Abstract
tRNA is a central component of the protein synthesis machinery in the cell. In living cells, tRNAs undergo numerous post-transcriptional modifications. In particular, modifications at the anticodon loop play an important role in ensuring efficient protein synthesis, maintaining protein homeostasis, and helping cell adaptation and survival. Hypo-modification of the wobble position of the tRNA anticodon loop is of particular relevance for translation regulation and is implicated in various human diseases. In this review we summarize recent evidence of how methyl and thiol modifications in eukaryotic tRNA at position 34 affect cellular fitness and modulate regulatory circuits at normal conditions and under stress.
KEYWORDS: decoding, HIV-1, ribosomal frameshifting, ribosome profiling, translation regulation, tRNA modifications
Introduction
In all organisms tRNAs are post-transcriptionally modified. For example, cytoplasmic tRNAs in yeast Saccharomyces cerevisiae bear as much as 7 to 17 modifications per tRNA (Fig. 1), which contribute to the thermodynamic stability and folding of tRNA and ensure its proper interactions with aminoacyl-tRNA synthetases (ARS), mRNA, and the ribosome. Some modifications are common to all tRNAs, while others are specific to certain tRNA species.1-8 tRNA modifications at or near the anticodon loop are particularly important, as they regulate the efficiency and fidelity of translation. Position 34 (X34) in tRNA, which reads the third nucleotide in the mRNA codon, is a hot spot for modifications that restrict or facilitate wobble base pairing, thereby influencing codon recognition. Recent data provide insights into how modifications at the tRNA wobble position fine-tune decoding at the ribosome,9,10 thereby shaping the proteome and regulating cellular fitness.11,12,14-17 In addition to their function in translation, tRNA modifications play roles in non-canonical functions of tRNA, e.g., in priming reverse transcription of human immunodeficiency virus type-1 (HIV-1) by human tRNALys3(UUU).18,19 In this review we discuss advances in understanding the roles of modifications at U34 or C34 wobble positions in a subset of eukaryotic tRNAs, in particular m5C and mcm5s2U modifications in yeast (Table 1), their impact on canonical and non-canonical tRNA functions, and the effect on cellular fitness and ability to respond to stress. More general aspects of modifications in eukaryotic and bacterial tRNAs are discussed in recent reviews.14,20,21
Figure 1.
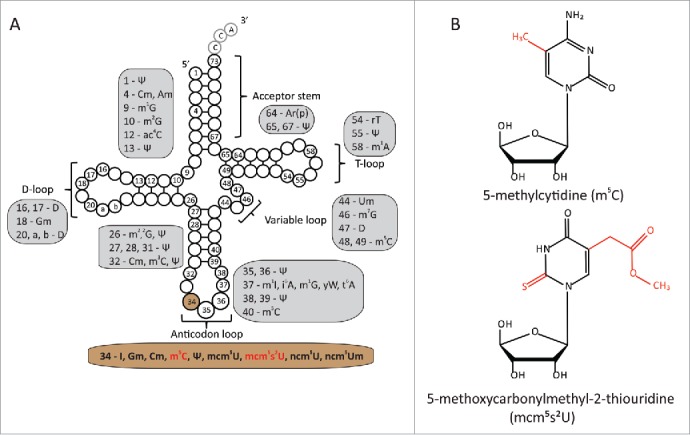
Modifications in cytoplasmic tRNAs in S. cerevisiae. A. Positions of various modifications in tRNA. Nucleotides are shown as circles, the residues 34, 35, and 36 in the anticodon are labeled in addition to several other positions for better orientation. Abbreviations used: Ψ - pseudouridine; Cm - 2′-O-methylcytidine; Am - 2′-O-methyladenosine; m1G - 1-methylguanosine; m2G - N2-methylguanosine; ac4C - N4-acetylcytidine; D - dihydrouridine; Gm – 2′-O-methylguanosine; m2,2G – N2,N2-dimethylguanosine; m3C – 3-methylcytidine; I – inosine; m5C – 5-methylcytidine; mcm5U – 5-methoxycarbonylmethyluridine; mcm5s2U - 5-methoxycarbonylmethyl-2-thiouridine; ncm5U - 5-carbonylmethyluridine; ncm5Um - 5-carbonylmethyl-2′-O-methyluridine; m1I – 1-methylinosine; i6A – N6-isopentenyladenosine; yW – wybutosine; t6A – N6-threonylcarbamoyladenosine; m7G – 7-methylguanosine; Um – 2′-O-methyluridine; m1A – 1-methyladenosine; rT – ribothymidine; Ar(p) – 2′-oribosyladenosine (phosphate). B. Chemical structures of m5C and mcm5s2U tRNA modifications. Unmodified nucleosides and nucleobases are depicted in black. Changes introduced by the modification are shown in red.
Table 1.
Modifications in eukaryotic tRNAs discussed in this review
| Modification and Position | Target tRNAs | Gene | Organism |
|---|---|---|---|
| m5C at C34 | Leu(CAA) | TRM4 | S. cerevisiae |
| mcm5U/mcm5s2U at U34 | Lys(UUU), Gln(UUG), Glu(UUC), Arg(UCU) | Trm9/Trm112 SIN3/ELP3 | S. cerevisiae S. pombe |
| s2U at U34 | Lys(UUU), Gln(UUG), Glu(UUC) | URM1 | S. cerevisiae |
| mcm5s2U at U34 | hLys(UUU), hGln(UUG), hGlu(UUC) | hABH8/hCTU2 | H. sapiens |
Modification pathways
In yeast, 11 out of 42 tRNAs are modified at U34 with 5-carboxymethyl derivatives (xcm5U) and one tRNA is modified with 5-methylcytidine (m5C) at C34. The synthesis of mcm5U and mcm5s2U is very complex and requires at least 15 gene products for mcm5 addition and 11 gene products for s2 group addition (Fig. 2). Synthesis of cm5U intermediate relies on elongator complex (Elp1p-Elp6p) together with killer toxin-insensitive gene products (Kti11p-Kti14p), suppressor of transcription initiation (Sit4p), and associated factors (Sap185p, Sap190p) in the presence of acetyl-CoA. Subsequent conversion of cm5U to mcm5U requires tRNA-methyltransferases Trm9p and Trm112p with S-adenosyl methionine (SAM) as the methyl donor.22,23 Finally, addition of s2U requires ubiquitin-related modifier 1 (Urm1p) along with its activating protein, Uba4p, and other associated gene products including cysteine desulfurase (Nfs1p), thiouridine modification protein (Tum1p), iron-sulfur cluster binding proteins (Cfd1p, Isu1p, Isu2p, Nbp35p, Cia1p), and s2U ligase (Ncs2p, Ncs6p). Cysteine is the main source of sulfur for s2U synthesis.24,25 By comparison, m5C modification is a simple reaction requiring a single enzyme tRNA-methyltransferase Trm4p with SAM as a methyl donor (Fig. 2).
Figure 2.
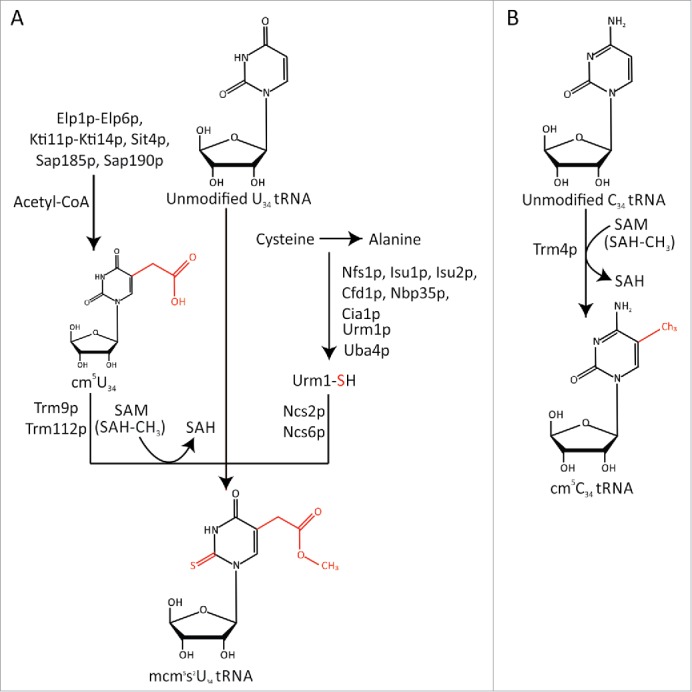
Modification pathway for mcm5s2U34- and m5C34-containing tRNAs. A. Proteins required for mcm5 modification (left) and s2 modification (right) of U34. Acetyl-CoA acts as a donor for cm5 modification, and SAM as a methyl donor to form mcm5U34. For s2 modification, cysteine acts as sulfur donor. Modified side groups are shown in red. B. Trm4p catalyzed cm5C34 modification where SAM acts as a methyl donor. Modified side group is shown in red.
Aminoacylation and interactions with the ribosome
In yeast and higher eukaryotes, U34 is ubiquitously modified to 5-methyl-2-thio (mcm5s2U34) in tRNALys(UUU), tRNAGln(UUG) and tRNAGlu(UUC) (Fig. 1, Table 1). These tRNAs read mRNA codons ending with A that belong to split codon boxes where U- and C-ending codons code for a different amino acid than A- and G-ending codons. In addition to codon reading, modifications of U34 can act as a determinant for tRNA aminoacylation. s2U34 modifications in tRNAGlu and tRNAGln improve the catalytic efficiency of the aminoacylation reaction by increasing the affinity of the tRNA for the synthetase in Escherichia coli.26-28 The crystal structure of GlnRS in complex with s2U34 tRNAGln(UUG) shows a binding pocket of the ARS that is selective for sulfur in the modification and likely repels oxygen at this position. This explains the higher binding affinity for fully modified tRNAs to GlnRS compared to the tRNA lacking the modification at position 34.
On the ribosome, modifications at tRNA position 34 are implicated in maintaining accurate decoding and translational processivity by introducing decoding capacities that exceed the wobble rules and by modulating decoding efficiency, the dwell time of the ribosome at each codon, and frequencies of misincorporation and frameshifting (Fig. 3).9,29-31 Mechanistic studies on how the modifications of eukaryotic tRNAs affect decoding are not available and the underlying principles are inferred from the work on bacterial tRNAs. In bacteria, tRNAAla with a cmo5U34 modification can read not only the codons ending with A,G, and U, as dictated by the wobble rules, but also a C-ending codon, albeit as reduced efficiency,9 indicating that modification can significantly broaden decoding capacity. s2U34 modification in tRNAGln(UUG) from E. coli affects the rates of GTP hydrolysis by EF-Tu and dipeptide formation.32 Either the binding of the ternary complex EF-Tu–GTP–Gln-tRNAGln to the ribosome or GTP hydrolysis itself (or both) are facilitated by the presence of the modification, but the selectivity of the cognate codon CAA vs. the near-cognate codon CAC prior to GTP hydrolysis is not changed. Because the ratio between the rates of GTP hydrolysis and peptide bond formation is similar for s2U34 tRNAGln(UUG) and unmodified tRNAGln(UUG), s2U34 modification may preferentially affect GTP hydrolysis and appears to have little effect on the subsequent steps in decoding.32 Together, these data suggest the effect of tRNA thiolation at the initial steps of decoding, e.g., codon recognition or GTPase activation. Mammalian tRNAGln also contains s2U34; given the fundamental conservation of the decoding mechanism,29 one may expect a similar effect of the modification on the initial steps of decoding in eukaryotes.
Figure 3.
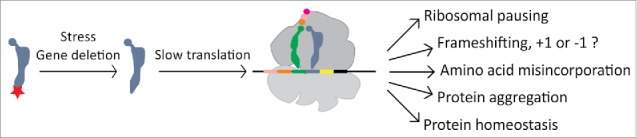
Cellular outcomes of tRNA hypomodification. Loss of tRNA modification upon stress or gene deletion leads to slow translation, which can cause recoding, synthesis of incorrect proteins and changes in the cellular proteome.
In S. cerevisiae mcm5s2U34 modifications of tRNALys(UUU), tRNAGln(UUG) and tRNAGlu(UUC) affect translation of a subset of mRNAs enriched for codons that are read by these tRNAs.33 In vitro studies comparing the decoding properties of tRNAs with and without s2 or mcm5 modifications at the wobble position show that each modification increases the affinity of the cognate tRNA binding to the A site of the ribosome. Also the rate of peptide bond formation at saturating concentrations of the ternary complex is slower in the absence of s2U34 or mcm5U34 modifications. Altered decoding properties appear to affect translation in vivo: When the Urm1p or Elp3p enzymes are deleted, the in vivo expression of translation reporters containing clusters of codons that are read by the modified tRNA is affected differently, suggesting that both s2U34 (modified by Urm1p) and mcm5s2U34 (that requires both enzymes) are required for proper decoding and protein synthesis.33 Another example of selective translation by modified tRNAs is provided by the Trm4-modified tRNALeu(CAA) containing m5C34. When the modification efficiency is increased upon H2O2-induced oxidative stress, mRNAs enriched in the UUG codon are selectively translated, which emphasizes the importance of the modification in efficient decoding of cognate codons.34 Similarly, trm4Δ cells, which lack the m5C34 modification, are hypersensitive to oxidative stress due to inability to efficiently translate UUG-containing stress-response mRNAs.34
On a global level, the loss of s2U34 in urm1Δ or mcm5U34 in elp3Δ cells impairs protein translation for genes enriched for AAA, CAA and GAA codons, and perturbs cellular signaling. Ribosome profiling (Ribo-seq) in yeast cells lacking functional Urm1p or Elp3p, which detects ribosome distribution on mRNAs at single codon resolution,35 reveals mild ribosome pausing at these codons when they are in the A site of ribosome.36 This does not, however, appear to lead to ribosome queuing, even when a cluster of such codons is encountered. Decoding of the AAG, CAG and GAG codons (which are normally read by a different tRNA) is not affected, although they belong to the same codon box as AAA, CAA and GAA codons and could thus be read as almost-cognate9 by the respective hypomodified tRNAs.36,37 The results of ribosome profiling analysis are consistent with the data that show modification-dependent effects on decoding of a single AAA codon in vitro and in vivo.33 The ribosome pausing due to the lack of modification is transient and does not elicit rescue responses that are activated in response to irreversible stalling, such as that induced by premature stop codons or on a truncated (non-stop) mRNA. Consistent with this notion, ribosome occupancy remained unaffected in the absence of Dom34-Hbs1,38 the proteins that rescue ribosomes after stalling.36
In addition to the effects on cognate decoding, the lack of tRNA modifications can also lead to recoding or increased error frequency in translation. In search of a cognate aa-tRNA, the ribosome screens different candidates in the total aminoacyl-tRNA pool, and the error frequency is dictated by the competition between cognate, near-cognate, and non-cognate aa-tRNAs. Changes in the decoding properties of unmodified tRNA not only affect reading of cognate codons, but may affect competition between cognate and near-cognate tRNAs resulting in changes of error frequency of translation. In trm9Δ cells that lack mcm5 modification of tRNAArg(UCU) and mcm5s2 modification of tRNAGlu(UUC), translation becomes error-prone,39 likely due to the hypomodified tRNA reading near-cognate AGU/C triplets coding for Ser in addition to cognate AGA/G triplets coding for Arg. Furthermore, weak interactions of unmodified tRNA with the ribosome may facilitate ribosomal frameshifting. In fact, a recent study shows increased +1 frameshifting upon mcm5s2U34 hypomodification, supporting the role of the modification in reading frame maintenance.40 Inserting a stretch of GAG triplets coding for Glu in a dual-luciferase reporter system results in increased −1 frameshifting in trm9Δ compared to wild-type cells.39 Thus, the modifications attenuate the efficiency and fidelity of translation, regulate the processivity of translation and can affect adaptation to stress (Fig. 3).
Link between tRNA modifications and stress response
Cells have to respond to permanently changing external conditions, and they adapt to these stimuli by an array of different mechanisms leading to changes in gene expression and the composition of the proteome. Examples of well-studied stress-induced pathways include heat shock and unfolded protein response, adaptation to nutrient starvation, reactive oxygen species (ROS) and DNA damage responses.41,42 The modifications at position 34 of tRNA appear to play a key role in a number of these responses, and in this section we will summarize the observations that the modifications affect the ability of cells to adapt to stress.
A comprehensive analysis of how modifications at position 34 respond to elevated temperature in yeast demonstrates a reversible decrease of s2U34 modification for tRNALys(UUU), tRNAGln(UUG) and tRNAGlu(UUC).43-45 Upon return to normal conditions, the modification level is restored. Under stress, the newly transcribed tRNA is not modified and the mature tRNA already available becomes unmodified, but seems not to be degraded to a significant extent.43-45 Also the level of s2U34 synthesis is regulated by nutrient availability. Because the URM1 modification pathway utilizes sulfur from sulfur-containing amino acids, intracellular methionine and cysteine availability during cell growth regulates the thiolation pattern of tRNA.46 Mass spectrometry and Northern blot analysis show a decrease in s2U34 levels when yeast cells are grown at permissive temperatures without methionine as a source of sulfur. Interestingly, the s2U34 modification pattern remained unaffected at elevated temperatures with varying methionine supply, indicating that attenuation of tRNA modification is independent of nutrient availability under heat challenge.44
Cells lacking genes for tRNA modifications show diverse phenotypes in response to various types of stress such as the presence of drugs (diamide, paromomycin, rapamycin), DNA damaging agents methylmethanosulfonate (MMS) or hydroxyurea (HU), or oxidative stress by hydrogen peroxide (H2O2). Yeast cells lacking Trm4p, an enzyme responsible for the m5C34 modification of tRNALeu(CAA), are sensitive to MMS and H2O2. Notably, expression of Trm4p confers resistance to H2O2 exposure, which rules out another mutation other than trm4 deletion as the cause of the phenotype. Moreover, quantitative mass spectrometry analysis showed that tRNA modifications were altered upon oxidative stress with H2O2.34,47,48 tRNAs without mcm5U34 are sensitive to a large variety of drugs, such as rapamycin (antibiotic targeting a key serine/threonine protein kinase mTOR), paromomycin (aminoglycoside antibiotic), diamide (thiol oxidizing agent) or cycloheximide (inhibitor of protein synthesis).25,30,37,39,45,49-53 Generally, gene deletions lead to pleiotropic negative effects on cell growth and survival, possibly because the lack of tRNA modification itself leads to a proteotoxic stress (see below). However, in some cases the lack of tRNA thiolation may become advantageous, e.g., by conferring resistance to endoplasmic reticulum stress.45 Taken together, these data suggest that alterations in the tRNA modification pattern at the wobble position 34 mediate the response to a variety of stresses. The mechanism of how the tRNA modifications modulate stress response is not clear; in the following we will summarize potential pathways by which they may be connected.
Effect on the proteome composition
Cell homeostasis and the ability to adapt to stress are ensured by the coordinated processes of transcription, translation and protein folding, and tRNA modifications appear to play an important role in maintaining this coordination. The loss of s2U34 tRNA modification in urm1Δ cells affects protein expression of a subset of genes, while the global levels of transcription and translation are not affected.33 Quantitative mass spectrometry with gene ontology (GO) analysis has revealed that several classes of proteins are significantly down-regulated in cells deficient in tRNA modification, such as ribosomal proteins, proteins involved in rRNA synthesis and processing, ribosome biogenesis, tRNA synthesis and modification, electron transport chain and oxidative phosphorylation, and translation regulation.33,34,36,44 Bioinformatic analysis revealed that s2U34 modification is required for the efficient expression of mRNAs rich in the AAA, CAA, and GAA codons that are read by the thiolated tRNA, with slow AAA decoding being the major cause of differential translation.33
While slow translation of codons that depend on the specific modified tRNA provides a simple explanation for the reduced expression of some genes, other effects are more difficult to understand. Expression of some protein is upregulated in urm1Δ cells, e.g., heat shock proteins (HSPs), which are a hallmark of proteotoxic stress, and the ubiquitin-proteasome system (UPS), suggesting activation of chaperone-assisted protein folding and degradation of misfolded proteins when tRNA modifications are compromised.36,44 The loss of tRNA modification due to heat stress results in upregulation of Hsp104p (a disagregase), and Hsp26p, Hsp42p and Hsp12p (that increase solubility of denatured proteins), suggesting a mechanism where Hsp104p and Hsp42p contribute to Q-body formation with soluble, misfolded proteins en route to degradation.44,54,55 The effect is specific to tRNA modification, because elevated temperature (37°C) alone does not cause protein aggregation.56 These results suggest that the lack of tRNA modification has a profound effect on the proteome, beyond the decreased stoichiometry of some proteins (Fig. 3).
Another example suggesting that loss of tRNA modification can lead to protein misfolding and aggregation was reported for ncs2Δelp6Δ cells.31 Notably, 70% of these aggregates coincide with those found in cells that lack ribosome-associated chaperones Ssb1p and Ssb2p.31 These chaperones assist in co-translational folding of nascent polypeptides substantially enriched for β-sheets,57 preventing aggregation which frequently results from incorrect folding. The aggregates formed in ssbΔ cells are ubiquitinated and directed for degradation.57 This suggests that abolishing any of the NCS2 or ELP6 pathways results in the formation of metastable proteins that might influence protein homeostasis and thereby reduce cellular fitness. Moreover, upon drug stress in ncs2Δelp6Δ, Hsp104 foci were upregulated implying formation of soluble misfolded proteins. These foci are eliminated in WT cells under stress indicating proteasome-assisted degradation. Paradoxically, in tRNA modification-deficient cells, the UPS is upregulated, while degradation of foci remained unaffected upon stress.36 Taking these data together, it is tempting to speculate that alterations in the translation processivity due to the lack of tRNA modifications results in changes in protein homeostasis and may lead to protein aggregation (Fig. 3). It is not clear whether increased misfolding in cells lacking tRNA modifications is caused by increased error frequency or changes in translation processivity. Protein folding can begin co-translationally and may be influenced by the rate of translation and transient translational pauses.58 tRNA modifications may be part of the machinery that ensures orchestration of periods of rapid synthesis vs. transient pauses; hence, protein aggregation may be due to altered local translation rates as a result of slower or faster decoding by hypomodified tRNAs or an increase in error frequency.
Altered expression levels of some proteins as a result of tRNA hypomodification may lead to severe changes in cell physiology. For example, altered translation levels of a subset of mRNAs affect cell cycle progression in trm9Δ cells.59 One mRNA target is the ribonucleotide reductase (Rnr) which catalyzes deoxynucleotides (dNTP) biosynthesis for DNA replication during cell cycle transition.60-62 The loss of Trm9p or exposure to MMS reduces Rnr1p levels in cells synchronized in different cell cycle phases. In S-phase cells, mcm5U34 modifications increase significantly upon DNA damage, suggesting efficient DNA synthesis by promoting RNR1 translation. In addition, when cells are treated with MMS or HU or exposed to ionizing radiations, overexpression of TRM9 in trm9Δ cells restores progression of cells into S-phase. Furthermore, Rnr1p overexpression or expression of codon-optimized Rnr1p (where codons regulated by Trm9-catalyzed tRNA modifications are changed to synonymous codons) in trm9Δ cells boosts G1-S phase transition upon DNA damage. These data demonstrate how tRNA modification can regulate cell fate.
Overexpression of unmodified tRNAs rescues hypomodification phenotypes
The growth phenotype of cells lacking tRNA modifying genes are rescued by over-expression of the respective unmodified tRNAs. For example, urm1Δ cells lacking s2U34 tRNA modification or elpΔ cells lacking mcm5U34 modification show striking growth defects at various stress conditions that are rescued by providing excess of the respective unmodified tRNAs, overexpressed either individually or in combination. Overexpression of tRNALys or tRNAGln (individually) alleviates viability defects to a different extent suggesting at least 2 different rescue mechanisms.25,34,45,63,64 When unmodified tRNALys(UUU), tRNAGln(UUG) and tRNAGlu(UUC) are overexpressed in WT cells, the proteome composition is restored, although tRNA thiolation is not increased.33,44 Overexpression of unmodified tRNAs under stress conditions not only rescues the protein aggregation phenotype, but also alleviates ribosome pausing, suggesting that cells resume normal translation and escape proteotoxic stress.33,36,44 Although the exact mechanism of rescue is not known, it is likely that hypomodified tRNAs are recognized by the ribosome, with high concentrations of the unmodified tRNAs compensating for their lower affinities. Furthermore, the rescue effect can be indirect: Given that (i) the concentrations of translation initiation factors (eIF1, eIF1a, eIF2α,β,γ, eIF5 and eIF5B) are limiting for translation, (ii) these proteins are prone to aggregation under conditions of tRNA hypomodification, and (iii) their levels can be restored by tRNA overexpression, it is conceivable that tRNA overexpression can lead to upregulation of translation initiation, allowing for a more efficient overall translation.
Human tRNALys3(UUU) and HIV-1 reverse transcription
In humans, among the 3 Lys tRNAs isoacceptors, htRNALys3(UUU) is responsible for decoding of AAA and AAG codons. This tRNA also has a non-canonical function acting as a primer for human HIV-1 reverse transcription.65-69 htRNALys3(UUU) is extensively modified at the anticodon loop compared to htRNALys1 and htRNALys2. Fully-modified htRNALys3(UUU) contains mcm5s2U34 at position 34, 2-methylthio-N6-threonylcarbonyladenosine (ms2t6A37) at position 37, and a pseudouridine at position 39 (Ψ39). These modifications enhance the binding of the synthetic anticodon stem and loop (ASL) domain of htRNALys3(UUU) to the cognate (AAA) and near-cognate (AAG) codons. Spectroscopic and structural studies suggested that the fully modified ASL had a more ordered structure compared to the unmodified ASL, suggesting that the presence of modifications enhances the codon-anticodon interaction by affecting the structure of the modified tRNAs.70
During HIV-1 replication, the nucleocapsid protein NCp7 recognizes and facilitates recruitment of the host cell htRNALys3(UUU).71-73 htRNALys3(UUU) is destabilized upon interaction with NCp7, which promotes annealing to HIV RNA, and in the subsequent infection cycle htRNALys3(UUU) acts as the primer for reverse transcriptase. Modifications of htRNALys3(UUU) increase its binding affinity to NCp7, while the fully-modified ASL specifically recognizes NCp7 peptide mimics from a peptide phage display library.74,75 A 15-amino acid signature peptide derived from NCp7 (R-W-Q/N-H-X2-F-Pho-X-G/A-W-R-X2-G, where X = any amino acids and Pho = hydrophobic) specifically recognizes modified htRNALys3(UUU) with a binding affinity 10-fold higher than that of a random sequence.76 Thus, modifications of htRNALys3(UUU) stabilize the native structure of the anticodon loop and provide a recognition element that is utilized both in normal translation and non-canonical tRNA functions.
Perspectives
In this review, we summarized the emerging view of tRNA modifications as regulators of translation and protein homeostasis at normal growth conditions and upon stress. While i6A37, t6A37, yW37, m1G37 and I34 are the most studied tRNA modifications,3,10 it will be interesting to study the interplay between the modifications at positions 37 and 34. One interesting aspect is the suggested role of modifications in protein folding and proteotoxic stress, including removing protein aggregates and inducing the unfolded protein response; however, strong data to support this idea are still lacking. From this perspective, it would be worthwhile to study the role of tRNA modification in co-translational folding of slowly growing polypeptide chains.
In light of the data showing −1 and +1 frameshifting and increased ribosome occupancy in the absence of tRNA modifications at position 34, it appears worthwhile to explore the involvement of these modifications in reading frame maintenance. Many viruses use programmed frameshifting to ensure that virus-encoded proteins are synthesized in the correct ratios for particle assembly and maturation. Thus, understanding the mechanism of ribosomal frameshifting induced by tRNA modifications may be of great importance to fully characterize the translational potential of DNA sequences, translational regulation, and might be of particular interest for antiviral therapeutics. Finally, the non-canonical function of htRNALys3(UUU) as a primer for HIV-1 reverse transcription bears the potential to be used as inhibitors of RNA-protein interactions related to diseases. Translation is a major hub in cell adaptation to changing environmental conditions and tRNA modification add an important layer of regulation to this fundamental cellular process.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful for the helpful discussion and invaluable comments from members of M.V.R. laboratory. We thank Evan Mercier for critical reading of the manuscript.
Funding
Research in M.V.R. laboratory is supported by the Max Planck Society and the Deutsche Forschungsgemeinschaft.
References
- [1].Alexandrov A, Chernyakov I, Gu W, Hiley SL, Hughes TR, Grayhack EJ, Phizicky EM. Rapid tRNA decay can result from lack of nonessential modifications. Mol Cell 2006; 21:87-96; PMID:16387656; http://dx.doi.org/ 10.1016/j.molcel.2005.10.036 [DOI] [PubMed] [Google Scholar]
- [2].Motorin Y, Helm M. tRNA stabilization by modified nucleotides. Biochemistry 2010; 49:4934-44; PMID:20459084; http://dx.doi.org/ 10.1021/bi100408z [DOI] [PubMed] [Google Scholar]
- [3].Phizicky EM, Alfonzo JD. Do all modifications benefit all tRNAs? FEBS Lett 2010; 584:265-71; PMID:19931536; http://dx.doi.org/ 10.1016/j.febslet.2009.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Motorin Y, Grosjean H. Multisite-specific tRNA:m5C-methyltransferase (Trm4) in yeast Saccharomyces cerevisiae: identification of the gene and substrate specificity of the enzyme. RNA 1999; 5:1105-18; PMID:10445884; http://dx.doi.org/ 10.1017/S1355838299982201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Edelheit S, Schwartz S, Mumbach MR, Wurtzel O, Sorek R. Transcriptome-wide mapping of 5-methylcytidine RNA modifications in bacteria, archaea, and yeast reveals m5C within archaeal mRNAs. PLoS Genet 2013; 9:e1003602; PMID:23825970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].King MY, Redman KL. RNA methyltransferases utilize two cysteine residues in the formation of 5-methylcytosine. Biochemistry 2002; 41:11218-25; PMID:12220187; http://dx.doi.org/ 10.1021/bi026055q [DOI] [PubMed] [Google Scholar]
- [7].Bjork GR, Huang B, Persson OP, Bystrom AS. A conserved modified wobble nucleoside (mcm5s2U) in lysyl-tRNA is required for viability in yeast. RNA 2007; 13:1245-55; PMID:17592039; http://dx.doi.org/ 10.1261/rna.558707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Songe-Moller L, van den Born E, Leihne V, Vågbø CB, Kristoffersen T, Krokan HE, Kirpekar F, Falnes PØ, Klungland A. Mammalian ALKBH8 possesses tRNA methyltransferase activity required for the biogenesis of multiple wobble uridine modifications implicated in translational decoding. Mol Cell Biol 2010; 30:1814-27; PMID:20123966; http://dx.doi.org/ 10.1128/MCB.01602-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kothe U, Rodnina MV. Codon reading by tRNAAla with modified uridine in the wobble position. Mol Cell (2007) 25:167-74; PMID:17218280; http://dx.doi.org/ 10.1016/j.molcel.2006.11.014 [DOI] [PubMed] [Google Scholar]
- [10].Gustilo EM, Vendeix FA, Agris PF. tRNA's modifications bring order to gene expression. Curr Opin Microbiol 2008; 11:134-40; PMID:18378185; http://dx.doi.org/ 10.1016/j.mib.2008.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Murphy FVT, Ramakrishnan V, Malkiewicz A, Agris PF. The role of modifications in codon discrimination by tRNA(Lys)UUU. Nat Struct Mol Biol 2004; 11:1186-91; PMID:15558052; http://dx.doi.org/ 10.1038/nsmb861 [DOI] [PubMed] [Google Scholar]
- [12].Murphy FVT, Ramakrishnan V. Structure of a purine-purine wobble base pair in the decoding center of the ribosome. Nat Struct Mol Biol 2004; 11:1251-2; PMID:15558050; http://dx.doi.org/ 10.1038/nsmb866 [DOI] [PubMed] [Google Scholar]
- [13].Urbonavicius J, Qiang Q, Durand JM, Hagervall TG, Björk GR. Improvement of reading frame maintenance is a common function for several tRNA modifications. EMBO J 2001; 20:4863-73; PMID:11532950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Phizicky EM, Hopper AK. tRNA biology charges to the front. Genes Dev 2010; 24:1832-60; PMID:20810645; http://dx.doi.org/ 10.1101/gad.1956510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Agris PF, Vendeix FA, Graham WD. tRNA's wobble decoding of the genome: 40 years of modification. J Mol Biol 2007; 366:1-13; PMID:17187822; http://dx.doi.org/ 10.1016/j.jmb.2006.11.046 [DOI] [PubMed] [Google Scholar]
- [16].Johansson MJ, Esberg A, Huang B., Bjork GR, Bystrom AS. Eukaryotic wobble uridine modifications promote a functionally redundant decoding system. Mol Cell Biol 2008; 28:3301-12; PMID:18332122; http://dx.doi.org/ 10.1128/MCB.01542-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Takai K, Yokoyama S. Roles of 5-substituents of tRNA wobble uridines in the recognition of purine-ending codons. Nucl Acids Res 2003; 31:6383-91; PMID:14602896; http://dx.doi.org/ 10.1093/nar/gkg839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Barat C, Lullien V, Schatz O, Keith G, Nugeyre MT, Grüninger-Leitch F, Barré-Sinoussi F, LeGrice SF, Darlix JL. HIV-1 reverse transcriptase specifically interacts with the anticodon domain of its cognate primer tRNA. EMBO J 1989; 8:3279-85; PMID:2479543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kleiman L. tRNA(Lys3): the primer tRNA for reverse transcription in HIV-1. IUBMB Life 2002; 53:107-14; PMID:12049192; http://dx.doi.org/ 10.1080/15216540211469 [DOI] [PubMed] [Google Scholar]
- [20].Shepherd J, Ibba M. Bacterial transfer RNAs. FEMS Microbiol Rev 2015; 39:280-300; PMID:25796611; http://dx.doi.org/ 10.1093/femsre/fuv004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bjork GR, Hagervall TG. Transfer RNA modification. Annu Rev Biochem 1987; 56:263-87; PMID:3304135; http://dx.doi.org/ 10.1146/annurev.bi.56.070187.001403 [DOI] [PubMed] [Google Scholar]
- [22].Karlsborn T, Tükenmez H, Mahmud AK, Xu F, Xu H, Byström AS. Elongator, a conserved complex required for wobble uridine modifications in eukaryotes. RNA Biol 2014; 11:1519-28; PMID:25607684; http://dx.doi.org/ 10.4161/15476286.2014.992276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Letoquart J, van Tran N, Caroline V, Aleksandrov A, Lazar N, van Tilbeurgh H, Liger D, Graille M. Insights into molecular plasticity in protein complexes from Trm9-Trm112 tRNA modifying enzyme crystal structure. Nucl Acids Res 2015; 43:10989-1002; PMID:26438534; http://dx.doi.org/ 10.1093/nar/gkv1009; E publ October 4, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pedrioli PG, Leidel S, Hofmann K. Urm1 at the crossroad of modifications. ‘Protein Modifications: Beyond the Usual Suspects’ Review Series. EMBO Rep 2008; 9:1196-202; PMID:19047990; http://dx.doi.org/ 10.1038/embor.2008.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Leidel S, Liu M, Qiu R, Ji C. Ubiquitin-related modifier Urm1 acts as a sulphur carrier in thiolation of eukaryotic transfer RNA. Nature 2009; 458:228-32; PMID:19145231; http://dx.doi.org/ 10.1038/nature07643 [DOI] [PubMed] [Google Scholar]
- [26].Rogers MJ, Spears JL, Gaston KW, Limbach PA, Gamper H, Hou YM, Kaiser R, Agris PF, Perona JJ. The recognition of E. coli glutamine tRNA by glutaminyl-tRNA synthetase. Nucleic Acids Symp Ser 1993; 211-3; PMID:7504247 [PubMed] [Google Scholar]
- [27].Sylvers LA, Rogers KC, Shimizu M, Ohtsuka E, Soll D. A 2-thiouridine derivative in tRNAGlu is a positive determinant for aminoacylation by Escherichia coli glutamyl-tRNA synthetase. Biochemistry 1993; 32:3836-41; PMID:8385989; http://dx.doi.org/ 10.1021/bi00066a002 [DOI] [PubMed] [Google Scholar]
- [28].Weygand-Durasevic I, Rogers MJ, Soll D. Connecting anticodon recognition with the active site of Escherichia coli glutaminyl-tRNA synthetase. J Mol Biol 1994; 240:111-8; PMID:8027995; http://dx.doi.org/ 10.1006/jmbi.1994.1425 [DOI] [PubMed] [Google Scholar]
- [29].Plant EP, Nguyen P, Russ JR, Pittman YR, Nguyen T, Quesinberry JT, Kinzy TG, Dinman JD. Differentiating between near- and non-cognate codons in Saccharomyces cerevisiae. PLoS One 2007; 2:e517; PMID:17565370; http://dx.doi.org/ 10.1371/journal.pone.0000517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Noma A, Sakaguchi Y, Suzuki T. Mechanistic characterization of the sulfur-relay system for eukaryotic 2-thiouridine biogenesis at tRNA wobble positions. Nucleic Acids Res 2009; 37:1335-52; PMID:19151091; http://dx.doi.org/ 10.1093/nar/gkn1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rodnina MV, Gromadski KB, Kothe U, Wieden HJ. Recognition and selection of tRNA in translation. FEBS Lett 2005; 579:938-42; PMID:15680978; http://dx.doi.org/ 10.1016/j.febslet.2004.11.048 [DOI] [PubMed] [Google Scholar]
- [32].Rodriguez-Hernandez A, Spears JL, Gaston KW, Limbach PA, Gamper H, Hou YM, Kaiser R, Agris PF, Perona JJ. Structural and mechanistic basis for enhanced translational efficiency by 2-thiouridine at the tRNA anticodon wobble position. J Mol Biol 2013; 425:3888-906; PMID:23727144; http://dx.doi.org/ 10.1016/j.jmb.2013.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rezgui VA, Tyagi K, Ranjan N, Konevega AL, Mittelstaet J, Rodnina MV, Peter M, Pedrioli PG. tRNA tKUUU, tQUUG, and tEUUC wobble position modifications fine-tune protein translation by promoting ribosome A-site binding. Proc Natl Acad Sci U S A 2013; 110:12289-94; PMID:23836657; http://dx.doi.org/ 10.1073/pnas.1300781110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chan CT, Pang YL, Deng W, Babu IR, Dyavaiah M, Begley TJ, Dedon PC. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat Commun 2012; 3:937; PMID:22760636; http://dx.doi.org/ 10.1038/ncomms1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 2009; 324:218-23; PMID:19213877; http://dx.doi.org/ 10.1126/science.1168978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nedialkova DD, Leidel SA. Optimization of codon translation rates via tRNA modifications maintains proteome integrity. Cell 2015; 161:1606-18; PMID:26052047; http://dx.doi.org/ 10.1016/j.cell.2015.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zinshteyn B, Gilbert WV. Loss of a conserved tRNA anticodon modification perturbs cellular signaling. PLoS Genet 2013; 9:e1003675; PMID:23935536; http://dx.doi.org/ 10.1371/journal.pgen.1003675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Shoemaker CJ, Green R. Translation drives mRNA quality control. Nat Struct Mol Biol 2012; 19:594-601; PMID:22664987; http://dx.doi.org/ 10.1038/nsmb.2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Patil A, Chan CT, Dyavaiah M, Rooney JP, Dedon PC, Begley TJ. Translational infidelity-induced protein stress results from a deficiency in Trm9-catalyzed tRNA modifications. RNA Biol 2012; 9:990-1001; PMID:22832247; http://dx.doi.org/ 10.4161/rna.20531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tukenmez H, Xu H, Esberg A, Bystrom AS. The role of wobble uridine modifications in +1 translational frameshifting in eukaryotes. Nucl Acids Res 2015; 43:9489-99; PMID:26283182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dedon PC, Begley TJ. A system of RNA modifications and biased codon use controls cellular stress response at the level of translation. Chem Res Toxicol 2014; 27:330-7; PMID:24422464; http://dx.doi.org/ 10.1021/tx400438d [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell 2000; 11:4241-57; PMID:11102521; http://dx.doi.org/ 10.1091/mbc.11.12.4241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Alings F, Sarin LP, Fufezan C, Drexler HC, Leidel SA. An evolutionary approach uncovers a diverse response of tRNA 2-thiolation to elevated temperatures in yeast. RNA 2015; 21:202-12; PMID:25505025; http://dx.doi.org/ 10.1261/rna.048199.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tyagi K, Pedrioli PG. Protein degradation and dynamic tRNA thiolation fine-tune translation at elevated temperatures. Nucl Acids Res 2015; 43:4701-12; PMID:25870413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Damon JR, Pincus D, Ploegh HL. tRNA thiolation links translation to stress responses in Saccharomyces cerevisiae. Mol Biol Cell 2015; 26:270-82; PMID:25392298; http://dx.doi.org/ 10.1091/mbc.E14-06-1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Laxman S, Sutter BM, Wu X, Kumar S, Guo X, Trudgian DC, Mirzaei H, Tu BP. Sulfur amino acids regulate translational capacity and metabolic homeostasis through modulation of tRNA thiolation. Cell 2013; 154:416-29; PMID:23870129; http://dx.doi.org/ 10.1016/j.cell.2013.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chan CT, Dyavaiah M, DeMott MS, Taghizadeh K, Dedon PC, Begley TJ. A quantitative systems approach reveals dynamic control of tRNA modifications during cellular stress. PLoS Genet 2010; 6:e1001247; PMID:21187895; http://dx.doi.org/ 10.1371/journal.pgen.1001247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Begley TJ, Rosenbach AS, Ideker T, Samson LD. Hot spots for modulating toxicity identified by genomic phenotyping and localization mapping. Mol Cell 2004; 16:117-25; PMID:15469827; http://dx.doi.org/ 10.1016/j.molcel.2004.09.005 [DOI] [PubMed] [Google Scholar]
- [49].Klassen R, Grunewald P, Thüring KL, Eichler C, Helm M, Schaffrath R. Loss of anticodon wobble uridine modifications affects tRNA(Lys) function and protein levels in Saccharomyces cerevisiae. PLoS One 2015; 10:e0119261; PMID:25747122; http://dx.doi.org/ 10.1371/journal.pone.0119261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Huang B, Johansson MJ, Bystrom AS. An early step in wobble uridine tRNA modification requires the Elongator complex. RNA 2005; 11:424-36; PMID:15769872; http://dx.doi.org/ 10.1261/rna.7247705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Schlieker CD, Van der Veen AG, Damon JR, Spooner E, Ploegh HL. A functional proteomics approach links the ubiquitin-related modifier Urm1 to a tRNA modification pathway. Proc Natl Acad Sci U S A 2008; 105:18255-60; PMID:19017811; http://dx.doi.org/ 10.1073/pnas.0808756105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Huang B, Lu J, Bystrom AS. A genome-wide screen identifies genes required for formation of the wobble nucleoside 5-methoxycarbonylmethyl-2-thiouridine in Saccharomyces cerevisiae. RNA 2008; 14:2183-94; PMID:18755837; http://dx.doi.org/ 10.1261/rna.1184108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Nakai Y, Nakai M, Hayashi H. Thio-modification of yeast cytosolic tRNA requires a ubiquitin-related system that resembles bacterial sulfur transfer systems. J Biol Chem 2008; 283:27469-76; PMID:18664566; http://dx.doi.org/ 10.1074/jbc.M804043200 [DOI] [PubMed] [Google Scholar]
- [54].Sontag EM, Vonk WI, Frydman J. Sorting out the trash: the spatial nature of eukaryotic protein quality control. Curr Opin Cell Biol 2014; 26:139-46; PMID:24463332; http://dx.doi.org/ 10.1016/j.ceb.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Escusa-Toret S, Vonk WI, Frydman J. Spatial sequestration of misfolded proteins by a dynamic chaperone pathway enhances cellular fitness during stress. Nat Cell Biol 2013; 15:1231-43; PMID:24036477; http://dx.doi.org/ 10.1038/ncb2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Nathan DF, Vos MH, Lindquist S. In vivo functions of the Saccharomyces cerevisiae Hsp90 chaperone. Proc Natl Acad Sci U S A 1997; 94:12949-56; PMID:9371781; http://dx.doi.org/ 10.1073/pnas.94.24.12949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Willmund F, del Alamo M, Pechmann S, Chen T, Albanèse V, Dammer EB, Peng J, Frydman J. The cotranslational function of ribosome-associated Hsp70 in eukaryotic protein homeostasis. Cell 2013; 152:196-209; PMID:23332755; http://dx.doi.org/ 10.1016/j.cell.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Pechmann S, Willmund F, Frydman J. The ribosome as a hub for protein quality control. Mol Cell 2013; 49:411-21; PMID:23395271; http://dx.doi.org/ 10.1016/j.molcel.2013.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Patil A, Dyavaiah M, Joseph F, Rooney JP, Chan CT, Dedon PC, Begley TJ. Increased tRNA modification and gene-specific codon usage regulate cell cycle progression during the DNA damage response. Cell Cycle 2012; 11:3656-65; PMID:22935709; http://dx.doi.org/ 10.4161/cc.21919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chabes A, Stillman B. Constitutively high dNTP concentration inhibits cell cycle progression and the DNA damage checkpoint in yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 2007; 104:1183-8; PMID:17227840; http://dx.doi.org/ 10.1073/pnas.0610585104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yao R, Zhang Z, An X, Bucci B, Perlstein DL, Stubbe J, Huang M. Subcellular localization of yeast ribonucleotide reductase regulated by the DNA replication and damage checkpoint pathways. Proc Natl Acad Sci U S A 2003; 100:6628-33; PMID:12732713; http://dx.doi.org/ 10.1073/pnas.1131932100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Begley U, Dyavaiah M, Patil A, Rooney JP, DiRenzo D, Young CM, Conklin DS, Zitomer RS, Begley TJ. Trm9-catalyzed tRNA modifications link translation to the DNA damage response. Mol Cell 2007; 28:860-70; PMID:18082610; http://dx.doi.org/ 10.1016/j.molcel.2007.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Esberg A, Huang B, Johansson MJ, Bystrom AS. Elevated levels of two tRNA species bypass the requirement for elongator complex in transcription and exocytosis. Mol Cell 2006; 24:139-48; PMID:17018299; http://dx.doi.org/ 10.1016/j.molcel.2006.07.031 [DOI] [PubMed] [Google Scholar]
- [64].Fernandez-Vazquez J, Vargas-Pérez I, Sansó M, Buhne K, Carmona M, Paulo E, Hermand D, Rodríguez-Gabriel M, Ayté J, Leidel S, et al.. Modification of tRNA(Lys) UUU by elongator is essential for efficient translation of stress mRNAs. PLoS Genet 2013; 9:e1003647; PMID:23874237; http://dx.doi.org/ 10.1371/journal.pgen.1003647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Isel C, Marquet R, Keith G, Ehresmann C, Ehresmann B. Modified nucleotides of tRNA(3Lys) modulate primer/template loop-loop interaction in the initiation complex of HIV-1 reverse transcription. J Biol Chem 1993; 268:25269-72; PMID:7503978 [PubMed] [Google Scholar]
- [66].Isel C, Lanchy JM, Le Grice SF, Ehresmann C, Ehresmann B, Marquet R. Specific initiation and switch to elongation of human immunodeficiency virus type 1 reverse transcription require the post-transcriptional modifications of primer tRNA3Lys. EMBO J 1996; 15:917-24; PMID:8631312 [PMC free article] [PubMed] [Google Scholar]
- [67].Raba M, Limburg K, Burghagen M, Katze JR, Simsek M, Heckman JE, Rajbhandary UL, Gross HJ. Nucleotide sequence of three isoaccepting lysine tRNAs from rabbit liver and SV40-transformed mouse fibroblasts. Eur J Biochem 1979; 97:305-18; PMID:225173; http://dx.doi.org/ 10.1111/j.1432-1033.1979.tb13115.x [DOI] [PubMed] [Google Scholar]
- [68].Pavon-Eternod M, Wei M, Pan T, Kleiman L. Profiling non-lysyl tRNAs in HIV-1. RNA 2010; 16:267-73; PMID:20007329; http://dx.doi.org/ 10.1261/rna.1928110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jiang M, Mak J, Ladha A, Cohen E, Klein M, Rovinski B, Kleiman L. Identification of tRNAs incorporated into wild-type and mutant human immunodeficiency virus type 1. J Virol 1993; 67:3246-53; PMID:8497049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Vendeix FA, Murphy FV 4th, Cantara WA, Leszczyńska G, Gustilo EM, Sproat B, Malkiewicz A, Agris PF. Human tRNA(Lys3)(UUU) is pre-structured by natural modifications for cognate and wobble codon binding through keto-enol tautomerism. J Mol Biol 2012; 416:467-85; PMID:22227389; http://dx.doi.org/ 10.1016/j.jmb.2011.12.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Levin JG, Mitra M, Mascarenhas A, Musier-Forsyth K. Role of HIV-1 nucleocapsid protein in HIV-1 reverse transcription. RNA Biology 2010; 7:754-74; PMID:21160280; http://dx.doi.org/ 10.4161/rna.7.6.14115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Fassati A. Multiple roles of the capsid protein in the early steps of HIV-1 infection. Virus Res 2012; 170:15-24; PMID:23041358; http://dx.doi.org/ 10.1016/j.virusres.2012.09.012 [DOI] [PubMed] [Google Scholar]
- [73].Chan B, Weidemaier K, Yip WT, Barbara PF, Musier-Forsyth K. Intra-tRNA distance measurements for nucleocapsid proteindependent tRNA unwinding during priming of HIV reverse transcription. Proc Natl Acad Sci U S A 1999; 96:459-64; PMID:9892655; http://dx.doi.org/ 10.1073/pnas.96.2.459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Eshete M, Marchbank MT, Deutscher SL, Sproat B, Leszczynska G, Malkiewicz A, Agris PF. Specificity of phage display selected peptides for modified anticodon stem and loop domains of tRNA. Protein J 2007; 26:61-73; PMID:17237992; http://dx.doi.org/ 10.1007/s10930-006-9046-z [DOI] [PubMed] [Google Scholar]
- [75].Graham WD, Barley-Maloney L, Stark CJ, Kaur A, Stolarchuk C, Sproat B, Leszczynska G, Malkiewicz A, Safwat N, Mucha P, et al.. Functional recognition of the modified human tRNALys3(UUU) anticodon domain by HIV's nucleocapsid protein and a peptide mimic. J Mol Biol 2011; 410:698-715; PMID:21762809; http://dx.doi.org/ 10.1016/j.jmb.2011.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Spears JL, Xiao X, Hall CK, Agris PF. Amino acid signature enables proteins to recognize modified tRNA. Biochemistry 2014; 53:1125-33; PMID:24483944; http://dx.doi.org/ 10.1021/bi401174h [DOI] [PMC free article] [PubMed] [Google Scholar]


