Abstract
For maternally transmitted microbes, a female-biased host sex ratio is of reproductive advantage. Here we found a strong female bias in a field population of the green lacewing, Mallada desjardinsi (Insecta; Neuroptera). This bias was attributed to the predominance of individuals harboring a maternally inherited male-killing bacterium that was phylogenetically closely related to the plant-pathogenic Spiroplasma phoeniceum and Spiroplasma kunkelii. Among 35 laboratory-reared broods produced by wild-caught females, 21 broods (60%)—all infected with Spiroplasma—consisted of only females (940 individuals). Among 14 broods consisting of both males and females (516 and 635 individuals, respectively), 4 broods were doubly infected with Spiroplasma and Rickettsia, 6 broods were singly infected with Rickettsia, and 3 broods were uninfected (remaining one brood was unknown). Mortality during embryonic and larval development was prominent in all-female broods but not in normal sex ratio broods. Following antibiotic treatment on all-female broods, mortality was significantly reduced and the sex ratio was restored to 1:1. Strong expression and high prevalence of this male-killer is remarkable considering its low density (~10−5–10−4 cells per host mitochondrial gene copy based on quantitative PCR). In addition, a bacterium closely related to Rickettsia bellii was present in 25 of 34 broods (73.5%), irrespective of the sex ratio, with the infection density comparable to other cases of endosymbiosis (~10−2–10−1 cells per mitochondrial gene copy). Higher density of Rickettsia than Spiroplasma was also demonstrated by electron microscopy which visualized both Spiroplasma-like cells and Rickettsia-like cells inside and outside the ovarian cells.
Introduction
Unlike vertebrates, many invertebrates allow massive proliferation of certain groups of microbes, such as Wolbachia, Rickettsia, and Spiroplasma, within cytoplasm of their somatic and germ cells [1]. Because cytoplasmic elements are transmitted to offspring only through mothers, male hosts are non-profitable for such microbes.
Attention has been paid to some of these microbes because they can distort their host sex ratio toward female—the sex they are transmitted from—even at the expense of host fitness. Such sex ratio distortions, including male-killing, feminization, and parthenogenesis-induction, together with cytoplasmic incompatibility, are understood as selfish behaviors of microbes [2, 3].
Among these host manipulations, male-killing is induced by a wide diversity of microbes—a variety of bacteria such as Spiroplasma, Wolbachia, Rickettsia, and Arsenophonus [4]; microsporidian protists [5]; and a putative RNA virus [6]. Microbe-induced male-killing has previously been found from four insect orders (i.e., Diptera, Lepidoptera, Hymenoptera, and Coleoptera) and pseudoscorpions (Arachnida; Pseudoscorpionida). In most of these cases of male-killing, however, male-killers are harbored only by a small portion of individuals in a population, and therefore, ecological and evolutionary impact of male-killers are elusive (but see exceptions in male-killing Wolbachia in butterflies [7, 8]).
Here we report that a strong female-bias in a population of the lacewing Mallada desjardinsi (Neuroptera; Chrysopidae)—a species attracting attention because its larvae escape from the attack of aphid-tending ants by carrying aphid carcasses on their backs [9]—is caused by an endosymbiotic Spiroplasma bacteria. In addition, M. desjardinsi was found to be infected with Rickettsia bacterium that is not associated with sex ratio distortion. This is the first report of the occurrence of male-killing and the presence of Rickettsia and Spiroplasma in the insect order Neuroptera [10, 11], wherein XX/XY sex determination is considered to be common [12]. Lacewing larvae are voracious consumers of aphids and insect eggs and are used as a biological control agent in agriculture [13]. Our findings highlight the effects of male-killing Spiroplasma on sex ratios, and possibly on sexual behaviors and population demography of this agriculturally important insect.
Materials and Methods
Insects
Sixty-four adults of M. desjardinsi (Insecta; Neuroptera; Chrysopidae) were collected by a sweeping net under street lamps near trees and bushes in the campus of Chiba University, Matsudo, Chiba Pref., Japan, at night (20:00–22:00) from May to October in 2011. M. desjardinsi is not an endangered or protected species. Specific permission is not required for insect collection for students and faculty members in the campus of Chiba University. Females were brought into the laboratory and individually allowed to lay eggs in plastic containers for 15 days. During egg collection, females were fed with 50% honey solution and dried yeast. After egg collection, females were stored at −40°C until DNA extraction. To prevent cannibalism, an egg was placed in each well of the 24-well plate (cat. no., 142475, Nunc™ Cell-Culture Treated Multidishes, Thermo Fisher Scientific K.K., Yokohama, Japan) together with Ephestia kuehniella (Lepidoptera; Pyralidae; Agrisect Inc., Ibaraki, Japan) eggs, as larval diet. Insects were reared under a 16-h:8-h light:dark photoperiod at 25 ± 1°C. Adults were sexed according to the abdominal tip morphology.
Antibiotic treatment
Six female adults of the line MK20 were fed with 50% honey solution containing tetracycline hydrochloride (0.1% w/w) for 7–8 days. The females were individually coupled with males and allowed to lay eggs for 1 month. Eggs laid by the females were reared as described above. As a control, we conducted the same procedure for six females of the same line using 50% honey solution that does not contain tetracycline hydrochloride.
DNA extraction and diagnostic polymerase chain reaction (PCR)
DNA was extracted from adult abdomen using DNeasy® Blood & Tissue Kit (Qiagen, Hilden, Germany). To assure DNA quality, partial sequence of mitochondrial cytochrome c oxidase subunit I (COI) gene was amplified by PCR using DNA barcoding primers (S1 Table). For the PCR detection of specific bacteria, partial sequence of Spiroplasma spoT, a gene sequence similar to that of the (p)ppGpp 39-pyrophosphohydrolase spoT gene, Rickettsia 16S ribosomal RNA (16S rRNA) gene, and Wolbachia surface protein (wsp) gene were amplified by respective primers (S1 Table). PCRs were conducted with Go-Taq® (Promega KK, Tokyo, Japan).
Quantification of bacterial densities by realtime PCR (qPCR)
Real-time fluorescence detection quantitative PCR (qPCR) was performed using SYBR Green and a LightCycler® 480 System (Roche Diagnostics K.K., Tokyo, Japan). Spiroplasma-specific sequence was amplified using a primer pair, spoT-MDF designed for M. desjardinsi Spiroplasma (S1 Table) and spoT-r, Rickettsia-specific citrate synthase gene (gltA) sequence was amplified using glt375-F and glt574-R (S1 Table), and host mitochondrial COII sequence was amplified using C2-J3399 and C2-N3665 (S1 Table). For each of the reactions, standard samples, i.e., dilution series of PCR products (108, 107, 106, 105, 104 and 103 copies per microliter), were included in order to estimate the absolute copy numbers of the target sequence in the samples. To prepare standard samples, PCR products were gel-excised and purified by Wizard SV (Promega). Copy numbers of the standard samples were estimated by the concentration measured by a spectrophotometer, regarding that the molecular weight of a nucleotide as 309 g/mol. In each qPCR reaction, two replicates were performed with similar results. All qPCR reactions were performed using a temperature profile of 40 cycles of 95°C for 10 s, 57°C for 10 s, and 72°C for 10 s. The qPCR data were analyzed by the Absolute Quantification analysis using the Second Derivative Maximum method implemented in the LightCycler® 480 Instrument Operator Software Version 1.5 (Roche).
Cloning and sequencing
From the whole-insect DNA of M. desjardinsi, almost the entire length of the bacterial 16S rRNA gene (about 1.5 kb) was amplified by PCR with the primers 16SA1 and 16SB1 (S1 Table) under the temperature profile of 94°C for 10 min, followed by 35 cycles consisting of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. The PCR product was electrophoresed in a 1.5% agarose gel, excised, purified by using a Wizard SV (Promega), and cloned with the TA cloning vector pGEM®-T Easy Vector Systems (Promega) and Escherichia coli DH5 competent cells (Takara Bio Inc., Kusatsu, Japan), in which ampicillin and 5-bromo-4-chloro-3-indolyl-beta-D-galactopyranoside (X-Gal) were used for the blue-white selection system. Products of colony PCR were filtered using S-300 (GE Healthcare Japan, Tokyo, Japan) and were subjected to sequencing reactions using BigDye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific) and the following sequencing primers: T7 and SP6 in the flanking regions of the vector and internal primers 16SA2 and 16SB2 (S1 Table). The sequencing products, filtered using Sephadex G-50 (GE Healthcare), were subjected to a DNA Analyzer (model 3730xl; Applied Biosystems). In addition, the RNA polymerase B (rpoB) gene amplified by the primers RpoBF1 and RpoBR4 (S1 Table) and (p)ppGpp 39-pyrophosphohydrolase spoT gene [14] amplified by the primers spoT-f and spoT-r (S1 Table) were gel-excised, purified, and directly sequenced.
Transmission electron microscopy (TEM)
Ovaries of M. desjardinsi female adults were carefully dissected and excised with fine forceps under a stereo dissecting microscope. The ovaries were fixed in 2.5% glutaraldehyde (Nacalai Tesque, Kyoto, Japan) in 0.06 M phosphate buffer at 4°C for 2 h. After rinsing with the same buffer, the samples were postfixed with 2% osmium tetroxide at 4°C for 1 h and washed three times with 0.1 M sodium acetate for 10 min each. The samples were stained with 1% uranyl acetate for 20 min at room temperature and then sequentially dehydrated on ice twice with 70% ethanol for 5 min each and twice with 95% ethanol for 5 min each. The dehydrated samples were placed in Quetol 651 (Nisshin EM, Tokyo, Japan), a water-miscible resin, and embedded in a Quetol 651 resin mixture according to the manufacturer’s protocol. Ultrathin sections were stained with TI blue (Nisshin EM) and Sato’s lead solution, and observed under a JEM-1010 transmission electron microscope (JEOL, Tokyo, Japan).
Phylogenetic analyses
To infer the phylogenetic position of the Spiroplasma among the other Spiroplasma species found to date, the nucleotide sequences of the 16S rRNA, rpoB, and spoT genes were used. Sequence alignments were performed using Clustal W [15] implemented in BioEdit [16]. The evolutionary history was inferred by using the Maximum Likelihood method based on the General Time Reversible model using MEGA6 [17]. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. A discrete Gamma distribution was used to model evolutionary rate differences among sites.
The nucleotide sequences of other Spiroplasma and Rickettsia were taken from the GenBank database (http://www.ncbi.nlm.nih.gov/).
Statistical analyses
Survival data: Analyses on binary outcomes (i.e., alive or dead) were performed with generalized linear mixed models (GLMMs) with binomial error distribution to assess the effects of mothers on survival rates. Generalized linear mixed models are an extension to the generalized linear models [18] in which the linear predictor contains random effects in addition to the fixed effects [19]. To account for stochastic among-brood variation (i.e., repeated observation within single broods), we included the random effects of brood identity in the models. As some of the data sets contain hierarchical error structures (i.e., effects of mothers), they were included as the hierarchical random effects in the models. All these statistical analyses were calculated by the function glmer of the program package lme4 using the software R version 3.0.1 [20].
Bacterial density data: The qPCR data (either Spiroplasma spoT copies per COII copy or Rickettsia gltA copies per COII copy) were analyzed with generalized linear models using the software R version 3.0.1. Using the function glm originally implemented in R, we adopted a generalized linear model for Gaussian, inverse Gaussian, or gamma distributions, which was selected according to the Akaike information criterion. Multiple comparisons were performed with Bonferroni corrections.
Results
Female-biased sex ratio and the prevalence of Spiroplasma and Rickettsia
Of 64 wild-caught M. desjardinsi adults, 57 were female and 7 were male. Thirty-five females bore offspring and 34 females were subjected to diagnostic PCR after oviposition (DNA extracted from one female was lost). Among 34 females, 19 females were positive for both Spiroplasma and Rickettsia (S+R+), 6 females were positive only for Spiroplasma (S+R−), 6 females were positive only for Rickettsia (S−R+) and 3 females were negative for both Spiroplasma and Rickettsia (S-R-) (Table 1). None of the 34 females was positive for Wolbachia. Total number of laboratory-reared F1 offspring were 1575 females and 516 males (S2 Table), wherein the number of males was disproportionately higher compared to the sex ratio of wild-caught adults (P = 0.0111 by Fisher’s exact probability test), which may suggest, in this population, that males take less time for searching for females than in population with 1:1 sex ratio [7].
Table 1. Infection status of wild-caught M. desjardinsi females and the sex ratio of their offspring.
| Infection status | Number of broods | |||
|---|---|---|---|---|
| All-female | Female-biaseda | Normalb | Total | |
| S+R+ | 15 | 2 | 2 | 19 |
| S+R− | 6 | − | − | 6 |
| S−R+ | − | 1 | 5 | 6 |
| S−R− | − | − | 3 | 3 |
S+R+: doubly infected with Spiroplasma and Rickettsia.
S+R−: singly infected with Spiroplasma.
S−R+: singly infected with Rickettsia.
S−R−: non-infected.
a Contain males but sex ratio significantly deviated from 1:1 (P < 0.05 by chi-squared test).
b Sex ratio not significantly deviated from 1:1 (P > 0.05 by chi-squared test).
Spiroplasma as a sex-ratio distorter
Among 35 broods produced by wild-caught females, 21 broods consisted of only females (n = 940), whereas 14 broods consisted of both males and females (516 and 635 individuals, respectively). All the 21 all-female-producing females were positive for Spiroplasma (Table 1; S2 Table). In contrast, Rickettsia was detected from 18 of 24 female-biased broods and 7 of 10 normal broods, suggesting that Rickettsia is irrelevant to the sex ratio distortion (Table 1; S2 Table). Moreover, cloning of the bacterial 16S rRNA gene from the all-female-producing female (#5) shows that all the 25 E. coli colonies contain a unique sequence of Spiroplasma (S4 Table), suggesting that other bacteria cannot be the cause of the all-female trait.
Male-killing as the likely mechanism for all-female trait
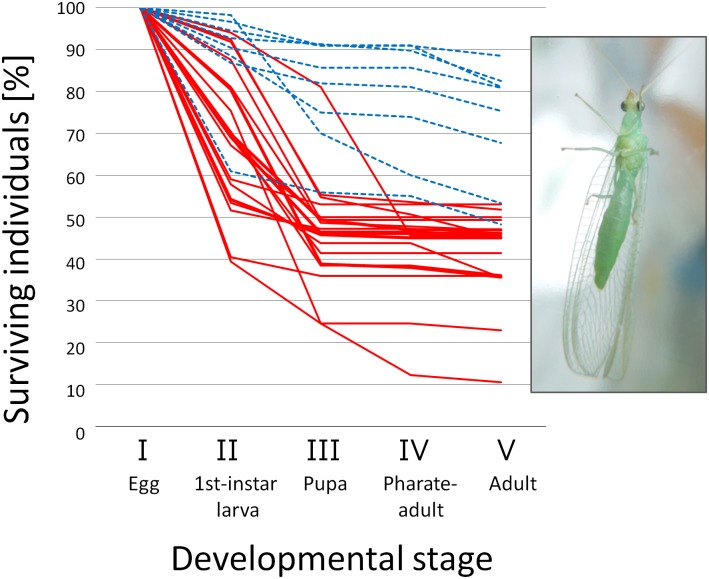
All-female broods suffered high mortality during embryonic and/or larval development, leading to nearly 50% or lower survival rate, whereas survival rates of normal sex ratio broods were higher than 50% except for one brood showing a survival rate of 48.3% (Fig 1). Survival rates of all-female broods were significantly lower than those of normal broods (P = 1.461 × 10−7).
Fig 1. Survivorship of offspring produced by wild-caught females.
Proportion of surviving individuals at the first-instar larval stage (II), the pupal stage (III), the pharate-adult (i.e., moving pupal) stage (IV), and the adult stage (V) among embryos (I). A solid line (red) represents a brood that developed to only females. A broken line (blue) represents a brood that developed to males and females with a ratio not significantly deviated from 1:1 (P > 0.05). A photograph of an adult female is given in the inset.
Antibiotic treatment on females of the line MK20 during the adult stage significantly improved the survival rate during whole developmental stages (P = 0.0003), the egg hatch rate (P = 0.0003), but not the larval survival rate (P = 0.6921) of their offspring, resulting in nearly 1:1 sex ratio (Table 2). Diagnostic PCR showed that these offspring were free from Spiroplasma. Therefore, at least in MK20, male-killing during the embryonic stage is likely to be the mechanism for the all-female trait. Considering the variable timing of death in the progeny of the wild-caught females (Fig 1), timing of male-killing may not be restricted to the embryonic stage.
Table 2. Effects of tetracycline on survival rates and sex ratio of the matriline MK20.
| Treatment | Brood | Eggs laid | No. of hatched larvae | No. adults | Proportion of females | Egg hatch rates1 | Larval survival rates2 | Survival rates3 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Total | ||||||||
| (a) tetracycline-treated | ||||||||||
| a | 53 | 45 | 18 | 22 | 40 | 0.450 | 0.849 | 0.889 | 0.755 | |
| b | 58 | 57 | 23 | 29 | 52 | 0.442 | 0.983 | 0.912 | 0.897 | |
| c | 81 | 38 | 22 | 11 | 33 | 0.667 | 0.469 | 0.868 | 0.407 | |
| d | 52 | 44 | 19 | 19 | 38 | 0.500 | 0.846 | 0.864 | 0.731 | |
| e | 46 | 41 | 18 | 16 | 34 | 0.529 | 0.891 | 0.829 | 0.739 | |
| f | 80 | 72 | 33 | 35 | 68 | 0.485 | 0.900 | 0.944 | 0.850 | |
| (b) non-treated | ||||||||||
| g | 28 | 11 | 7 | 0 | 7 | 1.000 | 0.393 | 0.636 | 0.250 | |
| h | 39 | 14 | 12 | 0 | 12 | 1.000 | 0.359 | 0.857 | 0.308 | |
| i | 121 | 61 | 52 | 0 | 52 | 1.000 | 0.504 | 0.852 | 0.430 | |
| j | 69 | 33 | 30 | 0 | 30 | 1.000 | 0.478 | 0.909 | 0.435 | |
| k | 166 | 76 | 70 | 0 | 70 | 1.000 | 0.458 | 0.921 | 0.422 | |
| l | 47 | 15 | 14 | 0 | 14 | 1.000 | 0.319 | 0.933 | 0.298 | |
1No. of hatched larvae/ no. of eggs laid
2No. of adults/ no. of hatched larvae
3No. of adults/ no. of eggs laid
Despite the absence of Spiroplasma, a wild-caught female (#2) infected with Rickettsia produced progeny with a significantly female-biased sex ratio (70 females and 12 males; P = 1.286 × 10−6 by chi-squared test) (S2 and S4 Tables), possibly reflecting the sex-ratio-distorting ability of Rickettsia, which, however, remains elusive because this line was not maintained in the laboratory for further analyses (cf. Rickettsia was shown to be transmitted vertically to female-biased offspring; S5 Table). Low egg hatch rate (0.519) in brood #2 suggests that male-killing during embryogenesis might be the cause of the female-biased sex ratio (S2 Table).
Localization and morphology of Spiroplasma and Rickettsia
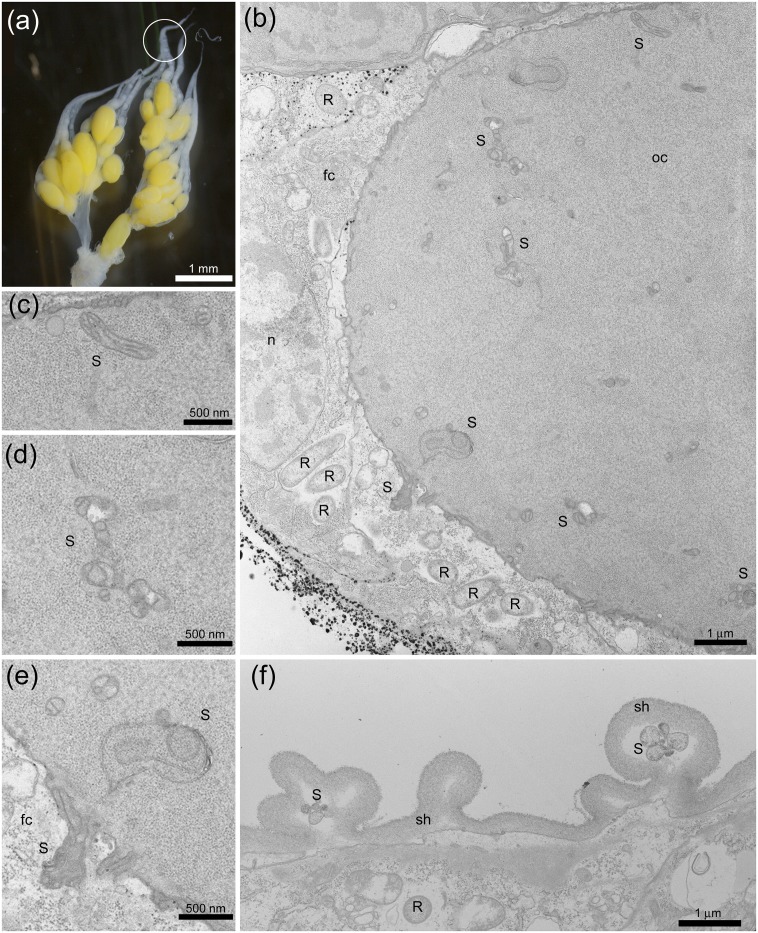
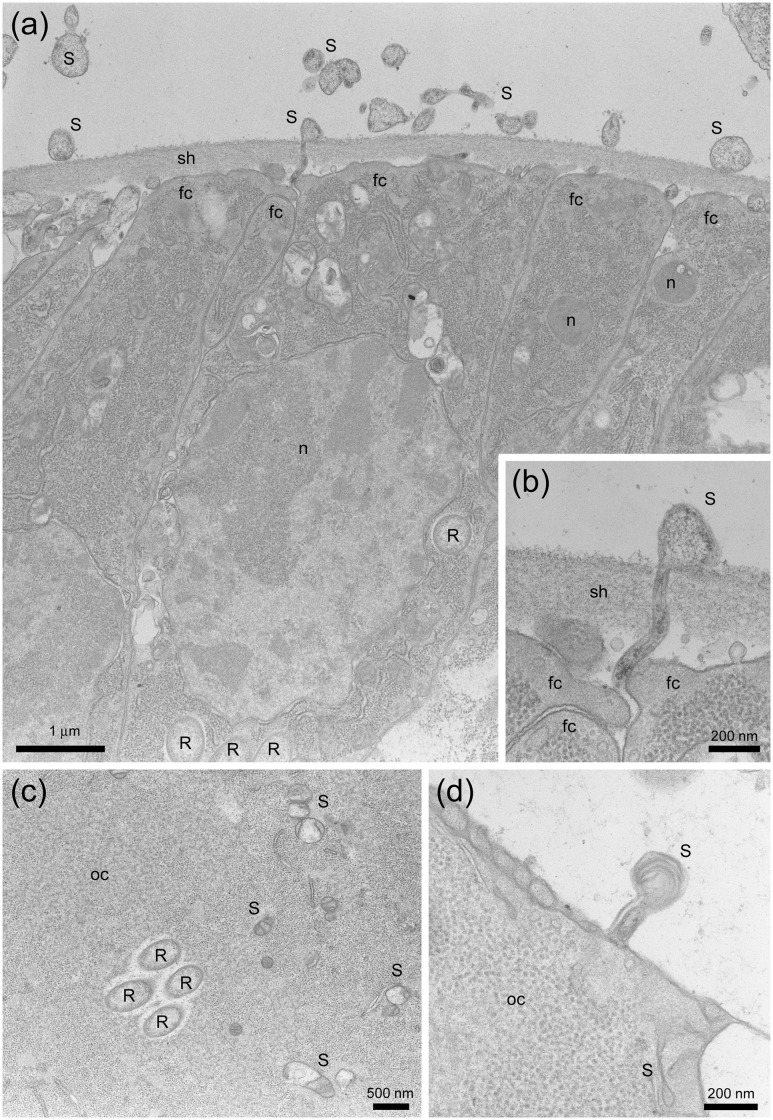
In undifferentiated parts of ovarioles (i.e., germarium) (located at the upper end within the circle in Fig 2a), a round-shaped Rickettsia-like structure [21] was abundantly observed in the follicle cells but no such structure was observed in oocytes (Fig 2b). In well-differentiated parts of ovarioles (located at the bottom end within the circle in Fig 2a), however, the Rickettsia-like structure was also observed in oocytes (Fig 3c), as well as in follicle cells (Fig 2f) and nurse cells (Fig 3a), possibly suggesting that Rickettsia intrude into the cytoplasm during egg maturation, which has been demonstrated in Spiroplasma poulsonii infecting Drosophila melanogaster [22].
Fig 2. Transmission electron micrographs of Spiroplasma and Rickettsia in the female reproductive tissues of M. desjardinsi.
a: Ovaries of a female M. desjardinsi. A circle represents a portion subjected to electron microscopy. b: Undifferentiated part of an ovariole, with Rickettsia-like structure in the follicle cells and Spiroplasma-like structure in the oocyte. c,d, e: Magnified images of (b), showing Spiroplasma-like structure. f: Differentiating part of an ovariole, with Rickettsia-like structure in the oocyte and Spiroplasma-like structure in the sheath. S: Spiroplasma; R: Rickettsia; oc: oocyte; fc: follicle cell; sh: sheath; n: nucleus.
Fig 3. Further images of Spiroplasma and Rickettsia in the female reproductive tissues of M. desjardinsi observed by transmission electron microscopy.
a: Differentiating part of an ovariole, with Spiroplasma-like structure outside and inside the sheath and Rickettsia-like structure in the follicle cells. b: Magnified image of (a), showing the presence of Spiroplasma-like structure in the intercellular space and the sheath, possibly at the timing of intrusion into a follicle cell. c: Cytoplasm of an oocyte, showing Rickettsia and Spiroplasma-like structure. d: Spiroplasm-like structure possibly at the timing of intrusion into the oocyte. S: Spiroplasma; R: Rickettsia; oc: oocyte; fc: follicle cell; sh: sheath; n: nucleus.
Although much less in frequency, Spiroplasma-like pleomorphic structure [10, 23]—often recognized as twisted and half collapsed—was present in the oocytes (Fig 2b, 2c, 2d and 2e) in the undifferentiated parts of ovarioles. The Spiroplasma-like structure was also observed in the sheath cells (Figs 2f, 3a and 3b) and nurse cells (Fig 3a), as well as in the intercellular space (Fig 3b and 3d).
Phylogenetic position of Spiroplasma and Rickettsia
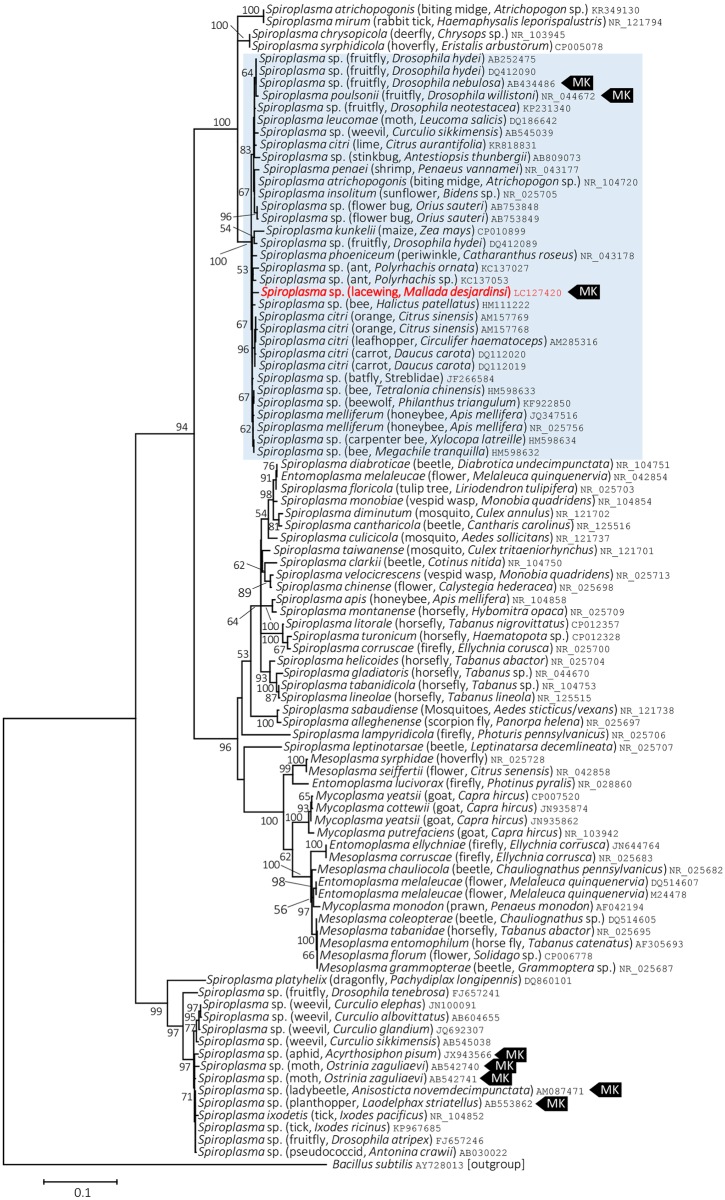
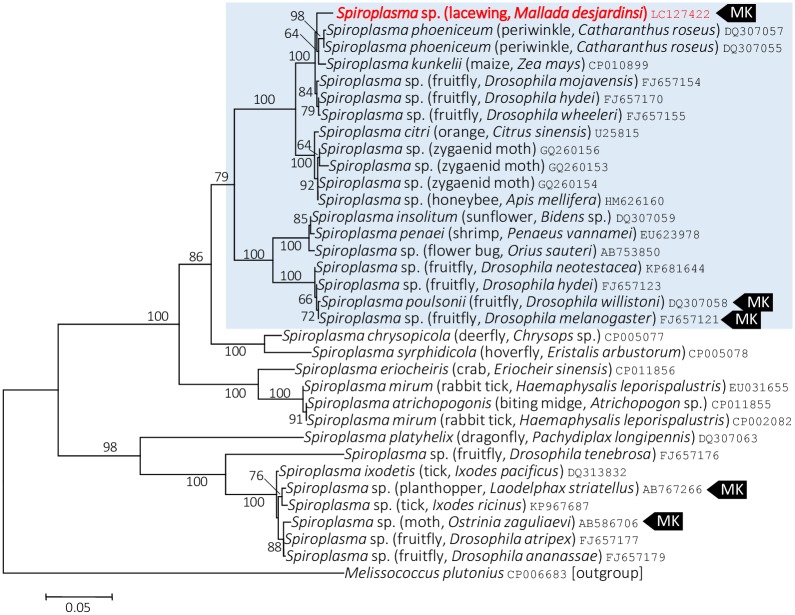
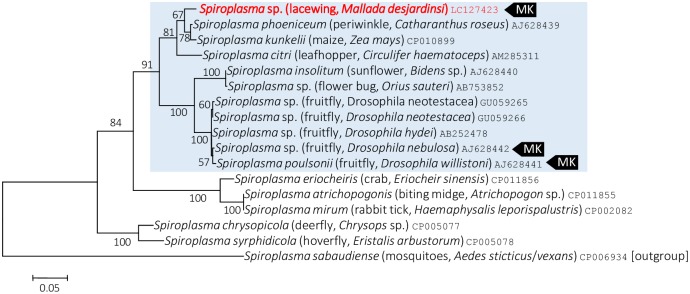
On the Maximum Likelihood tree based on the 16S rRNA gene sequences, Spiroplasma present in M. desjardinsi falls in the Citri-Poulsonii clade (Fig 4), a large group consisting of S. citri, S. poulsonii, S. phoeniceum, S. kunkelii, S. insolitum, as well as S. melliferum. Within this large clade, M. desjardinsi Spiroplasma was placed outside the clade consisting S. poulsonii and S. insolitum, but the relationship with other Spiroplasma members was unclear (Fig 4; S1 Fig). However, Maximum Likelihood tress based on rpoB and spoT gene sequences gave a better resolution—they consistently showed that Spiroplasma present in M. desjardinsi is closely related to S. phoeniceum and S. kunkelii (Figs 5 and 6). The same results were obtained by Neighbor Joining and Maximum Parsimony methods.
Fig 4. Phylogenetic position of M. desjardinsi Spiroplasma among Spiroplasma bacteria and relatives based on 16S rRNA gene sequences.
A maximum likelihood tree (lnL = −6745.9487) is shown. Bootstrap probabilities are given at the nodes. M. desjardinsi Spiroplasma is depicted as red. The scale bar indicates the number of nucleotide substitutions per site. A clade containing S. poulsonii, S. insolitum, S. kunkelii, S. phoeniceum, S. citri and S. melliferum is shaded with light blue. A host organism is given in parenthesis. An accession number is given at the end of each OTU. MK represents a male-killer.
Fig 5. Phylogenetic position of M. desjardinsi Spiroplasma among Spiroplasma bacteria and relatives based on rpoB gene sequences.
A maximum likelihood tree (lnL = −5057.9807) is shown. Bootstrap probabilities are given at the nodes. M. desjardinsi Spiroplasma is depicted as red. The scale bar indicates the number of nucleotide substitutions per site. A clade containing S. poulsonii, S. insolitum, S. kunkelii, S. phoeniceum, S. citri and S. melliferum is shaded with light blue. A host organism is given in parenthesis. An accession number is given at the end of each OTU. MK represents a male-killer.
Fig 6. Phylogenetic position of M. desjardinsi Spiroplasma among Spiroplasma bacteria and relatives based on spot gene sequences.
A maximum likelihood tree (lnL = −2525.6794) is shown. Bootstrap probabilities are given at the nodes. M. desjardinsi Spiroplasma is depicted as red. The scale bar indicates the number of nucleotide substitutions per site. A clade containing S. poulsonii, S. insolitum, S. kunkelii, S. phoeniceum, S. citri and S. melliferum is shaded with light blue. A host organism is given in parenthesis. An accession number is given at the end of each OTU. MK represents a male-killer.
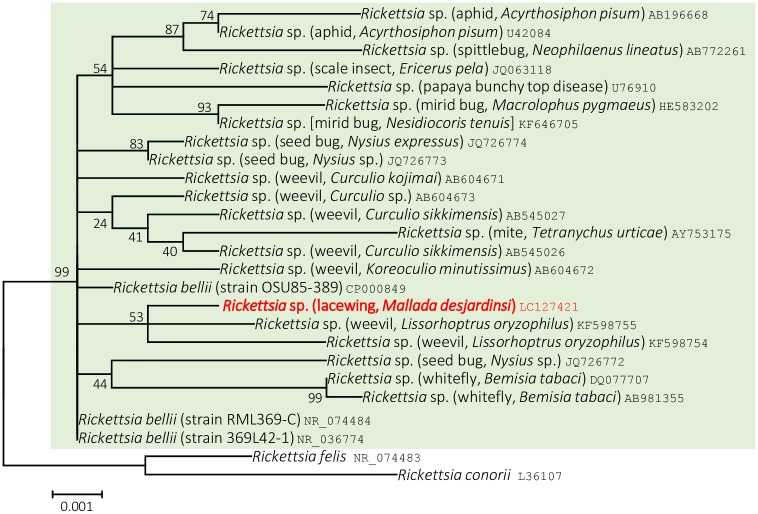
Maximum Likelihood tree based on 16S rRNA gene clearly showed that Rickettsia present in M. desjardinsi is a member of Rickettsia bellii relatives (Fig 7), which was also supported by Neighbor Joining and Maximum Parsimony methods.
Fig 7. Phylogenetic position of M. desjardinsi Rickettsia among R. bellii relatives.
A maximum likelihood tree (lnL = −2633.5879) based on 16S rRNA gene sequences. Bootstrap probability is shown next to the branches. M. desjardinsi Rickettsia is depicted as red. The scale bar (0.001) indicates the number of nucleotide substitutions per site. R. bellii and its relatives are shaded with light green. A host organism is given in parenthesis. An accession number is given at the end of each OTU.
Densities of Spiroplasma and Rickettsia
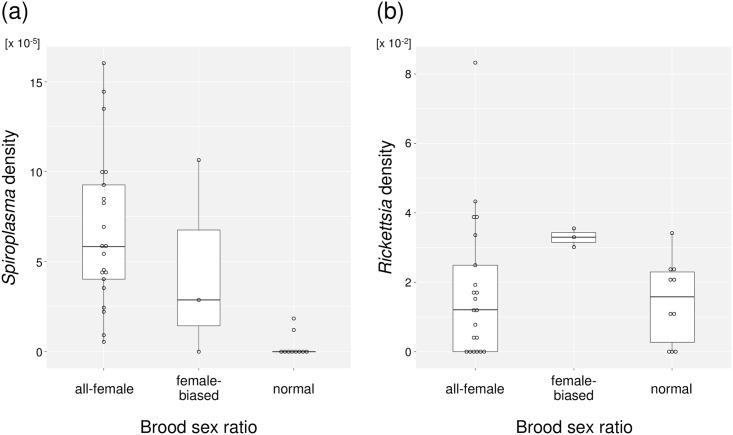
According to the qPCR data (S3 Table), although two normal-sex-ratio-producing females (#21 and #46) showed significantly smaller Spiroplasma density than all-female-producing females (P = 0.0087), they also showed higher Spiroplasma density than two of the all-female-producing females (#10 and #20), which showed lowest Spiroplasma densities (Fig 8a). Hence, the density threshold may not explain the lack of male-killing expression in the two broods. Rather, suppressors against male-killing might exist in the genetic background of M. desjardinsi. In contrast, qPCR data showed that Rickettsia densities were not significantly different between mothers producing all-female and normal sex-ratio progeny (Fig 8b; P = 0.6059), supporting the view that the presence of Rickettsia was irrelevant to the host sex ratio.
Fig 8. qPCR-estimates of endosymbiont population densities in mothers that are categorized by their offspring sex ratio.
(a) Spiroplasma density in mothers (i.e., Relative values of Spiroplasma spoT gene copies per mitochondrial COII gene copy). (b) Rickettsia density in mothers (i.e., Relative values of Rickettsia gltA gene copies per mitochondrial COII gene copy).
Infection status examined for some of the F1 offspring showed that both Spiroplasma and Rickettsia were stably inherited in all-female broods (S5 Table). Notably, in a female-biased brood (#9), all the examined females (n = 12) were positive for both Spiroplasma and Rickettsia, while all the males (n = 7) were positive only for Rickettsia (S5 Table). Failure of vertical transmission of Spiroplasma may be the cause for the rescue of males in this brood. However, another female-biased brood produced by a doubly infected mother (#30) did not show such pattern; among 12 females and 12 males examined, all but one female were doubly infected with Spiroplasma and Rickettsia (S5 Table).
Discussion
This study revealed that the frequent occurrence in M. desjardinsi of male-killing is the likely cause of female-biased population sex ratio of this species. The male-killing in M. desjardinsi is most likely caused by a Spiroplasma endosymbiont. According to previous reports, infection frequencies of male-killing Spiroplasma among host populations are variable; 30.77% in a ladybird beetle Anisosticta novemdecimpunctata [24], 0%–70% in another ladybird beetle Adalia bipunctata [25], 2%–3% in Drosophila flies [10, 26], 40% in a butterfly Danaus chrysippus [27], and only one instance in a moth Ostrinia zaguliaevi [28]. Considering the markedly high male-killing frequency (61.76%), the Spiroplasma–M. desjardinsi relationship would be an interesting biological system to investigate the evolutionary forces acting on insect sex ratio. Under such a highly female-biased condition, suppressors against male-killing and/or male-biased sex-ratio distorters would be expected to spread when they arise. The disproportionately female-biased sex ratio in wild-caught individuals in comparison with the breeding data may suggest the alteration of sexual behavior—males may spend less time for searching females, and hence, be less conspicuous—under the highly female-biased condition in M. desjardinsi. Moreover, genetic alterations related to sexual traits may be caused by persistence of female-biased sex ratio [29]. Alteration of sexual behaviors due to the Wolbachia-mediated scarcity of males have been suggested for a butterfly [7].
On the basis of molecular phylogenetic analyses, Spiroplasma found in the present study—a male-killer of M. desjardinsi—was unexpectedly closely related to S. phoeniceum and S. kunkelii. Both S. phoeniceum and S. kunkelii are plant pathogens, causing periwinkle yellowing disease and corn stunt disease, respectively [30, 31], and are closely related to S. citri, the causal agent of citrus stubborn disease. Although S. citri and S. kunkelii are known to be transmitted by leafhoppers, wherein spiroplasmas can proliferate in the hemolymph [32], it is unknown whether these spiroplasmas can be vertically (i.e., cytoplasmically) transmitted from mothers to offspring in the leafhoppers. Then, why is Spiroplasma in M. desjardinsi closely related to the plant pathogens? Although M. desjardinsi is a predatory insect and feeds on small insects in larval stages, it feeds on flower nectar in adult stage [13]. We consider that the common ancestors of M. desjardinsi Spiroplasma and its closely related plant pathogenic spiroplasmas might be at the interface between horizontal and vertical transmission of insect endosymbionts, and between plant pathogenicity and insect pathogenicity (i.e., male-killing). Further exploration of other spiroplasmas may lead to a better understanding of the evolution of these distinct phenotypic traits of spiroplasmas.
All the male-killing Spiroplasma discovered to date fall into S. ixodetis clade (moth, butterfly, and ladybird beetle) or S. poulsonii clade (Drosophila). Therefore, our finding of male-killing Spiroplasma in a new clade is suggestive of the possible occurrence of male-killers also from other Spiroplasma clades.
The timing of male-killing in M. desjardinsi was not restricted to the embryonic stage but was extended to later larval stages, suggesting that it may not be appropriate to be called early male-killing—the most common form of male-killing [33]. Such ambiguous timing of male-killing has also been reported in the Wolbachia-infected moths of the genus Ostrinia [34, 35], while timing of male-killing is restricted to embryonic stage in Spiroplasma-infected Ostrinia zaguliaevi [28]. Kageyama et al. [36] demonstrated, in Spiroplasma-infected Drosophila melanogaster, that the timing of male-killing is dependent on maternal age, and hence Spiroplasma titer. In M. desjardinsi, however, the timing of male-killing does not seem to be attributed to the Spiroplasma titers in mothers (S3 Table).
The low titer of Spiroplasma in M. desjardinsi revealed in the present study is reminiscent of the sex-ratio-distorting Wolbachia (strain wFem) in the butterfly Eurema mandarina [37], which shows 10−6–10−9 copies of Wolbachia wsp gene per mitochondrial COI copy [38]. These results may suggest that a small amount of bacteria-derived products are sufficient to manipulate insect sex ratios. Low density within insect cells may render the Spiroplasma prone to fail in vertical transmission, where a strong bottleneck is assumed. This drawback might be counter-balanced by the reproductive advantage through male-killing [4, 33] or other possible effects on the host [39].
The 1:1 sex ratio exhibited in the broods produced by two of the Spiroplasma-positive females was not likely to be attributed to the Spiroplasma titers. Considering the fact that nucleotide sequences of the three genes (16S rRNA, spoT and RpoB) were indistinguishable between Spiroplasma in females producing all-female and 1:1 sex ratio progeny, we suspect the presence of host suppressors against male-killing as has been reported in a butterfly Hypolymnas bolina [40], which, however, remains unproven by genetic analyses in M. desjardinsi. At present, therefore, some mutations in the Spiroplasma genome cannot be excluded as the cause of the 1:1 sex ratio.
High prevalence of Rickettsia infection in M. desjardinsi is also intriguing. Although no apparent effects on host fitness were detected under laboratory condition in the present study, some unknown context-dependent effects on hosts [39, 41] might be attributed to the maintenance of this bacterium in the M. desjardinsi population.
In sum, a maternally transmitted male-killing Spiroplasma is the main cause of female-biased sex ratio observed in M. desjardinsi. Close relatedness of this Spiroplasma to plant-pathogenetic S. phoeniceium, S. kunkelii and S. citri based on molecular phylogenetic analyses may suggest that M. desjardinsi Spiroplasma is at the interface between horizontal and vertical transmission of insect endosymbionts, and between plant pathogenicity and insect pathogenicity (i.e., male-killing). Strikingly low density of Spiroplasma (10−5–10−4 cells per host mitochondrial gene copy) in M. desjardinsi is suggestive when considering the evolution of endosymbiosis and host manipulation. Presence of some individuals that escape male-killing may suggest the existence of suppressors against male-killing, which should be examined in future studies. In addition, a portion of M. desjardinsi individuals harbor Rickettsia endosymbiont which seems to have no effects on host sex ratio.
Supporting Information
(TIF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank Dr. Atsushi Mochizuki for his valuable advice on insect collection and culture.
Data Availability
Nucleotide sequence data are available from the DNA Databank of Japan database (http://getentry.ddbj.nig.ac.jp/), under accession numbers LC127420-LC127423.
Funding Statement
This work was financially supported by KAKENHI, a Grant-in-Aid for Scientific Research (https://www.jsps.go.jp/english/e-grants/), grant No. 25450492 for DK, and by internal grants from the National Institute of Agrobiological Sciences and Chiba University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There was no additional external funding received for this study.
References
- 1.Duron O, Bouchon D, Boutin S, Bellamy L, Zhou L, Engelstädter J, et al. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 2008; 6:27 10.1186/1741-7007-6-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008; 6: 741–751. 10.1038/nrmicro1969 [DOI] [PubMed] [Google Scholar]
- 3.Kageyama D, Narita S, Watanabe M. Insect sex determination manipulated by their endosymbionts: incidences, mechanisms and implications. Insects. 2012; 3: 161–199. 10.3390/insects3010161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurst GD, Jiggins FM Male-killing bacteria in insects: mechanisms, incidence, and implications. Emerg Inf Dis. 2000; 6: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunn AM, Terry RS, Smith JE. Transovarial transmission in the microsporidia. Adv Parasitol. 2001; 48: 57–100. [DOI] [PubMed] [Google Scholar]
- 6.Nakanishi K, Hoshino M, Nakai M, Kunimi Y. Novel RNA sequences associated with late male killing in Homona magnanima. Proc Biol Sci. 2008; 275:1249–1254. 10.1098/rspb.2008.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiggins FM, Hurst GD, Majerus ME. Sex-ratio-distorting Wolbachia causes sex-role reversal in its butterfly host. Proc Biol Sci. 2000; 267:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dyson EA, Hurst GDD. Persistence of an extreme sex-ratio bias in a natural population. PNAS 2004; 101: 6520–6523. 10.1073/pnas.0304068101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi M, Nomura M. Larvae of the green lacewing Mallada desjardinsi (Neuroptera: Chrysopidae) protect themselves against aphid-tending ants by carrying dead aphids on their backs. Appl. Entomol. Zool. 2011; 46: 407–413. [Google Scholar]
- 10.Whitcomb RF, Tully JG. The Mycoplasmas, Volume V: Spiroplasmas, Acholeplasmas, and Mycoplasmas of Plants and Arthropods, 1st Edition, Elsevier; 1989 [Google Scholar]
- 11.Dunn AK, Stabb EV. Culture-independent characterization of the microbiota of the ant lion Myrmeleon mobilis (Neuroptera: Myrmeleontidae). Appl Environ Microbiol. 2005; 71: 8784–8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Resh VH, Cardé RT. Encyclopedia of Insects, 2nd Edition Academic Press; 2009 [Google Scholar]
- 13.McEwen PK, New TR, Whittington AE. Lacewings in the Crop Environment. Cambridge University Press; 2007 [Google Scholar]
- 14.Jacob C, Nouzieres F, Duret S, Bove JM, Renaudin J. Isolation, characterization, and complementation of a motility mutant of Spiroplasma citri. J Bacteriol. 1997; 179: 4802–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson JD, Higgins DG, Gibson TJ. ClustalW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nuc Acid Res. 1994; 22:4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nuc Acid Symp Ser. 1999; 41: 95–98. [Google Scholar]
- 17.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013; 30: 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCullagh P, Nelder JA. Generalized Linear Models, 2nd ed Chapman and Hall, London, United Kingdom: 1989 [Google Scholar]
- 19.Schall R. Estimation in generalized linear models with random effects. Biometrika 1991; 78: 719–727. [Google Scholar]
- 20.R Development Core Team. R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: 2005. [Google Scholar]
- 21.Brumin M, Levy M, Ghanim M. Transovarial transmission of Rickettsia spp. and organ-specific infection of the whitefly Bemisia tabaci. Appl Environ Microbiol. 2012; 78: 5565–5574. 10.1128/AEM.01184-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herren JK, Paredes JC, Schüpfer F, Lemaitre B. Vertical transmission of a Drosophila endosymbiont via cooption of the yolk transport and internalization machinery. mBio 2013; 4: e00532–12. 10.1128/mBio.00532-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe K, Yukuhiro F, Matsuura Y, Fukatsu T, Noda H. Intrasperm vertical symbiont transmission. PNAS 2014; 111: 7433–7437. 10.1073/pnas.1402476111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tinsley MC, Majerus ME. A new male-killing parasitism: Spiroplasma bacteria infect the ladybird beetle Anisosticta novemdecimpunctata (Coleoptera: Coccinellidae). Parasitology 2006; 132:757–765. [DOI] [PubMed] [Google Scholar]
- 25.Ryder JJ, Hoare MJ, Pastok D, Bottery M, Boots M, Fenton A, Atkinson D, Knell RJ and Gregory D. D. Hurst GDD. Disease epidemiology in arthropods is altered by the presence of nonprotective symbionts. Am. Nat. 2014; 183:E89–E104. 10.1086/674827 [DOI] [PubMed] [Google Scholar]
- 26.Montenegro H, Solferini VN, Klaczko LB, Hurst GD. Male-killing Spiroplasma naturally infecting Drosophila melanogaster. Insect Mol Biol. 2005; 14:281–287. [DOI] [PubMed] [Google Scholar]
- 27.Jiggins FM, Hurst GD, Jiggins CD, v d Schulenburg JH, Majerus ME. The butterfly Danaus chrysippus is infected by a male-killing Spiroplasma bacterium. Parasitology 2000; 120:439–446. [DOI] [PubMed] [Google Scholar]
- 28.Tabata J, Hattori Y, Sakamoto H, Yukuhiro F, Fujii T, Kugimiya S, et al. Male killing and incomplete inheritance of a novel Spiroplasma in the moth Ostrinia zaguliaevi. Microb Ecol. 2011; 61:254–263. 10.1007/s00248-010-9799-y [DOI] [PubMed] [Google Scholar]
- 29.Reuter M, Linklater JR, Lehmann L, Fowler K, Chapman T, Hurst GDD. Adaptation to experimental alterations of the operational sex ratio in populations of Drosophila melanogaster. Evolution 2008; 62: 401–412. 10.1111/j.1558-5646.2007.00300.x [DOI] [PubMed] [Google Scholar]
- 30.Saillard C, Vignault JC, Bové JM, Raie A, Tully JG, Williamson DL, et al. Spiroplasma phoeniceum sp. nov. a new plant-pathogenic species from Syria. Int J Syst Bacteriol. 1987; 37:106–115. [Google Scholar]
- 31.Whitcomb RF, Chen TA, Williamson DL, Liao C, Tully JG, Bové JM, et al. Spiroplasma kunkelii sp. nov.: characterization of the etiological agent of corn stunt disease. Int J Syst Bacteriol. 1986; 36:170–178. [Google Scholar]
- 32.Bové JM, Renaudin J, Saillard C, Foissac X, Garnier M. Spiroplasma citri, a plant pathogenic Mollicute: Relationships with its two hosts, the plant and the leafhopper vector. Ann Rev Phytopathol. 2003; 41:483–500. [DOI] [PubMed] [Google Scholar]
- 33.Hurst GDD and Majerus MEN. Why do maternally inherited microorganisms kill males?. Heredity 1993; 71: 81–95. [Google Scholar]
- 34.Kageyama D, Traut W. Opposite sex-specific effects of Wolbachia and interference with sex determination of its host Ostrinia scapulalis. Proc. Biol. Sci. 2004; 271: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakamoto H, Kageyama D, Hoshizaki S, Ishikawa Y. Sex-specific death in Ostrinia furnacalis (Asian corn borer moth) infected with Wolbachia occurs across the larval development. Genome 2007; 50: 645–652. [DOI] [PubMed] [Google Scholar]
- 36.Kageyama D, Anbutsu H, Shimada M, Fukatsu T. Spiroplasma infection causes either early or late male killing in Drosophila, depending on maternal host age. Naturwissenschaften 2007; 94: 333–337. [DOI] [PubMed] [Google Scholar]
- 37.Kern P, Cook JM, Kageyama D, Riegler M. Double trouble: combined action of meiotic drive and Wolbachia feminization in Eurema butterflies. Biol Lett. 2015; 11:20150095 10.1098/rsbl.2015.0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narita S, Nomura M, Kageyama D. Naturally occurring single and double infection with Wolbachia strains in the butterfly Eurema hecabe: transmission efficiencies and population density dynamics of each Wolbachia strain. FEMS Microbiol Ecol. 2007; 61:235–245. [DOI] [PubMed] [Google Scholar]
- 39.Xie J, Vilchez I, Mateos M. Spiroplasma bacteria enhance survival of Drosophila hydei attacked by the parasitic wasp Leptopilina heterotoma. PLoS ONE 2010; 5: e12149 10.1371/journal.pone.0012149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hornett EA, Charlat S, Duplouy AM, Davies N, Roderick GK, Wedell N, Hurst GD. Evolution of male-killer suppression in a natural population. PLoS Biol. 2006; 4: e283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Łukasik P, van Asch M, Guo H, Ferrari J, Godfray HC. Unrelated facultative endosymbionts protect aphids against a fungal pathogen. Ecol Lett. 2013; 16:214–218. 10.1111/ele.12031 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
Nucleotide sequence data are available from the DNA Databank of Japan database (http://getentry.ddbj.nig.ac.jp/), under accession numbers LC127420-LC127423.