing
Examples of 1H-MRS spectra are provided in Figure 1. Metabolite concentrations were estimated using commercially available and fully automated LCModel software (V6.3-1 C).25 This software applies zero-order and first-order corrections for phase, estimates a baseline, corrects for ppm shift and eddy currents as needed, and determines metabolite concentrations, without user interaction. Data were fitted with linear combinations of 45 model spectra of known concentration, which included: phosphocreatine, free creatine, glucose, citrate, lactate, alanine, N-acetyl-aspartate (NAA), acetoacetate, acetone, β-hydroxybutyrate, glutamate, glutamine, γ-aminobutyric acid (GABA), n-acetyl-apartyl glutamate (NAAG), glycine, taurine, and glutathione (i.e. metabolites of interest), as well as acetate, carnitine, cysteic acid, ethanolamine, fucose, glycerophosphocholine, phosphocholine, 2-hydroxyglutarate, myoinositol, phosphoethanolamine, propylene glycol, pyruvate, scyllo-inositol, succinate, threonine, guanadinoacetate, CH3-protons (lipids), CH2-CH2- protons (lipids), CH = CH-protons (lipids), polyunsaturated fatty acids, and macromolecules at ∼0.9, 1.2, 1.4, 1.7, and 2.0 ppm (see below for further details on metabolites of interest).
For quantitation, the unsuppressed water signal was used as a concentration reference, with tissue water content estimated at a standardized value (86%, i.e. 47.8 M) based on published reference data 26 (see also Figure S2 from Bluml et al.27). To ensure reliability, spectra of poor quality were excluded a priori based on stringent thresholds for linewidth (< 0.05 ppm, i.e. < 6.4 Hz) and signal to noise (≥10), which resulted in 5 out of 210 acquired spectra being excluded from analyses. For most metabolites, Cramér Rao Lower Bounds (CRLB) were <50%, and generally <20%, including phosphocreatine, free creatine, glucose, lactate, NAA, glutamate, glutamine, aspartate, NAAG, taurine, and glutathione (Table S1). However, a few metabolites, which were present in low concentration, had higher CRLB values (>50%), including citrate, acetoacetate, acetone, β-hydroxybutyrate, GABA, and glycine. These were retained in all subsequent analyses because higher CRLB values only serve to decrease statistical power and increase the risk of a type II error (failing to reject a false null hypothesis); there was no increased risk of type I error (falsely rejecting a true null hypothesis). However, caution is warranted as actual concentrations for these metabolites could be higher or lower than the values reported in the tables below.
Metabolites
Phosphocreatine and free creatine, which generate partially overlapping signals at ∼3.02 and 3.94 ppm (Figure S1), are metabolically inert compounds, critical for cellular energy homeostasis.28 In situations of increased energy demand, phosphocreatine functions as a cellular energy reserve by transferring its high-energy phosphate to ADP to maintain cellular ATP levels in a reversible reaction catalyzed by creatine kinase:
Glucose, which generates two small, complex peaks at ∼3.43 and 3.8 ppm, is the primary fuel for energy metabolism in the human brain and vital for the synthesis of many neurotransmitters, including glutamate, aspartate, and GABA.29 Under physiologic conditions, most cerebral glucose is metabolized aerobically, yielding carbon dioxide and water, or used in biosynthetic processes; however, in healthy neonates as much as 5% of glucose may be metabolized anaerobically to lactate.30
Lactate and alanine, which generate doublets at ∼1.33 and 1.48 ppm, respectively, are byproducts of anaerobic metabolism, which help maintain glycolysis, and in turn, ATP production when oxidative phosphorylation is insufficient, such as in the setting of hypoxia-ischemia.31
Citrate, which generates a multiplet centered at ∼2.6 ppm, is an intermediate product of the tricarboxylic acid (TCA) cycle.
NAA, which generates a prominent peak at ∼2.02 ppm as well as complex signals between ∼2.3 and 2.8 ppm, is synthesized in the mitochondria of neurons and axons from the amino acid aspartic acid and acetyl-coA.32,33 Although NAA is widely considered a marker for neuronal/axonal integrity,34 in this study, it is more appropriate to regard it as a marker of mitochondrial activity.
Acetoacetate (∼2.26 and 3.46 ppm), acetone (2.2 ppm), and β-hydroxybutyrate (1.2 ppm) are ketone bodies generated during the metabolism of fatty acids. Although typically non-detectable with MRS under physiological conditions due to inherently low concentrations, in healthy neonates, approximately 10% of cerebral oxidative metabolism stems from the consumption of ketone bodies.30,35,36
Glutamate, which generates complex signals at ∼2.36, 3.76, and 2.08 ppm (Figure S2), is the principal excitatory neurotransmitter in the human brain.37 It is synthesized from α-ketoglutarate, an intermediary in the TCA cycle, and is itself, an intermediary in the synthesis of GABA.38 It is estimated that as many as 60–70% of synapses in the mammalian brain utilize glutamate as their primary neurotransmitter.37
Glutamine, which generates complex signals at ∼2.45, 3.78 and 2.15 ppm, is an intermediary metabolite in multiple pathways supporting energy metabolism and neurotransmission. Under normal, physiological conditions excess glutamate released into the synaptic cleft is readily transported into perisynaptic astrocytes and converted to glutamine, which in turn, may be transported back into neurons and converted back into glutamate, i.e. the glutamate–glutamine cycle. Additionally, in astrocytes, glutamine can be synthesized from glucose or other energy substrates or utilized for energy metabolism or utilized as a precursor for the synthesis of glutathione.39
Aspartate, which generates complex signals at ∼2.7 and 3.9 ppm, is an excitatory neurotransmitter. Like glutamate, it is synthesized from a TCA-cycle intermediary, oxaloacetate.
GABA, which generates multiplets centered at ∼1.89, 2.3, and 3.01 ppm, is generally regarded as the principal inhibitory neurotransmitter40; however, in fetal and neonatal brains, GABA can function as an excitatory neurotransmitter triggering depolarization rather than hyperpolarization in the postsynaptic cell.41
NAAG, which generates a small peak at approximately 2.04 ppm, is the most abundant neuropeptide in the brain. Although the functions of NAAG are incompletely understood, NAAG binds to R3 metabotropic glutamatergic receptors on astrocytes and likely provides signals about the state of neurostimulation or changing requirements for vascular energy supply.42
Glycine, which generates a singlet at ∼3.56 ppm, is an inhibitory neurotransmitter predominantly localized to the spinal cord and subcortical regions43; however, it is also required as a co-agonist N-methyl-D-aspartate (NMDA) receptors, suggesting that it also plays a key role in excitatory neurotransmission and excitotoxic brain injury.44
Taurine, which generates a multiplet centered at ∼3.35 ppm, is widely regarded as an inhibitory neurotransmitter45 and osmolite.46,47
Glutathione, which generates a series of multiplets centered at ∼2.15, 2.55, 2.96, and 3.78 ppm, is an endogenous antioxidant.
Statistical analyses
Statistical analyses were performed using SPSS Software Version 21 (IBM Corporation) and carried out separately for each region of interest (thalamus, basal ganglia, cortical grey matter and white matter). Paired t tests were used to compare metabolite concentrations during and after TH. A p value of < 0.05 was considered statistically significant. Effect size was calculated using Cohen’s d, which can be interpreted as small (≥0.2), medium (≥0.5), or large (≥0.8).48
Results
Clinical and demographic data for the study cohort are summarized in Table 1. Overall, the characteristics of this sample are similar to those in the large-scale clinical trials for TH,2,4,6,7 with the exception that there are fewer infants here with severe Sarnat-stage HIE due in large part to the confounding fact that those infants are more often clinically too unstable to undergo MRI during hypothermia.
Energy reserves were higher during hypothermia while aerobic and anaerobic metabolism were reduced
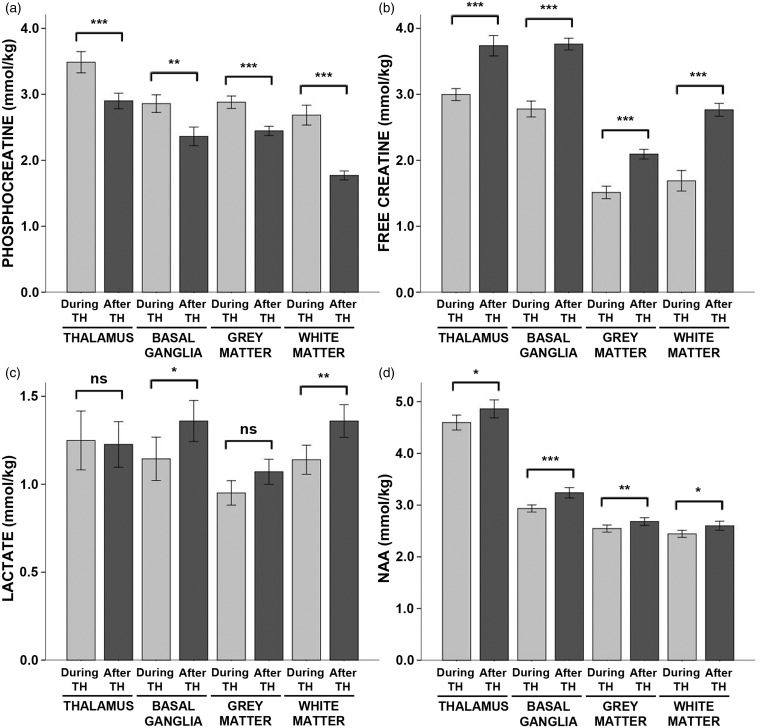
There were large effects of hypothermia on energy metabolites. Specifically, during hypothermia, phosphocreatine concentrations were increased by ∼20% in the thalamus, basal ganglia and cortical grey matter and by 50% in the cerebral white matter as compared to after rewarming, while free creatine concentrations were reduced by a similar degree (Figure 2; see also Table S2 for exact values, including means, standard deviations, and effect size for each contrast). Similarly, glucose concentrations were reduced by 22% in the thalamus while lactate was reduced by ∼16% in the basal ganglia and white matter during hypothermia. NAA was reduced by 5–10% in all four regions. Β-hydroxybutyrate concentrations were reduced slightly in the grey matter, but elevated in the white matter, during hypothermia relative to after rewarming. There were no differences in the concentrations citrate, acetoacetate, acetone, or alanine (Table S2).
Figure 2.
Energy metabolite concentrations during hypothermia versus after rewarming. Mean concentrations of (a) phosphocreatine, (b) free creatine, (c) lactate, and (d) NAA measured during hypothermia (light grey bars) and after rewarming (dark grey bars) are shown above, stratified by brain region. Error bars indicate ± standard error of the mean. ***p < 0.001; **p < 0.01; *p < 0.05; ns: non-significant; TH: therapeutic hypothermia.
Excitatory neurotransmitter concentrations were reduced during hypothermia
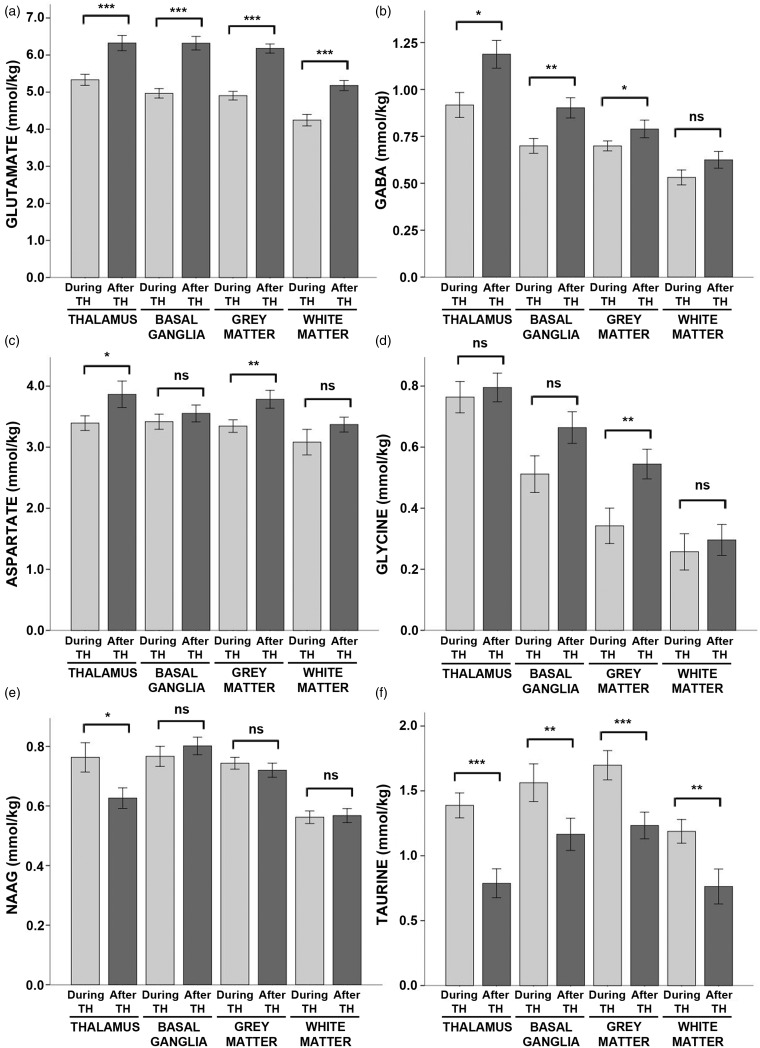
As expected, there were large effects of hypothermia on neurotransmitter concentrations. Glutamate was reduced by ∼20% across all regions during hypothermia as compared to after rewarming (Figure 3, see also Table S3). Likewise, GABA was reduced by ∼20% in the thalamus and basal ganglia and by 11% in the cortical grey matter. Aspartate was reduced by ∼11% in the thalamus and the cortical grey matter, and glycine was reduced by 37% in the cortical grey matter.
Figure 3.
Neurotransmitter concentrations during hypothermia versus after rewarming. Mean concentrations of (a) glutamate, (b) GABA, (c) aspartate, (d) glycine, (e) NAAG, and (f) taurine measured during hypothermia (light grey bars) and after rewarming (dark grey bars) are shown above, stratified by brain region. Error bars indicate ± standard error of the mean. ***p < 0.001; **p < 0.01; *p < 0.05; ns: non-significant; TH: therapeutic hypothermia.
In contrast, taurine was markedly increased during hypothermia in all regions, ranging from ∼35% in the basal ganglia and grey matter to 57% in the white matter and 76% in the thalamus. NAAG was elevated by 22% in the thalamus during hypothermia.
Antioxidant concentrations were elevated during hypothermia
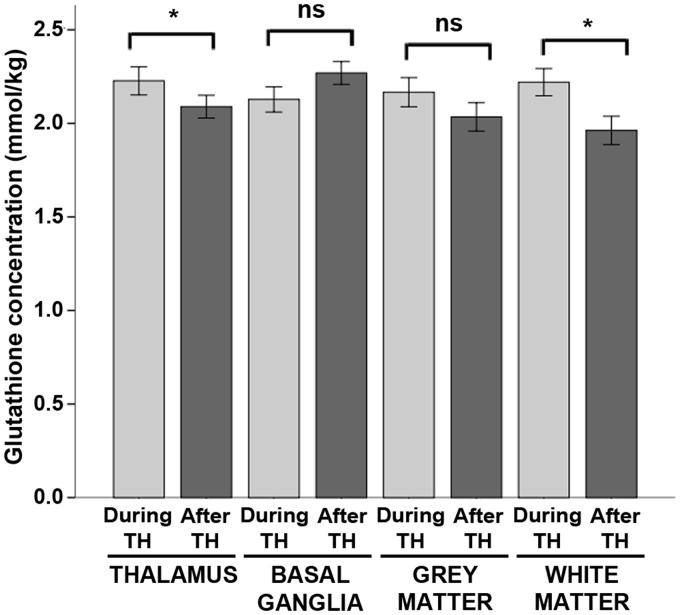
Glutathione concentrations were elevated by 7% during hypothermia in the thalamus and by 13% in the white matter (Figure 4). However, there were no significant effects of hypothermia on glutathione concentrations in the basal ganglia or cortical grey matter (Table S4).
Figure 4.
Glutathione concentrations during hypothermia versus after rewarming. Mean concentrations of glutathione measured during hypothermia (light grey bars) and after rewarming (dark grey bars) are shown above, stratified by brain region. Error bars indicate ± standard error of the mean. ***p < 0.001; **p < 0.01; *p < 0.05; ns: non-significant; TH: therapeutic hypothermia.
Discussion
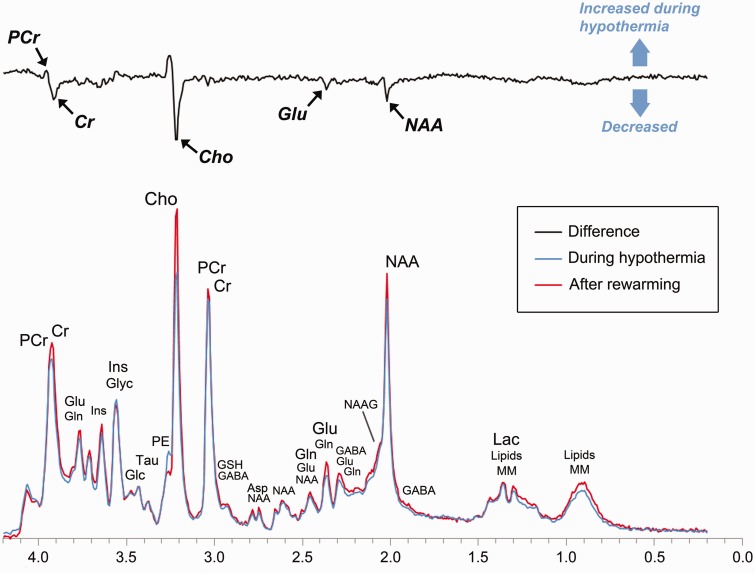
The primary aim of this study was to characterize the effects of hypothermia on cerebral metabolism in human neonates with HIE. Using serial 1H-MRS, we contrasted the concentrations of key energy metabolites, neurotransmitters, and antioxidants during and after hypothermia. As summarized in Figure 5, we found that phosphocreatine concentrations were significantly higher during hypothermia as compared to after rewarming, while free creatine, NAA, and excitatory neurotransmitters, such as glutamate, were reduced.
Figure 5.
The effect of hypothermia on cerebral metabolism in neonates with HIE. To illustrate the most prominent effects of hypothermia, we calculated a difference spectrum by first averaging all spectra acquired in the thalamus during hypothermia (blue) and after rewarming (red) and then subtracting the resultant spectra. The difference spectrum (black) clearly demonstrates that phosphocreatine concentrations were higher during hypothermia, while glutamate, creatine, and choline (a membrane metabolite not analyzed in the present study) were reduced. For reference, the prominent metabolites associated with each peak are labeled above. PCr: phosphocreatine; Cr: creatine; Glu: glutamate; Gln: glutamine; Glyc: glycine; Ins: myoinositol (not analyzed); Glc: glucose; Tau: taurine; Cho: choline (not analyzed); GSH: glutathione; GABA: γ-aminobutyric acid; Asp: aspartate; NAAG: n-acetyl-aspartyl-glutamate; NAA: n-acetyl-aspartate; Lac: lactate; Lipids: lipids; MM: macromolecules.
Phosphocreatine plays a key role in cellular energy homeostasis by supplying high energy phosphates for the synthesis of ATP from ADP, in essence, buffering large fluctuations in ATP synthesis and utilization.28 Additionally, phosphocreatine, together with ATP, regulates energy metabolism by inhibiting the phosphorylation of fructose-6-phosphate during glycolysis.49 In neonatal HIE, phosphocreatine concentrations fall during the period of secondary energy failure10,12–14 while anaerobic and aerobic glycolysis rise, stimulated by the energy deficit.12,13,50 Multiple studies have shown that hypothermia decreases the cerebral metabolic rate for glucose and oxygen.16,17,51,52 Still, it remains a paradox as to whether or how reducing energy metabolism (i.e. ATP production) engenders neuroprotection during the latent phase of HIE.53
Here, we show for the first time that phosphocreatine concentrations were increased by ∼20% or more during hypothermia as compared to after rewarming in neonates with HIE. At the same time, lactate concentrations were typically lower—reaching statistical significance in the basal ganglia and white matter—while glucose concentrations were nominally lower in the thalamus during hypothermia. Most important, we observed significant reductions, ∼11–35%, in the concentrations of glutamate, glutamine, GABA, and aspartate during hypothermia. Glutamate and aspartate are synthesized directly from TCA cycle intermediates, and prior studies have established that their rate of synthesis is stoichiometrically coupled to the rate of energy metabolism.29 Thus, in line with animal models,16,18,20 our data suggest that a key effect of hypothermia is not only a reduction in energy metabolism, but also a reduction in the synthesis of glutamate and other excitatory neurotransmitters.
Critically, a decrease in excitatory neurotransmitters is expected to not only exert a neuroprotective effect by mitigating excitotoxicity, but also inhibit seizure activity, or at least decrease the likelihood of seizures relative to after rewarming, which is consistent with clinical and laboratory observations.54–56 Perhaps most substantially, because neurotransmission consumes between one-third and one-half of the brain’s energy,57 a reduction in excitatory neurotransmission should, in effect, reduce energy utilization. In short, our results suggest that hypothermic neuroprotection is achieved, at least in part, by realizing a specific balance between energy metabolism and neurotransmission.
Finally, in line with animal models,58 we observed a marked increase, ∼35–75%, in taurine concentrations during hypothermia. Taurine is widely regarded as an inhibitory neurotransmitter45 and an osmolite.46 However, recent laboratory studies have shown that taurine can increase mitochondrial function in the setting of glutamate excitotoxicity and thereby act as an endogenous neuroprotective agent.59,60 Although our methods do not allow us to discern the specific effects of taurine, they do suggest that further research may be warranted to determine whether taurine facilitates neuroprotection in neonates with HIE.
Finally, we observed a modest effect of hypothermia on glutathione. At term equivalency, most antioxidants are underexpressed relative to their adult concentrations and do not respond to oxidative stress as robustly as in the adult brain.61 However, elevations in glutathione peroxidase have been observed in the white matter at term-equivalency,62 and we found a modest elevation in glutathione concentration in the white matter during hypothermia, as well as in the thalamus. Further research is needed to determine whether adjuvant therapies such as recombinant erythropoietin or melatonin, which are proposed to increase antioxidant mechanisms,15 allow for a more robust effect on glutathione in neonates with HIE.
This study reports on cerebral metabolism during and after TH. In an earlier report, we demonstrated that there is variability in cerebral temperature across patients and brain regions during hypothermia and after rewarming.22 It remains to be determined whether long-term neurodevelopmental outcomes can be enhanced by optimizing hypothermia protocols to achieve targeted brain temperatures in neonates with HIE. Furthermore, neurodevelopmental follow-up is ongoing in the present cohort. Accordingly, while our findings are in line with previous animal models, our interpretation of these results in relation to neuroprotection is limited at the present time.
In MRS, the visualized peaks typically represent overlapping signals arising from more than one cerebral metabolite with many metabolites contributing to more than one peak. In practice, reliable quantitation of individual metabolites is facilitated by the fact that no two metabolites generate identical resonances. Moreover, in high-quality datasets, small differences in chemical shift can be exploited, yielding reliable quantitation even in situations where metabolite resonances partial overlap (see Figures S1 and S2). To ensure reliability of our metabolite quantitation, we used stringent empirical standards for spectra quality (linewidth <0.05 ppm and signal to noise ≥10) and excluded spectra a priori that did not meet those standards. This approach, together with the inherent advantages of 3 T, which increases signal to noise by ∼50% over 1.5 T and doubles spectral resolution, allowed for the separate quantitation of key cerebral metabolites, including phosphocreatine and free creatine, in a clinical setting, and thus, demonstrates the feasibility of this technique for application in clinical trials.
Conclusions
In the last decade, clinical research has established the utility of MRS for predicting long-term disability in neonatal HIE.63–65 Building on this, we used modern MRS techniques to show a significant effect of TH on phosphocreatine concentrations, as well as glutamate, GABA, glycine, taurine, NAAG, lactate, free creatine, and NAA in neonates with HIE. These results, which are in line with animal models,16,18,20 support that hypothermia helps mitigate secondary energy failure by decreasing the availability of excitatory neurotransmitters (thereby suggesting a key mechanism for decreasing cellular energy demand). Given advances in MR technology, including the wide availability of 3 T MR systems and commercial software for metabolite quantitation, these results demonstrate the potential for using 1H-MRS, acquired during therapy, as an early biomarker to evaluate and contrast potential neuroprotective therapies in human clinical trials.
Supplementary Material
Acknowledgement
The authors would like to thank all of the families who took part in this research and our colleagues in the Newborn and Infant Critical Care Unit (NICCU) and the Department of Radiology at Children’s Hospital Los Angeles, including Robert Giesler, Lisa Villanueva, Daisy Zazueta Peña, Julia Castro, and all of the nurses, respiratory therapists and MR technologists whose efforts make this work possible.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the Gerber Foundation and the Rudi Schulte Research Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
Conceived and designed experiments: JLW, T-WW, CM, PF, AP, SB; Performed the experiments: JLW, T-WW, AJR, CM, PF, DV, EH, MDN, SB; Analyzed the data: JLW, SB; Interpreted the data: JLW, T-WW, AP, SB; Contributed reagents/materials/analysis tools: SB; Wrote the manuscript: JLW; Provided edits to the manuscript: all authors.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data.
References
- 1.Lawn J, Shibuya K, Stein C. No cry at birth: global estimates of intrapartum stillbirths and intrapartum-related neonatal deaths. Bull World Health Organ 2005; 83: 409–417. [PMC free article] [PubMed] [Google Scholar]
- 2.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 2005; 353: 1574–1584. [DOI] [PubMed] [Google Scholar]
- 3.Shankaran S, Pappas A, McDonald SA, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med 2012; 366: 2085–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009; 361: 1349–1358. [DOI] [PubMed] [Google Scholar]
- 5.Azzopardi D, Strohm B, Marlow N, et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med 2014; 371: 140–149. [DOI] [PubMed] [Google Scholar]
- 6.Edwards AD, Brocklehurst P, Gunn AJ, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 2010; 340: c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah PS. Hypothermia: a systematic review and meta-analysis of clinical trials. Semin Fetal Neonatal Med 2010; 15: 238–246. [DOI] [PubMed] [Google Scholar]
- 8.Higgins RD, Raju T, Edwards AD, et al. Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr 2011; 159: 851–858.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NICE. Therapeutic hypothermia with intracorporeal temperature monitoring for hypoxic perinatal brain injury | Guidance and guidelines | NICE, London: NICE, 2010. [Google Scholar]
- 10.Hope PL, Costello AM, Cady EB, et al. Cerebral energy metabolism studied with phosphorus NMR spectroscopy in normal and birth-asphyxiated infants. Lancet 1984; 2: 366–370. [DOI] [PubMed] [Google Scholar]
- 11.Penrice J, Cady EB, Lorek A, et al. Proton magnetic resonance spectroscopy of the brain in normal preterm and term infants, and early changes after perinatal hypoxia-ischemia. Pediatr Res 1996; 40: 6–14. [DOI] [PubMed] [Google Scholar]
- 12.Vannucci RC, Yager JY, Vannucci SJ. Cerebral glucose and energy utilization during the evolution of hypoxic-ischemic brain damage in the immature rat. J Cereb Blood Flow Metab 1994; 14: 279–288. [DOI] [PubMed] [Google Scholar]
- 13.Lorek A, Takei Y, Cady EB, et al. Delayed (“secondary”) cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatr Res 1994; 36: 699–706. [DOI] [PubMed] [Google Scholar]
- 14.Penrice J, Lorek A, Cady EB, et al. Proton magnetic resonance spectroscopy of the brain during acute hypoxia-ischemia and delayed cerebral energy failure in the newborn piglet. Pediatr Res 1997; 41: 795–802. [DOI] [PubMed] [Google Scholar]
- 15.Johnston MV, Fatemi A, Wilson MA, et al. Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol 2011; 10: 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laptook AR, Corbett RJ, Sterett R, et al. Quantitative relationship between brain temperature and energy utilization rate measured in vivo using 31P and 1H magnetic resonance spectroscopy. Pediatr Res 1995; 38: 919–925. [DOI] [PubMed] [Google Scholar]
- 17.Dehaes M, Aggarwal A, Lin PY, et al. Cerebral oxygen metabolism in neonatal hypoxic ischemic encephalopathy during and after therapeutic hypothermia. J Cereb Blood Flow Metab 2014; 34: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canevari L, Console A, Tendi EA, et al. Effect of postischaemic hypothermia on the mitochondrial damage induced by ischaemia and reperfusion in the gerbil. Brain Res 1999; 817: 241–245. [DOI] [PubMed] [Google Scholar]
- 19.Yenari MA, Iwayama S, Cheng D, et al. Mild hypothermia attenuates cytochrome c release but does not alter Bcl-2 expression or caspase activation after experimental stroke. J Cereb Blood Flow Metab 2002; 22: 29–38. [DOI] [PubMed] [Google Scholar]
- 20.Nakashima K, Todd MM. Effects of hypothermia on the rate of excitatory amino acid release after ischemic depolarization. Stroke 1996; 27: 913–918. [DOI] [PubMed] [Google Scholar]
- 21.Capani F, Loidl CF, Piehl LL, et al. Long term production of reactive oxygen species during perinatal asphyxia in the rat central nervous system: effects of hypothermia. Int J Neurosci 2003; 113: 641–654. [DOI] [PubMed] [Google Scholar]
- 22.Wu T-W, McLean C, Friedlich P, et al. Brain temperature in neonates with hypoxic-ischemic encephalopathy during therapeutic hypothermia. J Pediatr 2014; 165: 1129–1134. [DOI] [PubMed]
- 23.Wu TW, McLean C, Friedlich P, et al. Maintenance of whole-body therapeutic hypothermia during patient transport and magnetic resonance imaging. Pediatr Radiol 2014; 44: 613–617. [DOI] [PubMed] [Google Scholar]
- 24.Bonifacio SL, Glass HC, Vanderpluym J, et al. Perinatal events and early magnetic resonance imaging in therapeutic hypothermia. J Pediatr 2011; 158: 360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 2001; 14: 260–264. [DOI] [PubMed] [Google Scholar]
- 26.Lentner C. Geigy scientific tables, Basel, Switzerland: Ciba-Geigy, 1981. [Google Scholar]
- 27.Bluml S, Wisnowski JL, Nelson MD, Jr, et al. Metabolic maturation of white matter is altered in preterm infants. PLoS One 2014; 9: e85829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallimann T, Wyss M, Brdiczka D, et al. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit' for cellular energy homeostasis. Biochem J 1992; 281: 21–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sibson NR, Dhankhar A, Mason GF, et al. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci U S A 1998; 95: 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Settergren G, Lindblad BS, Persson B. Cerebral blood flow and exchange of oxygen, glucose ketone bodies, lactate, pyruvate and amino acids in anesthetized children. Acta Paediatr Scand 1980; 69: 457–465. [DOI] [PubMed] [Google Scholar]
- 31.Duffy TE, Nelson SR, Lowry OH. Cerebral carbohydrate metabolism during acute hypoxia and recovery. J Neurochem 1972; 19: 959–977. [DOI] [PubMed] [Google Scholar]
- 32.Clark JB. N-acetyl aspartate: a marker for neuronal loss or mitochondrial dysfunction. Dev Neurosci 1998; 20: 271–276. [DOI] [PubMed] [Google Scholar]
- 33.Bhakoo KK, Williams IT, Williams SR, et al. Proton nuclear magnetic resonance spectroscopy of primary cells derived from nervous tissue. J Neurochem 1996; 66: 1254–1263. [DOI] [PubMed] [Google Scholar]
- 34.Oz G, Alger JR, Barker PB, et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology 2014; 270: 658–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward Platt M, Deshpande S. Metabolic adaptation at birth. Semin Fetal Neonatal Med 2005; 10: 341–350. [DOI] [PubMed] [Google Scholar]
- 36.Edmond J, Auestad N, Robbins RA, et al. Ketone body metabolism in the neonate: development and the effect of diet. Fed Proc 1985; 44: 2359–2364. [PubMed] [Google Scholar]
- 37.Fonnum F. Glutamate: a neurotransmitter in mammalian brain. J Neurochem 1984; 42 1–11. [DOI] [PubMed] [Google Scholar]
- 38.Schousboe A, Westergaard N, Waagepetersen HS, et al. Trafficking between glia and neurons of TCA cycle intermediates and related metabolites. Glia 1997; 21: 99–105. [PubMed] [Google Scholar]
- 39.McKenna MC. The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain. J Neurosci Res 2007; 85: 3347–3358. [DOI] [PubMed] [Google Scholar]
- 40.McCormick DA. GABA as an inhibitory neurotransmitter in human cerebral cortex. J Neurophysiol 1989; 62: 1018–1027. [DOI] [PubMed] [Google Scholar]
- 41.Owens DF, Boyce LH, Davis MB, et al. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci 1996; 16: 6414–6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Neill J, Levitt JG, Alger JR. Magnetic resonance spectroscopy studies of attention deficit hyperactivity disorder. In: Bluml S, Panigrahy A. (eds). Magnetic resonance spectroscopy of pediatric brain disorders, New York, NY: Springer, 2013, pp. 229–275. [Google Scholar]
- 43.Legendre P. The glycinergic inhibitory synapse. Cell Mol Life Sci 2001; 58: 760–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnston MV. Excitotoxicity in perinatal brain injury. Brain Pathol 2005; 15: 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okamoto K, Kimura H, Sakai Y. Evidence for taurine as an inhibitory neurotransmitter in cerebellar stellate interneurons: selective antagonism by TAG (6-aminomethyl-3-methyl-4H,1,2,4-benzothiadiazine-1,1-dioxide). Brain Res 1983; 265: 163–168. [DOI] [PubMed] [Google Scholar]
- 46.Wade JV, Olson JP, Samson FE, et al. A possible role for taurine in osmoregulation within the brain. J Neurochem 1988; 51: 740–745. [DOI] [PubMed] [Google Scholar]
- 47.Bluml S, Wisnowski JL, Nelson MD, Jr, et al. Metabolic maturation of the human brain from birth through adolescence: insights from in vivo magnetic resonance spectroscopy. Cereb Cortex 2013; 23: 2944–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen J. Statistical power analysis for the behavioral sciences, 2nd ed Hillsdale, NJ: L. Erlbaum Associates, 1988. [Google Scholar]
- 49.Krzanowski J, Matschinsky FM. Regulation of phosphofructokinase by phosphocreatine and phosphorylated glycolytic intermediates. Biochem Biophys Res Commun 1969; 34: 816–823. [DOI] [PubMed] [Google Scholar]
- 50.Blumberg RM, Cady EB, Wigglesworth JS, et al. Relation between delayed impairment of cerebral energy metabolism and infarction following transient focal hypoxia-ischaemia in the developing brain. Exp Brain Res 1997; 113: 130–137. [DOI] [PubMed] [Google Scholar]
- 51.Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in mammalian central nervous system. J Cereb Blood Flow Metab 2003; 23: 513–530. [DOI] [PubMed] [Google Scholar]
- 52.De Vis JB, Hendrikse J, Petersen ET, et al. Arterial spin-labelling perfusion MRI and outcome in neonates with hypoxic-ischemic encephalopathy. Eur Radiol 2015; 25: 113–121. [DOI] [PubMed] [Google Scholar]
- 53.Tusor N and Edwards AD. Birth asphyxia: 100 years of progress. J Pediatr 2014; 165: 1081–1083. [DOI] [PubMed]
- 54.Gerrits LC, Battin MR, Bennet L, et al. Epileptiform activity during rewarming from moderate cerebral hypothermia in the near-term fetal sheep. Pediatr Res 2005; 57: 342–346. [DOI] [PubMed] [Google Scholar]
- 55.Simbruner G, Mittal RA, Rohlmann F, et al. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics 2010; 126: e771–778. [DOI] [PubMed] [Google Scholar]
- 56.Shankaran S, Pappas A, Laptook AR, et al. Outcomes of safety and effectiveness in a multicenter randomized, controlled trial of whole-body hypothermia for neonatal hypoxic-ischemic encephalopathy. Pediatrics 2008; 122: e791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 2001; 21: 1133–1145. [DOI] [PubMed] [Google Scholar]
- 58.Chan KW, Chow AM, Chan KC, et al. Magnetic resonance spectroscopy of the brain under mild hypothermia indicates changes in neuroprotection-related metabolites. Neurosci Lett 2010; 475: 150–155. [DOI] [PubMed] [Google Scholar]
- 59.El Idrissi A, Trenkner E. Growth factors and taurine protect against excitotoxicity by stabilizing calcium homeostasis and energy metabolism. J Neurosci 1999; 19: 9459–9468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El Idrissi A. Taurine increases mitochondrial buffering of calcium: role in neuroprotection. Amino Acids 2008; 34: 321–328. [DOI] [PubMed] [Google Scholar]
- 61.Ferriero DM. Neonatal brain injury. N Engl J Med 2004; 351: 1985–1995. [DOI] [PubMed] [Google Scholar]
- 62.Folkerth RD, Haynes RL, Borenstein NS, et al. Developmental lag in superoxide dismutases relative to other antioxidant enzymes in premyelinated human telencephalic white matter. J Neuropathol Exp Neurol 2004; 63: 990–999. [DOI] [PubMed] [Google Scholar]
- 63.Zhu W, Zhong W, Qi J, et al. Proton magnetic resonance spectroscopy in neonates with hypoxic-ischemic injury and its prognostic value. Transl Res 2008; 152: 225–232. [DOI] [PubMed] [Google Scholar]
- 64.Alderliesten T, de Vries LS, Benders MJ, et al. MR imaging and outcome of term neonates with perinatal asphyxia: value of diffusion-weighted MR imaging and (1)H MR spectroscopy. Radiology 2011; 261: 235–242. [DOI] [PubMed] [Google Scholar]
- 65.Thayyil S, Chandrasekaran M, Taylor A, et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics 2010; 125: e382–395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.