Abstract
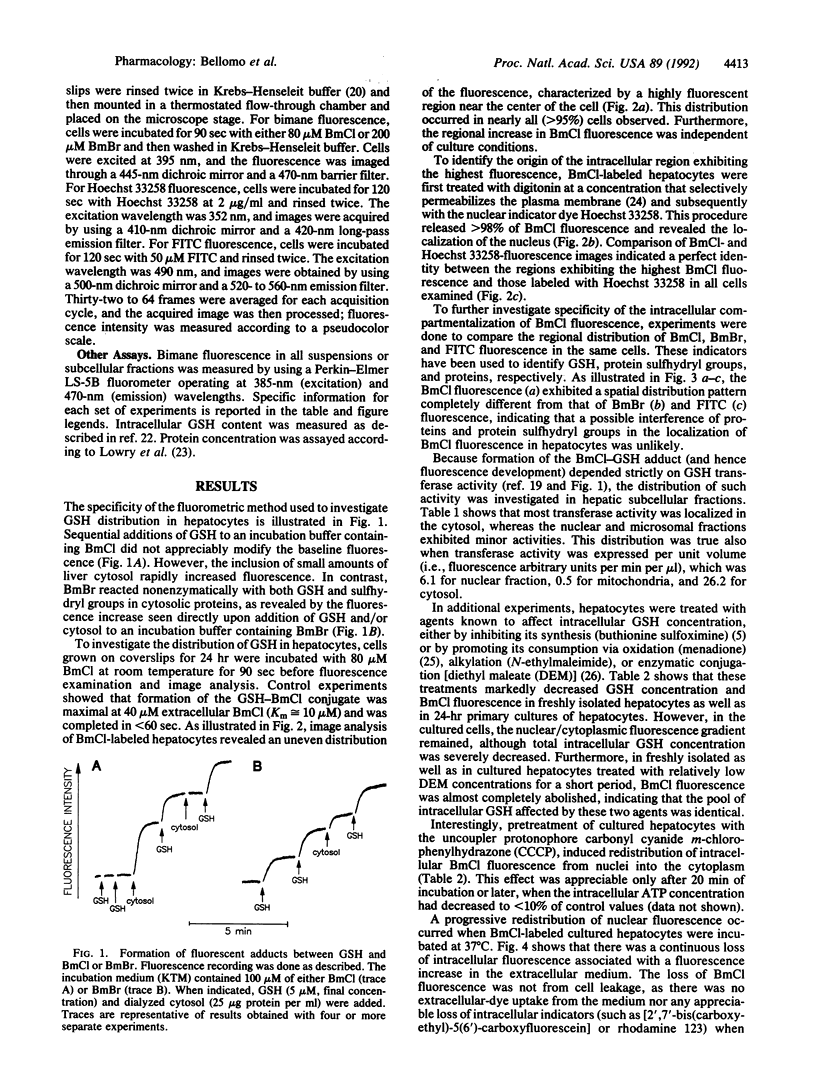
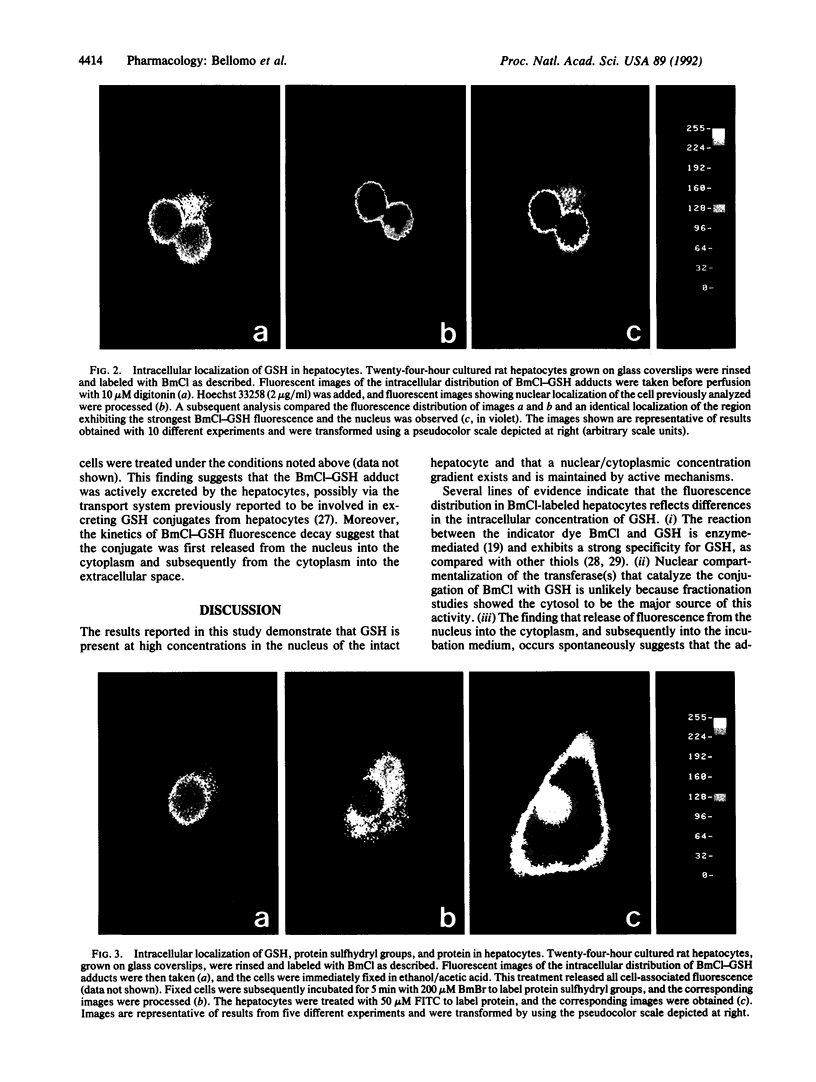
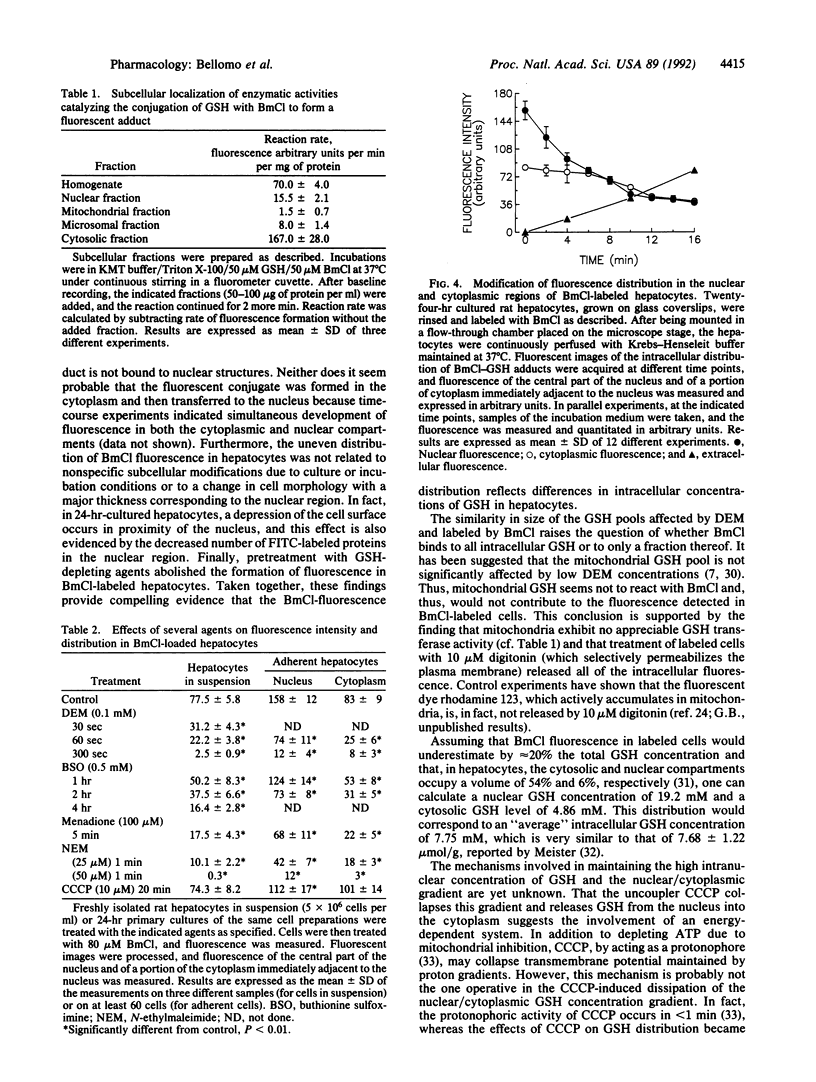
The intracellular distribution of glutathione (GSH) in cultured hepatocytes has been investigated by using the compound monochlorobimane (BmCl), which interacts specifically with GSH to form a highly fluorescent adduct. Image analysis of BmCl-labeled hepatocytes predominantly localized the fluorescence in the nucleus; the nuclear/cytoplasmic concentration gradient was approximately three. This concentration gradient was collapsed by treatment of the cells with ATP-depleting agents. The uneven distribution of BmCl fluorescence was not attributable to (i) nonspecific interaction of BmCl with protein sulfhydryl groups, (ii) any selective nuclear localization of the GSH transferase(s) catalyzing formation of the GSH-BmCl conjugate, or (iii) any apparent alterations in cell morphology from culture conditions, suggesting that this distribution did, indeed, reflect a nuclear compartmentalization of GSH. That the nuclear pool of GSH was found more resistant to depletion by several agents than the cytoplasmic pool supports the assumption that GSH is essential in protecting DNA and other nuclear structures from chemical injury.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellomo G., Fulceri R., Albano E., Gamberucci A., Pompella A., Parola M., Benedetti A. Ca(2+)-dependent and independent mitochondrial damage in hepatocellular injury. Cell Calcium. 1991 May;12(5):335–341. doi: 10.1016/0143-4160(91)90049-k. [DOI] [PubMed] [Google Scholar]
- Bellomo G., Thor H., Orrenius S. Modulation of cellular glutathione and protein thiol status during quinone metabolism. Methods Enzymol. 1990;186:627–635. doi: 10.1016/0076-6879(90)86158-r. [DOI] [PubMed] [Google Scholar]
- Britten R. A., Green J. A., Broughton C., Browning P. G., White R., Warenius H. M. The relationship between nuclear glutathione levels and resistance to melphalan in human ovarian tumour cells. Biochem Pharmacol. 1991 Feb 15;41(4):647–649. doi: 10.1016/0006-2952(91)90642-i. [DOI] [PubMed] [Google Scholar]
- Cook J. A., Iype S. N., Mitchell J. B. Differential specificity of monochlorobimane for isozymes of human and rodent glutathione S-transferases. Cancer Res. 1991 Mar 15;51(6):1606–1612. [PubMed] [Google Scholar]
- De Capoa A., Ferraro M., Lavia P., Pelliccia F., Finazzi-Agrò A. Silver staining of the nucleolus organizer regions (NOR) requires clusters of sulfhydryl groups. J Histochem Cytochem. 1982 Sep;30(9):908–911. doi: 10.1177/30.9.6182186. [DOI] [PubMed] [Google Scholar]
- Dijkwel P. A., Wenink P. W. Structural integrity of the nuclear matrix: differential effects of thiol agents and metal chelators. J Cell Sci. 1986 Aug;84:53–67. doi: 10.1242/jcs.84.1.53. [DOI] [PubMed] [Google Scholar]
- Edgren M., Révész L. Compartmentalised depletion of glutathione in cells treated with buthionine sulphoximine. Br J Radiol. 1987 Jul;60(715):723–724. doi: 10.1259/0007-1285-60-715-723. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Scheer U., Krohne G., Jarasch E. D. The nuclear envelope and the architecture of the nuclear periphery. J Cell Biol. 1981 Dec;91(3 Pt 2):39s–50s. doi: 10.1083/jcb.91.3.39s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemont P. S., Hanson I. M., Trowsdale J. A novel cysteine-rich sequence motif. Cell. 1991 Feb 8;64(3):483–484. doi: 10.1016/0092-8674(91)90229-r. [DOI] [PubMed] [Google Scholar]
- Gores G. J., Nieminen A. L., Wray B. E., Herman B., Lemasters J. J. Intracellular pH during "chemical hypoxia" in cultured rat hepatocytes. Protection by intracellular acidosis against the onset of cell death. J Clin Invest. 1989 Feb;83(2):386–396. doi: 10.1172/JCI113896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5606–5610. doi: 10.1073/pnas.76.11.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Origin and turnover of mitochondrial glutathione. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4668–4672. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert P. B., Yakubu S. I. Monobromobimane: a substrate for the fluorimetric assay of glutathione transferase. J Pharm Pharmacol. 1983 Jun;35(6):384–386. doi: 10.1111/j.2042-7158.1983.tb02962.x. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M., Newton G. L., Ranney H. M. Bimane fluorescent labels: labeling of normal human red cells under physiological conditions. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3382–3386. doi: 10.1073/pnas.76.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meredith M. J., Reed D. J. Status of the mitochondrial pool of glutathione in the isolated hepatocyte. J Biol Chem. 1982 Apr 10;257(7):3747–3753. [PubMed] [Google Scholar]
- Mårtensson J., Lai J. C., Meister A. High-affinity transport of glutathione is part of a multicomponent system essential for mitochondrial function. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7185–7189. doi: 10.1073/pnas.87.18.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera P., Baldi C., Svensson S. A., Larsson R., Bellomo G., Orrenius S. Glutathione S-conjugates stimulate ATP hydrolysis in the plasma membrane fraction of rat hepatocytes. FEBS Lett. 1985 Jul 22;187(1):121–125. doi: 10.1016/0014-5793(85)81226-6. [DOI] [PubMed] [Google Scholar]
- Nicotera P., McConkey D. J., Jones D. P., Orrenius S. ATP stimulates Ca2+ uptake and increases the free Ca2+ concentration in isolated rat liver nuclei. Proc Natl Acad Sci U S A. 1989 Jan;86(2):453–457. doi: 10.1073/pnas.86.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. C., Bump E. A., Shrieve D. C., Lee W., Kovacs M. Quantitative analysis of cellular glutathione by flow cytometry utilizing monochlorobimane: some applications to radiation and drug resistance in vitro and in vivo. Cancer Res. 1986 Dec;46(12 Pt 1):6105–6110. [PubMed] [Google Scholar]
- Sandström B. E., Marklund S. L. Effects of variation in glutathione peroxidase activity on DNA damage and cell survival in human cells exposed to hydrogen peroxide and t-butyl hydroperoxide. Biochem J. 1990 Oct 1;271(1):17–23. doi: 10.1042/bj2710017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrieve D. C., Bump E. A., Rice G. C. Heterogeneity of cellular glutathione among cells derived from a murine fibrosarcoma or a human renal cell carcinoma detected by flow cytometric analysis. J Biol Chem. 1988 Oct 5;263(28):14107–14114. [PubMed] [Google Scholar]
- Spelsberg T. C., Knowler J. T., Moses H. L. Specific methods for the isolation of nuclei from chick oviduct. Methods Enzymol. 1974;31:263–279. doi: 10.1016/0076-6879(74)31028-2. [DOI] [PubMed] [Google Scholar]
- Staal F. J., Roederer M., Herzenberg L. A., Herzenberg L. A. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthanthiran M., Anderson M. E., Sharma V. K., Meister A. Glutathione regulates activation-dependent DNA synthesis in highly purified normal human T lymphocytes stimulated via the CD2 and CD3 antigens. Proc Natl Acad Sci U S A. 1990 May;87(9):3343–3347. doi: 10.1073/pnas.87.9.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. H., Meyer D. J., Gillies N., Ketterer B. Detoxification of DNA hydroperoxide by glutathione transferases and the purification and characterization of glutathione transferases of the rat liver nucleus. Biochem J. 1988 Sep 15;254(3):841–845. doi: 10.1042/bj2540841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Thor H., Smith M. T., Hartzell P., Bellomo G., Jewell S. A., Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J Biol Chem. 1982 Oct 25;257(20):12419–12425. [PubMed] [Google Scholar]
- Tirmenstein M. A., Reed D. J. Characterization of glutathione-dependent inhibition of lipid peroxidation of isolated rat liver nuclei. Arch Biochem Biophys. 1988 Feb 15;261(1):1–11. doi: 10.1016/0003-9861(88)90097-5. [DOI] [PubMed] [Google Scholar]
- Tirmenstein M. A., Reed D. J. Role of a partially purified glutathione S-transferase from rat liver nuclei in the inhibition of nuclear lipid peroxidation. Biochim Biophys Acta. 1989 Apr 6;995(2):174–180. doi: 10.1016/0167-4838(89)90077-0. [DOI] [PubMed] [Google Scholar]
- Tirmenstein M. A., Reed D. J. The glutathione status of rat kidney nuclei following administration of buthionine sulfoximine. Biochem Biophys Res Commun. 1988 Sep 15;155(2):956–961. doi: 10.1016/s0006-291x(88)80589-8. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Harootunian A. T. Practical design criteria for a dynamic ratio imaging system. Cell Calcium. 1990 Feb-Mar;11(2-3):93–109. doi: 10.1016/0143-4160(90)90063-z. [DOI] [PubMed] [Google Scholar]
- Ursini F., Bindoli A. The role of selenium peroxidases in the protection against oxidative damage of membranes. Chem Phys Lipids. 1987 Jul-Sep;44(2-4):255–276. doi: 10.1016/0009-3084(87)90053-3. [DOI] [PubMed] [Google Scholar]
- Wahlländer A., Soboll S., Sies H., Linke I., Müller M. Hepatic mitochondrial and cytosolic glutathione content and the subcellular distribution of GSH-S-transferases. FEBS Lett. 1979 Jan 1;97(1):138–140. doi: 10.1016/0014-5793(79)80069-1. [DOI] [PubMed] [Google Scholar]
- Wahlländer A., Soboll S., Sies H., Linke I., Müller M. Hepatic mitochondrial and cytosolic glutathione content and the subcellular distribution of GSH-S-transferases. FEBS Lett. 1979 Jan 1;97(1):138–140. doi: 10.1016/0014-5793(79)80069-1. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Becker P. L., Fay F. S. Regional changes in calcium underlying contraction of single smooth muscle cells. Science. 1987 Mar 27;235(4796):1644–1648. doi: 10.1126/science.3103219. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Fogarty K. E., Tsien R. Y., Fay F. S. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature. 1985 Dec 12;318(6046):558–561. doi: 10.1038/318558a0. [DOI] [PubMed] [Google Scholar]