Abstract
Soluble and membrane-bound inorganic pyrophosphatases (sPPase and H+-PPase, respectively) of the purple nonsulfur bacterium Rhodospirillum rubrum are differentially regulated by environmental growth conditions. Both proteins and their transcripts were found in cells of anaerobic phototrophic batch cultures along all growth phases, although they displayed different time patterns. However, in aerobic cells that grow in the dark, which exhibited the highest growth rates, Northern and Western blot analyses as well as activity assays demonstrated high sPPase levels but no H+-PPase. It is noteworthy that H+-PPase is highly expressed in aerobic cells under acute salt stress (1 M NaCl). H+-PPase was also present in anaerobic cells growing at reduced rates in the dark under either fermentative or anaerobic respiratory conditions. Since H+-PPase was detected not only under all anaerobic growth conditions but also under salt stress in aerobiosis, the corresponding gene is not invariably repressed by oxygen. Primer extension analyses showed that, under all anaerobic conditions tested, the R. rubrum H+-PPase gene utilizes two activator-dependent tandem promoters, one with an FNR-like sequence motif and the other with a RegA motif, whereas in aerobiosis under salt stress, the H+-PPase gene is transcribed from two further tandem promoters involving other transcription factors. These results demonstrate a tight transcriptional regulation of the H+-PPase gene, which appears to be induced in response to a variety of environmental conditions, all of which constrain cell energetics.
Inorganic pyrophosphate (PPi) is a by-product of cellular metabolism whose removal is essential to allow anabolic reactions to proceed in the direction of biosynthesis (34). Two main types of PPi-hydrolyzing enzymes have been characterized to date: soluble and membrane-bound inorganic pyrophosphatases (sPPases and H+-PPases, respectively) (EC 3.6.1.1). sPPases are ubiquitous and simply hydrolyze PPi, releasing heat (31), whereas H+-PPases that are present in photosynthetic organisms, protists, and certain prokaryotes couple the energy of PPi hydrolysis to proton movement across biological membranes (48). H+-PPases have been characterized at biochemical and genetic levels in many higher plants (18, 35, 40, 51, 60) and some eubacteria (5, 42, 45), archaea (8, 20), and protozoa, both parasite and free-living forms (37, 44). These integral membrane proteins can be classified into two groups according to their sensitivity to K+: (i) K+-stimulated H+-PPases, reported to occur in the tonoplast of higher plants (19), protists' acidocalcisomes (31), and the hyperthermophilic bacterium Thermotoga maritima (45); and (ii) K+-insensitive H+-PPases, found in Golgi membranes of higher plants (38), in the parasitic protozoan Plasmodium falciparum (37), in some archaea (8, 20), and in the intracytoplasmic membrane systems of many photosynthetic anaerobic bacteria (3, 4).
In the purple nonsulfur bacterium Rhodospirillum rubrum, the PPi generated in the cytosol can be hydrolyzed by both a sPPase and a membrane-bound H+-PPase, with the latter located in pigment-containing intracellular membranes called chromatophores (3, 4). The H+-PPase of this phototrophic prokaryote has been extensively studied and consists of a single polypeptide with an apparent molecular mass of 56 kDa as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, a value clearly lower than the 71.6 kDa calculated from the gene-deduced sequence, which can mediate both PPi hydrolysis coupled to proton translocation and PPi synthesis at the expense of a previously generated proton gradient (3-5, 42, 47, 54). It has been postulated that a major function of this protein in R. rubrum is to maintain a substantial protonmotive force under circumstances of a low level of energy (e.g., darkness) by using stored PPi, probably in acidocalcisome-like structures (41, 53); on the other hand, under conditions of sufficient energy supply (e.g., high light), it would recover this intracellular PPi pool (41). However, the concurrent presence of an sPPase raised doubts about this proposal.
The remarkable metabolic versatility of R. rubrum allows it to grow under quite different nutritional metabolic conditions, namely, anaerobic photosynthesis, aerobic respiration, fructose fermentation, and finally, anaerobic respiration with fructose as the electron donor and several agents, such as dimethyl sulfoxide (DMSO) or trimethylamine N-oxide, as terminal electron acceptors (52). In prokaryotes, several regulatory factors are involved in the differential expression of genes in response to growth-impairing environmental changes, such as a shift from aerobic to anaerobic conditions. Some of these response regulators, like FNR, respond to oxygen deprivation favoring RNA polymerase activity (7). FNR controls the transcription of many target genes involved in anaerobic functions in enteric bacteria, such as the operons encoding fumarate or nitrate reductase (7). Other factors form two-component regulatory systems such as the RegA-RegB system of purple α-proteobacteria, which is responsible for anaerobic transcriptional induction of genes involved in photosynthesis and inorganic carbon and nitrogen assimilation (6, 26, 33, 46). In this work, different patterns of expression of R. rubrum sPPase and H+-PPase, encoded by the single-copy genes ppa and vpp, respectively, have been found under diverse trophic and stress conditions. Results demonstrate that these proteins are subjected to a differential regulation, with H+-PPase being tightly regulated at the transcriptional level. Primer extension analyses of the vpp gene have been performed under different environmental conditions, indicating that two different sets of tandem promoters were used in anaerobic and salt-stressed aerobic cells. Sequence analysis suggests that anaerobic tandem promoters may be dependent on response regulators FNR and RegA. This complex mechanism of transcriptional regulation suggests a pivotal function for H+-PPase in PPi-based bacterial energetics prevailing under diverse energy-constraining conditions.
MATERIALS AND METHODS
Strains and growth media.
R. rubrum (Proteobacteria; Alphaproteobacteria; Rhodospirillales; Rhodospirillaceae) strain S1 was grown under different conditions at 25°C in liquid culture medium containing malate and citrate as the organic substrates, as described previously elsewhere (10). Photosynthetic batch cultures were grown under incandescent white light (85 microeinsteins cm−2 s−1) under complete anaerobiosis. Fermentative batch cultures were grown in the dark under anaerobic conditions in medium supplemented with 11 mM fructose. The latter conditions were also used in cultures in which anaerobic respiration was being performed, with a further addition of 80 mM DMSO as the terminal respiratory electron acceptor. Fermentative and anaerobic respiration cultures were supplemented with 0.05% NaHCO3 to increase the initial pH up to 7.1 to 7.2 (52), thus reducing the lag period in the latter case. Anaerobiosis was obtained by bubbling dinitrogen through the cultures which were allowed to develop in tightly closed glass bottles. Aerobic cultures were grown with vigorous shaking (250 rpm) and complete darkness in conical flasks (culture-to-flask volume ratio, 1:10) containing basal medium. All cultures were started by inoculation with cells previously grown to saturation under the same growth conditions.
Bacteriochlorophyll, optical density, and total protein measurements.
Bacteriochlorophyll was estimated by using the in vivo extinction coefficient at 880 nm of 140 mM−1 cm−1 as previously described (13). Optical densities of the cultures were monitored by measuring absorbance at 680 nm. Total protein content of the cultures was estimated by a modification of Lowry's method (36).
Northern blot analyses.
Cells were harvested by centrifugation at 8,000 rpm for 10 min in a Sorvall centrifuge (SS34 rotor). Pellets were washed twice with 10 mM Tris-HCl (pH 7.5), and total RNA was isolated, subjected to electrophoresis (20 μg per lane) on 1% agarose gels in the presence of 2% formaldehyde, and transferred to nylon membranes as previously described (28, 39). Membranes were hybridized at 65°C with two different probes: a 600-bp-long DNA fragment corresponding to a region close to the 3′ terminus of the R. rubrum vpp gene (44) and a DNA fragment of 540 bp corresponding to the full-length R. rubrum ppa gene, both obtained by PCR amplification. In the case of vpp, a plasmid bearing the full coding sequence (5) was used as a template with a pair of degenerated oligonucleotides described previously (44). Two strict oligonucleotides, PPA1 (5′-GTCGACAACATGGATATCAAGAAAATTCC-3′) and PPA2 (5′-ACTAGTTTAGACCTTCTT-GTGGGC-3′), designed according to preliminary sequence data for the ppa gene from the R. rubrum genome database (see below), were used for PCR amplification of the full coding sequence of this gene with genomic DNA as a template; the single DNA band thus amplified was cloned into the pGEM-T vector (Promega), thus obtaining plasmid pRLppa. After restriction analysis of the 0.54-kb insert, this plasmid was subsequently used as a template for PCRs with the same pair of oligonucleotides to obtain the probe. rRNAs of known molecular sizes were used as standards for transcript size determination. Preliminary genome sequence data from R. rubrum were obtained from the U.S. Department of Energy Joint Genomic Institute (DOE JGI) Microbial Sequencing Program website (http://genome.ornl.gov/microbial/rrub/) and analyzed by using the BLAST algorithm (2).
Probes were labeled with [32P]dCTP by using a Ready-To-Go DNA labeling kit (Amersham Pharmacia) according to the manufacturer's instructions. Images of radioactive filters were obtained and quantified with a Cyclone Storage Phosphor system equipped with Optiquant image analysis software (Packard Instruments Co., Wellesley, Mass.). Data were normalized by using methylene blue-stained rRNAs; images were obtained with Biocapt software and quantified as described above.
Primer extension analyses and promoter region cloning of R. rubrum H+-PPase gene.
Highly pure RNA was isolated with an RNeasy Mini kit (QIAGEN) according to the manufacturer's instructions. Primer extension analyses were carried out by using a previously described procedure (55) with some modifications. Two micrograms of total RNA was used as a template for cDNA synthesis with two antisense primers; primer TSP1 (5′-TGAATGGGCCTAACGGC-3′) corresponded to a region from −36 to −20 bp upstream from the start codon of the R. rubrum vpp gene, whereas primer TSP2 (5′-GCCATGATTGTTTTGATGGTG-3′) was located from 64 to 85 bp downstream from the start codon. The thermophilic enzyme Tth205-DNA polymerase (Ecogen) was used in order to perform the reverse transcription reaction at 65°C, thus minimizing problems derived from the formation of RNA secondary structures due to the high G+C content (61.5%) of the R. rubrum template. Deoxynucleotides, including [32P]dCTP, were added to a final concentration of 1 mM.
In order to clone a DNA fragment carrying the promoter of the H+-PPase gene, 10 μg of R. rubrum genomic DNA digested with BclI was subjected to electrophoresis in a 0.7% agarose gel in Tris-borate-EDTA and transferred to a nylon membrane as described above. The membrane was hybridized with a 32P-radiolabeled 595-bp-long DNA probe containing the 5′ end of the vpp gene. A 1.9-kb-long fragment of R. rubrum genomic DNA, presumably containing about 700 bp of noncoding region upstream from vpp, was thus identified. This fragment was cloned by running another 10-μg aliquot of BclI-digested R. rubrum DNA in an 0.7% agarose gel, isolating DNA bands of about 1.9 kb, and ligating the latter into the unique BamHI site of plasmid pBluescript KS(+) (Stratagene), thus obtaining plasmid pRLM1. This plasmid was subsequently digested with SphI and SpeI and religated after treatment with T4 DNA polymerase (Amersham Biosciences), yielding plasmid pRLM2. After manual and automated sequencing, plasmid pRLM2 was shown to contain an insert of 1,078 bp comprising the 5′ end of the vpp coding sequence down to the first SphI site (385 bp) (5) and 693 bp of its 5′ noncoding flanking region.
Automated sequencing was carried out by using an ABI PRISM Big Dye Terminator cycle sequencing ready reaction kit version 3.0 (PE Biosystems), and the data were processed with Editview 1.0.1 software. For manual sequencing, a T7 sequencing kit (Amersham Biosciences) was used according to the manufacturer's instructions. Sequencing reactions were supplemented with 21% DMSO (5), radiolabeled with [35S]dATP, and run in 6% polyacrylamide gels containing 8 M urea. Manual sequencing reactions were routinely used for primer extension analysis. Images of radiolabeled gels were obtained and quantified as described above.
Preparation of cell extracts and membranes isolation.
Cells were harvested by centrifugation at 8,000 rpm for 6 min (Sorvall SS34 rotor), washed twice with water, and resuspended in 3 ml of ice-cold working buffer per g (fresh weight) of cells. Working buffer contained 25 mM Tris-HCl, pH 8, 10% (vol/vol) glycerol, 4 mM β-mercaptoethanol, 2 mM dithiothreitol, 2 mM EDTA, 10 mM MgCl2, and a protease inhibitor cocktail (1 mM benzamidine, 2 mM ɛ-aminocaproic acid, 1 mM phenylmethylsulfonyl fluoride). Cells were disrupted by sonication, and unbroken cells and debris were removed by centrifugation at 2,500 × g for 5 min. Total membranes were sedimented by centrifugation at 30,000 rpm (Beckman 60 Ti rotor) for 30 min. The supernatant (soluble protein fraction) was used as the crude soluble extract. The pellet was washed once with working buffer supplemented with 3 M KCl and twice with working buffer to remove contamination by sPPase. After resuspension in working \'0fbuffer, homogenization, and storage at −20°C, the pellet was used for subsequent membrane-bound PPase determinations.
Protein content estimation, Western blot analyses, and activity assays.
Immunoblot assays of protein samples were performed as previously reported (55) by using a monospecific affinity-purified polyclonal antibody raised against the R. rubrum H+-PPase, generously provided by M. Baltscheffsky, for membrane fractions and a monospecific polyclonal antibody raised against the sPPase of the cyanobacterium Synechocystis sp. strain PCC 6803 (23) for soluble protein extracts. This antibody readily cross-reacted with sPPases of a wide range of phototrophic prokaryotes (M. R. Gómez-García and A. Serrano, unpublished results). The protein amount was estimated by the Bradford method (11) with ovalbumin as a standard. Both soluble and membrane-bound PPase activities were assayed spectrophotometrically at 30°C as previously described (29, 42).
Nucleotide sequence accession number.
The sequence of the promoter region of the R. rubrum vpp gene has been assigned DDBJ/EMBL/GenBank accession number AJ549291.
RESULTS
Differential regulation of R. rubrum sPPase and H+-PPase by diverse environmental conditions.
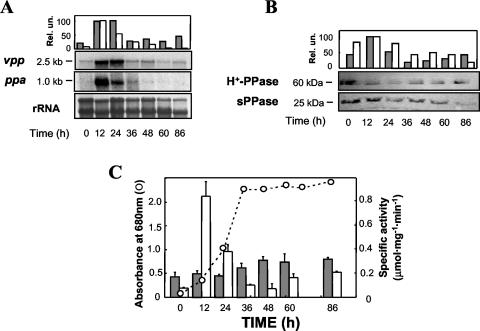
Batch cultures developed under different environmental (nutritional and stress) conditions were analyzed for sPPase and H+-PPase transcript and protein levels. The vpp (H+-PPase) transcript was detected in cells grown under anaerobic photosynthetic conditions along all developmental phases of the cultures up to the late stationary phase, with maximal levels 4achieved at the mid-exponential phase (Fig. 1A and C). A similar pattern followed the immunodetected protein (Fig. 1B). The size of the vpp transcript (ca. 2.5 kb) identified it as a monocistronic mRNA containing the reported 2.1-kb open reading frame (5). Membrane-bound PPase-specific activity did not correlate well with vpp transcript and H+-PPase protein patterns at initial growth phases, as a slight and progressive increase with culture development was observed (Fig. 1C). In contrast to the H+-PPase, the 1-kb-long ppa (sPPase) transcript exhibited a dramatic drop with time, being virtually undetectable at the stationary phase and showing its highest level at the early exponential phase, when maximal cell anabolic activity is expected (Fig. 1A and C). Western blot analysis also showed a decreasing protein pattern, the sPPase band (ca. 25 kDa), detected in all growth phases (Fig. 1B). PPase-specific activity in soluble extracts presented a parallel progression, with a sharp peak at the beginning of the logarithmic growth phase and a further decrease with time so that only residual activity was found at early stationary phase and up to late stationary phase, when a small recovery of activity could be detected (Fig. 1B). Bacteriochlorophyll content in these phototrophic cultures was typically around 22 to 25 μg ml−1.
FIG. 1.
Time course of H+-PPase and sPPase levels in R. rubrum anaerobic phototrophic batch cultures. (A) Northern blots performed with 20 μg of total RNA obtained at different times and hybridized with specific probes for R. rubrum vpp and ppa genes and normalized quantification of the resulting hybridization bands (see Materials and Methods). (B) Immunoblot analyses of membrane preparations and soluble cell extracts (100 μg of protein per lane) performed with monospecific antibodies against H+-PPase and sPPase, respectively, and quantification of the detected protein bands. (C) Growth curve and specific activity levels of membrane-bound and soluble PPase at selected times. Data are means ± standard error of the mean (SEM) of three independent experiments. Quantitative results presented in all three panels as gray and white bars refer to H+-PPase and sPPase, respectively. Rel. un., relative units.
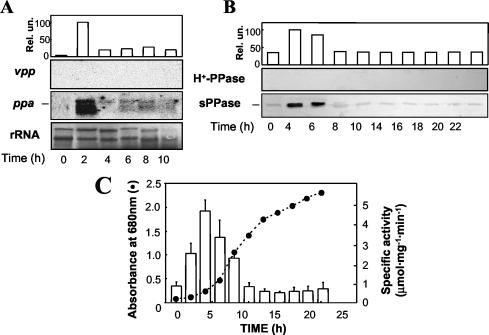
Under aerobic heterotrophic conditions, R. rubrum cultures exhibited growth rates about fivefold higher than those exhibited under anaerobic phototrophic conditions (Fig. 1C and 2C). It is noteworthy that aerobic heterotrophic cells showed no detectable levels of vpp transcript (Fig. 2A) and were virtually devoid of bacteriochlorophyll (15; data not shown). By contrast, the ppa transcript was detected in all growth phases, with a clear maximum being observed at the early exponential phase (Fig. 2A). Due to the marked instability of the mRNA preparations from these cultures, no Northern blot analyses could be performed for latter growth phases. Immunochemical analysis of membrane fractions showed no band of the expected molecular mass for the H+-PPase polypeptide at any growth phase (Fig. 2B), and consistently, no membrane-associated PPase activity was detected. On the other hand, analysis of soluble extracts with the anti-sPPase antibody showed the presence of the 25-kDa sPPase polypeptide at all growth stages (Fig. 2B). sPPase protein content and activity showed a marked peak at the early exponential phase followed by a dramatic drop (Fig. 2B and C), in clear parallelism with the evolution of the ppa transcript. Maximal levels of sPPase activity in aerobic cells were more than fourfold higher than those for photosynthetic cells. This result is consistent with the higher metabolic rates and the absence of the H+-PPase observed under aerobiosis.
FIG. 2.
Time course of sPPase and H+-PPase levels in R. rubrum aerobic heterotrophic batch cultures. Samples were taken every 2 h and processed as described in the legend of Fig. 1. (A) Northern blot analysis normalized with methylene blue-stained rRNA quantification. (B) Immunoblot analysis. (C) Growth curve and specific enzyme activity measurements; activity values are means ± SEM. Note the complete absence of both vpp transcript and H+-PPase protein, indicating a tight regulation at the genetic level; therefore, quantifications refer to only the ppa transcript and the sPPase protein.
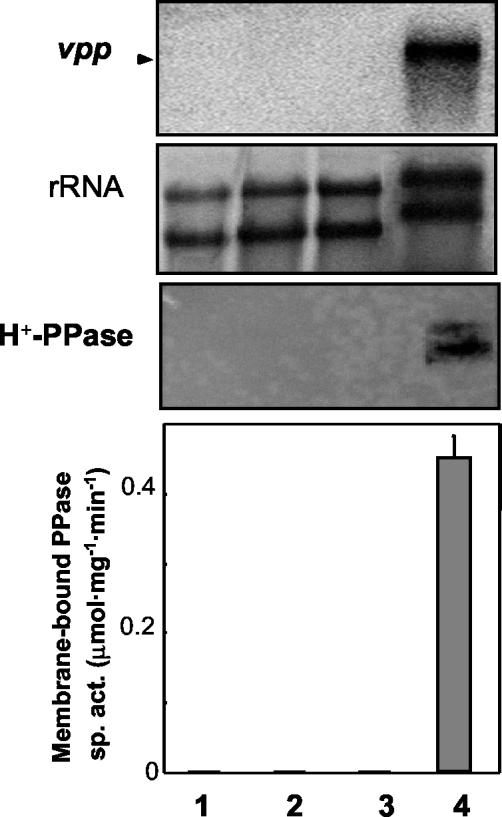
In order to investigate whether oxygen and/or light was among the signals triggering the transcriptional regulation of both H+-PPase and sPPase, further experiments were performed. An aerobic R. rubrum culture grown in darkness up to the mid-exponential phase was divided into two parallel cultures, one of them maintained under anaerobic phototrophic conditions and the other maintained under heterotrophic conditions as a control. Both vpp transcript and H+-PPase (protein function and activity) levels quickly responded (concurrently with the appearance of photosynthetic pigments [data not shown]) to phototrophic conditions, and the typical high levels of photosynthetic cells were reached after 12 h (Fig. 3). However, no induction was observed on vpp transcript expression or on H+-PPase synthesis (Fig. 3), as well as on photosynthetic pigment production (data not shown), by transfer from aerobic-dark to anaerobic-dark or aerobic-light conditions. Another point worthy of consideration is that small amounts of both vpp transcript and H+-PPase protein were found in R. rubrum aerobic cultures not subjected to intense aeration (shaking under 250 rpm) or when the culture-to-flask volume ratio was increased above 1:10, which was typically used in our experiments (data not shown). Small amounts of bacteriochlorophyll were also detected under these conditions (3 to 5 μg ml−1 versus 22 to 25 μg ml−1 in photosynthetic cultures).
FIG. 3.
Effect of transition from aerobic-dark to aerobic or anaerobic dark or light conditions on R. rubrum H+-PPase. An aerobic heterotrophic culture of R. rubrum grown in the dark was divided into four aliquots and subjected to different conditions. Samples were taken after 12 h. Lane 1, aerobiosis-dark (control); lane 2, aerobiosis light; lane 3, anaerobiosis-dark; lane 4, anaerobiosis light. (Top) Northern blot analysis and radioactive detection of the vpp transcript. (Center) rRNA methylene blue staining. (Bottom) Western blot analysis of the H+-PPase protein. Membrane-bound PPase specific activity (sp. act.) levels are also presented. Note that vpp transcript and H+-PPase protein function and activity were found exclusively under anaerobic light conditions (Fig. 1).
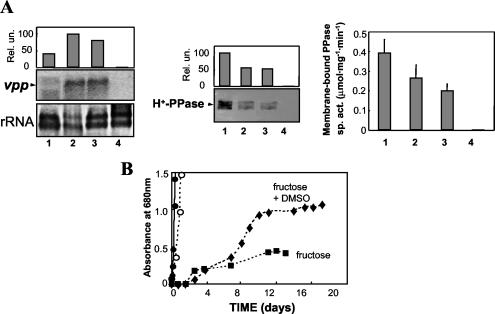
R. rubrum has a remarkable metabolic versatility; it is capable of growing in the dark on malate and/or fructose through anaerobic respiratory mechanisms in the presence of terminal electron acceptors such as trimethylamine N-oxide or DMSO or fermenting fructose without accessory oxidants (52). Under our experimental conditions, growth on malate and DMSO was achieved only in the presence of fructose. When grown with fructose alone, absorbance values at 680 nm of around 0.8 could be obtained, provided that the culture pH was initially adjusted to 7.1 to 7.2 with NaHCO3. This initial pH value was also required for cultures growing through anaerobic respiration in order to reduce the lag period. Growth rates under these two dark anaerobic conditions were clearly much lower than under aerobic or photosynthetic conditions. Indeed, generation times for anaerobic respiration cultures were about 10 times lower than those of phototrophic cultures and more than 45 times lower than those for aerobiosis. For fermentative cultures, the differences in growth rates were even more marked, and generation times were determined to be about 20 times lower than that for photosynthesis and a bit less than 100 times lower than those observed under aerobic conditions (Fig. 4B). It is noteworthy that significant amounts of H+-PPase and its transcript were detected in these dark anaerobic-growing cells (Fig. 4A). Whereas vpp transcript levels appeared to be higher than those in phototrophically grown cells, protein levels were lower, as were bacteriochlorophyll levels (7 to 10 μg ml−1). Membrane-bound PPase activity measurements were in agreement with immunodetected protein data, with levels 1.3- to 1.8-fold higher in phototrophic cells than in anaerobic respiratory or fermentative cells (Fig. 4A).
FIG. 4.
Comparative analysis of H+-PPase in R. rubrum cultures grown under different trophic conditions. (A) Samples were taken for each culture at the mid-exponential growth phase. Lane 1, anaerobic phototrophic; lane 2, fermentative; lane 3, anaerobic respiratory; lane 4, aerobic heterotrophic. Northern blot analysis normalized with rRNA quantification (left), Western blot analysis of membrane preparations (middle), and membrane-bound H+-PPase specific activity (sp. act.) (right) are shown; values are means ± SEM. (B) Growth curves under the different conditions tested. ○, phototrophic; •, aerobic heterotrophic; ▪, fermentative; ▴, anaerobic respiratory. Generation times for each culture were as follows: for phototrophic cultures, 9.8 h; for aerobic heterotrophic cultures, 1.9 h; for fermentative cultures, 186.6 h; for anaerobic respiratory cultures, 90.0 h.
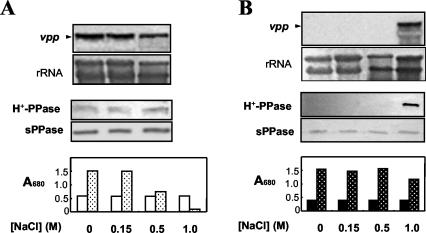
In order to look for growth-limiting conditions that may induce vpp expression in aerobic R. rubrum cells and to test the possible involvement of H+-PPase in a salt stress response, cells were cultured under both anaerobic phototrophic and aerobic heterotrophic conditions in the presence of increasing amounts of NaCl. No effect was produced by 150 mM NaCl either on growth or on the vpp transcript and H+-PPase protein levels under any of the nutritional conditions tested (Fig. 5). However, in the presence of 0.5 M NaCl, photosynthetically grown cells became growth arrested, although no significant changes in transcript and protein levels were observed after 12 h of salt treatment (Fig. 5A). Cellular death rapidly occurred in photosynthetic cultures in the presence of 1 M NaCl but not in aerobic cultures that continued growing, although at somewhat reduced rates (Fig. 5B). It is noteworthy that a dramatic induction of H+-PPase was observed under this severe ionic stress in aerobiosis by both Northern and Western blot analyses (Fig. 5B), and membrane-bound PPase-specific activity levels similar to those of photosynthetic cultures were reached (data not shown).
FIG. 5.
Salt stress induction of H+-PPase in R. rubrum. Cultures grown under anaerobic phototrophic (A) or aerobic heterotrophic (B) conditions up to the mid-exponential phase were then supplemented with either water or a concentrated NaCl solution. In each case, Northern (upper panels) and Western (middle panels) blot analyses were performed after 12 h of salt treatment. Growth during this time interval is also shown as bar histograms (lower panels). Open bars, onset of growth; dotted bars, growth after 12 h of treatment. The concentration of salt added (up to 1 M) is indicated in each case.
Determination of the TSP of the R. rubrum vpp gene.
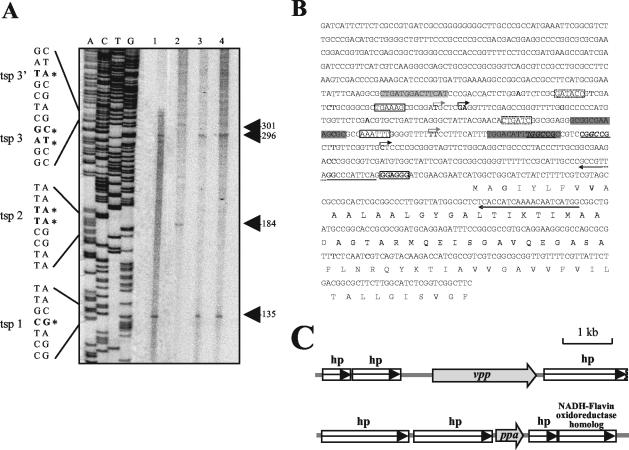
Primer extension analysis experiments were carried out in order to determine the transcription start point (TSP) of the R. rubrum H+-PPase transcript under the different physiological conditions tested in this work. For this purpose, a 923-bp-long DNA fragment containing the promoter region and part of the 5′ coding sequence (see Materials and Methods) of the vpp gene was cloned and automatically sequenced, as it was virtually identical to the corresponding sequence (contig 127, minus strand, nucleotides [nt] 172937 to 172014) in the R. rubrum unfinished genome project website (DOE JGI). Manual sequencing reactions of this fragment were used to determine the length of the primer extension products obtained with two different primers hybridizing around the putative ATG codon (4) of the vpp gene (Fig. 6A). Although photosynthesis, fermentation, and anaerobic respiration represent very different metabolic scenarios, two common tandem promoters were found for all these anaerobic cultures, with TSPs at positions −135 (TSP1) and −296 (TSP3) from the translation start codon (Fig. 6B). An analysis of the corresponding promoter regions was carried out in order to identify putative consensus sequences typical of anaerobic promoters. For TSP1, two sequences almost identical to the consensus for the binding site of the anaerobic response regulator RegA of Rhodobacter sphaeroides (29) were found at positions −23 to −37 and −67 to −79 (Fig. 6B, dark shaded boxes). Putative σ54 binding sites at positions −13 and −24, containing the TGGC-GC motif characteristic of this transcription factor, were also identified (Fig. 6B, boldface underlined letters) (49). A 14-bp sequence centered at position −63 from TSP3 was found (Fig. 6A, light shaded boxes), which is similar to the consensus sequence for FNR (8). In this case, no Escherichia coli consensus −35 region could be defined, but a putative −10 σ70 binding site is proposed (Fig. 6B). Primer extension analyses for aerobic cultures subjected to severe salt stress were also performed, allowing the identification of two further tandem promoters under this growth-arresting condition. The first one at position −184 (TSP2) was completely different from those found under anaerobic conditions, while the second one at position −301 (TSP3′) is located very close to TSP3. Another putative −10 sequence was identified for TSP2 (Fig. 6B, arrows above the sequence), but as is the case for TSP3, no E. coli consensus −35 box could be clearly established. Alternative −35 regions appropriate for other sigma factors different from σ70, like σB or σS, usually involved in the transcription of stress-related genes (1, 25, 56), could be identified for both TSP2 and TSP3 (Fig. 6). The noncoding downstream region of the R. rubrum vpp gene (contig 127, minus strand, nt 170149 to 169949 [DOE JGI; see Materials and Methods]) contained an inverted repeat (13-bp, 6-bp loop) followed by an oligo(T) tract found 25 bp from the translational termination codon TAA (data not shown). The calculated ΔG of the potential stem-loop structure formed by this inverted repeat is −78 kJ mol−1. These features suggest the existence of a factor-independent eubacterial transcription terminator. Overall, these results agree with the transcript size (ca. 2.5 kb) found in Northern blots, so the vpp gene (coding sequence, ca. 2.1 kb) should be transcribed as a monocistronic mRNA. Consistent with this, the genetic organization of the R. rubrum genome regions around both vpp and ppa genes, constructed from preliminary genome project data, indicates that these genes are indeed most probably not included in operons (Fig. 6C).
FIG. 6.
Promoter region sequence and primer extension analysis of R. rubrum H+-PPase gene. (A) Primer extension analysis showing the DNA sequence around the TSPs and the primer extension products for the vpp gene (indicated by boldface letters and asterisks) obtained by using primers designed from the 5′ noncoding region and total RNA from phototrophic cultures (lane 1), salt-stressed aerobic cultures (lane 2), fermentative cultures (lane 3), and anaerobic respiratory cultures (lane 4). (B) Sequence of a 923-bp-long genomic DNA fragment that includes 693 bp of noncoding sequence upstream from the vpp gene containing the promoter region. Nucleotides shown in boldface type and marked by asterisks correspond to the positions found for the TSPs. The Shine-Dalgarno motif proposed previously (4) is boxed and in boldface type. The two primers used for TSP experiments are indicated by arrows under the sequence. Sequences displaying similarity to E. coli −10 consensus sequences are indicated with a box. Putative −35 regions for alternative sigma factors different from σ70 are shown inside dotted boxes. A tentative FNR binding site is shaded in light gray. Putative RegA recognition sequences are shaded in dark gray. Boldface underlined letters represent a possible binding site for σ54 factor; the TGGC-GC motif characteristic of this transcription factor is further indicated in italics. (C) Genetic organization of the regions around the R. rubrum vpp and ppa genes. Preliminary sequence data of the R. rubrum genome were obtained from the corresponding DOE JGI microbial genome website.
DISCUSSION
In this report, experiments have been performed that were aimed at elucidating whether the sPPase and the H+-PPase, encoded by single-copy genes in R. rubrum, are differentially regulated in response to environmental conditions that exert a deep influence on cellular energetic status and metabolism. Two distinct enzymes capable of hydrolyzing PPi have been identified and characterized in this purple nonsulfur bacterium: a cytosolic sPPase (ca. 25-kDa subunit), with a Km for MgPPi of 20 to 30 μM (29), and an H+-PPase (ca. 60 kDa subunit), an integral membrane protein whose Km for MgPPi is in the range of 0.1 to 0.2 mM (47). In contrast to the situation in photosynthetic eukaryotic tissues that lack a cytosolic sPPase (57), PPi generated in bacterial cell anabolism is presumably accessible to both PPases, since a coordinated regulation is needed in order to maintain an adequate \'0fintracellular level of this metabolite (0.5 to 1.0 mM) (32) and a suitable proton gradient across membranes. Our results demonstrate that this is indeed the case. Although both proteins are present in anaerobic phototrophic cultures along all growth phases, maximal activities are reached at different times. This finding supports the idea of a differential and coordinated regulation. An increase in sPPase activity in late exponential phase was detected. This effect has also been observed for other prokaryotes (24) and could be related to a lower turnover of the R. rubrum protein at this culture phase. The progressive increase in H+-PPase activity along culture development did not correlate with the presence of maximal transcript and protein levels at the exponential phase, suggesting a posttranslational regulation of this protein. On the other hand, in aerobic heterotrophic cultures, neither the H+-PPase nor its transcript could be detected, and consequently, H+-PPase activity could not be measured, clearly demonstrating a differential genetic regulation of this protein with respect to sPPase, which showed high levels under these growth conditions.
Several results presented in this work show a positive correlation between photosynthetic pigments and H+-PPase levels in R. rubrum, which suggests a common regulation. On one side, when aerobic-dark cultures (for which no vpp transcript or H+-PPase protein function and activity could be detected) were transferred to anaerobic light conditions, a clear induction of the vpp transcript, as well as of the synthesis of bacteriochlorophyll, was observed. This result is consistent with previous reports showing that R. rubrum cells induce formation of intracytoplasmic membranes (including the photosynthetic apparatus) when transferred from high aeration to either low aeration in the dark or anaerobiosis in the light (43). It has also been reported that pigment synthesis is inhibited when photosynthetically grown cells are transferred to an aerobic environment, with oxygen tension being a regulatory factor (14, 15). On the other side, under our experimental conditions, bacteriochlorophyll was far more abundant, and consequently, the amount of H+-PPase was also much higher in photosynthetic cultures than in dark-grown anaerobic cultures, namely, under fermentative and oxidant-dependent respiration conditions. Furthermore, the presence of low amounts of bacteriochlorophyll and vpp transcript in cultures grown in low aeration further supported a common regulation. These latter observations could also explain why H+-PPase activity has been previously reported to occur in membranes of aerobically grown R. rubrum cells (50). On the other hand, a possible concurrent regulation of sPPase in vivo by changes in the levels of certain intermediaries involved in energy-linked central metabolism cannot be ruled out. Indeed, previous reports demonstrated that R. rubrum sPPase activity is inhibited in vitro by different compounds related to energy-linked metabolism, such as fructose-1,6-biphosphate-2-phosphoglycerate or NAD(P)H (29, 30).
The H+-PPase is located in the vacuolar membrane of higher plants, playing a role in ion homeostasis and related abiotic stress situations, i.e., salinity, drought, etc. (22). In the case of R. rubrum, our results demonstrate that the H+-PPase is also involved in the response of this prokaryote to salt stress, being dramatically induced under aerobic conditions in the presence of NaCl. Therefore, although the R. rubrum H+-PPase was present under all the anaerobic conditions tested, the absence of oxygen does \'0fnot seem to be the only requirement for vpp gene transcription. Transcriptional regulation of plant H+-PPases under conditions of chilling or anoxia has been reported (12), and an increase in protein and activity was observed under conditions of chilling and mineral nutrient deficiency or in the presence of NaCl (16, 17, 27). However, to our knowledge, this is the first report of transcriptional induction of H+-PPase by stress in a prokaryote.
Primer extension experiments indicate that there are two tandem promoters controlling the expression of the vpp gene under anaerobic conditions, with TSPs at positions −135 (TSP1) and −296 (TSP3). Different consensus sequences that could be involved in transcriptional control under anaerobic conditions have been tentatively identified for these two promoters. A possible FNR binding site was identified, which suggests that TSP3 is a class I FNR-dependent promoter (9), and a putative binding site for RegA was found for TSP1. Since RegA is a transcriptional activator for many genes involved in anoxygenic photosynthesis and anaerobic metabolism (21, 26, 46), this is consistent with the results presented in this work. In aerobic cultures under salt stress, which were completely devoid of pigments, two further tandem promoters with TSPs at positions −184 (TSP2) and −301 (TSP3′) are active, showing −35 regions characteristic of alternative sigma factors usually expressed under stress conditions (1, 25, 56). Although no bacterial transcription factor specific of gene induction upon salt stress has been described so far, induction of membrane proteins upon this type of stress has previously been reported for purple photosynthetic bacteria (58, 59). The genetic organization of the R. rubrum genome regions around both vpp and ppa genes (Fig. 6C) indicates that these genes are most probably not included in operons. The quite long intergenic distance of vpp with its upstream neighboring open reading frame is consistent with the likely complex molecular mechanisms of transcriptional regulation of this gene envisaged by promoter analyses. A comparative sequence analysis with the neighboring region upstream of vpp in the genome of the closely related phototrophic α-proteobacterium Rhodopseudomonas palustris (scaffold 1, nt 4589887 to 4590565; Rhodopseudomonas palustris DOE JGI genome project database [http://genome.jgi-psf.org/finished_microbes/rhopa/rhopa.home.html]) revealed putative regulatory sequences analogous to those found for R. rubrum (data not shown), suggesting that H+-PPase multifaceted regulation may be a common feature of proteobacteria.
Unlike higher plants, which lack cytosolic sPPase (57), the PPi generated in the cytosol of this photosynthetic prokaryote is in principle subjected to hydrolysis by two different PPases. The results presented in this work demonstrate that R. rubrum sPPase and H+-PPase are differentially regulated under the diverse growth conditions tested. Furthermore, the dramatic regulation of the membrane-bound PPase at the transcriptional level may be an element of general response to conditions severely disturbing the energetic status of R. rubrum cells. Thus, H+-PPase might not be needed \'0fwhen R. rubrum cells are grown under conditions of high energy supply and availability of nutrients, that is, an adequate carbon source and high aeration. However, it is readily induced when the cells are subjected to the stress inherent to a number of physiological conditions (anaerobic photosynthesis and respiration, fermentation, and high salt stress) under which diverse degrees of energy constraint are expected. In these metabolic scenarios, the use of PPi as a “low-cost” energy source useful for cell bioenergetics, a process in which H+-PPase plays a key role, should be an important adaptative advantage.
Acknowledgments
We gratefully thank M. Baltscheffsky (Stockholm University, Stockholm, Sweden) for the generous gifts of an antibody against R. rubrum H+-PPase and the R. rubrum vpp clone and E. Flores for critical reading of the manuscript and valuable advice.
Preliminary sequence data of R. rubrum genome were obtained freely for use in this publication only from the website of the DOE JGI.
This work was supported by research grants from the Spanish Ministry of Science and Technology (BMC2001-563) and the Andalusian Regional Government (III PAI, group CVI-261).
REFERENCES
- 1.Akbar, S., S. Y. Lee, S. A. Boylan, and C. W. Price. 1999. Two genes from Bacillus subtilis under the sole control of the general stress transcription factor σB. Microbiology 145:1069-1078. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, W. Miller, and J. Lipman. 1997. Gapped BLAST and PSI-BLAST, a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baltscheffsky, H., L. V. Von Steding, H. W. Heldt, and M. Klingenberg. 1966. Inorganic pyrophosphate formation in bacterial photophosphorylation. Science 153:1120-1124. [DOI] [PubMed] [Google Scholar]
- 4.Baltscheffsky, M., A. Schultz, and H. Baltscheffsky. 1999. H+-PPases: a tightly membrane-bound family. FEBS Lett. 457:527-533. [DOI] [PubMed] [Google Scholar]
- 5.Baltscheffsky, M., S. Nadanaciva, and A. Schultz. 1998. A pyrophosphate synthase gene: molecular cloning and sequencing of the cDNA encoding the inorganic pyrophosphate synthase from Rhodospirillum rubrum. Biochim. Biophys. Acta 1364:301-306. [DOI] [PubMed] [Google Scholar]
- 6.Bauer, C. E., D. A. Young, and B. L. Marrs. 1988. Analysis of the Rhodobacter capsulatus puf operon: location of the oxygen-regulated promoter region and the identification of an additional puf-encoded gene. J. Biol. Chem. 263:4820-4827. [PubMed] [Google Scholar]
- 7.Bauer, C. E., S. Elsen, and T. H. Bird. 1999. Mechanisms for redox control of gene expression. Annu. Rev. Microbiol. 53:495-523. [DOI] [PubMed] [Google Scholar]
- 8.Bäumer, S., S. Lentes, G. Gottschalk, and U. Deppenmeier. 2002. Identification and analysis of proton-translocating pyrophosphatases in the methanogenic archaeon Methanosarcina mazei. Archaea 1:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blake, T., A. Barnard, S. J. W. Busby, and J. Green. 2002. Transcription activation by FNR: evidence for a functional activating region 2. J. Bacteriol. 184:5855-5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bose, S. K., H. Gest, and J. G. Ormerod. 1961. Light-activated hydrogenase activity in a photosynthetic bacterium: a permeability phenomenon. J. Biol. Chem. 236:PC13-PC14.
- 11.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 12.Carystinos, G. D., H. R. MacDonald, A. F. Monroy, R. S. Dhindsa, and R. J. Poole. 1995. Vacuolar H+-translocating pyrophosphatase is induced by anoxia or chilling in seedlings of rice. Plant Physiol. 108:641-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clayton, R. K. 1963. Absorption spectra of photosynthetic bacteria and their chlorophylls, p. 495-500. In H. Gest, A. San Pietro, and L. P. Vernon (ed.), Bacterial photosynthesis. Antioch Press, Yellow Springs, Ohio.
- 14.Cohen-Bazire, G., and R. Kunisawa. 1963. The fine structure of Rhodospirillum rubrum cell. J. Cell Biol. 16:401-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen-Bazire, G., W. R. Sistrom, and R. Y. Stanier. 1957. Kinetic studies of pigment synthesis by nonsulfur purple bacteria. J. Cell. Comp. Physiol. 49:25-68. [DOI] [PubMed] [Google Scholar]
- 16.Colombo, R., and R. Cerana. 1993. Enhanced activity of tonoplast pyrophosphatase in NaCl grown cells of Daucus carota. J. Plant Physiol. 142:226-229. [Google Scholar]
- 17.Darley, C. P., J. M. Davies, and D. Sanders. 1995. Chill-induced changes in the activity and abundance of the vacuolar proton-pumping pyrophosphatase from Mung bean hypocotyls. Plant Physiol. 109:659-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drozdowicz, Y. M., J. C. Kissinger, and P. A. Rea. 2000. AVP2, a sequence-divergent, K+-insensitive H+-translocating inorganic pyrophosphatase from Arabidopsis. Plant Physiol. 123:353-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drozdowicz, Y. M., and P. A. Rea. 2001. Vacuolar H+ pyrophosphatase: from the evolutionary backwaters into the mainstream. Trends Plant Sci. 6:206-211. [DOI] [PubMed] [Google Scholar]
- 20.Drozdowicz, Y. M., Y.-P. Lu, V. Patel, S. Fitz-Gibbon, J. H. Miller, and P. A. Rea. 1999. A thermostable vacuolar-type membrane pyrophosphatase from the archaeon Pyrobaculum aerophilum: implications for the origins of pyrophosphate-energized pumps. FEBS Lett. 460:505-512. [DOI] [PubMed] [Google Scholar]
- 21.Du, S., T. H. Bird, and C. E. Bauer. 1998. DNA binding characteristics of RegA: a constitutively active anaerobic activator of photosynthetic gene expression in Rhodobacter capsulatus. J. Biol. Chem. 273:18509-18513. [DOI] [PubMed] [Google Scholar]
- 22.Gaxiola, R. A., J. Li, S. Undurraga, L. M. Dang, G. J. Allen, and S. L. Alper. 2001. Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc. Natl. Acad. Sci. USA 98:11444-11449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gómez-García, M. R., and A. Serrano. 2002. Expression studies of two paralogous ppa genes encoding distinct family I pyrophosphatases in marine unicellular cyanobacteria reveal inactivation of the typical cyanobacterial gene. Biochem. Biophys. Res. Commun. 295:890-897. [DOI] [PubMed] [Google Scholar]
- 24.Gómez-García, M. R., M. Losada, and A. Serrano. 2003. Concurrent transcriptional activation of ppa and ppx genes by phosphate deprivation in the cyanobacterium Synechocystis sp. strain PCC 6803. Biochem. Biophys. Res. Commun. 302:284-292. [DOI] [PubMed] [Google Scholar]
- 25.Hengge-Aronis, R. 2002. Signal transduction and regulatory mechanisms involved in control of the σS (RpoS) subunit of RNA polymerase. Microbiol. Mol. Biol. Rev. 66:373-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joshi, H. M., and F. R. Tabita. 1996. A global two component signal transduction system that integrates the control of photosynthesis, carbon dioxide assimilation, and nitrogen fixation. Proc. Natl. Acad. Sci. USA 93:14515-14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasai, M., T. Nakamura, N. Kaodi, H. Sato, M. Maeshima, and S. Sawada. 1998. The activity of the root vacuolar H+-pyrophosphatase in rye plants grown under conditions deficient in mineral nutrients. Plant Cell Physiol. 39:890-894. [DOI] [PubMed] [Google Scholar]
- 28.Kempter, B., P. Luppo, and D. Neumeier. 1991. A short procedure for Southern blotting on neutral and anionic membranes. Trends Genet. 7:109-110. [DOI] [PubMed] [Google Scholar]
- 29.Klemme, J.-H., and H. Gest. 1971. Regulatory properties of an inorganic pyrophosphatase from the photosynthetic bacterium Rhodospirillum rubrum. Proc. Natl. Acad. Sci. USA 68:721-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klemme, J. H., and H. Gest. 1971. Regulation of the cytoplasmic inorganic pyrophosphatase of Rhodospirillum rubrum. Eur. J. Biochem. 22:529-537. [DOI] [PubMed] [Google Scholar]
- 31.Kukko-Kalse, E., and J. Heinonen. 1985. Inorganic pyrophosphate and inorganic pyrophosphatases in Escherichia coli. Int. J. Biochem. 17:575-580. [DOI] [PubMed] [Google Scholar]
- 32.Kukko-Kalse, E., M. Lintunen, M. K. Inen, R. Lahti, and J. Heinonen. 1989. Intracellular PPi concentration is not directly dependent on amount of inorganic pyrophosphatase in Escherichia coli K-12 cells. J. Bacteriol. 171:4498-4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laguri, C., M. K. Phillips-Jones, and M. P. Williamson. 2003. Solution structure and DNA binding of the effector domain from the global regulator PrrA (RegA) from Rhodobacter sphaeroides: insights into DNA binding specificity. Nucleic Acids Res. 31:6778-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lahti, R., T. Pitkäranta, E. Valve, I. Ilta, E. Kukko-Kalse, and J. Heinonen. 1988. Cloning and characterization of the gene encoding inorganic pyrophosphatase of Escherichia coli K-12. J. Bacteriol. 170:5901-5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maeshima, M., and S. Yoshida. 1989. Purification and properties of vacuolar membrane proton-translocating inorganic pyrophosphatase from Mung bean. J. Biol. Chem. 196:11-17. [PubMed] [Google Scholar]
- 36.Markwell, M. A., S. M. Haas, L. L. Bieber, and N. E. Tolbert. 1987. Modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 180:152-157. [DOI] [PubMed] [Google Scholar]
- 37.McIntosh, M. T., and A. B. Vaidya. 2002. Vacuolar type H+ pumping pyrophosphatases of parasitic protozoa. Int. J. Parasitol. 32:1-14. [DOI] [PubMed] [Google Scholar]
- 38.Mitsuda, N., K. Enami, M. Nakata, K. Takayasu, and M. H. Sato. 2001. Novel type Arabidopsis thaliana H+-PPase is localized to the Golgi apparatus. FEBS Lett. 488:29-33. [DOI] [PubMed] [Google Scholar]
- 39.Muro-Pastor, A. M., A. Valladares, E. Flores, and A. Herrero. 2002. Mutual dependence of the expression of the cell differentiation regulatory protein HetR and the global nitrogen regulator NtcA during heterocyst development. Mol. Microbiol. 44:1377-1385. [DOI] [PubMed] [Google Scholar]
- 40.Nakanishi, Y., and M. Maeshima. 1998. Molecular cloning of vacuolar H+-pyrophosphatase and its developmental expression in growing hypocotyl of Mung bean. Plant Physiol. 100:718-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nyrén, P., and Å. Strid. 1991. Hypothesis: the physiological role of the membrane-bound proton-translocating pyrophosphatase of some phototrophic bacteria. FEMS Microbiol. Lett. 77:265-270. [Google Scholar]
- 42.Nyrén, P., B. F. Nore, and Å. Strid. 1991. Proton-pumping N,N′-dicyclohexylcarbodiimide-sensitive inorganic pyrophosphate synthase from Rhodospirillum rubrum: purification, characterization and reconstitution. Biochemistry 30:2883-2887. [DOI] [PubMed] [Google Scholar]
- 43.Oelze, J. 1976. Early formation of intracytoplasmic membranes in Rhodospirillum rubrum. Biochim. Biophys. Acta 436:95-100. [DOI] [PubMed] [Google Scholar]
- 44.Pérez-Castiñeira, J. R., J. Alvar, L. M. Ruiz-Pérez, and A. Serrano. 2002. Evidence for the wide occurrence of proton-translocating pyrophosphatase genes in parasitic and free-living protozoa. Biochem. Biophys. Res. Commun. 294:567-573. [DOI] [PubMed] [Google Scholar]
- 45.Pérez-Castiñeira, J. R., R. L. López-Marqués, M. Losada, and A. Serrano. 2001. A thermostable K+-stimulated vacuolar-type pyrophosphatase from the hyperthermophilic bacterium Thermotoga maritima. FEBS Lett. 496:6-11. [DOI] [PubMed] [Google Scholar]
- 46.Qian, Y., and F. R. Tabita. 1996. A global signal transduction system regulates aerobic and anaerobic CO2 fixation in Rhodobacter sphaeroides. J. Bacteriol. 178:12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Randahl, H. 1979. Characterization of the membrane-bound inorganic pyrophosphatase in Rhodospirillum rubrum. Eur. J. Biochem. 102:251-256. [DOI] [PubMed] [Google Scholar]
- 48.Rea, P. A., and R. J. Poole. 1993. Vacuolar H+-translocating pyrophosphatase. Annu. Rev. Plant Physiol. Plant Mol. Biol. 44:157-180. [Google Scholar]
- 49.Reitzer L., and B. L. Schneider. 2001. Metabolic context and possible physiological themes of σ54-dependent genes in Escherichia coli. Microbiol. Mol. Biol. Rev. 65:422-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romero, I., A. Gómez-Priego, and H. Celis. 1991. A membrane-bound pyrophosphatase from respiratory membranes of Rhodospirillum rubrum. J. Gen. Microbiol. 137:2611-2616. [Google Scholar]
- 51.Sarafian, V., Y. Kim, R. J. Poole, and P. A. Rea. 1992. Molecular cloning and sequence of cDNA encoding the pyrophosphate-energized vacuolar-membrane proton-pump of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 89:1775-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schultz, J. E., and P. F. Weaver. 1982. Fermentation and anaerobic respiration by Rhodospirillum rubrum and Rhodopseudomonas capsulata. J. Bacteriol. 149:181-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seufferheld, M., M. C. F. Vieira, F. A. Ruiz, C. O. Rodrigues, S. N. J. Moreno, and R. Docampo. 2003. Identification of organelles in bacteria similar to acidocalcisomes of unicellular eukaryotes. J. Biol. Chem. 278:29971-29978. [DOI] [PubMed] [Google Scholar]
- 54.Strid, Å., I.-M. Karlsson, and M. Baltscheffsky. 1987. Demonstration of ΔpH and ΔΨ-induced synthesis of inorganic pyrophosphate in chromatophores from Rhodospirillum rubrum. FEBS Lett. 224:348-352. [Google Scholar]
- 55.Valverde, F., M. Losada, and A. Serrano. 1997. Functional complementation of an Escherichia coli gap mutant supports an amphibolic role for NAD(P)-dependent glyceraldehyde-3-phosphate dehydrogenase of Synechocystis sp. strain PCC 6803. J. Bacteriol. 179:4513-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Viollier, P. H., G. H. Kelemen, G. E. Dale, K. T. Nguyen, M. J. Buttner, and C. J. Thompson. 2003. Specialized osmotic stress response systems involve multiple SigB-like sigma factors in Streptomyces coelicolor. Mol. Microbiol. 47:699-714. [DOI] [PubMed] [Google Scholar]
- 57.Weiner, H., M. Stitt, and H. W. Heldt. 1987. Subcellular compartmentation of pyrophosphate and alkaline pyrophosphatase in leaves. Biochim. Biophys. Acta 893:13-21. [Google Scholar]
- 58.Xu, X., M. Abo, A. Okubo, and S. Yamakazi. 2001. Salt-stress-responsive membrane proteins in Rhodobacter sphaeroides f. sp. denitrificans IL106. J. Biosci. Bioeng. 91:228-230. [DOI] [PubMed] [Google Scholar]
- 59.Xu, X. Y., H. Kadokura, A. Okubo, K. Kitamoto, and S. Yamakazi. 2001. Cloning and sequencing of a gene encoding a novel salt stress-induced membrane protein from Rhodobacter sphaeroides f. sp. denitrificans. Appl. Microbiol. Biotechnol. 56:442-447. [DOI] [PubMed] [Google Scholar]
- 60.Zheng, R.-G., E. Kim, and P. A. Rea. 1997. The molecular and biochemical basis of pyrophosphate-energized proton translocation at the vacuolar membrane. Adv. Bot. Res. 25:297-337. [Google Scholar]